Abstract
This study was conducted to investigate the effect of supplementing solubles from steam-exploded pine particles (SSPP) on mitigating the adverse effects of cyclic heat stress (CHS) in broilers which were distributed into 3 dietary treatment groups and 2 temperature conditions. Heat stress (HS) exposure for 6 h daily for 7 d adversely affected performance parameters and rectal temperature of chickens. The absolute and relative weights of the liver and bursa of Fabricius decreased in the CHS group while the relative lengths of the jejunum and ileum increased, which was rescued by dietary supplementation with SSPP. The expression of mucin2 (MUC2) and occludin (OCLN) genes was decreased in CHS birds. The expression of heat shock protein -70 and -90 increased in 0% HS compared to that in 0% NT. Birds supplemented with 0.4% SSPP had higher NADPH oxidase -1 expression than birds in the 0% and 0.1% SSPP treatments. Beta diversity of gut microbiota evaluated through unweighted UniFrac distances was significantly different among treatments. Bacteroidetes was among the 2 most abundant phyla in the cecum, which decreased with 0.1% NT and increased with 0.1% HS in comparison to 0% NT. A total of 13 genera were modified by HS, 5 were altered by dose, and nine showed an interaction effect. In conclusion, CHS adversely affects performance and gut health which can be mitigated with dietary SSPP supplementation that modifies the cecal microbiota in broilers.
Key words: broiler, cecum microbiota, cyclic heat stress, gene expression, solubles from steam-exploded pine particles
INTRODUCTION
Global warming has had a significant impact on livestock production with continuous increase in environmental temperature negatively influencing domesticated animals reared for meat production. Meat-producing broilers constitute a major proportion of the poultry sector in the livestock industry and are strongly affected by heat stress (HS) conditions, leading to retarded growth, challenged immunity, and enhanced mortality rate (Awad, et al., 2020). Previous studies have suggested the beneficial role of dietary feed additives in mitigating the adverse effects of HS (Wang, et al., 2018a; Mohammed, et al., 2019). Agricultural and industrial by-products, which can be problematic due to accumulation, can be used as feed additives in the livestock industry. Utilization of wood waste to prepare feed additives can provide low-cost nutrient sources and solve the problem of waste accumulation (Goel, et al., 2021a). In particular, for countries that are solely dependent on the import of animal feed constituents, the benefits of employing such waste could be overwhelmingly large.
In general, plant-derived ingredients are rich in fiber, with wood powder containing both lignin and hemicellulose. Other constituents in wood that may help modify the growth performance, microbiota, and antioxidant status of challenged farm animals are phenolic compounds (Ahmed, et al., 2013). The processing of wood waste by heat treatment could be beneficial for increasing its utility in terms of nutrient content by depolymerizing lignin and hemicellulose into soluble monomers and oligomers (Li, et al., 2007; Aarum, et al., 2018). Recently, steam-exploded pine particles (SPP) were prepared to improve their physical properties by breaking the glycosidic and hydrogen bonds in the fiber and enhancing the release of reducing sugars by up to 73%, thus, decreasing the antinutritional factors (Wang, et al., 2019; Jung, et al., 2022). SPP produced in this way has been used in poultry feed (Goel, et al., 2021a) as soluble dietary fiber has better fermentability and solubility than insoluble fiber (Chen, et al., 2019; Moczkowska, et al., 2019). The importance of solubles from shredded, steam-exploded pine particles (SSPP) in chicken feeding has been greatly enhanced as little information is available on the use of SSPP in livestock feeding.
Nutrient absorption occurs in different parts of the gut. The surface area of the jejunum increases to enhance the nutrient uptake (Alyileili, et al., 2020) while the cecum plays an important role in the breakdown of fibrous substances due to anaerobic fermentation. HS adversely affects gut health by modifying the morphology, permeability, and gut microbiota of chickens (Shi, et al., 2019; Nanto-Hara, et al., 2020).
The role of SPP in modulating the proliferation of fibrolytic bacteria has already been elucidated (Goel, et al., 2021a). But the use of SSPP for feeding chickens has not been explored. Furthermore, its role in broiler chickens exposed to chronic cyclic heat stress (CHS) is yet to be explained. The present study was conducted to evaluate the effect of SSPP-supplemented chicken diets on performance parameters, gene expression profiling, and cecum microbiota in broiler chickens exposed to CHS for 7 d. We hypothesized that when broilers are exposed to chronic cyclic HS, dietary supplementation with SSPP may modify gut health and development by fueling the beneficial microbiota in the cecum and modifying the gut length to develop heat resistance.
MATERIALS AND METHODS
The present experiment was conducted at the research facility of Gyeongsang National University, Korea. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU-200916-C0057).
Preparation of Solubles From Shredded, Steam-Exploded Pine Particles (SSPP)
SSPP was prepared as previously described (Goel, et al., 2022a). Briefly, pinewood chips of approximately 2 × 2 × 0.5 cm3 were exploded with steam at 200°C for 11.5 min. The produced particles were used in our previous studies (Goel, et al., 2021a; Goel, et al., 2021b). In this study, we prepared water extract of SSPP by mixing these particles with water at a ratio of 46:100 (water to substance, v/w) and extracted at 80°C for 213 min. This extract was then stored at 4°C until use after filtering it with Whatman filter paper, grade 2 (Z177601, Sigma-Aldrich Inc., Seoul, Korea). The SSPP thus produced contained approximately 9.2% acid-insoluble lignin, 4.9% phenolic compounds, and 75.2% carbohydrates.
Experimental Birds and Housing
On the eighth day of age, Ross 308 broiler chicks (n = 180) with similar body weights were distributed in the 3 dietary treatment groups. On the 29th day of age, the three groups were exposed to 2 different temperature conditions. Two identical adjoining rooms with automated climate-controlled facilities were prepared to provide different temperature conditions. Both rooms had similar dimensions with no difference in terms of size, temperature, light, ventilation rate, and so on. To normalize the room effects, all 3 treatments were equally distributed in both rooms. Birds were reared in cages. Each room received an equal number of birds, with 3 dietary treatment groups containing 6 replicates per treatment of 5 birds each. Birds were fed with grower and finisher feed (Supplementary Table 1) after adding 0%, 0.1%, and 0.4% SSPP from the 8th day to the 36th d of age. Until the 28th d of age, the temperature of the rooms was maintained as per the recommendation of the Ross 308 broiler guide. On the 29th d of age, the CHS experiment was initiated after weighing each bird. The temperatures of the two rooms were either maintained at the recommended thermoneutral temperature (NT) (21°C) or gradually increased for providing CHS conditions (Figure 1). The heat treatment started from 9:00 AM with an increment of approximately 3°C/h to reach 31°C within the first 3 h and was maintained thereafter for another 3 h before it was brought back to a thermoneutral temperature. Birds were reared for 7 d until the 35th d of age. Feed and water were provided ad libitum. Average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were calculated based on the data recorded before and after the experiment in each cage, and mortality was recorded daily.
Figure 1.
The temperature of thermoneutral control and heat stress rooms during the period of the experiment.
Rectal Temperature Measurement
Rectal temperature (RT) was recorded using a digital thermometer (HI 91610; Hanna Instruments Inc., Padova, Italy) by inserting the probe 3 cm inside the rectum before and after HS on the final day (35th d of age) of CHS.
Collection of Samples and Measurement
On the 36th d of age, 7 birds from each group were humanely sacrificed to collect blood samples in heparinized vacutainers for plasma processing, which were then stored at −20°C until further processing. The weights of internal organs such as the liver, spleen, and bursa of Fabricius (bursa) were recorded and expressed as absolute and relative to body weights. Similarly, the intestinal length (duodenum, jejunum, ileum, and cecum) was recorded and expressed as absolute and relative measures. The whole cecum and jejunum of each chick were snap-frozen in liquid nitrogen for metagenomic and gene expression studies.
Plasma Biochemical Analysis
Blood components such as glucose, total protein, triglycerides, and total cholesterol were determined in the plasma samples using a VetTest Chemistry Analyzer (IDEXX Co., Ltd., Westbrook, ME) with dry-slide technology. Briefly, slides (IDEXX Co., Ltd., Westbrook, ME) for each biochemical parameter were installed in the analyzer, and the plasma samples were loaded through an automated pipettor. The results were then recorded.
Quantitative Real-time PCR (qPCR)
The total RNA of jejunum samples was extracted using TRIzol reagent (Thermo Fisher Scientific, Massachusetts, Waltham, MA) following the manufacturer's instructions. The concentration and purity of the RNA samples were determined using a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA). Each RNA sample was reverse-transcribed using the Verso cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer's instructions. Differential gene expression analysis (Supplementary Table 2) was performed using StepOnePlus real-time PCR systems (Life Technologies, Carlsbad, CA) as follows: 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The 20 μL of RT-PCR reaction contained Power SYBR green PCR master mix (Life Technologies, Carlsbad, CA), 10 pmol concentration of forward and reverse primers specific for a particular gene, and cDNA. The geometric mean of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin were used as housekeeping genes for normalization. The relative expression was determined using the 2−ΔΔct algorithm (Livak and Schmittgen, 2001).
DNA Extraction and Sequencing
Total genomic DNA was extracted from the cecal samples using a DNeasyPowerSoil Kit (Qiagen, Hilden, Germany). These were quantified using a Quant-IT PicoGreen (Invitrogen, Waltham, MA). The metagenome was estimated by constructing a 16S metagenomic sequencing library using a Herculase II Fusion DNA Polymerase Nextera XT Index Kit V2 (Illumina, San Diego, CA). The Illumina platform was used to sequence the library at Macrogen, Inc. (Seoul, Korea). Quality profiling, adapter trimming, and read filtering were performed by creating FASTQ files followed by assembling paired-end reads into one sequence using the fastp program and FLASH (v1.2.11) software (Magoč and Salzberg, 2011; Chen, et al., 2018). The CD-HIT-EST and BLAST+ (v2.9.0) programs were used to determine the number of operational taxonomic units (OTUs) and their similarity to the reference database (NCBI 16S Microbial) (Zhang, et al., 2000; Li, et al., 2012). OTU abundance and taxonomic information were determined using the QIIME (v1.9) software. Alpha diversity was determined using OTU, Chao1, Shannon, Goods Coverage, and Inverse Simpson Indices. Beta diversity was evaluated using unweighted/weighted UniFrac distance.
Statistical Analysis
Analysis was performed using a completely randomized design. The data were analyzed using the general linear model (GLM) procedure of 2-way ANOVA using IBM SPSS statistics software package 25. 0 (IBM software, Chicago, IL). Each cage was treated as an experimental unit for growth studies, whereas an individual bird was treated as the experimental unit for organ weight, length, and body temperature. All data are expressed as the mean ± SEM. Duncan's multiple-range test was used to determine whether an interaction existed. Differences were considered statistically significant at P < 0. 05. Alpha-diversity (community richness and diversity) and taxonomic analysis (phylum and genus) were analyzed using the Scheirer Ray Hare test, and the Dunn test was used when P values were significant. The “Rcompanion” package of the R software version 4.0.3 (R Core Team, 2020) was used. PERMANOVA was performed to analyze beta diversity. The correlation among microbial communities was determined using the igraph package of the Rsoftware and is presented as a network analysis. Graphs were prepared using Prism-GraphPad software.
RESULTS
Broiler Performance
Table 1 presents the effect of different doses of SSPP in diets on the growth performance parameters of birds kept in either NT or HS. The initial body weight (IBW) before starting the CHS experiment was similar in all the treatment groups. However, after 7 d of CHS, a temperature effect was observed and birds exposed to a higher temperature (HS) had lower (P = 0.001) final body weight (FBW) and percent difference in body weight (PDBW) in comparison to birds kept at NT. Similarly, growth parameters such as ADG, ADFI, and FCR also showed a temperature effect; ADG and ADFI were decreased (P = 0.001) while FCR was increased (P = 0.001) in CHS birds in comparison to NT. No interaction or dose effect was observed in the ADG, ADFI, and FCR of chickens fed with increasing concentrations of SSPP in the diets (Table 1).
Table 1.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the growth performance parameters of thermoneutral and cyclic heat-stressed broiler chickens.
| IBW (g) | FBW (g) | % difference in BW | ADG | ADFI | FCR | ||
|---|---|---|---|---|---|---|---|
| 0% | NT | 1672 ± 27 | 2416 ± 26 | 44.62 ± 1.15 | 106.4 ± 1.6 | 182.4 ± 2.0 | 1.72 ± 0.02 |
| HS | 1660 ± 30 | 2192 ± 33 | 32.24 ± 3.24 | 80.5 ± 5.5 | 162.3 ± 5.7 | 2.04 ± 0.09 | |
| 0.10% | NT | 1710 ± 24 | 2477 ± 36 | 44.87 ± 0.61 | 109.6 ± 2.1 | 184.9 ± 3.3 | 1.69 ± 0.01 |
| HS | 1720 ± 17 | 2283 ± 42 | 32.78 ± 2.08 | 85.2 ± 3.9 | 167.8 ± 3.3 | 1.98 ± 0.07 | |
| 0.40% | NT | 1680 ± 5 | 2409 ± 15 | 43.37 ± 0.69 | 104.1 ± 1.8 | 183.3 ± 3.7 | 1.76 ± 0.03 |
| HS | 1684 ± 30 | 2242 ± 54 | 33.08 ± 2.08 | 84.4 ± 2.7 | 167.0 ± 4.3 | 1.98 ± 0.04 | |
| Main effects | |||||||
| Temp | NT | 1687 ± 12 | 2434 ± 16 | 44.3 ± 0.5 | 106.7 ± 1.1 | 183.5 ± 1.7 | 1.72 ± 0.01 |
| HS | 1688 ± 15 | 2239 ± 26 | 32.7 ± 1.4 | 83.4 ± 2.3 | 165.7 ± 2.5 | 2.00 ± 0.04 | |
| Dose | 0% | 1666 ± 19 | 2304 ± 39 | 38.4 ± 2.5 | 93.4 ± 4.8 | 172.3 ± 4.2 | 1.88 ± 0.07 |
| 0.1% | 1715 ± 14 | 2380 ± 39 | 38.8 ± 2.1 | 97.4 ± 4.2 | 176.3 ± 3.4 | 1.84 ± 0.06 | |
| 0.4% | 1682 ± 15 | 2325 ± 37 | 38.2 ± 1.9 | 94.3 ± 3.4 | 175.2 ± 3.6 | 1.87 ± 0.04 | |
| p values | |||||||
| Temp. | 0.965 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Dose | 0.129 | 0.116 | 0.949 | 0.444 | 0.572 | 0.682 | |
| Temp. * Dose | 0.901 | 0.739 | 0.837 | 0.618 | 0.874 | 0.593 | |
Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0 ◦C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Data show mean ± SEM (n = 6).
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; FBW, final body weight; FCR, feed conversion ratio; HS, heat stress; IBW, initial body weight; NT, normal temperature; Temp, temperature.
Rectal Temperature
RT was recorded before and after HS on the final day of the experiment (Table 2). Birds exposed to CHS had a lower (P = 0.001) RT than NT birds before heat exposure on the final day of the CHS experiment. However, the RT after HS on the same day and ΔT was higher (P = 0.001) in CHS birds than in NT birds. No dose or interaction effects were observed in terms of RT when birds were fed different doses of SSPP and reared under NT and CHS conditions (Table 2).
Table 2.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the rectal temperature of thermoneutral and cyclic heat-stressed broiler chickens.
| Rectal temperature |
||||
|---|---|---|---|---|
| Before HS | After HS | ΔT | ||
| 0% | NT | 41.30 ± 0.09 | 41.25 ± 0.15 | −0.05 ± 0.15 |
| HS | 41.10 ± 0.06 | 42.83 ± 0.18 | 1.73 ± 0.22 | |
| 0.1% | NT | 41.35 ± 0.08 | 41.15 ± 0.09 | −0.20 ± 0.07 |
| HS | 41.15 ± 0.04 | 42.88 ± 0.20 | 1.73 ± 0.19 | |
| 0.4% | NT | 41.30 ± 0.09 | 41.25 ± 0.11 | −0.05 ± 0.14 |
| HS | 41.07 ± 0.03 | 42.60 ± 0.10 | 1.53 ± 0.13 | |
| Main effects | ||||
| Temp | NT | 41.32 ± 0.05 | 41.22 ± 0.07 | −0.10 ± 0.07 |
| HS | 41.11 ± 0.03 | 42.77 ± 0.10 | 1.67 ± 0.10 | |
| Dose | 0% | 41.20 ± 0.06 | 42.04 ± 0.26 | 0.84 ± 0.30 |
| 0.1% | 41.25 ± 0.05 | 42.02 ± 0.28 | 0.77 ± 0.31 | |
| 0.4% | 41.18 ± 0.06 | 41.93 ± 0.22 | 0.74 ± 0.25 | |
| p values | ||||
| Temp | 0.001 | 0.001 | 0.001 | |
| Dose | 0.610 | 0.702 | 0.805 | |
| Temp * Dose | 0.962 | 0.422 | 0.544 | |
Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Data show mean ± SEM (n = 6). Abbreviations: HS, heat stress; NT, normal temperature; Temp, temperature.
Organ Weight and Length
Absolute and relative organ weights are shown in Supplementary Table 3. No dose-dependent effect was observed in the absolute and relative liver and bursa weights on the final day of the CHS experiment. However, a temperature effect was observed in both measures for the 2 organs, indicating higher (P = 0.009; P = 0.001) liver weight and lower (P = 0.001; P = 0.005) bursa weight in CHS birds than in birds kept at NT. No interaction, dosage, or temperature effect was observed on the absolute and relative weights of the spleen in chickens (Supplementary Table 3).
The effect of dietary SSPP supplementation on absolute and relative organ length when birds were exposed to NT or CHS conditions is presented in Supplementary Table 4. No temperature, dose, or interaction effects were observed in the absolute length of the different portions of the intestine (duodenum, jejunum, ileum, cecum, and total gut length) of the chickens. Similarly, there were no observable effects on the relative length of the duodenum. However, the relative jejunum length showed a temperature (P = 0.003) and dosage (P = 0.041) effect and was found to be higher in CHS birds than in NT birds. Birds fed 0.4% SSPP had a lower relative jejunum length than those fed the 0% and 0.1% SSPP-supplemented diets. The relative ileum length showed a temperature (P = 0.012) effect and was higher in CHS birds than in NT birds. The relative cecum length showed a dosage effect (P = 0.034) and was decreased by 0.4% SSPP compared to birds fed 0% SSPP. The relative total gut length also showed a temperature (P = 0.007) and dosage (P = 0.034) effect, with the birds exposed to CHS having higher relative total gut length in comparison to birds kept at NT, whereas 0.4% SSPP-supplemented chicks had lower relative total gut length in comparison to birds fed 0% SSPP (Supplementary Table 4).
Plasma Biochemical Parameters
The effects of dietary SSPP supplementation on the biochemical parameters of plasma, such as glucose, total protein, triglyceride, and cholesterol when birds were exposed to NT or HS conditions are presented in Table 3. No temperature effect, dose effect, or interaction (P > 0.05) was observed among the different treatment groups.
Table 3.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the plasma biochemicals of thermoneutral and cyclic heat-stressed broiler chickens.
| Glucose | Total protein | Triglyceride | Cholesterol | ||
|---|---|---|---|---|---|
| 0% | NT | 291.1 ± 14.2 | 3.5 ± 0.2 | 48.9 ± 7.9 | 151.7 ± 10.4 |
| HS | 304.6 ± 15.6 | 4.4 ± 0.2 | 65.7 ± 9.2 | 187.9 ± 8.7 | |
| 0.1% | NT | 306.1 ± 16.1 | 3.8 ± 0.2 | 55.9 ± 9.1 | 170.1 ± 9.6 |
| HS | 285.3 ± 17.3 | 3.7 ± 0.1 | 58.9 ± 8.2 | 161.6 ± 7.9 | |
| 0.4% | NT | 290.6 ± 24.1 | 3.6 ± 0.2 | 50.7 ± 5.8 | 149.9 ± 8.3 |
| HS | 292.0 ± 23.6 | 3.7 ± 0.3 | 60.7 ± 9.7 | 173.4 ± 16.1 | |
| Main effects | |||||
| Temp | NT | 296.0 ± 10.3 | 3.6 ± 0.1 | 51.8 ± 4.3 | 157.2 ± 5.6 |
| HS | 294.0 ± 10.6 | 3.9 ± 0.1 | 61.8 ± 5.0 | 174.3 ± 6.8 | |
| Dose | 0% | 297.9 ± 10.3 | 3.9 ± 0.2 | 57.3 ± 6.3 | 169.8 ± 8.2 |
| 0.1% | 295.7 ± 11.7 | 3.7 ± 0.1 | 57.4 ± 5.9 | 165.9 ± 6.1 | |
| 0.4% | 291.3 ± 16.2 | 3.6 ± 0.2 | 55.7 ± 5.6 | 161.6 ± 9.3 | |
| p values | |||||
| Temp | 0.898 | 0.132 | 0.156 | 0.055 | |
| Dose | 0.939 | 0.393 | 0.976 | 0.744 | |
| Temp * Dose | 0.658 | 0.128 | 0.715 | 0.106 | |
Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Data show mean ± SEM (n = 7).
Abbreviations: HS, heat stress; NT, normal temperature; Temp, temperature.
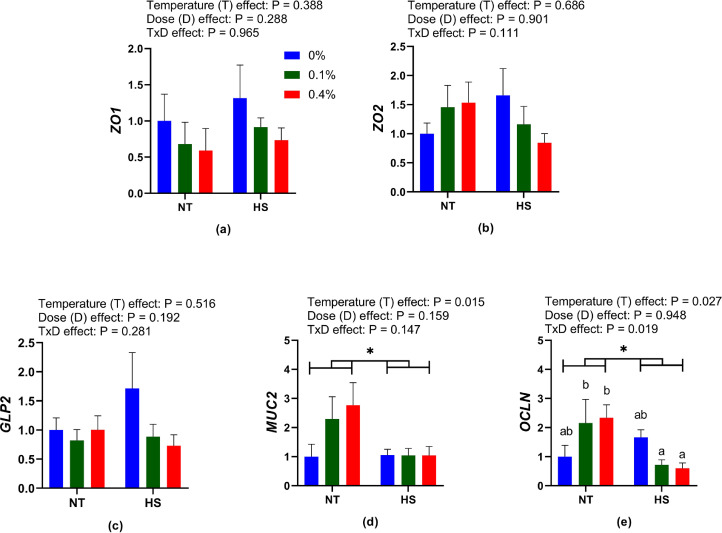
Gene Expression
The expression of the gut health-related genes is shown in Figure 2. No temperature, dose, or interaction effects were observed in the expression of the zona occludens (ZO) 1 and -2, and glucagon-like peptide (GLP) 2 genes when birds were supplemented with dietary SSPP and exposed to NT or HS. The expression of mucin2 (MUC2) and occludin (OCLN) genes showed a temperature effect and was found to be lower (P < 0.05) in CHS birds than in NT birds. The expression of the OCLN gene showed a nonsignificant increasing trend (P > 0.05) in 0.1% NT and 0.4% NT compared to 0% NT but decreased significantly (P < 0.05) in 0.1% HS and 0.4% HS compared to 0.1% NT and 0.4% NT (Figure 2).
Figure 2.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the expression of intestinal health-related genes (a) ZO1 (b) ZO2 (c) GLP2 (d) MUC2 and (e) OCLN respectively, in the jejunum of broilers reared under normal or cyclic heat stress conditions. Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Data show mean ± SEM (n = 7). Abbreviations: HS, heat stress; NT, normal temperature.
Figure 3 shows the expression of stress-related genes in chickens. The expression of heat shock protein (HSP) -70 and -90 genes was significantly increased (P < 0.05) in 0% HS compared to that in 0% NT. No temperature or dose effects were observed on the expression of HSP70 and -90 genes. The expression of the NADPH oxidase (NOX) 1 gene showed a dose-dependent effect and was found to be significantly increased (P < 0.05) by 0.4% in comparison to 0% and 0.1% SSPP-supplemented birds. No temperature, dose, or interaction effects were observed in the expression of NOX4, superoxide dismutase (SOD), and catalase (CAT) genes when broilers were supplemented with SSPP in diets and exposed to NT or HS.
Figure 3.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the expression of stress-related genes (a) HSP70 (b) HSP90 (c) NOX1 (d) NOX4 (e) CAT and (f) SOD respectively, in the jejunum of broilers reared under normal or cyclic heat stress conditions. Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Data show mean ± SEM (n = 7). Abbreviations: NT, normal temperature; HS, heat stress.
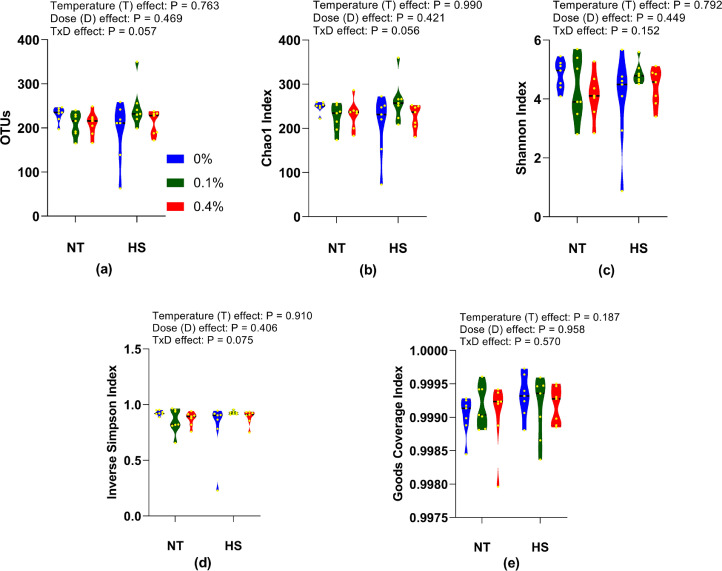
Cecum Microbiota
Various alpha-diversity indices such as OTUs, Chao1, Shannon, Inverse Simpson, and Good's Coverage were determined to evaluate the community richness and diversity in the cecum microbiome of the birds (Figure 4). No temperature, dose, or interaction effects (P > 0.05) were observed in the alpha diversity indices. Beta diversity was determined using unweighted and weighted UniFrac distance. The unweighted UniFrac distances were significantly different (P < 0.05) among the treatment groups. No significant variations (P > 0.05) were observed in weighted UniFrac distances (Figure 5).
Figure 4.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the alpha-diversity indices representing (a) OTUs (b) Chao1 index (c) Shannon index (d) Inverse Simpson index, and (e) Goods Coverage index in the cecum of broilers reared under normal or cyclic heat stress conditions. Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. The number of samples (n = 7). Abbreviations: HS, heat stress; NT, normal temperature.
Figure 5.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the beta diversity representing (a) Unweighted and (b) Weighted UniFrac distances in the cecum of broilers reared under normal or cyclic heat stress conditions. Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Red filled circle; 0% NT, blue filled triangle; 0.1% NT, green filled square; 0.4% NT, purple filled diamond; 0% HS, orange empty circle; 0.1% HS and yellow empty triangle; 0.4% HS. The number of samples (n = 7). Abbreviations: HS, heat stress; NT, normal temperature.
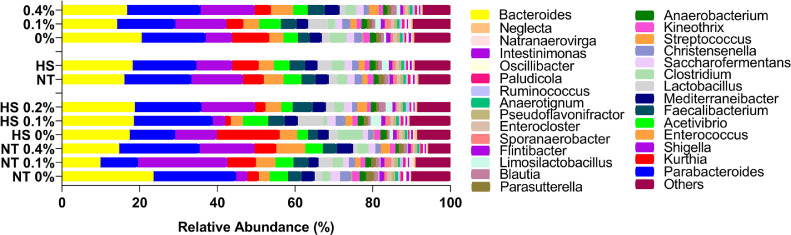
The cecum microbiota in terms of bacterial phyla is presented in Figure 6. Firmicutes and Bacteroidetes were the dominant phyla in chicken cecum. The abundance of Bacteroidetes was significantly lower in 0.1% NT group than in the 0% NT group. Furthermore, the abundance of Bacteroidetes was recovered and found to be higher in 0.1% HS than in 0.1% NT. No temperature, dose, or interaction effects were observed for the other bacterial phyla present in the cecum of dietary SSPP-supplemented birds exposed to NT or HS.
Figure 6.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the (a) relative abundance of phylum and (b) Bacteroidetes abundance (significantly modified phylum) in the cecum of broilers reared under normal or cyclic heat stress conditions. Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. The number of samples (n = 7). Abbreviations: HS, heat stress; NT, normal temperature.
The 30 most abundant bacterial genera present in the cecum of dietary SSPP-supplemented birds when they were exposed to NT or CHS are presented in Figure 7. Bacteroides and Parabacteroides were the most abundant genera in the cecum. The abundance of Bacteroides was lower in SSPP-supplemented birds (0.1% SSPP: 14.1%; 0.4% SSPP: 16.7%) than in 0% SSPP-supplemented birds (20.4%).
Figure 7.
Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the relative abundance of the genus in the cecum of broilers reared under normal or cyclic heat stress conditions. Chickens were fed diets containing 0% (control), 0.1%, and 0.4% solubles from shredded, steam-exploded pine particles from the 8th day to the 35th day of age. From 28th day to the 35th day of age, birds were either kept at a thermoneutral temperature (21.0°C) or exposed to cyclic heat stress at 31.0°C for 6 h daily. Data show mean (n = 7). Abbreviations: HS, heat stress; NT, normal temperature.
The abundance of the bacterial genera Rothia, Subdoligranulum, Desulfitobacterium, Leptotrichia, Porphyromonas, and Parasutterella decreased, while that of Pseudathrobacter, Prevotella, Limosilactobacillus, Frigidibacter, Undibacterium, Gallionella, and Pseudomonas increased under CHS (Figures S1 and S4). Streptococcus had a dose-dependent increase with the highest abundance in 0.4% SSPP. Sphingomonas and Desulfonispora increased by 0.1% compared to 0% and 0.4% SSPP. Escherichia abundance increased with 0.4% SSPP compared to 0% and 0.1% SSPP. The abundance of Porphyromonas was higher at 0% SSPP than at 0.4% SSPP (Figures S2 and S4). Mycolicibacterium and Sphingomonas increased by 0.1% HS, whereas Escherichia increased by 0.4% NT, compared to all other treatments. Anaerobium, Lachnoclostridium, and Oscillibacter decreased in 0% HS compared with that in 0% NT. Anaerobium was also decreased by 0.4% NT compared with that of 0% NT. The abundance of Acutalibacter was lower in 0.4% NT than in 0% NT and 0.4% HS while that of Turicibacter was higher in the 0.1% HS group than in the 0.1% NT group. Parasutterella increased in abundance with 0.1% HS compared to 0% HS, whereas it decreased in 0% HS compared to 0% NT, 0.1% NT, and 0.4% NT, and decreased in 0.4% HS compared to 0% NT (Figures S3 and S4).
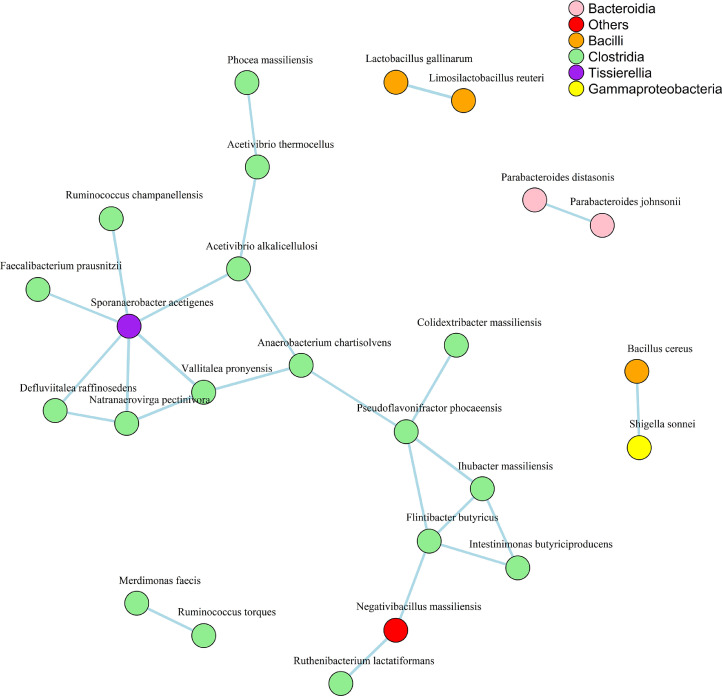
The correlation network analysis presented in Figure 8 revealed that the bacterial species Sporanaerobacter acetigenes from the class Tissierellia and Negativibacillus massiliensis from others were positively correlated with the species of class Clostridia. Similarly, Shigella sonnei from the class Gammaprotobacteria was positively correlated with Bacillus cereus from the class Bacilli. In addition, most species were positively correlated within the same class. No negative correlation was observed when the correlation coefficient was 0.7.
Figure 8.
The network pattern of cecum microbiota at class and species levels in broiler chickens following spearman correlation. The blue lines among nodes stand for positive correlations and the thickness of lines corresponds to correlation coefficients taken as 0.7. The color of the circle indicates class and each circle independent of color represent species.
DISCUSSION
Broilers are a favorable source of meat and play an essential role in protein production, which may be affected by increasing farm temperatures owing to global warming (Quinteiro-Filho, et al., 2010; Awad, et al., 2020). In the present study, CHS negatively affected parameters such as FBW, PDBW, ADG, ADFI, and FCR. Performance parameters may be correlated with respiration rate and panting in chickens. The panting behavior increases with temperature (Kang, et al., 2020). Higher respiration rates keep the bird away from being fed, resulting in a decrease in ADFI and a further reduction in body weight and related parameters. The absence of negative effects on the growth performance of broilers supplemented with SSPP in the present study is a positive sign of its use in poultry feed.
RT has been effectively used as an indicator of HS in chickens (Chen, et al., 2013). On the final day of CHS experiment, the RT was lower before heat exposure but increased when birds were exposed to CHS. Previous studies have also suggested an increase in RT when birds were exposed to acute or chronic HS (Barrett, et al., 2019; Goel, et al., 2021b). Continuous heat exposure initially increases RT and stabilizes thereafter, indicating adaptation (Chen, et al., 2013). Thus, the lower RT in the CHS birds compared to NT on the final day of CHS experiment before heat exposure might be due to an adaptation to develop heat tolerance.
The liver is an important organ for metabolism and hemostasis under stressful conditions (Emami, et al., 2020) while the bursa is an important immune organ that may play a role in gut barrier dysfunction in HS. In the present study, the absolute and relative weights of the liver increased, while those of the bursa decreased under CHS. This corroborates previous studies where liver weight increased and bursa weight decreased in HS birds (Quinteiro-Filho, et al., 2010; Chegini, et al., 2018; Lu, et al., 2019a). This could be due to the increase in glycogenolysis, gluconeogenesis, glycosylation, glutathione production, and fat deposition in the liver and enhanced lesion scores leading to atrophy and bursa damage in heat-exposed birds (Pamok, et al., 2009; Jastrebski, et al., 2017).
In general, the small intestine is responsible for the digestion and absorption of nutrients in chickens. Reduced feed intake is one of the implications of HS (Awad, et al., 2020). Adaptive responses occur in a living system to counter adverse situations. Thus, an increase in relative intestinal length is expected to maintain nutrient absorption and digestibility during feed deprivation. In the present study, the relative lengths of the jejunum and ileum increased in CHS birds with lower FI. Furthermore, enhanced simple sugar due to exogenous enzyme supplementation is associated with lower small intestine and cecum relative length in chickens (Wu, et al., 2004; Zhang, et al., 2014). A decrease in the jejunum and cecum length of birds fed 0.4% SSPP compared to those fed 0% and 0.1% SSPP could be attributed to the availability of higher simple sugars in the SSPP-supplemented diets.
The ZO family is responsible for the connection between tight junctions and the cytoskeleton while occludin mediates the binding between two epithelial cells by sealing the paracellular space (Ulluwishewa, et al., 2011). Previous studies have reported no variation in the ZO1 gene expression after 24 and 72 h of HS in broilers (Uerlings, et al., 2018) which correlates with our findings. This could be due to the lower severity of heat exposure and comparatively stronger binding between tight junctions and the cytoskeleton. Furthermore, in the present study, the expression of MUC2 and OCLN genes decreased in CHS birds. This is in agreement with previous studies, where the jejunal expression of OCLN and MUC2 was decreased in HS broilers (Song, et al., 2014; Liu, et al., 2022). Mucin protects the intestine from pathogenic penetration (Forder, et al., 2012). A decrease in both the genes indicates the dysfunction of the tight junction present between the paracellular space of the epithelium, whcih acts as the first line of defense that may further lead to pathogenic invasion. GLP family-related genes are linked with appetite and its administration results in the suppression of food intake (Dalvi and Belsham, 2012). It was expected that the decrease in ADFI might be related to GLP2. However, in the present study, no significant variation was observed in the expression of GLP2 in CHS chickens. Previous studies have also suggested that GLP levels in the jejunum, ileum, and serum were similar in fasted and heat-exposed chickens (Gilani, et al., 2018; Wang, et al., 2021). This could be due to the adaptation of birds to CHS as they were exposed to heat for 6 h daily and returned to thermoneutral temperatures for the rest of the day leading to recovery. A decrease in feed intake might be due to panting behavior under HS, which keeps the birds away from feed, ultimately adversely affecting performance parameters.
Genes related to the HSP family act as markers under HS as their expression increases under stress conditions (Chegini, et al., 2018; Roushdy, et al., 2018). The expression of HSP-related genes was significantly higher in 0% HS than in 0% NT. The beneficial effect of dietary SSPP has been observed in heat-exposed birds, as the expression of HSP-related genes showed a decreasing trend (P > 0.05) in comparison to non-SSPP-supplemented diets. Members of the NOX family are linked to superoxide and reactive oxygen species (ROS) generation (Katsuyama, 2010). The expression of NOX family-related genes increases under HS (Kikusato, et al., 2015). Antioxidant-related genes play crucial roles in the control of ROS production. The expression of antioxidant genes is modulated under HS (Roushdy, et al., 2018). In the present study, the expression of the NOX1 was higher in the 0.4% SSPP group than in the 0% and 0.1% SSPP-supplemented birds. Previous studies conducted on dietary supplementation with extracts prepared using steam also reported enhanced expression of NOX family related genes in chickens (Mavrommatis, et al., 2021). Thus, it can be concluded that the enhanced expression of NOX1 could be related to dietary intervention using the steam explosion method. An increase in the expression of NOX family-related genes might be associated with adaptation to withstand a higher level of ROS (Goel, et al., 2021c). Consequently, CHS did not affect the expression of NOX or antioxidant-related genes.
The intestine is crucial for nutrient digestion which is partly supported by beneficial microorganisms colonizing the gut for optimal growth and health of birds. Many factors influence the chicken microbiota. For instance, environmental factors such as enhanced temperature modulate the gut microbiota in chickens (Wang, et al., 2018b). The type of HS, intensity of temperature, and time of exposure are relatively important to depict the adverse effects on intestinal health. At 31°C, short-term acute HS for 6 h had no effect, but long-term heat exposure for 14 d significantly modified alpha-diversity indices in broilers (Wang, et al., 2018b; Goel, et al., 2021b). No significant variation was observed in the alpha-diversity indices in this study. The discrepancy in the results might be attributed to the shorter period of HS imposed in the cyclic form for seven days. In terms of beta diversity, the unweighted UniFrac distances that considered the presence and absence of microbial species were significantly different among the treatment groups. This might be due to the presence of beneficial bacteria in SSPP-supplemented diets. The increased presence of pathogenic bacteria in CHS birds due to heat exposure can not be ignored.
Firmicutes and Bacteroidetes were the major phyla identified in the chicken cecum. This is in agreement with previous studies (Goel, et al., 2021b; Goel, et al., 2022b). Bacteroidetes grow on polysaccharides and their abundance increases in heat-exposed chickens (McBride, et al., 2009; Wang, et al., 2020). The increase in the abundance of Bacteroidetes in 0.1% HS compared with 0.1% NT in this study might be attributed to the effect of HS.
Bacteroides and Parabacteroides were the most abundant bacterial genera in the cecum. This is consistent with the results of previous studies (Saati‐Santamaría, et al., 2022). Rothia is a frequently detected genus, whereas Leptotrichia is dominant in chicken with low FCR (Shah, et al., 2019; Kursa, et al., 2022). Subdoligranulum is a butyrate-producing genus with probiotic activity in chickens (Polansky, et al., 2015). Desulfitobacteria grow on decomposition products of lignin (Mingo, et al., 2014). A decrease in the abundance of Rothia, Leptotrichia, Subdoligranulum, and Desulfitobacteria in CHS birds indicates poor gut health and can be correlated with reduced performance parameters including feed intake and FCR in chickens. The absence of Desulfitobacteria in the 0% HS could be due to the lack of lignin, which requires further investigations.
Limosilactobacillus reuteri has probiotic activity and a protective effect in heat-exposed birds (Mohammed, et al., 2019). In the present study, its abundance was significantly increased in CHS birds, similar to our previous study, where we reported an increase in the abundance of Limosilactobacillus in heat-exposed birds supplemented with steam-exploded pine particles (Goel, et al., 2021b). Although not significant, the numerically higher abundance of Limosilactobacillus in heat-exposed SSPP-supplemented birds might be due to the same source of pine wood used in the preparation of SSPP.
Prevotella and Undibacterium are associated with infection and low body weight in chickens (Falagas and Siakavellas, 2000; Zhang, et al., 2022). An increase in the abundance of Prevotella and Undibacterium in CHS birds indicates the adverse effect of heat exposure, which was also correlated with a decrease in body weight in the present study.
Pseudarthrobacter is a cold-resistant bacterial genus that is least abundant in the gut and is present in chicken meat treated with chilled air (Chen, et al., 2020). Its enhanced abundance in the cecum of heat exposed birds is unclear. Pseudomonas is a multidrug-resistant bacterial genus whose prevalence is extensively related to the spoilage of chicken meat (Lee, et al., 2017). A higher abundance of Pseudomonas in the cecum of CHS birds enhanced the chances of cross-contamination of the carcass leading to a higher risk of poor carcass quality and reduced shelf life of chicken meat.
Streptococcus can degrade fibers and beta-glucans (Beckmann, et al., 2006). An increase in its abundance was observed in the SSPP-supplemented diet (0.1% and 0.4%) compared with that in control (0% SSPP). Previous studies have also reported an increased Streptococcus abundance in high-fiber-containing diets (Venardou, et al., 2021). The dose-dependent increase in the abundance of Streptococcus in the present study could be attributed to the presence of fibers and lignin in the SSPP.
Desulfonispora ferments organosulfonate to produce acetate and ammonia, which have a detrimental effect on pathogenic microorganisms such as salmonella (Lawhon, et al., 2002; Beckmann, et al., 2006). A higher Desulfonispora abundance in the 0.1% compared to the 0% and 0.4% SSPP-supplemented diets indicated better gut health due to reduced pathogenic loads.
The abundance of genera Lachnoclostridium, Oscillibacter, and Parasutterella were significantly decreased in 0% HS compared to 0% NT. Generally, energy demand increases under HS conditions. The genera Lachnoclostridium and Oscillibacter are responsible for butyrate production for energy (Polansky, et al., 2015; Ríos-Covián, et al., 2016). However, a decrease in their abundance may account for the reduced energy levels in HS chickens. Furthermore, Parasutterella abundance was negatively influenced by CHS, but dietary SSPP supplementation increased its abundance (0.1% HS compared to 0% HS) indicating its role in mitigating the effects of CHS.
In the present study, Turicibacter abundance was increased in HS birds (0% HS compared to 0% NT) but decreased significantly in 0.1% HS compared to 0.1% NT. Enhanced Turicibacter abundance was accompanied by a decrease in MUC2 and OCLN expression, suggesting impaired gut health in CHS birds. Additionally, decreased Turicibacter abundance (0.1% HS compared with 0% NT) could also be related to the lower ADFI in CHS birds. As a result, Turicibacter from the family Turicibacteraceae is inversely correlated with tight junction in mice and feed intake in chickens (Metzler-Zebeli, et al., 2019; Liu, et al., 2020).
Chronic heat stress compromises liver function by enhancing lipid deposition leading to hepatic steatosis (Lu, et al., 2019b). The genus Sphingomonas reduces liver fat by synthesizing sphingosine that forms sphingomyelin with fatty acid derivatives (Li, et al., 2020). Increased Sphingomonas abundance in 0.1% HS indicates that SSPP supplementation may protect the liver from the excessive deposition of fat under HS in chickens.
The abundance of Escherichia increases under HS in chickens (Song, et al., 2013). In the present study, it was absent in CHS birds and was only detected in 0.4% NT showing dose and interaction effects. The reasons for this are not clear and require further investigation.
The genera Frigidibacter, Gallionella, Mycolicibacterium, Anaerobium, Acutalibacter, and Porphyromonas also differ significantly, but the lack of information about their role emphasizes the need for further investigations.
Sporanaerobacter acetigenes from the class Tissierellia utilize sugars, peptides, amino acids, and ferment glucose to produce acetate (Ziganshina, et al., 2014). Faecalibacterium prausnitzii is a butyrate-producing bacterium that modifies goblet cells and mucin to strengthen the mucosal layer and inhibit pathogenic penetration in the gut (Duncan, et al., 2002; Wrzosek, et al., 2013). The exact role of S. acetigenes is yet to be explored, but its positive correlation with F. prausnitzii indicates its beneficial role in the chicken gut. Negativibacillus is positively correlated with the growth performance of chickens (Liu, et al., 2021). Furthermore, the abundance of Ruthenibacterium lactatiformans is higher in small birds than in larger ones (Lundberg, et al., 2021). In contrast, in the present study, N. massiliensis was positively correlated with R. lactatiformans which requires further investigation. S. sonnei and B. cereus cause disease in chickens (Shi, et al., 2014; Tahmasebi, et al., 2014) and were positively correlated with each other in the current study. Two more species from the class Bacilli, namely Lactobacillus gallinarum and Limosilactobacillus reuteri were found to be positively correlated with each other. These species are beneficial bacteria that protect the gut from pathogenic invasion by colonizing intestinal epithelial cells and are thus used as probiotics in chickens (Spivey, et al., 2014; Neveling, et al., 2017; Nii, et al., 2020).
Thus, CHS decreased performance parameters and enhanced RT in broiler chickens. Gut health is negatively influenced by CHS, as indicated by the suppressed expression of intestinal health-related genes (MUC2 and OCLN) and modified cecum microbiota. Dietary supplementation with SSPP helps mitigate CHS effects by proliferating the beneficial bacterial genera in the chicken cecum. Furthermore, the expression of stress-related genes (HSP70 and HSP90) was increased in unsupplemented heat-exposed birds but was similar in SSPP-supplemented heat-exposed birds compared to birds kept at thermoneutral temperatures, confirming the beneficial effects of SSPP.
ACKNOWLEDGMENTS
The authors are thankful to the Korea Forest Service (Korea Forestry Promotion Institute), and the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) for their support.
Funding: This work was supported in part by the Forest Science Technology R&D Program (2020193C10-2022-BA01) provided by Korea Forest Service (Korea Forestry Promotion Institute), and by the Brain Pool Program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (2019H1D3A1A01071142).
Authors contributions: J.K.Y. and Y.H.C. conception and funding. All authors, methodology. A.G. and C.M.N. software. A.G. and Y.H.C. validation and data curation. A.G. writing—original draft preparation. A.G. and Y.H.C. writing—review, and editing. Y.H.C. supervision. All authors read and approved the final manuscript.
Data availability statement: The dataset analyzed in the current study is available from the corresponding author upon reasonable request.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.102498.
Appendix. Supplementary materials
REFERENCES
- Aarum I., Devle H., Ekeberg D., Horn S.J., Stenstrøm Y. Characterization of pseudo-lignin from steam exploded birch. ACS Omega. 2018;3:4924–4931. doi: 10.1021/acsomega.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.T., Hossain M.E., Kim G.M., Hwang J.A., Ji H., Yang C.J. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian-Australas. J. Anim. Sci. 2013;26:683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyileili S.R., El-Tarabily K.A., Belal I.E.H., Ibrahim W.H., Sulaiman M., Hussein A.S. Intestinal development and histomorphometry of broiler chickens fed Trichoderma reesei degraded date seed diets. Front. Vet. Sci. 2020;7:349. doi: 10.3389/fvets.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad E.A., Najaa M., Zulaikha Z.A., Zulkifli I., Soleimani A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian-Australas. J. Anim. Sci. 2020;33:778–787. doi: 10.5713/ajas.19.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N.W., Rowland K., Schmidt C.J., Lamont S.J., Rothschild M.F., Ashwell C.M., Persia M.E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 2019;98:6684–6692. doi: 10.3382/ps/pez541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann L., Simon O., Vahjen W. Isolation and identification of mixed linked beta -glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-beta -glucanase activities. J. Basic Microbiol. 2006;46:175–185. doi: 10.1002/jobm.200510107. [DOI] [PubMed] [Google Scholar]
- Chegini S., Kiani A., Rokni H. Alleviation of thermal and overcrowding stress in finishing broilers by dietary propolis supplementation. Ital. J. Anim. Sci. 2018;17:377–385. [Google Scholar]
- Chen B., Cai Y., Liu T., Huang L., Deng X., Zhao Q., Zhao M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physical-chemical treatments. Food Hydrocoll. 2019;93:167–175. [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.Y., Wei P.P., Xu S.Y., Geng Z.Y., Jiang R.S. Rectal temperature as an indicator for heat tolerance in chickens. Anim. Sci. J. 2013;84:737–739. doi: 10.1111/asj.12064. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang J., Yu L., Xu T., Zhu N. Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci. Rep. 2020;10:5382. doi: 10.1038/s41598-020-60135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi P.S., Belsham D.D. Glucagon-like peptide-2 directly regulates hypothalamic neurons expressing neuropeptides linked to appetite control in vivo and in vitro. Endocrinology. 2012;153:2385–2397. doi: 10.1210/en.2011-2089. [DOI] [PubMed] [Google Scholar]
- Duncan S.H., Hold G.L., Harmsen H.J.M., Stewart C.S., Flint H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M.E., Siakavellas E. Bacteroides, Prevotella, and Porphyromonas species: a review of antibiotic resistance and therapeutic options. Int. J. Antimicrob. Agents. 2000;15:1–9. doi: 10.1016/s0924-8579(99)00164-8. [DOI] [PubMed] [Google Scholar]
- Forder R.E., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Nattrass G., Kitessa S.M., Barekatain R., Forder R.E.A., Tran C.D., Hughes R.J. Gene expression and morphological changes in the intestinal mucosa associated with increased permeability induced by short-term fasting in chickens. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:e653–e661. doi: 10.1111/jpn.12808. [DOI] [PubMed] [Google Scholar]
- Goel A., Kim B.-J., Ncho C.-M., Jeong C.-M., Gupta V., Jung J.-Y., Ha S.-Y., Lee D.-H., Yang J.-K., Choi Y.-H. Utilization of shredded steam-exploded pine particles as a dietary ingredient to modify cecal microbiota in broilers. Agriculture. 2021;11:1196. [Google Scholar]
- Goel A., Kim B.J., Ncho C.M., Jeong C.M., Gupta V., Jung J.Y., Ha S.Y., Lee D.H., Yang J.K., Choi Y.H. Dietary supplementation of shredded, steam-exploded pine particles decreases pathogenic microbes in the cecum of acute heat-stressed broilers. Animals. 2021;11:2252. doi: 10.3390/ani11082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Ncho C.-M., Jeong C.-M., Gupta V., Jung J.-Y., Ha S.-Y., Yang J.-K., Choi Y.-H. Effects of dietary supplementation of solubles from shredded, steam-exploded pine particles on the performance and cecum microbiota of acute heat-stressed broilers. Microorganisms. 2022;10:1795. doi: 10.3390/microorganisms10091795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Jeong C.M., Choi Y.H. Embryonic thermal manipulation and in ovo gamma-aminobutyric acid supplementation regulating the chick weight and stress-related genes at hatch. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.807450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Kim B.-J., Jeong C.-M., Gupta V., Jung J.-Y., Ha S.-Y., Yang J.-K., Choi Y.-H. Dietary shredded steam-exploded pine particle supplementation as a strategy to mitigate chronic cyclic heat stress by modulating gut microbiota in broilers. Sci. Rep. 2022;12:19704. doi: 10.1038/s41598-022-24031-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrebski S.F., Lamont S.J., Schmidt C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.Y., Ha S.Y., Goel A., Jung C., Choi Y.-H., Yang J.-K. The chemical and physical properties of steam-exploded wood at different temperatures and times at the same severity as a dietary fiber source. BioResources. 2022;17:2129. [Google Scholar]
- Kang S., Kim D.-H., Lee S., Lee T., Lee K.-W., Chang H.-H., Moon B., Ayasan T., Choi Y.-H. An acute, rather than progressive, increase in temperature-humidity index has severe effects on mortality in laying hens. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama M. NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J. Pharmacol. Sci. 2010;114:134–146. doi: 10.1254/jphs.10r01cr. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Yoshida H., Furukawa K., Toyomizu M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 2015;52:8–13. doi: 10.1016/j.jtherbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Kursa O., Tomczyk G., Adamska K., Chrzanowska J., Sawicka-Durkalec A. The microbial community of the respiratory tract of commercial chickens and turkeys. Microorganisms. 2022;10:987. doi: 10.3390/microorganisms10050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhon S.D., Maurer R., Suyemoto M., Altier C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 2002;46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Kwon M., Heo S., Kim M.G., Kim G.B. Characterization of the biodiversity of the spoilage microbiota in chicken meat using next generation sequencing and culture dependent approach. Korean J. Food Sci. Anim. Resour. 2017;37:535–541. doi: 10.5851/kosfa.2017.37.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Henriksson G., Gellerstedt G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion. Bioresour. Technol. 2007;98:3061–3068. doi: 10.1016/j.biortech.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Li S., Yan C., Liu T., Xu C., Wen K., Liu L., Zhao M., Zhang J., Geng T., Gong D. Research Note: Increase of bad bacteria and decrease of good bacteria in the gut of layers with vs. without hepatic steatosis. Poult. Sci. 2020;99:5074–5078. doi: 10.1016/j.psj.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Fu L., Niu B., Wu S., Wooley J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012;13:656–668. doi: 10.1093/bib/bbs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Lv H., Wang H., Yang H., Li Y., Qian J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:1284–1292. doi: 10.1093/gerona/glz263. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Pan Z.Y., Zhao Y., Guo Y., Qiu S.J., Balasubramanian B., Jha R. Effects of heat stress on production performance, redox status, intestinal morphology and barrier-related gene expression, cecal microbiome, and metabolome in indigenous broiler chickens. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.890520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.S., Li S., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021;100:273–282. doi: 10.1016/j.psj.2020.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Dietary taurine supplementation decreases fat synthesis by suppressing the liver X receptor α pathway and alleviates lipid accumulation in the liver of chronic heat-stressed broilers. J. Sci. Food Agric. 2019;99:5631–5637. doi: 10.1002/jsfa.9817. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X.F., Ma B.B., Zhang L., Li J.L., Jiang Y., Zhou G.H., Gao F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019;98:3695–3704. doi: 10.3382/ps/pez056. [DOI] [PubMed] [Google Scholar]
- Lundberg R., Scharch C., Sandvang D. The link between broiler flock heterogeneity and cecal microbiome composition. Anim. Microbiome. 2021;3:54. doi: 10.1186/s42523-021-00110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrommatis A., Giamouri E., Myrtsi E.D., Evergetis E., Filippi K., Papapostolou H., Koulocheri S.D., Zoidis E., Pappas A.C., Koutinas A., Haroutounian S.A., Tsiplakou E. Antioxidant status of broiler chickens fed diets supplemented with vinification by-products: a valorization approach. Antioxidants. 2021;10 doi: 10.3390/antiox10081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride M.J., Xie G., Martens E.C., Lapidus A., Henrissat B., Rhodes R.G., Goltsman E., Wang W., Xu J., Hunnicutt D.W., Staroscik A.M., Hoover T.R., Cheng Y.Q., Stein J.L. Novel features of the polysaccharide-digesting gliding bacterium Flavobacterium johnsoniae as revealed by genome sequence analysis. Appl. Environ. Microbiol. 2009;75:6864–6875. doi: 10.1128/AEM.01495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Siegerstetter S.C., Magowan E., Lawlor P.G., Petri R.M., NE O.C., Zebeli Q. Feed restriction modifies intestinal microbiota-host mucosal networking in chickens divergent in residual feed intake. mSystems. 2019;4:e00261. doi: 10.1128/mSystems.00261-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingo F.S., Studenik S., Diekert G. Conversion of phenyl methyl ethers by Desulfitobacterium spp. and screening for the genes involved. FEMS Microbiol. Ecol. 2014;90:783–790. doi: 10.1111/1574-6941.12433. [DOI] [PubMed] [Google Scholar]
- Moczkowska M., Karp S., Niu Y., Kurek M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed–a physicochemical approach. Food Hydrocoll. 2019;90:105–112. [Google Scholar]
- Mohammed A.A., Jiang S., Jacobs J.A., Cheng H.W. Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poult. Sci. 2019;98:4408–4415. doi: 10.3382/ps/pez246. [DOI] [PubMed] [Google Scholar]
- Nanto-Hara F., Kikusato M., Ohwada S., Toyomizu M. Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 2020;57:284–290. doi: 10.2141/jpsa.0190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling D.P., van Emmenes L., Ahire J.J., Pieterse E., Smith C., Dicks L.M.T. Safety assessment of antibiotic and probiotic feed additives for Gallus gallus domesticus. Sci. Rep. 2017;7:12767. doi: 10.1038/s41598-017-12866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T., Jirapat J., Isobe N., Yoshimura Y. Effects of oral administration of Lactobacillus reuteri on mucosal barrier function in the digestive tract of broiler chicks. J. Poult. Sci. 2020;57:67–76. doi: 10.2141/jpsa.0190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamok S., Aengwanich W., Komutrin T. Adaptation to oxidative stress and impact of chronic oxidative stress on immunity in heat-stressed broilers. J. Therm. Biol. 2009;34:353–357. [Google Scholar]
- Polansky O., Sekelova Z., Faldynova M., Sebkova A., Sisak F., Rychlik I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2015;82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., de Los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roushdy E.M., Zaglool A.W., El-Tarabany M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018;74:337–343. doi: 10.1016/j.jtherbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Saati-Santamaría Z., Revilla-Martín I., García-Fraile P., Palacios-Riocerezo C. Evolution and predicted functions of the microbiota of the medium-slow growing chicken during the first 4 weeks of chick development. Ann. Appl. Biol. 2022;181:9. [Google Scholar]
- Shah T.M., Patel J.G., Gohil T.P., Blake D.P., Joshi C.G. Host transcriptome and microbiome interaction modulates physiology of full-sibs broilers with divergent feed conversion ratio. NPJ Biofilms Microbiomes. 2019;5:24. doi: 10.1038/s41522-019-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., Li Q., Liu C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019;98:2405–2413. doi: 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- Shi R., Yang X., Chen L., Chang H.T., Liu H.Y., Zhao J., Wang X.W., Wang C.Q. Pathogenicity of Shigella in chickens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Jiao L., Xiao K., Luan Z., Hu C., Shi B., Zhan X. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed Sci. Technol. 2013;185:175–181. [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Spivey M.A., Dunn-Horrocks S.L., Duong T. Epithelial cell adhesion and gastrointestinal colonization of Lactobacillus in poultry. Poult. Sci. 2014;93:2910–2919. doi: 10.3382/ps.2014-04076. [DOI] [PubMed] [Google Scholar]
- Tahmasebi H., Talebi R., Zarif B. Isolated of Bacillus cereus in chicken meat and investigation β-lactamase antibiotic-resistant in Bacillus cereus from chicken meat. Adv. life Sci. 2014;4:200–206. [Google Scholar]
- Uerlings J., Song Z.G., Hu X.Y., Wang S.K., Lin H., Buyse J., Everaert N. Heat exposure affects jejunal tight junction remodeling independently of adenosine monophosphate-activated protein kinase in 9-day-old broiler chicks. Poult. Sci. 2018;97:3681–3690. doi: 10.3382/ps/pey229. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Venardou B., O'Doherty J.V., Vigors S., O'Shea C.J., Burton E.J., Ryan M.T., Sweeney T. Effects of dietary supplementation with a laminarin-rich extract on the growth performance and gastrointestinal health in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Li X., Zhou Y., Feng J., Zhang M. Effects of heat stress on gut-microbial metabolites, gastrointestinal peptides, glycolipid metabolism, and performance of broilers. Animals. 2021;11:1286. doi: 10.3390/ani11051286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Lin X., Jiao H., Uyanga V., Zhao J., Wang X., Li H., Zhou Y., Sun S., Lin H. Mild heat stress changes the microbiota diversity in the respiratory tract and the cecum of layer-type pullets. Poult. Sci. 2020;99:7015–7026. doi: 10.1016/j.psj.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.C., Yan F.F., Hu J.Y., Amen O.A., Cheng H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J., Feng J.H., Zhang M.H., Li X.M., Ma D.D., Chang S.S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 2018;97:2153–2158. doi: 10.3382/ps/pey032. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gong X., Hu X., Zhou N. Lignin monomer in steam explosion assist chemical treated cotton stalk affects sugar release. Bioresour. Technol. 2019;276:343–348. doi: 10.1016/j.biortech.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Wrzosek L., Miquel S., Noordine M.L., Bouet S., Joncquel Chevalier-Curt M., Robert V., Philippe C., Bridonneau C., Cherbuy C., Robbe-Masselot C., Langella P., Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.B., Ravindran V., Thomas D.G., Birtles M.J., Hendriks W.H. Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolisable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. Br. Poult. Sci. 2004;45:76–84. doi: 10.1080/00071660410001668897. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xu J., Lei L., Jiang Y., Gao F., Zhou G.H. Effects of xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in different sections of the gastrointestinal tract of broilers fed wheat-based diets. Asian-Australas. J. Anim. Sci. 2014;27:855–861. doi: 10.5713/ajas.2014.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Akhtar M., Chen Y., Ma Z., Liang Y., Shi D., Cheng R., Cui L., Hu Y., Nafady A.A., Ansari A.R., Abdel-Kafy E.M., Liu H. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome. 2022;10:107. doi: 10.1186/s40168-022-01299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Schwartz S., Wagner L., Miller W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- Ziganshina E.E., Belostotskiy D.E., Shushlyaev R.V., Miluykov V.A., Vankov P.Y., Ziganshin A.M. Microbial community diversity in anaerobic reactors digesting turkey, chicken, and swine wastes. J. Microbiol. Biotechnol. 2014;24:1464–1772. doi: 10.4014/jmb.1404.04043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.