Ambient air pollution is the most important environmental risk factor globally due to its well-established burden of respiratory and cardiovascular diseases (1). Within the complex mixture of polluted ambient air, fine ambient particles defined as mass of particles smaller or equal to 2.5 µm in aerodynamic diameter (PM2.5) are an established causal factor. Epidemiological evidence is accumulating that PM2.5 is linked to cognitive decline (2, 3). Recent evidence highlighted that PM2.5 is also associated with neurodegenerative diseases, specifically with Alzheimer’s and Parkinson’s disease (4). In this issue of the journal, Shi et al. investigated the association between constituents of PM2.5 and the incidence of dementia and Alzheimer’s disease (5). Their analyses are based on a cohort assembled from the Medicare and Medicaid records including all inhabitants of the contiguous United States aged 65 y and older for the time period 2000 to 2017. They constructed two separate cohorts: They identified 5.8 Mio cases of incident dementia among 18.5 Mio individuals and 2.8 Mio cases of incident Alzheimer’s disease among 19.2 Mio individuals. They report consistent associations for constituents of PM2.5, namely black carbon, organic matter, sulfates (SO42−), and ammonium (NH4+). They conclude that annual average PM2.5 concentrations from traffic and fossil fuel combustion are significantly associated with the development of dementia and Alzheimer’s disease (5).
PM2.5 is a complex mixture both with respect to their size ranging down to several nanometers and their composition (6). The composition of combustion-related particles is determined by three factors, the composition of the fuel, the chemical reactions when forming the primary particles, and the substances that are absorbed on the particle surfaces while being dispersed in air. The constituents of PM2.5 assessed by Shi et al. (5) describe the sources of the particles and the toxicological properties. Black carbon is a measure of soot particles produced in combustion processes. As primary particles, they are part of the ultrafine particles (UFP) defined as particles smaller than 100 nm. In this size range, they are characterized by high number concentration and surface area. Particles agglomerate as they age, so that primary ultrafine particles grow into the fine particle fraction. The majority of sulfates, nitrates, and ammonium in PM2.5 are secondary constituents formed from their gaseous precursors and absorbed on the particles. Their concentrations have been consistently associated with adverse health effects, while the toxicity of these constituents is low (6). They are indicators for combustion-related, aged, and regionally transported PM2.5.
Shi et al. have used two independent approaches to characterize the spatial variation of annual averages of PM2.5 constituents (5). First, they estimated the annual averages based on an integrated approach combing satellite data, chemical transport models, and ground-based observation with a resolution of 1 km by 1 km. Second, they estimated the annual averages based on nearly 1,000 measurement stations and hundreds of additional predictor variables with a resolution of 50 m by 50 m in urban areas and 1 km by 1 km in rural areas. Both methods yielded comparable correlations between the PM2.5 constituents and PM2.5 mass. The resulting spatial maps provide comparable spatial distributions for all constituents but black carbon. For black carbon, the method relying on chemical transport models assigned the largest values to the industrial southeast and suggests long-range transport of soot-containing particles. In contrast, the method putting most weight on measured constituents implicated that the metropolitan areas had the highest annual averages of black carbon.
A key finding of the study by Shi et al (5) is that the hazard ratio for dementia increased 12% (95% CI: 11 to 14%) per 1 µg/m3 black carbon for the chemical transport-based model and 25% (95% CI: 22 to 27%) per 1 µg/m3 black carbon for the second model with finer spatial resolution. The hazard ratio for Alzheimer’s disease increased 23% (95% CI: 21 to 25%) per 1 µg/m3 black carbon for the for the chemical transport-based model and 39% (95% CI: 36 to 43%) based on the second model with finer spatial resolution. The estimates based on the second model with finer resolution are robust against adjustments for the remaining variation in PM2.5 mass. The estimates based on chemical transport-based model are reduced to a null effect when adjusting for the remaining variation in PM2.5 mass. This highlights two important points: First, the observed associations are to a large degree driven by Alzheimer’s disease, and second, the second model captures the role of locally emitted soot particles better than the chemical transport-based model.
Considering sulfates as a key indicator for regionally transported, aged PM2.5, the differences are less striking. One µg/m3 SO42− is associated with an increased hazard ratio for dementia of 5.9 % (95% CI: 5.6 to 6.2%) based on the chemical transport-based model and of 6.2 % (95% CI: 5.8 to 6.5%) based on the second model with finer spatial resolution. The risk for Alzheimer’s disease associated with an increase of 1 µg/m3 SO42 was estimated to be 7.4 % (95% CI: 6.9 to 7.9%) based on the chemical transport-based model and 8.4 % (95% CI: 7.9 to 9.0%) based on the second model with finer resolution. These findings imply that first, the observed increased risks are attributable to Alzheimer’s disease and other dementias and second, both models derive consistent results for regionally transported fine particles.
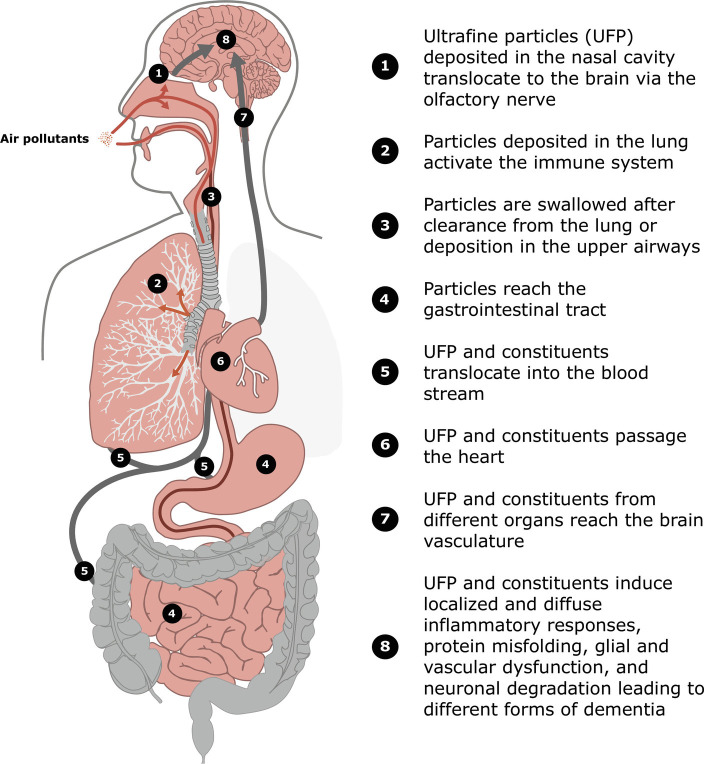
There are multiple ways how fine particles and their constituents are hypothesized to impact the brain and initiate and promote neurodegenerative diseases (Fig. 1). Particles are deposited in the upper and lower airways and reach the lung as well as the gastrointestinal tract (Fig. 1: 1 to 4). UFP are able to enter into cells (7, 8) and to reach the brain via the olfactory nerve (8) (Fig. 1:1). Consequently, UFP could contribute to Alzheimer’s disease development by translocation to the cortex regions where Alzheimer’s disease is initiated (9, 10) (Fig. 1:8). Alzheimer’s disease is characterized by progression of protein miss-folding and plaques that start to develop in distinct brain regions before the entire cortex is affected. Indeed, animal experiments with diesel exhaust containing high numbers of UFP showed protein miss-folding and plaques in addition to oxidative stress (11). By these mechanisms, UFP could contribute to the observed Alzheimer’s disease-specific associations of black carbon by Shi et al. (5). Furthermore, UFP and other constituents of PM2.5 such as transition metals or semivolatile organic compounds translocate from the lung or the gastrointestinal tract to the bloodstream and reach the brain vasculature (Fig. 1:5 to 8). It has been demonstrated by experimental studies that UFP are able to pass the blood–brain-barrier (12). Thereby, more diffuse impacts including microglia activation and reactive astrocytes inducing neuronal inflammation and degeneration as well as oligodendrocyte dysfunction have been described (13). Promotion of neurodegeneration in the entire brain including the cortex would be a consequence (Fig. 1:8). This would be consistent with the finding that aged and regionally transported PM2.5 is robustly associated with dementia and Alzheimer’s disease (5). Further support is provided by a study in children and young adults from Mexico City. (14). UFP were detected along with evidence for neurovascular damage in several brain regions using imaging modalities such as transmission electron microscopy. Furthermore, inflammatory processes induced by PM2.5 in multiple barrier organs could result in systemic oxidative stress and inflammation (Fig. 1:5). Systemic oxidative stress and inflammation are among the hallmarks of environmental insults (15) and could thereby contribute to the progression of dementia. We recently demonstrated the impact of PM2.5 on the gut microbiome (16) (Fig. 1:4), and in general, the gut–brain axis is discussed as relevant for dementia development and progression (17). Finally, a robust association has been documented between PM2.5 and vascular dysfunction (18). Therefore, it is also plausible that PM2.5 impairs endothelia in the brain and induces vascular dementia (Fig. 1:8). Taken together, the hypothesized pathways could promote various types of dementia (10, 17).
Fig. 1.
Schematic overview on ambient fine and ultrafine particle deposition and interaction with organs and the circulation contributing to the development and progression of dementia and neurodegeneration. Orange lines and arrows: Particle paths through the upper airways into the lung. Dark red lines: Particle paths through the esophagus into the gastrointestinal tract. Gray lines and arrows: Paths of ultrafine particles and constituents from the barrier organs to the brain.
“In PNAS, Shi et al. investigated the association between constituents of PM2.5 and the incidence of dementia and Alzheimer’s disease.”
There is the need for experimental studies to pursue these aspects further. In particular, toxicological studies are needed to understand the role of UFP and constituents of PM2.5 for initiation and progression of dementia including Alzheimer’s disease. Within epidemiological studies, measuring the incidence of Alzheimer’s disease and related dementia is a challenge. There is evidence for substantial misclassification and late detection of the disease. However, using the nationwide Medicare and Medicaid data provides a comprehensive and consistent approach. It is unlikely that a large proportion of the early stages of Alzheimer’s disease are captured. To further advance the understanding, large prospective population-based cohorts are needed including longitudinal brain imaging, cognitive function assessments, biomarker measurements, and genotyping to advance the understanding of Alzheimer’s disease initiation and progression by environmental factors such as air pollution.
The population around the world is growing and aging. Consequently, age-related diseases are increasing globally. Today, Alzheimer’s disease and other dementias are globally the seventh leading cause of mortality according to the World Health Organization (19). Of the approximately 55 Mio cases, 60 to 70% are Alzheimer’s disease. These numbers highlight that the paper by Shi et al. (5) has important implications for regulatory action. The finding that black carbon particles per µg/m3 have an approximately 10-fold larger effect size than the PM2.5 mixture calls for action to further limit emissions of soot particles from their sources. It also calls in my mind for intensified monitoring of ultrafine particles. The finding that aged regional transported particles are associated with dementia including Alzheimer’s disease strongly highlights that air pollution mitigation strategies need to be part of regional and national agendas.
Acknowledgments
Author contributions
A.P. designed research; performed research; and wrote the paper.
Competing interest
The author declares no competing interest.
Footnotes
See companion article, “Incident dementia and long-term exposure to constituents of fine particle air pollution: A national cohort study in the United States,” 10.1073/pnas.2211282119.
References
- 1.GBD Risk Factor Collaborators, Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 396, 1223–1249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weuve J., et al. , Exposure to air pollution in relation to risk of dementia and related outcomes: An updated systematic review of the epidemiological literature. Environ. Health Perspect. 129, 96001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgado-Saborit J. M., et al. , A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci. Total Environ. 757, 143734 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Shi L., et al. , Long-term effects of PM(2.5) on neurological disorders in the American medicare population: A longitudinal cohort study. Lancet Planet Health 4, e557–e565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi L., Incident dementia and long-term exposure to constituents of fine particle air pollution: A national cohort study in the United States. Proc. Natl. Acad. Sci. U.S.A. 120, e2211282119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassee F. R., Heroux M. E., Gerlofs-Nijland M. E., Kelly F. J., Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 25, 802–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters A., et al. , Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 3, 13 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone V., et al. , Nanomaterials versus ambient ultrafine particles: An opportunity to exchange toxicology knowledge. Environ. Health Perspect. 125, 106002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Piela D. R., Durisek G. R. III, Escobar Y. H., Mackos A. R., Wold L. E., Particulate matter and Alzheimer’s disease: An intimate connection. Trends Mol. Med. 28, 770–780 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doty R. L., Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrate? Lancet Neurol. 16, 478–488 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Hullmann M., et al. , Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part Fibre Toxicol. 14, 35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusinkveld H. J., et al. , Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56, 94–106 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Nicholson S., Baccarelli A., Prada D., Role of brain extracellular vesicles in air pollution-related cognitive impairment and neurodegeneration. Environ. Res. 204, 112316 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderon-Garciduenas L., et al. , Environmentally toxic solid nanoparticles in noradrenergic and dopaminergic nuclei and cerebellum of metropolitan Mexico City children and young adults with neural quadruple misfolded protein pathologies and high exposures to nano particulate matter. Toxics 10, 164 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters A., Nawrot T. S., Baccarelli A. A., Hallmarks of environmental insults. Cell 184, 1455–1468 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommer A. J., et al. , A randomization-based causal inference framework for uncovering environmental exposure effects on human gut microbiota. PLoS Comput. Biol. 18, e1010044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calderon-Garciduenas L., Stommel E. W., Rajkumar R. P., Mukherjee P. S., Ayala A., Particulate air pollution and risk of neuropsychiatric outcomes. What we breathe, swallow, and put on our skin matters. Int. J. Environ. Res. Public Health 18, 11568 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munzel T., et al. , Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 39, 3543–3550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO, Dementia (2022). https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 12/08/2022.