Abstract
Objective:
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease characterized by neuropsychiatric symptoms (e.g., anxiety, depression), where individuals suffer high levels of stress from the social, physical, and cognitive burden of the disease. The current study examined two factors associated with increased risk for symptoms of anxiety and depression: executive function skills (inhibitory control/attention and working memory) and skills to cope with stress.
Method:
Adults with HD completed the NIH Toolbox measures of inhibitory control/attention and working memory, as well as self-report measures of coping with HD-related stress and symptoms of anxiety and depression. Path analyses were used to test direct and indirect associations among the subtypes of executive functioning, coping, and symptoms.
Results:
No significant associations were found in the full sample (n=47), due to a significant portion of the sample with very low executive function abilities. Additional analyses were conducted on a subset of the sample (participants in the top three quartiles on both measures of executive functioning, n=32). Significant indirect associations emerged among inhibitory control/attention skills, secondary control coping (e.g., acceptance, reappraisal), and symptoms of anxiety and depression in the subsample. Higher inhibitory control/attention skills were associated with greater use of secondary control coping, and greater use of these coping skills was related to lower symptoms of anxiety and depression. No direct or indirect associations were found among working memory skills, coping, and symptoms of anxiety and depression.
Conclusions:
Implications for interventions to enhance executive function and coping skills in adults with HD are highlighted.
Keywords: Huntington’s disease, executive function, anxiety, depression, coping
Introduction
Huntington’s disease (HD) is a progressive neurodegenerative disease caused by an expanded cytosine-adenine-guanine (CAG) repeat on the huntingtin gene (The Huntington’s Disease Collaborative Research Group, 1993). The onset of HD is characterized by progressive cognitive, motor, and neuropsychiatric dysfunction. Adults with HD experience significant levels of chronic stress related to the disease, including the timing and course of symptoms, uncertainty about the future, and gradual loss of independence (Ho & Hocaoglu, 2011). The majority of stressors faced by adults with HD are uncontrollable and unpredictable, and these high levels of stress may further contribute to the development of neuropsychiatric symptoms, especially anxiety and depression (Missner & Anderson, 2018; Paulsen et al., 2005). Given the high neuropsychiatric burden in HD, it is imperative to understand the factors associated with their onset and progression (Goh et al., 2018). Two potential aspects related to neuropsychiatric symptoms in this population include executive function abilities and skills needed to cope with stress.
Executive function skills encompass a set of complex cognitive processes including updating (e.g., working memory), shifting (e.g., cognitive flexibility) and inhibition (Miyake & Friedman, 2011; Miyake et al., 2000). Extensive research has established a relationship between executive function skills and psychiatric symptoms. For example, a meta-analysis synthesizing data from over 20,000 individuals found that symptoms of anxiety, whether self-reported or experimentally induced, were consistently related to poorer performance on measures of working memory (Moran, 2016). More recent work has shown that among individuals with high trait anxious behaviors, state anxiety impaired inhibitory control, as measured in an anti-saccade task (Myles et al., 2020). Similar associations have been found for depression. Compared to healthy controls, individuals with major depressive disorder reliably demonstrate deficits on various measures of executive functioning (Snyder, 2013). Previous research has also established the reverse relationship (i.e., impairments in executive functioning can lead to or exacerbate psychiatric symptoms). It has been argued, for example, that deficits in inhibitory control may be associated with increased risk for anxiety by compromising an individual’s ability to inhibit and regulate symptoms of anxiety (e.g., worry; White et al. 2009). Furthermore, a recent review by LeMoult and Gotlib (2019) posits that deficits in cognitive control processes, including executive functioning, working memory, and processing speed, may underlie cognitive biases/emotion regulation strategies that lead to depression. Taken together, these findings suggest that executive function skills are a critical factor in understanding psychiatric symptoms.
Impairments in aspects of executive functioning, specifically inhibitory control and working memory, have been documented in individuals with HD and other neurodegenerative diseases (Lahr et al., 2018; Mörkl et al., 2016; You et al., 2014). For example, Rao et al. (2014) reported increased deficits in response inhibition as proximity to a diagnosis of HD neared. In addition, associations between working memory and psychiatric symptoms, specifically depression, have been documented in pre-symptomatic individuals with HD (Nehl et al., 2001). Relevant to the current study, Petkus et al. (2019) found worse performance across a variety of cognitive domains was associated with higher state and trait anxiety and depression symptoms in a sample of adults with movement disorders (Parkinson’s disease).
In addition to deficits in executive function skills, adults with HD face both controllable and uncontrollable sources of stress presented by the disease. Controllable sources of stress include making the decision to undergo genetic testing, the challenges of maintaining a healthy lifestyle, and illness perceptions (Arran et al., 2014). Many of the stressors adults with HD face, however, are uncontrollable and unpredictable in nature including the timing and course of symptoms, uncertainty about one’s own future, and the future of family members (Ho & Hocaoglu, 2011; Vamos et al., 2007). Furthermore, uncontrollable sources of stress are more likely to be associated with psychiatric symptoms (Compas et al., 2017).
Extensive research has shown individuals who use greater secondary control coping strategies in response to uncontrollable stressors experience fewer symptoms of anxiety and depression (Compas et al., 2017; Connor-Smith et al., 2000; Tu et al., 2016). Secondary control coping responses focus on adapting to a source of stress rather than trying to solve or change the stressor, and include skills such as acceptance, cognitive reappraisal, positive thinking, and distraction. Executive function skills, including inhibitory control and working memory, that operate under non-emotional “cold” conditions can be drawn upon to regulate thoughts and behavior under “hot” conditions of heightened emotional arousal or stress. For example, working memory skills involve holding, updating, and manipulating information in a short time frame, which may then aid in the generation and maintenance of cognitive appraisal in response to stress (Zaehringer et al., 2018). Cognitive reappraisal requires an individual to think about a stressor while simultaneously viewing it from an alternative, less negative perspective. In addition, inhibitory control skills may contribute to successful reappraisal by inhibiting automatic negative thoughts and allowing for more positive cognitions (Schmeichel & Tang, 2015). Although limited research has examined how adults with HD cope with the disease, there is some evidence that these individuals use less effective coping skills in response to stress (Helder et al., 2002). Therefore, given exposure to uncontrollable sources of stress and known impairments in aspects of executive functioning, it is particularly important to understand the coping strategies adults with HD utilize and their relationship with symptoms of anxiety and depression.
There is evidence that the association of executive function skills with symptoms of anxiety and depression is mediated by or accounted for in part by coping with stress. For example, several studies have shown secondary control coping strategies account for the association between working memory and symptoms of depression and anxiety, including individuals coping with leukemia (Campbell et al., 2009), brain tumors (Robinson et al., 2015), sickle cell disease (Prussien et al., 2018), and depression (Reising et al., 2018). Most relevant to the current study, Ciriegio et al. (2020) found support for an indirect pathway between working memory, secondary control coping, and symptoms of anxiety and depression in a sample of adolescents and young adults at-risk for HD. However, no studies have investigated these complex relationships in adults with HD.
The current study used path analyses to examine the associations among two aspects of executive functioning, inhibitory control/attention and working memory skills, the use of secondary control coping strategies, and symptoms of anxiety and depression in a sample of adults with HD. First, we hypothesized performance on standardized measures of inhibitory control/attention and working memory would be positively associated with reports of secondary control coping. Second, we hypothesized inhibitory control/attention and working memory skills would be negatively associated with symptoms of anxiety and depression. Third, we hypothesized there would be a negative association between secondary control coping skills and symptoms of anxiety and depression. Fourth, we hypothesized there would be an indirect relationship from inhibitory control/attention and working memory to symptoms of anxiety and depression through secondary control coping.
Method
Participants
Participants (n = 47) included adults with HD ages 31 to 69 years [mean (SD) = 46.83 (9.37) years; 53% female] with a mean CAG repeat length of 43.72 (2.99). Motor symptoms in adults with HD are assessed via the Diagnostic Confidence Level (DCL) from the Unified Huntington’s Disease Rating Scale (UHDRS; Huntington Study Group, 1996). The criterion for the DCL is as follows: 0 = normal (no motor abnormalities); 1 = nonspecific motor abnormalities; 2 = motor abnormalities that may be signs of HD (50–89% confidence); 3 = motor abnormalities that are likely signs of HD (90–98% confidence); 4 = motor abnormalities are unequivocal signs of HD (≥ 99% confidence). The majority of participants (63%) in the current sample were motor manifest with a DCL of 3 or 4, while the remaining 37% of participants were considered prodromal with a DCL of 2. Most of the sample (98%) identified as White. Although HD can affect individuals of all ethnic groups, it occurs predominantly among those of European descent (Bates et al., 2015). Participants came from a range of educational backgrounds (no high school diploma to a doctoral degree) with a mean 14.49 years of education. An additional five adults consented and participated in aspects of the research study, but they were excluded from the final full sample analyses due to missing data (n = 2 did not complete self-report surveys and n = 3 only had partial executive functioning data due to time constraints during the study visit).
Procedure
Participants were recruited through a Huntington Disease Society of America Level 1 Center of Excellence (COE) between October 2018 and October 2021. Eligibility requirements for participants included (1) fluent in English, (2) confirmed diagnosis of HD and (3) must have at least one child ages 7–39 years. The Medical Director of the COE oversaw recruitment of eligible individuals, and a member of the clinical team made the initial study introduction. This study was reviewed and approved by the Vanderbilt University Institutional Review Board. Informed consent was obtained from all participants prior to study enrollment and participation. Questionnaires were administered through a secure, online website (REDCap; Harris et al., 2009). Participants were compensated for their time.
Measures
Demographic and clinical information.
Participants provided information on their age, sex, race, and highest level of education. Information on CAG repeat length, anti-depressant usage at study entry, as well as total motor scores and total functional capacity scores (TFC) from the UHDRS (Huntington Study Group, 1996), a standardized assessment of key motor features of HD and independent daily functioning, were extracted from medical records.
Coping Responses.
The Responses to Stress Questionnaire-Huntington’s Disease Version (RSQ-HD; Ciriegio et al., 2020; Connor-Smith et al., 2000) is a self-report measure used to identify the coping strategies used in response to stress from having HD. The RSQ includes 57-items that provide three factors of coping: primary control coping, secondary control coping, and disengagement coping. Primary control coping is defined as efforts aimed at directly altering a stressor or one’s reaction to the stressor, while secondary control coping includes efforts focused on adapting to a problem or stressor. Conversely, disengagement coping is defined as responses that are oriented away from a stressor or one’s reactions to a stressor.
Each item on the RSQ is rated on a 4-point scale from 1 “Not at all” to 4 “A lot”. There is a tendency towards the more stress an individual is under, the more coping strategies are reported. To control for possible response biases in reports of total amounts of coping strategies used in response to stress, the standard scoring method for the RSQ was used in which proportion scores were calculated for each factor by dividing the total score for each factor by the total RSQ score (see Connor-Smith et al., 2000). Because much of the stress faced by adults with HD is uncontrollable, the current study focused on secondary control coping skills including acceptance (e.g., I realize I just have to live with things the way they are), cognitive reappraisal (e.g., I think about the things I’m learning from having HD, or that something good will come from it), positive thinking (e.g., I tell myself that I can get through this), and distraction (e.g., I keep my mind off of the stressful parts of HD by doing something else). The RSQ has demonstrated excellent test-retest reliability, and convergent and construct validity. Internal consistency for the secondary control coping scale in the current sample was α=.88.
Anxiety and depression symptoms.
Despite high prevalence rates of psychiatric symptoms in the HD population, consensus regarding recommended self-report measures of mental health in adults with HD has not yet been established within the field (Mestre et al., 2016). In the current study, symptoms of anxiety and depression were assessed using the Generalized Anxiety Disorder 7-item Scale (GAD-7; Spitzer et al., 2006) and Patient Health Questionnaire (PHQ-9; Kroenke et al., 2001), respectively. These scales were selected given their established diagnostic cut-off scores, concise number of items, and their demonstration as reliable and valid measures in a variety of clinical samples (Kroenke et al., 2010). Furthermore, the GAD-7 and PHQ-9 have been utilized previously in HD research (Carlozzi et al., 2019; Stopford, Ferrer-Duch, Moldovan, & MacLeod, 2020).
The GAD-7 is a self-report screening tool that assesses generalized anxiety related symptoms over the past two weeks. Similarly, the PHQ-9 is a self-report measure of the nine symptoms of Major Depressive Disorder based on DSM-IV criteria. Both scales asked participants to rate each item from 0 “Not at all” to 3 “Nearly every day” based on how much a symptom bothered them in the past two weeks. Cut-offs for the GAD-7 are minimal (0–4), mild (5–9), moderate (10–14), and severe anxiety (15–21). Cut-offs for the PHQ-9 are minimal (1–4), mild (5–9), moderate (10–14), moderately severe (15–19), and severe (20–27) depression. Internal consistency for the GAD-7 and PHQ-9 in the current sample was α = .90 and α = .85, respectively.
Given that the measures of anxiety and depression symptoms in the current study share approximately 64% of variance (r = .80) and in accordance with models presented in previous research (e.g., Ciriegio et al., 2020), we created a composite measure of anxiety and depression by transforming GAD-7 and PHQ-9 scores into z-scores for each participant. The mean of these z-scores was used for all analyses.
Executive functioning skills.
Aspects of executive functioning were assessed using subtests from the NIH Toolbox Cognition Battery (Gershon et al., 2010; Tulsky et al., 2014), which was selected for ease of administration and the availability of normative data across the full age range of participants included in the current study. To our knowledge, this is one of the first studies to utilize the NIH Toolbox with adults with HD. Although the NIH Toolbox Cognition Battery is a novel way of measuring executive functioning in the HD population, many of its subtests share similarities with other established measures. For example, Mestre et al. (2018) designated the Stroop color word interference test (part of the UHDRS cognitive assessment battery) as a “suggested” measure in HD. The Stroop color word interference test assesses multiple cognitive domains including psychomotor speed, cognitive flexibly, response inhibition and selective attention, which is similar to the domains assessed in the Flanker Task of the NIH Toolbox.
Inhibitory control/attention skills were assessed using the Flanker Inhibitory Control and Attention Test (FICAT), which measures attention and inhibitory control by requiring the participant to focus on a given stimulus (middle arrow) while inhibiting attention to other stimuli (row of arrows). The middle stimulus can either point the same way as the flanking arrows (congruent) or point in the opposite direction as the flankers (incongruent). Twenty trials are conducted, and scoring is based on a combination of accuracy and reaction time. If accuracy levels for the participant are less than or equal to 80%, the final “total” computed score is equal to the accuracy score. If accuracy levels for the participant reach more than 80%, the reaction time score and accuracy score are combined (Gershon et al., 2010).
Working memory was assessed using the List Sorting Working Memory Task (LSWMT), which requires the immediate recall and sequencing of presented stimuli. Pictures of different foods and animals are displayed with accompanying audio recording, and the participant is asked to recall aloud the items in size order from smallest to biggest, first within a single dimension (either animals or food, 1-List) and then on two dimensions (foods and then animals, 2-List). A task score is calculated by summing the total number of items correctly recalled and sequenced on the 1- and 2-Lists.
The FICAT and LSWMT scores were converted to age-corrected standard scores (M=100, SD=15), which compares the individual’s score to a nationally representative normative sample of adults (n = 1,038) distributed across specific age bands: 18–29 (n = 174), 30–39 (n = 212), 40–49 (n = 183), 50–59 (n = 143), 60–69 (n = 120), 70–79 (n = 112), and 80–85 (n = 94). These tasks have been shown to be developmentally appropriate and recommended for ages 7–85 years old (Casaletto et al., 2015). Administration of the NIH Toolbox Cognition Battery was completed on an iPad and conducted by trained research assistants during in-person study visits.
Statistical Analyses
Working memory and inhibitory control/attention mean age-corrected standard scores in a subsample of participants (see Preliminary Analyses below) were compared to normative scores using independent sample t-tests. PROCESS macro (v3.5; Hayes, 2017) for SPSS was used to evaluate both the direct association between two aspects of executive function, inhibitory control/attention and working memory, and symptoms of anxiety and depression as well as the indirect association accounted for by secondary control coping. PROCESS macro is an Ordinary Least Squares and logistic regression path analysis-modeling tool that incorporates a bootstrapping procedure. The total direct (path c, c’) and indirect (path ab) effects can be described through standardized regression coefficients (Hayes, 2017). Current analyses were conducted with a 95% confidence interval for all effects.
Results
Preliminary Analyses
Because impairments in executive functioning can be severe in later stages of HD (Paulsen, 2011), we conducted an initial analysis to determine if the hypothesized effects could be tested in the full sample or if there were minimal levels of executive functioning needed to detect associations with coping and symptoms of anxiety and depression. That is, it is plausible that individuals with severe impairments in executive functioning may not be able to enact complex coping strategies and may be unable to reliably report on their coping on the self-report measure used in the current study. In preliminary analyses with the full sample (n = 47), neither of the measures of executive functioning were significantly correlated with coping or symptoms of anxiety and depression. Therefore, the full sample was divided into quartiles separately based on scores for inhibitory control/attention (FICAT) and for working memory (LSWMT) subtests from the NIH Toolbox Cognition Battery. Participants who scored in the bottom quartile on either the FICAT or the LSWMT subtests were excluded from the analyses (n = 15).
Taken together, 32 participants scored in the top three quartiles on both the inhibitory control/attention and working memory measures and were retained for further analyses. Statistical power analyses indicated that with a subsample of n = 32, there was adequate power (β=.80, α=.05) to detect effect sizes of f2 ≥ .33. A total of 15 participants in the sample scored in the bottom quartile on either the FICAT or the LSWMT and these participants were excluded from further analyses. The excluded participants had a mean standard score of 62.40 on the FICAT, which is 2.5 standard deviations below the normative mean (M = 100), at <1st percentile. Furthermore, the excluded participants had a mean standard score of 68.80 on the LSWMT, which is greater than 2 standard deviations below the normative mean (M = 100), at <1st percentile. In addition, those in the bottom quartile on either the FICAT or the LSWMT had significantly higher UHDRS motor scores compared to the participants in the top three quartiles indicating more advanced disease among those who were excluded, t(40) = 2.87, p< .01.
Descriptive Statistics
Descriptive statistics for demographic and clinical data are presented for the full sample (n = 47), as well as the top three quartiles subsample (n = 32) in Table 1. Mean scores on the GAD-7 and PHQ-9 for the subsample reflected symptoms in the mild to moderate range, and 19% of adults in the subsample reported symptoms in the severe range for anxiety and 22% of the subsample reported symptoms in the moderately severe range for depression. Furthermore, 80% of participants in the top three quartiles subsample were prescribed an antidepressant medication. There were no differences on key study variables as a function of medication status.
Table 1.
Descriptive statistics for demographic and clinical data
| Full Sample (n = 47) | Subsample (n = 32) | |||
|---|---|---|---|---|
|
| ||||
| Range | M (SD) | Range | M (SD) | |
| Age | 31 – 69 | 46.83(9.37) | 31 – 69 | 47.13 (9.36) |
| UHDRS Motor Score | 0 – 68 | 20.12 (17.36) | 0 – 42 | 15.82 (13.53) |
| NIH Toolbox Flanker Inhibitory Control and Attention Test (FICAT) | 54 – 115 | 74.87 (13.54) | 68 – 115 | 80.72 (11.56) |
| NIH Toolbox List Sorting Working Memory Task (LSWMT) | 54 – 124 | 86.04 (18.74) | 69 – 124 | 94.13 (14.84) |
| GAD-7 Total Score | 0 – 22 | 6.98 (5.97) | 0 – 22 | 7.22 (5.98) |
| PHQ-9 Total Score | 0 – 18 | 8.45 (5.74) | 0 – 18 | 9.06 (5.61) |
| Secondary Control Coping | .11 – .39 | .26 (.06) | .11 – .39 | .26 (.07) |
Scores for the NIH Toolbox measures are age-corrected standard scores (M = 100, SD = 15).
Secondary control coping scores as measured on the RSQ-HD are reported in ratio scores.
Inhibitory Control/Attention Subsample Analyses
In support of the hypotheses, indirect effects of inhibitory control/attention with symptoms of anxiety and depression were significant (see Figure 1). Analyses of the full model revealed the direct association between inhibitory control/attention and symptoms of anxiety and depression (path c) was negative and approached significance (β = −.30, p = .09). The direct association between inhibitory control/attention and secondary control coping (path a) also approached significance (β = .31, p = .08). The direct effect of secondary control coping on symptoms of anxiety and depression was negative and significant at the p < .001 level (path b; β = −.67). In the full model, the effect of inhibitory control/attention was not significant when coping was included in the model (path c’; β = −.10). In the test of our fourth and primary hypothesis, the total indirect effect of inhibitory control/attention on symptoms of anxiety and depression through secondary control coping (path ab) was significant, β = −0.21 (standard error [SE] = 0.09; 95% CI [−0.41, −0.06]). Of note, our analyses are guided by the conceptual frameworks put forth by MacKinnon et al. (2007) and Hayes (2009) who stated that there are many cases where significant mediation exists, but the requirement of a significant relation of X to Y (path c) is not obtained (e.g., MacKinnon, Fairchild & Fritz, 2007). An indirect effect of X on Y through a mediator variable (path ab) in the absence of a direct effect between X and Y is possible once it is considered that a total effect is the sum of many different paths of influence (e.g., direct and indirect; Hayes, 2009).
Figure 1.
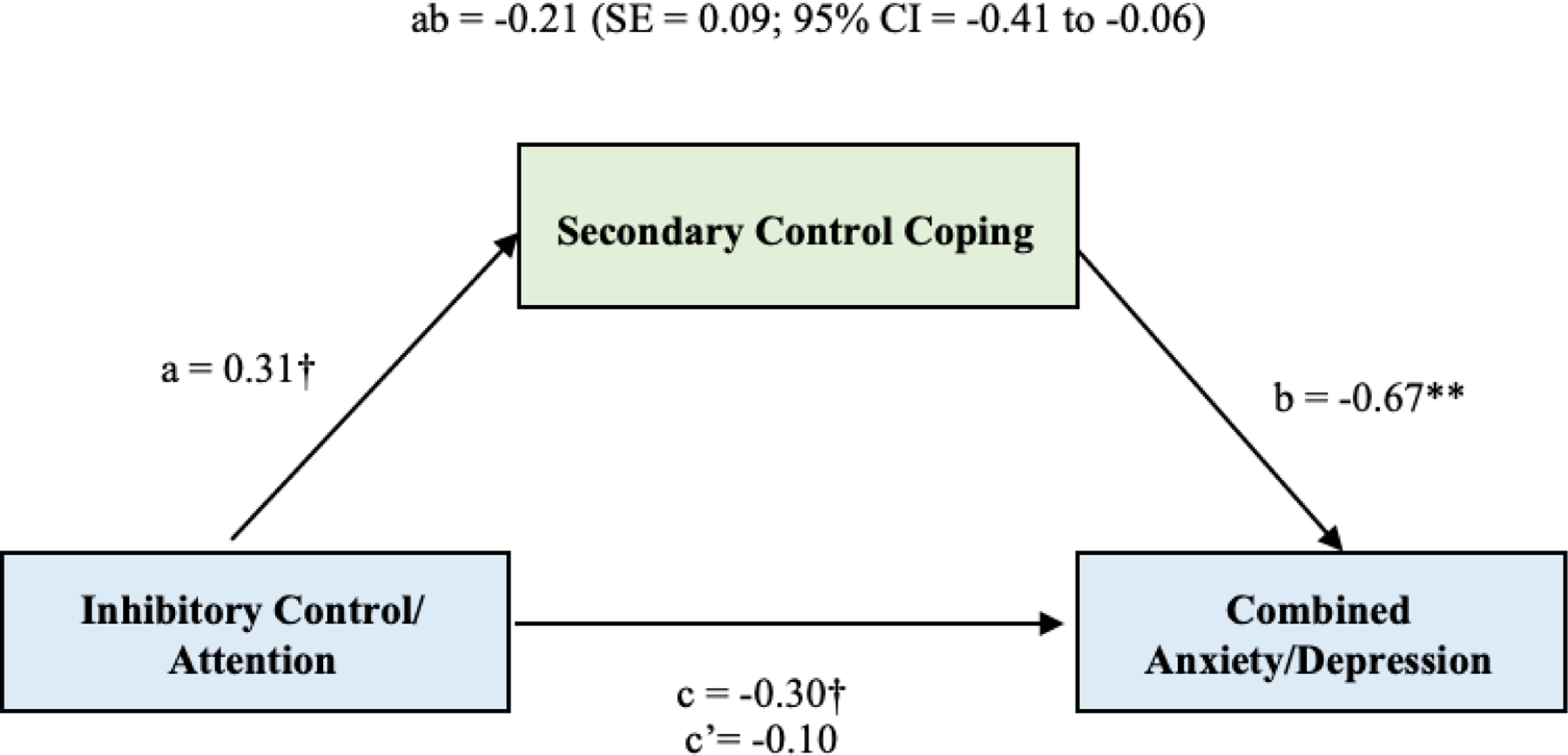
Direct and indirect associations of inhibitory control/attention and symptoms of anxiety and depression through secondary control coping. Standardized path coefficients are given.
** p < 0.01; * p < 0.05; † p < .10
Working Memory Subsample Analyses
Contrary to our hypotheses, neither the direct nor indirect models for the working memory subsample were significant. That is, no evidence was found for an indirect path from working memory to symptoms of anxiety and depression through secondary control coping in the subsample.
Discussion
Adults with HD are faced with significant sources of stress that may contribute to symptoms of anxiety and depression. The identification of sources of risk for these symptoms that serve as avenues for interventions to increase resilience in these individuals is a high priority. The current study provides evidence for two important factors related to symptoms of anxiety and depression in adults with HD. Specifically, we found a significant indirect path from inhibitory control/attention skills to symptoms of anxiety and depression through their association with the use of secondary control coping in response to HD-related stress. Thus, inhibitory control/attention skills may provide a foundation for enacting cognitively complex secondary control coping skills that are, in turn, related to lower symptoms of anxiety and depression.
Consistent with findings from previous research, the full sample of adults with HD (n = 47) exhibited significant deficits in two aspects of executive function skills. Even when participants scoring in bottom quartile on either measure of executive functioning were dropped from the sample, those remaining in the top three quartiles still had mean standard scores that were greater than one standard deviation below the normative mean for a measure of inhibitory control/attention. These findings provide further evidence of deficits in important higher order executive function skills in HD, and to our knowledge, provide the first evidence for these deficits on the NIH Toolbox Cognition Battery (Gershon et al., 2010) in this population. Further, as expected, symptoms of anxiety and depression were elevated in the subsample with 19% of adults reporting symptoms in the severe range for anxiety and 22% reporting symptoms in the moderately severe range for depression. In the PROCESS model, a significant association was found between secondary control coping and the composite measure of symptoms of anxiety and depression in the subsample. That is, greater use of these skills in response to HD-related stress was associated with lower levels of neuropsychiatric symptoms.
Consistent with the primary hypotheses for this study, there was support for the indirect association of inhibitory control/attention with a composite measure of symptoms of anxiety and depression through secondary control coping skills in a subsample of adults with HD (i.e., those in the top three quartiles on scores of executive functioning). Deficits in inhibitory control are well documented in adults with HD and the current sample appeared to be significantly impaired in this domain of executive function. There may be a wide range of factors associated with these deficits including hyper responsivity to reward and impulsive behavior (Kalkhoven et al., 2014, Reynolds et al., 2019). The current study extends prior research and suggests one important correlate may be impairment in the use of complex coping strategies that include cognitive reappraisal, acceptance, and the ability to use positive activities and thoughts to distract from sources of stress.
We did not find support for similar associations that were hypothesized for working memory skills in either the full sample or in the subsample of individuals who scored in the top three quartiles. Working memory was not directly associated with either secondary control coping skills or with the composite measure of anxiety and depression. Further, no evidence was found for indirect paths from working memory to anxiety and depression through secondary control coping. These findings differ from, but add to, those reported by Ciriegio et al. (2020) who found support for direct and indirect associations between working memory, secondary control coping, and symptoms of anxiety and depression in a sample of adolescent and young adult offspring of parents with HD, including both those with and without expansions of the CAG repeat that is associated with HD. Taken together with the findings from the current study, this suggests that impairments in working memory may be associated with anxiety and depression in individuals at risk for HD, and impairments in inhibitory control/attention may emerge as an important correlate of coping and symptoms of anxiety and depression in adults with manifest symptoms of HD. This suggests a possible shift in the correlates of deficits in domains of executive function over the course of the emergence of HD.
The current study builds on a growing body of evidence on the associations among executive functioning, skills for coping with stress, and symptoms of anxiety and depression. Previous research has found evidence for direct and indirect associations among executive function skills, coping, and neuropsychiatric symptoms in typically developing samples (Andreotti et al., 2013) and individuals with a range of different chronic health conditions (e.g., Prussien et al., 2018; Robinson et al., 2015). This study reports the first evidence for these processes in adults with a neurodegenerative disease.
These findings have potentially important implications for interventions to reduce anxiety and depression in adults with HD. First, there is support for the efficacy of interventions (e.g., mindfulness, cognitive training) to enhance specific aspects of executive functioning in typical populations (Whitfield et al., 2021) and those with other neurogenerative diseases (e.g., Multiple Sclerosis and Parkinson’s disease; Manglani et al., 2020; Ghielen et al., 2019). Second, there is ample evidence to support the efficacy of cognitive behavioral interventions to enhance skills that comprise secondary control coping skills, including acceptance and cognitive reappraisal, in the treatment and prevention of depression and anxiety. Evidence for these types of interventions in adults with HD is relatively limited. Andrews et al. (2015), for example, reviewed evidence from three small scale studies which show some beneficial effects of cognitive intervention when delivered in multidisciplinary rehabilitation clinics. Taken together with the current findings, evidence suggests that these types of interventions may be an important avenue for future research. However, the efficacy of cognitive behavioral and mindfulness interventions may be constrained by the level of an individual’s executive functioning and cognitive training interventions may be needed as a first step to enhance executive function skills prior to delivering other psychological interventions.
This study had several limitations that can provide directions for future research. First, these findings are cross-sectional and consequently the direction of effects among executive functioning, coping, and symptoms of anxiety and depression cannot be determined. Future research using longitudinal designs will be important to disentangle potential causal pathways among these variables. Second, coping skills and symptoms of anxiety and depression were measured using patient self-reports and the association between these variables may have been inflated due to shared method variance. Future research should include other methods and sources to assess these factors (e.g., diagnostic interviews of psychiatric symptoms, interviewer-based measures of stress, or reports from caregivers and other informants). Third, the associations among the key variables were not found in the full sample and were only significant when adults in the top three quartiles were retained in the analyses. Future research with larger samples is needed to understand possible curvilinear associations among these variables.
Given the limited sample size in the present study and in light of previous research showing that individuals who use secondary control coping skills in response to uncontrollable stress experience fewer psychiatric symptoms (for a review see Compas et al., 2017), we chose to focus exclusively on secondary control coping in the current analyses. However, future research that includes models with additional types of coping (e.g., primary control coping or disengagement coping) may also be important in understanding the complex relationship between executive functioning and emotional distress in the HD population. Another direction for future research may be to test models in which a combined measure of executive functioning is associated with coping and neuropsychiatric symptoms, as opposed to investigating subunits of executive functioning.
These limitations notwithstanding, the findings presented here provide valuable new insights into factors that are associated with increased risk for symptoms of anxiety and depression in adults with HD and suggest avenues to bolster resilience in this population.
Key Points.
Question: What is the relationship between aspects of executive functioning, specifically inhibitory control/attention and working memory skills, coping with stress, and neuropsychiatric symptoms in adults with Huntington’s disease (HD)?
Findings: Better performance on an inhibitory control/attention task was associated with greater use of secondary control coping skills (e.g., acceptance, cognitive reappraisal, positive thinking), which in turn was related to lower symptoms of anxiety and depression in a subsample of adults with HD.
Importance: These findings have potentially important implications for interventions to reduce anxiety and depression in adults with HD by enhancing executive function skills and skills to cope with stress.
Next Steps: Future research is needed to better understand the developmental path of the associations among executive function skills, coping, and anxiety and depression over the course of the progression of HD.
Acknowledgments
This research was supported by funds from the Griffin Family Foundation, the CHDI Foundation, and grant T32-MH018921 from the National Institute of Mental Health.
Footnotes
There are no conflicts of interest to declare.
References
- Andreotti C, Thigpen JE, Dunn MJ, Watson K, Potts J, Reising MM, …Compas BE (2013). Cognitive reappraisal and secondary control coping: associations with working memory, positive and negative affect, and symptoms of anxiety/depression. Anxiety, Stress & Coping, 26(1), 20–35. 10.1080/10615806.2011.631526 [DOI] [PubMed] [Google Scholar]
- Andrews SC, Domínguez JF, Mercieca EC, Georgiou-Karistianis N, & Stout JC (2015). Cognitive interventions to enhance neural compensation in Huntington’s disease. Neurodegenerative Disease Management, 5(2), 155–164. 10.2217/nmt.14.58 [DOI] [PubMed] [Google Scholar]
- Arran N, Craufurd D, & Simpson J (2014). Illness perceptions, coping styles and psychological distress in adults with Huntington’s disease. Psychology, Health & Medicine, 19(2). 10.1080/13548506.2013.802355 [DOI] [PubMed] [Google Scholar]
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, …Tabrizi SJ. (2015). Huntington disease. In Nature Reviews Disease Primers (Vol. 1) Nature Publishing Group. 10.1038/nrdp.2015.5 [DOI] [PubMed] [Google Scholar]
- Campbell LK, Scaduto M, van Slyke D, Niarhos F, Whitlock JA, & Compas BE (2009). Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. Journal of Pediatric Psychology, 34(3), 317–327. 10.1093/jpepsy/jsn080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Goodnight S, Kratz AL, Stout JC, McCormack MK, Paulsen JS… Ready RE. (2019). Validation of Neuro-QoL and PROMIS mental health patient reported outcome measures in persons with Huntington disease. Journal of Huntington’s Disease, 8(4), 467–482. 10.3233/JHD-190364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, & Heaton RK (2015). Demographically corrected normative standards for the English version of the NIH Toolbox Cognition Battery. Journal of the International Neuropsychological Society, 21(5). 10.1017/S1355617715000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriegio AE, Pfalzer AC, Hale L, McDonell KE, Claassen DO, & Compas BE (2020). Investigating the interplay of working memory, affective symptoms, and coping with stress in offspring of parents with Huntington’s disease. Neuropsychology, 34(7). 10.1037/neu0000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Jaser SS, Bettis AH, Watson KH, Gruhn MA, Dunbar JP, … Thigpen JC (2017). Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychological Bulletin, 143(9), 939–991. 10.1037/bul0000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, & Saltzman H (2000). Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology, 68(6), 976–992. 10.1037/0022-006X.68.6.976 [DOI] [PubMed] [Google Scholar]
- Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, & Wagster MV (2010). Assessment of neurological and behavioural function: the NIH Toolbox. In The Lancet Neurology (Vol. 9, Issue 2, pp. 138–139). 10.1016/S1474-4422(09)70335-7 [DOI] [PubMed] [Google Scholar]
- Ghielen I, Rutten S, Boeschoten RE, Houniet-de Gier M, van Wegen EEH, van den Heuvel OA, & Cuijpers P (2019). The effects of cognitive behavioral and mindfulness-based therapies on psychological distress in patients with multiple sclerosis, Parkinson’s disease and Huntington’s disease: Two meta-analyses. Journal of Psychosomatic Research, 122, 43–51. 10.1016/j.jpsychores.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Goh AMY, Wibawa P, Loi SM, Walterfang M, Velakoulis D, & Looi JCL (2018). Huntington’s disease: Neuropsychiatric manifestations of Huntington’s disease. Australasian Psychiatry, 26(4), 366–375. 10.1177/1039856218791036 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. [Google Scholar]
- Hayes AF (2017). Introduction to Mediation, Moderation, and Conditional Process Analysis Second Edition. The Guilford Press. [Google Scholar]
- Helder DI, Kaptein AA, van Kempen GMJ, Weinman J, van Houwelingen HC, & Roos RAC (2002). Living with Huntington’s disease: Illness perceptions, coping mechanisms, and patients’ well-being. British Journal of Health Psychology, 7(4), 449–462. 10.1348/135910702320645417 [DOI] [PubMed] [Google Scholar]
- Ho A, & Hocaoglu M (2011). Impact of Huntington’s across the entire disease spectrum: The phases and stages of disease from the patient perspective. Clinical Genetics, 80(3), 235–239. 10.1111/j.1399-0004.2011.01748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington Study Group. (1996). Unified Huntington’s Disease Rating Scale: Reliability and Consistency. Movement Disorders, 11(2), 136–142. [DOI] [PubMed] [Google Scholar]
- Kalkhoven C, Sennef C, Peeters A, & van den Bos R (2014). Risk-taking and pathological gambling behavior in Huntingtons disease. Frontiers in Behavioral Neuroscience, 8. 10.3389/fnbeh.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9). 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, & Löwe B (2010). The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. General Hospital Psychiatry, 32(4). 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Lahr J, Minkova L, Tabrizi SJ, Stout JC, Klöppel S, & Scheller E (2018). Working memory-related effective connectivity in Huntington’s disease patients. Frontiers in Neurology, 9. 10.3389/fneur.2018.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J & Gotlib IH (2019). Depression: A cognitive perspective. Clinical Psychology Review, 69, 51–66. 10.1016/j.cpr.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, & Fritz MS (2007). Mediation Analysis. Annual Review of Psychology, 58, 593–614. 10.1146/annurev.psych.58.110405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglani HR, Samimy S, Schirda B, Nicholas JA, & Prakash RS (2020). Effects of 4-week mindfulness training versus adaptive cognitive training on processing speed and working memory in multiple sclerosis. Neuropsychology, 34(5), 591–604. 10.1037/neu0000633 [DOI] [PubMed] [Google Scholar]
- Mestre TA, van Duijn E, Davis AM, Bachoud-Levi AC, Busse M, Anderson KE…. Members of the MDS Committee on Rating Scales Development. (2016). Rating scales for behavioral symptoms in Huntington’s disease: Critique and recommendations. Movement Disorders, 31(10), 1466–1478. 10.1002/mds.26675 [DOI] [PubMed] [Google Scholar]
- Mestre TA, Bachoud-Lévi AC, Marinus J, Stout JC, Paulsen JS, Como P, … Members of the MDS Committee on Rating Scales Development. (2018). Rating scales for cognition in Huntington’s disease: Critique and recommendations. Movement Disorders, 33(2):187–195. doi: 10.1002/mds.27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missner S, & Anderson K (2018). F42 Prevalence of psychiatric morbidity in the huntington’s disease community. Journal of Neurology, Neurosurgery & Psychiatry, 89(Suppl 1), A55-. 10.1136/jnnp-2018-EHDN.146 [DOI] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Moran TP (2016). Anxiety and working memory capacity: A meta-analysis and narrative review. Psychological Bulletin, 142(8), 831–864. 10.1037/bul0000051.supp [DOI] [PubMed] [Google Scholar]
- Mörkl S, Müller NJ, Blesl C, Wilkinson L, Tmava A, Wurm W, … Painold A (2016). Problem solving, impulse control and planning in patients with early- and late-stage Huntington’s disease. European Archives of Psychiatry and Clinical Neuroscience, 266(7), 663–671. 10.1007/s00406-016-0707-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles O, Grafton B, & MacLeod C (2020). Anxiety & inhibition: dissociating the involvement of state and trait anxiety in inhibitory control deficits observed on the anti-saccade task. Cognition and Emotion, 34(8). 10.1080/02699931.2020.1802229 [DOI] [PubMed] [Google Scholar]
- Nehl C, Ready RE, Hamilton J, & Paulsen JS (2001). Effects of depression on working memory in presymptomatic Huntington’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(3). 10.1176/jnp.13.3.342 [DOI] [PubMed] [Google Scholar]
- Paulsen JS (2011). Cognitive impairment in Huntington disease: Diagnosis and treatment. Current Neurology and Neuroscience Reports, 11(5). 10.1007/s11910-011-0215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Ferneyhough Hoth K, Kanz JE, Benjamin M, Conybeare R, … The Huntington Study Group. (2005). Depression and stages of Huntington’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(4), 496–502. http://neuro.psychiatryonline.org [DOI] [PubMed] [Google Scholar]
- Petkus AJ, Vincent Filoteo J, Schiehser DM, Gomez ME, & Petzinger G (2019). Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson’s disease: A bidirectional analysis. Neuropsychology, 33(1), 35–46. 10.1037/neu0000498 [DOI] [PubMed] [Google Scholar]
- Prussien KV, DeBaun MR, Yarboi J, Bemis H, McNally C, Williams E, & Compas BE (2018). Cognitive function, coping, and depressive symptoms in children and adolescents with sickle cell disease. Journal of Pediatric Psychology, 43(5), 543–551. 10.1093/jpepsy/jsx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JA, Harrington DL, Durgerian S, Reece C, Mourany L, Koenig K, …Rao SM (2014). Disruption of response inhibition circuits in prodromal Huntington disease. Cortex, 58, 72–85. 10.1016/j.cortex.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reising MM, Bettis AH, Dunbar JP, Watson KH, Gruhn M, Hoskinson KR, & Compas BE (2018). Stress, coping, executive function, and brain activation in adolescent offspring of depressed and nondepressed mothers. Child Neuropsychology, 24(5), 638–656. 10.1080/09297049.2017.1307950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BW, Basso MR, Miller AK, Whiteside DM, & Combs D (2019). Executive function, impulsivity, and risky behaviors in young adults. Neuropsychology, 33(2), 212–221. 10.1037/neu0000510 [DOI] [PubMed] [Google Scholar]
- Robinson KE, Pearson MM, Cannistraci CJ, Anderson AW, Kuttesch JF, Wymer K, …Compas BE. (2015). Functional neuroimaging of working memory in survivors of childhood brain tumors and healthy children: Associations with coping and psychosocial outcomes. Child Neuropsychology, 21(6), 779–802. 10.1080/09297049.2014.924492 [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, & Tang D (2015). Individual differences in executive functioning and their relationship to emotional processes and responses. Current Directions in Psychological Science, 24(2), 93–98. 10.1177/0963721414555178 [DOI] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, & Löwe B (2006). A brief measure for assessing Generalized Anxiety Disorder. Archives of Internal Medicine, 166(10). 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Stopford C, Ferrer-Duch M, Moldovan R, & MacLeod R (2020). Improving follow up after predictive testing in Huntington’s disease: Evaluating a genetic counselling narrative group session. Journal of Community Genetics, 11, 47–58. 10.1007/s12687-019-00416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell, 72, 971–983. [DOI] [PubMed] [Google Scholar]
- Tu KM, Erath SA, & El-Sheikh M (2016). Coping responses moderate prospective associations between marital conflict and youth adjustment. Journal of Family Psychology, 30(5). 10.1037/fam0000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi N, Chiaravalloti ND, Beaumont JL, Kisala PA, Mungas D, …Gershon R. (2014). NIH Toolbox Cognition Battery (NIHTB-CB): List sorting test to measure working memory. Journal of the International Neuropsychological Society, 20(6), 599–610. 10.1017/S135561771400040X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamos M, Hambridge J, Edwards M, & Conaghan J (2007). The Impact of Huntington’s disease on family life. Psychosomatics, 48(5), 400–404. [DOI] [PubMed] [Google Scholar]
- White LK, Helfinstein SM, Reeb-Sutherland BC, Degnan KA, & Fox NA (2009). Role of attention in the regulation of fear and anxiety. Developmental Neuroscience, 31(4), 309–317. 10.1159/000216542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield T, Barnhofer T, Acabchuk R et al. The effect of mindfulness-based programs on cognitive function in adults: A systematic review and meta-analysis. (2021, published online). Neuropsychology Review 10.1007/s11065-021-09519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- You SC, Geschwind MD, Sha SJ, Apple A, Satris G, Wood KA, …Possin KL. (2014). Executive functions in premanifest Huntington’s disease. Movement Disorders, 29(3), 405–409. 10.1002/mds.25762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehringer J, Falquez R, Schubert A-L, Nees F, & Barnow S (2018). Neural correlates of reappraisal considering working memory capacity and cognitive flexibility. Brain Imaging and Behavior, 12(6). 10.1007/s11682-017-9788-6 [DOI] [PubMed] [Google Scholar]


