Abstract
On 26 August 2022, the European Commission asked EFSA to provide scientific and technical assistance according to Article 21(2) of Regulation (EC) No 1107/2009 concerning the review of the approval of the active substance pirimicarb and to deliver a statement on whether the applicable approval criteria may still be considered fulfilled, taking into consideration the information submitted by the applicant and the assessment of the rapporteur Member State, Sweden and, where applicable, the results of a discussion with experts from Member States. The current statement contains a summary of the main findings of the assessment of the risks to consumers from the exposure to metabolites of pirimicarb through dietary intake, the risks to human health through non‐dietary exposure and the acute risk to birds from the representative uses of pirimicarb assessed for the first approval and additionally, from the representative uses as submitted as part of the renewal of approval. Concerns are reported where identified.
Keywords: pirimicarb, peer review, risk assessment, pesticide, insecticide, genotoxicity of metabolites, dietary and non‐dietary exposure
Summary
Pirimicarb is deemed to have been approved on 1 February 2007 under Regulation (EC) No 1107/2009 by Commission Directive 2006/39/EC, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulations (EU) No 541/2011, 2021/566 and 2022/378.
An application for the renewal of the approval of pirimicarb was submitted to the original rapporteur Member State (RMS), the United Kingdom, and the co‐rapporteur Member State, Sweden, in accordance with Commission Implementing Regulation (EU) No 844/2012. Following the departure of the United Kingdom from the EU, Sweden was designated as RMS. The peer review process of the renewal assessment remains ongoing. In accordance with Article 13(3a) of Commission Implementing Regulation (EU) No 844/2012, EFSA requested further information related to the implementation of the scientific criteria for the determination of endocrine‐disrupting (ED) properties introduced by Regulation (EU) No 2018/605.
The RMS subsequently informed the European Commission that during the peer review process concerns have been identified about the genotoxicity of some metabolites of pirimicarb to which consumers may be exposed through dietary intake, and for some metabolites, a conclusion on general toxicity could not be made. Furthermore, following an exchange with EFSA, it also became apparent that for some of the representative uses of pirimicarb, non‐dietary exposure exceeds the reference values established during the peer review. In addition, an acceptable acute risk to birds could not be demonstrated for any of the representative field uses.
Given those concerns, there are indications that pirimicarb may no longer satisfy the approval criteria in Article 4 of Regulation (EC) No 1107/2009.
Therefore, pursuant to Article 21(1) of Regulation (EC) No 1107/2009, the European Commission informed the applicant about the concerns and invited it to provide comments or information. The applicant submitted further information in January 2022, which has been evaluated by the designated RMS after Brexit, Sweden, who submitted its evaluation to the European Commission and EFSA on 16 June 2022.
In accordance with Article 21(2) of Regulation (EC) No 1107/2009, in August 2022, EFSA was requested to provide scientific and technical assistance concerning the review of the approval of the active substance pirimicarb and to deliver a statement on whether the applicable approval criteria may still be considered fulfilled, taking into consideration the information submitted by the applicant and the assessment of the RMS and, where applicable, the results of a discussion with experts from Member States. Specifically, EFSA was requested to consider (i) the risks to consumers from the exposure to metabolites of pirimicarb through dietary intake, (ii) the risks to human health through non‐dietary exposure and (iii) the acute risk to birds from the representative uses of pirimicarb assessed for the first approval and additionally, from the representative uses reflecting the currently authorised uses as submitted as part of the renewal of approval. The following conclusions are derived.
As regards the dietary exposure, the consumer risk assessment for none of the uses proposed under the renewal of the approval could be finalised due to insufficient data to address the genotoxic potential of the metabolites R34885, R31805, R16210, R34865 and R406405 and general toxicity of R406405, found relevant in plant and/or in livestock. Furthermore, no sufficient trials were available in primary and rotational crops to investigate the magnitude of residues. As regards the representative uses for the first approval, the consumer risk assessment could also not be finalised in view of the outstanding data from the renewal of the approval with impact on the consumer risk assessment.
As regards the human health assessment through non‐dietary exposure, no risks were identified for the existing uses on wheat crops assessed for the first approval and for the renewal uses on wheat crops, sugar beet crops, and ornamentals in greenhouse (in case of downward spraying) when appropriate risk mitigation measures are adopted, while risks were identified for the use on ornamentals in greenhouse (in case of upward spraying) for operators, bystanders and residents. As regards the acute risk to birds, low acute risk to birds could be concluded for the representative greenhouse uses in ornamentals in permanent structures, whereas high risk was indicated for all uses in wheat (from the first approval and renewal of approval), the uses in sugar beet and eventual uses in ornamentals in non‐permanent greenhouses.
1. Introduction
Pirimicarb is deemed to have been approved on 1 February 2007 under Regulation (EC) No 1107/20091 by Commission Directive 2006/39/EC2, in accordance with Commission Implementing Regulation (EU) No 540/20113, as amended by Commission Implementing Regulations (EU) No 541/20114, 2021/5665 and 2022/3786.
An application for the renewal of the approval of pirimicarb was submitted to the original rapporteur Member State (RMS), the United Kingdom, and the co‐rapporteur Member State, Sweden, in accordance with Commission Implementing Regulation (EU) No 844/2012. Following the departure of the United Kingdom from the EU, Sweden was designated as RMS. The peer review process of the renewal assessment remains ongoing. In accordance with Article 13(3a) of Commission Implementing Regulation (EU) No 844/2012, EFSA requested further information related to the implementation of the scientific criteria for the determination of endocrine‐disrupting (ED) properties introduced by Regulation (EU) No 2018/605, setting a deadline of 13 July 2022 for providing the requested information.
The RMS subsequently informed the European Commission that during the peer review process, concerns have been identified about the genotoxicity of some metabolites of pirimicarb to which consumers may be exposed through dietary intake, and for some metabolites, a conclusion on general toxicity could not be made. EFSA had already asked the applicant (Syngenta) to provide additional information on those metabolites during the renewal peer review, in accordance with Article 13(3) of Implementing Regulation (EU) No 844/2012; however, concerns remained or cannot be excluded following evaluation of the additional information provided and following expert discussion.
Furthermore, following an exchange with EFSA, it also became apparent that for some of the representative uses of pirimicarb, non‐dietary exposure exceeds the reference values established during the peer review. In addition, an acceptable acute risk to birds could not be demonstrated for any of the representative field uses.
Given those concerns, there are indications that pirimicarb may no longer satisfy the approval criteria in Article 4 of Regulation (EC) No 1107/2009.
Therefore, pursuant to Article 21(1) of Regulation (EC) No 1107/2009, the European Commission informed the applicant about the concerns and invited it to provide comments or information. The applicant submitted further information in January 2022, which has been evaluated by the designated RMS after Brexit, Sweden, who submitted its evaluation to the European Commission and EFSA on 16 June 2022.
On 26 August 2022, EFSA was requested by the European Commission to provide scientific and technical assistance concerning the review of the approval of the active substance pirimicarb and to deliver a statement on whether the applicable approval criteria may still be considered fulfilled, taking into consideration the information submitted by the applicant and the assessment of the RMS and, where applicable, the results of a discussion with experts from Member States, within 3 months following such consultations have been completed or once EFSA receives the updated assessment by the RMS following those consultations, if such update is needed.
More specifically, EFSA was requested to consider (i) the risks to consumers from the exposure to metabolites of pirimicarb through dietary intake, (ii) the risks to human health through non‐dietary exposure and (iii) the acute risk to birds from the representative uses of pirimicarb assessed for the first approval and additionally, from the representative uses reflecting the currently authorised uses as submitted as part of the renewal of approval (see Appendix A).
Based on that mandate, an expert consultation was organised in September 2022 and subsequently, following receipt of the revised assessment from the RMS, EFSA prepared a draft statement in October 2022 which was circulated to all Member States for commenting via a written procedure.
A key supporting document to this statement is the peer review report (EFSA, 2022a), which is a compilation of the documentation developed to evaluate and address all issues raised in the course of the peer review of the renewal of the active substance pirimicarb, from the initial commenting phase to the preparation of this statement. For reasons of completeness and transparency, the peer review report comprises all background documents, which were developed and finalised during the ongoing renewal process up to the production of the present statement and relate to all sections of the risk assessment, as follows:
the comments received on the RAR;
the reporting table (13 July 2018);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA statement.
Given the importance of the RAR, including its revisions prepared in the context of the renewal up to the production of the present statement (United Kingdom/Sweden, 2022) as well as the stand‐alone addendum prepared in the context of the Art 21 review (Sweden, 2022), and the peer review report, all these documents are considered as background documents to this statement and thus are made publicly available.
It is recommended that this statement and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this statement is based.
1.1. Terms of Reference as provided by the requestor
In accordance with Article 21(2) of Regulation (EC) No 1107/2009, on 26 August 2022, EFSA was mandated by the European Commission to provide scientific and technical assistance concerning the review of the approval of the active substance pirimicarb and to deliver a statement on whether the applicable approval criteria may still be considered fulfilled, taking into consideration the information submitted by the applicant and the assessment of the RMS and, where applicable, the results of a discussion with experts from Member States.
Specifically, EFSA was requested to consider the following aspects for the representative uses of pirimicarb assessed for the first approval (European Commission, 2006) and for the representative uses reflecting the currently authorised uses, as submitted as part of the renewal of approval:
the risks to consumers from the exposure to metabolites of pirimicarb through dietary intake;
the risks to human health through non‐dietary exposure;
the acute risk to birds.
Given the need for discussion of some aspects with experts from Member States, the outcome of which could be relevant for the assessment, EFSA was requested to provide its scientific assistance in the form of a statement within a 3‐month time period starting once such consultations have been completed or once EFSA received the updated assessment by the RMS following those consultations, if such update was needed. An updated assessment after the expert consultation was provided by the RMS on 3 October 2022 (Sweden, 2022).
Following request from the European Commission in accordance with Article 21(1) of Regulation (EC) No 1107/2009, the applicant submitted to EFSA and all Member States the information it provided to the Commission and the RMS in January 2022.
The active substance and the formulation for representative uses
Pirimicarb is the ISO common name for 2‐(dimethylamino)‐5,6‐dimethylpyrimidin‐4‐yl dimethylcarbamate (IUPAC).
The formulation for representative uses for the evaluation of the first approval and renewal of approval was ‘Pirimor’, a water dispersible granule (WG) containing 500 g/kg pirimicarb.
The representative uses evaluated were foliar spray applications on wheat (winter and summer), sugar beet and ornamentals (pot plants, permanent greenhouse7) for control of aphids. Full details of the good agricultural practice (GAPs) can be found in Appendix A.
2. Assessment
2.1. Toxicological reference values of pirimicarb and assessment of metabolites
The toxicological reference values of pirimicarb were discussed at the Pesticides Peer Review Experts' Meetings 190 in January 2019 and PREV 07 in June 2019 in the context of the renewal process, and at the Experts' Teleconference 89 in September 2022 in the context of the review under Article 21 of Regulation (EC) No 1107/2009.
Based on the available data in June 2019, the experts8 agreed to increase the uncertainty factors (UF) for the derivation of the toxicological reference values, in order to account for the lack of specific data addressing the sensitivity of pup to acetylcholinesterase (AChE) inhibition. The resulting acceptable daily intake (ADI) and acceptable operator exposure level (AOEL) are 0.007 mg/kg body weight (bw) per day, based on the no‐observed adverse effect level (NOAEL) of 3.5 mg/kg bw per day from the 1‐year dog study supported by the 2‐year rat study, and applying an overall UF of 500 (and no correction of the AOEL for oral absorption). The resulting acute reference dose (ARfD) and acute acceptable operator exposure level (AAOEL) are 0.01 mg/kg bw, based on the NOAEL of 10 mg/kg bw from the acute neurotoxicity study, and applying an overall UF of 1,000.
As part of the additional information submitted by the applicant in January 2022, new in vitro data for AChE enzyme inhibition and in vivo data with pirimicarb were assessed by the RMS (Sweden, 2022) and discussed by the experts.9 These data were concluded as supplementary, and insufficient to justify the removal of the extra uncertainty factors. Consequently, for the derivation of the toxicological reference values, the experts confirmed the applicability of an increased UF in order to take into account for the lack of specific data addressing the sensitivity of pups to AChE inhibition.
The toxicological profile of the metabolites of pirimicarb was discussed at the Pesticides Peer Review Experts' Meeting 190 in January 2019 and at the Pesticides Peer Review Experts' Teleconference 89 in September 2022. The discussion in the second experts' teleconference 89 was based on the additional information and assessment provided by the applicant for the metabolites R34885, R31680, R31805, R34865, R406405 and R16210.
For the metabolite R34836 10, the majority of the experts agreed that it was unlikely to be genotoxic (by read across from the parent), and that the reference values of pirimicarb could be applied for the consumer risk assessment.
For the metabolite R34885, three new genotoxicity tests were provided. The negative results in the in vitro mammalian cell gene mutation test and in the in vitro micronucleus test were not considered sufficient to address the mutagenic potential based on the equivocal results in the Ames test (data gap).
For the metabolite R31680, the three new genotoxicity tests (Ames, in vitro mammalian cell gene mutation test, in vitro micronucleus test) were all negative. R31680 is therefore considered unlikely to be genotoxic, and confirmed to be of lower systemic toxicity than the parent compound based on acute toxicity results.
For the metabolite R31805, positive results had been observed in the in vitro mouse lymphoma assay. The negative results in the new in vitro mammalian cell gene mutation test, submitted instead of an in vivo Comet assay (as agreed during the peer review) were considered insufficient to conclude on the genotoxicity potential (mutagenic endpoint) of the metabolite (data gap).
For the metabolite R34865, positive results had been observed in the in vitro mouse lymphoma assay. The negative results in the new in vitro mammalian cell gene mutation test, submitted instead of an in vivo Comet assay (as agreed during the peer review 10 ) were considered insufficient to conclude on the genotoxicity potential (mutagenic endpoint) of the metabolite (data gap). Pending on the outcome of the genotoxicity assessment, the toxicological reference values agreed during the peer review could be applied.
For the metabolite R16210, as no data have been made available, the assessment of its genotoxic potential cannot be concluded (data gap).
For the metabolite R406405, as no experimental data have been made available and the applicability of the read across for genotoxicity (from the metabolites R31805 and R34865) could not be agreed, 10 both the genotoxic and general toxicity assessment cannot be concluded (data gap).
2.2. Risks to consumers from the exposure to metabolites of pirimicarb through dietary intake
The assessment in this section is based on the following guidance documents: OECD (2009, 2011), European Commission (2011) and JMPR (2004, 2007).
Pirimicarb was discussed at the Pesticides Peer Review Experts' meeting 191 in January–February 2019 in the context of the renewal process.
To address the mandate, the representative uses in wheat evaluated for the first approval (European Commission, 2006) was considered for the consumer dietary risk assessment. Under the peer review process for renewal, the representative uses in wheat, sugar beet and ornamentals were assessed for the consumer risk assessment via dietary intake (see relevant GAPs in the Appendix A).
In the context of the current mandate, the applicant did not provide new data in the Residue section compared to the data submitted in the framework of the renewal process.
The available primary crops metabolism studies in lettuce, potatoes, apples and wheat by foliar application on pyrimidine ring showed that pirimicarb was the major residue in lettuce, apple fruits and wheat grain and in limited amount in potatoes (1.7% TRR). Metabolite R34836 was found in significant amounts in lettuce (21% TRR) while in the rest of the crops, it was below 10% TRR. Although metabolites R34885 and R31805 in most of the investigated crops were recovered at levels below 10% TRR, the absolute amount was found to be relevant (0.14 mg/kg and 1 mg/kg in lettuce respectively); metabolite R34865 was found up to 0.72 mg/kg in lettuce only. Metabolite R16210 was recovered at 32% TRR in potato tubers although the total radioactivity of residues in potato tubers was very low (0.04 mg/kg). It was noted that the genotoxic profile of R16210 was not resolved (see Section 2.1).
In the two rotational crops metabolism studies conducted in lettuce, radish, wheat and millet, similar metabolic patterns were observed as in primary crops. Pirimicarb was recovered in radish roots (up to 20% TRR) but also in the other crops in limited amounts. Metabolites R34836, R31805, R34865 and R34885 were recovered in relevant amounts either in relative or in absolute amounts of TRR in most of the crops (e.g. 0.13 mg/kg in radish leaves for R34865). It is also noted from the data submitted under the renewal that R31805, R34865 and R34885 are persistent compounds in soil.
During the renewal peer review process, two rotational crops field trials conducted in the US at sufficiently high concentration (i.e. 2,240 g/ha) were submitted; they cover the application rate and the soil plateau concentration of pirimicarb. In those trials, only pirimicarb, R34836 and R238177 were analysed, and the results confirmed the occurrence of pirimicarb and R34836 residues in rotational crops. Metabolites R31805, R34865 and R34885 were not investigated in the field rotational crops studies, although, based on the results from the metabolism studies and in view of their persistency in soil, residues are expected in rotational crops. Consequently, a data gap was identified during the expert's meeting11 for sufficient rotational field trials analysed for parent and metabolites: R31805, R34865, R34836 and R34885. It is also noted that the genotoxic profile of several metabolites R31805, R34865 and R34885 was not complete (see Section 2.1). Considering all the above information from the plant metabolism studies, the outstanding data in field rotational trials and the outcome from the mammalian toxicology section, the risk assessment residue definition is proposed as sum of pirimicarb and R34836, expressed as pirimicarb. This proposal is provisional pending the submission of the additional toxicological data needed in mammalian toxicology for the above‐mentioned metabolites and the additional rotational crop trials.
As regards the livestock assessment studies, four metabolism studies were submitted in laying hens and lactating goats. In laying hens, pirimicarb was extensively metabolised to R34865 (up to 48% TRRs in eggs white) and R31680 (up to 50% TRR in muscle). In ruminants, R34865, R31680, R406405 were found in milk up to 29%, 16% and 16% TRR, respectively. It is highlighted that the genotoxic potential of R34865 and R406405 cannot be excluded (see Section 2.1). Considering the occurrence of R406405 in milk and pending its genotoxicity profile, its inclusion in the risk assessment residue definition should be reconsidered. Currently, the residue definition for the risk assessment in ruminants provisionally includes residues of R34865 and R31680, expressed as pirimicarb. It is noted that the fate of R34836 in animal matrices was not investigated although triggered for ruminants only by the representative uses assessed under the renewal. Therefore, information on the fate of R34836 in ruminants needs to be provided (data gap). Further to that, the investigation of R34836 in poultry is not needed, since the metabolism studies conducted with pirimicarb were highly overdosed and residues > 0.01 mg/kg are not expected in poultry matrices. The risk assessment residue definition in poultry is proposed as pirimicarb by default.
Field trials to support the representative uses under renewal were sufficient only for wheat grain. For the wheat straw, only six trials were submitted for Northern Europe (NEU) use, thus two additional GAP compliant trials are needed (data gap). No residue trials were submitted for sugar beet leaves to support the representative use in NEU and need to be provided (data gap).
The stability of pirimicarb residues and R34836 was demonstrated in several commodities up to 18 months when the samples are stored at least at −15°C. Although storage stability data were provided in cabbage and lettuce (high water content), the sugar beet leaves cannot be considered as covered by available data since the stability of residues should be demonstrated in three different crops with high water content to cover the whole group of commodities (data gap).
An indicative consumer risk assessment for the representative uses as part of the renewal was conducted by using the toxicological reference values, ADI of 0.007 mg/kg bw per day and the ARfD of 0.01 mg/kg bw derived during the renewal peer review process, the available residue trials on wheat, sugar beet and leaves by using the PRIMo rev. 3.1. The chronic exposure (TMDI) resulted in maximum 7% of the ADI (GEMs/Food G06 diet),12 while the acute intake (IESTI) was 11% of the ARfD (NL adults). However, the overall consumer risk assessment cannot be finalised for all the uses due to the non‐finalised risk assessment residue definitions in plant and livestock, the outstanding data in primary and rotational crops and the lack of conclusion on the genotoxic potential of R31805, R34885, R34865, R406405 and R16210 and lack of data on general toxicity on R406405 (see Section 2.1). Regarding the representative use on ornamentals in greenhouse, although they are not foodstuff, they could be grown in rotation with other food crops. Thus, the consumer exposure via dietary intake cannot be excluded for the ornamentals use that leads to the consumer risk assessment not finalised also for ornamentals.
An indicative consumer risk assessment was conducted also for the uses evaluated under the first approval with PRIMo rev. 3.1 and the toxicological reference values of ADI of 0.007 mg/kg bw per day and ARfD of 0.01 mg/kg bw derived during the peer review process for renewal, and the results from the residue trials in wheat grain. The chronic exposure (TMDI) resulted in maximum 6% of the ADI (GEMs/Food G06 diet), while the acute intake (IESTI) was 9% of the ARfD (UK children). However, also for these uses, the consumer risk assessment could not be finalised considering the lack of conclusion on the genotoxic potential of R31805, R34885, R34865, R406405 and R16210 and lack of data on general toxicity on R406405, found in relevant amounts in plant and animal commodities.
2.3. Risks to human health through non‐dietary exposure
The non‐dietary exposure assessment of pirimicarb was discussed at the Pesticides Peer Review Experts' Teleconference 89 in September 2022, based on the following guidance documents: EFSA, 2014b, EFSA PPR Panel, 2017 and EFSA, 2022b.
With regard to dermal absorption (DA), the agreed values during the peer review meeting13 were 1% for the undiluted formulation and 14% for the dilution of 0.4 g a.s./L, based on experimental data and applicable to the uses of the existing approval (wheat). Pro‐rata corrections were agreed for the different spray concentrations in the renewal uses, including 19% for the use on winter wheat (0.3 g/L), 18% for the use on summer wheat (0.31 g/L), 15% for the use on sugar beet (0.375 g/L) and 22% for the use on ornamentals (0.25 g/L).
Based on two dislodgeable foliar residue (DFR) trials on indoor ornamentals, a refined DFR value of 1.74 μg/cm2/kg a.s./ha has been agreed for the use on ornamentals, and a refined DT 50 value of 4.65 days was concluded as applicable to pirimicarb for all uses.
2.3.1. Operator exposure
For the existing uses on wheat crops from the first approval, the predicted systemic exposure estimates using the EFSA calculator (EFSA, 2014b) are below the (A)AOEL with the use of drift reduction nozzles and appropriate personal protective equipment (PPE) (i.e. workwear and gloves during mixing, loading and application).
For the renewal uses on winter wheat, summer wheat and sugar beet crops, the predicted systemic exposure estimates using the EFSA calculator (EFSA, 2014b) are below the (A)AOEL with the use of drift reduction nozzles and appropriate PPE (i.e. workwear and gloves during mixing, loading and application).
For the renewal use on ornamentals in greenhouses, the predicted systemic exposure estimates using the new EFSA calculator (EFSA, 2022b) are above the AAOEL for upward spraying (high ornamentals, e.g. on shelves or staggered plant pots), and below the (A)AOEL for downward spraying (low ornamentals), considering appropriate PPE and respiratory protective equipment (RPE).
It is noted that the number of applications has no impact on the predicted exposure estimates for the operators, and that exposure estimates for use in greenhouses are for closed permanent structures (it is considered that non‐permanent greenhouses would be closed during the application of the product; therefore, operator exposure for permanent structures would also apply).
An overview of the risk assessment for operators can be found in Table 1, while detailed results are provided in Appendix B.1.
Table 1.
Risk assessment (RA) for the operators
|
Pirimor WG – Representative uses from the existing approval (European Commission, 2006) Model: EFSA 2015 (last version of calculator from EFSA 2014b) | |||
| Use: Wheat, Southern EU, outdoor use, 0.21 kg a.s. per ha | |||
| Application method | PPE/RPE * | Short‐term RA (%AOEL) | Acute RA (%AAOEL) |
| Vehicle‐mounted, downward spray | Workwear (Ww) | 93 | 419 |
| Ww + gloves + RPE (MLA**) | 10 | 114 | |
| Ww + gloves (MLA**) + drift reduction | 21 | 52 | |
| Use: Wheat, Northern EU, outdoor use, 0.15 kg a.s. per ha | |||
| Vehicle‐mounted, downward spray | Workwear (Ww) | 71 | 336 |
| Ww + gloves + RPE (MLA**) | 8 | 108 | |
| Ww + gloves (MLA**) + drift reduction | 18 | 51 | |
|
Pirimor WG – Representative uses from the renewal process Model: EFSA (2015) for outdoor uses (last version of calculator from EFSA, 2014b) and EFSA, 2022b for indoor uses (ornamentals) | |||
| Use: Winter wheat, outdoor use, 0.12 kg a.s. per ha | |||
| Vehicle‐mounted, downward spray | Workwear (Ww) | 71 | 363 |
| Ww + gloves + RPE (MLA**) | 9 | 140 | |
| Ww + gloves (MLA**) + drift reduction | 17 | 50 | |
| Use: Summer (spring) wheat, outdoor use, 0.125 kg a.s. per ha | |||
| Vehicle‐mounted, downward spray | Workwear (Ww) | 71 | 358 |
| Ww + gloves + RPE (MLA**) | 9 | 133 | |
| Ww + gloves (MLA**) + drift reduction | 17 | 50 | |
| Use: sugar beet, outdoor use, 0.15 kg a.s. per ha | |||
| Vehicle‐mounted, downward spray | Workwear (Ww) | 74 | 353 |
| Ww + gloves + RPE (MLA**) | 9 | 115 | |
| Ww + gloves (MLA**) + drift reduction | 18 | 50 | |
|
Use: Low ornamentals (pot plants), greenhouse, 0.3 kg a.s. per ha Input parameters: normal crop density | |||
| Hand‐held application, downward spray | Workwear (Ww) | 94 | > 100 |
| Ww + gloves (MLA**) | < 94 | 45 | |
|
Use: High ornamentals (pot plants), greenhouse, 0.3 kg a.s. per ha Input parameters: normal crop density | |||
| Hand‐held application, upward spray | Ww + gloves + RPE (MLA**) | > 100 | > 100 |
| Trolley application, upward spray | Ww + gloves (MLA**) | 87 | > 100 |
| Ww + gloves + RPE (MLA**) | < 87 | > 100 | |
PPE/RPE: Personal/respiratory (FFP2, P2 and similar) protective equipment.
MLA: during mixing/loading and application tasks.
2.3.2. Worker exposure
For the existing uses on wheat crops from the first approval, the predicted systemic exposure estimates using the EFSA calculator (EFSA, 2014b) and the refined DT50 value of 4.65 days are below the AOEL for a worker performing crop inspection activity and wearing normal workwear.
For the renewal uses on winter/summer (spring) wheat and sugar beet crops, the predicted systemic exposure estimates using the EFSA calculator (EFSA, 2014b) are below the AOEL for a worker performing crop inspection activity and wearing normal workwear.
For the renewal use on ornamentals in greenhouses, the predicted systemic exposure estimates using the EFSA calculators (EFSA, 2014b, 2022b) are above the AOEL for a worker performing cutting and harvesting activities when wearing normal workwear and using gloves. A period of 9 days after one application and 12 days after three applications is required to result in predicted systemic exposure below the AOEL. For a worker performing inspection and maintenance activities (8 h, wearing normal workwear and gloves), a re‐entry interval of 8 days after one application and 10 days after three applications is required to obtain a predicted systemic exposure below the AOEL (EFSA, 2022b). For a worker performing inspection and irrigation activities (2 h, wearing normal workwear), a systemic exposure below the AOEL is predicted for a 14‐day interval between applications (EFSA, 2022b) or when only one application is performed (and re‐entry is done on the day of application after the product has dried).
It is noted that in the case of non‐permanent greenhouse, the exposure estimates for workers are expected to be lower than for permanent greenhouse.
An overview of the risk assessment for workers can be found in Table 2, while detailed results are provided in Appendix B.2.
Table 2.
Risk assessment for the workers
|
Pirimor WG – Representative uses from the existing approval (European Commission, 2006) Model: EFSA (2015) (last version of calculator from EFSA, 2014b) | ||
|
Use: Wheat, Southern EU, outdoor use, 2 × 0.21 kg a.s. per ha Input parameters: 7‐day interval between applications, DT50 4.65 days | ||
| Worker task | PPE | %AOEL |
| Inspection | Ww | 80 |
|
Use: Wheat, Northern EU, outdoor use, 2 × 0.15 kg a.s. per ha Input parameters: 7‐day interval between applications | ||
| Inspection | Ww | 57 |
|
Pirimor WG – Representative uses from the renewal process Model: EFSA (2015) (last version of calculator from EFSA, 2014b) and also EFSA, 2022b for the ornamentals | ||
|
Use: Winter wheat, outdoor use, 1 × 0.12 kg a.s. per ha, Input parameters: DT50 4.65 days | ||
| Inspection | Ww | 46 |
|
Use: Summer (spring) wheat, outdoor use, 2 × 0.125 kg a.s. per ha Input parameters: 14‐day interval between applications, DT50 4.65 days | ||
| Inspection | Ww | 51 |
|
Use: Sugar beet, outdoor use, 1 × 0.15 kg a.s. per ha Input parameters: DT50 4.65 days | ||
| Inspection | Ww | 45 |
|
Use: Low/high ornamentals (pot plants), greenhouse, 3 × 0.3 kg a.s. per ha Input parameters: 7‐day interval between applications, DT50 4.65 days, DFR 1.74 μg/cm2/kg as/ha | ||
| Cutting and harvesting activities* | Ww + gloves | > 500 |
|
Cutting and harvesting activities** (DAA*** 0) (DAA*** 12) |
Ww + gloves |
> 500 < 100 |
|
Inspection and maintenance (8 h)** (DAA*** 0) (DAA*** 10) |
Ww + gloves |
413 < 100 |
|
Inspection and irrigation (2 h)** (DAA*** 0) (DAA*** 1) |
Ww + gloves |
103 < 100 |
|
Use: Low/high ornamentals (pot plants), greenhouse, 1 × 0.3 kg a.s. per ha Input parameters: DT50 4.65 days, DFR 1.74 μg/cm2/kg as/ha | ||
| Cutting and harvesting activities* | Ww + gloves | 364 |
|
Cutting and harvesting activities** (DAA*** 0) (DAA*** 9) |
Ww + gloves |
366 < 100 |
|
Inspection and maintenance (8 h)** (DAA*** 0) (DAA*** 8) |
Ww + gloves |
282 < 100 |
| Inspection and irrigation (2 h)** (DAA*** 0) | Ww | 79 |
|
Use: Low/high ornamentals (pot plants), greenhouse, 3 × 0.3 kg a.s. per ha Input parameters: 14‐day interval between applications, DT50 4.65 days, DFR 1.74 μg/cm2/kg as/ha | ||
|
Inspection and maintenance (8 h)** (DAA*** 0) (DAA*** 8) |
Ww + gloves |
320 < 100 |
| Inspection and irrigation (2 h)** (DAA*** 0) | Ww | 90 |
Based on EFSA calculator 2015 (last version of calculator from EFSA, 2014b).
Based on EFSA calculator 2022b.
DAA: day after application.
2.3.3. Bystander and resident exposure
For the existing use on wheat (Southern EU) from the first approval, predicted systemic exposure estimates below the (A)AOEL are obtained when drift reduction technology is used, a buffer zone of 10 m is implemented, and an interval of 14 days between applications is observed. Alternatively, a single application with the same drift reduction and buffer zone could be sufficient. For the use on wheat (Northern EU) from the first approval, only drift reduction is required.
For the renewal uses on wheat and sugar beet crops, the predicted systemic exposure estimates are below the (A)AOEL with the use of drift reduction technology (already required for operators).
For the renewal use on ornamentals in permanent greenhouses, the predicted systemic exposure estimates in case of high ornamentals (upward spraying14) are above the (A)AOEL for both bystanders and residents (adult and child), even considering increased buffer zone (10 m) or single application. In case of low ornamentals (downward spraying 14 ), they are below the (A)AOEL with the use of a buffer zone of 5 m.
It is noted that in the case of non‐permanent greenhouse, the systemic exposure for bystanders and residents is expected to be higher.
An overview of the risk assessment for residents and bystanders can be found in Table 3, while detailed results are provided in Appendices B.3 and B.4.
Table 3.
Risk assessment for bystanders and residents
|
Pirimor WG – Representative uses from the existing approval (European Commission, 2006) Model: EFSA (2015)* | |||||
|
Use: Wheat, Southern EU, outdoor use, 2 × 0.21 kg a.s. per ha Input parameters: 7‐day interval between applications, DT50 4.65 days, drift reduction | |||||
| Category | % (A)AOEL by exposure pathway | ||||
| Drift | Vapour | Deposits | Re‐entry | All (mean) | |
| Bystander child/adult | 45/12 | 11/2 | 12/4 | 67/37 | – |
| Resident child/adult | 28/7 | 15/3 | 6/2 | 96/53 | 112/50 |
| Input parameters: 14‐day interval between applications, DT50 4.65 days, drift reduction, buffer zone 10 m | |||||
| Bystander child/adult | 24/5 | 11/2 | 2/0.8 | 56/31 | – |
| Resident child/adult | 16/3 | 15/3 | 1/0.4 | 80/44 | 88/40 |
| Input parameters: 1 application, DT50 4.65 days, drift reduction, buffer zone 10 m | |||||
| Bystander child/adult | 24/5 | 11/2 | 2/0.7 | 50/28 | – |
| Resident child/adult | 16/3 | 15/3 | 1/0.3 | 71/39 | 81/37 |
|
Use: Wheat, Northern EU, outdoor use, 2 × 0.15 kg a.s. per ha Input parameters: 7‐day interval between applications, DT50 4.65 days, drift reduction | |||||
| Bystander child/adult | 32/9 | 11/2 | 8/3 | 48/27 | – |
| Resident child/adult | 20/5 | 15/3 | 4/1 | 68/38 | 84/37 |
|
Pirimor WG – Representative uses from the renewal process Model: EFSA (2015)* and also EFSA, 2022b for the ornamentals | |||||
|
Use: Winter wheat, outdoor use, 1 × 0.12 kg a.s. per ha Input parameters: DT50 4.65 days, drift reduction | |||||
| Bystander child/adult | 47/13 | 11/2 | 6/2 | 38/21 | – |
| Resident child/adult | 29/7 | 15/3 | 3/1 | 55/31 | 77/32 |
|
Use: Summer (spring) wheat, outdoor use, 2 × 0.125 kg a.s. per ha Input parameters: 14‐day interval between applications, DT50 4.65 days, drift reduction | |||||
| Bystander child/adult | 46/12 | 11/2 | 7/3 | 43/24 | – |
| Resident child/adult | 29/7 | 15/3 | 3/1 | 61/34 | 82/34 |
|
Use: Sugar beet, outdoor use, 1 × 0.15 kg a.s. per ha, Input parameters: DT50 4.65 days, drift reduction | |||||
| Bystander child/adult | 46/12 | 11/2 | 7/2 | 38/21 | – |
| Resident child/adult | 29/7 | 15/3 | 3/1 | 54/30 | 77/31 |
|
Use: Low ornamentals (pot plants), greenhouse, 3 × 0.3 kg a.s. per ha (EFSA calculator 2022b) Input parameters: 7‐day interval between applications, DFR 1.74 μg/cm2 foliage per kg a.s./ha, DT50 4.65 days | |||||
| Bystander child/adult | 101/27 | 8/2.7 | 26/10 | – | – |
| Resident child/adult | 64/15 | 11/4 | 13/5 | – | 56/14 |
| Input parameters: 7‐day interval between applications, DFR 1.74 μg/cm2 foliage per kg a.s./ha, DT50 4.65 days, buffer zone 5 m | |||||
| Bystander child/adult | 66/13 | 8/2.7 | 11/4 | – | – |
| Resident child/adult | 43/8 | 11/4 | 5/2 | – | 39/9 |
|
Use: High ornamentals (pot plants), greenhouse, 3 × 0.3 kg a.s. per ha (EFSA calculator 2022b) Input parameters: 7‐day interval between applications, DFR 1.74 μg/cm2 foliage per kg a.s./ha, DT50 4.65 days | |||||
| Bystander child/adult | 526/291 | 8/2.7 | 11/4 | – | – |
| Resident child/adult | 328/182 | 11/4 | 7/3 | – | 232/125 |
| Input parameters: 7‐day interval between applications, DFR 1.74 μg/cm2 foliage per kg a.s./ha, DT50 4.65 days, buffer zone 10 m | |||||
| Bystander child/adult | 526/291 | 8/2.7 | 4/1.5 | – | – |
| Resident child/adult | 328/182 | 11/4 | 2/1 | – | 229/123 |
|
Use: High ornamentals (pot plants), greenhouse, 1 × 0.3 kg a.s. per ha (EFSA calculator 2022b) Input parameters: DFR 1.74 μg/cm2 foliage per kg a.s./ha, DT50 4.65 days, buffer zone 10 m | |||||
| Bystander child/adult | 526/291 | 8/2.7 | 2.6/1 | – | – |
| Resident child/adult | 328/182 | 11/4 | 1.6/0.6 | – | 228/123 |
Based on EFSA calculator 2015 (last version of calculator from EFSA, 2014b).
2.4. Acute risk to birds
Following a mandate from the European Commission, the acute risk to birds was assessed for the representative uses of pirimicarb evaluated for the first approval and for the representative uses reflecting the currently authorised uses, submitted as part of the renewal of approval. The EFSA guidance for birds and mammals (EFSA, 2009) was used for this assessment.
The greenhouse use in ornamentals is indicated for closed permanent structures. For that use, low acute risk could be concluded for birds based on limited exposure to pirimicarb. Nevertheless, for eventual uses in non‐permanent greenhouse uses in ornamentals, a risk assessment is also presented.
The screening risk assessment for all field and eventual non‐permanent greenhouse uses falling under the mandate is summarised in Table 4.
Table 4.
Screening acute risk assessment for birds for the representative field and eventual non‐permanent greenhouse uses of pirimicarb evaluated for the first approval and for those submitted as part of the renewal of approval
| Crop | Application rate (g a.s./ha) | MAF | Indicator species | Shortcut value | DDD (mg a.s./kg bw) | LD50 (mg/kg bw) | TER (a) | |
|---|---|---|---|---|---|---|---|---|
| Existing approval (European Commission, 2006) | Wheat | 2 × 210 (b) | 1.4 | Small omnivorous bird | 158.8 | 46.69 | 20.9 | 0.4 |
| 1 × 210 | 1.0 | 33.35 | 0.6 | |||||
| 2 × 150 (b) | 1.4 | 33.35 | 0.6 | |||||
| 1 × 150 | 1.0 | 23.82 | 0.9 | |||||
| Renewal of approval | Winter wheat | 1 × 120 | 1.0 | 19.06 | 1.1 | |||
| Summer (Spring) wheat | 2 × 125 (c) | 1.2 | 23.82 | 0.9 | ||||
| 1 × 125 | 1.0 | 19.85 | 1.0 | |||||
| Sugar beet | 1 × 150 | 1.0 | 23.82 | 0.9 | ||||
| Ornamentals (pot plants) (d) | 3 × 300 | 2.0 | Small insectivorous bird | 46.8 | 28.08 | 0.7 |
DDD: daily dietary dose; LD50: 50% lethal dose; MAF: multiple application factor; TER: toxicity/exposure ratio.
TER values which are below the trigger value of 10 are highlighted in bold.
Minimum interval between applications of 7 days.
Minimum interval between applications of 14 days.
The greenhouse use in ornamentals is indicated for closed permanent structures. For that use, low acute risk could be concluded for birds based on limited exposure to pirimicarb. Nevertheless, for eventual uses in non‐permanent greenhouse uses in ornamentals, TER values have also been estimated.
High risk could not be excluded for any of the representative field and eventual non‐permanent greenhouse uses. This triggered the need for a tier 1 acute risk assessment. The tier 1 risk assessment required for the generic focal species following EFSA (2009) is presented in Table 5.
Table 5.
Tier 1 acute risk assessment for birds for the representative field and eventual non‐permanent greenhouse uses of pirimicarb evaluated for the first approval and for those uses submitted as part of the renewal of approval
| Crop | Application rate (g a.s./ha) | BBCH | MAF90 | Generic focal species | SV90 | DDD (mg a.s./kg bw) | LD50 (mg/kg bw) | TER (a) | |
|---|---|---|---|---|---|---|---|---|---|
| Existing approval (European Commission (2006) | Wheat (b) | 2 × 210 | 65–85 | 1.4 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 2.12 | 20.9 | 9.9 |
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 16.93 | 1.2 | ||||||
| 1 × 210 | 65–85 | 1.0 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 1.51 | 13.8 | |||
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 12.10 | 1.7 | ||||||
| 2 × 150 | 65–85 | 1.4 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 1.51 | 13.8 | |||
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 12.10 | 1.7 | ||||||
| 1 × 150 | 65–85 | 1.0 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 1.08 | 19.4 | |||
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 8.64 | 2.4 | ||||||
| Renewal of approval | Winter wheat | 1 × 120 | 51–89 | 1.0 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 0.86 | 24.2 | |
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 6.91 | 3.0 | ||||||
| Summer (Spring) wheat (c) | 2 × 125 | 51–89 | 1.2 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 1.08 | 19.4 | ||
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 8.64 | 2.4 | ||||||
| 1 × 125 | 51–89 | 1.0 | Small omnivorous bird ‘lark’ (BBCH ≥ 40) | 7.2 | 0.90 | 23.2 | |||
| Small insectivorous bird ‘passerine’ (BBCH 71–89) | 57.6 | 7.20 | 2.9 | ||||||
| Sugar beet | 1 × 150 | 12–49 | 1.0 | Small insectivorous bird ‘wagtail’ (BBCH 10–19) | 10.9 | 1.64 | 12.8 | ||
| Small insectivorous bird ‘wagtail’ (BBCH 20–49) | 7.7 | 1.16 | 18.1 | ||||||
| Small insectivorous bird ‘wagtail’ (BBCH 20–49) | 25.2 | 3.78 | 5.5 | ||||||
| Small omnivorous bird ‘lark’ (BBCH 10–19) | 24.0 | 3.60 | 5.8 | ||||||
| Small granivorous bird ‘finch’ (BBCH 30–49) | 24.7 | 3.71 | 5.6 | ||||||
| Ornamentals (pot plants) (d) | 1 × 300 | 12–92 | 1 | Small insectivorous bird ‘tit’ | 46.8 | 14.04 | 1.5 | ||
| Small insectivorous/worm feeding species ‘thrush’ | 7.4 | 2.22 | 9.4 | ||||||
| 2 × 300 | 1.6 | Small insectivorous bird ‘tit’ | 46.8 | 22.46 | 0.9 | ||||
| Small insectivorous/worm feeding species ‘thrush’ | 7.4 | 3.55 | 5.9 | ||||||
| 3 × 300 | 2.0 | Small insectivorous bird ‘tit’ | 46.8 | 28.08 | 0.7 | ||||
| Small insectivorous/worm feeding species ‘thrush’ | 7.4 | 4.44 | 4.7 |
DDD: daily dietary dose; LD50: 50% lethal dose; MAF: multiple application factor; SV90: Shortcut value for 90th percentile residues; TER: toxicity/exposure ratio.
TER values which are below the trigger value of 10 are highlighted in bold.
Minimum interval between applications of 7 days.
Minimum interval between applications of 14 days.
Minimum interval between applications of 7 days.
Based on the available data and tier‐1 risk assessment, high acute risk was indicated for all representative field uses of pirimicarb. The risk assessment was further refined for those scenarios for which TER values were lower than the trigger value of 10. To do so, the applicant proposed several options:
-
–
Updated residue unit dose (RUD) values in the relevant matrices, based on the database developed by Lahr et al. (2018);
-
–
Updated deposition values for spray applications, based on the guidance document to obtain DegT50 values (EFSA, 2014a);
-
–
Selection of the yellow wagtail, Motacilla flava, as relevant focal insectivorous species and a mixed diet of plant‐ and ground‐dwelling arthropods (50% each).
The use of updated RUD and deposition values were accepted to refine the acute risk to birds.15
Regarding the selection of focal species, it was noted that the yellow wagtail had been previously accepted as focal insectivorous species in wheat and sugar beet at a Pesticide Peer Review experts' meeting in the context of the renewal of approval of pirimicarb.16 However, specific studies investigating the dietary composition of the yellow wagtail were not provided and there was no relevant evidence from published data supporting a 50/50% diet of plant‐ and ground‐dwelling arthropods in both crops. Besides, additional focal insectivorous species other than the yellow wagtail might be needed to cover the uses in wheat in Southern Europe (see e.g. Dietzen et al., 2014). Therefore, this refinement option was not accepted for the higher tier risk assessment.
Toxicity/exposure ratios for all pertinent scenarios were calculated by using the updated RUD and deposition values (Appendix C). The outcome of the higher tier risk assessment, presented in Table 6, indicated high acute risk to birds for all representative field and eventual non‐permanent uses of pirimicarb.
Table 6.
Higher tier acute risk assessment for the representative field and eventual non‐permanent greenhouse uses of pirimicarb considering updated residue unit dose and deposition values
| Crop (Regulatory zone) | Application rate (g a.s./ha) | BBCH | Risk (a) | |
|---|---|---|---|---|
| Existing approval in European Commission (2006) |
Wheat (SEU) |
2 × 210 | 65–85 | High |
| 1 × 210 | High | |||
|
Wheat (NEU) |
2 × 150 | 65–85 | High | |
| 1 × 150 | High | |||
| Renewal of approval |
Winter wheat (All zones) |
1 × 120 | 51–89 | High |
|
Summer (Spring) wheat (All zones) |
2 × 125 | 51–89 | High | |
| 1 × 125 | High | |||
|
Sugar beet (SEU) |
1 × 150 (b) | 12–49 | High | |
|
Ornamentals (pot plants) (All zones) |
1 × 300 | 12–92 | High | |
| 2 × 300 | High | |||
| 3 × 300 | High |
NEU: Northern Europe; SEU: Southern Europe.
Toxicity/exposure ratio values are reported in Appendix C.
To mitigate the risk for the uses in sugar beet, the applicant proposed a modification of the GAP by reducing the application rate from 150 g pirimicarb/ha to 86.1 g pirimicarb/ha (i.e. 42.6% reduction). This was the rate resulting in a TER exceeding the trigger value of 10 using the yellow wagtail as focal species and a mixed diet of ground‐ and plant‐dwelling arthropod species. Since this refinement option was not accepted, low risk cannot be indicated even at an application rate of 86.1 g pirimicarb/ha. In any case, it should be noted that there was no evidence supporting the efficacy of pirimicarb to control aphids at the reduced rate proposed by the applicant.
Based on the available data, accepted refinement options and risk assessment,
-
–
Low risk was concluded for the permanent greenhouse uses in ornamentals;
-
–
High risk was indicated for all uses in wheat and the uses in sugar beet, and for the eventual non‐permanent greenhouse uses in ornamentals.
3. Conclusions
For all the representative uses submitted as part of the renewal of approval, the overall consumer risk assessment cannot be finalised due to the non‐finalised risk assessment residue definitions in plant and livestock, the outstanding data in primary and rotational crops, the lack of conclusion on the genotoxic potential of R31805, R34885, R34865, R406405 and R16210 and lack of data on general toxicity on R406405 (see Section 2.1) found in relevant amounts in plant and animal commodities for the renewal of approval.
Regarding the existing approval, the consumer risk assessment can also not be finalised taking into account the new information on the metabolites mentioned above found relevant in plant and animal commodities.
As regards the human health assessment through non‐dietary exposure, no risks were identified for the existing uses in wheat crops assessed for the first approval and for the renewal uses on wheat crops, sugar beet crops and ornamentals in greenhouse (in case of downward spraying) when appropriate risk mitigation measures are adopted, while risks were identified for the use on ornamentals in greenhouse (in case of upward spraying) for operators, bystanders and residents. It is noted that for non‐permanent greenhouses, likewise the risk is also identified for operators, bystanders and residents (in case of upward spraying) while workers are covered by the use in permanent greenhouses.
Based on the available data, accepted refinement options and risk assessment, low acute risk to birds could be concluded for the representative greenhouse uses in ornamentals in permanent structures, whereas high risk was indicated for all uses in wheat (from the first approval and renewal of approval), the uses in sugar beet and eventual greenhouse uses in ornamentals in non‐permanent structures (Table 7).
Table 7.
Overview of concerns reflecting the issues not finalised and the risks identified for each representative use considered
| Representative use (a) | Existing approval in European Commission (2006) | Renewal of approval | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Wheat (SEU) 2 × 210 |
Wheat (SEU) 1 × 210 |
Wheat (NEU) 2 × 150 |
Wheat (NEU) 1 × 150 |
Winter wheat 1 × 120 |
Summer (Spring) wheat 1 × 125 |
Summer (Spring) wheat 2 × 125 |
Sugar beet 1 × 150 |
Ornamentals (pot plants) 1–3 × 300 |
Ornamentals (pot plants) 1 × 300 |
Ornamentals (pot plants) 2 × 300 |
Ornamentals (pot plants) 3 × 300 |
||
| Field use | Field use | Field use | Field use | Field use | Field use | Field use | Field use | Green house (non‐permanent structures) | Greenhouse use (permanent structures) | Greenhouse use (permanent structures) | Greenhouse use (permanent structures) | ||
| Operator risk | Risk identified | X (b) | X (b) | X (b) | X (b) | ||||||||
| Assessment not finalised | |||||||||||||
| Worker risk | Risk identified | ||||||||||||
| Assessment not finalised | |||||||||||||
| Resident/bystander risk | Risk identified | X (b) | X (b) | X (b) | X (b) | ||||||||
| Assessment not finalised | |||||||||||||
| Consumer risk | Risk identified | ||||||||||||
| Assessment not finalised | X | X | X | X | X | X | X | X | X (c) | X (c) | X (c) | X (c) | |
| Acute risk to birds | Risk identified | X | X | X | X | X | X | X | X | X | |||
| Assessment not finalised | |||||||||||||
NEU: Northern Europe; SEU: Southern Europe.
Application rate in g pirimicarb/ha.
Risk identified for upward spraying (high ornamentals, e.g. on shelves or staggered plant pots).
The consumer exposure via dietary intake is expected from the food crops that might be grown in rotation with the ornamentals.
Abbreviations
- λ
wavelength
- μg
microgram
- μm
micrometre (micron)
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- AChE
acetylcholinesterase
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- AUC
area under the blood concentration/time curve
- AV
avoidance factor
- BCF
bioconcentration factor
- bp
base pair
- BUN
blood urea nitrogen
- bw
body weight
- CABI
Centre for Agricultural Bioscience International
- CI
confidence interval
- cm
centimetre
- d
day
- DAA
days after application
- DDD
daily dietary dose
- DNA
deoxyribonucleic acid
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EAS
oestrogen, androgen and steroidogenesis modalities
- EbC50
effective concentration (biomass)
- EC50
effective concentration
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- EINECS
European Inventory of Existing Commercial Chemical Substances
- ELINCS
European List of New Chemical Substances
- ELISA
Enzyme‐linked immunosorbent assay
- EMDI
estimated maximum daily intake
- ER50
emergence rate/effective rate, median
- ErC50
effective concentration (growth rate)
- ERO
ecological recovery option
- ERSTTA
Stably Transfected Human Oestrogen Receptor‐alpha Transcriptional Activation Assay
- ETO
ecological threshold option
- FAO
Food and Agriculture Organisation of the United Nations
- g
gram
- GAP
Good Agricultural Practice
- GPC
gel permeation chromatography
- h
hour(s)
- ha
hectare
- Hb
haemoglobin
- Hct
haematocrit
- hL
hectolitre
- HPG
hypopharygeal glands
- ISO
International Organization for Standardization
- iv
intravenous
- kg
kilogram
- L
litre
- m
metre
- M
mol
- mg
milligram
- M/L
mixing and loading
- MAF
multiple application factor
- mL
millilitre
- mm
millimetre (also used for mean measured concentrations)
- mN
milli‐Newton
- ng
nanogram
- OECD
Organisation for Economic Co‐operation and Development
- Pa
pascal
- PCR
polymerase chain reaction
- PHI
preharvest interval
- pKa
negative logarithm (to the base 10) of the dissociation constant
- Pow
partition coefficient between n‐octanol and water
- PPE
personal protective equipment
- ppm
parts per million (10−6)
- r2
coefficient of determination
- RAR
Renewal Assessment Report
- RPE
respiratory protective equipment
- RUD
residue per unit dose
- S
svedberg, S (10−13 s)
- t1/2
half‐life (define method of estimation)
- TC
technical material
- TER
toxicity exposure ratio
- TERA
toxicity exposure ratio for acute exposure
- TERLT
toxicity exposure ratio following chronic exposure
- TERST
toxicity exposure ratio following repeated exposure
- TRR
total radioactive residue
- w/w
weight per unit weight
- WHO
World Health Organization
Appendix A – List of representative uses considered in the assessment as requested in the mandate
List of uses from the existing approval in European Commission ( 2006 )
| Crop and/or situation(a) | Member State or Country | Product name | F G or I(b) | Pests or Group of pests controlled(c) | Formulation | Application | Application rate per treatment | PHI (days)(m) | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Type (d–f) |
Conc. of as(i) | method kind(f–h) | growth stage & season(j) | number min max(k) | interval between applicati ons (min) |
kg as/hl min–max(l) |
water l/ha min–max | kg as/ha min–max(l) | |||||||
| Wheat | Southern Europe | Pirimor | F | Aphids | WG | 50% w/w | Tractor‐ mounted foliar spray |
1st app: BBCH 65–77 2nd app: BBCH 83–85 |
1–2 | Min 7 days, typical interval 14 days | – | 200–300 | 0.21 | N/A | Since growth stage is specified for second application, PHI is not appropriate |
| Wheat | Northern Europe | Pirimor | F | Aphids | WG | 50% w/w | Tractor‐ mounted foliar spray |
1st app: BBCH 65–77 2nd app: BBCH 83–85 |
1–2 | Min 7 days, typical interval 14 days | – | 200–300 | 0.15 | N/A | Since growth stage is specified for second application, PHI is not appropriate |
Summary of representative uses evaluated under the peer review of the renewal of pirimicarb
| Crop and/or situation (a) | Member State or Country | Product name | F G or I (b) | Pests or Group of pests controlled (c) | Preparation | Application | Application rate per treatment | PHI (days) (m) | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Type (d–f) |
Conc. a.s. (i) | Method kind (f) , (g) , (h) | range of growth stages & season (j) | number min–max (k) | Interval between application (min) | kg a.s/hL min–max (l) | Water L/ha min–max | kg a.s./ha min–max (l) | |||||||
| Winter Wheat | EU | Pirimor | F | Aphids | WG | 500 | Foliar spray | BBCH 51–89 | 1 | N/A | 0.03–0.08 | 150–400 | 0.12 | 28 | |
| Summer (spring) wheat | EU | Pirimor | F | Aphids | WG | 500 | Foliar spray | BBCH 51–89 | 1–2 | N/A | 0.031–0.083 | 150–400 | 0.125 | 28 | |
| Sugar beet | EU | Pirimor | F | Aphids | WG | 500 | Foliar spray | BBCH 12–49 | 1 | N/A | 0.0375–0.1 | 150–400 | 0.15 | 21 | |
| Ornamentals (pot plants) | EU | Pirimor | G* | Aphids | WG | 500 | Foliar spray | BBCH 12–92 | 1–3 | 7–14 | 0.025–0.15 | 200–1,200 | 0.3 | N/A | |
During the renewal, it was clarified that the greenhouse use for ornamentals is considered to be in permanent structures (cf Reporting Table point 5(235), 2018‐07‐13).
For crops, the EU and Codex classifications (both) should be taken into account; where relevant, the use situation should be described (e.g. fumigation of a structure).
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
e.g. biting and sucking insects, soil born insects, foliar fungi, weeds.
e.g. wettable powder (WP), emulsifiable concentrate (EC), granule (GR).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
All abbreviations used must be explained.
Method, e.g. high volume spraying, low volume spraying, spreading, dusting, drench.
Kind, e.g. overall, broadcast, aerial spraying, row, individual plant, between the plant and type of equipment used must be indicated.
g/kg or g/L. Normally the rate should be given for the active substance (according to ISO) and not for the variant in order to compare the rate for same active substances used in different variants (e.g. fluroxypyr). In certain cases, where only one variant is synthesised, it is more appropriate to give the rate for the variant (e.g. benthiavalicarb‐isopropyl).
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including where relevant, information on season at time of application.
Indicate the minimum and maximum number of applications possible under practical conditions of use.
The values should be given in g or kg whatever gives the more manageable number (e.g. 200 kg/ha instead of 200,000 g/ha or 12.5 g/ha instead of 0.0125 kg/ha).
PHI – minimum preharvest interval.
Appendix B – Non‐dietary exposure estimates, detailed calculations
B.1 OPERATOR
B.1.1 Existing approval – Use on wheat (Southern Europe)
Model: EFSA calculator 2014b (version of 30 Mar 2015)
Input parameters: no specific PPE/RPE (personal/respiratory protective equipment)
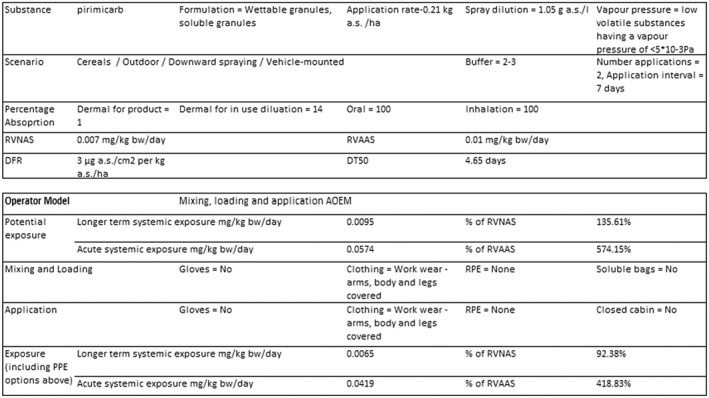
Input parameters: gloves and RPE during mixing/loading and application (MLA)

Input parameters: drift reduction technology and gloves during MLA

B.1.2 Existing approval – Use on wheat (Northern Europe)
Model: EFSA calculator 2014 (version of 30 March 2015)
Input parameters: no specific PPE/RPE

Input parameters: gloves and RPE during MLA

Input parameters: drift reduction technology and gloves during MLA

B.1.3 Renewal – Use on winter wheat
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: no specific PPE/RPE

Input parameters: gloves and RPE during MLA

Input parameters: drift reduction technology and gloves during MLA

B.1.4 Renewal – Use on summer wheat
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: no specific PPE/RPE.

Input parameters: gloves and RPE during MLA

Input parameters: drift reduction technology and gloves during MLA

B.1.5 Renewal – Use on sugar beet
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: no specific PPE/RPE.

Input parameters: gloves and RPE during MLA

Input parameters: drift reduction technology and gloves during MLA

B.1.6 Renewal – Use on ornamentals (greenhouse)
Model: EFSA calculator 2022b (v.0.3.22, 25/04/2022).
Short‐term exposure: downward spraying, manual hand‐held, three applications.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| Low ornamentals/Indoor/Downward spraying/Manual‐hand held/75th percentileCrop density: Normal | |||
| Pirimicarb | Number of applications and application rate: 3 × 0.3 kg a.s./ha; Dermal absorption (concentrate): 1%; Dermal absorption (in‐use dilution): 22% | ||
| M/L: Workwear App: Workwear | 0.007 | 94.2 | |
Acute exposure: downward spraying, manual hand‐held, three applications.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw] | % of systemic AAOEL |
|---|---|---|---|
| Low ornamentals/Indoor/Downward spraying/Manual‐hand held/95th percentileCrop density: Normal | |||
| Pirimicarb | Number of applications and application rate: 3 × 0.3 kg a.s./ha; Dermal absorption (concentrate): 1%; Dermal absorption (in‐use dilution): 22% | ||
| M/L: Workwear + Protected hands App: Workwear + Protected hands | 0.004 | 45 | |
Short‐term exposure: upward spraying, manual hand‐held, three applications.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| High ornamentals/Indoor/Upward spraying/Manual‐hand held/75th percentile Crop density: Normal | |||
| Pirimicarb | Number of applications and application rate: 3 × 0.3 kg a.s./ha; Dermal absorption (concentrate): 1%; Dermal absorption (in‐use dilution): 22% | ||
| M/L: Workwear + Protected hands + FP2, P2 and similar App: Workwear + Protected hands + FP2, P2 and similar | 0.02 | No safe use! | |
Acute exposure: upward spraying, manual hand‐held, three applications.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw] | % of systemic AAOEL |
|---|---|---|---|
| High ornamentals/Indoor/Upward spraying/Manual‐hand held/95th percentileCrop density: Normal | |||
| Pirimicarb | Number of applications and application rate: 3 × 0.3 kg a.s./ha; Dermal absorption (concentrate): 1%; Dermal absorption (in‐use dilution): 22% | ||
| M/L: Workwear + Protected hands + FP2, P2 and similar App: Workwear + Protected hands + FP2, P2 and similar | 0.1 | No safe use! | |
Short term exposure: upward spraying, manual‐trolley, three applications.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| High ornamentals/Indoor/Upward spraying/Manual‐trolley/75th percentileCrop density: Normal | |||
| Pirimicarb | Number of applications and application rate: 3 × 0.3 kg a.s./ha; Dermal absorption (concentrate): 1%; Dermal absorption (in‐use dilution): 22% | ||
| M/L: Workwear + Protected hands App: Workwear + Protected hands | 0.006 | 87 | |
Acute exposure: upward spraying, manual‐trolley, three applications.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw] | % of systemic AAOEL |
|---|---|---|---|
| High ornamentals/Indoor/Upward spraying/Manual‐trolley/95th percentileCrop density: Normal | |||
| Pirimicarb | Number of applications and application rate: 3 × 0.3 kg a.s./ha; Dermal absorption (concentrate): 1%; Dermal absorption (in‐use dilution): 22% | ||
| M/L: Workwear + Protected hands + FP2, P2 and similar App: Workwear + Protected hands + FP2, P2 and similar | 0.02 | No safe use! | |
B.2 WORKER
B.2.1 Existing approval – Use on wheat (Southern Europe)
Model: EFSA calculator 2014b (version of 30 March 2015).

B.2.2 Existing approval – Use on wheat (Northern Europe)
Model: EFSA calculator 2014b (version of 30 March 2015).
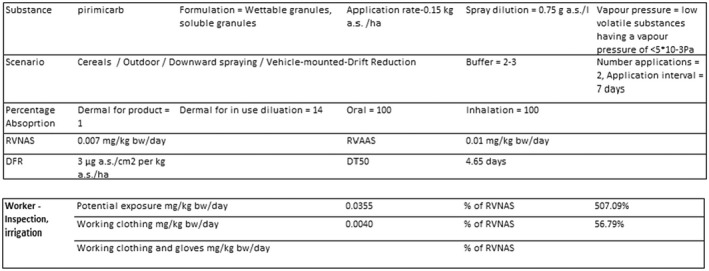
B.2.3 Renewal – Use on winter wheat
Model: EFSA calculator 2014b (version of 30 March 2015).

B.2.4 Renewal – Use on summer wheat
Model: EFSA calculator 2014b (version of 30 March 2015).

B.2.5 Renewal – Use on sugar beet
Model: EFSA calculator 2014b (version of 30 March 2015).

B.2.6 Renewal – Use on ornamentals (greenhouse)
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: three applications at 7‐day interval.

Input parameters: one application.

Model: EFSA calculator 2022b (v.0.3.22, 25/04/2022)
Input parameters: cutting/harvesting activities, three applications at 7‐day interval.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Cutting, sorting, bundling, carrying/harvesting, including cutting and bundling/indoor; Work rate: 8 h/day; interval: 7 days; body weight: 60 kg; TC (potential): 14000 cm2/h; TC (workwear (arms, body and legs covered)): 5000 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1400 cm2/h; TC (gloves): NA cm2/h; TSF: 0.1 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days; DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.3 | 4,592 | 26 |
| Workwear | 0.1 | 1,678 | 20 |
| Workwear and gloves | 0.04 | 513 | 12 |
| Hands covered, no workwear | |||
Input parameters: cutting/harvesting activities (8 h/day), one application.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Cutting, sorting, bundling, carrying/harvesting, including cutting and bundling/indoor; work rate: 8 h/day; interval: 7 days; body weight: 60 kg; TC (potential): 14000 cm2/h; TC (workwear (arms, body and legs covered)): 5000 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1400 cm2/h; TC (gloves): NA cm2/h; TSF: 0.1 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 1 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days; DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.2 | 3,122 | 24 |
| Workwear | 0.08 | 1,153 | 17 |
| Workwear and gloves | 0.03 | 366 | 9 |
| Hands covered, no workwear | |||
Input parameters: general maintenance (8 h/day), three applications at 7‐day interval.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Inspection, irrigation/general maintenance/indoor; work rate: 8 h/day; interval: 7 days; body weight: 60 kg; TC (potential): 12500 cm2/h; TC (workwear (arms, body and legs covered)): 1400 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1250 cm2/h; TC (gloves): NA cm2/h; TSF: 0.01 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22% DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.3 | 4,055 | 25 |
| Workwear | 0.03 | 461 | 11 |
| Workwear and gloves | 0.03 | 413 | 10 |
| Hands covered, no workwear | |||
Input parameters: general maintenance (8 h/day), one application.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Inspection, irrigation/general maintenance/indoor; work rate: 8 h/day; interval: 7 days; body weight: 60 kg; TC (potential): 12500 cm2/h; TC (workwear (arms, body and legs covered)): 1400 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1250 cm2/h; TC (gloves): NA cm2/h; TSF: 0.01 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 1 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days; DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.2 | 2,742 | 23 |
| Workwear | 0.02 | 314 | 8 |
| Workwear and gloves | 0.02 | 282 | 8 |
| Hands covered, no workwear | |||
Input parameters: inspection/irrigation (2 h/day), three applications at 7‐day interval.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Inspection, irrigation/inspection, watering/indoor; work rate: 2 h/day; interval: 7 days; body weight: 60 kg; TC (potential): 12500 cm2/h; TC (workwear (arms, body and legs covered)): 1400 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1250 cm2/h; TC (gloves): NA cm2/h; TSF: 0.01 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days; DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.07 | 1,014 | 16 |
| Workwear | 0.008 | 115 | 1 |
| Workwear and gloves | 0.007 | 103 | 1 |
| Hands covered, no workwear | |||
Input parameters: inspection/irrigation (2 h/day), one application.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Inspection, irrigation/inspection, watering/indoor; work rate: 2 h/day; interval: 7 days; body weight: 60 kg; TC (potential): 12500 cm2/h; TC (workwear (arms, body and legs covered)): 1400 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1250 cm2/h; TC (gloves): NA cm2/h; TSF: 0.01 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 1 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days; DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.05 | 686 | 13 |
| Workwear | 0.006 | 78.6 | 0 |
| Workwear and gloves | 0.005 | 70.4 | 0 |
| Hands covered, no workwear | |||
Input parameters: inspection/irrigation (2 h/day), three applications at 14‐day interval.
| Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL | Re‐entry restriction [days] |
|---|---|---|---|
| Inspection, irrigation/inspection, watering/indoor; work rate: 2 h/day; interval: 14 days; body weight: 60 kg; TC (potential): 12500 cm2/h; TC (workwear (arms, body and legs covered)): 1400 cm2/h; TC (workwear (arms, body and legs covered) and gloves): 1250 cm2/h; TC (gloves): NA cm2/h; TSF: 0.01 mg a.s./h/kg a.s./ha | |||
| Pirimicarb (refined DFR & DT 50 foliar) | Number of applications & application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22% DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50 foliar: 4.65 days; DT50 air: 30 days; DT50 soil: 30 days | ||
| Potential | 0.05 | 781 | 14 |
| Workwear | 0.006 | 89.3 | 0 |
| Workwear and gloves | 0.006 | 80 | 0 |
| Hands covered, no workwear | |||
B.3 RESIDENT
B.3.1 Existing approval – Use on wheat (Southern Europe)
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: drift reduction technology, two applications at 7‐day interval, 200 L water/ha.


Input parameters: drift reduction technology, buffer zone 10 m, two applications at 14‐day interval, 200 L water/ha.


Input parameters: drift reduction technology, buffer zone 10 m, one application, 200 L water/ha.


B.3.2 Existing approval – Use on wheat (Northern Europe)
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: drift reduction technology, two applications at 7‐day interval, 200 L water/ha.

B.3.3 Renewal – Use on winter wheat
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: drift reduction technology, one application, 150 L water/ha.

B.3.4 Renewal – Use on summer wheat
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: drift reduction technology, two applications at 14‐day interval, 150 L water/ha.

B.3.5 Renewal – Use on sugar beet
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: drift reduction technology, one application, 150 L water/ha.
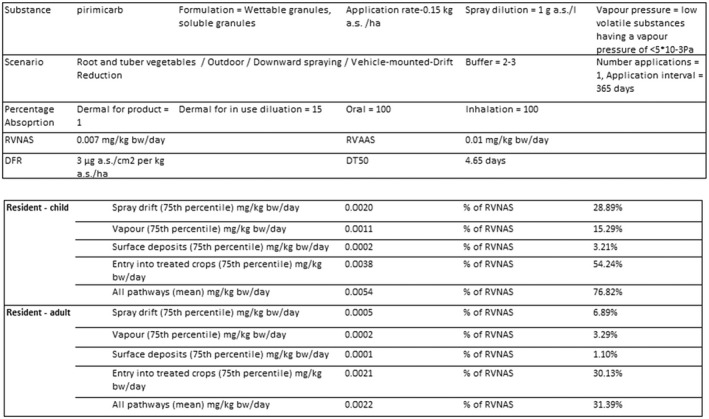
B.3.6 Renewal – Use on ornamentals (greenhouse)
Model: EFSA calculator 2022b (v.0.3.22, 25/04/2022).
Input parameters: low ornamentals, three applications at 7‐day interval.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| Season: Not relevant; Buffer zone: 2–3 m Interval between treatments: 7 days Minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Resident child Body weight: 10 kg | Drift (75th perc.) | 0.004 | 64 |
| Vapour (75th perc.) | 0.0008 | 11.4 | |
| Deposits (75th perc.) | 0.0009 | 12.7 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.004 | 55.7 | |
| Resident adult Body weight: 60 kg | Drift (75th perc.) | 0.001 | 15.2 |
| Vapour (75th perc.) | 0.0003 | 3.9 | |
| Deposits (75th perc.) | 0.0003 | 4.8 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.001 | 14.4 | |
Input parameters: low ornamentals, three applications at 7‐day interval, buffer zone 5 m.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| Season: Not relevant; buffer zone: 5 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Resident child Body weight: 10 kg | Drift (75th perc.) | 0.003 | 42.7 |
| Vapour (75th perc.) | 0.0008 | 11.4 | |
| Deposits (75th perc.) | 0.0004 | 5.2 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.003 | 38.8 | |
| Resident adult body weight: 60 kg | Drift (75th perc.) | 0.0005 | 7.8 |
| Vapour (75th perc.) | 0.0003 | 3.9 | |
| Deposits (75th perc.) | 0.0001 | 2 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.0006 | 9.3 | |
Input parameters: high ornamentals, three applications at 7‐day interval.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| Season: Not relevant; buffer zone: 5 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Resident child Body weight: 10 kg | Drift (75th perc.) | 0.02 | 328 |
| Vapour (75th perc.) | 0.0008 | 11.4 | |
| Deposits (75th perc.) | 0.0005 | 7 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.02 | 232 | |
| Resident adult Body weight: 60 kg | Drift (75th perc.) | 0.01 | 182 |
| Vapour (75th perc.) | 0.0003 | 3.9 | |
| Deposits (75th perc.) | 0.0002 | 2.6 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.009 | 125 | |
Input parameters: high ornamentals, three applications at 7‐day interval, buffer zone 10 m.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| Season: Not relevant; buffer zone: 10 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Resident child Body weight: 10 kg | Drift (75th perc.) | 0.02 | 328 |
| Vapour (75th perc.) | 0.0008 | 11.4 | |
| Deposits (75th perc.) | 0.0002 | 2.3 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.02 | 229 | |
| Resident adult Body weight: 60 kg | Drift (75th perc.) | 0.01 | 182 |
| Vapour (75th perc.) | 0.0003 | 3.9 | |
| Deposits (75th perc.) | 6 e‐05 | 0.9 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.009 | 123 | |
Input parameters: high ornamentals, one application, buffer zone 10 m.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AOEL |
|---|---|---|---|
| Season: Not relevant; Buffer zone: 10 m; interval between treatments: NA; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 1 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Resident child Body weight: 10 kg | Drift (75th perc.) | 0.02 | 328 |
| Vapour (75th perc.) | 0.0008 | 11.4 | |
| Deposits (75th perc.) | 0.0001 | 1.6 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.02 | 228 | |
| Resident adult Body weight: 60 kg | Drift (75th perc.) | 0.01 | 182 |
| Vapour (75th perc.) | 0.0003 | 3.9 | |
| Deposits (75th perc.) | 4 e‐05 | 0.6 | |
| Re‐entry (75th perc.) | |||
| Sum (mean) | 0.009 | 123 | |
B.4 BYSTANDER
B.4.1 Existing approval – Use on wheat (Southern Europe)
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: drift reduction technology, two applications at 7‐day interval, 200 L water/ha.

Input parameters: drift reduction technology, buffer zone 10 m, two applications at 14‐day interval, 200 L water/ha.

Input parameters: drift reduction technology, buffer zone 10 m, one application, 200 L water/ha.

B.4.2 Existing approval – Use on wheat (Northern Europe)
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: drift reduction technology, two applications at 7‐day interval, 200 L water/ha.

B.4.3 Renewal – Use on winter wheat
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: drift reduction technology, one application, 150 L water/ha.

B.4.4 Renewal – Use on summer wheat
Model: EFSA calculator 2014b (version of 30 Mar 2015).
Input parameters: drift reduction technology, two applications at 14‐day interval, 150 L water/ha.

B.4.5 Renewal – Use on sugar beet
Model: EFSA calculator 2014b (version of 30 March 2015).
Input parameters: drift reduction technology, one application, 150 L water/ha.

B.4.6 Renewal – Use on ornamentals (greenhouse)
Model: EFSA calculator 2022b (v.0.3.22, 25/04/2022).
Input parameters: low ornamentals, three applications at 7‐day interval.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AAOEL |
|---|---|---|---|
| Season: Not relevant; buffer zone: 2–3 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Bystander child Body weight: 10 kg | Drift (95th perc.) | 0.01 | 101 |
| Vapour (95th perc.) | 0.0008 | 8 | |
| Deposits (95th perc.) | 0.003 | 26.3 | |
| Re‐entry (95th perc.) | |||
| Bystander adult Body weight: 60 kg | Drift (95th perc.) | 0.003 | 27.4 |
| Vapour (95th perc.) | 0.0003 | 2.7 | |
| Deposits (95th perc.) | 0.001 | 10 | |
| Re‐entry (95th perc.) | |||
Input parameters: low ornamentals, three applications at 7‐day interval, buffer zone 5 m.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AAOEL |
|---|---|---|---|
| Season: Not relevant; buffer zone: 5 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Bystander child Body weight: 10 kg | Drift (95th perc.) | 0.007 | 65.7 |
| Vapour (95th perc.) | 0.0008 | 8 | |
| Deposits (95th perc.) | 0.001 | 10.8 | |
| Re‐entry (95th perc.) | |||
| Bystander adult body weight: 60 kg | Drift (95th perc.) | 0.001 | 12.9 |
| Vapour (95th perc.) | 0.0003 | 2.7 | |
| Deposits (95th perc.) | 0.0004 | 4.1 | |
| Re‐entry (95th perc.) | |||
Input parameters: high ornamentals, three applications at 7‐day interval.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AAOEL |
|---|---|---|---|
| Season: Not relevant; Buffer zone: 5 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha Dermal absorption: 22% DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Bystander child Body weight: 10 kg | Drift (95th perc.) | 0.05 | 526 |
| Vapour (95th perc.) | 0.0008 | 8 | |
| Deposits (95th perc.) | 0.001 | 11.2 | |
| Re‐entry (95th perc.) | |||
| Bystander adult Body weight: 60 kg | Drift (95th perc.) | 0.03 | 291 |
| Vapour (95th perc.) | 0.0003 | 2.7 | |
| Deposits (95th perc.) | 0.0004 | 4.3 | |
| Re‐entry (95th perc.) | |||
Input parameters: high ornamentals, three applications at 7‐day interval, buffer zone 10 m.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AAOEL |
|---|---|---|---|
| Season: Not relevant; Buffer zone: 10 m; interval between treatments: 7 days; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 3 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Bystander child Body weight: 10 kg | Drift (95th perc.) | 0.05 | 526 |
| Vapour (95th perc.) | 0.0008 | 8 | |
| Deposits (95th perc.) | 0.0004 | 3.8 | |
| Re‐entry (95th perc.) | |||
| Bystander adult Body weight: 60 kg | Drift (95th perc.) | 0.03 | 291 |
| Vapour (95th perc.) | 0.0003 | 2.7 | |
| Deposits (95th perc.) | 0.0001 | 1.5 | |
| Re‐entry (95th perc.) | |||
Input parameters: high ornamentals, one application, buffer zone 10 m.
| Model data | Level of PPE | Total absorbed dose [mg/kg bw per day] | % of systemic AAOEL |
|---|---|---|---|
| Season: Not relevant; buffer zone: 10 m; interval between treatments: NA; minimum volume of water: 200 l | |||
| Pirimicarb (refined DFR & DT 50 ) | Number of applications and application rate: 1 × 0.3 kg a.s./ha; dermal absorption: 22%; DFR: 1.74 μg/cm2 foliage per kg a.s./ha; DT50: 4.65 days | ||
| Bystander child Body weight: 10 kg | Drift (95th perc.) | 0.05 | 526 |
| Vapour (95th perc.) | 0.0008 | 8 | |
| Deposits (95th perc.) | 0.0003 | 2.6 | |
| Re‐entry (95th perc.) | |||
| Bystander adult Body weight: 60 kg | Drift (95th perc.) | 0.03 | 291 |
| Vapour (95th perc.) | 0.0003 | 2.7 | |
| Deposits (95th perc.) | 0.0001 | 1 | |
| Re‐entry (95th perc.) | |||
Appendix C – Higher tier acute risk assessment to birds for the representative field uses of pirimicarb under evaluation following EFSA (2009) and using updated residue unit dose and deposition values
| Crop | Application (mg a.s./ha) | MAF90 | Generic focal species | FIR/bw | Diet composition | RUD (a) | Deposition value (b) | DDD (mg/kg bw/day) | DDDtotal (mg/kg bw/day) | LD50 (mg/kg bw) | TER (c) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter wheat | 1 × 120 | 1.0 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 3.15 | 3.15 | 20.9 | 6.6 |
| Spring wheat | 2 × 125 | 1.2 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 3.94 | 3.94 | 20.9 | 5.3 |
| 1 × 125 | 1.0 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 3.29 | 3.29 | 20.94 | 6.4 | |
| Sugar beet | 1 × 105 | 1.0 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 3.94 | 3.94 | 20.9 | 5.3 |
| Small omnivorous bird ‘lark’ (BBCH 10–19) | 0.52 | 25% crop leaves | 84.8 | 1 | 1.65 | 3.64 | 20.9 | 5.7 | |||
| 25% weed seeds | 87.0 | 0.8 | 1.36 | ||||||||
| 50% ground‐dwelling arthropods | 20.2 | 0.8 | 0.63 | ||||||||
| Small granivorous bird ‘finch’ (BBCH 30–49) | 0.28 | 100% weed seeds | 87.0 | 0.3 | 1.10 | 1.10 | 20.9 | 19.1 | |||
| Wheat | 2 × 210 | 1.4 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 7.73 | 7.73 | 20.9 | 2.7 |
| 1 × 210 | 1.0 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 5.52 | 5.52 | 20.9 | 3.8 | |
| 2 × 150 | 1.4 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 5.52 | 5.52 | 20.9 | 3.8 | |
| 1 × 150 | 1.0 | Small insectivorous bird ‘passerine’ (BBCH 71–89) | 1.06 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 3.94 | 3.94 | 20.9 | 5.3 | |
| Ornamentals (d) | 1 × 300 | 1.0 | Small insectivorous bird ‘tit’ | 0.86 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 6.40 | 6.40 | 20.9 | 3.3 |
| Small insectivorous/worm feeding species ‘thrush’ | 0.76 | 100% soil‐dwelling arthropods | 20.2 | 1 | 4.61 | 4.61 | 20.9 | 4.5 | |||
| 2 × 300 | 1.6 | Small insectivorous bird ‘tit’ | 0.86 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 10.24 | 10.24 | 20.9 | 2.0 | |
| Small insectivorous/worm feeding species ‘thrush’ | 0.76 | 100% soil‐dwelling arthropods | 20.2 | 1 | 7.37 | 7.37 | 20.9 | 2.8 | |||
| 3 × 300 | 2.0 | Small insectivorous bird ‘tit’ | 0.86 | 100% foliar‐dwelling arthropods | 24.8 | 1 | 12.80 | 12.80 | 20.9 | 1.6 | |
| Small insectivorous/worm feeding species ‘thrush’ | 0.76 | 100% soil‐dwelling arthropods | 20.2 | 1 | 9.21 | 9.21 | 20.9 | 2.3 |
Based on Lahr et al. (2018).
Based on EFSA (2014a).
TER values which are below the trigger value of 10 are highlighted in bold.
The greenhouse use in ornamentals is indicated for closed permanent structures. For that use, low acute risk could be concluded for birds based on limited exposure to pirimicarb. Nevertheless, for eventual uses in non‐permanent greenhouse uses in ornamentals, TER values have also been estimated.
Appendix D – PRIMo 3.1
D.1 PRIMo calculation for the representative uses assessed under the renewal process

D.2 PRIMo calculation for the representative uses assessed under the first approval

Appendix E – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
| Pirimicarb |
2‐(dimethylamino)‐5,6‐dimethylpyrimidin‐4‐yl dimethylcarbamate Cc1nc(nc(OC(=O)N(C)C)c1C)N(C)C YFGYUFNIOHWBOB‐UHFFFAOYSA‐N |

|
| R34885 |
5,6‐dimethyl‐2‐(N‐methylformamido)pyrimidin‐4‐yl dimethylcarbamate Cc1nc(nc(OC(=O)N(C)C)c1C)N(C)C=O GDEAMEURJBBCOQ‐UHFFFAOYSA‐N |
|
| R31680 |
2‐amino‐5,6‐dimethylpyrimidin‐4‐ol Cc1c(O)nc(N)nc1C APWRLAZEMYLHKZ‐UHFFFAOYSA‐N |
|
| R31805 |
2‐(dimethylamino)‐5,6‐dimethylpyrimidin‐4‐ol Cc1nc(nc(O)c1C)N(C)C MUEHLDAHWSCFAG‐UHFFFAOYSA‐N |
|
| R34865 |
5,6‐dimethyl‐2‐(methylamino)pyrimidin‐4‐ol Cc1nc(NC)nc(O)c1C IFOLNWVBRSCJOJ‐UHFFFAOYSA‐N |
|
| R16210 |
N,N‐dimethylguanidine N=C(N)N(C)C SWSQBOPZIKWTGO‐UHFFFAOYSA‐N |

|
| R406405 |
5‐(hydroxymethyl)‐6‐methyl‐2‐(methylamino)pyrimidin‐4‐ol Cc1nc(NC)nc(O)c1CO NTGVGPFDHAOUOY‐UHFFFAOYSA‐N |
|
| R34836 |
5,6‐dimethyl‐2‐(methylamino)pyrimidin‐4‐yl dimethylcarbamate Cc1nc(NC)nc(OC(=O)N(C)C)c1C GTKRZJVAXAQBMB‐UHFFFAOYSA‐N |
|
| R238177 |
2‐(dimethylamino)‐6‐(hydroxymethyl)‐5‐methylpyrimidin‐4‐yl dimethylcarbamate CN(C)c1nc(CO)c(C)c(OC(=O)N(C)C)n1 YLZGFEXCEZDUAQ‐UHFFFAOYSA‐N |

|
The compound name in bold is the name used in the statement.
ACD/Name 2021.1.3 ACD/Labs 2021.1.3 (File Version N15E41, Build 123,232, 7 July 2021).
ACD/ChemSketch 2021.1.3 ACD/Labs 2021.1.3 (File Version C25H41, Build 123,835, 28 August 2021).
Suggested citation: EFSA (European Food Safety Authority) , 2023. Statement concerning the review of the approval of the active substance pirimicarb. EFSA Journal 2023;21(2):7807, 79 pp. 10.2903/sp.efsa.2023.7807
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00584
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 21 December 2022
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, pp. 1–50.
Commission Directive 2006/39/EC of 12 April 2006 amending Council Directive 91/414/EEC to include clodinafop, pirimicarb, rimsulfuron, tolclofos‐methyl and triticonazole as active substances. OJ L 104, 13.4.2006, pp. 30–35.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, pp. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, pp. 187–188.
Commission Implementing Regulation (EU) 2021/566 of 30 March 2021 amending Implementing Regulation (EU) No 540/2011 as regards the extension of the approval periods of the active substances abamectin, Bacillus subtilis (Cohn 1872) strain QST 713, Bacillus thuringiensis subsp. Aizawai strains ABTS‐1857 and GC‐91, Bacillus thuringiensis subsp. Israeliensis (serotype H‐14) strain AM65‐52, Bacillus thuringiensis subsp. Kurstaki strains ABTS 351, PB 54, SA 11, SA12 and EG 2348, Beauveria bassiana strains ATCC 74040 and GHA, clodinafop, clopyralid, Cydia pomonella Granulovirus (CpGV), cyprodinil, dichlorprop‐P, fenpyroximate, fosetyl, mepanipyrim, Metarhizium anisopliae (var. anisopliae) strain BIPESCO 5/F52, metconazole, metrafenone, pirimicarb, Pseudomonas chlororaphis strain MA342, pyrimethanil, Pythium oligandrum M1, rimsulfuron, spinosad, Streptomyces K61 (formerly ‘S. griseoviridis’), Trichoderma asperellum (formerly ‘T. harzianum’) strains ICC012, T25 and TV1, Trichoderma atroviride (formerly ‘T. harzianum’) strain T11, Trichoderma gamsii (formerly ‘T. viride’) strain ICC080, Trichoderma harzianum strains T‐22 and ITEM 908, triclopyr, trinexapac, triticonazole and ziram. OJ L 118, 7.4.2021, pp. 1–5.
Commission Implementing Regulation (EU) 2022/378 of 4 March 2022 amending Implementing Regulation (EU) No 540/2011 as regards the extension of the approval periods of the active substances abamectin, Bacillus subtilis (Cohn 1872) strain QST 713, Bacillus thuringiensis subsp. Aizawai strains ABTS‐1857 and GC‐91, Bacillus thuringiensis subsp. Israeliensis (serotype H‐14) strain AM65‐52, Bacillus thuringiensis subsp. Kurstaki strains ABTS 351, PB 54, SA 11, SA12 and EG 2348, Beauveria bassiana strains ATCC 74040 and GHA, clodinafop, Cydia pomonella Granulovirus (CpGV), cyprodinil, dichlorprop‐P, fenpyroximate, fosetyl, malathion, mepanipyrim, metconazole, metrafenone, pirimicarb, Pseudomonas chlororaphis strain MA342, pyrimethanil, Pythium oligandrum M1, rimsulfuron, spinosad, Trichoderma asperellum (formerly T. harzianum) strains ICC012, T25 and TV1, Trichoderma atroviride (formerly T. harzianum) strain T11, Trichoderma gamsii (formerly T. viride) strain ICC080, Trichoderma harzianum strains T‐22 and ITEM 908, triclopyr, trinexapac, triticonazole and ziram. OJ L 72, 7.3.2022, pp. 2–6.
The greenhouse use in ornamentals is indicated for closed permanent structures. Nevertheless, for completeness, considerations for eventual uses in non‐permanent greenhouse are also presented, where relevant.
Refer to experts' consultation 2.11 in the Report of Pesticides Peer Review Experts' Meeting 07 session 1 in June 2019 (EFSA, 2022a).
Refer to experts' consultation 2.1 in the Report of the Pesticides Peer Review Experts' Teleconference 89 in September 2022 (EFSA, 2022a).
Refer to experts' consultation 2.10 in the Report of the Pesticides Peer Review Experts' Meeting 190 in January 2019 (EFSA, 2022a).
See the Report of the Pesticides Peer Review Experts' Meeting 191 (28 January −1 February 2019) (EFSA, 2022a).
General population cluster diet 06 covers Greece.
Refer to experts' consultation 2.12 in the Report of the Pesticides Peer Review Experts' Meeting 190 in January 2019 (EFSA, 2022a).
From the EFSA guidance and calculator 2022b.
It is noted that the RUD values based on Lahr et al. (2018) are not yet accepted for tier 1 spray applications and that more details on how RUD values were extracted from the database should have been provided. However, updated RUD values for plant‐ and ground‐dwelling arthropods and plants were accepted for refining the acute risk for pirimicarb as the data set was considered more robust than that used in EFSA (2009).
Refer to experts' consultation 5.1 in the Report of the Pesticide Peer Review Experts' meeting 192 – Session 2 (February 2019) (EFSA, 2022a).
References
- Dietzen C, Edwards PJ, Wolf C, Ludwigs J‐D and Luttikk R, 2014. Focal species of birds in European crops for higher tier pesticide risk assessment. Integrated Environmental Assessment and Management, 10, 247–259. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014a. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014b. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874. Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Charistou A, Coja T, Craig P, Hamey P, Martin S, Sanvido O, Chiusolo A, Colas M and Istace F, 2022a. Peer Review Report to the statement regarding the pesticide risk assessment of the active substance pirimicarb. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) , Charistou A, Coja T, Craig P, Hamey P, Martin S, Sanvido O, Chiusolo A, Colas M and Istace F, 2022b. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment of plant protection products. EFSA Journal 2022;20(1):7032, 134 pp. 10.2903/j.efsa.2022.7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , EFSA (European Food Safety Authority) , Buist H, Craig P, Dewhurst I, Hougaard Bennekou S, Kneuer C, Machera K, Pieper C, Court Marques D, Guillot G, Ruffo F and Chiusolo A, 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 60 pp. 10.2903/j.efsa.2017.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2006. Review report for the active substance pirimicarb finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 27 January 2006 in view of the inclusion of pirimicarb in Annex I of Directive 91/414/EEC. SANCO/10529/05 – rev. 5, 10 February 2006.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9 March 2011. pp. 1–46.
- JMPR (Joint Meeting on Pesticide Residues) , 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues) , 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland. 18–27 September 2007, 164 pp.
- Lahr, J , Krämer, W , Mazerolles, V , Poulsen, V , Jölli, D , Müller, M , McVey, E , Wassenberg, J , Derkx, R , Brouwer, A , Deneer, D , Beltman, W , Lammertsma, D , Jansman, H and Buij, R , 2018. Data collection for the estimation of ecological data (specific focal species, time spent in treated areas collecting food, composition of diet), residue level and residue decline on food items to be used in the risk assessment for birds and mammals. EFSA supporting publication 2018;15(11):EN‐1513, 155 pp. 10.2903/sp.efsa.2018.EN-1513 [DOI] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- Sweden , 2022. Addendum Article 21 of Regulation (EC) No 1107/2009 on pirimicarb (genotoxicity of metabolites, residue definition, and acceptability of the acute risk to birds); June 2022, updated in September and December 2022. Available online: www.efsa.europa.eu
- United Kingdom/Sweden , 2022. Revised Renewal Assessment Report (RAR) on pirimicarb prepared by the initial rapporteur Member State United Kingdom and designated new RMS after Brexit Sweden in the framework of Commission Implementing Regulation (EU) No 844/2012, initial version: November 2017, latest updates prepared until production of the Statement: October 2022. Available online: www.efsa.europa.eu