Abstract
Introduction
Radiation-induced cognitive decline (RICD) occurs in 50%–90% of adult patients 6 months post-treatment. In patients with low-grade and benign tumours with long expected survival, this is of paramount importance. Despite advances in radiation therapy (RT) treatment delivery, better understanding of structures important for RICD is necessary to improve cognitive outcomes. We hypothesise that RT may affect network topology and microstructural integrity on MRI prior to any gross anatomical or apparent cognitive changes. In this longitudinal cohort study, we aim to determine the effects of RT on brain structural and functional integrity and cognition.
Methods and analysis
This study will enroll patients with benign and low-grade brain tumours receiving partial brain radiotherapy. Patients will receive either hypofractionated (>2 Gy/fraction) or conventionally fractionated (1.8–2 Gy/fraction) RT. All participants will be followed for 12 months, with MRIs conducted pre-RT and 6-month and 12 month post-RT, along with a battery of neurocognitive tests and questionnaires. The study was initiated in late 2018 and will continue enrolling through 2024 with final follow-ups completing in 2025. The neurocognitive battery assesses visual and verbal memory, attention, executive function, processing speed and emotional cognition. MRI protocols incorporate diffusion tensor imaging and resting state fMRI to assess structural connectivity and functional connectivity, respectively. We will estimate the association between radiation dose, imaging metrics and cognitive outcomes.
Ethics and dissemination
This study has been approved by the Research Subjects Review Board at the University of Rochester (STUDY00001512: Cognitive changes in patients receiving partial brain radiation). All results will be published in peer-reviewed journals and at scientific conferences.
Trial registration number
ClinicalTrials.gov NCT04390906.
Keywords: radiotherapy, neurological oncology, magnetic resonance imaging, radiation oncology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Limiting the study to participants with only benign and low-grade brain tumours helps mitigate confounding factors such as variation in tumour biology and normal tissue infiltration.
Prospective design with baseline evaluation prior to radiation allows capture of longitudinal changes in imaging and cognitive outcomes.
Use of open-source software assures transparency, reproducibility and implementation of the proposed protocol by other investigators.
Inclusion of patients receiving hypofractionated radiation, which has increasingly been used in benign and low-grade brain tumours, is highly important since much of the data focuses on conventionally fractionated radiation.
Heterogeneity of patient population including tumour type, size and location, radiation techniques, patient clinical factors including age, other cancer treatments including chemotherapy and surgery is a significant limitation; however, these factors will be adjusted for in analysis and improve generalisability of results.
Introduction
Rationale and evidence gaps
Cognitive impairment in patients with brain tumours has a major impact on quality of life and on the ability to function at work and in daily life.1–4 Deficits manifest clinically as impairments in multiple cognitive domains including memory, attention and executive function.5 6 The aetiology is often multifactorial; contributing factors may include anxiety and/or depression, tumour location and pathology, comorbidities and age, as well as effects from treatment (chemotherapy, surgery and/or radiation therapy (RT)7). Notably, radiation-induced cognitive decline (RICD) is observed in more than 30% of patients at 4 months after partial or whole brain RT and in more than 50% at 6 months.8 RICD is particularly important in patients with low-grade and benign tumours who are expected to have long-term survivals. In these patients, treatment selection to maximise quality of life and minimise cognitive deficits is imperative. Considerable efforts have been directed toward understanding and preventing RICD, an important late effect of RT.5 9 10 To date, multiple mechanisms underlying RICD have been elucidated, including damage to sites of neurogenesis,11 12 neuroinflammation,13 14 neuronal dysfunction15 and vascular changes.16–18
RICD can occur in the absence of any gross anatomical changes. Advanced MRI techniques, however, may be able to detect effects from RT early on and may help elucidate mechanisms of radiation damage in RICD.19 MRI can examine volumetric and connectivity changes (both functional and structural) as well as changes in brain vasculature and perfusion. MRI may ultimately provide tools to identify patients at risk for RICD and help to direct efforts to prevent or ameliorate cognitive decline. Accordingly, accurate modelling of neurocognitive function with neuropsychological tests and correlation with in vivo imaging findings may help to identify putative biomarkers for routine quantitative evaluation of cognitive changes in patients with RICD. Novel MRI biomarkers of RICD are essential to improve understanding of how RT affects the brain structurally and functionally, to identify potential targets and therapeutics to mitigate RICD, and to improve initial RT plans to decrease complication rates.
RT affects both grey and white matter structures, yet the functional implications of these changes are actively being investigated. Studies have shown that cranial RT is associated with dose-dependent atrophy of the cortex,20 hippocampus21 and amygdala22 on T1-weighted (T1w) MRI. The hippocampus in particular has garnered attention as a vulnerable structure in the setting of RT; where RT has been shown to reduce neurogenesis23 24 and the pool of neural stem cells in the dentate gyrus.25 26 Additionally, radiation dose to the hippocampus has also been shown to predict Hopkins Verbal Learning Test scores after brain irradiation.27 Currently, the hippocampus is the only intracranial structure for which validated dose constraints are used in standard treatment planning.28–31 NRG Oncology CC001 showed that conformal avoidance of the bilateral hippocampi (important structures in learning and memory) during whole brain RT reduced the risk of cognitive decline at 6 months from 68.2% to 59.5%.32 Despite advances in understanding of the role of the hippocampus in RICD, however, nearly 60% of patients still experience diminished cognitive function after RT despite conformal avoidance of the hippocampus. Moreover, recent studies have shown that radiation dose to the corpus callosum and surrounding white matter tracts can impact attention and processing speed at 6 months post-RT,33 executive function with radiation damage to the anterior cingulate cortex,34 damage to perisylvian white matter can predict language dysfunction,35 and damage to the hippocampus, temporal pole and entorhinal cortex can predict changes in visuospatial memory.36 Thus, while the hippocampus is undoubtedly an important structure in memory formation, the singular focus on this region likely belies the complexity of structures and networks involved in memory formation and ignores the contribution of other anatomic structures to cognitive deficits seen post-therapy.
Novelty and innovation
While there have been some strides made in understanding RICD, there remain significant gaps in our knowledge, which our study hopes to address. These include applications of rs-fMRI in prediction of RICD, evaluation of cognitive outcomes after hypofractionated radiation for low-grade and benign brain tumours, evaluation of novel areas of interest that could contribute to cognitive decline, and integration of established autosegmentation software such as Freesurfer with radiation dose information.
Whole brain networks can be evaluated by analysing structural and functional connections within the brain and their connections to function and behaviour.37 Structural connectivity (SC) in the brain is measured by tracing white matter tracts derived from diffusion tensor imaging (DTI).38 DTI evaluates the direction and magnitude of water molecular diffusion in a three-dimensional space (diffusion tensor) and can provide information on anisotropic diffusion. Additional quantitative metrics such as the fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD) and radial diffusivity (RD) can be obtained from DTI and can help describe different disease states such as demyelination.39 40 Studies using DTI have shown that RT results in atrophy, demyelination of white matter and gliosis41 particularly in patients with a history of demyelinating diseases.42 Partial brain RT has also been shown to result in decreased AD and increased RD within the parahippocampal cingulum, where these changes are correlated with declines in verbal memory and fluency.43 Notably, reconstruction of fibre tracts in brain tumours and surrounding tissues is confounded by false continuities within the tumour and surrounding oedema.44 Accordingly, advanced diffusion methods have recently been developed to model and eliminate free water with single-shell diffusion weighted imaging data, to more accurately model the tissue microstructure of surrounding normal brain tissue.45 However, similar to gross volumetric changes, apparent evidence of white matter atrophy and demyelination may not be discernable prior to 6 months or 1-year post-RT.46 47 Resting state functional MRI (rs-fMRI) can be used to evaluate functional connectivity (FC). In particular, graph-theory analysis of FC has revealed topological organisation of brain networks,48 which has been used to investigate how network topology is affected in development,49 ageing50 and pathology.51–53 Graph theory-based approaches treat the brain as a network of nodes and edges, where nodes can be a region of interest (ROI) or a single voxel. Edges are the connections between each node. These graphical relationships can then be modelled as a correlation matrix, in which cross correlation is performed to determine the strength between pairs of nodes. Analysis of these matrices has revealed the brain to be highly modular,54 55 with specific network hubs (areas of many connections to other nodes),56 which have been shown to change in the setting of pathology and RT.57 Nevertheless, it is not known whether functional network changes can predict RICD or precede structural changes on MRI.
There has been limited evaluation of whether rs-fMRI can be used to predict early radiation changes. This may be partially due to difficulty in using rs-fMRI in high-grade glioma.33 Other studies have consistently demonstrated that IDH wild type gliomas (ie, gliomas with more aggressive histology) have greater impact on FC metrics such as global FC derived from rs-fMRI58 as well as impact baseline cognitive status to a greater degree prior to any treatment.59 When limited to select patients, this modality may be useful as an MRI biomarker in early grade and benign brain tumour patients receiving radiation. Further studies focused on that population and excluding high-grade glioma patients, such as this one, are needed. Whole brain metrics such as FC may provide early identification of participants who are at risk of decline and can be targeted with novel therapeutics, either by using advanced RT techniques to improve RT plans or use of radioprotective pharmaceuticals. Preliminary studies are limited but suggest that rs-fMRI and FC represent a promising modality with which to develop dose constraints and mitigate cognitive decline after RT.60–62
Study aims
Investigation into structures outside the hippocampus that can be spared in order to improve cognitive outcomes remains an area of active study. We have the most data for RICD related to radiation dose to the corpus callosum33 63 and hippocampus.31 32 However, we currently have no valid dose constraints for the structures outside the hippocampus including the corpus callosum. Development of dose constraints and investigation of which structures can be avoided and lead to improvement in clinical outcomes is an area of active research as we seek to understand the complex structural and functional relationships that lead to RICD.64
Additionally, radiosurgery and fractionated radiosurgery are important modalities used frequently in the treatment of benign brain tumours. With the increased use of hypofractionation and radiosurgery, it is important to establish dose constraints that are valid in the setting of high dose per fraction.65 As of now, we have little data on dose constraints for cognitive avoidance structures in the setting of hypofractionation, and much is extrapolated from studies of conventional fractionation.
Accordingly, this study will evaluate the effects of RT in patients with benign and low-grade brain tumours using multimodal neuroimaging and a battery of neurocognitive tests. We hypothesise that radiation induced damage will manifest prior to gross anatomical changes via alterations in network topology and microstructural integrity. Ultimately, we aim to establish structures beyond the hippocampus that are vulnerable to RT and develop dose constraints to minimise the risk and progression of RICD in this vulnerable patient population.
Methods and analysis
Study design
A total of 75 patients with benign and low-grade brain tumours planned to receive partial brain RT, either hypofractionated (>2 Gy/fraction) or conventionally fractionated (1.8–2 Gy/fraction), will be enrolled at the Wilmot Cancer Institute. All participants provide written informed consent according to the Institutional Review Board (IRB) approved protocol prior to any evaluation. Participants are followed for 12 months.
Key inclusion criteria include (1) age ≥18 years; (2) patients with benign or low-grade brain tumours including grade 2 IDH-mutant astrocytoma, grade 2 oligodendroglioma, grade 1 and 2 meningiomas, vestibular schwannomas, pituitary adenomas, craniopharyngiomas, haemangiopericytomas or other benign or low-grade brain tumours; (3) planned to receive either conventional or hypofractionated RT and (4) no contraindication to gadolinium-enhanced MRI. Surgical excision and/or chemotherapy prior to enrolment is permitted.
Key exclusion criteria include: (1) prior cranial RT; (2) inability to participate in neurocognitive testing; (3) intractable seizures; (4) non-English-speaking and (5) aphasia limiting ability to participate in neurocognitive testing.
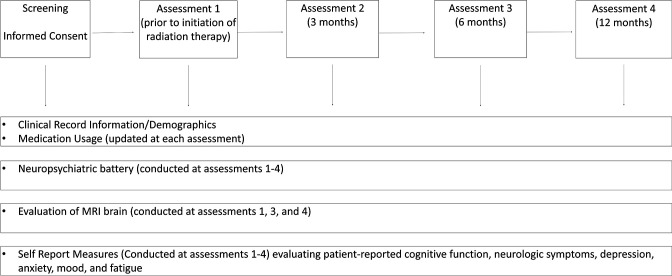
Participants will undergo three comprehensive evaluations (baseline, 6-month and 12-month time points) that include clinical evaluation, MRI, a battery of neurocognitive tests, and questionnaires which evaluate patient-reported cognition, fatigue, anxiety and depression. An additional 3-month time point includes questionnaires and neurocognitive testing only (figure 1).
Figure 1.
Study schema.
Patient and public involvement
Patients will be involved in the design and conduct of this research as follows: After completion of this study, we will plan to further tailor the study design for a larger study by conducting interviews with participants. Once the results have been published, participants will be informed via email.
Neurocognitive testing
Assessments of neurocognitive and functional performance are performed to evaluate neurocognitive changes post-RT. The components of the neurocognitive testing battery are described in table 1. The battery includes the standard tests recommended by the International Cognition and Cancer Task Force66 testing verbal memory (Hopkins Verbal Learning Test-Revised, HVLT-R),67 68 verbal fluency (Controlled Oral Word Associated Test)69 and executive function (Trail Making Test).70 71 However, the battery additionally includes the Brief Visuospatial Memory Test-Revised, which has a similar format to the HVLT-R but focuses on visuospatial learning and memory,36 as well as additional iPad-based tests from Cambridge Cognition which have been shown to be valid and sensitive in the assessment of cancer-related cognitive impairment.72–74 Neuropsychological testing is administered by trained study coordinators using a standardised testing manual; study coordinators are supervised by the study team with expertise in neurology, neuropsychology and cognitive science. Raw scores will be used in analysis and adjusted for covariates such as age. Testing is performed in a quiet, comfortable room without distractions.
Table 1.
Description and platform of tests in cognitive battery
| Test | Description | Cognitive domain | Platform |
| Wide Range Achievement Test-4 | Word reading | Cognitive reserve, education level | Paper based |
| Hopkins Verbal Learning Test-Revised | Immediate and delayed recall of a word list | Verbal learning and memory | Paper based |
| Controlled Oral Word Associated Test | Number of words the participant can provide in a category over 1 min | Verbal fluency | Paper based |
| Trail Making Test A and B (TMT-A and TMT-B) | Connect circles containing a series of numbers (A) or numbers and letters (B) in a pattern | Executive function | Paper based |
| Brief Visuospatial Memory Test-Revised | Immediate and delayed recall of a series of shapes and designs | Visuospatial learning and memory | Paper based |
| Emotional Recognition Task | Identification of the emotion indicated by a facial expression | Emotional and social cognition | Cambridge cognition |
| Spatial Working Memory | Use of strategy to find a yellow token behind coloured boxes | Executive function, visuospatial working memory | Cambridge cognition |
| Paired Associates Learning | Match the pattern to the box where it was previously displayed | Visuospatial episodic memory and new learning | Cambridge cognition |
| Delayed Matching to Sample | Matching of complex visual patterns | Visual matching ability and short-term visual recognition memory | Cambridge cognition |
| Reaction Time Task | Select a circle in which a yellow dot appears | Assessment of motor and mental response speed | Cambridge cognition |
Patient-reported outcomes
Patient-reported outcome measures of symptoms that may influence cognition are recorded longitudinally so that they can be studied and accounted for in analyses. These symptoms include fatigue, anxiety and depression using validated measures including the Functional Assessment of Cancer Therapy-Brain (FACT-Br, neurological symptoms in brain tumour patients,75 Functional Assessment of Chronic Illness Therapy-Fatigue), symptoms of fatigue,76 Patient Health Questionnaire-9, symptoms of depression,77 State-Trait Anxiety Inventory78 and Short Form of the Profile of Mood States-2, subscales of anger-hostility, confusion-bewilderment, depression-dejection, fatigue-inertia, tension-anxiety, vigor-activity and friendliness.79 Subjective cognition is measured using the FACT-Cognition (FACT-Cog),80 for comparison with scores on objective neurocognitive testing.
Demographic and clinical information
Patient characteristics that may affect cognitive outcomes and trajectories are recorded, including age, education level, comorbidities including diabetes, hypertension, autoimmune disease, tumour hemisphere, tumour site, tumour pathology, prior surgeries, employment status, smoking status, alcohol use, sex/gender, hypopituitarism, menopausal status, steroid use, use of medications that can affect cognition and mood, and exposure to chemotherapy.
Radiotherapy planning
Each patient is planned and treated per standard of care by their treating radiation oncologist. RT plans for single fraction or fractionated radiosurgery are created using BrainLAB Elements planning software. All other plans were created using Varian Eclipse treatment planning software. For consistency, all radiation dose maps were calculated in Eclipse for all patients using a 1 mm × 1 mm grid.
MRI acquisition
All imaging is performed on a 3T GE Discovery 750 MRI system (Milwaukee, Wisconsin, USA), equipped with an 8-channel head coil. High-resolution T1w anatomical images are acquired using a 3D BRAVO FSPGR sequence with the following parameters: repetition time (TR)=8.2 ms, echo time (TE)=3.2 ms, field of view (FOV) 256 mm2, resolution 1×1×1 mm3.
Blood oxygen-level dependent (BOLD) rs-fMRI is acquired using a BOLD sensitive gradient-echo echo planar imaging (EPI) sequence with the following parameters: TR=2000 ms, TE=30 ms, FOV=192 mm2, resolution 3×3×3 mm3, 150 volumes.
In order to evaluate white matter integrity and microstructural changes, we make use of a standard clinical DTI protocol with 30 diffusion directions, demonstrated to be sufficient for reconstructing white matter fibre tracts and not to affect test–retest reliability.81 DTI is acquired using a two-dimensional axial single-shot dual spin-echo EPI sequence with the following parameters: TR=10 000 ms, TE=81 ms, FOV=256 mm2, resolution 2×2×2 mm3, 30 diffusion weighted directions with b=1000 s/mm2 and 4 b=0 reference images.
MRI data processing
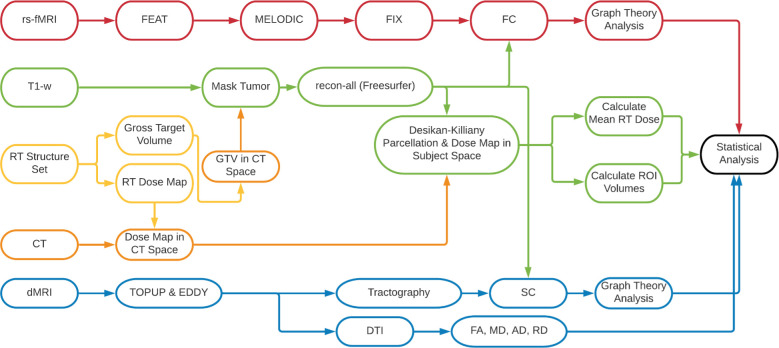
Here, we describe a comprehensive image analysis pipeline, which includes preprocessing, data cleaning and postprocessing for each imaging modality, including advanced modelling and calculation of quantitative imaging biomarkers (figure 2).
Figure 2.
MRI data processing pipeline. AD, axial diffusivity; dMRI, diffusion MRI; DTI, diffusion tensor imaging; FA, fractional anisotropy; FC, functional connectivity; FEAT, FMRI Expert Analysis Tool; FIX, FMRIB’s ICA-based Xnoisefier; GTV, Gross Target Volume; MD, mean diffusivity; MELODIC, Multivariate Exploratory Linear Optimised Decomposition into Independent Components; RD, radial diffusivity; rs-fMRI, resting state functional MRI; RT, radiotherapy; SC, structural connectivity.
All image processing is completed within URMC servers in the Centre for Integrated Research Computing, using BHWARD, a HIPAA compliant server.
RT dose calculations
The RT dose map is first scaled and mapped with CT images using pydicom (V.1.4). T1w images are registered to patient-specific CT space using FMRIB’s Linear Image Registration Tool (FLIRT).82 Patient-specific parcellations derived from the Desikan-Killiany83 atlas using Freesurfer are registered with the RT dose map. The mean, maximum and minimum RT doses are extracted from each ROI, and the 2 Gy/fraction equivalent dose (EQD2) is calculated using the linear quadratic model,84 with an equal to 3, to model the radiosensitivity of normal brain tissue.85
T1 weighted
T1w images are processed by first masking out the tumour using the gross target volume (GTV) as contoured by the primary radiation oncologist from the RT structure set,86 delineated on planar MRI and CT imaging, using nibabel (https://nipy.org, V.3.1.1). This is achieved by mapping the GTV to the CT images in patient space, and then performing an affine transform to register the T1-w images to the patient specific CT images (figure 3). Thereafter, segmentation is performed using the tumour masked T1-w in Freesurfer (V.6.0.0, http://surfer.nmr.harvard.edu). The Freesurfer pipeline is run independently for each participant at each time point (pre-RT, 6 months and 12 months post-RT). Briefly, processing includes skull-stripping and removal of non-brain tissue, motion correction, intensity normalisation, automated Talairach transformation, white matter segmentation and cortical parcellation using the Desikan-Killiany atlas,83 which includes cortical and subcortical ROIs. Subsequent segmentation of thalamic nuclei are also performed using a probabilistic atlas based on ex vivo MRI and histology.87 ROIs will include whole brain grey and white matter, cerebral hemispheres and subcortical grey matter (hippocampus, amygdala, caudate, putamen, thalamus, nucleus basalis of Meynert), as well as white matter tracts including cingulum, fornix, parahippocampal white matter and corpus callosum.
Figure 3.
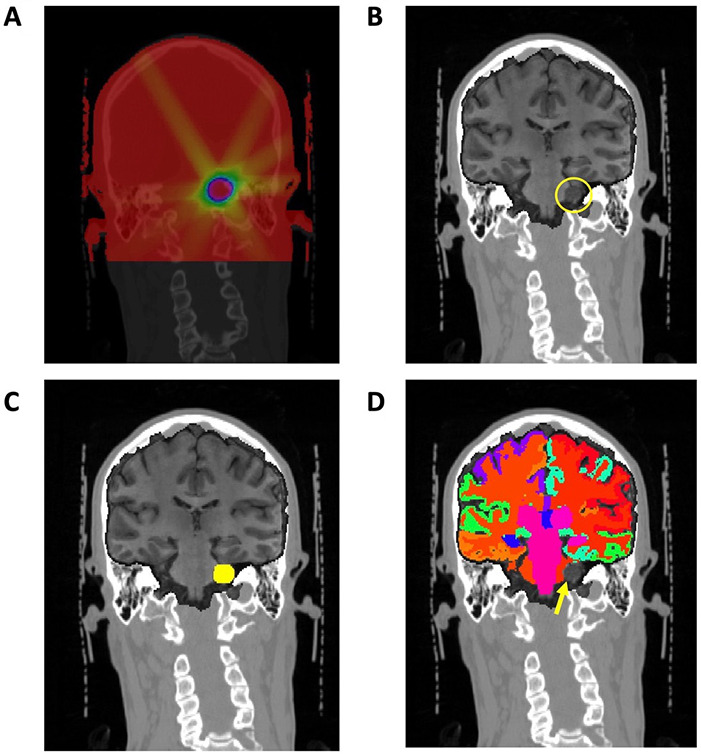
Representative images from participant with vestibular schwannoma. (A) RT dose map from RT structure set, mapped to CT image and scaled. (B) T1w structural image coregistered with CT image and RT dose map via affine transformation (yellow circle shows acoustic schwannoma). (C) T1w image with gross target volume (GTV, yellow circle) used to mask tumour prior to processing. (D) Subcortical and cortical structures obtained from brain parcellation, with vestibular schwannoma excluded (yellow arrow). RT, radiation therapy.
Diffusion imaging
DTI data are preprocessed using FMRIB’s Software Library (FSL,88–91 diffusion toolbox (FDT, http://fsl.fmrib.ox.ac.uk/fsl). Briefly, intervolume patient motion, brain extraction using BET91 and eddy-current induced distortion correction are performed using EDDY.92 The diffusion tensors are then fit on eddy-corrected data using DTIFIT.93 DTIFIT fits a diffusion tensor model at each voxel and provides the three principal eigenvectors and eigenvalues of the diffusion tensor, from which the FA, MD, AD and RD can be measured.93 All images are then registered to MNI standard space and interpolated to 1 mm3 voxels. Binary GTV masks are then used to mask out abnormal tissue in processed maps. The JHU white-matter tractography atlas, composed of 20 structures identified using probabilistic tractography, is then used to extract mean FA, MD, AD and RD values from ROI.94
Functional imaging
rs-fMRI data are processed using FSL’s FMRI Expert Analysis Tool (FEAT, V.6.00).95 Registration to high resolution structural space is carried out using a two-stage registration. First rs-fMRI data are registered to high-resolution structural space using FMRIB’s Linear Image Registration Tool (FLIRT),82 and then registration to MNI standard space96 is further refined using FMRIB’s Nonlinear Image Registration Tool (FNIRT).97 Since field maps were not acquired as part of the clinical scan protocol, a 12 df affine transformation was used for linear and nonlinear registration. All registrations are visually inspected during processing. Further, preprocessing includes skull stripping using BET,91 motion correction using MCFLIRT,82 slice-time correction, spatial smoothing using a Gaussian kernel of FWHM 5 mm and high-pass temporal filtering.
Single-session independent component analysis (ICA) is then performed for each participant using probabilistic ICA implemented in FSL’s Multivariate Exploratory Linear Optimised Decomposition into Independent Components (MELODIC, V.3.15). MELODIC decomposes input data into separate time courses and spatial maps using probabilistic principal component analysis. FMRIB’s ICA-based Xnoisifier (FIX)98 99 is then used to further denoise functional data by automatically classifying signal versus noise components from the time series data. FIX is run using the standard pretrained data (TR=3 s, 3.5×3.5×3.5 mm resolution, 6 min) which was preprocessed using default FEAT processing. All images and components are visually inspected for accuracy prior to further processing.
Once all fMRI data have been preprocessed, the denoised functional data are used to construct participant specific FC matrices. Participant-specific atlases generated using Freesurfer are then registered to the MNI standard space and used to extract the mean time series from each ROI. This is done to ensure that only functional regions outside of the tumour are used to construct FC matrices. FC matrices are then generated by computing the cross correlation between all pairs of nodes (ROIs), using the Pearson correlation coefficient (figure 4).
Figure 4.
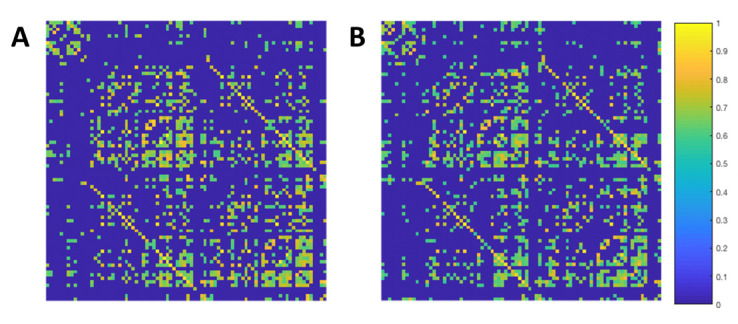
Representative functional connectivity correlation matrices. Matrices are computed using the Pearson correlation coefficient between every time course for all pairs of nodes. Matrices are thresholded at 0.5 and normalised. The average of all patient specific correlation matrices at baseline (A) and 6 months post-RT (B). The colour bar represents the normalised correlation coefficient between pairs of nodes. RT, radiation therapy.
Participant-specific FC matrices are then analysed using the Brain Connectivity Toolbox100 in MATLAB (R2020a). Participant-specific correlation matrices are thresholded to yield weighted undirected networks, and analysed using graph theory to yield measures of functional integration and segregation. Global measures of integration, including global efficiency, transitivity and modularity, are then computed for further statistical analysis.101 Local measures of segregation, including clustering coefficient and local efficiency (table 2), are also computed for each ROI for further statistical analysis. A more thorough review of graph theory-based measures for rs-fMRI may be found in Rubinov and Sporns.102
Table 2.
Overview of graph theory measures used to analysis resting state functional connectivity and structural connectivity obtained from diffusion tractography
| Measurement | Definition | Equation |
| Node degree | The no of connections between one node and the rest of the network | |
| Clustering coefficient | The no of connections between the neighbours of a node |
k is the node degree; t is the fraction of triangles around a node. |
| Efficiency | Inverse of path length (minimum no of edges to traverse from one node to another) |
Where is the shortest path length between nodes i and j |
| Modularity | Areas of highly interconnected nodes, with few connections to nodes in other modules |
A more in depth review of graph theory and graph theory measures can be found in Rubinov and Sporns.102
We estimate that 52 evaluable patients (a total of 75 participants allowing for 30% of participants with missing or incomplete data) will have ≥80% power to detect at least 0.4 SD change on the delayed recall measure of the HVLT-R post-RT. The power analysis is based on a paired t-test with a two-sided significance level of 0.05.
Analysis plan
Graphical methods will be used to explore the cognitive test and imaging data, to visually describe and compare distributions of continuous variables, and to visualise results of statistical analyses. Quantitative imaging metrics (cortical thickness, subcortical volume, FA, MD, AD, RD, local efficiency and clustering coefficient) will be analysed to investigate their relationships with RT dose and cognitive measures. Comparisons between raw scores on cognitive tests and imaging metrics pre-RT and post-RT will be performed using paired t-tests and Wilcoxon signed-rank tests. Pearson and Spearman correlation analyses will be used to assess associations between pairs of continuous measures. Multivariate mixed effect regression models will be used to evaluate the relationships of cognitive tests at 6-month and 12-month visits with RT dose to ROIs known to be instrumental in the specific cognitive domain adjusting for the baseline cognitive test, imaging parameters, age, gender, tumour laterality and tumour type. During the analyses, false discovery rate method will be used to account for multiple comparisons.103 All statistical analyses will be performed using R (R Foundation for Statistical Computing, Vienna, Austria) or SAS V.9.4.
RICD is an important target of efforts to use more sophisticated radiation techniques such as intensity modulated RT and proton therapy in order to decrease side effects.104 While validated dose constraints exist for structures such as the brainstem, cochlea, optic nerves and chiasm, and pituitary gland,28 development of dose constraints for intracranial structures involved in cognition is a new and exciting area of research that promises to improve radiation outcomes. Despite conformal dose reduction to the hippocampi, RICD occurs in a large percentage of patients, reflecting the complexity of memory formation and the need to identify non-hippocampal structures involved in higher cognitive functions. The pathology underlying RICD likely begins prior to any gross anatomical changes or noticeable differences in cognition observed by the patient. Accordingly, development of quantitative in vivo biomarkers is essential for developing dose constraints and monitoring RICD.
This study uses conventional and advanced MRI, neurocognitive testing and dosimetry information to provide a comprehensive description of RICD in patients with brain tumours receiving RT. The proposed analyses will provide insight into which intracranial structures are particularly susceptible to RT and how they modulate changes in cognition via aberrant network topology. The results of this study will help to provide dose constraints to better avoid cognitive decline that can ultimately be used to create radiation plans associated with less cognitive change. Incorporation of rs-fMRI into treatment planning and monitoring has the potential to improve cognitive outcomes in the setting of RT and provide personalised treatment. Additionally, utilisation of graph theory will be able to identify specific nodes and hubs within brain networks that are susceptible to RT at the population and individual level.105
This protocol and analysis pipeline will aid researchers interested in combining MRI data including segmentation of intracranial structures not used in standard radiation planning with radiation dosimetry information to advance our understanding of RICD. We provide detailed information on study design, clinical and imaging protocols and analysis pipeline with which to investigate RT-induced cognitive changes on intracranial structures that are not segmented with standard radiation planning software. This is an important and complex process which should be transparent, and one of our goals with this paper is to promote utilisation of open software packages in a useful and standardised way for the radiation oncology community.
Ethics and dissemination
Studies involving human participants are reviewed and approved by the Research Subjects Review Board at the University of Rochester. All patients/participants provide their written informed consent to participate in this study. All results will be presented at relevant conferences and published in relevant peer-reviewed journals.
Supplementary Material
Acknowledgments
Support from the Department of Radiation Oncology at University of Rochester.
Footnotes
SJH and AJF contributed equally.
Contributors: SH: Protocol development and design, principal investigator, manuscript editing. AJF: Development of MRI pipeline for processing and analysis, manuscript editing. EC: Development of statistical plan and power analysis, manuscript editing. KU: Mentorship on clinical aspects of protocol and recruitment, manuscript editing. JZ: MRI protocol development, manuscript editing. MCJ-B: Research mentorship in cognitive assessment and cohort studies, help with protocol development, manuscript editing. GS: Research mentorship in neuroimaging, help with protocol development and manuscript editing. MM: Research mentorship in evaluation of radiation toxicity, help with protocol development and manuscript editing. NM: Research mentorship in neuro-oncology, help with protocol development and manuscript editing. MT: MRI protocol development, manuscript editing. EL: MRI protocol development, manuscript editing. MW: Clinical neuropsychology, development of cognitive battery and interpretation of cognitive data, manuscript editing.
Funding: This work is supported by the Department of Radiation Oncology (internal funding), Wilmot Fellowship Award to SH, Schmitt Foundation Grant number GR505052 (PI: SH), DP2195765 and R01CA231014 (PI: Michelle Janelsins), R01MH118020 and R01AG054328 (PI: GS), and the Cancer Control Clinical Research Training Programme in the Dept. of Surgery. The project described in this publication was also supported by the University of Rochester CTSA award number UL1TR002001 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol 2004;3:159–68. 10.1016/S1474-4422(04)00680-5 [DOI] [PubMed] [Google Scholar]
- 2.Klaver KM, Duijts SFA, Engelhardt EG, et al. Cancer-Related cognitive problems at work: experiences of survivors and professionals. J Cancer Surviv 2020;14:168–78. 10.1007/s11764-019-00830-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison RA, Wefel JS. Neurocognitive function in adult cancer patients. Neurol Clin 2018;36:653–74. 10.1016/j.ncl.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 4.Salans M, Tibbs MD, Huynh-Le M-P, et al. Quality of life is independently associated with neurocognitive function in patients with brain tumors: analysis of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2021;111:754–63. 10.1016/j.ijrobp.2021.05.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazzaglia S, Briganti G, Mancuso M, et al. Neurocognitive decline following radiotherapy: mechanisms and therapeutic implications. Cancers (Basel) 2020;12:146. 10.3390/cancers12010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy SJ, Krull KR, Wefel JS, et al. Cognitive changes in cancer survivors. Am Soc Clin Oncol Educ Book 2018;38:795–806. 10.1200/EDBK_201179 [DOI] [PubMed] [Google Scholar]
- 7.Coomans MB, van der Linden SD, Gehring K, et al. Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol 2019;31:540–7. 10.1097/CCO.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makale MT, McDonald CR, Hattangadi-Gluth JA, et al. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2017;13:52–64. 10.1038/nrneurol.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res 2013;19:2294–300. 10.1158/1078-0432.CCR-11-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment -- from bench to bedside. Neuro Oncol 2012;14 Suppl 4:iv37–44. 10.1093/neuonc/nos196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaelidesová A, Konířová J, Bartůněk P, et al. Effects of radiation therapy on neural stem cells. Genes (Basel) 2019;10:640. 10.3390/genes10090640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellinzona M, Gobbel GT, Shinohara C, et al. Apoptosis is induced in the subependyma of young adult rats by ionizing irradiation. Neurosci Lett 1996;208:163–6. 10.1016/0304-3940(96)12572-6 [DOI] [PubMed] [Google Scholar]
- 13.Lumniczky K, Szatmári T, Sáfrány G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front Immunol 2017;8:517.:517. 10.3389/fimmu.2017.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constanzo J, Midavaine É, Fouquet J, et al. Brain irradiation leads to persistent neuroinflammation and long-term neurocognitive dysfunction in a region-specific manner. Prog Neuropsychopharmacol Biol Psychiatry 2020;102:109954. 10.1016/j.pnpbp.2020.109954 [DOI] [PubMed] [Google Scholar]
- 15.Wu PH, Coultrap S, Pinnix C, et al. Radiation induces acute alterations in neuronal function. PLoS One 2012;7:e37677. 10.1371/journal.pone.0037677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010;55:1237–9. 10.1016/j.jacc.2009.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesulu BP, Mahadevan LS, Aliru ML, et al. Radiation-Induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl Sci 2018;3:563–72. 10.1016/j.jacbts.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatesulu BP, Sanders KL, Hsieh CE, et al. Biomarkers of radiation-induced vascular injury. Cancer Rep (Hoboken) 2019;2:e1152. 10.1002/cnr2.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene-Schloesser D, Robbins ME, Peiffer AM, et al. Radiation-induced brain injury: a review. Front Oncol 2012;2:73. 10.3389/fonc.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunamuni R, Bartsch H, White NS, et al. Dose-dependent cortical thinning after partial brain irradiation in high-grade glioma. Int J Radiat Oncol Biol Phys 2016;94:297–304. 10.1016/j.ijrobp.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seibert TM, Karunamuni R, Bartsch H, et al. Radiation dose-dependent hippocampal atrophy detected with longitudinal volumetric magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2017;97:263–9. 10.1016/j.ijrobp.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh-Le M-P, Karunamuni R, Moiseenko V, et al. Dose-dependent atrophy of the amygdala after radiotherapy. Radiother Oncol 2019;136:44–9. 10.1016/j.radonc.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang J, Kim W, Seo H, et al. Radiation-Induced overexpression of transthyretin inhibits retinol-mediated hippocampal neurogenesis. Sci Rep 2018;8:8394. 10.1038/s41598-018-26762-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji S, Ding X, Ji J, et al. Cranial irradiation inhibits hippocampal neurogenesis via DNMT1 and Dnmt3a. Oncol Lett 2018;15:2899–904. 10.3892/ol.2017.7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Abdallah NM-B, Slomianka L, Lipp H-P. Reversible effect of x-irradiation on proliferation, neurogenesis, and cell death in the dentate gyrus of adult mice. Hippocampus 2007;17:1230–40. 10.1002/hipo.20358 [DOI] [PubMed] [Google Scholar]
- 26.Mineyeva OA, Bezriadnov DV, Kedrov AV, et al. Radiation induces distinct changes in defined subpopulations of neural stem and progenitor cells in the adult hippocampus. Front Neurosci 2018;12:1013. 10.3389/fnins.2018.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okoukoni C, McTyre ER, Ayala Peacock DN, et al. Hippocampal dose volume histogram predicts Hopkins verbal learning test scores after brain irradiation. Adv Radiat Oncol 2017;2:624–9. 10.1016/j.adro.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scoccianti S, Detti B, Gadda D, et al. Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol 2015;114:230–8. 10.1016/j.radonc.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 29.Kazda T, Jancalek R, Pospisil P, et al. Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol 2014;9:139. 10.1186/1748-717X-9-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “ how-to ” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1244–52. 10.1016/j.ijrobp.2010.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol 2010;97:370–6. 10.1016/j.radonc.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondi V, Deshmukh S, Brown PD, et al. Preservation of neurocognitive function (ncf) with conformal avoidance of the hippocampus during whole-brain radiotherapy (ha-wbrt) for brain metastases: preliminary results of phase III trial NRG oncology CC001. Int J Radiat Oncol Biol Phys 2018;102:1607. 10.1016/j.ijrobp.2018.08.056 [DOI] [Google Scholar]
- 33.Huynh-Le M-P, Tibbs MD, Karunamuni R, et al. Microstructural injury to corpus callosum and intrahemispheric white matter tracts correlate with attention and processing speed decline after brain radiation. Int J Radiat Oncol Biol Phys 2021;110:337–47. 10.1016/j.ijrobp.2020.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tringale KR, Nguyen T, Bahrami N, et al. Identifying early diffusion imaging biomarkers of regional white matter injury as indicators of executive function decline following brain radiotherapy: a prospective clinical trial in primary brain tumor patients. Radiother Oncol 2019;132:27–33. 10.1016/j.radonc.2018.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibbs MD, Huynh-Le M-P, Karunamuni R, et al. Microstructural injury to left-sided perisylvian white matter predicts language decline after brain radiation therapy. Int J Radiat Oncol Biol Phys 2020;108:1218–28. 10.1016/j.ijrobp.2020.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tringale KR, Nguyen TT, Karunamuni R, et al. Quantitative imaging biomarkers of damage to critical memory regions are associated with post-radiation therapy memory performance in brain tumor patients. Int J Radiat Oncol Biol Phys 2019;105:773–83. 10.1016/j.ijrobp.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol 2005;1:e42. 10.1371/journal.pcbi.0010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baliyan V, Das CJ, Sharma R, et al. Diffusion weighted imaging: technique and applications. World J Radiol 2016;8:785–98. 10.4329/wjr.v8.i9.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med 2011;65:1532–56. 10.1002/mrm.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–46. 10.1002/jmri.1076 [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Wu EX, Qiu D, et al. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res 2009;69:1190–8. 10.1158/0008-5472.CAN-08-2661 [DOI] [PubMed] [Google Scholar]
- 42.Milic M, Rees JH. Acute demyelination following radiotherapy for glioma: a cautionary tale. Pract Neurol 2017;17:35–8. 10.1136/practneurol-2016-001432 [DOI] [PubMed] [Google Scholar]
- 43.Chapman CH, Zhu T, Nazem-Zadeh M, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol 2016;120:234–40. 10.1016/j.radonc.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson F, Abdullah KG, Verma R, et al. Tractography and the connectome in neurosurgical treatment of gliomas: the premise, the progress, and the potential. Neurosurg Focus 2020;48:E6. 10.3171/2019.11.FOCUS19785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker D, Ould Ismail AA, Wolf R, et al. Freewater estimator using interpolated initialization (FERNET): characterizing peritumoral edema using clinically feasible diffusion MRI data. PLoS One 2020;15:e0233645. 10.1371/journal.pone.0233645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman CH, Nagesh V, Sundgren PC, et al. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys 2012;82:2033–40. 10.1016/j.ijrobp.2011.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagesh V, Tsien CI, Chenevert TL, et al. Radiation-Induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys 2008;70:1002–10. 10.1016/j.ijrobp.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- 49.Suprano I, Delon-Martin C, Kocevar G, et al. Topological modification of brain networks organization in children with high intelligence quotient: a resting-state fmri study. Front Hum Neurosci 2019;13:241. 10.3389/fnhum.2019.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varangis E, Habeck CG, Razlighi QR, et al. The effect of aging on resting state connectivity of predefined networks in the brain. Front Aging Neurosci 2019;11:234. 10.3389/fnagi.2019.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Openneer TJC, Marsman J-B, van der Meer D, et al. A graph theory study of resting-state functional connectivity in children with tourette syndrome. Cortex 2020;126:63–72. 10.1016/j.cortex.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 52.Khazaee A, Ebrahimzadeh A, Babajani-Feremi A. Identifying patients with alzheimer’s disease using resting-state fmri and graph theory. Clin Neurophysiol 2015;126:2132–41. 10.1016/j.clinph.2015.02.060 [DOI] [PubMed] [Google Scholar]
- 53.Sjoerds Z, Stufflebeam SM, Veltman DJ, et al. Loss of brain graph network efficiency in alcohol dependence. Addict Biol 2017;22:523–34. 10.1111/adb.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caparelli EC, Ross TJ, Gu H, et al. Graph theory reveals amygdala modules consistent with its anatomical subdivisions. Sci Rep 2017;7:14392. 10.1038/s41598-017-14613-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meunier D, Lambiotte R, Fornito A, et al. Hierarchical modularity in human brain functional networks. Front Neuroinform 2009;3:37. 10.3389/neuro.11.037.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Power JD, Schlaggar BL, Lessov-Schlaggar CN, et al. Evidence for hubs in human functional brain networks. Neuron 2013;79:798–813. 10.1016/j.neuron.2013.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bahrami N, Seibert TM, Karunamuni R, et al. Altered network topology in patients with primary brain tumors after fractionated radiotherapy. Brain Connect 2017;7:299–308. 10.1089/brain.2017.0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derks J, Kulik S, Wesseling P, et al. Understanding cognitive functioning in glioma patients: the relevance of IDH-mutation status and functional connectivity. Brain Behav 2019;9:e01204. 10.1002/brb3.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wefel JS, Noll KR, Rao G, et al. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol 2016;18:1656–63. 10.1093/neuonc/now165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell TJ, Seitzman BA, Ballard N, et al. Human brain functional network organization is disrupted after whole-brain radiation therapy. Brain Connect 2020;10:29–38. 10.1089/brain.2019.0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffau H. Why brain radiation therapy should take account of the individual structural and functional connectivity: toward an irradiation “ à la carte.” Crit Rev Oncol Hematol 2020;154:103073. 10.1016/j.critrevonc.2020.103073 [DOI] [PubMed] [Google Scholar]
- 62.Li M, Zhang Q, Yang K. Role of MRI-based functional imaging in improving the therapeutic index of radiotherapy in cancer treatment. Front Oncol 2021;11:645177. 10.3389/fonc.2021.645177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redmond KJ, Hildreth M, Sair HI, et al. Association of neuronal injury in the genu and body of corpus callosum after cranial irradiation in children with impaired cognitive control: a prospective study. Int J Radiat Oncol Biol Phys 2018;101:1234–42. 10.1016/j.ijrobp.2018.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Redmond KJ, Milano MT, Kim MM, et al. Reducing radiation-induced cognitive toxicity: sparing the hippocampus and beyond. Int J Radiat Oncol Biol Phys 2021;109:1131–6. 10.1016/j.ijrobp.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 65.Milano MT, Grimm J, Niemierko A, et al. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys 2021;110:68–86. 10.1016/j.ijrobp.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wefel JS, Vardy J, Ahles T, et al. International cognition and cancer Task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 2011;12:703–8. 10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- 67.Shapiro AM, Benedict RH, Schretlen D, et al. Construct and concurrent validity of the Hopkins verbal learning test-revised. Clin Neuropsychol 1999;13:348–58. 10.1076/clin.13.3.348.1749 [DOI] [PubMed] [Google Scholar]
- 68.Rasmusson DX, Bylsma FW, Brandt J. Stability of performance on the Hopkins verbal learning test. Arch Clin Neuropsychol 1995;10:21–6. [PubMed] [Google Scholar]
- 69.Benton AL HK, Sivan AB. Controlled oral word association multilingual aphasia examination professional manual. Lutz, FL, 1978. [Google Scholar]
- 70.Gaudino EA, Geisler MW, Squires NK. Construct validity in the TRAIL making test: what makes Part B harder? J Clin Exp Neuropsychol 1995;17:529–35. 10.1080/01688639508405143 [DOI] [PubMed] [Google Scholar]
- 71.Goul WR, Brown M. Effects of age and intelligence on TRAIL making test performance and validity. Percept Mot Skills 1970;30:319–26. 10.2466/pms.1970.30.1.319 [DOI] [PubMed] [Google Scholar]
- 72.Janelsins MC, Heckler CE, Peppone LJ, et al. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol 2018;36:JCO2018786624. 10.1200/JCO.2018.78.6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fray PJ, Robbins TW. Cantab battery: proposed utility in neurotoxicology. Neurotoxicol Teratol 1996;18:499–504. 10.1016/0892-0362(96)00027-x [DOI] [PubMed] [Google Scholar]
- 74.Robbins TW, James M, Owen AM, et al. Cambridge neuropsychological test automated battery (Cantab): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266–81. 10.1159/000106735 [DOI] [PubMed] [Google Scholar]
- 75.Thavarajah N, Bedard G, Zhang L, et al. Psychometric validation of the functional assessment of cancer therapy -- brain (FACT-br) for assessing quality of life in patients with brain metastases. Support Care Cancer 2014;22:1017–28. 10.1007/s00520-013-2060-8 [DOI] [PubMed] [Google Scholar]
- 76.Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 2009;20:17–25. 10.1093/annonc/mdn537 [DOI] [PubMed] [Google Scholar]
- 77.Hinz A, Mehnert A, Kocalevent R-D, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry 2016;16:22. 10.1186/s12888-016-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the state-trait anxiety inventory. Consulting Psychologists Press, 1983. [Google Scholar]
- 79.Baker F, Denniston M, Zabora J, et al. A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology 2002;11:273–81. 10.1002/pon.564 [DOI] [PubMed] [Google Scholar]
- 80.Dyk KV, Crespi CM, Petersen L, et al. Identifying cancer-related cognitive impairment using the FACT-cog perceived cognitive impairment. JNCI Cancer Spectr 2020;4:pkz099. 10.1093/jncics/pkz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang JY, Abdi H, Bakhadirov K, et al. A comprehensive reliability assessment of quantitative diffusion tensor tractography. Neuroimage 2012;60:1127–38. 10.1016/j.neuroimage.2011.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–41. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- 83.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 84.McMahon SJ. The linear quadratic model: usage, interpretation and challenges. Phys Med Biol 2018;64:01TR01. 10.1088/1361-6560/aaf26a [DOI] [PubMed] [Google Scholar]
- 85.van Leeuwen CM, Oei AL, Crezee J, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol 2018;13:96. 10.1186/s13014-018-1040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Law MYY, Liu B. Informatics in radiology: DICOM-RT and its utilization in radiation therapy. Radiographics 2009;29:655–67. 10.1148/rg.293075172 [DOI] [PubMed] [Google Scholar]
- 87.Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 2018;183:314–26. 10.1016/j.neuroimage.2018.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in fsl. Neuroimage 2009;45(1 Suppl):S173–86. 10.1016/j.neuroimage.2008.10.055 [DOI] [PubMed] [Google Scholar]
- 89.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural Mr image analysis and implementation as fsl. Neuroimage 2004;23 Suppl 1:S208–19. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 90.Jenkinson M, Beckmann CF, Behrens TEJ, et al. Fsl. Neuroimage 2012;62:782–90. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 91.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–55. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016;125:1063–78. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247–54. 10.1006/jmrb.1994.1037 [DOI] [PubMed] [Google Scholar]
- 94.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage 2008;39:336–47. 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Woolrich MW, Ripley BD, Brady M, et al. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage 2001;14:1370–86. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- 96.Evans AC, Janke AL, Collins DL, et al. Brain templates and atlases. Neuroimage 2012;62:911–22. 10.1016/j.neuroimage.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 97.Kim J-W, Andersson JL, Seifert AC, et al. Incorporating non-linear alignment and multi-compartmental modeling for improved human optic nerve diffusion imaging. Neuroimage 2019;196:102–13. 10.1016/j.neuroimage.2019.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salimi-Khorshidi G, Douaud G, Beckmann CF, et al. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 2014;90:449–68. 10.1016/j.neuroimage.2013.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffanti L, Salimi-Khorshidi G, Beckmann CF, et al. ICA-based artefact removal and accelerated fmri acquisition for improved resting state network imaging. Neuroimage 2014;95:232–47. 10.1016/j.neuroimage.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sporns O. The human connectome: a complex network. Ann N Y Acad Sci 2011;1224:109–25. 10.1111/j.1749-6632.2010.05888.x [DOI] [PubMed] [Google Scholar]
- 101.Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci 2013;15:247–62. 10.31887/DCNS.2013.15.3/osporns [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 103.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 104.Cho B. Intensity-Modulated radiation therapy: a review with a physics perspective. Radiat Oncol J 2018;36:1–10. 10.3857/roj.2018.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farahani FV, Karwowski W, Lighthall NR. Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci 2019;13:585. 10.3389/fnins.2019.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.