Abstract
Drug-induced testicular injury (DITI) is one of the often-observed and challenging safety issues seen during drug development. Semen analysis and circulating hormones currently utilized have significant gaps in their ability to detect testicular damage accurately. In addition, no biomarkers enable a mechanistic understanding of the damage to the different regions of the testis, such as seminiferous tubules, Sertoli, and Leydig cells. MicroRNAs (miRNAs) are a class of non-coding RNAs that modulate gene expression post-transcriptionally and have been indicated to regulate a wide range of biological pathways. Circulating miRNAs can be measured in the body fluids due to tissue-specific cell injury/damage or toxicant exposure. Therefore, these circulating miRNAs have become attractive and promising non-invasive biomarkers for assessing drug-induced testicular injury, with several reports on their use as safety biomarkers for monitoring testicular damage in preclinical species. Leveraging emerging tools such as ‘organs-on-chips’ that can emulate the human organ’s physiological environment and function is starting to enable biomarker discovery, validation, and clinical translation for regulatory qualification and implementation in drug development.
Keywords: miRNAs, Drug-induced testicular injury, DITI, TransBioLine, PSTC
Introduction
The testis is a common target organ identified in standard nonclinical toxicity studies conducted during drug development. Depending on the safety margins of the testicular finding, indication, and patient population, testicular injury can lead to the termination of the drug development program or may advance with the addition of clinical monitoring (such as semen analysis). Therefore, reliable biomarkers can benefit early in the discovery program to screen compounds in animal studies with better safety profiles and to predict the onset and progression of testicular injury in humans. The 2018 U.S. Food and Drug Administration (FDA) ‘Testicular Toxicity Evaluation’ guidance describes that semen analysis and circulating hormones such as testosterone, follicle-stimulating hormone (FSH), and luteinizing (LH) can be utilized for monitoring testis damage during clinical trials (FDA 2018). However, the traditional methods, such as sperm count, motility, morphology analysis, and testosterone hormone measurement in biological fluids, cannot accurately and specifically indicate testicular injury severity and progression (Dere et al. 2013). This is due to their high variability and the fact that there is often a delay between the initial testicular injury and detection of a change in sperm endpoints, which are typically more of an “end-stage” effect. In addition, evaluation of hormone levels is only helpful for compounds for which the primary mechanism of toxicity is disruption of hormone production or signaling. Serum protein biomarkers such as inhibin B in combination with FSH have also been considered biomarkers for testicular injury (von Eckardstein et al. 1999). Other literature-reported potential protein biomarkers for testicular injury are summarized in Table 1. However, due to the insensitivity and non-specificity of these protein biomarkers, it is challenging to accurately utilize these biomarkers in toxicology studies to select compounds devoid of testicular injury. Testis histopathology remains the “gold standard” for identifying and characterizing testicular injury. Given that this method is invasive, time-consuming, costly, and unable to be utilized as a translatable biomarker (McDuffie et al. 2016), it is imperative to develop reliable, sensitive, noninvasive, and easily attainable biomarkers of testicular injury that would serve as an indicator of testicular injury to de-risk testicular toxicities in animals and also potentially serve as improved monitorable clinical biomarkers.
Table 1.
Literature-reported protein biomarkers for evaluation of testicular injury
| Biomarkers | Pros | Cons | References |
|---|---|---|---|
| Androgen binding protein (ABP) | Easy to measure in serum/plasma; Correlated with sperm concentration | Lack of sensitivity; Nonspecific; Not reflect Sertoli cell damage | Reader et al. (Reader et al. 1991), Rehnberg et al. (Rehnberg et al. 1989) |
| Anti-Mullerian hormone (AMH) | Easy to measure in serum/plasma | They are only reported in preclinical studies, e.g., rodents and horses, Insensitive to mild/moderate testicular injury |
Pozor et al. (Pozor et al. 2018), Levi et al. (Levi et al. 2015) |
| Creatine | Easy to measure in urine | Associated with another tissue injury, e.g., liver, heart, and muscle; Lack of sensitivity; High variation in humans | Moore et al. (Moore et al. 1992, 1998), Butterworth et al. (Butterworth et al. 1995), Timbrell et al. (Timbrell 2000) |
| Inhibin B in combination with Follicle-stimulating hormone (FSH) | Easy to measure in serum; Associated with Sertoli cell function | Lack of sensitivity; Not sensitive as histopathology | Von Eckardstein et al. (von Eckardstein et al. 1999), Coulson et al. (Coulson et al. 2013) |
| Lactate dehydrogenase-C4 (LDH-C4) | Easy to measure in serum/plasma; Correlated with sperm count, motility | Associated with lung cancer; Lack of sensitivity | Draper et al. (Draper et al. 1996), Reader et al. (Reader et al. 1991) |
| SP22 | Highly conserved protein across the species | Low abundant in serum; Challenge in assay development | Klinefelter et al. (Klinefelter et al. 1999, 1997) |
| Testosterone | Easy to measure in serum | Insensitive to mild/moderate testicular injury | Rehnberg et al. (Rehnberg et al. 1989) |
In addition to the testicular biomarker gap, there are no biomarkers that can guide mechanistic understanding of the damage to different testicular compartments. Briefly, the testis has two functional compartments: highly convoluted seminiferous tubules and the interstitial space comprised mainly of Leydig cells responsible for testosterone production. Seminiferous tubules have a uniform structure and are the site of spermatogenesis, where germ cells develop into spermatozoa in close interaction with Sertoli cells (Mruk and Cheng 2004). It is hypothesized that proteins/miRNAs can leak from seminiferous tubules into testicular interstitial fluid and subsequently into the blood due to either loss of blood-testis barrier (BTB) integrity or germ cell-specific damage as a result of drug or toxicant exposure (McDuffie et al. 2016). miRNAs, a class of small non-coding RNAs with 18 to 24 nucleotides, play critical regulatory roles in many cellular processes and are highly stable in body fluids such as blood, urine, saliva, milk, and cerebrospinal fluid (CSF) (O’Brien et al. 2018; Schofield et al. 2021). miRNA has high tissue or cell-type specificity and much lower complexity than proteins. Moreover, miRNA sequences are highly conserved across species; therefore, miRNAs have become attractive, novel accessible biomarker candidates (O'Brien et al. 2018).
Extensive research in recent years has suggested that miRNAs can detect and predict drug toxicity and enhance drug safety assessment (Bailey and Glaab 2018; Marrone et al. 2015; Schofield et al. 2021). miR-122, one of the most demonstrated miRNA biomarkers, is a crucial regulator of liver physiology and disease biology and has been reported as a predictive biomarker for drug-induced liver toxicity (DILI) (Howell et al. 2018; Llewellyn et al. 2021; Wang et al. 2009). Similarly, miRNAs monitorable in biofluids and specific to cardiotoxicity, kidney toxicity, neurotoxicity, pancreas toxicity, skeletal muscle toxicity, and dermal toxicity have been comprehensively reviewed (Koturbash et al. 2015; Marrone et al. 2015; Schofield et al. 2021). More specifically, miR-122, miR-133a, miR-124 and miR-217 have been established as novel biomarkers for early detection of drug induced injuries in liver, muscle, central nervous system, and pancreas, respectively (Schofield et al. 2021). However, drug-induced testicular injury or drug-induced testicular injury-related miRNAs have received limited to no attention.
Testicular-specific miRNAs were discovered and have been demonstrated to be differentially expressed in testicular cell types during testis development and are responsible for the normal function of testis and spermatogenesis (Kamalidehghan et al. 2020; Kasimanickam and Kasimanickam 2015; Kotaja 2014; Mobasheri and Babatunde 2019). In this review, we have summarized and discussed current knowledge on testicular-specific/enriched miRNAs, emerging methods for miRNA profiling, and the application of circulating miRNA as a potential biomarker for toxicant-induced testicular injury (Bouhallier et al. 2010; Goldstein et al. 2022; Matthews et al. 2020).
Current methodologies for circulating miRNAs profiling
Several methods can be used to identify and quantify circulating miRNAs, including qRT-PCR, droplet digital PCR (ddPCR), microarray, NanoString nCounter, and sRNA-Seq (Moldovan et al. 2014; Pritchard et al. 2012). Each method has advantages and limitations regarding its sensitivity and throughput (Fig. 1). Following sections will describe briefly targeted and discovery or hypothesis generation-based approaches.
Fig. 1.
Methodologies for circulating miRNA profiling. miRNAs are extracted from various body fluids samples such as plasma/serum, urine, saliva, milk, and cerebrospinal fluid (CSF) and can be profiled by several listed methods with different sensitivity and throughput. qRT-PCR: quantitative real-time polymerase chain reaction; ddPCR droplet digital polymerase chain reaction; sRNA-Seq small RNA sequencing
Targeted approach
First, there is no technically standardized miRNA method from the total RNA isolation to miRNA expression analysis. The results could vary by researchers, assay platforms, and normalization methods, making it difficult to compare the data/results from study to study directly. Using this method, candidate miRNA’s can be easily validated and tested in suitable matrix and fold changes can be interpreted in animal studies or humans. It is also possible that this approach can be used after suitable miRNAs are discovered and prioritized from discovery-based platforms (see below).
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
The most established method to identify and quantify circulating miRNAs is conventional qRT-PCR. qRT-PCR is a promising technique for spreading miRNA identification, which can quantify the number of miRNA copies using a standard curve or relatively quantify the level of miRNA expression using the ratio of cycle threshold (Ct) value of miRNA to endogenous controls. However, traditional qRT-PCR lacks uniformity and reproducibility in the criteria for measuring the circulating miRNAs (Pritchard et al. 2012).
Droplet digital polymerase chain reaction (ddPCR)
The ddPCR technique, an automated droplet flow-cytometer-based platform, is increasingly considered one of the most robust methods for quantifying circulating miRNAs (Hindson et al. 2011). ddPCR has been shown to have superior precision and sensitivity and is less affected by PCR inhibitors. ddPCR is particularly useful for detecting low-abundance miRNAs and acquiring the absolute quantification of specific circulating miRNAs (Ferracin and Negrini 2018). Despite the sensitivity of qRT-PCR/ddPCR and the availability of methods for measuring miRNA expression, PCR-based platforms are considered low to moderate throughput technologies for quantifying circulating miRNAs.
Although targeted techniques used for miRNA workflows are non-GLP, a qRT-PCR method has been validated for extracellular miRNA quantification in blood samples (Fauth et al. 2019). The combination of external control spike-in cel-miR-39 and internal reference miRNA control such as miR-103 could be a good choice for normalization. Second, the testicular-specific and enriched miRNAs must be resealed into the circulating biofluids to be detected in the samples during the testicular injury. Whether mild-to-moderate testicular damage can cause reproducible miRNA leakage from testicular cells. If miRNA leakage/release occurs only after more severe testis damage, this could impact the sensitivity of miRNAs as biomarkers. Third, although some of the miRNAs are highly expressed in the testis, they may not specifically be indicative of testicular injury and may be associated with other organ toxicities. (Cummings and Kinney 2022; Hendrix et al. 2021; Stephenson et al. 2019).
Discovery-based approaches
Leverages newer sequencing technologies to discover novel candidates and utilize bioinformatics to identify uniquely altered miRNAs in target tissues. Which can then be prioritized for targeted approaches.
Microarrays
Microarrays are a widely used, high-throughput hybridization-based technology capable of simultaneously detecting the expression of thousands of miRNAs within samples (Liu et al. 2008). However, microarrays cannot be used as an absolute quantification method for miRNAs and have lower sensitivity and specificity than qRT-PCR (Gant 2007). A short length and similar sequence among clusters of miRNAs also influence microarrays. This platform requires a pre-amplification process, which could ameliorate the miRNA level of low-expressed transcripts (Pradervand et al. 2010).
NanoString next generation counter (nanostring nCounter)
NanoString nCounter, a gene expression profiling platform, is based on direct molecular barcoding and digital detection of target molecules using unique color-coded probe pairs for each target of interest. nCounter does not utilize printed chips or rely on enzymes for reverse transcription or amplification, and it is becoming another powerful high-throughput tool for circulating miRNA profiling (Moldovan et al. 2014).
Small ribonucleic acid sequencing (sRNA-Seq)
sRNA-Seq technology is another recently developed tool for detecting novel high-throughput and extremely sensitive miRNAs. It is increasingly used to profile circulating miRNAs (Coenen-Stass et al. 2018). However, sRNA-Seq is a high-cost technology and often requires bioinformatics support to analyze the data. In recent years, costs are drastically reduced, and bioinformatics pipelines are well utilized. The significantly higher data output outweighs the cost from this technology. Moreover, it has been reported that miRNA sequences could vary from the microRNA database (miRBase) reference sequence due to RNA editing, which can influence comparison to the validated effect miRBase (Moldovan et al. 2014).
Despite the high cost and potential database variation, these RNA-sequencing technologies offering high-sensitive full panel miRNAs profiling and allowing for novel miRNAs identification, rather than a target qPCR-based assays, have made it possible to accelerate the discovery of circulating miRNAs that could be developed as biomarkers for nonclinical and clinical use. Indeed, combining discovery sequencing platforms and target assay tools could further enhance the future miRNAs biomarker discovery and application.
Species specific testicular miRNAs
The roles of testicular-specific miRNAs in regulating spermatogenesis are well-documented in rodents, monkeys, and men (Kotaja 2014). Several miRNA expression profiling studies using small RNA sequencing (sRNA-Seq), microarrays, and quantitative real-time PCR (qRT-PCR) have identified several miRNAs that are exclusively or highly expressed in several cell types within the testis (Fig. 2). These testicular-specific/enriched miRNAs are involved in spermatogenesis, particularly in spermatogonia stem cell renewal, regulating spermatocyte meiosis, and Sertoli cell and germ cell development. The following sections will summarize specific miRNAs relevant to preclinical toxicology species and human.
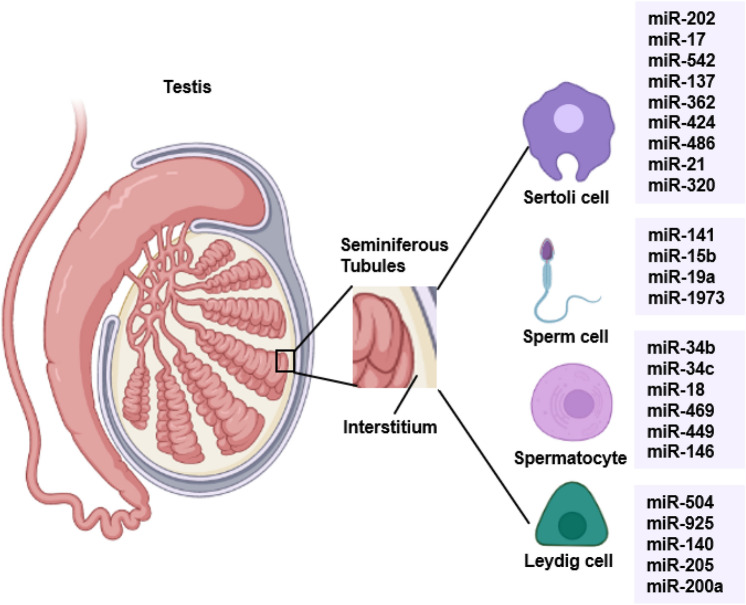
Fig. 2.
Testicular cell-specific and enriched miRNAs. The specific miRNAs from different testicular cell types include the Sertoli cell, sperm cell, spermatocyte in the seminiferous tubular, and Leydig cell in the interstitium. These miRNAs are involved in spermatogenesis and Sertoli/germ cell development
Rats
Three miRNAs (miR-320, miR-134, and miR-188) were confirmed in rat testes by miRNA microarray technology in combination with qRT-PCR (Fukushima et al. 2011). These three miRNAs were increased tenfold with the treatment of testicular toxicant ethylene glycol monomethyl ether (EGME), suggesting that these miRNAs have an essential role in testicular function. Male Sprague Dawley rat plasma samples collected, as detailed in Breslin et al. (Breslin et al. 2013), following a short-term repeat administration of carbendazim (CBZ), 1,3-dinitrobenzene (DNB), and mono(2-Ethylhexyl) phthalate (MEHP) (a metabolite of di-(2ethylhexyl) phthalate (DEHP)) were analyzed, in parallel, using RT-qPCR methodologies, by five Predictive Safety Testing Consortium (PSTC) member pharmaceutical companies (Goldstein et al. 2022). Over 20 circulating candidate miRNAs identified by Smith et al. (2016) were evaluated, and miR-202-5p was identified as a sensitive biomarker of testicular injury (Goldstein et al. 2022). Following treatment of Wistar male rats with Kaempferia parviflora (KP) (aka black ginger or Thai ginseng), in vitro, antioxidant activity, sperm functional analysis, serum testosterone, and circulating levels of miRNAs were evaluated respectively using colorimetry, ELISA, and real-time RT-PCR analysis (Al-Rawaf et al. 2021). Compared to nontreated rats, in KP-treated rats, circulating levels of miR-328 and miR-19b significantly decreased, and miR-34 increased considerably.
Mice
Eleven putative novel pubertal testicular miRNAs, including miR-149, miR-139, miR-19, miR-190, and miR-196, were identified in C57B6 mice testes by sRNA-seq and have been proposed to be involved in early germ cell development (Buchold et al. 2010a, 2010b). Similarly, 37 abundantly expressed miRNAs have been identified by northern blot analysis in mouse testes, associated explicitly with spermatocyte and spermatogonia (Marcon et al. 2008). Various mouse tissues with the expression of miR-34c were examined (Bouhallier et al. 2010) using the methods detailed by Raymond et al. (Raymond et al. 2005) and documented a high miR-34c expression in pachytene spermatocytes and round spermatids. Only a weak expression of miR-34c was found in Sertoli cells, which led to the conclusion that miR-34c was highly expressed in germ cells and only very weakly present in somatic testis cells.
Dogs
A serum-based qRT-PCR panel of tissue specific miRNAs for Beagle dogs and Sprague Dawley rats were reported in 2016 (Koenig et al. 2016). As part of this publication, authors reported the first Beagle dog miRNA tissue atlas (Koenig et al. 2016) and catalogued 15 tissue enriched miRNAs specific to liver, heart, skeletal muscle, pancreas, testes, and brain. Among the miRNAs, highly testis enriched miRNAs included miR-202, miR-34b, miR34c, miR-449a, miR-506, miR-507b, miR-508a, miR-508b, miR-8831, miR-8908a-3p, miR-8908a-5p, miR-8908b, miR-8908c, miR-34b, miR-34c, and miR-449b. Other moderate testes enriched miRNAs but not exclusive to tastes tissue included miR-106a, miR-205, miR-146b, miR-335, and miR-873. Of the 16 testes enriched miRNAs, 10 were also expressed in other tissues, including miR-202 and miR-508b. Apart from miR-34b/c, which has been reported to be involved in the late steps of spermatogenesis (Bouhallier et al. 2010), thus far, other miRNAs have not been reported to be associated with testes. However, to consider miR34b/c as a candidate biomarker specific to dog testes, carefully designed studies with castrated and non-castrated dogs will be required.
A recent study profiling miRNAs from Beagle dog serum samples indicated that miR-146b could be a potential biomarker candidate for drug-induced testicular injury (Shing et al. 2021). In this study, 2–3-year-old male beagle dogs were dosed daily with ethylene glycol monomethyl ether (EGME) at 50 mg/kg for 14 to 28 days. The compound induced seminiferous tubular degeneration/atrophy and loss of germ cells, including spermatocytes, spermatogonia, and Sertoli cells. The authors used sRNA-seq to profile the serum miRNAs and identified 13 elevated and 14 depleted miRNAs in dogs dosed with EGME. miR-146b was elevated ~ eightfold over baseline after 1-week EGME treatment. Interestingly, the serum level was unchanged in EGME-treated castrated dogs, suggesting that miR-146b is related explicitly to toxicant-induced testicular injury (Shing et al. 2021).
Monkeys
In rhesus monkeys, differential expression of miRNAs from immature and mature rhesus monkey testis was evaluated by miRNAs microarray profiling. Four miRNAs, miR-154, miR-181c, miR-181d, and miR-487b, had higher expression in immature primate testis than in mature primate testes (Yan et al. 2009). In addition, eleven miRNAs showed higher expression in mature primate testis, including miR-34b, miR-34c, and miR-449a. Many of these differentially expressed miRNAs are highly expressed in Sertoli cells and regulate germ cell differentiation and spermatogenesis by targeting cell survival/differentiation-related genes such as NOTCH1 (Yan et al. 2009), which is regulated by miR-34b, miR-34c, and miR-449a.
Following testicular hyperthermia (TH) treatment of cynomolgus monkeys, testicular injury miRNAs were profiled using next-generation sequencing (NGS), microarray, and reverse transcription-quantitative real-time-PCR (RT-qPCR). Results showed miR-34c-5p, miR-202-5p, miR-449a and miR-508-3p to be testicular specific and modulated following TH treatment (Sakurai et al. 2016). The same group, using the same technology, also studied EGME-induced testicular toxicity model in cynomolgus monkeys (Sakurai et al. 2015). Results showed down regulation of miR-34b-5p and miR-449a specific to pachytene spermatocytes.
Pigs/Minipigs
Both pigs and minipigs have been used in surgical and physiological research due to their similarities to humans in metabolism, their digestive tract, and their genetics (Svendsen 2006). Experimental monkey shortage generated by the COVID-19 lockdown and the commercial airlines not transporting monkeys from China has resulted in research organization resorting to minipigs (Feyen et al. 2016; Tian 2021). Data on miRNA in relation to testicular toxicity in minipigs are very limited or absent. However, in pigs, miRNA expression profiles from Sertoli cells have been assessed by sRNA-seq (Chen et al. 2020). Mature Sertoli cells were isolated from 5-month-old pigs, and the Sertoli cell RNA was further analyzed by sRNA-seq. Eighteen miRNAs were reported to be highly expressed in Sertoli cells, such as miR-7173, miR-217, miR-362, miR-202, and miR-149, which participate in cell proliferation, cell cycle signaling, and apoptosis. Accordingly, the miRNA profiling from pig germ cells was also assessed. The study demonstrated that miR-10a, miR-125b, let-7f, and miR-186 were highly expressed in pig germ cells, including spermatogonia, pachytene spermatocytes, round spermatids, and spermatozoa, which were purified using STA-PUT apparatus before sequencing (Chen et al. 2017b).
Human
A review of over 30 publications focusing on miRNA performance and their role in testicular germ cell tumors showed that levels of miR-371a-3p correlated with primary tumor mass and the clinical stage of the tumors (Leao et al. 2021). A similar conclusion was reported by Chavarriaga and Hamilton (Chavarriaga and Hamilton 2022) as a biomarker of choice for clinicians caring for patients with testicular germ cell tumor. A miRNA profiling analysis study from formalin-fixed paraffin-embedded (FFPE) testis tissue in azoospermic men used microarray and qRT-PCR to identify four miRNAs (miR-34b, miR-34c, miR-449a, and miR-449b) involved in the spermatogenesis process (Abu-Halima et al. 2014). These four miRNAs were downregulated in the testicular tissue of infertile men, suggesting that alteration of their expression contributes to azoospermia. Similarly, 174 miRNAs were reported to be distinctly expressed in Sertoli cells between Sertoli-cell-only syndrome patients and obstructive azoospermia patients with normal spermatogenesis (Yao et al. 2016). Among these 174 miRNAs, miR-133b was statistically upregulated in the Sertoli cell of Sertoli-cell-only syndrome patients and has been suggested as a mechanism of enhanced human Sertoli cell proliferation by targeting GLI3 and activating Cyclin B1 and D1 (Yao et al. 2016). Combination of miR-10b-3p and miR-34b-5p was identified as predictive biomarkers of azoospermia in humans (Zhang et al. 2020). Indeed, the investigation of testicular-specific/enriched miRNAs in nonclinical species and humans increases our understanding of the role of miRNAs in testicular function and provides excellent value for evaluating the translational circulating miRNA biomarkers for testicular injury in humans.
Toxicant-induced testicular injury
Testicular toxicants can be important tool compounds for miRNA biomarker discovery and validation including understanding the mechanistic basis of testicular injury. Testicular toxicants act via various mechanisms on different cell types within the testis to induce testis damage/injury that manifests as seminiferous tubule degeneration, Sertoli cell vacuolation, Leydig cell necrosis, and germ cell degeneration and depletion (Creasy and Chapin 2013). The cell targets and mechanism of action of some classical testis toxicants are summarized in Table 2.
Table 2.
Summary of testis toxicants and mechanism of action
| Toxicants | Target cell types | Mechanism of action (MOA) | References |
|---|---|---|---|
| Ethane dimethanesulfonate (EDS) | Leydig cell | Leydig cell apoptosis/necrosis with secondary germ cell death | Morris et al. (Morris et al. 1997, 1986; Morris 1985), Kelce et al. (Kelce and Zirkin 1993) |
| Cadmium chloride | Sertoli cell, Leydig cell, Germ cell | Induce DNA damage, Disrupt the tight junctions in BTB, Cause damage to the seminiferous tubules, Loss of sperm | Siu et al. (Siu et al. 2009), Zhe et al. (Zhu et al. 2020), Ren et al. (Ren et al. 2019) |
| Ethylene glycol monomethyl ether (EGME) |
Germ cell Sertoli cell |
Disruption of sperm synthesis, Vacuolations in seminiferous tubules | Somade et al. (Somade et al. 2020), Fukushima et al. (Fukushima et al. 2011), Sakurai et al. (Sakurai et al. 2016), Matsuyama et al. (Matsuyama et al. 2018) |
| 1,3-dinitrobenzene (1,3-DNB) | Sertoli cell, | Sertoli cell vacuolation/apoptosis | Brown et al. (Brown et al. 1997) |
| 2,5-hexanedione (2,5-HD) | Sertoli cell, Germ cell | Germ cell loss, Sertoli cell vacuolations | Boekelheide et al. (Boekelheide et al. 2003), Blanchard et al. (Blanchard et al. 1996) |
| Carbendazim (CBZ) | Sertoli cell, Germ cell | Germ cell apoptosis, Seminiferous tubules vacuolations | Moffit et al. (Moffit et al. 2007) |
| Methoxyacetic acid (MAA) | Germ cell | Spermatocyte apoptosis | Bagchi and Waxman(Bagchi and Waxman 2008) |
Sertoli cells are essential for spermatogenesis by controlling the environment within the seminiferous tubules, supporting the germ cell progenitor cells, and transferring nutrients from nearby capillaries (Griswold 1998). Damage to Sertoli cells disrupts their support of germ cells and can result in secondary germ cell degeneration and loss. Toxicants may also disrupt Leydig cells, the interstitial cells in the testis that produce testosterone in response to the luteinizing hormone (Vasta et al. 2006). In addition, toxicants may directly target germ cells. However, it can be challenging to determine the primary cell target of a testis toxicant since the appearance of the lesion may not necessarily reflect the primary cell target. The challenge to understanding the mechanism of testicular injury is to distinguish whether germ cell injury is caused directly by the toxicant or as a consequence of Sertoli cell or Leydig cell injury (Murphy and Richburg 2014). Since it can be challenging to delineate the specific mechanism of testis injury, monitorable biomarkers of testis injury is generally sensitivity to detect injury irrespective of the mechanism of testicular damage. In such a scenario, a panel of multiple biomarkers may be needed to identify injury caused by different mechanisms, mainly when the intention is to utilize the biomarkers in cases when the agent and primary cell target may not be known.
Circulating candidate miRNAs as potential biomarkers of testicular injury
miRNAs can be passively released from tissue-specific cells following different types of injury, secreted into the extracellular fluids, and transported to target cells via vesicles such as exosomes (O’Brien et al. 2018). Therefore, testicular-specific miRNAs could be secreted into the circulation following toxicant-induced testicular injury. Many recent studies have demonstrated that miRNA in biofluids can be specific and sensitive biomarkers of testicular injury in non-clinical species. A summary of the potential circulating miRNAs (and their human homologs) that could serve as indicators of testicular injury is provided in Table 3 and is further discussed in this section.
Table 3.
Summary of circulating miRNAs as potential non-invasive biomarkers for drug-induced testicular injury
| miRNAs | Species in the studies | Human homologs | Testicular toxicant | Matrix | Approach | Potential mechanism of action | References |
|---|---|---|---|---|---|---|---|
| miR-146b | Dog | hsa-miR-146b-3p: GCCCUGUGGACUCAGUUCUGGU | EGME | Serum | NGS, qRT-PCR | We are regulating the SMAD pathway, Targeting cycle-related genes such as PCNA, CDK2, CyclinD1, and p21 | Shing et al. (Shing et al. 2021), Gao et al. (Gao et al. 2020) |
| miR-202 | Rat | hsa-miR-202-3p: AGAGGUAUAGGGCAUGGGAA | 1,3-DNB, CBZ | Plasma | RT-PCR | They regulate cell differentiation proliferation and apoptosis pathways, such as Cyclin D1 and Wnt/β-Catenin pathways | Dere et al. (Dere et al. 2013), Yan et al. (Yang et al. 2019) |
| miR-34b/c, miR449a | Monkey |
hsa-miR-34b-3p: CAAUCACUAACUCCACUGCCAU hsa-miR-34c-3p: AAUCACUAACCACACGGCCAGG hsa-miR-449a: UGGCAGUGUAUUGUUAGCUGGU |
EGME | Plasma | Microarray, qRT-PCR |
Regulating cell proliferation by targeting the CDK6 pathway, Activating the p53 signaling pathway through sirtuin 1 |
Sakurai et al. (Sakurai et al. 2015, 2016), Lize et al. (Lize et al. 2010), Lee et al. (Lee and Kemper 2010) |
| miR-423, miR128 | Rat |
hsa-miR-423-3p: AGCUCGGUCUGAGGCCCCUCAGU hsa-miR-128-3p: UCACAGUGAACCGGUCUCUUU |
EGME, CBZ | Serum exosome | NGS | Regulating cell proliferation and apoptosis pathways, such as MAPK14 | Kawata et al. (Kawata et al. 2020), Rao et al. (Rao et al. 2017) |
| miR-200c, miR-486, miR-1892 | Mouse |
has-miR-200c-3p: UAAUACUGCCGGGUAAUGAUGGA hsa-miR-486-3p: CGGGGCAGCUCAGUACAGGAU |
Doxorubicin | Sperm | Microarray, qRT-PCR | Regulating cell apoptosis pathway through PTEN and p53 pathway | Sakai et al. (Sakai et al. 2021), Akinjo et al. (Akinjo et al. 2016, 2018) |
EGME Ethylene glycol monomethyl ether; 1,3-DNB 1,3-dinitrobenzene; CBZ Carbendazim; NGS Next-generation sequencing; qRT-PCR Quantitative real-time polymerase chain reaction
miR-146b
miR-146b is enriched in the testis and involved in testicular development and spermatogenesis by regulating the SMAD family member four protein and spermatid perinuclear RNA-binding protein (Kasimanickam and Kasimanickam 2015). It has been reported that overexpression of miR-146b inhibited the proliferation and promoted the apoptosis of bovine spermatogonia stem cells (SSCs) (Gao et al. 2020). Furthermore, exogenous miR-146b decreased the expression of cell cycle-related genes such as PCNA, CDK2, CyclinD1, and p21 during the bovine SSC’s growth stage (Gao et al. 2020), indicating a critical role in spermatogenesis.
miR-202
miR-202 is a conserved miRNA highly expressed in embryonic gonads across animal species and maintains mouse SSCs by inhibiting cell cycle regulators and RNA binding Fox protein 2 (RBFOX2) (Chen et al. 2017a; Wainwright et al. 2013). Recently it has been shown that miR-202 prevents spermatogonia differentiation and inhibits meiosis during mouse spermatogenesis. In miR-202 knockout mice, loss of miR-202 results in premature expression of STRA8 and DMRT6, two essential genes regulating cell differentiation and meiosis (Chen et al. 2021). Furthermore, overexpression of miR-202 marks mouse gonad differentiation and functions downstream of testis-determining factor SOX9 (Wainwright et al. 2013). Interestingly, the elevated circulating miR-202 has been observed in a rat study with testicular toxicants1,3-DNB and CBZ (Dere et al. 2013). With an improved RT-qPCR sensitivity and a standardized rat plasma assay protocol, five pharmaceutical companies (AbbVie, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, and Pfizer) worked collaboratively with Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium (PSTC) scientists and established miR-202-5p as a sensitive biomarker for early detection of drug-induced testicular injuries in rats (Goldstein et al. 2022).
Additionally, miR-202 is involved in mediating the proliferation, apoptosis, and synthesis of human Sertoli cells by mediating LRP6 and Cyclin D1 of the Wnt/β-Catenin Signaling pathway (Yang et al. 2019). Overexpression of miR-202 in human Sertoli cells suppressed cell proliferation and increased apoptosis, whereas inhibition of miR-202 promoted proliferation and reduced Sertoli cell apoptosis. These data further suggest that miR-202 is involved in testicular cell function.
miR-34b/c and miR-449a
miR-34b/c and miR-449a are preferentially expressed in the testis of mice and are essential for spermatogenesis (Wu et al. 2014). miR-449a structurally resembles the miR-34 family and can regulate cell proliferation and cell cycle progression by reducing Cyclin-Dependent Kinase 6 (CDK6) expression (Lize et al. 2010). Sakurai and colleagues performed miRNA microarray analysis with plasma and testis samples. They reported that testicular-specific miR-34b and miR-449a were significantly downregulated in the testis and plasma of monkeys administered 300 mg/kg/d EGME for four days resulting in the depletion of pachytene spermatocytes and round spermatids in testis (Sakurai et al. 2015). The decreased expression of testicular miR-34b and miR-449a resulting from EGME administration was confirmed by qRT-PCR. It was reported that both miR-34b/c and miR-449a could activate the p53 signaling pathway through sirtuin 1 (SIRT1) (Lee and Kemper 2010), and double knockout of miR-34b/c and miR-449a can disrupt spermatogenesis in mice (Wu et al. 2014). The downregulation of miR-34b/c and miR-449a in the testis could repress p53, which leads to decreased cell proliferation and spermatogenesis.
Furthermore, in the testicular hyperthermia (TH)-induced testicular injury monkey model, miR-34c and miR-449a were also enriched in the testis and downregulated in TH-treated testes (Sakurai et al. 2016). However, the plasma level of miR-34c remained unchanged in the TH-treated group compared to control groups, suggesting that leakage of miR-34c from damaged testis did not occur or had little impact on circulating levels.
miR-128 and miR-423
Exosomes, abundant in body fluids, contain proteins, lipids, and miRNAs responsible for delivering signaling molecules between specific cells. In a recent study, miR-128 and miR-423 were identified from serum exosomes in rats as potential biomarkers for testicular injury (Kawata et al. 2020). In this study, rats were treated with the testicular toxicants EGME and CBZ, which induce spermatocyte degeneration, Sertoli cell vacuolation, and seminiferous tubule dilation in the testes. The exosomes were isolated from serum, and exosomal small RNAs were sequenced by sRNA-seq. 51 and 22 differentially expressed miRNAs were identified in EGME and CBZ-treated rats; miR-128 and miR-423 were the most increased with these two toxicants (Kawata et al. 2020). miR-128 and miR-423, highly expressed in mouse testis, were negatively correlated with germ cell apoptosis (Rao et al. 2017). However, the serum level of miR-128 and miR-423 remained unchanged with EGME and CBZ in rats (Kawata et al. 2020), suggesting that exosomal miRNAs are more sensitive biomarkers than serum biomarkers for testicular injury. Despite the excellent advantages, low throughput and large sample volume requirements for exosome isolation are the most significant challenges to using exosomal miRNAs as biomarkers. Furthermore, the techniques of exosome isolation are complicated and time-consuming.
miR-200c, miR-486, and miR-1892
Sperm miRNAs have been reported to be involved in spermatogenesis and sperm maturation. Therefore, their expression profile can serve as sensitive and non-invasive biomarkers for testicular function, including drug-induced testicular injury. Recently, an sRNA-seq analysis of sperm from doxorubicin-treated mice has indicated that the expression of small non-coding RNA, including miRNA, was deregulated under the condition of doxorubicin-induced testicular injury (Sakai et al. 2021). Three miRNAs, including miR-200c, miR-486, and miR-1892, have been consistently reported to be significantly altered in a spermatogonia cell line (GC1) treated with doxorubicin (Akinjo et al. 2018), which is consistent with in vivo doxorubicin-induced miRNAs changes (Akinjo et al. 2016). These data suggest a critical role of sperm miRNA in maintaining testis function.
Translation from preclinical species to humans
The miRNA sequence homology is approximately 90% conserved among humans and rodents in general (McCallie et al. 2010), indicating that miRNA profiling data from rodents would help investigate the mechanism of toxicant-induced testicular injury and potential translational testicular biomarkers in humans. Considering the physiological similarity between nonhuman primates and humans, these miRNAs identified in monkeys could provide valuable insight into the mechanism of testicular toxicity in humans. Considering the patient safety in clinical trials, when pre-clinical evidence of testicular toxicity is present in pre-clinical species, male subjects are minimized, or restricted and female subjects are enrolled in first-in-human (FIH) studies (FDA 2018). Alternatively, male subjects completed family planning and undergone vasectomy could be enrolled in the FIH studies (FDA 2018). However, a few studies have profiled human testicular miRNAs linked to testicular tumors to possibly translate to drug-induced testicular toxicity (Leao et al. 2021; Song et al. 2022; Zhang et al. 2020).
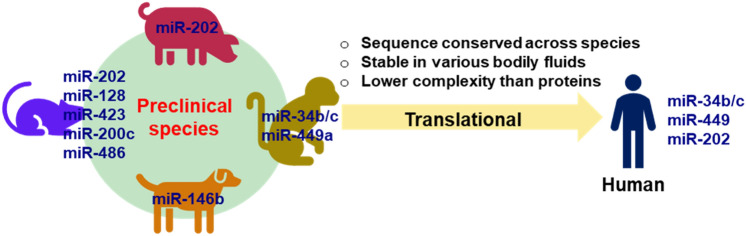
So far, the utilization of circulating miRNAs as testicular injury biomarkers has only been demonstrated in nonclinical studies. However, the sequence of these circulating miRNAs discussed above is conserved from preclinical species to humans (Table 3), suggesting that these miRNAs could be potentially applied in clinical studies to monitor testicular injury in patients. In rodents and pigs, miR-202 has been established as a sensitive biomarker for early detection of drug-induced testicular injuries (Dere et al. 2013; Goldstein et al. 2022; Sun et al. 2021). The miR-34b/c and miR-449a identified from the nonhuman primate studies might be excellent miRNA biomarkers for evaluating testicular damage in humans due to the physiological similarity between monkeys and humans (Sakurai et al. 2015, 2016). Further investigation will be needed to assess the association between these circulating miRNA levels and the severity of the testicular injury, especially in human trials (Fig. 3).
Fig. 3.
Translation of circulating miRNA as a testicular safety biomarker
Current regulatory framework around DITI
International conferences and publications have facilitated various drug development procedures to be harmonized and resulted in various DITI guidance documents for pharmaceutical industry, including those from U. S. Food and Drug Administration (FDA) (FDA 2018) as well as the European Medicines Agency (EMA) (EMA 2020) and International Conference on Harmonization (ICH). The EMA/ICH guidance document provide little information about DITI and guidance on how to handle toxicity related to DITI.
Among them, FDA’s 2018 guidance document titled “Testicular Toxicity: Evaluation During Drug Development” provides a clear framework to evaluate clinical stage testicular injury by semen analysis, serum testosterone and serum gonadotropin concentrations (FDA 2018). For preclinical evaluations of DITI, the same document provides the guidance to complete histopathological evaluations of the testes, seminal vesicle, epididymis, and prostate with appropriate fixation and staining of the testes. Detecting DITI in humans in real time is nearly impossible because of the lapse between an injury and the time when that injury can be detected using semen analysis. miRNA biomarkers discussed in this review can provide early reading related to DITI and likely eliminate the dependency on semen analysis and very late detection of DITI. Clearly guidance does not reflect the incorporation of newer biomarkers into the safety mitigation and further justifies the need for miRNA as biomarker tool for incorporation into early discovery toxicology studies and clinical monitoring settings. In this context, substantial scientific knowledge, regulatory precedence, and years of collaboration are required for revisions of these guidance documents and to include novel biomarkers to facilitate DITI detection early in preclinical or clinical studies.
Consortia impact on DITI regulatory framework and standardization
Regulatory guidance documents are available for preclinical risk assessment before bringing a potential testicular toxicant into the clinic and guidelines to monitor DITI in patients to assure safety. Several international consortia have been established to provide a forum for regulators, academic researchers, pharmaceutical scientists, clinicians and CRO colleagues to qualify, validate and utilize novel biomarkers of safety (Fader et al. 2021; Gerlach et al. 2018; Schomaker et al. 2019). Among the global consortia, (1) the Predictive Safety Testing Consortium (PSTC) and (2) the Translational Safety Biomarker Pipeline (TransBioLine) consortium are very active in conducting preclinical and clinical studies, qualifying novel biomarkers and implementing in preclinical and clinical studies to assure safety.
The Critical Path Institute (C-Path) was identified as the coordinating organization, and the formation PSTC was announced by C-Path and the FDA in March 2006 (Dieterle et al. 2010; Goodsaid et al. 2007; Mattes and Walker 2009; Stephenson and Sauer 2014). Over the years, C-Path and PSTC have developed the framework for data sharing between participating pharmaceutical companies and the regulatory agencies. In a collaboration with participating companies and regulatory agencies, PSTC has completed several biomarker qualification efforts, including serum glutamate dehydrogenase (GLDH) as a specific biomarker for hepatocellular injury (Schomaker et al. 2020) and multiple kidney safety biomarkers present in urine (Tengstrand et al. 2019). In search of better biomarkers for testicular toxicity that can translate from preclinical to clinical space, PSTC partnered with AbbVie, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, and Pfizer to scout for over 22 rat testis specific biomarkers reported previously (Smith et al. 2016). About 100 × improved qRT-PCR sensitivity for miRNAs and a standardized rat plasma assay protocol paved the way for re-analysis of rat plasma samples collected following administration of known toxicants, CBZ, DNB, and MEHP (Breslin et al. 2013). PSTC and the participating scientists established miR-202-5p as a sensitive biomarker for early detection of drug-induced testicular injuries in rats (Goldstein et al. 2022). Overall, PSTC, member pharmaceutical companies, and regulators have made progress in several fronts in the development and qualification of novel biomarkers but there still is a great amount work to be done to discover, qualify, and validate novel biomarkers of DITI.
Translational Safety Biomarker Pipeline (TransBioLine) consortium was funded by the Innovative Medicines Initiative (IMI) to bring together global regulators, pharmaceutical scientists and European Federation of Pharmaceutical Industries and Associations (EFPIA). The goal of this consortium is to discover, qualify and validate novel biomarkers for five organ systems: kidney, liver, pancreas, vascular, and central nervous system (Huehnchen et al. 2022). Evaluation and qualification of miRNAs in liquid biopsies is also part of the effort through the consortia, which has helped to improve the knowledge of the ‘translational gap’ of miRNAs biomarkers in drug safety assessment and increased the confidence in utilizing miRNAs as safety biomarkers in the clinical setting. Another goal of this consortium is to establish inter- and intra-individual variability of circulating miRNAs among healthy volunteers. To achieve this goal, a detailed protocol has been developed by TransBioLine for plasma sample collection for the purpose of miRNA analysis (Schofield et al. 2021). In addition, TransBioLine members have established robust assays for identification and quantification of miRNAs (Khamina et al. 2022). Although testicular toxicity is not in the scope of this project, the learnings will enable advancement of miRNA work in other areas such as testicular toxicity.
Conclusions and perspectives
Under the 21st Century Cures Act, the FDA will now rely more on biomarkers to accelerate regulatory decisions. Biomarker-related collaborative research and regulatory interactions have expanded in recent years based on publications and consortium related engagements. Under this ecosystem, there is the opportunity to expedite the development of reliable biomarker (s) that can accurately predict testicular injury in preclinical/clinical settings due to the multiple potential cell targets and mechanisms of testicular injury. While we advance miRNA biomarker research, it is critical to access the relevant and appropriate biofluids and technology in addition to developing an objective replication and data analysis approaches to obtain reproducible biomarker results. This will need significant investments and can be accomplished by strong partnerships between industries, academia, and regulators as part of a precompetitive consortia to advance biomarkers from discovery to regulatory qualification. From a translation standpoint, although most miRNA biomarkers thus far are identified from nonclinical species, identifying, and replicating biomarker datasets from well annotated clinical samples will provide insights into translation considering that the translation of miRNA biomarkers from preclinical findings to human clinical studies remains unclear. One of the challenges for difficulty in preclinical to clinical translation of testicular toxicity can be attributed to the physiological and metabolic differences between the species. With the cutting-edge technologies such as ‘organs-on-chips’ (Zommiti et al. 2022) that can emulate the human organ’s physiological environment and function, there is potential to enhance our understanding of the translatability of biomarker miRNAs from nonclinical to clinical settings (Akinjo et al. 2018; Park et al. 2022). Leveraging such emerging tools will enable biomarker discovery, validation and clinical translation for regulatory qualification and implementation for a specific context of use in drug development.
Author contributions
Wrote or contributed to the writing of the manuscript: JZ, SC, NC, JR, KP, KR, and RR.
Data availability
As this manuscript is a literature review, all data discussed are based on cited references. JZ and RR contributed to generating the figures and tables.
Declarations
Conflict of interest
Zhang, Campion, Catlin, Reagan, Palyada, Ramaiah, and Ramanathan are employees of Pfizer Inc.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Halima M, Backes C, Leidinger P, et al. MicroRNA expression profiles in human testicular tissues of infertile men with different histopathologic patterns. Fertil Steril. 2014;101(1):78–862 e2. doi: 10.1016/j.fertnstert.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Akinjo OO, Gant TW, Marczylo EL. Perturbation of epigenetic processes by doxorubicin in the mouse testis. Toxicol Res (camb) 2016;5(4):1229–1243. doi: 10.1039/c6tx00078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinjo OO, Gant TW, Marczylo EL. Perturbation of microRNA signalling by doxorubicin in spermatogonial, leydig and sertoli cell lines in vitro. Toxicol Res (camb) 2018;7(5):760–770. doi: 10.1039/c7tx00314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rawaf HA, Gabr SA, Alghadir AH. The potential role of circulating microRNAs in male rat infertility treated with Kaempferia parviflora. Evid Based Complement Alternat Med. 2021;2021:9622494. doi: 10.1155/2021/9622494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi G, Waxman DJ. Toxicity of ethylene glycol monomethyl ether: impact on testicular gene expression. Int J Androl. 2008;31(2):269–274. doi: 10.1111/j.1365-2605.2007.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey WJ, Glaab WE. Accessible miRNAs as novel toxicity biomarkers. Int J Toxicol. 2018;37(2):116–120. doi: 10.1177/1091581817752405. [DOI] [PubMed] [Google Scholar]
- Blanchard KT, Allard EK, Boekelheide K. Fate of germ cells in 2,5-hexanedione-induced testicular injury. I. Apoptosis is the mechanism of germ cell death. Toxicol Appl Pharmacol. 1996;137(2):141–8. doi: 10.1006/taap.1996.0066. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Fleming SL, Allio T, et al. 2,5-hexanedione-induced testicular injury. Annu Rev Pharmacol Toxicol. 2003;43:125–147. doi: 10.1146/annurev.pharmtox.43.100901.135930. [DOI] [PubMed] [Google Scholar]
- Bouhallier F, Allioli N, Lavial F, et al. Role of miR-34c microRNA in the late steps of spermatogenesis. RNA. 2010;16(4):720–731. doi: 10.1261/rna.1963810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin WJ, Paulman A, Sun-Lin D, Goldstein KM, Derr A. The inhibin B (InhB) response to the testicular toxicants mono-2-ethylhexyl phthalate (MEHP), 1,3 dinitrobenzene (DNB), or carbendazim (CBZ) following short-term repeat dosing in the male rat. Birth Defects Res B Dev Reprod Toxicol. 2013;98(1):72–81. doi: 10.1002/bdrb.21043. [DOI] [PubMed] [Google Scholar]
- Brown CD, Jacobson CF, Miller MG. Metabolism and testicular toxicity of 1,3-dinitrobenzene in the rat: evaluation of the stage-synchrony model. Reprod Toxicol. 1997;11(1):57–67. doi: 10.1016/S0890-6238(96)00197-9. [DOI] [PubMed] [Google Scholar]
- Buchold G, Zhu HF, Coarfa C, Gunaratne P, Matzuk M. Analysis of microRNA expression in the prepubertal testis. J Androl. 2010;1:53–53. doi: 10.1371/journal.pone.0015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchold GM, Coarfa C, Kim J, Milosavljevic A, Gunaratne PH, Matzuk MM. Analysis of microRNA expression in the prepubertal testis. PLoS One. 2010;5(12):e15317. doi: 10.1371/journal.pone.0015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth M, Creasy D, Timbrell JA. The detection of subchronic testicular damage using urinary creatine: studies with 2-methoxyethanol. Arch Toxicol. 1995;69(3):209–211. doi: 10.1007/s002040050160. [DOI] [PubMed] [Google Scholar]
- Chavarriaga J, Hamilton RJ. miRNAs for testicular germ cell tumours: contemporary indications for diagnosis, surveillance, and follow up. Andrology. 2022 doi: 10.1111/andr.13337. [DOI] [PubMed] [Google Scholar]
- Chen J, Cai T, Zheng C, et al. MicroRNA-202 maintains spermatogonial stem cells by inhibiting cell cycle regulators and RNA binding proteins. Nucleic Acids Res. 2017;45(7):4142–4157. doi: 10.1093/nar/gkw1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Che D, Zhang P, et al. Profiling of miRNAs in porcine germ cells during spermatogenesis. Reproduction. 2017;154(6):789–798. doi: 10.1530/REP-17-0441. [DOI] [PubMed] [Google Scholar]
- Chen X, Zheng Y, Li X, et al. Profiling of miRNAs in porcine sertoli cells. J Anim Sci Biotechnol. 2020;11:85. doi: 10.1186/s40104-020-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gao C, Lin X, et al. The microRNA miR-202 prevents precocious spermatogonial differentiation and meiotic initiation during mouse spermatogenesis. Development. 2021 doi: 10.1242/dev.199799. [DOI] [PubMed] [Google Scholar]
- Coenen-Stass AML, Magen I, Brooks T, et al. Evaluation of methodologies for microRNA biomarker detection by next generation sequencing. RNA Biol. 2018;15(8):1133–1145. doi: 10.1080/15476286.2018.1514236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson M, Bickerton S, Betts CJ, et al. Analytic evaluation of a human ELISA kit for measurement of inhibin B in rat samples. Birth Defects Res B Dev Reprod Toxicol. 2013;98(1):4–16. doi: 10.1002/bdrb.21047. [DOI] [PubMed] [Google Scholar]
- Creasy DM, Chapin RE. Male Reproductive System. Haschek Rousseauxs Handbook Toxicolc Pathol. 2013;1-3:2493–2598. doi: 10.1016/B978-0-12-415759-0.00059-5. [DOI] [Google Scholar]
- Cummings J, Kinney J. Biomarkers for alzheimer’s disease: context of use, qualification, and roadmap for clinical implementation. Medicina (Kaunas) 2022 doi: 10.3390/medicina58070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Anderson LM, Coulson M, McIntyre BS, Boekelheide K, Chapin RE. SOT symposium highlight: translatable indicators of testicular toxicity: inhibin B, microRNAs, and sperm signatures. Toxicol Sci. 2013;136(2):265–273. doi: 10.1093/toxsci/kft207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat Biotechnol. 2010;28(5):455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- Draper RP, Creasy DM, Timbrell JA. Comparison of urinary creatine with other biomarkers for the detection of 2-methoxyethanol-induced testicular damage. Biomarkers. 1996;1(3):190–195. doi: 10.3109/13547509609079356. [DOI] [PubMed] [Google Scholar]
- EMA (2020) ICH S5 (R3) guideline on reproductive toxicology: Detection of Toxicity to Reproduction for Human Pharmaceuticals.
- Fader KA, Zhang J, Menetski JP, et al. A Biomarker-centric approach to drug discovery and development: lessons learned from the coronavirus disease 2019 pandemic. J Pharmacol Exp Ther. 2021;376(1):12–20. doi: 10.1124/jpet.120.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth M, Hegewald AB, Schmitz L, Krone DJ, Saul MJ. Validation of extracellular miRNA quantification in blood samples using RT-qPCR. FASEB Bioadv. 2019;1(8):481–492. doi: 10.1096/fba.2019-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2018) Testicular Toxicity: Evaluation during Drug Development Guidance for Industry. [DOI] [PubMed]
- Ferracin M, Negrini M. Quantification of circulating microRNAs by droplet digital PCR. Methods Mol Biol. 2018;1768:445–457. doi: 10.1007/978-1-4939-7778-9_25. [DOI] [PubMed] [Google Scholar]
- Feyen B, Penard L, van Heerden M, et al. ”All pigs are equal” Does the background data from juvenile gottingen minipigs support this? Reprod Toxicol. 2016;64:105–115. doi: 10.1016/j.reprotox.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Taki K, Ise R, Horii I, Yoshida T. MicroRNAs expression in the ethylene glycol monomethyl ether-induced testicular lesion. J Toxicol Sci. 2011;36(5):601–611. doi: 10.2131/jts.36.601. [DOI] [PubMed] [Google Scholar]
- Gant TW. Novel and future applications of microarrays in toxicological research. Expert Opin Drug Metab Toxicol. 2007;3(4):599–608. doi: 10.1517/17425225.3.4.599. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wu F, Ren Y, et al. MiRNAs expression profiling of bovine (Bos taurus) testes and effect of bta-miR-146b on proliferation and apoptosis in bovine male germline stem cells. Int J Mol Sci. 2020 doi: 10.3390/ijms21113846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach CV, Derzi M, Ramaiah SK, Vaidya VS. Industry perspective on biomarker development and qualification. Clin Pharmacol Ther. 2018;103(1):27–31. doi: 10.1002/cpt.919. [DOI] [PubMed] [Google Scholar]
- Goldstein KM, Lin H, Smith AT, et al. (2022) Identification of microRNA-202–5p as a novel biomarker of testicular toxicity for use in nonclinical safety testing in rats. In Review
- Goodsaid FM, Frueh FW, Mattes W. The predictive safety testing consortium: a synthesis of the goals, challenges and accomplishments of the critical path. Drug Discov Today Technol. 2007;4(2):47–50. doi: 10.1016/j.ddtec.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9(4):411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Hendrix SB, Mogg R, Wang SJ, et al. Perspectives on statistical strategies for the regulatory biomarker qualification process. Biomark Med. 2021;15(9):669–684. doi: 10.2217/bmm-2020-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson BJ, Ness KD, Masquelier DA, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LS, Ireland L, Park BK, Goldring CE. MiR-122 and other microRNAs as potential circulating biomarkers of drug-induced liver injury. Expert Rev Mol Diagn. 2018;18(1):47–54. doi: 10.1080/14737159.2018.1415145. [DOI] [PubMed] [Google Scholar]
- Huehnchen P, Schinke C, Bangemann N, et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight. 2022 doi: 10.1172/jci.insight.154395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalidehghan B, Habibi M, Afjeh SS, et al. The importance of small non-coding RNAs in human reproduction: a review article. Appl Clin Genet. 2020;13:1–11. doi: 10.2147/TACG.S207491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasimanickam VR, Kasimanickam RK. Differential expression of microRNAs in sexually immature and mature canine testes. Theriogenology. 2015;83(3):394–398 e1. doi: 10.1016/j.theriogenology.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Kawata R, Kagawa T, Koya Y, Kajiyama H, Oda S, Yokoi T. Exploration of small RNA biomarkers for testicular injury in the serum exosomes of rats. Toxicology. 2020;440:152490. doi: 10.1016/j.tox.2020.152490. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Zirkin BR. Mechanism by which ethane dimethanesulfonate kills adult rat leydig cells: involvement of intracellular glutathione. Toxicol Appl Pharmacol. 1993;120(1):80–88. doi: 10.1006/taap.1993.1089. [DOI] [PubMed] [Google Scholar]
- Khamina K, Diendorfer AB, Skalicky S, et al. A MicroRNA next-generation-sequencing discovery assay (miND) for Genome-scale analysis and absolute quantitation of circulating microRNA biomarkers. Int J Mol Sci. 2022 doi: 10.3390/ijms23031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinefelter GR, Laskey JW, Ferrell J, Suarez JD, Roberts NL. Discriminant analysis indicates a single sperm protein (SP22) is predictive of fertility following exposure to epididymal toxicants. J Androl. 1997;18(2):139–150. [PubMed] [Google Scholar]
- Klinefelter G, Suarez J, Roberts N, Strader L. The sperm biomarker SP22 is highly correlated with infertility resulting from the testicular toxicant bromochloroacetic acid. Biol Reprod. 1999;60:152–152. [Google Scholar]
- Koenig EM, Fisher C, Bernard H, et al. The beagle dog MicroRNA tissue atlas: identifying translatable biomarkers of organ toxicity. BMC Genomics. 2016;17:649. doi: 10.1186/s12864-016-2958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja N. MicroRNAs and spermatogenesis. Fertil Steril. 2014;101(6):1552–1562. doi: 10.1016/j.fertnstert.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Koturbash I, Tolleson WH, Guo L, et al. microRNAs as pharmacogenomic biomarkers for drug efficacy and drug safety assessment. Biomark Med. 2015;9(11):1153–1176. doi: 10.2217/bmm.15.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao R, Albersen M, Looijenga LHJ, et al. Circulating microRNAs, the next-generation serum biomarkers in testicular germ cell tumours: a systematic review. Eur Urol. 2021;80(4):456–466. doi: 10.1016/j.eururo.2021.06.006. [DOI] [PubMed] [Google Scholar]
- Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (albany NY) 2010;2(8):527–534. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, Hasky N, Stemmer SM, Shalgi R, Ben-Aharon I. Anti-Mullerian hormone is a marker for chemotherapy-induced testicular toxicity. Endocrinology. 2015;156(10):3818–3827. doi: 10.1210/en.2015-1310. [DOI] [PubMed] [Google Scholar]
- Liu CG, Calin GA, Volinia S, Croce CM. MicroRNA expression profiling using microarrays. Nat Protoc. 2008;3(4):563–578. doi: 10.1038/nprot.2008.14. [DOI] [PubMed] [Google Scholar]
- Lize M, Pilarski S, Dobbelstein M. E2F1-inducible microRNA 449a/b suppresses cell proliferation and promotes apoptosis. Cell Death Differ. 2010;17(3):452–458. doi: 10.1038/cdd.2009.188. [DOI] [PubMed] [Google Scholar]
- Llewellyn HP, Vaidya VS, Wang Z, et al. Evaluating the sensitivity and specificity of promising circulating biomarkers to diagnose liver injury in humans. Toxicol Sci. 2021;181(1):23–34. doi: 10.1093/toxsci/kfab003. [DOI] [PubMed] [Google Scholar]
- Marcon E, Babak T, Chua G, Hughes T, Moens PB. miRNA and piRNA localization in the male mammalian meiotic nucleus. Chromosome Res. 2008;16(2):243–260. doi: 10.1007/s10577-007-1190-6. [DOI] [PubMed] [Google Scholar]
- Marrone AK, Beland FA, Pogribny IP. The role for microRNAs in drug toxicity and in safety assessment. Expert Opin Drug Metab Toxicol. 2015;11(4):601–611. doi: 10.1517/17425255.2015.1021687. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Yabe K, Kuwata C, et al. Transcriptional profile of ethylene glycol monomethyl ether-induced testicular toxicity in rats. Drug Chem Toxicol. 2018;41(1):105–112. doi: 10.1080/01480545.2017.1320406. [DOI] [PubMed] [Google Scholar]
- Mattes WB, Walker EG. Translational toxicology and the work of the predictive safety testing consortium. Clin Pharmacol Ther. 2009;85(3):327–330. doi: 10.1038/clpt.2008.270. [DOI] [PubMed] [Google Scholar]
- Matthews O, Morrison EE, Tranter JD, et al. Transfer of hepatocellular microRNA regulates cytochrome P450 2E1 in renal tubular cells. EBioMedicine. 2020;62:103092. doi: 10.1016/j.ebiom.2020.103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallie B, Schoolcraft WB, Katz-Jaffe MG. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil Steril. 2010;93(7):2374–2382. doi: 10.1016/j.fertnstert.2009.01.069. [DOI] [PubMed] [Google Scholar]
- McDuffie JE, Olaharski AJ, Brandon DJ, Will Y. Drug discovery toxicology: from target assessment to translational biomarkers. John Wiley & Sons; 2016. [Google Scholar]
- Mobasheri MB, Babatunde KA. Testicular miRNAs in relation to spermatogenesis, spermatogonial stem cells and cancer/testis genes. Sci Afr. 2019;3:e00067. doi: 10.1016/j.sciaf.2019.e00067. [DOI] [Google Scholar]
- Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol. 2007;35(5):719–727. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med. 2014;18(3):371–390. doi: 10.1111/jcmm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore NP, Creasy DM, Gray TJ, Timbrell JA. Urinary creatine profiles after administration of cell-specific testicular toxicants to the rat. Arch Toxicol. 1992;66(6):435–442. doi: 10.1007/BF02035135. [DOI] [PubMed] [Google Scholar]
- Moore NP, Gray TJ, Timbrell JA. Creatine metabolism in the seminiferous epithelium of rats. II. Effect of modulators of cellular biochemical function on creatine secretion by cultured sertoli cells. J Reprod Fertil. 1998;112(2):331–6. doi: 10.1530/jrf.0.1120331. [DOI] [PubMed] [Google Scholar]
- Morris ID. Leydig cell resistance to the cytotoxic effect of ethylene dimethanesulphonate in the adult rat testis. J Endocrinol. 1985;105(3):311–316. doi: 10.1677/joe.0.1050311. [DOI] [PubMed] [Google Scholar]
- Morris ID, Phillips DM, Bardin CW. Ethylene dimethanesulfonate destroys leydig cells in the rat testis. Endocrinology. 1986;118(2):709–719. doi: 10.1210/endo-118-2-709. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Taylor MF, Morris ID. Leydig cell apoptosis in response to ethane dimethanesulphonate after both in vivo and in vitro treatment. J Androl. 1997;18(3):274–280. [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25(5):747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Murphy CJ, Richburg JH. Implications of Sertoli cell induced germ cell apoptosis to testicular pathology. Spermatogenesis. 2014;4(2):e979110. doi: 10.4161/21565562.2014.979110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim JS, Lee R, Song H. Cisplatin induces apoptosis in mouse neonatal testes organ culture. Int J Mol Sci. 2022 doi: 10.3390/ijms232113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozor M, Conley AJ, Roser JF, et al. Anti-Mullerian hormone as a biomarker for acute testicular degeneration caused by toxic insults to stallion testes. Theriogenology. 2018;116:95–102. doi: 10.1016/j.theriogenology.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Pradervand S, Weber J, Lemoine F, et al. Concordance among digital gene expression, microarrays, and qPCR when measuring differential expression of microRNAs. Biotechniques. 2010;48(3):219–222. doi: 10.2144/000113367. [DOI] [PubMed] [Google Scholar]
- Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13(5):358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M, Zeng Z, Tang L, Cheng G, Xia W, Zhu C. Next-generation sequencing-based microRNA profiling of mice testis subjected to transient heat stress. Oncotarget. 2017;8(67):111672–111682. doi: 10.18632/oncotarget.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Roberts BS, Garrett-Engele P, Lim LP, Johnson JM. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA. 2005;11(11):1737–1744. doi: 10.1261/rna.2148705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader SC, Shingles C, Stonard MD. Acute testicular toxicity of 1,3-dinitrobenzene and ethylene glycol monomethyl ether in the rat: evaluation of biochemical effect markers and hormonal responses. Fundam Appl Toxicol. 1991;16(1):61–70. doi: 10.1016/0272-0590(91)90135-q. [DOI] [PubMed] [Google Scholar]
- Rehnberg GL, Cooper RL, Goldman JM, Gray LE, Hein JF, McElroy WK. Serum and testicular testosterone and androgen binding protein profiles following subchronic treatment with carbendazim. Toxicol Appl Pharmacol. 1989;101(1):55–61. doi: 10.1016/0041-008x(89)90211-1. [DOI] [PubMed] [Google Scholar]
- Ren Y, Shao W, Zuo L, et al. Mechanism of cadmium poisoning on testicular injury in mice. Oncol Lett. 2019;18(2):1035–1042. doi: 10.3892/ol.2019.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hiradate Y, Hara K, Tanemura K. Potential of sperm small non-coding RNAs as biomarkers of testicular toxicity in a doxorubicin-induced mouse model. Biochem Biophys Rep. 2021;28:101160. doi: 10.1016/j.bbrep.2021.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Mikamoto K, Shirai M, et al. MicroRNA profiling in ethylene glycol monomethyl ether-induced monkey testicular toxicity model. J Toxicol Sci. 2015;40(3):375–382. doi: 10.2131/jts.40.375. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Mikamoto K, Shirai M, et al. MicroRNA profiles in a monkey testicular injury model induced by testicular hyperthermia. J Appl Toxicol. 2016;36(12):1614–1621. doi: 10.1002/jat.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield AL, Brown JP, Brown J, et al. Systems analysis of miRNA biomarkers to inform drug safety. Arch Toxicol. 2021;95(11):3475–3495. doi: 10.1007/s00204-021-03150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomaker S, Ramaiah S, Khan N, Burkhardt J. Safety biomarker applications in drug development. J Toxicol Sci. 2019;44(4):225–235. doi: 10.2131/jts.44.225. [DOI] [PubMed] [Google Scholar]
- Schomaker S, Potter D, Warner R, et al. Serum glutamate dehydrogenase activity enables early detection of liver injury in subjects with underlying muscle impairments. PLoS One. 2020;15(5):e0229753. doi: 10.1371/journal.pone.0229753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing JC, Schaefer K, Grosskurth SE, et al. Small RNA sequencing to discover circulating microRNA biomarkers of testicular toxicity in dogs. Int J Toxicol. 2021;40(1):26–39. doi: 10.1177/1091581820961515. [DOI] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238(3):240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Calley J, Mathur S, et al. The Rat microRNA body atlas; evaluation of the microRNA content of rat organs through deep sequencing and characterization of pancreas enriched miRNAs as biomarkers of pancreatic toxicity in the rat and dog. BMC Genomics. 2016;17:694. doi: 10.1186/s12864-016-2956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somade OT, Ajayi BO, Adeyi OE, Adeshina AA, James AS, Ayodele PF. Ethylene glycol monomethyl ether-induced testicular oxidative stress and time-dependent up-regulation of apoptotic, pro-inflammatory, and oncogenic markers in rats. Metabol Open. 2020;7:100051. doi: 10.1016/j.metop.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WP, Gu SJ, Tan XH, et al. Proteomic analysis and miRNA profiling of human testicular endothelial cell-derived exosomes: the potential effects on spermatogenesis. Asian J Androl. 2022;24(5):478–486. doi: 10.4103/aja202190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D, Sauer JM. The predictive safety testing consortium and the coalition against major diseases. Nat Rev Drug Discov. 2014;13(11):793–794. doi: 10.1038/nrd4440. [DOI] [PubMed] [Google Scholar]
- Stephenson D, Hill D, Cedarbaum JM, et al. The qualification of an enrichment biomarker for clinical trials targeting early stages of parkinson’s disease. J Parkinsons Dis. 2019;9(3):553–563. doi: 10.3233/JPD-191648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhao Y, He J, et al. Small RNA expression patterns in seminal plasma exosomes isolated from semen containing spermatozoa with cytoplasmic droplets versus regular exosomes in boar semen. Theriogenology. 2021;176:233–243. doi: 10.1016/j.theriogenology.2021.09.031. [DOI] [PubMed] [Google Scholar]
- Svendsen O. The minipig in toxicology. Exp Toxicol Pathol. 2006;57(5–6):335–339. doi: 10.1016/j.etp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tengstrand E, Zhang H, Liu N, Dunn K, Hsieh F. A multiplexed UPLC-MS/MS assay for the simultaneous measurement of urinary safety biomarkers of drug-induced kidney injury and phospholipidosis. Toxicol Appl Pharmacol. 2019;366:54–63. doi: 10.1016/j.taap.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Tian CY. China is facing serious experimental monkey shortage during the COVID-19 lockdown. J Med Primatol. 2021;50(4):225–227. doi: 10.1111/jmp.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbrell JA. Urinary creatine as a biochemical marker of chemical induced testicular damage. Arh Hig Rada Toksikol. 2000;51(3):295–303. [PubMed] [Google Scholar]
- Vasta V, Shimizu-Albergine M, Beavo JA. Modulation of leydig cell function by cyclic nucleotide phosphodiesterase 8A. Proc Natl Acad Sci U S A. 2006;103(52):19925–19930. doi: 10.1073/pnas.0609483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eckardstein S, Simoni M, Bergmann M, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84(7):2496–2501. doi: 10.1210/jcem.84.7.5855. [DOI] [PubMed] [Google Scholar]
- Wainwright EN, Jorgensen JS, Kim Y, et al. SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biol Reprod. 2013;89(2):34. doi: 10.1095/biolreprod.113.110155. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106(11):4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bao J, Kim M, et al. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci U S A. 2014;111(28):E2851–E2857. doi: 10.1073/pnas.1407777111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Lu Y, Sun H, et al. Microarray profiling of microRNAs expressed in testis tissues of developing primates. J Assist Reprod Genet. 2009;26(4):179–186. doi: 10.1007/s10815-009-9305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yao C, Tian R, et al. miR-202-3p regulates sertoli cell proliferation, synthesis function, and apoptosis by targeting LRP6 and Cyclin D1 of Wnt/beta-catenin signaling. Mol Ther Nucleic Acids. 2019;14:1–19. doi: 10.1016/j.omtn.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Sun M, Yuan Q, et al. MiRNA-133b promotes the proliferation of human sertoli cells through targeting GLI3. Oncotarget. 2016;7(3):2201–2219. doi: 10.18632/oncotarget.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Zhang Z, Hong K, et al. Altered microRNA profiles of testicular biopsies from patients with nonobstructive azoospermia. Asian J Androl. 2020;22(1):100–105. doi: 10.4103/aja.aja_35_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Li X, Ge RS. Toxicological effects of cadmium on mammalian testis. Front Genet. 2020;11:527. doi: 10.3389/fgene.2020.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zommiti M, Connil N, Tahrioui A, et al. Organs-on-chips platforms are everywhere: a zoom on biomedical investigation. Bioengineering (Basel) 2022 doi: 10.3390/bioengineering9110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this manuscript is a literature review, all data discussed are based on cited references. JZ and RR contributed to generating the figures and tables.