Abstract
(R,S)-ketamine (ketamine) and its enantiomer (S)-ketamine (esketamine) can produce rapid and substantial antidepressant effects. However, individual response to ketamine/esketamine is variable, and there are no well-accepted methods to differentiate persons who are more likely to benefit. Numerous potential peripheral biomarkers have been reported, but their current utility is unclear. We conducted a systematic review/meta-analysis examining the association between baseline levels and longitudinal changes in blood-based biomarkers, and response to ketamine/esketamine. Of the 5611 citations identified, 56 manuscripts were included (N = 2801 participants), and 26 were compatible with meta-analytical calculations. Random-effect models were used, and effect sizes were reported as standardized mean differences (SMD). Our assessments revealed that more than 460 individual biomarkers were examined. Frequently studied groups included neurotrophic factors (n = 15), levels of ketamine and ketamine metabolites (n = 13), and inflammatory markers (n = 12). There were no consistent associations between baseline levels of blood-based biomarkers, and response to ketamine. However, in a longitudinal analysis, ketamine responders had statistically significant increases in brain-derived neurotrophic factor (BDNF) when compared to pre-treatment levels (SMD [95% CI] = 0.26 [0.03, 0.48], p = 0.02), whereas non-responders showed no significant changes in BDNF levels (SMD [95% CI] = 0.05 [−0.19, 0.28], p = 0.70). There was no consistent evidence to support any additional longitudinal biomarkers. Findings were inconclusive for esketamine due to the small number of studies (n = 2). Despite a diverse and substantial literature, there is limited evidence that blood-based biomarkers are associated with response to ketamine, and no current evidence of clinical utility.
INTRODUCTION
Major depressive disorder (MDD) and bipolar depression are common, disabling, and often difficult to treat. Placebo-controlled trials reveal that only about half of individuals with MDD or bipolar depression treated with conventional/commonly-used agents have significant (at least 50%) improvement in depressive symptoms [1–5]. Even when effective, conventional treatments may take weeks to provide significant symptomatic relief [5, 6], extending the personal and social burden of depression and increasing risk of negative consequences such as suicide [7]. Several hypotheses have been proposed to explain the low success rates of current antidepressant treatment, including the intrinsic heterogeneity of depression, and the largely monothematic mechanism of action of conventional drugs by modulation of monoamines (serotonin, norepinephrine, and dopamine) [8–10].
There is growing evidence that (R,S)-ketamine (ketamine) and its (S)-ketamine enantiomer (esketamine) have rapid and substantial antidepressant effects in major depressive episodes (MDE) in MDD or bipolar disorder (BD) [11, 12], including in subjects previously unresponsive to conventional treatments. Initially developed in the early 1960s as an anesthetic agent [13], ketamine is a NMDA receptor antagonist with a unique antidepressant mechanism of action that may ultimately result in enhanced glutamatergic neurotransmission and strengthening of excitatory synapses [14, 15]. Esketamine has also been shown to have efficacy in treating MDD [16–18], and its intranasal formulation was approved in 2019 by the United States Food and Drug Administration (FDA) for use in treatment-resistant depression (TRD) [19]. However, the use of ketamine or esketamine can be associated with potentially problematic side effects including short term dissociative, psychotomimetic, and cardiovascular symptoms (particularly elevated blood pressure). Moreover, approximately 30 to 60% of the individuals with treatment-resistant depression treated with ketamine or esketamine show limited response to treatment [16, 20–27], highlighting the importance of potentially identifying the individuals for whom the risk-benefit ratio of ketamine and esketamine treatment may be most favorable.
The ability to predict which treatment will be most beneficial for a specific individual is a central tenet of precision medicine [28]. However, despite a growing research base, there remains no well replicated means to identify which depressed individuals are more likely to benefit from specific therapeutic interventions, including ketamine and esketamine [29–31]. Clinical variables, such as prior longitudinal course or current symptom profile, have so far shown modest utility in predicting therapeutic response [32–38]. As a result, treatment with both traditional and rapid-acting medications still largely relies on a trial-and-error approach [29, 30]. Consequently, there has been increasing interest in identifying potential biomarkers that could help identify subsets of depressed patients who are more likely to respond to a specific treatment [39, 40].
Biomarkers, defined as any “characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention” [41], may predict response to treatment in two ways: (1) as baseline (pre-treatment) markers that identify individuals who are more likely to respond to a future intervention (i.e., before the initiation of a treatment), or (2) as dynamic markers that are longitudinally measured and reflect biological changes potentially related to therapeutic mechanisms of action (i.e., indicators of target engagement) [41]. Importantly, the study of biomarkers associated with rapid antidepressant effect presents the novel opportunity to feasibly study either state or mediating biomarkers, and to correlate them with clinical improvement [31, 42, 43].
Several biomarker modalities have been studied in psychiatric disorders (including depression and its treatment), and they can be broadly divided into peripheral (generally blood-based studies) and brain-based (electrophysiological, neuroimaging) biomarkers. Despite the appeal of brain-based studies, blood-based predictors have the significant advantages of being more easily obtained, more widely accepted by patients, and more likely to be more cost-effective [44, 45]. There is now a significant number of studies on biomarkers of response to ketamine, most of which consist of blood-based assays [31, 42]. Initial studies have provided some evidence for blood-based biomarkers as potential moderators and/or mediators of response to ketamine and/or esketamine [46–56], however, there has been significant inconsistency in findings to date. Hence, in the current study, we have performed a pre-registered systematic review and meta-analysis addressing the following questions:
1) Are baseline (pre-treatment) levels of blood-based biomarkers associated with antidepressant response to ketamine and/or esketamine in individuals with a MDE in MDD and BD?
2) Are longitudinal changes in blood-based biomarkers associated with antidepressant response to ketamine and/or esketamine in individuals with a MDE in MDD and BD?
METHODS
The protocol of this systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews – PROSPERO [57] database prior to initiation of the screening process (ID: CRD42020210941). This study followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [58].
Search strategy
The search strategy was run in the electronic databases MEDLINE (PubMed), Embase (Embase.com), The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials - CENTRAL, Cochrane Methodology Register), PsycINFO (EBSCOhost), and Web of Science (Science and Social Science Citation Index). Searches were initially run on September 8, 2020 and were updated on August 27, 2021. For the search strategies designed for MEDLINE (PubMed), the Cochrane Library, PsycINFO, and Embase, controlled vocabulary terms were identified and combined with keyword synonyms. Web of Science was searched using keyword terms only. Results were limited to human studies in PubMed and Embase. There were no restrictions in publication date in any of the databases. See Supplementary Table 1 for additional information regarding search terms.
The electronic searches were complemented by manual searches. We manually evaluated the references of the included articles, review papers and grey literature obtained in the initial electronic searches (backward manual search). Using Google Scholar, we also examined manuscripts that cited the articles included in this review (forward manual search). In addition, studies obtained through personal communication were included.
Inclusion and exclusion criteria
This systematic review and meta-analysis included manuscripts that (1) examined adult human subjects (i.e. individuals aged 18 years or older), (2) studied individuals with a MDE in MDD or BD, (3) were published in English, (4) reported on clinical trials (randomized controlled and/or open label) that administered at least one dose of ketamine or its S-enantiomer esketamine, (5) measured improvement of depressive symptoms with a standardized depression tool, and (6) assessed the association between levels of blood-based biomarkers and improvement of depressive symptoms after ketamine/esketamine treatment.
We excluded articles that (1) investigated individuals with current psychotic symptoms, (2) examined persons with depressive symptoms due to other disorders besides MDD and BD, (3) allowed concurrent neuromodulatory treatments including repetitive transcranial magnetic stimulation, electroconvulsive therapy, and vagal nerve stimulation (concurrent medications and psychotherapy were allowed), (4) studied individuals with serious comorbid medical or neurological diseases such as conditions requiring treatment in an intensive care unit or with palliative care, Parkinson’s disease, Alzheimer’s disease, central nervous system (CNS) neoplasia, epilepsy, multiple sclerosis, significant traumatic brain injury, (5) assessed individuals in surgical/perioperative settings, and 6) reported on non-treatment related (naturalistic) studies.
Screening and selection
Two investigators (GCM and FSG) independently screened the titles and abstracts and, then, the full-text manuscripts that seemed eligible for inclusion. Discrepancies between the two reviewers were solved by consensus discussion. The Covidence [59] software was used to facilitate the screening process. If there were duplicated publications reporting on the same findings in the same sample (e.g., conference abstract and full-research article), only the more comprehensive report was included. Two independent investigators (GCM and FSG) extracted the main findings of the manuscripts. Other data (assay, design, sample, age, gender, depression measure, etc) were extracted by GCM and verified by FSG.
Risk of bias assessment
Risk of bias was determined by the Quality in Prognostic Studies (QUIPS) tool, which is a standardized assessment of six domains of bias (participants, attrition, prognostic factor measurement, outcome measurement, confounders, and analysis/report) as well as the overall risk of bias [60]. The QUIPS tool categorizes the risk of bias as “low risk”, “moderate risk” or “high risk”. Two reviewers (GCM and WP) independently assessed the risk of bias of the included studies, and disagreements were solved by consensus discussion.
Outcomes
The main goal of this study was to investigate associations between blood-based biomarkers and improvement of depressive symptoms in individuals who were treated with ketamine or esketamine. In order to address our research questions, we reported two different types of associations: (1) associations between baseline levels of blood-based biomarkers and improvement of depressive symptoms, and (2) associations between longitudinal changes in blood-based biomarkers and improvement of depressive symptoms, i.e., comparison between baseline (pre-treatment) and post-treatment levels.
In the qualitative summary, we reported on the association between levels of blood-based biomarkers and any measure of improvement, including continuous improvement in depression scores (such as absolute and percentage changes in depression scores) and categorical improvement (such as response and remission). In the quantitative summary, results across studies were compared using response status as primary outcome (i.e., responder versus non-responder where response is defined as at least 50% improvement in standardized depression measures).
If the data needed to conduct meta-analytical calculations were available in graphic format, the values were extracted using WebPlotDigitizer [61]. If there was a need for additional data not reported in the manuscript or for further clarifications, we contacted the corresponding authors.
Statistical analyses
Meta-analytical calculations were conducted if there were at least three comparable studies on the same blood-based biomarker. If there were studies with overlapping samples, only the study with the largest sample was included in the quantitative summary. Baseline levels and longitudinal changes of blood-based biomarkers were compared between responders and non-responders using standardized mean differences (SMD) and 95% confidence intervals (CI). SMD values of 0.2, 0.5, and 0.8 are considered thresholds for small, medium, and large effect size, respectively [62]. A significant degree of heterogeneity between studies was previously anticipated, therefore, random-effects models were used (inverse-variance weighting).
If only log-transformed values were provided, logarithmic values were converted to raw scale using the methods previously described by Higgins et al. (2008) [63]. If standard deviation (SD) was zero, the value was estimated using the average variance of other similar studies examining the same biomarker [64]. Heterogeneity between studies was examined using I2 where values between 0 and 40% are considered trivial heterogeneity, values between 30 and 60% are considered moderate heterogeneity, values between 50 and 90% are considered substantial heterogeneity, and between 75 and 100% are seen as considerable heterogeneity [65].
Publication bias was visually assessed by inspection of funnel plots, and objectively measured by Egger’s tests. Meta-regressions were performed to identify potential effect modifiers, and individually examined the impact of (1) number of treatments (single versus multiple), (2) blood fraction (serum versus plasma), (3) primary diagnosis (MDD versus bipolar depression), and (4) overall risk of bias. As recommended by Cochrane, meta-regressions were conducted only if the number of studies was ten or more in the meta-analytical calculation [65]. In order to assess the impact of each study on SMD estimates, sensitivity analyses were conducted by serially excluding individual studies. Statistical analyses were performed using the softwares R studio version 4.0.3 (“metafor” package – version 3.0–2), and Review Manager 5.4.
RESULTS
The initial search identified 5611 citations (database searching: 5481; manual searching: 116; personal communication: 14), of which 103 were potentially eligible for inclusion and were reviewed in full (Fig. 1). Ultimately, 56 manuscripts met criteria for inclusion in the qualitative evaluation, and 26 articles had available data compatible with a quantitative synthesis. The total combined sample of all manuscripts included in the study was 2801 individuals. Analyses of the 56 included studies revealed that the research base on blood-based biomarkers of response to ketamine/esketamine is progressively growing with the number of publications per year increasing over time (F = 18.89, p = 0.001).
Fig. 1. PRISMA flow diagram of systematic searches for studies assessing blood-based biomarkers of antidepressant response to ketamine and esketamine.
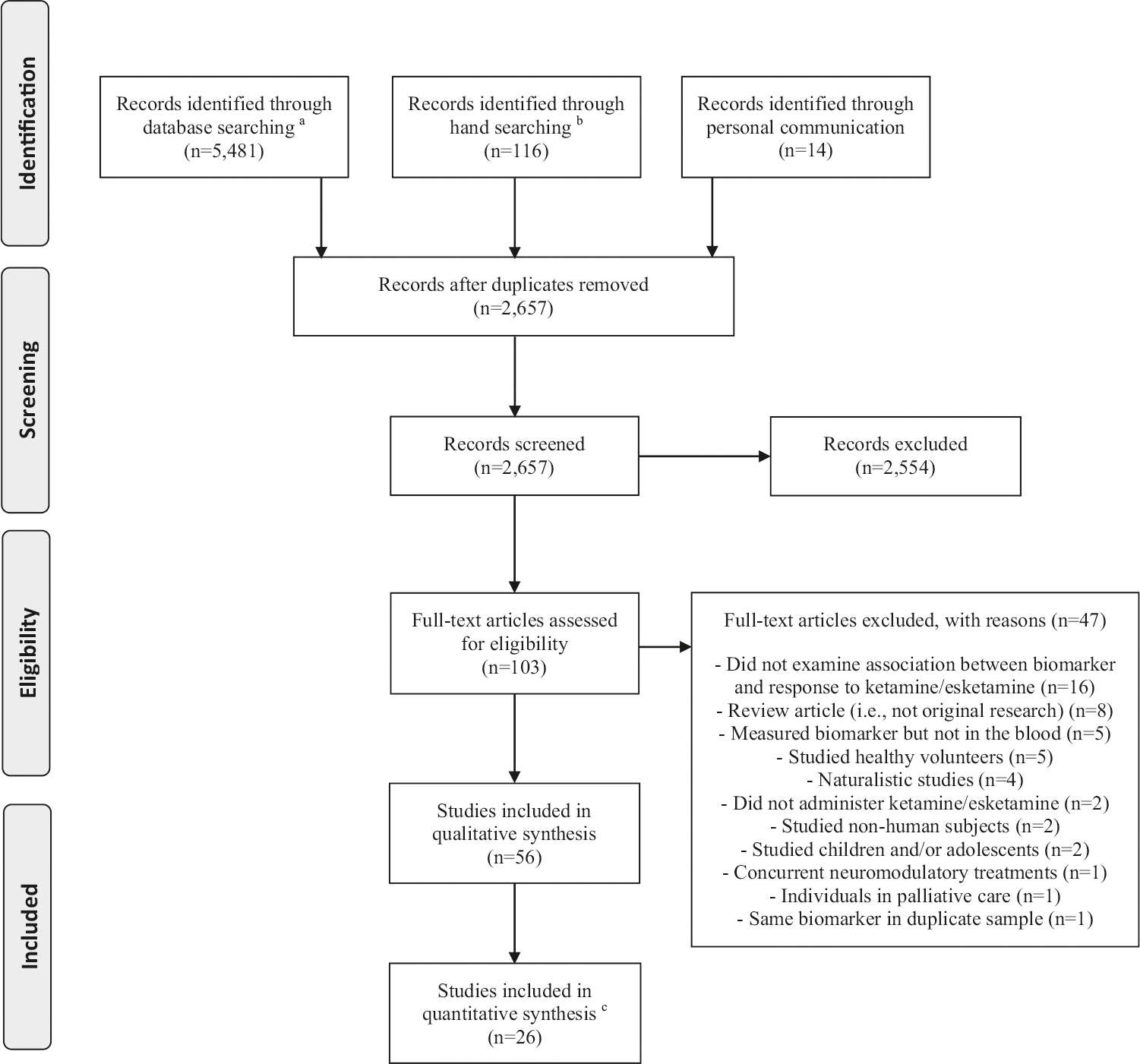
aDatabases used in the searches: Medline (PubMed), Cochrane Library, Embase, PsycInfo, and Web of Science. bHand searching included screening of review articles, grey literature, included papers and manuscripts that cited the included papers. cMeta-analytical calculations were conducted only if there were comparable data on the same blood-based biomarker for at least three studies. If a biomarker was examined in studies with overlapping samples, only the study with largest sample size was included.
Fifty-four studies (96%) reported on the association between blood-based biomarkers and response to ketamine, one study (2%) reported only on response to esketamine [66], and one study (2%) reported on responses to both ketamine and esketamine [67]. All studies investigating ketamine used intravenous (n = 55) delivery. The two manuscripts that reported on esketamine examined the intranasal formulation. The included studies described results on baseline levels of 464 biomarkers, and on longitudinal changes of 470 biomarkers. The most frequently studied groups of biomarkers were neurotrophic factors (n = 15, 27%), ketamine related metabolites (n = 13, 23%), inflammatory markers (n = 12, 21%), metabolites of the tryptophan-kynurenine pathway (n = 10, 18%), genetic markers (n = 7, 12%), and amino acids and derivates (n = 5, 9%). Thirty studies (54%) reported both baseline and longitudinal changes in biomarkers, fourteen (25%) reported only longitudinal changes, and twelve (21%) only baseline levels. Supplementary Table 2 summarizes the characteristics of the included articles.
Forty-three studies (74%) examined a single infusion of ketamine and the most frequent regimen was a dose of 0.5 mg/kg delivered over 40 min (40 of the 43 single-infusion studies included at least one treatment arm with this regimen). The timepoint most commonly used to define response was 1 day post-infusion(s) (25%, 14 studies). After accounting for overlapping samples, the combined response rate after a single ketamine infusion (0.5 mg over 40 min) at 1 day post-infusion was 46% (n = 95/208). At this timepoint, there were no statistically significant differences in response between individuals with MDD and bipolar depression (response rates, respectively, of 45% and 48%, odds-ratio [95% CI] = 0.90 (0.38, 2.16), p = 0.83).
The risk of bias assessment revealed that the overall risk of bias was moderate in 21 studies (37%), and high in 35 studies (63%) (Supplementary Fig. 1). Specifically, the most problematic dimensions of bias were: (1) potential study confounding due to factors such as insufficient assessment and control of important confounders including concomitant psychiatric and medical treatments; (2) insufficient description of study participants due to factors such as limited description of recruitment procedures; (3) missing biomarker levels in a significant proportion of the participants; and (4) variable measurement of prognostic factors due to lack of standardization in blood collection, and insufficient description of laboratory procedures.
ASSOCIATION BETWEEN BLOOD-BASED BIOMARKERS AND ANTIDEPRESSANT RESPONSE TO KETAMINE Neurotrophic factors
Many preclinical models of ketamine’s mechanism of action identified the effect of increased synaptic plasticity and enhanced production of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) [68–71]. Although the preclinical studies are primarily based on rodent brain tissues, studies in humans have tested the assumption that such changes would also be reflected by changes in neurotrophic factors in blood.
Fifteen studies reported the relationship between antidepressant response to ketamine and blood levels of neurotrophic factors [21, 47, 50, 72–82]. All 15 reported on baseline levels, and 14 examined longitudinal changes. The most studied neurotrophic factors were BDNF (15 studies) and vascular endothelial growth factor (VEGF) (5 studies). The qualitative synthesis found significant associations between baseline levels of BDNF and improvement of depression in only two of 15 studies and, in both cases, higher baseline levels of BDNF were correlated with a greater improvement in depression scores [79, 81] (Supplementary Table 3). Nominally significant associations between longitudinal changes in the levels of BDNF and improvement of depression were observed in three of 14 studies, however, the specific findings were inconsistent [50, 74, 81] (Supplementary Table 4). Studies of VEGF and other neurotrophic factors were more limited and no clear pattern was observed. Of these 15 studies, 12 were appropriate for meta-analyses, with comparable data and non overlapping participants. These included ten studies of BDNF (N = 332) and three of VEGF (N = 154). As shown below (Table 1, Supplementary Fig. 2), meta-analyses showed no statistically significant associations between baseline levels of BDNF or VEGF and response status.
Table 1.
Meta-analytical calculations comparing baseline levels of blood-based biomarkers in responders and non-responders to ketamine.
| Blood-based biomarker | Number of studies | Sample size (responders/non-responders) | SMD (95% confidence interval) | p value | I2 |
|---|---|---|---|---|---|
| Neurotrophic factors | |||||
| Brain-derived neurotrophic factor (pg/ml) | 11 | 332 (161/171) | −0.04 (−0.30, 0.23) | 0.77 | 16% |
| Vascular endothelial growth factor (pg/ml) | 3 | 154 (88/66) | 0.08 (−0.25, 0.42) | 0.62 | 0% |
| Inflammatory markers | |||||
| Interleukin 6 (pg/ml) | 6 | 260 (128/132) | −0.04 (−0.36, 0.29) | 0.83 | 29% |
| Tumor necrosis factor α (pg/ml) | 6 | 260 (128/132) | −0.10 (−0.40, 0.21) | 0.54 | 22% |
| Interleukin 10 (pg/ml) | 4 | 196 (101/95) | −0.18 (−0.47, 0.11) | 0.23 | 0% |
| Interleukin 8 (pg/ml) | 3 | 186 (92/94) | −0.07 (−0.36, 0.22) | 0.62 | 0% |
| C-reactive protein (CRP) (mg/L) | 3 | 113 (47/66) | −0.28 (−0.67, 0.10) | 0.15 | 0% |
Responder = participant with least 50% improvement in depression.
Positive values for standardized mean difference (SMD) indicate that baseline levels of the biomarker are higher in responders than in non-responders while negative SMD(s) indicate that baseline levels of the biomarker are lower in responders than in non-responders.
However, quantitative analyses of longitudinal changes in BDNF levels in responders and non-responders (11 studies, N = 331) revealed that responders (SMD [95% CI] = 0.26 [0.03, 0.48], p = 0.02) but not non-responders (SMD [95% CI] = 0.05 [−0.19, 0.28], p = 70) had increased post-treatment levels of BDNF (Fig. 2). There was no evidence for between-study heterogeneity (I2 = 0) and no statistically significant evidence of publication bias (p for Egger’s test = 0.58; funnel plots for all comparisons are displayed in Supplementary Figs. 3 and 4).
Fig. 2. Meta-analytical calculations comparing post-treatment and baseline blood levels of brain-derived neurotrophic factor (pg/ml) in responders and non-responders to ketamine (n = 331, 11 studies).
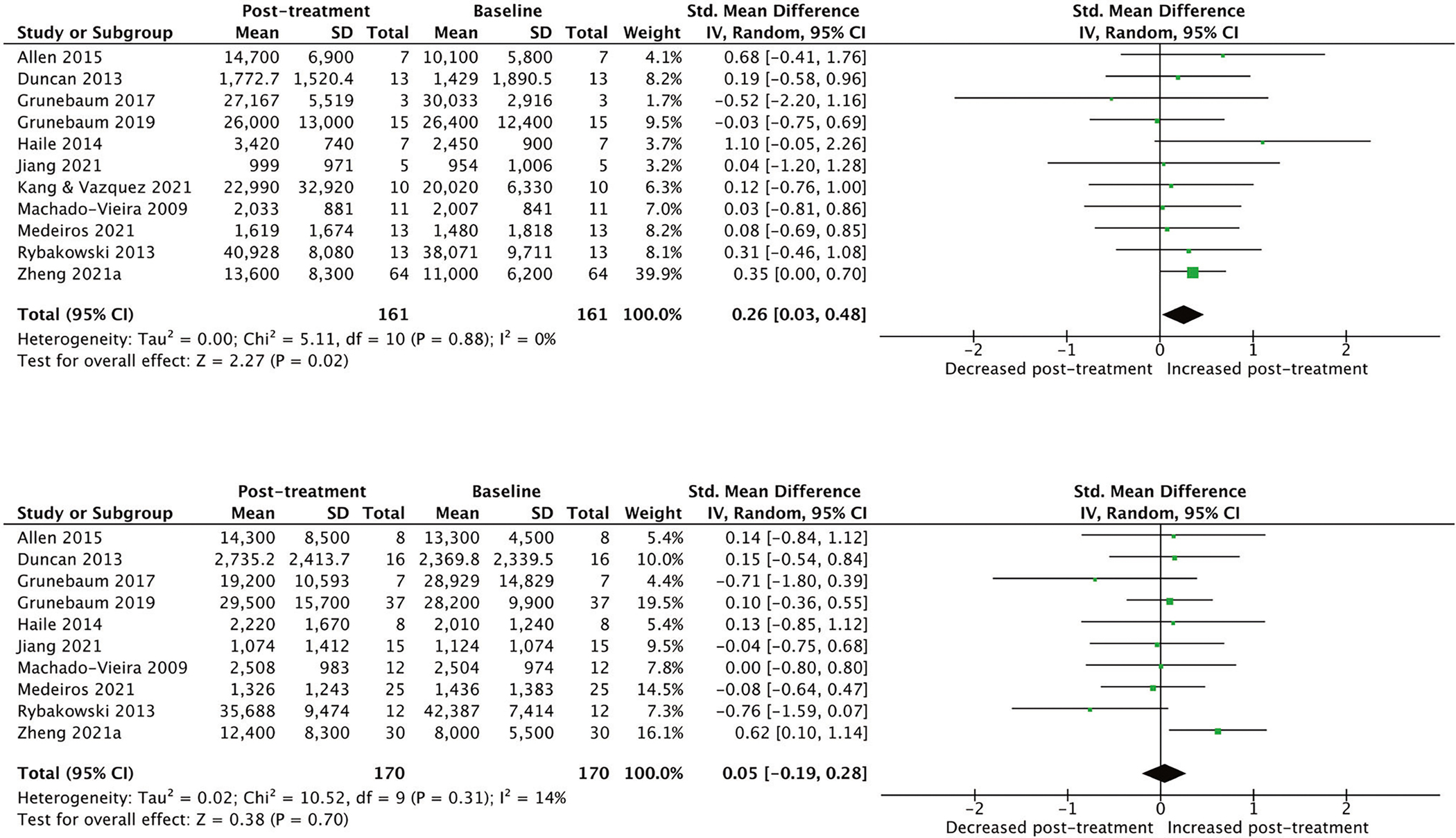
*Responder = participant with least at 50% improvement in depression scores. **The manuscript by Kang & Vasquez (2021) had only one non-responder, therefore, it was not possible to conduct meta-analytical calculations for the non-responder group in their study.
Further analyses in responders revealed that the magnitude of the increases in BDNF levels was greater in studies with multiple infusions (SMD [95% CI] = 0.35 [0.04, 0.66], 3 studies) than in studies with a single infusion (SMD [95% CI] = 0.16 [−0.15, 0.47], 8 studies, Supplementary Fig. 5). However, the difference was not statistically significant (β = 0.19, p = 0.41) since the number of studies in each group (single vs multiple) was relatively small. Other potential effect modifiers investigated were also not significantly different (plasma versus serum: p = 0.69; primary diagnosis: p = 0.63; and overall risk of bias: p = 0.85). Sensitivity analyses individually excluding each study found that the exclusion of either the Haile et al. (SMD [95% CI = 0.22 [0.00, 0.45], p = 0.05) or Zheng et al. studies (SMD [95% CI] = 0.19 [−0.19, 0.48], p = 0.19) made the overall findings of increase of BDNF in responders non-significant; however, the effect size estimates were similar.
Levels of ketamine and ketamine metabolites
Ketamine is rapidly and stereoselectively metabolized by liver p450 enzymes to a number of metabolites, including at first norketamine, and then to either dehydronorketamine (DHNK) or one of the 12 hydroxynorketamines (HNKs) [83]. Thirteen studies have investigated whether blood levels of ketamine or its metabolites were associated with antidepressant response to ketamine [21, 25, 84–94]. All thirteen studies measured levels of ketamine and norketamine, while DHNK was investigated in four studies, and additional select hydroxynorketamines were assessed in only two studies. Ten studies failed to find statistically significant associations between levels of ketamine or ketamine metabolites and response to ketamine (Supplementary Table 4). However, two metabolites showed similar evidence of association in more than one study: (1) two studies reported that higher levels of ketamine were correlated with greater improvement in depression scores [91, 92], and (2) two studies observed that lower levels of (2R-6R)-hydroxynorketamine were correlated with greater improvement in depression scores [22, 91].
Meta-analysis of data for nine studies (N = 286) did not find statistically significant associations between levels of ketamine or norketamine and response status (Fig. 3).
Fig. 3. Meta-analytical calculations comparing the blood levels of ketamine (pg/ml) and norketamine (pg/ml) in responders and non-responders to ketamine (n = 286, 9 studies).
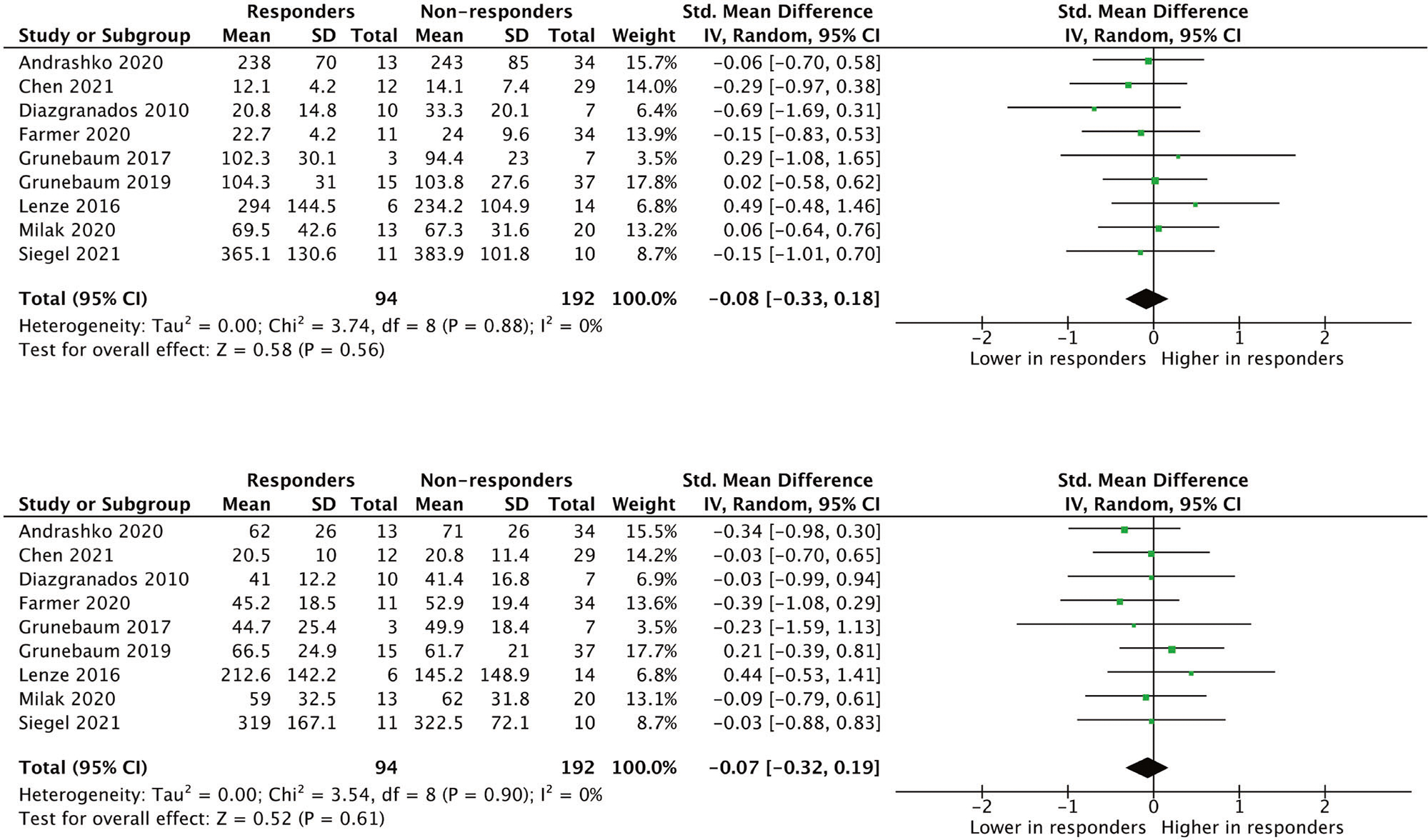
*Responder = participant with at least 50% improvement in depression scores. **Blood levels of ketamine and norketamine were measured at the same timepoint for responders and non-responders in individual studies. However, the timepoints were not the same across all studies (varied from during the infusion to 24 h post-infusion). The specific timepoints were: Andrashko et al. (2020) = immediately post-infusion; Chen et al. (2021) = 200 min post-infusion; Diazgranados et al., (2010) = 190 min post-infusion; Farmer et al. (2020) = 190 min post-infusion; Grunebaum et al., (2017) = immediately post-infusion; Grunebaum et al., (2019) = immediately post-infusion; Lenze et al. (2016) = 24 h post-infusion; Milak et al. (2020) = area under the curve calculated as the sum of the blood levels at 50 and 80 min post-infusion; Siegel et al. (2021) = 24 h post-infusion.
Inflammatory markers
Ketamine has anti-inflammatory effects [12, 95, 96], motivating several studies (N = 12) of biomarkers related to immune function, mostly pro-inflammatory markers, as predictors of antidepressant response [30, 46–49, 79, 97–102]. Based on this hypothesis, studies have tested whether ketamine may normalize the elevated levels of pro-inflammatory markers found in a subset of individuals with depression [30, 46–49, 97, 99, 103]. Pro-inflammatory markers studied included several interleukins (IL-1β, IL-2, IL-6, IL-8, IL-10), tumor necrosis factor alpha (TNF-α) and c-reactive protein (CRP).
Eleven manuscripts reported on the association between baseline levels of inflammatory markers and response to ketamine with no consistent pattern in the findings (Supplementary Table 3). Among studies looking at longitudinal changes of pro-inflammatory markers and response to ketamine, only three of the ten studies found an association between improvement of depressive symptoms and lowered levels of pro-inflammatory factors (Supplementary Table 4). However, no individual inflammatory biomarker was significantly associated in more than one study.
Meta-analysis included six studies (N = 260). At baseline, responders had non-significantly lower levels of pro-inflammatory factors compared to non-responders (Table 1, Supplementary Fig. 6). CRP was the baseline pro-inflammatory marker with the strongest association with response but the meta-analytic effect size was modest and non-significant (CRP: SMD [95% CI] = −0.28 [−0.67, 0.10], p = 0.15, 3 studies). There were no statistically significant longitudinal changes in inflammatory markers in responders or non-responders (Fig. 4).
Fig. 4. Meta-analytical calculations comparing post-treatment and baseline blood levels of pro-inflammatory markers in responders and non-responders to ketamine.
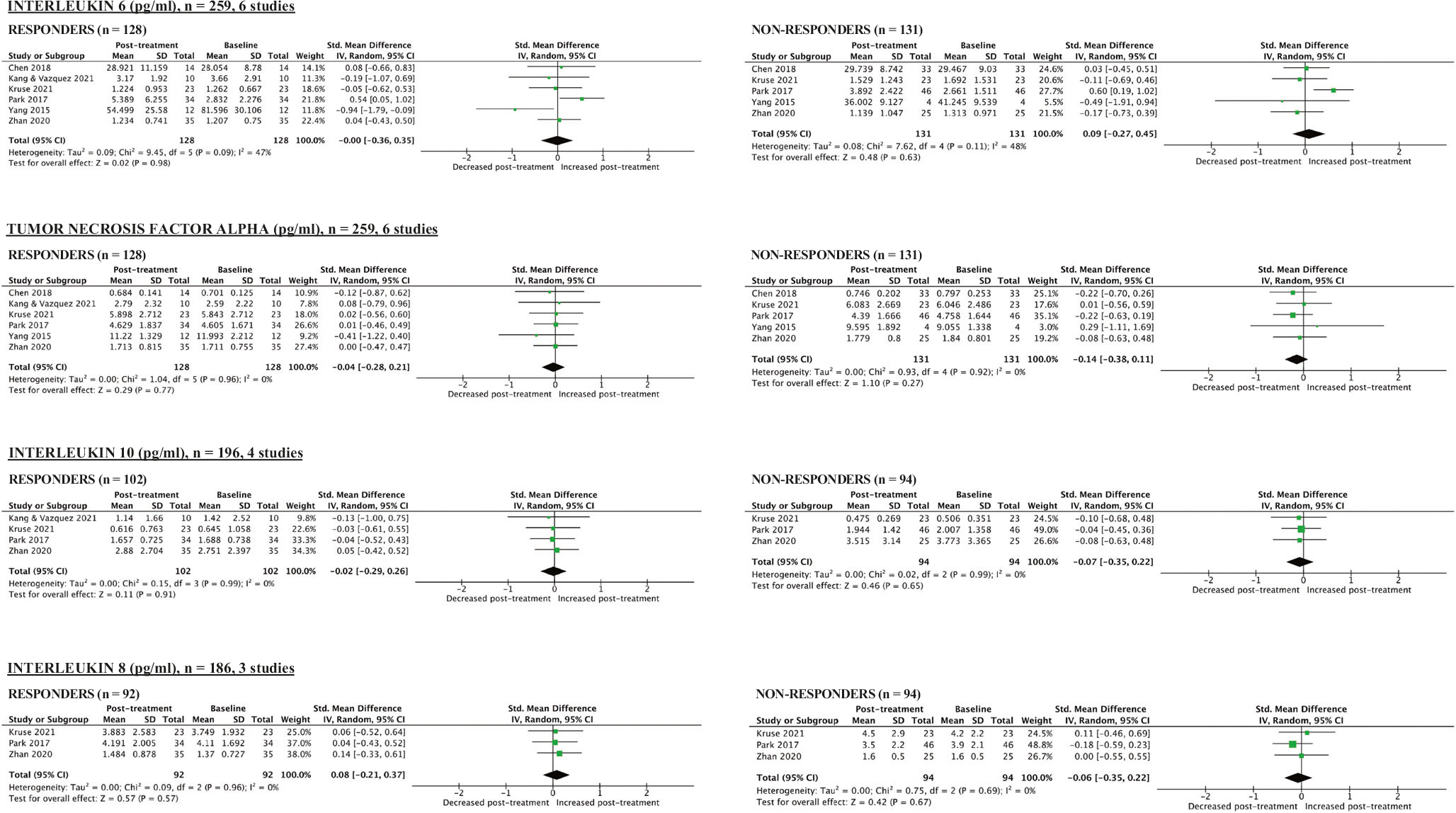
*Responder = participant with at least 50% improvement in depression scores. **The manuscript by Kang & Vasquez (2021) had only one non-responder, therefore, it was not possible to conduct meta-analytical calculations for the non-responder group in their study.
Tryptophan-kynurenine pathway
Preclinical studies have suggested that ketamine may mitigate potentially neurotoxic effects of kynurenine and its metabolites (quinolinic acid and anthranilic acid) [104, 105], which have been linked to glutamatergic excitation and the potential reduction of effective synapses [54, 105]. Therefore, it has been hypothesized that ketamine, potentially via its action as an NMDA glutamate receptor antagonist, may be protective against these neurotoxic effects [104]. Ten studies examined the association between antidepressant response to ketamine and levels of metabolites of the tryptophan-kynurenine pathway [46, 52, 54, 67, 97, 99, 100, 104, 106, 107]. The most studied molecules were tryptophan (nine studies), kynurenine (nine studies), kynurenic acid (seven studies) and quinolinic acid (three studies) as well as tryptophan/kynurenine and kynurenic acid/kynurenine ratios.
The qualitative synthesis revealed that only one of the seven baseline studies of the tryptophan-kynurenine pathway found a statistically significant association (Supplementary Table 3). Specifically, Verdonk and colleagues (2019) observed that lower baseline KynA/QA ratio was associated with greater improvement in depression scores (p = 0.016). There were no consistent patterns of results on the relationship between longitudinal changes in levels of metabolites of the tryptophan-kynurenine pathway and response to ketamine (Supplementary Table 4).
Genetic markers
Seven studies have tested the association between common genetic markers and response to ketamine [25, 56, 86, 93, 108–110]. Two studies examined the BDNF single nucleotide polymorphism (SNP), one investigated a polymorphism in the μ-opioid receptor 1 SNP, one examined several polymorphisms in the CYP450 enzymes, and three studies used a genome-wide approach. As a test of the neurotrophin hypothesis, the BDNF candidate gene studies evaluated the impact of the Val66Met single nucleotide polymorphism (SNP) on antidepressant response to ketamine [25, 56]. In a study at the National Institute of Mental Health (NIMH), Laje and collaborators (2012) analyzed 62 individuals with MDD or BD treated with a single ketamine infusion finding that individuals with MDD and the Val/Val genotype had more reductions in depression scores than individuals with MDD who were Met carriers (F = 5.59, p = 0.0007). However, Su and colleagues (2017) failed to observe a statistically significant association between response to ketamine and the Val66Met polymorphism in 71 Chinese individuals who received a single infusion of ketamine (0.2 mg/kg, n = 23; ketamine 0.5 mg/kg, n = 24) or placebo (normal saline, n = 24).
Grunebaum and colleagues (2020) examined 71 Caucasians with MDD and failed to find an association between response to ketamine and a common loss-of-function SNP in the OPRM1 gene, which encodes the μ-opioid receptor 1 [110]. The study that assessed the association between SNPs in CYP450 enzymes and response to ketamine investigated 67 participants with MDD (n = 45) or bipolar depression (n = 22), and failed to observe statistically significant relationships [86].
There also were exploratory genome-wide association studies investigating the association between SNPs and response to ketamine. However, the sample size of the GWAS studies were relatively small and there were no genome-wide significant findings [93, 108, 109].
Amino acids and derivates
Amino acids can have varied functional effects on the CNS. Some amino acids (such as glutamate, aspartate, glycine, and GABA) have specific neurotransmitter activity, while others (such as phenylalanine, tyrosine, arginine, and serine) serve as precursors of neurotransmitters [111, 112]. Five studies have assessed the association between levels of amino acids and derivates, and response to ketamine [53, 67, 76, 107]. Three studies reported on baseline levels (Supplementary Table 3) and four studies examined longitudinal changes (Supplementary Table 4). No statistically significant association was replicated.
Other biomarkers
Other groups of biomarkers investigated include micronutrients [76, 113–115], gene expression [116, 117], targeted proteins [51, 78, 118], and metabolomic patterns [52, 67, 107]. In general, the results were inconsistent, and no individual biomarker association showed replication across two or more studies.
ASSOCIATION BETWEEN BLOOD-BASED BIOMARKERS AND ANTIDEPRESSANT RESPONSE TO ESKETAMINE
Only two studies have tested the association between blood-based biomarkers and antidepressant response to esketamine [66, 67] (Supplementary Table 5). Rotroff and colleagues (2016) used two complimentary metabolomics platforms (>400 individual biomarkers investigated) to assess response in 20 participants randomized to esketamine 0.2 or 0.4 mg/kg. The authors did not find any statistically significant correlations with response to esketamine. Li and collaborators (2020) conducted a GWAS with 527 individuals with MDD and observed an association between reduction of depressive symptoms after 8 infusions of esketamine (28 mg, 56 mg or 84 mg) and the IRAK3 gene, which encodes a kinase linked to interleukin-1 receptor signaling, and NME7, which encodes a protein that regulates microtubule-nucleating activity [66]. The authors did not find an association between response to esketamine (percentage reduction in Montgomery–Åsberg Depression Rating Scale score) and the BDNF Val66Met polymorphism [66].
DISCUSSION
We conducted a comprehensive qualitative (56 studies) and quantitative synthesis (26 studies) of blood-based biomarkers of response to ketamine and esketamine. More than 460 individual blood-based biomarkers were examined. Our results revealed no consistent associations between baseline levels of blood-based biomarkers and response to ketamine. In the longitudinal analyses, we found statistically significant but modest effect association between response and increased treatment-associated levels of BDNF compared to baseline (pre-treatment) levels. However, even for the meta-analysis of of BDNF there was significant variability between studies, indicating the need for further research with larger samples sizes and more attention to potential confounds. There was no consistent evidence to support other longitudinal biomarkers. Although ketamine and esketamine might have similar mechanisms of action, the number of studies assessing specifically the association between blood-based biomarkers and response to esketamine was limited (n = 2). Therefore, this study has mostly summarized the literature related to antidepressant response to racemic ketamine.
Our meta-analysis indicates that increased levels of BDNF may be associated with ketamine’s antidepressant effects, and provides preliminary evidence in humans for a mechanism that has been widely studied in pre-clinical literature [68, 69, 71]. Ketamine is hypothesized to activate post-synaptic AMPA receptors, causing downstream glutamatergic potentiation that triggers intracellular pathways ultimately resulting in increased BDNF release [69, 119]. The elevation in the levels of BDNF in the CNS has been similarly hypothesized to promote synaptogenesis, and potentially reversing impaired neuroplasticity and atrophic changes associated with MDD [120]. Peripheral levels of BDNF have also been shown to be increased by treatment with conventional antidepressants [121]. Preliminary pre-clinical evidence suggests that CNS and peripheral levels of BDNF are partially correlated [122]; however, it is still unclear the degree to which peripheral levels of BDNF relate to CNS levels of BDNF and synaptic function in humans. Additional studies in larger samples and with more controlled methodology are needed to further validate the association between blood-based post-treatment increases in BDNF and response to ketamine.
The relationship between therapeutic drug levels and clinical response in psychiatry is often complex, and there exists ongoing controversy in the literature as to whether the blood levels of ketamine and its metabolites are correlated with antidepressant response [92, 123]. Our study conducted the largest analysis to date by combining data from nine clinical trials, and did not observe a statistically significant association between blood levels of ketamine or norketamine and antidepressant response. These findings suggest that monitoring levels of ketamine and norketamine may have limited utility in clinical practice. However, future studies should examine whether ketamine and its metabolites have a minimum effective concentration or therapeutic range required to exert significant antidepressant effects.
We also failed to observe a statistically significant association between baseline or longitudinal levels of pro-inflammatory markers and response to ketamine. Despite previous evidence indicating possible anti-inflammatory properties of ketamine [12, 69], our results question whether individuals with elevated levels of pro-inflammatory markers, compared to those without, benefit more from antidepressant treatment with ketamine.
As reflected in our risk of bias analyses, this systematic review and meta-analysis should be interpreted in the context of several limitations. First, most sample sizes have been relatively small (the median sample size included in this study was 33 individuals), which are likely to lead to imprecise effect sizes that replicate poorly [28, 124]. This is particularly relevant for a heterogenous condition such as MDD or bipolar depression [125], where effect sizes of potential biomarkers are likely to be modest. Second, many studies did not account for potential confounding factors (Supplementary Table 6) such as the use of concurrent medications, smoking, time of the day when the blood was obtained, medical comorbidities, and fasting status [126, 127]. Third, while biomarkers may be measured more reliably than clinical variables, the included studies have typically not reported basic measurement properties such as assay reliability and specific covariates used in the primary analyses [128]. Finally, the studies included in this systematic review and meta-analysis showed significant heterogeneity, with differences in study design, sample characteristics, treatment schedule, timepoints assessing antidepressant response and laboratory procedures. Taken together, these factors highlight the importance of promoting more open data sharing to facilitate standardized individual-level meta-analyses [129].
CONCLUSIONS
Ketamine’s rapid antidepressant effect has been described as one of the most impactful recent discoveries in biological psychiatry. Despite a growing literature, there is currently limited evidence that blood-based biomarkers may serve as predictors of ketamine treatment efficacy. Our meta-analysis found that responders, but not non-responders, had statistically significant small effect increases in post-treatment levels of brain-derived neurotrophic factor (BDNF), highlighting a potential dynamic biomarker of ketamine’s antidepressant effects. However, there was no consistent evidence in our systematic literature review or meta-analysis to support the other more than 460 biomarkers, including metabolites of ketamine and inflammation-related biomarkers. Risk of bias was generally high amongst the included studies, highlighting the need to conduct larger trials with more comprehensive consideration of confounders.
Supplementary Material
FUNDING
This systematic review/meta-analysis has not been directly funded by any legal entities or organizations. We received operational support from the Johns Hopkins School of Medicine. TDG is supported by NIH R01-MH107615 and RAI145211A, and VA Merit Awards 1I01BX004062 and 101BX003631-01A1. FSG received partial support from the Johns Hopkins Catalyst Award.
Footnotes
COMPETING INTERESTS
TDG is listed as co-author on patent and patent applications related to the pharmacology and use of (2 R,6 R)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorder. He has assigned his patent rights to the University of Maryland Baltimore, but will share a percentage of any royalties that may be received. TDG has received research funding from Allergan and Roche Pharmaceuticals, and has served as a consultant for FSV7 LLC, during the preceding 3 years. NBF holds patents pertaining to improved use of NMDA antagonists as therapeutic agents. EDA has served on advisory boards for Alkermes, Janssen, Lundbeck/Otsuka, Roche, Sunovion and Teva and reports previous stock holdings in AstraZeneca, Johnson & Johnson, Moderna, and Pfizer. EDA has received research support from Alkermes, Astellas, Biogen, Boehringer-Ingelheim, InnateVR, Janssen, National Network of Depression Centers, Neurocrine Biosciences, Novartis, Otsuka, Pear Therapeutics, Takeda and serves on the SMI Adviser LAI Center of Excellence (unpaid). BS reports research time support from Medibio (unrelated to the current study); grant support from Mayo Clinic. SS has received grants/research support from NIMH R21 (1R21MH119441 – 01A1) and SAMHSA (FG000470-01) and research supplement funds from The University of Texas Health Science Center at Houston. SS has received speaking honoraria from British Medical Journal Publishing Group and received research support from Compass pathways, Janssen and LivaNova. RMV has received consulting fees from Eurofarma Pharmaceuticals. Abbott and BioStrategies group, and has a research contract for trials with Janssen and Boehringer-Ingelheim Pharmaceuticals. RMV has also received speaker fees from Otsuka, Lundbeck, EMS, and Cristalia and is a member of the scientific board of Symbinas Pharmaceuticals and Allergan. SVP has received honoraria for consulting or research funds from Assurex (Myriad), Sage, Otsuka, Takeda, Janssen, Aifred, Mensante, Canadian Institutes for Health Research, Ontario Brain Institute, and the Flinn Foundation. MAF has received Grant Support from Assurex Health, and Mayo Foundation. He also has financial interests in Chymia LLC. CAZ is a full-time U.S government employee. He is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation. CAZ is listed as a coinventor on a patent for the use of (2 R,6 R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. CAZ is listed as co-inventor on a patent application for the use of (2 R,6 R)-hydroxynorketamine and (2 S,6 S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. CAZ has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. FSG has received research grant support from Janssen Therapeutics. GCM, WLP, JN, and MFG do not have conflicts of interest to report.
ADDITIONAL INFORMATION
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41380-022-01652-1.
REFERENCES
- 1.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 2016;26:979–93. [DOI] [PubMed] [Google Scholar]
- 4.Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Therapeutic Adv Psychopharmacol. 2016;6:99–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J bipolar Disord. 2020;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazer A, Benmansour S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 2002;7:S23–8. [DOI] [PubMed] [Google Scholar]
- 7.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA Jr. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depression. J Clin Psychiatry. 2008;69:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–86. [DOI] [PubMed] [Google Scholar]
- 9.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus. 2018;16:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs LM, Gould TD. Ketamine and the future of rapid-acting antidepressants. Annu Rev Clin Psychol. 2021;17:207–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kryst J, Kawalec P, Mitoraj AM, Pilc A, Lasoń W, Brzostek T. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta-analysis of randomized clinical trials. Pharmacol Rep. 2020;72:543–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould TD, Zarate CA Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molero P, Ramos-Quiroga J, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana J. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–20. [DOI] [PubMed] [Google Scholar]
- 17.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175:620–30. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression-first FDA-approved antidepressant in a new class. N. Engl J Med. 2019;381:1–4. [DOI] [PubMed] [Google Scholar]
- 20.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: A pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19:176–83. [DOI] [PubMed] [Google Scholar]
- 22.Grunebaum MF, Galfalvy HC, Choo T-H, Keilp JG, Moitra VK, Parris MS, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9. [DOI] [PubMed] [Google Scholar]
- 24.Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–34. [DOI] [PubMed] [Google Scholar]
- 25.Su T-P, Chen M-H, Li C-T, Lin W-C, Hong C-J, Gueorguieva R, et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42:2482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009–January 2019. J Affect Disord. 2020;277:831–41. [DOI] [PubMed] [Google Scholar]
- 28.Bzdok D, Varoquaux G, Steyerberg EW. Prediction, not association, paves the road to precision medicine. JAMA Psychiatry. 2021;78:127–8. [DOI] [PubMed] [Google Scholar]
- 29.Rong C, Park C, Rosenblat JD, Subramaniapillai M, Zuckerman H, Fus D, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health. 2018;15:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park M, Newman LE, Gold PW, Luckenbaugh DA, Yuan P, Machado-Vieira R, et al. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J Psychiatr Res. 2017;84:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadriu B, Ballard ED, Henter ID, Murata S, Gerlus N, Zarate CA Jr. Neurobiological biomarkers of response to ketamine. Adv Pharmacol (San Diego, Calif). 2020;89:195–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D trial. Am J Psychiatry. 2015;172:743–50. [DOI] [PubMed] [Google Scholar]
- 33.Saveanu R, Etkin A, Duchemin A-M, Goldstein-Piekarski A, Gyurak A, Debattista C, et al. The international Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2015;61:1–12. [DOI] [PubMed] [Google Scholar]
- 34.Chan HN, Rush AJ, Nierenberg AA, Trivedi M, Wisniewski SR, Balasubramani G, et al. Correlates and outcomes of depressed out-patients with greater and fewer anxious symptoms: a CO-MED report. Int J Neuropsychopharmacol. 2012;15:1387–99. [DOI] [PubMed] [Google Scholar]
- 35.Medeiros GC, Prueitt WL, Rush AJ, Minhajuddin A, Czysz AH, Patel SS, et al. Impact of childhood maltreatment on outcomes of antidepressant medication in chronic and/or recurrent depression. J Affect Disord. 2021;291:39–45. [DOI] [PubMed] [Google Scholar]
- 36.Perna G, Alciati A, Daccò S, Grassi M, Caldirola D. Personalized psychiatry and depression: the role of sociodemographic and clinical variables. Psychiatry Investig. 2020;17:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niciu MJ, Ionescu DALDF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien B, Lijffijt M, Lee J, Kim YS, Wells A, Murphy N, et al. Distinct trajectories of antidepressant response to intravenous ketamine. J Affect Disord. 2021;286:320–9. [DOI] [PubMed] [Google Scholar]
- 39.Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, et al. Biomarkers to predict antidepressant response. Curr Psychiatry Rep. 2010;12:553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gadad BS, Jha MK, Czysz A, Furman JL, Mayes TL, Emslie MP, et al. Peripheral biomarkers of major depression and antidepressant treatment response: current knowledge and future outlooks. J Affect Disord. 2018;233:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US); Bethesda (MD): National Institutes of Health (US) www.ncbi.nlm.nih.gov/books/NBK326791/ (2016). [PubMed] [Google Scholar]
- 42.Zarate CA Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol Psychiatry. 2013;73:1142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriksen K, O’Bryant SE, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimer’s Dement. 2014;10:115–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimer’s Dement. 2017;13:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J-J, Wang N, Yang C, Shi J-Y, Yu H-Y, Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry. 2015;77:e19–e20. [DOI] [PubMed] [Google Scholar]
- 47.Kiraly D, Horn S, Van Dam N, Costi S, Schwartz J, Kim-Schulze S, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7:e1065–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M-H, Li C-T, Lin W-C, Hong C-J, Tu P-C, Bai Y-M, et al. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatry Res. 2018;269:207–11. [DOI] [PubMed] [Google Scholar]
- 49.Zhan Y, Zhou Y, Zheng W, Liu W, Wang C, Lan X, et al. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl Psychiatry. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen AP, Naughton M, Dowling J, Walsh A, Ismail F, Shorten G, et al. Serum BDNF as a peripheral biomarker of treatment-resistant depression and the rapid antidepressant response: a comparison of ketamine and ECT. J Affect Disord. 2015;186:306–11. [DOI] [PubMed] [Google Scholar]
- 51.Ortiz R, Niciu MJ, Lukkahati N, Saligan LN, Nugent AC, Luckenbaugh DA, et al. Shank3 as a potential biomarker of antidepressant response to ketamine and its neural correlates in bipolar depression. J Affect Disord. 2015;172:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology. 2018;235:3017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moaddel R, Luckenbaugh DA, Xie Y, Villaseñor A, Brutsche NE, Machado-Vieira R, et al. D-serine plasma concentration is a potential biomarker of (R, S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology. 2015;232:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdonk F, Petit A-C, Abdel-Ahad P, Vinckier F, Jouvion G, de Maricourt P, et al. Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain, Behav, Immun. 2019;81:361–73. [DOI] [PubMed] [Google Scholar]
- 55.Machado-Vieira R, Gold P, Luckenbaugh D, Ballard E, Richards E, Henter I, et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry. 2017;22:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.PROSPERO IPRoSR-. National Institute for Health Research. Centre for Reviews and Dissemination - University of York. 2021.
- 58.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org. [Google Scholar]
- 60.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- 61.Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41:323–39. [DOI] [PubMed] [Google Scholar]
- 62.Cohen J Statistical power analysis for the behavioral sciences: Academic press; 2013. [Google Scholar]
- 63.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol. 2018;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deeks JJ, Higgins JP, Altman DG. Cochrane Statistical Methods Group. Analyzing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions. 2019;23:241–84. [Google Scholar]
- 66.Li QS, Wajs E, Ochs-Ross R, Singh J, Drevets WC. Genome-wide association study and polygenic risk score analysis of esketamine treatment response. Sci Rep. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rotroff D, Corum D, Motsinger-Reif A, Fiehn O, Bottrel N, Drevets W, et al. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: new mechanistic insights for rapid acting antidepressants. Transl Psychiatry. 2016;6:e894–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deyama S, Duman RS. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol Biochem Behav. 2020;188:172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-F, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duncan WC Jr, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A. Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol: Clin Exp. 2013;28:87–90. [DOI] [PubMed] [Google Scholar]
- 75.Haile C, Murrough J, Iosifescu D, Chang L, Al Jurdi R, Foulkes A, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Permoda-Osip A, Dorszewska J, Bartkowska-Sniatkowska A, Chlopocka-Wozniak M, Rybakowski J. Vitamin B12 level may be related to the efficacy of single ketamine infusion in bipolar depression. Pharmacopsychiatry. 2013;46:227–8. [DOI] [PubMed] [Google Scholar]
- 77.McGrory CL, Ryan KM, Gallagher B, McLoughlin DM. Vascular endothelial growth factor and pigment epithelial-derived factor in the peripheral response to ketamine. J Affect Disord. 2020;273:380–3. [DOI] [PubMed] [Google Scholar]
- 78.Jiang H, Veldman ER, Tiger M, Ekman C-J, Lundberg J, Svenningsson P. Plasma levels of brain-derived neurotrophic factor and S100B in relation to antidepressant response to ketamine. Front Neurosci. 2021:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang MJ, Vazquez G. A Pilot Study: An Open-Label Biomarker Development of Ketamine for Unipolar Refractory Depression. Biol Psychiatry. 2021;89:S92–3. [Google Scholar]
- 80.Medeiros GC, Greenstein D, Kadriu B, Yuan P, Park LT, Gould TD, et al. Treatment of depression with ketamine does not change plasma levels of brain-derived neurotrophic factor or vascular endothelial growth factor. J Affect Disord. 2020;280:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng W, Zhou Y-L, Wang C-Y, Lan X-F, Zhang B, Zhou S-M, et al. Plasma BDNF concentrations and the antidepressant effects of six ketamine infusions in unipolar and bipolar depression. PeerJ. 2021;9:e10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng W, Zhou Y-L, Wang C-Y, Lan X-F, Zhang B, Zhou S-M, et al. Association of plasma VEGF levels and the antidepressant effects of ketamine in patients with depression. Therapeutic Advances in. Psychopharmacology. 2021;11:20451253211014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Highland JN, Zanos P, Riggs LM, Georgiou P, Clark SM, Morris PJ, et al. Hydroxynorketamines: pharmacology and potential therapeutic applications. Pharmacol Rev. 2021;73:763–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ibrahim L, DiazGranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SLV, Ramamoorthy A, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol Lett. 2013;34:101–7. [PubMed] [Google Scholar]
- 88.Lenze EJ, Farber NB, Kharasch E, Schweiger J, Yingling M, Olney J, et al. Ninety-six hour ketamine infusion with co-administered clonidine for treatment-resistant depression: a pilot randomised controlled trial. World J Biol Psychiatry. 2016;17:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grunebaum MF, Galfalvy HC, Choo T-H, Parris MS, Burke AK, Suckow RF, et al. Ketamine metabolite pilot study in a suicidal depression trial. J Psychiatr Res. 2019;117:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andrashko V, Novak T, Brunovsky M, Klirova M, Sos P, Horacek J. The antidepressant effect of ketamine is dampened by concomitant benzodiazepine medication. Front Psychiatry. 2020;11:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farmer CA, Gilbert JR, Moaddel R, George J, Adeojo L, Lovett J, et al. Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milak MS, Rashid R, Dong Z, Kegeles LS, Grunebaum MF, Ogden RT, et al. Assessment of relationship of ketamine dose with magnetic resonance spectroscopy of Glx and GABA responses in adults with major depression: a randomized clinical trial. JAMA Netw Open. 2020;3:e2013211–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen M-H, Kao C-F, Tsai S-J, Li C-T, Lin W-C, Hong C-J, et al. Treatment response to low-dose ketamine infusion for treatment-resistant depression: A gene-based genome-wide association study. Genomics. 2021;113:507–14. [DOI] [PubMed] [Google Scholar]
- 94.Siegel JS, Palanca BJ, Ances BM, Kharasch ED, Schweiger JA, Yingling MD, et al. Prolonged ketamine infusion modulates limbic connectivity and induces sustained remission of treatment-resistant depression. Psychopharmacology. 2021;238:1157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eldufani J, Nekoui A, Blaise G. Nonanesthetic effects of ketamine: a review article. Am J Med. 2018;131:1418–24. [DOI] [PubMed] [Google Scholar]
- 96.Tan S, Wang Y, Chen K, Long Z, Zou J. Ketamine alleviates depressive-like behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol Pharm Bull. 2017;40:1260–7. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Y-L, Wu F-C, Wang C-Y, Zheng W, Lan X-F, Deng X-R, et al. Relationship between hippocampal volume and inflammatory markers following six infusions of ketamine in major depressive disorder. J Affect Disord. 2020;276:608–15. [DOI] [PubMed] [Google Scholar]
- 98.Permoda-Osip A, Skibinska M, Bartkowska-Sniatkowska A, Kliwicki S, Chlopocka-Wozniak M, Rybakowski JK. Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr Pol. 2014;48:35–47. [PubMed] [Google Scholar]
- 99.Allen AP, Naughton M, Dowling J, Walsh A, O’Shea R, Shorten G, et al. Kynurenine pathway metabolism and the neurobiology of treatment-resistant depression: comparison of multiple ketamine infusions and electroconvulsive therapy. J Psychiatr Res. 2018;100:24–32. [DOI] [PubMed] [Google Scholar]
- 100.Kadriu B, Farmer CA, Yuan P, Park LT, Deng Z-D, Moaddel R, et al. The kynurenine pathway and bipolar disorder: intersection of the monoaminergic and glutamatergic systems and immune response. Mol Psychiatr. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ryan K, Gallagher B. Abstract# 4393 Analysis of mRNA levels of inflammatory mediators in samples from the KARMA-dep (Ketamine as an adjunctive therapy for major depression) Trial. Brain, Behav, Immun. 2019;81:52. [Google Scholar]
- 102.Kruse JL, Vasavada MM, Olmstead R, Hellemann G, Wade B, Breen EC, et al. Depression treatment response to ketamine: sex-specific role of interleukin-8, but not other inflammatory markers. Transl psychiatry. 2021;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Medeiros GC, Rush AJ, Jha M, Carmody T, Furman JL, Czysz AH, et al. Positive and negative valence systems in major depression have distinct clinical features, response to antidepressants, and relationships with immunomarkers. Depress Anxiety. 2020;37:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, et al. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain, Behav, Immun. 2018;74:205–12. [DOI] [PubMed] [Google Scholar]
- 105.Hunt BC, e Cordeiro TM, Robert S, de Dios C, Leal VAC, Soares JC, et al. Effect of mmune activation on the kynurenine pathway and depression symptoms–a systematic review and meta-analysis. Neurosci Biobehav Rev. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Y, Liu W, Zheng W, Wang C, Zhan Y, Lan X, et al. Predictors of response to repeated ketamine infusions in depression with suicidal ideation: An ROC curve analysis. J Affect Disord. 2020;264:263–71. [DOI] [PubMed] [Google Scholar]
- 107.Villaseñor A, Ramamoorthy A, Silva dos Santos M, Lorenzo M, Laje G, Zarate C Jr, et al. A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol. 2014;171:2230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo W, Machado-Vieira R, Mathew S, Murrough JW, Charney DS, Grunebaum M, et al. Exploratory genome-wide association analysis of response to ketamine and a polygenic analysis of response to scopolamine in depression. Transl Psychiatry. 2018;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bao Z, Zhao X, Li J, Zhang G, Wu H, Ning Y, et al. Prediction of repeated-dose intravenous ketamine response in major depressive disorder using the GWAS-based machine learning approach. J Psychiatr Res. 2021;138:284–90. [DOI] [PubMed] [Google Scholar]
- 110.Grunebaum MF, Galfalvy HC, Liu J, Huang Y-Y, Marcott S, Burke AK, et al. Opioid receptor μ−1 and ketamine effects in a suicidal depression trial: a post hoc exploration. J Clin Psychopharmacol. 2020;40:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2012;15:1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parker G, Brotchie H. Mood effects of the amino acids tryptophan and tyrosine: ‘Food for Thought’III. Acta Psychiatr Scandinavica. 2011;124:417–26. [DOI] [PubMed] [Google Scholar]
- 113.Lundin N, Niciu M, Luckenbaugh D, Ionescu D, Richards E, Voort JV, et al. Baseline vitamin B12 and folate levels do not predict improvement in depression after a single infusion of ketamine. Pharmacopsychiatry. 2014;47:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Słupski J, Cubała WJ, Górska N, Słupska A, Gałuszko-Węgielnik M. Copper concentrations in ketamine therapy for treatment-resistant depression. Brain Sci. 2020;10:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Górska N, Cubała WJ, Słupski J, Wiglusz MS, Gałuszko-Węgielnik M, Kawka M, et al. Magnesium in ketamine administration in treatment-resistant depression. Pharmaceuticals. 2021;14:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gururajan A, Naughton M, Scott KA, O’connor R, Moloney G, Clarke G, et al. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl Psychiatry. 2016;6:e862–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bevilacqua L, Cathomas F, Ramakrishnan A, Schneider M, Shen L, Russo S, et al. Gene expression and molecular pathways associated with rapid antidepressant response to ketamine in patients with treatment resistant depression. Biol Psychiatry. 2020;87:S176–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Veldman ER, Mamula D, Jiang H, Tiger M, Ekman C-J, Lundberg J, et al. P11 (S100A10) as a potential predictor of ketamine response in patients with SSRI-resistant depression. J Affect Disord. 2021;290:240–4. [DOI] [PubMed] [Google Scholar]
- 119.McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178:383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levy MJ, Boulle F, Steinbusch HW, van den Hove DL, Kenis G, Lanfumey L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018;235:2195–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Björkholm C, Monteggia LM. BDNF–a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–53. [DOI] [PubMed] [Google Scholar]
- 123.Farmer CA, Gilbert JR, Moaddel R, George J, Adeojo L, Lovett J, et al. Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology. 2020;45:1398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tajika A, Ogawa Y, Takeshima N, Hayasaka Y, Furukawa TA. Replication and contradiction of highly cited research papers in psychiatry: 10-year follow-up. Br J Psychiatry. 2015;207:357–62. [DOI] [PubMed] [Google Scholar]
- 125.Fried E Moving forward: how depression heterogeneity hinders progress in treatment and research. Taylor & Francis; 2017. [DOI] [PubMed] [Google Scholar]
- 126.Bus B, Molendijk ML, Penninx B, Buitelaar JK, Kenis G, Prickaerts J, et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 2011;36:228–39. [DOI] [PubMed] [Google Scholar]
- 127.Bathina S, Das UN . Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci: AMS. 2015;11:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moriarity DP, Alloy LB. Back to basics: the importance of measurement properties in biological psychiatry. Neurosci Biobehavioral Rev. 2021;123:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Byrd JB, Greene AC, Prasad DV, Jiang X, Greene CS. Responsible, practical genomic data sharing that accelerates research. Nat Rev Genet. 2020;21:615–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


