Highlights
-
•
Collagen type II is the major constituent of cartilage tissue.
-
•
Collagen type I devices in cartilage engineering are associated with suboptimal functional therapeutic outcomes.
-
•
In vitro and preclinical data clearly illustrate the potential of collagen type II as building block in cartilage engineering approaches.
Keywords: Collagen type II, Articular cartilage, Chondrocytes, Chondrogenic induction
Abstract
Collagen type II is the major constituent of cartilage tissue. Yet, cartilage engineering approaches are primarily based on collagen type I devices that are associated with suboptimal functional therapeutic outcomes. Herein, we briefly describe cartilage's development and cellular and extracellular composition and organisation. We also provide an overview of collagen type II biosynthesis and purification protocols from tissues of terrestrial and marine species and recombinant systems. We then advocate the use of collagen type II as a building block in cartilage engineering approaches, based on safety, efficiency and efficacy data that have been derived over the years from numerous in vitro and in vivo studies.
1. Introduction
Articular cartilage is a specialised connective tissue of the joints [1, 2]. Hyaline cartilage provides a smooth and lubricated surface for articulation and facilitates the transmission of loads with low frictional coefficient [3, 4]. As articular cartilage lacks blood vessels, lymphatics and nerves, it has limited capacity for intrinsic healing and repair [5, 6]. Articular cartilage injuries are frequently caused by sports and recreational activities and, if left untreated, articular cartilage lesions form fibrocartilage and lead to osteoarthritis (OA).
OA is a whole joint disease, involving structural alterations in the hyaline articular cartilage, subchondral bone, ligaments, capsule synovium and periarticular muscles [7]. OA affects over 250 million people worldwide [8] and financially drains healthcare systems. For example, medical costs account for 1.0% to 2.5% of gross domestic product of high-income countries [9] and in USA alone, the annual insurer spending for OA-related medical care is estimated to be US$ 185.5 billion [10]. OA, due to the imbalance between the repair and the damage of joint cartilage, leads to structural destruction and failure of the synovial joint [11]. The pathogenesis of OA involves compositional changes and structural / integrity losses of cartilage [12]. Initially, disruption, caused by physical forces, happens at the cartilage surface and is followed by the expansion of the calcified zone into the radial zone. Then, the hypertrophic chondrocytes synthesise extracellular matrix (ECM) degradation products and proinflammatory mediators. Subsequently, the bone turnover is increased in the subchondral bone and vascular invasion takes place towards the cartilage region [13]. Clinically, the knee, hip, hand, spine and foot are the most common sites of OA, followed by the wrists, shoulders and ankles [14]. OA incidents gradually increase over the years, due to the combined effects of ageing, obesity and heavy work activities [15], [16], [17]. In fact, by 2032, the proportion of the population aged >45 with any doctor-diagnosed OA is estimated to increase from 26.6% to 29.5% [18]. OA is more prevalent in women than in men, with female-to-male ratio ranging from 1.5 to 4.0 [19]. Exercise, weight loss (in the case of overweight patients) and walking aids are widely recommended to improve daily activities of OA patients [20, 21]. Pharmacological agents [22], [23], [24], [25], [26], [27], [28], [29], surgical procedures [30], [31], [32], [33] and advanced therapy medicinal products [34], [35], [36], [37] have shown variable degree of efficiency and effectiveness, considering their complexity, cost of goods and regulatory hurdles. To this end, biomaterial-based therapies are continuously gaining pace, especially for large defects [38], [39], [40], [41].
Herein, we provide a brief description of cartilage's development, cellular and extracellular composition and organisation and then focus on collagen type II biosynthesis, extraction protocols, scaffold fabrication and in vitro, in vivo and clinical data, in light of recent studies that demonstrate the inability of collagen type I scaffolds to yield functional therapeutic outcomes [42, 43].
2. Cartilage development, cellular and extracellular composition and organisation
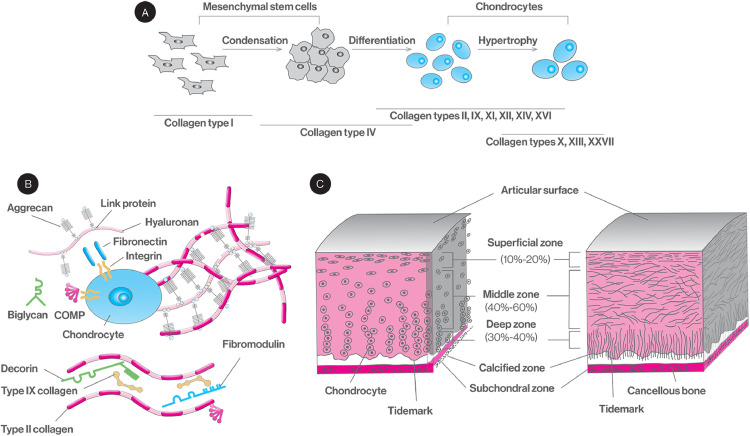
Cartilage morphogenesis [44], [45], [46], [47], [48] is a complex and well-orchestrated sequence of numerous intracellular and extracellular spatiotemporal events, responsible for the tissue composition and structure (Fig. 1). Articular cartilage and long bones are formed by endochondral ossification that is initiated from the lateral growth plate that contains mesenchymal stem cells that secrete hyaluronan and collagen type I. As these stem cells move towards the centre of the limb, they aggregate; stop proliferating and expressing collagen type I; and start expressing N-cadherin, tenascin-C and cell adhesion molecules. Formation of tight aggregates marks the start of the condensation processes that involves stem cell aggregation and increased hyaluronidase activity that in turn decreases hyaluronan and cell movement and increases cell-cell interactions. These increased cell-cell interactions trigger signalling pathways responsible for the initiation of chondrogenic differentiation. An array of small proteoglycans (PGs, e.g. versican, perlecan), growth factors (e.g. fibroblast growth factors, FGF; bone morphogenetic proteins, BMPs; transforming growth factor-β, TGF-β), transcription factors (e.g. Sox5, Sox6, Sox9), signalling molecules (e.g. sonic hedgehog, Indian hedgehog) and ECM molecules (e.g. matrilins, fibronectin) contribute in chondrogenic differentiation and cartilage formation. Subsequently, the cells cease the expression of adhesion molecules, resume proliferation via action of growth hormone, parathyroid hormone-related peptide and insulin-like growth factor-1 (IGF-1), initiate ECM synthesis (e.g. collagen types II, IX, X and XI; aggrecan; decorin; annexin II, V and VI; tenascins; thrombospondins; cartilage oligomeric matrix protein) and decrease production of fibronectin. A series of maturation steps then takes place for the differentiation of committed chondrocytes to pre-hypertrophic, hypertrophic and matrix-mineralising chondrocytes. Hypertrophic chondrocytes increase in size, start to synthesise calcified matrix rich in collagen type X and alkaline phosphatase, synthesise an array of terminal differentiation molecules (e.g. matrix metalloproteinase-13; Runx2, Runx3, BMP-6, BMP-2, BMP-7, aggrecan, hyaluronan) and cease to synthesise others (e.g. Sox9, collagen type II).
Fig. 1.
Sequential stem cell differentiation to chondrocytes and associated ECM changes (A). Chondrocyte secreted ECM and interactions (B). Chondrocyte cellular (left) and extracellular (right) organisation (C).
Articular cartilage contains a highly specialised cell population, the chondrocytes, which is responsible for the production, organisation and maintenance of the cartilage ECM [49, 50]. It also contains a small number of mesenchymal progenitor cells, the number of which increases in osteoarthritic articular cartilage [51, 52]. A diverse range of collagen types are encountered in cartilage with distinct functions [53], [54], [55], [56]. For example, collagen type II is the predominant component of the cartilage ECM, forms a fibrillar network primarily responsible for the mechanical integrity of the tissue and plays a significant role in chondrocyte differentiation and hypertrophy during normal cartilage development and OA pathogenesis [57, 58]. Loss of collagen type II has been shown to accelerate chondrocyte hypertrophy and OA progression, through the BMP-SMAD1 pathway [59]. Collagen type III is extensively crosslinked to collagen type II and regulates collagen fibrillar structure and biomechanics in cartilage tissue [60, 61]. Collagen type VI is expressed in both healthy and OA cartilage tissues, is the major component of the chondrocyte pericellular matrix and enhances cartilage regeneration via stimulation of chondrocyte proliferation [62, 63]. Collagen type IX covalently crosslinks to collagen type II with the collagenous (called COL3) and the non-collagenous (called NC4) domains of the molecules projecting at periodic distances away from the surface of the fibril. These projections allow it to interact with numerous components of cartilage tissue (e.g. cartilage oligomeric protein, heparin, fibromodulin), ultimately stabilising and organising the fibrillar collagen network in cartilage [64], [65], [66], [67], [68]. Collagen type X is a short chain, non-fibril-forming collagen, primarily synthesised by hypertrophic chondrocytes, that enables endochondral ossification by regulating matrix mineralisation and is essential for mesenchymal stem cell cartilage formation and endochondral ossification [69], [70], [71], [72], [73]. Collagen type XI interacts with various cartilage components (e.g. collagen type II, collagen type IX, perlecan, heparan sulphate) to form a meshwork that provides cartilage matrix stabilisation, mechanical resilience and homeostasis. The ratio of collagen type XI to collagen type II regulates fibre diameter, with thick fibres having more collagen type II [74], [75], [76], [77]. Articular cartilage also contains a variety of PGs (e.g. aggrecan, decorin, biglycan and fibromodulin) and glycosaminoglycans (GAGs, e.g. chondroitin sulphate, keratan sulphate and hyaluronan) that represent ∼ 10% of tissue dry weight, subject to age and disease state. PGs and GAGs play significant role in both normal tissue function and arthritis manifestation and progression [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89]. Indeed, physiological PGs and GAGs synthesis and composition contribute towards normal cartilage function and properties; control the release and protect against proteolysis of bounded cytokines, chemokines and growth factors; and modulate various signalling cascades that facilitate cell attachment and motility and cell-cell and cell-ECM interactions. For example, under physiological conditions, the major PG found in cartilage is aggrecan that interacts with hyaluronan to occupy the interfibrillar space of the cartilage ECM and provide cartilage with its osmotic properties to resist compressive loads [90]. On the other hand, in arthritis, PGs and GAGs are significantly degraded by matrix metalloproteinases and their breakdown products are released into synovial fluid, eliciting an inflammatory response [91], [92], [93]. Overall, ECM synthesis and degradation are regulated by the change of chondrocyte proliferation and metabolism under normal and OA conditions [94], [95], [96], [97], [98]; the effect of hormones [99], [100], [101] and growth factors [102], [103], [104]; aging [105], [106], [107]; oxygen tension [108], [109], [110], [111]; and mechanical loading [112], [113], [114], [115], [116].
Structurally speaking, articular cartilage is divided into the superficial, transitional, radial and calcified zones from the joint surface to the subchondral bone, with distinct composition and architectural features [117], [118], [119], [120]. The superficial zone is the gliding surface of the joint; contains high concentration of collagen and low concentration of PGs; and adjoins a layer of elongated chondrocytes organised parallel to the articular surface. The transitional zone contains collagen fibres larger than those in the superficial zone, which are arranged randomly within this zone; is composed of spheroid-shaped chondrocytes; and has higher concentration of PGs compared to the superficial zone. The radial zone contains the largest collagen fibres, which are organised in a columnar pattern, perpendicularly to the joint surface; has the lowest concentration of chondrocytes; and has high PG content. Between the radial and the calcified zone, there is a wavy and irregular line, termed tidemark, that prevents the collagen fibres from being sheared of anchorage to the calcified zone. The calcified zone separates the radial zone of cartilage from subchondral bone ensuring a cohesive connection between them; has no PGs; and contains spheroid-shaped chondrocytes, which present a hypertrophic phenotype and synthesise collagen type X.
3. Collagen type II biosynthesis, extraction and synthesis via recombinant technologies
Collagen type II exhibits a triple stranded, coiled rod-like structure, is expressed as a homotrimer (i.e. [a1(II)]3) and is synthesised exclusively by chondrocytes [121, 122]. During synthesis, the procollagen chains undergo proline and lysine hydroxylation by prolyl-3-hydroxylase, prolyl-4-hydroxylase and lysyl hydroxylase [123]. The modification of proline and lysine hydroxylation requires ascorbic acid, iron and 2-oxo-glutarate. These steps occur prior to the formation of the triple helical structure as hydroxyproline is critical for the stabilisation of the collagen triple helix. Prolyl-4-hydroxylase triggers protein disulphide-isomerase activity, which leads to the formation of the collagen triple helix in the endoplasmic reticulum, as the association of collagen chains requires correct disulphide bond formation in the C-propeptide region of procollagen. Lysyl hydroxylase catalyses the hydroxylation of proline and lysine at both helical and non-helical regions of procollagen polypeptide chains. Moreover, some of the hydroxylysine residues then undergo glycosylation mediated by hydroxylysyl galactosyltransferase and galactosylhydroxylysyl glucosyltransferase [124, 125]. Procollagen processing and crosslinking occurs in the extracellular space. The C-terminal and N-terminal non-helical propeptides of secreted procollagen molecules are removed by procollagen C-proteinases and members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family of proteases (N-proteinases). Consequently, the removal of propeptides results in a decreased solubility of procollagen molecules, which assemble into a collagen triple helical structure with a higher organisation. Subsequent intra- and inter- molecular crosslinking enables the formation of insoluble and closely packed fibrils of 50 nm in diameter, able to withhold mechanical loads [126, 127].
Over the years, various tissues have been used and extraction protocols have been proposed to obtain collagen type II, albeit with variable degree of efficiency with respect to purity, from terrestrial and marine species. From the terrestrial species, bovine [128, 129], porcine [130, 131] and chicken [132, 133] tissues are preferred for collagen type II extraction. It is interesting to note that it has been suggested that collagen type II retains memory of the tissue that is extracted from, with articular cartilage derived collagen type II to be more effective than auricular and tracheal cartilage derived collagen type II in inducing chondrogenic differentiation of human stem cells [131]. With respect to marine species, squid [134], jellyfish [135, 136], amur sturgeon [137], hoki [138] and chondrichthyes (e.g. sharks [139], [140], [141], skates [142], rays [143]), a diverse group of cartilaginous fish that lack true bone and exhibit a skeleton solely comprised of unmineralized cartilage, have been used for collagen type II extraction. In general, high yield, pure collagen type II preparations are produced by acid solubilisation, pepsin digestion and repeated salt precipitation / acid solubilisation and finally dialysis methods (Fig. 2). Considering though the antibiotic usage in animal breeding, religious tenets and the potential for interspecies disease transmission [144], [145], [146], recombinant collagen technologies have been developed for biomedical applications [147], [148], [149]. In this frontier, cells [150], [151], [152], yeast [153] and baculovirus-silkworm [154] systems have been used to express procollagen type II. Compared with the yeast expression system, the insect cell expression system has lower background interference and facilitates post-translational processing and modification [154, 155]. Nonetheless, recombinant technologies are still of low yield and are primarily utilised for niche biomedicine areas [156].
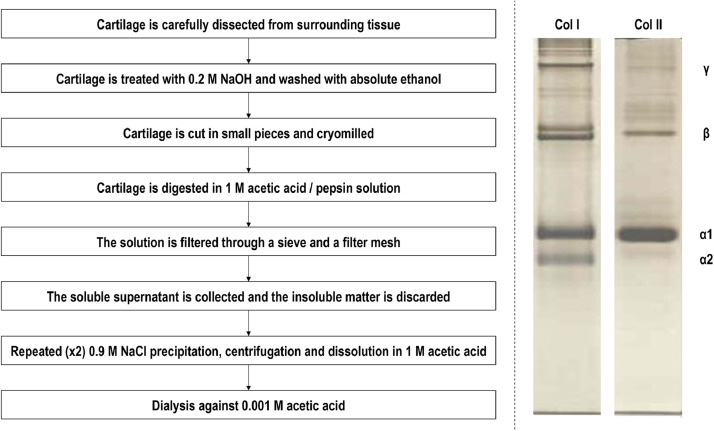
Fig. 2.
Collagen type II extraction flow chart from cartilage tissues (the detailed protocol can be found here [131, 143]) and electrophoretic mobility of collagen type I and collagen type II (the detailed protocol can be found here [223]). Notes: As the same protocol is used to extract collagen type I [224], [225], [226], attention should be paid during dissection to remove all not cartilaginous tissues. Tissue to acetic acid / pepsin solution ratio: 1:1 g/l. Tissue to pepsin ratio: 10:1 w/w. High activity pepsin (e.g. 3200-4500 units per mg protein) is recommended. Sieve: approximately 1,000 μm in diameter. Filter mesh: 100 μm in diameter. Centrifugation details: 20 min, 8000 rpm, < 8 °C. After the second NaCl precipitation, dissolution is conducted in minimum amount of acetic acid in order to produce a high in concentration collagen type II solution. All experiments are conducted at 4-8 ºC to avoid collagen denaturation.
4. Collagen type II scaffolds in cartilage engineering
Mammalian, marine and recombinant collagen type II-based hydrogels and sponges (primary scaffold conformations used in cartilage engineering) have been shown to both maintain and induce chondrogenic phenotype in vitro (Table 1). To enhance mechanical integrity, crosslinking (e.g. poly(ethylene glycol) ether tetrasuccinimidyl glutarate [157], carbodiimide [158], [159], [160], genipin [161]) and/or blending with other polymers (e.g. polyvinyl alcohol [162], poly(L-lactide) and poly(lactide-co-glycolide) [163, 164], chitosan [165]) is traditionally employed. Although structural configurational differences between collagen type II hydrogels and sponges have been shown to induce different chondrocyte response (with respect to morphology, proliferation and gene expression), in the end, both scaffolds have been shown to stimulate comparable chondrogenesis [166]. It is also worth noting that collagen type II electrospun scaffolds have also been developed [167], [168], [169], but the unavoidable denaturation of collagen prior or during the process [170, 171] has restricted their use. Advances in engineering (e.g. bioprinted collagen type II hydrogels with cell density gradient [172], alginate / collagen type II microbeads [173], hyaluronic acid / collagen type II microspheres [174]) and/or functionalisation technologies (e.g. chondroitin sulphate [175, 176], hyaluronic acid [158], glycosaminoglycan [177]) have made available elegant collagen type II scaffolds with enhanced in vitro chondrogenic potential. In preclinical setting, collagen type II scaffolds (with / without functional molecules and/or with / without cells) have been shown to stimulate hyaline neocartilage formation in chondral and osteochondral defects of a diverse range of animal species (Table 2).
Table 1.
Indicative examples of mammalian, marine and recombinant collagen type II scaffolds that have been shown to maintain and/or induce chondrogenic phenotype in vitro. Abbreviations: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide-sulfo-N-hydroxy-succinimide: EDC-NHS; Adipose derived stem cells: ADSCs; Bone marrow stem cells: BMSCs; Cartilage oligomeric matrix protein: COMP; Chondroitin sulphate: CS; Dehydrothermal: DHT; Extracellular matrix: ECM; Fibroblast growth. factor: FGF; Glycosaminoglycan: GAG; Hyaluronan: HA; Insulin-like growth factor: IGF; Matrix metalloproteinase: MMP; Proteoglycan: PG; Sex-determining region Y-type box transcription factor 9: SOX9; Ultraviolet irradiation: UV.
| Scaffold conformation | Major findings, Reference |
|---|---|
| Bovine collagen type II, CS and HA spongesGenipin crosslinkedHuman chondrocytes | Chondrocytes maintained round morphology after 14 days of culture. Increased gene expression of aggrecan, collagen type II and COMP and greater accumulation of PGs was seen on scaffolds with CS and HA than those without CS and HA [161] |
| Bovine collagen type II spongesGenipin crosslinkedHuman chondrocytes | The administration of GAGs to culture medium improved cell differentiation tendency to functional hyaline cartilage, as evidenced by the upregulation of GAG biosynthesis rate and gene expression of aggrecan and collagen type II after 28 days of culture [201] |
| Bovine collagen type II and CS spongesNo crosslinkerHuman BMSCs | The cells produced abundant collagen type II on type II scaffolds and collagen type I on type I scaffolds. The addition of CS upregulated the gene expression of collagen type II, compared to type I and type II alone scaffolds [175] |
| Bovine collagen type II and CS spongesEDC-NHS crosslinkedBovine chondrocytes | Chondrocytes maintained round morphology, the cells loaded scaffolds were surfaced with a cartilaginous-like layer and collagen type II scaffolds contained occasionally clusters of cells inside the sponges in contrast to collagen type I sponges after 14 days of culture [202] |
| Bovine collagen type II spongesUV crosslinkedMurine chondrocytes | The primary chondrocytes in the scaffolds maintained chondrogenic phenotype after 3 weeks of culture. The gene expression of collagen type II, collagen type XI, and SOX9 in de-differentiated chondrocytes cultured in the scaffolds decreased when compared to that in primary chondrocytes after 4 weeks of culture [203] |
| Bovine collagen type II coated chitosan fibresPolyglycolic acid mesh was used as a reference groupNo crosslinkerMurine BMSCs | The cell number, the matrix production (dry weight, GAG quantifications), and the chondrogenic marker gene expression (aggrecan, collagen type II) were upregulated in collagen type II coated chitosan scaffolds compared to pure chitosan scaffolds and polyglycolic acid scaffolds after 21 days of culture [165] |
| Porcine collagen type II hydrogelsNo crosslinkerRabbit chondrocytes | Chondrocytes maintained chondrogenic phenotype and the cell density gradient distribution resulted in a ECM gradient distribution in the scaffolds after 3 weeks of culture [172] |
| Porcine collagen type II and CS hydrogelsEDC-NHS crosslinkedRabbit chondrocytes | Chondrocytes maintained round morphology, the collagen fibres became thicker and arranged neatly with the increase of CS in the scaffolds and displayed periodic alternation of light and shade after 7 days of culture [176] |
| Porcine collagen type II and GAG sheetsDHT and carbodiimide crosslinkedCanine chondrocytes | The addition of 5 ng/ml FGF-2 to the culture medium increased the biosynthetic activity of the cells and the accumulation of GAGs compared to the addition of 25 ng/ml FGF-2, 100 ng/ml IGF-1, 5 ng/ml FGF-2 plus 100 ng/ml IGF-1 after 2 weeks of culture [177] |
| Porcine collagen type II spongesEDC/NHS crosslinkedCanine chondrocytes | Most of the chondrocytes were around the periphery of the sponges, the cells tend to be elongated along the periphery of the scaffolds and round inside the scaffolds. α-smooth muscle actin is present in the cytoplasm of the cells after 4 weeks of culture [200] |
| Porcine collagen type II spongesUV crosslinkedCanine chondrocytes | Chondrocytes maintained chondrogenic morphology and displayed less shrinkage, higher biosynthetic activity and more hyaline cartilage-like tissue formation compared to collagen type I scaffolds after 21 days of culture [204] |
| Porcine collagen type II and GAG spongesUV crosslinkedCanine chondrocytes | After 4 weeks of culture, a range of pore diameter from 25-257 μm did not affect cell-mediated scaffold contraction and α-smooth muscle actin was present in the cytoplasm of the seeded chondrocytes [205] |
| Porcine collagen type II and GAG sheetsDHT and EDC-NHS crosslinkedCanine chondrocytes | Scaffolds with low cross-link densities (DHT and low EDC/NHS treatment) enhanced cell proliferation, chondrogenic maintenance and collagen type II synthesis and increased the rate of scaffold degradation compared to scaffolds with high cross-link densities (high EDC/NHS treatment) after 2 weeks of culture [206] |
| Porcine collagen type II sheetsDHT and EDC-NHS crosslinkedCanine chondrocytes | Static compressions of 50% strain decreased the biosynthetic activity of the chondrocytes (the accumulation rate of 3H-proline-labeled protein and 35S-sulfate-labeled PG over a 24 h period). Dynamic compression (3% strain, 0.1 Hz superimposed on 10% strain offset) upregulated protein and PG biosynthesis compared to statically compressed and uncompressed controls after 7 days of culture [207] |
| Porcine male and female and articular, tracheal and auricular cartilage collagen type II sponges 4-arm polyethylene glycol succinimidyl glutarateHuman ADSCs | Articular cartilage derived sponges exhibited significantly higher resistance to enzymatic degradation and biomechanical properties in comparison to tracheal and auricular cartilage sponges. Articular cartilage sponges induced the highest sulphated GAG synthesis and aggrecan and collagen type II mRNA expression [208] |
| Chicken collagen type II and chondroitin sulphate spongesEDC-NHS crosslinkedRabbit chondrocytes | Chondrocytes maintained round morphology. The cell proliferation, the accumulation of proteoglycans and collagen type II were enhanced in collagen type II and CS scaffolds compared to pure collagen type II scaffolds after 14 days of cell culture. A cartilaginous-like layer was formed at the periphery of the scaffolds [160] |
| Chicken collagen type II hydrogelsNo crosslinkerRabbit chondrocytes | The cells in collagen type II scaffolds maintained chondrogenic phenotype and displayed increased PGs synthesis compared to the cells on polystyrene. > 50% of the newly synthesized PGs were recovered from collagen type II scaffolds compared to 13-16% of those recovered from polystyrene [209] |
| Squid collagen type II coatingNo crosslinkerMurine chondrocytes | Chondrocytes cultured in the conditioned medium from collagen type II treated M1 macrophages mostly maintained round morphology and displayed mild increase in the expression of MMP13, compared with those in the conditioned medium from untreated M1 macrophages [134] |
| Lesser spotted dogfish, thorn back ray, cuckoo ray and blonde ray collagen type II sponges 4-arm polyethylene glycol succinimidyl glutarateHuman ADSCs | The lesser spotted dogfish sponges induced the highest collagen α1(I), collagen α1(III) (in comparison to thorn back ray and blonde ray), COMP (in comparison to cuckoo ray and blonde ray) and aggrecan (in comparison to cuckoo ray) gene expression, indicative of highest chondrogenic induction potential [131] |
| Recombinant human collagen type II hydrogelsNo crosslinkerHuman BMSCs | The cells in the scaffolds displayed similar GAGs deposition and similar chondrogenic marker gene expression and upregulated gene expression of metallopeptidases compared to the high-density cell pellet after 84 days of culture [210] |
| Recombinant human collagen type II hydrogelsNo crosslinkerBovine chondrocytes | Chondrocytes maintained round morphology and the accumulation of GAGs and collagen type II increased after 4 weeks of culture. The gene expression of aggrecan and collagen type II was increased since week 1 [211] |
| Collagen type II (species is not mentioned) hydrogelsNo crosslinkerRabbit chondrocytes | The dedifferentiated auricular chondrocytes were converted to articular chondrogenic phenotype in a collagen type II coated environment after 14 days of culture. The converted auricular chondrocytes expressed similar histological and biomechanical features as articular chondrocytes in the scaffolds after 28 days of culture [212] |
Table 2.
Indicative examples of mammalian, marine and recombinant collagen type II scaffolds in preclinical models. Abbreviations: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide-sulfo-N-hydroxy-succinimide: EDC-NHS; Adipose derived stem cells: ADSCs; Chondroitin sulphate: CS; Dehydrothermal: DHT; Bone marrow stem cells: BMSCs; Glycosaminoglycan: GAG; Osteoarthritis: OA; Ultraviolet irradiation: UV
| Scaffold conformation | Model, Major findings, Reference |
|---|---|
| Bovine collagen type II, cadherin 11 and recombinant fibronectin spongesGlutaraldehyde crosslinkedRabbit BMSCs | Rabbit chondral defectThe cell loaded scaffold induced cartilage formation 12 weeks post-surgery [213] |
| Bovine collagen type II and CS spongesGenipin crosslinkedRabbit BMSCs | Rabbit chondral defectLacuna formation 4 weeks post-surgery and high collagen type II and aggrecan and low collagen type I gene expression 24 weeks post-surgery [214] |
| Bovine collagen type I and collagen type II and CS spongesCarbodiimide crosslinkedNo cells | Rabbit chondral defectCollagen type I scaffolds attracted progenitor cells into the defect and induced fibro-cartilage repair, whilst collagen type II scaffolds attracted less cells into the defected, but the invaded cells adopted a chondrogenic phenotype and increased the amount of superficial cartilage-like tissue 12 weeks post-surgery [188] |
| Bovine collagen type II hydrogelsPentaerythritol polyethylene glycol ether tetrasuccinimidyl glutarate crosslinkedRabbit chondrocytes | Rabbit chondral defectCartilage repair was improved in cell-scaffold treated groups and collagen type I was not detected 24 weeks post-surgery [215] |
| Bovine collagen type II spongesGenipin crosslinkedRabbit BMSCs | Rabbit osteochondral defectThe implanted cells became chondrocytes in the implanted area and cartilage structure, same as normal cartilage, was observed in the repair site 24 weeks post-surgery [216] |
| Porcine collagen type I, collagen type II and collagen type III blend spongesNo crosslinkerAutologous ovine chondrocytes | Ovine chondral defectScaffolds with chondrocytes and with microfracture into the subchondral plate resulted in hyaline-like cartilage regeneration 16 weeks post-surgery [217] |
| Porcine collagen type II spongesEDC-NHS crosslinkedAutologous chondrocytes | Canine chondral defectScaffolds cultured with chondrocytes for 4 weeks prior implantation increased the amount of reparative hyaline cartilage tissue after 15 weeks [200] |
| Porcine collagen type II or Arg-Gly-Asp sequence with poly(L-lactide) or poly(D,L-lactide-co-glycolide) spongesCarbodiimide crosslinkedRabbit chondrocytes | Rabbit chondral defectCollagen type II prevented infiltration by host tissue and capsule formation, showed no inflammation and resulted in partial or full repair with equal cellularity and 75-80% matrix contents of a normal rabbit articular cartilage 8 weeks post-surgery [218] |
| Porcine collagen type II sponges and filmsUV crosslinkedAutologous canine chondrocytes | Canine chondral defectTotal defect filling ranged 56-86%, with the greatest amount found in scaffolds with cells and microfracture compared to scaffolds alone with microfracture and microfracture alone 15 weeks post-surgery, the tissue filling the defect was predominantly fibrocartilage [199] |
| Porcine collagen type II spongesNo crosslinkerNo cells | Rabbit chondral defectScaffolds displayed quicker effusion absorption, greater newly formed cartilage-like areas than the empty group 18 weeks post-surgery, sporadic cartilage signals first appeared at 6 weeks in the scaffolds [219] |
| Collagen type II hydrogelsNo crosslinkerRabbit chondrocytes | Rabbit osteochondral defectCells seeded collagen type II hydrogels displayed better cartilage repair compared to sham, cell pellet and scaffolds alone groups [212] |
| Collagen type II-GAG sponges reconstituted from porcine cartilage and bovine collagen type I sponges with shark CSDHT and UV crosslinkedAutologous canine chondrocytes | Canine chondral defectBoth cell-seeded scaffolds exhibited comparable cartilage regeneration potential and increased cartilaginous tissue in chondral defects and adjacent subchondral bone space compared to empty group 15 weeks post-surgery [189] |
| Chicken collagen type II and fibrin sealant hydrogelsNo crosslinkerHuman ADSCs | Rabbit chondral defectImproved overall repair of chondral defects, cellular organisation and collagen fibre alignment 12 weeks post-surgery [220] |
| Chicken collagen type II and rat collagen type I blend hydrogelsNo crosslinkerAutologous rabbit BMSCs | Rabbit chondral defectCell-seeded collagen type I/II scaffolds exhibited better cartilage repair outcomes in trochlear groove defects compared to pure collagen type I hydrogels and empty chondral defects 13 weeks post-surgery [187] |
| Squid collagen type II intra-articular injectionNo crosslinkerNo cells | Suppressed pro-inflammatory macrophage phenotype, prevented hypertrophic chondrocyte phenotype and alleviated inflammation in an OA rat model 6 weeks after OA induction [134] |
| Shark collagen type II was administered orallyNo crosslinkerNo cells | Facilitated recovery of articular membranes in the ankle joint and suppressed rheumatoid arthritis in a complete Freund's adjuvant-induced rheumatoid arthritis rat model 2 weeks after rheumatoid arthritis induction [221] |
| Recombinant collagen type II hydrogelsNo crosslinkerAutologous rabbit chondrocytes | Rabbit osteochondral defectCell-scaffold treated group exhibited a slight but insignificant improvement in cartilage repair compared to spontaneous repair group and both groups had lower modified O'Driscoll's score than intact cartilage 24 weeks post-surgery [222] |
| Recombinant collagen type II and polylactide spongesCarbodiimide crosslinkedAutologous porcine chondrocytes | Porcine chondral defectHyaline cartilage formed most frequently in the recombinant collagen type II / polylactide / cells group, which also improved biomechanically properties only over the spontaneous repair group and showed less adverse subchondral reactions than the Chondro-Gide® (a bilayer collagen type I / collagen type III membrane) / cells group, but not in comparison to the spontaneous repair group 16 weeks post-surgery [190] |
Despite all the available data-to-date that have comprehensively shown the importance of collagen type II in chondrogenic induction or maintenance and in cartilage repair and regeneration, numerous studies still utilise collagen type I in cartilage engineering [178], [179], [180], [181]. This is surprising, as the clear superiority of collagen type II over collagen type I in cartilage engineering has been well-documented in the literature, possibly due to biochemical signals (i.e. the lack of collagen type I and the presence of collagen type II and other bounded cartilage-specific constituents) [131]. In in vitro setting, for example, collagen type II, as opposed to collagen type I, scaffolds have been shown to maintain round chondrocyte morphology and to significantly increase DNA and collagen type II and GAG synthesis [182, 183]. Collagen type II, as opposed to collagen type I, scaffolds have also been shown to more effectively induce chondrogenic induction of adipose derived stem cells [184] and bone marrow stem cells [175, 185], as judged by round cell morphology (via the integrin β1-mediated Rho A/Rock signalling pathway [184]), upregulation of chondrogenic genes (e.g. collagen type II, collagen type X, aggrecan, COMP, SOX6, SOX9) and increased synthesis of cartilage matrix (e.g. collagen type II, PG, GAG). It is also worth noting that increased collagen type II, as opposed to collagen type I, scaffolds induced differentiation of adipose derived stem cells to nucleus pulposus cells, as judged by increased SOX9, aggrecan and collagen type II gene expression; increased sulphated PG synthesis; expression of KRT19 marker; and increased phosphorylated Smad3 expression [186]. These in vitro observations were also verified in preclinical models. For example, in cartilage defects in the femurs of rabbits, 13 weeks post implantation, collagen type I / collagen type II hydrogels showed statistically higher cartilage repair score than either collagen type I alone hydrogels (both loaded with bone marrows stem cells) or empty defect controls (pure collagen type II hydrogels were not used) [187]. In full-thickness defects in the femoral trochlea of adolescent rabbits, although collagen type I scaffolds induced higher than collagen type II scaffolds cell migration into the defect, the collagen type II scaffolds more effectively than collagen type I scaffolds directed invaded cells towards chondrocyte phenotype and 12 weeks post implantation, the cartilage contours in defects with collagen type I scaffolds were repaired with fibro-cartilage tissue, whilst defects treated with collagen type II scaffolds, although the original contour was not completely restored in all animals, showed an increase in the amount of superficial cartilage-like tissue [188]. In the trochlea grooves of the knees of dogs, 15 weeks post implantation, groups treated with collagen type II scaffolds and chondrocytes showed the greatest total amount of reparative tissue and the tissue at subchondral region of defects was positive for collagen type II, GAGs and PGs [189]. In a 4-month-old domestic pig full-thickness cartilage lesion model, 4 months post operation, a human recombinant collagen type II / polylactide scaffold most frequently formed hyaline cartilage than the spontaneous healing group and a collagen type I / collagen type III scaffold [190].
In the commercial arena, it is interesting to note that, to the best of our knowledge, only a handful of companies provide high purity collagen type II (e.g. porcine articular cartilage derived collagen type II, Symatese) and that no single collagen type II-based device is available, whilst numerous collagen type I devices are available for cartilage engineering (e.g. Chondro-Gide®, Geistlich Pharma AG; Novocart® Basic, TETEC AG; MeRG®, Bioteck Srl), despite the fact that collagen type I scaffolds have failed to demonstrate efficiency in healing of human osteochondral [43] and large cartilage [42] defects. This limited technology transfer of collagen type II can be attributed to two main reasons. Firstly, we believe that commercialisation of collagen type II-based devices has been compromised by early studies that showed native collagen type II from human, chick, murine and bovine cartilage to induce inflammatory arthritis in rats [191], [192], [193] and in non-human primates [194]; and antibodies of native and denatured collagen type II to be present in patients with early rheumatoid arthritis and chronic gouty arthritis [195], [196], [197]. It is worth noting though that effectively crosslinked collagen type II does not induce arthritis in rats [198] and studies have demonstrated collagen type II devices to promote efficient defect filling and hyaline neocartilage formation. For example, 15 weeks post operation, defects in trochlear grooves of adult dogs (that were treated with microfracture, microfracture with collagen type II scaffold and collagen type II scaffold loaded chondrocytes) resulted in 56% to 86% total defect filling, with the microfracture with collagen type II scaffold treatment group inducing the highest defect filling capacity [199]. When collagen type II scaffold were seeded for 4 weeks with chondrocytes (after 3 weeks of monolayer expansion) and then implanted in a canine trochlear groove defect model, 15 weeks post implantation, although the repaired tissue formed had significantly lower compressive stiffness than the native cartilage, the total defect filling ranged from 70% to 100%, with hyaline cartilage accounting for 42 ± 10% of the defect area [200]. The second issue that may be responsible for the limited use of collagen type II in medical device development is the difficulty in producing high amounts of high purity and high yield, all in comparison to collagen type I, collagen type II preparations. Obviously, the main reason behind this is the articular cartilage tissue availability, in comparison to skin, for example, tissue. Having said that, a typical cartilage defect is a lot smaller than a typical skin defect and therefore extraction of collagen type II constitutes a value for money proposition.
5. Conclusions
Cartilage injuries and pathophysiologies continuously increase and financially drain healthcare systems worldwide. In the quest of the optimal building block for cartilage engineering scaffolds, collagen type II has been overlooked due to either outdated data or economic drivers, despite being the most abundant extracellular matrix component of cartilage. This review clearly illustrates the beneficial effects of collagen type II in cartilage engineering and urges the adaptation of a more rational and biomimetic approach in designing biomaterial-based therapeutic strategies for functional cartilage repair and regeneration.
Conflict of Interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work forms part of the Teagasc Walsh Fellowship (grant agreement No. 2014045) and the ReValueProtein Research Project (grant agreement No. 11/F/043) supported by the Department of Agriculture, Food and the Marine under the National Development Plan 2007-2013 funded by the Irish Government. This publication has emanated from research supported by grants from Science Foundation Ireland (SFI) under grant numbers 15/CDA/3629 and 19/FFP/6982 and Science Foundation Ireland (SFI) and European Regional Development Fund (ERDF) under grant number 13/RC/2073_2. This work has also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme, grant agreement No. 866126.
References
- 1.Kazemnejad S., Khanmohammadi M., Baheiraei N., Arasteh S. Current state of cartilage tissue engineering using nanofibrous scaffolds and stem cells. Avicenna J Med Biotechnol. 2017;9:50–65. [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg A., Mitchell K., Soans J., Kim L., Zaidi R. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res. 2017;12:39. doi: 10.1186/s13018-017-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly P., O'Connor J. Transmission of rapidly applied loads through articular cartilage Part 1: Uncracked cartilage. Proc Inst Mech Eng H. 1996;210:27–37. doi: 10.1243/PIME_PROC_1996_210_388_02. [DOI] [PubMed] [Google Scholar]
- 5.Pickett A.M., Hensley Jr D.T. Knee cell-based cartilage restoration. J Knee Surg. 2019;32:127–133. doi: 10.1055/s-0038-1676378. [DOI] [PubMed] [Google Scholar]
- 6.Reissis D., Tang Q.O., Cooper N.C., Carasco C.F., Gamie Z., Mantalaris A., Tsiridis E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther. 2016;16:535–557. doi: 10.1517/14712598.2016.1145651. [DOI] [PubMed] [Google Scholar]
- 7.Poole A.R. Osteoarthritis as a whole joint disease. HSS J. 2012;8:4–6. doi: 10.1007/s11420-011-9248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson A.K., Rawle R.A., Adams E., Greenwood M.C., Bothner B., June R.K. Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem Biophys Res Commun. 2018;499:182–188. doi: 10.1016/j.bbrc.2018.03.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 10.Kotlarz H., Gunnarsson C.L., Fang H., Rizzo J.A. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 11.Fu K., Robbins S.R., McDougall J.J. Osteoarthritis: the genesis of pain. Rheumatology. 2018;57 doi: 10.1093/rheumatology/kex419. iv43-iv50. [DOI] [PubMed] [Google Scholar]
- 12.Horton W., Bennion P., Yang L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:379–381. [PubMed] [Google Scholar]
- 13.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–420. doi: 10.1055/s-0040-1712946. PMID: 32483798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman A.B., Haggerty C.L., Goodpaster B., Harris T., Kritchevsky S., Nevitt M., Miles T.P., Visser M. Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 15.Coggon D., Reading I., Croft P., McLaren M., Barrett D., Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25:622–627. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 16.Silverwood V., Blagojevic-Bucknall M., Jinks C., Jordan J., Protheroe J., Jordan K. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:507–515. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Harris E.C., Coggon D. Hip osteoarthritis and work. Best Prac Res Clin Rheumatol. 2015;29:462–482. doi: 10.1016/j.berh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turkiewicz A., Petersson I.F., Björk J., Hawker G., Dahlberg L.E., Lohmander L.S., Englund M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthritis Cartilage. 2014;22:1826–1832. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Arden N., Nevitt M.C. Osteoarthritis: Epidemiology. Best Prac Res Clin Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., Callahan L., Copenhaver C., Dodge C., Felson D. American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2019;72(2020):149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson A.E., Allen K.D., Golightly Y.M., Goode A.P., Jordan J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the US bone and joint initiative. Semin Arthritis Rheum. 2014;43:701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 22.da Costa B.R., Reichenbach S., Keller N., Nartey L., Wandel S., Jüni P., Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 23.Zeng C., Wei J., Persson M.S., Sarmanova A., Doherty M., Xie D., Wang Y., Li X., Li J., Long H. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642–650. doi: 10.1136/bjsports-2017-098043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z.Y., Shi S.Y., Li S.J., Chen F., Chen H., Lin H.Z., Lin J.M. Efficacy and safety of duloxetine on osteoarthritis knee pain: a meta-analysis of randomized controlled trials. Pain Med. 2015;16:1373–1385. doi: 10.1111/pme.12800. [DOI] [PubMed] [Google Scholar]
- 25.Lohmander L.S., Hellot S., Dreher D., Krantz E.F., Kruger D.S., Guermazi A., Eckstein F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66:1820–1831. doi: 10.1002/art.38614. [DOI] [PubMed] [Google Scholar]
- 26.Deshmukh V., Hu H., Barroga C., Bossard C., Kc S., Dellamary L., Stewart J., Chiu K., Ibanez M., Pedraza M. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2018;26:18–27. doi: 10.1016/j.joca.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Sampson E.R., Hilton M.J., Tian Y., Chen D., Schwarz E.M., Mooney R.A., Bukata S.V., O'Keefe R.J., Awad H., Puzas J.E. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra193. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosloski M.P., Goss S., Wang S.X., Liu J., Loebbert R., Medema J.K., Liu W., Dutta S. Pharmacokinetics and tolerability of a dual variable domain immunoglobulin ABT-981 against IL-1α and IL-1β in healthy subjects and patients with osteoarthritis of the knee. J Clin Pharmacol. 2016;56:1582–1590. doi: 10.1002/jcph.764. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z., Ding C., Li T., Yu S.P.-C. Current status and future prospects for disease modification in osteoarthritis. Rheumatology. 2018;57 doi: 10.1093/rheumatology/kex496. iv108-iv123. [DOI] [PubMed] [Google Scholar]
- 30.Kraeutler M.J., Aliberti G.M., Scillia A.J., McCarty E.C., Mulcahey M.K. Microfracture versus drilling of articular cartilage defects: a systematic review of the basic science evidence. Orthop J Sports Med. 2020;8 doi: 10.1177/2325967120945313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao L., Goebel L.K., Orth P., Cucchiarini M., Madry H. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018;11 doi: 10.1242/dmm.034280. dmm034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gowd A.K., Cvetanovich G.L., Liu J.N., Christian D.R., Cabarcas B.C., Redondo M.L., Verma N.N., Yanke A.B., Cole B.J. Management of chondral lesions of the knee: analysis of trends and short-term complications using the national surgical quality improvement program database. Arthroscopy. 2019;35:138–146. doi: 10.1016/j.arthro.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 33.DeFroda S.F., Bokshan S.L., Yang D.S., Daniels A.H., Owens B.D. Trends in the surgical treatment of articular cartilage lesions in the United States from 2007 to 2016. J Knee Surg. 2020 doi: 10.1055/s-0040-1712946. May 29. Epub ahead of print. PMID: 32483798. [DOI] [PubMed] [Google Scholar]
- 34.Graceffa V., Vinatier C., Guicheux J., Stoddart M., Alini M., Zeugolis D.I. Chasing chimeras–the elusive stable chondrogenic phenotype. Biomaterials. 2019;192:199–225. doi: 10.1016/j.biomaterials.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Graceffa V., Vinatier C., Guicheux J., Evans C.H., Stoddart M., Alini M., Zeugolis D.I. State of art and limitations in genetic engineering to induce stable chondrogenic phenotype. Biotechnol Adv. 2018;36:1855–1869. doi: 10.1016/j.biotechadv.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Pourakbari R., Khodadadi M., Aghebati-Maleki A., Aghebati-Maleki L., Yousefi M. The potential of exosomes in the therapy of the cartilage and bone complications; Emphasis on osteoarthritis. Life Sci. 2019;236 doi: 10.1016/j.lfs.2019.116861. [DOI] [PubMed] [Google Scholar]
- 37.Confalonieri D., Schwab A., Walles H., Ehlicke F. Advanced therapy medicinal products: a guide for bone marrow-derived MSC application in bone and cartilage tissue engineering. Tissue Eng Part B. 2018;24:155–169. doi: 10.1089/ten.TEB.2017.0305. [DOI] [PubMed] [Google Scholar]
- 38.Wei W., Ma Y., Yao X., Zhou W., Wang X., Li C., Lin J., He Q., Leptihn S., Ouyang H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact Mater. 2020;6:998–1011. doi: 10.1016/j.bioactmat.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz E.N., Zeugolis D.I. Electrospun polymers in cartilage engineering—state of play. Front Bioeng Biotechnol. 2020;8:77. doi: 10.3389/fbioe.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armiento A., Stoddart M., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Campos Y., Almirall A., Fuentes G., Bloem H.L., Kaijzel E.L., Cruz L.J. Tissue engineering: an alternative to repair cartilage. Tissue Eng Part B. 2019;25:357–373. doi: 10.1089/ten.TEB.2018.0330. [DOI] [PubMed] [Google Scholar]
- 42.Schüttler K.-F., Götschenberg A., Klasan A., Stein T., Pehl A., Roessler P., Figiel J., Heyse T., Efe T. Cell-free cartilage repair in large defects of the knee: increased failure rate 5 years after implantation of a collagen type I scaffold. Arch Orthop Trauma Surg. 2019;139:99–106. doi: 10.1007/s00402-018-3028-4. [DOI] [PubMed] [Google Scholar]
- 43.Christensen B.B., Foldager C.B., Jensen J., Jensen N.C., Lind M. Poor osteochondral repair by a biomimetic collagen scaffold: 1-to 3-year clinical and radiological follow-up. Knee Surg Sports Traumatol Arthrosc. 2016;24:2380–2387. doi: 10.1007/s00167-015-3538-3. [DOI] [PubMed] [Google Scholar]
- 44.Goldring M., Tsuchimochi K., Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 45.Mackie E., Ahmed Y., Tatarczuch L., Chen K., Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Gadjanski I., Spiller K., Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev Rep. 2012;8:863–881. doi: 10.1007/s12015-011-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsang K., Tsang S., Chan D., Cheah K. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res C. 2014;102:52–73. doi: 10.1002/bdrc.21060. [DOI] [PubMed] [Google Scholar]
- 48.Chijimatsu R., Saito T. Mechanisms of synovial joint and articular cartilage development. Cell Mol Life Sci. 2019;76:3939–3952. doi: 10.1007/s00018-019-03191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lotz M., Loeser R.F. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akkiraju H., Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 2015;3:177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fickert S., Fiedler J., Brenner R.E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6:1–11. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alsalameh S., Amin R., Gemba T., Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 53.Eyre D.R., Weis M.A., Wu J.-J. Articular cartilage collagen: an irreplaceable framework. Eur Cell Mater. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 54.Aigner T., Stöve J. Collagens—major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55:1569–1593. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Luo Y., Sinkeviciute D., He Y., Karsdal M., Henrotin Y., Mobasheri A., Önnerfjord P., Bay-Jensen A. The minor collagens in articular cartilage. Protein Cell. 2017;8:560–572. doi: 10.1007/s13238-017-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bielajew B.J., Hu J.C., Athanasiou K.A. Collagen: Quantification, biomechanics and role of minor subtypes in cartilage. Nat Rev Mater. 2020;5:730–747. doi: 10.1038/s41578-020-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C., Rui H., Mosler S., Notbohm H., Sawaryn A., Müller P. Collagen II from articular cartilage and annulus fibrosus: structural land functional implication of tissue specific posttranslational modifications of collagen molecules. Eur J Biochem. 1993;213:1297–1302. doi: 10.1111/j.1432-1033.1993.tb17881.x. [DOI] [PubMed] [Google Scholar]
- 58.Nah H.D., Swoboda B., Birk D.E., Kirsch T. Type IIA procollagen: expression in developing chicken limb cartilage and human osteoarthritic articular cartilage. Dev Dyn. 2001;220:307–322. doi: 10.1002/dvdy.1109. [DOI] [PubMed] [Google Scholar]
- 59.Lian C., Wang X., Qiu X., Wu Z., Gao B., Liu L., Liang G., Zhou H., Yang X., Peng Y. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019;7:1–15. doi: 10.1038/s41413-019-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C., Brisson B.K., Terajima M., Li Q., Han B., Goldberg A.M., Liu X.S., Marcolongo M.S., Enomoto-Iwamoto M., Yamauchi M. Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus. Matrix Biol. 2020;85:47–67. doi: 10.1016/j.matbio.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J.-J., Weis M.A., Kim L.S., Eyre D.R. Type III collagen, a fibril network modifier in articular cartilage. J Biol Chem. 2010;285:18537–18544. doi: 10.1074/jbc.M110.112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smeriglio P., Dhulipala L., Lai J.H., Goodman S.B., Dragoo J.L., Smith R.L., Maloney W.J., Yang F., Bhutani N. Collagen VI enhances cartilage tissue generation by stimulating chondrocyte proliferation. Tissue Eng Part A. 2015;21:840–849. doi: 10.1089/ten.TEA.2014.0375. [DOI] [PubMed] [Google Scholar]
- 63.Pullig O., Weseloh G., Swoboda B. Expression of type VI collagen in normal and osteoarthritic human cartilage. Osteoarthritis Cartilage. 1999;7:191–202. doi: 10.1053/joca.1998.0208. [DOI] [PubMed] [Google Scholar]
- 64.Müller-Glauser W., Humbel B., Glatt M., Sträuli P., Winterhalter K.H., Bruckner P. On the role of type IX collagen in the extracellular matrix of cartilage: Type IX collagen is localized to intersections of collagen fibrils. J Cell Biol. 1986;102:1931–1939. doi: 10.1083/jcb.102.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diab M., Wu J., Eyre D. Collagen type IX from human cartilage: a structural profile of intermolecular cross-linking sites. Biochem J. 1996;314:327–332. doi: 10.1042/bj3140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parsons P., Gilbert S.J., Vaughan-Thomas A., Sorrell D.A., Notman R., Bishop M., Hayes A.J., Mason D.J., Duance V.C. Type IX collagen interacts with fibronectin providing an important molecular bridge in articular cartilage. J Biol Chem. 2011;286:34986–34997. doi: 10.1074/jbc.M111.238188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brewton R., Wright D., Mayne R. Structural and functional comparison of type IX collagen-proteoglycan from chicken cartilage and vitreous humor. J Biol Chem. 1991;266:4752–4757. [PubMed] [Google Scholar]
- 68.Blumbach K., Bastiaansen-Jenniskens Y., DeGroot J., Paulsson M., Van Osch G., Zaucke F. Combined role of type IX collagen and cartilage oligomeric matrix protein in cartilage matrix assembly: cartilage oligomeric matrix protein counteracts type IX collagen–induced limitation of cartilage collagen fibril growth in mouse chondrocyte cultures. Arthritis Rheum. 2009;60:3676–3685. doi: 10.1002/art.24979. [DOI] [PubMed] [Google Scholar]
- 69.Von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 70.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11–17. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 71.Aigner T., Reichenberger E., Bertling W., Kirsch T., Stöss H., Von der Mark K. Type X collagen expression in osteoarthritic and rheumatoid articular cartilage. Virchows Arch B. 1993;63:205–211. doi: 10.1007/BF02899263. [DOI] [PubMed] [Google Scholar]
- 72.von der Mark K., Frischholz S., Aigner T., Beier F., Belke J., Erdmann S., Burkhardt H. Upregulation of type X collagen expression in osteoarthritic cartilage. Acta Orthop Scand Suppl. 1995;66:125–129. [PubMed] [Google Scholar]
- 73.Knuth C., Sastre E.Andres, Fahy N., Witte-Bouma J., Ridwan Y., Strabbing E., Koudstaal M., van de Peppel J., Wolvius E., Narcisi R. Collagen type X is essential for successful mesenchymal stem cell-mediated cartilage formation and subsequent endochondral ossification. Eur Cell Mater. 2019;38:106–122. doi: 10.22203/eCM.v038a09. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez R., Seegmiller R., Stark M., Bridgewater L. A type XI collagen mutation leads to increased degradation of type II collagen in articular cartilage. Osteoarthritis Cartilage. 2004;12:314–320. doi: 10.1016/j.joca.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Smith S.M., Melrose J. Type XI collagen–perlecan–HS interactions stabilise the pericellular matrix of annulus fibrosus cells and chondrocytes providing matrix stabilisation and homeostasis. J Mol Histol. 2019;50:285–294. doi: 10.1007/s10735-019-09823-1. [DOI] [PubMed] [Google Scholar]
- 76.Lawrence E.A., Kague E., Aggleton J.A., Harniman R.L., Roddy K.A., Hammond C.L. The mechanical impact of col11a2 loss on joints; Col11a2 mutant zebrafish show changes to joint development and function, which leads to early-onset osteoarthritis. Philos Trans R Soc Lond B. 2018;373 doi: 10.1098/rstb.2017.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garofalo S., Metsäranta M., Ellard J., Smith C., Horton W., Vuorio E., de Crombrugghe B. Assembly of cartilage collagen fibrils is disrupted by overexpression of normal type II collagen in transgenic mice. Proc Natl Acad Sci U S A. 1993;90:3825–3829. doi: 10.1073/pnas.90.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eyre D. Articular cartilage and changes in arthritis: collagen of articular cartilage. Arthritis Res Ther. 2001;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuiper N., Sharma A. A detailed quantitative outcome measure of glycosaminoglycans in human articular cartilage for cell therapy and tissue engineering strategies. Osteoarthritis Cartilage. 2015;23:2233–2241. doi: 10.1016/j.joca.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Watanabe H., Cheung S.C., Itano N., Kimata K., Yamada Y. Identification of hyaluronan-binding domains of aggrecan. J Biol Chem. 1997;272:28057–28065. doi: 10.1074/jbc.272.44.28057. [DOI] [PubMed] [Google Scholar]
- 81.Horkay F., Basser P.J., Hecht A.M., Geissler E. Structure and properties of cartilage proteoglycans. Macromol Symp. 2017;372:43–50. doi: 10.1002/masy.201700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vynios D.H. Metabolism of cartilage proteoglycans in health and disease. Biomed Res Int. 2014;2014:1–9. doi: 10.1155/2014/452315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knudson C.B., Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 84.Roughley P.J., Lee E.R. Cartilage proteoglycans: Structure and potential functions. Microsc Res Tech. 1994;28:385–397. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]
- 85.J. Bertrand, A. Held, Role of proteoglycans in osteoarthritis, Cartilage, Springer 2017, pp. 63-80.
- 86.Muir H. Proteoglycans of cartilage. J Clin Pathol Suppl. 1978;12:67–81. [PMC free article] [PubMed] [Google Scholar]
- 87.Carney S., Muir H. The structure and function of cartilage proteoglycans. Physiol Rev. 1988;68:858–910. doi: 10.1152/physrev.1988.68.3.858. [DOI] [PubMed] [Google Scholar]
- 88.Mankin H.J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971;50:1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hjertquist S.-O., Lemperg R. Identification and concentration of the glycosaminoglycans of human articular cartilage in relation to age and osteoarthritis. Calcif Tissue Res. 1972;10:223–237. doi: 10.1007/BF02012552. [DOI] [PubMed] [Google Scholar]
- 90.Han E., Chen S., Klisch S., Sah R. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J. 2011;101:916–924. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roughley P. Articular cartilage and changes in arthritis: noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res. 2001;3:342–347. doi: 10.1186/ar326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghosh P., Smith M. Osteoarthritis, genetic and molecular mechanisms. Biogerontology. 2002;3:85–88. doi: 10.1023/a:1015219716583. [DOI] [PubMed] [Google Scholar]
- 93.Fosang A., Last K., Maciewicz R. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 1996;98:2292–2299. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lippiello L., Hall D., Mankin H. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977;59:593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nelson F., Dahlberg L., Laverty S., Reiner A., Pidoux I., Ionescu M., Fraser G.L., Brooks E., Tanzer M., Rosenberg L.C. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maldonado M., Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013:1–10. doi: 10.1155/2013/284873. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldring M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:269–285. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rim Y.A., Nam Y., Ju J.H. The role of chondrocyte hypertrophy and senescence in osteoarthritis initiation and progression. Int J Mol Sci. 2020;21:2358. doi: 10.3390/ijms21072358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Talwar R.M., Wong B.S., Svoboda K., Harper R.P. Effects of estrogen on chondrocyte proliferation and collagen synthesis in skeletally mature articular cartilage. J Oral Maxillofac Surg. 2006;64:600–609. doi: 10.1016/j.joms.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 100.Stevens D.A., Williams G.R. Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol. 1999;151:195–204. doi: 10.1016/s0303-7207(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 101.He H., Wang C., Tang Q., Yang F., Xu Y. Elucidation of possible molecular mechanisms underlying the estrogen-induced disruption of cartilage development in zebrafish larvae. Toxicol Lett. 2018;289:22–27. doi: 10.1016/j.toxlet.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 102.Fortier L.A., Barker J.U., Strauss E.J., McCarrel T.M., Cole B.J. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ellman M., Yan D., Ahmadinia K., Chen D., An H., Im H. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114:735–742. doi: 10.1002/jcb.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patil A., Sable R., Kothari R. An update on transforming growth factor-β (TGF-β): sources, types, functions and clinical applicability for cartilage/bone healing. J Cell Physiol. 2011;226:3094–3103. doi: 10.1002/jcp.22698. [DOI] [PubMed] [Google Scholar]
- 105.DeGroot J., Verzijl N., Bank R.A., Lafeber F.P., Bijlsma J.W., TeKoppele J.M. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: The role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 106.Madej W., van Caam A., Davidson E.B., Hannink G., Buma P., van der Kraan P. Ageing is associated with reduction of mechanically-induced activation of Smad2/3P signaling in articular cartilage. Osteoarthritis Cartilage. 2016;24:146–157. doi: 10.1016/j.joca.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 107.Rahmati M., Nalesso G., Mobasheri A., Mozafari M. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 108.Fermor B., Christensen S., Youn I., Cernanec J., Davies C., Weinberg J. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. [DOI] [PubMed] [Google Scholar]
- 109.Buckley C.T., Vinardell T., Kelly D.J. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage. 2010;18:1345–1354. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 110.Qu C., Lindeberg H., Ylärinne J.H., Lammi M.J. Five percent oxygen tension is not beneficial for neocartilage formation in scaffold-free cell cultures. Cell Tissue Res. 2012;348:109–117. doi: 10.1007/s00441-012-1366-z. [DOI] [PubMed] [Google Scholar]
- 111.Shi Y., Ma J., Zhang X., Li H., Jiang L., Qin J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr Biol. 2015;7:289–297. doi: 10.1039/c4ib00273c. [DOI] [PubMed] [Google Scholar]
- 112.Bleuel J., Zaucke F., Brüggemann G.-P., Niehoff A. Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jørgensen A.E.M., Kjær M., Heinemeier K.M. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol. 2017;44:410–417. doi: 10.3899/jrheum.160226. [DOI] [PubMed] [Google Scholar]
- 114.Meinert C., Schrobback K., Hutmacher D.W., Klein T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-16523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bian L., Fong J.V., Lima E.G., Stoker A.M., Ateshian G.A., Cook J.L., Hung C.T. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781–1790. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mauck R., Byers B., Yuan X., Tuan R. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 117.Bhosale A.M., Richardson J.B. Articular cartilage: Structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 118.Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970;52:554–563. [PubMed] [Google Scholar]
- 119.Chen R., Chen S., Chen X., Long X. Study of the tidemark in human mandibular condylar cartilage. Arch Oral Biol. 2011;56:1390–1397. doi: 10.1016/j.archoralbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 120.James C.-B., Uhl T.L. A review of articular cartilage pathology and the use of glucosamine sulfate. J Athl Train. 2001;36:413–419. [PMC free article] [PubMed] [Google Scholar]
- 121.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frazer A., Bunning R.A., Thavarajah M., Seid J.M., Russell R.G.G. Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-β and interleukin-1β. Osteoarthritis Cartilage. 1994;2:235–245. doi: 10.1016/s1063-4584(05)80075-5. [DOI] [PubMed] [Google Scholar]
- 123.Kivirikko K.I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: Prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989;3:1609–1617. [PubMed] [Google Scholar]
- 124.Prockop D.J., Kivirikko K.I., Tuderman L., Guzman N.A. The biosynthesis of collagen and its disorders. N Engl J Med. 1979;301:77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- 125.Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22:15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 126.Siegel R.C. Biosynthesis of collagen crosslinks: Increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Natl Acad Sci U S A. 1974;71:4826–4830. doi: 10.1073/pnas.71.12.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eyre D.R., Paz M.A., Gallop P.M. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 128.Herbage D., Bouillet J., Bernengo J. Biochemical and physiochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161:303–312. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bishop P., Crossman M., McLeod D., Ayad S. Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem J. 1994;299:497–505. doi: 10.1042/bj2990497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eyre D., Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975;151:595–602. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu Z., Korntner S., Mullen A., Skoufos I., Tzora A., Zeugolis D. In the quest of the optimal tissue source (porcine male and female articular, tracheal and auricular cartilage) for the development of collagen sponges for articular cartilage. Biomed Eng Adv. 2021;1 [Google Scholar]
- 132.Cao H., Xu S.-Y. Purification and characterization of type II collagen from chick sternal cartilage. Food Chem. 2008;108:439–445. doi: 10.1016/j.foodchem.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 133.Akram A., Zhang C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105053. [DOI] [PubMed] [Google Scholar]
- 134.Dai M., Liu X., Wang N., Sun J. Squid type II collagen as a novel biomaterial: Isolation, characterization, immunogenicity and relieving effect on degenerative osteoarthritis via inhibiting stat1 signaling in pro-inflammatory macrophages. Mater Sci Eng C. 2018;89:283–294. doi: 10.1016/j.msec.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 135.Rigogliuso S., Salamone M., Barbarino E., Barbarino M., Nicosia A., Ghersi G. Production of injectable marine collagen-based hydrogel for the maintenance of differentiated chondrocytes in tissue engineering applications. Int J Mol Sci. 2020;21:5798. doi: 10.3390/ijms21165798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sewing J., Klinger M., Notbohm H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J Tissue Eng Regen Med. 2017;11:916–925. doi: 10.1002/term.1993. [DOI] [PubMed] [Google Scholar]
- 137.Zhang X., Adachi S., Ura K., Takagi Y. Properties of collagen extracted from Amur sturgeon Acipenser schrenckii and assessment of collagen fibrils in vitro. Int J Biol Macromol. 2019;137:809–820. doi: 10.1016/j.ijbiomac.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 138.Cumming M., Hall B., Hofman K. Isolation and characterisation of major and minor collagens from hyaline cartilage of hoki (Macruronus novaezelandiae) Mar Drugs. 2019;17:223. doi: 10.3390/md17040223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jeevithan E., Bao B., Bu Y., Zhou Y., Zhao Q., Wu W. Type II collagen and gelatin from silvertip shark (Carcharhinus albimarginatus) cartilage: Isolation, purification, physicochemical and antioxidant properties. Mar Drugs. 2014;12:3852–3873. doi: 10.3390/md12073852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kittiphattanabawon P., Benjakul S., Visessanguan W., Shahidi F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus) LWT-Food Sci Technol. 2010;43:792–800. [Google Scholar]
- 141.Merly L., Smith S.L. Collagen type II, alpha 1 protein: a bioactive component of shark cartilage. Int Immunopharmacol. 2013;15:309–315. doi: 10.1016/j.intimp.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 142.Mizuta S., Hwang J., Yoshinaka R. Molecular species of collagen in pectoral fin cartilage of skate (Raja kenojei) Food Chem. 2003;80:1–7. [Google Scholar]
- 143.Wu Z., Korntner S., Mullen A., Zeugolis D. In the quest of the optimal chondrichthyan for the development of collagen sponges for articular cartilage. J Sci-Adv Mater Dev (In Press) 2021 [Google Scholar]
- 144.Hashim P., Ridzwan M.M., Bakar J., Hashim M.D. Collagen in food and beverage industries. Int Food Res J. 2015;22:1–8. [Google Scholar]
- 145.Avila Rodríguez M.I., Rodriguez Barroso L.G., Sánchez M.L. Collagen: a review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17:20–26. doi: 10.1111/jocd.12450. [DOI] [PubMed] [Google Scholar]
- 146.Silvipriya K., Kumar K.K., Bhat A., Kumar B.D., John A., Lakshmanan P. Collagen: animal sources and biomedical application. J Appl Pharm Sci. 2015;5:123–127. [Google Scholar]
- 147.Chen Z., Fan D., Shang L. Exploring the potential of the recombinant human collagens for biomedical and clinical applications: a short review. Biomed Mater. 2020;16 doi: 10.1088/1748-605X/aba6fa. [DOI] [PubMed] [Google Scholar]
- 148.Olsen D., Yang C., Bodo M., Chang R., Leigh S., Baez J., Carmichael D., Perälä M., Hämäläinen E.-R., Jarvinen M. Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev. 2003;55:1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 149.Fertala A. Three decades of research on recombinant collagens: reinventing the wheel or developing new biomedical products? Bioengineering. 2020;7:155. doi: 10.3390/bioengineering7040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fertala A., Holmes D.F., Kadler K.E., Sieron A.L., Prockop D.J. Assembly in vitro of thin and thick fibrils of collagen II from recombinant procollagen II. The monomers in the tips of thick fibrils have the opposite orientation from monomers in the growing tips of collagen I fibrils. J Biol Chem. 1996;271:14864–14869. doi: 10.1074/jbc.271.25.14864. [DOI] [PubMed] [Google Scholar]
- 151.Fertala A., Sieron A.L., Ganguly A., Li S.-W., Ala-Kokko L., Anumula K.R., Prockop D. Synthesis of recombinant human procollagen II in a stably transfected tumour cell line (HT1080) Biochem J. 1994;298:31–37. doi: 10.1042/bj2980031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ala-Kokko L., Hyland J., Smith C., Kivirikko K.I., Jimenez S.A., Prockop D.J. Expression of a human cartilage procollagen gene (COL2A1) in mouse 3T3 cells. J Biol Chem. 1991;266:14175–14178. [PubMed] [Google Scholar]
- 153.Xi C., Liu N., Liang F., Zhao X., Long J., Yuan F., Yun S., Sun Y., Xi Y. Molecular assembly of recombinant chicken type II collagen in the yeast Pichia pastoris. Sci China Life Sci. 2018;61:815–825. doi: 10.1007/s11427-017-9219-4. [DOI] [PubMed] [Google Scholar]
- 154.Qi Q., Yao L., Liang Z., Yan D., Li Z., Huang Y., Sun J. Production of human type II collagen using an efficient baculovirus-silkworm multigene expression system. Mol Genet Genomics. 2016;291:2189–2198. doi: 10.1007/s00438-016-1251-7. [DOI] [PubMed] [Google Scholar]
- 155.Ikonomou L., Schneider Y.-J., Agathos S. Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol. 2003;62:1–20. doi: 10.1007/s00253-003-1223-9. [DOI] [PubMed] [Google Scholar]
- 156.Browne S., Zeugolis D.I., Pandit A. Collagen: Finding a solution for the source. Tissue Eng Part A. 2013;19:1491–1494. doi: 10.1089/ten.tea.2012.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Collin E.C., Grad S., Zeugolis D.I., Vinatier C.S., Clouet J.R., Guicheux J.J., Weiss P., Alini M., Pandit A.S. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–2870. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 158.Taguchi T., Ikoma T., Tanaka J. An improved method to prepare hyaluronic acid and type II collagen composite matrices. J Biomed Mater Res. 2002;61:330–336. doi: 10.1002/jbm.10147. [DOI] [PubMed] [Google Scholar]
- 159.Yang K., Sun J., Wei D., Yuan L., Yang J., Guo L., Fan H., Zhang X. Photo-crosslinked mono-component type II collagen hydrogel as a matrix to induce chondrogenic differentiation of bone marrow mesenchymal stem cells. J Mater Chem B. 2017;5:8707–8718. doi: 10.1039/c7tb02348k. [DOI] [PubMed] [Google Scholar]
- 160.Cao H., Xu S.-Y. EDC/NHS-crosslinked type II collagen-chondroitin sulfate scaffold: characterization and in vitro evaluation. J Mater Sci Mater Med. 2008;19:567–575. doi: 10.1007/s10856-007-3281-5. [DOI] [PubMed] [Google Scholar]
- 161.Ko C.-S., Huang J.-P., Huang C.-W., Chu I.-M. Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J Biosci Bioeng. 2009;107:177–182. doi: 10.1016/j.jbiosc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 162.Lan W., Xu M., Zhang X., Zhao L., Huang D., Wei X., Chen W. Biomimetic polyvinyl alcohol/type II collagen hydrogels for cartilage tissue engineering. J Biomater Sci Polym Ed. 2020;31:1179–1198. doi: 10.1080/09205063.2020.1747184. [DOI] [PubMed] [Google Scholar]
- 163.Hsu S.h., Tsai C.L., Tang C.M. Evaluation of cellular affinity and compatibility to biodegradable polyesters and type-II collagen-modified scaffolds using immortalized rat chondrocytes. Artif Organs. 2002;26:647–658. doi: 10.1046/j.1525-1594.2002.06889.x. [DOI] [PubMed] [Google Scholar]
- 164.Yen H.-J., Tseng C.-S., Hsu S.-h., Tsai C.-L. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed Microdev. 2009;11:615–624. doi: 10.1007/s10544-008-9271-7. [DOI] [PubMed] [Google Scholar]
- 165.Ragetly G., Griffon D.J., Chung Y.S. The effect of type II collagen coating of chitosan fibrous scaffolds on mesenchymal stem cell adhesion and chondrogenesis. Acta Biomater. 2010;6:3988–3997. doi: 10.1016/j.actbio.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 166.Zhang J., Yang Z., Li C., Dou Y., Li Y., Thote T., Wang D.-a., Ge Z. Cells behave distinctly within sponges and hydrogels due to differences of internal structure. Tissue Eng Part A. 2013;19:2166–2175. doi: 10.1089/ten.tea.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shields K.J., Beckman M.J., Bowlin G.L., Wayne J.S. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng. 2004;10:1510–1517. doi: 10.1089/ten.2004.10.1510. [DOI] [PubMed] [Google Scholar]
- 168.Barnes C.P., Pemble IV C.W., Brand D.D., Simpson D.G., Bowlin G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007;13:1593–1605. doi: 10.1089/ten.2006.0292. [DOI] [PubMed] [Google Scholar]
- 169.Matthews J.A., Boland E.D., Wnek G.E., Simpson D.G., Bowlin G.L. Electrospinning of collagen type II: a feasibility study. J Bioact Compat Polym. 2003;18:125–134. [Google Scholar]
- 170.Yang L., Fitié C.F., van der Werf K.O., Bennink M.L., Dijkstra P.J., Feijen J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials. 2008;29:955–962. doi: 10.1016/j.biomaterials.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 171.Zeugolis D.I., Khew S.T., Yew E.S., Ekaputra A.K., Tong Y.W., Yung L.-Y.L., Hutmacher D.W., Sheppard C., Raghunath M. Electro-spinning of pure collagen nano-fibres–just an expensive way to make gelatin? Biomaterials. 2008;29:2293–2305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 172.Ren X., Wang F., Chen C., Gong X., Yin L., Yang L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet Disord. 2016;17:1–10. doi: 10.1186/s12891-016-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Annamalai R.T., Mertz D.R., Daley E.L., Stegemann J.P. Collagen Type II enhances chondrogenic differentiation in agarose-based modular microtissues. Cytotherapy. 2016;18:263–277. doi: 10.1016/j.jcyt.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang S.J., Kuo S.M., Manousakas I., Niu G.C.-C., Chen J.P. Preparation and characterization of hyaluronan/collagen II microspheres under an electrostatic field system with disc electrodes. Acta Biomater. 2009;5:101–114. doi: 10.1016/j.actbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 175.Tamaddon M., Burrows M., Ferreira S., Dazzi F., Apperley J., Bradshaw A., Brand D., Czernuszka J., Gentleman E. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Sci Rep. 2017;7:43519. doi: 10.1038/srep43519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao Y., Li B., Kong W., Yuan L., Guo L., Li C., Fan H., Fan Y., Zhang X. Injectable and self-crosslinkable hydrogels based on collagen type II and activated chondroitin sulfate for cell delivery. Int J Biol Macromol. 2018;118:2014–2020. doi: 10.1016/j.ijbiomac.2018.07.079. [DOI] [PubMed] [Google Scholar]
- 177.Veilleux N., Spector M. Effects of FGF-2 and IGF-1 on adult canine articular chondrocytes in type II collagen–glycosaminoglycan scaffolds in vitro. Osteoarthritis Cartilage. 2005;13:278–286. doi: 10.1016/j.joca.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 178.Szychlinska M.A., Calabrese G., Ravalli S., Dolcimascolo A., Castrogiovanni P., Fabbi C., Puglisi C., Lauretta G., Di Rosa M., Castorina A. Evaluation of a cell-free collagen type I-based scaffold for articular cartilage regeneration in an orthotopic rat model. Materials. 2020;13:2369. doi: 10.3390/ma13102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Calabrese G., Gulino R., Giuffrida R., Forte S., Figallo E., Fabbi C., Salvatorelli L., Memeo L., Gulisano M., Parenti R. In vivo evaluation of biocompatibility and chondrogenic potential of a cell-free collagen-based scaffold. Front Physiol. 2017;8:984. doi: 10.3389/fphys.2017.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang J., Chen X., Yuan T., Yang X., Fan Y., Zhang X. Regulation of the secretion of immunoregulatory factors of mesenchymal stem cells (MSCs) by collagen-based scaffolds during chondrogenesis. Mater Sci Eng C. 2017;70:983–991. doi: 10.1016/j.msec.2016.04.096. [DOI] [PubMed] [Google Scholar]
- 181.Cao C., Zhang Y., Ye Y., Sun T. Effects of cell phenotype and seeding density on the chondrogenic capacity of human osteoarthritic chondrocytes in type I collagen scaffolds. J Orthop Surg Res. 2020;15:1–11. doi: 10.1186/s13018-020-01617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nehrer S., Breinan H.A., Ramappa A., Shortkroff S., Young G., Minas T., Sledge C.B., Yannas I.V., Spector M. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997;38:95–104. doi: 10.1002/(sici)1097-4636(199722)38:2<95::aid-jbm3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 183.Veilleux N., Yannas I., Spector M. Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng. 2004;10:119–127. doi: 10.1089/107632704322791763. [DOI] [PubMed] [Google Scholar]
- 184.Lu Z., Doulabi B., Huang C., Bank R., Helder M. Collagen type II enhances chondrogenesis in adipose tissue–derived stem cells by affecting cell shape. Tissue Eng Part A. 2010;16:81–90. doi: 10.1089/ten.TEA.2009.0222. [DOI] [PubMed] [Google Scholar]
- 185.Bosnakovski D., Mizuno M., Kim G., Takagi S., Okumura M., Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 186.Tao Y., Zhou X., Liu D., Li H., Liang C., Li F., Chen Q. Proportion of collagen type II in the extracellular matrix promotes the differentiation of human adipose-derived mesenchymal stem cells into nucleus pulposus cells. Biofactors. 2016;42:212–223. doi: 10.1002/biof.1266. [DOI] [PubMed] [Google Scholar]
- 187.Kilmer C.E., Battistoni C.M., Cox A., Breur G., Panitch A., Liu J.C. Collagen type I and II blend hydrogel with autologous mesenchymal stem cells as a scaffold for articular cartilage defect repair. ACS Biomater Sci Eng. 2020;6:3464–3476. doi: 10.1021/acsbiomaterials.9b01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buma P., Pieper J.S., van Tienen T., van Susante J.L., van der Kraan P.M., Veerkamp J.H., van den Berg W.B., Veth R.P., van Kuppevelt T.H. Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects—A study in rabbits. Biomaterials. 2003;24:3255–3263. doi: 10.1016/s0142-9612(03)00143-1. [DOI] [PubMed] [Google Scholar]
- 189.Nehrer S., Breinan H., Ramappa A., Hsu H., Minas T., Shortkroff S., Sledge C., Yannas I., Spector M. Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials. 1998;19:2313–2328. doi: 10.1016/s0142-9612(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 190.Muhonen V., Salonius E., Haaparanta A.M., Järvinen E., Paatela T., Meller A., Hannula M., Björkman M., Pyhältö T., Ellä V. Articular cartilage repair with recombinant human type II collagen/polylactide scaffold in a preliminary porcine study. J Orthop Res. 2016;34:745–753. doi: 10.1002/jor.23099. [DOI] [PubMed] [Google Scholar]
- 191.Trentham D.E., Townes A.S., Kang A.H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Holmdahl R., Vingsbo C., Malmström V., Jansson L., Holmdahl M. Chronicity of arthritis induced with homologous type II collagen (CII) in rats is associated with anti-CII B-cell activation. J Autoimmun. 1994;7:739–752. doi: 10.1006/jaut.1994.1058. [DOI] [PubMed] [Google Scholar]
- 193.Ofosu-Appiah W., Morgan K., Holt P.Lennox. Native type II collagen-induced arthritis in the rat. III. Relationship between the cellular immune response to native type II collagen and arthritis. Ann Rheum Dis. 1983;42:331–337. doi: 10.1136/ard.42.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cathcart E., Hayes K., Gonnerman W., Lazzari A., Franzblau C. Experimental arthritis in a nonhuman primate. I. Induction by bovine type II collagen. Lab Invest. 1986;54:26–31. [PubMed] [Google Scholar]
- 195.Cook A.D., Rowley M.J., Mackay I.R., Gough A., Emery P. Antibodies to type II collagen in early rheumatoid arthritis. Correlation with disease progression. Arthritis Rheum. 1996;39:1720–1727. doi: 10.1002/art.1780391015. [DOI] [PubMed] [Google Scholar]
- 196.Stuart J.M., Townes A.S., Kang A.H. Collagen autoimmune arthritis. Ann Rev Immunol. 1984;2:199–218. doi: 10.1146/annurev.iy.02.040184.001215. [DOI] [PubMed] [Google Scholar]
- 197.Kim H.A., Seo Y.-I., Lee J., Jung Y.O. Detection of anti-type II collagen antibodies in patients with chronic gouty arthritis: findings from a pilot study. J Clin Rheumatol. 2016;22:360–363. doi: 10.1097/RHU.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 198.Thompson H., Henderson B., Spencer J., Hobbs S., Peppard J., Staines N. Tolerogenic activity of polymerized type II collagen in preventing collagen-induced arthritis in rats. Clin Exp Immunol. 1988;72:20–25. [PMC free article] [PubMed] [Google Scholar]
- 199.Breinan H.A., Martin S.D., Hsu H.P., Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18:781–789. doi: 10.1002/jor.1100180516. [DOI] [PubMed] [Google Scholar]
- 200.Lee C., Grodzinsky A., Hsu H.P., Spector M. Effects of a cultured autologous chondrocyte-seeded type II collagen scaffold on the healing of a chondral defect in a canine model. J Orthop Res. 2003;21:272–281. doi: 10.1016/S0736-0266(02)00153-5. [DOI] [PubMed] [Google Scholar]
- 201.Wu C.-H., Ko C.-S., Huang J.-W., Huang H.-J., Chu I.-M. Effects of exogenous glycosaminoglycans on human chondrocytes cultivated on type II collagen scaffolds. J Mater Sci Mater Med. 2010;21:725–729. doi: 10.1007/s10856-009-3889-8. [DOI] [PubMed] [Google Scholar]
- 202.Pieper J., Van Der Kraan P., Hafmans T., Kamp J., Buma P., Van Susante J., Van Den Berg W., Veerkamp J., Van Kuppevelt T. Crosslinked type II collagen matrices: preparation, characterization, and potential for cartilage engineering. Biomaterials. 2002;23:3183–3192. doi: 10.1016/s0142-9612(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 203.Mukaida T., Urabe K., Naruse K., Aikawa J., Katano M., Hyon S.-H., Itoman M. Influence of three-dimensional culture in a type II collagen sponge on primary cultured and dedifferentiated chondrocytes. J Orthop Sci. 2005;10:521–528. doi: 10.1007/s00776-005-0930-8. [DOI] [PubMed] [Google Scholar]
- 204.Nehrer S., Breinan H.A., Ramappa A., Young G., Shortkroff S., Louie L.K., Sledge C.B., Yannas I.V., Spector M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 205.Lee C.R., Breinan H.A., Nehrer S., Spector M. Articular cartilage chondrocytes in type I and type II collagen-GAG matrices exhibit contractile behavior in vitro. Tissue Eng. 2000;6:555–565. doi: 10.1089/107632700750022198. [DOI] [PubMed] [Google Scholar]
- 206.Vickers S.M., Squitieri L.S., Spector M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: dynamic pore reduction promotes cartilage formation. Tissue Eng. 2006;12:1345–1355. doi: 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- 207.Lee C., Grodzinsky A., Spector M. Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J Biomed Mater Res A. 2003;64:560–569. doi: 10.1002/jbm.a.10443. [DOI] [PubMed] [Google Scholar]
- 208.Wu Z., Korntner S., Mullen A., Skoufos I., Tzora A., Zeugolis D. In the quest of the optimal tissue source (porcine male and female articular, tracheal and auricular cartilage) for the development of collagen sponges for articular cartilage. Biomed Eng Adv. 2021;1 [Google Scholar]
- 209.Malemud C.J., Stevenson S., Mehraban F., Papay R.S., Purchio A.F., Goldberg V.M. The proteoglycan synthesis repertoire of rabbit chondrocytes maintained in type II collagen gels. Osteoarthritis Cartilage. 1994;2:29–41. doi: 10.1016/s1063-4584(05)80004-4. [DOI] [PubMed] [Google Scholar]
- 210.Muhonen V., Narcisi R., Nystedt J., Korhonen M., van Osch G.J., Kiviranta I. Recombinant human type II collagen hydrogel provides a xeno-free 3D micro-environment for chondrogenesis of human bone marrow-derived mesenchymal stromal cells. J Tissue Eng Regen Med. 2017;11:843–854. doi: 10.1002/term.1983. [DOI] [PubMed] [Google Scholar]
- 211.Pulkkinen H., Tiitu V., Valonen P., Hamalainen E., Lammi M., Kiviranta I. Recombinant human type II collagen as a material for cartilage tissue engineering. Int J Artif Organs. 2008;31:960–969. doi: 10.1177/039139880803101106. [DOI] [PubMed] [Google Scholar]
- 212.Wong C.-C., Chen C.-H., Chiu L.-H., Tsuang Y.-H., Bai M.-Y., Chung R.-J., Lin Y.-H., Hsieh F.-J., Chen Y.-T., Yang T.-L. Facilitating in vivo articular cartilage repair by tissue-engineered cartilage grafts produced from auricular chondrocytes. Am J Sports Med. 2018;46:713–727. doi: 10.1177/0363546517741306. [DOI] [PubMed] [Google Scholar]
- 213.Dong S., Guo H., Zhang Y., Li Z., Kang F., Yang B., Kang X., Wen C., Yan Y., Jiang B. rFN/Cad-11-modified collagen type II biomimetic interface promotes the adhesion and chondrogenic differentiation of mesenchymal stem cells. Tissue Eng Part A. 2013;19:2464–2477. doi: 10.1089/ten.tea.2012.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen W.C., Wei Y.H., Chu I., Yao C.L. Effect of chondroitin sulphate C on the in vitro and in vivo chondrogenesis of mesenchymal stem cells in crosslinked type II collagen scaffolds. J Tissue Eng Regen Med. 2013;7:665–672. doi: 10.1002/term.1463. [DOI] [PubMed] [Google Scholar]
- 215.Funayama A., Niki Y., Matsumoto H., Maeno S., Yatabe T., Morioka H., Yanagimoto S., Taguchi T., Tanaka J., Toyama Y. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci. 2008;13:225–232. doi: 10.1007/s00776-008-1220-z. [DOI] [PubMed] [Google Scholar]
- 216.Chen W.-C., Yao C.-L., Wei Y.-H., Chu I.-M. Evaluating osteochondral defect repair potential of autologous rabbit bone marrow cells on type II collagen scaffold. Cytotechnology. 2011;63:13–23. doi: 10.1007/s10616-010-9314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dorotka R., Windberger U., Macfelda K., Bindreiter U., Toma C., Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26:3617–3629. doi: 10.1016/j.biomaterials.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 218.Hsu S.h., Chang S.H., Yen H.J., Whu S.W., Tsai C.L., Chen D.C. Evaluation of biodegradable polyesters modified by type II collagen and Arg-Gly-Asp as tissue engineering scaffolding materials for cartilage regeneration. Artif Organs. 2006;30:42–55. doi: 10.1111/j.1525-1594.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 219.Chen H., Yang X., Liao Y., Zeng X., Liang P., Kang N., et al. MRI and histologic analysis of collagen type II sponge on repairing the cartilage defects of rabbit knee joints. J Biomed Mater Res B. 2011;96:267–275. doi: 10.1002/jbm.b.31762. [DOI] [PubMed] [Google Scholar]
- 220.Lazarini M., Bordeaux-Rego P., Giardini-Rosa R., Duarte A.S., Baratti M.O., Zorzi A.R., de Miranda J.B., Lenz Cesar C., Luzo Â., Olalla Saad S.T. Natural type II collagen hydrogel, fibrin sealant, and adipose-derived stem cells as a promising combination for articular cartilage repair. Cartilage. 2017;8:439–443. doi: 10.1177/1947603516675914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen L., Bao B., Wang N., Xie J., Wu W. Oral administration of shark type II collagen suppresses complete Freund’s adjuvant-induced rheumatoid arthritis in rats. Pharmaceuticals. 2012;5:339–352. doi: 10.3390/ph5040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pulkkinen H., Tiitu V., Valonen P., Jurvelin J., Rieppo L., Töyräs J., Silvast T., Lammi M., Kiviranta I. Repair of osteochondral defects with recombinant human type II collagen gel and autologous chondrocytes in rabbit. Osteoarthritis Cartilage. 2013;21:481–490. doi: 10.1016/j.joca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 223.Capella-Monsonís H., Coentro J.Q., Graceffa V., Wu Z., Zeugolis D.I. An experimental toolbox for characterization of mammalian collagen type I in biological specimens. Nat Protoc. 2018;13:507–529. doi: 10.1038/nprot.2017.117. [DOI] [PubMed] [Google Scholar]
- 224.Sorushanova A., Skoufos I., Tzora A., Mullen A.M., Zeugolis D.I. The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges. J Mater Sci Mater Med. 2021;32:1–12. doi: 10.1007/s10856-020-06485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zeugolis D., Paul R., Attenburrow G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: animal species and collagen extraction method. J Biomed Mater Res A. 2008;86:892–904. doi: 10.1002/jbm.a.31694. [DOI] [PubMed] [Google Scholar]
- 226.Delgado L.M., Shologu N., Fuller K., Zeugolis D.I. Acetic acid and pepsin result in high yield, high purity and low macrophage response collagen for biomedical applications. Biomed Mater. 2017;12 doi: 10.1088/1748-605X/aa838d. [DOI] [PubMed] [Google Scholar]