Highlights
-
•
Bioreactors are being widely developed and used for tissue engineering.
-
•
Introducing convective fluid flow in cell culture can boost physiological relevance.
-
•
The outcome of a culture is influenced by fluid flow and apparatus-specific factors.
-
•
Choice of design parameters allow tailoring of apparatus for specific applications.
-
•
Future development is key to improving performance of bioengineered tissues.
Keywords: Bioreactors, Tissue engineering, Fluid flow, Cell culture
Abbreviations: 3D, three-dimensional; ABS, acrylonitrile butadiene styrene; ALI, air-liquid interface; CFD, computational fluid dynamics; ECM, extracellular matrix; FDM, fused deposition modelling; PC, polycarbonate; PET, polyethylene terephthalate; PLA, polylactic acid; PTFE, polytetrafluoroethylene; SLA, stereolithography; UL, unstirred layer; UV, ultraviolet light
Abstract
One of the major aims of bio-engineering tissue equivalents in vitro is to create physiologically relevant culture conditions to accurately recreate the cellular microenvironment. This often includes incorporation of factors such as the extracellular matrix, co-culture of multiple cell types and three-dimensional culture techniques. These advanced techniques can recapitulate some of the properties of tissue in vivo, however fluid flow is a key aspect that is often absent. Fluid flow can be introduced into cell and tissue culture using bioreactors, which are becoming increasingly common as we seek to produce increasingly accurate tissue models. Bespoke technology is continuously being developed to tailor systems for specific applications and to allow compatibility with a range of culture techniques. For effective perfusion of a tissue culture many parameters can be controlled, ranging from impacts of the fluid flow such as increased shear stress and mass transport, to potentially unwanted side effects such as temperature fluctuations. A thorough understanding of these properties and their implications on the culture model can aid with a more accurate interpretation of results. Improved and more complete characterisation of bioreactor properties will also lead to greater accuracy when reporting culture conditions in protocols, aiding experimental reproducibility, and allowing more precise comparison of results between different systems. In this review we provide an analysis of the different factors involved in the development of benchtop flow bioreactors and their potential biological impacts across a range of applications.
1. Introduction
The microenvironment of cells in vivo is a highly complex and dynamic system which is regulated by a number of factors (Fig. 1). These factors have a significant impact on the structure and function of cells and therefore are key to the successful creation of tissue models, though are often absent in conventional in vitro culture methods. Techniques such as three-dimensional (3D) cell culture can be used to provide a more natural morphology for the cells [1,2], and often incorporate other components such as extracellular matrix and growth factors which can further aid in the formation of functional tissue models [3]. Co-culture of multiple cell types is also a commonly used technique, possible in either a paracrine or juxtacrine manner, to incorporate native tissue interactions such as that between stromal supports and epithelia which can lead to enhanced polarisation and functionality in epithelial cells [4,5], or between immune cells and the surrounding tissue to model the effects of complex pathways such as diseases and drug responses [6], [7], [8].
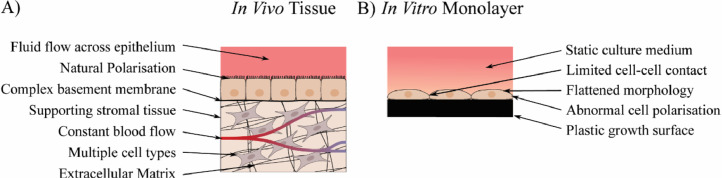
Fig. 1.
The cellular microenvironment in vivo and in vitro. (A) In the body, cells exist in a complex microenvironment surrounded by a wide range of stimuli (an example of an epithelial tissue is shown). These include mechanical stimulation such as from blood flow, contact between a large number of cells, both of the same type and different types, and contact with structural support such as the extracellular matrix and basement membranes. (B) Many of these factors are missing in standard in vitro monolayer culture. The lack of a 3D growth surface leads cells to flatten out, minimising contact with other cells. Cells adhere to the growth surface in a single direction, leading to an enforced polarisation which may not resemble that found in vivo.
By combining these methods, complex tissue equivalents can be generated for modelling a wide range of organs and tissues. These vary in complexity from utilising single cell types in a 3D structure to co-culture of several cell types and incorporation of physiologically relevant ECM. Full thickness models of the skin are a widely used example of a tissue model, due to the ethical concerns with animal models as well as the European Union ban on testing cosmetics on animals in 2013 [9]. These incorporate a stratified epidermis and supporting dermal compartment to allow interplay between the keratinocytes and fibroblasts [10]. Further complexity can be created through the inclusion of cell types such as melanocytes, Langerhans cells and endothelial cells, making it possible to model processes such as UV-induced tissue damage and investigate potential treatments [11,12]. Tissue equivalents have also been created for internal organs such as the lungs [13], kidneys [14], [15], [16], liver [17,18] and intestines [4,19,20]. Testing these models with drugs has been frequently performed and results often demonstrate an improved predictive response, though variable between different models, compared to 2D cultures [21,22]. Other factors such as the use of cell lines instead of primary cells can also significantly alter the outcomes of such experiments, making comparisons between studies more difficult [23,24]. Despite the potential sources of variation between these models, tissue equivalents represent an improvement over standard 2D cell cultures and are a step closer to accurately recapitulating the in vivo microenvironment [25].
These techniques, whilst able to recreate aspects of the in vivo microenvironment, often utilise a static culture medium which lacks many of the dynamic cues created by the movement of fluids in tissue. To address this shortfall, fluid flow may be introduced when culturing cell and tissue models using bioreactor technologies. These allow the creation of the dynamic conditions found in native tissue, such as blood and interstitial fluid flow. This can not only provide mechanical stimulation of cells, but also allows the levels of nutrients and metabolites in the medium to be maintained at more consistent levels, reducing the variability caused by factors such as medium changes and unstirred layers [26].
A wide variety of methods can be used to drive the fluid flow in a bioreactor, such as through the use of pumps or rotary motion. The level of complexity can also be hugely variable depending on the desired level of control, the tissue complexity, and the requirements for outputs such as real-time data readouts. This complexity brings with it many properties which need to be understood to ensure the systems reach their full potential from both the biological and engineering perspectives, and to make sure that data is correctly interpreted. Combined with the fact that the field of tissue engineering is highly interdisciplinary and requires researchers to have a broad knowledge of many fields means that there is potential for knowledge gaps which can be a hindrance to the development of effective bioreactor systems. Drawing this information together to give abroad overview of the different properties which may be encountered could therefore aid in the effective development of future bioreactors.
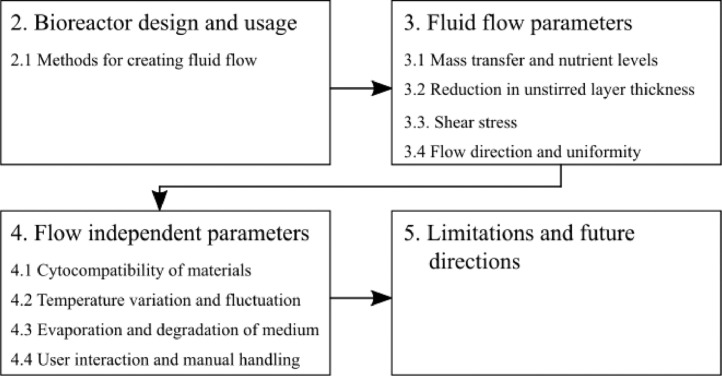
In this review we provide an overview of the parameters involved in the creation of bioreactor technology to support cell and tissue growth along with the impacts these factors can have on cultured cells and tissues. An outline of these parameters is shown in Fig. 2. In Section 2, the different types of bioreactor design and ways in which they have been used in literature will be discussed. In Section 3, different culture properties which are influenced by the fluid flow will be described. Section 4 will work through a series of other culture properties which are not affected by fluid flow, however, can be influenced by design choices such as the geometry of the bioreactor system. Finally, in Section 5 the limitations of current methods and future directions will be discussed. A thorough understanding of these factors can have the potential to help explain some unexpected results and aid with creation of a biological system which represents in vivo tissue accurately and effectively. The properties to be discussed in this review can be separated into two major categories: Fluid flow parameters and flow-independent properties. Within these two categories are different properties which will be discussed throughout this review.
Fig. 2.
An overview of the parameters presented in this review. Section 2 covers the different techniques for creating fluid flow. In Section 3 the different parameters which are affected by fluid flow are explored, followed by the parameters which are independent of the fluid flow in Section 4. Finally the limitations of current techniques and future directions will be discussed in Section 5.
2. Bioreactor design and usage
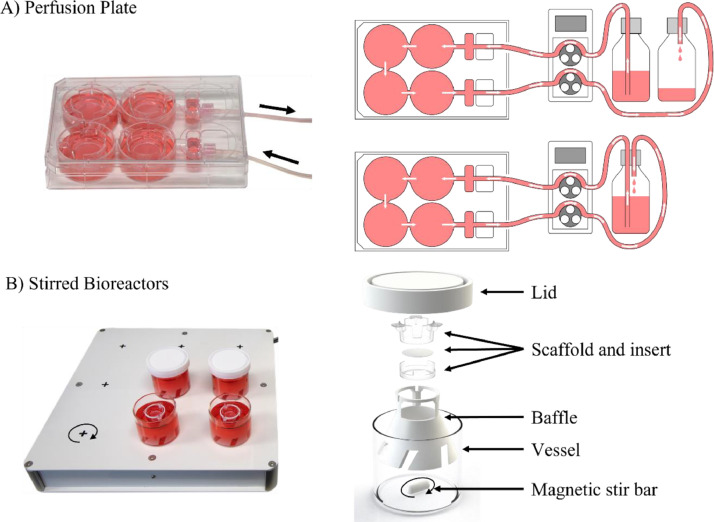
Bioreactors are increasingly used in cell culture for the creation of tissue equivalents in dynamic conditions with physiologically relevant fluid flow. These can range widely in complexity and utilise a range of additional components to generate the desired conditions. The main part of a bioreactor is the vessel in which the culture is held. This can be a simple, readily available piece of culture plasticware such as a multi-well plate or can be a bespoke vessel with complex control systems designed to direct fluid flow and perfusion of the tissue construct. The use of different three-dimensional cell culture techniques such as hydrogels and scaffolds in these systems is common. For example, a simple porous polystyrene scaffold used in 3D cell culture [27] is held in a cell culture insert and can be placed in a standard cell culture multi-well plate for static culture. Fig. 3 shows examples of two different designs that have been developed to maintain these 3D scaffold cultures in inserts whilst also providing fluid flow. These different techniques introduce fluid flow and of different types, demonstrating that flexibility that can be built into dynamic culture systems. A standard 6-well plate allows both submerged and air-liquid interface culture to be performed with ease. The perfusion plate shown in Fig. 3A utilises pumped flow through the wells in a continuous-fed manner to provide fluid flow across the 3D scaffold culture [28]. This allows inserts to be cultured in the same manner as a standard 6-well plate, minimising the variables which are changed. The rate of fluid flow is controlled through the pump whilst medium can be either recirculated or continually refreshed. The bioreactor system in Fig. 3B uses a batch-fed stirred tank system which can draw fluid both across and through the membrane [29]. This uses a large volume of batch-fed medium to minimise the need for additional changes, and the fluid flow rate is controlled by the stir speed. These demonstrate the flexibility of bioreactor systems for providing fluid flow with a range of properties and using different methods whilst able to support a standard culture insert. There are many alternative methods which can also be used to introduce fluid flow, some of which will be highlighted in the next section.
Fig. 3.
Fluid flow in cell culture inserts can be introduced in different ways. (A) A modified culture plate which allows medium to be pumped between 4 wells housing a scaffold insert. Additional equipment required includes a peristaltic pump and fresh and waste medium reservoirs. The inserts can be cultured in this system in the same way as with a standard 6-well plate. (B) A stirred bioreactor system for introducing fluid flow to scaffold inserts. This utilises a magnetic stirrer and uses a baffle to help direct the flow. A larger volume of medium is used to minimise the need for regular replenishment.
2.1. Methods used for creating fluid flow
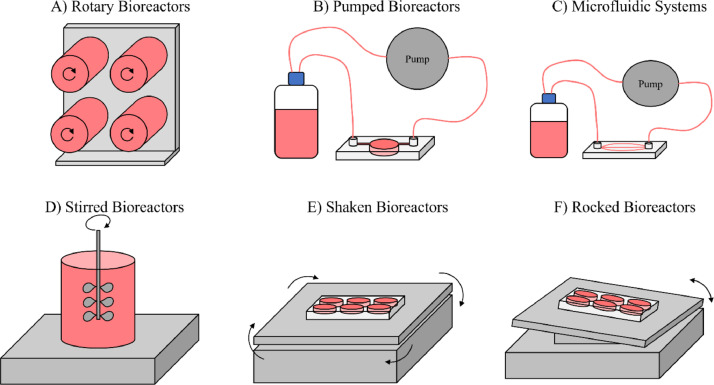
A variety of techniques can be used to introduce fluid flow into a cell culture system, with a range of commonly used approaches shown in Fig. 4. These methods can be used individually, or multiple methods can be incorporated into one system to give a higher level of control over the conditions. Further apparatus can be added including heat exchanges, humidifiers, bubble traps and oxygenators, which may be needed with some bioreactors if specific culture conditions are required. A range of monitoring systems can also be incorporated to measure properties such as pH, temperature, and oxygenation and provide insight into conditions within the bioreactor. The more complex apparatus typically has a trade-off in terms of cost, spatial requirements, and technical ability.
Fig. 4.
Commonly used technologies to introduce fluid flow into cell culture. Different techniques lead to flow patterns which can have desirable properties for modelling different tissues in vitro. The designs shown here are simple methods for creating fluid movement in cell culture. Variations exist for each design which vary widely in complexity. Simple systems may use conventional cell culture plasticware on equipment such as a mechanical rocker to produce fluid flow whilst maximising ease of use. More complex designs combine several of these technologies to maximise physiological relevance.
There are a range of commercially available bioreactor systems available for cell and tissue culture (Table 1). These also have a range of scales, from microfluidic chips to vessels which can hold litres of medium. There are also variations in complexity, from simple vessels with a minimal system to generate fluid flow to bioreactors which can monitor atmospheric conditions [30,31] or culture properties such as trans-epithelial electrical resistance of epithelial barriers [32].
Table 1.
Commercially available bioreactor systems for cell and tissue culture under fluid flow conditions. These table has a representation of different systems and is not exhaustive, with a wide range of different systems available for specific applications. ALI = Air-Liquid Interface, ECM = Extracellular Matrix.
| Manufacturer | System | Bioreactor Type | Set-up | Volume per culture | Basic Control Functions | Cell location | Refs. |
|---|---|---|---|---|---|---|---|
| Synthecon | Rotary Cell Culture System | Rotary | Single, dual, or quad rotary vessels | Customizable | Rotation speed | In suspension or on microcarriers | [33] |
| CelVivo | ClinoStar™ / ClinoReactor™ | Rotary | Six rotary vessels in a stand-alone incubator | 10 mL | Rotation Speed Temperature CO2 Level |
In suspension or on microcarriers | [30] |
| Reprocell | Alvetex® Perfusion Plate | Pumped | Plate with 4 interconnected wells | 10 mL | Flow rate | In scaffold, submerged or at ALI | [34] |
| Kirkstall | Quasi Vivo® | Pumped / Microfluidic | Single or multiple connected chambers | 2 - 4 mL | Flow rate | In scaffold, submerged or at ALI, barrier model option | [35] |
| ibidi | Pump System | Pumped / Microfluidic | Microslides with chambers | 60 µL | Flow rate | Channel surface | [36] |
| Emulate | Human Emulation System | Pumped / Microfluidic | Stretchable dual channel microchips with a porous membrane | 15 - 35 µL | Flow rate Cyclic stretch |
Porous membrane, channel surface | [31] |
| ABLE® Biott® | Magnetic Stir System | Stirred | Impeller stirred bioreactors | 5 −100 mL | Stir speed | In suspension or on microcarriers | [37] |
| Corning™ | Spinner Flasks | Stirred | Impeller stirred bioreactors | 125 mL – 36 L | Stir speed | In suspension or on microcarriers | [38] |
| Mimetas | OrganoFlow® / OrganoPlate® | Rocked / Microfluidic | Multichannel microchips with oscillatory perfusion | 50 µL | Rocker speed Rocker angle |
On channel surface, on or in ECM |
[39] |
Although many studies utilise bespoke bioreactor systems, the use of commercially available bioreactors has a number of advantages. Key amongst these is the reproducibility benefit, with laboratories around the world able to access these systems to carry out experiments. There has also been a large amount of characterisation already performed by different research groups for some of these bioreactors, and often literature is available for applications of interest, providing a starting point to build upon rather than having to perform full optimisation before using the systems.
Rotary bioreactors (Fig. 4A), such as the Rotary Cell Culture System produced by Synthecon [33] and the ClinoReactor™ by CelVivo [30], allow the formation of aggregate cultures in a dynamic environment across a range of volumes. The speed of rotation allows for control over mass transfer and shear stress, as well as giving control over the average size of the aggregates formed [40,41]. Further complexity can be incorporated into the cultures using materials such as the use of microcarriers [42,43] and scaffolds [44] to provide extracellular matrix components and give additional spatial control over tissue structures. On top of their simplicity, these systems can also be readily scaled between millilitre and litre volumes, with both single use and autoclavable vessels available at a range of sizes. Additional features such as atmospheric control and real-time imaging of cultures are also available with some systems [30].
Pumped bioreactors (Fig. 4B) can be designed to use existing culture ware or made as bespoke systems. These utilise a pump, often peristaltic, to provide a flow of medium across a culture. These also usually employ either one or two reservoirs to provide a supply of medium, which allows either recirculation of the same medium or a constant supply of fresh medium. A wide range of different systems have been designed, incorporating different cell supports such as porous membranes [45], synthetic or extracellular matrix (ECM) scaffolds [46], [47], [48] and hydrogels [49,50]. An advantage of these systems is the ease of incorporating them into standard static 3D culture methods, allowing complex tissue equivalents to readily be perfused with medium without needing to simplify other aspects of the culture, as has been demonstrated with full-thickness skin equivalents [51,52]. These systems are available in a range of sizes, such as the 6-well plate format of the Alvetex® Perfusion Plate [34] or as microfluidic and organ-on-a-chip systems which require much smaller volumes of medium [31,35].
Microfluidic systems (Fig. 4C) are typically a subset of pumped bioreactors, utilising a fluidic chip to house the cell culture. These use much smaller volumes (microtiters) and flow rates, therefore presenting an effective system for fields such as drug discovery, where quantities of the test compounds may be limited [53]. A wide range of microfluidic chips are commercially available and production of custom chips using materials such as polydimethylsiloxane or polymethylmethacrylate can be performed with ease using several simple techniques [54]. These systems tend to be more sophisticated than other systems due to the need for a large number of components and tubing to connect them and the subsequent control requirements. Many opportunities are feasible using this technique such as linking multiple cultures together to create complex multi-cellular systems designed to simulate crosstalk between different tissues [55,56].
Stirred bioreactors (Fig. 4D) is a technique commonly found for producing larger quantities of cells and producing products such as antibodies or proteins but can also have utility for engineering cultured tissue models. Commercially available examples of these systems are commonly in the form of spinner flasks, such as the Able® Biott® and Corning™ systems [37,38]. These can come in a range of sizes to allow for scale-up production and typically use a larger volume of medium than other methods alongside batch feeding to maintain adequate nutrient levels across the culture period. The stir speed and shape of the stirrer allows for significant control over the mass transfer and shear stress in the system. Variation of the stir speed in this manner has been shown to impact the levels of different ECM components secreted by chondrocytes [57] and affect properties such as albumin secretion in HepG2 hepatocellular carcinoma cells [58]. One of the most common uses in tissue engineering is for the expansion of stem cells by maintaining stem cell pluripotency and offering an alternative to methods such as feeder layers [59], [60], [61]. This technique can also be used alongside 3D scaffold cultures to allow dynamic fluid flow to support tissue equivalents with a greater degree of structural control [62], [63], [64], [65].
Rocked and shaken bioreactors (Fig. 4E, F) are some of the simplest methods for creating fluid motion. These utilise low-cost equipment commonly found in the laboratory that can be housed within a cell culture incubator. They can be used in a variety of ways, such as with standard cell culture ware to provide additional mass transfer or bespoke plasticware can be designed for purposes such as allowing cultures to oscillate between contact with air and medium. These systems are also highly scalable due to the ability to stack multiple plates vertically without additional modification of the system. Pumpless organ-on-a-chip technology is also available in the form of the Mimetas OrganoFlow system [39]. This uses a rocker-based system to create fluid motion, removing the need for pumps and tubing and therefore requiring less complex apparatus. Studies have shown the utility of rocked perfusion to enhance mineral deposition with osteogenic progenitor cells [66,67], as well as for the maintenance of precision-cut liver slices through enhanced oxygen delivery [68,69]. Due to their simplicity and compatibility with standard culture plates, shaken bioreactors have been used in many studies to investigate the effects of shear stress on endothelial cells in 2D culture [70], [71], [72]. Some studies have also been demonstrated compatibility with Transwell® inserts, both using endothelial cells [73] and renal tubular epithelial cells [74]. One of the main limitations with this method is the lack of control over the direction of the fluid flow and the variability of the shear stress, with levels increasing with distance from the centre of the plate [75,76].
These different bioreactor designs demonstrate the range of ways that fluid flow can be introduced into cell and tissue culture, with the choice often dependant on cell type, 2D or 3D model requirements and the desired emulated physiological conditions. Suspension cells are commonly cultured in stirred or rotated systems, where the exact positioning of the cell relative to the fluid flow direction is not crucial. Instead, fluid flow is intended to facilitate mass transfer to support proliferation and aggregate formation. Selection of stirring speed is used to tune aggregate size and shape but is highly cell type and application dependant. Higher stirring speeds in spinner flask systems used for human induced pluripotent stem cells have been shown to form more homogenous round-shaped aggregates at higher stirring speeds without losing their pluripotency [77] whilst increasing the stirring speed too much can correlated to a reduction in aggregate size and increasing levels of cellular detachment from microcarriers [78,79]. As shear stress, velocity and pressure vary within stirred systems, shear stress values for suspension cells are not global but location dependant which should be taken in account when planning stirred suspension cultures [80]. A general assumption for suspension cells is that they can withstand high shear stress values, if they are naturally exposed to high flow rates in vivo like erythrocytes or leukocytes [81]. However, stem cells are often expanded in stirred systems and are more susceptible to shear stress, requiring fine tuning of the parameters to avoid loss of pluripotency [80].
Adherent cells require a substrate to grow on, which will affect perfusion characteristics. Although microcarriers for cell adhesion in stirred suspension cultures have been used, these systems do not allow for a tight control of a delicate microenvironment which is often necessary to archive desired tissue morphology and function. Adherent cells often exhibit polarization with a distinctive apical and basal side crucial for proper cell function and fluid flow affects this polarization [82]. Separation of these distinct compartments also allows for investigations into epithelial barrier and transport properties [83], studies which are of high value in areas such as intestinal drug absorption [84]. It is therefore favourable to use systems that allow for controlled flow regimes, like pumped or gravity driven systems with directed fluid flow. These forms of fluid flow allow for the incorporation of complex scaffolds and cellular supports, providing a basis for physiologically relevant recreations of tissue architecture [85]. Other cell type specific characteristics that should be taken into consideration when choosing a perfusion system, are metabolic activity, influencing how much medium is required, the frequency of medium changes and build-up of harmful metabolites as well as specific cues needed for certain differentiation processes such as an air-liquid interface for the differentiation of keratinocytes in models of the epidermis [86].
3. Fluid flow parameters
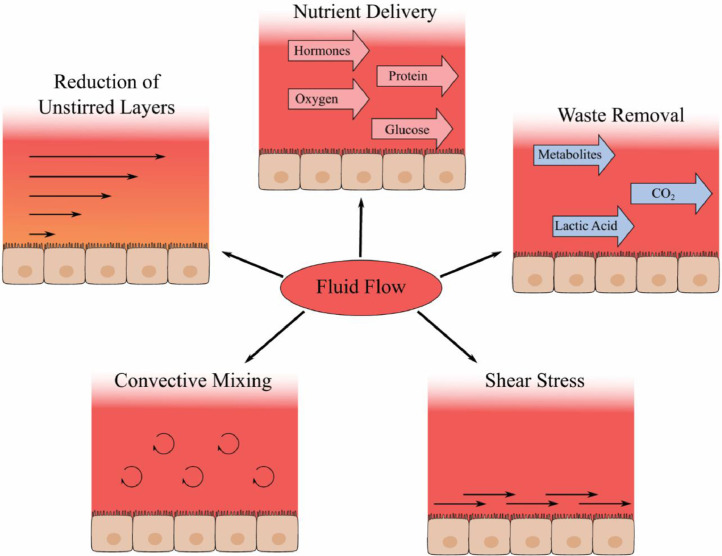
Bioreactor functions are centred around the controlled introduction of fluid flow to a cell or tissue culture. The addition of fluid flow has a major impact on a wide range of cell types with the exact properties of the fluid flow leading to different effects between varying cell types and tissues [87]. Some of the key impacts of fluid flow, as shown in Fig. 5, are on the mass transfer both in the form of nutrient delivery and waste removal [88,89], shear stress experienced by cells adjacent to the fluid flow [90,91], the convective mixing and resultant homogeneity of the media and the reduction in the size of the unstirred layers close to the cell boundary [92].
Fig. 5.
Direct impacts of fluid flow in cell culture. Fluid flow aids with healthy growth of cells through a variety of mechanisms. The supply of fresh nutrients, as well as the removal of metabolic waste can reduce nutritional stress on the cells. A reduction of the unstirred layer thickness and convective mixing aid with this process whilst also being useful for research such as transport studies. Shear stress presented to the cell surfaces creates mechanical signals which the cells respond to and can aid with maintaining physiological function however can have a detrimental effect at supraphysiological levels.
3.1. Mass transfer and nutrient levels
As cells take up media constituents such as glucose, proteins and vitamins for metabolic purposes, the levels of such molecules need to be replenished to maintain consistent culture conditions and healthy cells [93]. Supraphysiological levels of nutrients such as glucose, pyruvate and glutamine are often used for cell culture medium to allow for this reduction over time. While these concentrations were often derived empirically to maximise viability and proliferation, they do not necessarily mimic the environment in vivo and can induce further unwanted artifacts [94]. Many studies have demonstrated an induction of apoptosis in high glucose medium with a range of cell types [95], [96], [97], whilst improved medium formulations have been shown to produce more physiologically relevant metabolic levels and drug response in cancer cells [98], [99], [100]. On top of this, metabolic waste products such as lactic acid and ammonia can be damaging to cells and affect properties such as pH, with the levels of these building up over time in culture in the absence of frequent media changes [101].
Within bioreactors, motion of the fluid improves the level of mass transfer through convective mixing, on top of the diffusive mixing found in static culture. The mass transfer consists of the transport of both nutrients to tissue and of metabolites and waste products away from tissue [102]. Providing adequate mass transfer to cells can improve their proliferation, viability and function [26,103]. This is particularly relevant for the culture of stem cells which can have varied viability and differentiation states depending on nutrient availability [104] and for ex vivo tissue slices such as liver slices where high levels of oxygenation are typically required for long-term maintenance [105]. Effective mass transfer, both of nutrients to tissue and of metabolites and waste products away from tissue is therefore one of the key goals of fluid bioreactors for tissue engineering [102].
One of the most direct methods for increasing mass transfer is through an increase in fluid flow rate in a bioreactor. This can lead to an increase in convective mixing, as well as greater replenishment of medium around the cell surfaces therefore aiding mass transfer to and from the culture. Measurement of the relationship between fluid flow and mass transfer has been carried out for shaken bioreactors [106], impeller-based systems [107], [108], [109] however is rarely performed for bioreactors used in tissue engineering. The impact of fluid flow rates on cultures is often measured through cell viability or proliferation [110,111], though other changes can also be readily performed in some cases, such as albumin secretion in liver models [112,113] or the thickness and maturity of the epidermal barrier of skin models [114]. A beneficial impact typically tends to correlate with increasing flow rate until the point in which shear stress starts to have harmful impacts and outweighs the benefits of mass transfer, after which a decrease is measurable [111,112]. The impacts of shear stress within the system will be covered in more depth in Section 3.3.
Another factor which affects mass transfer is the geometry of a system. This can be beneficial or detrimental, depending on the bioreactor type and method of fluid movement. A key example of where the geometry can increase mass transfer is found in stirred bioreactor systems, in which the geometry of the impeller or stirrer has a major impact on the mass flow rate. The number of blades and blade angle, along with the impeller size, can lead to significant changes in mass transfer rates which in turn affects the performance of the cells within these systems [109,115,116]. Other geometric features which significantly alter the direction or rate of fluid flow, such as sharp corners, can lead to a shift from laminar flow towards turbulent flow. While generally regarded as less desirable in bioreactors than laminar flow, turbulent flow allows a higher level of convective mixing within the fluid layers. It can therefore be useful in regions of medium which are not in contact with cells, such as reservoirs, to maintain an even mix of molecules in the bulk medium.
The spatial position of cells also plays a role in the mass transfer and nutrient supply in a bioreactor system. Cells in direct contact with the fluid will see increased mass transfer whereas cells within the centre of a tissue mass are primarily supplied by diffusion through the tissue. Diffusion of oxygen is a relatively slow process and in tissue it has typically been measured to reach a maximum depth of between 100 and 200 µm from the vasculature and therefore presents a limiting factor for the size of tissue equivalents in the absence of vascularisation or fluid flow [117,118]. This is a problem commonly found in aggregates and spheroids, where interior regions suffer from a depletion of nutrients alongside a build-up of waste products, leading to a necrotic core [119,120]. Structures such as scaffolds or membranes used to support cells can also suffer from nutrient deficiency due to the reduced diffusion caused by the presence of the scaffold hence adequate mass transfer also plays an important role when using such materials [121]. The use of scaffolds with pores or channels to allow the perfusion of medium though the tissue construct can be an effective way to overcome this, with structural properties such as pore size and tortuosity impacting the mass transfer of solutes through the material [26,122]. Effective examples of this are hollow fibre bioreactors which use permeable fibres to transport medium through the culture. A precisely controlled distance between the interior cells and the flow of medium maintains effective mass transfer to all cells without limitations of diffusion distance. On top of this, the fibres also offer protection from shear stress, allowing high mass transport whilst minimising shear stress-related damage to cells [123,124].
3.1.1. Oxygenation
One specific example of the importance of mass transfer and nutrient supply in vitro can be found in the case of oxygen. Oxygen is essential to maintain viable growth and function however there is a wide variability in the dissolved oxygen levels seen in vivo, both between different tissues and different cells within a tissue. Regions such as the peri‑sinusoidal bone marrow have been found to experience an oxygen tension as low as and 0.64 kPa [125] whilst on the other end of the spectrum renocortical tissue and periportal liver tissue can both have levels reaching around 8–10 kPa [126,127]. Cell culture medium in a 5% CO2, humidified incubator typically has a dissolved oxygen level of around 18–19 kPa, though this value is lower in the pericellular region due to the cellular depletion and has been found to be near zero for cells with high oxygen consumption such as hepatoma cell lines and renal epithelial cells [128,129]. Meanwhile, the value is around 13 kPa for arterial blood [130]. This demonstrates the large complexity of mimicking physiologically relevant oxygen concentrations in vitro and why there is not a “one size fits all” approach to bioreactor design.
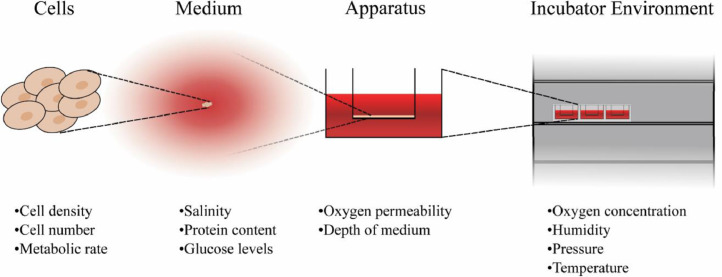
Fig. 6 shows some of the different properties of cell culture systems which can impact the oxygen received by cells. At the cellular level, factors such as cell density, quantity and metabolic rate all influence dissolved oxygen levels. Large quantities of cells or highly metabolic tissues deplete oxygen at a faster rate which may be higher than the diffusion rate of oxygen to the cells leading to reduced oxygen levels over time [131]. A second problem with large quantities of cells occurs when considering the diffusion distance of oxygen. As mentioned in the previous section, it is generally observed that the diffusion distance for oxygen within tissues is less than 200 µm. When creating tissues significantly larger than this a necrotic core is frequently reported and this distance therefore presents a size limitation for avascular in vitro tissues [132], [133], [134]. For medium, different formulations have different rates of oxygen solubility and diffusion depending on properties such as salinity and protein content [118]. The vessel used to hold cultures can lead to variability in oxygen levels due to factors such as the diffusion of oxygen through the vessel walls. This effect has been investigated for increasing the oxygen levels using highly gas-permeable materials in both static and dynamic culture systems [135], [136], [137], [138]. The depth of the culture within the medium also leads to changes in oxygen concentration, with the major limiting factor being the diffusion rate through the media, the further a culture is from the air the lower the dissolved oxygen levels will be. Atmospheric pressure and oxygen levels also impact the dissolution of oxygen into the media whilst aspects such as media depth and flow rate affect the diffusion and transport of oxygen through the media.
Fig. 6.
Properties which can impact the levels of dissolved oxygen in culture. The different properties can be divided by the scale upon which they act. At the smallest scale, the properties of the cells can change the oxygen properties through varying the rates of depletion in the surrounding media. Medium properties such as the molecular constituents have an impact through the changes in the oxygen solubility. Culture geometry such as the distance to the air-liquid interface and the permeability of vessel walls influence uptake into the medium. The atmosphere that the culture is in also has an impact, with factors such as humidity, pressure and temperature affecting local levels of oxygen concentration.
Additional oxygenation systems are often used in conjunction with perfusion bioreactors to achieve optimal conditions. These can range from placement of the bioreactor in an oxygenated incubator to self-contained oxygenation systems specific for the bioreactor [139]. Alternatively methods such as disruption of the gas-liquid boundary of the media has been used to increase oxygen concentration without an additional oxygen supply [140]. For applications which require lower oxygen levels a commonly used method is to reduce the partial pressure of atmospheric oxygen with technology such as hypoxic chambers. Alternatively reductions in oxygen delivery to media, either actively through the control of flow rates or passively through oxygen-permeable membranes, can be another useful tool particularly if access to a gas supply is limited [138,[141], [142], [143], [144]]. This leads to wide variability in the effectiveness of oxygenation systems and characterisation of oxygenation levels is important if results can be accurately replicated [131].
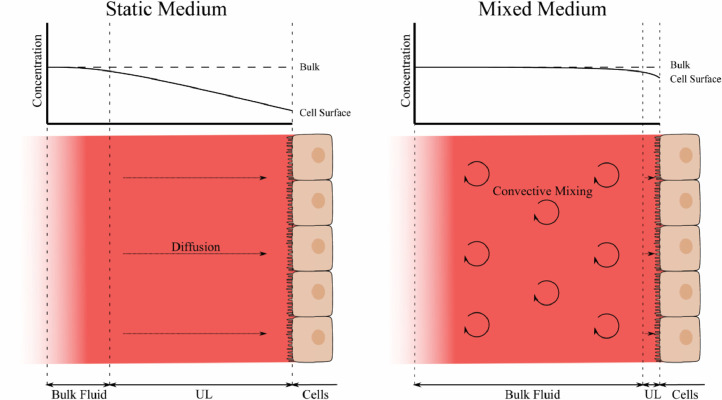
3.2. Reduction in unstirred layer thickness
Unstirred water layers are regions close to membranes in which limited convective mixing occurs and so the majority of mixing occurs by diffusion of molecules [145]. These vary in size and impact depending on the particular solute, the viscosity of the fluid and any convective mixing that the fluid is undergoing. Fig. 7 shows the effect that a larger unstirred layer in static culture medium has on the solute concentration for nutrients at the cell surface compared to in mixed medium. The addition of convective mixing reduces the thickness of the unstirred layer and therefore increases the concentration at the cell surface. The impact on tissue culture is therefore unique to each individual tissue model and the exact impacts can be difficult to predict. The first potential problem caused by these is the diffusion of nutrients such as oxygen to the cells. Whilst uptake of oxygen into the cells depletes the concentration in these regions, the limited diffusion can lead to reduction in oxygen available to the cells and therefore lead to a greater level of oxygen stress than expected from the oxygen concentration in the bulk medium [146]. The size of the unstirred layers is generally regarded to be inversely proportional to the diffusion coefficient for a molecule [147]. Oxygen, with a high diffusion coefficient, can therefore be predicted to have a large unstirred layer with a thickness up to several hundred microns, as has been found for similar solutes such as carbon monoxide [148], and is therefore a far more likely rate-limiting step for uptake into cells than barriers such as the cell membrane.
Fig. 7.
Unstirred layers in static and dynamic cell culture conditions. In static culture the medium is mixed through diffusion which can lead to a depletion in concentration close to the cell surface, a region termed the unstirred layer (UL). The application of convective mixing to the medium, such as with fluid flow, greatly increases the distribution of molecules within the medium and reduces the thickness of the unstirred layer, where diffusion is predominant. This in turn leads to greater levels of solute concentrations at the cell surface.
As well as potential nutrient and metabolite diffusion problems, another example of an issue which can be caused by unstirred water layers is additional inaccuracy in measuring cellular transport for drug metabolism studies. The limitations to uptake caused by the unstirred layers can lead to a significant underprediction of uptake rates into cells. This can lead to errors in the measurement of membrane transport, but can also be problematic when calculating drug metabolism and clearance due to becoming the major rate limiting step [149,150]. These effects have been heavily studied in the context of gut permeability and hepatocyte drug clearance with results showing that perfusion or agitation of the medium can be an effective way to reduce the impact of unstirred layers on permeability measurements [151], [152], [153].
3.3. Shear stress
Shear stress is caused by the flow of fluid across a cell and is proportional to the fluid flow rate parallel to the cell surface. The level of shear stress is a key property of fluid flow in native tissue and can vary over many orders of magnitude between different regions in a tissue, such as blood or interstitial fluid. Cells can detect and respond to shear stresses, and therefore an accurate replication of in vivo shear stress levels can be beneficial for physiologically relevant function [154,155]. The mass transfer is also proportional to the fluid flow rate so this can lead to the difficult position of balancing a high mass transfer with the typically low levels of shear stress required for many cells [156]. The use of baffles, porous membranes and specific topographies to shield the cells from the bulk levels of fluid flow can be an effective way to maintain adequate mass transfer whilst keeping shear stress at a reasonable level [63,157,158].
In vivo, high shear stress is primarily experienced by endothelial cells lining blood vessels and these protect many of the other cell types from being directly in contact with the blood flow. The level of shear stress that endothelial cells receive varies hugely between different regions of vasculature with arterial endothelial cells experiencing levels around 1.5 Pa while in smaller vessels such as the liver sinusoids a range of around 5 to 10 mPa is experienced [159,160]. Factors such as variations in blood vessel diameter and bends in the vessels also contribute to further variation in the shear stress levels. Shear stress applied to endothelial cells at these levels has been shown to have some beneficial effects such as promoting angiogenesis and inhibiting inflammatory responses [161].
Due to the shielding function of the endothelial cells, most non-endothelial cells experience levels of shear stress which are many orders of magnitude lower and bioreactors often utilise shear stresses in the micropascal region [162,163]. Higher arterial values can still be useful with these cells, however, for researching the effects of mechanical stimulation and response to injury, for example mimicking damage to the endothelial wall and subsequent cellular responses [164], [165], [166]. Values of shear stress experienced by many cell types are difficult to acquire, either experimentally or computationally, due to the protection offered by the endothelial cells and therefore a range of shear stresses are often explored to experimentally determine the optimum values. Shear stress experienced by cells through interstitial fluid flow outside the vasculature has been the subject of numerous investigations, using in vivo, in vitro and in silico studies [166], [167], [168], [169], [170]. Depending on the application, extensive reviews are available which provide helpful compilations of experimentally acquired shear stress data for various cell types, particularly in the context of endothelial cells [164,[171], [172], [173]]. For example, a recent publication on organ-on-a-chip applications refers experimentally derived shear stress values for different cell types, which could be used as a starting point for initial experiments to empirically determine which levels of shear stress are beneficial for the desired outcome [174]. In the case of cell types with limited literature available, shear stress values from cell types residing in a similar environment to that being investigated could provide an initial estimation.
The liver, as an example with a high level of vasculature, is often used in fluid flow systems and many studies have performed this analysis to experimentally determine optimum conditions in vitro in the absence of accurate in vivo values [162,175,176]. Computational fluid dynamics (CFD) can be used alongside experimental methods to confirm that the levels of shear stress are within a similar range to estimated physiological levels, and this analysis has been performed previously for many commercial and bespoke bioreactor systems [177,178]. As well as shear stress, these simulations can be used to provide an insight into distribution and transport of nutrients such as oxygen, making them a powerful tool for understanding the complex conditions within a bioreactor system [179]. The ability to fine-tune the shear stress of a bioreactors in this way allows for accurate replication of in vivo conditions as well as expanding the possible applications and therefore increases the research value of a system [63,180,181]. While the use of CFD allows a bioreactor to be optimised for specific tissues, in practice this is not always performed as effectively as it could be. One recent review, for example, highlights that many bioreactors for bone tissue engineering are designed for generic bone tissue engineering, rather than for use modelling specific bones, which could therefore limit their utility due to the wide variability of conditions in different locations [182].
Viscosity of culture medium also has an impact on the shear stress experienced by cells under flow, however this is less well-studied compared to many of the factors mentioned previously. For the purposes of computational modelling, medium formulations are often assumed to have a viscosity close to that of water however addition of components such as proteins can lead to significantly increased viscosity. Addition of 10% foetal bovine serum, as is often used in cell culture, has been measured to cause an increase in the viscosity of the medium by 20–30% whilst further changes over time can also be caused by secretion of proteins from cells during culture [183]. The presence of serum can also cause the medium to have the non-Newtonian behaviour of shear thinning, adding further complexity to shear stress modelling through a reduction in viscosity at higher shear stresses [184].
3.4. Flow direction and uniformity
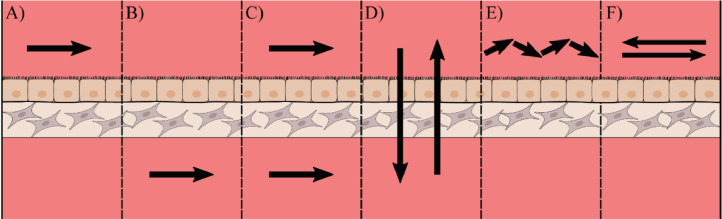
The direction of the flow may need to be altered to cater for different tissues as illustrated in Fig. 4. The two most basic types of fluid flow are across the tissue or through the tissue and these can either be continuous or pulsatile. Flow across a tissue can be performed in specific compartments of a tissue: for example fluid flow over the epithelium (Fig. 8A) has been used for modelling intestinal epithelia [46]; perfusion below the stromal tissue (Fig. 8B) is widely used and has shown positive results for applications such as skin models and renal models [185,186]; flow all around a tissue can be useful for delivery of nutrients to tissues with high nutritional needs [187]. Flow through a tissue (Fig. 8D) can be more useful for perfusion of larger constructs as this method can be used to aid diffusion of molecules such as oxygen into the centre of these tissues [134,188].
Fig. 8.
Methods for introducing fluid flow to an epithelial submucosal tissue model. With certain complex tissues consisting of an epithelial layer and a stromal compartment there are many possible ways to introduce flow. (A) Flow along the epithelial surface such as that found in the intestinal lumen (B) Flow along the stromal surface partially mimicking blood supply to a tissue (C) Flow across all surfaces of the model (D) Flow perfused through the tissue which can be used to improve the diffusion of nutrients such as oxygen to the centre of thicker tissue constructs. (E) Pulsatile flow, such as that produced by a peristaltic pump, can be used to mimic the pumping motion of the heart for modelling structures such as arteries. (F) Reciprocal oscillatory flow, which changes direction over a set period, allows convective mixing to be introduced using typically very simple apparatus such as a mechanical rocker.
Further increases to the complexity and physiological relevance of flow patterns can be the introduction of pulsatile or oscillatory flow. Pulsatile flow (Fig. 8E), such as that created by peristaltic pumps, is a more accurate representation of the flow of blood in regions such as larger arteries, replicating the conditions produced through the pumping mechanism of the heart. The addition of pulsatile flow to cultures can be straightforward to implement and has been shown to create beneficial conditions for arterial cell culture, with enhancements to endothelial cell function and angiogenesis [189], [190], [191]. As an alternative to pulsatile flow, reciprocal oscillatory flow can also be used, in which the flow alternates in direction over a designated period (Fig. 8F). Oscillatory flow patterns are highly relevant in vivo, especially in connective tissue, like bone tissue, and the vasculature. The effect of oscillatory flow perfusion regimes, either continuous, intermittent or varying in flow rate, has been studied for various cell types and tissue models. Intermittent oscillatory flow stimulations of mesenchymal stem cells during otherwise static conditions, have been shown to robustly upregulate osteogenic gene expression, drive lineage commitment and enhance collagen secretion and mineral deposition [192], [193], [194]. The application of continuous oscillatory flow regimes at low rates enhanced cell viability, uniform cell distribution and early osteogenesis in scaffold-supported osteoblast-like cell-based models [195,196]. In a combinatorial approach, bone-tissue models were continuously perfused at a low oscillatory rate with intermittent high-rate bouts to archive mechanical stimulation which significantly increased prostaglandin E2 levels, an important factor for bone formation [197].
Oscillatory flow can be induced using equipment such as a rocker creating a simple way to perfuse a model for media mixing whilst reducing the volume of addition equipment required. Simple oscillatory flow in this way has been used successfully for the differentiation of mesenchymal stem cells and the maintenance of precision-cut liver slices [67,68]. Modifications of these methods can also be used to create more complex systems for specific applications such as stacked substrates for patterned co-culture [198] and cultures with adjustable medium levels for raising cells to the air-liquid interface [199].
Complex geometries, in particular the presence of sharp angles, can lead to irregularities in the fluid flow and therefore mass transfer and shear stress [200], [201], [202], as well as leading to an increase in turbulence. Tissue culture bioreactors frequently utilise laminar flow, in which the motion of fluid particles is parallel to the bulk flow of the fluid, to create the desired conditions due to its uniformity and ease of modelling. Blood flow in vivo is also typically regarded as being laminar. Turbulent flow, which is more chaotic and disordered, leads to irregularities in the fluid flow and increases the complexity of the systems from a modelling perspective, though modern computational fluid dynamics software can be used to model these systems with relative ease [203]. Turbulent flow in vivo can be found in curved regions of the vasculature as well as at branch points and has been associated with increased risk of disease such as atherosclerosis [204,205]. The usefulness of turbulence within a tissue culture bioreactor is therefore dependant on the application, with turbulent regions effective for keeping the bulk medium mixed, though typically less desirable in regions of fluid that are in contact with cells due to the disruptive effects of the shear stress.
4. Flow-independent parameters
Aside from fluid flow parameters there are many other properties which can influence the outcomes of perfusion bioreactor techniques. Cytocompatibility issues can be caused when working with certain materials. The operation of a bioreactor and some design parameters can also cause deleterious effects such as fluctuations in culture temperature or a constant deviation from the desired temperature. Some feeding regimes can lead to issues such as media degradation, particularly if media changes are carried out infrequently as in some batch-fed systems. High levels of user interaction and manual handling can also be problematic by introducing additional variability to cultures which may impact reproducibility. Designing a bioreactor with an appreciation of these problems and with an aim to work around them as much as possible can lead to systems which more accurately agree with the theoretical outcomes of the system and are therefore of improved research value.
4.1. Cytocompatibility of materials
Routine cell culture ware has typically been made from materials which are known to have high cytocompatibility, with polystyrene being the most common material used for items such as multi-well plates and flasks. This is a material which is known to be very biologically inert and compatible with a wide range of cell types which makes it ideal for culture of cells with minimal side effects [206]. However other materials exist that have been thoroughly characterised for biocompatibility and may represent alternative options for use in bioreactor design [85,207,208].
Whilst polystyrene is a suitable material compatible with mass production techniques such as injection moulding, there are many other materials which are easier to work with at the scale of individual laboratories. Hard plastics such as polycarbonate (PC), polyethylene terephthalate (PET) and polytetrafluoroethylene (PTFE) are commonly used in bioreactors due to their ease of manufacture and minimal biological activity. PC and PET are regularly used as they have reasonable chemical resistance, high biocompatibility and can be readily treated to enhance cellular adherence [209,210]. This has led to many cell culture membranes, such as Transwell® and Millicell®, being made from these polymers. PTFE is highly inert and has a high melting temperature making it suitable for repeated autoclaving. It is a useful material for creating bioreactor components and is often used for the outer layer of magnetic stir bars. PTFE is also highly hydrophobic and has an extremely low coefficient of friction. This makes it useful in the case of moving parts to minimise wear, however it also means that PTFE requires further surface modification to be viable as a growth substrate for cells [211,212].
Rapid prototyping methods such as fused deposition modelling (FDM) or stereolithography (SLA) offer the ability to create custom designs within a short turnaround time. However, some of the materials typically used for these techniques are of limited value for cell culture. Acrylonitrile butadiene styrene (ABS) and MED610 have both been found to cause changes to cell viability and gene expression, such as an upregulation of oestrogen-response genes, in some cell lines, which shows that materials should be carefully chosen to ensure there are no specific biological effects with the cells of interest [213]. Polylactic acid (PLA) is another material which is widely used for FDM and has been shown by many studies to have a high level of biocompatibility [214,215]. Much like polystyrene used for conventional cell culture, PLA is a hydrophobic polymer and can benefit from surface modification to boost its adherent properties if cells are to be grown directly onto it [216].
A second problem found with techniques such as SLA is the leeching of compounds such as photoinitiators and plasticizers into the medium. Even with polymers which have been certified to be biocompatible, toxicity issues have been identified with cell culture. The continual release of photoinitiator into the medium for up to 7 days of culture has been demonstrated, along with a consequent reduction in viability [217,218]. In some cases these issues can be reduced by post-manufacture treatment with solvents such as ethanol [219].
Non-plastic materials are also often found in bioreactors. Glass and stainless steel are two common examples of these. Glass has been widely used for cell culture since its inception as both a growth surface and as a material for vessels. A high level of chemical resistance, minimal biological activity make glass a useful material for cell culture, and its high melting temperature makes it compatible with a range of heat sterilisation methods such as autoclaving and flaming, allowing glass components to be easily sterilised and reused. For this reason, glass is still commonly used for 2D culture on coverslips and slides. Drawbacks of glass are its fragility, which means that handling can be more difficult, as well as being more difficult to manufacture at small quantities, particularly in complex designs, which can lead to high costs for prototyping. Stainless steel can also be used for components of bioreactors and is commonly found in large-scale stirred tank systems due to its mechanical properties, chemical resistance and ease of cleaning making it suitable for repeated use [220]. For smaller bioreactor systems plastics are typically used instead of stainless steel due to their lower cost and ease of manufacturing, though some laboratories still use stainless steel for many components of their bioreactors [221].
Other issues can occur from materials that may seem on the surface to be ideal candidates for the creation of bioreactors. Commonly used sterilisation and disinfection procedures can cause changes to the chemical properties for a material potentially leading to unwanted biological effects [222,223]. For example, treatment of some polymers with laboratory disinfectant can lead to cytotoxicity from residual disinfectant absorbed into the polymer [224] whilst ultraviolet light (UV) treatment can also cause problems with some plastics, leading to changes in surface properties if exposed for periods of several hours [225]. Autoclaving generally leads to minimal increases in cytotoxicity and in some cases has even caused materials to have improved biocompatibility after autoclaving, however it is not compatible with plastics with low melting points such as conventional polystyrene cultureware [226]. Due to the wide range of available sterilisation methods, it is typically possible to find a method which is non-disruptive to the properties of the apparatus, allowing the preferred materials to be used.
4.2. Temperature variation and fluctuation
Cells and tissues require precise temperature control, typically at 37 °C, to maintain normal function and to promote reproducibility. Cell cultures are very sensitive to increases in media temperature and an increase of a few degrees can significantly reduce cell proliferation and viability over prolonged periods [227], [228], [229]. The effect of changing temperature goes beyond viability and proliferation, with many metabolic processes also affected. Other metabolic events can be altered by the changing temperature leading to inaccurate results in drug toxicity experiments [230] whilst expression of a range of proteins varies with the extent of the temperature increase [231].
The use of electronic equipment for powering perfusion in some bioreactor systems can impact the cultures through the effects it has on the temperature. With equipment that is thermally separated from the culture, for example power supplies which are outside the incubator or at a distance from the culture vessels, the impact of this is negligible. When equipment is in direct contact with the culture vessel, for example in the case of stirred or shaken bioreactors, undesirable additional heat can be transferred into the culture system. Whilst the impact of this is likely to only changed the temperature by a few degrees, as discussed in the previous paragraph this can have major repercussions over prolonged culture periods. As well as simply affecting cellular properties, this can also introduce variability between experiments, particularly if the contact regions have unequal heat distribution or if multi-culture systems are under different levels of load.
The impact of lower temperatures on cultures tends to be less heavily studied than increased temperatures but it has been shown to have effects such as a decreased metabolic rates and reduced proliferation [232,233]. Differentiation can also be altered with reduced temperature, with one study demonstrating effects such as delayed early differentiation and enlargement of granular cells in the epidermis of a full thickness skin model when cultured at temperatures of 33 °C and 35 °C [234]. The use of small fluid volumes or long lengths of tubing without adequate insulation or jacketing can lead to greater temperature fluctuation and reductions below the desired temperature. Whilst measurement of this effect is carried out infrequently, studies have shown that it can be done with relative ease to confirm that growth conditions are maintained at desirable levels for a range of system scales [45,235,236].
4.3. Evaporation and degradation of culture medium
The design and operation of a bioreactor system can have an impact on the quality of the culture medium over time. Long culture periods without media changes or use of small volumes can lead to high levels of evaporation which can be problematic for cell cultures, even in a humidified environment [237]. Large media volumes can be beneficial due to the more homogeneous media composition achieved without media changes. However, this can be impacted by evaporation and the resultant increase in the concentration of dissolved compounds over time. Use of media gas supplies such as oxygenators can also increase evaporation due to the addition of dry gas lowering the relative humidity, and bubble humidifiers are therefore often used [238,239]. Evaporative loss of medium still occurs in unsealed systems regardless of whether the environment is humidified. One study using a 24-well bioreactor system found an evaporation rate of roughly 0.6% per day over a 9-day culture period [103] whilst another study showed an evaporation rate of roughly 0.4 mL/day in a perfusion circuit at 20% relative humidity, whilst in the absence of humidity this was increased to around 1.4 mL/day [240].
Another potential issue with larger media volumes with minimal media changes aside from evaporation is the degradation of media constituents. Common media supplements such as L-glutamine and ascorbic acid are heat sensitive and therefore degrade over time in culture [241,242]. The degree to which this is a problem varies depending on specific conditions such as the culture period however it can also be very variable between cell types, with some cell lines being shown to be unaffected by L-glutamine degradation whilst others suffer a loss in viability [243]. This can be avoided by topping up the supplements periodically either directly or through partial media changes. In some cases supplements which are more stable can be used, for example in the case of L-glutamine a range of products using the L-alanyl-L-glutamine are available which have reduced build-up of ammonia compared to L-glutamine [244,245]. It is worth noting that not all supplements should be periodically topped up due to potential cytotoxic effects from large quantities of their degradation products [246,247].
Whilst replacement of the medium is beneficial for keeping nutrient and metabolic waste within desirable limits, it can also have undesirable effects on the function of cells. Over time in culture a variety of secreted molecules comprising the cell secretome influence the cellular function, and this communication has a key role for determining culture fate [248]. Secreted factors influence the viability, proliferation and function of cells, as well as being able to direct differentiation [249]. With pluripotent stem cells, for example, autocrine factors are secreted by the cells which aid with proliferation and maintenance of the undifferentiated state, whilst paracrine factors from other cell types have ability to lead embryonic stem cells down different pathways depending on the cells used for co-culture [250], [251], [252]. Whenever the medium is changed, these secreted factors are lost, and levels have to build back up in the fresh medium. Recirculation of medium can allow these factors to remain, whilst the use of conditioned medium also represents an alternative method to provide cells with necessary factors, even between media changes.
4.4. User interaction and manual handling
One problem which can occur in cell culture is the presence of biological contamination within the system. This can be in forms such as microbial contamination or cross-contamination with different cell lines [253], [254], [255]. Microbial contamination in cell culture, such as by bacteria or yeast, is an occasional occurrence in many laboratories and is often caused by poor aseptic technique during handling [256]. Even with the use of antibiotic and antimycotic agents it is still possible to get a contamination of these types, which negatively impact the experiments and lead to incorrect results, wasting time and money [257].
Careful aseptic technique is used to reduce the chances of contaminations and means that for the majority of the time these are not a problem during cell culture, however all handling of a sterile system increases the risk of contamination. Complex techniques and apparatus often require increased user interaction and therefore one challenge in the design of bioreactor systems is reducing the amount of handling of sterile components that is required to minimise the risk of contamination. Through careful optimisation of the design parameters, systems have been developed which can minimise these aspects of handling to maintain a high level of sterility [188].
One of the key uses of bioreactors for tissue engineering is to measure and control the conditions within the system, allowing fine tuning for tissues and cells of interest. Recent designs are frequently using continuous or non-invasive measurement systems to reduce the risk of handling cultures for data collection. Such probes can be maintained in the apparatus for the entire period and can be read through direct electrical connections or contactless methods. These can often be readily miniaturised and are now widely used in microfluidic culture systems for measurement of properties such as dissolved oxygen and pH [258], [259], [260].
As well as the contamination risk, manual handling can also have an impact on the reproducibility of the results. The reproducibility of published results is increasingly found to be a problem in cell biology and the creation of bioreactors which keep processes as simple as possible is key to generating systems in which results can be accurately reproduced between different individuals and laboratories [261], [262], [263]. Studies have shown that culture handling can be a potential source of error for assay results [264], and therefore minimal handling during ongoing culture is preferable to enhance the reproducibility of work and is something that should be factored into the design of bioreactors.
5. Limitations and future directions
While this review aimed to cover some of the major factors which influence the function and physiological relevance of bioreactor technology for the field of tissue engineering, there are many more properties which did not fit within the scope of this work. In a theoretical ‘perfect’ bioreactor system we might aim to control every environmental property which affect the cultures, with factors not mentioned here such as pressure and pH all at tightly controlled physiological levels whilst other factors such as oxygen might have their in vivo tissue oxygen gradients recreated. The reality is that with each additional factor controlled for, the subsequent complexity of the system increases and as such there is a trade-off between control and the time, cost and expertise required to develop and use the bioreactor.
A common concomitant of advancing system complexity is manual handling, increasing the contamination risk and disruption of the cell culture environment. While basic perfusion can be introduced rather simple with a rocker, more elaborate flow patterns may require pumps with adequate control units that can be space consuming and technically complex. High system complexity is also more prone to error, especially in long-term experiments that require steady and tightly controlled conditions. Each additional component of a system has to be compatible with the cell culture environment and is a possible source of variation which has to be considered and accounted for in experimental planning. Introduction of complex flow patterns also requires extensive testing to validate correct fluid flow within the system.
Further complexity is found through the interplay between different properties. Much like how mass transfer and shear stress are intrinsically linked to flow rate, so are other factors together such as carbon dioxide levels and the pH of the medium. These relationships make the creation of ideal conditions more difficult, with the movement of one towards more physiological levels having the potential to move the other further from them, and this is something that has not been covered in this review. In such cases it is often that a compromise must be found which promotes the physiological function of cells as best as possible within the limits of the system. New culture platforms are being created at great speed to push these boundaries and ongoing technological advances are further supporting the capacity for replication of physiological conditions and monitoring these in real time. Non-invasive monitoring of factors such as pH, glucose and oxygen concentration in the medium can give a real-time view of conditions over an entire culture period [265] whilst new sensors are being developed to allow a wider range of proteins to be continually monitored [266,267]. Highly penetrative imaging using multiphoton microscopy can allow detailed analysis of thick tissue equivalents and can be minimally invasive through the use of methods like second harmonic generation, which can allow label-free visualisation of proteins including collagen I [268,269].
Another potential limitation is the difficulty determining the ideal fluid flow conditions for a given organ due to the complexity of tissue structure. As a prime example, in the liver the commonly used cells for modelling drug metabolism in vitro are hepatocytes. These cells experience low levels of shear stress due to the protective effect of the sinusoidal endothelial cells however also have high levels of mass transfer due to the highly permeable, fenestrated surfaces of the sinusoidal endothelial cells [270]. These low shear stress conditions are significantly different to the experience of sinusoidal endothelial cells, as well as Kupffer cells, the resident liver macrophages, which reside within the sinusoidal lumen [271]. The variability is further complicated by the nutrient gradient present in the liver lobule, with periportal cells experiencing high levels of oxygenation whilst pericentral cells experience it at reduced levels, a factor which helps to determine the specific cell function [272]. These cell-specific conditions are key to accurately recapitulating a tissue in vitro, however, also introduce significant technical challenges. Studies often develop models for studying a specific physiological condition and can therefore minimise the complexity in terms of number of cell types and spatial organisation. This makes it easier to provide biomimetic conditions to the cells that are included however can limit the transferability of models between different applications.
6. Conclusion
The development of perfusion bioreactors is a fast progressing field and new systems being produced for a wide range of different applications the requirement for characterising the properties of systems in as much depth as possible is increasingly important to ensure reproducibility and applicability of results between systems [263]. In a perfusion bioreactor a key aspect is the properties of the fluid flow. With properties such as flow rate, shear stress and fluid mixing all having significant impacts on the health of tissues these need to be tailored or the tissue of interest. Modelling of the fluid flow is a powerful tool for both characterisation and development of a system and is now routinely carried out for newly developed bioreactor systems [273], [274], [275], [276]. The ability to accurately model the flow in a system computationally without the need for time intensive prototyping and testing can speed up the initial stages of design and allow issues to be picked up early in the development process.
A series of other properties can impact the tissue health. Factors such as cytotoxicity of materials are universal to the design of any system and therefore care should be taken to select materials which are most appropriate and effective for the requirements of a system. Other factors tend to be specific to particular methods being utilised such as evaporation of media, temperature fluctuations and media degradation which are of concern for systems with low volumes or long culture periods. Meanwhile, some properties are far more subjective and need to be weighed against the criteria for the system. High levels of user interaction can have an impact on the success and reproducibility of culture techniques, and therefore minimising the need for interaction where possible is generally preferable. While some of these parameters may be of less importance in the early development stages of a bioreactor, reproducibility, ease of use and cost should generally be regarded as key aims for systems which are aiming for wider adoption.
The factors considered herein aim to cover a range of possible requirements for benchtop perfusion bioreactor systems. The range of applications for these systems coupled with individual constraints such as readily available equipment and space means that there is no one-size-fits-all approach to creating perfusion bioreactors for tissue engineering. There is also a range of properties found in vivo which can be controlled on top of those dealt with specifically by bioreactor systems and can have an impact on cell and tissue culture outcomes such as medium composition, heterocellularity and cellular patterning. These are all factors which also need to be taken into account when designing the optimum conditions to encompass the huge complexity of physiologically tissue microenvironment. By discussing these key factors, and examining the complex interactions between them, this review aims to promote the future development of benchtop bioreactor systems for culturing tissue equivalents. A reduction in development cost, both financially and in time spent on optimisation, through incorporating these properties in the early stages of the design process will support more novel bioreactor technology to reach routine use in laboratory experiments. Further to this, with the wide range of different bioreactor systems in use, thorough understanding and reporting of culture conditions can improve comparability between studies and boost the research impact of future studies.
Conflict of interest
Author S.P. is affiliated with the company Reprocell Europe. All other authors declare no competing interests.
Funding
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) through the Centre for Doctoral Training in Molecular Sciences for Medicine programme (MoSMed CDT, Grant number EP/S022791/1)
References
- 1.Yamada K.M., Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Knight E., Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 2015;227:746–756. doi: 10.1111/joa.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urbanczyk M., Layland S.L., Schenke-Layland K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2020;85–86:1–14. doi: 10.1016/j.matbio.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Darling N.J., Mobbs C.L., González-Hau A.L., Freer M., Przyborski S. Bioengineering novel in vitro co-culture models that represent the human intestinal mucosa with improved caco-2 structure and barrier function. Front Bioeng Biotechnol. 2020;8:1–15. doi: 10.3389/fbioe.2020.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause S., Maffini M.V., Soto A.M., Sonnenschein C. A novel 3D in vitro culture model to study stromal–epithelial interactions in the mammary gland. Tissue Eng Part C Methods. 2008;14:261–271. doi: 10.1089/ten.tec.2008.0030. [DOI] [PubMed] [Google Scholar]
- 6.Ullm F., Riedl P., Machado de Amorim A., Patzschke A., Weiß R., Hauschildt S., Franke K., Anderegg U., Pompe T. 3D scaffold-based macrophage fibroblast coculture model reveals IL-10 dependence of wound resolution phase. Adv Biosyst. 2020;4 doi: 10.1002/adbi.201900220. [DOI] [PubMed] [Google Scholar]
- 7.Li F., Cao L., Parikh S., Zuo R. Three-dimensional spheroids with primary human liver cells and differential roles of Kupffer Cells in drug-induced liver injury. J Pharm Sci. 2020;109:1912–1923. doi: 10.1016/j.xphs.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Dwyer A.R., Ellies L.G., Holme A.L., Pixley F.J. A three-dimensional co-culture system to investigate macrophage-dependent tumor cell invasion. J Biol Methods. 2016;3:e49. doi: 10.14440/jbm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yun Y.E., Jung Y.J., Choi Y.J., Choi J.S., Cho Y.W. Artificial skin models for animal-free testing. J Pharm Investig. 2018;48:215–223. doi: 10.1007/s40005-018-0389-1. [DOI] [Google Scholar]
- 10.Roger M., Fullard N., Costello L., Bradbury S., Markiewicz E., O'Reilly S., Darling N., Ritchie P., Määttä A., Karakesisoglou I., Nelson G., von Zglinicki T., Dicolandrea T., Isfort R., Bascom C., Przyborski S. Bioengineering the microanatomy of human skin. J Anat. 2019;234:438–455. doi: 10.1111/joa.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duval C., Schmidt R., Regnier M., Facy V., Asselineau D., Bernerd F. The use of reconstructed human skin to evaluate UV-induced modifications and sunscreen efficacy. Exp Dermatology. 2003;12:64–70. doi: 10.1034/j.1600-0625.12.s2.10.x. Suppl. [DOI] [PubMed] [Google Scholar]
- 12.Goyer B., Pereira U., Magne B., Larouche D., Kearns-Turcotte S., Rochette P.J., Martin L., Germain L. Impact of ultraviolet radiation on dermal and epidermal DNA damage in a human pigmented bilayered skin substitute. J Tissue Eng Regen Med. 2019;13:2300–2311. doi: 10.1002/term.2959. [DOI] [PubMed] [Google Scholar]
- 13.Sen C., Freund D., Gomperts B.N. Three-dimensional models of the lung: past, present and future: a mini review. Biochem Soc Trans. 2022;50:1045–1056. doi: 10.1042/BST20190569. [DOI] [PubMed] [Google Scholar]
- 14.King S.M., Higgins J.W., Nino C.R., Smith T.R., Paffenroth E.H., Fairbairn C.E., Docuyanan A., Shah V.D., Chen A.E., Presnell S.C., Nguyen D.G. 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front Physiol. 2017;8:1–18. doi: 10.3389/fphys.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuffin J., Chesor M., Kuzmuk V., Johnson T., Satchell S.C., Welsh G.I., Saleem M.A. GlomSpheres as a 3D co-culture spheroid model of the kidney glomerulus for rapid drug-screening. Commun Biol. 2021;4 doi: 10.1038/s42003-021-02868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DesRochers T.M., Suter L., Roth A., Kaplan D.L. Bioengineered 3D human kidney tissue, a platform for the determination of nephrotoxicity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell C.C., Hendriks D.F.G., Moro S.M.L., Ellis E., Walsh J., Renblom A., Fredriksson Puigvert L., Dankers A.C.A., Jacobs F., Snoeys J., Sison-Young R.L., Jenkins R.E., Nordling Å., Mkrtchian S., Park B.K., Kitteringham N.R., Goldring C.E.P., Lauschke V.M., Ingelman-Sundberg M. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep. 2016;6:1–13. doi: 10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen D.G., Funk J., Robbins J.B., Crogan-Grundy C., Presnell S.C., Singer T., Roth A.B. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macedo M.H., Martínez E., Barrias C.C., Sarmento B. Development of an improved 3D in vitro intestinal model to perform permeability studies of paracellular compounds. Front Bioeng Biotechnol. 2020;8:1–17. doi: 10.3389/fbioe.2020.524018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden L.R., Nguyen T.V., Garcia-Mojica S., Shah V., Le A.V., Peier A., Visconti R., Parker E.M., Presnell S.C., Nguyen D.G., Retting K.N. Bioprinted 3D primary human intestinal tissues model aspects of native physiology and ADME/Tox functions. IScience. 2018;2:156–167. doi: 10.1016/j.isci.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda J.P., Serras A.S., Rodrigues J.S., Cipriano M., Rodrigues A.V., Oliveira N.G. A critical perspective on 3D liver models for drug metabolism and toxicology studies. Front Cell Dev Biol. 2021;9:203. doi: 10.3389/fcell.2021.626805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu P., Duan Z., Liu S., Pachon I., Ma J., Hemstreet G.P., Zhang Y. Drug-induced nephrotoxicity assessment in 3D cellular models. Micromachines. 2022;13:1–23. doi: 10.3390/mi13010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerets H.H.J., Tilmant K., Gerin B., Chanteux H., Depelchin B.O., Dhalluin S., Atienzar F.A. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;28:69–87. doi: 10.1007/s10565-011-9208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkening S., Stahl F., Bader A. Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 25.Moysidou C.M., Barberio C., Owens R.M. Advances in engineering human tissue models. Front Bioeng Biotechnol. 2021;8 doi: 10.3389/fbioe.2020.620962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouwkema J., Koopman B.F.J.M., Blitterswijk C.A.V., Dhert W.J.A., Malda J. Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev. 2009;26:163–178. doi: 10.5661/bger-26-163. [DOI] [PubMed] [Google Scholar]
- 27.Romo-Morales A., Knight E., Przyborski S. Technology Platforms for 3D Cell Culture: A User's Guide. 2017. Alvetex®, a highly porous polystyrene scaffold for routine three-dimensional cell culture; pp. 223–249. [DOI] [Google Scholar]
- 28.Reprocell, Reinnervate perfusion plate dynamic circulation and perfusion of culture medium within a multi-welled plate, (n.d.). https://resources.reprocell.com/hubfs/website/resources/application-notes/AN-SC-08_AppNote_Scaffold_08-A4.pdf (accessed February 4, 2022).
- 29.H.W. Hoyle, C.L. Mobbs, S.A. Przyborski, Applying stirred perfusion to 3D tissue equivalents to mimic the dynamic in vivo microenvironment BT - bioreactors in stem cell biology: methods and protocols, in: K. Turksen (ed.), Springer US, New York, NY, 2022: pp. 241–56. doi: 10.1007/7651_2021_442. [DOI] [PubMed]
- 30.CelVivo, ClinoStar, (n.d.). https://celvivo.com/products/clinostar/ (accessed March 17, 2022).
- 31.Emulate, Human emulation system | emulate, (n.d.). https://emulatebio.com/human-emulation-system/ (accessed March 17, 2022).
- 32.Nicolas A., Schavemaker F., Kosim K., Kurek D., Haarmans M., Bulst M., Lee K., Wegner S., Hankemeier T., Joore J., Domansky K., Lanz H.L., Vulto P., Trietsch S.J. High throughput transepithelial electrical resistance (TEER) measurements on perfused membrane-free epithelia. Lab Chip. 2021;21:1676–1685. doi: 10.1039/d0lc00770f. [DOI] [PubMed] [Google Scholar]
- 33.Synthecon, Rotary cell culture systems, (n.d.). https://synthecon.com/pages/rotary_cell_culture_systems_13.asp (accessed March 17, 2022).
- 34.Reprocell, Products: alvetex perfusion plate, (n.d.). https://www.reprocell.com/product-catalog/alvetex-3d-cell-culture-systems/alvetex-perfusion-plate (accessed March 17, 2022).
- 35.Kirkstall, Quasi Vivo® | The many benefits of the organ-on-a-chip Technology | Kirkstall Ltd., (n.d.). https://www.kirkstall.com/why-quasi-vivo/ (accessed March 17, 2022).
- 36.Ibidi, ibidi pump system | Perform cell-based flow assays, (n.d.). https://ibidi.com/perfusion-system/112-ibidi-pump-system.html (accessed March 17, 2022).
- 37.Reprocell, Products: ABLE® Biott® bioreactor systems, (n.d.). https://www.reprocell.com/product-catalog/able-biott-bioreactor-systems (accessed March 17, 2022).
- 38.Corning, Corning® disposable spinner flasks | corning, (n.d.). https://ecatalog.corning.com/life-sciences/b2c/US/en/Bioprocess-and-Scale-up/Disposable-Spinner-Flasks/Corning®-Disposable-Spinner-Flasks/p/corningDisposableSpinnerFlasks (accessed March 17, 2022).
- 39.Mimetas, OrganoFlow®, (n.d.). https://www.mimetas.com/en/organoflow-/ (accessed March 17, 2022).
- 40.Ingram M., Techy G.B., Saroufeem R., Yazan O., Narayan K.S., Goodwin T.J., Spaulding G.F., Al I.E.T. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell Dev Biol. 1997;33:459–466. doi: 10.1007/s11626-997-0064-8. [DOI] [PubMed] [Google Scholar]
- 41.Hammond T.G., Hammond J.M. Optimized suspension culture: the rotating-wall vessel. Am J Physiol Ren Physiol. 2001;281:12–25. doi: 10.1152/ajprenal.2001.281.1.f12. [DOI] [PubMed] [Google Scholar]
- 42.Mitteregger R., Vogt G., Rossmanith E., Falkenhagen D. Rotary cell culture system (RCCS): a new method for cultivating hepatocytes on microcarriers. Int J Artif Organs. 1999;22:816–822. doi: 10.1177/039139889902201207. [DOI] [PubMed] [Google Scholar]
- 43.Radtke A.L., Herbst-Kralovetz M.M. Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J Vis Exp. 2012:1–10. doi: 10.3791/3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N., Rickel A.P., Sanyour H.J., Hong Z. Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells towards vascular cells on vascular extracellular matrix scaffold under mechanical stimulation in a rotary bioreactor. J Mater Chem B. 2019;7:2703–2713. doi: 10.1039/c8tb03348j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert E., Mosher M., Gottipati A., Elder S. A novel through-thickness perfusion bioreactor for the generation of scaffold-free tissue engineered cartilage. Processes. 2014;2:658–674. doi: 10.3390/pr2030658. [DOI] [Google Scholar]
- 46.Zhou W., Chen Y., Roh T., Lin Y., Ling S., Zhao S., Lin J.D., Khalil N., Cairns D.M., Manousiouthakis E., Tse M., Kaplan D.L. Multifunctional bioreactor system for human intestine tissues. ACS Biomater Sci Eng. 2018;4:231–239. doi: 10.1021/acsbiomaterials.7b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouet G., Cruel M., Laurent C., Vico L., Malaval L., Marchat D. Validation of an in vitro 3D bone culture model with perfused and mechanically stressed ceramic scaffold. Eur Cells Mater. 2015;29:250–267. doi: 10.22203/eCM.v029a19. [DOI] [PubMed] [Google Scholar]
- 48.Magrofuoco E., Flaibani M., Giomo M., Elvassore N. Cell culture distribution in a three-dimensional porous scaffold in perfusion bioreactor. Biochem Eng J. 2019;146:10–19. doi: 10.1016/j.bej.2019.02.023. [DOI] [Google Scholar]
- 49.Panek M., Antunović M., Pribolšan L., Ivković A., Gotić M., Vukasović A., Mihalić K.C., Pušić M., Jurkin T., Marijanović I. Bone tissue engineering in a perfusion bioreactor using dexamethasone-loaded peptide hydrogel. Materials. 2019;16 doi: 10.3390/ma12060919. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huber B., Hoch E., Calderon I., Borchers K., Kluger P.J. A versatile perfusion bioreactor and endothelializable photo cross-linked tubes of gelatin methacryloyl as promising tools in tissue engineering. Biomed Tech. 2019;64:397–406. doi: 10.1515/bmt-2018-0015. [DOI] [PubMed] [Google Scholar]
- 51.Sriram G., Alberti M., Dancik Y., Wu B., Wu R., Feng Z., Ramasamy S., Bigliardi P.L., Bigliardi-Qi M., Wang Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater Today. 2018;21:326–340. doi: 10.1016/j.mattod.2017.11.002. [DOI] [Google Scholar]
- 52.Rimal R., Marquardt Y., Nevolianis T., Djeljadini S., Marquez A.B., Huth S., Chigrin D.N., Wessling M., Baron J.M., Möller M., Singh S. Dynamic flow enables long-term maintenance of 3-D vascularized human skin models. Appl Mater Today. 2021;25 doi: 10.1016/j.apmt.2021.101213. [DOI] [Google Scholar]
- 53.Coluccio M.L., Perozziello G., Malara N., Parrotta E., Zhang P., Gentile F., Limongi T., Raj P.M., Cuda G., Candeloro P., Di Fabrizio E. Microfluidic platforms for cell cultures and investigations. Microelectron Eng. 2019;208:14–28. doi: 10.1016/j.mee.2019.01.004. [DOI] [Google Scholar]
- 54.Fiorini G.S., Chiu D.T. Disposable microfluidic devices: fabrication, function, and application. BioTechniques. 2005;38:429–446. doi: 10.2144/05383RV02. [DOI] [PubMed] [Google Scholar]
- 55.Picollet-D'hahan N., Zuchowska A., Lemeunier I., Le Gac S. Multiorgan-on-a-chip: a systemic approach to model and decipher inter-organ communication. Trends Biotechnol. 2021;39:788–810. doi: 10.1016/j.tibtech.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y., Kankala R.K., Bin Wang S., Chen A.Z. Multi-organs-on-chips: towards long-term biomedical investigations. Molecules. 2019;24 doi: 10.3390/molecules24040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.García Cruz D.M., Salmerón-Sánchez M., Gómez-Ribelles J.L. Stirred flow bioreactor modulates chondrocyte growth and extracellular matrix biosynthesis in chitosan scaffolds. J Biomed Mater Res Part A. 2012;100 A:2330–2341. doi: 10.1002/jbm.a.34174. [DOI] [PubMed] [Google Scholar]
- 58.Khodabakhshaghdam S., Khoshfetrat A.B., Rahbarghazi R. Alginate-chitosan core-shell microcapsule cultures of hepatic cells in a small scale stirred bioreactor: impact of shear forces and microcapsule core composition. J Biol Eng. 2021;15:1–12. doi: 10.1186/s13036-021-00265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohani L., Borys B.S., Razian G., Naghsh P., Liu S., Johnson A.A., Machiraju P., Holland H., Lewis I.A., Groves R.A., Toms D., Gordon P.M.K., Li J.W., So T., Dang T., Kallos M.S., Rancourt D.E. Stirred suspension bioreactors maintain naïve pluripotency of human pluripotent stem cells. Commun Biol. 2020;3:1–22. doi: 10.1038/s42003-020-01218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kehoe D.E., Jing D., Lock L.T., Tzanakakis E.S., Ph D. Scalable stirred-suspension bioreactor culture. Tissue Eng Part A. 2010;16:405–421. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krawetz R., Taiani J.T., Liu S., Meng G., Li X., Kallos M.S., Rancourt D.E. Large-scale expansion of pluripotent human embryonic stem cells in stirred-suspension bioreactors. Tissue Eng Part C Methods. 2010;16:573–582. doi: 10.1089/ten.tec.2009.0228. [DOI] [PubMed] [Google Scholar]
- 62.Sikavitsas V.I., Bancroft G.N., Mikos A.G. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 63.Hoyle H.W., Smith L.A., Williams R.J., Przyborski S.A. Applications of novel bioreactor technology to enhance the viability and function of cultured cells and tissues. Interface Focus. 2020;10 doi: 10.1098/rsfs.2019.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vunjak-Novakovic G., Obradovic B., Martin I., Bursac P.M., Langer R., Freed L.E. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 65.Stiehler M., Bünger C., Baatrup A., Lind M., Kassem M., Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res Part A. 2009;89:96–107. doi: 10.1002/jbm.a.31967. [DOI] [PubMed] [Google Scholar]
- 66.Delaine-Smith R.M., MacNeil S., Reilly G.C. Matrix production and collagen structure are enhanced in two types of osteogenic progenitor cells by a simple fluid shear stress stimulus. Eur Cells Mater. 2012;24:162–174. doi: 10.22203/eCM.v024a12. [DOI] [PubMed] [Google Scholar]
- 67.Puwanun S., Delaine-Smith R.M., Colley H.E., Yates J.M., MacNeil S., Reilly G.C. A simple rocker-induced mechanical stimulus upregulates mineralization by human osteoprogenitor cells in fibrous scaffolds. J Tissue Eng Regen Med. 2018;12:370–381. doi: 10.1002/term.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paish H.L., Reed L.H., Brown H., Bryan M.C., Govaere O., Leslie J., Barksby B.S., Garcia Macia M., Watson A., Xu X., Zaki M.Y.W., Greaves L., Whitehall J., French J., White S.A., Manas D.M., Robinson S.M., Spoletini G., Griffiths C., Mann D.A., Borthwick L.A., Drinnan M.J., Mann J., Oakley F. A bioreactor technology for modeling fibrosis in human and rodent precision-cut liver slices. Hepatology. 2019;0:1–15. doi: 10.1002/hep.30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu X., Roberto J.B., Knupp A., Kenerson H.L., Truong C.D., Yuen S.Y., Brempelis K.J., Tuefferd M., Chen A., Horton H., Yeung R.S., Crispe I.N. Precision-cut human liver slice cultures as an immunological platform. J Immunol Methods. 2018;455:71–79. doi: 10.1016/j.jim.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asada H., Paszkowiak J., Teso D., Alvi K., Thorisson A., Frattini J.C., Kudo F.A., Sumpio B.E., Dardik A. Sustained orbital shear stress stimulates smooth muscle cell proliferation via the extracellular signal-regulated protein kinase 1/2 pathway. J Vasc Surg. 2005;42:772–780. doi: 10.1016/j.jvs.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 71.Kraiss L.W., Alto N.M., Dixon D.A., McIntyre T.M., Weyrich A.S., Zimmerman G.A. Fluid flow regulates E-selectin protein levels in human endothelial cells by inhibiting translation. J Vasc Surg. 2003;37:161–168. doi: 10.1067/mva.2003.67. [DOI] [PubMed] [Google Scholar]
- 72.Haga M., Yamashita A., Paszkowiak J., Sumpio B.E., Dardik A. Oscillatory shear stress increases smooth muscle cell proliferation and Akt phosphorylation. J Vasc Surg. 2003;37:1277–1284. doi: 10.1016/S0741-5214(03)00329-X. [DOI] [PubMed] [Google Scholar]
- 73.Potter C.M.F., Lundberg M.H., Harrington L.S., Warboys C.M., Warner T.D., Berson R.E., Moshkov A.V., Gorelik J., Weinberg P.D., Mitchell J.A. Role of shear stress in endothelial cell morphology and expression of cyclooxygenase isoforms. Arterioscler Thromb Vasc Biol. 2011;31:384–391. doi: 10.1161/ATVBAHA.110.214031. [DOI] [PubMed] [Google Scholar]
- 74.Ferrell N., Cheng J., Miao S., Roy S., Fissell W.H. Orbital shear stress regulates differentiation and barrier function of primary renal tubular epithelial cells. ASAIO J. 2018;64:766–772. doi: 10.1097/MAT.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salek M.M., Sattari P., Martinuzzi R.J. Analysis of fluid flow and wall shear stress patterns inside partially filled agitated culture well plates. Ann Biomed Eng. 2012;40:707–728. doi: 10.1007/s10439-011-0444-9. [DOI] [PubMed] [Google Scholar]
- 76.Velasco V., Gruenthal M., Zusstone E., Thomas J.M.D., Berson R.E., Keynton R.S., Williams S.J. An orbital shear platform for real-time, in vitro endothelium characterization. Biotechnol Bioeng. 2016;113:1336–1344. doi: 10.1002/bit.25893. [DOI] [PubMed] [Google Scholar]
- 77.Kwok C.K., Ueda Y., Kadari A., Günther K., Ergün S., Heron A., Schnitzler A.C., Rook M., Edenhofer F. Scalable stirred suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors. J Tissue Eng Regen Med. 2018;12:e1076–e1087. doi: 10.1002/term.2435. [DOI] [PubMed] [Google Scholar]
- 78.He H., He Q., Xu F., Zhou Y., Ye Z., Tan W.S. Dynamic formation of cellular aggregates of chondrocytes and mesenchymal stem cells in spinner flask. Cell Prolif. 2019;52:1–10. doi: 10.1111/cpr.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta P., Ismadi M.Z., Verma P.J., Fouras A., Jadhav S., Bellare J., Hourigan K. Optimization of agitation speed in spinner flask for microcarrier structural integrity and expansion of induced pluripotent stem cells. Cytotechnology. 2016;68:45–59. doi: 10.1007/s10616-014-9750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gareau T., Lara G.G., Shepherd R.D., Krawetz R., Rancourt D.E., Rinker K.D., Kallos M.S. Shear stress influences the pluripotency of murine embryonic stem cells in stirred suspension bioreactors. J Tissue Eng Regen Med. 2014;8:268–278. doi: 10.1002/term. [DOI] [PubMed] [Google Scholar]
- 81.Brindley D., Moorthy K., Lee J.H., Mason C., Kim H.W., Wall I. Bioprocess forces and their impact on cell behavior: implications for bone regeneration therapy. J Tissue Eng. 2011;2:1–13. doi: 10.4061/2011/620247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fois C.A.M., Schindeler A., Valtchev P., Dehghani F. Dynamic flow and shear stress as key parameters for intestinal cells morphology and polarization in an organ-on-a-chip model. Biomed Microdevices. 2021;23:1–12. doi: 10.1007/s10544-021-00591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeste J., Illa X., Alvarez M., Villa R. Engineering and monitoring cellular barrier models. J Biol Eng. 2018;12:1–19. doi: 10.1186/s13036-018-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fedi A., Vitale C., Ponschin G., Ayehunie S., Fato M., Scaglione S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: a systematic review. J Control Release. 2021;335:247–268. doi: 10.1016/j.jconrel.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 85.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 86.Bernstam L.I., Vaughan F.L., Bernstein I.A. Keratinocytes grown at the air-liquid interface. In Vitro Cell Dev Biol. 1986;22:695–705. doi: 10.1007/BF02621086. [DOI] [PubMed] [Google Scholar]
- 87.Huber D., Oskooei A., Casadevall Solvas X., Demello A., Kaigala G.V. Hydrodynamics in cell studies. Chem Rev. 2018;118:2042–2079. doi: 10.1021/acs.chemrev.7b00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malda J., Radisic M., Levenberg S., Woodfield T., Oomens C., Baaijens F., Svalander P., Vunjak-Novakovic G. Cell nutrition. van Blitterswijk C., Thomsen P., Lindahl A., Hubbell J., Williams D.F., Cancedda R., de Bruijn J.D., Sohier J., editors. Cell nutritionTissue Engineering. 2008 doi: 10.1016/B978-0-12-370869-4.00012-4. Eds. 327–362. [DOI] [Google Scholar]
- 89.Rouwkema J., Rivron N.C., van Blitterswijk C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Hua J., Erickson L.E., Yiin T.Y., Glasgow L.A. A review of the effects of shear and interfacial phenomena on cell viability. Crit Rev Biotechnol. 1993;13:305–328. doi: 10.3109/07388559309075700. [DOI] [PubMed] [Google Scholar]
- 91.Jin G., Yang G.H., Kim G. Tissue engineering bioreactor systems for applying physical and electrical stimulations to cells. J Biomed Mater Res Part B Appl Biomater. 2015;103:935–948. doi: 10.1002/jbm.b.33268. [DOI] [PubMed] [Google Scholar]
- 92.Endeward V., Gros G. Extra- and intracellular unstirred layer effects in measurements of CO2 diffusion across membranes–a novel approach applied to the mass spectrometric 18O technique for red blood cells. J Physiol. 2009;587:1153–1167. doi: 10.1113/jphysiol.2008.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weegman B.P., Nash P., Carlson A.L., Voltzke K.J., Geng Z., Jahani M., Becker B.B., Papas K.K., Firpo M.T. Nutrient regulation by continuous feeding removes limitations on cell yield in the large-scale expansion of mammalian cell spheroids. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao T., Asayama Y. Animal-cell culture media: history, characteristics, and current issues. Reprod Med Biol. 2017;16:99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krętowski R., Borzym-Kluczyk M., Stypułkowska A., Brańska-Januszewska J., Ostrowska H., Cechowska-Pasko M. Low glucose dependent decrease of apoptosis and induction of autophagy in breast cancer MCF-7 cells. Mol Cell Biochem. 2016;417:35–47. doi: 10.1007/s11010-016-2711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poulsen R.C., Knowles H.J., Carr A.J., Hulley P.A. Cell differentiation versus cell death: extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis. 2014;5:1–12. doi: 10.1038/cddis.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kageyama S.I., Yokoo H., Tomita K., Kageyama-Yahara N., Uchimido R., Matsuda N., Yamamoto S., Hattori Y. High glucose-induced apoptosis in human coronary artery endothelial cells involves up-regulation of death receptors. Cardiovasc Diabetol. 2011;10:1–11. doi: 10.1186/1475-2840-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ackermann T., Tardito S. Cell culture medium formulation and its implications in cancer metabolism. Trends Cancer. 2019;5:329–332. doi: 10.1016/j.trecan.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhuang Y., Chan D.K., Haugrud A.B., Miskimins W.K. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vande Voorde J., Ackermann T., Pfetzer N., Sumpton D., Mackay G., Kalna G., Nixon C., Blyth K., Gottlieb E., Tardito S. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci Adv. 2019;5 doi: 10.1126/sciadv.aau7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Glacken M.W., Fleischaker R.J., Sinskey A.J. Reduction of waste product excretion via nutrient control: possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells. Biotechnol Bioeng. 1986;28:1376–1389. doi: 10.1002/bit.260280912. [DOI] [PubMed] [Google Scholar]
- 102.A.W. Nienow, Mass transfer and mixing across the scales in animal cell culture, in: M. Al-Rubeai (editor), Animal Cell Culture cell eng., Springer, Cham, 2015: pp. 137–67. 10.1007/978-3-319-10320-4.
- 103.Betts J.P.J., Warr S.R.C., Finka G.B., Uden M., Town M., Janda J.M., Baganz F., Lye G.J. Impact of aeration strategies on fed-batch cell culture kinetics in a single-use 24-well miniature bioreactor. Biochem Eng J. 2014;82:105–116. doi: 10.1016/j.bej.2013.11.010. [DOI] [Google Scholar]
- 104.Rodrigues C.A.V., Fernandes T.G., Diogo M.M., da Silva C.L., Cabral J.M.S. Stem cell cultivation in bioreactors. Biotechnol Adv. 2011;29:815–829. doi: 10.1016/j.biotechadv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 105.Drobner C., Glöckner R., Müller D. Optimal oxygen tension conditions for viability and functioning of precision-cut liver slices. Exp Toxicol Pathol. 2000;52:335–338. doi: 10.1016/S0940-2993(00)80059-7. [DOI] [PubMed] [Google Scholar]
- 106.Klöckner W., Gacem R., Anderlei T., Raven N., Schillberg S., Lattermann C., Büchs J. Correlation between mass transfer coefficient kLa and relevant operating parameters in cylindrical disposable shaken bioreactors on a bench-to-pilot scale. J Biol Eng. 2013;7:1–14. doi: 10.1186/1754-1611-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sivaprakasam S., Mahadevan S., Gopalaraman S. Oxygen mass transfer studies on batch cultivation of P. aeruginosa in a biocalorimeter. Electron J Biotechnol. 2008;11 doi: 10.2225/vol11-issue1-fulltext-15. [DOI] [Google Scholar]
- 108.Lopes M., Mota M., Belo I. Oxygen mass transfer rate in a pressurized lab-scale stirred bioreactor. Chem Eng Technol. 2013;36:1779–1784. doi: 10.1002/ceat.201300082. [DOI] [Google Scholar]
- 109.Karimi A., Golbabaei F., Mehrnia M.R., Neghab M., Mohammad K., Nikpey A., Pourmand M.R. Oxygen mass transfer in a stirred tank bioreactor using different impeller configurations for environmental purposes, Iran. J Environ Heal Sci Eng. 2013;10:1. doi: 10.1186/1735-2746-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cimetta E., Flaibani M., Mella M., Serena E., Boldrin L., De Coopi P., Elvassore N. Enhancement of viability of muscle precursor cells on 3D scaffold in a perfusion bioreactor. Int J Artif Organs. 2007;30:415–428. doi: 10.1177/039139880703000509. [DOI] [PubMed] [Google Scholar]
- 111.Cartmell S.H., Porter B.D., García A.J., Guldberg R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 112.Baptista P.M., Moran E.C., Vyas D., Ribeiro M.H., Atala A., Sparks J.L., Soker S. Fluid flow regulation of revascularization and cellular organization in a bioengineered liver platform. Tissue Eng Part C Methods. 2016;22:199–207. doi: 10.1089/ten.tec.2015.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baudoin R., Alberto G., Paullier P., Legallais C., Leclerc E. Parallelized microfluidic biochips in multi well plate applied to liver tissue engineering. Sens Actuators B Chem. 2012;173:919–926. doi: 10.1016/j.snb.2012.06.050. [DOI] [Google Scholar]
- 114.Strüver K., Friess W., Hedtrich S. Development of a perfusion platform for dynamic cultivation of in vitro skin models. Skin Pharmacol Physiol. 2017;30:180–189. doi: 10.1159/000476071. [DOI] [PubMed] [Google Scholar]
- 115.Scargiali F., Busciglio A., Grisafi F., Brucato A. Mass transfer and hydrodynamic characteristics of unbaffled stirred bio-reactors: influence of impeller design. Biochem Eng J. 2014;82:41–47. doi: 10.1016/j.bej.2013.11.009. [DOI] [Google Scholar]
- 116.Odeleye A.O.O., Marsh D.T.J., Osborne M.D., Lye G.J., Micheletti M. On the fluid dynamics of a laboratory scale single-use stirred bioreactor. Chem Eng Sci. 2014;111:299–312. doi: 10.1016/j.ces.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olive P.L., Vikse C., Trotter M.J. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int J Radiat Oncol Biol Phys. 1992;22:397–402. doi: 10.1016/0360-3016(92)90840-e. [DOI] [PubMed] [Google Scholar]
- 118.Place T.L., Domann F.E., Case A.J. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic Biol Med. 2017;113:311–322. doi: 10.1016/j.freeradbiomed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin R.Z., Chang H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 120.Franko A.J., Sutherland R.M. Oxygen diffusion distance and development of necrosis in multicell spheroids author Allan J. Franko and Robert M. Sutherland published by : radiation research society stable. Radiat Res. 1979;79:439–453. [PubMed] [Google Scholar]
- 121.Bergemann C., Elter P., Lange R., Wei�mann V., Hansmann H., Klinkenberg E.D., Nebe B. Cellular nutrition in complex three-dimensional scaffolds: a comparison between experiments and computer simulations. Int J Biomater. 2015;2015 doi: 10.1155/2015/584362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Offeddu G.S., Mohee L., Cameron R.E. Scale and structure dependent solute diffusivity within microporous tissue engineering scaffolds. J Mater Sci Mater Med. 2020;31:1–11. doi: 10.1007/s10856-020-06381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eghbali H., Nava M.M., Mohebbi-Kalhori D., Raimondi M.T. Hollow fiber bioreactor technology for tissue engineering applications. Int J Artif Organs. 2016;39:1–15. doi: 10.5301/ijao.5000466. [DOI] [PubMed] [Google Scholar]
- 124.Wung N., Acott S.M., Tosh D., Ellis M.J. Hollow fibre membrane bioreactors for tissue engineering applications. Biotechnol Lett. 2014;36:2357–2366. doi: 10.1007/s10529-014-1619-x. [DOI] [PubMed] [Google Scholar]
- 125.Spencer J.A., Ferraro F., Roussakis E., Klein A., Wu J., Runnels J.M., Zaher W., Mortensen L.J., Alt C., Turcotte R., Yusuf R., Côté D., Vinogradov S.A., Scadden D.T., Lin C.P. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muller M., Padberg W., Schindler E., Sticher J., Osmer C., Friemann S., Hempelmann G. Renocortical Tissue oxygen pressure measurements in patients undergoing living donor kidney transplantation. Anesth Analg. 1998;87:474–476. doi: 10.1097/00000539-199808000-00045. [DOI] [PubMed] [Google Scholar]
- 127.Jungermann K., Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 128.Metzen E., Wolff M., Fandrey J., Jelkmann W. Pericellular PO2 and O2 consumption in monolayer cell cultures. Respir Physiol. 1995;100:101–106. doi: 10.1016/0034-5687(94)00125-J. [DOI] [PubMed] [Google Scholar]
- 129.Wolff M., Fandrey J., Jelkmann W. Microelectrode measurements of pericellular PO2 in erythropoietin- producing human hepatoma cell cultures. Am J Physiol Cell Physiol. 1993;265:266–270. doi: 10.1152/ajpcell.1993.265.5.c1266. [DOI] [PubMed] [Google Scholar]
- 130.Williams A.J. Assessing and interpreting arterial blood gases and acid-base balance. BMJ. 1998;317:1213–1216. doi: 10.1136/bmj.317.7167.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-Ani A., Toms D., Kondro D., Thundathil J., Yu Y., Ungrin M. Oxygenation in cell culture: critical parameters for reproducibility are routinely not reported. PLoS One. 2018;13:1–13. doi: 10.1371/journal.pone.0204269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barisam M., Saidi M.S., Kashaninejad N., Nguyen N.T. Prediction of necrotic core and hypoxic zone of multicellular spheroids in a microbioreactor with a U-shaped barrier. Micromachines. 2018;9:1–19. doi: 10.3390/mi9030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berger E., Magliaro C., Paczia N., Monzel A.S., Antony P., Linster C.L., Bolognin S., Ahluwalia A., Schwamborn J.C. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip. 2018;18:3172–3183. doi: 10.1039/c8lc00206a. [DOI] [PubMed] [Google Scholar]
- 134.Volkmer E., Drosse I., Otto S., Stangelmayer A., Stengele M., Kallukalam B.C., Mutschler W., Schieker M. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A. 2008;14:1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- 135.Kim S.M., Kim D.H., Oh D.J. Development and Evaluation of Cell culture devices with the gas-permeable membrane. Biotechnol Bioprocess Eng. 2020;25:62–70. doi: 10.1007/s12257-019-0149-8. [DOI] [Google Scholar]
- 136.Papas K.K., Avgoustiniatos E.S., Tempelman L.A., Weir G.C., Colton C.K., Pisania A., Rappel M.J., Friberg A.S., Bauer A.C., Hering B.J. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37:3412–3414. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 137.Sugiura S., Sakai Y., Nakazawa K., Kanamori T. Superior oxygen and glucose supply in perfusion cell cultures compared to static cell cultures demonstrated by simulations using the finite element method. Biomicrofluidics. 2011;5:1–11. doi: 10.1063/1.3589910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmid J., Schwarz S., Meier-Staude R., Sudhop S., Clausen-Schaumann H., Schieker M., Huber R. A perfusion bioreactor system for cell seeding and oxygen-controlled cultivation of three-dimensional cell cultures. Tissue Eng Part C Methods. 2018;24:585–595. doi: 10.1089/ten.tec.2018.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Westra I.M., Mutsaers H.A.M., Luangmonkong T., Hadi M., Oosterhuis D., De Jong K.P., Groothuis G.M.M., Olinga P. Human precision-cut liver slices as a model to test anti fibrotic drugs in the early onset of liver fibrosis. TIV. 2016;35:77–85. doi: 10.1016/j.tiv.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 140.Bai Y., Moo-Young M., Anderson W.A. A mechanistic model for gas–liquid mass transfer prediction in a rocking disposable bioreactor. Biotechnol Bioeng. 2019;116:1986–1998. doi: 10.1002/bit.27000. [DOI] [PubMed] [Google Scholar]
- 141.Saini S., Wick T.M. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10:825–832. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 142.Jomezadeh Kheibary N., Abolfazli Esfahani J., Mousavi Shaegh S.A. Analysis of oxygen transport in microfluidic bioreactors for cell culture and organ-on-chip applications. Eng Rep. 2020;2:1–13. doi: 10.1002/eng2.12062. [DOI] [Google Scholar]
- 143.Brennan M.D., Rexius-Hall M.L., Elgass L.J., Eddington D.T. Oxygen control with microfluidics. Lab Chip. 2014;14:4305–4318. doi: 10.1039/c4lc00853g. [DOI] [PubMed] [Google Scholar]
- 144.Wulftange W.J., Rose M.A., Garmendia-Cedillos M., da Silva D., Poprawski J.E., Srinivasachar D., Sullivan T., Lim L., Bliskovsky V.V., Hall M.D., Pohida T.J., Robey R.W., Morgan N.Y., Gottesman M.M. Spatial control of oxygen delivery to three-dimensional cultures alters cancer cell growth and gene expression. J Cell Physiol. 2019;234:20608–20622. doi: 10.1002/jcp.28665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dainty J., Preston R.D. Academic Press; 1963. Water relations of plant cells; pp. 279–326. Ed. [DOI] [Google Scholar]
- 146.Wenger R., Kurtcuoglu V., Scholz C., Marti H., Hoogewijs D. Frequently asked questions in hypoxia research. Hypoxia. 2015;35 doi: 10.2147/hp.s92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pohl P., Saparov S.M., Antonenko Y.N. The size of the unstirred layer as a function of the solute diffusion coefficient. Biophys J. 1998;75:1403–1409. doi: 10.1016/S0006-3495(98)74058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Levitt M.D., Kneip J.M., Levitt D.G. Use of laminar flow and unstirred layer models to predict intestinal absorption in the rat. J Clin Invest. 1988;81:1365–1369. doi: 10.1172/JCI113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Verkman A.S., Dix J.A. Effect of unstirred layers on binding and reaction kinetics at a membrane surface. Anal Biochem. 1984;142:109–116. doi: 10.1016/0003-2697(84)90524-4. [DOI] [PubMed] [Google Scholar]
- 150.Barry P.H., Diamond J.M. Effects of unstirred layers on membrane phenomena. Physiol Rev. 1984;64:763–872. doi: 10.1152/physrev.1984.64.3.763. http://physrev.physiology.org/content/64/3/763.abstract [DOI] [PubMed] [Google Scholar]
- 151.Naruhashi K., Tamai I., Li Q., Sai Y., Tsuji A. Experimental demonstration of the unstirred water layer effect on drug transport in Caco-2 cells. J Pharm Sci. 2003;92:1502–1508. doi: 10.1002/jps.10409. [DOI] [PubMed] [Google Scholar]
- 152.Korjamo T., Heikkinen A., Monkkonen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci. 2009;98:4469–4479. doi: 10.1002/jps. [DOI] [PubMed] [Google Scholar]
- 153.Wood F.L., Brian Houston J., Hallifax D. Importance of the unstirred water layer and hepatocyte membrane integrity in vitro for quantification of intrinsic metabolic clearance. Drug Metab Dispos. 2018;46:268–278. doi: 10.1124/dmd.117.078949. [DOI] [PubMed] [Google Scholar]
- 154.Roux E., Bougaran P., Dufourcq P., Couffinhal T. Fluid shear stress sensing by the endothelial layer. Front Physiol. 2020;11:1–17. doi: 10.3389/fphys.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rutkowski J.M., Swartz M.A. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 156.Martin Y., Vermette P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials. 2005;26:7481–7503. doi: 10.1016/j.biomaterials.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 157.Park J., Berthiaume F., Toner M., Yarmush M.L., Tilles A.W. Microfabricated grooved substrates as platforms for bioartificial liver reactors. Biotechnol Bioeng. 2005;90:632–644. doi: 10.1002/bit.20463. [DOI] [PubMed] [Google Scholar]
- 158.Lawrence B.J., Devarapalli M., Madihally S.V. Flow dynamics in bioreactors containing tissue engineering scaffolds. Biotechnol Bioeng. 2009;102:935–947. doi: 10.1002/bit.22106. [DOI] [PubMed] [Google Scholar]
- 159.Ballermann B.J., Dardik A., Eng E., Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;54:100–108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 160.Lalor P.F., Adams D.H. Adhesion of lymphocytes to hepatic endothelium. J Clin Pathol Mol Pathol. 1999;52:214–219. doi: 10.1136/mp.52.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Hear Circ Physiol. 2007;292 doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 162.Rashidi H., Alhaque S., Szkolnicka D., Flint O., Hay D.C. Fluid shear stress modulation of hepatocyte-like cell function. Arch Toxicol. 2016;90:1757–1761. doi: 10.1007/s00204-016-1689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vinci B., Duret C., Klieber S., Gerbal-Chaloin S., Sa-Cunha A., Laporte S., Suc B., Maurel P., Ahluwalia A., Daujat-Chavanieu M. Modular bioreactor for primary human hepatocyte culture: medium flow stimulates expression and activity of detoxification genes. Biotechnol J. 2011;6:554–564. doi: 10.1002/biot.201000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wittkowske C., Reilly G.C., Lacroix D., Perrault C.M. In vitro bone cell models: impact of fluid shear stress on bone formation. Front Bioeng Biotechnol. 2016;4 doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garanich J.S., Mathura R.A., Shi Z.D., Tarbell J.M. Effects of fluid shear stress on adventitial fibroblast migration: implications for flow-mediated mechanisms of arterialization and intimal hyperplasia. Am J Physiol Hear Circ Physiol. 2007;292:3128–3135. doi: 10.1152/ajpheart.00578.2006. [DOI] [PubMed] [Google Scholar]
- 166.Shi Z.D., Tarbell J.M. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng. 2011;39:1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim K.M., Choi Y.J., Hwang J.H., Kim A.R., Cho H.J., Hwang E.S., Park J.Y., Lee S.H., Hong J.H. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yao W., Ding G.H. Interstitial fluid flow: simulation of mechanical environment of cells in the interosseous membrane. Acta Mech Sin Xuebao. 2011;27:602–610. doi: 10.1007/s10409-011-0439-7. [DOI] [Google Scholar]
- 169.Yao W., Shen Z., Ding G. Simulation of interstitial fluid flow in ligaments: comparison among stokes, brinkman and darcy models. Int J Biol Sci. 2013;9:1050–1056. doi: 10.7150/ijbs.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tada S., Tarbell J.M. Flow through internal elastic lamina affects shear stress on smooth muscle cells (3D simulations) Am J Physiol Hear Circ Physiol. 2002;282:1589–1597. doi: 10.1152/ajpheart.00751.2001. [DOI] [PubMed] [Google Scholar]
- 171.Meng F., Cheng H., Qian J., Dai X., Huang Y., Fan Y. In vitro fluidic systems: applying shear stress on endothelial cells. Med Nov Technol Devices. 2022;15 doi: 10.1016/j.medntd.2022.100143. [DOI] [Google Scholar]
- 172.Gijsen F., Katagiri Y., Barlis P., Bourantas C., Collet C., Coskun U., Daemen J., Dijkstra J., Edelman E., Evans P., Van Der Heiden K., Hose R., Koo B.K., Krams R., Marsden A., Migliavacca F., Onuma Y., Ooi A., Poon E., Samady H., Stone P., Takahashi K., Tang D., Thondapu V., Tenekecioglu E., Timmins L., Torii R., Wentzel J., Serruys P. Expert recommendations on the assessment of wall shear stress in human coronary arteries: existing methodologies, technical considerations, and clinical applications. Eur Heart J. 2019;40:3421–3433. doi: 10.1093/eurheartj/ehz551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stolberg S., McCloskey K.E. Can shear stress direct stem cell fate? Biotechnol Prog. 2009;25:10–19. doi: 10.1002/btpr.124. [DOI] [PubMed] [Google Scholar]
- 174.Kurth F., Györvary E., Heub S., Ledroit D., Paoletti S., Renggli K., Revol V., Verhulsel M., Weder G., Loizeau F. In: Organ-on-a-chip. Hoeng J., Bovard D., Peitsch M.C., editors. Academic Press; 2020. Chapter 3 - organs-on-a-chip engineering; pp. 47–130. Eds. [DOI] [Google Scholar]
- 175.Tilles A.W., Baskaran H., Roy P., Yarmush M.L., Toner M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng. 2001;73:379–389. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]
- 176.Tanaka Y., Yamato M., Okano T., Kitamori T., Sato K. Evaluation of effects of shear stress on hepatocytes by a microchip-based system. Meas Sci Technol. 2006;17:3167–3170. doi: 10.1088/0957-0233/17/12/S08. [DOI] [Google Scholar]
- 177.Pedersen J.M., Shim Y.S., Hans V., Phillips M.B., Macdonald J.M., Walker G., Andersen M.E., Clewell H.J., Yoon M. Fluid dynamic modeling to support the development of flow-based hepatocyte culture systems for metabolism studies. Front Bioeng Biotechnol. 2016;4 doi: 10.3389/fbioe.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wasson E.M., Dubbin K., Moya M.L. Go with the flow: modeling unique biological flows in engineered: in vitro platforms. Lab Chip. 2021;21:2095–2120. doi: 10.1039/d1lc00014d. [DOI] [PubMed] [Google Scholar]
- 179.Hutmacher D.W., Singh H. Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol. 2008;26:166–172. doi: 10.1016/j.tibtech.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 180.Davidovich N.E.T., Kloog Y., Wolf M., Elad D. Mechanophysical stimulations of mucin secretion in cultures of nasal epithelial cells. Biophys J. 2011;100:2855–2864. doi: 10.1016/j.bpj.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Warren S.M., Sailon A.M., Allori A.C., Davidson E.H., Reformat D.D., Allen R.J. A novel flow-perfusion bioreactor supports 3D dynamic cell culture. J Biomed Biotechnol. 2009 doi: 10.1155/2009/873816. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pires T., Dunlop J.W.C., Fernandes P.R., Castro A.P.G. Challenges in computational fluid dynamics applications for bone tissue engineering. Proc R Soc A Math Phys Eng Sci. 2022;478 doi: 10.1098/rspa.2021.0607. https://royalsocietypublishing.org/doi/10.1098/rspa.2021.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Poon C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. J Mech Behav Biomed Mater. 2022;126 doi: 10.1016/j.jmbbm.2021.105024. [DOI] [PubMed] [Google Scholar]
- 184.Wyma A., Martin-Alarcon L., Walsh T., Schmidt T.A., Gates I.D., Kallos M.S. Non-Newtonian rheology in suspension cell cultures significantly impacts bioreactor shear stress quantification. Biotechnol Bioeng. 2018;115:2101–2113. doi: 10.1002/bit.26723. [DOI] [PubMed] [Google Scholar]
- 185.Sekiya S., Kikuchi T., Shimizu T. Perfusion culture maintained with an air-liquid interface to stimulate epithelial cell organization in renal organoids in vitro. BMC Biomed Eng. 2019;1:1–12. doi: 10.1186/s42490-019-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schimek K., Hsu H.H., Boehme M., Kornet J.J., Marx U., Lauster R., Pörtner R., Lindner G. Bioengineering of a full-thickness skin equivalent in a 96-well insert format for substance permeation studies and organ-on-a-chip applications. Bioengineering. 2018;5:1–19. doi: 10.3390/bioengineering5020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paish H.L., Reed L.H., Brown H., Bryan M.C., Govaere O., Leslie J., Barksby B.S., Garcia Macia M., Watson A., Xu X., Zaki M.Y.W., Greaves L., Whitehall J., French J., White S.A., Manas D.M., Robinson S.M., Spoletini G., Griffiths C., Mann D.A., Borthwick L.A., Drinnan M.J., Mann J., Oakley F. A bioreactor technology for modeling fibrosis in human and rodent precision-cut liver slices. Hepatology. 2019;70:1377–1391. doi: 10.1002/hep.30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Piola M., Soncini M., Cantini M., Sadr N., Ferrario G., Fiore G.B. Design and functional testing of a multichamber perfusion platform for three-dimensional scaffolds. Sci World J. 2013;2013:12–14. doi: 10.1155/2013/123974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Prim D.A., Potts J.D., Eberth J.F. Pulsatile perfusion bioreactor for biomimetic vascular impedances. J Med Device. 2018;12 doi: 10.1115/1.4040648. [DOI] [Google Scholar]
- 190.Song L., Zhou Q., Duan P., Guo P., Li D., Xu Y., Li S., Luo F., Zhang Z. Successful development of small diameter tissue-engineering vascular vessels by our novel integrally designed pulsatile perfusion-based bioreactor. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cullen J.P., Sayeed S., Sawai R.S., Theodorakis N.G., Cahill P.A., Sitzmann J.V., Redmond E.M. Pulsatile flow-induced angiogenesis: role of Gi subunits. Arterioscler Thromb Vasc Biol. 2002;22:1610–1616. doi: 10.1161/01.ATV.0000034470.37007.58. [DOI] [PubMed] [Google Scholar]
- 192.Stavenschi E., Labour M.N., Hoey D.A. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: the effect of shear stress magnitude, frequency, and duration. J Biomech. 2017;55:99–106. doi: 10.1016/j.jbiomech.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 193.Jaasma M.J., O'Brien F.J. Mechanical stimulation of osteoblasts using steady and dynamic fluid flow. Tissue Eng Part A. 2008;14:1213–1223. doi: 10.1089/ten.tea.2007.0321. [DOI] [PubMed] [Google Scholar]
- 194.Chen G., Xu R., Zhang C., Lv Y. Responses of MSCs to 3D scaffold matrix mechanical properties under oscillatory perfusion culture. ACS Appl Mater Interfaces. 2017;9:1207–1218. doi: 10.1021/acsami.6b10745. [DOI] [PubMed] [Google Scholar]
- 195.Du D., Furukawa K.S., Ushida T. Oscillatory perfusion culture of CaP-based tissue engineering bone with and without dexamethasone. Ann Biomed Eng. 2009;37:146–155. doi: 10.1007/s10439-008-9586-9. [DOI] [PubMed] [Google Scholar]
- 196.Du D., Furukawa K.S., Ushida T. 3D culture of osteoblast-like cells by unidirectional or oscillatory flow for bone tissue engineering. Biotechnol Bioeng. 2009;102:1670–1678. doi: 10.1002/bit.22214. [DOI] [PubMed] [Google Scholar]
- 197.Vance J., Galley S., Liu D.F., Donahue S.W. Mechanical Stimulation of MC3T3 Osteoblastic Cells. Tissue Eng. 2005;11:1832–1839. doi: 10.1089/ten.2005.11.1832. [DOI] [PubMed] [Google Scholar]
- 198.Park J., Li Y., Berthiaume F., Toner M., Yarmush M., Tilles A. Radial flow hepatocyte bioreactor using stacked microfabricated grooved substrates. Biotechnol Bioeng. 2007;99:455–467. doi: 10.1002/bit. [DOI] [PubMed] [Google Scholar]
- 199.Helmedag M.J., Weinandy S., Marquardt Y., Baron J.M., Pallua N., Suschek C.V., Jockenhoevel S. The effects of constant flow bioreactor cultivation and keratinocyte seeding densities on prevascularized organotypic skin grafts based on a fibrin scaffold. Tissue Eng Part A. 2015;21:343–352. doi: 10.1089/ten.tea.2013.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Leo H.L., Xia L., Ng S.S., Poh H.J., Zhang S.F., Cheng T.M., Xiao G.F., Tuo X.Y., Yu H. Proceedings of the 13th international conference on biomedical engineering Vols 1-3. Vol. 23. 2009. Computational fluid dynamics investigation of the effect of the fluid-induced shear stress on hepatocyte sandwich perfusion culture; pp. 1405–1408. [Google Scholar]
- 201.Israelowitz M., Weyand B., Rizvi S., Vogt P.M., Von Schroeder H.P. Development of a laminar flow bioreactor by computational fluid dynamics. J Healthc Eng. 2012;3:455–476. doi: 10.1260/2040-2295.3.3.455. [DOI] [Google Scholar]
- 202.Hidalgo-Bastida L.A., Thirunavukkarasu S., Griffiths S., Cartmell S.H., Naire S. Modeling and design of optimal flow perfusion bioreactors for tissue engineering applications. Biotechnol Bioeng. 2012;109:1095–1099. doi: 10.1002/bit.24368. [DOI] [PubMed] [Google Scholar]
- 203.Li C., Xia J.Y., Chu J., Wang Y.H., Zhuang Y.P., Zhang S.L. CFD analysis of the turbulent flow in baffled shake flasks. Biochem Eng J. 2013;70:140–150. doi: 10.1016/j.bej.2012.10.012. [DOI] [Google Scholar]
- 204.Prado C.M., Ramos S.G., Elias J., Rossi M.A. Turbulent blood flow plays an essential localizing role in the development of atherosclerotic lesions in experimentally induced hypercholesterolaemia in rats. Int J Exp Pathol. 2008;89:72–80. doi: 10.1111/j.1365-2613.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cunningham K.S., Gotlieb A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab Investig. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 206.Lerman M.J., Lembong J., Muramoto S., Gillen G., Fisher J.P. The evolution of polystyrene as a cell culture material. Tissue Eng Part B Rev. 2018;24:359–372. doi: 10.1089/ten.teb.2018.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ahadian S., Rahal R., Ramón-Azcón J., Obregón R., Hasan A. Tissue engineering for artificial organs: regenerative medicine, smart diagnostics and personalized medicine. 2016. Biomaterials in Tissue Engineering; pp. 35–83. [DOI] [Google Scholar]
- 208.Lee I.J., Kasper F.K., Mikos A.G. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42:323–337. doi: 10.1007/s10439-013-0859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Recek N., Resnik M., Zaplotnik R., Mozetic M., Motaln H., Lah-Turnsek T., Vesel A. Cell proliferation on polyethylene terephthalate treated in plasma created in SO2/O2 mixtures. Polymers. 2017;9:1–16. doi: 10.3390/polym9030082. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chang S.J., Kuo S.M., Lan J.W., Wang Y.J. Amination of polycarbonate surface and its application for cell attachment. Artif Cells Blood Substit Immobil Biotechnol. 1999;27:229–244. doi: 10.3109/10731199909117696. [DOI] [PubMed] [Google Scholar]
- 211.Ahad I.U., Butruk B., Ayele M., Budner B., Bartnik A., Fiedorowicz H., Ciach T., Brabazon D. Extreme ultraviolet (EUV) surface modification of polytetrafluoroethylene (PTFE) for control of biocompatibility. Nucl Instrum Methods Phys Res Sect B Beam Interact with Mater Atoms. 2014;364:98–107. doi: 10.1016/j.nimb.2015.08.093. [DOI] [Google Scholar]
- 212.Gumpenberger T., Heitz J., Bäuerle D., Kahr H., Graz I., Romanin C., Svorcik V., Leisch F. Adhesion and proliferation of human endothelial cells on photochemically modified polytetrafluoroethylene. Biomaterials. 2003;24:5139–5144. doi: 10.1016/S0142-9612(03)00460-5. [DOI] [PubMed] [Google Scholar]
- 213.Schmelzer E., Over P., Gridelli B., Gerlach J.C. Response of primary human bone marrow mesenchymal stromal cells and dermal keratinocytes to thermal printer materials in vitro. J Med Biol Eng. 2016;36:153–167. doi: 10.1007/s40846-016-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Borowiec J., Hampl J., Gebinoga M., Elsarnagawy T., Elnakady Y.A., Fouad H., Almajhadi F., Fernekorn U., Weise F., Singh S., Elsarnagawy D., Schober A. Thermoforming techniques for manufacturing porous scaffolds for application in 3D cell cultivation. Mater Sci Eng C. 2015;49:509–516. doi: 10.1016/j.msec.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 215.M.C. Wurm, T. Möst, B. Bergauer, D. Rietzel, F. Wilhelm Neukam, S.C. Cifuentes, C. von Wilmowsky, In-vitro evaluation of polylactic acid (PLA) manufactured by fused deposition modeling, (n.d.). 10.1186/s13036-017-0073-4 2022. [DOI] [PMC free article] [PubMed]
- 216.Serra T., Mateos-Timoneda M.A., Planell J.A., Navarro M. 3D printed PLA-based scaffolds: a versatile tool in regenerative medicine. Organogenesis. 2013;9:239–244. doi: 10.4161/org.26048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bail R., Patel A., Yang H., Rogers C.M., Rose F.R.A.J., Segal J.I., Ratchev S.M. The effect of a type I photoinitiator on cure kinetics and cell toxicity in projection-microstereolithography. Procedia CIRP. 2013;5:222–225. doi: 10.1016/j.procir.2013.01.044. [DOI] [Google Scholar]
- 218.M. Carve, D. Wlodkowic, 3D-printed chips: compatibility of additive manufacturing photopolymeric substrata with biological applications, Micromachines. 9 (2018). 10.3390/mi9020091. [DOI] [PMC free article] [PubMed]
- 219.MacDonald N.P., Zhu F., Hall C.J., Reboud J., Crosier P.S., Patton E.E., Wlodkowic D., Cooper J.M. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip. 2016;16:291–297. doi: 10.1039/c5lc01374g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang D., Liu W., Han B., Xu R. The bioreactor: a powerful tool for large-scale culture of animal cells. Curr Pharm Biotechnol. 2005;6:397–403. doi: 10.2174/138920105774370580. [DOI] [PubMed] [Google Scholar]
- 221.Song K., Liu T., Cui Z., Li X., Ma X. Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res Part A. 2008;86:323–332. doi: 10.1002/jbm.a.31624. [DOI] [PubMed] [Google Scholar]
- 222.Ahmed M., Punshon G., Darbyshire A., Seifalian A.M. Effects of sterilization treatments on bulk and surface properties of nanocomposite biomaterials. J Biomed Mater Res Part B Appl Biomater. 2013;101:1182–1190. doi: 10.1002/jbm.b.32928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shearer H., Ellis M.J., Perera S.P., Chaudhuri J.B. Effects of common sterilization methods on the structure and properties of Poly(D,L Lactic-Co-Glycolic Acid) scaffolds. Tissue Eng. 2006;12:2717–2727. doi: 10.1089/ten.2006.12.2717. [DOI] [PubMed] [Google Scholar]
- 224.Ryu M., Kobayashi T., Kawamukai E., Quan G., Furuta T. Cytotoxicity assessment of residual high-level disinfectants. Biocontrol Sci. 2013;18:217–220. doi: 10.4265/bio.18.217. [DOI] [PubMed] [Google Scholar]
- 225.Fischbach C., Tessmar J., Lucke A., Schnell E., Schmeer G., Blunk T., Göpferich A. Does UV irradiation affect polymer properties relevant to tissue engineering? Surf Sci. 2001;491:333–345. doi: 10.1016/S0039-6028(01)01297-3. [DOI] [Google Scholar]
- 226.Bittner G.D., Yang C.Z., Stoner M.A. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environ Heal A Glob Access Sci Source. 2014;13 doi: 10.1186/1476-069X-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dewhirst M.W., Viglianti B.L., Lora-Michiels M., Hanson M., Hoopes P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperth. 2003;19:267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 228.Armour E.P., McEachern D., Wang Z., Corry P.M., Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Res. 1993;53:2740–2744. [PubMed] [Google Scholar]
- 229.Zhu S., Wang J., Xie B., Luo Z., Lin X., Liao D.J. Culture at a higher temperature mildly inhibits cancer cell growth but enhances chemotherapeutic effects by inhibiting cell-cell collaboration. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0137042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hildebrandt B., Wust P., Ahlers O., Dieing A., Sreenivasa G., Kerner T., Felix R., Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 231.Mantso T., Vasileiadis S., Anestopoulos I., Voulgaridou G.P., Lampri E., Botaitis S., Kontomanolis E.N., Simopoulos C., Goussetis G., Franco R., Chlichlia K., Pappa A., Panayiotidis M.I. Hyperthermia induces therapeutic effectiveness and potentiates adjuvant therapy with non-targeted and targeted drugs in an in vitro model of human malignant melanoma. Sci Rep. 2018;8:1–16. doi: 10.1038/s41598-018-29018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Masterton R.J., Smales C.M. The impact of process temperature on mammalian cell lines and the implications for the production of recombinant proteins in CHO cells. Pharm Bioprocess. 2014;2:49–61. doi: 10.4155/pbp.14.3. [DOI] [Google Scholar]
- 233.Furukawa K., Ohsuye K. Effect of culture temperature on a recombinant CHO cell line producing a C-terminal α-amidating enzyme. Cytotechnology. 1998;26:153–164. doi: 10.1023/A:1007934216507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mieremet A., van Dijk R., Boiten W., Gooris G., Bouwstra J.A., El Ghalbzouri A. Characterization of human skin equivalents developed at body's core and surface temperatures. J Tissue Eng Regen Med. 2019;13:1122–1133. doi: 10.1002/term.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Abeille F., Mittler F., Obeid P., Huet M., Kermarrec F., Dolega M.E., Navarro F., Pouteau P., Icard B., Gidrol X., Agache V., Picollet-D'Hahan N. Continuous microcarrier-based cell culture in a benchtop microfluidic bioreactor. Lab Chip. 2014;14:3510–3518. doi: 10.1039/c4lc00570h. [DOI] [PubMed] [Google Scholar]
- 236.Mäki A.J., Verho J., Kreutzer J., Ryynänen T., Rajan D., Pekkanen-Mattila M., Ahola A., Hyttinen J., Aalto-Setälä K., Lekkala J., Kallio P. A portable microscale cell culture system with indirect temperature control. SLAS Technol. 2018;23:566–579. doi: 10.1177/2472630318768710. [DOI] [PubMed] [Google Scholar]
- 237.Halldorsson S., Lucumi E., Gómez-Sjöberg R., Fleming R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 2015;63:218–231. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 238.Wang T., Gardiner B.S., Lin Z., Rubenson J., Kirk T.B., Wang A., Xu J., Smith D.W., Lloyd D.G., Zheng M.H. Bioreactor design for tendon/ligament engineering. Tissue Eng Part B Rev. 2013;19:133–146. doi: 10.1089/ten.teb.2012.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mahfouzi S.H., Amoabediny G., Doryab A., Safiabadi-Tali S.H., Ghanei M. Noninvasive Real-Time Assessment of Cell Viability in a Three-Dimensional Tissue. Tissue Eng. - Part C Methods. 2018;24:197–204. doi: 10.1089/ten.tec.2017.0371. [DOI] [PubMed] [Google Scholar]
- 240.de Bournonville S., Lambrechts T., Vanhulst J., Luyten F.P., Papantoniou I., Geris L. Towards self-regulated bioprocessing: a compact benchtop bioreactor system for monitored and controlled 3D cell and tissue culture. Biotechnol J. 2019;14 doi: 10.1002/biot.201800545. [DOI] [PubMed] [Google Scholar]
- 241.Khan K., Elia M. Factors affecting the stability of L-glutamine in solution. Clin Nutr. 1991;10:186–192. doi: 10.1016/0261-5614(91)90037-D. [DOI] [PubMed] [Google Scholar]
- 242.Golubitskii G.B., Budko E.V., Basova E.M., Kostarnoi A.V., Ivanov V.M. Stability of ascorbic acid in aqueous and aqueous-organic solutions for quantitative determination. J Anal Chem. 2007;62:742–747. doi: 10.1134/S1061934807080096. [DOI] [Google Scholar]
- 243.Jagušić M., Forčić D., Brgles M., Kutle L., Šantak M., Jergović M., Kotarski L., Bendelja K., Halassy B. Stability of minimum essential medium functionality despite l-glutamine decomposition. Cytotechnology. 2016;68:1171–1183. doi: 10.1007/s10616-015-9875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.ThermoFisher GlutaMAX media Keep your cells healthier for longer. Co Leafl. 2017:1–3. https://assets.thermofisher.com/TFS-Assets/LSG/brochures/glutamax-product-brochure.pdf [Google Scholar]
- 245.Imamoto Y., Tanaka H., Takahashi K., Konno Y., Suzawa T. Advantages of AlaGln as an additive to cell culture medium: use with anti-CD20 chimeric antibody-producing POTELLIGENTTM CHO cell lines. Cytotechnology. 2013;65:135–143. doi: 10.1007/s10616-012-9468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schnellbaecher A., Binder D., Bellmaine S., Zimmer A. Vitamins in cell culture media: stability and stabilization strategies. Biotechnol Bioeng. 2019;116:1537–1555. doi: 10.1002/bit.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Heeneman S., Deutz N.E.P., Buurman W.A. The concentrations of glutamine and ammonia in commercially available cell culture media. J Immunol Methods. 1993;166:85–91. doi: 10.1016/0022-1759(93)90331-Z. [DOI] [PubMed] [Google Scholar]
- 248.Vis M.A.M., Ito K., Hofmann S. Impact of culture medium on cellular interactions in in vitro co-culture systems. Front Bioeng Biotechnol. 2020;8:1–8. doi: 10.3389/fbioe.2020.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mittal N., Voldman J. Non-mitogenic survival-enhancing autocrine factors including cyclophilin A contribute to density-dependent mESC growth. Stem Cell Res. 2011;6:168–176. doi: 10.1016/j.scr.2010.10.001.Non-Mitogenic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Peerani R., Rao B.M., Bauwens C., Yin T., Wood G.A., Nagy A., Kumacheva E., Zandstra P.W. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wexler E.M., Paucer A., Kornblum H.I., Palmer T.D., Geschwind D.H. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bendall S.C., Stewart M.H., Menendez P., George D., Vijayaragavan K., Werbowetski-Ogilvie T., Ramos-Mejia V., Rouleau A., Yang J., Bossé M., Lajoie G., Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 253.Markovic O., Markovic N. Cell cross-contamination in cell cultures: the silent and neglected danger. In Vitro Cell Dev Biol Anim. 1998;34:1–8. doi: 10.1007/s11626-998-0040-y. [DOI] [PubMed] [Google Scholar]
- 254.Fogh J., Holmgren N.B., Ludovici P.P. A review of cell culture contaminations. In Vitro. 1971;7:26–41. doi: 10.1007/BF02619002. http://www.jstor.org/stable/4291579 [DOI] [PubMed] [Google Scholar]
- 255.Mirjalili A., Parmoor E., Moradi Bidhendi S., Sarkari B. Microbial contamination of cell cultures: a 2 years study. Biologicals. 2005;33:81–85. doi: 10.1016/j.biologicals.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 256.Vierck J.L., Byrne K., Mir P.S., Dodson M.V. Ten commandments for preventing contamination of primary cell cultures. Methods Cell Sci. 2000;22:33–41. doi: 10.1023/A:1009826012986. [DOI] [PubMed] [Google Scholar]
- 257.Geraghty R.J., Capes-Davis A., Davis J.M., Downward J., Freshney R.I., Knezevic I., Lovell-Badge R., Masters J.R.W., Meredith J., Stacey G.N., Thraves P., Vias M. Guidelines for the use of cell lines in biomedical research. Br J Cancer. 2014;111:1021–1046. doi: 10.1038/bjc.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Azimzadeh M., Khashayar P., Amereh M., Tasnim N., Hoorfar M., Akbari M. Microfluidic-Based Oxygen (O2) Sensors for on-chip monitoring of cell, tissue and organ metabolism. Biosensors. 2022;12 doi: 10.3390/bios12010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Magnusson E.B., Halldorsson S., Fleming R.M.T., Leosson K. Real-time optical pH measurement in a standard microfluidic cell culture system. Biomed Opt Express. 2013;4:1749. doi: 10.1364/boe.4.001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kieninger J., Weltin A., Flamm H., Urban G.A. Microsensor systems for cell metabolism-from 2D culture to organ-on-chip. Lab Chip. 2018;18:1274–1291. doi: 10.1039/c7lc00942a. [DOI] [PubMed] [Google Scholar]
- 261.Niepel M., Hafner M., Mills C.E., Subramanian K., Williams E.H., Chung M., Gaudio B., Barrette A.M., Stern A.D., Hu B., Korkola J.E., Shamu C.E., Jayaraman G., Azeloglu E.U., Iyengar R., Sobie E.A., Mills G.B., Liby T., Jaffe J.D., Alimova M., Davison D., Lu X., Golub T.R., Subramanian A., Shelley B., Svendsen C.N., Ma'ayan A., Medvedovic M., Gray J.W., Birtwistle M.R., Heiser L.M., Sorger P.K. A multi-center study on the reproducibility of drug-response assays in mammalian cell lines. Cell Syst. 2019;9:35–48. doi: 10.1016/j.cels.2019.06.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pamies D., Bal-Price A., Chesné C., Coecke S., Dinnyes A., Eskes C., Grillari R., Gstraunthaler G., Hartung T., Jennings P., Leist M., Martin U., Passier R., Schwamborn J.C., Stacey G.N., Ellinger-Ziegelbauer H., Daneshian M. Advanced good cell culture practice for human primary, stem cell-derived and organoid models as well as microphysiological systems. ALTEX. 2018;35:353–378. doi: 10.14573/altex.1710081. [DOI] [PubMed] [Google Scholar]
- 263.Hirsch C., Schildknecht S. In vitro research reproducibility: keeping up high standards. Front Pharmacol. 2019;10:1–9. doi: 10.3389/fphar.2019.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Elliott J.T., Rösslein M., Song N.W., Toman B., Kinsner-Ovaskainen A., Maniratanachote R., Salit M.L., Petersen E.J., Sequeira F., Romsos E.L., Kim S.J., Lee J., Von Moos N.R., Rossi F., Hirsch C., Krug H.F., Suchaoin W., Wick P. Toward achieving harmonization in a nanocytotoxicity assay measurement through an interlaboratory comparison study. ALTEX. 2017;34:201–218. doi: 10.14573/altex.1605021. [DOI] [PubMed] [Google Scholar]
- 265.Steinwedel T., Dahlmann K., Solle D., Scheper T., Reardon K.F., Lammers F. Single-use technology in biopharmaceutical manufacture. John Wiley & Sons, Ltd; 2019. Sensors for disposable bioreactor systems; pp. 69–82. [DOI] [Google Scholar]
- 266.Shin S.R., Kilic T., Zhang Y.S., Avci H., Hu N., Kim D., Branco C., Aleman J., Massa S., Silvestri A., Kang J., Desalvo A., Hussaini M.A., Chae S.K., Polini A., Bhise N., Hussain M.A., Lee H.Y., Dokmeci M.R., Khademhosseini A. Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell secretomes. Adv Sci. 2017;4:1–14. doi: 10.1002/advs.201600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Riahi R., Shaegh S.A.M., Ghaderi M., Zhang Y.S., Shin S.R., Aleman J., Massa S., Kim D., Dokmeci M.R., Khademhosseini A. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci Rep. 2016;6:1–14. doi: 10.1038/srep24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Vielreicher M., Schürmann S., Detsch R., Schmidt M.A., Buttgereit A., Boccaccini A., Friedrich O. Taking a deep look: modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine. J R Soc Interface. 2013;10 doi: 10.1098/rsif.2013.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Borile G., Sandrin D., Filippi A., Anderson K.I., Romanato F. Label-free multiphoton microscopy: much more than fancy images. Int J Mol Sci. 2021;22:1–20. doi: 10.3390/ijms22052657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Poisson J., Lemoinne S., Boulanger C., Durand F., Moreau R., Valla D., Rautou P.E. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017;66:212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 271.Dixon L.J., Barnes M., Tang H., Pritchard M.T., Laura E., Clinic C. Kupffer cells in the liver. Compr Physiol. 2016;3:785–797. doi: 10.1002/cphy.c120026.Kupffer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kietzmann T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 2017;11:622–630. doi: 10.1016/j.redox.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fragomeni G., Iannelli R., Falvo D'Urso Labate G., Schwentenwein M., Catapano G. Validation of a novel 3D flow model for the optimization of construct perfusion in radial-flow packed-bed bioreactors (rPBBs) for long-bone tissue engineering. N Biotechnol. 2019;52:110–120. doi: 10.1016/j.nbt.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 274.Pearce D., Fischer S., Huda F., Vahdati A. Applications of computer modeling and simulation in cartilage tissue engineering. Tissue Eng Regen Med. 2020;17:1–13. doi: 10.1007/s13770-019-00216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Burova I., Wall I., Shipley R.J. Mathematical and computational models for bone tissue engineering in bioreactor systems. J Tissue Eng. 2019;10:1–25. doi: 10.1177/2041731419827922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhao F., van Rietbergen B., Ito K., Hofmann S. Flow rates in perfusion bioreactors to maximise mineralisation in bone tissue engineering in vitro. J Biomech. 2018;79:232–237. doi: 10.1016/j.jbiomech.2018.08.004. [DOI] [PubMed] [Google Scholar]