Abstract
A growing number of geroprotectors have demonstrated healthspan extension in young animals, but the effectiveness of these therapies when commenced in midlife or later has been under-studied. We and others have shown that much like calorie restriction (CR), restriction of specific nutrients, including total protein, the three branched-chain amino acids leucine, isoleucine, and valine, or isoleucine alone, can promote lifespan and metabolic health. While CR is less efficacious when starting in late life, the effects of interventions restricting amino acids in late life on healthy aging is unknown. Here, we investigate the metabolic, molecular, and physiological effects of consuming diets with a 67% reduction of either all amino acids (Low AA) or of isoleucine alone (Low Ile) in male and female C57BL/6J.Nia mice starting at 20 months of age. We find that both diets reduce adiposity in aged mice; however, these diets decreased lean mass, and did not show significant improvements in frailty or fitness. The glucose tolerance of both male and female mice consuming Low Ile and Low AA diets were improved. We also observed a moderate increase in energy expenditure and respiratory exchange ratio induced by the two dietary interventions. In the hearts of aged female mice, Low Ile reversed age-associated changes in heart rate and stroke volume, returning cardiac function to similar levels as observed in young mice. We found that both Low AA and Low Ile diets promoted a more youthful molecular cardiac profile, preventing age-dependent increases in phosphatidylglycerols. These results demonstrate that Low AA and Low Ile diets can improve aspects of metabolic health in aged mice of both sexes, and has positive effects on cardiac health in aged females, suggesting that these dietary interventions are translationally promising for promoting healthy aging even in older people.
Keywords: protein restriction, isoleucine restriction, metabolic health, frailty, mice
Introduction
As the world’s population rapidly greys, there is a critical need to develop effective means to prevent or delay age-related diseases. Rather than targeting the diverse array of age-related diseases individually, addressing the aging process directly may be the most effective way to promote healthy aging. One of the most robust and affordable ways to promote healthspan and extend lifespan in diverse species is calorie restriction (CR), in which calories are restricted, with animals typically receiving 60–80% of their ad libitum caloric intake in a single meal each day (C. L. Green, D. W. Lamming, et al., 2022; Pak et al., 2021). While CR is a highly effective geroprotective treatment that delays or prevents many different age-related diseases in animal models, long-term adherence to a reduced-calorie diet is difficult for many individuals. Further, CR is much more efficacious when begun early in life than when initiated in older animals (Hahn et al., 2019).
An appealing alternative to reducing calories may be manipulating dietary macronutrients. Contrary to the conventional wisdom that calories from different sources are equivalent, a number of retrospective and prospective clinical trials have found that eating diets with lower levels of protein is associated with lower rates of age-related diseases including cancer and diabetes, and an overall reduction of mortality in those under age 55 (Levine et al., 2014; Sluijs et al., 2010). While the effect of long-term protein restriction (PR) on human aging has not been tested in a randomized clinical trial (RCT), short-term RCTs in overweight or diabetic humans has found that protein restriction (PR) promotes metabolic health (Ferraz-Bannitz et al., 2022; Fontana et al., 2016). Finally, PR diets have been repeatedly shown to increase the healthspan and lifespan of model organisms, including flies and rodents (Green & Lamming, 2019; Mair et al., 2005; Solon-Biet et al., 2014; Solon-Biet et al., 2015; Speakman et al., 2016).
While the mechanisms that drive the benefits of PR remain uncertain, PR diets necessarily reduce levels of the nine dietary essential amino acids (EAAs). The EAAs are critical regulators of the metabolic response to PR in mice, and restriction of the nine EAAs is required for the benefits of a CR diet on lifespan (Yoshida et al., 2018). The three branched-chain amino acids (BCAAs; leucine, isoleucine and valine) are highly correlated with metabolic diseases, with blood levels of BCAAs higher in obese and insulin-resistant humans and rodents (Fontana et al., 2016; Lynch & Adams, 2014). We and others have shown that restricting dietary BCAAs improves metabolic health in mice and rats, improving glucose tolerance and reducing adiposity in both lean and diet-induced obese animals (Cummings et al., 2018; Richardson et al., 2021; White et al., 2016; Yu et al., 2021). The restriction of BCAAs extends lifespan and reduces frailty in male mice, while dietary supplementation with additional BCAAs shortens lifespan in both male and female mice (Richardson et al., 2021; Solon-Biet et al., 2019).
While most studies have investigated the physiological role of the BCAAs as a group, the individual BCAAs have distinct metabolic effects. In particular, we have shown that restriction of isoleucine is both necessary and sufficient for the effects of PR on metabolic health in young mice, with restriction of isoleucine improving glucose tolerance, reducing adiposity, and altering energy balance by increasing both food consumption and energy expenditure (Yu et al., 2021). In humans, dietary isoleucine levels, but not levels of leucine or valine, are strongly correlated with BMI (Yu et al., 2021). Finally, restriction of isoleucine beginning in adult mice extends the lifespan of both male and female mice (Cara L. Green et al., 2022), and in humans, blood levels of isoleucine, but not leucine or valine, are positively associated with mortality (Deelen et al., 2019). Thus, there is significant evidence that dietary isoleucine is a critical regulator of metabolic health and aging in both mice and humans.
Many geroprotective interventions have reduced efficacy when starting in mid-life or later as opposed to early in life or as young adults. Notably, CR begun at 24 months of age has a reduced ability to extend lifespan (Hahn et al., 2019). Other interventions, such as rapamycin, remain fully efficacious when initiated at 20 months of age (Harrison et al., 2009; Strong et al., 2016). Mice from 18–21 months of age seem to benefit metabolically from PR, but with a loss of lean mass in males; the effects on fitness and frailty of late life PR intervention are unknown (Dommerholt et al., 2021; C. L. Green, H. H. Pak, et al., 2022). As the appeal of a geroprotective treatment is greatly increased if it can be initiated in middle-aged and older individuals, we decided to evaluate the efficacy of PR and isoleucine restriction in aged male and female mice.
Here, we tested the effect of restricting either all amino acids (Low AA) or specifically isoleucine (Low Ile) by 67% starting at 20 months of age in C57BL/6J.Nia male and female mice, roughly equivalent to 60 year old humans (Flurkey et al., 2007). We found that as in young mice, Low AA and Low Ile diet have robust benefits to metabolic health when begun in old mice. We tracked weight, body composition, and fitness/frailty longitudinally, examined the effect of the interventions on glycemic control, and tested the effects on heart function using echocardiography. We found that in both sexes, Low AA and Low Ile diets promote metabolic health, with mixed effects on frailty and fitness. A Low Ile diet favorably remodeled the aged female heart, with particular alterations in phosphatidylglycerol lipid content. Our findings suggest that restricting protein or isoleucine may be a promising translational therapy for older individuals.
Results
A low isoleucine diet reduces body weight and adiposity in aged male and female mice
To examine the effects of PR and isoleucine restriction as a late life intervention, we placed 20-month-old male and female C57BL/6J.Nia mice from the National Institute on Aging (NIA) Aged Mouse Colony and randomized them at the cage level to one of three treatment groups of equivalent body weight, adiposity, and frailty. Each group was placed on an amino acid (AA) defined diet containing all twenty common amino acids; the diet composition of the Control diet (Control; TD.140711) reflects that of a natural chow diet in which 21% of calories of derived from protein. The other two groups were placed on diets in which isoleucine was specifically reduced by 67% (Low Ile; TD.160734), or in which all twenty amino acids were reduced by 67% (Low AA; TD.140712). All three diets are isocaloric, with identical levels of fat; in the case of the Low Ile diet, non-essential AAs were increased to keep the calories derived from AAs constant, while carbohydrate levels were increased in the Low AA diet to maintain caloric density. All three of these diets have been used previously (Yu et al., 2021), and the detailed composition of the diets can be found in Table 1. An additional group of 6-month-old mice from the NIA Aged Mouse Colony was placed on the Control diet as a young control group (Young Control).
Table 1.
Specific composition of the amino acids-defined diets utilized in this manuscript.
| Diet | Control | Low Ile | Low AA |
|---|---|---|---|
| Teklad ID | TD.140711 | TD.160734 | TD.140712 |
| Kcal/g | 3.9 | 3.9 | 3.9 |
| kcal protein | 22.0% | 22.0% | 7.1% |
| kcal CHO | 59.4% | 59.4% | 74.4% |
| kcal fat | 18.6% | 18.6% | 18.5% |
| Amino acids g/kg | |||
| L-Alanine | 9.38 | 9.83 | 3.05 |
| L-Arginine | 6.30 | 6.30 | 2.05 |
| L-Asparagine | 20.58 | 20.91 | 6.70 |
| L-Aspartic Acid | 20.58 | 21.25 | 6.70 |
| L-Cystine | 7.20 | 7.20 | 2.34 |
| L-Glutamic Acid | 28.97 | 29.71 | 9.43 |
| L-Glutamine | 33.77 | 34.14 | 11.00 |
| Glycine | 2.96 | 3.34 | 0.96 |
| L-Histindine HCl, monohydrate | 4.60 | 4.60 | 1.50 |
| L-Isoleucine | 7.80 | 2.54 | 2.54 |
| L-Leucine | 25.40 | 25.40 | 8.27 |
| L-Lysine HCl | 20.38 | 20.38 | 6.64 |
| L-Methionine | 6.70 | 6.70 | 2.18 |
| L-Phenylalanine | 6.60 | 6.60 | 2.15 |
| L-Proline | 7.41 | 7.99 | 2.41 |
| L-Serine | 7.41 | 7.94 | 2.41 |
| L-Threonine | 9.70 | 9.70 | 3.16 |
| L-Tryptophan | 3.40 | 3.40 | 1.10 |
| L-Tyrosine | 6.90 | 6.90 | 2.25 |
| L-Valine | 8.40 | 8.40 | 2.74 |
| Sucrose | 291.25 | 291.25 | 291.25 |
| Corn Starch | 150.00 | 150.61 | 232.43 |
| Maltodextrin | 150.00 | 150.61 | 232.43 |
| Corn Oil | 52.00 | 52.00 | 52.00 |
| Olive Oil | 29.00 | 29.00 | 29.00 |
| Cellulose | 30.00 | 30.00 | 30.00 |
| Mineral Mix, AIN-93M-MX (94049) | 35.00 | 35.00 | 35.00 |
| Calcium Phosphate, monobasic, monohydrate | 8.20 | 8.20 | 8.20 |
| Vitamin Mix, Teklad (40060) | 10.00 | 10.00 | 10.00 |
| BHQ, antioxidant | 0.012 | 0.012 | 0.012 |
| Food color (0.1 g/kg) | Red | Orange | Blue |
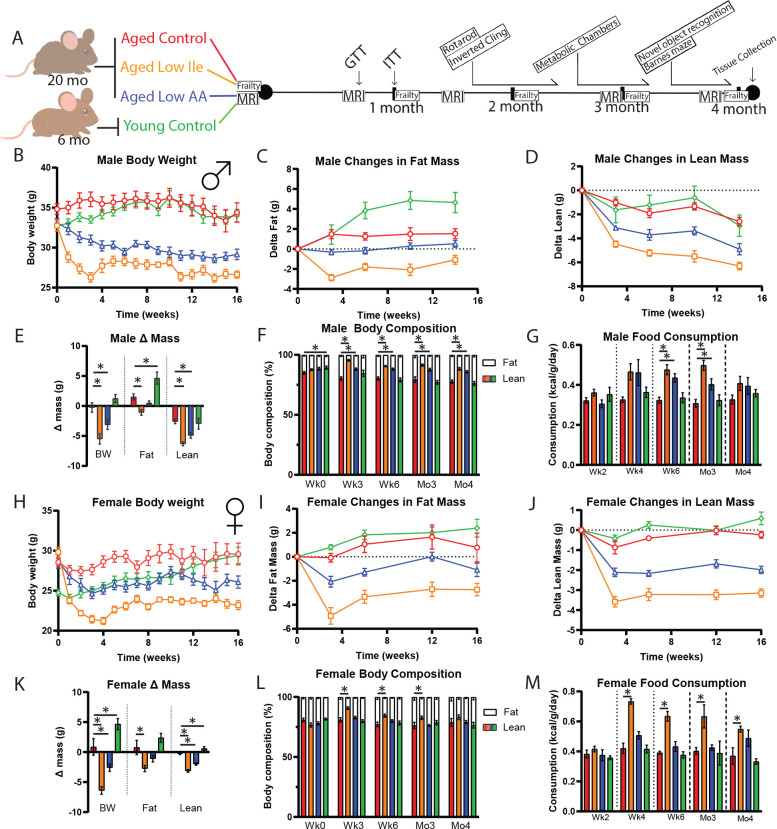
We monitored the mice longitudinally for 4 months with periodic assessment of weight and body composition (Fig. 1A). Old male mice placed on the Control diet maintained their weight during the course of the experiments, while old males fed either the Low Ile or Low AA diets had a significant decrease in body weight, with the Low Ile group experiencing the most weight loss; this weight loss occurred rapidly, with body weight largely stabilizing within 1 month (Fig. 1B). We assessed body composition by EchoMRI; weight loss in the Low Ile-fed males resulted from decreases in both fat mass and lean mass, whereas the lesser amount of weight loss in Low AA-fed mice was due to decreased lean mass only (Figs. 1C–E). Both Low Ile and Low AA-fed males had an overall decrease in adiposity at all time points examined (Fig. 1F). Periodic food consumption monitoring revealed that the changes in weight were not associated with reduced food intake; at all times, the weight-normalized caloric intake of Low Ile- and Low AA-fed mice was equal to or greater than aged Control-fed animals (Fig. 1G).
Figure 1. Both Low Ile and Low AA diets promote leanness in aged C57BL/6J.Nia mice.
A) Experimental scheme. Three different amino acids defined diets were utilized: Control (red), Low AA (blue), and Low Ile (orange). Three groups of aged mice at 20 months of age were fed each diet. A fourth group of young mice at 6 months of age was fed the Control diet (green).
B-F) Body weight of the male mice during the duration of the experiment (B), with the change in fat mass (C) and lean mass (D) over time. E) Final changes in body weight, fat, and lean mass of the male mice and (F) body composition percentage (B-F, N = 10–13/group; E-F, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test vs. Aged Control-fed mice).
G) Food consumption of the male mice throughout the experiment (N = 5–6/group, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test vs. Aged Control-fed mice).
H-M) Similar observations were made in female mice, presented in the same order. H-J) N = 10–11/group. K-M) N = 10–11/group. M) N = 4/group, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test vs. Aged Control-fed mice.
Data presented as mean ± SEM.
We observed similar effects of Low Ile and Low AA diets on the weight, fat mass, and lean mass of aged female mice as in the aged males (Figs. 1H–K). Notably, the overall effect of the Low Ile diet was similar in females, with an overall reduction in adiposity, while in contrast to males the effect of a Low AA diet on the total body composition was minimal (Fig. 1L). As in males, the weight loss of Low Ile and Low AA-fed female mice was not the results of reduced food intake, as the weight-normalized caloric intake of these groups was at all times equal to or greater than aged Control-fed animals (Fig. 1M).
Late-life Low Ile diet does not improve frailty, fitness, or cognition
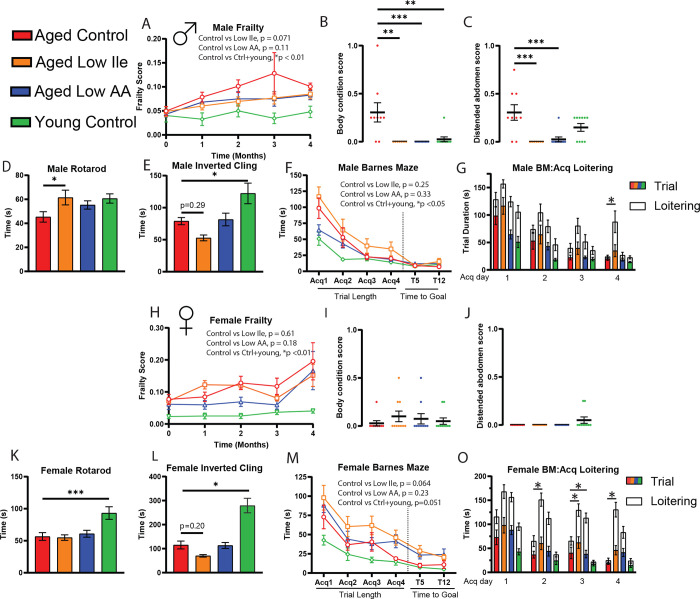
Decreased protein intake is associated with frailty and sarcopenia in older adults (Coelho-Junior et al., 2020; Coelho-Junior et al., 2018), and as we observed a significant reduction in lean mass in mice fed Low Ile and Low AA diets, we evaluated frailty and strength throughout the dietary intervention. We utilized a validated mouse frailty index that quantifies frailty through the accumulation of deficits (Whitehead et al., 2014). As we anticipated, the frailty score increases in both male and female mice as the mice age (Fig. 2A, H). While we did not observe an overall significant difference in frailty of Low Ile or Low AA mice of either sex, there was a clear trend towards lower frailty in Low Ile-fed males (p=0.071) and Low AA-fed males and females (p=0.18 and p=0.11, respectively). The categorical parameters and the individual measures that contributed the most to the group trends are provided (Sup. Fig 1). Most notably, aged Control-fed male mice had significantly worse body condition and distended abdomen scores than aged Low Ile-fed or Low AA-fed mice (Fig. 2B, C).
Figure 2. Healthspan evaluations of Low Ile and Low AA in aged male and female C57BL/6J.Nia mice.
A-C) In male mice, the average frailty score between 20 and 24 months of age (N = 10–13/group, pair-wise two-way ANOVA). B-C) Selected individual frailty categories, presented as the average of 3- and 4-month scores. At the beginning of the experiments N = 10–13 each group. *p < 0.05, one-way ANOVA with Dunnett’s post-hoc test.
D-E) Male rotarod (D) and inverted cling (E) performance were assessed between 22–23 months of age (N = 8–11/group, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test vs, aged Control-fed mice).
F-G) Male Barnes maze performance at 24 months of age (F). N = 7–10 each group, pair-wise two-way ANOVA. G) Male Barnes maze acquisition trial duration with loitering (test on loitering time, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test vs aged Control-fed mice).
H-O) Similar observations were observed in female mice in the same order. H-J) N = 10–11/group. K-L) N = 8–11/group. M-O) N = 8–10.
Data presented as mean ± SEM.
We assessed neuromuscular coordination and muscle strength with an accelerating rotarod test and an inverted cling test, respectively. As we expected, young Control-fed mice performed better on these assays than aged Control-fed mice (Figs. 2D–E, K–L). Interestingly, aged Low Ile-fed male mice performed significant better in the rotarod test compared to the aged Control-fed males, and comparably to the young Control-fed group (Fig. 2D). There was no effect of diet on the rotarod performance of aged females (Fig. 2K). In an inverted cling test, we observed a non-significant trend (p=0.29 and p=0.2) towards decreased cling time in aged Low Ile-fed male and female mice, respectively (Figs. 2E, L). This is surprising, as the Low Ile-fed mice of both sexes were lighter than aged Control-fed mice, and if muscle strength was equivalent, Low Ile-fed mice would have been expected to cling on for longer, not shorter, periods of time. Interestingly, Low AA-fed mice did not show this decrease in cling time (Fig. 2E, L). In ANCOVA analysis of rotarod and cling performance with body weight as a covariate, the cling time of aged Low Ile-fed female mice was significantly decreased compared to Control-fed aged females (Sup. Figs. 2A–D). To evaluate whether this trend of diet-induced loss in grip strength is age-dependent, a separate cohort of young 3-month-old male mice was fed the Low Ile diet for 2 months; we observed a similar negative effect of a Low Ile diet on cling time despite their young age and decreased body weight (Sup. Figs. 2E–F).
Finally, C57BL/6J mice have previously been shown to suffer from age-related cognitive decline (Majumder et al., 2012), and we examined the impact of Low Ile and Low AA diets on cognitive performance by performing a Novel Object Recognition test (NOR) and Barnes Maze test (BM) at approximately 23 months of age. During the habituation phase for the NOR, we evaluated the amount of movement in each group in the open field (Sup. Fig. 3A, B). Interestingly, aged Control-fed females exhibited increased velocity compared to Low Ile-fed aged females and the Young Control females; this effect of diet and age was not observed in males (Sup. Fig. 3A, B). In the NOR test, we did not observe a difference in NOR performance between Young Control and aged Control-fed mice of either sex, and thus is it not surprising that we also did not observe significant differences between groups during the acquisition phase, the short term memory (STM) test, or the long-term memory (LTM) test (Sup Figs. 3C–J). Summing all three phases, the total time investigating both objects was highest in Young Control males and significantly lower in aged Control-fed males, while Low Ile-fed males investigated for even less time than aged Control-fed mice (Sup Fig. 3F).
In the BM, we observed that during the acquisition phase there was an age-related deficit in time to find the escape hole in both sexes of mice (Figs. 2F, M). Interestingly, aged Low Ile-fed mice of both sexes performed more poorly than aged Control-fed mice (p=0.064 in females). The aged mice spent a significant amount of time hesitating after finding the escape hole, with the ‘escape’ as defined by remaining within the area for 3 seconds, after which the program terminates the automatic tracking trial. We manually measured this duration between the end of the trials and the time when mice definitively enters the escape box. We determined that this loitering time period was persistently increased in mice of both sexes fed the Low Ile diet and in female mice fed the Low AA diet. This was in sharp contrast to the loitering time in Control-fed mice of both sexes, which clearly diminishes over the acquisition sessions (Figs. 2G, O).
Low Ile and Low AA diets promote glycemic control and energy expenditure in aged mice
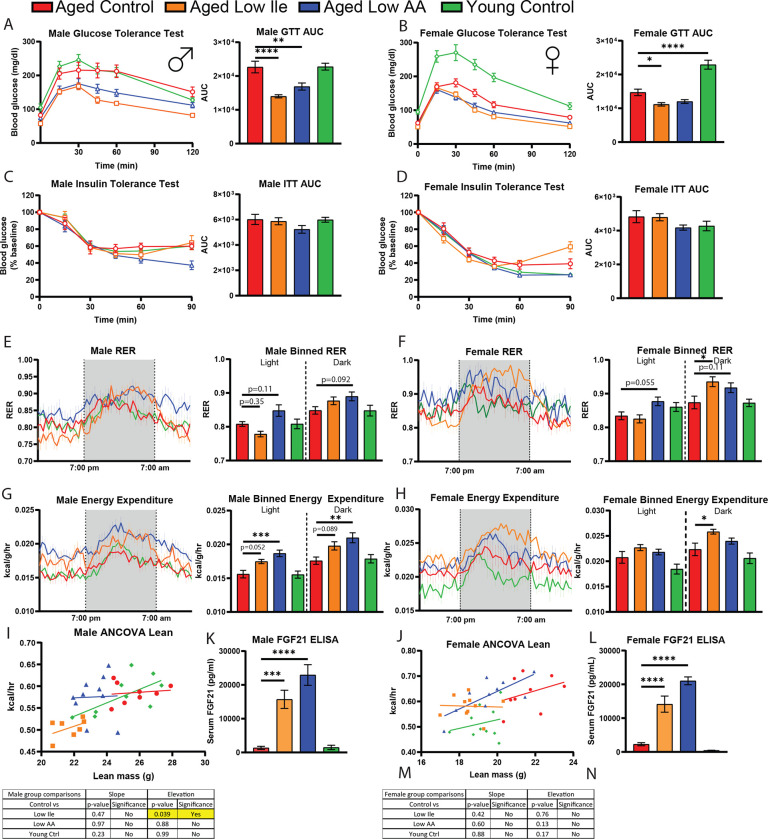
Glucose dysregulation is a precursor to many pertinent age-driven diseases, and we and others have shown that restriction of protein, BCAAs, or isoleucine can promote glucose tolerance in young male mice. Here, we examined how Low Ile and Low AA diets impacts blood sugar control in aged mice. Three weeks after the animals were placed on their respective diets, we performed a glucose tolerance test (GTT). Similar to what we have previously observed in young males, we found that both male and female aged mice fed a Low Ile diet had significantly improved glucose tolerance relative to aged Control-fed mice; similarly, a Low AA diet significantly improved glucose tolerance, reaching statistical significance in the case of Low AA-fed males (Figs. 3A–B). While conducting these assays, we observed that the Low Ile-fed male mice had significantly lower fasting blood glucose after 16 hr (Sup. Figs. 4A–B). In a separate cohort of animals at 25-months of age and similarly on the interventional diets for 3 weeks, glucose tolerance in Low Ile-fed males and females also improved, with a trend towards improved glucose tolerance in Low AA-fed mice as well, indicating that the window of effectiveness for initiating Low Ile or Low AA to improve glucose tolerance extends until at least 25 months of age (Sup. Fig 4C–D). Consistent with our previous results in young males, no diet groups exhibited significantly altered response to intraperitoneal injection of insulin in a tolerance test (ITT) (Figs. 3C–D).
Figure 3. Metabolic evaluation of late life dietary intervention found improved glycemic control and increased energy expenditure.
A-D) Glucose tolerance test in male (A) and female (B) mice fed the indicated diets. Insulin tolerance test in male (C) and female (D) mice fed the indicated diets. N = 10–13/group, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test vs. aged Control-fed mice.
E-H) Respiratory exchange ratio was determined using metabolic cages over 24 hours in male (e) and females (f). Energy expenditure normalized to body weight was determined over 24 hours in males (g) and females (h). N = 7–10/group, *p < 0.05, one-way ANOVA conducted separately for the light and dark cycles, Dunnett’s post-hoc test vs. Aged Control-fed mice.
I, J) The ANCOVA of energy expenditure with lean mass as a covariate.
K, L) The serum FGF21 level at the end of the experiment, after 16 hr fasting overnight and 3 hr refeeding. N = 5–7/group. *p < 0.05, two-way ANOVA, Dunnett’s post-hoc test.
Data presented as mean ± SEM.
We have previously found that restriction of protein or isoleucine increases the respiratory exchange ratio (RER) and energy expenditure of young male mice (Yu et al., 2021). We observed a trend towards increased RER in aged Low AA-fed mice, particularly in males, but this did not reach statistical significance (Figs. 3E–F). Interestingly, aged Low Ile-fed males had a lower RER than the aged Control-fed males during the light cycle, and a slightly higher one during the dark cycle; this may be the result of a right-shifting of the entire RER curve (Fig. 3E). In contrast, in aged Low Ile-fed female mice, RER values were significantly higher in the dark cycle (Fig. 3F).
Energy expenditure of male mice fed a Low Ile or Low AA diet was, as we previously observed for young male mice, higher than that of Control-fed mice, with the Low AA diet energy expenditure significantly increased during both the light and dark cycles (Fig. 3G). In females, energy expenditure was higher in Low Ile-fed mice during the active dark cycle (Fig. 3H). Using ANCOVA analysis using lean mass as a covariate, we found that aged Low Ile-fed males have reduced total energy expenditure independent of their lean mass compared to aged Control-fed males (Figs. 3I–J).
We have previously shown that FGF21 is induced by Low Ile and Low AA diets in young male mice, and here we see this is the case in aged mice of both sexes (Figs. 3K–L). The activity levels of all diet groups were comparable (Sup. Figs. 4E–F).
Low Ile diet induces sex-specific cardiac remodeling
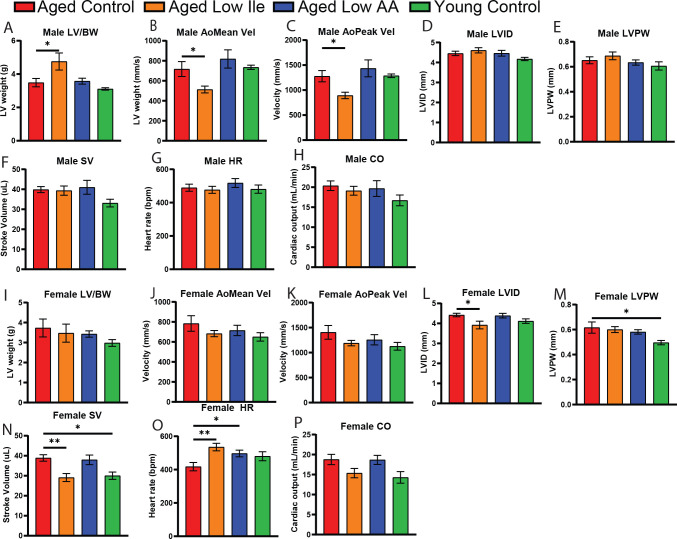
Cardiovascular disease is a top cause of death in aging adults (Tsao et al., 2022). The aging heart exhibits ventricular thickening associated with a primary loss of vessel flexibility and increased blood pressure. BCAA catabolism is highly implicated in various aspects of heart failure (Karwi & Lopaschuk, 2022), and recent work has shown that dietary BCAAs can directly induce the growth of cardiomyocytes, contributing to worsening heart function (Latimer et al., 2021). To determine if a Low Ile or Low AA diet impact cardiac function, we performed echocardiography to evaluate heart function in a separate cohort of animals at 25 months of age after 6 weeks of dietary intervention.
We observed a significant increase in body weight-normalized left ventricle (LV) mass in Low Ile-fed males (Fig. 4A). In addition, mean and peak aortic flow velocity is decreased by Low Ile (Figs 4B–C). We observed different effects in aged female mice. We did not observe significant changes in LV mass or mean and peak aortic flow velocity in response to diet (Figs. 4I–K). However, more in line with our expectations of cardiac aging, Low Ile-fed females had a significant decrease in diastolic LV inner diameter (Figs. 4L), while the diastolic LV posterior wall is increased in size in the aged female animals but unchanged by the diets (Fig. 4M). Interestingly, we observed an age-dependent increase in stroke volume that was prevented by a Low Ile diet (Fig. 4N), and both Low Ile and Low AA-fed mice had increased heart rates as compared to aged Control-fed females, to levels similar to the Young Control (Fig. 4O). There was no significant changes in the cardiac output, although there was a trend of age-dependent increase and an abatement by the Low Ile diet (Fig. 4P). These trends and changes were not observed in the male mice counterparts (Fig. 4D–H). As the ejection fraction and cardiac output are not significantly altered in any of the groups, it appears that the gross function of the hearts are unchanged, but the long-term consequences of the dietary interventions are yet to be determined. We suggest that a Low Ile diet may selectively induce some aspects of a youthful cardiac physiology in the aged female heart. The entirety of this dataset is provided in Table 2.
Figure 4. Echocardiogram evaluation found cardiac restructuring induced by the dietary interventions.
A-H) Echocardiogram evaluation of male mice at 25 months of age. Presented in order A) body weight-normalized left ventricle mass, B) mean aortic flow velocity, C) peak aortic velocity, D) left ventricle inner diameter, E) left ventricle posterior wall diameter, F) stroke volume, G) heart rate, H) cardiac output.
I-J) Echocardiogram evaluation of female mice at 25 months of age, presented in the same order as males. A-J) N = 5–10/group, *p < 0.05, one-way ANOVA, Dunnett’s post-hoc test.
Data presented as mean ± SEM.
Table 2.
Male and female echocardiogram dataset in mice after 6 weeks of dietary intervention starting from 24 months of age.
| Male Echocardiogram Dataset | |||||||||
| Aged Control | Aged Low Ile | Aged Low AA | Young Control | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Sig. vs Control | |
| Weight (g) | 31.57 | 1.38 | 25.86 | 1.22 | 29.40 | 0.81 | 29.20 | 0.80 | Aged Low Ile |
| LV Mass (AW) (mg) | 109.33 | 6.74 | 121.50 | 12.18 | 105.32 | 7.15 | 90.72 | 2.86 | |
| LV Mass/BW (mg) | 3.49 | 0.25 | 4.76 | 0.51 | 3.58 | 0.18 | 3.11 | 0.07 | Aged Low Ile |
| LVID;d (mm) | 4.45 | 0.11 | 4.61 | 0.13 | 4.46 | 0.14 | 4.17 | 0.08 | |
| LVPW;d (mm) | 0.65 | 0.03 | 0.69 | 0.03 | 0.63 | 0.02 | 0.61 | 0.03 | |
| LVID;s (mm) | 3.48 | 0.15 | 3.69 | 0.17 | 3.47 | 0.11 | 3.31 | 0.06 | |
| LVPW;s (mm) | 0.80 | 0.04 | 0.81 | 0.03 | 0.78 | 0.02 | 0.74 | 0.03 | |
| LVAW;d (mm) | 0.66 | 0.02 | 0.66 | 0.03 | 0.64 | 0.03 | 0.64 | 0.02 | |
| LVAW;s (mm) | 0.80 | 0.04 | 0.81 | 0.03 | 0.82 | 0.01 | 0.75 | 0.02 | |
| LV Vol;d (μL) | 90.70 | 5.44 | 98.46 | 6.68 | 91.19 | 7.11 | 77.69 | 3.61 | |
| LV Vol;s (μL) | 50.84 | 5.05 | 59.13 | 6.64 | 50.20 | 4.20 | 44.57 | 1.82 | |
| EF (%) | 44.54 | 2.66 | 40.84 | 3.26 | 44.90 | 1.75 | 42.56 | 0.72 | |
| FS (%) | 22.04 | 1.54 | 20.04 | 1.83 | 22.18 | 1.03 | 20.68 | 0.43 | |
| IVRT (ms) | 19.12 | 1.47 | 17.27 | 1.68 | 20.11 | 1.82 | 17.28 | 1.11 | |
| Stroke Volume (μL) | 39.86 | 1.52 | 39.34 | 2.31 | 40.99 | 3.47 | 33.12 | 1.91 | |
| Heart Rate (bpm) | 489.49 | 21.43 | 476.81 | 21.14 | 517.75 | 26.71 | 480.92 | 24.60 | |
| Cardiac Output (mL/min) | 20.37 | 1.21 | 19.11 | 1.10 | 19.67 | 1.96 | 16.71 | 1.35 | |
| Ao Area (cm) | 1.49 | 0.20 | 2.24 | 0.38 | 1.25 | 0.18 | 1.06 | 0.07 | |
| Ao Mean Vel (mm/s) | 717.31 | 74.51 | 512.96 | 34.50 | 818.39 | 91.26 | 734.91 | 21.09 | Aged Low Ile |
| Ao Mean Grad (mmHg) | 2.17 | 0.47 | 1.08 | 0.14 | 2.78 | 0.60 | 2.17 | 0.13 | |
| Ao Peak Vel (mm/s) | 1277.76 | 112.74 | 892.86 | 64.41 | 1434.55 | 168.96 | 1287.00 | 36.04 | Aged Low Ile |
| Ao Peak Grad (mmHg) | 6.79 | 1.24 | 3.27 | 0.47 | 8.58 | 1.98 | 6.65 | 0.38 | |
| Ejection Time (ms) | 44.12 | 3.15 | 38.47 | 2.68 | 40.35 | 2.63 | 43.69 | 1.87 | |
| Female Echocardiogram Dataset | |||||||||
| Control | Low Ile | Low AA | Young Ctrl | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Sig. vs Control | |
| Weight (g) | 28.63 | 1.73 | 23.13 | 0.48 | 28.20 | 0.85 | 24.00 | 1.52 | Aged Low Ile |
| LV Mass (AW) (mg) | 103.13 | 9.38 | 79.45 | 9.35 | 96.27 | 4.32 | 71.29 | 5.47 | Young Control |
| LV Mass/BW (mg) | 3.73 | 0.45 | 3.47 | 0.45 | 3.43 | 0.16 | 2.98 | 0.17 | |
| LVID;d (mm) | 4.42 | 0.09 | 3.92 | 0.19 | 4.39 | 0.11 | 4.11 | 0.11 | Aged Low Ile |
| LVPW;d (mm) | 0.62 | 0.04 | 0.60 | 0.02 | 0.58 | 0.02 | 0.50 | 0.02 | Young Control |
| LVID;s (mm) | 3.47 | 0.08 | 3.08 | 0.21 | 3.45 | 0.10 | 3.31 | 0.17 | |
| LVPW;s (mm) | 0.76 | 0.04 | 0.72 | 0.02 | 0.72 | 0.01 | 0.61 | 0.01 | Young Control |
| LVAW;d (mm) | 0.64 | 0.03 | 0.60 | 0.02 | 0.63 | 0.02 | 0.54 | 0.02 | Young Control |
| LVAW;s (mm) | 0.79 | 0.03 | 0.73 | 0.01 | 0.74 | 0.01 | 0.69 | 0.03 | Young Control |
| LV Vol;d (μL) | 88.96 | 4.07 | 68.42 | 8.30 | 87.88 | 5.10 | 75.21 | 5.05 | |
| LV Vol;s (μL) | 50.07 | 2.80 | 39.32 | 7.00 | 49.91 | 3.38 | 45.20 | 5.77 | |
| EF (%) | 43.88 | 1.05 | 44.68 | 3.34 | 43.44 | 1.56 | 40.68 | 3.87 | |
| FS (%) | 21.59 | 0.59 | 21.97 | 1.90 | 21.34 | 0.86 | 19.73 | 2.12 | |
| IVRT (ms) | 20.72 | 2.32 | 16.63 | 1.72 | 20.50 | 1.60 | 19.33 | 1.88 | |
| Stroke Volume (μL) | 38.90 | 1.64 | 29.10 | 2.00 | 37.97 | 2.40 | 30.01 | 1.83 | Aged Low Ile, Young Control |
| Heart Rate (bpm) | 417.71 | 24.83 | 535.23 | 22.28 | 496.71 | 19.80 | 480.11 | 26.98 | Aged Low Ile, Aged Low AA |
| Cardiac Output (mL/min) | 18.77 | 1.28 | 15.37 | 1.16 | 18.67 | 1.14 | 14.29 | 1.45 | |
| Ao Area (cm) | 1.08 | 0.11 | 1.00 | 0.06 | 1.30 | 0.11 | 1.10 | 0.08 | |
| Ao Mean Vel (mm/s) | 783.91 | 78.03 | 682.80 | 30.99 | 714.48 | 51.60 | 650.10 | 42.24 | |
| Ao Mean Grad (mmHg) | 2.63 | 0.57 | 1.91 | 0.17 | 2.14 | 0.28 | 1.72 | 0.22 | |
| Ao Peak Vel (mm/s) | 1405.10 | 137.05 | 1189.40 | 53.96 | 1256.36 | 102.35 | 1126.18 | 77.47 | |
| Ao Peak Grad (mmHg) | 8.43 | 1.76 | 5.78 | 0.51 | 6.70 | 0.95 | 5.17 | 0.72 | |
| Ejection Time (ms) | 48.47 | 2.56 | 42.01 | 1.60 | 42.64 | 1.56 | 42.11 | 2.17 | |
Statistics - 1-way ANOVA, Dunnett’s Multiple Comparison.
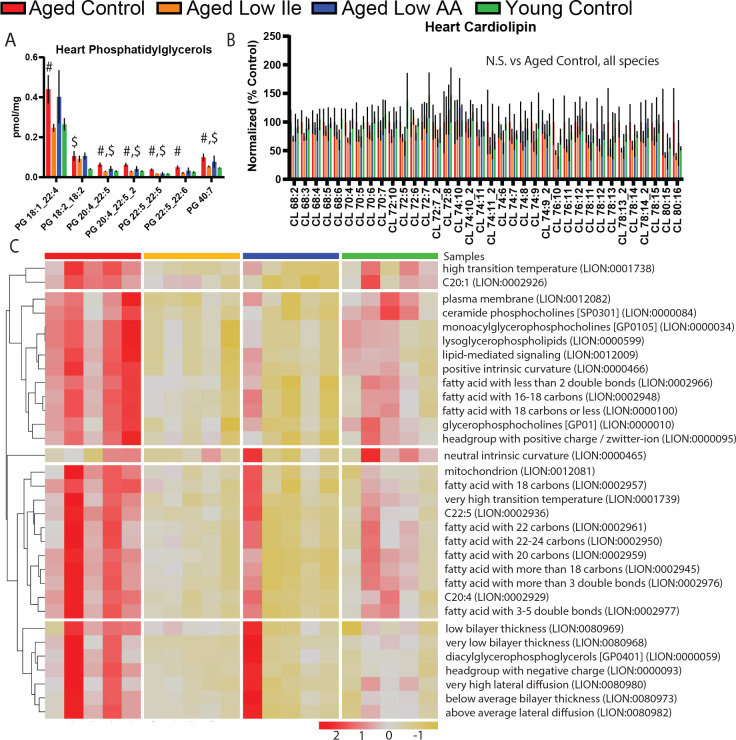
In order to obtain an unbiased and comprehensive assessment of the effects of Low Ile and Low AA diets on the heart, we performed lipidomic profiling on the hearts of female mice collected following 4 months of dietary intervention at 24 months of age. We observed a significant age-dependent increase in several phosphatidyglycerol species that was mostly ameliorated in Low Ile-fed mice, but not in Low AA-fed mice (Fig. 5A). As phosphatidylglycerol is the primary component of the mitochondrial outer membrane, we wanted to also explore the lipid most abundant in the mitochondrial inner membrane, cardiolipin. Interestingly, cardiolipin species were unchanged (Fig. 5B). We performed lipid ontology enrichment analysis (LION); pathways of the significantly altered lipid species are shown in (Fig. 5C). Overall, we observed a rejuvenating effect of the Low Ile and Low AA diets, with pathways showing age-related increases in aged Control-fed mice being restored to a more youthful level by Low Ile and Low AA feeding.
Figure 5. Lipidomic analysis of the aged female heart.
A-B) Statistically significant phosphatidylglycerols (A) and all cardiolipins (B), which were not significant, in female mice heart at 24 months of age after 4 months of dietary intervention. Multiple t-tests significances are shown; Aged Control vs Low Ile (#) or vs Young Control ($); p < 0.05. N = 5 each group.
C) LION lipid ontology analysis of significantly altered lipid species in the female mice heart.
Data presented as mean ± SEM.
Low Ile and Low AA induce specific senomorphic changes in the aged liver
Cellular senescence plays a large role in promoting several aging phenotypes of the liver, including fibrosis and non-alcoholic fatty liver disease (Matthew et al., 2017). To characterize the molecular remodeling that occurs in the aged liver induced by the two dietary interventions, we performed real time quantitative PCR (rt-qPCR) for gene expression level changes of well-established senescence-associated markers. The results found that compared to the aged Control mice, Low Ile significantly increased p21 and Low AA significantly reduced Ile-1a and TNF-α (Sup. Fig. 5). Of note, the trends in the changes of senescence markers induced by Low Ile and Low AA are distinctly senomorphic, as they each alter the profile of expression of senescence markers in a different manner and not inducing a general elevation or suppression.
Discussion
Recent work from our laboratory and others has demonstrated that a calorie is not “just a calorie,” and that the macronutrient composition of the diet is a key regulator of human and animal health (C. L. Green, D. W. Lamming, et al., 2022). In particular, it has now become clear that dietary levels of protein or BCAAs impacts the metabolic health and longevity of both rodents and humans (Fontana et al., 2016; Levine et al., 2014; Richardson et al., 2021; Solon-Biet et al., 2019; Solon-Biet et al., 2014). As the BCAAs are normally consumed together and are catabolized via highly similar processes, they have often been studied in combination, but it is now becoming clear that the individual BCAAs have distinct roles on metabolic health (Jang et al., 2016; Richardson et al., 2021). In particular, we have previously shown that 67% restriction of isoleucine is necessary for the metabolic benefits of a low protein diet, and is sufficient to promote glucose tolerance, hepatic insulin sensitivity, increased energy expenditure and to reverse the effects of Western diet-induced obesity on body composition, glycemic control, and hepatic steatosis (Yu et al., 2021). In recent studies, we have shown that a Low Ile diet increases the median and maximum lifespan of genetically heterogeneous mice when fed to 6-month-old adult mice (Cara L. Green et al., 2022).
While this is very promising, to be of maximum clinical use geroprotective drugs and diets should be able to promote healthy aging even when begun in late life. We and others have proposed the use of a comprehensive metabolic, physical, and cognitive phenotyping pipeline in aged mice (Bellantuono et al., 2020), and here we have used this pipeline to examine the effects of Low Ile and Low AA diets started late in life on healthspan. We find that Low Ile and Low AA diets started late in life have dramatic effects on weight, body composition, glycemic control, and energy balance in aged mice of both sexes. Interestingly, the effects of a Low Ile and Low AA diets are distinct, which we previously observed in a study of mice started as young adults (Cara L. Green et al., 2022).
Overall, many of the effects of Low Ile and Low AA diets in aged mice are similar to those we observed in younger age groups. In both sexes, Low Ile and Low AA diets begun in late life induce weight loss, improve glucose tolerance, and increase energy expenditure while also increasing food intake. In contrast to our previous studies in young male animals, aged mice consuming either a Low Ile or Low AA diet have a significant loss of lean mass. This decline in lean mass could have contributed to the unanticipated decline in inverted cling performance that we observed. Overall, while there are beneficial effects of Low Ile and Low AA diet in both sexes, there are also potentially troubling negative sex-specific effects on physical performance, frailty, and cognition that require further investigation before the use of these types of dietary interventions in the elderly can be recommended.
Further, sex-specific effects of a Low Ile diet on heart function were observed. In agreement with previous work, we found that female mice had certain age-related deficits in cardiac parameters (Dai et al., 2014), although we did not observe a statistically significant effect of age on left ventricle mass (Kane et al., 2020). We found that in aged females, a Low Ile diet restored stroke volume and heart rate to levels comparable to young Control-fed mice, and that the effects of age on phosphatidylglycerol, and significantly altered lipid features in general, were reversed by a Low Ile diet, an encouraging sign that a Low Ile diet may promote cardiac health. Broadly, this work is consistent with emerging data suggesting that high blood levels of BCAAs are deleterious for cardiac function (Chen et al., 2019; Latimer et al., 2021; Portero et al., 2021; Uddin et al., 2019), although the specific role of the individual BCAAs in cardiac function and the aging heart has not previously been examined.
Many of the metabolic benefits of protein restriction (PR) have been linked to induction of the FGF21; here, we show that FGF21 is induced by both Low Ile and Low AA diets in aged C57BL/6J mice of both sexes. While our recent work has shown that induction of FGF21 is not required in certain strains and sexes, it has been recently demonstrated that FGF21 is required for the metabolic and lifespan extension effects of PR (Hill et al., 2022). Thus, the involvement of FGF21 in the late life response to Low Ile and Low AA diets remains to be determined. Notably, FGF21 can regulate muscle fiber type and reduce fiber size (Sun et al., 2021), and plasma FGF21 is associated with lower muscle mass in aging humans (Roh et al., 2021). As maintaining skeletomuscular function is critical in the elderly, future work should examine if late life Low Ile and Low AA diets reduce muscle function, and determine the role of FGF21 in this response.
Efforts attempting to manipulate BCAA catabolic flux to simulate either a BCAA restriction or supplementation diet have not been conclusive. Enhancing BCAA catabolism in mice has been shown to improve obesity-associated insulin resistance (Zhou et al., 2019). Recently, a small cohort randomized placebo-controlled clinical trial with T2D patients also found a robust improvement in their peripheral insulin sensitivity (Vanweert et al., 2022). On the other hand, BCAA catabolism has also been shown to permit obesity and block adipose tissue browning via downstream acetylation, which can be reversed with genetic or pharmacological methods (Ma et al., 2022). Interestingly, a recent study suggests that increased BCAA catabolism may grant protection from cardiac failure, although the mechanisms of action is likely indirect (Murashige et al., 2022). While it is clear that BCAA flux and its manipulation plays a critical role in metabolic health, further work is necessary for clarifying its most prominent functions and the contribution of individual BCAAs.
In conclusions, we have shown dietary restriction of all dietary amino acids, or specific restriction of isoleucine, can benefit the healthspan of mice even when begun late in life. Metabolic improvements are observed in both sexes, but the effects on cardiac function and molecular effects are female specific. Additional research will be required to examine the potentially negative effects of isoleucine or protein restriction on lean mass and muscle strength in aged mice, and how optimal levels of isoleucine on lifespan and healthspan vary with age and sex, as well as genetic background. Overall, these results suggest that late life initiation of isoleucine or protein restriction must be approached with caution, but may still be beneficial overall for older individuals who are physically robust but are suffering from obesity.
Materials & Methods
Animals
All procedures were performed in accordance to institutional guidelines and were approved by the Institutional Animal Care and Use Committee of William S. Middleton Memorial Veterans Hospital (Madison, WI, USA). C57BL/6J.NIA mice from the NIA Aged Rodent Colony were obtained at 20 months of age and at the young adult age of 6 months of age. Animals were provided ad libitum access to Laboratory Rodent Diet 5001 diet for 1–2 weeks and housed as 2–3 animals per cage before beginning their respective dietary interventions. All mice were maintained at a temperature of approximately 22°C, and health checks were performed daily by the facility staff. Mice were housed in a SPF facility in static microisolator cages under 12:12 light cycle conditions with ad libitum access to food and water unless specified below for upcoming experiments.
At the experiment start, aged animals were randomized at the cage level to groups of approximately equivalent weight, body composition and average frailty scores to one of three diets groups: Control with 21% calories derived from amino acids (TD.140711; Envigo), Low Ile with 67% less isoleucine content than the Control diet (TD.160734), and a Low AA diets with 7% calories derived from amino acids (TD.140714). The three diets are isocaloric, with supplemental non-essential amino acids used to balance the protein content in the Low Ile diet, and supplemental carbohydrates used to balance the calories content in the Low AA diet.
Metabolic chambers indirect calorimetry was carried out using Oxymax/CLAMs metabolic chamber system (Columbus Instruments) for ~48 continuous hours. The first ~24 hours of data was discarded as acclimation period with a subsequent continuous 24 hours period utilized for data analysis. Food consumption monitoring was carried out in home cages over 2–4 days and analyzed using total animal mass in the cage. Body composition was determined using an EchoMRI Body Composition Analyzer.
In vivo procedures
Glucose and insulin tolerance tests were performed by fasting the mice overnight for 16 hours or 4 hours in the morning respectively. In respective tests, mice were injected with glucose (1 g/kg; Sigma, G7021) or insulin (0.75 U/kg; Novolin) intraperitoneally (i.p.). Blood glucose was monitored via a Bayer Contour glucometer and test strips (Bayer, Leverkusen, Germany). All behavior tracking is done automatically with EthoVision XT program (Noldus). All following behavior/fitness testing follow previously described protocols (Bellantuono et al., 2020).
Novel Object Recognition.
Briefly, an habituation/open field test is followed by the acquisition trial. Short-term memory test (STM) is carried out 1 hr after the acquisition trial, and the long-term memory test is carried out 23 hr from the acquisition trial. The habituation, acquisition, short-, and long-term memory test are 5 min each.
Barnes Maze.
Briefly, the test is carried out on a maze table with 20 possible exit holes with distinct visible landmarks outside of the arena. Each animal are exposed to four acquisition days with a maximum trial time of 180 seconds. On day 5, a test trial takes place for short-term memory, then the mice are not experimented with until day 12, when a long-term memory test trial takes place.
Rotarod.
Animals are trained in slow moving 4 rpm. On test trials, the accelerating rotarod (Rotamex 5, Columbus Instruments) is set to increase by 0.5 rpm per 4 seconds. There is a minimum rest period of 30 min between training and trials for each animal. The average trial performance for 3 test trials is taken for statistical analysis.
Inverted cling.
Animals are placed onto a wire-grid bounded with masking tape and inverted onto the test bin. Mice naïve to the experiment are first trained through 3 trials of 30 seconds gripping prior to test trials. A minimum 30 min rest between training and, for each animal, 3 test trials are averaged for analysis.
FGF21 ELISA.
Circulating FGF21 is measured using blood plasma obtained after 16 hr overnight fast and 3 hr refeed at 4 months after diet start, age 24 months for aged mice and age 10 months for young adult mice. Circulating FGF21 was quantified using a mouse/rat FGF21 quantikine ELISA kit (MF2100; R&D Systems, Minneapolis, MN, USA).
Quantative real-time PCR.
rt-qPCR was carried out according to protocols described previously (Calubag et al., 2022) using TRI Reagent according to the manufacturer’s protocol. The primers that were used are as follows. P21 Fwd GAGACTAAGGCAGAAGATGTAGAG, Rev GCAGACCAGCATGACAGAT; P16 Fwd TGAGCTTTGGTTCTGCCATT, Rev AGCTGTCGACTTCATGACAAG; Il-1a Fwd GAGGCCTTACGCATACCTCC, Rev ACAAGTGCGTCGTCAAAACG; Il-1b Fwd AGCCATGGCAGAAGTACCTG, Rev TGAAGCCCTTGCTGTAGTGG; Mcp-1 Fwd GATCTCAGTGCAGAGGCTCG, Rev TTTGCTTGTCCAGGTGGTCC; Il-10 Fwd ATAACTGCACCCACTTCCCA, Rev GGGCATCACTTCTACCAGGT; TNF-α Fwd ATGAGAAGTTCCCAAATGGC, Rev CTCCACTTGGTGGTTTGCTA; Il-6 Fwd CTGGGAAATCGTGGAAT, Rev CCAGTTTGGTAGCATCCATC;
Echocardiogram.
Mice used for echocardiography were separate from the main study at a later age as indicated. Transthoracic echocardiography was performed using a Visual Sonics Vevo 770 ultrasonograph with a 30-MHz transducer as detailed previously (Harris et al., 2002). For acquisition of two-dimensional guided M-mode images at the tips of papillary muscles and Doppler studies, mice were sedated with 1% isoflurane administered through a facemask, hair removed, and maintained on a heated platform. Blood velocities across the mitral, aortic and pulmonary valves were measured using Doppler pulsed-wave imaging, angling the probe to obtain a nearly parallel orientation to the blood flow. End diastolic and systolic left ventricular (LV) diameter, as well as anterior and posterior wall (AW and PW, respectively) thickness were measured on line from M-mode images obtained in a parasternal long-axis view using the leading-edge-to-leading-edge convention. All parameters were measured over at least three consecutive cardiac cycles and averaged. Left ventricular FS was calculated as: ((LV diameterdias − LV diametersys)/LV diameterdias) × 100; ejection fraction: ((7.0/(2.4 + LV diameterdias)(LV diameterdias)3 − (7.0/(2.4 + LV diametersys) (LV diametersys)3/(7.0/(2.4 + LV diameterdias) (LV diameterdias)3 × 100; and LV mass: (1.05 × ((PWdias + AWdias + LV diameterdias)3 − (LV diameterdias)3)). Heart rate was determined from at least three consecutive intervals from the pulsed-wave Doppler tracings of the LV outflow tract. Ejection time was measured from the same outflow track tracings from the onset of flow to the end of flow. Isovolumic relaxation time was measured as the time from the closing of the aortic valve to the opening of the mitral valve from pulsed-wave Doppler tracings of the LV outflow tract and mitral inflow region. The same investigator obtained all images and measures.
Heart Lipidomics.
MTBE extraction will be performed with standards from SPLASH Lipidomix from Avanti, using methods outlined previously (Jain et al., 2022). Signal from the processed blank will be subtracted as background during data processing. For positive mode, samples were diluted to 15X in MeOH and 3uL were injected. Samples were run undiluted in negative mode and 5uL were injected. MS/MS data from pooled samples was run through Agilent LipidAnnotator, which were then used to processes MS1 data with Profinder (v8.0). Data was normalized to the internal standards and tissue weight, and further processed using R.
Statistical Analyses
All statistical analyses were conducted using Prism, version 9 (GraphPad Software Inc., San Diego, CA, USA). Tests involving multiple factors were analyzed by either a two-way analysis of variance (ANOVA) with Time and Group as categorical variables or by one-way ANOVA with Group as the categorical variable followed by a Dunnett’s post hoc test for multiple comparisons against the Aged Control. Data distribution was assumed to be normal but was not formally tested.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all members of the Lamming lab. The Lamming laboratory is supported in part by the NIH/NIA (AG056771, AG062328, and AG061635 to D.W.L.), NIH/NIDDK (DK125859 to D.W.L.) and startup funds from the University of Wisconsin-Madison School of Medicine and Public Health and Department of Medicine to D.W.L. C-Y. Y. was supported by a training grant (T32AG000213) and a NIA F32 postdoctoral fellowship (F32AG077916). M.F.C. was supported in part by a Diana Jacobs Kalman/AFAR Scholarships for Research in the Biology of Aging.
Support for this research was provided by the University of Wisconsin - Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. This work was supported in part by the U.S. Department of Veterans Affairs (I01-BX004031), and this work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
COMPETING INTERESTS
D.W.L has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. The remaining authors declare no competing interests.
References
- Bellantuono I., de Cabo R., Ehninger D., Di Germanio C., Lawrie A., Miller J., Mitchell S. J., Navas-Enamorado I., Potter P. K., Tchkonia T., Trejo J. L., & Lamming D. W. (2020). A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc, 15(2), 540–574. 10.1038/s41596-019-0256-1 [Record #39 is using a reference type undefined in this output style.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Gao C., Yu J., Ren S., Wang M., Wynn R. M., Chuang D. T., Wang Y., & Sun H. (2019). Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure. J Am Heart Assoc, 8(11), e011625. 10.1161/JAHA.118.011625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Junior H. J., Calvani R., Picca A., Goncalves I. O., Landi F., Bernabei R., Cesari M., Uchida M. C., & Marzetti E. (2020). Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients, 12(2). 10.3390/nu12020508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Junior H. J., Rodrigues B., Uchida M., & Marzetti E. (2018). Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients, 10(9). 10.3390/nu10091334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings N. E., Williams E. M., Kasza I., Konon E. N., Schaid M. D., Schmidt B. A., Poudel C., Sherman D. S., Yu D., Arriola Apelo S. I., Cottrell S. E., Geiger G., Barnes M. E., Wisinski J. A., Fenske R. J., Matkowskyj K. A., Kimple M. E., Alexander C. M., Merrins M. J., & Lamming D. W. (2018). Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol, 596(4), 623–645. 10.1113/JP275075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D. F., Karunadharma P. P., Chiao Y. A., Basisty N., Crispin D., Hsieh E. J., Chen T., Gu H., Djukovic D., Raftery D., Beyer R. P., MacCoss M. J., & Rabinovitch P. S. (2014). Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell, 13(3), 529–539. 10.1111/acel.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J., Kettunen J., Fischer K., van der Spek A., Trompet S., Kastenmuller G., Boyd A., Zierer J., van den Akker E. B., Ala-Korpela M., Amin N., Demirkan A., Ghanbari M., van Heemst D., Ikram M. A., van Klinken J. B., Mooijaart S. P., Peters A., Salomaa V., Sattar N., Spector T. D., Tiemeier H., Verhoeven A., Waldenberger M., Wurtz P., Davey Smith G., Metspalu A., Perola M., Menni C., Geleijnse J. M., Drenos F., Beekman M., Jukema J. W., van Duijn C. M., & Slagboom P. E. (2019). A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun, 10(1), 3346. 10.1038/s41467-019-11311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommerholt M. B., Blankestijn M., Vieira-Lara M. A., van Dijk T. H., Wolters H., Koster M. H., Gerding A., van Os R. P., Bloks V. W., Bakker B. M., Kruit J. K., & Jonker J. W. (2021). Short-term protein restriction at advanced age stimulates FGF21 signalling, energy expenditure and browning of white adipose tissue. The FEBS Journal, 288(7), 2257–2277. 10.1111/febs.15604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz-Bannitz R., Beraldo R. A., Peluso A. A., Dall M., Babaei P., Foglietti R. C., Martins L. M., Gomes P. M., Marchini J. S., Suen V. M. M., de Freitas L. C. C., Navegantes L. C., Pretti M. A. M., Boroni M., Treebak J. T., Mori M. A., Foss M. C., & Foss-Freitas M. C. (2022). Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial. Nutrients, 14(13). 10.3390/nu14132670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K., Currer J., & Harrison D. (2007). The Mouse in Aging Research. In JG F (Ed.), The Mouse in Biomedical Research 2nd Edition (pp. 637–672). American College Laboratory Animal Medicine. [Google Scholar]
- Fontana L., Cummings N. E., Arriola Apelo S. I., Neuman J. C., Kasza I., Schmidt B. A., Cava E., Spelta F., Tosti V., Syed F. A., Baar E. L., Veronese N., Cottrell S. E., Fenske R. J., Bertozzi B., Brar H. K., Pietka T., Bullock A. D., Figenshau R. S., Andriole G. L., Merrins M. J., Alexander C. M., Kimple M. E., & Lamming D. W. (2016). Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep, 16(2), 520–530. 10.1016/j.celrep.2016.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., & Lamming D. W. (2019). Regulation of metabolic health by essential dietary amino acids. Mech Ageing Dev, 177, 186–200. 10.1016/j.mad.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., Lamming D. W., & Fontana L. (2022). Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol, 23(1), 56–73. 10.1038/s41580-021-00411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., Pak H. H., Richardson N. E., Flores V., Yu D., Tomasiewicz J. L., Dumas S. N., Kredell K., Fan J. W., Kirsh C., Chaiyakul K., Murphy M. E., Babygirija R., Barrett-Wilt G. A., Rabinowitz J., Ong I. M., Jang C., Simcox J., & Lamming D. W. (2022). Sex and genetic background define the metabolic, physiologic, and molecular response to protein restriction. Cell Metab, 34(2), 209–226 e205. 10.1016/j.cmet.2021.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., Trautman M. E., Babygirija R., Jain R., Chaiyakul K., Pak H. H., Bleicher A., Novak G., Sonsalla M. M., Yeh C.-Y., Calubag M. F., Liu T. T., Flores V., Newman S., Ricke W. A., Matkowskyj K. A., Ong I. M., Simcox J., & Lamming D. W. (2022). Dietary restriction of isoleucine increases healthspan and lifespan of genetically heterogeneous mice. bioRxiv, 2022.2010.2006.511051. 10.1101/2022.10.06.511051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn O., Drews L. F., Nguyen A., Tatsuta T., Gkioni L., Hendrich O., Zhang Q., Langer T., Pletcher S., Wakelam M. J. O., Beyer A., Gronke S., & Partridge L. (2019). A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat Metab, 1(11), 1059–1073. 10.1038/s42255-019-0121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. P., Bartley C. R., Hacker T. A., McDonald K. S., Douglas P. S., Greaser M. L., Powers P. A., & Moss R. L. (2002). Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res, 90(5), 594–601. 10.1161/01.res.0000012222.70819.64 [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., & Miller R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. M., Albarado D. C., Coco L. G., Spann R. A., Khan M. S., Qualls-Creekmore E., Burk D. H., Burke S. J., Collier J. J., Yu S., McDougal D. H., Berthoud H. R., Munzberg H., Bartke A., & Morrison C. D. (2022). FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat Commun, 13(1), 1897. 10.1038/s41467-022-29499-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Wade G., Ong I., Chaurasia B., & Simcox J. (2022). Determination of tissue contributions to the circulating lipid pool in cold exposure via systematic assessment of lipid profiles. J Lipid Res, 63(7), 100197. 10.1016/j.jlr.2022.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C., Oh S. F., Wada S., Rowe G. C., Liu L., Chan M. C., Rhee J., Hoshino A., Kim B., Ibrahim A., Baca L. G., Kim E., Ghosh C. C., Parikh S. M., Jiang A., Chu Q., Forman D. E., Lecker S. H., Krishnaiah S., Rabinowitz J. D., Weljie A. M., Baur J. A., Kasper D. L., & Arany Z. (2016). A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med, 22(4), 421–426. 10.1038/nm.4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane A. E., Bisset E. S., Heinze-Milne S., Keller K. M., Grandy S. A., & Howlett S. E. (2020). Maladaptive Changes Associated With Cardiac Aging Are Sex-Specific and Graded by Frailty and Inflammation in C57BL/6 Mice. The Journals of Gerontology: Series A, 76(2), 233–243. 10.1093/gerona/glaa212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwi Q. G., & Lopaschuk G. D. (2022). Branched-Chain Amino Acid Metabolism in the Failing Heart. Cardiovasc Drugs Ther. 10.1007/s10557-022-07320-4 [DOI] [PubMed] [Google Scholar]
- Latimer M. N., Sonkar R., Mia S., Frayne I. R., Carter K. J., Johnson C. A., Rana S., Xie M., Rowe G. C., Wende A. R., Prabhu S. D., Frank S. J., Rosiers C. D., Chatham J. C., & Young M. E. (2021). Branched chain amino acids selectively promote cardiac growth at the end of the awake period. J Mol Cell Cardiol, 157, 31–44. 10.1016/j.yjmcc.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. E., Suarez J. A., Brandhorst S., Balasubramanian P., Cheng C. W., Madia F., Fontana L., Mirisola M. G., Guevara-Aguirre J., Wan J., Passarino G., Kennedy B. K., Wei M., Cohen P., Crimmins E. M., & Longo V. D. (2014). Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab, 19(3), 407–417. 10.1016/j.cmet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J., & Adams S. H. (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology, 10(12), 723–736. 10.1038/nrendo.2014.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. X., Zhu W. Y., Lu X. C., Jiang D., Xu F., Li J. T., Zhang L., Wu Y. L., Chen Z. J., Yin M., Huang H. Y., & Lei Q. Y. (2022). BCAA-BCKA axis regulates WAT browning through acetylation of PRDM16. Nat Metab, 4(1), 106–122. 10.1038/s42255-021-00520-6 [DOI] [PubMed] [Google Scholar]
- Mair W., Piper M. D., & Partridge L. (2005). Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol, 3(7), e223. 10.1371/journal.pbio.0030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S., Caccamo A., Medina D. X., Benavides A. D., Javors M. A., Kraig E., Strong R., Richardson A., & Oddo S. (2012). Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell, 11(2), 326–335. 10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew S., Jacqueline A. S., Milad M., Bryan G., Zach H., Jacqueline F., Amrita M., Henri B., & Juan S. (2017). Cellular Senescence and Their Role in Liver Metabolism in Health and Disease: Overview and Future Directions. In Costin Teodor S, Cristin Constantin V, & Ion R(Eds.), Hepatocellular Carcinoma (pp. Ch. 4). IntechOpen. 10.5772/intechopen.71659 [DOI] [Google Scholar]
- Murashige D., Jung J. W., Neinast M. D., Levin M. G., Chu Q., Lambert J. P., Garbincius J. F., Kim B., Hoshino A., Marti-Pamies I., McDaid K. S., Shewale S. V., Flam E., Yang S., Roberts E., Li L., Morley M. P., Bedi K. C. Jr., Hyman M. C., Frankel D. S., Margulies K. B., Assoian R. K., Elrod J. W., Jang C., Rabinowitz J. D., & Arany Z. (2022). Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab, 34(11), 1749–1764.e1747. 10.1016/j.cmet.2022.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak H. H., Haws S. A., Green C. L., Koller M., Lavarias M. T., Richardson N. E., Yang S. E., Dumas S. N., Sonsalla M., Bray L., Johnson M., Barnes S., Darley-Usmar V., Zhang J., Yen C. E., Denu J. M., & Lamming D. W. (2021). Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat Metab, 3(10), 1327–1341. 10.1038/s42255-021-00466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portero V., Nicol T., Podliesna S., Marchal G. A., Baartscheer A., Casini S., Tadros R., Treur J. L., Tanck M. W. T., Cox I. J., Probert F., Hough T. A., Falcone S., Beekman L., Muller-Nurasyid M., Kastenmuller G., Gieger C., Peters A., Kaab S., Sinner M. F., Blease A., Verkerk A. O., Bezzina C. R., Potter P. K., & Remme C. A. (2021). Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc Res. 10.1093/cvr/cvab207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson N. E., Konon E. N., Schuster H. S., Mitchell A. T., Boyle C., Rodgers A. C., Finke M., Haider L. R., Yu D., Flores V., Pak H. H., Ahmad S., Ahmed S., Radcliff A., Wu J., Williams E. M., Abdi L., Sherman D. S., Hacker T., & Lamming D. W. (2021). Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging, 1(1), 73–86. 10.1038/s43587-020-00006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh E., Hwang S. Y., Yoo H. J., Baik S. H., Cho B., Park Y. S., Kim H. J., Lee S. G., Kim B. J., Jang H. C., Kim M., Won C. W., & Choi K. M. (2021). Association of plasma FGF21 levels with muscle mass and muscle strength in a national multicentre cohort study: Korean Frailty and Aging Cohort Study. Age Ageing, 50(6), 1971–1978. 10.1093/ageing/afab178 [DOI] [PubMed] [Google Scholar]
- Sluijs I., Beulens J. W., van der A. D., Spijkerman A. M., Grobbee D. E., & van der Schouw Y. T. (2010). Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care, 33(1), 43–48. 10.2337/dc09-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S. M., Cogger V. C., Pulpitel T., Wahl D., Clark X., Bagley E., Gregoriou G. C., Senior A. M., Wang Q. P., Brandon A. E., Perks R., O’Sullivan J., Koay Y. C., Bell-Anderson K., Kebede M., Yau B., Atkinson C., Svineng G., Dodgson T., Wali J. A., Piper M. D. W., Juricic P., Partridge L., Rose A. J., Raubenheimer D., Cooney G. J., Le Couteur D. G., & Simpson S. J. (2019). Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab, 1(5), 532–545. 10.1038/s42255-019-0059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S. M., McMahon A. C., Ballard J. W., Ruohonen K., Wu L. E., Cogger V. C., Warren A., Huang X., Pichaud N., Melvin R. G., Gokarn R., Khalil M., Turner N., Cooney G. J., Sinclair D. A., Raubenheimer D., Le Couteur D. G., & Simpson S. J. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab, 19(3), 418–430. 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S. M., Mitchell S. J., Coogan S. C., Cogger V. C., Gokarn R., McMahon A. C., Raubenheimer D., de Cabo R., Simpson S. J., & Le Couteur D. G. (2015). Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell Rep, 11(10), 1529–1534. 10.1016/j.celrep.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J. R., Mitchell S. E., & Mazidi M. (2016). Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp Gerontol, 86, 28–38. 10.1016/j.exger.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Strong R., Miller R. A., Antebi A., Astle C. M., Bogue M., Denzel M. S., Fernandez E., Flurkey K., Hamilton K. L., Lamming D. W., Javors M. A., de Magalhães J. P., Martinez P. A., McCord J. M., Miller B. F., Müller M., Nelson J. F., Ndukum J., Rainger G. E., Richardson A., Sabatini D. M., Salmon A. B., Simpkins J. W., Steegenga W. T., Nadon N. L., & Harrison D. E. (2016). Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell, 15(5), 872–884. 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Sherrier M., & Li H. (2021). Skeletal Muscle and Bone - Emerging Targets of Fibroblast Growth Factor-21. Front Physiol, 12, 625287. 10.3389/fphys.2021.625287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C. W., Aday A. W., Almarzooq Z. I., Alonso A., Beaton A. Z., Bittencourt M. S., Boehme A. K., Buxton A. E., Carson A. P., Commodore-Mensah Y., Elkind M. S. V., Evenson K. R., Eze-Nliam C., Ferguson J. F., Generoso G., Ho J. E., Kalani R., Khan S. S., Kissela B. M., Knutson K. L., Levine D. A., Lewis T. T., Liu J., Loop M. S., Ma J., Mussolino M. E., Navaneethan S. D., Perak A. M., Poudel R., Rezk-Hanna M., Roth G. A., Schroeder E. B., Shah S. H., Thacker E. L., VanWagner L. B., Virani S. S., Voecks J. H., Wang N.-Y., Yaffe K., & Martin S. S. (2022). Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation, 145(8), e153–e639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- Uddin G. M., Zhang L., Shah S., Fukushima A., Wagg C. S., Gopal K., Al Batran R., Pherwani S., Ho K. L., Boisvenue J., Karwi Q. G., Altamimi T., Wishart D. S., Dyck J. R. B., Ussher J. R., Oudit G. Y., & Lopaschuk G. D. (2019). Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol, 18(1), 86. 10.1186/s12933-019-0892-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanweert F., Neinast M., Tapia E. E., van de Weijer T., Hoeks J., Schrauwen-Hinderling V. B., Blair M. C., Bornstein M. R., Hesselink M. K. C., Schrauwen P., Arany Z., & Phielix E. (2022). A randomized placebo-controlled clinical trial for pharmacological activation of BCAA catabolism in patients with type 2 diabetes. Nature Communications, 13(1), 3508. 10.1038/s41467-022-31249-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Lapworth A. L., An J., Wang L., McGarrah R. W., Stevens R. D., Ilkayeva O., George T., Muehlbauer M. J., Bain J. R., Trimmer J. K., Brosnan M. J., Rolph T. P., & Newgard C. B. (2016). Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab, 5(7), 538–551. 10.1016/j.molmet.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead J. C., Hildebrand B. A., Sun M., Rockwood M. R., Rose R. A., Rockwood K., & Howlett S. E. (2014). A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci, 69(6), 621–632. 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Yamahara K., Kume S., Koya D., Yasuda-Yamahara M., Takeda N., Osawa N., Chin-Kanasaki M., Adachi Y., Nagao K., Maegawa H., & Araki S. I. (2018). Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell, 17(4), e12796. 10.1111/acel.12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Richardson N. E., Green C. L., Spicer A. B., Murphy M. E., Flores V., Jang C., Kasza I., Nikodemova M., Wakai M. H., Tomasiewicz J. L., Yang S. E., Miller B. R., Pak H. H., Brinkman J. A., Rojas J. M., Quinn W. J. 3rd, Cheng E. P., Konon E. N., Haider L. R., Finke M., Sonsalla M., Alexander C. M., Rabinowitz J. D., Baur J. A., Malecki K. C., & Lamming D. W. (2021). The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab, 33(5), 905–922 e906. 10.1016/j.cmet.2021.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Shao J., Wu C. Y., Shu L., Dong W., Liu Y., Chen M., Wynn R. M., Wang J., Wang J., Gui W. J., Qi X., Lusis A. J., Li Z., Wang W., Ning G., Yang X., Chuang D. T., Wang Y., & Sun H. (2019). Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes, 68(9), 1730–1746. 10.2337/db18-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.