Abstract
Neuroticism/Negative Emotionality (N/NE)—the tendency to experience and express more frequent, intense, or persistent negative affect—is a fundamental dimension of childhood temperament and adult personality, with profound consequences for health, wealth, and wellbeing. Elevated N/NE is associated with a panoply of adverse outcomes, from reduced socioeconomic attainment and divorce to mental illness and premature death. Yet our understanding of the underlying neurobiology remains surprisingly speculative. Work in animals suggests that N/NE reflects heightened reactivity to uncertain threat in the central extended amygdala (EAc)—including the central nucleus of the amygdala (Ce) and bed nucleus of the stria terminalis (BST)—but the relevance of these discoveries to the complexities of the human brain and temperament have remained unclear. Here we used a combination of psychometric, psychophysiological, and neuroimaging approaches to understand the relevance of the EAc to individual differences in N/NE in a racially diverse sample of adults selectively recruited to capture a broad spectrum of N/NE. A series of cross-validated, robust regression analyses demonstrated that trait-like individual differences in N/NE are uniquely associated with heightened BST activation during the uncertain anticipation of a genuinely distressing threat. In contrast, N/NE was unrelated to BST activation during the certain anticipation of threat, Ce activation during the anticipation of either threat, or activation in either region during the presentation of threat-related faces. While the BST is often associated with anxiety, analyses showed that heightened BST reactivity to uncertain threat is broadly associated with the internalizing facets of N/NE, including depression. Implicit in much of the neuroimaging literature is the assumption that different threat paradigms are quasi-interchangeable probes of individual differences in circuit function, yet our analyses revealed negligible evidence of convergence between popular threat-anticipation and threat-perception (emotional faces) tasks in the EAc. These observations provide a framework for conceptualizing emotional traits and the development of emotional disorders; for guiding the design and interpretation of biobank and other neuroimaging studies of psychiatric risk, disease, and treatment; and for informing mechanistic research in humans and animals.
Keywords: neuroticism, fear and anxiety, temperament and personality, extended amygdala, bed nucleus of the stria terminalis (BST/BNST)
INTRODUCTION
Neuroticism/Negative Emotionality (N/NE)—the tendency to experience and express more intense, persistent, or frequent negative affect—is a fundamental dimension of childhood temperament and adult personality with profound consequences for health, wealth, and wellbeing1,2. Individuals with a more negative disposition show diminished socioeconomic attainment3–6. They are more likely to experience interpersonal conflict, unemployment, and divorce; to have difficulty adjusting to major life transitions; to feel lonely, dissatisfied, and burned out; to engage in unhealthy behaviors; to develop chronic disease; to be hospitalized; and to die prematurely3,4,7–18. The deleterious consequences of N/NE are especially robust in the sphere of mental health19. Individuals with a negative disposition are more likely to develop pathological anxiety and depression; and, among those who do, to experience more severe, recurrent, and treatment-resistant symptoms13,20–32. Despite this burden, the neural systems underlying variation in this risk-conferring phenotype remain incompletely understood, impeding the development of more effective or tolerable biological interventions.
It is widely believed that N/NE reflects a neurobiological tendency to overreact to novelty, threat, and other ‘trait-relevant’ challenges, increasing the likelihood or intensity of distress, arousal, and defensive behaviors when stressors or potential threats are encountered33–37. While a number of neural systems have been implicated1,38,39, the central extended amygdala (EAc) has received the most empirical scrutiny and occupies a privileged position in most theoretical models of N/NE3,40,41. The EAc is a neuroanatomical macrocircuit encompassing the dorsal amygdala in the region of the central nucleus (Ce) and the neighboring bed nucleus of the stria terminalis (BST)42. Mechanistic studies demonstrate that the EAc is critical for orchestrating adaptive defensive responses to a wide variety of threats in rodents, monkeys, and humans3,43. Neuroimaging studies in monkeys show that Ce and BST reactivity to uncertain threat covaries with trait-like variation in anxious temperament and behavioral inhibition, core biobehavioral facets of N/NE44,45. But the relevance of these discoveries to the complexities of the human brain and temperament remains unclear. In stark contrast to animal studies, only a handful of human neuroimaging studies have used genuinely distress-eliciting threats to examine relations between N/NE and EAc function and nearly all have focused exclusively on the amygdala proper (Table S1). Studies focused on the acute presentation of aversive photographs and Pavlovian threat cues have yielded inconsistent results. Even less is known about the BST, the other major division of the EAc. Only one small-scale (N=50) study has directly addressed this question, providing preliminary evidence that individuals with a more negative disposition show heightened BST activation during the uncertain anticipation of aversive stimulation46. Although modest sample sizes, limited neuroanatomical resolution, and a lack of attention to the BST preclude decisive inferences, these observations motivate the hypothesis that N/NE reflects heightened recruitment of the BST, and possibly the dorsal amygdala (Ce), during the anticipation of aversive stimulation and suggest that these associations may be more pronounced when threat is uncertain in timing or likelihood.
Here we used fMRI to quantify EAc reactivity to the Maryland Threat Countdown task—a well-established and genuinely distressing threat-anticipation paradigm—and test its relevance to variation in N/NE in a racially diverse (38.6% BIPOC) sample of 220 emerging adults (Figure 1 and Figure S1). A best-practices fMRI pipeline and spatially unsmoothed data enhanced our ability to resolve the Ce and BST relative to earlier studies (Table S1). Participants were selectively recruited from a pool of 6,594 pre-screened individuals, ensuring a broad spectrum of N/NE and addressing a key limitation of prior work focused on self-selected convenience samples54. We focused on ‘emerging adulthood’ because it is a time of profound, often stressful transitions55. Indeed, more than half of undergraduate students report moderate-to-severe symptoms of anxiety and depression, with many experiencing the first emergence or recurrence of frank internalizing illness (pathological anxiety or depression) during this turbulent developmental chapter56–58. Prior neuroimaging studies of N/NE have relied on analytic approaches that produce overly optimistic estimates of brain-phenotype association59. Here we instead rely on a priori anatomically defined EAc (Ce and BST) regions-of-interest (ROIs) and cross-validated robust estimates of brain-temperament associations (Figure 1). To further enhance power, we used a composite measure of N/NE—aggregated across two scales and three measurement occasions—minimizing state-like fluctuations in responding (‘noise’) that can attenuate brain-phenotype associations (Figure S2)60,61. Study hypotheses and general approach were pre-registered as a further guard against questionable research practices62.
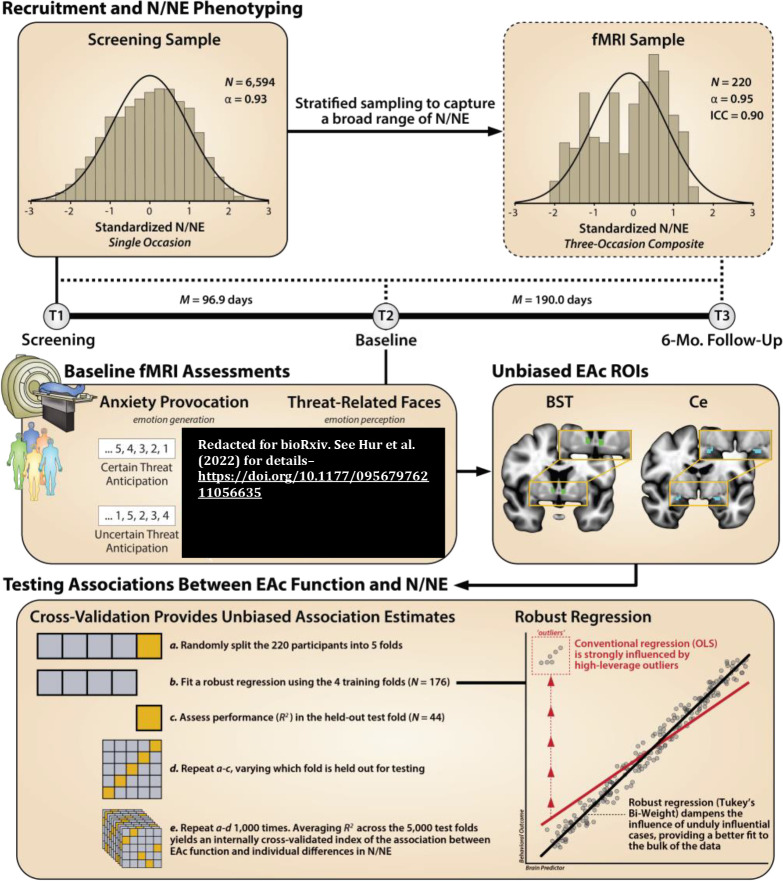
Figure 1. Study overview.
Recruitment and N/NE Phenotyping. To ensure a broad spectrum of N/NE, participants were selectively recruited from an ethnoracially diverse pool of 6,594 pre-screened individuals. N/NE was assessed at screening (T1), at the baseline laboratory session (T2), and at the 6-month follow-up session (T3). To maximize reliability and power, analyses leveraged a composite measure of N/NE that was aggregated across 2 scales and 3 measurement occasions (see also Supplementary Figure S2). Upper inset panels indicate the distribution (histogram), internal-consistency reliability (α), and test-retest reliability of N/NE in the screening (left) and fMRI (right) samples. Timeline indicates the interval between assessments. Baseline fMRI Assessments. Anxiety Provocation. Following the baseline laboratory assessment (T2), participants completed an fMRI assessment. All participants completed the Maryland Threat Countdown task, a well-established anxiety-provocation paradigm. As detailed in Supplementary Figure S1, the MTC takes the form of a 2 (Valence: Threat/Safety) × 2 (Temporal Certainty: Certain/Uncertain) factorial design. On threat trials, subjects saw a stream of integers that terminated with the temporally certain or uncertain presentation of a noxious electric shock, unpleasant photograph, and thematically related audio clip. Safety trials were similar, but terminated with the delivery of benign stimuli. Hypothesis testing focused on neural activation associated with the anticipation of temporally certain and uncertain threat, relative to safety. A total of 220 individuals provided usable imaging data. Threat-Related Faces. A subset of 213 participants also completed a ‘threat-related’ (fearful/angry) faces fMRI paradigm. As detailed in Supplementary Figure S3, participants viewed short blocks of photographs, alternating between blocks of faces and benign everyday scenes (e.g. park, office). Hypothesis testing focused on activation associated with threat-related faces, relative to scenes. Unbiased EAc ROIs. Anatomically defined regions-of-interest (ROIs) enabled us to rigorously test the central hypothesis that N/NE reflects heightened recruitment of the BST (green), and potentially the Ce (dorsal amygdala; cyan), during aversive anticipation and explore the possibility that these associations are more evident when the timing of threat encounters is uncertain. Unlike conventional whole-brain voxelwise analyses—which screen thousands of voxels for statistical significance and yield optimistically biased associations—anatomically defined ROIs ‘fix’ the measurements-of-interest a priori, providing statistically unbiased estimates of brain-phenotype associations. Standardized regression coefficients were extracted and averaged across voxels for each combination of ROI, task contrast, and participant. Testing Associations Between EAc Function and N/NE. Cross-Validation Provides Unbiased Association Estimates. Conventional regression approaches use all available data for model fitting (‘training’), yielding optimistically biased estimates of model performance (R2) that do not generalize well to unseen data (‘overfitting’). As shown in the bottom-left panel, we used a well-established cross-validation framework (i.e. repeated 5-fold) to compute unbiased associations. Robust Regression. As shown in the bottom-right panel, conventional regression is sensitive to high-leverage outliers (red). Here we used robust regression (Tukey’s bi-weight) to reduce the influence of unduly influential cases, providing a better fit to the bulk of the data, and reducing volatility across the cross-validated training (N=176) and test (N=44) folds. The same analytic framework was used for the faces dataset. Abbreviations—α, Cronbach’s alpha (internal-consistency reliability); BST, bed nucleus of the stria terminalis; Ce, dorsal amygdala in the region of the central nucleus; EAc, central extended amygdala; ICC, intraclass correlation (test-retest reliability); M, mean; Mo., months; N, number of observations; N/NE, neuroticism/negative emotionality; OLS, ordinary least squares; ROI, region of interest.
To provide a more direct link with on-going research, we performed parallel analyses using data from a subset of 213 participants who also completed an emotional-faces fMRI paradigm. Variants of the emotional-faces paradigm are widely used as probes of amygdala function—often in the guise of quantifying variation in Research Domain Criteria (RDoC) ‘Negative Valence Systems’64—and have been incorporated into many prominent biobank studies (e.g., ABCD, UK Biobank). Although there is abundant evidence that photographs of ‘threat-related’ (fearful/angry) facial expressions robustly recruit the amygdala65, they do not elicit substantial distress in typical adults and, therefore, are better conceptualized as a measure of threat perception, rather than the experience or expression of negative emotion3. Here, we tested whether EAc (Ce and BST) reactivity to threat-related faces is associated with variation in N/NE.
The inclusion of two neuroimaging tasks also afforded the opportunity to determine whether they are statistically interchangeable. It is often tacitly assumed that different experimental tasks targeting a common psychological function (e.g., ‘threat’) are quasi-equivalent probes of individual differences in circuit function (i.e., threat-of-shock ≈ threat-related faces). Yet this assumption of rank-order fungibility has rarely been examined empirically, never in a large sample, and never in the BST66. Here, we formally quantified the degree to which the threat-anticipation and threat-perception tasks show statistical interchangeability (‘convergent validity’) in the EAc (Ce and BST).
N/NE has been conceptualized as the single most important psychological risk factor in public health, yet the underlying neurobiology remains surprisingly speculative2. Addressing this fundamental question has the potential to refine basic scientific and clinical models of temperament and personality; inform the design, use, and interpretation of biobank data; and guide mechanistic work aimed at developing improved biological interventions for extreme N/NE19.
RESULTS
Threat anticipation amplifies subjective distress and objective arousal
We used a series of repeated-measures general linear models (GLMs) to confirm that the threat-anticipation paradigm had the intended impact on anxious distress and arousal. Mean-centered N/NE was included as a fully crossed dimensional covariate, allowing us to explore the possibility that N/NE alters reactivity to this well-controlled emotional challenge1.
As shown in Figure 2a, fearful and anxious feelings were significantly elevated during the anticipation of Threat compared to Safety, and this was particularly evident when the timing of aversive encounters was uncertain (Valence: F(1,218)=1,135.06, p<0.001; Certainty: F(1,218)=212.95, p<0.001; Valence × Certainty: F(1,218)=31.75, p<0.001; Threat, Uncertain vs. Certain: F(1,218)=148.90, p<0.001; Safety, Uncertain vs. Certain: F(1,218)=78.78, p<0.001).
Figure 2. The threat-anticipation and threat-perception tasks are valid probes of EAc function.
a. Threat anticipation evokes subjective distress. Fear and anxiety were increased during the anticipation of Threat compared to Safety, and this was particularly evident for Uncertain Threat (p<0.001). b. Threat anticipation evokes objective signs of arousal. A similar pattern was evident for skin conductance level (p<0.001). c. Threat-anticipation and threat-perception recruit the EAc. As shown in the top three rows, the anticipation of Threat, Uncertain Threat, and Certain Threat activated the BST and the dorsal amygdala in the region of the Ce, when compared to their respective reference conditions (q<0.05, corrected). As shown in the bottom row, the acute presentation of threat-related faces was also associated with significant activation in the region of the BST and the dorsal amygdala (Ce), relative to the reference condition. See the Supplement for complete whole-brain voxelwise results. Bars indicate the means (colored bars), Bayesian 95% highest density intervals (gray bands), and individual participants (black points). Abbreviations—BST, bed nucleus of the stria terminalis; Ce, dorsal amygdala in the region of the central nucleus; FDR, false discovery rate; HDI, highest density interval; t, Student’s t; WB, whole-brain-corrected.
As shown in Figure 2b, the same general pattern was evident for skin conductance, an objective psychophysiological index of arousal (Valence: F(1,216)=790.55, p<0.001; Certainty: F(1,216)=138.95, p<0.001; Valence × Certainty: F(1,216)=661.63, p<0.001; Threat, Uncertain vs. Certain: F(1,216)=455.78, p<0.001; Safety, Uncertain vs. Certain: F(1,216)=270.03, p<0.001). These observations confirm the validity of the threat-anticipation paradigm as an experimental probe of fear and anxiety, replicating and extending prior work using the Maryland Threat Countdown task67–69.
N/NE amplifies distress evoked by the threat-anticipation paradigm
Exploratory analyses demonstrated that individuals with a more negative disposition experienced pervasively elevated distress across the four conditions of the threat-anticipation paradigm—both aversive and benign (F(1,218)=33.56, p<0.001)—and modestly potentiated reactivity to the anticipation of Threat compared to Safety, and to the anticipation of Uncertain compared to Certain outcomes (N/NE × Valence: F(1,218)=6.35, p=.01; N/NE × Certainty: F(1,218)=6.03, p=.02; Figure S4). No other moderator effects were significant for either subjective distress or objective arousal (p>0.57). In short, individuals with a more negative disposition show a combination of indiscriminately elevated (‘overgeneralized’), threat-potentiated, and uncertainty-potentiated anticipatory distress, in broad accord with prior work1,70.
Threat anticipation and perception robustly recruit the EAc
We used whole-brain voxelwise GLMs to confirm that the threat-anticipation and threat-perception (emotional-faces) paradigms had the intended consequences on brain function. As expected, the anticipation of Threat, Uncertain Threat, and Certain Threat significantly recruited both the BST and the dorsal amygdala in the region of the Ce (FDR q<.05, whole-brain corrected; Figure 2c). Beyond the EAc, each of these contrasts was associated with significant activation across a widely distributed network of regions previously implicated in the expression and regulation of human fear and anxiety71, including the midcingulate cortex, anterior insula/frontal operculum, dorsolateral prefrontal cortex, and periaqueductal grey (Tables S3–S4). Analyses focused on the presentation of threat-related faces revealed significant activation in the BST and the dorsal amygdala, consistent with prior work (Figure 2c and Table S5)43. Together, these observations demonstrate that both tasks are both robust probes of EAc function.
N/NE is uniquely associated with BST activation during the uncertain anticipation of threat
We used statistically unbiased anatomical ROIs and spatially unsmoothed fMRI data to test the hypothesis that individuals with a more negative disposition will show exaggerated recruitment of the EAc (BST and/or Ce) during threat anticipation, and test whether this association is more evident when the timing of the threat encounter is uncertain (Figure 1). As a precursor to hypothesis testing, we used a series of ttests to confirm that the anatomically defined BST and Ce ROIs are recruited by the threat-anticipation and threat-perception (emotional-faces) tasks. Consistent with the voxelwise results (Figure 2c), both anatomically defined ROIs evinced nominally significant activation (ts(219)>3.10, ps<0.002, uncorrected; Figure S5 and Tables S6–S7).
As shown schematically in Figure 1, cross-validated robust GLMs were used for hypothesis testing. Results revealed that general EAc threat reactivity—aggregating across the anticipation of certain and uncertain aversive stimulation—was unrelated to individual differences in N/NE (BST: β=0.12, t(218)=1.57, p=0.12; Ce: β=0.02, t(218)=0.32, p=0.75; Figure 3a). Prior work suggests that relations between N/NE and EAc function may be magnified when threat encounters are uncertain in their timing or likelihood46. To test this, we computed robust regressions between N/NE and EAc reactivity to uncertain threat, separately for each region. To clarify specificity, models controlled for activation during certain threat anticipation. Results demonstrated that heightened BST activation during uncertain-threat anticipation was significantly and selectively associated with trait-like variation in N/NE (Uncertain: β=0.24, t(217)=2.61, p=0.01; Certain: β=−0.09, t(217)=−1.00, p=0.32; Figures 3a–3b). Leveraging a simpler bivariate model, we estimated that BST reactivity to uncertain threat explained, on average, 5.1% of the variance in N/NE in out-of-sample test folds (β=0.19, t(218)=2.59, p=0.01, R2CV=0.051). BST reactivity was unrelated to individual differences in task-related distress (p>0.21; Table S8).
Figure 3. Individual differences in N/NE are uniquely associated with heightened BST activation during the uncertain anticipation of a genuinely distressing threat.
a. Standardized robust regression coefficients. Heightened BST reactivity to uncertain-threat anticipation (orange) was associated with variation in N/NE (p=0.01). Other effects were not significant (p>0.11). Bars depict standardized coefficients for each robust regression model. Whiskers indicate standard errors. Significant associations are marked by an asterisk. b. Scatterplot. Orange line depicts the robust association (Tukey’s bi-weight) between BST reactivity to uncertain-threat anticipation and variation in the composite measure of N/NE, while controlling for differences in BST reactivity to certain-threat anticipation. Abbreviations—BST, bed nucleus of the stria terminalis ROI; Ce, central nucleus of the amygdala ROI; N/NE, neuroticism/negative emotionality.
In contrast to the BST, Ce reactivity to the threat-anticipation task was unrelated to variation in N/NE, regardless of threat certainty (Certain: β=0.04, t(217)=0.45, p=0.65; Uncertain: β=−0.10, t(217)= −0.99, p=0.32; Figure 3a). EAc reactivity to the threat-perception (emotional-faces) task was also unrelated to N/NE (BST: β=0.03, t(211)=0.37, p=0.72; Ce: β=0.03, t(211)=0.38, p=0.70; Figure 3a). Consistent with these nil effects, the association between BST reactivity to uncertain threat and N/NE remained significant in models that controlled for either BST reactivity to threat-related faces or Ce reactivity to uncertain-threat anticipation (t>2.59, p<0.02). In sum, individual differences in N/NE are uniquely associated with heightened BST activation during the uncertain anticipation of a genuinely distressing threat.
BST reactivity to uncertain threat is broadly associated with the internalizing facets of N/NE
Epidemiological, psychiatric, and biological studies typically focus on coarse ‘broadband’ measures of N/NE1,3. Yet it is clear that N/NE is a complex phenotype that subsumes several narrower traits—including dispositional anxiety, depression/sadness, and emotional volatility63,72,73—each characterized by a mixture of shared and unique psychological associations and biological correlates39,74–76. While our composite N/NE instrument has many psychometric strengths (Figure 1), it cannot address which of these traits is most relevant to BST function. To do so, we leveraged the revised Big Five Inventory (BFI-2), a well-established, hierarchically organized scale that was expressly constructed to enable rigorous facet-level analyses63. The BFI-2 was administered at the baseline (T2) and 6-month follow-up (T3) sessions (Figure 1). Paralleling the approach used for broadband N/NE, facet scores were averaged across assessments to minimize occasion-specific fluctuations (‘noise’). Cross-validated robust GLMs were used to quantify associations between BST reactivity to uncertain-threat anticipation and each facet of N/NE, while controlling for BST reactivity to certain threat. Results revealed significant associations with dispositional Anxiety and Depression/Sadness, but not Emotional Volatility (Anxiety: β=0.20, t(217)=2.19, p=0.03; Depression/Sadness: β=0.22, t(217)=2.45, p=0.02; Volatility: β=0.10, t(217)=1.10, p=0.27). Consistent with the broadband results, BST reactivity to certain-threat anticipation was unrelated to the three narrow traits (p>0.21). In cross-validated bivariate models, variation in BST reactivity to uncertain-threat anticipation explained an average of ~4% of the variance in the Anxiety and Depression/Sadness facets of N/NE in out-of-sample test data (Anxiety: β=0.15, t(218)=2.13, p=0.04, R2CV=0.041; Depression/Sadness: β=0.16, t(218)=2.17, p=0.03, R2CV=0.040). While the BST is often conceptualized as playing a central role in anxiety-related states, traits, and disorders43,71,77, these findings demonstrate that heightened BST reactivity to uncertain threat is more broadly associated with the ‘internalizing’ facets of N/NE. They also indicate that our major conclusions generalize across trait instruments.
EAc reactivity to the threat-anticipation and threat-perception tasks shows negligible convergence
It is often assumed that different experimental tasks targeting ‘threat’ are quasi-interchangeable probes of individual differences in circuit function (i.e., threat-of-shock ≈ threat-related faces). Yet this tacit assumption of convergent validity has never been tested in a larger sample or in the BST66. As shown in Table S9, robust GLMs revealed negligible associations between BST reactivity to the threat-anticipation and threat-perception (emotional faces) tasks (p>0.06). The same pattern of null associations was evident for the Ce (p>0.11). The absence of robust cross-task correlations raises important questions about the equivalence of two popular fMRI threat tasks—one centered on the cued anticipation of aversive stimulation, the other focused on the perception of angry and fearful facial expressions—and caution against relying exclusively on emotional-face paradigms to probe individual differences in threat-related EAc function.
DISCUSSION
N/NE is a fundamental dimension of mammalian temperament, with profound consequences for human health and wellbeing1,3. Yet our understanding of the underlying neurobiology remains far from complete. Mechanistic and neuroimaging research in rodents and monkeys suggests that N/NE reflects exaggerated EAc reactivity to uncertain dangers, but the translational relevance of this work has remained speculative1,43. Here we used a novel combination of psychometric, psychophysiological, and neuroimaging approaches to understand the relevance of the EAc to trait-like individual differences in N/NE in a racially diverse sample of 220 adults selectively recruited to capture a broad spectrum of N/NE (Figure 1). Results demonstrated that the threat-anticipation paradigm elicited robust symptoms of distress and signs of arousal, replicating work in smaller samples67–69 (Figure 2). Fearful and anxious feelings were more intense and indiscriminate among individuals with a more negative disposition, with elevated distress evident while waiting to receive benign stimulation (Figure S4). Both the threat-anticipation and threat-perception paradigms strongly recruited the Ce and BST (Figure 2). Leveraging statistically unbiased anatomical ROIs and spatially unsmoothed fMRI data, we used a series of cross-validated, robust GLMs to show that N/NE is uniquely associated with heightened BST activation during the uncertain anticipation of aversive stimulation (Figure 3). In contrast, N/NE was unrelated to variation in BST activation during the anticipation of certain threat, Ce activation during the anticipation of either threat, or EAc reactivity to threat-related faces. While the BST is often associated with anxiety43,71,77,78, follow-up analyses showed that heightened BST reactivity to uncertain threat is broadly associated with the ‘internalizing’ facets of N/NE, including the tendency to feel depressed, sad, or insecure63. Implicit in much of the neuroimaging literature is the assumption that different threat paradigms are equivalent probes of individual differences in EAc function (i.e., threat-of-shock ≈ threat-related faces), yet our results revealed negligible evidence of cross-task convergence in the EAc (Table S9). In the section that follows, we outline the basic, clinical, and methodological implications of these observations.
Our results provide compelling evidence that individual differences in N/NE are associated with heightened BST reactivity to the uncertain anticipation of a genuinely distressing threat, replicating and extending prior neuroimaging observations in young monkeys and a comparatively small convenience sample of Dartmouth undergraduates44–46. Individual differences in N/NE are heritable, and work in monkeys suggests that individual differences in BST reactivity to uncertain threat may be particularly relevant to the heritable variance of this risk-conferring trait3,44. Invasive anatomical tracing studies in rodents and monkeys indicate that the BST is poised to assemble behavioral, psychophysiological, and neuroendocrine signs of negative affect via dense mono- and polysynaptic projections to brainstem and subcortical effector regions79,80, with many of these regions also showing robust functional connectivity with the BST in human neuroimaging studies81–83. Collectively, these observations reinforce the hypothesis that the BST is a key component of the distributed neural circuitry governing N/NE. A key challenge for the future will be to clarify causation. Although the mechanistic contribution of the BST to dispositional fear and anxiety has yet to be explored in humans or other primates, work in rodents provides compelling evidence that specific cellular populations in the BST exert bi-directional control over defensive behaviors elicited by a range of uncertain threats, consistent with a causal role84–89. Among humans, N/NE is stable, but not immutable, and can change in response to positive and negative life experiences, providing opportunities for enhancing mechanistic insight1,3,19,90–93. In a comprehensive meta-analysis of 199 studies, Roberts and colleagues found marked reductions in N/NE following psychosocial or pharmacological treatment for internalizing disorders(Cohen’s d=0.59–0.69)94. It will be fruitful to determine whether this salubrious effect is associated with diminished BST reactivity to uncertain threat.
N/NE is a well-established risk factor for future internalizing illnesses. The magnitude of these associations is substantial and remains evident even when eliminating overlapping item content or adjusting for baseline symptoms95,96. For example, the Zurich Cohort Study (N=591) reported that a one standard-deviation increase in N/NE increased the odds of developing an anxiety disorder by 32% and a major depressive episode by 41% across the twenty-year follow-up period97. Likewise, a comprehensive meta-analysis revealed medium-to-large associations between N/NE and future symptoms and diagnoses, for both anxiety (Cohen’s d=0.48–0.68) and depression (d=0.50–0.74)96. Our results provide a framework for understanding the mechanisms underlying this transdiagnostic liability3,98. Co-morbidity between anxiety and depression disorders is rampant and ~75% of patients with DSM-5 Major Depressive Disorder show clinically significant anxiety symptoms98–101. From a biological perspective, anxiety, depression, and N/NE show robust genetic correlations and overlapping pharmacological effects (e.g., respond to anti-depressants)3,75,94,98,102–104. While these findings are suggestive of a common neurobiological substrate98, identifying the relevant neural systems has proven challenging105. Our observation that heightened BST reactivity to uncertain threat is associated with both the anxious and the depressive facets of N/NE suggests that alterations in BST threat reactivity might contribute to this still-enigmatic shared substrate. While this hypothesis remains to be tested, the available circumstantial evidence is supportive. A recent meta-analysis demonstrated that the BST is hyper-reactive to unpleasant emotional challenges among individuals with anxiety disorders71,106. Both anxiety disorders and depression are often treated via chronic administration of selective serotonin reuptake inhibitors (SSRIs) or, in the case of anxiety, acute administration of benzodiazepines103,104. Both treatments have been shown to reduce defensive responses to uncertain threat in humans and rats and to dampen threat-evoked BST activity in rats107,108. From the standpoint of understanding the etiology of internalizing disorders, a key avenue for future research will be to use prospective-longitudinal data to determine whether exaggerated BST reactivity to uncertain threat increases the likelihood of experiencing pathological anxiety and depression.
Our results also have implications for the design and interpretation of neuroimaging studies of psychiatric risk, disease, and treatment development. Much of this work relies on emotional-face tasks as the sole probe of fear, anxiety, and related ‘Negative Valence Systems’64. Yet our results indicate that EAc (Ce and BST) reactivity to ‘threat-related’ (fearful/angry) faces is unrelated to the risk-conferring N/NE phenotype, despite a relatively well-powered sample. Although there are a number of possible explanations, these null effects are not unprecedented. Three other recent well-powered studies failed to detect significant associations between amygdala reactivity to threat-related faces and individual differences in N/NE (Duke Neurogenetics Study, N=1,256; Human Connectome Project, N=319; Minnesota Twin Study, N=548)109–111. Our results also make it clear that the perception of threat-related faces and the anticipation of aversive stimulation are fundamentally distinct assays of individual differences in EAc function, in broad accord with prior work66. These observations highlight the hazards of continuing to rely on a single or small number of ‘workhorse’ neuroimaging paradigms to understand and predict emotion, temperament, and psychopathology77,112,113. Our results also caution against muddling the distinction between threat perception and the actual experience and expression of fear and anxiety114, a practice that has become routine in experimental therapeutics neuroimaging research115–118.
The present results indicate that BST reactivity to uncertain-threat anticipation predicted 5.1% of the variance in N/NE in out-of-sample data. The size of this effect—while far too modest to be useful for screening, diagnostic, or treatment-development purposes—compares favorably with other psychiatrically relevant biological associations, including prospective associations between ventral striatum reward reactivity and depression (1%) and amygdala reactivity to threat-related faces and internalizing symptoms (2.7%)105. It exceeds the out-of-sample performance (1.5–4.2%) of polygenic scores derived from large-scale GWAS of N/NE, but is half that of the cross-validated association reported by Marek, Tervo-Clemmens and colleagues for the variance in general cognitive ability explained by activation in the dorsal-attention network during a working-memory task (11.6%)59,119,120. From a mechanistic or therapeutics-development perspective, the small-but-reliable ‘hits’ uncovered by adequately powered association studies are useful for prioritizing targets for perturbation and recording studies in animals and neuromodulation studies in humans. It merits comment that small-but-reliable brain-behavior associations do not preclude much larger effects with targeted biological interventions121,122. Indeed, EAc perturbations can have dramatic consequences for anxiety-related behaviors43.
Our results also have implications for psychological theories of N/NE. Consider this fundamental question, What does N/NE negativity do? Decades ago, the influential theorist, Gordon Allport, answered by writing that, “traits are cortical [or] subcortical … dispositions having the capacity to gate or guide specific phasic reactions” 123. Today, most theories remain rooted in the idea that differences in N/NE, behavioral inhibition, and trait anxiety reflect hyper-sensitivity to novelty, threat, and other ‘trait-relevant’ challenges, amplifying the intensity of distress, arousal, and defensive behaviors when such challenges are encountered2,33–36,124–126. Consistent with this perspective, we found that high-N/NE experienced potentiated fear and anxiety when anticipating threat (R2=2.9%; Figure S4b). But they also reported elevated distress when anticipating outcomes—whether aversive or benign—that were uncertain in timing (R2=2.6%; Figure S4c), consistent with models emphasizing the centrality of uncertainty to typical and pathological anxiety70. But by far the strongest effect of N/NE was indiscriminately elevated distress across threat and safety trials (R2=13.7%; Figure S4a). The latter observation is consistent with several recent reports of overgeneralized distress in cued threat-anticipation paradigms127,128 and extend work focused on more naturalistic distress provocations129,130 and self-report measures of daily experience1,68,131. Taken together, these observations provide compelling empirical support for conceptual models that emphasize the importance of pervasive, contextually inappropriate negative affect1,132–135. From a psychiatric perspective, overgeneralized responses to threat are particularly interesting, because have been shown to promote instrumental avoidance, a key sign of pathological anxiety136–138; to distinguish anxiety patients from controls139; and to confer heightened risk for future internalizing symptoms and disorders140–142.
Clearly, a number of other important challenges remain for the future. First, our study was focused on a racially diverse sample of emerging adults. Moving forward, it will be useful to expand this to encompass more nationally representative samples143. Second, although our results highlight the importance of the BST, N/NE is a complex phenotype that likely reflects multiple distributed networks. Moving forward, it will be important to understand how functional interactions between the BST and other regions implicated in the expression and regulation of negative affect support trait-like variation in N/NE.
Elevated N/NE is associated with a multitude of practically important outcomes—from satisfaction and wealth to divorce and disease—and has been conceptualized as the single most important psychological risk factor in public health2. Yet the underlying neurobiology has remained surprisingly speculative. Our observations provide rigorous evidence that individual differences in N/NE are significantly and selectively associated with heightened activation during the anticipation of an uncertain, genuinely distressing threat in the BST, but not the Ce. EAc reactivity to threat-related faces was also unrelated to variation in N/NE. These observations provide a framework for conceptualizing N/NE and lay the groundwork for the kinds of prospective-longitudinal and mechanistic studies that will be necessary to determine causation and, ultimately, to develop improved intervention strategies for extreme N/NE. A comparatively large, diverse, and carefully phenotyped sample (Table S1); well-controlled tasks; and a pre-registered, best-practices approaches to data acquisition, processing, and analysis enhance confidence in the robustness and translational relevance of these results.
Supplementary Material
ACKNOWLEDGMENTS
Authors acknowledge assistance and critical feedback from A. Antonacci, L. Friedman, J. Furcolo, M. Gamer, C. Grubb, R. Hum, C. Kaplan, T. Kashdan, J. Kuang, C. Lejuez, D. Limon, B. Nacewicz, L. Pessoa, S. Rose, J. Swayambunathan, A. Vogel, J. Wedlock, members of the Affective and Translational Neuroscience laboratory, the staff of the Maryland Neuroimaging Center, and the Office of the Registrar at the University of Maryland. This work was partially supported by the ASAP Foundation; California National Primate Center; National Institutes of Health (AA030042, DA040717, MH107444, MH121409, MH121735, MH128336, MH129851, OD011107, MH131264, MH132280); National Research Foundation of Korea (2021R1F1A1063385 and 2021S1A5A2A03070229); University of California, Davis; University of Maryland; and Yonsei Signature Research Cluster Program (2021-22-0005). Authors declare no conflicts of interest.
Footnotes
PREREGISTRATION
Our general approach and hypotheses were preregistered (https://osf.io/wzhdm).
RESOURCE SHARING
Raw data and select materials are publicly available at the National Institute of Mental Health Data Archive (https://nda.nih.gov/edit_collection.html?id=2447). Processed data are available at the Open Science Framework (https://osf.io/w5cdk). Key neuroimaging maps are available at NeuroVault.Org (https://neurovault.org/collections/13109).
REFERENCES
- 1.Shackman A. J. et al. Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin 142, 1275–1314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahey B. B. Public health significance of neuroticism. American Psychologist 64, 241–256, doi: 10.1037/a0015309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hur J., Stockbridge M. D., Fox A. S. & Shackman A. J. Dispositional negativity, cognition, and anxiety disorders: An integrative translational neuroscience framework. Progress in Brain Research 247, 375–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soto C. J. Do links between personality and life outcomes generalize? Testing the robustness of trait–outcome associations across gender, age, ethnicity, and analytic approaches. Social Psychological and Personality Science 12, 118–130, doi: 10.1177/1948550619900572 (2021). [DOI] [Google Scholar]
- 5.Hoff K. A., Einarsdóttir S., Chu C., Briley D. A. & Rounds J. Personality changes predict early career outcomes: Discovery and replication in 12-Year longitudinal studies. Psychological Science 32, 64–79, doi: 10.1177/0956797620957 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Spry E. A. et al. Parental personality and early life ecology: a prospective cohort study from preconception to postpartum. Sci Rep 13, 3332, doi: 10.1038/s41598-023-29139-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buecker S., Maes M., Denissen J. J. A. & Luhmann M. Loneliness and the Big Five personality traits: A meta-analysis. European Journal of Personality 34, 8–28, doi: 10.1002/per.2229 (2020). [DOI] [Google Scholar]
- 8.Swider B. W. & Zimmerman R. D. Born to burnout: A meta-analytic path model of personality, job burnout, and work outcomes. Journal of Vocational Behavior 76, 487–506, doi: 10.1016/j.jvb.2010.01.003 (2010). [DOI] [Google Scholar]
- 9.Jokela M. et al. Personality, disability-free life years, and life expectancy: Individual participant meta-analysis of 131,195 individuals from 10 cohort studies. Journal of Personality 88, 596–605, doi: 10.1111/jopy.12513 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Liu M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics 51, 237–244, doi: 10.1038/s41588-018-0307-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puterman E. et al. Predicting mortality from 57 economic, behavioral, social, and psychological factors. Proceedings of the National Academy of Sciences USA 117, 16273–16282, doi: 10.1073/pnas.1918455117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J. et al. Personality and health: Disentangling their between-person and within-person relationship in three longitudinal studies. Journal of Personality and Social Psychology 122, 493–522, doi: 10.1037/pspp0000399 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimfeld K. et al. The winding roads to adulthood: A twin study. JCPP Advances 1, e12053, doi: 10.1002/jcv2.12053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbs W. R. & Ong A. D. For living well, behaviors and circumstances matter just as much as psychological traits. Proc Natl Acad Sci U S A 120, e2212867120, doi: 10.1073/pnas.2212867120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olaru G., van Scheppingen M. A., Bleidorn W. & Denissen J. J. A. The link between personality, global, and domain-specific satisfaction across the adult lifespan. Journal of Personality and Social Psychology, doi: 10.1037/pspp0000461 (in press; ). [DOI] [PubMed] [Google Scholar]
- 16.Schunk F. & Trommsdorff G. Longitudinal associations of neuroticism with life satisfaction and social adaptation in a nationally representative adult sample. Journal of personality doi: 10.1111/jopy.12783 (in press; ). [DOI] [PubMed] [Google Scholar]
- 17.Willroth E. C. et al. Personality traits and health care use: A coordinated analysis of 15 international samples. Journal of Personality and Social Psychology, doi: 10.1037/pspp0000465 (in press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephan Y., Sutin A. R., Luchetti M., Aschwanden D. & Terracciano A. Personality and risk of incident stroke in 6 prospective studies. Stroke, doi: 10.1161/strokeaha.123.042617 (in press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer-Zavala S. E. & Barlow D. H. Neuroticism. A new framework for emotional disorders and their treatment. (Guilford Press, 2021). [Google Scholar]
- 20.Bucher M. A., Suzuki T. & Samuel D. B. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clinical Psychology Review 70, 51–63 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Brouwer M. E. et al. Psychological theories of depressive relapse and recurrence: A systematic review and meta-analysis of prospective studies. Clinical Psychology Review 74, 101773, doi: 10.1016/j.cpr.2019.101773 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Fullana M. A. et al. Risk and protective factors for anxiety and obsessive-compulsive disorders: an umbrella review of systematic reviews and meta-analyses. Psychological Medicine 50, 1300–1315, doi: 10.1017/S0033291719001247 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Kostyrka-Allchorne K., Wass S. V. & Sonuga-Barke E. J. S. Research Review: Do parent ratings of infant negative emotionality and self-regulation predict psychopathology in childhood and adolescence? A systematic review and meta-analysis of prospective longitudinal studies. Journal of Child Psychology and Psychiatry 61, 401–416, doi: 10.1111/jcpp.13144 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Hovenkamp-Hermelink J. H. M., Jeronimus B. F., Myroniuk S., Riese H. & Schoevers R. A. Predictors of persistence of anxiety disorders across the lifespan: a systematic review. The Lancet Psychiatry 8, 428–443, doi: 10.1016/S2215-0366(20)30433-8 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Lynch S. J., Sunderland M., Newton N. C. & Chapman C. A systematic review of transdiagnostic risk and protective factors for general and specific psychopathology in young people. Clinical Psychology Review 87, 102036, doi: 10.1016/j.cpr.2021.102036 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Schneider G., Köhnke C., Teismann H. & Berger K. Childhood trauma and personality explain more variance in depression scores than sociodemographic and lifestyle factors - Results from the BiDirect Study. J Psychosom Res 147, 110513, doi: 10.1016/j.jpsychores.2021.110513 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Scholten W. et al. Recurrence of anxiety disorders and its predictors in the general population. Psychol Med, doi: 10.1017/s0033291721002877 (in press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ten Have M. et al. Duration of anxiety disorder and its associated risk indicators: Results of a longitudinal study of the general population. Depress Anxiety 38, 328–336, doi: 10.1002/da.23103 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Toenders Y. J. et al. Predicting depression onset in young people based on clinical, cognitive, environmental, and neurobiological data. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 7, 376–384, doi: 10.1016/j.bpsc.2021.03.005 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Waszczuk M. A. et al. The prognostic utility of personality traits versus past psychiatric diagnoses: Predicting future mental health and functioning. Clinical Psychological Science 10, 734–751, doi: 10.1177/21677026211056596 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conway C. C. et al. Neuroticism is selectively associated with longitudinal increases in broadband internalizing symptoms, but not narrow-band positive affect or anxious arousal, in emerging adulthood. Clinical Psychological Science [Preprint at PsyArXiv; ], doi: 10.31234/osf.io/2awth (accepted in principle). [DOI] [Google Scholar]
- 32.Chavanne A. V. et al. Anxiety onset in adolescents: a machine-learning prediction. Mol Psychiatry 28, 639–646, doi: 10.1038/s41380-022-01840-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eysenck H. J. The biological basis of personality. (Charles C. Thomas, 1967). [Google Scholar]
- 34.Kagan J., Reznick J. S. & Snidman N. Biological bases of childhood shyness. Science 240, 167–171 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Spielberger C. D. in Anxiety and behavior (ed Spielberger C. D.) 3–22 (Academic Press, 1966). [Google Scholar]
- 36.Goldsmith H. H. et al. Roundtable: what is temperament? Four approaches. Child Dev 58, 505–529 (1987). [PubMed] [Google Scholar]
- 37.Gray J. A. & McNaughton N. The neuropsychology of anxiety. 2nd edn, (Oxford University Press, 2000). [Google Scholar]
- 38.Kim W. & Kim M. J. Morphological similarity of amygdala-ventral prefrontal pathways represents trait anxiety in younger and older adults. Proc Natl Acad Sci U S A 119, e2205162119, doi: 10.1073/pnas.2205162119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein-Flügge M. C. et al. Relationship between nuclei-specific amygdala connectivity and mental health dimensions in humans. Nat Hum Behav, 1705–1722, doi: 10.1038/s41562-022-01434-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagan J. Temperamental and theoretical contributions to clinical psychology. Annual Review of Clinical Psychology 18, 1–18, doi: 10.1146/annurev-clinpsy-071720-014404 (2022). [DOI] [PubMed] [Google Scholar]
- 41.DeYoung C. G. et al. Personality neuroscience: An emerging field with bright prospects. Personality science 3, e7269, doi: 10.5964/ps.7269 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shackman A. J. & Fox A. S. Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience 36, 8050–8063, doi: 10.1523/JNEUROSCI.0982-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox A. S. & Shackman A. J. The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters 693, 58–67, doi: 10.1016/j.neulet.2017.11.056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox A. S. et al. Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences USA 112, 9118–9122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shackman A. J. et al. Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Molecular Psychiatry 22, 724–732 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somerville L. H., Whalen P. J. & Kelley W. M. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68, 416–424, doi: 10.1016/j.biopsych.2010.04.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann L. et al. Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage 166, 110–116 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Indovina I., Robbins T. W., Núñez-Elizalde A. O., Dunn B. D. & Bishop S. J. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69, 563–571, doi: 10.1016/j.neuron.2010.12.034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirlic N. et al. Latent variable analysis of negative affect and its contributions to neural responses during shock anticipation. Neuropsychopharmacology 44, 695–702, doi: 10.1038/s41386-018-0187-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klumpers F., Kroes M. C. W., Baas J. & Fernandez G. How human amygdala and bed nucleus of the stria terminalis may drive distinct defensive responses. J Neurosci 37, 9645–9656, doi: 10.1523/JNEUROSCI.3830-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuyler B. S. et al. Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc Cogn Affect Neurosci 9, 176–181, doi: 10.1093/scan/nss131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjouwerman R., Scharfenort R. & Lonsdorf T. B. Individual differences in fear acquisition: Multivariate analyses of different Emotional Negativity scales, physiological responding, subjective measures, and neural activation. bioRxiv, 233528, doi: 10.1101/233528 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Visser R. M., Bathelt J., Scholte H. S. & Kindt M. Robust BOLD responses to faces but not to conditioned threat: Challenging the amygdala’s reputation in human fear and extinction learning. J Neurosci 41, 10278–10292, doi: 10.1523/jneurosci.0857-21.2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charpentier C. J. et al. How representative are neuroimaging samples? Large-scale evidence for trait anxiety differences between fMRI and behaviour-only research participants. Social Cognitive and Affective Neuroscience (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shackman A. J. et al. Dispositional negativity in the wild: Social environment governs momentary emotional experience. Emotion 18, 707–724, doi:10.1037/emo0000339 10.1037/emo0000339.supp (Supplemental) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 396, 1204–1222. (interactive dashboard at https://vizhub.healthdata.org/gbd-compare/), doi: 10.1016/S0140-6736(20)30925-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ACHA. American College Health Association-National College Health Assessment (ACHA-NCHA III). (American College Health Association, 2020). [Google Scholar]
- 58.Solmi M. et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry 27, 281–295, doi: 10.1038/s41380-021-01161-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marek S. et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660, doi: 10.1038/s41586-022-04492-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolaidis A. et al. Suboptimal phenotypic reliability impedes reproducible human neuroscience. bioRxiv, doi: 10.1101/2022.07.22.501193 (2022). [DOI] [Google Scholar]
- 61.Fox A. S., Lapate R. C., Davidson R. J. & Shackman A. J. in The nature of emotion. Fundamental questions (eds Fox A. S., Lapate R. C., Shackman A. J., & Davidson R. J.) 403–417 (Oxford University Press, 2018). [Google Scholar]
- 62.Grogans S. E., Smith J. F. & Shackman A. J. Understanding the relevance of extended amygdala reactivity to dispositional negativity, <https://osf.io/wzhdm> (2020). [Google Scholar]
- 63.Soto C. J. & John O. P. The next Big Five Inventory (BFI-2): Developing and assessing a hierarchical model with 15 Facets to enhance bandwidth, fidelity, and predictive power. J Pers Soc Psychol 113, 117–143, doi: 10.1037/pspp0000096 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Clark L. A., Cuthbert B., Lewis-Fernandez R., Narrow W. E. & Reed G. M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol Sci Public Interest 18, 72–145, doi: 10.1177/1529100617727266 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Miller K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19, 1523–1536, doi: 10.1038/nn.4393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villalta-Gil V. et al. Convergent individual differences in visual cortices, but not the amygdala across standard amygdalar fMRI probe tasks. Neuroimage 146, 312–319, doi: 10.1016/j.neuroimage.2016.11.038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hur J. et al. Anxiety and the neurobiology of temporally uncertain threat anticipation. Journal of Neuroscience 40, 7949–7964, doi: 10.1101/2020.02.25.964734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hur J. et al. Anxiety-related frontocortical activity is associated with dampened stressor reactivity in the real world. Psychological Science 33, 906–924, doi: 10.1101/2021.03.17.435791 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim H. C. et al. Acute nicotine abstinence amplifies subjective withdrawal symptoms and threat-evoked fear and anxiety, but not extended amygdala reactivity. PLoS One 18, e0288544, doi: 10.1371/journal.pone.0288544 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grupe D. W. & Nitschke J. B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14, 488–501, doi:nrn3524 [pii] 10.1038/nrn3524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shackman A. J. & Fox A. S. Two decades of anxiety neuroimaging research: New insights and a look to the future American Journal of Psychiatry 178, 106–109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caspi A., Roberts B. W. & Shiner R. L. Personality development: Stability and change. Annual Review of Psychology 56, 453–484, doi: 10.1146/annurev.psych.55.090902.141913 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Kalokerinos E. K. et al. Neuroticism may not reflect emotional variability. Proceedings of the National Academy of Sciences USA 117, 9270–9276, doi: 10.1073/pnas.1919934117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khoo S. et al. The predictive validity of consensual and unique facets of neuroticism, conscientiousness, and agreeableness in five personality inventories. Assessment 30, 1182–1199, doi: 10.1177/10731911221089037 (2023). [DOI] [PubMed] [Google Scholar]
- 75.Thorp J. G. et al. Symptom-level modelling unravels the shared genetic architecture of anxiety and depression. Nat Hum Behav 5, 1432–1442, doi: 10.1038/s41562-021-01094-9 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Watson D., Clark L. A., Simms L. J. & Kotov R. Classification and assessment of fear and anxiety in personality and psychopathology. Neuroscience & Biobehavioral Reviews 142, 104878, doi: 10.1016/j.neubiorev.2022.104878 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Grogans S. E. et al. The nature and neurobiology of fear and anxiety: State of the science and opportunities for accelerating discovery. Neuroscience & Biobehavioral Reviews 151, 105237, doi: 10.1016/j.neubiorev.2023.105237 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avery S. N., Clauss J. A. & Blackford J. U. The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141, doi: 10.1038/npp.2015.185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox A. S., Oler J. A., Tromp D. P., Fudge J. L. & Kalin N. H. Extending the amygdala in theories of threat processing. Trends Neurosci 38, 319–329, doi: 10.1016/j.tins.2015.03.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davis M. & Whalen P. J. The amygdala: vigilance and emotion. Mol Psychiatry 6, 13–34 (2001). [DOI] [PubMed] [Google Scholar]
- 81.Torrisi S. et al. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Hum Brain Mapp 36, 4076–4088, doi: 10.1002/hbm.22899 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorka A. X., Torrisi S., Shackman A. J., Grillon C. & Ernst M. Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroimage 168, 392–402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tillman R. M. et al. Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping 39, 1291–1312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jennings J. H. et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228, doi:nature12041 [pii] 10.1038/nature12041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mazzone C. M. et al. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatry 23, 143–153, doi: 10.1038/mp.2016.218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glangetas C. et al. NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun 8, 14456, doi: 10.1038/ncomms14456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis M., Walker D. L., Miles L. & Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135, doi:npp2009109 [pii] 10.1038/npp.2009.109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S. Y. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223, doi:nature12018 [pii] 10.1038/nature12018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahrens S. et al. A central extended amygdala circuit that modulates anxiety. J Neurosci 38, 5567–5583, doi: 10.1523/JNEUROSCI.0705-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schalet B. D. et al. Specific pharmacological effects of paroxetine comprise psychological but not somatic symptoms of depression. PLoS One 11, e0159647, doi: 10.1371/journal.pone.0159647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Widiger T. A. et al. Personality in a hierarchical model of psychopathology. Clinical Psychological Science 7, 77–92, doi: 10.1177/2167702618797105 (2019). [DOI] [Google Scholar]
- 92.Stieger M. et al. Changing personality traits with the help of a digital personality change intervention. Proc Natl Acad Sci U S A 118, e2017548118, doi: 10.1073/pnas.2017548118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zemestani M., Ommati P., Rezaei F. & Gallagher M. W. Changes in neuroticism-related constructs over the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in patients on an optimal dose of SSRI. Personality Disorders: Theory, Research, and Treatment 13, 171–181, doi: 10.1037/per0000482 (2022). [DOI] [PubMed] [Google Scholar]
- 94.Roberts B. W. et al. A systematic review of personality trait change through intervention. Psychol Bull 143, 117–141, doi: 10.1037/bul0000088 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Uliaszek A. A. et al. An examination of content overlap and disorder-specific predictions in the associations of neuroticism with anxiety and depression. J Res Pers 43, 785–794, doi: 10.1016/j.jrp.2009.05.009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeronimus B. F., Kotov R., Riese H. & Ormel J. Neuroticism’s prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychol Med 46, 2883–2906, doi: 10.1017/S0033291716001653 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Hengartner M. P., Ajdacic-Gross V., Wyss C., Angst J. & Rossler W. Relationship between personality and psychopathology in a longitudinal community study: a test of the predisposition model. Psychol Med 46, 1693–1705, doi: 10.1017/S0033291716000210 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Barlow D. H., Sauer-Zavala S., Carl J. R., Bullis J. R. & Ellard K. K. The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science 2, 344–365, doi: 10.1177/2167702613505532 (2013). [DOI] [Google Scholar]
- 99.Hasin D. S. et al. Epidemiology of adult DSM-5 Major Depressive Disorder and its specifiers in the United States. JAMA Psychiatry, doi: 10.1001/jamapsychiatry.2017.4602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caspi A. et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin birth cohort study. JAMA Network Open 3, e203221–e203221, doi: 10.1001/jamanetworkopen.2020.3221 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barr P. B., Bigdeli T. B. & Meyers J. L. Prevalence, comorbidity, and sociodemographic correlates of psychiatric disorders reported in the All of Us Research program. JAMA Psychiatry 79, 622–628, doi: 10.1001/jamapsychiatry.2022.0685 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levey D. F. et al. Reproducible genetic risk loci for anxiety: Results from ~200,000 participants in the Million Veteran Program. Am J Psychiatry 177, 223–232, doi: 10.1176/appi.ajp.2019.19030256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Craske M. G. et al. Anxiety disorders. Nature Reviews Disease Primers 3, 17024, doi: 10.1038/nrdp.2017.24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otte C. et al. Major depressive disorder. Nat Rev Dis Primers 2, 16065, doi: 10.1038/nrdp.2016.65 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Grogans S. E., Fox A. S. & Shackman A. J. The amygdala and depression: A sober reconsideration. Am J Psychiatry 179, 454–457, doi: 10.1176/appi.ajp.20220412 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chavanne A. V. & Robinson O. J. The overlapping neurobiology of adaptive and pathological anxiety: a meta-analysis of functional neural activation. American Journal of Psychiatry 178, 156–164, doi: 10.1176/appi.ajp.2020.19111153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bechtholt A. J., Valentino R. J. & Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology 33, 2117–2130, doi: 10.1038/sj.npp.1301616 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Grillon C. & Ernst M. A way forward for anxiolytic drug development: Testing candidate anxiolytics with anxiety-potentiated startle in healthy humans. Neurosci Biobehav Rev 119, 348–354, doi: 10.1016/j.neubiorev.2020.09.024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacDuffie K. E., Knodt A. R., Radtke S. R., Strauman T. J. & Hariri A. R. Self-rated amygdala activity: an auto-biological index of affective distress. Personal Neurosci 2, e1, doi: 10.1017/pen.2019.1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silverman M. H. et al. Trait neuroticism and emotion neurocircuitry: Functional magnetic resonance imaging evidence for a failure in emotion regulation. Development and Psychopathology 31, 1085–1099, doi: 10.1017/S0954579419000610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.West H. V., Burgess G. C., Dust J., Kandala S. & Barch D. M. Amygdala activation in cognitive task fMRI varies with individual differences in cognitive traits. Cognitive, Affective, & Behavioral Neuroscience 21, 254–264, doi: 10.3758/s13415-021-00863-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holmes A. J. & Patrick L. M. The myth of optimality in clinical neuroscience. Trends in Cognitive Sciences 22, 241–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosenberg M. D., Casey B. J. & Holmes A. J. Prediction complements explanation in understanding the developing brain. Nature Communications 9, 589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davidson R. J. & Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences 3, 11–21 (1999). [DOI] [PubMed] [Google Scholar]
- 115.Schwandt M. L. et al. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: Translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology 41, 2818–2829, doi: 10.1038/npp.2016.61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paulus M. P. et al. The effects of FAAH inhibition on the neural basis of anxiety-related processing in healthy male subjects: a randomized clinical trial. Neuropsychopharmacology 46, 1011–1019, doi: 10.1038/s41386-020-00936-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwako L. E. et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 40, 1053–1063, doi: 10.1038/npp.2014.306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bloomfield M. A. P. et al. The acute effects of cannabidiol on emotional processing and anxiety: a neurocognitive imaging study. Psychopharmacology (Berl) 239, 1539–1549, doi: 10.1007/s00213-022-06070-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baselmans B. M. L. et al. Multivariate genome-wide analyses of the well-being spectrum. Nature Genetics 51, 445–451, doi: 10.1038/s41588-018-0320-8 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Nagel M. et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nature Genetics 50, 920–927 (2018). [DOI] [PubMed] [Google Scholar]
- 121.Shackman A. J. & Fox A. S. Getting serious about variation: Lessons for clinical neuroscience. Trends in Cognitive Sciences 22, 368–369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nielson D. M. et al. Great expectations: A critical review of and suggestions for the Study of reward processing as a cause and predictor of depression. Biological Psychiatry 89, 134–143, doi: 10.1016/j.biopsych.2020.06.012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allport G. W. Traits revisited. American Psychologist 21, 1–10 (1966). [Google Scholar]
- 124.Zuckerman M. in Emotions and anxiety: new concepts, methods and applications (eds Zuckerman M. & Spielberger C. D.) 133–174 (Lawrence Erlbaum, 1976). [Google Scholar]
- 125.Reiss S. Trait anxiety: it’s not what you think it is. J Anxiety Disord 11, 201–214 (1997). [DOI] [PubMed] [Google Scholar]
- 126.Mineka S. et al. Five-year prospective neuroticism-stress effects on major depressive episodes: Primarily additive effects of the general neuroticism factor and stress. J Abnorm Psychol 129, 646–657, doi: 10.1037/abn0000530 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stegmann Y., Reicherts P., Andreatta M., Pauli P. & Wieser M. J. The effect of trait anxiety on attentional mechanisms in combined context and cue conditioning and extinction learning. Sci Rep 9, 8855, doi: 10.1038/s41598-019-45239-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sep M. S. C., Steenmeijer A. & Kennis M. The relation between anxious personality traits and fear generalization in healthy subjects: A systematic review and meta-analysis. Neurosci Biobehav Rev 107, 320–328, doi: 10.1016/j.neubiorev.2019.09.029 (2019). [DOI] [PubMed] [Google Scholar]
- 129.Gross J. J., Sutton S. K. & Ketelaar T. Relations between affect and personality: Support for the affect-level and affective reactivity views. Personality and Social Psychology Bulletin 24, 279–288 (1998). [Google Scholar]
- 130.Thake J. & Zelenski J. M. Neuroticism, BIS, and reactivity to discrete negative mood inductions. Personality and Individual Differences 54, 208–213, doi: 10.1016/j.paid.2012.08.041 (2013). [DOI] [Google Scholar]
- 131.Bolger N. & Schilling E. A. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. J Pers 59, 355–386 (1991). [DOI] [PubMed] [Google Scholar]
- 132.Davidson R. J., Fox A. S. & Kalin N. H. in Handbook of emotion regulation. (ed Gross J. J.) 47–68 (Guilford Press, 2007). [Google Scholar]
- 133.Perkins A. M., Arnone D., Smallwood J. & Mobbs D. Thinking too much: self-generated thought as the engine of neuroticism. Trends Cogn Sci 19, 492–498, doi: 10.1016/j.tics.2015.07.003 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Suls J. & Martin R. The daily life of the garden-variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. Journal of Personality 73, 1485–1509 (2005). [DOI] [PubMed] [Google Scholar]
- 135.Watson D. & Clark L. A. Negative affectivity: the disposition to experience aversive emotional states. Psychological Bulletin 96, 465–490 (1984). [PubMed] [Google Scholar]
- 136.Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry 51, 851–858, doi:S0006322301013701 [pii] (2002). [DOI] [PubMed] [Google Scholar]
- 137.van Meurs B., Wiggert N., Wicker I. & Lissek S. Maladaptive behavioral consequences of conditioned fear-generalization: a pronounced, yet sparsely studied, feature of anxiety pathology. Behav Res Ther 57, 29–37, doi: 10.1016/j.brat.2014.03.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hunt C., Cooper S. E., Hartnell M. P. & Lissek S. Distraction/Suppression and Distress Endurance diminish the extent to which generalized conditioned fear is associated with maladaptive behavioral avoidance. Behaviour Research & Therapy 96, 90–105 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Duits P. et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and Anxiety 32, 239–253 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Craske M. G. et al. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: evidence from a longitudinal investigation. J Abnorm Psychol 121, 315–324, doi: 10.1037/a0025738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lenaert B. et al. Aversive learning and generalization predict subclinical levels of anxiety: a six-month longitudinal study. J Anxiety Disord 28, 747–753, doi: 10.1016/j.janxdis.2014.09.006 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Barker T. V. et al. Contextual startle responses moderate the relation between behavioral inhibition and anxiety in middle childhood. Psychophysiology 52, 1544–1549, doi: 10.1111/psyp.12517 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.LeWinn K. Z., Sheridan M. A., Keyes K. M., Hamilton A. & McLaughlin K. A. Sample composition alters associations between age and brain structure. Nat Commun 8, 874, doi: 10.1038/s41467-017-00908-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.