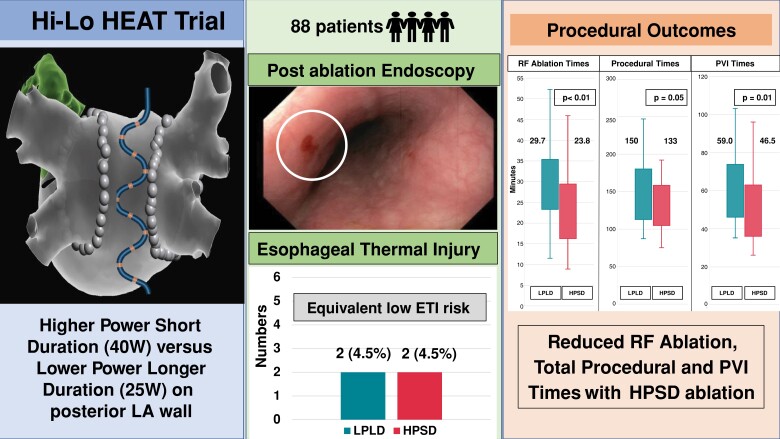
Abstract
Aims
Radiofrequency (RF) ablation for pulmonary vein isolation (PVI) in atrial fibrillation (AF) is associated with the risk of oesophageal thermal injury (ETI). Higher power short duration (HPSD) ablation results in preferential local resistive heating over distal conductive heating. Although HPSD has become increasingly common, no randomized study has compared ETI risk with conventional lower power longer duration (LPLD) ablation. This study aims to compare HPSD vs. LPLD ablation on ETI risk.
Methods and results
Eighty-eight patients were randomized 1:1 to HPSD or LPLD posterior wall (PW) ablation. Posterior wall ablation was 40 W (HPSD group) or 25 W (LPLD group), with target AI (ablation index) 400/LSI (lesion size index) 4. Anterior wall ablation was 40–50 W, with a target AI 500–550/LSI 5–5.5. Endoscopy was performed on Day 1. The primary endpoint was ETI incidence. The mean age was 61 ± 9 years (31% females). The incidence of ETI (superficial ulcers n = 4) was 4.5%, with equal occurrence in HPSD and LPLD (P = 1.0). There was no difference in the median value of maximal oesophageal temperature (HPSD 38.6°C vs. LPLD 38.7°C, P = 0.43), or the median number of lesions per patient with temperature rise above 39°C (HPSD 1.5 vs. LPLD 2, P = 0.93). Radiofrequency ablation time (23.8 vs. 29.7 min, P < 0.01), PVI duration (46.5 vs. 59 min, P = 0.01), and procedure duration (133 vs. 150 min, P = 0.05) were reduced in HPSD. After a median follow-up of 12 months, AF recurrence was lower in HPSD (15.9% vs. LPLD 34.1%; hazard ratio 0.42, log-rank P = 0.04).
Conclusion
Higher power short duration ablation was associated with similarly low rates of ETI and shorter total/PVI RF ablation times when compared with LPLD ablation. Higher power short duration ablation is a safe and efficacious approach to PVI.
Keywords: Atrial fibrillation, Radiofrequency, Pulmonary vein isolation, HPSD, LPLD, Oesophageal thermal injury
Graphical Abstract
Graphical Abstract.
What’s new?
No previous randomized trial has demonstrated the safety of higher power short duration (HPSD) ablation in relation to oesophageal thermal injury (ETI) risk, as evaluated with post-ablation endoscopy.
Hi-Lo HEAT showed that HPSD ablation is associated with low ETI incidence, similar to conventional lower power longer duration (LPLD) ablation.
Oesophageal temperature dynamics were not significantly different between HPSD or LPLD ablation approaches.
HPSD resulted in improved acute procedural outcomes and less arrhythmia recurrence over a 12-month follow-up period.
Introduction
Pulmonary vein isolation (PVI) is the cornerstone of catheter ablation and superior to medical therapy in randomized trials in patients with atrial fibrillation (AF).1 Radiofrequency (RF) ablation involves the cauterization of cardiac tissue through thermal energy transfer and is the predominant energy modality utilized. Factors that affect the durability of an RF lesion include the RF current delivered (power), ablation duration, and catheter stability.2,3 Contact force sensing catheters are now increasingly utilized and provide an acute RF ablation endpoint based on a combination of these indices.1
Radiofrequency ablation is not without risk of complications. In particular, the oesophagus is in close proximity to the posterior wall (PW) of the left atrium (LA), and ablation in this area may result in oesophageal thermal injury (ETI). Rarely, it can evolve into atrio-oesophageal fistula (AEF), a life-threatening complication with a mortality rate of up to 75%.1 Ablation strategies to minimize the risk of developing ETI may include lowering RF power in the posterior LA, luminal oesophageal temperature monitoring (ETM),1 and minimizing ablation in close vicinity to the oesophagus.
In recent years, higher power short duration (HPSD) RF ablation (40–50 W) has become popularized.3 Non-randomized studies have reported shorter procedural times, and decreased RF energy delivery, with no increase in complication rates with HPSD ablation.3 Thermal biophysics suggests HPSD delivers shallower, broader ablation lesions due to preferential resistive heating of more proximal myocardial tissue compared with greater conductive distal heating with lower power longer duration (LPLD) which has the potential to impact extra cardiac structures.4
While prior studies suggest that HPSD ablation is an effective strategy, further evidence is needed to determine the safety of this approach on the posterior LA where traditionally power has been reduced to minimize the risk of oesophageal injury. Prior HPSD ablation studies are mostly limited by non-randomized design.2,5 In particular, no statistically powered randomized controlled study to date has been undertaken to compare HPSD vs. LPLD ablation with regard to ETI outcomes. Although high-power ablation has become popularized, there are few randomized studies to determine whether it is superior to LPLD in improving freedom from AF with catheter ablation.6 The aims of the present randomized study were to compare the impact of HPSD vs. LPLD ablation on (i) ETI and (ii) procedural outcomes including freedom from AF after a minimum follow-up of 12 months.
Methods
Study design and participants
This randomized, multi-centre, prospective, double-blinded, controlled trial was conducted in Melbourne, Australia. Patients with paroxysmal AF (AF lasting <7 days) and persistent AF (AF lasting ≥7 days) undergoing their first catheter RF ablation procedure were recruited. Major exclusion criteria include the following: patients with long-standing persistent AF, AF secondary to an obvious reversible cause, severe valvular heart disease, severe renal/liver impairment, severe gastro-oesophageal reflux disease, and hypertrophic cardiomyopathy. The study was approved by the Alfred Hospital Human Research and Ethics Committee and registered under the Australian New Zealand Clinical Trials Registry (ACTRN12619001603101).
Randomization and blinding
Treatment group randomization was computer generated. Double blinding was utilized, with participants and clinicians involved in participant follow-up blinded to treatment allocation. It was considered futile to blind the operator to treatment allocation given clear temporal differences in achieving ablation lesion targets with higher vs. lower powers.
Catheter ablation procedure
AADs were discontinued approximately five half-lives before the procedure and in the case of amiodarone, at least 1 month before the ablation where possible. Direct oral anticoagulant agents were generally uninterrupted although this was left to the discretion of the operator. All procedures were performed under general anaesthesia with an intra-operative transesophageal echocardiogram to guide transseptal puncture. Therapeutic heparin was administered to achieve an activated clotting time of at least 350 s intra-procedurally.
Three-dimensional (3D) mapping was performed with either CARTO (Biosense Webster), or Ensite Precision/Velocity (Abbott Medical). Irrigated contact force sensing ablation catheter was utilized in all cases. Wide antral ablation was completed with the end point of PVI. Ablation lesions were performed point by point, and ablation targets were based on ablation index (AI) (CARTO, Biosense Webster), or lesion size index (LSI) (Ensite, Abbott Medical).
Additional ablation such as PW isolation in persistent AF patients was permitted at the operator’s discretion. However, the ablation power and duration settings were kept as per randomization, that is the floor line and ablation inside the box was 25 W in LPLD vs. 40 W in HPSD. Post ablation all participants received 1 month of a proton pump inhibitor (PPI).
Randomization groups and ablation targets
A higher power was defined as 40–50 W with a force range of 10–20 g to permit AI/LSI-guided ablation given 6–7 s of RF is required before an AI/LSI parameter is generated. All participants underwent ablation on the anterior wall of the LA at 40–50 W power. Ablation targets were set at AI of 500–550, or LSI of 5–5.5. On the PW of the LA, participants underwent ablation according to randomization (see Supplementary material online, Figure S1). In the HPSD ablation group, power was set at 40 W. In the LPLD ablation group, power was set at 25 W. In both groups, ablation was terminated when either of the following occurred: (i) AI of 400 or LSI of 4 was achieved; or (ii) luminal oesophageal temperature exceeded ≥38°C, or there was a steep rise of >1°C within 5 s whereby ablation was ceased immediately by the operator. Ablation was resumed once the oesophageal temperature had decreased to <37°C. Whether ablation was resumed adjacent to the previous lesion with temperature rise, or at a further site first before returning to the area with temperature rise was left to the operator’s discretion.
Oesophageal temperature monitoring
A 10-French multi-sensor oesophageal temperature probe (Circa S-Cath™, Circa Scientific) was used to continuously record the intraluminal oesophageal temperature during PVI. Any site of significant oesophageal temperature rise was annotated with the maximum oesophageal temperature on the atrial map. At the end of each AF ablation case, the distance from the patient’s incisor teeth to the exact Circa electrode(s) with significant temperature rise was recorded. This measurement was used to correlate any visualized oesophageal lesions during endoscopy (also measured from the patient’s incisor teeth) to atrial sites which recorded temperature rises.
Post-ablation endoscopy
Endoscopy was performed in all participants on Day 1 post procedure to assess for the presence of ETI. Lesions were classified as ETI if they occurred at the anterior side of the oesophagus and at a level consistent with the measured distance on the Circa probe (allowed margin of deviation of 2 cm). Oesophageal lesions were described based on the Kansas City Classification system.7 Participants with Type 1 and Type 2 ETI were treated with a PPI at 40 mg twice a day. Any repeat endoscopy to reassess ETI healing was left to the gastroenterologist’s recommendation.
Follow-up
All participants were followed up for a minimum of 12 months after their ablation procedure, with clinical reviews undertaken at 3, 6, and 12 months. AADs were generally not recommenced following PVI unless symptomatic AF was recurrent. During the COVID-19 pandemic, telephone/video telehealth reviews were permitted. All patients underwent comprehensive rhythm monitoring throughout the course of the study. Heart rhythm monitoring was performed via Kardia AliveCor® electronic rhythm monitoring system or pre-existing implantable cardiac devices. Participants with the AliveCor® electronic rhythm monitoring system transmitted their rhythm twice daily via the Kardia application on a smartphone. The rhythm strips were reviewed daily by study investigators who were blinded to the treatment allocation. Three monthly 24-h Holter monitors were used when participants were not compliant with single-lead electrocardiogram (ECG) transmissions. Twelve lead ECGs were also acquired during clinical visits, hospitalizations, or any patient-reported symptoms. All arrhythmia recurrences post 3-month blanking period were adjudicated by a committee.
Study outcomes
The primary study outcome was the incidence of ETI in the HPSD and LPLD groups. Secondary outcomes include the following:
Freedom from AF after a single procedure of antiarrhythmic drug at 12 months.
Acute procedural outcomes.
-
Oesophageal temperature dynamic endpoints which were pre-specified as:
number of temperature spikes of ≥39°C;
number of steep temperature rises of >1°C within 5 s; and
cumulative amount of time in which temperature was ≥ 38°C.
Statistical analysis
This study was designed to investigate whether HPSD was superior to LPLD ablation on the PW in reducing the incidence of ETI. A previous study of RF ablation at 25–30 W reported an ETI rate of 21%.8 More recently a 2.5% incidence of ETI was reported in a HPSD cohort.9 To detect an 18.5% reduction in ETI rates with a Type 1 error rate of 0.05 and power of 80%, the minimum sample size needed was 88 patients. As all participants were expected to undergo post-ablation endoscopy during their inpatient stay, drop-outs were not expected.
Continuous data were expressed as mean ± standard deviation if normally distributed (interquartile range if skewed data). Categorical data were presented as numbers and percentages. Differences in variables between groups were analysed using the χ2 test for categorical data, and the Student’s t-test or Mann–Whitney U test for normally distributed and skewed continuous data, respectively. Arrhythmia recurrence was described using Kaplan–Meier survival curves. Cox regression was used to examine variables predictive of late arrhythmia recurrence. Univariate variables with P < 0.20 were included in the multivariate model. Statistical significance was defined as a P value of <0.05. Statistical analyses were done using SPSS version 27 (IBM, Armonk, NY, USA).
Results
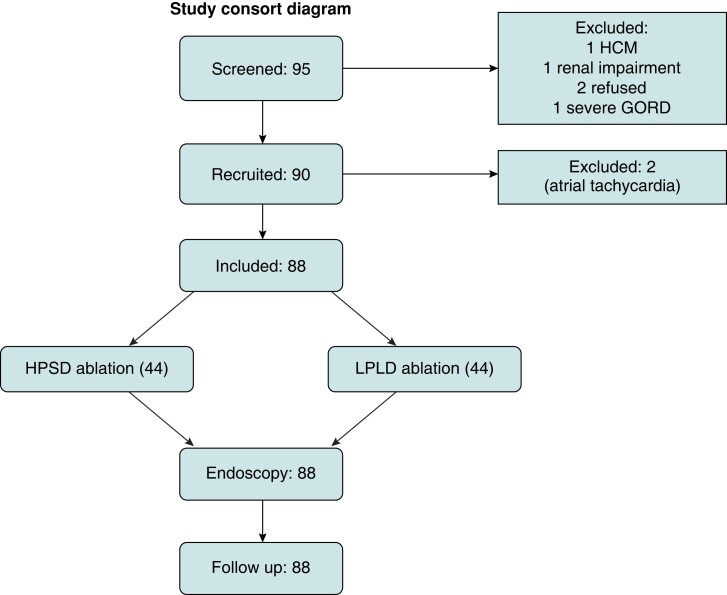
Ninety-five patients were screened, with 90 meeting the eligibility criteria and recruited into the study. Two patients were excluded as atrial tachycardia was the primary arrhythmia at the time of ablation and PVI was not performed. The final 88 patients were randomized 1:1 into the HPSD vs. LPLD groups (Figure 1). The mean age of the cohort was 61 years, with 30.7% females. Baseline characteristics were similar across both groups, with the exception of hypertension which was more prevalent in HPSD (56.8 vs. 31.8%, P = 0.02) (Table 1). Ablation on the anterior wall was performed at 40 W in 72 (81.8%), 45 W in 1 (1.1%), and 50 W in 15 (17.1%).
Figure 1.
Study consort diagram. GORD, gastro-oesophageal reflux disease; HCM, hypertrophic cardiomyopathy; HPSD, higher power short duration; LPLD, lower power longer duration.
Table 1.
Baseline cohort characteristics
| LPLD (44 patients) | HPSD (44 patients) | P | |
|---|---|---|---|
| Age (mean, SD) | 59.7 ± 10.0 | 62.9 ± 8.2 | 0.11 |
| Females (n, %) | 14 (31.8%) | 13 (29.5%) | 0.50 |
| PAF (n, %) | 21 (47.7%) | 15 (34.1%) | 0.42 |
| CHADSVASc score | 1.5 ± 1.5 | 2 ± 2 | 0.44 |
| BMI (kg/m2) | 29.2 ± 4.8 | 29.2 ± 5.6 | 0.66 |
| Hypertension (n, %) | 14 (31.8%) | 25 (56.8%) | 0.02 |
| IHD (n, %) | 7 (15.9%) | 5 (11.4%) | 0.38 |
| Stroke (n, %) | 2 (4.5%) | 1 (2.3%) | 0.50 |
| Congestive cardiac failure (n, %) | 12 (27.3%) | 11 (25%) | 0.50 |
| Diabetes mellitus (n, %) | 2 (4.5%) | 3 (6.8%) | 0.48 |
| OSA (n, %) | 2 (4.5%) | 7 (15.9%) | 0.08 |
| LAVI (mL/m3) | 40.0 ± 11.2 | 43.0 ± 21.2 | 0.31 |
| LVEDD (mm) | 51.3 ± 6.5 | 50.4 ± 6.7 | 0.56 |
| LVESD (mm) | 35.8 ± 8.8 | 34.9 ± 8.9 | 0.71 |
| LVEF (%) | 54.6 ± 11.4 | 55.4 ± 13.0 | 0.79 |
| E/e′ ratio | 9.8 ± 4.2 | 9.4 ± 2.9 | 0.57 |
| RVSP (mmHg) | 27.9 ± 7.4 | 28.5 ± 9.7 | 0.82 |
| Anticoagulation pre ablation (n, %) | 37 (84.1%) | 41 (93.2%) | 0.09 |
| Antiplatelet use (n, %) | 5 (11.4%) | 2 (4.5%) | 0.22 |
| ACE/ARB use (n, %) | 16 (36.4%) | 22 (50%) | 0.14 |
| Mineralcorticoid use (n, %) | 3 (7.8%) | 6 (13.6%) | 0.24 |
| Anti-arrhythmic drug use (n, %) | 35 (79.5%) | 35 (79.5%) | 1.00 |
| Flecainide | 9 (20.5%) | 13 (6.8%) | 0.46 |
| Sotalol | 15 (34.1%) | 15 (34.1%) | 1.00 |
| Amiodarone | 10 (22.7%) | 8 (18.2%) | 0.79 |
| Rhythm monitoring (n, %) | 0.94 | ||
|
38 (86.4%) | 39 (88.6%) | |
|
5 (11.3%) | 4 (9.1%) |
Bold indicates P < 0.05.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; HPSD, higher power short duration; IHD, ischaemic heart disease; LAVI, left atrial indexed volume; LPLD, lower power longer duration; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnoea; PAF, paroxysmal AF; RVSP, right ventricular systolic pressure.
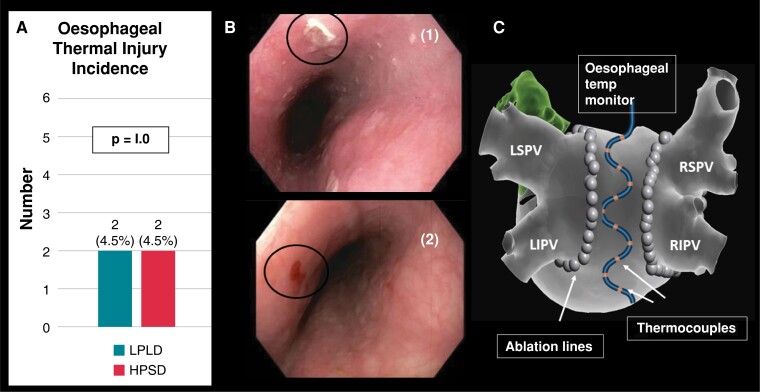
Oesophageal thermal injury
The primary endpoint of ETI occurred in four participants (4.5%), with equal occurrence in both HPSD (n = 2) and LPLD (n = 2) groups. All ETI cases were classified as superficial ulcers (Type 2A) (Figure 2), and treated conservatively with PPI therapy with complete recovery without clinical sequelae. No patient required a repeat endoscopy. Five participants (5.6%) had oesophageal lesions which were not adjacent to the heart; as determined by the distance and orientation of these lesions from the incisor teeth in relation to the Circa electrode(s) with recorded temperature rises. As such these lesions were adjudicated to be caused by oesophageal instrumentation from either the transoesophageal echocardiogram (TOE) or the Circa probes. In the HPSD group, they consisted of one mucosal erythema and two superficial ulcers, and in the LPLD group, there was one case each of a superficial ulcer and mucosal erythema (see Supplementary material online, Figure S2).
Figure 2.
(A) incidence of ETI (primary endpoint) was 4.5% which was similar across both HPSD and LPLD groups; (B) ETI examples included (i) which demonstrates superficial ulcer; (ii) which demonstrates mucosal erythema with superficial ulcer; (C) multi-sensor ETM probe. HPSD, higher power short duration; LIPV, left inferior pulmonary vein; LPLD, lower power longer duration; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Oesophageal temperature dynamics
There was no difference between the HPSD and LPLD groups on the median value of maximal temperature reached (HPSD 38.6 ± 3.9°C vs. LPLD 38.7 ± 3.4°C, P = 0.23), the median number of lesions per patient with a significant temperature rise of ≥39°C (HPSD 1.5 vs. LPLD 2, P = 0.93), and the median duration in which oesophageal heating remained ≥38°C (HPSD 34.7 s vs. LPLD 33.0 s, P = 0.46). Furthermore, no significant differences were seen in the median number of lesions with rapid temperature rises across both groups (Table 2).
Table 2.
Oesophageal temperature dynamics
| LPLD | HPSD | P value | |
|---|---|---|---|
| Maximum temperature (°C) (median, IQR) | 38.7 ± 0.3 | 38.6 ± 1.0 | 0.23 |
| Lesions with temperature ≥ 39°C per patient (median, IQR) | 2 ± 5.5 | 1.5 ± 3.0 | 0.93 |
| Lesions with rapid temperature rise per patient | |||
|
4 ± 6 | 5 ± 4 | 0.78 |
|
2 ± 4.75 | 2 ± 3.75 | 0.68 |
| Total time above 38°C (s) | 33.0 ± 25.2 | 34.7 ± 21.0 | 0.46 |
| RF duration (median, IQR) | 12 ± 8 | 8 ± 4 | <0.01 |
HPSD, higher power short duration; IQR, interquartile range; LPLD, lower power longer duration.
Procedural outcomes
Pulmonary vein isolation was achieved in all participants (Table 3). There was a significant reduction in PVI time (46.5 vs. 59.0 min, P = 0.01), total RF ablation time (23.8 vs. 29.7 min, P < 0.01), PVI RF time (18.5 vs. 25.3 min, P < 0.01), and the number of PVI applications (67 vs. 76, P = 0.02) with HPSD compared with LPLD (Figure 3). Total procedural time was reduced in the HPSD group (133 vs 150 min, P = 0.05). Fluoroscopy time was not significantly different between the two groups. There was no difference in first-pass isolation rates and acute PV reconnection rates between the groups. No procedural complication was encountered. The mean LSI values on the posterior antral lines during PVI were significantly higher in the HPSD compared with the LPLD group. On the right PV posterior antral line, the mean LSI was 4.1 ± 0.2 for HPSD vs. 3.8 ± 0.3 for LPLD (P < 0.01). The mean LSI for the left PV posterior antral line was 4.2 ± 0.4 (HPSD) vs. 3.6 ± 0.3(LPLD), P < 0.01. Mean AI on the left PV posterior antral line was higher in the HPSD group (382.4 ± 20.2 vs. 352.1 ± 33.4, P < 0.01), although there was no difference in AI values on the right PV posterior antral line between both groups (HPSD 379.1 ± 27.3 vs. LPLD 380.2 ± 24.9, P = 0.91) (see Supplementary material online, Table S2).
Table 3.
Procedural outcomes
| LPLD | HPSD | P value | |
|---|---|---|---|
| PVI only (n, %) | 37 (84.1%) | 31 (70.5%) | 0.10 |
| CTI ablation (n, %) | 5 (11.4%) | 8 (18.2%) | 0.28 |
| Rhythm at time of ablation (n, %) | |||
|
32 (72.7%) | 28 (63.6%) | 0.45 |
|
12 (27.3%) | 15 (34.1%) | |
|
0 (0%) | 1 (2.3%) | |
| Total procedure time (min) | 150.9 ± 45.8 | 133.3 ± 37.6 | 0.05 |
| Total PVI time (min)a | 59.0 ± 28.0 | 46.5 ± 26.8 | 0.01 |
| Total RF time (min) | 29.7 ± 9.2 | 23.8 ± 9.45 | <0.01 |
| PVI RF time (min) | 25.3 ± 6.4 | 18.5 ± 6.0 | <0.01 |
| PVI RF time (posterior antral line) (min) | 10.3 ± 3.0 | 5.5 ± 1.8 | <0.01 |
| PVI application number (n, IQR)a | 76 ± 19.8 | 67 ± 23.3 | 0.02 |
| Fluoroscopy time (min)a | 13.1 ± 8.1 | 11.2 ± 4.3 | 0.17 |
| LPV first pass isolation (n, %) | 38 (86.4%) | 39 (88.6%) | 0.50 |
| RPV first pass isolation (n, %) | 31 (70.5%) | 28 (63.6%) | 0.33 |
| LPV intervenous ridge ablation (n, %) | 5 (11.4%) | 4 (9.1%) | 0.50 |
| RPV intervenous ridge ablation (n, %) | 12 (29.5%) | 13 (29.5%) | 0.50 |
| Acute PV reconnection (n, %) | 15 (34.1%) | 12 (27.3%) | 0.64 |
| PVI success (n, %) | 44 (100%) | 44 (100%) | |
| Complications (n, %) | 0 (0%) | 0 (0%) |
Bold indicates P < 0.05.
CTI, cavotricuspid isthmus; HPSD, higher power short duration; LPLD, lower power longer duration; LPV, left pulmonary vein; PVI, pulmonary vein isolation; RF, radiofrequency; RPV, right pulmonary vein.
Data expressed as median and interquartile range.
Figure 3.
HPSD ablation resulted in shorter RF ablation time, PVI time, and procedural duration compared with LPLD ablation. HPSD, higher power short duration; LPLD, lower power longer duration; PVI, pulmonary vein isolation; RF, radiofrequency.
Follow-up
There were two deaths in the study cohort during follow-up, which were unrelated to their AF ablation. One patient died from urinary sepsis with multiorgan failure at 158 days, and another patient died from thrombosis of a pre-existing basilar stent at 118 days. The number of patients with early (90-day blanking period) arrhythmia recurrence was similar between both groups (LPLD 16 vs. HPSD 19, P = 0.66). The predominant rhythm monitoring method utilized in the study was twice daily ECGs using the Kardia AliveCor® device (87.5% compliance).
Over a median follow-up of 12 months, freedom from AF, off anti-arrhythmic therapy, was (84.1% in the HPSD group vs. 65.9% in LPLD group, P = 0.04) (see Supplementary material online, Figure S3). Atrial flutter recurrence was similar across both groups (3 in HPSD vs. 5 in LPLD, P = 0.71). Direct currrent (DC) cardioversion for atrial arrhythmia was performed in four HPSD patients vs. two LPLD patients (P = 0.67). At 12 months, 5 HPSD patients (2 on amiodarone, 3 on sotalol), and 13 LPLD patients (8 on flecainide, 3 on sotalol, 2 on amiodarone) were on AAD (P = 0.06).
Predictors of arrhythmia outcome post atrial fibrillation ablation
Following univariate analysis, age, obesity, persistent AF, left atrial indexed volume (LAVI), AF at the start of the procedure, early arrhythmia recurrence, and HPSD were identified as predictive of arrhythmia recurrence following AF ablation. On multivariate analysis, early arrhythmia recurrence (within the blanking period) (P = 0.03), and persistent AF (P = 0.02) were predictive of late arrhythmia recurrence. Higher power short duration ablation was protective against arrhythmia recurrence (P = 0.002) (see Supplementary material online, Table S2).
Discussion
In this prospective randomized study, comparing HPSD with LPLD on the PW for AF ablation using multi-sensor ETM, we demonstrated the following findings:
Higher power (40 W) short-duration ablation resulted in a similarly low incidence of minor ETI to LPLD ablation. This is the first randomized study to directly compare ETI rates between HPSD and LPLD ablation.
Oesophageal temperature dynamics were not significantly different between the two approaches.
HPSD resulted in improved acute procedural outcomes including reduced PVI time/RF ablation times/total procedural time, with no evidence of increased complication.
Oesophageal thermal injury
The present study demonstrates a low incidence of ETI with HPSD ablation in all patients undergoing endoscopy. Prior studies are largely limited by non-randomized design and endoscopy reserved for selected patients with oesophageal heating. Prospective cohort studies involving AI guided ablation reported ETI ranging between 3.5 and 7% at endoscopy.10–12 Chen et al.10 reported mild mucosal erosions in 3.5% of 122 consecutive patients undergoing ablation at 50 W with multi-sensor ETM, although endoscopy was confined to patients with marked oesophageal heating with a mean peak temperature elevation of 41.2°C. Muller et al.12 reported an ETI incidence of 6% including a 0.1% incidence of AEF in 953 consecutive patients who underwent AI-guided ablation at 50 W without ETM. There was no significant difference in mean AI values between the ETI and the non-ETI patients. Kaneshiro et al.11 reported a non-randomized comparison of 271 patients with no difference in the incidence of ETI (7% with 50 W vs. 8% with 20–30 W). In the randomized POWER-AF trial, ablation at 45 W was compared with 35 W with ablation targets of ≥550 at the anterior wall and ≥400 everywhere else. Oesophageal temperature monitoring was utilized, with ablation discontinued if temperature exceeded 38.5°C and endoscopy was performed in selected patients with temperature elevation of >38.5°C, with an equivalent ETI incidence of 4.5% in both groups.6 Wolf et al. reported AI-guided ablation at 35 W with ETM, with a reduced AI on PW to 300 if oesophageal temperature exceeded 38.5°C. The incidence of ETI was 1.2%, although endoscopy was performed on average 9 days post ablation. The median maximum temperature was 39.9°C with average AI value of 351 in regions of oesophageal heating.13 Schade et al. compared contact force guided vs. AI-guided ablation at 30–35 W anteriorly and 20–25 W posteriorly, without ETM, and reported an ETI incidence of 5% in the AI-guided group. There was no difference in mean AI values between patients with ETI vs. those without. An AI >520 on the PW was an adverse predictor of ETI.14 In the present study the routine use of ETM may have contributed to the low rates of ETI seen as operators would immediately cease ablation with steep rises in temperature, if the temperature exceeded 38°C and would await normalization of oesophageal temperature before further ablation was applied.
Atrio-oesophageal fistula is a rare complication of AF ablation. A retrospective series reported an incidence of 0.008% in ablations performed at 45–50 W on the PW and 0.11% in ablations at 35 W.3 Notably two out of the three AEF cases in the 35 W group occurred in the absence of ETM. It is challenging to design a study with a sufficient sample size to detect a difference in such a rare complication. As such, ETI was selected as the primary endpoint in this study as it is considered a surrogate for the risk of serious oesophageal perforation, in particular AEF.15 Halbfass et al. reported ETI (mucosal erythema/erosion or ulcers) in 18% of 832 patients who underwent endoscopy within 7 days of AF ablation. Oesophageal perforating complications were seen in five (3%), including two AEF. Importantly, only those with an oesophageal ulcer at baseline progressed to oesophageal perforation, with an absolute risk of 9.6%.15
The mechanistic underpinnings behind the purported safety of HPSD have been described in animal studies. By increasing RF power and reducing ablation time, resistive heating is maximized while conductive heating is minimized.4,16 Bourier et al.4 compared lesion metrics from RF ablation on ex vivo porcine thigh muscle preparations utilizing different HPSD settings (70 W at 7 s, 60 W at 10 s, or 50 W at 13 s) vs. standard settings of 30 W at 30 s, and reported wider but shallower lesions with higher powers compared with narrower and deeper lesions at 30 W. In contrast, Nakagawa et al.17 compared lesion geometries from irrigated ablation on canine thigh muscle preparations at 90 W for 4 s vs. 50 W for 10 s and 30 W for 30 s, and showed that lesion diameter, depth, and volume increased with longer duration ablation despite lowering power determined by a greater energy deposit. The ablation targets in the present study were based on AI/LSI values and were the same in both groups, thus resulting in equivalent energy deposits and plausibly explaining the similarly low rate of oesophageal injury. We reported no difference in oesophageal temperature dynamics between HPSD and LPLD. Yavin et al.18 had reported oesophageal temperature dynamics in 20 patients by delivering RF lesions alternating between 50 and 25 W at 15–20 s in each patient. There was no significant difference in the magnitude of temperature rise, and time for oesophageal temperature to return to baseline. The present larger study extends these findings further through the randomized comparison of ablation power settings and does not support a reduction in power in regions of oesophageal temperature rises.
Lastly, a further 5.6% of patients had oesophageal lesions remote to the heart likely related to the use of the TOE or ETM probe itself. This reminds clinicians of the risk of oesophageal injury from instrumentation itself, separate from that posed by thermal injury from AF ablation.19
Clinical outcomes of higher power ablation
Randomized controlled trials have demonstrated shorter PVI times, procedural times, and RF ablation times with HPSD compared with LPLD,6,20 which was corroborated in our study. We did not find that HPSD ablation improved first-pass PVI rate nor acute PV reconnections, in keeping with the findings from the POWER-AF study.6 Two recent studies reported significantly higher first-pass PVI rates with a higher power.21,22 In contrast to the present study the definition of ‘first pass isolation’ in earlier studies allowed for intervenous ridge ablation during circumferential encirclement.21,22 Leo et al.21 compared 20 W/LSI 4 with 40 W/LSI 4, akin to our study groups, with no difference in first-pass isolation rates.
The impact of higher power ablation on freedom from AF after catheter ablation has been mixed.6,20,21 Shin et al. reported no difference in AF recurrence in 150 patients randomized to three study arms, with 12-lead ECGs performed during follow-ups or with symptoms and 24 h Holters at 3, 6, and 12 months. In the randomized POWER-AF study, which included 96 patients who underwent ECGs at 1, 3, and 6 months, there was no significant difference in AF recurrence between 45 vs. 35 W.6 In contrast in the present study, 97.7% of the cohort underwent intensive surveillance. Higher power short duration was associated with a significant reduction in arrhythmia recurrence (15.9%) compared with 34.1% with LPLD at a median follow-up of 12 months.
Higher power ablation remained a significant predictor of improved arrhythmia-free survival after adjusting for potential confounding factors. Plausible explanations to account for the superiority of higher power are broader shallower, overlapping lesions which may reduce the later development of gaps in ablation lines during follow-up.18,23 Additionally ablation lesions targets are achieved more rapidly with higher power potentially important at sites where catheter stability is challenging. The well-accepted predictors of early relapse of arrhythmia during the blanking period24 and persistent AF history were adverse predictors of arrhythmia recurrence.
Limitations
Participants did not undergo endoscopy prior to AF ablation. As such the possibility that any detected lesions were present prior to ablation could not be entirely excluded. However, all detected lesions were adjudicated by the committee based on the anatomical location of the lesions and correlation with temperature rises on the multi-sensor temperature probe. We did not compare HPSD vs. LPLD across the entire PVI ring but focused on the posterior left atrial wall only. A larger study population would be preferable given the low incidence of ETI.
Conclusions
Higher power ablation using multi sensor ETM was safe with equivalent low rates of ETI when compared with lower power ablation. Higher power short duration ablation significantly shortens PVI duration and total RF ablation time. Higher power short duration ablation was associated with improved long-term freedom from recurrent arrhythmias and should be considered part of the routine approach to AF ablation.
Supplementary Material
Contributor Information
David Chieng, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia.
Louise Segan, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia.
Hariharan Sugumar, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia.
Ahmed Al-Kaisey, School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia.
Joshua Hawson, School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia.
Benjamin M Moore, Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia.
Michael C Y Nam, Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia.
Aleksandr Voskoboinik, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia.
Sandeep Prabhu, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia.
Liang-Han Ling, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia.
Jer Fuu Ng, Department of Gastroenterology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia.
Gregor Brown, Department of Gastroenterology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia.
Geoffrey Lee, Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia.
Joseph Morton, Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia.
Henry Debinski, Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia.
Jonathan M Kalman, School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3050, Australia; School of Medicine, Monash University, Wellington Road, Clayton, Victoria 3800, Australia.
Peter M Kistler, Clinical Electrophysiology Research, Baker Heart and Diabetes Research Institute, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; School of Medicine, University of Melbourne, Parkville, Victoria 3010, Australia; Department of Cardiology, Cabrini Hospital, 181/183 Wattletree Road, Malvern, Victoria 3144, Australia; School of Medicine, Monash University, Wellington Road, Clayton, Victoria 3800, Australia.
Supplementary material
Supplementary material is available at Europace online.
Funding
None declared.
Data availability
Data from the study will be made available upon reasonable request to the corresponding author.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2017;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pambrun T, Durand C, Constantin M, Masse A, Marra C, Meillet Vet al. . High-power (40–50 W) radiofrequency ablation guided by unipolar signal modification for pulmonary vein isolation. Experimental findings and clinical results. Circ Arrhythm Electrophysiol 2019;12:e007304. [DOI] [PubMed] [Google Scholar]
- 3. Winkle RA, Mohanty S, Patrawala RA, Mead RH, Kong MH, Engel Get al. . Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm 2019;16:165–9. [DOI] [PubMed] [Google Scholar]
- 4. Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa Met al. . High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol 2018;29:1570–5. [DOI] [PubMed] [Google Scholar]
- 5. Kanj MH, Wazni O, Fahmy T, Thal S, Patel D, Elayi Cet al. . Pulmonary vein antral isolation using an open irrigation ablation catheter for the treatment of atrial fibrillation. J Am Coll Cardiol 2007;49:1634–41. [DOI] [PubMed] [Google Scholar]
- 6. Wielandts JY, Kyriakopoulou M, Almorad A, Hilfiker G, Strisciuglio T, Phlips Tet al. . Prospective randomized evaluation of high power during CLOSE-guided pulmonary vein isolation: the POWER-AF study. Circ Arrhythm Electrophysiol 2021;14:e009112. [DOI] [PubMed] [Google Scholar]
- 7. Yarlagadda B, Deneke T, Turagam M, Dar T, Paleti S, Parikh Vet al. . Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation catheter ablation. Heart Rhythm 2019;16:204–12. [DOI] [PubMed] [Google Scholar]
- 8. Kuwahara T, Takahashi A, Okubo K, Takagi K, Yamao K, Nakashima Eet al. . Oesophageal cooling with ice water does not reduce the incidence of oesophageal lesions complicating catheter ablation of atrial fibrillation: randomized controlled study. Europace 2014;16:834–9. [DOI] [PubMed] [Google Scholar]
- 9. Chen S, Schmidt B, Seegeer A, Bordignon S, Tohoku S, WIllems Fet al. . Catheter ablation of atrial fibrillation using ablation index guided high power (50 W) for pulmonary vein isolation: with or without esophageal temperature probe (the AI-HP ESO II). Heart Rhythm 2020;17:1833–40. [DOI] [PubMed] [Google Scholar]
- 10. Chen S, Chun KRJ, Tohoku S, Bordignon S, Urbanek L, Willems Fet al. . Esophageal endoscopy after catheter ablation of atrial fibrillation using ablation-index guided high-power. Frankfurt AI-HP ESO-I. JACC Clin Electrophysiol 2020;6:1253–61. [DOI] [PubMed] [Google Scholar]
- 11. Kaneshiro T, Kamioka M, Hijioka N, Yamada S, Yokokawa T, Misaka Tet al. . Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short- duration setting. Circ Arrhythm Electrophysiol 2020;13:008602. [DOI] [PubMed] [Google Scholar]
- 12. Muller J, Berkovitz A, Halbfass P, Nentwich K, Ene E, Sonne Ket al. . Acute oesophageal safety of high-power short duration with 50 W for atrial fibrillation ablation. Europace 2022;24:928–37. [DOI] [PubMed] [Google Scholar]
- 13. Wolf M, Haddad ME, Wilde VD, Phlips T, Pooter JD, Almorad Aet al. . Endoscopic evaluation of the esophagus after catheter ablation of atrial fibrillation using contiguous and optimized radiofrequency applications. Heart Rhythm 2019;16:1013–20. [DOI] [PubMed] [Google Scholar]
- 14. Schade A, Costello-Boerrigter L, Deneke T, Steinborn F, Chapran M, Vathie Ket al. . Oesophageal safety in voltage-guided atrial fibrillation ablation using ablation index or contact force only: a prospective comparison. Europace 2022;24:1909–16. [DOI] [PubMed] [Google Scholar]
- 15. Halbfass P, Pavlov B, Müller P, Nentwich K, Sonne K, Barth Set al. . Progression from esophageal thermal asymptomatic lesion to perforation complicating atrial fibrillation ablation: a single-center registry. Circ Arrhythm Electrophysiol 2017;10:e005233. [DOI] [PubMed] [Google Scholar]
- 16. Enomoto Y, Nakamura K, Ishii R, Toyoda Y, Asami M, Takagi Tet al. . Lesion size and adjacent tissue damage assessment with high power and short duration radiofrequency ablation: comparison to conventional radiofrequency ablation power setting. Heart Vessels 2021;36:1438–44. [DOI] [PubMed] [Google Scholar]
- 17. Nakagawa H, Ikeda A, Sharma T, Govari A, Ashton J, Maffre Jet al. . Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high power–short duration and moderate power–moderate duration. Effects of thermal latency and contact force on lesion formation. Circ Arrhythm Electrophysiol 2021;14:e009899. [DOI] [PubMed] [Google Scholar]
- 18. Yavin HD, Leshem E, Shapira-Daniels A, Sroubek J, Barkagan M, Haffajee CIet al. . Impact of high-power short-duration radiofrequency ablation on long-term lesion durability for atrial fibrillation ablation. JACC Clin Electrophysiol 2020;6:973–85. [DOI] [PubMed] [Google Scholar]
- 19. Kumar S, Brown G, Sutherland F, Morgan J, Andrews D, Ling LHet al. . The transesophageal echo probe may contribute to esophageal injury after catheter ablation for paroxysmal atrial fibrillation under general anesthesia: a preliminary observation. J Cardiovasc Electrophysiol 2015;26:119–26. [DOI] [PubMed] [Google Scholar]
- 20. Shin DG, Ahn J, Han SJ, Lim HE. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace 2020;22:1495–501. [DOI] [PubMed] [Google Scholar]
- 21. Leo M, Pedersen M, Rajappan K, Ginks MR, Hunter RJ, Bowers Ret al. . Power, lesion size index and oesophageal temperature alerts during atrial fibrillation ablation. A randomised study. Circ Arrhythm Electrophysiol 2020;13:e008316. [DOI] [PubMed] [Google Scholar]
- 22. Okamatsu H, Koyama J, Sakai Y, Negishi K, Hayashi K, Tsurugi Tet al. . High power application is associated with shorter procedure time and higher rate of first-pass pulmonary vein isolation in ablation index-guided atrial fibrillation ablation. Circ Rep 2021;3:559–68. [DOI] [PubMed] [Google Scholar]
- 23. Otsuka N, Okumura Y, Kuorkawa S, Nagashima K, Wakamatsu Y, Hayashida Set al. . Actual tissue temperature during ablation index-guided high-power short-duration ablation versus standard ablation: implications in terms of the efficacy and safety of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2022;33:55–63. [DOI] [PubMed] [Google Scholar]
- 24. Kim YG, Boo KY, Choi JI, Choi YY, Choi HY, Roh SYet al. . Early recurrence is reliable predictor of late recurrence after radiofrequency catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2021;7:343–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the study will be made available upon reasonable request to the corresponding author.