Abstract
Aims
Subcutaneous implantable cardioverter defibrillators (S-ICDs) are well established. However, inappropriate shocks (IAS) remain a source of concern since S-ICDs offer very limited troubleshooting options. In our multicentre case series, we describe several patients who experienced IAS due to a previously unknown S-ICD system issue.
Methods and results
We observed six patients suffering from this novel IAS entity. The IAS occurred exclusively in primary or alternate S-ICD sensing vector configuration (therefore called ‘Sense-B-noise’). IAS were caused by non-physiologic oversensing episodes characterized by intermittent signal saturation, diminished QRS amplitudes, and disappearance of the artefacts after the IAS. Noise/oversensing could not be provoked by manipulation, X-ray did not show evidence for lead/header issues and impedance measurements were within normal limits. The pooled experience of our centres implies that up to ∼5% of S-ICDs may be affected. The underlying root cause was discussed extensively with the manufacturer but remains unknown and is under further investigation.
Conclusion
Sense-B-noise is a novel cause for IAS due to non-physiologic signal oversensing, arising from a previously unknown S-ICD system issue. Sense-B-noise may be suspected if episodes of signal saturation in primary or alternate vector configuration are present, oversensing cannot be provoked, and X-ray and electrical measurements appear normal. The issue can be resolved by reprogramming the device to secondary sensing vector.
Keywords: Subcutaneous defibrillator, S-ICD, Inappropriate shock, Oversensing, Device failure
What’s new?
We describe a novel entity of inadequate shocks in S-ICDs, which has not been described previously.
Inadequate shocks are caused by oversensing of non-physiologic signals due to an S-ICD system issue with unknown root cause.
The issue exclusively occurs in primary or alternate sensing vector configuration and can be resolved by reprogramming the device.
Introduction
Subcutaneous implantable cardioverter defibrillators (S-ICDs) overcome key limitations of conventional transvenous ICDs. Lead endocarditis, lead-induced tricuspid valve regurgitation, and some implantation-related complications may be avoided using S-ICDs. Thus, they should be considered as an alternative to transvenous systems particularly in patients not requiring pacing. They may also be implanted if venous access is difficult to obtain or after device removal due to infection. One important downside of S-ICDs, however, is the inappropriate shock (IAS) rate. Initially, 1-year IAS rates had reached 8–13%.1,2 More recent studies report a lower incidence of IAS3 in newer device generations with introduction of SMART pass filter technology, more conservative device programming, and different patient baseline characteristics in the respective studies. Long-term follow-up reports now show an annual IAS rate of ∼2%,4 which may not be different compared with transvenous ICDs.5 Nonetheless, IAS are a particular source of concern in S-ICDs, since these devices offer only very limited troubleshooting options and system revision may be deemed necessary (up to 5.6% 2 years after implantation).6 Common sources for IAS in S-ICDs encompass T-wave oversensing, myopotential oversensing, misinterpretation of supraventricular arrhythmias, lead fractures, and air entrapment.7 In this case series, we present a novel enigmatic entity of IAS (called Sense-B-noise, related to the involvement of the node B sensing circuit in the S-ICD), which has not been described previously.
Case description
Subsequently, we provide an extensive case-by-case description of the patients that experienced IAS due to Sense-B-noise. All cases and their management were extensively discussed with the device manufacturer.
Case 1
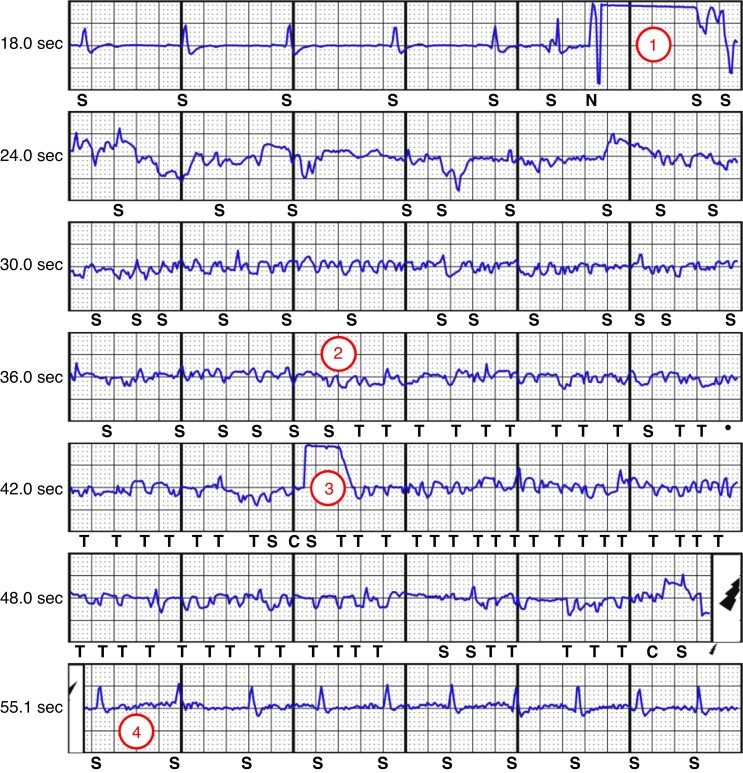
A 29-year-old male had received an Emblem A219 S-ICD with a 3501 lead (Boston Scientific, Marlborough, MA, USA) in July 2019 due to idiopathic ventricular fibrillation (VF). Two months after implantation, he received two shocks due to VF. After 16 months, while working on the computer, the patient received an IAS (Figure 1; SMART pass on, primary sensing vector, gain 2×, shock impedance 64Ω). Device manipulation and provocation manoeuvers showed no suspicious findings. The patient underwent system revision and the complete S-ICD was sent to the manufacturer for analysis. The analysis revealed that no lead defect was present. However, changes in the generator’s impedance pathway of the primary sensing vector were identified, which may have caused the noise oversensing according to the manufacturer.
Figure 1.
Inappropriate shock due to oversensing (Case #1). The episode begins with a sudden artefact driving the signal into saturation (1). Subsequently, the cardiac signal becomes largely unrecognizable with low-amplitude signals (2), and the S-ICD starts to charge (3, charging artefact). The shock delivery is ensued by normal QRS signal amplitudes (4).
Case 2
A 25-year-old woman had received an Emblem A219 S-ICD with a 3501 lead in February 2020 due to malignant mitral valve prolapse (primary prevention). Six months later, she experienced an IAS (Figure 2A). Another untreated episode had been registered two weeks prior (Figure 2B). During shock delivery (SMART pass on, primary sensing vector, gain 1×, shock impedance 59Ω), she was cleaning the floor. Extensive device manipulation and provocation manoeuvers at rest and during exercise could not reproduce oversensing. The X-ray showed correct connection of the lead inside the header without signs of lead fracture or kinks. Lead revision was considered but—based on the manufacturer’s suggestion—we only reprogrammed the device to secondary sensing vector and installed remote monitoring. The patient was successfully rescued from VF by an appropriate shock delivered 15 months after device reprogramming. During those 15 months and until today, no oversensing was observed.
Figure 2.
Patient Case #2. Panel (A) inappropriate shock due to artefact oversensing. Signal saturation episodes are visible (asterisks), the low-amplitude QRS becomes better visible after the IAS. Panel (B) similar to Figure 1, the episode is triggered by a sudden artefact (1). The ensuing artefact-rich signal shows episodes of signal saturation and oversensing.
Case 3
A 55-year-old female SCD survivor with long QT syndrome had received an Emblem A219 S-ICD with a 3501 lead in October 2019 after extractions of transvenous ICD systems (one due to lead fracture and another one due to superior vena cava syndrome). Twenty-seven months after S-ICD implantation, she received an IAS while being in atrial fibrillation and at rest (Figure 3A; SMART pass on, alternate sensing vector, gain 2×, shock impedance 90Ω). The IAS induced VF, which was successfully defibrillated after 27 s (Figure 3B). The device was re-programmed to secondary sensing vector and remote monitoring was installed. During follow-up, the SMART pass filter was automatically deactivated due to low-amplitude signals. Moreover, intermittent QRS undersensing and T-wave oversensing occurred (Figure 3C). The patient is under close observation and on remote monitoring and somewhat lost confidence in device therapy after multiple adverse events with transvenous ICDs and the S-ICD.
Figure 3.
Patient Case #3. Panel (A) patient with IAS inducing ventricular fibrillation. The shock is preceded by an artefact-rich signal with episodes of signal saturation (asterisks). Panel (B) shows the following second shock. Panel (C) remote monitoring of the patient in secondary vector. Episodes as shown in panel (A) were not registered anymore. However, intermittent T-wave oversensing (1) and QRS undersensing (2) occurred. Myopotential noise was appropriately classified by the device (3).
Case 4
A 42-year-old male had received an Emblem A219 S-ICD with a 3401 lead in September 2016 after survival of sudden cardiac death (SCD, Chagas heart disease). Sixteen months after implantation, he received an IAS due to signal dropout (Figure 4A, SMART pass off, primary sensing vector, gain 1×, shock impedance 72Ω). Similar non-sustained episodes with aborted shocks had been stored a minute before and on the preceding evening. Secondary and alternate sensing vectors showed low-amplitude signals, so the primary sensing vector was maintained, with re-activation of SMART pass. Two days later, the patient presented with a new IAS, again due to signal dropout (Figure 4B), again with automatic inactivation of SMART pass due to low signal amplitudes. Since there was no satisfying explanation, the patient underwent system explantation. Perioperatively, the suture sleeve was properly fixated and did not cover the proximal electrode, the lead pin was correctly inserted and showed no signs of failure. Technical analysis of the device by the manufacturer was unable to determine the root cause since the device function was unremarkable.
Figure 4.
Patient Case #4. Panel (A) heavily diminished ECG signal amplitudes causing oversensing and shock delivery. Panel (B) similar episode 2 days later. Both episodes (panel B and C) showing QRS amplitude restoration after shock delivery.
Case 5
A 44-year-old male had received an Emblem A219 S-ICD with a 3501 lead in July 2021 due to dilated cardiomyopathy with severely reduced left ventricular ejection fraction. Eight months later, he received an IAS after episodes of signal saturation and diminished QRS amplitudes (SMART pass on, primary sensing vector, gain 1×, shock impedance 105Ω). Artefacts could not be provoked during troubleshooting, the X-ray was unremarkable. The technical support of the manufacturer confirmed that also this patient suffered from so-called Sense-B-noise and recommended reprogramming to secondary sensing vector.
Case 6
A 52-year-old male had received an Emblem A219 S-ICD with a 3501 lead in August 2017 for primary prophylaxis due to hypertropic obstructive cardiomyopathy. Fifty-six months after implantation, he received an IAS due to signal dropout (SMART pass on, primary sensing vector, gain 1×, shock impedance 66Ω). During troubleshooting, no artefacts could be provoked and due to a suitable secondary vector, Boston Scientifics’ technical service recommended to use this vector instead. Since the reprogramming and starting of remote monitoring, no further shocks have occurred.
Discussion
We present six patients, who experienced IAS due to ‘Sense-B-noise’, an enigmatic source of S-ICD oversensing. The issue has been labelled ‘Sense-B-noise’ by the manufacturer due to the exclusive involvement of the node B sensing circuit involving the proximal electrode (i.e. the alternate and primary vector) and the associated connectors and circuitry. More than 300 similar cases have been identified worldwide, resulting in an annual incidence of <0.1% according to the manufacturer. However, detailed information regarding the issue is lacking so far. All cases share some common features that are inconsistent with other non-cardiac sources of oversensing.
How to diagnose sense-B-noise
According to the manufacturer and in line with our experience, Sense-B-noise is characterized by:
Occurrence exclusively in primary or alternate sensing vector configuration (no similar cases in secondary vector according to the manufacturer). Resolution of oversensing episodes with secondary vector during follow-up.
Electrogram showing episodes of signal saturation (‘square signal’, which was absent in Case #4 but could have been missed as the onset of the abnormal electrogram had not been recorded) and diminished QRS amplitudes without sufficient alternative explanation (e.g. conductor fracture, trapped air after implantation, intermittent contact to sternal cerclages). Reduction of QRS amplitude with restoration of normal QRS waveforms and disappearance of the artefacts immediately after shock delivery.
Noise/oversensing cannot be provoked by device manipulation or other manoeuvers. There are rare exceptions (<3% of all known cases according to the manufacturer) where artefacts can be provoked by manoeuvers.
No fluoroscopic evidence for lead fracture, incompletely inserted lead in the device header, or loose set screw.
Intact high-voltage circuitry with normal device impedances.
The above features allow distinguishing oversensing episodes from other sources of S-ICD oversensing. Myopotential oversensing is typically characterized by high-frequency low-amplitude signals (Figure 3C), which can be provoked by exercise and are potentially accompanied by baseline wander. Air entrapment after implantation may also cause signal saturation, low-amplitude QRS complexes and baseline wander.7–9 Changing the sensing vector can in some instances resolve such issues.9 However, air gets absorbed within a few days to weeks after implantation7 and is unlikely present many months after implantation.
A challenging differential diagnosis is (partial) lead fracture, which may exhibit similar findings as described above.10 Lead fractures early after implantation are unlikely, but the manufacturer has released an advisory11 due to lead body fractures distal to the proximal sensing ring of the model 3501, which has recently been replaced by a reinforced model.12 When programmed in primary vector, this failure mode will not cause non-physiologic artefacts but if the device is programmed in alternate vector and only the distal sense conductor is fractured, similar artefacts with normal impedances may be seen. Chest X-ray does not necessarily reveal fracture of only some filars. Thus, if oversensing episodes can be provoked, lead or header issues have to be suspected and a system revision is required. IAS not only cause intense discomfort to patients but may result in the induction of ventricular tachyarrhythmia (Figure 3A). A flowchart summarizing the differential diagnosis and management of inadequate S-ICD shocks of non-cardiac origin is shown in Figure 5.
Figure 5.
Simplified flowchart for the diagnosis and management of inadequate S-ICD shocks of non-cardiac origin. Initial diagnostic steps (yellow boxes) mostly allow identifying the cause for the IAS (red boxes). Dashed arrows indicate diagnostic key hints. If the findings remain unclear or unsuspicious, the manufacturer’s technical service should be involved. Green boxes show general management recommendations. Abbreviations: AC, alternating current; IAS, inadequate shock.
How to troubleshoot sense-B-noise
The troubleshooting strategy in this case series was discussed on an individual basis with the manufacturer. Re-programming to secondary sensing vector accompanied by remote monitoring is an option in case of true Sense-B-noise if other causes of device failure have (largely) been ruled out after discussion with the manufacturer’s technical support. The technical service of the manufacturer may provide additional information in the form of advanced remote monitoring or quantitative signal analyses. Whenever the secondary sensing vector cannot be used due to poor signals [Cases #3 (Figure 3C) and #4], device revision is recommended. However, Boston Scientific has not yet released specific general management recommendations.
The underlying root cause and incidence of sense-B-noise
The root cause of the described issue is still unclear although other similar cases have been identified worldwide. An underlying hardware problem (electronic circuitry or device-electrode connection in the header) causing impedance pathway changes seems likely. However, which part of the system is responsible for the issue is still being investigated and preliminary results are kept confidential by the manufacturer. The node B sensing circuit involves different parts, which may, alone or in combination, be responsible for the observed issue. It cannot be ruled out, that different failure mechanisms may result in Sense-B-noise, which would then be more an umbrella term and a clinical phenomenon than a failure of a specific device part. Furthermore, the frequency of the problem is unclear. The pooled experience of our centres implies that ∼5% of S-ICDs may be affected (quotient of patients with Sense-B-noise and all implanted S-ICDs at our centres), which seems high due to the absence of previous publications regarding this issue. According to the manufacturer (Boston Scientific Corporation, Quality System Data), a 60-month rate of occurrence of 0.42% has been observed worldwide. Underreporting is likely because the issue has not been described in medical literature yet and may, thus, have been missed by implanters. A search by the authors in the Manufacturer and User Facility Device Experience (MAUDE) database showed some cases that appear to resemble the patients of our report. These issues were labelled ‘Sense B node noise’, ‘noise attributed to the Sense B node’, ‘Sense B node impairment’ and alike. Due to the limited details provided in MAUDE, we cannot draw firm conclusions on the overall incidence of the issue.
Potential technical implications
The enigmatic cause and incidence of Sense-B-noise are a significant challenge for patients and physicians. The manufacturer is strongly encouraged to further investigate the issue and communicate results and improvements of the technology to healthcare professionals. The current management recommendation to reprogram devices to secondary sensing vector may be considered to be a temporary measure until the underlying root cause is identified. The authors assume that the manufacturer will modify the device header since a disturbance of the Sense-B-contacts in the header seems to be a likely root cause. Moreover, other features may improve diagnosis and management of Sense-B-noise and other sources for IAS in S-ICDs:
Tachycardia confirmation algorithms might be implemented, which use a combination of the three available sensing vectors or information on previous signal saturation episodes.
Advanced remote monitoring capabilities such as the analysis of discarded signals, electrode impedance trends, and system status may also be helpful to distinguish Sense-B-noise from other issues. To date, accessing Latitude™ data for advanced analysis is only possible for the manufacturer’s technical service. Automatic transmission of alerts is also desirable (currently, the system only transmits scheduled or patient-initiated interrogations).
During device interrogation, precise measurement of the signal amplitudes and/or signal-to-noise ratios of all vectors should be possible. This would allow assessing the suitability of other sensing vectors better and could prevent follow-up complications. Currently, Boston Scientific’s technical service provides this information at special request.
Limitations
This is a preliminary technical case series including a limited number of patients, who experienced IAS due to an enigmatic root cause. The severity of the issue is recognized by the manufacturer and remains under investigation. General caution is warranted regarding the described diagnostic features of ‘Sense-B-noise’ and the suggested troubleshooting options in this article. The ongoing investigation and future findings may change management recommendations.
Conclusion
We describe a technical case series of S-ICDs implanted for 6–27 months with enigmatic IAS due to non-physiologic signal oversensing in primary or alternate sensing vector configuration, which is likely to arise from a previously unknown S-ICD system issue. Sense-B-noise may be suspected if episodes of signal saturation are present (accompanied by QRS amplitude reduction reverting to normal directly after an IAS), oversensing cannot be provoked and lead fractures, lead underinsertion or entrapped air are unlikely. The issue may be resolved by reprogramming the device to secondary sensing vector.
Acknowledgements
We acknowledge the helpful discussions and the support provided by Stefano Accinelli, Amy Brisben, Maria Macuare Gorden, Wyatt Stahl, Leila Cammoun, and Vittoria Storni (all from Boston Scientific).
Contributor Information
Andreas Haeberlin, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; ARTORG Center for Biomedical Engineering Research, University of Bern, Bern, Switzerland.
Haran Burri, University Hospital of Geneva, Cardiology Department, Geneva, Switzerland.
Beat Schaer, Department of Cardiology, University Hospital, University of Basel, Basel, Switzerland.
Pascal Koepfli, Cantonal Hospital Baden, Baden, Switzerland.
Christian Grebmer, Department of Cardiology, Kantonsspital Lucerne, Lucerne, Switzerland.
Alexander Breitenstein, Department of Cardiology, University Hospital Zurich, Zurich, Switzerland.
Tobias Reichlin, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Fabian Noti, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Funding
No funding declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–53. [DOI] [PubMed] [Google Scholar]
- 2. Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil Pet al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol 2017;70:830–41. [DOI] [PubMed] [Google Scholar]
- 3. Gold MR, Lambiase PD, El-Chami MF, Knops RE, Aasbo JD, Bongiorni MG et al. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021;143:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambiase PD, Theuns DA, Murgatroyd F, Barr C, Eckardt L, Neuzil Pet al. Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. Eur Heart J 2022. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knops RE, Olde Nordkamp LRA, Delnoy P-PHM, Boersma LVA, Kuschyk J, El-Chami MF et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–36. [DOI] [PubMed] [Google Scholar]
- 6. Noel A, Ploux S, Bulliard S, Strik M, Haeberlin A, Welte N et al. Oversensing issues leading to device extraction: when subcutaneous implantable cardioverter-defibrillator reached a dead-end. Heart Rhythm 2020;17:66–74. [DOI] [PubMed] [Google Scholar]
- 7. Ali H, Lupo P, Foresti S, De Ambroggi G, De Lucia C, Penela Det al. Air entrapment as a potential cause of early subcutaneous implantable cardioverter defibrillator malfunction: a systematic review of the literature. Europace 2022;24:1608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishinarita R, Kishihara J, Matsuura G, Arakawa Y, Kobayashi S, Shirakawa Yet al. Early inappropriate shock in a subcutaneous cardiac defibrillator due to subcutaneous air. J Arrhythm 2019;35:682–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang YC, Aung TT, Bailin SJ, Rhodes TE. Air entrapment causing inappropriate shock from a subcutaneous implantable cardioverter defibrillator. Cardiol Res 2019;10:128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kella DK, Stambler BS. Subcutaneous implantable cardioverter-defibrillator electrode fracture: follow-up, troubleshooting, and evaluation. J Cardiovasc Electrophysiol 2021;32:1452–7. [DOI] [PubMed] [Google Scholar]
- 11. Boston Scientific Inc. Important Medical Device Advisory. Marlborough, Massachusetts, United States 2020.
- 12. Viani S, Migliore F, Ottaviano L, Biffi M, Ammendola E, Ricciardi Get al. Longevity of model-3501 subcutaneous implantable defibrillator leads in clinical practice. Heart Rhythm 2022. online. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.