Abstract
Aims
Three Tesla (T) magnetic resonance imaging (MRI) provides critical imaging information for many conditions. Owing to potential interactions of the magnetic field, it is largely withheld from patients with cardiac implantable electronic devices (CIEDs). Therefore, we assessed the safety of 3T MRI in patients with ‘3T MRI-conditional’ and ‘non-3T MRI-conditional’ CIEDs.
Methods and results
We performed a retrospective single-centre analysis of clinically indicated 3T MRI examinations in patients with conventional pacemakers, cardiac resynchronization devices, and implanted defibrillators from April 2020 to May 2022. All CIEDs were interrogated and programmed before and after scanning. Adverse events included all-cause death, arrhythmias, loss of capture, inappropriate anti-tachycardia therapies, electrical reset, and lead or generator failure during or shortly after MRI. Changes in signal amplitude and lead impedance were systematically assessed. Statistics included median and interquartile range. A total of 132 MRI examinations were performed on a 3T scanner in 97 patients. Thirty-five examinations were performed in patients with ‘non-3T MRI-conditional’ CIEDs. Twenty-six scans were performed in pacemaker-dependent patients. No adverse events occurred during or shortly after MRI. P-wave or R-wave reductions ≥ 50 and ≥ 25%, respectively, were noted after three (2.3%) scans, all in patients with ‘3T MRI-conditional’ CIEDs. Pacing and shock impedance changed by ± 30% in one case (0.7%). Battery voltage and stimulation thresholds did not relevantly change after MRI.
Conclusion
Pending verification in independent series, our data suggest that clinically indicated MRI scans at 3T field strength should not be withheld from patients with cardiac pacemakers or defibrillators.
Keywords: Magnetic resonance imaging, Cardiac implantable electronic devices, Implantable cardioverter defibrillator, Pacemakers, Tesla
Graphical Abstract
Graphical Abstract.
Abbreviations: CIEDs, cardiac implantable electronic devices; MRI, magnetic resonance imaging; T, Tesla
What’s new?
In this study, we assessed the safety of 3T MRI in patients with cardiac pacemakers and defibrillators as 3T MRI instead of 1.5T MRI which is increasingly performed in clinical practice due to its higher diagnostic demand and excellent image quality.
In patients with ‘3T MRI conditional’ and with ‘non-3T MRI conditional’ cardiac devices, stimulation thresholds and battery voltage did not significantly change after MRI. Although for both groups, changes of ≥50 Ω in stimulation impedance were noted after almost a quarter of MRI scans, only one case exceeded an alteration of ± 30%.
In this real-world cohort, we found that patients with cardiac pacemakers or defibrillators had a good safety profile and should not be denied access to clinically indicated 3T MRI in both ‘3T MRI conditional’ and ‘non-3T MRI conditional’ cardiac devices.
Abbreviations
- CIEDs
cardiac implantable electronic devices
- CRT
cardiac resynchronization therapy
- ICD
internal cardioverter defibrillator
- IQR
interquartile range
- LV
left ventricle
- MRI
magnetic resonance imaging
- RA
right atrium
- RV
right ventricle
- T
Tesla
- V
volt
Introduction
Magnetic resonance imaging (MRI) is a fundamental diagnostic tool for assessing a multitude of diseases including tumours, musculoskeletal diseases, and neurological disorders. Each year, ∼120 MRI exams are performed per 10 000 inhabitants in Germany.1 Patients with cardiac implantable electronic devices (CIEDs) have been denied access to MRI for many years due to safety concerns.2 Effects of the static and gradient magnetic and radiofrequency field on CIEDs theoretically may lead to loss of capture, induction of arrhythmias, battery depletion or electrical reset.3Ex vivo, several medical devices demonstrated potentially hazardous interactions not only with 1.5 Tesla (T) MRI but especially with 3T MRI.4 Therefore, generators and leads specifically for the magnetic resonance environment were designed and tested in trials in 1.5T MRI and 3T MRI without major adverse events.5,6 Many CIEDs now carry a ‘MRI-conditional’ label confirming their safety for use in MRI scanners. Growing evidence suggests that 1.5T MRI can be performed safely in patients with MRI-conditional CIEDs and with ‘MRI-non-conditional’ CIEDs.7–9 MRI is increasingly performed at 3T to improve image quality.10 Data on the safety of MRI imaging at 3T in patients with CIEDs are scarce.11
Therefore, the aim of this study was to evaluate the safety of clinically indicated 3T MRI in patients with CIEDs.
Methods
Study design
We performed a retrospective single-centre analysis of consecutive patients admitted to the University Medical Center Hamburg-Eppendorf in Germany from April 2020 to May 2022.
Eligible patients underwent clinically indicated 3T MRI scans of different thoracic and non-thoracic body regions. MRI was performed using scanners of two different manufacturers (Ingenia, Philips Medical Systems and MAGNETOM Vida, Siemens Healthineers). MRI was performed according to standard institutional protocols for the region of interest.
MRI-conditional labelling was determined for each device using manufacturer approval information. If one component (lead or generator) of the CIED was not approved for 3T MRI or 1.5T MRI by the manufacturer, the CIED was considered ‘MRI non-conditional’. Written informed consent was obtained from all patients with an amendment for ‘MRI non-conditional’ and ‘1.5T MRI-conditional’ devices. All possible risks were discussed.
Data were anonymized prior to analysis. The project is registered at the local Ethics committee (2022-300206-WF, State of Hamburg Chamber of Medical Practitioners). This analysis adheres to local regulations (§12 HmbKHG) and to the Declaration of Helsinki.
MRI monitoring
During MRI, pulse oximetry and cardiac rhythm were continuously monitored with an MRI-compatible system, enabling the capture of short and asymptomatic arrhythmias. A physician trained in advanced cardiac life support was onsite during MRI scans in patients with an ICD or in pacemaker-dependent patients with ‘non-3T MRI-conditional’ CIEDs.
Device programming
Devices were interrogated before and after MRI according to our standard protocol including measurements of battery power, P-wave and R-wave amplitudes, stimulation thresholds, and impedances. The recommended MRI mode according to the manufacturer’s recommendations was activated for the duration of the MRI scan. Pacing was programmed to an MRI-safe mode during MRI, including deactivation of pacing in non-pacemaker-dependent patients or asynchronous pacing modes V00 or D00 at 20–30 bpm above the intrinsic rhythm. Antitachycardia therapies were inactivated during MRI.
Statistical analysis and endpoints
Continuous parameters are reported as median and interquartile range (IQR). Device interrogation parameters were compared before and after MRI. Alterations of stimulating thresholds of ≥ 0,5V were considered relevant. Relevant changes concerning sensing were defined as ≥ 50% reduction in P-wave or ≥ 25% reduction in R-wave amplitude. Thresholds for relevant changes concerning stimulating impedance were set at ≥ 50 Ω, shock impedance at ≥ 3 Ω, in line with major publications.9,12 In addition, the number of devices exceeding a relative change of ±30% in lead impedance was calculated.
Adverse events included all-cause death, arrhythmias, loss of capture, inappropriate therapies, electrical reset and lead or generator failure.
Results
Study population
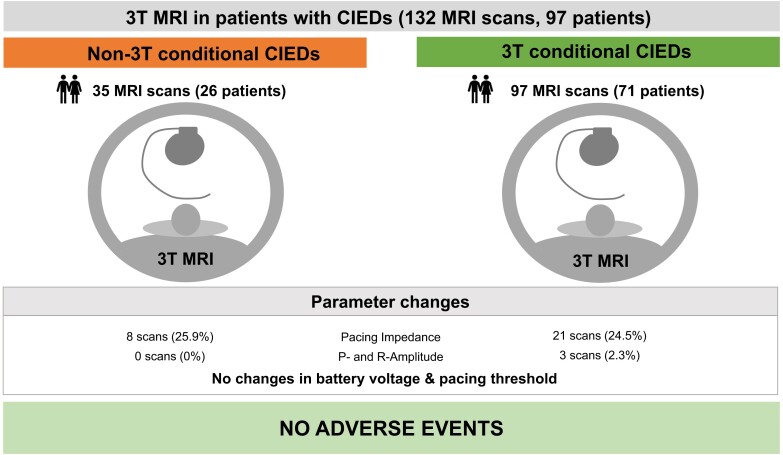
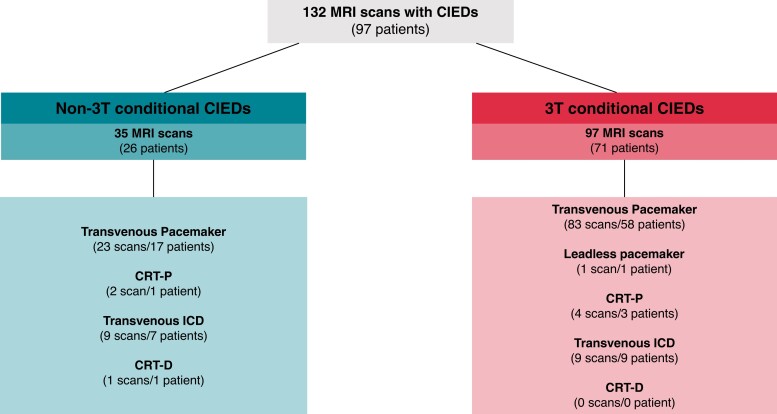
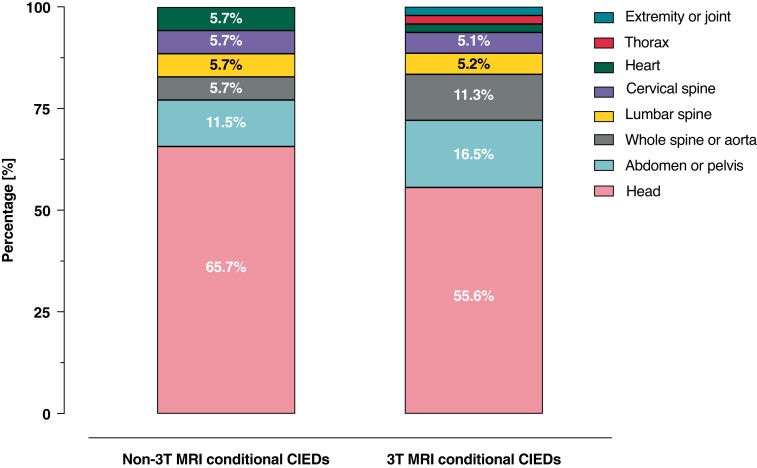
Ninety-seven patients [median age 72, (IQR: 63–79), n = 35 (36.1%) female] underwent 132 MRI scans (Graphical Abstract, Figure 1). Baseline characteristics are presented in Table 1. Thirty-five (26.5%) 3T MRI scans were performed in patients with ‘non-3T MRI-conditional’ devices, and 97 (73.5%) scans in patients with ‘3T MRI-conditional’ devices (Figure 1, see Supplementary material online, Tables S1 and S2). Patients were pacemaker-dependent in 26 (19.7%) MRI scans, of which 11 (8.3%) scans were conducted in patients with ‘non-3T MRI-conditional’ CIEDs. Patients with CIEDs underwent MRI of all body regions: scans of the head presented the majority followed by abdomen and pelvis (Figure 2).
Figure 1.
Three tesla (T) magnetic resonance imaging (MRI) in patients with non-3T MRI and 3T MRI conditional cardiac implantable electronic devices (CIEDs). Abbreviations: CIED, cardiac implantable devices; CRT-P/-D, cardiac resynchronization therapy pacemaker/defibrillator; ICD, implantable cardioverter defibrillator; MRI, magnetic resonance imaging; T, Tesla.
Table 1.
Baseline characteristics
| Non-3T MRI conditional | 3T MRI conditional | |
|---|---|---|
| Scans no. | 35 | 97 |
| Patients no. | 26 | 71 |
| Demographics | ||
| Age (years), mean IQR | 72 (62, 79) | 72 (63, 79) |
| Women no. (%) | 9 (34.6) | 26 (36.6) |
| BMI (kg/m2), mean IQR | 24.9 (21.0, 30.6) | 25.8 (22.5, 29.4) |
| Comorbidities | ||
| Arterial hypertension no. (%) | 16 (36.0) | 38 (55.1) |
| Diabetes Type 1 or Type 2 (%) | 5 (22.0) | 15 (22.1) |
| Coronary artery disease no. (%) | 9 (36.0) | 23 (34.3) |
| Heart failure a no. (%) | 7 (41.0) | 17 (25) |
| Device indications | ||
| Sick-sinus-syndrome no. (%) | 8 (30.8) | 32 (45.1) |
| Atrioventricular block no. (%) | 11 (42.3) | 23 (23.4) |
| Bradyarrhythmia absoluta no. (%) | 0 (0) | 4 (5.6) |
| Resynchronisation therapy only no. (%) | 0 (0) | 4 (85.6) |
| Primary prevention no. (%) | 2 (7.7) | 4 (5.6) |
| Secondary prevention no. (%) | 5 (19.2) | 4 (5.6) |
| Device characteristics | ||
| Pacemaker-dependent no. (%) | 11 (31.4) | 15 (15.5) |
| Pacemaker no. (%) | 23 (65.7) | 83 (85.6) |
| Leadless pacemaker no. (%) | 0 (0) | 1 (1) |
| CRT-P no. (%) | 2 (5.71) | 4 (4.1) |
| CRT-D no. (%) | 1 (2.9) | 0 (0) |
| ICD no. (%) | 9 (25.7) | 9 (9.3) |
| Generator manufacturer | ||
| Abbott/St. Jude Medical no. (%) | 15 (42.9) | 6 (6.3) |
| Biotronik no. (%) | 9 (25.7) | 17 (17.7) |
| Boston Scientific no. (%) | 3 (8.6) | 13 (13.5) |
| Medtronic no. (%) | 8 (22.9) | 60 (62.5) |
| Microport/ELA/Sorin no. (%) | 0 | 0 |
| Lead and generator characteristics | ||
| RA leads no. | 26 | 84 |
| RV leads no. | 35 | 93 |
| LV leads no. | 3 | 4 |
| Time since RA lead implantation (years) | 2.38 | 2.96 |
| Time since RV lead implantation (years) | 2.96 | 2.62 |
| Time since LV lead implantation (years) | 0.67 | 0.73 |
| Time since generator implantation (years) | 2.57 | 2.41 |
Diagnosis based
Abbreviations: BMI, body mass index; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LV, left ventricular; MRI, magnetic resonance imaging; RA, right atrial; RV, right ventricular; T, Tesla
Figure 2.
Number of MRI scans per body region. Patients with ‘non-3T MRI-conditional’ CIEDs underwent MRI scans of: head (65.7%, n = 23), abdomen or pelvis (11.5%, n = 4), whole spine or aorta (5.7%, n = 2), lumbar spine (5.7%, n = 2), cervical spine (5.7%, n = 2), and heart (5.7%, n = 2). Patients with ‘3T MRI-conditional’ CIEDs underwent MRI scans of the head (55.6%, n = 54) followed by abdomen or pelvis (16.5%, n = 16), whole spine or aorta (11.3%, n = 11), lumbar spine (5.2%, n = 5), cervical spine (5.1%, n = 5), heart (2.1%, n = 2), thorax (2.1%, n = 2), extremity and joint (2.1%, n = 2). Abbreviations: MRI, magnetic resonance imaging; T, Tesla.
Adverse events
There were no deaths, arrhythmias, loss of capture, inappropriate anti-tachycardia therapies, electrical reset or lead or generator failure during or shortly after MRI scans (Graphical Abstract).
Generator and lead parameters before and after MRI
Device interrogation before and after MRI revealed several alterations in device R-wave and P-wave sensing as well as impedance (Table 2). Device programming was not adjusted in any patient due to these changes. Relevant P-wave and R-wave reduction of 50 and 25%, respectively, occurred in 3 (2.3%) cases, all in patients with ‘3T MRI-conditional’ CIEDs. After MRI in patients with ‘3T MRI-conditional’ CIEDs, right atrial lead impedance had changed by 50 Ω or more in 12 (14.8%) devices, right ventricular lead impedance in nine (9.7%) devices. Eight cases of altered lead impedances were registered among patients with ‘non-3T MRI-conditional’ CIEDs. High-voltage impedances demonstrated relevant alterations in 1 (12.5%) ‘non-3T MRI-conditional’ ICD and in 3 (33.3%) ‘3T MRI-conditional’ ICDs. Changes of at least 30% in lead impedance occurred only in one case: one atrial lead of a ‘non-3T MRI-conditional’ device demonstrated a drop of 312 Ω (45.7%) after MRI but was still within normal limits. Relevant changes in battery voltage were not noted. Stimulation thresholds did not exceed the predefined threshold of at least 0.5 V increase in any patient.
Table 2.
ȃDevice parameter changes and numbers exceeding predefined thresholds after magnetic resonance imaging (MRI) scans
| Prespecified threshold | Non-3T MRI conditional | 3T MRI conditional | |||
|---|---|---|---|---|---|
| no. (%) | Median change (IQR) | no. (%) | Median change (IQR) | ||
| Amplitudes | |||||
| ȃP-Wave amplitude | − 50% | 0 (0) | 0 (−9.3, 7.7) | 1 (1.19) | 0 (−3.1, 0) |
| ȃR-Wave amplitude | − 25% | 0 (0) | 0 (−1.0, 0) | 2 (2.41) | 0 (−2.7, 0) |
| Pacing lead thresholds | |||||
| ȃRA | + 0.5 V | 0 (0) | 0 (0, 0) | 0 (0) | 0 (0, 0) |
| ȃRV | + 0.5 V | 0 (0) | 0 (0, 0) | 0 (0) | 0 (0, 0) |
| ȃLV | + 0.5 V | 0 (0) | 0 (0, 0) | 0 (0) | 0 (0, 0) |
| Pacing lead impedance | |||||
| ȃRA | ± 50 Ω | 2 (8.3) | 0 (−10.0, 6.0) | 12 (14.8) | 0 (−7.5, 3.0) |
| ȃRV | ± 50 Ω | 6 (17.6) | 0 (−9.0, 1.5) | 9 (9.7) | 0 (−19, 0) |
| ȃLV | ± 50 Ω | 0 (0) | 0 (−21.5, 9.5) | 0 (0) | 0 (0, 9.5) |
| ȃHigh voltage (HV) | ± 3 Ω | 1 (12.5) | 0 (0, 0) | 3 (33.3) | 0 (−1.3, 0.3) |
| Battery | |||||
| ȃBattery voltage | − 0.04 V | 0 (0) | 0 (0, 0) | 0 (0) | 0 (0, 0) |
Abbreviations: HV, high voltage; IQR, interquartile range; LV, left ventricular; MRI, magnetic resonance imaging; RA, right atrial RV, right ventricular; T, Tesla.
Discussion
In this consecutive series, 3T MRI scans were safe in patients with ‘3T MRI-conditional’ and ‘non-3T MRI-conditional’ CIEDs. Although changes in lead parameters were detected in approximately one of five cases, no adverse events occurred. To our knowledge, this is the largest series reporting safety of 3T MRI in patients with CIEDs.
No adverse events during or after 3T MRI
This report extends the evidence on the safety of MRI scans in patients with implanted devices to 3T MRI scans. Our study included MRI scans of different body regions in patients with conventional pacemakers, a leadless pacemaker, ICDs, and cardiac resynchronization therapy (CRT) devices. Importantly, adverse events were not only absent in patients with ‘3T MRI-conditional’ CIEDs but also in those with ‘non-3T MRI-conditional’ CIEDs. A significant number of MRI scans were conducted in pacemaker-dependent patients. Recently, Bhuva et al.12 proposed magnetic resonance conditional labelling of all pacemaker and defibrillator lead and provided evidence for 1.5T MRI scans. Pushing the safety boundary, two hundred 1.5T MRI scans were safely performed even in patients with abandoned leads, which was considered an absolute contraindication for MRI before.13 Accordingly, two small studies from 2008 with a total of only 67 mainly brain 3T MRI scans in patients with non-MRI conditional CIEDs found no relevant safety issues either.14,15 In line with these results, our data suggest that 3T MRI of various body regions might be safe in most patients with CIEDs without abandoned or epicardial leads if a strict protocol is followed. However, even patients with MRI-conditional CIEDs still experience significant barriers in obtaining MRI scans as data from English hospitals demonstrated.2 Therefore, our findings add important safety data possibly reducing the reluctance of providers to offer 3T MRI scans to most CIED patients.
Impact of 3T MRI on functional CIED parameters
Changes in lead impedance, sensing, and thresholds after 1.5T MRI scans have been reported before in large registries and might be attributed to heating of the generator and leads.7,9,16 In line, we documented a significant proportion of cases with altered functional parameters, although 3T instead of 1.5T MRIs were conducted. Importantly, functional parameters did not relevantly differ between cases performed in patients with ‘3T MRI-conditional’ vs. ‘non-3T MRI-conditional’ CIEDs. Lead impedance was the parameter with the largest number of cases exceeding the predefined threshold of 50 Ω in 23% of cases. This number was doubled compared with results from a multicentre registry of 1.5T MRI scans including 1148 in both patients with MRI-conditional and non-MRI-conditional CIEDs: in approximately 10% of cases relevantly altered lead impedance was reported.12 In the large MagnaSafe registry of 1.5T MRI scans in non-MRI conditional CIEDs only 3.3% of cases exceeded a change in impedance of at least 50 Ω after MRI.9 Interestingly, the proportion of leads exceeding a change in impedance of 50 Ω did not relevantly differ between cases in patients with ‘3T MRI-conditional’ vs. ‘non-3T MRI-conditional’ CIEDs in our study. However, the clinical relevance of a 50 Ω change remains unclear, as e.g. normal lead impedance might vary by more than 100 Ω upon the usage of different pacing system analysers.17 Importantly, only one device exceeded the predefined threshold of ± 30% change of lead or shock impedance.
Relevant changes in sensing thresholds after MRI occurred in 2.3% of cases and only in ‘3T MRI-conditional’ CIEDs. Accordingly, Bhuva et al. report rates between 1 and 5% of cases exceeding the same predefined thresholds but with a slightly higher rate of cases in non-MRI conditional CIEDs. Possible reasons for those deviations in sensing after MRI include direct impact of local 3T MRI-induced myocardial heating at the lead tips. Alternatively, and maybe the reason why relevant changes only occurred in ‘3T MRI-conditional’ CIEDs in our study, the findings might be coincidental as a result of e.g. premature ventricular or atrial contractions.
Accordingly, it remains unclear if the observed changes in our study may be a result of 3T MRI and might have been attenuated if a 1.5T MRI scan would have been conducted instead. In the MagnaSafe registry of 1.5T MRI, most cases of altered device parameters returned to pre-MRI measurements at a 3-month follow-up.9 Therefore, although significant changes after 3T MRI scans were observed in some patients, these findings seem to be unlikely to translate into a negative clinical impact.
Limitations
Our study incorporates several limitations which have to be considered. First of all, we report real-world data from only a single centre and a limited number of patients with heterogeneous devices and leads. Body regions examined by MRI demonstrated a high heterogeneity including only a very limited number of thoracic examinations. Data collection was conducted retrospectively. Follow-up data exceeding the time period immediately after the MRI scan were not available, although patients with repetitive scans were included. The MRI scanners used were from two different manufacturers possibly limiting the external validity of our results.
Conclusions
In this study, 132 examinations on a 3T MRI scanner were safely conducted in patients with ‘3T MRI-conditional’ and ‘non-3T MRI- conditional’ CIEDs. Lead impedance and sensing were relevantly altered in a significant number of patients after MRI. These alterations were irrespective of being labelled as ‘3T MRI-conditional’ or ‘non-3T MRI-conditional’ by the manufacturer. However, no adverse events occurred. Therefore, warranting prospective validation in larger clinical cohorts, our data suggest that patients with cardiac pacemakers or defibrillators should not be denied access to clinically indicated 3T MRI.
Supplementary Material
Acknowledgements
None
Contributor Information
Nina Fluschnik, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr 52, 20251 Hamburg, Germany.
Enver Tahir, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20251 Hamburg, Germany.
Jennifer Erley, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20251 Hamburg, Germany.
Kai Müllerleile, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany.
Andreas Metzner, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany.
Jan-Per Wenzel, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr 52, 20251 Hamburg, Germany.
Helena Guerreiro, Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany.
Gerhard Adam, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20251 Hamburg, Germany.
Stefan Blankenberg, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr 52, 20251 Hamburg, Germany.
Paulus Kirchhof, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr 52, 20251 Hamburg, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK.
Tobias Tönnis, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany.
Julius Nikorowitsch, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr 52, 20251 Hamburg, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
No funding declared
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. OECD (2022), Magnetic resonance imaging (MRI) exams (indicator). doi: 10.1787/1d89353f-en(Accessed on 20 January 2022). OECD; 2021. [DOI]
- 2. Sabzevari K, Oldman J, Herrey AS, Moon JC, Kydd AC, Manisty C. Provision of magnetic resonance imaging for patients with ‘MR-conditional’ cardiac implantable electronic devices: an unmet clinical need. Europace 2017;19:425–31. [DOI] [PubMed] [Google Scholar]
- 3. Deshpande S, Kella D, Padmanabhan D. MRI In patients with cardiac implantable electronic devices: a comprehensive review. PACE—Pacing Clin Electrophysiol 2021;44(2):360–72. [DOI] [PubMed] [Google Scholar]
- 4. Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-tesla MR system. J Magn Reson Imaging 2002;16:721–32. [DOI] [PubMed] [Google Scholar]
- 5. Zbinden R, Wollmann C, Brachmann J, Michaelsen J, Steinwender C, Kovoor Pet al. . Clinical safety of the ProMRI implantable cardioverter-defibrillator systems during head and lower lumbar magnetic resonance imaging at 3 T: results of the ProMRI 3T ENHANCED master study. Europace 2019;21(11):1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilkoff BL, Bello D, Taborsky M, Vymazal J, Kanal E, Heuer Het al. . Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Hear Rhythm 2011;8:65–73. [DOI] [PubMed] [Google Scholar]
- 7. Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek Eet al. . Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med 2017;377:2555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munawar DA, Chan JEZ, Emami M, Kadhim K, Khokhar K, O’shea Cet al. . Magnetic resonance imaging in non-conditional pacemakers and implantable cardioverter-defibrillators: a systematic review and meta-analysis. Europace 2020;22(2):288–98. [DOI] [PubMed] [Google Scholar]
- 9. Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RWWet al. . Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med 2017;376(8):755–64. [DOI] [PubMed] [Google Scholar]
- 10. Wardlaw JM, Brindle W, Casado AM, Shuler K, Henderson M, Thomas Bet al. . A systematic review of the utility of 1.5 versus 3 tesla magnetic resonance brain imaging in clinical practice and research. Eur Radiol 2012;22:2295–303. [DOI] [PubMed] [Google Scholar]
- 11. Ning X, Li X, Fan X, Chen K, Hua W, Liu Zet al. . 3.0 T magnetic resonance imaging scanning on different body regions in patients with pacemakers. J Interv Card Electrophysiol 2021;61:545–50. [DOI] [PubMed] [Google Scholar]
- 12. Bhuva AN, Moralee R, Brunker T, Lascelles K, Cash L, Patel KPet al. . Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices. Eur Heart J 2021;0044:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaller RD, Brunker T, Riley MP, Marchlinski FE, Nazarian S, Litt H. Magnetic resonance imaging in patients with cardiac implantable electronic devices with abandoned leads. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naehle CP, Meyer C, Thomas D, Remerie S, Krautmacher C, Litt Het al. . Safety of brain 3-T MR imaging with transmit-receive head coil in patients with cardiac pacemakers: pilot prospective study with 51 Examinations1. Radiol Soc North Am 2008;249(3):991–1001. [DOI] [PubMed] [Google Scholar]
- 15. Gimbel JR. Magnetic resonance imaging of implantable cardiac rhythm devices at 3.0 tesla. Pacing Clin Electrophysiol John Wiley & Sons, Ltd2008;31:795–801. [DOI] [PubMed] [Google Scholar]
- 16. Bhuva AN, Moralee R, Moon JC, Manisty CH. Making MRI available for patients with cardiac implantable electronic devices: growing need and barriers to change. Eur Radiol Springer2020;30:1378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichiyanagi ÃH, Shiga ÃY, Ooiwa ÃN, Nishiki ÃK, Hara ÃK, Sato ÃY, et al. Variation in Lead Impedance according to Pacemaker Analyzing Systems.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.