Abstract
Aims
Premature ventricular contractions (PVCs) are a common form of arrhythmia associated with an unfavourable prognosis in patients with structural heart disease. However, the prognostic significance in absence of heart disease is debated. With this study, we aim to investigate whether subjects with PVC, without structural heart disease, have a worse prognosis than the general population.
Methods and results
Patients evaluated for PVC at a secondary care centre in Stockholm County from January 2010 to December 2016 were identified. We included patients without history of previous heart disease who had undergone echocardiography and exercise test with normal findings. Based on sex and age, we matched the PVC cohort to a four times bigger control group from the general population and compared the outcome in terms of mortality and cardiovascular morbidity during a median follow-up time of 5.2 years. We included 820 patients and 3,264 controls. Based on a non-inferiority analysis, the PVC group did not have a higher mortality than the control group (0.44, CI 0.27–0.72). Sensitivity analysis with propensity score matching confirmed this result.
Conclusions
PVC patients, who after thorough evaluation showed no signs of structural heart disease, did not have a worse prognosis when compared to an age- and sex- control group based on the general population.
Keywords: Premature, Ventricular, Contractions, Complex, Arrhythmia
What’s new?
Premature ventricular contractions are associated with a poor prognosis in individuals with structural heart disease, while their significance amongst healthy individuals is uncertain.
Patients with premature ventricular contractions who have undergone a medical evaluation including echocardiogram and exercise test without signs of structural heart disease do not have a worse prognosis than a sex- and age-matched control group.
This study can contribute to a better understanding of prognosis and evaluation of patients with premature ventricular contractions.
Background
Premature ventricular contractions (PVCs) are cardiac contractions due to depolarizations originating from any area of the ventricles and, though their reported prevalence ranges widely, between 1 and 40%, are common in clinical practice1–4. This large variation depends mainly on different ways of recording and storing electrocardiogram (ECG), different study populations and inclusion criteria. Nevertheless, it is reasonable to believe that PVCs are common in the general population.
The association between PVCs and a negative prognosis in presence of structural heart disease is well established.5–8 However, the evidence amongst subjects without obvious heart disease is much more uncertain; studies exploring the issue vary in design and present conflicting results.4,9,10
Meta-analyses of the prognostic implications of PVCs in individuals without pre-existing clinically apparent heart disease were missing until 2012 when Lee et al.9 published a study which was rapidly followed by Ataklte et al.10 The pooled results from both meta-analyses showed an association between PVC prevalence and poor prognostic outcome. However, the authors suggested an insufficient adjustment for possible confounders in some of the included studies.
More recent data continue to show conflicting results. Some studies suggest that asymptomatic or low-symptomatic PVCs have a benign natural course in subjects with normal ventricular function,11,12 and others show that PVCs increase the risk for structural heart disease in healthy individuals or on a population basis.13,14 While it is reasonable to believe that PVCs are mostly benign in healthy individuals, it is currently unclear whether PVC patients with normal findings at structural examinations may be reassured about their prognosis.
Consequently, the aim of this study is to investigate the association between PVCs and prognostic outcome among individuals without obvious structural heart disease.
Methods
We identified and included consecutive patients receiving a PVC diagnosis both as a primary and non-primary diagnosis by conducting a search in the database of three major hospitals in the Stockholm area. The search was limited to the period between January 2010 and December 2016 and was conducted using QlikView software (Radnor, USA) with ICD-10-code I49.3 (PVC). The patients were followed prospectively until 31 December 2018. The hospitals were secondary centres with a total catchment area of about 1.5 million people. The patients were referred from primary care, emergency care, or after prior evaluation of a cardiologist due to various reasons. After identification of patients through specific ICD-code (I49.3), the correctness of the PVC diagnosis was verified by an experienced cardiologist through scrutiny of their medical records. Even patients with asymptomatic PVCs, but in which PVCs had been noticed and evaluated, were included.
PVC patients with previous cardiovascular disease (CVD) (myocardial infarction, heart failure, sustained ventricular tachycardia, survived cardiac arrest, moderate or severe valvular dysfunction, previous cardiac surgery, or percutaneous revascularization) were not included. We linked PVC patients and controls to the National Patient Registry and the Swedish Prescribed Drugs Registry, both run by the National Board of health and Welfare. These data covered all in-patient hospital care and hospital associated out-patient care in Sweden for the years 1997–2018.
As a second step, we demanded that patients had been evaluated with ECG recording (at least 24-hour Holter monitoring or telemetry), echocardiography, and exercise test and that echocardiography and exercise test (or, when needed, completing tests to rule out coronary artery disease) had shown normal findings. Normal findings at echocardiography were defined as left ventricular ejection fraction equal or higher than 55%, absence of moderate to severe valvular heart disease, normal ventricular dimensions, and normal ventricular wall thickness. Normal exercise test was defined as absence of electrocardiographic finding indicating possible coronary artery disease, such as exercise-induced depression of the ST-interval or exercise-induced ventricular arrhythmia. In presence of these findings, we included patients only if an additional exam (myocardial scintigraphy, magnetic resonance tomography, or coronary angiography, according to clinical practice) ruled out coronary artery disease.
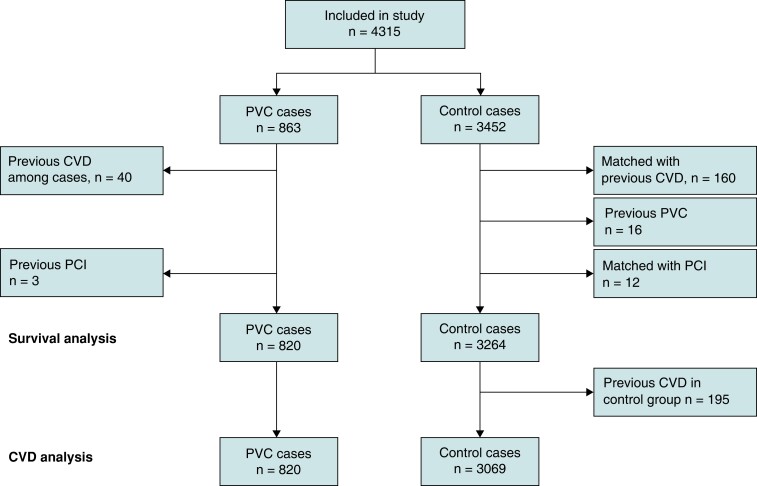
After linkage with the National Patient Registry, we found that 40 of the included patients had previously received a cardiovascular diagnosis, which led to their exclusion.
In summary, patients were included if they were confirmed to have PVCs and found to have no signs of structural heart disease after examination with echocardiography and exercise test. In a few cases, we also performed an additional examination with myocardial scintigraphy, magnetic resonance tomography or coronary angiography to exclude structural heart disease.
The controls were identified by Statistics Sweden (Statistiska Centralbyrån) from a random sample of the general Swedish population and matched on sex and age at diagnosis (each control was included the same date as the case of referral). Four controls were individually matched to each person in our PVC cohort. Amongst them, 16 persons had a previous PVC diagnosis and were therefore excluded.
This inclusion/selection process yielded a cohort of 820 PVC patients and 3 264 controls. This was the study base for survival analysis. For the analysis of other cardiovascular outcomes, we also excluded controls with a previous cardiovascular diagnosis, which left 820 PVC-patients and 3 069 controls for these analyses.
The whole process is summarized with a flow-chart in Figure 1.
Figure 1.
Graphical summary of the inclusion and selection process .
Outcomes and statistics
Our hypothesis was that a PVC population, in which structural heart disease was thoroughly excluded, did not have a worse prognosis than the general population. Following this, we ran a power calculation based on the hypothesis of non-inferiority. The calculation indicated that the study would be able to identify a mortality risk among cases with 90% power if it was more than two times higher than among the controls. The assumption was based on 700 patients during a median follow-up of 3.5 years.
The considered outcomes were total mortality and cardiovascular morbidity. Cardiovascular morbidity was defined as diagnosis of ischaemic heart disease (International Classification of Diseases-10 codes I20-I25), cardiomyopathy (I42, I43), cardiac arrest (I46), ventricular tachycardia (I47.2), ventricular fibrillation (I49.0), and heart failure (I50).
Continuous variables are presented as medians and quartiles while categorical variables are presented as counts and proportions. For outcome analyses, we used a Cox proportional hazard model. For each outcome, three regressions models were used. Model 1 was only adjusted for the matching variables (age and sex); model 2 added adjustment for hypertension, cerebrovascular disease, diabetes, malignancy, hyperlipidaemia, and atrial fibrillation; model 3 further adjusted for previous medication use (beta blockers, diuretics, and calcium channels blockers). The proportional hazard assumption was tested by proportional hazard test and visual inspection. When a covariate violated the proportional hazard assumption, we stratified the analysis on that variable. Results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The secondary analysis on cardiovascular morbidity was calculated with Fine-Grey sub-distribution hazard regression with mortality as competing risk.
Data management and statistical analyses were performed in R version 4.0.3 (R Foundation, Vienna, Austria). The study is registered at ClinicalTrials.gov with identifier NCT03370679.
Patients and public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Ethics
The study was approved by the regional ethics committee (diary number 2014/670–31/4) and complied with the Declaration of Helsinki.
Results
Baseline characteristics are presented in Table 1. Compared to controls, the PVC cohort had a higher share of individuals with previous cancer (13.7% vs. 9.7%), hypertension (21.1% vs. 16.4%), atrial fibrillation (6.1% vs. 4.2%), and hyperlipidaemia (8.0% vs. 4.9%).
Table 1.
Baseline characteristics of the primary analytic sample
| PVC cohort (n = 820) | Controls (n = 3 264) | P | SMD | |
|---|---|---|---|---|
| Age, median (IQR) | 59.0 (45.0–70.0) | 59.0 (45.0–70.0) | 0.983 | <0.001 |
| Men, n (%) | 347 (42.3) | 1376 (42.2) | 0.965 | 0.003 |
| Ischaemic heart disease, n (%) | 0 (0.0) | 155 (4.7) | <0.001 | 0.316 |
| Heart failure, n (%) | 0 (0.0) | 60 (1.8) | <0.001 | 0.194 |
| Cancer, n (%) | 112 (13.7) | 317 (9.7) | 0.001 | 0.123 |
| Hypertension, n (%) | 173 (21.1) | 535 (16.4) | 0.002 | 0.121 |
| Diabetes, n (%) | 39 (4.8) | 212 (6.5) | 0.076 | 0.076 |
| Cerebrovascular disease, n (%) | 23 (2.8) | 128 (3.9) | 0.158 | 0.062 |
| Hyperlipidaemia, n (%) | 66 (8.0) | 161 (4.9) | 0.001 | 0.127 |
| Atrial fibrillation, n (%) | 50 (6.1) | 137 (4.2) | 0.025 | 0.086 |
| Beta blockers, n (%) | 410 (50.0) | 760 (23.3) | <0.001 | 0.577 |
| Anticoagulants and platelet inhibitors, n (%) | 238 (29.0) | 752 (23.0) | <0.001 | 0.137 |
| Antiarrhythmic drug, class 1, n (%) | 14 (1.7) | 9 (0.3) | <0.001 | 0.145 |
| Antiarrhythmic drug, class 3, n (%) | 11 (1.3) | 3 (0.1) | <0.001 | 0.149 |
| Calcium channel blockers, n (%) | 216 (26.3) | 611 (18.7) | <0.001 | 0.183 |
| Diuretics, n (%) | 311 (37.9) | 983 (30.1) | <0.001 | 0.165 |
| Digitalis, n (%) | 6 (0.7) | 27 (0.8) | 0.956 | 0.011 |
| ACE inhibitors, n (%) | 159 (19.4) | 602 (18.4) | 0.567 | 0.024 |
| Angiotensin receptor blockers, n (%) | 108 (13.2) | 401 (12.3) | 0.531 | 0.027 |
In the secondary analytic sample, controls with previous cardiovascular diagnosis were excluded. The sample consisted of 820 PVC cases and 3 069 controls. Baseline characteristics are presented in Table 2.
Table 2.
Baseline characteristics of the secondary analytic sample
| PVC cohort (N = 820) | Controls (N = 3069) | P | SMD | |
|---|---|---|---|---|
| Age, median (IQR) | 59.0 (45.0–70.0) | 59.0 (45.0–69.0) | 0.590 | 0.018 |
| Men, n (%) | 347 (42.3) | 1249 (40.7) | 0.425 | 0.033 |
| Ischaemic heart disease, n (%) | 0 (0.0) | 0 (0.0) | NA | <0.001 |
| Heart Failure, n (%) | 0 (0.0) | 0 (0.0) | NA | <0.001 |
| Cancer, n (%) | 112 (13.7) | 265 (8.6) | <0.001 | 0.160 |
| Hypertension, n (%) | 173 (21.1) | 398 (13.0) | <0.001 | 0.218 |
| Diabetes, n (%) | 39 (4.8) | 161 (5.2) | 0.635 | 0.022 |
| Cerebrovascular disease, n (%) | 23 (2.8) | 92 (3.0) | 0.862 | 0.011 |
| Hyperlipidaemia, n (%) | 66 (8.0) | 96 (3.1) | <0.001 | 0.215 |
| Atrial Fibrillation, n (%) | 50 (6.1) | 87 (2.8) | <0.001 | 0.158 |
| Beta Blockers, n (%) | 410 (50.0) | 585 (19.1) | <0.001 | 0.688 |
| Anticoagulants and platelet-inhibitors, n (%) | 238 (29.0) | 570 (18.6) | <0.001 | 0.247 |
| Antiarrhythmic drug, class 1, n (%) | 14 (1.7) | 8 (0.3) | <0.001 | 0.147 |
| Antiarrhythmic drug, class 3, n (%) | 11 (1.3) | 1 (0.0) | <0.001 | 0.159 |
| Calcium channel blockers, n (%) | 216 (26.3) | 520 (16.9) | <0.001 | 0.230 |
| Diuretics, n (%) | 311 (37.9) | 865 (28.2) | <0.001 | 0.208 |
| Digitalis, n (%) | 6 (0.7) | 8 (0.3) | 0.094 | 0.067 |
| ACE inhibitors, n (%) | 159 (19.4) | 472 (15.4) | 0.007 | 0.106 |
| Angiotensin receptor blockers, n (%) | 108 (13.2) | 335 (10.9) | 0.081 | 0.069 |
Overall mortality
During a median follow-up time of 5.2 years [interquartile range (IQR) 3.9–6.4 years], 24 patients in the PVC group died, resulting in a mortality rate of 5.7/1 000 person-years. In the control group, 194 patients died, resulting in a mortality rate of 11.9/1 000 person years. The causes of death in the two groups are summarized in Table 3.
Table 3.
Causes of death in PVC group and controls according to ICD classification
| PVC patients (n) | Controls (n) | |
|---|---|---|
| Infectious diseases | 0 | 5 |
| Tumours | 12 | 55 |
| Endocrine and metabolic diseases | 0 | 5 |
| Psychiatric diseases | 0 | 10 |
| Neurological diseases | 1 | 12 |
| CVDs | 9 | 69 |
| Respiratory diseases | 1 | 16 |
| Diseases of the digestive system | 1 | 5 |
| External causes | 0 | 15 |
| Other causes | 0 | 2 |
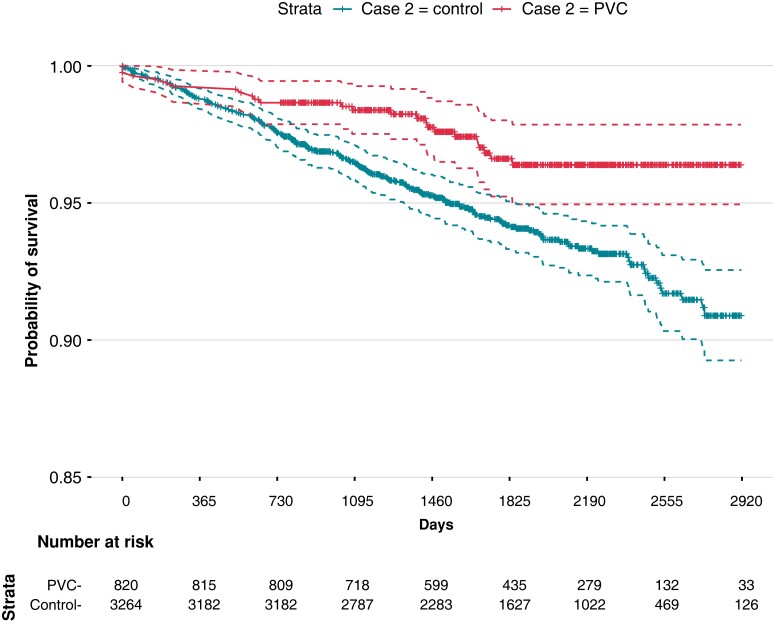
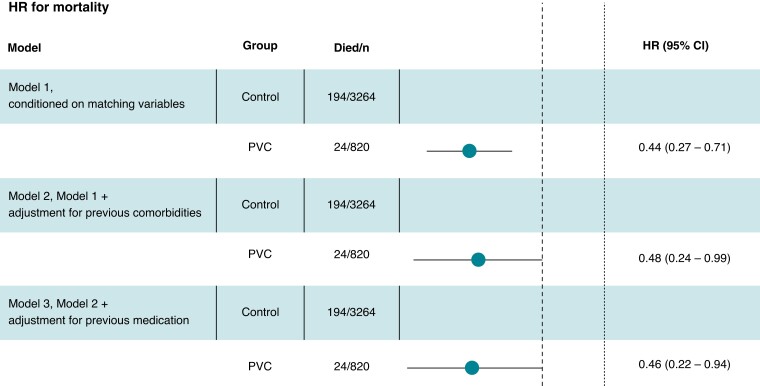
The conditional HR for overall mortality in the PVC group was 0.44 (CI 0.27–0.71). The control patients started to diverge from the PVC patients after 1 year of follow-up (Figure 2). Adjustment for potential confounders did not affect the association between PVC and survival with similar HR in all models, as shown in Figure 3.
Figure 2.
Kaplan–Meier plot for survival probability in PVC and control groups.
Figure 3.
Hazard ratio for overall mortality in the PVC group compared to control group according to different conditioning models. The dotted line corresponds to the non-inferiority limit (HR = 2.0).
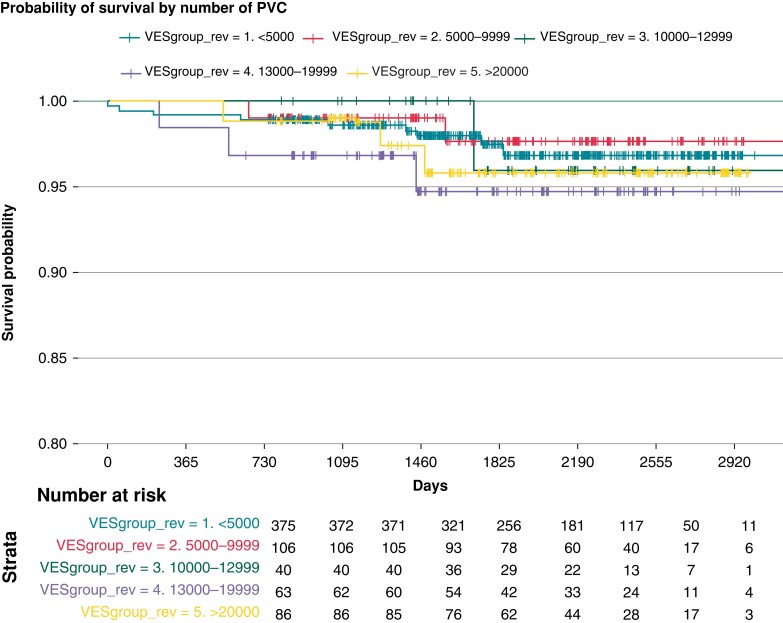
We had access to information about the PVC burden in 670 patients and classified them according to the number of PVCs per day. The data were obtained through inpatient or outpatient registration, and 76.3% had at least 1 000 PVCs/day. We stratified the PVC patients according to PVC burden. Survival analysis could not show any difference in mortality between the strata. The results are summarized in Figure 4.
Figure 4.
Survival probability according to PVC-burden in 670 patients in which data about number of PVCs/day were available.
Cardiovascular morbidity
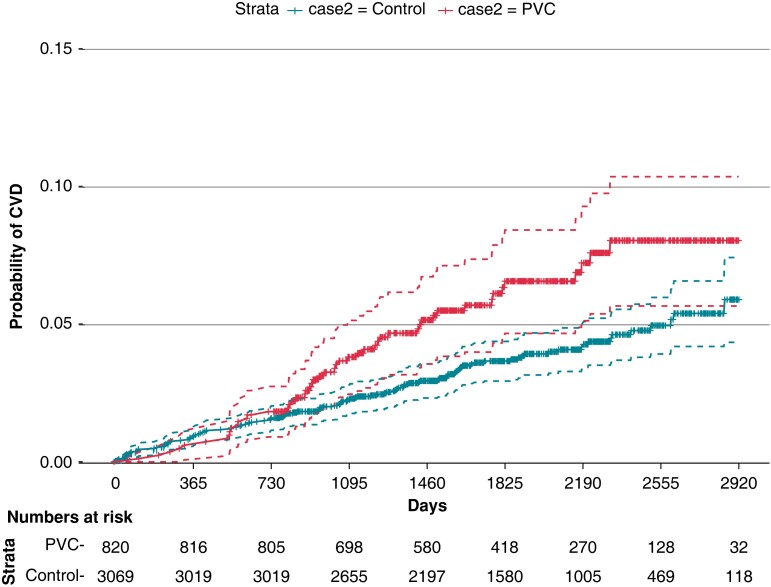
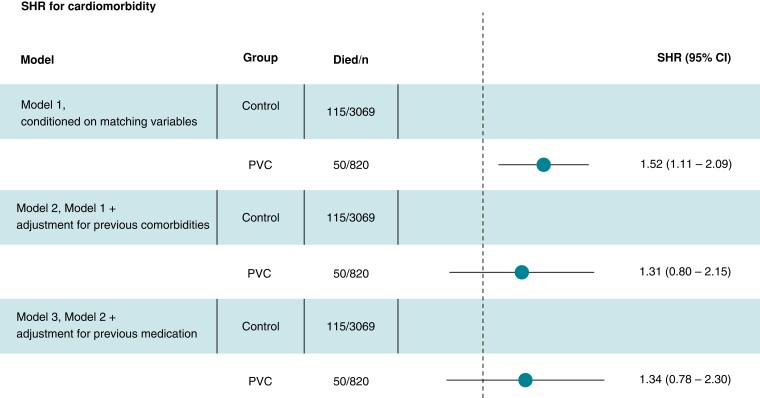
Registry data allowed us to identify controls with previous CVD. After exclusion of these individuals the subset consisted of 820 PVC patients and 3 069 controls (for details, see Figure 1). The morbidity rate was 12.1/1 000 person-years among PVC patients (median follow-up time of 5.1 years, IQR 3.8–6.4) and 7.4/1 000 person-years among controls (follow-up time 5.1 years, IQR 3.8–6.3). The survival curves start to diverge after approximately 1.5 years of follow-up (Figure 5). The conditional HR for PVC patients was 1.53 (CI 1.06–2.21). Addition of comorbidity data modified the HR to 1.31 (CI 0.72–2.37). In the third model, where previous medications were added, the HR was 1.34 (CI 0.71–2.53), as shown in Figure 6.
Figure 5.
Kaplan–Meier plot for probability of receiving a cardiovascular diagnosis in PVC and control groups.
Figure 6.
Sub-distribution hazard ratio (fine-grey) for cardiovascular morbidity in the PVC group compared to control group according to different conditioning models.
A sensitivity analysis after propensity score matching showed similar results as in the main analysis regarding all-cause mortality (HR 0.32, CI 0.20–0.50). For cardiovascular morbidity, the propensity-score-matched analysis did not show a significant difference between cases and controls (HR 1.37, CI 0.90–2.09).
Discussion
Overall mortality
It is well established that PVCs are a risk marker in patients with structural heart disease, while their prognostic significance in healthy subjects is debated. The topic is important because PVCs are common in clinical praxis. The 1985 paper by Kennedy et al.4 has been a landmark study for many years. It was based on a mean follow-up of 6.5 years and included 70 asymptomatic healthy subjects with ventricular ectopy, who did not show increased risk for death. As mentioned before, following studies in this topic have shown conflicting results. In our study, including patients with frequent PVCs but without detectable structural heart disease who were compared to an age- and sex-matched sample from the general Swedish population, the prognosis for the PVC group was not worse than for the control group.
Previous meta-analysis has highlighted the risk of insufficient examination leading to inclusion of individuals with undetected structural heart disease.9,10 More recently, Dukes et al.15 found that a higher frequency of PVCs was associated with increased risk for heart failure and death. However, all participants in this study were 65 years of age or older, and exercise test was not included in initial evaluation. This is why it is possible that a significant number of individuals with silent coronary artery disease were included.
In our study, the prognosis for PVC patients with normal echocardiogram and exercise test was not worse than the one for the control group. The evaluation routine was compliant to current ESC guidelines from 2015, in which ECG monitoring, exercise test, and echocardiography receive a class I recommendation in non-invasive evaluation of patients with ventricular arrhythmia.16 In more recent papers, the role of exercise test was toned down, and authors suggested evaluating with cardiac MRI in special situations.14,17
There were differences between the two groups at baseline. In the PVC group, no patients had heart failure or ischaemic heart disease, according to study design, while the prevalence in the control group was, respectively, 1.8 and 6%. On the other hand, the PVC group had a higher prevalence of cancer, hypertension, diabetes mellitus, hyperlipidaemia, and atrial fibrillation. However, after adjusting for comorbidities, the PVC group did not have a higher mortality than controls during the follow-up time.
Cardiovascular morbidity
Previous study has shown a link between PVCs and increased risk for cardiovascular morbidity,13,15 although they have not always assessed included patients with echocardiography and exercise test and thus potentially have included subjects with structural heart diseases.13 In our study, when testing for cardiovascular morbidity, we excluded controls with previous heart disease. The subsequent analysis showed that the PVC cohort had a higher risk for receiving a cardiovascular diagnosis during follow-up. As this may contrast with the mortality results, a reasonable explanation for the discrepancy is that PVC subjects were often followed by a cardiologist, which could lead to both a higher and/or earlier detection of cardiovascular conditions during follow-up. Another possible contributing factor is the PVC cohort being identified in three hospitals in the Stockholm area. As controls were Swedish citizens from anywhere in the country, they possibly had different heath care access possibilities and contact patterns than the Stockholm-based cohort. However, after adjusting for previous diseases and medications, we could state no statistically significant difference in cardiovascular morbidity between the two groups.
PVC burden
A relation between frequency of PVC and clinical outcome, including a possible cut-off value in PVC/day has been discussed in several studies.15,18,19 In the study by Dukes et al.,15 a correlation was found between number of PVCs, decline in left ventricular function and mortality. Baman et al.18 reported that among patients who had been referred for PVC-ablation, those with reduced ejection fraction, had higher PVC-burden than those with normal left ventricular function. In the study by Lin,20 a cut off PVC frequency of 12/day for increased mortality risk was identified, while Lie identified the optimal cut off at 8% of total beats. In our study a sub-group analysis after stratification of the PVC group according to PVC burden did not show any difference in mortality. However, it is possible that we did not have sufficient power to show such a difference, albeit we included more patients than some of the studies above.
Control group
When designing this study, we chose a control group, matched for sex and age, from the general population. Including control subjects with previous CVD would mean that the control group would better represent the general population and potentially give the study a high external validity. However, it could also lead to a falsely favourable outcome for the PVC group given that it is not always possible to completely adjust for pre-existing conditions during data analysis. On the contrary, comparing the PVC group to a control group without cardiovascular diagnosis could decrease the risk for confounding but put the results farther away from ‘real life’ settings. We opted to include controls with heart disease in our survival analysis and adjust for pre-existing conditions. Adjustment did not alter the result, showing an overall good prognosis for the PVC group. When running the Cox regression for cardiovascular morbidity we could state that the requirement for proportional hazard was not fulfilled, as a proportion of the sample already had previous CVD diagnosis which led to violation of the proportional hazard assumption. Excluding controls with CVD handled this violation. The following regression analysis showed a higher incidence of CVD for the PVC group. However, sub-distribution HR for morbidity did not significantly differ after adjustment for previous comorbidities and medication, and sensitivity analysis with propensity score matching confirmed the good general survival prognosis for the PVC group while showing no differences in cardiovascular morbidity.
Clinical evaluation
Our results suggest that individuals with PVCs without signs of CVD after a diagnostic work-up including echocardiogram and exercise test seem to have a good prognosis. These findings are consistent with results and recommendations from other studies.11,21 It is possible that previous studies showing a negative prognostic effect of PVCs in healthy individuals did not have sufficiently strict exclusion criteria to exclude structural heart disease, several of them identifying structural heart disease only by history and physical examination. This was one of the conclusions from previously mentioned meta-analysis.9 Moreover, the authors of this paper examined the quality of the included studies. The results of this analysis showed that the increase in Odds Ratio for PVC patients was less pronounced in studies with higher quality, potentially meaning that a study that managed to identify and exclude patients with structural heart diseases would show a favourable prognosis for the included PVC patients.
Limitations
This study is based on a cohort of PVC patients identified at secondary health care centres. The population studied represented both symptomatic and asymptomatic patients with PVC and normal findings at exercise tests and echocardiography. It does not necessarily represent a broader population of PVC patients received care at primary centres or who did not seek care at all.
We did not have complete access to the included individuals’ demographic data, so we are not able to state whether socio-economic status could by any means affect the outcome. As stated in the Discussion chapter, the PVC cases were identified in three hospitals of the Stockholm area of Sweden while the control group potentially consisted of individuals from the entire country.
The accuracy of the PVC diagnosis was verified in the PVC cohort at inclusion. While identification through ICD code can be seen as a limitation and we cannot exclude that there were controls with PVCs (but without an ICD-diagnosis) there is a large set of evidence that suggests ICD-codes have a high specificity, sensitivity, and positive predictive value for CVDs.22–27
Albeit the waiting time from referral to specialist evaluation is usually short (less than 30 days), we do not have information about deaths on the waiting list and the possible positive selection effect they ensue. However, while this is a general problem with hospital-based data, it is reasonable to believe that very few, if any, deaths occurred while on waiting list.
According to ESC guidelines from 2015, we evaluated all patients in our PVC cohort with echocardiography and exercise test. Recently the role of exercise test in evaluation of cardiac ischaemia has been questioned, with imaging methods such as resonance imaging and computed tomography being the alternative. However, none of the included patients had current history of angina, and exercise test must still be considered a viable tool for risk assessment of patients with ventricular arrhythmia.
Conclusions
PVC patients who have had a thorough evaluation that ruled out structural heart disease should be given a reassuring message regarding their mid-term prognosis. More studies are needed to fully comprehend the overall impact of PVCs and identify potentially vulnerable subgroups of patients.
Supplementary Material
Contributor Information
Raffaele Scorza, Department of Clinical Sciences, Karolinska Institutet, Cardiovascular Unit, Danderyd University Hospital, Stockholm, Sweden.
Martin Jonsson, Department for Clinical Science and Education, Karolinska Institutet, Södersjukhuset, Center for Resuscitation Science, Stockholm, Sweden.
Leif Friberg, Department of Clinical Sciences, Karolinska Institutet, Cardiovascular Unit, Danderyd University Hospital, Stockholm, Sweden.
Mårten Rosenqvist, Department of Clinical Sciences, Karolinska Institutet, Cardiovascular Unit, Danderyd University Hospital, Stockholm, Sweden.
Viveka Frykman, Department of Clinical Sciences, Karolinska Institutet, Cardiovascular Unit, Danderyd University Hospital, Stockholm, Sweden.
Funding
This study was supported by grants from Stockholms Läns Landsting, Stiftelsen Hjärta and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden).
Data availability
The data that support the findings of this study are available from the corresponding author, RS, upon reasonable request.
References
- 1. Cheriyath P, He F, Peters I, Li X, Alagona P Jr, Wu C. et al. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the atherosclerosis risk in communities ARIC study). Am J Cardiol 2011;107:151–5. [DOI] [PubMed] [Google Scholar]
- 2. Abdalla ISH, Prineas RJ Neaton JD, Jacobs DR Crow RS.. Relation between ventricular premature complexes and sudden cardiac death in apparently healthy-men. Am J Cardiol 1987;60:1036–42. [DOI] [PubMed] [Google Scholar]
- 3. Yang J, Dudum R, Mandyam MC, Marcus GM. Characteristics of unselected high-burden premature ventricular contraction patients. Pacing Clin Electrophysiol 2014;37:1671–80. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy HL Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med 1985;312:193–7. [DOI] [PubMed] [Google Scholar]
- 5. Maggioni AP, Zuanetti G, Franzosi MG, Rovelli F, Santoro E, Staszewsky L et al. Prevalence and prognostic-significance of ventricular arrhythmias after acute myocardial-infarction in the fibrinolytic era - GISSI-2 results. Circulation 1993;87:312–22. [DOI] [PubMed] [Google Scholar]
- 6. Bigger JT, Weld FM. Analysis of prognostic-significance of ventricular arrhythmias after myocardial-infarction - shortcomings of lown grading system. Br Heart J 1981;45:717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bikkina M, Larson MG, Levy D. Asymptomatic ventricular arrhythmias and mortality risk in subjects with left-ventricular hypertrophy. J Am Coll Cardiol 1993;22:1111–6. [DOI] [PubMed] [Google Scholar]
- 8. Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death - epidemiology, transient risk, and intervention assessment. Ann Intern Med 1993;119:1187–97. [DOI] [PubMed] [Google Scholar]
- 9. Lee V, Hemingway H, Harb R, Crake T, Lambiase P. The prognostic significance of premature ventricular complexes in adults without clinically apparent heart disease: a meta-analysis and systematic review. Heart 2012;98:1290–8. [DOI] [PubMed] [Google Scholar]
- 10. Ataklte F, et al. Erqou S, Laukkanen J, Kaptoge S. Meta-Analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol 2013;112:1263–70. [DOI] [PubMed] [Google Scholar]
- 11. Lee AKY, Andrade J, Hawkins NM, Alexander G, Bennett MT, Chakrabarti S. et al. Outcomes of untreated frequent premature ventricular complexes with Normal left ventricular function. Heart 2019;105:1408–13. [DOI] [PubMed] [Google Scholar]
- 12. Nomura Y, Seki S, Hazeki D, Ueno K, Tanaka Y, Masuda K. et al. Risk factors for development of ventricular tachycardia in patients with ventricular premature contraction with a structurally normal heart. J Arrhythm 2020;36:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YG, Choi YY, Han KD, Min KJ, Choi HY, Shim J. et al. Premature ventricular contraction increases the risk of heart failure and ventricular tachyarrhythmia. Sci Rep. 2021 Jun 16;11(1):12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee A, Walters TE, Gerstenfeld EP, Haqqani HM. Frequent ventricular ectopy: implications and outcomes. Heart Lung Circ 2019;28:178–90. [DOI] [PubMed] [Google Scholar]
- 15. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS. et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Priori SG, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM et al. . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793. [DOI] [PubMed] [Google Scholar]
- 17. Marcus GM. Evaluation and management of premature ventricular complexes. Circulation 2020;141:1404–18. [DOI] [PubMed] [Google Scholar]
- 18. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C,. et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865–9. [DOI] [PubMed] [Google Scholar]
- 19. Parreira L, Marinheiro R, Amador P, Mesquita D, Farinha J, Lopes A. et al. Frequent premature ventricular contractions. Association of burden and complexity with prognosis according to the presence of structural heart disease. Ann Noninvasive Electrocardiol 2021;26(1):e12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin CY, Chang SL, Lin YJ, Chen YY, Lo LW, Hu YF. et al. An observational study on the effect of premature ventricular complex burden on long-term outcome. Medicine 2017;96:e5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brienesse SC, Sverdlov AL. Premature ventricular complexes: benign, pathogenic or just a marker of myocardial disease? Heart Lung Circ 2019;28:351–3. [DOI] [PubMed] [Google Scholar]
- 22. Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf 2012;21:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: A systematic review. Plos One 2014Mar 28;9(3):e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying ventricular arrhythmias using administrative and claims data. Pharmacoepidemiol Drug Saf 2012;21:148–53. [DOI] [PubMed] [Google Scholar]
- 25. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, Blacks, and Whites. Circulation 2013;128:2470–7. [DOI] [PubMed] [Google Scholar]
- 27. Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I et al. . Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RS, upon reasonable request.