Abstract
Two intergenic spacers cpDNA barcoding regions were used to assess the genetic diversity and phylogenetic structure of a collection of 25 Prunus accessions. The trnH-psbA and trnL-trnF intergenic spacers were able to distinguish and identify only four Prunus species. The average aligned length was 316–352 bp and 701–756 bp for trnH-psbA and trnL-trnF, respectively. The overall evolutionary divergence was higher in trnH-psbA than trnL-trnF. The transition/transversion bias (R) recorded as 0.59 in trnL-trnF and 0.89 in trnH-psbA. The number of invariable sites, nucleotide diversity (Pi), and the average number of nucleotide differences (k) was higher in the trnH-psbA region. The trnL-trnF records was above the other region in the number of variable sites, number of singleton variable sites, and the parsimony informative sites. Phylogenetic relationships among the 25 accessions of Prunus species were investigated. Most of the different Prunus species clustered in a homogenized distribution in both regions, except for the plum (P. domestica) accession (African Rose) was assigned with the peach (P. persica) accessions. The two intergenic cpDNA trnH-psbA and trnL-trnF were able to distinguish and identify the four Prunus species accessions.
Keywords: DNA barcode, Prunus species, Prunus armanica, Prunus persica, Prunus persica var. nucipersica, Prunus domestica L., P. salicina
Introduction
The first crucial step in conserving plant genetic resources is the correct identification of the targeted species. A potential method to meet this identification is DNA barcoding, which is the identification of species by a short universal DNA sequence that exhibits a sufficient level of variation to discriminate among species [1, 2]. The emergence of DNA barcoding has had a positive impact on biodiversity classification and identification [3]. The primary goals of DNA barcoding technique are species identification of known specimens and discovery of overlooked species for enhancing taxonomy for the benefit of science and society [4]. Using DNA barcoding, a species can be identified from a tiny amount of tissue, seeds, or fragmentary materials [5]. After an extensive inventory of gene regions in the mitochondrial, plastid, and nuclear genomes of plants, four primary gene regions (rbcL, matK, trnH-psbA, and ITS) have generally been agreed upon as the standard DNA barcodes of choice in most applications for plants [6–9]. Recently, research interest has spread through the DNA barcoding for economically important species of plants [10].
Prunus (or stone fruits) belongs to family Rosacea, is an economically important genus with approximately 200 species, grown in moderate regions [11]. The most common important cultivated species are; european plum (P. domestica L.), japanese plum (P. salicina Lindl.), sweet cherry (P. avium L.), sour cherry (P. cerasus L.), peach (P. persica (L.) Batsch), nectarine (P. persica var. nucipersica (Suckow) C. K. Schneid.), almond (P. dulcis (Mill.) D. A. Webb.), and apricot (P. armeniaca L.) [12]. Prunus persica includes peach and nectarine. The nectarine (P. perscica var. nucipersica) is a mutant strain of peach (P. persica), with special unique fruit characteristics [13]. Prunus genome is relatively small with about 250–300 Mbp [14]. The basic number of Prunus chromosomes is (x = 8). Almond (P. dulcis), peach (P. persica), apricot (P. armeniaca L.), sweet cherry (P. avium L.), Japanese plum (P. salicina Lindl.) are diploids (2n = 2 × = 16). Unless the European plum (P. domestica L.) is hexaploidy (2n = 6 × = 48), it is supposed resulted from the tetraploid species (P. spinosa L.) and the diploid species (P. cerasifera Ehrh.) [15]. The correct identification and characterization of plant genetic resources (PGR) is important for germplasm utilization [16]. Using modern DNA-based markers is necessary for gene bank management [17].
The overall goal of this study is to assess the genetic diversity and phylogenetic structure of a collection of 25 Prunus accessions grown in Egypt conserved in the National Gene Bank, utilizing two intergenic DNA barcoding regions (trnH-psbA and trnL-trnF).
Materials and Methods
Plant Materials
The current research conducted using 25 Prunus genotypes belonging to 5 species grown in Egypt, collected from different locations. The twenty-five Prunus accessions were collected, conserved, and maintained in the gene bank greenhouses. The samples used in this study are demonstrated in Table 1.
Table 1.
Prunus species and cultivar/variety name of Prunus accession samples
| Prunus species | Accessions sample name |
|---|---|
| Almond (Prunus dulcis (Mill.)) | Sweet almond, old-local cultivar “Hash” |
| Sweet almond, old-local cultivar “Adm” | |
| Sweet almond, local variety | |
| Bitter almond, local variety | |
| Apricot (Prunus armeniaca L.) | Old-local variety “Ammar01-clone1” |
| Old-local variety “Ammar02-clone2” | |
| Commercial variety “Hammway” | |
| Commercial local variety “El-Amal” | |
| Commercial local variety “Hayed” | |
| Commercial variety “Canino” | |
| Peach (Prunus persica (L.) Batsch) | Old-local variety “Balady” |
| Old-local variety “Mit Ghamar” | |
| Commercial variety “Early Grand” | |
| Commercial variety “Early Swelling” | |
| Commercial variety “Desert Red” | |
| Commercial variety “Florida Prince” | |
| Nectarine (Prunus persica var. nucipersica (Suckow) C. K. Schneid.) | Commercial variety |
| European plum (Prunus domestica L.) | Old-local variety “Succari” |
| Old-local variety “Bokra” | |
| Commercial variety “Hollywood” | |
| Commercial variety “Santa Rosa” | |
| Commercial variety “Pioneer” | |
| Commercial variety “African Rose” | |
| Commercial variety “English” | |
| Japanese plum (Prunus salicina Lindl.) | Commercial variety “Japanese” |
DNA Isolation, PCR Thermocycling Profile of Prunus DNA Barcoding Identification
The genomic DNA (gDNA) of the samples was extracted using Qiagen DNeasy kit (cat No. 69104). The DNA was quantified using NanoDrop™ OneC (cat No. 840-329700) and adjusted to 50 ng/µl and used in the reactions. The twenty-five different Prunus samples were identified using two chloroplast DNA intergenic regions (trnH-psbA and trnL-trnF). The PCR reaction amplifications were performed on BioRad™ T100 thermal Cycler (No. 1861096), in 25 µl reaction volume, containing 2X of EmeraldAmp® MAX PCR mix (RR320A), 50 ng gDNA, and 20pMol for each primer. The primer sequence and thermocycling profile of PCR are demonstrated in Table 2.
Table 2.
DNA chloroplast region, primer name and sequence, PCR thermocycling profile, and reference
| DNA chloroplast region | Primer forward name and sequence | Primer reverse name and sequence | PCR thermocycling profile | Reference |
|---|---|---|---|---|
| trnH-psbA | trnHGUG: CGCGCATGGTGGATTCACAATCC | psbA: GTTATGCATGAACGTAATGCTC | 94 °C for 3 min, 34 cycles (94 °C for 30 s, 50 °C for 2 min, 72 °C for 5 min), and final extension for 5 min | [18] |
| trnL-trnF | trn-c: CGAAATCGGTAGACGCTACG | trn-f: ATTTGAACTGGTGACACGAG | 94 °C for 3 min, 34 cycles (94 °C for 30 s, 61.2 °C for 2 min, 72 °C for 5 min), and final extension for 5 min | [19] |
DNA sequencing was carried out by Potsdam, Institute of Biochemistry and Biology (Potsdam, Germany) using an ABI sequencer. All sequences were submitted to NCBI GenBank, USA. GenBank provided accession numbers for the nucleotide sequences of each accession for each of the two loci, as demonstrated in Table 3.
Table 3.
Prunus accessions name, Genbank accession numbers for the for the two barcoding loci (trnH-psbA and trnL-trnF)
| No | Prunus species | Prunus accessions name | NCBI Genbank accession number | |
|---|---|---|---|---|
| trnH-psbA | trnL-trnF | |||
| 1 | Almond (P. dulcis (Mill.)) | Sweet almond, old-local cultivar “Hash” | OM328809 | OM720097 |
| 2 | Sweet almond, old-local cultivar “Adm” | OM328810 | OM720098 | |
| 3 | Sweet almond, local variety | OM328811 | OM720099 | |
| 4 | Bitter almond, local variety | OM328812 | OM720100 | |
| 5 | Apricot (P. armeniaca L.) | Old-local variety “Ammar01-clone 1” | OM416742 | OM720101 |
| 6 | Old-local variety “Ammar02-clone 2” | OM416743 | OM720102 | |
| 7 | Commercial variety “Hammway” | OM416744 | OM720103 | |
| 8 | Commercial local variety “El-Amal” | OM416745 | OM720104 | |
| 9 | Commercial local variety “Hayed” | OM416746 | OM720105 | |
| 10 | Commercial variety “Canino” | OM416747 | OM720097 | |
| 11 | Peach (P. persica (L.) Batsch) | Old-local variety “Balady” | OM416748 | OM720106 |
| 12 | Old-local variety “Mit Ghamar” | OM416749 | OM720107 | |
| 13 | Commercial variety “Early Grand” | OM416750 | OM720108 | |
| 14 | Commercial variety “Early Swelling” | OM416751 | OM720109 | |
| 15 | Commercial variety “Desert Red” | OM416752 | OM720110 | |
| 16 | Commercial variety “Florida Prince” | OM416753 | OM720111 | |
| 17 | Nectarine (P. persica var. nucipersica (Suckow) C. K. Schneid.) | Nectarine, commercial variety | OM416754 | OM720112 |
| 18 | European plum (P. domestica L.) | Old-local variety “Succari” | OM416755 | OM720113 |
| 19 | Old-local variety “Bokra” | OM416756 | OM720114 | |
| 20 | Commercial variety “Hollywood” | OM416757 | OM720115 | |
| 21 | Commercial variety “Santa Rosa” | OM416759 | OM720117 | |
| 22 | Commercial variety “Pioneer” | OM416760 | OM720118 | |
| 23 | Commercial variety “African Rose” | OM416761 | OM720119 | |
| 24 | Commercial variety “English” | OM416762 | OM720120 | |
| 25 | Japanese plum (P. salicina Lindl.) | Commercial variety “Japanese” | OM416758 | OM720116 |
The Sequences Alignment and Phylogenetic Trees
The sequences of trnH-psbA and trnL-trnF for the two loci were subjected to NCBI–BLASTN online tool http://blast.ncbi.nlm.nih.gov/Blast.cgi [20] to check the sequence similarity against sequences in the nucleotide collection (nr/nt) database. BLASTN default parameters were used and the organism selected was Prunus species in this database. Alignments of sequence were achieved by MUSCLE algorithm [21]. The evolutionary rate parameters, the pattern of nucleotide substitutions, and the average of evolutionary divergence over all the sequences, and phylogenetic trees were generated based on the Maximum Likelihood (ML) model, using MEGA version 11 software [22], other parameters of sequence diversity were calculated using DnaSP version5 [23].
Results and Discussion
The average aligned length was 316–352 bp and 701–756 bp, for trnH-psbA and trnL-trnF loci, respectively. The trnH-psbA over all evolutionary divergence was higher (0.05) than in trnL-trnF (0.007). The transition/transversion bias (R) recorded as 0.59 and 0.89 in trnL-trnF and trnH-psbA, respectively.
The number of invariable sites was higher in trnH-psbA than in trnL-trnF (670 and 214, respectively). While, the number of variable (polymorphic) and singleton variable sites were lower (18 and 6) in trnH-psbA than in the other loci (77 and 47). The nucleotide diversity (Pi) and the average number of nucleotide differences (k) in trnH-psbA was lower than the other region. Meanwhile, the number of parsimony informative sites was higher (30) in trnL-trnF than the other region (12), Table 4 represent the results.
Table 4.
Nucleotide sequence parameters for trnH-psbA and trnL-trnF regions, based on calculations of DnaSP-5 software
| Sequence parameter | trnH-psbA | trnL-trnF |
|---|---|---|
| Number of invariable (monomorphic) sites | 670 | 214 |
| Number of variable (polymorphic) sites | 18 | 77 |
| Number of singleton variable sites | 6 | 47 |
| Number of parsimony informative sites | 12 | 30 |
| Sequence conservation (C) | 0.967 | 0.695 |
| Nucleotide diversity (Pi) | 0.00592 | 0.03652 |
| Average number of nucleotide differences (k) | 4.070 | 10.627 |
trnH-psbA Loci Sequence Analyses
The trnH-psbA loci length across the twenty-five Prunus accessions ranged from 316 to 352 bp. The nucleotide frequencies for A, T, C and G was 37.6%, 37.6%, 12.4% and 12.4%, respectively. The rate of different transitional substitutions from G to A was equal to those from C to T (16.73). On the other hand, the transversionsal substitution rates was equal as it recorded 10.44 for transversion from T to A, from C to A, and from G to T. While, it reached 3.44 in transversion from G to C, results shown in Table 5.
Table 5.
ML estimate of the pattern of nucleotide substitution for trnH-psbA loci sequences across the twenty-five Prunus accessions, as calculated by MEGA version 11
| A | T | C | G | |
|---|---|---|---|---|
| A | – | |||
| T | 10.44 | |||
| C | 10.44 | 16.73 | – | |
| G | 16.73 | 10.44 | 3.44 | – |
Each entry is the probability of substitution (r) from one base (row) to another base (column). Rates of different transitional substitutions are shown in bold and those of transversionsal substitutions are shown in italics. Substitution pattern and rates were estimated under the Tamura (1992) model (+ G). A discrete Gamma distribution was used to model evolutionary rate differences among sites. Evolutionary analyses were conducted in MEGA11
trnH-psbA Phylogenetic Tree
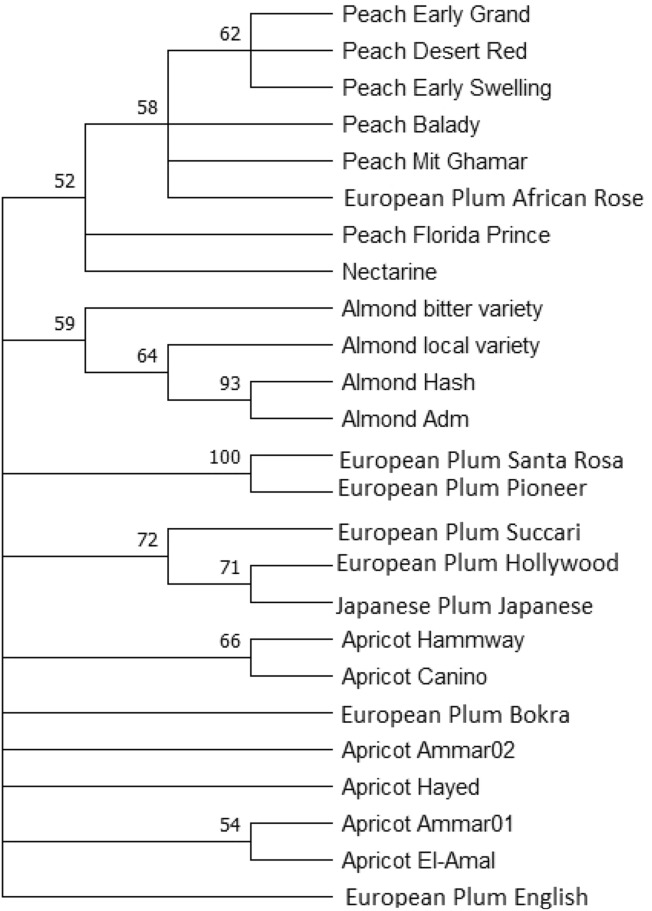
The phylogentic tree computed from the trnH-psbA chloroplast region (Fig. 1) for the different Prunus species, assigned the peach, almond, and apricot to its relative species.
Fig. 1.
ML phylogeny tree based on trnH-psbA sequences, showing the relationships among the twenty-five Prunus accessions. Bootstrap values were indicated for each node (500 replicates), cut-off value for consensus tree is 50%, as calculated by MEGA version 11
The Japanese plum accession (P. salicina) was assigned among the European plum (P. domestica) species accessions in the phylogenetic tree. European plum accessions (Bokra, and English) were clustered away from the related species accessions. Also, African Rose European plum accession was clustered among the peach accessions. Almond (P. dulcis) samples were homogenized and grouped together in the same group, where the two local accessions (Hash and Adm) were closer to each other than the other two samples. Peach (P. persica) and Nectarine (P. persica var. nucipersica) were grouped in the similar group. The apricot (P. armeniaca) accessions (Hammaway and Canino) constructed together, as they were closer to each other than the other accessions.
trnL-trnF Region Sequence Analyses
The trnL-trnF chloroplast region length across the different Prunus sequences length ranged from 701 to 756 bp. The nucleotide frequencies for trnL-trnF region sequence was as equal for T and A (32.99%), and equal in G and C as 17.01%. The lowest rate of transitional substitution events was 5.14 for transition substitution from G to C. While, it was equal rate (9.98) in the transition substitution from T to A, from C to A, and from G to T. The transversion substitution from C to T had the equal value (13.04) as for the value of transversion from G to A (results shown in Table 6). The estimates of average evolutionary divergence over all sequences for trnL-trnF region was 0.007.
Table 6.
ML estimate of the pattern of nucleotide substitution for trnL-trnF loci sequences across the twenty-five Prunus accessions, as calculated by MEGA version 11
| A | T | C | G | |
|---|---|---|---|---|
| A | – | |||
| T | 9.98 | – | ||
| C | 9.98 | 13.04 | – | |
| G | 13.04 | 9.98 | 5.14 | – |
Each entry is the probability of substitution (r) from one base (row) to another base (column). Rates of different transitional substitutions are shown in bold and those of transversionsal substitutions are shown in italics. Substitution pattern and rates were estimated under the Tamura (1992) model (+ G). A discrete Gamma distribution was used to model evolutionary rate differences among sites. Evolutionary analyses were conducted in MEGA11
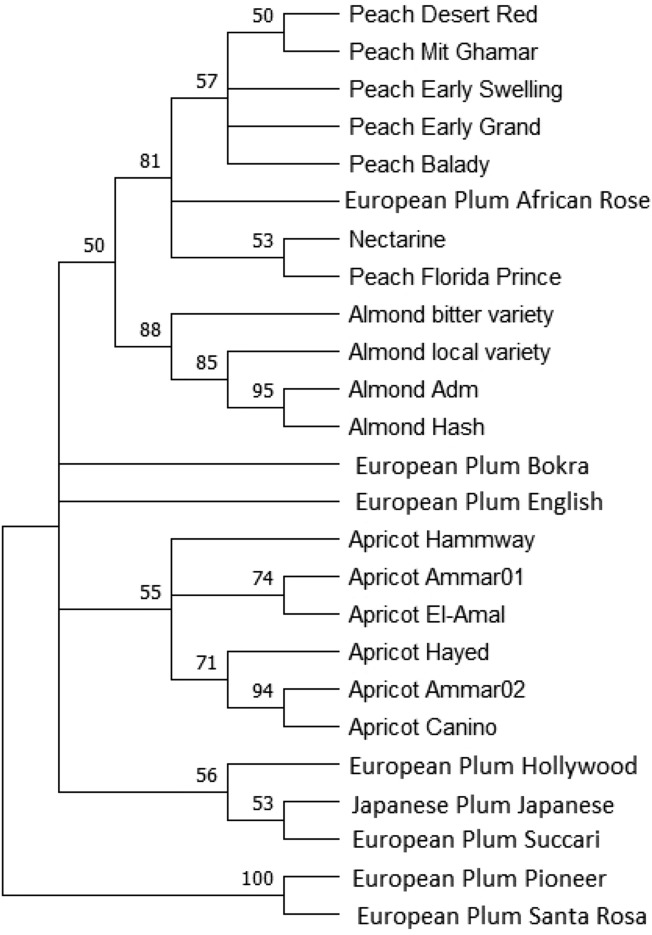
trnL-trnF Phylogenetic Tree
The trnL-trnF-based phylogenetic (Fig. 2) clustered most the Prunus species properly, with two exceptions. First: the African Rose European plum accession, was clustered distantly away from related species near to peach species (P. persica) accessions. Second: the Japanese plum (P. salicina) was assigned amomg the European plum (P. domstica) species accessions. The apricot (P. armeniaca) accessions clustered together in two groups, as accessions (Hammway, El-Amal and Ammar01) clustered in the first group, while accessions (Hayed, Ammar02, and Canino) clustered in the second. The European plum (P. domestica) species accessions were clustered in a homogenized groups, except the Japanese plum accession (P. salicina) was assigned with the succari European plum species accession. The almond (P. dulcis) species accessions were grouped together in a related cluster. The peach (P. persica) and nectarine (P. persica var. nucipersica) accessions were grouped in a related groups, where the nectarine (P. persics var. nucipesica) acession was in the same group with Florida Prince, and Early Grand peach. the two accessions (Balady and Early Swelling) were clustered in a distant groups. The African Rose (European plum) species accession was clustered in the same group with Florida Prince, Early Grand, and Nectarine peach accessions.
Fig. 2.
ML phylogenetic tree based on trnL-trnF region sequences, showing the relationships among the twenty-five Prunus accessions. Bootstrap values are indicated for each node (500 replicates), cut-off value for consensus tree is 50%, as calculated by MEGA version 11
Concatenated Sequences-Based Phylogenetic Tree
The concatenated (combined) sequences were assembled and aligned from trnH-psbA and trnL-trnF sequences for the twenty-five Prunus accessions.
The concatenated-based phylogentic tree (Fig. 3) demonstrated an overview for the combined sequences of the two chloroplast intergenic regions across the five Prunus species for the 25 Prunus accessions. The most noted observation was that most Prunus species clustered together with the same relative species. Except, the European plum (P. domestica) accession (African Rose) which was assigned with the peach (P. persica) accessions. Also, the Japanese plum (P. salicina) accession assigned with the European plum (P. domestica) accessions.
Fig. 3.
ML phylogenetic tree based on the concatenated sequences of both trnH-psbA and trnL-trnF sequences, showing the relationships among the twenty-five Prunus species. Bootstrap values are indicated for each node (500 replicates), cut-off value for consensus tree is 50%, as calculated by MEGA version 11
The two accessions of European plum (Bokra and English) grouped away from the other relative European plum accessions, as thesse accessions were used only for pollination not for commercial purposes. The almond (P. dulcis) accessions were clustered together, as the local accessions (Adm and Hash) were near to each other. The apricot (P. armeniaca) accessions samples were clustered together at the same group. The peach (P. persica) and the nectarine (P. persica var. nucipersica) accession samples were related to each other.
Teberlet et al. [24] proposed six primers for three non-coding chloroplast regions. These primers were tested and reused as universal primers for wide range of taxonomic plant groups. These regions were latter used by many researchers to investigate the systematics and phylogentic relationships of Prunus species [18, 19, 25, 26]. Meanwhile, Uncu [27] used trnH-psbA region sucessefully to detect the fraud of apricot kernels to the almond valuable oil.
In the present study, the intergenic chloroplast regions trnLUAA-trnFGAA and trnH-psbA, which was first proposed by Teberlet et al. [24], were able to identify the different Prunus species, and were able to characterize the different accessions. The trnL-trnF region had higher values in number of polymorphic sites, number of singleton variable sites, number of parsimony informative sites, nuclotide diversity, and average number of nucleotide differences. Meanwhile, trnH-psbA had evolutionary divergence, transition/transversion bias, monomorphic sites, and sequence conservation values higher than the second region.
The two intergenic regions were able to identify only four species, and were not able to identify P. salicina species, as P. salicina species was assigned with P. domestica species. The most notable observation in the phylogentic clusters was that the African Rose European plum accession, was distantly away from the related species, near to peach species accessions. Since this accession breeding ancestors had peach parents (data not published). The Japanese plum accession (P. salicina) is less resolved here as it was assigned among the European plum species (P. domestica) accessions, it could be for the selections proceeded for this adapted old-local variety. The nectarine accession (P. persica var. nucipersica) was assigned properly with peach species (P. persica) accessions, as nectarine is a mutant strain of peach [13]. It was observe that across the three constructed phylogenetic trees that almond (P. dulcis) and peach (P. persica) is closer to each other, as it was evolutionary hybridized [28]. Bortiri et al. [25] used trnL-trnF regions to identify different Prunus species, indicated little variations because of the monophyletic divergence of Prunus. Batnini et al. [26] used trnL-trnF and trnH-psbA regions in studying the genetic diversity among different Prunus species, resulting in high variability among studied species, with higher average than our obtained results.
Conclusion/Future Perspectives
The current research constructed the phylogentic relationships of Prunus collection. This step is a cornerstone in identifying the conserved Punus germplasm, which will help in the crop development, sustainable use and impeovement of Prunus.
Acknowledgements
The authors wish to thank the Science, Technology and Innovation Funding Authority (STIFA), Egypt, US Jouint (Grant No. 1119) for the financial support.
Funding
Open access funding provided by The Science, Technology and Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Conflict of interest
The authors declare that they have no conflict of interests, and contributed equally.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2003;270(1512):313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcaccia G, Lucchin M, Cassandro M. DNA barcoding as a molecular tool to track down mislabeling and food piracy. Diversity. 2015;8:2. doi: 10.3390/d8010002. [DOI] [Google Scholar]
- 3.Gregory TR. DNA barcoding does not compete with taxonomy. Nature. 2005;434:1067. doi: 10.1038/4341067b. [DOI] [PubMed] [Google Scholar]
- 4.Kress WJ, Erickson DL. DNA barcodes: Genes, genomics, and bioinformatics. Proceedings of the National Academy of Sciences of the United States of America (PNAS) 2008;105(8):2761–2762. doi: 10.1073/pnas.0800476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentini A, Pompanon F, Taberlet P. DNA barcoding for ecologist. Trends in Ecology and Evolution. 2008;24(2):110–117. doi: 10.1016/j.tree.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Kress WJ. Plant DNA barcodes: Applications today and in the future. Journal of Systematics and Evolution. 2017;55(4):291–307. doi: 10.1111/jse.12254. [DOI] [Google Scholar]
- 7.Li X, Yang Y, Henry RJ, Rossetto M, Wang Y, Chen S. Plant DNA barcoding: From gene to genome. Biological Reviews. 2015;90(1):157–166. doi: 10.1111/brv.12104. [DOI] [PubMed] [Google Scholar]
- 8.Li DZ, Gao LM, Li HT, Wang H, Ge XJ, Liu JQ, Chen ZD, Zhou SL, Chen SL, Yang JB, Fu CX, Zeng CX, Yan HF, Zhu YJ, Sun YS, Chen SY, Zhao L, Wang K, Yang T, Duan GW. Comparative analysis of a large dataset indicates that internal transcribed spacer ITS should be incorporated into the core barcode for seed plants. Proceedings of the National Academy of Sciences (PNAS) 2011;108(49):19641–19646. doi: 10.1073/pnas.1104551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CBOL Plant Working Group A DNA barcode for land plants. Proceedings of the National Academy of Sciences (PNAS) 2009;106(31):12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed SM, Fadl M. Investigating hybridization and variability between Ficus species in Saudi Arabia through DNA barcoding approach and morphological characters. Pakistan Journal of Botany. 2019;51(4):1–8. doi: 10.30848/PJB2019-4(2). [DOI] [Google Scholar]
- 11.Shi S, Li J, Sun J, Yu J, Zhou S. Phylogeny and classification of Prunus sensu lato (Rosaceae) Journal of Integrative Plant Biology. 2013;55(11):1069–1079. doi: 10.1111/jipb.12095. [DOI] [PubMed] [Google Scholar]
- 12.Bouhadida M, Martin JP, Eremin G, Pinochet J, Moreno MA, Gogorcena Y. Chloroplast DNA diversity in Prunus and its implication on genetic relationships. Journal of the American Society for Horticultural Science. 2007;132(5):670–679. doi: 10.21273/JASHS.132.5.670. [DOI] [Google Scholar]
- 13.Gil MI, Tomas F, Betty BA, Pierce H, Kader AA. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. Journal of Agricultural and Food Chemistry. 2002;50(17):4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- 14.Dirlewanger E, Graziano E, Joobeur T, Garriga-Calderé F, Cosson P, Howad W, Arús P. Comparative mapping and marker-assisted selection in Rosaceae fruit crops. Proceedings of the National Academy of Sciences (PNAS) 2004;101(26):9891–9896. doi: 10.1073/pnas.0307937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer JD. Historical geography of crop plants. CRC Press; 1993. [Google Scholar]
- 16.Govindaraj M, Vetriventhan M, Srinivasan M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genetics Research International. 2015;2015:14. doi: 10.1155/2015/431487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Börner A, Khlestkina EK, Chebotar S, Nagel M, Arif MA, Neumann K, Kobiljski B, Lohwasser U, Röder MS. Molecular markers in management of ex situ PGR—A case study. Journal of Biosciences. 2012;37(5):871–877. doi: 10.1007/s12038-012-9250-2. [DOI] [PubMed] [Google Scholar]
- 18.Quan X, Zhou S. Molecular identification of species in Prunus sect. Persica (Rosaceae), with emphasis on evaluation of candidate barcodes for plants. Journal of Systematics and Evolution. 2011;49(2):138–145. doi: 10.1111/j.1759-6831.2010.00112.x. [DOI] [Google Scholar]
- 19.Cheong EJ, Cho M, Kim S, Kim C. Chloroplast noncoding DNA sequences reveal genetic distinction and diversity between wild and cultivated Prunus yedoensis. Journal of the American Society for Horticultural Science. 2017;142(6):434–443. doi: 10.21273/JASHS.142.6.434. [DOI] [Google Scholar]
- 20.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 21.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Stecher G, Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution. 2021;38(7):3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 24.Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 25.Bortiri E, Oh S, Jiang J, Baggett S, Granger A, Weeks C, Buckingham M, Potter D, Parfit DE. Phylogeny and systematics of Prunus (Rosaceae) as determined by sequence analysis of ITS and the chloroplast trnL-trnF spacer DNA. Systematic Botany. 2001;26(4):797–807. [Google Scholar]
- 26.Batnini MA, Bourguiba H, Trifi-Farah N, Krichen L. Molecular diversity and phylogeny of Tunisian Prunus armeniaca L. by evaluating three candidate barcodes of the chloroplast genome. Scientia Horticulturae. 2019;245:99–106. doi: 10.1016/j.scienta.2018.09.071. [DOI] [Google Scholar]
- 27.Uncu AO. A trnH-psbA barcode genotyping assay for the detection of common apricot (Prunus armeniaca L.) adulteration in almond (Prunus dulcis Mill.) CYTA—Journal of Food. 2020;18(1):187–194. doi: 10.1080/19476337.2020.1727961. [DOI] [Google Scholar]
- 28.Palmer JD. Chloroplast DNA and molecular phylogeny. Bioassays. 1985;2:263–267. doi: 10.1002/bies.950020607. [DOI] [Google Scholar]
- 29.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversionand G+C-content biases. Molecular Biology and Evolution. 1992;9(4):678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]