Version Changes
Revised. Amendments from Version 1
This second version incorporates edits made in response to the input from two reviewers. These include: 1. The term polyphyletic is no longer used, accepting the reviewer comment that our data are not sufficient to do so; instead, we refer to or describe the deeply divided phylogenetic tree topology of the genus Giuris. 2. We replaced Gobioidei (including one mis-spelt occurrence) with Gobiiformes to comply with recent changes in taxonomic usage. 3. We accepted a reviewer suggestion regarding the way in which we refer to the NCBI Basic Local Alignment Search Tool routine used, and amended the methods section accordingly. 4. We clarified the further research recommended in the conclusion, as a response to reviewer input. 5. We deleted the estimated volume of Lake Bolano Sau, as this may not be very accurate. 6. We accepted the reviewer suggestion to use standard (rather than italic) font for local language common names of organisms. 7. We made spelling corrections and improvements in terms of English language usage, some on the advice of reviewers and some based on our own review of the text.
Abstract
Background: The freshwater ichthyofauna of Wallacea is diverse and understudied. A baseline survey of Bolano Sau Lake in Parigi Moutong District, Central Sulawesi Province, Indonesia in 2019 found an eleotrid goby (local name payangka) with characters conforming to the genus Giuris, long considered monophyletic as G. margaritacea/G. margaritaceus but recently found to comprise at least eight species. This study focused on the molecular (DNA barcoding) identification and phenotypic characters of the payangka.
Methods: Payangka samples were collected from August to December 2019 in collaboration with local fishermen, weighed and measured, and preserved in 75% ethanol. Length, weight, sex (n=111) and 17 morphometric characters/six meristic counts (n=42) were recorded. DNA barcoding was performed on a fin clipping preserved in 96% ethanol. Homologous nucleotide sequences were obtained from public (GenBank and BOLD) databases, analysis conducted in MEGA X, and phylogenetic trees edited in the Interactive Tree of Life (iToL).
Results: Within the deeply divided Giuris clade, the payangka sequence resolved into a sub-clade identified as Giuris laglaizei (Sauvage 1880), a recently resurrected taxon, based on a sequence provided by Philippe Keith. The length-weight relationship (L = 0.0087∙W3.162) indicated mildly allometric positive growth. Size distribution differed significantly between male and female fish with significantly larger mean size of males (13.56 cm) than females (11.62 cm). The meristic formula was: D VI-I,8 A I,8 P 13 V I,5 C15. Phylogenetic analysis indicated four Giuris species in wetlands around Tomini Bay and five in Sulawesi.
Conclusions: This first record of G. laglaizei in Indonesia advances knowledge of Wallacean and Indo-Pacific Gobiiformes biogeography and highlights the need for a revision of the conservation status of the taxa currently grouped under Giuris margaritacea/G. margaritaceus in the IUCN Red List and FishBase databases. The data will inform biodiversity and fisheries management at local and regional levels.
Keywords: Eleotridae, Giuris margaritacea, amphidromy, phylogeny, meristic, morphometric, Tomini Bay, Wallacea
Background
Indonesian freshwater ichthyofauna is highly diverse, comprising primary freshwater and diadromous fishes. 1 While the ichthyofauna of western Indonesia (Sundaland biogeographic province) is dominated by cyprinids (Cyprinidae), the gobies (Gobiiformes) predominate in the Wallacea biogeographic province. 1 , 2 The freshwater ichthyofauna of Sulawesi, the largest island in Wallacea, is characterised by endemic freshwater species flocks and diadromous (mostly amphidromous sensu 3 , 4 ) taxa with marine larval stages. 1 These include gobies (Gobiiformes) of the families Gobiidae and Eleotridae, the latter also known as sleepers or gudgeons. 1 , 5 – 9 Although the number of diadromous (mostly amphidromous) gobies reported from the lakes, rivers and coastal waters around Sulawesi is increasing, with several recently described species and range extensions, 10 – 18 the ichthyofauna of most Sulawesian waterbodies is still largely unstudied. 9
Typically multi-species shoals of amphidromous goby postlarvae, with local names including nike, penja and duwo, are heavily fished in coastal waters around Sulawesi as they migrate to freshwater habitats, both as the main fish catch and as bycatch in anguillid glass eel fisheries. 11 , 12 , 19 – 23 Goby postlarvae are also present and intensively fished in some inland waters, in particular Tondano Lake in North Sulawesi. 10 , 24 – 28 The adults of some diadromous gobies are also locally important as food fish, including gudgeons (Eleotridae) generally known as payangka or (more rarely) payangga. 10 , 29 , 30 Sulawesian payangka populations have been identified as northern mud gudgeon Ophiocara porocephala (Valenciennes 1837) 31 – 33 and snakehead gudgeon Giuris margaritacea (Valenciennes 1837). 25 , 26 , 34 – 36 Both taxa are thought to be amphidromous. 37
In recent decades the Indonesian Government has promoted so-called “re-stocking” of inland waterbodies. These programs almost always involve the release of non-native (alien) species, often with negative impacts on native aquatic species in Indonesia 38 , 39 including in Sulawesi. 40 – 43 Increasing concern for native aquatic species has prompted surveys of inland waterbodies in Sulawesi, including those with a history of such introductions.
Bolano Sau Lake is one of a series of three small lakes close to the Tomini Bay coast of the northern arm of Sulawesi, in Bolano Subdistrict, Parigi Moutong District, Central Sulawesi Province. A baseline survey of Bolano Sau Lake in 2019 found an ichthyofauna dominated by introduced (alien) fish species. 44 , 45 Three native species were caught during sampling, one of which was a gudgeon with the local name payangka, tentatively identified as Giuris margaritacea, 45 the current valid name of the snakehead gudgeon in FishBase, the Global Database of Fishes. According to Kottelat (2013) the genus name Giuris is masculine in gender, and therefore the correct nomenclature is Giuris margaritaceus, the current organism name in the NCBI GenBank and BOLD nucleotide sequence databases. 46
Within the order Gobiiformes, cryptic and morphologically similar species can complicate identification based on external morphology, 46 – 49 and there have been many taxonomic revisions, including within the family Eleotridae. There are at least 10 “non valid” synonyms of G. margaritacea (Valenciennes 1837) (originally Eleotris margaritacea Valenciennes 1837) listed in FishBase, including Ophieleotris aporos (originally Eleotris aporos Bleeker 1854) and junior synonyms under the genera Eleotris (n=7), Hypseleotris (n=1), and Ophieleotris (n=1). Ophieleotris aporos in particular is still commonly used, 14 , 30 , 50 – 52 and a recent checklist of fishes from two Sulawesian islands 13 lists G. margaritacea (from East and West Timor) and O. aporos (from lakes in North Sulawesi) as separate species, with the further addition of Ophieleotris aff. aporos (from Buton Island in Southeast Sulawesi). Recent research has demonstrated that G. margaritacea has been erroneously and confusingly applied to a taxonomic group comprising at least eight species. 15 , 53
Such taxonomic uncertainty seriously complicates accurate species determination and the search for valid information on aspects such as species distribution, biology, ecology and status. As pointed out by, 54 the ability to precisely identify the fish species in fisheries catches or waters is important for moving towards more sustainable exploitation of fish resources and better protection of fish diversity. An increasingly common molecular approach to species identification is DNA barcoding; this involves the sequencing of a fragment of DNA which is highly conserved within species but differs between species of the taxonomic group being studied. 55 For vertebrates, including fishes, a subset or fragment of the mitochondrial cytochrome oxidase I (COI) gene is the most commonly used barcoding region. 2 , 56 In addition to species identification, phenotypic traits are important for taxonomy and to support fisheries management. 54 , 57 , 58
From a biodiversity and responsible fisheries management point of view, it was considered important to determine the native species currently present in Bolano Sau Lake. This study combined molecular biology methods (DNA barcoding) to identify the payangka in Bolano Sau Lake with classic methods to describe phenotypic characters, in particular external morphology (morphometric and meristic characters) and growth pattern (length-weight relationship). The study will inform management of the payangka as well as contributing to knowledge of Giuris biogeography .
Methods
Ethical statement
This study complied with relevant ethical regulations in Indonesia and followed the ARRIVE guidelines. The use of the samples in this study did not require specific ethical approval for the following reasons:
-
1.
All fish specimens used were obtained under an ongoing collaboration between the Parigi Moutong District Marine and Fisheries Service (Dinas Kelautan dan Perikanan Kabupaten Parigi Moutong) and Universitas Tadulako following all applicable regulations.
-
2.
The fish were captured by local fishers operating legal fisheries using permitted artisanal fishing gears.
-
3.
All fish were euthanized following the standard guidelines in use for fish specimens at Universitas Tadulako. Based on standard internationally recognised protocols, 59 the procedures used were designed to minimise any suffering experienced by the specimens (through anaesthetising the fish before pithing), and were performed by experienced personnel.
-
4.
The IUCN Red List assessment lists Giuris margaritacea (the only listed Giuris taxon) in the Least Concern category (not considered at risk of extinction).
-
5.
There was no experimental component.
Study site
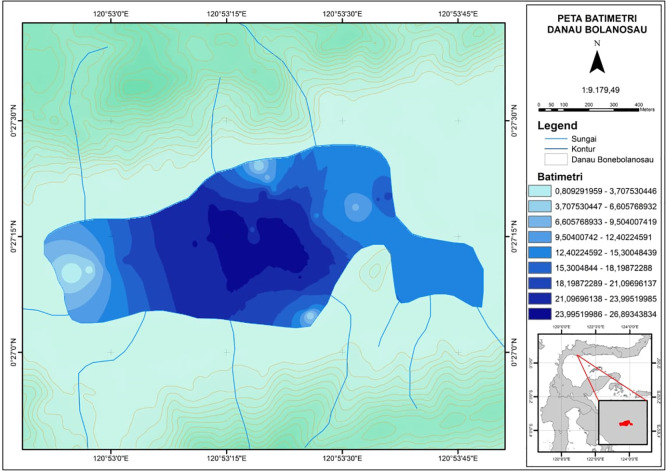
Bolano Sau is the largest of a three-lake complex in the coastal plain along the north coast of Tomini Bay in Parigi Moutong District, Central Sulawesi Province, Indonesia. Batudako Lake is further inland, and Laut Kecil Lake is closer to the coast. Bolano Sau Lake is situated just north of the equator in Bolano Barat Village, Bolano Subdistrict, between approximately 0° 27’ 12” to 0° 27’ 15” N and 120° 52’ 52” to 120° 53’ 49” E an elevation of 5 m above sea level with an area of around 76 Ha, an average depth of around 4.26 m and a maximum depth of less than 10 m ( Figure 1).
Figure 1. Bathymetric map of Bolano Sau Lake in Parigi Moutong District, Central Sulawesi, Indonesia.
Fish specimens
Payangka specimens for morphometric and meristic analysis were collected from August to December 2019 in collaboration with local fishermen using a throw net with mesh size 3.5” (n=42). Field identification followed. 6 , 60 Specimens were humanely euthanized (anaesthesia with 70% ethanol followed by pithing) following. 59 Each fish was then weighed (electronic scales, precision 1 g), measured (Cadwell fish ruler, precision 0.5 mm), labelled and preserved in 70% alcohol.
DNA extraction and barcoding
Prior to preservation, a fin clipping was taken from the right-hand pectoral fin of a payangka specimen (female, total length (TL)=13.32 mm) and placed in a 1.5 mL Eppendorf tube filled with 96% absolute ethanol. The sample was dispatched to the BIONESIA laboratory in Denpasar for DNA barcoding. Genomic DNA was extracted from the sample using the Qiagen DNeasy Blood & Tissue Kit following manufacturer’s protocols. A fragment of mitochondrial DNA (mtDNA) from the cytochrome oxidase I (COI) gene was amplified through polymerase chain reaction (PCR) using Fish_F1 and Fish_R1 primers. 61 The PCR profile comprised initial denaturation at 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 60 s; and final extension at 72 °C for 2 min. The PCR product was visualized via electrophoresis on 1% agarose gel stained with Nucleic Acid Gel Stain (GelRed ®). Sanger sequencing of the PCR product was performed by First Base (Singapore). The forward and reverse sequences were trimmed, aligned and combined in MEGA X 62 to produce a nucleotide sequence submitted to the NCBI GenBank repository as accession OM674613. 63 The nucleotide composition (adenine, thymine, guanine, cytosine bases) of the payangka mitochondrial cytochrome C oxidase subunit I (COI) gene sequence was determined in MEGA X. 62 The online National Center for Biotechnology Information (NCBI) standard nucleotide Basic Local Alignment Search Tool-nucleotide (BLAST ©) blastn routine and the Barcode of Life Database (BOLD) Identification routine were used to provide an initial identification.
Phylogenetic analysis
Homologous nucleotide sequences with at least 90% coverage were obtained from the NCBI blastn results (Gobiiformes in the 100 closest matches) and NCBI GenBank accession search using the terms Giuris margaritaceus, G. margaritacea, Ophieleotris aporos and Ophiocara porocephala, as well as from the BOLD Database using the keyword Giuris which yielded seven Barcode Index Numbers (BINs), with additional sequences obtained from the scientific literature (Table S1 – see Extended Data). The climbing perch Anabas testudineus was used as an outgroup: a sequence from Bolano Sau Lake, GenBank accession OM674614, 63 and GenBank accession MG407353. 64 All alignment, trimming and evolutionary analyses were conducted in MEGA X. 62 Evolutionary relationships were inferred and phylogenetic trees constructed using the Maximum Likelihood method and Kimura 2-parameter model 65 with default parameters, all codon positions, 100 × bootstrap test, and branch lengths representing the number of substitutions per site.
The first phylogenetic tree was constructed from an aligned dataset with 630 nucleotide positions containing 96 nucleotide sequences (GenBank accessions in Table S1). A second tree was constructed using all sequences in Table S1 with the genus level label Giuris (including BOLD records and non-deposited sequences) and other sequences nested within the Giuris clade in the first tree; there were 94 nucleotide sequences with 580 nucleotide positions in the aligned dataset. The payangka sequence was included in both analyses. The phylogenetic trees were exported from Mega X as Newick tree files and edited in the on-line interactive Tree of Life (iToL). 66 , 67 Pairwise evolutionary distances (number of base substitutions per site) within the Giuris and an outgroup ( Mogurnda adspersa) from the nearest Eleotridae clade were estimated using the Compute Pairwise Distances routine in Mega X, using the Maximum Composite Likelihood model. 68
Phenotypic characteristics of the Bolano Sau Lake payangka
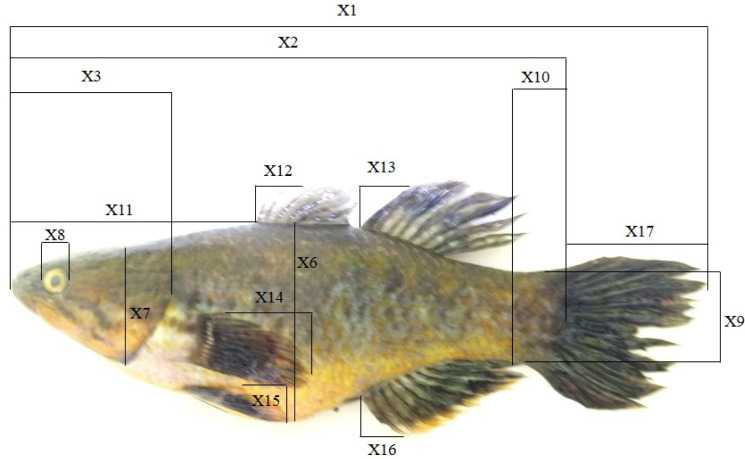
Morphometric and meristic characters ( Table 1) of the Bolano Sau Lake payangka (n=42) were measured or counted in the Aquatic Biology Laboratory, Universitas Tadulako, Palu. 69 Morphometric characters ( Figure 2) were measured using electronic callipers with a precision of 0.01 mm. Length, weight, sex and gonad maturity status data from a study on payangka reproductive biology (n=69; 25 females and 44 males) collected from Bolano Sau Lake in August and October 2019 45 were also included in some analyses. Data were tabulated in Microsoft Excel 2010 and analysed descriptively. The length-weight relation analysis (n=107) was performed in Microsoft Excel 2010 (RRID:SCR_016137) using the log 10-transformed version of the formula W= a∙L b, where W is total body weight (g); L is total length (cm); a is the antilog of the intercept and b is the slope of the linear regression of the Log 10 transformed data. Analysis of mean size and size class distribution were implemented in R version 3.6.0 (RRID:SCR_001905) 70 through the Rstudio version 1.1.456 interface (RRID:SCR_000432), 71 using code from 72 with a size class interval of 1 cm. Microsoft Excel spreadsheet algorithms based on 72 were used to estimate mean size at first maturity (L50) and sex-ratio by size-class, also with a size class interval of 1 cm. The meristic formula was based on median values of the 6 meristic characters (dorsal fins D1 and D2, anal fin A, pectoral fin P, ventral fin V, caudal fin C) with spine counts given in Roman numerals and ray counts in Arabic numerals. Selected characters were compared with data on other Giuris spp. populations.
Table 1. Morphometric and meristic characters of payangka used in this study.
| Morphometric characters (see Figure 2) | Meristic counts | ||||
|---|---|---|---|---|---|
| Code | Description | Code | Description | Code | Description |
| X1-TL | Total length | X10-LP | Length of caudal peduncle | C | Caudal fin rays |
| X2-SL | Standard length | X11-SD | Tip of snout to base of anterior dorsal fin | A | Anal fin spines/rays |
| X3-HL | Head length from tip of snout to operculum margin | X12-DA | Length of first anterior dorsal fin spine | D1 | Anterior dorsal fin spines/rays |
| X4-UJ | Upper jaw length | X13-DP | Length of first posterior dorsal fin spine | D2 | Posterior dorsal fin spines/rays |
| X5-LJ | Lower jaw length | X14-PF | Length of pectoral fin | P | Pectoral fin rays |
| X6-BD | Body depth (maximum) | X15-VF | Length of ventral fin | V | Ventral fin rays |
| X7-HH | Head height | X16-AF | Length of first anal fin spine | ||
| X8-ED | Eye diameter | ||||
| X9-CP | Height of caudal peduncle | X17-CL | Tail (caudal) length | ||
Figure 2. Morphometric characters of Bolano Sau Lake payangka measured in this study.
Biodiversity conservation and fisheries
Qualitative data on biodiversity and fisheries in Bolano Sau Lake were collected during the payangka sample and environmental data collection. Secondary data were sourced from the baseline survey carried out from August to October 2019, 44 scientific literature, and reputable on-line sources. Primary and secondary data were analysed with respect to implications for biodiversity, including taxon conservation status, and fisheries management.
Results
Species identification and phylogenetic analysis
The nucleotide composition (nitrogen bases) of the mitochondrial cytochrome C oxidase subunit I (COI) gene sequence was guanine 19.3%, cytosine 29.0%, adenine 23.9% and thymine 27.8%. The NCBI blastn and BOLD Identification routines assigned the goby or gudgeon, known locally as payangka, to the Gobiiformes, Family Eleotridae, genus Giuris and taxonomic group labelled as Giuris margaritacea. Ten snakehead gudgeon Giuris margaritaceus sequences from Taal Lake, Luzon in the Philippines (accessions HQ654732-HQ654740) originally deposited as Ophieleotris aporos 52 had a very high similarity (99.84%–99.85%) with the payangka sequence from Central Sulawesi, Indonesia, GenBank accession OM674613. The condensed tree in Figure 3 shows the payangka sequence (labelled BIOSUB77_03) nested within a G. margaritaceus sub-clade containing these sequences. Additional analyses using the Neighbor-Join option in MEGA X produced an equivalent structure. Seven other Philippine sequences had similarities of 98.45–99.38%, including accessions HQ682711 and HQ682712 from Laguna Lake, also in Luzon 73 and accessions MG407388 to MG407392 from Lanao Lake in Mindanao. 64
Figure 3. Evolutionary relationships of payangka from Bolano Sau Lake with several gobioid taxa and Anabas testudineus as an outgroup.
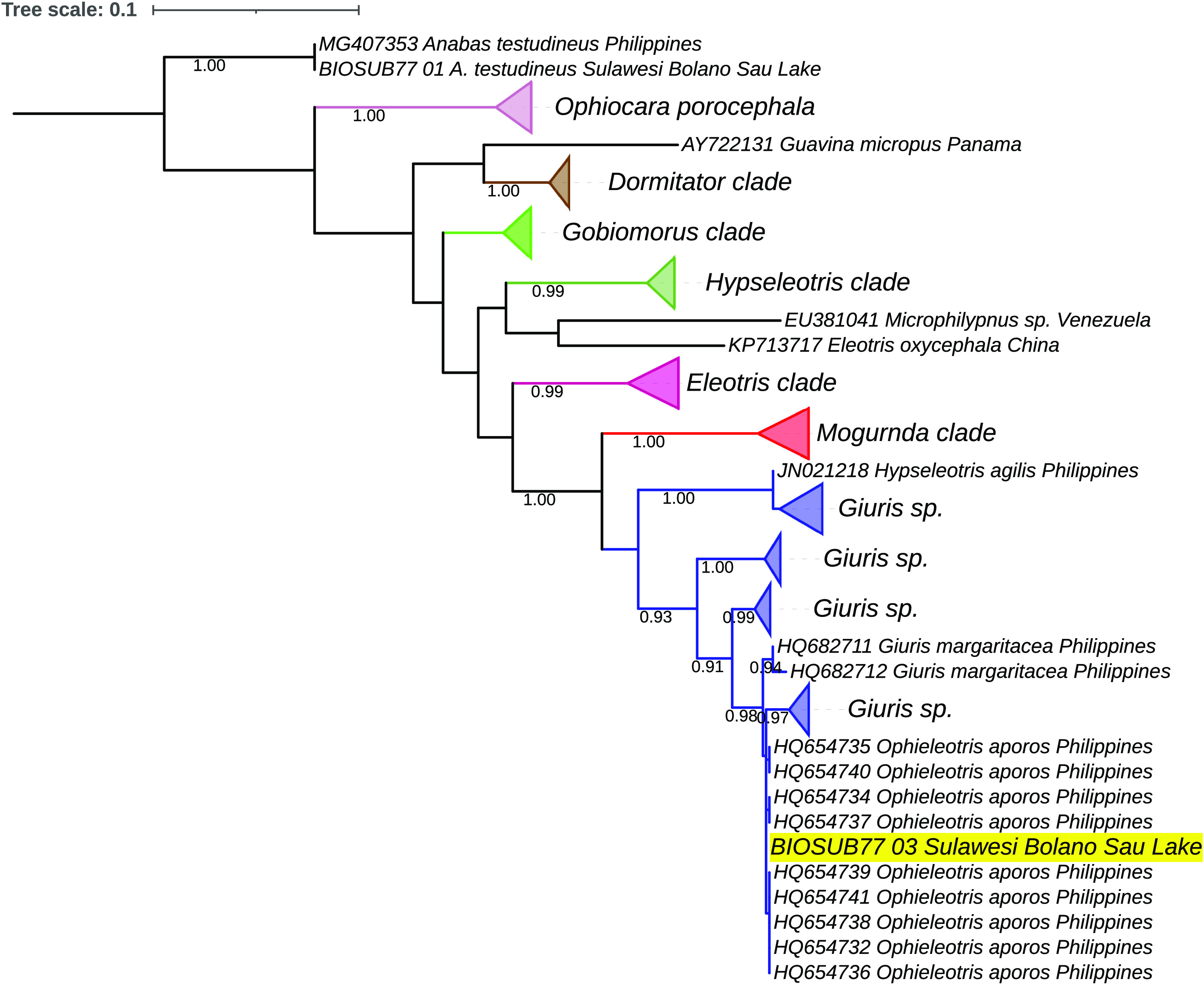
The Bolano Sau Lake payangka sequence is highlighted in yellow. Evolutionary relationships were estimated using the Maximum Likelihood routine in MEGA X. 62 The analysis comprised 96 sequences with 630 nucleotide positions and 100 bootstrap replicates. The blue clade comprises sequences deposited in GenBank or BOLD databases as Giuris margaritacea and synonyms including Ophieleotris aporos. The length of the triangles is proportional to the number of sequences. The branch scale is in number of substitutions per site.
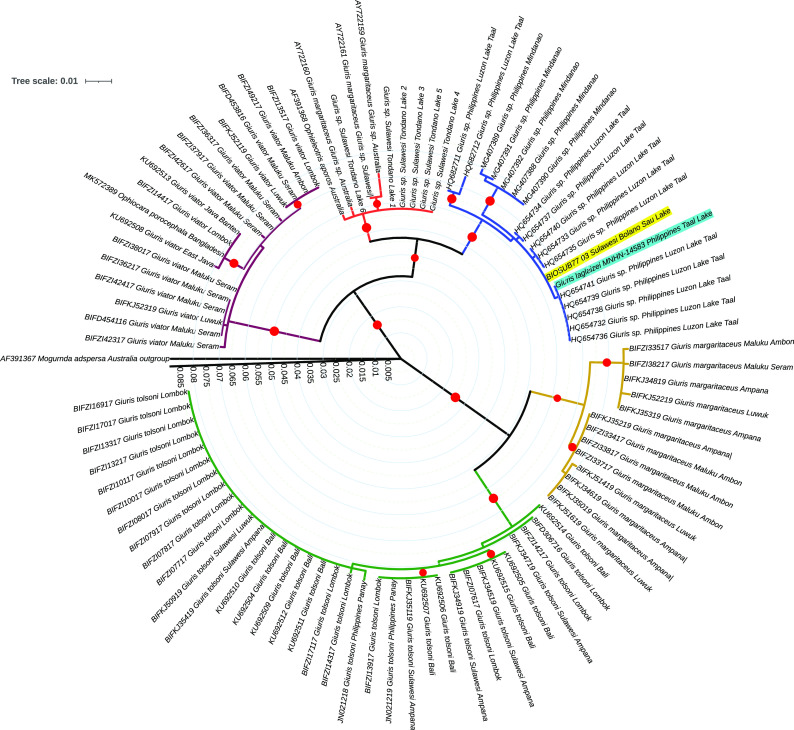
The analysis of the Giuris clade incorporating sequences from 15 retrieved from the BOLD database ( Figure 4) shows that the payangka from Bolano Sau Lake is not closely related to other Giuris specimens from Sulawesi or other regions in Indonesia. The number of base differences per site for representative sequences from each clade in Figure 4 ( Table 2) shows that the genetic distance between the payangka and Giuris sp. from the Philippines was 0.002 to 0.016. Meanwhile the genetic distance between payangka and sequences from Indonesia in other Giuris clades was between 0.064 and 0.126, a range consonant with congeneric rather than conspecific relationships.
Figure 4. Evolutionary relationships of the genus Giuris, with Mogurnda adspersa as outgroup.
The Bolano Sau Lake payangka sequence is highlighted in yellow and the Giuris laglaizei sequence MNHN-14583 from Philippe Keith is highlighted in turquoise. The Maximum Likelihood analysis in MEGA X 62 included 94 sequences with 580 positions and 100 bootstrap replicates. The red circles indicate node bootstrap values over 75%. The branch scale is in number of substitutions per site. Accession/Record details: see Table S1.
Table 2. Genetic distances between payangka and selected Giuris sp. from Philippines, Indonesia and Australia (part 1).
| No | Sequence details | Sequence number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession/Record | Taxon a | Origin b | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| 1 | BIOSUB77_03 | GS1 | CS-BS | |||||||||||
| 2 | HQ654740 | GS1 | PH-L | 0.002 | ||||||||||
| 3 | HQ654739 | GS1 | PH-L | 0.002 | 0.003 | |||||||||
| 4 | HQ682712 | GS1 | PH-L | 0.013 | 0.014 | 0.014 | ||||||||
| 5 | MG407390 | GS1 | PH-M | 0.013 | 0.014 | 0.014 | 0.026 | |||||||
| 6 | MG407389 | GS1 | PH-M | 0.016 | 0.018 | 0.018 | 0.029 | 0.003 | ||||||
| 7 | None | GS2 | NS-T | 0.029 | 0.031 | 0.031 | 0.039 | 0.032 | 0.032 | |||||
| 8 | AY722161 | GS2 | SUL | 0.034 | 0.036 | 0.036 | 0.044 | 0.037 | 0.037 | 0.005 | ||||
| 9 | AF391368 | GS2 | AUS | 0.031 | 0.032 | 0.032 | 0.041 | 0.034 | 0.034 | 0.002 | 0.006 | |||
| 10 | BIFKJ35219 | GM | CS-A | 0.122 | 0.124 | 0.124 | 0.135 | 0.120 | 0.122 | 0.120 | 0.126 | 0.122 | ||
| 11 | BIFKJ51419 | GM | CS-L | 0.126 | 0.128 | 0.128 | 0.139 | 0.124 | 0.126 | 0.120 | 0.126 | 0.122 | 0.003 | |
| 12 | BIFZI33517 | GM | MAL | 0.111 | 0.112 | 0.112 | 0.123 | 0.109 | 0.111 | 0.109 | 0.115 | 0.111 | 0.018 | 0.021 |
| 13 | BIFKJ52319 | GV | CS-L | 0.066 | 0.066 | 0.068 | 0.077 | 0.077 | 0.077 | 0.059 | 0.064 | 0.061 | 0.130 | 0.126 |
| 14 | BIFZI36317 | GV | MAL | 0.068 | 0.069 | 0.069 | 0.079 | 0.078 | 0.078 | 0.061 | 0.066 | 0.062 | 0.124 | 0.120 |
| 15 | BIFZI13517 | GV | LO | 0.066 | 0.068 | 0.068 | 0.077 | 0.076 | 0.076 | 0.062 | 0.068 | 0.064 | 0.126 | 0.122 |
| 16 | KU692513 | GV | J-B | 0.064 | 0.066 | 0.066 | 0.075 | 0.075 | 0.075 | 0.061 | 0.066 | 0.062 | 0.124 | 0.120 |
| 17 | KU692508 | GV | J-E | 0.066 | 0.068 | 0.068 | 0.077 | 0.076 | 0.076 | 0.062 | 0.068 | 0.064 | 0.126 | 0.122 |
| 18 | BIFKJ34919 | GT | CS-A | 0.121 | 0.119 | 0.122 | 0.133 | 0.121 | 0.123 | 0.115 | 0.121 | 0.117 | 0.051 | 0.054 |
| 19 | BIFKJ50919 | GT | CS-L | 0.119 | 0.117 | 0.121 | 0.131 | 0.119 | 0.121 | 0.113 | 0.119 | 0.115 | 0.054 | 0.058 |
| 20 | BIFZI08017 | GT | LO | 0.119 | 0.117 | 0.121 | 0.131 | 0.119 | 0.121 | 0.113 | 0.119 | 0.115 | 0.054 | 0.058 |
| 21 | KU692506 | GT | BAL | 0.124 | 0.123 | 0.126 | 0.137 | 0.124 | 0.126 | 0.119 | 0.125 | 0.121 | 0.054 | 0.054 |
| 22 | JN021219 | GT | PH-P | 0.124 | 0.123 | 0.126 | 0.137 | 0.124 | 0.126 | 0.119 | 0.125 | 0.121 | 0.054 | 0.054 |
| 23 | AF391367 | MA | AUS | 0.145 | 0.147 | 0.147 | 0.158 | 0.147 | 0.143 | 0.141 | 0.145 | 0.143 | 0.161 | 0.163 |
Taxon: GS = Giuris sp.; GM = Giuris margaritaceus; GT = Giuris tolsoni; GV = Giuris viator; MA = Mogurnda adspersa (outgroup).
Origin: AUS = Australia; AUS/S = Australia or Sulawesi (actual site unknown); PH= Philippines; LU = Luzon (Lake Taal); M = Mindanao; P = Panay; Indonesia: CS= Central Sulawesi: A = Ampana; BS = Lake Bolano Sau; L = Luwuk; NS-T = North Sulawesi, Tondano Lake; SUL = Sulawesi, location unknown; BAL = Bali; LO = Lombok; MAL = Maluku; J-B = Java, Banten; J-E = East Java.
Table 2. (continued) Genetic distances between payangka and selected Giuris sp. from Philippines, Indonesia and Australia (part 2).
| No | Sequence details | Sequence number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession/Record | Taxon a | Origin b | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |
| 1 | BIOSUB77_03 | GS1 | CS-BS | |||||||||||
| 2 | HQ654740 | GS1 | PH-L | |||||||||||
| 3 | HQ654739 | GS1 | PH-L | |||||||||||
| 4 | HQ682712 | GS1 | PH-L | |||||||||||
| 5 | MG407390 | GS1 | PH-M | |||||||||||
| 6 | MG407389 | GS1 | PH-M | |||||||||||
| 7 | None | GS2 | NS-T | |||||||||||
| 8 | AY722161 | GS2 | SUL | |||||||||||
| 9 | AF391368 | GS2 | AUS | |||||||||||
| 10 | BIFKJ35219 | GM | CS-A | |||||||||||
| 11 | BIFKJ51419 | GM | CS-L | |||||||||||
| 12 | BIFZI33517 | GM | MAL | |||||||||||
| 13 | BIFKJ52319 | GV | CS-L | 0.015 | ||||||||||
| 14 | BIFZI36317 | GV | MAL | 0.113 | 0.005 | |||||||||
| 15 | BIFZI13517 | GV | LO | 0.115 | 0.006 | 0.005 | ||||||||
| 16 | KU692513 | GV | J-B | 0.113 | 0.011 | 0.009 | 0.011 | |||||||
| 17 | KU692508 | GV | J-E | 0.115 | 0.013 | 0.011 | 0.013 | 0.002 | ||||||
| 18 | BIFKJ34919 | GT | CS-A | 0.061 | 0.133 | 0.130 | 0.129 | 0.127 | 0.125 | |||||
| 19 | BIFKJ50919 | GT | CS-L | 0.065 | 0.131 | 0.128 | 0.127 | 0.125 | 0.123 | 0.006 | ||||
| 20 | BIFZI08017 | GT | LO | 0.065 | 0.131 | 0.128 | 0.127 | 0.125 | 0.123 | 0.006 | 0.000 | |||
| 21 | KU692506 | GT | BAL | 0.065 | 0.129 | 0.127 | 0.125 | 0.123 | 0.121 | 0.006 | 0.006 | 0.006 | ||
| 22 | JN021219 | GT | PH-P | 0.065 | 0.129 | 0.127 | 0.125 | 0.123 | 0.121 | 0.006 | 0.006 | 0.006 | 0.000 | |
| 23 | AF391367 | MA | AUS | 0.163 | 0.141 | 0.137 | 0.135 | 0.141 | 0.143 | 0.161 | 0.155 | 0.155 | 0.155 | 0.155 |
Taxon: GS = Giuris sp.; GM = Giuris margaritaceus; GT = Giuris tolsoni; GV = Giuris viator; MA = Mogurnda adspersa (outgroup).
Origin: AUS = Australia; AUS/S = Australia or Sulawesi (actual site unknown); PH= Philippines; LU = Luzon (Lake Taal); M = Mindanao; P = Panay; Indonesia: CS= Central Sulawesi: A = Ampana; BS = Lake Bolano Sau; L = Luwuk; NS-T = North Sulawesi, Tondano Lake; SUL = Sulawesi, location unknown; BAL = Bali; LO = Lombok; MAL = Maluku; J-B = Java, Banten; J-E = East Java.
Phenotypic characters
The TL of Giuris sp. specimens (n=107) ranged from 7.9 cm to 16.3 cm, while weight ranged from 4.96 g to 61.0 g. The specimens in the morphometric and meristic study (n=42) ranged in size from 9.95 to 15.25 cm TL (mean 12.58 cm). In the sex-disaggregated length data set (n=69), variance was unequal between males and females (two-sample F-test, P < 0.01). The overall mean length was 12.95 cm. There was a highly significant ( P < 0.001, two-tail t-test assuming unequal variance) difference in mean length between males (13.56 cm TL, n=44) and females (11.62 cm TL, n=25), with overlapping length distributions ( Figure 5).
Figure 5. Total length (TL) distribution of male (n=44) and female (n=25) payangka ( Giuris laglaizei) from Bolano Sau Lake.
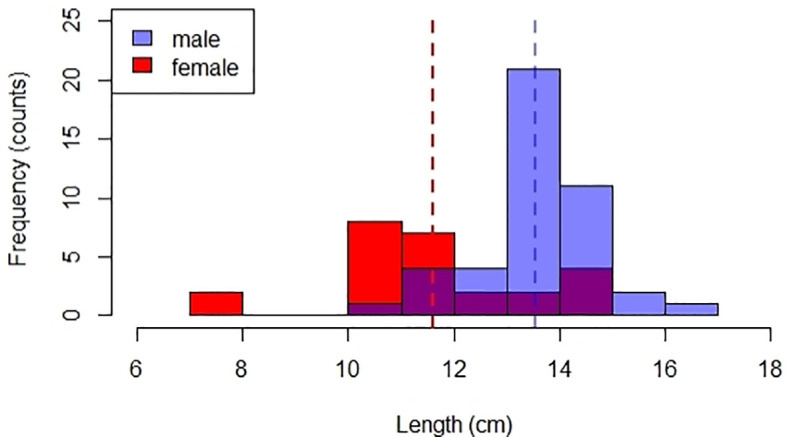
Dotted lines indicate mean TL for male (blue) and female (red) fish.
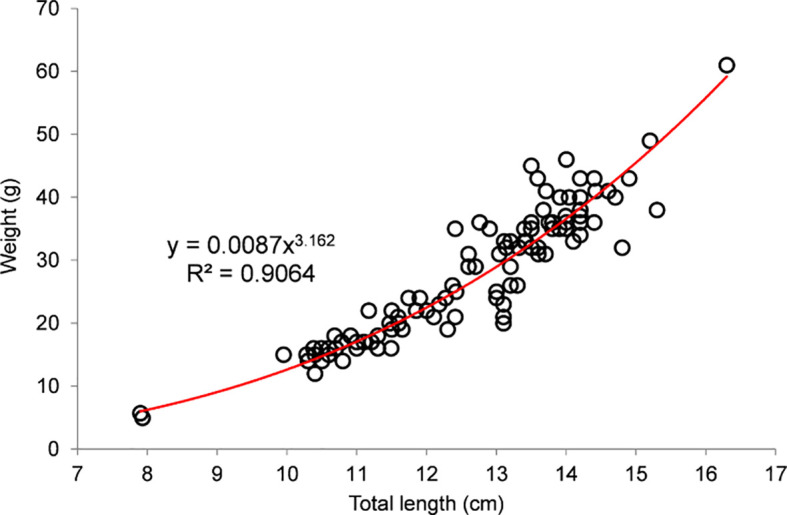
Mean length at maturity (L 50) was 9.3 cm TL for females and 11.5 TL for males. Sex ratio was significantly ( P < 0.05) different from 1:1 for all size classes except 12–13 cm TL, with female dominance below 12 cm TL and male dominance above 13 cm TL. The length-weight relationship was L=0.0087∙W 3.162 ( Figure 6), with a strong correlation (R 2=0.901) and b > 3, indicating a mildly allometric positive growth pattern.
Figure 6. Length-weight relationship of payangka ( Giuris laglaizei) from Bolano Sau Lake (n=107).
A synopsis of the 17 morphometric characters measured for Giuris laglaizei from Bolano Sau Lake ( Table 3) presents the data as absolute values (in mm) and as dimensionless ratios to TL. These data indicate considerable variability in most characters. The 6 meristic counts of Giuris laglaizei from Bolano Sau Lake ( Table 4) yield a meristic formula based on median values of D VI-I,8 A I,8 P 13 V I,5 C15.
Table 3. Bolano Sau Lake payangka (n=42) morphometric characters.
| Code ( Table 1) | Absolute values (mm) | Ratio to total length (X1-TL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | Minimum | Maximum | Mean | SD | |
| X1-TL | 99.5 | 152.5 | 125.8 | 14.6 | - | - | - | - |
| X2-SL | 80.1 | 80.1 | 101.0 | 12.4 | 72.1% | 90.0% | 80.3% | 2.8% |
| X3-HL | 24.9 | 48.1 | 33.2 | 5.0 | 20.1% | 31.5% | 26.4% | 2.0% |
| X4-UJ | 6.0 | 9.1 | 7.3 | 0.8 | 4.1% | 7.2% | 5.8% | 0.6% |
| X5-LJ | 6.5 | 9.7 | 7.8 | 0.8 | 4.7% | 7.8% | 6.3% | 0.6% |
| X6-BD | 16.7 | 39.6 | 27.4 | 4.9 | 15.9% | 27.4% | 21.7% | 2.1% |
| X7-HH | 11.2 | 37.6 | 22.7 | 5.7 | 10.7% | 27.0% | 17.9% | 3.3% |
| X8-ED | 4.5 | 7.8 | 5.9 | 0.6 | 3.6% | 6.0% | 4.7% | 0.5% |
| X9-CP | 13.5 | 22.1 | 17.9 | 2.5 | 10.4% | 16.5% | 14.2% | 1.0% |
| X10-LP | 19.3 | 31.7 | 25.1 | 3.1 | 16.6% | 26.7% | 20.0% | 1.9% |
| X11-SD | 27.9 | 54.6 | 45.4 | 5.2 | 26.9% | 39.4% | 36.1% | 2.2% |
| X12-DA | 13.2 | 41.1 | 20.6 | 4.9 | 12.7% | 27.0% | 16.3% | 2.9% |
| X13-DP | 21.2 | 51.7 | 34.5 | 8.2 | 16.9% | 36.0% | 27.4% | 5.4% |
| X14-PF | 16.6 | 31.2 | 23.6 | 3.2 | 15.2% | 21.4% | 18.7% | 1.3% |
| X15-VF | 14.0 | 30.1 | 21.3 | 3.5 | 13.3% | 20.4% | 16.8% | 1.5% |
| X16-AF | 13.3 | 33.2 | 22.3 | 4.9 | 12.3% | 22.4% | 17.6% | 2.7% |
| X17-CL | 18.3 | 34.4 | 26.7 | 4.1 | 17.4% | 25.7% | 21.2% | 1.7% |
Table 4. Bolano Sau Lake payangka meristic characters (n=42).
| Fin | Spines | Rays | |||
|---|---|---|---|---|---|
| Code | Description | Minimum | Maximum | Median | |
| D1 | Anterior dorsal | VI * | - | - | - |
| D2 | Posterior dorsal | I | 7 | 8 | 8 |
| A | Anal | I | 8 | 10 | 9 |
| P | Pectoral | - | 12 | 15 | 13 |
| V | Ventral | I | 4 | 5 | 5 |
| C | Caudal | 0 | 11 | 17 | 15 |
One specimen V.
Environmental and fisheries data
Water quality parameters tended to vary between sampling stations and times ( Table 5). The ranges considered normal for Indonesian freshwater bodies used for fisheries (classes 2 and 3) according to Government Regulation No. 82/2001 (RI 2001) are also provided for the parameters covered under this regulation. During the collection of payangka samples and water quality data, qualitative data on environmental conditions were noted. These included visual records (photographs) of general conditions; for excerpts from the visual record see Figure 7. Originally Bolano Sau Lake was surrounded by lowland tropical rainforest and sago palm dominated wetlands. Observations showed considerable anthropogenic impacts, including extensive land-use change leading to erosion and sedimentation. Extensive areas around the lake had been converted for agriculture and human habitation ( Figure 7a), and few sago palms remained in the riparian wetlands ( Figure 7b).
Table 5. Water quality data for Bolano Sau Lake (August-December 2019).
| No | Parameter | Unit | Range | Standard 109 |
|---|---|---|---|---|
| 1 | Water temperature | °C | 31.30-33.42 | Normal ± 3°C |
| 2 | Visibility | m | 0.22-0.67 | - |
| 3 | pH | 8.06-8.57 | 6-9 | |
| 4 | Total suspended solids (TSS) | mg/L | 75.04-183,99 | - |
| 5 | Dissolved oxygen (DO) | mg/L | 2.70-3.90 | 3-4 |
| 6 | Total alkalinity | mg/L | 1.5-11.5 | - |
| 7 | Hardness | mg/L | 174.28-222.54 | - |
| 8 | Total ammonia nitrogen TAN (NH3+NH4 +) | mg/L | 0.05-0.07 | - |
| 9 | Nitrate (NO3-N) | mg/L | <0.01-0.6 | <0.02 |
| 10 | Orthophosphate (PO4-P) | mg/L | <0.01 | - |
Figure 7. Photographs of Bolano Sau Lake in Central Sulawesi, Indonesia show extensive deforestation (a) and riparian wetlands with few remaining sago palms (b).
(Photographs taken by Samliok Ndobe).
During the baseline survey, 44 fish samples collected through experimental fishing in collaboration with local fishermen using 3½″ gillnets. 45 Six species were reported: Nile tilapia Oreochromis niloticus (comprising nearly 77% of the experimental catch), payangka, striped snakehead ( Channa striata), climbing perch ( Anabas testudineus), gourami ( Trichogaster sp.), a tank goby ( Glossogobius sp.), and the Mozambique tilapia ( Oreochromis mossambicus). Local fishermen also reported catching common carp ( Cyprinus carpio) and freshwater eels ( Anguilla sp.) in the lake. This survey also revealed a predominantly sandy (67-73%) lake substrate with some silt (14-22%) and clay (11-13%). Phytoplankton concentration was 14,580/L, dominated by Cylindrospermopsis spp., Dinobryon spp., and Cyclotella spp. Mean zooplankton density was 28,890/L, dominated by unidentified larvae (Annelida, Crustacea (shrimps), Mollusca (bivalves) and fish) and Paramecia.
Discussion
Giuris phylogeny, biogeography and first species record
Barcode sequences (COI gene sequence fragments) over 600 bp are considered sufficient for differentiating between animal species, with 98-100% similarity generally indicating species identity. 56 The NCBI blastn and BOLD Identity routine results place the specimen collected (GenBank accession OM674614) within the taxon named as Giuris margaritacea (Chordata, Actinopterygii, Gobiiformes, Eleotridae) in these databases. Some unexpected sequence placements may have been due to errors in specimen identification, as might be expected within this taxonomic group. 47 , 48 Examples include the nesting of Xenisthmus sp. from Australia (accession AF391372 47 ) in the Mogurnda clade, Ophiocara porocephala from Bangladesh (accession MK572389 74 ) in a Giuris margaritaceus sub-clade, and well-defined clades with a mixture of species names in the genera Eleotris and Gobiomorus. The nesting of O. porocephala from Bangladesh in the G. margaritacea clade lends credence to the possibility that some reports of payangka as O. porocephala may be similar cases, and point to a possible history of mistaken identity between two taxa which are not closely related genetically.
First recorded and described from the Solomon Islands, 75 the snakehead gudgeon G. margaritaceus has been thought to have a widespread distribution in the Indo-Pacific region. 76 The gudgeon specimen examined by Valenciennes and named as Giuris margaritacea Valenciennes 1837, with the French common name éléotris perlé, was 6 inches long and collected from Vanikolo in the Solomon Islands by Quoy and Gaimard. 75 G. margaritaceus is reported from Madagascar 77 and the Indian sub-continent 78 to Papua New Guinea and Pacific Islands, 75 , 77 , 79 – 82 and from the Philippines 52 , 83 to northern Queensland and north-eastern Australia. 47 , 77 , 84 , 85 Considered native to Indonesia, it has been reported from Sumatra 86 in the west to Papua 51 in the east, including Sulawesi. 6 , 14
Historically, the large number of G. margaritaceus synonyms appears related to the geographical distribution of this taxon. Original references ( e.g. Cuvier and Valenciennes, 1837 75 and Sauvage, 1880 87 ) tend to give different species names to specimens collected from different islands or regions, even when they note a high level of similarity between morphological traits, with descriptions typically based on a small sample (in many cases just one specimen). Subsequent studies lead to a consensus view of the genus Giuris as monophyletic, with G. margaritacea or G. margaritaceus as the most senior (and hence valid) species name, as reflected in databases including the NCBI GenBank and BOLD, FishBase, the World Register of Marine Species (WoRMS), and Eschmeyer's Catalog of Fishes. However, Kottelat 46 noted that: “The wide distribution and the observed variability of G. margaritaceus suggests that more than one species might be confused under this name”. The structure of the trees in this study ( Figures 3 and 4) not only show deep divisions consonant with multiple species within Giuris but also indicate widespread misidentification within Eleotridae and a need for taxonomic revision within other eleotrid taxa.
The deep divisions within the putative G. margaritaceus clade in Figure 3 are similar to the deep divisions in Glossogobius giuris from India. 49 A study on the ichthyofauna of Java and Bali 88 reported two BOLD BINs for Giuris margaritacea from this region, with a genetic distance of 12.56%, indicating more than one species in this region of Indonesia. Recent studies on the genus Giuris within the Indonesian Archipelago 15 and other regions of the Indo-Pacific 53 collectively resurrect and redescribe three species previously synonymised with G. margaritaceus ( G. laglaizei Sauvage 1880; Giuris aporocephalus Macleay 1884; Giuris tolsoni Bleeker 1854), redescribe Giuris margaritaceus, and describe four new species ( Giuris charpini Keith & Mennesson 2020; Giuris yahayai Keith & Mennesson 2020; Giuris caussei Keith, Mennesson & Lord 2020; and Giuris viator Keith, Mennesson, Lord, Hubert 2020). However, the analyses in this study indicate the range distributions for Giuris species in Keith et al. (2020) and Keith & Mennesson (2020) 15 , 53 are still incomplete.
It is interesting that no Giuris sequence from Indonesia were closely related to the payangka from Bolano Sau Lake ( Figure 4), and evolutionary relationships within Giuris do not necessarily seem to follow readily discernible patterns based on past or present geographical distance. For example, as in Ref. 53, Australian and Philippine clades (the latter including payangka from Bolano Sao Lake) seem more closely related to each other than to species found in areas lying between these two regions ( Figure 4; Table 2), with one of the two barcoded lacustrine Giuris populations from the northern arm of Sulawesi (Bolano Sau and Tondano) belonging to each of these clades. The tree topography ( Figure 4) and location of the closest matching sequences (Taal Lake, Luzon, Philippines) strongly suggested that the payangka, as well as the Philippine sequences within which it is nested, are in fact Giuris laglaizei Sauvage 1880 (originally Eleotris (Giuris) laglaizei). This species was first described from a specimen with the local name poi-poi collected near Manila in the Philippines. 87 Unfortunately, sequences from Ref. 53 were not available in either GenBank or BOLD databases at the time of this study. However, Philippe Keith of the National Museum of Natural History of Paris kindly provided a sequence of G. laglaizei from the Philippines (Number 14583 in Ref. 53), which was not yet available in public databases. As expected, this sequence nested within the same Giuris clade as the payangka ( Figure 4). The identity was 99.83% (one nucleotide difference in the aligned dataset) with a genetic distance of ≈ 0.00136. Based on this result, the Bolano Sau Lake payangka can be identified as G. laglaizei, a first record for Sulawesi and Indonesia, considerably extending the known distribution of this species.
Comparison with Ref. 15 and Ref. 53 strongly suggests that the Australian sister clade to that containing the payangka in Figure 4 is most likely Giuris aporocephalus Macleay 1884. The six specimens from Tondano Lake resolved within this clade. If indeed the Giuris in Tondano Lake are descended from introduced payangka sourced from Limboto Lake over 100 years ago, then it is likely that the payangka in Limboto Lake also belong to this clade, and are therefore not the same species as the payangka in Bolano Sau Lake, even though these two lakes are relatively close to each other. However homologous barcode (mtDNA COI) sequences for Limboto Lake payangka are not yet available, despite recent research using the Cyt-b genetic marker. 36
Table 6 shows the known and (strongly) suspected species identities and distributions of species within the genus Giuris. This study brings the number of Giuris species in Indonesia and Sulawesi to five, with four species in freshwater ecosystems around the coasts of Tomini Bay.
Table 6. Species in the genus Giuris and their known (X) or suspected ( X) distributions.
| No | Giuris Species a | Indonesia b | Other Countries c | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | A | L | T | S | M | K | B | J | P | N | A | C | J | B | M | ||
| 1 | G. aporocephalus | X | X | X | X | ||||||||||||
| 2 | G. charpini | X | |||||||||||||||
| 3 | G. caussei | X | |||||||||||||||
| 4 | G. laglaizei | X | X | ||||||||||||||
| 5 | G. margaritaceus | X | X | X | X | X | X | ||||||||||
| 6 | G. tolsoni | X | X | X | X | X | X | X | X | ||||||||
| 7 | G. viator | X | X | X | X | X | X | ||||||||||
| 8 | G. yahayai | X | |||||||||||||||
Sources: No. 1-8: Ref. 53; No.1: inferred from Ref. 25 and Ref. 50; No. 4: this study; No. 5-7: Ref. 15; No. 7: Ref. 74.
O = Bolano Sau Lake (this study): A = Ampana; L = Luwuk; T = Tondano Lake, North Sulawesi (possibly also Limboto Lake in Gorontalo); S = Sulawesi, unknown site; M = Maluku; K = Lombok; B = Bali; J = Java; Grey shading and bold font = sites in Sulawesi.
P = Philippines; N = Papua New Guinea; A = Australia; C = Pacific islands; J = Taiwan and Japan (Okinawa); B = Bangladesh; M = Madagascar, Mayotte and Comoros.
Morphometric and meristic characters within Giuris
Data on the length-weight relation of Giuris sp. appear to be limited and confined to Indonesia. Length-weight relation parameters and size ranges of payangka ( Giuris laglaizei) from Bolano Sau Lake and other Giuris populations in Indonesia ( Table 7) show a general tendency towards allometric positive growth patterns. The mildly allometric positive length-weight relation for Bolano Sau payangka is within the range reported for other populations of Giuris sp. The value of b (3.162) is slightly lower than that reported from Tondano Lake (Makmur et al. 2019) and Santani Lake in Papua and close to the lower limit of Giuris sp. from Limboto Lake. However, a value of b > 3 indicates that food availability is unlikely to be a limiting factor.
Table 7. Length-weight parameters and size range of Giuris sp. from sites in Indonesia.
| Site | Sample | Length-weight parameters | Source | ||
|---|---|---|---|---|---|
| n | Size range (cm) | a | b | ||
| 1. Bolano Sau Lake | 107 | 7.9-16.3 | 0.0087 | 3.162 | This study |
| 2a. Tondano Lake (male) | 249 | 10.7-19.0 | 0.0054 | 3.27 | 10 |
| 2b.Tondano Lake (female) | 353 | 10.5-20.5 | 0.004 | 3.38 | |
| 3. Limboto Lake | 309 | no data | 0.003-0.005 | 3.13-3.36 | 108 |
| 4. Sentani Lake, Papua | 64 | no data | 0.0044 | 3.36 | ( http://www.fishbase.org) |
The length distribution of Giuris spp. specimens in this study was biased towards fish large enough to have potentially achieved sexual maturity. This is important because all samples were collected with gears (throw nets and gillnets) currently used by the Bolano Sau fishing community. With respect to the gillnet fishery, 67% of fish caught during the baseline survey of Bolano Sau Lake were found to be sexually mature, with a mean size at first maturity (both sexes combined) of 11.92 cm. 45 Re-analysing the data disaggregated by sex ( Figure 5), the mean size at capture was greater than the estimated size at sexual maturity for both sexes. This analysis also indicates that the fishery is selective for males, and could explain the male bias previously reported for this population. 45
A study of Giuris spp. (most likely G. aporocephalus) in Tondano Lake, North Sulawesi 10 also found a larger mean length in males (14.15 cm) than in females (13.75 cm), although the difference was less marked than for the Bolano Sau Lake payangka identified as G. laglaizei in this study. Furthermore, mean and maximum sizes sampled for both sexes in Tondano Lake were larger than in Bolano Sau Lake ( Table 7). The generally smaller size of payangka in Bolano Sau Lake compared with Tondano Lake 10 could be related to inter-specific differences and/or environmental factors. A comparison between selected morphometric characters of the Bolano Sau Lake payangka and eight Giuris species recently described or re-described is presented in Table 8 while Table 9 shows comparative data on meristic characters.
Table 8. Comparison of selected morphometric characters within the genus Giuris.
| No | Species a | n | Nearest whole percentage of standard length (SL) | ||||
|---|---|---|---|---|---|---|---|
| Head length | Body depth | CP b depth | Jaw length | Eye diameter | |||
| 1 | Giuris laglaizei ( payangka) | 42 | 27-40 | 19-35 | 12-22 c | 6-11 | 4-7 |
| 2 | G. aporocephalus | 12 | 31-36 | 20-25 | 13-16 | 9-11 | 5-7 |
| 3 | G. caussei | 2 | 36 | 22-25 | 14-16 | 10 | 4-6 |
| 4 | G. charpini | 8 | 31-33 | 19-25 | 13-16 | 10-11 | 5-7 |
| 5 | G. laglaizei | 7 | 30-35 | 22-27 | 14-17 | 8-11 | 5-6 |
| 6 | G. margaritaceus | 12 | 30-35 | 20-25 | 13-16 | 10-11 | 6-8 |
| 7 | G. tolsoni | 11 | 31-37 | 20-24 | 13-15 | 9-12 | 6-7 |
| 8 | G. viator | 10 | 31-35 | 20-24 | 14-15 | 10-12 | 6-8 |
| 9 | G. yahayai | 10 | 31-36 | 26-39 | 16-20 | 9-12 | 4-6 |
| 10a | G. margaritacea | 5 M | 32 | 24 | 12 | 6 | 6 |
| 10b | G. margaritacea | 5 F | 32 | 25 | 13 | 6 | 5 |
Table 9. Comparison of meristic characters within the genus Giuris.
| No | Species a | n | Fin spines and rays b | |||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | A | P | V | C | |||
| 1 | Giuris laglaizei ( payangka) | 42 | V-VI | I,7-8 | I,8-10 | 12-15 | I,4-5 | 11-17 |
| 2 | G. aporocephalus | 12 | VI | I,8 | I,9 | 14-15 | I,5 | 13-14 |
| 3 | G. caussei | 2 | VI | I,8-9 | I,9 | 14-15 | I,5 | 13 |
| 4 | G. charpini | 8 | VI | I,8 | I,8-9 | 13-14 | I,5 | 13-14 |
| 5 | G. laglaizei | 7 | VI | I,8 | I,9 | 15 | I,5 | 14-15 |
| 6 | G. margaritaceus | 12 | VI | I,8 | I,9 | 14-15 | I,5 | 13-14 |
| 7 | G. tolsoni | 11 | VI | I,8 | I,9 | 14 | I,5 | 13-15 |
| 8 | G. viator | 10 | VI | I,8 | I,9 | 14 | I,5 | 13-14 |
| 9 | G. yahayai | 10 | VI | I,8-9 | I,9 | 14 | I,5 | 15 |
| 10 | G. margaritacea (5 male, 5 female) | 10 | VI | I,9 | I,9 | 16 | - | - |
| 11 | G. margaritacea | no data | VI | I,8 | I,9 | 14-15 | - | - |
Sources: No. 1: This Study; No. 2-9: Ref. 53; No. 6-8: also Ref. 15; No. 10: Ref. 10; No. 11: http://www.fishbase.org.
D = dorsal fins (D1 = anterior; D2 = posterior); A = anal fin; P = pectoral fins; V = ventral (pelvic) fins; C = caudal fin.
Unlike the genetic (DNA barcoding) data, a comparison between morphometric and meristic characters of the Bolano Sau Lake payangka and the eight Giuris species recently described or re-described 15 , 53 in Tables 8 and 9 does not give a clear indication regarding the taxonomic identity of the Bolano Sau Lake Giuris population, although some characters are similar to the Philippine Giuris laglaizei. The wider range in Bolano Sau Lake payangka compared with most Giuris species for most of the characters could be related to the larger number of samples (42 compared with 2-12 fish). Although some morphometric characters have been used in discussing or determining the characteristics of species within the genus Giuris, in particular the resurrecting of synonymised species and the description of new species resulting in a total of eight now-recognised Giuris species, 15 , 53 there are many similarities. All eight are described as having a body shape which is more ovoid than elongated, with G. yahayai also having a somewhat backed appearance. Other common features include sexual dimorphism and known or suspected amphidromy.
Life history of payangka (genus Giuris)
Amphidromy as described by McDowall (2007) is characterised by “reproduction in fresh water, passage to sea by newly hatched larvae, a period of feeding and growing at sea usually a few months long, return to fresh water of well-grown juveniles, a further period of feeding and growing in fresh water, followed by reproduction there” and can be obligate or facultative. 89 The presence of all life stages including adults (payangka) and larvae (nike) in Tondano Lake 25 indicates that amphidromy is most likely facultative in at least some Giuris species. Mean length at first maturity in Tondano Lake payangka has been reported as 10.75 cm (females) 10 and 13.4 cm (males)/13.7 cm (females). 30 Reported values for fecundity range from 12,000 to 127,000, increasing with female size. 10 , 28 Eggs fertilised in the morning hatch the following night and the larvae begin swimming after about 10 minutes, even though fins have not yet developed. 28 Although in the past all or some of these larvae may have been carried to the sea and completed an amphidromous life-cycle, the Tondano Lake Giuris population seems to have adopted a fully freshwater lifecycle. It has been proposed that, although this non-migratory lifestyle may have evolved within the population over a more extended time, it may have become the prevailing mode of reproduction as an adaptation to the construction of three hydroelectric power plant dams preventing downstream and upstream migrations. 25 Alternatively, if the Tondano Lake payangka was indeed introduced in 1902 from Limboto Lake, 34 then the adaptation may date from this introduction. The lack of genetic variation in the COI barcode for the six specimens sequenced by Pangemanan et al. 25 ( Figure 4) may be the result of a small founder population followed by such selection.
Whether amphidromy is obligate or facultative is an important consideration with respect to the Bolano Sau Lake payangka population. Despite the lake’s proximity to Tomini Bay, there is no permanent feature enabling fish to move between the lake and the sea. However, according to local fishermen seasonal flooding occurs at times during the raining season, creating a temporary connection. No larvae or small juveniles were found during the survey. While this may be an artefact of the collection methods used and/or the timing of the sampling, local fishermen do not catch nike in the lake, and did not report large schools of larvae. Further observations (monitoring) of Bolano Sau Lake payangka reproductive patterns and identification of early life stages in and/or near to the lake could shed light on this question.
If the payangka in Bolano Sau Lake is amphidromous and found in other waterbodies nearby, there is hope for natural recruitment from the wider Tomini Bay population to boost the population in the lake. The facilitation of such a process through the transport of migrating larvae (nike) is unlikely to be a wise move, as shoals of nike are typically multispecies, comprising several genera of Gobiidae and/or Eleotridae 11 , 23 , 90 as well as other taxa such as glass eels of the genus Anguilla and crustacea. 12 , 19 – 21 Therefore, shoals of nike are unlikely to be composed solely of Giuris laglaizei or indeed other species currently present in Bolano Sau Lake.
It is also possible that the payangka arrived in Bolano Sau Lake at some period(s) in the past when geological and hydrological features were more conducive to the migrations of diadromous fishes, and then adopted a fully freshwater life cycle, as seems to be the case for the payangka in Tondano Lake. In this case, it might be necessary to support recovery of the severely depleted Giuris population through well-planned release of captive bred fish. Initial steps towards captive breeding of the Tondano Lake payangka ( Giuris sp.) have been taken, including ex-situ husbandry of larvae and juveniles. 28 If indeed the Giuris sp. in Tondano Lake became established after the introduction of payangka from Limboto Lake in 1902, 34 , 91 it would indicate that such an approach might be successful. However, any such moves should follow the national guidelines for re-stocking, 92 in particular with respect to biosecurity (e.g. pests and disease), as well as ensuring the fish used for re-stocking are indeed the same species and ideally come from populations with similar genetic and other characteristics.
From an ecological perspective, trophic relations are an important consideration. Species reported as preying on G. margaritacea and/or O. porocephala include the piscivorous goby Glossogobius giuris. 93 The payangka in Tondano Lake is omnivorous but appears to undergo an ontogenetic shift in dietary preference, becoming increasingly carnivorous. Juveniles in the 12-30 mm TL range are reported as planktivorous, with the proportion of zooplankton relative to phytoplankton increasing as the fish grow; they begin to prey on Caradina shrimp at around 30 mm TL and on molluscs (Gastropods) and fish (including smaller conspecifics) at around 36-40 mm TL. 31 However, such data are lacking for the payangka from Bolano Sau Lake, and indeed for the species G. laglaizei in the Philippines.
Conservation status and fisheries management
The IUCN Red List of Threatened Species re-assessment of the snakehead gudgeon G. margaritacea in 2019 76 lists the species under the Least Concern (LC) category, with the rationale that the species is widespread and common in parts of its range, and that it is found in a wide variety of habitats. With respect to fisheries, the assessment mentions that this taxon is harvested for the international aquarium trade and in localised areas is also used for subsistence level consumption and as a bait fish, but levels of exploitation are unknown. The assessment also notes the need for further taxonomic work to determine if the Western Pacific and Western Indian Ocean subpopulations are conspecific. Recent genetic evidence validates this concern as the newly described Giuris yahayai appears to be limited to the Indian Ocean, 53 while seven species have been identified in the Indo-West Pacific with at least three species present in eastern Central Sulawesi, the Moluccas, and the Philippines. 15 , 53 The boundaries of each species cannot as yet be determined with certainty; however, the known ranges of several species vary in extent and in some cases overlap. 53 Together with the identification of the payangka from Bolano Sau Lake as G. laglaizei, the reanalysis of Giuris sequences from Tondano Lake in Pangemanan et al., (2020) as belonging to a different clade from all other Sulawesian Giuris sequences (most likely G. aporocephalus) means that at least five Giuris species are present in Sulawesi, with four species found in Tomini Bay watersheds. Future barcoding studies may further increase the known range of one or several Giuris species.
The deep divisions in the genus Giuris call into question the validity of the LC status 76 for at least some of the species formerly considered as a single taxon, G. margaritacea. The fragmented nature and uneven size of known distributions indicate that some of the eight species currently apparent within this genus could be at risk from serial extirpation and even extinction. At least one Giuris population (species unknown) from the Proserpine River in Australia appears to have been lost. 76 , 85 It may never be known if this was a now extinct species or an extirpated population of a widespread species.
The principal threats mentioned in the Red List assessment 76 are subsistence and ornamental fisheries. However, alien or exotic invasive species introductions are considered a major threat to freshwater fish biodiversity worldwide. 94 In particular, wild Nile tilapia ( Oreochromis niloticus) populations are increasingly widespread across Indonesia 39 – 42 , 95 – 97 and have been implicated in the decline of native species in many inland waters across the Indonesian Archipelago. 39 , 40 , 97 – 99 Mechanisms through which introduced species, including the Nile tilapia, could affect the payangka and other native fishes include competition for food and habitat; the introduction and transmission of parasites and disease; predation, especially on eggs and larvae or juveniles; and behaviour leading to habitat degradation 39 , 41 , 97 , 100 – 102
The majority of data on Giuris fisheries are from Tondano Lake in North Sulawesi, approximately 500 km east of Bolano Sau Lake. Payangka has historically been the main fisheries species in Tondano Lake, comprising 35% of the total production volume in 1980. 28 , 100 All sizes from 9 mm to 200 mm are reported in fisheries catch 28 , 31 ; however the nike fisheries target postlarvae and fingerlings in the 12-30 mm TL range, while payangka catches were dominated by the size range 105-135 mm TL, with few larger individuals. While the nike fishery is economically viable, with relatively high income and profit margins, 27 concerns have been expressed regarding the aggregate ecological sustainability of the fisheries targeting payangka in the lake. 30 Strong indications of overfishing were already apparent around 30 years ago, with annual catch volume reduced to 25% of that in 1980-1985 by 1990. 31 The introduction of alien species has been implicated as a causal factor of declining Giuris catches and abundance. In North Sulawesi, introduced catfish ( Clarias sp.) are reported to have had negative impacts on payangka stocks in Tondano Lake. 100 However, it seems the payangka itself may be an introduced species in this waterbody. 34
With respect to the Bolano Sau Lake payangka, this once common and popular food fish has become increasingly rare since the introduction of alien species under government programs intended to increase fisheries production, although overfishing is also suspected as a factor. 45 In Bolano Sau Lake, government-supported “re-stocking” has occurred in the lake over several decades. 35 The most abundant alien species in 2019 was the Nile tilapia ( Oreochromis niloticus), while other introduced alien species included the Mozambique tilapia ( Oreochromis mossambicus) and gourami ( Trichogaster sp.). Two other species, the striped snakehead Channa striata and climbing perch Anabas testudineus, are widely considered as native fishes by Sulawesians and figure in many traditional dishes, although they may have been introduced to Sulawesi, possibly in prehistoric times. 6 , 103 , 104 With respect to introduced species, it is interesting to note that not all species introduced to Bolano Sau Lake have become established or invasive. For example, the common carp ( Cyprinus carpio) had been repeatedly introduced prior to 2016, 35 but none were seen during the surveys in 2019. Fishermen reported that after the introduction they did catch carp; but the numbers dwindled over time, in contrast to the Nile tilapia ( O. niloticus) which quickly became the dominant species in the lake.
The IUCN Red List assessment 76 describes the habitat of G. margaritaceus as streams, while in Northern Australia the most common habitat is described as small rainforest creeks and wetlands located close to the river mouth 85 ; lacustrine habitat is not mentioned. However, Giuris species have been found in both coastal streams and lakes, and would seem that lakes are a key habitat for at least some Giuris species in Indonesia, and specifically in Sulawesi, as well as in the Philippines and Papua New Guinea. 15 , 52 , 53 , 105 It would seem likely that G. laglaizei may be a predominantly lacustrine species, as the known Philippine populations are all lacustrine, as is the Sulawesi population in this study.
As in many regions worldwide, 94 lakes in this region are typically subject to significant anthropogenic disturbance leading to habitat alteration and degradation. 40 , 41 , 106 , 107 This includes lakes known to have Giuris populations such as Limboto and Tondano, 100 , 108 with negative impacts on payangka stocks including changes in condition factor. 100 Quantitative data ( Table 5) and qualitative observations indicate potential threats to the Bolano Sau Lake environment as a habitat for fish. Parameters of concern include temperature, consistently above 31°C with a maximum in excess of 33°C, and dissolved oxygen (DO). The latter was consistently below 4 mg/L and sometimes below 3 mg/L, the lower limit considered acceptable by Government Regulation No. 82/2001. 109 The low levels of DO may be related to the elevated temperature, as the capacity to retain oxygen is inversely correlated with water temperature. 110 The gill oxygen limitation theory (GOLT) proposed by Pauly 111 posits that fish growth and size are limited by the availability of oxygen; higher temperatures increase metabolic rates, lowering the size at which the limitation will be reached. Thus, the high temperature and low DO values recorded in Bolano Sau Lake could be a contributing factor to the relatively small maximum size of the payangka. Locally high levels of nitrate (NO3-N) may be due to sewage and/or fertiliser run-off, and could and act in synergy with the temperature and low oxygen conditions, especially as toxicity increases with temperature. 110 Combined with the observed land use/land cover changes, these data call for integrated watershed management.
Conclusion
The COI barcoding approach identified a fish locally called payangka from Bolano Sau Lake in Central Sulawesi Indonesia as the recently resurrected Giuris laglaizei. This represents the first record of G. laglaizei in Indonesia and indeed the first outside the Philippines. However, in contrast to the molecular approach, the phenotypic characters measured or counted in this study could not enable a definitive identification of the Giuris sp. in Bolano Sau Lake to species level. In addition, other characteristics noted during this study and other visits to the study site are ambiguous in terms of taxonomic identification. For example, both colour and general appearance vary between sexes, stage of the reproductive cycle, and even habitat characteristics within the lake. These results and considerations strongly indicate the advisability of further research using molecular biology methods to resolve the taxonomic identity of other Giuris sp. populations throughout the distribution of this genus, including the use of multiple molecular markers.
The phylogenetic analysis of Giuris highlights the complex biogeography of this genus in Indonesia, with at least four Giuris species present in the coastal regions around Tomini Bay and five in Sulawesi. These findings call into question the IUCN Red List Least Concern status of the taxonomic unit, until recently named Giuris margaritacea, now revealed as a genus comprising at least eight species. While identifying the species present is a first step towards managing and preserving fish biodiversity in inland waters, this needs to be followed by appropriate management. The threats to and sharp decline of the Bolano Sau Lake payangka population are reflected in similar threats and/or trends reported for other Giuris spp. populations, and could result in serial extirpations if unchecked. From both biodiversity and fisheries perspectives, there is a need to manage freshwater fisheries resources to conserve native fish species still present, including the Bolano Sau Lake payangka.
Data availability
Underlying data
Harvard Dataverse: Bolano Sau Lake Fish Data https://doi.org/10.7910/DVN/JL5JP9. 69
This project contains the following underlying data:
-
•
Giuris_payangka_data_2019.tab (dataset)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
NCBI Metazoan Mitochondrial COX1 SUB10960949 63 :
-
•
The partial Cytochrome C Oxidase subunit I gene mitochondrial DNA sequence of Giuris laglaizei, Accession OM674613 https://www.ncbi.nlm.nih.gov/nuccore/OM674613
-
•
The partial Cytochrome C Oxidase subunit I gene mitochondrial DNA sequence of Anabas testudineus, Accession OM674614 https://www.ncbi.nlm.nih.gov/nuccore/OM674614
Extended data
Harvard Dataverse: GenBank Accessions, BOLD Records (mitochondrial COI gene sequences) and other nucleotide sequences used for phylogenetic analyses of Eleotridae and Giuris spp. https://doi.org/10.7910/DVN/WMDHOJ. 112
This project contains the following extended data:
-
•
Table S1_mtDNA_COI_sequence_references.pdf (Table S1)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Reporting guidelines
Harvard Dataverse: ARRIVE checklist for ‘DNA barcoding detects resurrected taxon Giuris laglaizei (Sauvage 1880) in Sulawesi, Indonesia: Bolano Sau Lake payangka phylogeny, phenotypic characters and implications for Giuris spp. conservation’ https://doi.org/10.7910/DVN/JL5JP9. 69
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
The authors gratefully acknowledge a vital contribution from Philippe Keith of the National Museum of Natural History, Paris in the form of a Giuris laglaizei COI nucleotide sequence which was not yet available through public databases. The authors also wish to thank the Parigi Moutong District Marine and Fisheries Service, Central Sulawesi and the Faculty of Animal Husbandry and Fisheries, Tadulako University, Palu for support during this research.
Funding Statement
This study was partially supported by the Parigi Moutong District Marine and Fisheries Service, Central Sulawesi under the Bolano Sau Lake Baseline Survey Project in collaboration with Universitas Tadulako.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Hutama AA, Hadiaty RK, Hubert N: Biogeography of Indonesian Freshwater Fishes: Current Progress. Treubia. 2016;43:17–30. Reference Source [Google Scholar]
- 2. Hubert N, Kadarusman WA, Busson F, et al. : DNA Barcoding Indonesian freshwater fishes: challenges and prospects. DNA Barcodes. 2015;3(1):144–169. 10.1515/dna-2015-0018 [DOI] [Google Scholar]
- 3. Keith P: Biology and ecology of amphidromous Gobiidae of the Indo-Pacific and the Caribbean regions. J Fish Biol. 2003 Oct;63(4):831–847. 10.1046/j.1095-8649.2003.00197.x [DOI] [Google Scholar]
- 4. Keith P, Lord C: Tropical Freshwater Gobies: Amphidromy as a Life Cycle. Patzner RA, Tassell JL, Kovačić M, et al., editors. The Biology of Gobies. Boca Raton: CRC Press;2012; p.243–77. 10.1201/b11397 [DOI] [Google Scholar]
- 5. Whitten AJ, Bishop KD, Nash SV, et al. : One or More Extinctions from Sulawesi, Indonesia?. Conserv Biol. 1987;1(1):42–48. 10.1111/j.1523-1739.1987.tb00007.x [DOI] [Google Scholar]
- 6. Kottelat M, Whitten AJ, Kartikasari S, et al. : Freshwater fishes of Western Indonesia and Sulawesi. Singapore: Periplus TD;1993;428. [Google Scholar]
- 7. Hadiaty RK: Status taksonomi iktiofauna endemik perairan tawar Sulawesi (Taxonomical status of endemic freshwater ichthyofauna of Sulawesi). J Iktiologi Indones. 2018;18(2):175–190. 10.32491/jii.v18i2.428 [DOI] [Google Scholar]
- 8. Berra TM: Freshwater Fish Distribution. Chicago and London: The University of Chicago Press;2007;648. [Google Scholar]
- 9. Miesen FW, Droppelmann F, Hüllen S, et al. : An annotated checklist of the inland fishes of Sulawesi. Bonn Zool Bull. 2016;64(2):77–106. Reference Source [Google Scholar]
- 10. Makmur S, Muthmainnah D, Subagdja S: Biological characters of snakehead gudgeon ( Giuris margaritacea Valenciennes, 1837) in Tondano Lake, Minahasa, North Sulawesi, Indonesia. BIOVALENTIA Biol Res J. 2019;5(2):1–9. 10.24233/BIOV.5.2.2019.125 [DOI] [Google Scholar]
- 11. Sahami FM, Habibie SA: Exploration of adult phase of Nike fish to maintain its sustainability in Gorontalo Bay waters, Indonesia. AACL Bioflux. 2020;13(4):2829–2867. Reference Source [Google Scholar]
- 12. Nurjirana AM, Sufardin HA, Burhanuddin AI: Diversity and distribution freshwater ichthyofaunal of West Sulawesi. IOP Conf Ser Earth Environ Sci. 2020;486:012079. 10.1088/1755-1315/486/1/012079 [DOI] [Google Scholar]
- 13. Tweedley JR, Bird DJ, Potter IC, et al. : Species compositions and ecology of the riverine ichthyofaunas in two Sulawesian islands in the biodiversity hotspot of Wallacea. J Fish Biol. 2013 Jun [cited 2016 Dec 2];82(6):1916–1950. 10.1111/jfb.12121 [DOI] [PubMed] [Google Scholar]
- 14. Parenti LR, Hadiaty RK, Lumbantobing DN: Collection of freshwater and coastal fishes from Sulawesi Tenggara, Indonesia. J Iktiologi Indones. 2014;14(1):1–19. Reference Source [Google Scholar]
- 15. Keith P, Mennesson M, Sauri S, et al. : Giuris (Teleostei: Eleotridae) from Indonesia, with description of a new species. Cybium. 2020;44(4):317–329. Reference Source [Google Scholar]
- 16. Nurjirana BAI, Haris A: Diversity of penja fish (amphidromous goby) in Leppangan River, West Sulawesi, Indonesia. AACL Bioflux. 2019;12(1):246–249. Reference Source [Google Scholar]
- 17. Gani A, Wuniarto E, Khartiono LD, et al. : A note on Gobiidae from some rivers in Luwuk Banggai, Central Sulawesi, Indonesia. IOP Conf Ser Earth Environ Sci. 2020;473:012054. 10.1088/1755-1315/473/1/012054/pdf [DOI] [Google Scholar]
- 18. Gani A, Nurjirana BAA, Adriany DT, et al. : First record of Stiphodon annieae Keith & Hadiaty, 2015 (Teleostei, Oxudercidae) from Sulawesi Island, Indonesia. Check List. 2021;17(1):261–267. 10.15560/17.1.261 [DOI] [Google Scholar]
- 19. Muchsin I, Zairon, Ndobe S: Beberapa aspek biologi larva sidat ( Anguilla sp.) di muara Sungai Poso, Sulawesi Tengah. Prosiding Forum Nasional Sumberdaya Perikanan Sidat Tropik 2002. Jakarta: Badan Pengkajian dan Penerapan Teknologi;2002; p.78–83. [Google Scholar]
- 20. Sugianti Y, Saepulloh H: Keragaan alat tangkap ikan dan pengaruhnya terhadap sumberdaya ikan sidat ( Anguilla spp.). Prosiding Forum Nasional Pemacuan Sumber Daya Ikan III. 2011; p.POS-15:1-7. Reference Source
- 21. Ambo-Rappe R, Moore AM: Sulawesi Seas, Indonesia. Sheppard C, editor. World Seas: an Environmental Evaluation. 2nd edn. London, United Kingdom: Elsevier;2019; p.559–581. Reference Source [Google Scholar]
- 22. Sahami FM, Kepel RC, Olii AH, et al. : Morphometric and genetic variations of species composers of nike fish assemblages in Gorontalo Bay waters, Indonesia. Biodiversitas. 2020;21(10):4571–4581. 10.13057/biodiv/d211015 [DOI] [Google Scholar]
- 23. Nurjirana HA, Sahami FM, Keith P, et al. : Preliminary note on the morphological characters of penja (amphidromous goby postlarvae) in West Sulawesi and Gorontalo Bay. IOP Conf Ser Earth Environ Sci. 2019;370(1):012007. 10.1088/1755-1315/370/1/012007/pdf [DOI] [Google Scholar]
- 24. Makmur S, Subagdja M, Sudrajat A, et al. : Karakteristik lingkungan, keanekaragaman jenis ikan dan aktivitas penangkapan sumberdaya ikan Danau Tondano, Sulawesi Utara. Jakarta. 2015. Reference Source
- 25. Pangemanan NPL, Kepel RC, Bataragoa NE, et al. : Morphological and molecular identification of nike fish, Ophieleotris aporos in Tondano Lake, North Sulawesi, Indonesia. AACL Bioflux. 2020;13(3):1614–1621. Reference Source [Google Scholar]
- 26. Tamanampo JFWS, Bataragoa NE: Potensi dan Pengelolaan dari Juvenil ikan Payangka ( Ophieleotris aporos) di danau Tondano (Potential and Management of Juvenil Payangka fish Ophieleotris aporos in Lake Tondano). J Ilm Platax. 2017;5(2):264–272. 10.35800/jip.5.2.2017.17967 [DOI] [Google Scholar]
- 27. Pieter S, Pangemanan JF, Dien CR: Analisis kelayakan usaha penangkapan ikan nike ( Ophieleotris aporos) di Danau Tondano Desa Kaima Kecamatan Remboken Kabupaten Minahasa. Akulturasi. 2019;7(2):1365–1372. Reference Source [Google Scholar]
- 28. Susanto MK, Bataragoa NE, Moningkey RD: Size Distribution and Growth of young Payangka Fish, Ophieleotris aporos (Bleeker) from Lake Tondano. J Ilm Platax. 2017;5(2):189–197. 10.35800/jip.5.2.2017.15884 [DOI] [Google Scholar]
- 29. Rostitawati T, Wahyuddin NI, Obie M: The Poverty Puddles of the Cage Fishing Community at Limboto Lake Coast, Indonesia. J Sustain Dev. 2019;12(3):82–90. 10.5539/jsd.v12n3p82 [DOI] [Google Scholar]
- 30. Mamangkey JJ, Rogahang FHN, Adil E: Analisis struktur populasi dan tingkat kemantangan gonad ikan payangka ( Ophieleotris aporos) di Danau Tondano Sulawesi Utara. Front J Sains dan Teknol. 2019;2(3):211–219. Reference Source [Google Scholar]
- 31. Satria H, Kartamihardja ES: Distribusi panjang total dan kebiasaan makan yuwana ikan payangka ( Ophiocara porocephala). J Penelit Perikan Indones. 2002;8(l):41–50. 10.15578/jppi.8.1.2002.41-50 Reference Source [DOI] [Google Scholar]
- 32. Kartamihardja ES: Laju pertumbuhan, mortalitas, rekrutmen, eksploitasi stok ikan, dominan, dan total hasil tangkapan ikan di Danau Tondano, Sulawesi Utara. J Penelit Perikan lndonesia. 2000;6(2):1–12. 10.15578/jppi.6.2.2000.1-12 [DOI] [Google Scholar]
- 33. Satria H, Kartamihardja ES: Beberapa aspek biologi reproduksi ikan payangka ( Ophiopcara porocephala) dan manggabai ( Glossgobius giurus) di perairan Danau Limboto Sulawesi Utara. J Penelit Perikan Indones. 1995;2(3):72–79. 10.15578/jppi.2.3.1996.72-79 [DOI] [Google Scholar]
- 34. Umar C, Krismono.: Beberapa aspek limno-biologi dan perikanan di Danau Tondano, Sulawesi Utara. J Penelit Perikan Indones. 1995;4(4):1–10. 10.15578/jppi.4.4.1998.1-10 Reference Source [DOI] [Google Scholar]
- 35. Herjayanto M, Gani A, Adel YS, et al. : Iktiofauna air tawar beberapa danau dan sungai inletnya di Provinsi Sulawesi Tengah, Indonesia - Freshwater fish of lakes and it’s inlet rivers in Sulawesi Tengah Province, Indonesia. J Aquatropica Asia. 2019;4(1):1–9. 10.33019/aquatropica.v4i1.1679 [DOI] [Google Scholar]
- 36. Nuha U, Amin M, Lestari U: The molecular phylogenetic of payangga ( G. margaritacea), manggabai ( G. giuris) and hulu’u from Limboto Lake based on cytochrome B sequences. J Phys Conf Ser. 2020;1465:012006. Reference Source [Google Scholar]
- 37. Donaldson TJ, Myers RF: Insular freshwater fish faunas of Micronesia: patterns of species richness and similarity. Environ Biol Fishes. 2002;65:139–149. 10.1023/A:1020050931158 [DOI] [Google Scholar]
- 38. Dudgeon D, Smith REW: Exotic species, fisheries and conservation of freshwater biodiversity in tropical Asia: the case of the Sepik River, Papua New Guinea. Aquat Conserv Mar Freshw Ecosyst. 2006;16:203–215. 10.1002/aqc.713 [DOI] [Google Scholar]
- 39. Muchlisin ZA: First report on introduced freshwater fishes in the waters of Aceh. Indonesia. Arch Polish Fish. 2012;20(2):129–135. 10.2478/v10086-012-0015-1 [DOI] [Google Scholar]
- 40. Herder F, Schliewen U, Geiger M, et al. : Alien invasion in Wallace’s Dreamponds: records of the hybridogenic “flowerhorn” cichlid in Lake Matano, with an annotated checklist of fish species introduced to the Malili Lakes system in Sulawesi. Aquat Invasions. 2012 Nov;7(4):521–535. 10.3391/ai.2012.7.4.009 [DOI] [Google Scholar]
- 41. Serdiati N, Arfiati D, Sri Widodo M, et al. : Perspectives on sustainable management of the Poso Lake (Indonesia) endemic ricefish, Oryzias nigrimas (Actinopterygii: Adrianichthyidae). Rev Biol Trop. 2020 Nov 9;69(1):139–152. 10.15517/rbt.v69i1.42404 Reference Source [DOI] [Google Scholar]
- 42. Ndobe S, Rusaini MA, Serdiati N, et al. : Meristic characters and length-weight relation of climbing perch ( Anabas testudineus) from wetlands in Sigi District, Central Sulawesi, Indonesia. IOP Conf Ser Earth Environ Sci. 2019;370(1):012001. 10.1088/1755-1315/370/1/012001/pdf [DOI] [Google Scholar]
- 43. Yanuarita D, Inaku DF, Nurdin N, et al. : Aquatic invasive species distribution within Wallace region: A preliminary review. IOP Conf Ser Earth Environ Sci. 2020;564(1):012038. 10.1088/1755-1315/564/1/012038/pdf [DOI] [Google Scholar]
- 44. Parigi Moutong Fisheries Service : Kajian biodiversitas dan potensi sumberdaya ikan di Danau Bolano Sau Kabupaten Parigi Moutong [Study on the biodiversity and fisheries resources of Bolano Sau Lake, Parigi Moutong District]. Parigi. 2019. [Google Scholar]
- 45. Putra AE, Nurdin MS, Hasanah N, et al. : Sex Ratio and Size at First Maturity of Snakehead Gudgeon ( Giuris margaritacea) Caught with Gillnets at Bolano Sau Lake, Parigi Moutong District. J AgriSains. 2020;21(3):111–118. Reference Source [Google Scholar]
- 46. Kottelat M: The fishes of the inland waters of Southeast Asia: A catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull Zool. 2013;Supplement:1–663. Reference Source [Google Scholar]
- 47. Thacker CE: Molecular phylogeny of the gobioid fishes (Teleostei: Perciformes: Gobioidei). Mol Phylogenet Evol. 2003;26(3):354–368. 10.1016/S1055-7903(02)00361-5 [DOI] [PubMed] [Google Scholar]
- 48. Hammer MP, Adams M, Thacker CE, et al. : Comparison of genetic structure in co-occurring freshwater eleotrids (Actinopterygii: Philypnodon) reveals cryptic species, likely translocation and regional conservation hotspots. Mol Phylogenet Evol. 2019;139(June):106556. 10.1016/j.ympev.2019.106556 [DOI] [PubMed] [Google Scholar]
- 49. Laskar BA, Kumar V, Kundu S, et al. : DNA barcoding of Gobiid fishes (Perciformes: Gobiidae) from eastern and northeastern India with new record of a Gobionellinae species for the region. Mitochondrial DNA Part A DNA Mapping, Seq Anal. 2017;28(4):584–587. 10.3109/24701394.2016.1143470 [DOI] [PubMed] [Google Scholar]
- 50. Thacker CE, Hardman MA: Molecular phylogeny of basal gobioid fishes: Rhyacichthyidae, Odontobutidae, Xenisthmidae, Eleotridae (Teleostei: Perciformes: Gobioidei). Mol Phylogenet Evol. 2005;37(3):858–871. 10.1016/j.ympev.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 51. Hadiaty RK, Allen GR, Erdmannt MV: Keanekaragaman jenis ikan di Teluk Arguni, Kaimana, Papua Barat. Zoo Indones. 2012;21(2):35–42. Reference Source [Google Scholar]
- 52. Aquilino SVL, Tango JM, Fontanilla IKC, et al. : DNA barcoding of the ichthyofauna of Taal Lake, Philippines. Mol Ecol Resour. 2011 Jul 2;11(4):612–619. 10.1111/j.1755-0998.2011.03000.x [DOI] [PubMed] [Google Scholar]
- 53. Keith P, Mennesson M: Review of Giuris (Teleostei: Eleotridae) from Indo-Pacific islands, with description of three new species. Cybium. 2020;44(4):331–349. 10.26028/cybium/2020-444-004103 Reference Source [DOI] [Google Scholar]
- 54. Chang C-H, Shao K-T, Lin H-Y, et al. : DNA barcodes of the native ray-finned fishes in Taiwan. Mol Ecol Resour. 2017 Jul;17(4):796–805. 10.1111/1755-0998.12601 [DOI] [PubMed] [Google Scholar]
- 55. Hebert PDN, Gregory TR: The Promise of DNA Barcoding for Taxonomy. Savolainen V, editor. Syst Biol. 2005 Oct 1;54(5):852–859. 10.1080/10635150500354886 [DOI] [PubMed] [Google Scholar]
- 56. Hebert PDN, Cywinska A, Ball SL, et al. : Biological identifications through DNA barcodes. Proc R Soc B Biol Sci. 2003;270(1512):313–321. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ward TD, Algera DA, Gallagher AJ, et al. : Understanding the individual to implement the ecosystem approach to fisheries management. Conserv Physiol. 2016 Apr 7;4(1):cow005. 10.1093/conphys/cow005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pusey BJ, Kennard M, Arthington A: Freshwater Fishes of North-Eastern Australia. Collingwood: CSIRO Publishing;2004;699. [Google Scholar]
- 59. Pickles J: A Guide to Acceptable Procedures and Practices for Aquaculture and Fisheries Research. 4th Editio. NSW Fisheries Animal Care and Ethics Committee. Nelson Bay: NSW Department of Primary Industries;2015;61. Reference Source [Google Scholar]
- 60. Weber M, Beaufort LF: The fishes of the Indo-Australian Archipelago: Heteromi, Solenichthyes, Synentognathi, Percesoces, Labyrinthici, Microcyprini. Leiden: E. J. Brill Ltd.;1922; vol.4:434. Reference Source [Google Scholar]
- 61. Ward RD, Zemlak TS, Innes BH, et al. : DNA barcoding Australia’s fish species. Philos Trans R Soc B Biol Sci. 2005;360(1462):1847–1857. 10.1098/rstb.2005.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kumar S, Stecher G, Li M, et al. : MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ndobe S, Nurdin MS, Hasanah N, et al. : SUB10960949: Metazoan Mitochondrial COX1/Bolano Sau Lake fish COI Accessions OM674613-OM674615. NCBI GenBank;2022. Reference Source [Google Scholar]
- 64. Abdulmalik-Labe OP, Quilang JP: DNA barcoding of fishes from Lake Lanao, Philippines. Mitochondrial DNA Part B Resour. 2019;4(1):1890–1894. 10.1080/23802359.2019.1614890 [DOI] [Google Scholar]
- 65. Kimura M: A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–120. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- 66. Letunic I, Bork P: Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44(W1):W242–W245. 10.1093/nar/gkw290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Letunic I, Bork P: Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):W256–W259. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tamura K, Nei M, Kumar S: Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci. 2004 Jul 27;101(30):11030–11035. 10.1073/pnas.0404206101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ndobe S, Nurdin MS, Putra AE, et al. : Bolano Sau Lake Fish Data, V2. Harvard Dataverse. 2022. 10.7910/DVN/JL5JP9 [DOI] [Google Scholar]
- 70. R Core Team: R: A language and environment for statistical computing Version 3.6.0. Vienna, Austria: R Foundation for Statistical Computing;2019. Reference Source [Google Scholar]
- 71. RStudio Team: RStudio: Integrated Development for R. Boston, MA: RStudio Inc.;2016. Reference Source [Google Scholar]
- 72. Longenecker K, Langston R, Franklin EC: Standard operating procedure for histology-based rapid reproductive analysis of tropical fishes, Report prepared by the University of Hawaii at Manoa (UHM) for Tetra Tech’s Supplemental Technical Assistance to the USAID SEA Project and Walton Family Found. Manoa: University of Hawaii;2020;95. [Google Scholar]
- 73. Aquino LMG, Tango JM, Canoy RJC, et al. : DNA barcoding of fishes of Laguna de Bay, Philippines. Mitochondrial DNA. 2011 Aug 31;22(4):143–153. 10.3109/19401736.2011.624613 [DOI] [PubMed] [Google Scholar]
- 74. Rahman MM, Norén M, Mollah AR, et al. : Building a DNA barcode library for the freshwater fishes of Bangladesh. Sci Rep. 2019 Dec 28;9(1):9382. 10.1038/s41598-019-45379-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cuvier MLB, Valenciennes MA: Histoire Naturelle des Poissons. Douzieme T, editor. Paris: Chez F. G. Levrault;1837;540pages. Reference Source [Google Scholar]
- 76. Larson H: Giuris margaritacea, Snakehead gudgeon. The IUCN Red List of Threatened Species 2019. 2019; p.e.T196317A123380986. 10.2305/IUCN.UK.2019-2.RLTS.T196317A123380986.en [DOI]
- 77. Fricke R, Allen GR, Amon D, et al. : Checklist of the marine and estuarine fishes of New Ireland Province, Papua New Guinea, western Pacific Ocean, with 810 new records. Zootaxa. 2019 Apr 23;4588(1):1–360. 10.11646/zootaxa.4588.1.1 [DOI] [PubMed] [Google Scholar]
- 78. Azadi MA, Ul-Alam MA: Ichthyofauna of the River Halda, Chittagong, Bangladesh. Bangladesh J Zool. 2013;41(2):113–133. 10.3329/bjz.v41i2.23313 [DOI] [Google Scholar]
- 79. Allen GR, Coates D: An ichthyological survey of the Sepik River, Papua New Guinea. Rec West Aust Museum. 1990;34:31–116. Reference Source [Google Scholar]
- 80. Allen GR, Coates D: A new species of freshwater eleotridid fish from northern Papua New Guinea. Rec West Aust Museum Suppl. 1990;34:131–137. Reference Source [Google Scholar]
- 81. Coates D: Fish ecology and management of the Sepik-Ramu, New Guinea, a large contemporary tropical river basin. Environ Biol Fishes. 1993;38(4):345–368. 10.1007/BF00007528 [DOI] [Google Scholar]
- 82. Ohee HL, Ngamelubun G, Ansaka JJ, et al. : Ekologi dan Kelimpahan Ikan Sentani Gudgeon ( Oxyeleotris heterodon, Weber 1908) dan Snakehead Gudgeon ( Giuris margaritacea, Valenciennes 1837) di Danau Sentani, Papua. J Biol Papua. 2019;11(1):24–32. 10.31957/jbp.646 Reference Source [DOI] [Google Scholar]
- 83. Masagca JT, Ordonez JA: Chromosomes of the Phillipine snake-head gudgeon, Ophieleotris aporos (Eleotridae) from Lake Taal, Luzon Island. Biotropia (Bogor). 2007;14(1):1–8. 10.11598/btb.2007.14.1.20 [DOI] [Google Scholar]
- 84. Unmack PJ: Biogeography of Australian freshwater fishes. J Biogeogr. 2001;28(9):1053–1089. 10.1046/j.1365-2699.2001.00615.x [DOI] [Google Scholar]
- 85. Pusey BJ, Burrows DW, Kennard MJ, et al. : Freshwater fishes of northern Australia. Zootaxa. 2017;4253(1):1–104. 10.11646/zootaxa.4253.1.1 [DOI] [PubMed] [Google Scholar]
- 86. Hadiaty RK, Sauri S: Iktiofauna air tawar Pulau Enggano, Indonesia. J Iktiologi Indones. 2018;17(3):273. 10.32491/jii.v17i3.365 [DOI] [Google Scholar]
- 87. Sauvage MHE: Desription des Gobioides nouveux ou peu connus de la collection du Museum d’Histoire Naturelle. Bull la Société Philomath Paris. 1880;4(Series 7):40–58. Reference Source [Google Scholar]
- 88. Dahruddin H, Hutama A, Busson F, et al. : Revisiting the ichthyodiversity of Java and Bali through DNA barcodes: taxonomic coverage, identification accuracy, cryptic diversity and identification of exotic species. Mol Ecol Resour. 2017 Mar;17(2):288–299. 10.1111/1755-0998.12528 [DOI] [PubMed] [Google Scholar]
- 89. McDowall RM: On amphidromy, a distinct form of diadromy in aquatic organisms. Fish Fish. 2007;8(1):1–13. 10.1111/j.1467-2979.2007.00232.x [DOI] [Google Scholar]
- 90. Sahami FM, Kepel RC, Olii AH, et al. : Determination of morphological alteration based on molecular analysis and melanophore pattern of the migrating Nike fish in Gorontalo Bay, Indonesia. AACL Bioflux. 2019;12(4):1358–1365. Reference Source [Google Scholar]
- 91. Pontoh O: Analisis usaha perkembangan budidaya ikan dalam jaring apung di Desa Tandengan Kabupaten Minahasa (Analisis of fish culture development in floating net at Tandengan Village Minahasa Regency). Budid Perair. 2014;2(1):38–45. 10.35800/bdp.2.1.2014.3791 [DOI] [Google Scholar]
- 92. Sadili D, Haryono KMM, Sarmintohadi RI: Pedoman Umum Restoking Jenis Ikan Terancam Punah. Dermawan A, editor. Jakarta: Direktorat Konservasi Kawasan dan Jenis Ikan, Ditjen Kelautan, Pesisir dan Pulau-Pulau Kecil, Kementerian Kelautan dan Perikanan RI. 2015;xi+ 68.
- 93. Suryandari A, Krismono.: Beberapa aspek biologi ikan manggabai ( Glossogobius giuris) di Danau Limboto, Gorontalo. Bawal. 2011;3(5):329–336. 10.15578/bawal.3.5.2011.329-336 [DOI] [Google Scholar]
- 94. Dudgeon D, Arthington AH, Gessner MO, et al. : Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc. 2006;81(2):163–182. 10.1017/S1464793105006950 [DOI] [PubMed] [Google Scholar]
- 95. Hasan V, Mukti AT, Putranto TWC: Range expansion of the invasive Nile tilapia Oreochromis niloticus (Perciformes: Cichlidae) in Java Sea and first record for Kangean Island, Madura, East Java, Indonesia. Ecol Environ Conserv. 2019;25(July Suppl.Issue):S187–s189. Reference Source [Google Scholar]
- 96. Hasan V, Tamam MB: First record of the invasive Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) (Perciformes, Cichlidae), on Bawean Island, Indonesia. Check List. 2019;15(1):225–227. 10.15560/15.1.225 [DOI] [Google Scholar]
- 97. Insani L, Hasan V, Valen FS, et al. : Presence of the invasive Nile tilapia Oreochromis niloticus Linnaeus, 1758 (Perciformes, Cichlidae) in the Yamdena Island, Indonesia. Ecol Environ Conserv. 2020;26(3):1115–1118. Reference Source [Google Scholar]
- 98. Mokodongan DF: Oryzias nigrimas. Vol. T15576A909, The IUCN Red List of Threatened Species 2019. 2019 [cited 2019 Oct 10]; p.1–9. Reference Source
- 99. Mokodongan DF: Oryzias soerotoi. Vol. e.T1258534, The IUCN Red List of Threatened Species 2019. 2019 [cited 2019 Oct 10]; p.1–8. 10.2305/IUCN.UK.2019-2.RLTS.T125853495A125853502.en [DOI]
- 100. Tuapetel F: Perubahan kondisi tubuh ikan payangka ( Ophieleotris aporos Bleeker) di Danau Tondano. Amanisal. 2010;1(1):51–56. [Google Scholar]
- 101. Kiruba-Sankar R, Raj JP, Saravanan K, et al. : Invasive Species in Freshwater Ecosystems – Threats to Ecosystem Services. Sivaperuman C, Velmurugan A, Singh AK, et al., editors. Biodiversity and Climate Change Adaptation in Tropical Islands. Elsevier Inc.;2018. p.257–96. 10.1016/B978-0-12-813064-3/00009-0 [DOI] [Google Scholar]
- 102. Vicente IST, Fonseca-Alves CE: Pakistan Journal of Biological Sciences. Pakistan J Biol Sci. 2013;16(3):121–126. 10.3923/pjbs.2013.121.126 [DOI] [PubMed] [Google Scholar]
- 103. Ndobe S, Serdiati N, Moore A: Domestication and Length-Weight Relationship of Striped Snakehead Channa striata (Bloch). Proceeding of International Conference of Aquaculture Indonesia (ICAI) 2014. Bandung: Masyarakat Akuakultur Indonesia (MAI);2014. [Google Scholar]
- 104. Ndobe S, Masyahoro A, Serdiati N, et al. : Reproductive and morphometric characteristics of climbing perch Anabas testudineus in Sigi, Central Sulawesi, Indonesia. AACL Bioflux. 2020;13(1):167–182. Reference Source [Google Scholar]
- 105. Labatos JBV, Briones ND: Freshwater Fishes of Tikub Lake, Tiaong, Quezon, Philippines. Asian J Biodivers. 2014;5(1):41–53. Reference Source [Google Scholar]
- 106. Giesen W: Indonesia’s major freshwater lakes: A review of current knowledge, development processes and threats Wim. Int Vereinigung für Theor und Angew Limnol Mitteilungen. 1994;24(1):115–128. 10.1080/05384680.1994.11904030 [DOI] [Google Scholar]
- 107. Soeprobowati TR: Integrated Lake Basin Management For Save Indonesian Lake Movement. Procedia Environ Sci. 2015;23:368–374. 10.1016/j.proenv.2015.01.053 [DOI] [Google Scholar]
- 108. Auliyah N: Length-weight relationship and condition factor of huluu fish ( Giuris margaritacea) in Limboto Lake. IOP Conf Ser Mater Sci Eng. 2019;567(1):012027. 10.1088/1757-899X/567/1/012027/pdf [DOI] [Google Scholar]
- 109. RI: Peraturan Pemerintah Republik Indonesia Nomor 82 Tahun 2001 tentang Pengelolaan Kualitas Air dan Pengendalian Pencemaran Air. Jakarta, Indonesia: Government of the Republic of Indonesia;2001; p.1–22+ Appendix. [Google Scholar]
- 110. Ficke AD, Myrick CA, Hansen LJ: Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish. 2007;17:581–613. 10.1007/s11160-007-9059-5 [DOI] [Google Scholar]
- 111. Pauly D: The gill-oxygen limitation theory (GOLT) and its critics. Sci Adv. 2021 Jan 6;7(2):eabc6050. 10.1126/sciadv.abc6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ndobe S, Nurdin MS, Hasanah N, et al. : GenBank Accessions, BOLD Records (mitochondrial COI gene sequences) and other nucleotide sequences used for phylogenetic analyses of Eleotridae and Giuris spp. Harvard Dataverse. 2022;4. 10.7910/DVN/WMDHOJ [DOI] [Google Scholar]