Abstract
Background
Garlic is one of the favorite herbs in traditional medicine that has been reported to have many medicinal features. The aim of the current study is to review the latest documents on the effect of garlic on diabetes, VEGF, and BDNF and, finally, to review the existing studies on the effect of garlic on diabetic retinopathy.
Main text
The therapeutic effect of garlic on diabetes has been investigated in various studies. Diabetes, especially in advanced stages, is associated with complications such as diabetic retinopathy, which is caused by the alteration in the expression of molecular factors involved in angiogenesis, neurodegeneration, and inflammation in the retina. There are different in-vitro and in-vivo reports on the effect of garlic on each of these processes. Considering the present concept, we extracted the most related English articles from Web of Science, PubMed, and Scopus English databases from 1980 to 2022. All in-vitro and animal studies, clinical trials, research studies, and review articles in this area were assessed and classified.
Result and conclusion
According to previous studies, garlic has been confirmed to have beneficial antidiabetic, antiangiogenesis, and neuroprotective effects. Along with the available clinical evidence, it seems that garlic can be suggested as a complementary treatment option alongside common treatments for patients with diabetic retinopathy. However, more detailed clinical studies are needed in this field.
Keywords: Garlic, Diabetes, Diabetic retinopathy, BDNF, VEGF
Background
Garlic (Allium sativum) is a species of flowering plant belonging to the genus Allium [1]. This edible plant is widely used as a seasoning and flavoring, and its medicinal properties have been mentioned in various human and animal studies [2–4]. Garlic contains sulfur-based compounds (such as allicin, ajoene, diallyl polysulfides, vinyldithiins, diallyl sulfide (DAS), diallyl disulfide (DADS), and S-allylcysteine) as well as non-sulfur-active compounds that exert the garlic’s biological properties. Biochemically, the main ingredient in garlic is allicin (alyl 2-propentio sulfate), which has a heat-sensitive structure and degrades rapidly into sulfur compounds in response to high temperatures. Biologically active compounds formed by the degradation of allicin can reduce reactive oxygen species and, therefore, may play a significant role in the immune enhancement and treating disease [5]. Several therapeutic benefits of garlic include anti-infective [6], antioxidant [7], antimicrobial [8], and anti-cancer [9] effects that have been reported in previous studies. Recent studies have shown that garlic contains more than 200 chemicals including organosulfur compounds, volatile oils, enzymes, vitamins and a range of other biologically active molecules that lead to its medicinal effects. Garlic can prevent the development of cardiovascular diseases, regulate blood pressure, reduce blood sugar and cholesterol levels, and provide antibiotic, antifungal, and antiviral properties. Moreover, garlic has the ability to remove free radicals and can exert anti tumoral properties [10, 11]. A number of studies have investigated the pharmacokinetic profile of garlic compounds [12, 13]. In one of the recent studies, the permeability parameters of some garlic-derived organosulfur compounds and their membrane interaction have been investigated using the artificial immobilized membrane chromatography technique [12]. Based on this study, the permeation ability of garlic-derived organosulfurs is mainly dependent on the lipophilic/polar interactions of the chemicals [12] (Table 1).
Table 1.
Molecular formula, chemical structure and some pharmacokinetic parameters of some garlic -derived organosulfursa
| OSCs | MF | ChS | RT (min) | HIA% | BBBP | pKa1 (acidic) | pKa2 (basic) | RB | TPSA | Tm | TE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S-allyl-l-cysteine | C6H11NO2S | 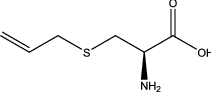 |
3.93 | 81.972 | No | 2.53 | 9.14 | 5 | 88.62 | 7.758 | 6.222 |
| Alliin | C6H11NO3S | 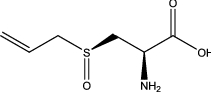 |
3.71 | 73.041 | No | 1.84 | 8.45 | 5 | 99.60 | 8.386 | 11.192 |
| Diallyl disulfide | C6H10S2 | 16.68 | 98.169 | Yes | b | b | 5 | 50.60 | 6.75 | 3.512 | |
| E-Ajoene | C9H14OS3 | 7.46 | 99.314 | No | 14.9 | b | 8 | 86.88 | 16.744 | 10.291 | |
| Z-Ajoene | C9H14OS3 | 7.41 | 99.314 | No | 14.9 | b | 8 | 86.88 | 13.945 | 10.421 | |
| Diallyl sulfide | C6H10S | 9.71 | 100.000 | Yes | b | b | 4 | 25.30 | 6.067 | 3.223 | |
| Allicin | C6H10OS2 | 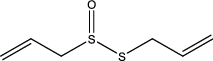 |
5.06 | 98.312 | Yes | b | b | 5 | 61.58 | 6.92 | 6.924 |
| Diallyl Trisulfide | C6H10S3 | 25.6 | 98.996 | Yes | b | b | 6 | 75.90 | 8.334 | 3.549 |
BBBP blood–brain barrier permeation, ChS chemical structure, HIA human gastrointestinal absorption, MF molecular formula, OSCs organosulfur compound, RB Rotatable bonds, RT Retention time, TE topographic electronic descriptor, Tm total size index/weighted by mass, TPSA topological polar surface area
aFor more information please see the work by Ramirez et al.[12]
bNon-ionizable compound
Garlic has been shown to play a protective role in cardiovascular disease [14]. In addition, garlic is advantageous for treating metabolic diseases such as diabetes [15]. Garlic can decrease the glucose level in mice and rats' serum [16]. The effect of garlic on blood glucose levels in diabetic mellitus (DM) has also been reported [17]. The focal point of this study is to review reports related to garlic's anti-diabetic, anti-angiogenesis, and neuroprotective impacts. Diabetic retinopathy (DR), the leading ocular problem in diabetic patients, is one of the world's most common causes of visual impairment [18]. The disease appears to be caused by retinal vasculopathy, retinal inflammation, and retinal neuropathy [19]. Hence, the new therapeutic strategies focus on controlling these processes at the molecular level. In this regard, the present study has focused on the effect of garlic on the expression of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) at the cellular and molecular levels. This study is specific in terms of reviewing these two factors and the antidiabetic effect simultaneously. Besides, the clinical and experimental studies investigating the effect of garlic on the improvement of DR and retinal abnormality have also been reviewed in this study.
Main text
In this study, after searching the Web of Science, PubMed, and Scopus English databases from 1980 to 2022, we extracted the most related English articles with these keywords: garlic, garlic extract, aged garlic extract, allium sativum, allyl compounds, DADS, diabetes, DM, type 2 diabetes, glucose parameters, anti-glycation, hypoglycaemic, insulin-resistance, DR, BDNF, neurogenesis, neuronal survival, VEGF, VEGF-related factor, and angiogenesis factor. All in-vitro and animal studies, clinical trials, research studies, and review articles, including the mentioned keywords, were assessed and classified.
Fresh garlic, aqueous extract of heat-treated garlic, garlic powder, aged garlic extract (AGE), and garlic oil (GO) were the most common forms of garlic under investigation in different studies. The enzyme alliinase, liable for converting from alliin (S-allyl cysteine sulfoxide) to allicin, is commonly inactivated by heat. As a result, the main component of the aqueous extract of heat-treated garlic is alliin. It has also been shown that the chemical composition of garlic powder is indistinguishable from fresh garlic [14]. AGE is delivered by placing the sliced garlic in 15–20% ethanol freshly soaked garlic at room temperature for a long time. The aging process diminishes the oil–solvent foul sulfur mixtures and upgrades the substance of water-dissolvable mixtures. The entire cycle should cause an impressive loss of allicin and expanded action of certain fresher mixtures, such as S-allylcysteine, S-allylmercaptocysteine, allixin, and selenium, which are steady, extremely bioavailable, and potentially antioxidant [20]. GO is generally prepared by the steam refining process. From more to less, steam-refined GO compounds include diallyl, allyl methyl, and dimethyl mono to hexasulfide [21].
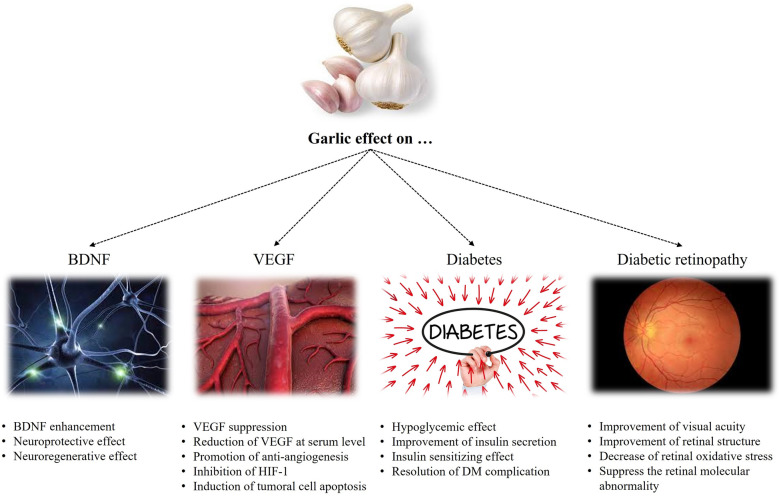
The papers focusing on at least one of the forms of garlic compounds for managing diabetes, DR, and BDNF/VEGF level were included in the present study. Figure 1 represents a schematic illustration of garlic effect on BDNF, VEGF, DM, and DR. The collected data were classified as represented separately below (Table 2).
Fig. 1.
A schematic illustration of garlic effect on BDNF, VEGF, DM, and DR
Table 2.
A brief review of reports on the effect of garlic on diabetes, diabetic retinopathy, BDNF, and VEGF
| Garlic (and its compounds) | The subject under study | Outcome | Refs. |
|---|---|---|---|
| Garlic effect on diabetes | |||
| Aged garlic extract | STZ-induced diabetic rat | BMI (−), GLU (−), insulin (+), CL (−), TG (−), UP (−) | [32] |
| Garlic + met | T2D patients |
Garlic: FBS (−), insulin (+), TC (−), risk of cardiovascular disease (−) Garlic + Met: more glycemic control and antihyperlipidemic activity |
[15] |
| Garlic oil | STZ-induced diabetic rat | UP (−), GTT (+), insulin (+) | [36] |
| Chewing vs swallowed garlic | T2D patient (uncontrolled dyslipidemia |
CL (−), TG (−), SBP (−), DBP (−), even with increasing fat intake; *Swallowed garlic had no significant effect on serum lipids |
[22] |
| Raw crushed garlic | Metabolic syndrome patient | FBS and other metabolic syndrome elements (−) | [24] |
| Garlic extract | STZ-induced diabetic rat | GLU (−), insulin (+) | |
| Raw garlic extract | STZ-induced diabetic rat | GLU (−), CL (−), TG (−), UP (−) | [38] |
| VO (3mpa)2 complex |
3T3-L1 preadipocytes STZ-induced diabetic mice |
PI3K/Akt pathway (−), GLUT4 translocation to the plasma membrane (+), GLU (−) | [39] |
| Garlic oil /DATS | Diabetic rat | Insulin (+), GTT (+) | [16] |
|
VO (alx)2 (Allixin isolated from dried garlic) |
rat adipocytes T1D model mice |
serum glucose (−), insulin resistance (+), GTT (+), *The plasma adiponectin level was not changed |
[43] |
| Aqueous extract of raw garlic | alloxan-induced diabetic male rabbit | GLU (−), CL (−) | [45] |
| Aged garlic extract | STZ-induced diabetic rat | GLU (−), GHb (−), Renoprotective effect (+) | [38, 46] |
| Alliin (S-allyl cysteine sulfoxide) | DIO mice | Insulin sensitivity (+), CL (−), TG (−) | [47] |
| Garlic extract | T2D patient | GLU (−), CL (−) | [49] |
| Aged garlic extract | Immobilization stress in mice | renal cell hypertrophy (−), corticosterone (−), GLU (−) | [57] |
| Garlic | Metabolic syndromes in fructose fed rat | insulin sensitivity (+) | [64] |
| s-allyl cysteine-enriched black garlic juice | STZ-induced insulin-deficient mice | BMI (−), GLU (−), insulin (+) | [129] |
| Aged black garlic | db/db mice | insulin resistance (−), TC (−), TG (−), HDL (+) | [130] |
| Garlic Effect on VEGF | |||
| Aged garlic extract | T2D patients at high cardiovascular risk | No significant change in endothelial cell function, vascular inflammation, oxidative stress or resistance to insulin | [23] |
| Aged garlic extract | Rat | Coronary arteries relaxation (+), myocardial contractility (−) | [77] |
| Alliin | Chick chorioallantoic membrane (CAM) model |
Human endothelial cell proliferation (−), VEGF-induced angiogenesis (−) *The effect was boosted by the antioxidant vitamins C and E |
[84] |
| Aged garlic solution | Chicken dorsum skin excisional lesion | Wound closure (+), re-epithelialization (+), dermal matrix regeneration (+), angiogenesis (+) | [88] |
| DAS | B16F-10 melanoma cell induced capillary formation in C57BL/6 mice | Tumor-directed capillary formation (−), IL-1 (−), IL-6 (−), TNF-α (−), GM-CSF (−) | [89] |
| Aged garlic extract | Colorectal carcinoma cells | Tumor growth (−), neovascularization (−), endothelial cell motility (−), endothelial cell proliferation (−), tube formation (−) | [91] |
| Garlic + lemon aqueous extraction | Mice with breast cancer | Angiogenesis (−), apoptosis (+) | [94] |
| DADS | Human prostate cancer cell line | PI3K/Akt (−), Ras/Raf (−), MMPs (−), pro-inflammatory/pro-angiogenic molecules (−), VEGF (−) | [96] |
| Garlic-derived extracellular vesicles | A549 human lung carcinoma cells |
VEGF (−), programmed cell death (+), *Normal cells were unaffected by treatment |
[98] |
| Garlic oil | Avian embryo chorioallantoic membranes | Microvessel (−) | [131] |
| Garlic powder | ductal breast carcinoma cells | VEGF (−), Angiogenesis (−) | [90] |
| Garlic stem extract | melanoma cells | Cell growth (−) cell migration (−) | [99] |
| Garlic effect on BDNF | |||
| Aged garlic extract | Cerebral ischemia rat | 8-OHdG (+), TNFα (+), COX-2 (+), The damage caused by neuronal injury (−) | [115] |
| DADS, DATS | Chronic constriction injury rat | Strong improvement in discomfort, H2S (+), BDNF (+), Nrf2 (+) | [116] |
| Allixin | Cultured neurons from fetal rat brain | Neurotrophic activity (+), nerve cells viability (+), division site per axon in hippocampal neurons (+) | [117] |
| Garlic essential oil | Mouse dentate gyrus | BDNF (+), AChE (−) | [118] |
| S-allyl-L-cysteine | Mouse dentate gyrus | Neuroblast proliferation (+), neuroblast differentiation (+) | [118] |
| Garlic essential oil | Rats utilizing the forced swimming test and unexpected chronic mild stress | The immobilization period (−), The sucrose preference index (+), 5-HT (+), DA (+), hippocampal BDNF (+), CREB (+), and protein kinase B (AKT) (+) | [119] |
| DADS | Hippocampal neural cell proliferation in the adult brain | Hippocampal neurogenesis (−), neurocognitive functions (−), BDNF (−), CREB (−), ERK (−) | [120] |
| DAS/DADS | Human malignant Neuroblastoma SH-SY5Y cells | Anti-apoptotic factors (−), calpain and intrinsic caspase cascade (+), hippocampal neurogenesis (−), neurocognitive functions (−), BDNF (−), CREB (−), ERK (−) | [121] |
| Garlic extract | Hippocampus cell in diabetic rat | hippocampus Na + /K + ATPase (+), Ca2 + ATPase (+) | [107] |
| DATS | diabetic rats heart tissue | NO2−(−), O2−(−), Bax, caspase-3 and -9 (−) | [113] |
| Garlic effect on DR | |||
| Garlic | Pre-diabetes induced retinal abnormalities in high fructose fed rat model | Functional, structural, and molecular abnormalities of the retina (−) | [125] |
| Aqueous garlic extract | Diabetic Wistar rat | Retinal oxidative stress serum glucose (−), TGF-β2 (−), IL-1β (−) | [124, 126] |
| Garlic | Patients with diabetic macular edema | Visual acuity (+), CMT (−), IOP (−) | [128] |
AChE Acetylcholinesterase, BMI body mass index, CL Cholesterol, CREB c-AMP response element-binding protein, DADS Diallyl disulfide, DAS diallyl sulfide, DATS Diallyl trisulfide, DBP diastolic blood pressure, db/db mice diabetic mice, DIO diet induced obese, FBS fasting blood sugar, GHb Glycosylated hemoglobin, GLU glucose, GTT glucose tolerance test, HDL high density lipoprotein cholesterol, Met metformin, Nrf2 nuclear factor erythroid 2-related factor 2, SBP systolic blood pressure, TC total cholesterol, TG triglyceride, T1D type 1 diabetes, T2D type 2 diabetes, UP urinary protein
Garlic and antidiabetic effects
The anti-diabetic effect of garlic has received much attention in the last two decades. [15, 22–30] The available documents have mainly investigated the effect of garlic on blood glucose control in healthy animals, animal models, and diabetic patients. [31] Diabetes was developed in animal models using chemical mixtures such as alloxan or streptozotocin (STZ). Alloxan or STZ do not cause actual type 1 DM due to differences in the immunologic mechanism or type 2 DM since it could not create actual insulin resistance. As an alternative, in such experimental investigations, DM was caused only by the injection of a toxic substance that affects the beta cells of the pancreas. The severity of this procedure depended on the dosing schedule and delivery technique. These types of studies had methodological limitations because these animals can live even without insulin injection, so they failed to create an entirely insulin-dependent state [16, 32–44].
Of 45 articles that were critically appraised for assessment of the anti-diabetic impact of garlic in this systematic review, 7 pieces of research were done in-vitro, 32 were done on the pharmacologically diabetic induced animals, 6 were clinical trials done on diabetic patients. The results of the antidiabetic impact of garlic are categorized in the 5-sub grouped as the discussion below.
Hypoglycemic effect of garlic
Lower blood glucose was meaningfully highlighted in the garlic-treated diabetic rats’ group compared to the control group. Raw garlic was shown to exert significant effects on hypoglycemia and hypocholesterolemia [45]. Eidi et al. reported lower serum glucose was obtained by oral administration of the garlic extract [33]. He described a persistent increase in blood glucose level in untreated control rats, while glucose level was significantly reduced in diabetic rats given 300 or 600 mg/kg of AGE. In contradiction with previous results, the blood glucose level in diabetic rats treated with a 100 mg/kg AGE dose was no longer substantially distinct from control ones. Glycosylated hemoglobin (GHb) ranges had been considerably reduced by administering the 300 and 600 mg/kg AGE doses. Treatment with the 100 mg/kg dose of AGE could no longer affect this parameter [38, 46]. This study found that the antidiabetic effect of AGE was dose-dependent.
Several investigations focused on the synergistic effects of garlic and anti-diabetic drugs like metformin on fasting blood glucose (FBS) levels in patients with type 2 diabetes [21, 26, 33]. Ashraf et al. described a statistically significant drop in FBS [15]. The effects of garlic extract and glibenclamide, a common anti-diabetic medication, were compared in diabetic rat models. The extract's anti-diabetic effect was more potent than glibenclamide's [33]. Therefore, garlic, combined with traditional anti-diabetic medication, has been demonstrated to enhance glucose control. FBS and other metabolic syndrome elements were considerably reduced when raw crushed garlic was used [24]. In alloxan-induced diabetic male rabbits, an aqueous extract of garlic -with very identical components to fresh garlic- provided a considerable drop in the elevated blood glucose level when tested to survey the physiological effect on serum glucose [45]. In addition to better glycaemic control, GO-treated diabetic rats revealed an increased in-vivo glycemic control and hypoglycemia sensitivity to insulin administration [29, 47]. The hypoglycemic action of GO is mediated by diallyl trisulfide (DATS), a fundamentally important component of garlic [16]. Scanga found that alliin is a substrate of LAT1, a cell membrane transporter that binds to alliin and plays a vital role in human metabolism, diabetes, and cancer [48]. Besides the investigation that shows garlic can help control diabetes, some studies suggest the contrary. According to Liu et al. study GO did not have antidiabetic effects immediately, but it took a few weeks for the result to appear [36].
Furthermore, DADS did not affect oral glucose tolerance [36]. Another study found that taking 900 mg of garlic orally, twice a day, did not reduce FBS or two-hour postprandial glucose levels in type 2 diabetes patients [49]. As more investigations indicate garlic has a favorable impact on blood sugar, the negative results of specific studies might be attributed to discrepancies in product preparation, consumption dosage, or duration.
Mechanisms for garlic as a hypoglycemic agent
The mixture of alliin, with usual antidiabetic drugs like glibenclamide and insulin, has been stated to have moderate effectiveness in regulating hyperglycemia [37]. Black solo garlic extract reduced the IL-1β, IL-6, and TNF-α level and increased IFN-γ in the STZ diabetic rats compared to glibenclamide. [50]. Recently Takim et al. showed that allium plays a role in the treatment of diabetes by enhancing the gene expression of caspase 3 and caspase 9 [51]. alliin modulates intestinal microbe composition, typically reducing Lachnospiraceae and augmented Ruminococcaceae in diet-induced obese (DIO) mice, and because of this effect, Zhui et al. concluded that garlic has a nutritional or therapeutic role in preventing diabetes [47]. In an animal study, Khare concluded that the use of allicin as an agonist of transient receptor potential ankyrin 1 (TRPA1), concurrently with a high-fat diet, could prevent GLP-1 dysregulation and glucose hemostasis [52]. It has also been hypothesized that the hypoglycemic effect of garlic is due to the presence and the impact of allylepropyl disulphide or diallyle disulphide and their effect on purine metabolism [16, 53, 54] Treating the rats with a combination of GO and DATS meaningfully increased the proportion of glucose to glycogen conversion. [16, 55]. In another study by Swanston, it was shown that the blood glucose regulating the activity of garlic extract was due to the presence of sulfur-containing combinations and flavonoids [56]. Kasuga. S also reported that garlic was effective in preventing the rise of corticosterone in response to adrenal hypertrophy, and because of that, it might avoid increasing blood glucose in diabetic mice [57].
Garlic and insulin secretagogues
Garlic is demonstrated to have insulin secretagogue properties, while the detailed mechanism is not apparent [15, 58]. Higher serum insulin levels resulted in diabetic rats that used the AGE (doses of 100, 300, or 600 mg/kg) but not in control rats [33, 38]. Moreover, insulin level was underlined to be increased in diabetic rats when given garlic in the form of oil [16] or garlic extract with a 200 mg/kg dose. Also, it may increase pancreas beta cell function by upregulation of the peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) gene [59] and alter histopathological features of the pancreas cells [60]. S-allylcysteine sulfoxide appeared to have a direct stimulatory effect on the insulin secretion ability of the pancreas cells [16, 34]. It can stimulate the glucose transporter-4 and increase insulin secretion [61]. A recent study highlighted the role of protein tyrosine phosphatase 1B (PTP1B) negative consequences in the insulin signal pathways. Garlic and its inhibitory effect on PTP1B may help in DM type 2 treatment [62].
Insulin sensitizing effect of garlic
Moreover, garlic was found to deliver hypoglycemic effects by preventing insulin inactivation by the sulfhydryl group and increasing insulin-sensitizing [63]. Antioxidant components of aged black garlic, such as phenols and flavonoids, also reduce insulin resistance [28]. Padiya et al. described garlic’s potential to improve insulin sensitivity in fructose-fed rats [64]. Recently Parham et al. reported a garlic-containing herbal medicine that could elevate insulin secretion, sensitivity, and diminished insulin resistance [27]. About which active ingredient in garlic is responsible for this effect, Zhai et al. suggested that alliin may increase insulin sensitivity [47]. In addition, GO or DATS was shown to enhance oral glucose tolerance and increase insulin sensitivity [16]. As suggested by previous studies, the role of the other components of garlic named VO (alx) 2 (allixin isolated from dried garlic)- in the normalized hyperglycemia in mice- was also highlighted. This role was obtained by improving insulin resistance without any changes in the plasma adiponectin levels [39, 43, 65].
Effect of garlic on the DM complications
Longstanding high blood glucose associated with DM has a temporary or long-term effect on organs and tissues. Cardiovascular disease, stroke, renal illness, ocular problems, microvascular abnormalities such as neuropathy and nerve injury, foot problems, cutaneous problems, gastrointestinal problems, and various types of mental illness are all recognized as DM consequences. Micro-and macrovascular diseases linked with type 1 and type 2 diabetes have been widely discovered [65, 66]. Endothelial cell function and blood perfusion to the peripheral tissue measured by acetylcholine (Ach) provocation were improved after the AGE consumption in the cases of arterial arteriolosclerosis [67].
Furthermore, it directly acts on the arterial wall [68] and can elevate cystathionine-γ-lyase expression in the myocardium [69]. A regulation in the liver peroxisome PGC-1α and irisin-encoding gene expression, along with the other inflammatory cytokines, showed that the garlic oil could lighten diabetic liver injury [70]. The study on nephropathy, a common microvascular complication in DM, showed that the diabetic rats treated with garlic remarkably lowered urinary protein levels and renal CD36, podocalyxin, and NGAL in diabetic rats compared to the control ones [71, 72]. In another study, Thomson et al. showed that raw garlic could decrease kidney damage by reducing the urine protein levels in STZ-induced diabetes [38]. GO may also affect decreasing proteinuria at the end of the 16th week of supplementation and improved oral glucose tolerance [36]. It seems that garlic can decrease the total cholesterol level by reducing LDL-C and thereby lower the risk of cardiovascular diseases [73]. Referring to the report of Ryu et al. aged black garlic could inhibit lipid oxidation by decreasing the free radicals [28]. Besides, it could effectively treat a patient with uncontrolled dyslipidemia (high cholesterol and triglyceride) and reduce blood pressure [25, 74]. Meanwhile, undamaged garlic (swallowed) did not have much effect on serum lipids [22]. Alongside raw garlic, AGE can also protect the cardiac and nervous systems and prevent clot formation [28]. Persaud H. reported that raw garlic consumption is associated with increased bleeding tendency [75]. In animal models' hearts, AGE improved ischemia–reperfusion by relaxing the outcome in coronary arteries [76, 77]. On the other hand, Atkin et al. developed a clinical trial on 26 subjects with type 2 diabetes who had high cardiovascular risk. The patients were treated with 1200 mg of AGE or placebo for 1 month, however, the results did not seem to confirm any improvement in the function of the endothelial cell, decreasing insulin resistance or oxidative stress [23].
Garlic and gestational DM (GDM)
Si et al. designed a randomized clinical trial to evaluate the effect of black garlic on GDM and concluded that insulin resistance was enhanced in the treatment group. The probiotic bacteria could improve the antioxidant effect of garlic in the GDM patient by converting the glucopyranoside in the fresh garlic to glucofuranoside, besides altering the intestinal’s normal flora [78].
Garlic and VEGF
VEGF is a growth factor and a homodimeric glycoprotein. Its gene is located on chromosome 6p21.1. Endothelial, neuronal, and glial cell proliferation, migration, and cell survival can be affected using VEGF [79]. Hypoxia conditions and ischemia can stimulate VEGF expression. The previous investigations revealed that VEGF receptors are mainly located on endothelial cells; also, they can encourage ocular neovascularization with the help of receptors on the retinal cells [80, 81]. Hypoxia has been shown to stimulate VEGF expression. The primary genes involved in this process include stimulating hypoxia-inducible factor-1 (HIF-1), nitric oxide synthase (NOS), and VEGF genes. Hypoxia is a known etiological factor in various systemic and eye diseases [82]. Alliin, as part of the garlic component, displayed significant suppression of VEGF, resulting in anti-angiogenesis in the chick chorioallantoic membrane. Oral uses of the S-allyl cysteine can reduce the plasma level of VEGF and other pro-inflammatory cytokines such as interleukins (IL-1β, IL-4, IL-5, IL-10). [83]. This inhibitory effect was significantly boosted by the antioxidant vitamins C and E [84, 85]. Garlic can enhance cardiac angiogenesis by enhancing the expression level of myocardial miR-126 and miR-210. [86] Recent studies have shown that GO might reduce the number of microvessels in the avian embryo chorioallantoic membranes [87]. Besides, the histological analysis showed that AGE could improve the wound healing process in chicken dorsum skin excisional lesions, which was associated with dose-dependent neovascularization in AGE-treated injuries [88]. In rats, studies on the anti-angiogenic activity of DAS (a garlic component) showed the inhibition of tumor-directed capillary formation and reduction of pro-inflammatory cytokine production [89]. Hussein et al. reported that daily garlic powder could reduce intratumoral angiogenesis by decreasing the level of VEGF in the ductal breast carcinoma [90]. The anti-angiogenic effect was also reported in AGE-treated colorectal carcinoma [91] and glioblastoma [92], resulting in tumor growth inhibition of endothelial cell motility and proliferation. There is also a report on the same effect in hepatocellular carcinoma [93]. Furthermore, Talib Wamidhe found that combining garlic and lemon aqueous extraction reduced angiogenesis and caused apoptosis in mice with breast cancer [94]. It was shown that DADS could downregulate the PI3K/Akt and Ras/Raf signaling pathways in the human prostate cancer cell line through its inhibitory effect on HIF-1. This, subsequently, led to the downregulation of MMPs and several pro-inflammatory/pro-angiogenic molecules, namely VEGF expression. Hence it can be suggested that garlic possesses anti-invasive and anti-metastatic properties [95–97]. Using A549 lung carcinoma cells in humans as models and normal dermal fibroblast cells as controls, Özkan et al. demonstrated that garlic-derived tiny extracellular vesicles cause cancer cells to die by programmed cell death, although normal cells are unaffected by the treatment [98]. There is the study on the effect of garlic stem extract on the inhibition of the cell growth and migration in the melanoma cells [99]. As previously mentioned, various studies investigating the relationship between garlic and VEGF found that the main anti-VEGF effect of garlic is on apoptosis in malignant cell death and reduced angiogenesis in the in-vitro cell models.
Garlic, BDNF, and neuroprotection
BDNF, a small size basic protein (with an isoelectric point of 9.6), is one of the most attractive members of the neurotrophin family [100]. BDNF plays a critical role in the growth and development of neurons. Its highest level of expression is in the human brain. BDNF has been shown to influence non-neuronal cells and their function in neuronal development. The liver, heart, and even lungs, express BDNF in smaller amounts [101]. Although it is commonly believed that newly produced hippocampal cells can be incorporated into neural networks during adolescence, neurogenesis usually occurs exclusively during embryonic development [102]. Hippocampal neurogenesis is critical for the generation of new synapses as well as the preservation of old ones. Numerous impulses that directly affect neurotransmitters, neurotrophic, and growth factors, such as BDNF, as well as a variety of environmental impulses, can control neurogenesis in adults [103, 104]. Herbal fortification can modulate hippocampal neurogenesis, both beneficially and adversely, even in adulthood [105, 106]. Semuyaba et al. noted the memory upgrading, increased hippocampus Ca2+ ATPase activity, and glutamine synthetase after ingesting a certain dosage of garlic in diabetic rats [107]. Numerous studies have been published to focus on the protective role of fresh garlic or AGE in neurodegenerative conditions such as Alzheimer's disease and cerebral ischemia [108–111]. Garlic might have a neuroprotective effect by enhancing tissue immunity against the oxidative stress caused by low-density lipoprotein [112], decreasing the free radicals such as NO2− and O2− [113], besides increasing mitochondrial function [114]. Colin. A. conducted a study on rats subjected to ischemia for 60 min plus 24 h of reperfusion. At the start of the reperfusion phase, the rats were supplemented with AGE (1.2 ml/kg weight). AGE reduced TNF levels as well as COX-2 protein amount and function. These findings imply that AGE's neuroprotective qualities are linked to both its anti-oxidant characteristics and ability to reduce TNF concentrations as well as COX-2 protein production and function. This study suggested that AGE could reduce the damage caused by neuronal injury [115]. In another study, DADS (25 and 50 mg/kg) and DATS (20 and 40 mg/kg) were given to chronic constriction injury (CCI) rats for 14 days. This study showed great discomfort after treating the rats with these garlic derivatives. Furthermore, H2S, BDNF, and nuclear factor erythroid 2-related factor 2 (Nrf2) amounts in the sciatic nerve and dorsal root ganglia were restored after administration of these organosulfur chemicals. DAS and DATS therapy of CCI-treated rats resulted in a considerable reduction in neuropathic pain. After nerve damage, the BDNF level in these areas decreased significantly. DAS and DATS therapy, on the other hand, resulted in a considerable recovery of the biochemical markers as well as a reduction in neuropathic pain [116]. Adding allixin (1–100 ng/ml) to the cell environment could markedly increase the viability of nerve cells obtained from different parts of the brain and expanded the number of division sites per axon in hippocampal neurons, as demonstrated by Moriguchi [117]. In another investigation, GO (10 ml/kg) was given orally to mice once a day, for three weeks. Subsequently, the analysis of the hippocampus homogenate confirmed a considerable elevation of BDNF concentration and a reduction in acetylcholinesterase (AChE) function [118]. The study of Huang Ju was the first to look into the antidepressant impacts of GO in rats utilizing the forced swimming test (FST) and unexpected chronic mild stress (UCMS). GO (25 and 50 mg/kg) effectively diminished the immobilization period in rats after 28 days of oral treatment. GO and DADS could also dramatically correct the reduction of sucrose preference index that was caused by five weeks of UCMS. With no hippocampal consequences, GO could substantially lower the frontal cortex recycling ratio of neurotransmitters such as serotonin and dopamine, raising their levels [119]. It has been shown that long-term administration of GO can augment hippocampal BDNF through monoamine neurotransmitter regulation and the BDNF-associated signaling cycle [119]. A study conducted on the dentate gyrus confirmed that S-allyl-L-cysteine, a constituent of the Allium class, can stimulate proliferation and differentiation in the neuroblast cells [118]. Despite the positive results of the effect of garlic on the increase of BDNF, some contradictory reports are also observed in this field. Recently the effect of sulfur components of garlic has been investigated on the proliferation of neural progenitor cells. This study showed that DADS remarkably downregulated the proliferation of these cells. Besides, the treatment of 10 mg/kg DADS decreased the hippocampus BDNF concentrations and subsequently reduced neurogenesis and lowered the function in the passive avoidance test. DADS could reduce the hippocampal level of BDNF, phosphorylated CREB signaling, and phosphorylated ERKs, all of that linked to neural stem proliferation and differentiation in the hippocampus. Furthermore, when compared to controls, DADS caused severe memory problems. Hence, DADS might have negative consequences on hippocampus neural cell proliferation and differentiation [120].
Garlic's effect on neurology system-related neoplasia has also been explored in keeping with its neuroprotective benefits. In neuroblastoma SHSY5Y cells in the human, Karmakar. et al. discovered that the GO components (DAS and DADS) stimulate the endogenous calpain-caspase pathway resulting in cell death. SH-SY5Y cells were treated with 50 and 100 M DAS or DADS for one day. This treatment led to the manifestation of cellular morphological hallmarks of apoptosis that was approved by Wright staining [121]. It seems that garlic can suppress neurological neoplasia.
Compared to non-diabetic adults, diabetic patients have lower levels of BDNF in their blood. Several studies have shown that the concentration of BDNF at the protein or mRNA level is reduced in the retina of diabetic rats [111, 122]. Considering the available evidence, it seems that garlic can enhance the neuroprotective effect in the management of diabetes in addition to improving the index related to glycemia. Although more studies in this field are needed, especially at the clinical level and in controlled conditions.
Garlic and diabetic retinopathy
Notwithstanding numerous papers on the anti-diabetic effects of garlic, limited information has been reported on the impact of garlic on diabetic retinopathy [123]. The garlic components can reduce retinal oxidative stress and diabetic retinopathy by the effect of the Nuclear Factor kappa B (NF-ĸB) pathway and downregulation of its mRNA expression [124] . As reported by Kommula et al., the early supplementation of garlic at a rate of 3% in the daily diet of pre-diabetic Wistar rat models could prevent functional, structural, and molecular abnormalities of the retina. Pre-diabetic rats (n=9, in each of the control and case groups) in this study were modeled by use of high fructose diet for ten months. In this study, known molecular markers were used to track the abnormalities of a diabetic retinopathy model. VEGF and glial fibrillary acidic protein (GFAP) expression indicated the process of angiogenesis and glial activation in the diabetic retina, and increased expression of carboxymethyl lysine (CML-KLH), and 4-hydroxynanoenol (4-HNE) represented the association of glycation and retinal oxidative stress. This study demonstrated that garlic intake could significantly decrease the expression of VEGF, GFAP, CML-KLH, and 4-HNE in the retinal cells of the diabetic rat models compared to their control ones. This study provided valuable evidence on the potential of garlic intake to postpone retinal abnormalities manifestations [125]. Another study on streptozotocin-induced diabetic rats investigated the protective value of garlic intake on retinal abnormalities. Diabetic albino rats (n = 20, in each control and case group) were supplemented with raw garlic (0.4 g/100 gram of body weight) for seven weeks. Then after retinal samples were examined for tracking of any histopathological and ultrastructural alteration. It was found that the treated samples had morphological and structural improvements [123]. The anti-oxidative and anti-inflammatory effect of aqueous garlic extract on the retinal tissues of rats affected with DM has also been recently reported. Male Wistar rats (n=6 in each of the control and case groups) were modeled for diabetes by applying streptozotocin and nicotinamide. The modeled rats were supplemented with aqueous garlic extract (200 mg/100 gram of body weight /day) for 5 weeks and further analyses were performed on the homogenate’s lysis of retinal samples. This study supported the hypnotized of the reducing effects of garlic on the standard parameters associated with retinal oxidative stress. Besides, the expression level of TGF-β2 and IL-1β was indicated to be significantly decreased in the garlic-supplemented group [126, 127]. Recently, in a randomized clinical trial on 117 eyes of diabetic patients, Afarid et al. have shown that garlic intake could remarkably improve visual acuity, decrease central macular thickness, and reduce intraocular pressure. This study suggested that garlic can be a complementary treatment for diabetic macular edema [128].
Conclusion
In conclusion, while the results of a few studies share several similarities in the impact of garlic on each of the antidiabetic, antiangiogenesis, and the neuroprotective effects were opposing and conflicting, the vast majority of many other research findings with a beneficial influence cannot be overlooked, and the positive impact of garlic can be supported in overall. This review was unique in that it looked at antidiabetic, neuroprotective, and antiangiogenic effects of garlic simultaneously. Also, the latest studies on garlic's therapeutic effect on DR were included in this study. According to the available evidence, it seems that garlic can be prescribed as a complementary treatment for DR patients. More research is needed to discover particular chemicals in garlic or garlic components accountable for most of its biological effects, such as antidiabetic, neuroprotective, and antiangiogenic actions.
Acknowledgements
The authors would like to thank the directors of Shiraz University of Medical Sciences for supporting this research.
Abbreviations
- 4-HNE
4-Hydroxynanoenol
- AChE
Acetylcholinesterase
- AGE
Aged garlic extract
- BDNF
Brain-derived neurotrophic factor
- CML-KLH
Carboxymethyl lysine
- DADS
Diallyl disulfide
- DATS
Diallyl trisulfide
- DAS
Diallyl sulfide
- DIO
Diet-induced obese
- DM
Diabetic mellitus
- DR
Diabetic retinopathy
- FBS
Fasting blood glucose
- FST
Forced swimming test
- GDM
Gestational DM
- GFAP
Glial fibrillary acidic protein
- GHb
Glycosylated hemoglobin
- GO
Garlic oil
- HIF-1
Hypoxia-inducible factor-1
- IL
Interleukin
- NF-ĸB
Nuclear Factor kappa B
- PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator-1α gene
- PTP1B
Protein tyrosine phosphatase 1B
- STZ
Streptozotocin
- TRPA1
Transient receptor potential ankyrin 1
- UCMS
Unexpected chronic mild stress
- VEGF
Vascular endothelial growth factor
Author contributions
FSJ and MA designed the study. FSJ and ZZ collected the data and wrote the article. All authors read the manuscript and confirmed the final version.
Funding
This study was supported by Shiraz University of Medical Science (Grant# 26401).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Ethics approval for this study was obtained from the Ethics Committee of Shiraz University of Medical sciences.
Consent for publication
Not applicable.
Competing interests
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fatemeh Sanie-Jahromi and Zahra Zia have the same contribution as correspondence
Contributor Information
Fatemeh Sanie-Jahromi, Email: fsanie@sums.ac.ir.
Zahra Zia, Email: Ziazahraa@yahoo.com.
Mehrdad Afarid, Email: afaridm@sums.ac.ir.
References
- 1.Khan MM, Khalilullah H, Eid EE, Azam F, Khan MA, Khan A, et al. A dig deep to scout the pharmacological and clinical facet of garlic (Allium sativum) Curr Tradit Med. 2022;8(1):1–19. [Google Scholar]
- 2.Cakmakci O, Sensoy S, Alan AR. Bioactive constituents of Allium vineale L. accessions from Eastern Turkey. Sci Hortic. 2022;303:111203. doi: 10.1016/j.scienta.2022.111203. [DOI] [Google Scholar]
- 3.Pérez-Rubio KG, Méndez-del Villar M, Cortez-Navarrete M. The role of garlic in metabolic diseases: a review. J Med Food. 2022;25(7):683–694. doi: 10.1089/jmf.2021.0146. [DOI] [PubMed] [Google Scholar]
- 4.Thomson M, Al-Qattan KK, Divya J, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;16(1):1–9. doi: 10.1186/s12906-016-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3):716S–S725. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 6.Odongo EA, Mutai PC, Amugune BK, Mungai NN. A systematic review of medicinal plants of Kenya used in the management of bacterial infections. Evid-based Complement Altern Med. 2022;2022:1–43. doi: 10.1155/2022/9089360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract-and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012;2012:1–16. doi: 10.1155/2012/907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goncagul G, Ayaz E. Antimicrobial effect of garlic (Allium sativum) Recent Pat Antiinfect Drug Discov. 2010;5(1):91–93. doi: 10.2174/157489110790112536. [DOI] [PubMed] [Google Scholar]
- 9.Tsubura A, Lai Y-C, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anti-Cancer Agents Med Chem. 2011;11(3):249–253. doi: 10.2174/187152011795347441. [DOI] [PubMed] [Google Scholar]
- 10.El-Saber Batiha G, Magdy Beshbishy A, Wasef LG, Elewa YH, Al-Sagan AA, Abd El-Hack ME, et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12(3):872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayaz E, Alpsoy HC. Garlic (Allium sativum) and traditional medicine. Turkiye Parazitol Derg. 2007;31(2):145–149. [PubMed] [Google Scholar]
- 12.Ramirez DA, Federici MF, Altamirano JC, Camargo AB, Luco JM. Permeability data of organosulfur garlic compounds estimated by immobilized artificial membrane chromatography: correlation across several biological barriers. Front Chem. 2021;9:690707. doi: 10.3389/fchem.2021.690707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C, Jiang X, Wang H, Zhao Z, Wang W. Drug metabolism and pharmacokinetics of organosulfur compounds from garlic. J Drug Metab Toxicol. 2013;4(5):1–10. [Google Scholar]
- 14.Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1(1):1–14. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashraf R, Khan RA, Ashraf I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak J Pharm Sci. 2011;24(4):565–570. [PubMed] [Google Scholar]
- 16.Liu C-T, Hse H, Lii C-K, Chen P-S, Sheen L-Y. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur J Pharmacol. 2005;516(2):165–173. doi: 10.1016/j.ejphar.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Oh KK. A network pharmacology study to investigate bioactive compounds and signaling pathways of garlic (Allium sativum L.) husk against type 2 diabetes mellitus. J Food Biochem. 2022;46(7):e14106. doi: 10.1111/jfbc.14106. [DOI] [PubMed] [Google Scholar]
- 18.Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair SH, Schwartz SS. Diabetic retinopathy–an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front Endocrinol. 2019;10:843. doi: 10.3389/fendo.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131(3):955S–S962. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 21.Lawson LD. Garlic: a review of its medicinal effects and indicated active compounds. Blood. 1998;179:62. [Google Scholar]
- 22.Ashraf R, Aamir K, Shaikh AR, Ahmed T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J Ayub Med Coll Abbottabad. 2005;17(3):60–64. [PubMed] [Google Scholar]
- 23.Atkin M, Laight D, Cummings MH. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J Diabetes Complicat. 2016;30(4):723–7. doi: 10.1016/j.jdiacomp.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Choudhary PR, Jani RD, Sharma MS. Effect of raw crushed garlic (Allium sativum L.) on components of metabolic syndrome. J Diet Suppl. 2018;15(4):499–506. doi: 10.1080/19390211.2017.1358233. [DOI] [PubMed] [Google Scholar]
- 25.Ghorbani A, Zarvandi M, Rakhshandeh H. A randomized controlled trial of a herbal compound for improving metabolic parameters in diabetic patients with uncontrolled dyslipidemia. Endocr Metab Immune Disord Drug. 2019;19(7):1075–1082. doi: 10.2174/1871530319666190206213420. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Mittal A, Babu D, Mittal A. Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev. 2021;17(4):437–456. doi: 10.2174/18756417MTExfMTQ1z. [DOI] [PubMed] [Google Scholar]
- 27.Parham M, Bagherzadeh M, Asghari M, Akbari H, Hosseini Z, Rafiee M, et al. Evaluating the effect of a herb on the control of blood glucose and insulin-resistance in patients with advanced type 2 diabetes (a double-blind clinical trial) Caspian J Intern Med. 2020;11(1):12. doi: 10.22088/cjim.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu JH, Kang D. Physicochemical properties, biological activity, health benefits, and general limitations of aged black garlic: a review. Molecules. 2017;22(6):919. doi: 10.3390/molecules22060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobenin IA, Nedosugova LV, Filatova LV, Balabolkin MI, Gorchakova TV, Orekhov AN. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: the results of double-blinded placebo-controlled study. Acta Diabetol. 2008;45(1):1–6. doi: 10.1007/s00592-007-0011-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X-H, Lowe D, Giles P, Fell S, Connock MJ, Maslin DJ. Gender may affect the action of garlic oil on plasma cholesterol and glucose levels of normal subjects. J Nutr. 2001;131(5):1471–1478. doi: 10.1093/jn/131.5.1471. [DOI] [PubMed] [Google Scholar]
- 31.Saikat ASM, Hossain R, Mina FB, Das S, Khan IN, Mubarak MS, et al. Antidiabetic effect of garlic. Rev bras farmacogn. 2021;32(1):1–11. doi: 10.1007/s43450-021-00193-y. [DOI] [Google Scholar]
- 32.Thomson M, Al-Qattan KK, Js D, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;16(1):1–9. doi: 10.1186/s12906-016-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13(9–10):624–9. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Islam MS, Choi H. Comparative effects of dietary ginger (Zingiber officinale) and garlic (Allium sativum) investigated in a type 2 diabetes model of rats. J Med Food. 2008;11(1):152–159. doi: 10.1089/jmf.2007.634. [DOI] [PubMed] [Google Scholar]
- 35.Kook S, Kim G-H, Choi K. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. J Med Food. 2009;12(3):552–560. doi: 10.1089/jmf.2008.1071. [DOI] [PubMed] [Google Scholar]
- 36.Liu C-T, Wong P-L, Lii C-K, Hse H, Sheen L-Y. Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem Toxicol. 2006;44(8):1377–1384. doi: 10.1016/j.fct.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Sheela C, Kumud K, Augusti K. Anti-diabetic effects of onion and garlic sulfoxide amino acids in rats. Planta Med. 1995;61(04):356–357. doi: 10.1055/s-2006-958099. [DOI] [PubMed] [Google Scholar]
- 38.Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2007;15(3):108–115. [Google Scholar]
- 39.Basuki W, Hiromura M, Adachi Y, Tayama K, Hattori M, Sakurai H. Enhancement of insulin signaling pathway in adipocytes by oxovanadium (IV) complexes. Biochem Biophys Res Commun. 2006;349(3):1163–1170. doi: 10.1016/j.bbrc.2006.08.162. [DOI] [PubMed] [Google Scholar]
- 40.El-Demerdash F, Yousef MI, Abou E-N. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43(1):57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Olefsky JM. Effects of streptozotocin-induced diabetes on insulin binding, glucose transport, and intracellular glucose metabolism in isolated rat adipocytes. Diabetes. 1979;28(2):87–95. doi: 10.2337/diab.28.2.87. [DOI] [PubMed] [Google Scholar]
- 42.Yasui H, Adachi Y, Katoh A, Sakurai H. Metallokinetic characteristics of antidiabetic bis (allixinato) oxovanadium (IV)-related complexes in the blood of rat. J Biol Inorg Chem. 2007;12(6):843–853. doi: 10.1007/s00775-007-0239-5. [DOI] [PubMed] [Google Scholar]
- 43.Adachi Y, Yoshida J, Kodera Y, Katoh A, Takada J, Sakurai H. Bis (allixinato) oxovanadium (IV) complex is a potent antidiabetic agent: studies on structure—activity relationship for a series of hydroxypyrone—vanadium complexes. J Med Chem. 2006;49(11):3251–3256. doi: 10.1021/jm060229a. [DOI] [PubMed] [Google Scholar]
- 44.Adachi Y, Yoshikawa Y, Yoshida J, Kodera Y, Katoh A, Takada J, et al. Improvement of diabetes, obesity and hypertension in type 2 diabetic KKAy mice by bis (allixinato) oxovanadium (IV) complex. Biochem Biophys Res Commun. 2006;345(3):945–950. doi: 10.1016/j.bbrc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Mahesar H, Bhutto M, Khand A, Narejo N. Garlic used as an alternative medicine to control diabetic mellitus in alloxan-induced male rabbits. Pak J Physiol. 2010;6(1):39–41. [Google Scholar]
- 46.Shiju T, Rajesh N, Viswanathan P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J Pharmacol. 2013;45(1):18. doi: 10.4103/0253-7613.106429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhai B, Zhang C, Sheng Y, Zhao C, He X, Xu W, et al. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci Rep. 2018;8(1):1–7. doi: 10.1038/s41598-018-21421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scanga R, Scalise M, Rovella F, Regina TMR, Galluccio M, Indiveri C. The nutraceutical Alliin from garlic is a novel substrate of the essential amino acid transporter LAT1 (SLC7A5) Front Pharmacol. 2022;13:951. doi: 10.3389/fphar.2022.877576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afkhami-Ardekani M, Kamali-Ardekani A, Shojaoddiny-Ardekani A. Effects of garlic on serum lipids and blood glucose of type 2 diabetic patients. Int J Diab Dev Ctries. 2006;26(2):86–88. doi: 10.4103/0973-3930.28279. [DOI] [Google Scholar]
- 50.Nani D, Proverawati A. Immunomodulatory effects of black solo garlic (Allium sativum L.) on streptozotocin-induced diabetes in Wistar rats. Heliyon. 2021;7(12):e08493. doi: 10.1016/j.heliyon.2021.e08493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takim K, Yigin A, Koyuncu I, Kaya R, Gülçin İ. Anticancer, anticholinesterase and antidiabetic activities of tunceli garlic (Allium tuncelianum): determining its phytochemical content by LC–MS/MS analysis. J Food Meas Charact. 2021;15(4):3323–3335. doi: 10.1007/s11694-021-00912-y. [DOI] [Google Scholar]
- 52.Khare P, Mahajan N, Singh DP, Kumar V, Kumar V, Mangal P, et al. Allicin, a dietary trpa1 agonist, prevents high fat diet-induced dysregulation of gut hormones and associated complications. Food Funct. 2021;12(22):11526–11536. doi: 10.1039/D1FO01792F. [DOI] [PubMed] [Google Scholar]
- 53.Goudappala P, Gowda CY, Kashinath R. Diallyl disulfide regulates purine metabolism and their metabolites in diabetes mellitus. Int J Res Pharm Sci. 2021;65(1):28–34. [Google Scholar]
- 54.Sujithra K, Srinivasan S, Indumathi D, Vinothkumar V. Allyl methyl sulfide, an organosulfur compound alleviates hyperglycemia mediated hepatic oxidative stress and inflammation in streptozotocin-induced experimental rats. Biomed Pharmacother. 2018;107:292–302. doi: 10.1016/j.biopha.2018.07.162. [DOI] [PubMed] [Google Scholar]
- 55.Goudappala P, Sukumar E, Kashinath R. Influence of diallyl disulphide on hepatic gluconeogenesis suppression by CREB Binding protein phosphorylation. Int J Res Pharm Sci. 2019;10:1327. doi: 10.26452/ijrps.v10i2.536. [DOI] [Google Scholar]
- 56.Swanston-Flatt S, Day C, Bailey C, Flatt P. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33(8):462–4. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 57.Kasuga S, Ushijima M, Morihara N, Itakura Y, Nakata Y. Effect of aged garlic extract (AGE) on hyperglycemia induced by immobilization stress in mice. Nihon Yakurigaku zasshi. 1999;114(3):191–197. doi: 10.1254/fpj.114.191. [DOI] [PubMed] [Google Scholar]
- 58.Mohana L, Sandhya R, Kiran U. A review on diabetes milletus and the herbal plants used for its treatment. Asian J Pharm Clin Res. 2012;5(4):15–21. [Google Scholar]
- 59.Shaker M, Khamisipour G, Sadeghipour H, Zar A, Naeimi B, Akbarzadeh S. Effect of resistance training and garlic extract on insulin sensitivity/resistance and biochemical parameters in diabetic rats. Comp Exerc Physiol. 2022;18(2):163–170. doi: 10.3920/CEP210031. [DOI] [Google Scholar]
- 60.Faradilla A, Rahman M. The effect of single type black garlic (Allium sativum L.) extract on the cell of langerhans islets and the kidney tubular microscopy in male wistar rats (rattus norvegicus) models of diabetes mellitus. Syst Rev Pharm. 2020;11(6):290–6. [Google Scholar]
- 61.Bhattacharya S, Maji U, Khan GA, Das R, Sinha AK, Ghosh C, et al. Antidiabetic role of a novel protein from garlic via NO in expression of Glut-4/insulin in liver of alloxan induced diabetic mice. Biomed Pharmacother. 2019;111:1302–1314. doi: 10.1016/j.biopha.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 62.Ojo OA, Adegboyega AE, Johnson GI, Umedum NL, Onuh K, Adeduro MN, et al. Deciphering the interactions of compounds from Allium sativum targeted towards identification of novel PTP 1B inhibitors in diabetes treatment: a computational approach. Inform Med Unlocked. 2021;26:100719. doi: 10.1016/j.imu.2021.100719. [DOI] [Google Scholar]
- 63.Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, Omelka R. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals. 2021;14(8):806. doi: 10.3390/ph14080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padiya R, Khatua TN, Bagul PK, Kuncha M, Banerjee SK. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr Metab. 2011;8(1):1–8. doi: 10.1186/1743-7075-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkman MS, McCarren M, Shah J, Duckworth W, Abraira C, Group VS The association between metabolic control and prevalent macrovascular disease in Type 2 diabetes: the VA Cooperative Study in diabetes. J Diabetes Complications. 2006;20(2):75–80. doi: 10.1016/j.jdiacomp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Stettler C, Allemann S, Jüni P, Cull CA, Holman RR, Egger M, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38. doi: 10.1016/j.ahj.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Lindstedt S, Wlosinska M, Nilsson AC, Hlebowicz J, Fakhro M, Sheikh R. Successful improved peripheral tissue perfusion was seen in patients with atherosclerosis after 12 months of treatment with aged garlic extract. Int Wound J. 2021;18(5):681–691. doi: 10.1111/iwj.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ribeiro M, Alvarenga L, Cardozo LF, Chermut TR, Sequeira J, Moreira LDSG, et al. From the distinctive smell to therapeutic effects: garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin Nutr. 2021;40(7):4807–19. doi: 10.1016/j.clnu.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Tsai C-Y, Wen S-Y, Shibu MA, Yang Y-C, Peng H, Wang B, et al. Diallyl trisulfide protects against high glucose-induced cardiac apoptosis by stimulating the production of cystathionine gamma-lyase-derived hydrogen sulfide. Int J Cardiol. 2015;195:300–310. doi: 10.1016/j.ijcard.2015.05.111. [DOI] [PubMed] [Google Scholar]
- 70.Eser N, Yoldas A, Turk A, Kalaycı Yigin A, Yalcin A, Cicek M. Ameliorative effects of garlic oil on FNDC5 and irisin sensitivity in liver of streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2021;73(6):824–834. doi: 10.1093/jpp/rgab023. [DOI] [PubMed] [Google Scholar]
- 71.Dragoș D, Manea MM, Timofte D, Ionescu D. Mechanisms of herbal nephroprotection in diabetes mellitus. J Diabetes Res. 2020;2020:1–31. doi: 10.1155/2020/5710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuvashree M, Ganesh RN, Viswanathan P. Potential application of nanoemulsified garlic oil blend in mitigating the progression of type 2 diabetes-mediated nephropathy in Wistar rats. 3 Biotech. 2020;10(6):1–15. doi: 10.1007/s13205-020-02262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashraf R, Sarwar M, Kamil N, Wahid S, Qureshi A. Analysis of dose and duration dependent effects of Allium sativum Linn and other hypocholesterolemic agents exhibited on dyslipidemia in patients with essential hypertension. Pak J Pharm Sci. 2022;35(3). [PubMed]
- 74.Xie C, Gao W, Li X, Luo S, Chye FY. Study on the hypolipidemic properties of garlic polysaccharide in vitro and in normal mice as well as its dyslipidemia amelioration in type2 diabetes mice. Food Biosci. 2022;47:101683. doi: 10.1016/j.fbio.2022.101683. [DOI] [Google Scholar]
- 75.Persaud H. A case study: raw garlic consumption and an increased risk of bleeding. J Herb Med. 2022;32:100544. doi: 10.1016/j.hermed.2022.100544. [DOI] [Google Scholar]
- 76.Asdaq SMB, Lokaraja S, Alamri AS, Alsanie WF, Alhomrani M, Almutiri AH, et al. Potential interaction of fresh garlic with metformin during ischemia-reperfusion induced cardiac injury in diabetic rats. Evid-based Complement Altern Med. 2021;2021:1–12. doi: 10.1155/2021/9739089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.García-Villalón A, Amor S, Monge L, Fernández N, Prodanov M, Muñoz M, et al. In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects. J Funct Foods. 2016;27:189–200. doi: 10.1016/j.jff.2016.08.062. [DOI] [Google Scholar]
- 78.Si L, Lin R, Jia Y, Jian W, Yu Q, Wang M, 2019. Lactobacillus bulgaricus improves antioxidant capacity of black garlic in the prevention of gestational diabetes mellitus: a randomized control trial. Biosci Rep. [DOI] [PMC free article] [PubMed]
- 79.Penn J, Madan A, Caldwell RB, Bartoli M, Caldwell R, Hartnett M. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kheirouri S, Naghizadeh S, Alizadeh M. Zinc supplementation does not influence serum levels of VEGF, BDNF, and NGF in diabetic retinopathy patients: a randomized controlled clinical trial. Nutr Neurosci. 2019;22(10):718–724. doi: 10.1080/1028415X.2018.1436236. [DOI] [PubMed] [Google Scholar]
- 81.Miller JW, Adamis AP, Shima DT, D'Amore PA, Moulton RS, O'Reilly MS, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Clin Pathol. 1994;145(3):574. [PMC free article] [PubMed] [Google Scholar]
- 82.Pożarowska D, Pożarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol. 2016;41(3):311–316. doi: 10.5114/ceji.2016.63132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ayeleso AO, Lembede BW, Nyakudya TT, Adepoju AE, Chegou NN, Mukwevho E. Administration of S-allyl cysteine to neonatal rats modulates inflammatory biomarkers in high-fructose-fed rats in adulthood. Trop J Pharm Res. 2020;19(5):1053–1058. doi: 10.4314/tjpr.v19i5.21. [DOI] [Google Scholar]
- 84.Mousa AS, Mousa SA. Anti-angiogenesis efficacy of the garlic ingredient alliin and antioxidants: role of nitric oxide and p53. Nutr Cancer. 2005;53(1):104–110. doi: 10.1207/s15327914nc5301_12. [DOI] [PubMed] [Google Scholar]
- 85.Block E, Bechand B, Gundala S, Vattekkatte A, Wang K, Mousa SS, et al. Fluorinated analogs of organosulfur compounds from garlic (Allium sativum): synthesis, chemistry and anti-angiogenesis and antithrombotic studies. Molecules. 2017;22(12):2081. doi: 10.3390/molecules22122081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Naderi R, Mohaddes G, Mohammadi M, Alihemmati A, Khamaneh A, Ghyasi R, et al. The effect of garlic and voluntary exercise on cardiac angiogenesis in diabetes: the role of MiR-126 and MiR-210. Arq Bras Cardiol. 2018;112:154–162. doi: 10.5935/abc.20190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramos GB, Arguelles CL, Herrera AA, Ragasa CV. Screening for potential anti-angiogenic property utilizing avian embryo chorioallantois membranes (CAMs) II Garlic (Allium sativum L.) oil. Asia Life Sci. 2010;20(1):13–23. [Google Scholar]
- 88.Ejaz S, Chekarova I, Cho JW, Lee SY, Ashraf S, Lim CW. Effect of aged garlic extract on wound healing: a new frontier in wound management. Drug Chem Toxicol. 2009;32(3):191–203. doi: 10.1080/01480540902862236. [DOI] [PubMed] [Google Scholar]
- 89.Thejass P, Kuttan G. Antiangiogenic activity of diallyl sulfide (DAS) Int Immunopharmacol. 2007;7(3):295–305. doi: 10.1016/j.intimp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Hussein HA, Hamzah H, Shari MR, Sabri J, Mustapha NM, Sithambaram S. Low levels of microvessel density and immunohistochemical expression of vascular endothelial growth factor in carcinogen-induced ductal mammary gland carcinoma of rats supplemented with garlic. Asian J Pharm Clin Res. 2016;9(4):143–146. [Google Scholar]
- 91.Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, et al. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006;136(3):842S–S846. doi: 10.1093/jn/136.3.842S. [DOI] [PubMed] [Google Scholar]
- 92.Wallace GC, Haar CP, Vandergrift WA, Giglio P, Dixon-Mah YN, Varma AK, et al. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J Neuro-Oncol. 2013;114(1):43–50. doi: 10.1007/s11060-013-1165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng KT, Guo DY, Cheng Q, Geng W, Ling CC, Li CX, et al. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS ONE. 2012;7(2):e31655. doi: 10.1371/journal.pone.0031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Talib WH. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. J Nutr. 2017;43:89–97. doi: 10.1016/j.nut.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Alhasan L, Addai ZR. Allicin-induced modulation of angiogenesis in lung cancer cells (A549) Trop J Pharm Res. 2018;17(11):2129–2134. doi: 10.4314/tjpr.v17i11.3. [DOI] [Google Scholar]
- 96.Arunkumar R, Singh P, Elumalai P, Sambantham S, Rani N, Dinakaran P, et al. Antiangiogenic and anti-invasive effect of diallydisulfide: an in-vitro investigation using prostate cancer cell line and in-vivo using zebrafish embryo model. J Bioequivalence Bioavailab. 2016;8(6):260–271. [Google Scholar]
- 97.Wei Z, Shan Y, Tao L, Liu Y, Zhu Z, Liu Z, et al. Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol Carcinog. 2017;56(10):2317–2331. doi: 10.1002/mc.22686. [DOI] [PubMed] [Google Scholar]
- 98.Özkan İ, Koçak P, Yıldırım M, Ünsal N, Yılmaz H, Telci D, et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-93876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gam D-H, Park J-H, Kim J-H, Beak D-H, Kim J-W. Effects of Allium sativum stem extract on growth and migration in melanoma Cells through inhibition of VEGF, MMP-2, and MMP-9 genes expression. Molecules. 2021;27(1):21. doi: 10.3390/molecules27010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizui T, Kojima M. Recent advances in the biology of BDNF and the newly identified pro-peptide. J Neurol Neuromed. 2018;3(6):1–4. doi: 10.29245/2572.942X/2018/6.1228. [DOI] [Google Scholar]
- 101.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Res. 2015;11(6):1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urbán N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 2014;8:396. doi: 10.3389/fncel.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12:52. doi: 10.3389/fnins.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 105.Farooqui T, Farooqui AA. Neuroprotective effects of garlic in model systems of neurodegenerative diseases. In: Farooqui T, Farooqui AA, editors. Role of the Mediterranean diet in the brain and neurodegenerative diseases. Academic Press; 2018. pp. 253–69. [Google Scholar]
- 106.Park HR, Lee J. Neurogenic contributions made by dietary regulation to hippocampal neurogenesis. Ann N Y Acad Sci. 2011;1229(1):23–28. doi: 10.1111/j.1749-6632.2011.06089.x. [DOI] [PubMed] [Google Scholar]
- 107.Semuyaba I, Safiriyu AA, Tiyo EA, Niurka RF. Memory improvement effect of ethanol garlic (A. Sativum) extract in streptozotocin-nicotinamide induced diabetic wistar rats is mediated through increasing of hippocampal sodium-potassium ATPase, glutamine synthetase, and calcium ATPase activities. Evid-based Complement Altern Med. 2017;2017:1–7. doi: 10.1155/2017/3720380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qu Z, Mossine VV, Cui J, Sun GY, Gu Z. Protective effects of AGE and its components on neuroinflammation and neurodegeneration. Neuromolecular Med. 2016;18(3):474–482. doi: 10.1007/s12017-016-8410-1. [DOI] [PubMed] [Google Scholar]
- 109.Song H, Cui J, Mossine VV, Greenlief CM, Fritsche K, Sun GY, et al. Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration. Exp Ther Med. 2020;19(2):1554–1559. doi: 10.3892/etm.2019.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tedeschi P, Nigro M, Travagli A, Catani M, Cavazzini A, Merighi S, et al. Therapeutic potential of allicin and aged garlic extract in Alzheimer’s disease. Int J Mol Sci. 2022;23(13):6950. doi: 10.3390/ijms23136950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chauhan N. Multiplicity of garlic health effects and Alzheimer's disease. J Nutr Health Aging. 2005;9(6):421–432. [PubMed] [Google Scholar]
- 112.He H, Ma Y, Huang H, Huang C, Chen Z, Chen D, et al. A comprehensive understanding about the pharmacological effect of diallyl disulfide other than its anti-carcinogenic activities. Eur J Pharmacol. 2021;893:173803. doi: 10.1016/j.ejphar.2020.173803. [DOI] [PubMed] [Google Scholar]
- 113.Jeremic JN, Jakovljevic VL, Zivkovic VI, Srejovic IM, Bradic JV, Bolevich S, et al. The cardioprotective effects of diallyl trisulfide on diabetic rats with ex vivo induced ischemia/reperfusion injury. Mol Cell Biochem. 2019;460(1):151–164. doi: 10.1007/s11010-019-03577-w. [DOI] [PubMed] [Google Scholar]
- 114.Leurcharusmee P, Sawaddiruk P, Chattipakorn N, Chattipakorn SC. Possible roles of garlic and its bioactive components on mitochondrial function in physiological and pathological conditions. In: Oliveira MRD, editor. Mitochondrial physiology and vegetal molecules. Academic Press; 2021. pp. 489–539. [Google Scholar]
- 115.Colín-González AL, Ortiz-Plata A, Villeda-Hernández J, Barrera D, Molina-Jijón E, Pedraza-Chaverrí J, et al. Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum Nutr. 2011;66(4):348–354. doi: 10.1007/s11130-011-0251-3. [DOI] [PubMed] [Google Scholar]
- 116.Wang G, Yang Y, Wang C, Huang J, Wang X, Liu Y, et al. Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats. Korean J Pain. 2020;33(3):216–225. doi: 10.3344/kjp.2020.33.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moriguchi T, Matsuura H, Itakura Y, Katsuki H, Saito H, Nishiyama N. Allixin, a phytoalexin produced by garlic, and its analogues as novel exogenous substances with neurotrophic activity. Life Sci. 1997;61(14):1413–1420. doi: 10.1016/S0024-3205(97)00687-5. [DOI] [PubMed] [Google Scholar]
- 118.Jung HY, Lee KY, Yoo DY, Kim JW, Yoo M, Lee S, et al. Essential oils from two Allium species exert effects on cell proliferation and neuroblast differentiation in the mouse dentate gyrus by modulating brain-derived neurotrophic factor and acetylcholinesterase. BMC Complement Altern Med. 2016;16(1):1–10. doi: 10.1186/s12906-016-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang Y-J, Lu K-H, Lin Y-E, Panyod S, Wu H-Y, Chang W-T, et al. Garlic essential oil mediates acute and chronic mild stress-induced depression in rats via modulation of monoaminergic neurotransmission and brain-derived neurotrophic factor levels. Food Funct. 2019;10(12):8094–8105. doi: 10.1039/C9FO00601J. [DOI] [PubMed] [Google Scholar]
- 120.Ji ST, Kim M-S, Park HR, Lee E, Lee Y, Jang YJ, et al. Diallyl disulfide impairs hippocampal neurogenesis in the young adult brain. Toxicol Lett. 2013;221(1):31–38. doi: 10.1016/j.toxlet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 121.Karmakar S, Banik NL, Patel SJ, Ray SK. Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Apoptosis. 2007;12(4):671–684. doi: 10.1007/s10495-006-0024-x. [DOI] [PubMed] [Google Scholar]
- 122.Afarid M, Namvar E, Sanie-Jahromi F. Diabetic retinopathy and BDNF: a review on its molecular basis and clinical applications. J Ophthalmol. 2020;2020:1–7. doi: 10.1155/2020/1602739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-brakati AY. Protective effect of garlic against diabetic retinopathy in adult albino rats. Res J Pharm Biol Chem Sci. 2016;7(5):2748–2759. [Google Scholar]
- 124.Lotfi F, Abbasalipourkabir R, Goodarzi MT, Ziamajidi N. Garlic extract attenuates retinal oxidative stress and IL-1 expression in STZ-induced diabetic rats. Med Plants Int J Phytomed Relat Ind. 2021;13(1):157–163. doi: 10.5958/0975-6892.2021.00018.6. [DOI] [Google Scholar]
- 125.Kommula SR, Chekkilla UK, Ganugula R, Patil MA, Vadakattu SS, Myadara S, et al. Garlic ameliorates long-term pre-diabetes induced retinal abnormalities in high fructose fed rat model. Indian J Exp Biol. 2020;58(7):452–460. [Google Scholar]
- 126.Ziamajidi N, Abbasalipourkabir R, Lotfi F, Goodarzi MT. Aqueous garlic extract alleviates oxidative stress and inflammation in retinal tissue of rats with diabetes type 2. J Adv Med Biomed Res. 2022;30(139):138–145. doi: 10.30699/jambs.30.139.138. [DOI] [Google Scholar]
- 127.Lotfi F, Abbasalipourkabir R, Goodarzi MT, Ziamajidi N. Garlic extract attenuates retinal oxidative stress and IL-1ƣ expression in STZ-induced diabetic rats. Med Plants Int J Phytomed Relat Ind. 2021;13(1):157–163. doi: 10.5958/0975-6892.2021.00018.6. [DOI] [Google Scholar]
- 128.Afarid M, Sadeghi E, Johari M, Namvar E, Sanie-Jahromi F. Evaluation of the effect of garlic tablet as a complementary treatment for patients with diabetic retinopathy. J Diabetes Res. 2022;2022:1–7. doi: 10.1155/2022/6620661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim JH, Yu SH, Cho YJ, Pan JH, Cho HT, Kim JH, et al. Preparation of S-allylcysteine-enriched black garlic juice and its antidiabetic effects in streptozotocin-induced insulin-deficient mice. J Agric Food Chem. 2017;65(2):358–363. doi: 10.1021/acs.jafc.6b04948. [DOI] [PubMed] [Google Scholar]
- 130.Seo Y-J, Gweon O-C, Im J-E, Lee Y-M, Kang M-J, Kim J-I. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. Prev Nutr Food Sci. 2009;14(1):1–7. doi: 10.3746/jfn.2009.14.1.001. [DOI] [Google Scholar]
- 131.Ramos GB, Mariano AP, Arguelles CL, Ples MB, De Vera MP, Ragasa CV, et al. Screening for potential anti-angiogenic property utilizing avian embryo's chorioallantoic membranes (CAMs): I. Pterocarpus indicus Willd.(Papilionaceae) leaf extract. Asia Life Sci. 2010;19(1):115–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Si L, Lin R, Jia Y, Jian W, Yu Q, Wang M, 2019. Lactobacillus bulgaricus improves antioxidant capacity of black garlic in the prevention of gestational diabetes mellitus: a randomized control trial. Biosci Rep. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.