Abstract
Background
The frequent coexistence of obesity and metabolic syndrome in patients with Androgenetic alopecia (AGA), may indicate a common pathogenetic pathway with adipokines being a possible implicating cytokine.
Objective
This study was conducted to investigate the changes in serum levels of adipokines, insulin resistance, vitamin D status and their relationship with AGA, and the relationship between serum levels of adipokines and insulin resistance.
Methods
80 male patients with AGA were selected as the experimental group and 60 healthy males served as the control group. Both the AGA group and healthy control group were divided into 2 groups according to the presence or absence of insulin resistance (IR): the IR group and the NIR group. Serum levels of leptin, adiponectin, resistin, visfatin, insulin and 25(OH)D were evaluated in all subjects.
Results
Compared with the control group, AGA patients showed higher serum levels of leptin and lower adiponectin/leptin (Adpn/Lep) ratio (P<0.05), and both were positively correlated with the severity of the disease. Compared with the AGA NIR group, serum leptin levels were increased in the AGA IR group (P<0.05). AGA IR group and AGA NIR group possessed lower Adpn/Lep ratio when compared with the healthy IR group and healthy NIR group respectively (P<0.05). The multi-factor logistic regression analysis results showed decreased Adpn/Lep level and increased leptin level as risk factors for AGA. AGA Patients had lower vitamin D levels than healthy controls (P<0.05).
Conclusion
Patients with AGA show an imbalance between pro- and anti-inflammatory adipokines, and probably be involved in AGA pathogenesis. Insulin resistance may influence levels of adipokines, but the present findings cannot indicate insulin resistance plays a role in the onset of AGA. The insufficiency and deficiency of vitamin D are common health concern in our subjects and may be involved in the dysfunction of adipocytes and the development of AGA.
Keywords: androgenetic alopecia, adipokines, leptin, vitamin D
Introduction
Androgenetic alopecia (AGA) is the most common chronic hair loss disorder, which presents progressive, hereditary, androgen-sensitive thinning of the scalp hair, accompanied by the excessive secretion of sebum. As the disease progresses, the length of anagen is shortened while telogen remains constant or is prolonged. Eventually, the anagen period is too short for the hair to grow from the skin surface. The miniaturization of hair follicles is a histological feature of AGA; therefore, hair loss is likely to be irreversible, suggesting the importance of early prevention and treatment. AGA is often accompanied by obesity, cardiovascular disease, and metabolic syndrome.1 Previous studies have shown an association between obesity and higher severity of hair loss so that obesity-induced metabolic changes may regulate AGA progression.2
Adipose tissue has now been recognized as an active endocrine organ with the secretion of various adipokines involved in energy expenditure, glucolipid metabolism and inflammatory response processes.3 Adipokines can be classified into pro-inflammatory (eg, leptin) and anti-inflammatory (eg, Adiponectin) types, which are balanced under normal conditions.4 In some instances, including obesity and high-calorie diets, an imbalance of adipocyte function is characterized by an increased expression of pro-inflammatory adipokines and decreased expression of anti-inflammatory adipokines, leading to a chronic, low-grade inflammatory state.5 The follicular papilla and inner root sheaths secrete leptin and have leptin receptors.6 Differential protease expression is observed during the hair cycle, showing a minimum in the early anagen phase, an upregulation in the late anagen phase and a maximum in the telogen phase, suggesting that leptin may regulate hair growth hair cycle progression.7 However, the exact regulatory mechanism by which leptin affects the hair cycle and hair growth is still not fully understood. According to Sumikawa Y et al, leptin is an anagen inducer that can induce hair growth around the injection site when injected topically into the shaved dorsal skin of wild-type mice,8 while Yang CC suggested that the differentially expressed leptin during the hair cycle contributes to adipocyte-mediated inhibition of anagen-phase and vibrissa hair cycle may be influenced primarily by the presence of systemic leptin in the blood.7 A previous study by Yang CC showed that a higher plasma leptin level is associated with a higher risk of developing AGA in men.9 The relationship between adiponectin and hair loss has been poorly investigated. Yang CC et al reported no differences in adiponectin levels between AGA patients and healthy controls. Adipose tissue strongly participates in insulin resistance. According to Matilainen et al, early androgenetic hair loss is a clinical indicator of insulin resistance,10 while controversies exist regarding the association of androgenetic alopecia (AGA) with insulin resistance and there is a lack of studies on adipokine changes in the state of insulin resistance among patients with AGA.
Vitamin D is important in regulating adipogenesis and adipose tissue functions.11 Vitamin D deficiency has been associated with aberrant adipogenesis. In contrast, Vitamin D deficiency disturbs adipocytokines secretion, metabolism, the regulation of inflammation, and oxidative stress balance.12 The deficiency of Vitamin D is also closely linked to several types of alopecia, including AGA, alopecia areata and telogen effluvium. Sanke et al suggested that vitamin D may play a role in the premature onset of androgenetic alopecia. Therefore, vitamin D levels should be assessed in patients with AGA.13
To date, there are limited reports on the role of adipose function in AGA. We aimed to investigate the association between serum adipocytokines levels, insulin resistance and vitamin D status with the risk and severity of AGA in male patients with AGA.
Materials and Methods
Patients
This study included 80 male patients with androgenetic alopecia and 60 healthy controls matched for age, sex and BMI. Diagnosis of androgenetic alopecia is based on a detailed medical history and clinical examination. Eligibility criteria included no treatment taken for the last 6 months. The disease course was 1 year to 10 years, with an average course of 4.4±2.573 years. The stage of individual participant alopecia was evaluated according to the Hamilton–Norwood scale. Alopecia is classified according to the severity of hair loss: mild alopecia (grade I, II, III), moderate alopecia (grade IV, V) and severe alopecia (grade VI, VII). The patients are divided into two groups according to the presence or absence of insulin resistance (IR).
The exclusion criteria were: (1) Patients with incomplete data. (2) Patients with other conditions known to cause hair loss. (3) Obese patients which was defined as BMI ≥30 kg/m2. (4) Patients who presence of other acute or chronic inflammations (5) Patients receiving hormone replacement therapy (6) Patients who have a history of disease including cardiovascular disease, hyperlipidemia, endocrine disease and various malignancies, etc. (7) Patients who took vitamins, minerals and other dietary supplements within the 3 months before the study.
Biochemical Measurements
All blood samples were collected after 8 hours of fasting and stored at − 80 °C. Leptin, adiponectin, resistin and visfatin were measured by enzyme-linked immunosorbent assays. Insulin, serum lipid parameter, and 25(OH)D were measured by the laboratories of Jinling hospital.
Calculations
The homeostasis model assessment for insulin resistance (HOMA-IR) was used as a proxy measurement for insulin sensitivity because it correlates well with the “gold standard” euglycemic-hyperinsulinemic clamp. HOMA-IR = fasting insulin (mU/l)×fasting glucose (mmol/l)/22.5.14 HOMA-IR < 2.5 was considered normal while HOMA-IR ≥ 2.5 indicated insulin resistance. The leptin/adiponectin ratio was calculated to measure adipose tissue dysfunction. The deficiency was defined as a 25(OH)D < 20 ng/mL, insufficiency as 25(OH)D values 20–30 ng/mL, and adequacy as a 25(OH)D ≥ 30 ng/mL.
Statistical Analysis
Kolmogorov–Smirnov normality tests and Shapiro–Wilk normality tests were used to check the normality of all continuous variables, with those obeying a normal distribution expressed as mean±standard deviation and those not satisfying a normal distribution expressed as M (P25, P75). T-test and one-way analysis of variance (ANOVA) was used to examine the measurement data that followed the normal distribution and the Mann–Whitney U-test was used for data that did not conform to a normal distribution. Correlations between variables were examined using Spearman’s rank test. Multivariable analysis with independent variables of age, BMI, Leptin, Adiponectin, Adpn/Lep Ratio and 25(OH)D was performed by Logistic regression analysis. Data were considered significant for p < 0.05. The analyses were performed with Statistica 26.0.
Results
There were 80 male participants with AGA [mean age 36.28±10.49 years, range 19–57] and 60[mean age 36.28±10.98 years, range 18–56] healthy subjects were included in the study. There were no statistically significant differences between the two groups in terms of age, BMI, fasting glucose, cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides (p = 0.996, p = 0.160, p = 0.235, p = 0.940, p = 0.380, p = 0.387 and p = 0.093, respectively). Detailed characteristics of patients and healthy controls are presented in Table 1.
Table 1.
Characteristic of Patients with Androgenetic Alopecia and Healthy Controls
| Parameter | Patients With AGA (n=80) | Healthy Controls (n=60) | P value |
|---|---|---|---|
| Age, mean ± SD (years) | 36.28±10.49 | 36.28±10.98 | 0.996 |
| BMI, mean±SD (kg/m2) | 24.29±2.41 | 23.59±2.42 | 0.160 |
| Fasting glucose, mean ± SD (mg/dl) | 5.08±0.55 | 4.97±0.43 | 0.235 |
| Cholesterol, mean ± SD (mg/dl) | 4.51±1.08 | 4.49±0.85 | 0.940 |
| Low-density lipoprotein, mean ± SD (mg/dl) | 2.37±0.74 | 2.41±0.63 | 0.380 |
| High-density lipoprotein, mean ± SD (mg/dl) | 1.28±0.23 | 1.24±0.23 | 0.387 |
| Triglycerides, mean ± SD (mg/dl) | 1.23±0.68 | 1.05±0.54 | 0.093 |
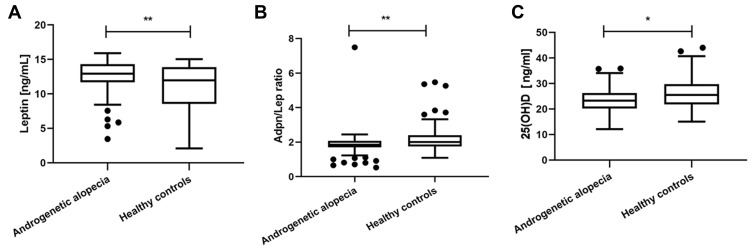
Mild alopecia was observed in 36 (45%) patients, moderate alopecia in 37 (46.3%) patients and severe alopecia in 7 (0.09%) patients. The plasma level of leptin was significantly higher in AGA subjects compared to non-AGA subjects (12.94 vs 11.98 ng/mL, P=0.006) (Figure 1a). The Adpn/Lep ratio mean levels were significantly lower in the patients with AGA compared to healthy controls (1.86 vs 2.01, P=0.006) (Figure 1b). Serum adiponectin, resistin, visfatin, insulin levels and HOMA-IR scores were not statistically significant in the AGA patients compared with the control subjects. Further details are listed in Table 2.
Figure 1.
Serum concentrations of leptin, Adpn/Lep ratio and 25(OH)D in patients with AGA and healthy controls. (a) In compare to healthy group, AGA patients showed higher leptin level. (b) In compare to healthy group, AGA patients showed lower Adpn/Lep ratio. (c) In compare to healthy group, AGA patients showed lower 25(OH)D level. *Indicates statistically significant difference, p value<0.05; **Indicates statistically significant difference, p value<0.01.
Table 2.
Serum Concentrations of Leptin, Adiponectin, Adpn/Lep Ratio, Resistin, Visfatin, 25(OH)D, Insulin and HOMA-IR in Patients with AGA and Healthy Controls
| Parameter | Patients With AGA (n=80) | Healthy Controls (n=60) | P value |
|---|---|---|---|
| Leptin (ng/mL) | 12.94(11.70, 14.32) | 11.98(8.55, 13.89) | 0.006 |
| Adiponectin (μg/mL) | 23.81(20.31, 26.20) | 24.49(20.25, 27.04) | 0.350 |
| Adpn/Lep Ratio | 1.86(1.70, 2.08) | 2.01(1.75, 2.40) | 0.006 |
| Resistin (ng/mL) | 38.31(33.14, 42.63) | 40.93(28.68, 45.59) | 0.241 |
| Visfatin (ng/mL) | 93.21(78.46, 105.50) | 99.62(83.42, 108.10) | 0.179 |
| 25(OH)D (ng/mL) | 23.64±5.12 | 25.76±6.37 | 0.031 |
| Insulin | 5.76(4.17, 11.34) | 5.87(4.21, 9.29) | 0.908 |
| HOMA-IR | 1.27(0.88, 2.83) | 1.25(0.94, 1.97) | 0.825 |
Note: Bold text indicates p < 0.05.
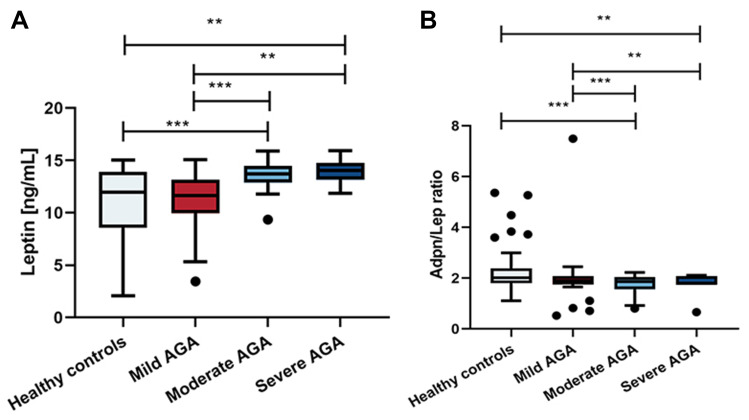
In the test for subgroup differences, there were large differences in leptin and the Adpn/Lep ratio relating to disease severity, both between moderate AGA versus healthy group and mild AGA, and between severe AGA versus healthy group and mild AGA, while moderate versus severe AGA showed no significant difference (Figure 2).
Figure 2.
Serum concentrations of leptin and Adpn/Lep ratio in patients with mild, moderate and severe AGA. (a) There were large differences in serum leptin level both between moderate AGA versus healthy group and mild AGA, and between severe AGA versus healthy group and mild AGA, while moderate versus severe AGA showed no significant difference. (b) There were large differences in Adpn/Lep ratio both between moderate AGA versus healthy group and mild AGA, and between severe AGA versus healthy group and mild AGA, while moderate versus severe AGA showed no significant difference. **Indicates statistically significant difference, p value<0.01, ***Indicates statistically significant difference, p value<0.001.
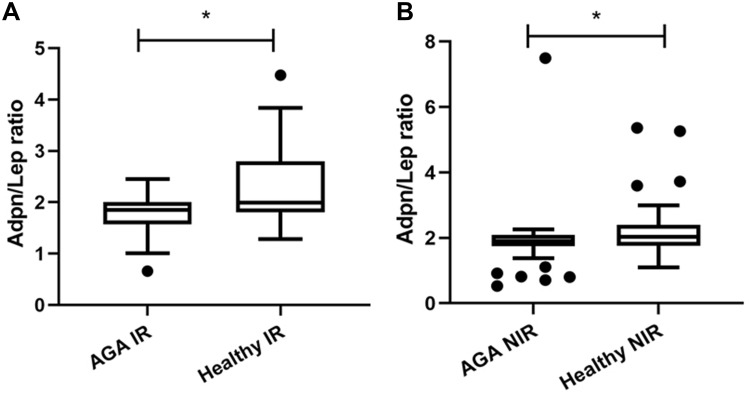
According to the HOMA-IR, IR was detected in 24 (30%) of the AGA patients and 12 (20%) of control group, while NIR was in 56 (70%) patients and in 48 (80%) healthy subjects. Compared with the AGA NIR group, the serum level of leptin of the AGA IR group was increased (14.19 vs 12.98 ng/mL, p = 0.014), while the serum level of adiponectin, resistin and visfatin showed no difference. When comparing AGA IR group versus healthy IR group, AGA IR group possessed a lower Adpn/Lep ratio (1.84 vs 1.99, P=0.041)(Figure 3a), no other statistically significant difference was observed in leptin, adiponectin, resistin and visfatin. Furthermore, AGA NIR group showed a lower Adpn/Lep ratio compared with the healthy NIR group (1.90 vs 2.03, P=0.012)(Figure 3b), while there were no significant differences in leptin, adiponectin, resistin and visfatin among these two groups.
Figure 3.
(a) AGA IR group versus healthy IR group, AGA IR group possessed a lower Adpn/Lep ratio. (b) AGA NIR group versus healthy NIR group, AGA NIR group showed a lower Adpn/Lep ratio. *Indicates statistically significant difference, p value<0.05.
Out of 140 participants, the majority (80%) of people had vitamin D deficiency or insufficiency. Vitamin D insufficiency was observed in 52 (65%) patients and 39 (65%) healthy subjects. There was vitamin D deficiency in 19 (23.75%) of cases compared with 10 (16.67%) of controls.
AGA patients show lower vitamin D levels compared to healthy controls (23.64 vs 25.76 ng/mL, p=0.031)(Figure 1c).
Furthermore, multiple factors logistic regression was performed to evaluate independent risk factors for AGA. The risk factors assessed in the logistic model included age, BMI, 25(OH)D, insulin resistance and Adpn/Lep ratio. As shown in Table 3, decreased Adpn/Lep ratio is an independent risk factor for AGA (OR=0.485, 95% CI 0.264–0.890, P=0.019). Because it was necessary to remove the strongly correlated parameters, we also performed logistic regression analysis of age, BMI, 25(OH)D, insulin resistance and leptin. The results showed that increased serum leptin level is also an independent risk factor for AGA (OR=1.374, 95% CI 1.150–1.641, P=0.000), results are presented in Table 4.
Table 3.
The Association of Insulin Resistance, Age, BMI, 25(OH)D and Adpn/Lep Ratio with the Development of Androgenetic Alopecia by Multivariate Analysis
| Parameter | P | OR | 95% CI |
|---|---|---|---|
| Adpn/Lep Ratio | 0.028 | 0.433 | 0.205–0.915 |
| Age | 0.638 | 0.991 | 0.957–1.027 |
| BMI | 0.101 | 1.179 | 0.968–1.436 |
| 25(OH)D | 0.064 | 0.938 | 0.876–1.004 |
| Insulin resistance | 0.498 | 1.345 | 0.571–3.169 |
Note: Bold text indicates p < 0.05.
Table 4.
The Association of Insulin Resistance, Age, BMI, 25(OH)D and Leptin with the Development of Androgenetic Alopecia by Multivariate Analysis
| Parameter | P | OR | 95% CI |
|---|---|---|---|
| Leptin | 0.00 | 1.378 | 1.150–1.641 |
| Age | 0.524 | 0.988 | 0.953–1.025 |
| BMI | 0.108 | 1.182 | 0.964–1.449 |
| 25(OH)D | 0.070 | 0.936 | 0.872–1.005 |
| Insulin resistance | 0.783 | 0.883 | 0.363–2.146 |
Note: Bold text indicates p < 0.05.
Discussion
The most common cause of non-scarring hair loss is AGA, which seriously affects the psychosocial manifestations of patients.15 In the Caucasian population, about 80% of men and 50% of women will develop symptoms of AGA by the age of 70.16 The prevalence of AGA is relatively low in Asian populations but shows an increasing trend. The etiology and pathogenesis of AGA are unclear, and it is currently accepted that there is a genetic predisposition and androgen dependence. In addition, the development of AGA is influenced by a variety of other factors, including perifollicular low-grade inflammatory state, psychological factors, lifestyle and dietary habits.17
It is worth noting that AGA is not only a cosmetic problem, as it is often associated with metabolic abnormalities. A statistically significant additive effect of obesity and hair loss has been found.18 According to Matilainen et al, early androgenetic alopecia can be a clinical marker of insulin resistance.10 Numerous studies have demonstrated the relationship between androgenetic alopecia, cardiovascular disease, and hypertension.19,20 These metabolic disorders and impaired metabolic states associated with androgenetic alopecia suggest that adipokines may play a role in the pathogenesis of the disease. However, a limited number of studies have evaluated the role of adipokines in AGA.
Leptin is encoded by the leptin gene (LEP) located on chromosome 7q31.3, which is mainly synthesized and secreted by white adipose tissue. In the hypothalamus, leptin acts mainly on the satiety center to suppress appetite and stimulate energy expenditure through negative feedback. In peripheral tissues, the wide distribution of leptin receptors determines the pleiotropic nature of leptin, such as stimulating angiogenesis, regulating hormone secretion and regulating the production of pro-inflammatory cytokines. Blood leptin concentration correlates directly with adipose tissue mass and BMI under normal physiological conditions. Circulating leptin levels rise due to obesity, high fat intake, and aging. This rise in leptin levels probably reflects leptin resistance, closely related to insulin resistance.21 In addition, leptin acts as an inflammatory mediator and induces the production of pro-inflammatory cytokines such as TNF-α and IL-6. Leptin also stimulates neutrophil chemotaxis and the release of oxygen free radicals, by affecting lymphocyte receptors, promoting a shift in Th1/Th2 balance towards Th1, leading to increased inflammation. In the present study, higher serum levels of leptin were revealed in non-obese AGA patients compared to those in healthy individuals. Moreover, a positive correlation was identified between the serum levels of leptin and the severity of hair loss. Elevated serum leptin and decreased Adpn/Lep ratio are independent risk factors for AGA. This trend is in line with the previous findings of YCC et al.9
Adiponectin is a serum protein produced mainly by the white adipose tissue. Adiponectin has insulin-sensitizing, anti-inflammatory and anti-atherogenic effects whose expression is inversely correlated with obesity.22 Reduced adiponectin synthesis in the presence of obesity and metabolic abnormalities may dysregulate anti-inflammatory mechanisms. This imbalanced inflammatory state is associated with an increased risk of cardiovascular disease (CVD), type II diabetes and other metabolic dysfunction.23 The adiponectin/leptin (Adpn/Lep) ratio has been proposed as a marker of adipose tissue dysfunction. This emerging biomarker decreases with an increasing number of metabolic risk factors for metabolic syndrome (MS) and has therefore been considered a predictive marker for MS, better than adiponectin or leptin alone.24 Moreover, the Adpn/Lep ratio is negatively correlated with markers of low-grade chronic inflammation, such as C-reactive protein (CRP) and serum amyloid A (SAA).25 The present study showed that the adiponectin concentration did not correspond to the onset of AGA and the severity of hair loss, while the Adpn/Lep ratio shows lower levels in patients with AGA and the values are inversely proportional to the severity of the disease.
Hyperinsulinemia secondary to insulin resistance may lead to AGA.18 30% of patients in our study had a state of insulin resistance (IR). IR is a chronic subclinical inflammatory process in which elevated leptin levels may affect insulin receptor and post-receptor signaling leading to an inhibition of glucose uptake, which leads to a decrease in insulin sensitivity and, therefore, compensatory insulin secretion. Elevated insulin levels, in turn, stimulate leptin secretion, which can further exacerbate IR.26 IR can lead to a decrease in Sex Hormone Binding Globulin (SHBG) concentrations, which increases free androgen levels that act on AGA-susceptible hair follicles, thereby leading to progressive follicular miniaturization and hair loss. In contrast, adiponectin can improve IR and reduce blood glucose in the body. Our study showed that serum leptin was higher in the IR group than in the NIR group, with a significant difference. The metabolic disorders caused by leptin and IR may contribute to the development of AGA.
Resistin and visfatin are also pro-inflammatory proteins in human lipid, glucose and insulin homeostasis. Resistin is primarily secreted by macrophages, not adipocytes. Visfatin is a recently discovered adipokine produced primarily by perivascular adipose tissue. Despite the probable involvement of resistin and visfatin in the pathogenesis of metabolic diseases, no differences between the serum concentrations of resistin and visfatin in patients with AGA and healthy controls were reported in the present study.
Vitamin D is primarily synthesized in the skin due to exposure to sunlight. Subsequently, pre-vitamin D is hydroxylated into 25-hydroxyvitamin D [25(OH)D] in the liver, which is the major circulating form of vitamin D and is used as an indicator of vitamin D status.27 However, the biologically active 1,25-dihydroxy-vitamin D [1,25(OH)2D] is mainly converted in the kidney. It is worth noting that Vitamin D is fat-soluble and stored in adipose tissue, and vitamin D receptor (VDR) plays a vital role in adipogenesis.28 Previous studies have shown that 1,25(OH)2D3 treatment can upregulate adiponectin in vitro and inhibit anti-inflammatory cytokine expression, and daily intake of fortified vitamin D can improve inflammation in T2DM.29 The evidence that vitamin D deficiency plays a role in hair loss is controversial. Nevertheless, there is growing evidence that vitamin D and its receptors are responsible for maintaining the homeostasis of calcium and the skin.30 In addition, current evidence indicates that inverse association between leptin level and 25(OH)D concentration, which can be additional proof of the association between adipokines with AGA progression.31 Therefore, the importance of vitamin D supplementation should be realized. In our study, we found that 85.7% of people who did not routinely take additional oral plain vitamin D supplementation had vitamin D deficiency or deficiency status. Patients with hair loss show lower vitamin D levels than healthy controls. We recommend that patients with low vitamin levels take VD supplements rather than active vitamin D and its analogues, such as osteopontin, as the risk of hypercalcemia is significantly higher with active vitamin D than with regular vitamin D.
Our study had some strengths and limitations that need to be addressed. Firstly, we controlled for a number of possible confounders such as BMI, age, lipid metabolism and taking vitamin supplements in this study and the sample size has been expanded compared to previous studies. Furthermore, to the best of our knowledge, this is the first time that Adpn/Lep ratio is proposed to be used as a biomarker for the risk of AGA. In addition, our study explored for the first time the role of resistin and visfatin in AGA. However, this study also had limitations. Firstly, considering the effect of sex hormones on adipokines, this study only focuses on the male population. Secondly, we could not obtain local tissue samples from the scalp for adipokine testing and, therefore, could not investigate the effect of local adipokines on hair follicles. Lastly, this study did not further investigate the mechanism for the over-expression of leptin in AGA, which needs further exploration.
Conclusion
In summary, the results of the present study support the hypothesis that the impaired secretion of certain adipokines may play an important role in the pathogenesis of AGA and its continuity. Patients with AGA are characterized by the dysfunction of adipose tissue, with abnormal increases in serum leptin levels and decreases in the Adpn/Lep ratio, which are proportional to the severity of the disease. Elevated serum leptin and decreased Adpn/Lep ratio are independent risk factors for AGA. Insulin resistance may influence levels of adipokines, but we found no significant relationship between insulin resistance and male AGA. AGA patients should be prevented from multiple metabolic disorders, suggesting that patients with AGA should improve their living habits and reduce high-fat diets. Furthermore, whether or not it works to improve hair loss, vitamin D supplementation is a good recommendation for AGA patients.
Acknowledgments
The authors would like to thank all the staff at the Dermatology Department of the Jinling Hospital for their help and guidance.
Funding Statement
This work was supported by Jiangsu Dermatology Innovation Team Foundation (grant number CXTDA2017038).
Abbreviations
BMI, body mass index; SD, standard deviation; AGA, Androgenetic alopecia; IR, insulin resistance; Adpn/Lep, adiponectin/leptin; CVD, cardiovascular disease; MS, metabolic syndrome.
Ethics and Consent Statements
This study was approved by the Research Ethics Committees of the Jinling Hospital (No.2021NZKY-065-01). This study adhered to the guidelines outlined in the Declaration of Helsinki and all subjects have signed an informed consent form.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sheikh FZ, Butt G, Hafeez R, Maqsood A, Altaf F, Hussain I. Association of early-onset androgenetic alopecia and metabolic syndrome. J Coll Physicians Surg Pak. 2021;31(2):123–127. [DOI] [PubMed] [Google Scholar]
- 2.Yang -C-C, Hsieh F-N, Lin L-Y, Hsu C-K, Sheu H-M, Chen W. Higher body mass index is associated with greater severity of alopecia in men with male-pattern androgenetic alopecia in Taiwan: a cross-sectional study. J Am Acad Dermatol. 2014;70(2):297–302.e1. doi: 10.1016/j.jaad.2013.09.036 [DOI] [PubMed] [Google Scholar]
- 3.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–920. doi: 10.1016/j.jaci.2005.02.023 [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104(4):541–549. doi: 10.1161/CIRCRESAHA.108.182998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iguchi M, Aiba S, Yoshino Y, Tagami H. Human follicular papilla cells carry out nonadipose tissue production of leptin. J Invest Dermatol. 2001;117(6):1349–1356. doi: 10.1046/j.0022-202x.2001.01606.x [DOI] [PubMed] [Google Scholar]
- 7.Yang CC, Sheu HM, Chung PL, et al. Leptin of dermal adipose tissue is differentially expressed during the hair cycle and contributes to adipocyte-mediated growth inhibition of anagen-phase vibrissa hair. Exp Dermatol. 2015;24(1):57–60. doi: 10.1111/exd.12566 [DOI] [PubMed] [Google Scholar]
- 8.Sumikawa Y, Inui S, Nakajima T, Itami S. Hair cycle control by leptin as a new anagen inducer. Exp Dermatol. 2014;23(1):27–32. doi: 10.1111/exd.12286 [DOI] [PubMed] [Google Scholar]
- 9.Yang CC, Chung PL, Lin LY, Hughes MW, Tsai YS. Higher plasma leptin is associated with higher risk of androgenetic alopecia in men. Exp Dermatol. 2017;26(6):524–526. doi: 10.1111/exd.13369 [DOI] [PubMed] [Google Scholar]
- 10.Matilainen V, Koskela P, Keinänen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. Lancet. 2000;356(9236):1165–1166. doi: 10.1016/S0140-6736(00)02763-X [DOI] [PubMed] [Google Scholar]
- 11.Nimitphong H, Park E, Lee M-J. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr Res Pract. 2020;14(6):553–567. doi: 10.4162/nrp.2020.14.6.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szymczak-Pajor I, Miazek K, Selmi A, Balcerczyk A, Śliwińska A. The action of vitamin d in adipose tissue: is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders? Int J Mol Sci. 2022;23(2):956. doi: 10.3390/ijms23020956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerkowicz A, Chyl-Surdacka K, Krasowska D, Chodorowska G. The role of vitamin D in non-scarring alopecia. Int J Mol Sci. 2017;18(12):2653. doi: 10.3390/ijms18122653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 15.Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17. doi: 10.1007/s12020-017-1280-y [DOI] [PubMed] [Google Scholar]
- 16.Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32(1):11–22. doi: 10.1111/jdv.14624 [DOI] [PubMed] [Google Scholar]
- 17.Heymann WR. The inflammatory component of androgenetic alopecia. J Am Acad Dermatol. 2022;86(2):301–302. doi: 10.1016/j.jaad.2021.11.013 [DOI] [PubMed] [Google Scholar]
- 18.González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol. 2009;71(4):494–499. doi: 10.1111/j.1365-2265.2008.03508.x [DOI] [PubMed] [Google Scholar]
- 19.Arias-Santiago S, Gutiérrez-Salmerón MT, Buendía-Eisman A, Girón-Prieto MS, Naranjo-Sintes R. Hypertension and aldosterone levels in women with early-onset androgenetic alopecia. Br J Dermatol. 2010;162(4):786–789. doi: 10.1111/j.1365-2133.2009.09588.x [DOI] [PubMed] [Google Scholar]
- 20.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: a comparative study. J Am Acad Dermatol. 2010;63(3):420–429. doi: 10.1016/j.jaad.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 21.Wauters M, Considine RV, Yudkin JS, Peiffer F, De Leeuw I, Van Gaal LF. Leptin levels in type 2 diabetes: associations with measures of insulin resistance and insulin secretion. Horm Metab Res. 2003;35(2):92–96. doi: 10.1055/s-2003-39054 [DOI] [PubMed] [Google Scholar]
- 22.Anandaraj AA, Syed Ismail PM, Namis SM, Bajnaid YJ, Shetty SB, Almutairi KM. Association of selected adipocytokines and inflammatory markers on body mass index in type 2 diabetes patients in Saudi Arabia and as risk factors to cardiovascular disease. Curr Diabetes Rev. 2017;13(3):330–335. doi: 10.2174/1573399812666160614014254 [DOI] [PubMed] [Google Scholar]
- 23.Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 24.Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7(1):57–62. doi: 10.1080/21623945.2017.1402151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frühbeck G, Catalán V, Rodríguez A, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;11(2):454. doi: 10.3390/nu11020454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marques-Oliveira GH, Silva TM, Lima WG, Valadares HMS, Chaves VE. Insulin as a hormone regulator of the synthesis and release of leptin by white adipose tissue. Peptides. 2018;106:49–58. doi: 10.1016/j.peptides.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 27.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87(4):1087S–1091S. doi: 10.1093/ajcn/87.4.1087S [DOI] [PubMed] [Google Scholar]
- 28.Abbas MA. Physiological functions of Vitamin D in adipose tissue. J Steroid Biochem Mol Biol. 2017;165(Pt B):369–381. doi: 10.1016/j.jsbmb.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 29.Peters KE, Davis WA, Beilby J, Hung J, Bruce DG, Davis TME. The relationship between circulating adiponectin, ADIPOQ variants and incident cardiovascular disease in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Res Clin Pract. 2018;143:62–70. doi: 10.1016/j.diabres.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Kechichian E, Ezzedine K. Vitamin D and the skin: an update for dermatologists. Am J Clin Dermatol. 2018;19(2):223–235. doi: 10.1007/s40257-017-0323-8 [DOI] [PubMed] [Google Scholar]
- 31.Hajimohammadi M, Shab-Bidar S, Neyestani TR. Vitamin D and serum leptin: a systematic review and meta-analysis of observational studies and randomized controlled trials. Eur J Clin Nutr. 2017;71(10):1144–1153. doi: 10.1038/ejcn.2016.245 [DOI] [PubMed] [Google Scholar]