INTRODUCTION
There are currently more than 1000 prescription medications available for use in the United States and more than 100,000 over‐the‐counter herbal and dietary supplements (HDS) available for purchase in retail stores and online. In addition, the average adult American receives more than six prescription medications per year.1,2 Many of these drugs and HDS products have been implicated as causes of DILI. Furthermore, DILI is a leading reason for regulatory actions regarding drugs in development as well as those in the marketplace.1 Confidently establishing a diagnosis of DILI is difficult because of the need to exclude more common competing causes of liver injury, the protean clinical manifestations from an individual agent, and the lack of a validated diagnostic biomarker.3–5
This guidance was developed with the support and oversight of the American Association for the Study of Liver Diseases Practice Guidelines Committee, who chose to commission a guidance, rather than a guideline, because of the paucity of randomized controlled trials on this topic. This document was developed by consensus of an expert panel and provides guidance statements based on formal review and analysis of the literature on the topics and questions related to the needs of patients with drug and supplement–induced liver injury.
The aim of this practice guidance is to provide recommendations regarding the common clinical, laboratory, and histological features seen in patients with DILI based on observational and epidemiological data reported in case series or DILI registries. In addition, expert opinion–based recommendations for patient management, including risk stratification, are provided to assist patients and practitioners.
DILI classification
DILI can be mechanistically classified as being either direct (i.e., dose‐dependent, intrinsic, and predictable) or idiosyncratic (largely dose‐independent, idiosyncratic, and unpredictable) (Table 1). Direct hepatotoxins such as acetaminophen (APAP) (N‐acetyl‐para‐aminophenol) can cause liver injury in nearly all exposed individuals if a threshold dose or duration is exceeded. In contrast, idiosyncratic hepatotoxins are usually neither dose‐related nor duration‐related but rather occur at varying times during or after drug administration.6 Idiosyncratic DILI is uncommon, with most approved drugs occurring in only 1 in 1000 to 1 in a million exposed individuals. Although most patients do not have rash, eosinophilia, or other hypersensitivity features at presentation, aberrant host immunity is implicated in most instances of idiosyncratic DILI.3
TABLE 1. Proposed classification of DILI.
| Mechanistic classification | Direct hepatotoxicity | Idiosyncratic hepatotoxicity | Indirect hepatotoxicity |
|---|---|---|---|
| Incidence | Common | Rare | Intermediate |
| Dose relatedness | Yes | No | No |
| Predictable | Yes | No | Partially |
| Reproduced in animal models | Yes | No | Not usually |
| Latency | Rapid (days) | Variable (days to years) | Delayed (months) |
| Phenotypes of injury | Serum AST, ALT, or ALP elevations, hepatic necrosis, acute fatty liver, nodular regeneration | Mixed or cholestatic hepatitis, bland cholestasis, chronic hepatitis | Immune‐mediated hepatitis, fatty liver, chronic hepatitis |
| Examples | Acetaminophen, niacin, intravenous methotrexate | Amoxicillin‐clavulanate, cephalosporins, isoniazid, nitrofurantoin | Immune checkpoint inhibitors, anti‐CD20 monoclonal Ab, protein kinase inhibitors |
| Touted mechanism of injury | Intrinsic hepatotoxicity that is dose‐dependent | Idiosyncratic host metabolic or immune reaction | Indirect effect on liver or host immunity |
Source: Adapted from Björnsson et al.5
A third mechanism of hepatotoxicity is called indirect DILI, which arises when the biological action of the drug affects the host immune system, leading to a secondary form of liver injury. Like idiosyncratic DILI, indirect hepatotoxins are generally independent of the dose of medication administered and have a latency of weeks to months with varying clinical manifestations. Examples of indirect hepatotoxicity include the immune‐mediated hepatitis (IMH) observed with immune checkpoint inhibitors (ICIs) and reactivation of HBV infection following rituximab infusions.7,8
Guidance statements.
Clinicians should be familiar with the three main types of hepatotoxicity when evaluating patients with suspected DILI.
Direct hepatotoxins such as APAP can cause liver injury in nearly all exposed individuals once a threshold dose or duration of use is exceeded.
Idiosyncratic DILI is largely independent of the dose and duration of medication use and characterized by a low incidence and variable drug latency and clinical and histological features.
Idiosyncratic DILI is believed to arise from an aberrant adaptive host immune response to the drug and/or its metabolite(s).
Indirect hepatotoxins are generally independent of the dose administered and have a variable latency and manifestations that arise from the biological action of the drug on the liver and/ or host immune system.
Epidemiology of idiosyncratic DILI
Idiosyncratic DILI is uncommon, with an estimated annual incidence in the general population of 14 to 19 events per 100,000 inhabitants or 60,000 cases per year in the general US population.9,10 The estimated incidence of idiosyncratic DILI also varies based on the case definition as well as the methods used for case ascertainment. For example, the incidence appears to be higher in exposure‐based studies using electronic medical records (32.8 per 100,000 adult patients who received one of the top implicated drugs in the United States and 40 per 100,000 patients at a pediatric hospital).11,12 The incidence of idiosyncratic DILI is even higher in hospitalized patients, being reported as high as 1.4% among medical inpatients.13–16
Results of ongoing DILI registry studies demonstrate that the spectrum of suspect drugs and demographics of afflicted patients substantially differ among countries and regions.17–24 These observations likely reflect differences in case definitions as well as differences in medication use, health care systems, and sociocultural and medical attributes in the various populations (Table 2).
TABLE 2. Etiologies and outcomes with DILI in different countries.
| Country | United States/DILIN, n = 899 | Spain, n = 843 | Iceland, n = 96 | Latin America, n = 311 | China, n = 25, 927 | India, n = 313/1288 |
|---|---|---|---|---|---|---|
| Study design | Prospective registry30 | Prospective registry29 | Prospective, population‐based9 | Prospective registry18 | Retrospective case series22 | Prospective case series21,31 |
| Publication year | 2015 | 2021 | 2013 | 2019 | 2019 | 2010/2021 |
| Age distribution, years | 49 ± 17 | 54 (11–91) | 55¥ (16–91) | 50 (11–91) | 43% (40–59 years) | 39 (12–84)/43 (1–86) |
| % Female | 59 | 48 | 56 | 61 | 49 | 42/48.6 |
| % Liver‐ and non‐liver‐related fatality | Liver‐related: 3.0; non‐liver‐related: 3.2 | Liver‐related: 2.1; non‐liver‐related: 1.7 | Overall fatality: 1 | Overall fatality: 4.9 | Liver‐related: 0.28a; non‐liver‐related: 0.11a | Overall fatality: 17.3/12.3 |
| % Liver transplant | 3.7 | 1.5 | 0 | 0 | 0.01 | 0 |
| Top 3 implicated drug classes | Antimicrobials, HDS, cardiovascular agents | Anti‐infectives, CNS drugs, musculoskeletal drugs (including NSAID) | Antibiotics, immuno‐suppressants, psychotropic drugs | Antibiotics,b NSAIDs,b antitubercularb | TCM or HDS, antitubercular, antineoplastic or immune modulators | Antitubercular, HDS, antiepileptics |
| Top 10 implicated agents | HDS, amoxicillin/clavulanate, isoniazid, nitrofurantoin, trimethoprim‐sulfamethoxazole, minocycline, cefazolin, azithromycin, ciprofloxacin, levofloxacin | Amoxicillin/clavulanate, antitubercular, HDS, ibuprofen, anabolic androgenic steroids, flutamide, isoniazid, atorvastatin, diclofenac, ticlopidine | Amoxicillin/clavulanate, diclofenac, infliximab, nitrofurantoin, isotretinoin, atorvastatin, doxycycline, azathioprine | Amoxicillin/clavulanate, nitrofurantoin, diclofenac, RIP + INH + PIZ, nimesulide, ibuprofen, cyproterone, carbamazepine, methyldopa, atorvastatin | Natural medicine, rifampicin, TCM, isoniazid, pyrazinamide, He Shou Wu, methimazole, propylthiouracil, atorvastatin, methotrexate | Antitubercular, phenytoin, dapsone, olanzapine, carbamazepine, cotrimoxazole, NSAIDs, atorvastatin, leflunomide, ayurvedic |
Note: The duration of follow‐up varied among studies. Age distributions are presented as ¥median (range), mean ± SD, or most prevalent age group (%).
Abbreviations: CNS, central nervous system; DILIN, Drug‐Induced Liver Injury Network; HDS, herbal and dietary supplement; INH, isoniazid; NSAID, nonsteroidal anti‐inflammatory drug; PIZ, pyrazinamide; RIP, rifampin; TCM, traditional Chinese medicine.
The case fatality rates (liver‐related vs. non‐liver‐related) were computed based on the cause of death in individual fatal cases: liver‐related (72 deaths due to DILI + 1 cirrhosis/DILI case) and non‐liver‐related (20 DILI‐contributing death +9 nonrelated death). The table follows the classification/terminology used in the individual manuscripts, except for the Latin America study,b to which categories were assigned based on the listed drugs.
Leading causes of idiosyncratic DILI worldwide
Although hundreds of medications can cause idiosyncratic DILI, several drug classes are more frequently implicated than others. For example, antimicrobials, central nervous system agents, immunomodulatory agents, and antineoplastic agents are more frequently implicated than antihypertensives.17–24 Also, striking geographic differences exist among the specific implicated drugs. For instance, HDS products surpass pharmaceuticals in China, Korea, and Singapore, accounting for 27%–62% of their DILI cases.22,25,26 In contrast, HDS products represent only a minority of cases in Japan, the United States, and Spain but with an increasing incidence over time.23,27–31 Amoxicillin‐clavulanate is the most frequently implicated individual agent in many western countries, whereas anti‐tuberculosis (TB) agents dominate in Asian countries (Table 2).
Risk determinants
An individual's risk of developing idiosyncratic DILI is determined by complex interactions among host, drug, and environmental factors.32
-
Drug properties: Although idiosyncratic DILI typically is independent of the total dose or duration of medication administered, most implicated drugs are given at a daily dose of > 50–100 mg per day.33 More than 80% of DILI cases that resulted in liver transplantation in the United States were caused by medications with daily doses exceeding 50 mg.34 In some instances, dose escalation may also increase the risk of developing idiosyncratic DILI as seen with azathioprine, whereas dose reduction or increasing the dosing interval may improve tolerability.35–37
Drugs with high lipophilicity and extensive metabolism in the liver (> 50%) are associated with an increased hepatotoxic potential, especially in combination with a high daily dose (> 100 mg daily).38,39 In addition, drugs that form reactive metabolites, exert mitochondrial toxicity, and inhibit bile acid transporters in in vitro test systems are associated with increased DILI risk in humans.32 Concomitant administration of multiple hepatotoxic drugs has also been associated with an increased risk of DILI in several studies.40–43
-
Host age, sex, and race and ethnicity: The impact of host age, sex, and race and ethnicity on DILI susceptibility is not well established because of the lack of large exposure‐based epidemiological studies to compare DILI incidence with drug‐treated controls. Although standardized DILI incidence increases with patient age, this may be explained, in part, by greater medication use with increasing age.9 Noticeable differences also exist between sexes, with women experiencing more frequent and severe hepatotoxicity.44,45 A French population‐based study showed that the standardized DILI incidence was more than 2 times higher in women than men older than 50 years, although no sex differences were noted under age 50.9,10 In addition, older subjects appear to be at increased risk of isoniazid and amoxicillin‐clavulanate hepatotoxicity, whereas younger individuals are more prone to develop DILI from anticonvulsants and minocycline.45,46 Finally, case series demonstrate an overrepresentation of women with diclofenac, macrolide, flucloxacillin, halothane, ibuprofen, interferon beta‐1a, and nitrofurantoin hepatotoxicity. Similarly, men appear to be overrepresented with azathioprine, anabolic steroid, and amoxicillin‐clavulanate hepatotoxicity.45–47
The Drug‐Induced Liver Injury Network (DILIN) has demonstrated that trimethoprim‐sulfamethoxazole is the most common suspect drug among African Americans, whereas amoxicillin‐clavulanate is the leading cause in White populations. In addition, African Americans were more likely to have adverse outcomes and develop chronic DILI.48,49 In contrast, Asian Americans were more likely to experience a liver‐related death or undergo liver transplant than the other racial groups.48,49 Because of the limited number of ethnic minorities included, additional studies are needed to confirm these data.
Medical comorbidities and environmental factors: Obesity has been associated with an increased risk of tamoxifen‐induced steatosis/steatohepatitis.50 Being overweight, having diabetes, alcohol use, and chronic viral hepatitis have also been associated with progressive fibrosis in methotrexate‐treated patients.51,52 However, the amount of alcohol consumed was not associated with clinical outcomes in consecutive patients enrolled in the DILIN Prospective registry.53 Furthermore, there are limited data exploring the impact of diet, tobacco use, and coffee consumption on DILI susceptibility. The mechanism by which chronic liver disease (e.g., NAFLD, viral hepatitis) impacts DILI susceptibility remains unclear.54 However, DILI caused by anti‐TB therapy has been associated with abnormal baseline serum aminotransferases, showing a stronger dose‐dependent association with the severity of liver enzyme elevation than older age.55
Host genetic risk factors: Various host genetic factors related to drug‐metabolizing enzymes and transporters have been reported as increasing DILI susceptibility56 (Table 3). A missense variant (rs2476601) in PTPN22, which has been associated with other autoimmune disorders, appears to be a risk factor for all‐cause DILI across multiple racial and ethnic groups with an OR of 1.4.57,58 Several genetic studies have also identified distinct human leukocyte antigen (HLA) alleles as risk factors for specific drugs or HDS products. In general, the identified HLA alleles have low positive predictive value, because of the low incidence of DILI in the general population, but a high negative predictive value. Therefore, pretreatment HLA testing will likely not prove useful in most circumstances to prevent DILI, but HLA testing may be helpful in DILI diagnosis and causality assessment.59,60
TABLE 3. Genetic polymorphisms associated with DILI susceptibility.
| Drug | HLA group | Genetic variants | OR | MAF in controlsa |
|---|---|---|---|---|
| Multiple drugs58,61 | Non‐HLA | PTPN22 (rs2476601) | 1.4 | 0.08 |
| rs72631567 (Chromosome 2) | 2.0 | 0.03 | ||
| Mixed/cholestatic | HLA‐I | A*33:01/rs114577328g | 5.0 | 0.01 |
| A*33:01/B*14:02/C*08:02. | 5.6 | 0.009 | ||
| Hepatocellular | Non‐HLA | rs28521457 (chromosome 4/LRBA) | 2.1 | 0.04 |
| Amoxicillin‐clavulanate62,63 | HLA‐I | A*02:01 (rs2523822) | 2.3 | 0.28/0.28b |
| A*30:02 | 6.7 (HC) | 0.029 | ||
| B*18:01 | 2.9 (HC) | 0.096 | ||
| HLA‐II | DRB1*15:01/DQB1*06:02 (rs3135388) | 2.8 | 0.14/0.05b | |
| rs9274407 | 3.1 | 0.15/0.081b | ||
| rs9267992 | 3.1 | 0.14/0.063b | ||
| Non‐HLA | PTPN22 (rs2476601) | 1.6 | 0.08 | |
| Flucloxacillin64,65 | HLA‐I | B*57:01 | 36.6 | 0.04 |
| B*57:03 | 79.2 | 0.0003 | ||
| Minocycline66 | HLA‐I | HLA‐B*35:02 | 29.6 | 0.006 |
| Trimethoprim‐sulfamethoxazole67 | HLA‐I | A*34:02 (EUR) | 47.5 | 0.001 |
| B*14:01 (EUR) | 9.2 | 0.009 | ||
| B*27:02 (EUR) | 13.5 | 0.002 | ||
| HLA‐B*35:01 (AA) | 2.8d | 0.087 | ||
| Isoniazid‐containing antitubercular treatments61,68 | Non‐HLA | rs72631567 (Chromosome 2) | 5.8 | 0.03 |
| rs117491755 (ASTN2: EUR) | 4.4 | 0.037 | ||
| NAT2*6/*6, *6/*7, or *7/*7 (ultraslow) (EUR/IND) | 2.0/1.8 | 0.10/0.19 | ||
| HLA‐I | C*12:02 (EUR) | 6.4 | 0.006 | |
| B*52:01 (EUR) | 6.4 | 0.007 | ||
| B*52:01‐C*12:02 (EUR/IND) | 6.7/1.8 | 0.01/0.07 | ||
| HLA‐II | DQA1*03:01(IND) | 2.6 | 0.06 | |
| Terbinafine69 | HLA‐I | A*33:01/rs114577328g | 40.5 | 0.01–0.03 |
| A*33:01/B*14:02/C*08:02 | 49.2 | 0.009 | ||
| Valproate70 | Non‐HLA | Mitochondrial DNA polymerase γ (POLG) | 23.6e | |
| p.Q1236H | ≤ 0.086 | |||
| p.E1143G | ≤ 0.04 | |||
| Allopurinol71 | HLA‐I | HLA‐A*34:02 (AA) | 8.0/4.5f | 0.033/0.057c |
| HLA‐B*53:01 (AA) | 4.1/2.5f | 0.120/0.184c | ||
| HLA‐B*58:01 (AA) | 5.6/13.3f | 0.046/0.020c | ||
| Green tea72 | HLA‐I | B*35:01 | 6.8 | 0.06 |
| C*04:01 | 3.7 | 0.12 | ||
| Polygonum multiflorum 73 | HLA‐I | B*35:01 | 30.4 | 0.027 |
Abbreviations: AA, African American; ASNT2, astrotactin 2; EUR, European descendants; HC, hepatocellular injury; HLA, human leukocyte antigen; IND, Indian; LRBA, LPS‐responsive vesicle trafficking, beach and anchor containing gene; MAF, minor allele frequency (presented as fractions).
Controls used in the analyses vary among the studies. Because allele frequencies significantly vary among racial groups, the provided allele frequencies should be interpreted cautiously.
Northwestern European/Spanish controls.
The Charles Bronfman Institute for Personalized Medicine BioMe, National Center for Biotechnology Information database of Genotypes and Phenotypes (phs000925.v1.p1)/non‐allopurinol DILI cases at Drug‐Induced Liver Injury Network.
Unadjusted OR due to the limited size of the cohort.
Combined odds.
Computed based on the reported data.
A proxy marker of HLA‐A*33:01.
Guidance statements.
-
6
The estimated annual incidence of idiosyncratic DILI in the general population is low (14–19/100,000) but higher in exposure‐based studies using electronic medical record data (33–40/100,000).
-
7
Antimicrobials, central nervous system agents, and anti‐inflammatory agents are the most commonly implicated agents in the DILI series worldwide. However, HDS are most commonly implicated in some Asian countries and are increasingly implicated in Western countries as well.
-
8
The daily dose of a medication, its lipophilicity, and extent of hepatic metabolism influence the risk of causing DILI when comparing medications.
-
9
Insufficient data exist to confirm subject age, sex, and race and ethnicity as reliable risk factors for DILI susceptibility. However, some drugs are more likely to cause DILI in older individuals (e.g., amoxicillin‐clavulanate, isoniazid), whereas others are more commonly implicated in children (valproate, minocycline).
-
10
Medical comorbidities such as obesity and diabetes are associated with increased incidence and severity of DILI with specific drugs. However, the role of alcohol, tobacco, and diet in DILI susceptibility is not established.
-
11
Patients with pre‐existing liver disease are at increased risk of developing liver injury with selected drugs (e.g., methotrexate, anti‐TB therapy). In addition, subjects with pre‐existing liver disease are at increased risk of poor outcomes with a DILI episode.
-
12
A polymorphism in PTPN22 is a genetic risk factor across multiple drugs and major ethnic groups. Various HLA alleles have also been associated with increased susceptibility to individual drugs, but the clinical utility of HLA testing in DILI diagnosis has yet to be determined.
Diagnostic approach to DILI
DILI is largely a clinical diagnosis of exclusion, relying on a detailed medical history including medication exposure, the pattern and course of liver biochemistry tests before and after drug discontinuation, and exclusion of other causes of liver disease. The initial laboratory testing for DILI includes serum aminotransferases (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP]) and total and direct bilirubin levels, whereas serum albumin and international normalized ratio (INR) levels are a marker of severity (Figure 1). Clinically significant DILI is commonly defined as any one of the following: (1) serum AST or ALT > 5× upper limit of normal (ULN) or ALP > 2× ULN (or pretreatment baseline if baseline is abnormal) on two separate occasions at least 24 h apart; (2) total serum bilirubin > 2.5 mg/dl along with elevated serum AST, ALT, or ALP level; or (3) INR > 1.5 with elevated serum AST, ALT, or ALP.30,74 Although DILI may present with lower levels of laboratory abnormalities, up to 20% of individuals in the general population have mildly increased liver biochemistries because of NAFLD, alcohol, and other common conditions.74
FIGURE 1.

Proposed diagnostic algorithm for patients with suspected DILI. A diagnosis of DILI relies on careful elicitation of clinical history and drug exposures along with exclusion of other more common causes of liver injury. Abbreviations: A1AT, alpha‐1‐antitrypsin; ALP, alkaline phosphatase; ALT alanine aminotransferase; AMA, anti‐mitochondrial antibody; ANA, antinuclear antibody; APAP, acetaminophen; ASMA, anti‐smooth muscle antibody; AST, aspartate aminotransferase; CK, creatine kinase; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HDS, herbal and dietary supplement; HSV, herpes simplex virus; INR, international normalized ratio; LDH, lactate dehydrogenase; T3, triiodothyronine; T4, thyroxine; TB, total bilirubin; TSH, thyroid stimulating hormone; TTG, tissue transglutaminase; ULN, upper limit of normal.
Medication history
A detailed medication history, including the use of HDS products, is critical in all suspected DILI cases. This information should include start and stop dates of the suspect agent(s), dose change (if any and when), prior use of the medication, dechallenge data (i.e., clinical course following drug discontinuation), and rechallenge results (i.e., response to re‐exposure). Typically, DILI appears within 6 months of starting a new medication, although certain drugs have longer latency periods (e.g., nitrofurantoin, methotrexate). In contrast, hypersensitivity reactions can have very short latency periods of only 24–72 h. Although DILI is often attributed to repeated exposure to an oral agent, it is important to recognize that exposure to an intravenous agent, such as monoclonal antibodies, may also cause DILI. However, topical formulations of medications to the skin, eyes, or ears rarely, if ever, cause DILI because of the low dose of medication absorbed.
Initial laboratory assessment
A clinical pattern of liver injury that matches what has been previously reported for a particular medication or HDS product can be helpful in deciding whether an agent is likely the cause of the injury. The biochemical pattern of liver injury also guides the evaluation for competing causes of liver disease (Figure 1). In general, the pattern of injury can be categorized as primarily hepatocellular, with a predominance of transaminase (ALT, AST) elevation; cholestatic, with a predominance of ALP elevation; or mixed. These patterns can be more precisely and quantitatively expressed through the R‐value, defined as serum ALT/ULN divided by serum ALP/ULN. An R value > 5 identifies cases of hepatocellular liver injury, whereas an R value < 2 categorizes cases of cholestatic liver injury, and an R value between 2 and 5 reflects a mixed liver injury pattern.75,76 The R‐value is best calculated at the time of presentation, but the pattern of injury can change as the condition progresses.77 Moreover, a given drug may be associated with more than one clinical profile.
Competing causes of liver injury
Testing for acute viral hepatitis is recommended for all patients with suspected DILI including hepatitis A IgM, HBsAg, anti–hepatitis B core antibody IgM, and HCV RNA to exclude acute hepatitis C infection (Figure 1). In fact, 1.3% of adjudicated cases in the initial analysis of the DILIN cohort tested positive for HCV RNA.30 Another mimicker of DILI is acute HEV infection, which is increasingly reported in developed nations because of exposure to HEV genotype 3 infections. Of note, anti‐HEV IgM seroprevalence was 3% in adjudicated cases in the DILIN database. Although there are concerns regarding reliability of the commercially available serologic tests, testing for acute HEV infection should be considered in selected instances, including cases without a clear suspect agent or in cases with very high aminotransferase values arising in older adults.78 All patients with suspected DILI should also undergo screening for sporadic autoimmune hepatitis (AIH), with testing for autoantibodies (e.g., antinuclear and anti–smooth muscle antibodies) and serum Ig levels, although there are some drugs that can manifest an AIH‐like picture.79–81
Patients with recent hypotension, sepsis, or heart failure are at risk for ischemic liver injury, usually characterized by rapid and a marked increase in serum aminotransferase values followed by rapid decline with normal or near‐normal bilirubin levels. In younger patients, Wilson disease can be considered using recommended testing.3,79 In cholestatic cases, testing for antimitochondrial antibody is recommended to assess for primary biliary cholangitis. In patients with a predominance of AST greater than ALT, alcohol‐associated hepatitis should be considered, especially if aminotransferase elevations are modest (e.g., AST generally < 300 U/L) and associated with high gamma‐glutamyl transpeptidase and erythrocyte macrocytosis. Furthermore, testing for serum creatinine phosphokinase levels in this setting is recommended. All patients with suspected DILI should undergo some type of liver imaging, typically starting with an abdominal ultrasound to assess for presence of cirrhosis, biliary obstruction, or other focal liver changes. Additional imaging, such as CT or MR cholangiography, may be used to assess for vascular abnormalities or pancreaticobiliary disease.82
Certain drugs have been associated with specific clinical and histologic phenotypes, also called “signatures,” such as autoimmune‐like hepatitis, granulomatous hepatitis, vanishing bile duct syndrome (VBDS), or sinusoidal obstruction syndrome (SOS).80 These signature phenotypes are summarized in Table 4. However, DILI can present with a multitude of clinical and histological phenotypes from the same drug, depending on host factors and timing of evaluation.
TABLE 4. Clinical and histological phenotypes of idiosyncratic DILI.
| Clinical phenotype | Histological phenotype | ||
|---|---|---|---|
| Pattern | Characteristic histology | Examples of associated drugs | |
| Hepatocellular | Acute hepatitis | Spotty necrosis, apoptosis, lobular inflammation, with or without portal inflammation and interface hepatitis | Phenytoin, dapsone, para‐aminosalicylate, isoniazid, sulfonamides |
| Panlobular hepatitis | Spotty or focal necrosis, acidophil bodies scattered throughout the lobule, hepatocytes with degenerative changes and lytic necrosis, lymphocytic infiltrates | Immune checkpoint inhibitors (e.g., ipilimumab, nivolumab) | |
| Zonal or nonzonal (confluent) necrosis | Coagulative necrosis in zone 3 or panlobular involvement with either submassive or massive necrosis | Acetaminophen, halothane, CCL4, cocaine, ferrous sulfate | |
| Granulomatous hepatitis | Noncaseating granulomas accompanied by significant inflammation; fibrin‐ring granulomas | Sulfonamides, sulfonylurea, phenytoin, carbamazepine, quinidine, hydralazine, interferon‐α, etanercept, ipilimumab | |
| Chronic hepatitis | Similar to chronic viral hepatitis or autoimmune hepatitis with portal inflammation, interface hepatitis, fibrosis, or cirrhosis | Atorvastatin, HDS, methotrexate, vinyl chloride | |
| Drug‐induced AIH | More prominent portal neutrophils than plasma cells along with cholestasis concurrently with the typical AIH histology of portal inflammation, interface hepatitis, rosette formation | Nitrofurantoin, diclofenac, α‐methyldopa, hydralazine, minocycline, HMG‐CoA reductase inhibitors, TNF inhibitors | |
| Cholestatic | Acute cholestasis/bland cholestasis | Bile accumulation in hepatocytes and/or bile canaliculi with little or no inflammation or hepatocyte injury | Anabolic and oral contraceptives |
| Chronic cholestasis | Bile accumulation, possibly bile duct loss/ductopenia, cholate stasis | Amoxicillin‐clavulanate, flucloxacillin, enalapril, antifungal terbinafine | |
|
Acute cholestatic hepatitis Mixed hepatocellular/cholestatic |
Bile accumulation in hepatocytes and/or bile canaliculi with more prominent inflammation and hepatocyte injury | Antibiotics (erythromycin, amoxicillin‐clavulanate), ACE inhibitors, phenothiazine neuroleptics | |
| Sclerosing cholangitis | Bile duct injury with intraepithelial lymphocytic infiltration and periductal fibrosis | Nivolumab | |
| Fatty liver (drug‐induced steatosis, drug‐induced steatohepatitis) | Pure microvesicular | Numerous small droplets, foamy cytoplasm, hepatocyte nuclei retained in the center | Acetylsalicylic acid (Reye syndrome), valproic acid, glucocorticoids, aspirin, NSAIDS, tetracycline, NRTI, cocaine |
| Macrovesicular | Medium‐sized or large‐sized fat droplets with hepatocyte nuclei displaced to the periphery | Glucocorticoids, methotrexate, NSAIDs, metoprolol, chlorinated hydrocarbons (e.g., CCL4 and chloroform), 5‐fluorouracil, cisplatin, irinotecan, tamoxifen | |
| Mixed macrovesicular and microvesicular | Combination of small and large droplet | Amiodarone, valproic acid, methotrexate | |
| Steatohepatitis | Presence of ballooning, inflammation, Mallory‐Denk hyalines, and fibrosis, in a background of steatosis | Amiodarone, methotrexate, 5‐floururacil, cisplatin, irinotecan, tamoxifen | |
| Vascular | Sinusoidal obstruction syndrome | Sinusoidal congestion with hepatocyte necrosis, red blood cells trapped in Disse spaces, perisinusoidal fibrosis, fibrous obliteration of terminal hepatic venules; sloughing of endothelial cells | Busulfan, cyclophosphamide, plants containing pyrrolizidine alkaloids |
| NRH and OPV | Small (1 mm) hyperplastic nodules bordered by atrophic hepatocyte plates (NRH); may require a reticulin stain. OPV will show either dilated and herniated portal veins or sclerotic lumina | Arsenic, copper sulfate, azathioprine, methotrexate, 6‐mercaptopurine, oxaliplatin, didanosine, stavudine | |
| Peliosis hepatis | Blood‐filled sinusoidal spaces | Androgens and oral contraceptives | |
| Chronic DILI | Fibrosis/cirrhosis | Progression of fibrosis similar to chronic viral hepatitis | Methotrexate, valproic acid, HDS, oral contraceptives, isoniazid, trimethoprim‐sulfamethoxazole, nitrofurantoin, methotrexate, diclofenac, fenofibrate, amoxicillin‐clavulanate |
| Miscellaneous | Ground‐glass cytoplasm (induction hepatocytes), Lafora body‐like inclusions | Homogeneous light pink cytoplasmic inclusions with displacement of the nuclei | Barbiturates, phenytoin, polypharmacy; immunosuppressive agents, antibiotics |
| Phospholipidosis | Enlarged, granular, or foamy cytoplasm; may require electron microscopy to check for lamellar bodies | Antibiotics, antipsychotic, antidepressants, antianginal, antimalarial, antiarrhythmic, cholesterol‐lowering agents; amiodarone | |
| Pigment deposition | Ceroid‐containing macrophages; lipofuscin | 6‐mercaptopurine, phenothiazine, aminopyrine, phenacetin, | |
| Neoplastic | Hepatocellular adenoma | All subtypes possible; most common are inflammatory and HNF‐1‐alpha mutated | Oral contraceptives, anabolic and male hormone steroids, danazol |
Abbreviations: ACE, angiotensin‐converting enzyme; AIH, autoimmune hepatitis; HCA, hepatocellular adenoma; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase; HNF, hepatocyte nuclear factor; NRH, nodular regenerative hyperplasia; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NSAID, nonsteroidal anti‐inflammatory drug; OPV, obliterative portal venopathy.
Finally, improvement of liver injury after drug discontinuation (dechallenge) is important in DILI diagnosis; resolution of injury after discontinuation helps confirm the causal relationship to the drug. Equally important is a comparison of the present suspect drug presentation with reported cases in public databases such as LiverTox (see https://www.ncbi.nlm.nih.gov/books/NBK547852).5 The LiverTox website provides a brief synopsis of the clinical features of idiosyncratic DILI due to more than 1000 prescription drugs and 60 HDS that are culled from the world's literature. In addition, LiverTox provides a likelihood scale summarizing how many reports of bona fide hepatotoxicity have been attributed to a product as follows: category A, 50 or more reports; category B, 12–49 cases; category C, 4–11 cases; category D, 1–3 plausible cases; category E, no reports of liver injury; and category X, newly approved agents.
Guidance statements.
-
13
Clinically significant DILI is typically defined as any one of the following: (1) serum AST or ALT > 5× ULN, or ALP > 2× ULN (or pretreatment baseline if baseline is abnormal) on two separate occasions; (2) total serum bilirubin > 2.5 mg/dl along with elevated AST, ALT, or ALP level; or (3) INR > 1.5 with elevated AST, ALT, or ALP.
-
14
Most hepatotoxic drugs cause liver injury within the first 6 months of use but occasionally have longer latency intervals or may even present after drug discontinuation (e.g., amoxicillin‐clavulanate). Therefore, evaluation of a patient with suspected DILI should include a detailed medication and HDS history within the 180 days before presentation.
-
15
Idiosyncratic DILI cases should be categorized by the R value at presentation (R = (ALT/ULN)/(ALP/ULN)) into hepatocellular (R ≥ 5), mixed (2 < R < 5), and cholestatic (R ≤ 2) profiles, which can help guide the evaluation of alternative causes of liver injury.
-
16
Excluding alternative causes of liver injury is required in all DILI cases, including testing for viral hepatitis, metabolic liver disease, AIH, and pancreaticobiliary disease.
-
17
Certain drugs have been associated with specific laboratory and histologic phenotypes, termed signatures which may be useful in causality assessment.
-
18
We recommend accessing the LiverTox website for a synopsis of the published literature on liver injury due to over 1000 prescription drugs and more than 60 HDS.
Liver biopsy in suspected DILI
Although a liver biopsy is not necessary to diagnose DILI, it can be helpful in excluding other causes of liver disease and in increasing the confidence in a diagnosis of DILI in cases of clinical uncertainty.83 Certain medications are associated with specific histological patterns of liver injury that can be confirmed on biopsy.84 Biopsy can also be useful when the liver biochemistries or symptoms do not improve with drug dechallenge or the patient remains jaundiced, and can be used to help assess the severity of liver injury.84–86 Finally, a liver biopsy may help identify other causes of underlying or concomitant diseases that can confound the clinical or biochemical presentation.86
Approach to liver biopsy interpretation
The first step in the evaluation of a liver biopsy for a patient with suspected DILI is to determine the pattern of injury, as there are various histological presentations of DILI.87,88 Approximately one‐third to one‐half of DILI cases will present with acute hepatocellular liver injury and accompanying necro‐inflammatory type of histology, which includes acute or chronic hepatitis with or without accompanying mild cholestasis.87 This histological pattern includes various degrees of lobular inflammation, portal inflammation, interface hepatitis, apoptosis, granulomas, coagulative necrosis, and confluent or bridging necrosis (Table 4).87 A diagnostic challenge occurs when trying to distinguish idiopathic AIH from drug‐induced AIH (DI‐AIH). Histologic features typically observed in AIH, such as interface hepatitis, emperipolesis (the presence of an intact cell within the cytoplasm of another), and rosette formation, are also observed among DILI cases (89%, 34%, and 40%, respectively, in DILI cases) and are not pathognomonic for AIH.89 DI‐AIH may show more portal neutrophilic infiltrates and be accompanied by cholestasis, whereas sporadic AIH may show a chronic “hepatitis” pattern, and the interface hepatitis will be dominated by plasma cells.89 The presence of fibrosis may aid in distinguishing AIH from DI‐AIH.90–94
DILI ICIs, referred to clinically as immune‐related adverse events (irAEs), have been increasingly reported. The predominant histological pattern in ICI DILI is hepatocellular injury, with approximately 70% showing panlobular hepatitis and approximately 20% with centrilobular coagulative necrosis on liver biopsy.95–97 Unlike AIH, plasma cell infiltration is not predominant, and the inflammatory infiltrate consists mostly of T lymphocytes, with CD8+ cells being greater in number than CD4+ cells. Sclerosing cholangitis is an uncommon manifestation of ICI DILI.96,97 Overall, jaundice and liver failure are rare in DILI because of ICI, and approximately a third of those with severe grades of DILI may even regress spontaneously.97
Cholestatic DILI histology includes acute cholestasis, chronic cholestasis, and acute cholestatic hepatitis. In the acute cholestatic type, cholestasis without accompanying inflammation (so‐called bland cholestasis) may be the sole histological presentation and manifests as bile present in dilated canaliculi and within the hepatocyte cytoplasm.98 Acute cholestatic hepatitis is the presence of cholestasis accompanied by more prominent lobular inflammation. In chronic cholestasis, the cholestasis persists and may have severe bile duct injury or progress to bile duct loss.88 If bile duct loss exceeds 50%, the condition is then termed VBDS.99
Less common histological manifestations of DILI include fatty liver disease, drug‐induced steatosis, and drug‐induced steatohepatitis. Steatosis may be purely microvesicular, which is primarily related to mitochondrial injury, mixed microvesicular and macrovesicular, or purely macrovesicular.100 Of note, microvesicular steatosis usually does not lead to increased echogenicity on ultrasound, nor does it manifest with hepatomegaly, and only liver biopsy can confirm its presence.101
DILI resulting in vascular injury may lead to the development of nodular regenerative hyperplasia (NRH), obliterative portal venopathy (OPV), and SOS (formerly known as veno‐occlusive disease).86,88 NRH and OPV may clinically present insidiously, whereas SOS may manifest as either acute or chronic disease. Peliosis hepatis appears as blood‐filled lacunar spaces, and its development is associated with androgens and oral contraceptive agents.102
Nonspecific histological features and minimal changes may be seen on a liver biopsy in a patient with suspected DILI. These changes may include activation of sinusoidal lining cells, ceroid‐laden macrophages, and ground‐glass‐like cytoplasm of hepatocytes (also known as induction hepatocytes).103 Induction hepatocytes are frequently noted in the setting of polypharmacy, or chronic intake of phenytoin and barbiturates.103 Phospholipidosis is another form of DILI seen as hepatocytes with foamy granular cytoplasm. Similar to induction hepatocytes, phospholipidosis represents an adaptive response to cationic amphophilic drugs like amiodarone and antimalarial agents, via the inhibition of lysosome‐specific phospholipase A2.100,101
DILI severity and prognosis
A liver biopsy can provide helpful prognostic information. The degree of necrosis and presence of prominent ductular reaction are associated with poor outcome, whereas the presence of eosinophils and granulomas is associated with better outcome.104 These observations were also noted in a meta‐analysis of DILI case reports.105 According to DILIN, chronic DILI is the perpetuation of liver damage after 6 months from DILI onset, independent of the pattern of liver injury, whereas the Spanish DILI Group considers 1 year as the best cutoff point.48,106 In contrast, a liver biopsy defines chronic liver disease when there is significant fibrosis or even cirrhosis noted on histology.104
Guidance statements.
-
19
Liver biopsy is not required to make a diagnosis of idiosyncratic DILI but may be useful in DILI cases with a severe or protracted course and in those with diagnostic uncertainty. However, a biopsy is usually not required in mild or self‐limited cases.
-
20
A liver biopsy can help identify the hepatotoxic drugs based on specific histological patterns and can exclude concurrent liver diseases.
-
21
A broad spectrum of histological patterns has been reported in patients with DILI, and a given drug may be associated with more than a single histopathological signature.
-
22
The presence of eosinophils and granulomas on a liver biopsy in a patient with suspected DILI is associated with a more favorable outcome, whereas those who have necrosis or fibrosis have poorer outcomes.
-
23
A liver biopsy from a patient with DILI may help determine the mechanism of injury, as was seen with the mitochondrial toxin fialuridine that led to microvesicular steatosis and necrosis.
Causality assessment
Causality assessment provides an organized approach to determining the likelihood that a given drug or HDS is the cause of liver injury by reviewing the timing, laboratory, and clinical features following exposure and exclusion of other more common causes of liver injury.107 A scoring system is then applied to the component data fields, and a summary causality score is generated that typically ranges from definite (highly probable) to excluded (unlikely).
Models of causality assessment
Several clinical tools have been developed for DILI causality assessment (Tables 5 and 6).
TABLE 5. Data fields in the RUCAM, CDS, and RECAM causality assessment instruments.
| Data field | Updated RUCAM108 score | CDS109 score | RECAM113 score |
|---|---|---|---|
| 1. Chronology (latency) | |||
| 1a. Drug start to liver injury onseta | +1 to +2 | +1 to +3 | −6 to +4 |
| 1b. Drug discontinuation to liver injury onseta | +1 | −3 to +3 | −6 to 0 |
| 2. Dechallengeb | −2 to +3 hepatocellular; 0 to +2 cholestatic/mixed | 0 to +3 | −6 to +4 |
| 3. Competing causes of liver injury | −3 to +2 | −3 to +3 | −6 to 0 |
| 4. Rechallenge | 0 to +3 | +3 | 0 or + 6 |
| 5. Track record of drug/HDS hepatotoxicity | 0 to +2 | −3 to +2 | 0 to +3 |
| Risk factors | 0 to +1 | N/A | N/Ac |
| 6. Concomitant medication | −3 to 0 | N/A | N/Ad |
| 7. Extrahepatic manifestations | – | 0 to +3 | – |
| Range of scores | −9 to +14 | −6 to 17 | −6 to +20 |
| DILI likelihood categories | |||
| Definite | ≥ 9 | > 17 | Highly likely/high probable ≥ 8 |
| Probable | 6–8 | 14–17 | 4–7 |
| Possible | 3–5 | 10–13 | −3 to +3 |
| Unlikely | 1–2 | 6–9 | Unlikely/excluded, < −4 |
| Excluded | ≤ 0 | ≤ 6 |
Note: Only scores from the updated RUCAM are shown and are composites derived from hepatocellular and mixed/cholestatic categories.110
Abbreviations: CDS, clinical diagnostic scale; NA, not applicable; RECAM, Revised Electronic Causality Assessment Method; RUCAM, Roussel‐Uclaf Causality Assessment Method.
Only 1 of those 2 (i.e., only 1a or 1b) is counted.
Stratified by hepatocellular versus mixed/cholestatic in early version.
In RECAM, risk factors were not assigned scores.
RECAM was developed only for single drug cases and does not account for concomitant medications.
TABLE 6. Drug‐Induced Liver Injury Network expert opinion scoring categories.
| Causality score | Likelihood, % | Description |
|---|---|---|
| 1. Definite beyond any reasonable doubt | > 95 | |
| 2 Highly likely | 75–95 | Clear and convincing data, but not definite |
| 3. Probable | 50–74 | Most data support causal relationship |
| 4. Possible | 25–49 | Most data suggest no causal relationship, but possibility remains |
| 5. Unlikely | < 25 | Causal relationship very unlikely, with alternative etiology more likely |
| 6. Insufficient data | Determinable | Missing key data |
Source: Adapted from Fontana et al.74
-
Structured causality assessment instruments: Causality may be determined using various instruments with predefined points awarded to features from the patient's history (Table 5):
The Roussel‐Uclaf Causality Assessment Method (RUCAM), also known as the Council for International Organizations of Medical Sciences scale, was first published in 1993.75,77 It provides a score varying from −10 to +14 points and groups the scores into five likelihood categories with stratification by hepatocellular versus cholestatic/mixed injury. The updated RUCAM score was published in 2016 and has several modifications that generates a score ranging from −9 to +14 points with the same five likelihood categories.108
The Maria‐Victorino Clinical Diagnostic Scale (CDS)109 uses similar variables to the RUCAM but excludes concomitant medications and includes points for extrahepatic manifestations. There are five likelihood categories, but the dynamic range of possible scores is more compressed compared with the RUCAM and the Revised Electronic Causality Assessment Method (RECAM). The CDS is not used widely in clinical practice because it was shown to be inferior to the RUCAM.110
The Digestive Disease Week–Japan 2004 (DDW‐J) score is a modification of the RUCAM with the inclusion of drug‐lymphocyte stimulation test (DLST) results and peripheral eosinophilia.111,112 Scores range from −5 to +17 points. Although the DDW‐J was shown to be superior to the original RUCAM in Japanese patients, it is not currently used outside of Japan because of the lack of widely available and reproducible DLST assays.112
The RECAM is currently available online. This semiautomated, computerized platform has a dynamic range of −6 to +20 points and performs at least as well as the RUCAM in independent data sets.113 The RECAM removed several risk factors and has an expanded list of competing causes to exclude, and diagnostic testing is categorical and menu‐driven to reduce interobserver variability.
Generic causality assessment models include the World Health Organization Collaborating Center for International Drug Monitoring system by the Uppsala Monitoring Center, which have not gained traction in DILI research or clinical practice because of their lack of liver specificity.114,115
Structured expert opinion: The semiquantitative scale developed by DILIN categorizes the likelihood of DILI into five probability groups that vary from < 25% to > 95% probability (Table 6).74,116,117 Advantages of expert opinion include the ability to account for atypical cases, interrupted drug exposure, and synthesis of subtle clues including liver histology in relationship to published literature. This approach has been shown to be as useful as the RUCAM, although expert opinion is rarely available in routine clinical practice.117
Limitations of causality assessment in DILI
There are several important challenges in DILI causality assessment, especially with the structured nonexpert opinion approaches. For example, patients may be taking multiple drugs or HDS products over the same time frame (e.g., multiple drugs for TB). In addition, compositional complexity and lack of label trustworthiness of HDS confounds assessment.118,119 An underlying chronic liver disease flare also is not accounted for by the current scales.120,121 Finally, structured assessments do not take into account evolving knowledge of and experience with hepatotoxicity due to drugs and HDS over time, which will add confidence to decision making. Causality assessment by expert opinion addresses the unique clinical features of a particular patient along with knowledge of the hepatotoxic potential of the suspect agent versus other causes of liver injury.122
Limitations regarding the RUCAM include the relative weighting of its domain scores, which were developed using a set of cases with drug rechallenge and not by evidence‐based or statistical weighting. Furthermore, consideration of other causes of liver injury may have been overlooked or unappreciated when the tools were first developed.75,118 For example, there is no requirement for testing for acute HCV or HEV infection, and there are few good data to justify inclusion of risk factors as listed in the RUCAM.9,29,30 Some limitations of the original have been addressed in the updated RUCAM, which stratifies causality assessment by the R‐value, expands the search for alternative diagnoses, specifies criteria for rechallenge, but still retains the risk factors of age and alcohol for all cases.108,119 The updated RUCAM also provides more specific guidance in ascertaining the hepatotoxicity profile of the suspect drug but is not intended for use in patients with chronic liver disease.
With RECAM now being available online, it is anticipated that this automated electronic platform may provide more rapid and reliable causality assessment using standardized, quantitative, and categorical data fields. Notwithstanding, RECAM has yet to be tested in regions of the world where the spectrum of DILI agents differs from that seen in the United States and Spain (Table 2) or in cases with more than a single suspect drug. Furthermore, the RECAM has not yet been tested in HDS‐induced liver injury cases, and its interrater and intrarater reliability needs to be determined.
Guidance statements.
-
24
Currently there are three commonly used causality assessment methods, and each has its own strengths and limitations.
-
25
Structured causality assessment instruments incorporate the dose, duration, and timing of suspect drug and other concomitant drug or HDS product use; an assessment of the laboratory, radiological, and histological features at presentation; and exclusion of competing causes of liver injury.
-
26
The semiquantitative expert opinion causality assessment scale developed by the DILIN is frequently used in clinical practice and in prospective research studies, but the need for specialized expertise limits its generalizability.
-
27
The updated RUCAM has improved user instructions and more complete diagnostic evaluation compared with the original RUCAM but retains risk factors of age, alcohol, and pregnancy that are of unclear value.
-
28
The RECAM is a newly developed, computerized causality assessment instrument that may prove more reproducible and reliable than RUCAM but further validation studies are needed.
-
29
Intentional suspect drug rechallenge is rarely undertaken in clinical practice, but when available, may prove useful in causality assessment.
HDS hepatotoxicity
HDS are used widely around the world on a daily basis. For example, more than 50% of adults over the age of 20 used dietary supplements in the preceding 30 days in a 2017–2018 study.123 Marketed supplements comprise single‐ingredient products as well as mixtures of many different ingredients that may be both natural and synthetic. Although herbals have been used for millennia by many different cultures for many purposes, contemporary HDS are commonly multi‐ingredient products that are marketed under the guise of delivering some improvement in appearance, performance, or sense of well‐being.124 Although most marketed supplements are safe, many instances of harm resulting from individual and multi‐ingredient products have been reported, including acute liver failure (ALF).
Epidemiology of HDS use and liver injury
American consumers spent more than $9.6 billion on herbal products in 2019.125 Based on DILIN Registry data, HDS comprise approximately 20% of all cases of liver injury encountered in adults.28 Regulation of products in the United States is minimal: Manufacturers are not compelled to prove that their product is safe, and only need to attest to the product's safety based on historical use. The 1994 Dietary Supplement Health and Education Act provides the current regulatory framework for supplement manufacturing and distribution in the United States.126 The regulatory environment in non‐US markets varies, as summarized in a recent review.127 For example, in the European Union, the allowance of a product on the market requires a demonstrated history of safe use, along with periodic chemical verification of the labeled ingredients.
Allegations of injury attributable to a dietary supplement can be reported by consumers and providers to the US Food and Drug Administration (FDA), through the MedWatch passive reporting system.128 These reports are investigated by the FDA's Center for Food Safety and Applied Nutrition, and, when the veracity of a report is verified, regulatory actions can be taken against the manufacturer, including withdrawal of a product from the market in the most extreme circumstance.
Special considerations in the diagnosis of HDS‐associated liver injury
Structured causality assessment tools are confounded by several factors unique to HDS. First, it is well known that supplements are vulnerable to intentional or inadvertent inclusion of ingredients. Botanical ingredients include plant parts and other herbs that are not listed on the product label. Nonbotanical ingredients include chemicals, pesticides, and heavy metals. Intentional adulteration usually results from the inclusion of substances, usually pharmaceuticals, to achieve some pharmacodynamic effect in keeping with the supplement's marketed purpose for use. An example is the inclusion of sildenafil in products marketed for sexual performance. Second, the composition of HDS may change over time as a result of varying growing conditions, leading to batch‐to‐batch variability. Third, latency of exposure to a product before the onset of injury can be quite variable because of the accumulation of product within the body. Finally, the lack of knowledge and awareness of potential liver injury from these widely used, over‐the‐counter supplements may cause the injury to go unrecognized by patients and providers.
HDS hepatotoxicity, susceptibility factors, and outcomes
Many of the most prominent instances of hepatotoxicity from HDS have resulted from multi‐ingredient products such as Hydroxycut, Herbalife, and Oxy‐Elite Pro.129 However, dietary supplements are ever‐changing, in that there is variability of ingredients that may come and go within the same supplement, such that the product sold with the same label at two time points could be substantially different. Furthermore, the DILIN has shown that supplements implicated in liver injury are frequently mislabeled.130 The DILIN's current efforts are being directed to understand the toxicity that may result from specific ingredients that are sold individually or as ingredients in product mixtures (Table 7).
TABLE 7. HDS products and ingredients implicated in hepatotoxicity.
| Ingredient | Chemical structure | Common uses | Hepatotoxicity phenotype | Expected outcome |
|---|---|---|---|---|
| Ashwagandha,135 Withania somnifera | Steroidal lactone | Neuroprotection, anti‐inflammatory | Cholestatic or mixed | Recovery expected |
| Green tea extract72,132 | Catechin‐polyphenol | Weight loss | Hepatocellular | Most recover; liver failure, transplant, death reported |
| Garcinia cambogia136 | (−)‐hydroxycitric acid | Weight loss | Hepatocellular | |
| Polygonum multiflorum 73,137 | Stilbenes and anthraquinones | Anti‐aging, intestinal function | Hepatocellular or mixed | Most recover; fatalities reported |
| Chinese skullcap, Scutellaria baicalensis; Scutellaria lateriflora | Flavonoid | Anxiety, insomnia, neurological disorders | Hepatocellular | Recovery typical |
| Kratom,138 Mitragyna speciosa | Tetracyclic indole and pentacyclic oxindole alkaloids | Anxiety, opiate effect, or withdrawal | Mixed | Recovery typical |
| Anabolic steroids139 | Steroid backbone | Bodybuilding, performance enhancement | Cholestasis | Prolonged jaundice, full recovery |
| Turmeric/curcumin140–142 | Polyphenol | Anti‐inflammatory, weight loss, anticancer, cardiovascular disease | Hepatocellular | Recovery expected; one case of AIH reported |
Through detailed analyses of hepatotoxicity due to specific ingredients, recognition of characteristic toxicity patterns arises. The polyphenolic catechins comprise the chemically active component of green tea extract (GTE). The polyphenolic backbone of the catechins is exploited for its antioxidant potential but is likely also responsible for liver injury. Several cases of liver injury due to GTE have been published, with the most convincing cases being those in which injury recurred following rechallenge.131,132 A focused analysis of GTE cases enrolled in the DILIN has led to recognition of the typical presentation of GTE as being hepatocellular and sometimes fatal, with a strong genetic association with HLA‐B*35:01.72 This same HLA risk allele has also been associated with hepatotoxicity in Han Chinese individuals attributed to Polygonum multiflorum, a popular herbal taken to enhance hair color and improve fertility.73
Recent studies have shown that patients with HDS hepatoxicity leading to liver failure are more likely to die or undergo transplantation compared to patients with drug hepatoxicity.133,134 This may be due to delayed recognition of the product as the cause of liver injury or reluctance of HDS consumers to seek medical care.
Guidance statements.
-
30
HDS are commonly used worldwide, with permissive standards of safety in the United States and other countries leading to the possibility of inaccurate labeling, adulteration, and contamination.
-
31
Supplements can cause severe hepatotoxicity that can have variable clinical, laboratory, and histological phenotypes.
-
32
Genetic polymorphisms in the HLA region and the conditions under which a product is consumed may influence the likelihood of an individual patient developing HDS hepatotoxicity.
-
33
HLA‐B 35:01 has been associated with hepatotoxicity attributed to GTE in White populations and P. multiflorum hepatotoxicity in Asian populations.
Natural history and management of idiosyncratic DILI
Most adults and children with idiosyncratic DILI present with a drug latency of 2–24 weeks, although some drugs have an ultrashort (< 7 days) latency.30 In multiple prospective registry studies, nearly 50% of patients have acute hepatocellular injury, whereas the remainder present with either an acute mixed or cholestatic injury pattern (Table 2). Once a diagnosis of idiosyncratic DILI is suspected, the suspect agent(s) should be immediately discontinued. Hospitalized patients with severe acute liver injury need to be carefully monitored for disease progression, and those with ALF (coagulopathy and encephalopathy) should be urgently referred to a liver transplant center because of their low likelihood (~25% chance) of spontaneous recovery.34,143
With drug discontinuation, most patients with DILI (80%) fully recover without long‐term sequelae.30 However, up to 10% of patients with severe hepatocellular DILI with jaundice may be at risk of death because of their liver condition or underlying medical comorbidities. Multiple studies have also demonstrated that patients with higher total bilirubin and INR levels as well as lower serum albumin levels at presentation are at greatest risk for adverse outcomes.143–146 In addition, recent prospective registries have demonstrated that patients with pre‐existing liver disease are at greater risk of adverse hepatic outcomes.29,30 As indicated in Table 8, a variety of prognostic indices and tools have been proposed to identify patients with DILI at increased risk of adverse hepatic outcomes. Similarly, some clinical features are associated with a greater likelihood of spontaneous recovery, such as the presence of granulomas and eosinophils on liver biopsy.88,107
TABLE 8. Prognostic indices for patients with idiosyncratic DILI.
| Model/parameter | Model components | Proposed thresholds for liver transplant/death | Comments |
|---|---|---|---|
| MELD score143 | Bilirubin, INR, and creatinine | AUROC = 0.83 | Developed for cirrhosis patients |
| Hy's law145 | ALT > 3× ULN and bilirubin > 2.5 mg/dl | PPV = 8%–20% | ALP should be < 2× ULN; not applicable to mixed/cholestatic cases |
| Modified Hy's law144 | R‐value > 5 and bilirubin > 2.5 mg/dl | PPV = 12%; AUROC = 0.73 | |
| Charlson Comorbidity Index and labs146 a | MELD score, albumin, Charlson > 2 | AUROC = 0.89 | Discovery and validation cohort used for 6‐month mortality |
Abbreviations: AUROC, area under the receive operating curve; MELD, Model for End‐Stage Liver Disease; PPV, positive predictive value.
Web‐based mortality calculator available at http://gihep.com/calculators/hepatology/dili‐CAM/.
Chronic DILI is typically defined as persistent elevation in serum liver biochemistries or the presence of radiological or histological evidence of ongoing injury 6–12 months after DILI onset.29,147 The incidence of chronic DILI in 598 subjects enrolled into the DILIN was 21% at 6 months, with African Americans and patients with a cholestatic liver injury at presentation being at increased risk.147 A minority of patients (i.e., < 1%) may also experience progressive loss of intrahepatic bile ducts leading to VBDS, which can be progressive and fatal.100 Other reported phenotypes of chronic DILI include hepatic steatosis from tamoxifen and NRH due to azathioprine or oxaliplatin, which may lead to complications of portal hypertension during long‐term follow‐up (Table 4).
Medical management of idiosyncratic patients with DILI
General supportive care is recommended for all patients with acute DILI, including the use of antiemetics, analgesics, antipruritics, and parenteral hydration as needed. Patients with severe nausea and vomiting, coagulopathy, mental status changes, or dehydration may require hospitalization for observation and monitoring (Table 9). A 3‐day course of N‐acetylcysteine (NAC) should be considered in adult patients with DILI‐related ALF in light of improved 3‐week outcomes in a large randomized controlled trial, particularly in patients with early‐stage encephalopathy.148 Another randomized trial of 102 patients with antitubercular DILI also demonstrated a shorter length of stay but no survival benefit with NAC.149 However, outcomes with a short course of parenteral NAC were poorer in children with non‐APAP ALF, limiting enthusiasm for its use in children.150 Corticosteroids at a dose of 1 mg/kg of methylprednisolone are frequently given to patients with severe immune‐mediated hypersensitivity reactions, including the syndrome known as drug reaction with eosinophilia and systemic symptoms (DRESS).151,152 In some instances, a short course of corticosteroids (i.e., 1–3 months) with rapid tapering may be of benefit in patients with autoimmune features on biopsy as well as for patients with DILI from ICIs and tyrosine kinase inhibitors (Table 9).153,154 Ursodeoxycholic acid may improve symptoms of pruritus and hasten DILI recovery, but large, randomized controlled trials are needed to determine the optimal dose and duration.155
TABLE 9. Recommended interventions for patients with idiosyncratic DILI.
| Intervention | Target population | Dosing | Comments |
|---|---|---|---|
| General intervention | |||
| APAP analgesics | Mild to moderate pain | 2 g maximum per day in divided doses | Consider short acting opiates if moderate to severe pain |
| Antiemetics | Moderate nausea/vomiting | Per package insert | |
| Ursodeoxycholic acid | Severe pruritus | 10–15 mg/kg in divided doses | Prospective efficacy data lacking; likely safe |
| Hospitalization | Dehydrated, coagulopathic, encephalopathic patients | NA | Transfer to transplant center if ALF |
| N‐acetylcysteine | Hospitalized with ALF | See Table 10 for dosing; 72‐h duration in studies | Requires cardiac monitoring (i.v.); greatest benefit in early‐stage ALF |
| Corticosteroids | Severe hypersensitivity reactions; DRESS; checkpoint inhibitor with ALT > 5× ULN; histology showing AIH‐like features | 1 mg/kg per day of methylprednisolone equivalents for ICI cases; 40–60 mg of prednisone for others | Optimal dose and duration not established but frequently can be tapered in 1–3 months |
| Drug‐specific interventions | |||
| l‐carnitine | Valproate with hyperammonemia (hospitalized children) | 100 mg/kg load followed by 50 mg/kg every 8 h | Short‐term use |
| Cholestyramine | Leflunomide cases with persistent cholestasis | 1 packet every 6–8 h for 14 days | Taper once cholestasis/pruritus resolves; give separately from other medications |
| Penicillin (i.v.)/silymarin and dialysis | Amanita mushroom toxicity | Hospitalized patients or ALF | Short‐term use to remove enterohepatic toxin |
| Defibrotide (i.v.) | Hematopoietic cell transplant recipients with severe sinusoidal obstruction syndrome | 6.25 mg/kg every 6 h for > 21 days up to a maximum of 60 days | Shown to improve survival in children and adults compared with historical controls |
Abbreviations: AIH, autoimmune hepatitis; ALF, acute liver failure; APAP, acetaminophen; DILI, drug‐induced liver injury; DRESS, drug reaction with eosinophilia and systemic symptoms; ICI, immune checkpoint inhibitor; IV, intravenous; NA, not applicable; ULN, upper limit of normal.
In addition to general supportive care, drug‐specific therapy may be recommended for selected scenarios. For example, there are uncontrolled data demonstrating clinical benefit with l‐carnitine therapy for children with hyperammonemia due to valproate hepatotoxicity.156 In addition, cholestyramine may be of value for patients with leflunomide hepatotoxicity because of its prolonged half‐life and enterohepatic circulation.157 Finally, defibrotide is a complex mixture of single‐stranded polydeoxyribonucleotides derived from porcine intestine that has antithrombotic and profibrinolytic activity. Its use has been associated with improved survival in patients with severe SOS following hematopoietic cell transplantation compared with historical controls.158
Guidance statements.
-
34
Most adults and children with idiosyncratic DILI present with an acute liver injury phenotype that may or may not be symptomatic but typically resolves within 6 months of onset without long-term sequelae in 80%.
-
35
In registry studies, 10% of patients with idiosyncratic DILI are at risk for adverse hepatic outcomes including ALF, liver transplantation, and death within 6 months of onset.
-
36
Because of the low likelihood of spontaneous survival in idiosyncratic DILI-related ALF of only 25%, early transfer of these individuals to a liver transplant center is recommended.
-
37
Chronic liver injury that persists beyond 6–12 months is observed in 10%–20% of patients with DILI and may be more commonly encountered in those with cholestatic DILI.
-
38
Individuals at increased risk for adverse outcomes include patients with DILI with higher bilirubin and INR values and lower serum albumin at presentation as well as those with severe necrosis and fibrosis on liver biopsy and those with medical comorbidities and pre-existing liver disease.
-
39
Discontinuation of the suspect drug along with supportive care of antiemetics, antipruritics, and hydration are the mainstay of idiosyncratic DILI management.
-
40
A short course of intravenous NAC may be of benefit in hospitalized adult patients with DILI-related ALF, but this therapy is not recommended for children.
-
41
Corticosteroids given for 1–3 months may be of benefit in selected patients with idiosyncratic DILI, including those with severe hypersensitivity features, DRESS, and autoimmune features on liver biopsy. However, the optimal dose and duration are unknown because of the lack of controlled clinical trials.
-
42
Ursodeoxycholic acid is not an established therapy for patients with DILI but is presumably safe to administer.
-
43
Defibrotide is a profibrolytic that is approved for use in adults and children undergoing hematopoietic cell transplantation with moderate to severe SOS.
-
44
Rechallenge with the suspect drug should generally be avoided unless the anticipated benefit is high for a severe or life-threatening condition.
APAP hepatotoxicity
APAP is used widely as a ubiquitous over‐the‐counter analgesic. In North America, APAP overdose is believed to result in 100,000 calls to poison control centers, 50,000 emergency room visits, and at least 500 deaths annually.159 The annual number of ALF cases from APAP dwarfs the number of ALF cases associated with all idiosyncratic reactions combined.160 The reason for this widespread toxicity is that, unlike drugs associated with idiosyncrasy, APAP is a dose‐related hepatotoxin, with all mammalian species susceptible to liver injury in doses only 2 to 3 times therapeutic dosing.161 Although APAP initially was noted to be a frequent cause of toxicity in attempts at self‐harm, increasing recognition of inapparent or unintentional overdosing has become apparent.162 Unintentional overdosing may occur in the setting of chronic pain or flu‐like symptoms because of the lack of awareness of dosing limitations and/or the simultaneous use of multiple APAP‐containing products.163 Other risk factors for APAP toxicity include fasting and malnutrition, which can lead to depletion of intrahepatic glutathione stores, as well as use of alcohol and other medications that can induce the cytochrome P‐450 system and lead to enhanced production of the toxic metabolite, N‐acetyl‐p‐benzoquinone imine.164 Recent data suggest that APAP hepatotoxicity may occur even when therapeutic doses are used, but particularly in association with these other cofactors.165 Histologically, APAP toxicity is characterized by a variable degree of pericentral necrosis.
A diagnosis of APAP overdose is based on a history of ingestion of excessive doses (usually > 4 g as a single time point) that can then lead to variable severity of acute hepatocellular liver injury with towering transaminase levels (often > 1000 U/L) within the first 24 h of observation (Table 10). Measurement of a serum APAP level after a single‐time‐point ingestion can help identify the patients at greatest risk of developing liver injury.161 More recently, detection of serum APAP‐protein adducts has been proposed as a more specific way to diagnose APAP hepatotoxicity particularly in patients presenting late or with an unintentional overdose, but this assay is not commercially available.166
TABLE 10. Diagnosis and management of APAP hepatotoxicity.
| Recommendation | Intentional overdose | Unintentional overdose |
|---|---|---|
| Diagnostic approach | ||
| Time of ingestion | Single time point | Several days of repeated use |
| Dose | Supratherapeutic (typically > 4 g over 24 h) | Repeated therapeutic (up to 4 g per day) or supratherapeutic dosing |
| Presence of coingestants | Diphenhydramine and other sedatives can lead to central nervous system depression | Opioids often used in combination |
| Liver injury parameters | From time of ingestion: 24–72 h: rapid rise in ALT to > 1000 IU/L associated with variable rise in INR; total bilirubin is typically < 10 mg/dl. 72–96 h: Biochemical elevations peak, and can progress to acute liver failure or rapid and full recovery | Presentation is often delayed, but still see rapid rise in ALT to > 1000 IU/L, associated with rise in INR. Comorbid conditions, such as alcohol use, can affect total bilirubin levels. Eventually, liver injury can progress to acute liver failure or recovery |
| Serum APAP level | Use modified Rumack‐Matthew nomogram to estimate risk of hepatotoxicity | Often undetectable at initial presentation. APAP‐protein adducts useful but assay not commercially available |
| Excluding other causes of acute liver injury | Review clinical history to exclude risk factors for hepatic ischemia and perform tests for acute viral hepatitis | |
| Management | ||
| GI decontamination | Activated charcoal (1 g/kg, max dose 50 g) if within 4 h of ingestion. Gastric lavage also used in some cases175 | Usually not helpful nor recommended |
| N‐acetylcysteine | Oral dosing: 140 mg/kg load followed by 70 mg/kg every 4 h; antiemetics as needed. Intravenous dosing176: preferred if intolerant of oral intake/ileus or pregnant; telemetry monitoring recommended 150 mg/kg load over 15–60 min, followed by 50 mg/kg (12.5 mg/kg/h) over the next 4 h then 100 mg/kg (6.25 mg/kg/h) over 16 h thereafter (total 300 mg/kg over 24 h). For those with evidence of liver injury, treatment is extended at 6.25 mg/kg/h until ALT is decreasing and INR is < 2 | |
| Evidence of acute liver failure (coagulopathy and encephalopathy) | Close monitoring in intensive care unit and consider prompt referral to a liver transplant center | |
Abbreviation: GI, gastrointestinal.
Management of APAP overdose
After a single‐time‐point APAP overdose, symptoms of nausea and vomiting ensue within 12–24 h, peaking at about 72 h, and resolving rapidly thereafter. The severity of necrosis is linked to the extent of excess dosing and can lead to hyperacute ALF because of its rapid onset. Administration of oral or intravenous NAC is an effective antidote, given as a loading dose followed by maintenance doses over several days.167 If NAC is administered within 12 h of ingestion, it virtually assures that the liver damage will be minimal. The characteristic laboratory profile of APAP hepatotoxicity includes very high aminotransferase levels with low bilirubin. The coagulopathy can be severe, and a prolonged INR is a bad prognostic sign.168
Management in the early hours after an APAP overdose includes activated charcoal by ingestion or gavage, and certainly NAC, even if given more than 12 h after APAP ingestion.169,170 For unintentional cases, NAC is also given, although its efficacy may be limited. Development of signs and symptoms of liver failure (encephalopathy, primarily) are concerning, and once they are present, nearly one‐third of patients either die or require a liver transplant. The remaining patients make a full and complete recovery within 7 days.
Prognosis
Several prognostic scores have been developed and evaluated including the King's College Hospital score, Model for End‐Stage Liver Disease score, and the Acute Liver Failure Study Group prognostic index.167 In countries in which the over‐the‐counter sale of APAP has been restricted, the incidence of serious APAP toxicity has fallen. Outcomes have also generally improved over the past two decades, likely because of improvements in intensive care, with only 8% of patients undergoing transplantation.168,169
Guidance statements.
-
45
APAP is a dose‐dependent hepatotoxin that leads to acute pericentral liver injury when doses exceeding 4 g are ingested within a 24‐h period or excessive doses over several days.
-
46
APAP overdose is the leading cause of ALF among adults in the United States.
-
47
A diagnosis of APAP hepatotoxicity relies on a history of excessive APAP ingestion, detection of an elevated serum APAP level following single‐time‐point ingestion, and exclusion of competing causes of acute hepatocellular liver injury.
-
48
Gastric lavage and activated charcoal should be given to all patients presenting within 4 h of a single‐time‐point APAP overdose.
-
49
Intravenous or orally administered NAC can prevent liver injury nearly completely if given within 12 h of ingestion, but is also recommended for patients presenting later.
-
50
The prognosis in APAP‐related ALF is related to the degree of encephalopathy, coagulopathy, and acidosis.
Early detection of DILI in clinical practice
The key to preventing clinically significant liver injury from DILI is early detection of the signal event before it becomes symptomatic or severe. Therefore, individuals taking a drug with a moderate to high likelihood of causing DILI should undergo laboratory and clinical monitoring using a validated surveillance program, but only a few bona fide protocols exist. Currently, the FDA advises practitioners to follow recommendations in the FDA product labels for a multitude of potential hepatic adverse events.171 In addition, patients taking potentially hepatoxic medications are advised to report any new or untoward symptoms to their provider.
FDA‐approved labels are available online and can be searched through the FDA database, Drugs@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm). Substantial differences have been identified between US (FDA) and European Medicines Agency drug labeling recommendations regarding hepatoxicity.172,173 For example, 8.7% of the warnings for drug hepatoxicity and 21.3% of the contraindications for patients with liver disease were disparate in a recent study.173
The package inserts of currently approved drugs may recommend (i) monitoring, with or without providing a schedule for testing or any instructions; (ii) therapy discontinuation if symptoms and/or signs of liver injury supervene; or (iii) medication discontinuation or interruption for specified laboratory abnormalities.174,175 According to Table S1, the specific recommendations vary substantially by agent. Although many medications have been associated with liver‐related fatalities, only a minority carry a black box warning for hepatotoxicity. Some recently approved drugs and biological agents have concrete recommendations for monthly laboratory monitoring during the first 12 months of therapy to detect acute hepatocellular injury. In contrast, VBDS was observed during clinical studies of pexidartinib, a monoclonal antibody used to treat tenosynovial giant cell tumor.176 To ensure prompt treatment modification or discontinuation in patients with early liver injury, a comprehensive risk evaluation and mitigation strategy has been instituted by the FDA for pexidartinib that requires the registration and clinical monitoring of all treated patients.176
Hepatotoxicity monitoring in routine clinical practice
A commonsense approach to monitoring is to target individuals who are taking medications that have a high likelihood of causing hepatotoxicity. Important considerations for liver biochemistry monitoring include (i) reference ranges for serum aminotransferase levels that may vary among laboratories, (ii) the presence of baseline elevations in patients with underlying liver disease, (iii) latency of enzyme elevations that may vary from days to months and, rarely, even years (e.g., with nitrofurantoin and minocycline),81 and (iv) transient and self‐limited aminotransferase elevations encountered with drugs like isoniazid and statins (Figure 2) that can resolve with continued dosing, presumably because of metabolic and or immunological adaptation.177,178
FIGURE 2.
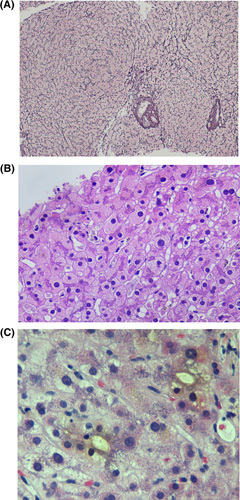
Examples of histological injury attributed to DILI. (A) Nodular regenerative hyperplasia can be seen with azathioprine and oxaliplatin. Reticulin stain highlights a nodular architecture with nodules made up of hyperplastic hepatocytes characterized by two‐cell‐thick plates that are bordered by atrophic hepatocyte plates. Note that the portal tract (arrow) is in the center of the nodule, termed reverse lobulation (original magnification ×10, reticulin stain). (B) Hepatocytes with ground‐glass like cytoplasm are characterized by smooth homogeneous light pink color as opposed to the typical grainy eosinophilic cytoplasm of normal hepatocytes. These hepatocytes are typically found in zone 3. The development of these is often due to polypharmacy (original magnification ×10, hematoxylin and eosin stain). (C) Photomicrograph showing dilated canaliculi containing bile but no inflammatory infiltrates are present and very rare hepatocytes are noted to be undergoing feathery degeneration. This pattern of injury is reported with drugs such as trimethoprim‐sulfamethoxazole (original magnification ×40, hematoxylin and eosin).
Monitoring strategies for four commonly used medications
Isoniazid
In the United States, an estimated 13 million individuals have latent TB, but > 10,000 individuals are treated for active TB each year.179 Although the incidence of severe DILI appears to be lower than previously appreciated,180 isoniazid continues to be a leading cause of DILI‐related ALF in the United States and worldwide (Table 2).181–184 The recommended treatment for latent TB has recently changed from 6 to 9 months of isoniazid monotherapy to regimens with a lower risk of hepatoxicity, including 3–4‐month regimens of isoniazid with other agents.185 Whereas the treatment for active TB still consists of isoniazid, rifamycin, pyrazinamide, and ethambutol, alternative strategies are now available that depend on various individual patient characteristics.186
Over the past 40 years, various recommendations for laboratory monitoring while receiving isoniazid have been proposed that begin with baseline liver assessments for all patients. However, this approach has not been shown to be better than assessing for clinical symptoms of hepatitis at detecting toxicity.181–184 Although the specific details are left to individual local and state programs to adopt, monthly liver test monitoring is generally reserved for those with baseline liver test abnormalities, viral hepatitis, heavy alcohol use, use of other hepatotoxic medications, underlying liver disease or HIV infection, and current or recently pregnant women. Periodic liver tests can also be performed in those older than 35 years of age. Underreporting and poor adherence to American Thoracic Society guidelines are common in cases of isoniazid hepatotoxicity and are associated with hospitalization, death, and liver transplantation.187,188 However, when patients are educated to self‐monitor and stop drugs when symptoms occur, ALF and death can be averted.189 Finally, reintroduction of isoniazid after a DILI episode leads to recurrent liver injury in only 10% of patients but should only be done for patients with active, drug‐resistant TB.190
Methotrexate
Long‐term methotrexate treatment can be associated with the insidious development of hepatic steatosis and fibrosis. Established risk factors for accelerated liver injury with methotrexate therapy include active alcohol consumption, pre‐existing liver disease, diabetes, hyperlipidemia, and obesity.191 Serial serum liver enzyme testing is part of all surveillance protocols devised by rheumatologists and dermatologists, and interval liver biopsy had previously been the mainstay to determine the extent and progression of fibrosis. When the liver biopsy guidelines in rheumatoid arthritis were relaxed, more frequent blood testing reduced the need for liver biopsies without sacrificing patient safety.192,193 The 2008 American College of Rheumatology guidelines for the treatment of rheumatoid arthritis advises laboratory monitoring at baseline and then every 2–4 weeks with the first 3 months, every 8–12 weeks for 3–6 months, and then every 12 weeks beyond 6 months of treatment.194 The updated 2021 guidelines further restrict the use of methotrexate in patients with suspected NAFLD to those with normal liver tests without advanced hepatic fibrosis (stage 3 or 4), detected by noninvasive testing.195 In contrast, the 2020 Academy of Dermatology guidelines for managing psoriasis recommends fibrosis‐4 serologic testing and transient elastography at baseline and annually while on methotrexate therapy in patients with risk factors for hepatotoxicity.196 Laboratory monitoring is recommended at baseline and every 3–6 months. Liver biopsy is reserved for those who have abnormal transient elastography results or those who have persistent liver test elevations. After 3.5–4.0 g of cumulative dose exposure, transient elastography and/or liver biopsy are recommended for all methotrexate recipients.
Statins
There are seven 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase inhibitors or “statin” drugs that are used on a daily basis by millions of patients with hyperlipidemia. In general, statins are safe to administer, but myalgias and myopathy may lead to early dose reduction or termination in up to 10% of treated patients.197 Early on there was concern of self‐limited serum aminotransferase elevations in up to 20% of patient receiving statins, but clinically significant hepatic dysfunction was very uncommon. In the DILIN study, only 22 of 1188 (1.8%) consecutively enrolled patients with DILI were attributed to a statin over an 8‐year period.93 Both acute cholestatic and hepatocellular injury were observed, as well as fewer patients with autoimmune features. Several randomized controlled trials have demonstrated no significant increase in the incidence of persistently elevated serum aminotransferase levels between statin and placebo therapy, including in patients with known chronic liver disease.198–200 In addition, other studies have suggested that statins in patients with compensated chronic liver disease and cirrhosis may even reduce the risk of hepatocellular cancer and decompensation.201 In 2012, the FDA altered the product labels of available statins so that baseline liver biochemistries be obtained but that on‐treatment liver biochemistry monitoring is not required unless clinically indicated.202 Therefore, we do not recommend checking liver biochemistries in patients receiving statins unless there are new or unexplained symptoms of hepatitis. However, statins should be avoided in patients with decompensated cirrhosis due to their hepatic metabolism, but low doses can be considered on an individual basis after assessing overall risk versus benefit.
Immunotherapy
ICIs are monoclonal antibodies given alone or in combination with other cancer treatments every 2–4 weeks. They are prescribed to more than 50% of oncology patients with advanced solid organ tumors.8,203 The severity of IMH and other irAEs has been stratified into five grades according to common terminology criteria for adverse events. The incidence of IMH ranges varies from 1%–15% in clinical trials and observational studies, respectively.204 Most patients with IMH develop asymptomatic injury in the first 6–12 weeks of treatment. Patients who receive cytotoxic T‐lymphocyte‐associated protein 4 antagonists particularly in combination with programmed cell death 1 and programmed cell death receptor ligand 1 inhibitors are at greatest risk of developing IMH. Recent studies suggest that bona fide DILI is only responsible for 30% of cases of demonstrable liver injury in patients with advanced cancer, whereas hepatic metastases, sepsis, and other causes of liver disease account for the remainder, emphasizing the importance of contrast‐enhanced CT and MR scanning in evaluation of these patients.205 Liver biopsy typically demonstrates lobular or periportal hepatitis and is generally not recommended unless patients have persistent grade 3 hepatotoxicity or jaundice despite corticosteroids.206
Monitoring for IMH and other irAEs begins with baseline clinical assessment and laboratory testing before each treatment cycle. For patients with grade 1 liver injury (ALT > 1–3× ULN and/or total bilirubin > 1–1.5× ULN), continued therapy with more frequent laboratory monitoring is advisable. For patients with an ALT 3–5x ULN and/or total bilirubin 1.5–3× ULN (grade 2 liver injury), the ICI should be withheld and consider oral prednisone 0.5–1.0 mg/kg per day (Table 9). For patients with grade 3 or higher hepatotoxicity (ALT 5–20× ULN and/or bilirubin 3–10× ULN or symptomatic liver dysfunction), the ICI should be permanently discontinued, and i.v. steroids at a dose of 1–1.5 mg/kg per day along with hospitalization for patients with jaundice should be considered. Mycophenolate mofetil or azathioprine can be used for steroidrefractory disease. After tapering of immunosuppression, the liver tests should continue to be monitored every 2–4 weeks because of the risk of rebound hepatitis. Fatalities arise in < 1% of patients with IMH and almost exclusively occur in those with jaundice.207 Rarely, ICI‐related sclerosing cholangitis can present with a cholestatic pattern of liver test elevations.
Guidance statements.
-
51
Early detection of DILI is best achieved by educating patients to report untoward symptoms to their providers along with prospective clinical and laboratory monitoring with certain high-risk drugs like the ICIs, isoniazid, and methotrexate.
-
52
All practitioners are encouraged to voluntarily report instances of suspected DILI to the FDA via the MedWatch system at https://www.fda. gov/safet y/medwatch.
-
53
Transient elevations of serum liver enzymes can be seen with drugs such as isoniazid that are self-limited despite continued dosing, presumably because of metabolic and immunological adaptation.
-
54
The FDA and LiverTox websites are a rich resource for information about drug hepatotoxicity and provide informative relevant documents and recommendations for surveillance that may be accessed online, including drug labeling and package inserts.
-
55
Recommendations for hepatotoxicity monitoring vary in detail, according to the background information available. Often, common sense must be applied and/or experts consulted.
-
56
Recommended monitoring for isoniazid hepatotoxicity includes patient education to report new symptoms suggestive of hepatitis.Monthly laboratory monitoring has not been shown to reduce the incidence of clinically significant liver injury and can lead to premature discontinuation of therapy in many patients. However, many specialty societies advise baseline and on-treatment laboratory monitoring in high-risk individuals.
-
57
Annual measurement of liver elastography is recommended as a noninvasive means to monitor the hepatotoxicity of drugs like methotrexate that tend to cause silent fibrosis but is not likely applicable to most other drugs that cause DILI.
-
58
Predosing liver biochemistries are recommended for all patients initiating statin therapy.However, routine on-treatment monitoring of liver biochemistries is not recommended because of the low risk of hepatotoxicity, including patients with liver disease.
-
59
Patients with known compensated chronic liver disease and cirrhosis can and should receive statins as clinically indicated. However, use of statins in people with decompensated cirrhosis should be individualized based on assessment of risk versus benefit.
-
60
Predosing and on-treatment laboratory monitoring is the standard of care for oncology patients receiving ICIs with a series of steps to withhold the drug, increase laboratory monitoring,and use corticosteroids based on the severity of liver injury.
SUMMARY/FUTURE DIRECTIONS
Areas of unmet need in DILI clinical care include the need for improved diagnostic and prognostic biomarkers, accurate and reliable causality assessment instruments, and studies of the epidemiology of DILI. International Classification of Diseases, Tenth Revision codes and natural language processing algorithms may help identify DILI cases from administrative databases, but further refinement is needed.208,209 In addition, improved understanding of the molecular pathogenesis of DILI is needed to minimize future morbidity and mortality and identify therapeutic targets for intervention.
DILI biomarkers
Currently available serum markers of liver injury (i.e., AST, ALT, ALP) are neither sensitive nor specific enough to detect early DILI, nor are they able to reliably predict clinical outcomes. DILI biomarkers in development broadly fall into four categories: (A) dynamic liver injury markers that quantify the extent or severity of hepatocyte damage; (B) mechanistic biomarkers that aim to elucidate the intracellular pathways of liver injury; (C) prognostic biomarkers; and (D) diagnostic biomarkers including single‐nucleotide polymorphisms. Currently, glutamate dehydrogenase and micro‐RNA‐122 show promise as being more sensitive and specific biomarkers for liver injury compared with ALT from clinical studies in patients with APAP overdose (Table 12).210,211 The apoptotic index, which incorporates full‐length serum cytokeratin 18 (CK18) and caspase‐cleaved CK18 levels, may also be more sensitive than serum ALT in detecting liver injury and also be of prognostic value.212,213 Release of damage‐associated molecular patterns (DAMPs) that activate immune cells to release cytokines and chemokines are believed to be important in DILI pathogenesis. In this regard, high‐mobility group box 1, a DAMP that can be detected in the serum in various isoforms, as well as MCSFR and osteopontin, demonstrate promise as prognostic biomarkers.210,214–216
To improve DILI diagnosis, several groups have proposed including the results of in vitro lymphocyte proliferation assays, wherein lymphocytes from the index patient are incubated with the suspect drug (Table 4).111,112 The DILIN tested a multiplex lymphocyte proliferation assay but did not obtain informative results.217 Other groups are exploring the development of in vitro test systems derived from circulating macrophages and human liver organoids, but further validation is needed.218,219 To facilitate DILI biomarker discovery and research, collection of biological samples using standardized protocols is strongly recommended, along with use of consistent case definitions and adjudication both in clinical trials and registry studies.5
The early intracellular events and mechanisms that lead to DILI are not well understood. Studies of infiltrating lymphocytes in the livers of patients with DILI have demonstrated unique cellular profiles, but further studies are needed to improve our understanding of the immunopathogenesis of DILI with the hope of preventing disease progression and identifying targets for therapeutic intervention.220
Guidance statements.
-
61
Currently available serum markers of liver injury such as serum AST, ALT, and ALP levels are not sensitive or specific enough to detect early DILI.
-
62
DILI research continues to be hampered by the lack of an objective, reliable laboratory test to confirm a particular drug as the correct suspect agent.
-
63
DILI biomarkers in development are currently being directed toward improved DILI diagnosis and prognosis as well as to provide mechanistic insight into DILI pathogenesis.
-
64
DILI registries worldwide should use standardized methods and protocols for clinical and biological sample collection and causality assessment to facilitate studies of DILI epidemiology, outcomes, and treatment.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
All of the authors contributed to the design, drafting, editing and finalization of the document.
ACKNOWLEDGMENTS
The authors are grateful for the valuable contributions of the American Association for the Study of Liver Diseases (AASLD) Practice Guideline Committee (PGC), particularly Matthew J. Stotts, MD. Members of the PGC include Elizabeth C. Verna (chair), George Ioannou, (chair), Rabab Ali, Scott W. Biggins, Roniel Cabrera, Henry Chang, Michael F. Chang, Po‐Hung Chen, Kathleen Corey, Archita Parikh Desai, Albert Do, David S. Goldberg, Saul J. Karpen (board liaison), W. Ray Kim (board liaison), Lindsay King, Cynthia Levy, Jeff McIntyre, Jessica L. Mellinger, Anthony J. Michaels, Andrew Moon, Mindie H. Nguyen, Nadia Ovchinsky, Anjana A. Pillai, Daniel S. Pratt, Ashwani K. Singal, Elizabeth Rand, Hugo R. Rosen, Adrienne Simmons, Matthew J. Stotts, Christopher J. Sonnenday, Puneeta Tandon, and Lisa B. VanWagner. The authors also wish to thank the AASLD staff and particularly Audrey Davis‐Owino for assistance in developing this guidance document.
CONFLICT OF INTEREST
Victor Navarro received grants from Zydus. Robert Fontana received grants from Gilead. William Lee consults for SeaGem, GSK, Forma, and Veristat. He received grants from Eiger, Alexion, Gilead, Boehringer Ingelheim, Lipocine, and Camurus.
Footnotes
Abbreviations: AIH, autoimmune hepatitis; ALF, acute liver failure; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APAP, acetaminophen; AST, aspartate aminotransferase; DI‐AIH, drug‐induced AIH; DILIN, Drug‐Induced Liver Injury Network; DRESS, drug reaction with eosinophilia and systemic symptoms; FDA, US Food and Drug Administration; GTE, green tea extract; HDS, herbal and dietary supplement; HLA, human leukocyte antigen; ICI, immune checkpoint inhibitor; IMH, immune‐mediated hepatitis; INR, international normalized ratio; irAE, immune‐related adverse event; NAC, N‐acetylcysteine; NRH, nodular regenerative hyperplasia; OPV, obliterative portal venopathy; RECAM, Revised Electronic Causality Assessment Method; RUCAM, Roussel‐Uclaf Causality Assessment Method; SOS, sinusoidal obstruction syndrome; TB, tuberculosis; ULN, upper limit of normal; VBDS, vanishing bile duct syndrome
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
REFERENCES
- 1.Bakke OM, Manocchia M, de Abajo F, Kaitlin KI, Lasagna L. Drug safety discontinuation in the UK, the United States, and Spain from 1974 to 1993: a regulatory perspective. Clinic Pharmacol Ther. 1995;58:108–17. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Servais J, Martin CB, Kohen D. Prescription drug use among adults aged 40–79 in the United States and Canada. NCHS Data Brief. 2019;334:1–8. [PubMed] [Google Scholar]
- 3.Fontana RJ, Seeff LB, Andrade RJ, Bjornsson E, Day CP, Serrano J, et al. Standardization of nomenclature and causality assessment in drug‐induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M, et al. Causality assessment in drug‐induced liver injury using a structured expert opinion process: comparison to the Roussel‐Uclaf causality assessment method. Hepatology. 2010;51(6):2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63:590–603. [DOI] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Björnsson ES. Drug‐induced liver injury—types and phenotypes. N Engl J Med. 2019;381:264–73. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardi A, Mondelli MU. Review article: immune checkpoint inhibitors and the liver, from therapeutic efficacy to side effects. Aliment Pharm Ther. 2019;50:872–84. [DOI] [PubMed] [Google Scholar]
- 9.Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug‐induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [DOI] [PubMed] [Google Scholar]
- 10.Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug‐induced hepatic injuries: a French population‐based study. Hepatology. 2002;36:451–5. [DOI] [PubMed] [Google Scholar]
- 11.Shin J, Hunt CM, Suzuki A, Papay JI, Beach KJ, Cheetham TC. Characterizing phenotypes and outcomes of drug‐associated liver injury using electronic medical record data. Pharmacoepidemiol Drug Saf. 2013;22:190–8. [DOI] [PubMed] [Google Scholar]
- 12.Sandritter TL, Goldman JL, Habiger CJ, Daniel JF, Lowry J, Fischer RT. An electronic medical records‐based approach to identify idiosyncratic drug‐induced liver injury in children. Sci Rep. 2019;9:18090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier Y, Cavallaro M, Roos M, Pauli‐Magnus C, Folkers G, Meier PJ, et al. Incidence of drug‐induced liver injury in medical inpatients. Eur J Clin Pharmacol. 2005;61:135–43. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Chen Y, Xu J, Zhou Q. Drug‐induced liver injury in hospitalized patients with notably elevated alanine aminotransferase. World J Gastroenterol. 2012;18:5972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Björnsson HK, Olafsson S, Bergmann OM, Björnsson ES. A prospective study on the causes of notably raised alanine aminotransferase (ALT). Scand J Gastroenterol. 2016;51:594–600. [DOI] [PubMed] [Google Scholar]
- 16.Sistanizad M, Peterson GM. Drug‐induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [DOI] [PubMed] [Google Scholar]
- 17.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García‐Ruiz E, et al. Drug‐induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10‐year period. Gastroenterology. 2005;129:512–21. [DOI] [PubMed] [Google Scholar]
- 18.Bessone F, Hernandez N, Mendizabal M, Sanchez A, Paraná R, Arrese M, et al. When the creation of a consortium provides useful answers: experience of the Latin American DILI network (LATINDILIN). Clin Liver Dis (Hoboken). 2019;13:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug‐induced liver injury in the United States. Gastroenterology. 2008;135:1924–34.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhaddad O, Elsabaawy M, Abdelsameea E, Abdallah A, Shabaan A, Ehsan N, et al. Presentations, causes and outcomes of drug‐induced liver injury in Egypt. Sci Rep. 2020;10:5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single‐center experience with drug‐induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [DOI] [PubMed] [Google Scholar]
- 22.Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and etiology of drug‐induced liver injury in mainland China. Gastroenterology. 2019;156:2230–41.e11. [DOI] [PubMed] [Google Scholar]
- 23.Takikawa H, Murata Y, Horiike N, Fukui H, Onji M. Drug‐induced liver injury in Japan: an analysis of 1676 cases between 1997 and 2006. Hepatol Res. 2009;39:427–31. [DOI] [PubMed] [Google Scholar]
- 24.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug‐induced liver disease. Hepatology. 2005;42:481–9. [DOI] [PubMed] [Google Scholar]
- 25.Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug‐induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380–7. [DOI] [PubMed] [Google Scholar]
- 26.Wai CT. Presentation of drug‐induced liver injury in Singapore. Singapore Med J. 2006;47:116–20. [PubMed] [Google Scholar]
- 27.Andrade RJ, Medina‐Caliz I, Gonzalez‐Jimenez A, Garcia‐Cortes M, Lucena ML. Hepatic damage by natural remedies. Semin Liver Dis. 2018;38:21–40. [DOI] [PubMed] [Google Scholar]
- 28.Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. drug‐induced liver injury network. Hepatology. 2014;60:1399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens C, Robles‐Diaz M, Medina‐Caliz I, Garcia‐Cortes M, Ortega‐Alonso A, Sanabria‐Cabrera J, et al. Comprehensive analysis and insights gained from long‐term experience of the Spanish DILI registry. J Hepatol. 2021;75:86–97. [DOI] [PubMed] [Google Scholar]
- 30.Chalasani N, Bonkovsky HL, Fontana RJ, Lee W, Stolz A, Talwalkar J, et al. Features and outcomes of 899 patients with drug‐induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devarbhavi H, Joseph T, Kumar NS, Rathi C, Thomas V, Singh SP, et al. The Indian network of drug‐induced liver injury: etiology, clinical features, outcome and prognostic markers in 1288 patients. J Clin Exp Hepatol. 2021;11:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug‐induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015;63:503–14. [DOI] [PubMed] [Google Scholar]
- 33.Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug‐induced liver injury: search for signals. Hepatology. 2008;47:2003–9. [DOI] [PubMed] [Google Scholar]
- 34.Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group. Drug‐induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrascosa MF, Salcines‐Caviedes JR, Lucena MI, Andrade RJ. Acute liver failure following atorvastatin dose escalation: is there a threshold dose for idiosyncratic hepatotoxicity? J Hepatol. 2015;62:751–2. [DOI] [PubMed] [Google Scholar]
- 36.Seki N, Uematsu K, Shibakuki R, Eguchi K. Promising new treatment schedule for gefitinib responders after severe hepatotoxicity with daily administration. J Clin Oncol. 2006;24:3213–5. [DOI] [PubMed] [Google Scholar]
- 37.Otani K, Kaneko S, Tasaki H, Fukushima Y. Hepatic injury caused by mianserin. BMJ. 1989;299:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug‐induced liver injury. Hepatology. 2013;58:388–96. [DOI] [PubMed] [Google Scholar]
- 39.McEuen K, Borlak J, Tong W, Chen M. Associations of drug lipophilicity and extent of metabolism with drug‐induced liver injury. Int J Mol Sci. 2017;18:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki A, Yuen NA, Ilic K, Miller RT, Reese MJ, Brown HR, et al. Comedications alter drug‐induced liver injury reporting frequency: data mining in the WHO VigiBase™. Regul Toxicol Pharmacol. 2015;72:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki A, Yuen N, Walsh J, Papay J, Hunt CM, Diehl AM. Co‐medications that modulate liver injury and repair influence clinical outcome of acetaminophen‐associated liver injury. Clin Gastroenterol Hepatol. 2009;7:882–888. [DOI] [PubMed] [Google Scholar]
- 42.De Abajo FJ, Montero D, Madurga M, García Rodríguez LA. Acute and clinically relevant drug‐induced liver injury: a population based case‐control study. Br J Clin Pharmacol. 2004;58:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez Gutthann S, García Rodríguez LA. The increased risk of hospitalizations for acute liver injury in a population with exposure to multiple drugs. Epidemiology. 1993;4:496–501. [PubMed] [Google Scholar]
- 44.Zopf Y, Rabe C, Neubert A, Gassmann KG, Rascher W, Hahn EG, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64:999–1004. [DOI] [PubMed] [Google Scholar]
- 45.George N, Chen M, Yuen N, Hunt CM, Suzuki A. Interplay of gender, age and drug properties on reporting frequency of drug‐induced liver injury. Regul Toxicol Pharmacol. 2018;94:101–7. [DOI] [PubMed] [Google Scholar]
- 46.Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7‐year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–23. [DOI] [PubMed] [Google Scholar]
- 47.DiPaola F, Molleston JP, Gu J, Cirulli ET, Chalasani N, Barnhart H, et al. Antimicrobials and antiepileptics are the leading causes of idiosyncratic drug‐induced liver injury in American children. J Pediatr Gastroenterol Nutr. 2019;69:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, et al. Idiosyncratic drug‐induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147:96–108.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, et al. Idiosyncratic drug induced liver injury in African‐Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol. 2017;112:1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, et al. Incidence and risk factors for non‐alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laharie D, Seneschal J, Schaeverbeke T, Doutre MS, Longy‐Boursier M, Pellegrin JL, et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case‐control study. J Hepatol. 2010;53:1035–40. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg P, Urwitz H, Johannesson A, Ros AM, Lindholm J, Kinnman N, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111–8. [DOI] [PubMed] [Google Scholar]
- 53.Dakhoul L, Ghabril M, Gu J, Navarro V, Chalasani N, Serrano J, et al. Heavy consumption of alcohol is not associated with worse outcomes in patients with idiosyncratic drug‐induced liver injury compared to non‐drinkers. Clin Gastroenterol Hepatol. 2018;16:722–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054–60. [PubMed] [Google Scholar]
- 55.Gray EL, Goldberg HF. Baseline abnormal liver function tests are more important than age in the development of isoniazid‐induced hepatoxicity for patients receiving preventive therapy for latent tuberculosis infection. Intern Med J. 2016;46:281–7. [DOI] [PubMed] [Google Scholar]
- 56.Khoury T, Rmeileh AA, Yosha L, Benson AA, Daher S, Mizrahi M. Drug induced liver injury: review with a focus on genetic factors, tissue diagnosis, and treatment options. J Clin Transl Hepatol. 2015;3:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung SA, Criswell LA. PTPN22: its role in SLE and autoimmunity. Autoimmunity. 2007;40:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, et al. A missense variant in PTPN22 is a risk factor for drug‐induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kullak‐Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug‐induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koido M, Kawakami E, Fukumura J, Noguchi Y, Ohori M, Nio Y. Polygenic architecture informs potential vulnerability to drug‐induced liver injury. Nat Med. 2020;26:1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, et al. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome‐wide association study. Gastroenterology. 2017;152:1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, et al. Susceptibility to amoxicillin‐clavulanate‐induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephens C, López‐Nevot MÁ, Ruiz‐Cabello F, Ulzurrun E, Soriano G, Romero‐Gómez M, et al. HLA alleles influence the clinical signature of amoxicillin‐clavulanate hepatotoxicity. PLoS ONE. 2013;8:e68111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, et al. HLA‐B*5701 genotype is a major determinant of drug‐induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–9. [DOI] [PubMed] [Google Scholar]
- 65.Nicoletti P, Aithal GP, Chamberlain TC, Coulthard S, Alshabeeb M, Grove JI, et al. Drug‐induced liver injury due to flucloxacillin: relevance of multiple human leukocyte antigen alleles. Clin Pharmacol Ther. 2019;106:245–53. [DOI] [PubMed] [Google Scholar]
- 66.Urban TJ, Nicoletti P, Chalasani N, Serrano J, Stolz A, Daly AK, et al. Minocycline hepatotoxicity: clinical characterization and identification of HLA‐B*35:02 as a risk factor. J Hepatol. 2017;67:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li YJ, Phillips EJ, Dellinger A, Nicoletti P, Schutte R, Li D, et al. Human leukocyte antigen B*14:01 and B*35:01 are associated with trimethoprim‐sulfamethoxazole induced liver injury. Hepatology. 2021;73:268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicoletti P, Devarbhavi H, Goel A, Venkatesan R, Eapen CE, Grove JI, et al. Genetic risk factors in drug‐induced liver injury due to isoniazid‐containing antituberculosis drug regimens. Clin Pharmacol Ther. 2021;109:1125–35. [DOI] [PubMed] [Google Scholar]
- 69.Fontana RJ, Cirulli ET, Gu J, Kleiner D, Ostrov D, Phillips E, et al. The role of HLA‐A*33:01 in patients with cholestatic hepatitis attributed to terbinafine. J Hepatol. 2018;69:1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart JD, Horvath R, Baruffini E, Ferrero I, Bulst S, Watkins PB, et al. Polymerase gamma gene POLG determines the risk of sodium valproate‐induced liver toxicity. Hepatology. 2010;52:1791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fontana RJ, Li YJ, Phillips E, Saeed N, Barnhart H, Kleiner D, et al. Allopurinol hepatotoxicity is associated with human leukocyte antigen class I alleles. Liver Int. 2021;41:1884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoofnagle JH, Bonkovsky HL, Phillips EJ, Li YJ, Ahmad J, Barnhart H, et al. HLA‐B*35:01 and green tea‐induced liver injury. Hepatology. 2021;73:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li C, Rao T, Chen X, Zou Z, Wei A, Tang J, et al. HLA‐B*35:01 allele is a potential biomarker for predicting Polygonum multiflorum‐induced liver injury in humans. Hepatology. 2019;70:346–57. [DOI] [PubMed] [Google Scholar]
- 74.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Drug‐induced liver injury network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danan G, Benichou C. Causality assessment of adverse reactions to drugs. Part I: a novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. [DOI] [PubMed] [Google Scholar]
- 76.Bénichou C. Criteria of drug‐induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–6. [DOI] [PubMed] [Google Scholar]
- 77.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs. Part II: an original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–6. [DOI] [PubMed] [Google Scholar]
- 78.Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, et al. Acute hepatitis E infection accounts for some cases of suspected drug‐induced liver injury. Gastroenterology. 2011;141:1665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agarwal VK, McHutchison JG, Hoofnagle JH, Drug‐Induced Liver Injury Network. Important elements for the diagnosis of drug‐induced liver injury. Clin Gastroenterol Hepatol. 2010;8:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug‐induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–8. [DOI] [PubMed] [Google Scholar]
- 81.De Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, et al. Features of autoimmune hepatitis in patients with drug‐induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–12.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad J, Rossi S, Rodgers SK, Ghabril M, Fontana RJ, Stolz A, et al. Sclerosing cholangitis‐like changes on magnetic resonance cholangiography in patients with drug induced liver injury. Clin Gastroenterol Hepatol. 2019;17:789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: the diagnosis and management of idiosyncratic drug‐induced liver injury. Am J Gastroenterol. 2014;109:950–66. [DOI] [PubMed] [Google Scholar]
- 84.Kleiner DE. Histopathological challenges in suspected drug‐induced liver injury. Liver Int. 2018;38:198–209. [DOI] [PubMed] [Google Scholar]
- 85.Gasmi B, Kleiner DE. Liver histology: diagnostic and prognostic features. Clin Liver Dis. 2020;24:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodman ZD. Drug hepatotoxicity. Clin Liver Dis. 2002;6:381–97. [DOI] [PubMed] [Google Scholar]
- 87.Kleiner DE. The pathology of drug‐induced liver injury. Semin Liver Dis. 2009;29:364–72. [DOI] [PubMed] [Google Scholar]
- 88.Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al. Hepatic histological findings in suspected drug‐induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug‐induced liver injury. Hepatology. 2011;54:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Licata A, Maida M, Cabibi D, Butera G, Macaluso FS, Alessi N, et al. Clinical features and outcomes of patients with drug‐induced autoimmune hepatitis: a retrospective cohort study. Dig Liver Dis. 2014;46:1116–20. [DOI] [PubMed] [Google Scholar]
- 91.Febres‐Aldana CA, Alghamdi S, Krishnamurthy K, Poppiti RJ. Liver fibrosis helps to distinguish autoimmune hepatitis from DILI with autoimmune features: a review of twenty cases. J Clin Transl Hepatol. 2019;7:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: experience of the drug‐induced liver injury network. Hepatology. 2014;60:679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Björnsson ES. Hepatotoxicity of statins and other lipid‐lowering agents. Liver Int. 2017;37:173–8. [DOI] [PubMed] [Google Scholar]
- 94.Meurer L, Cohen SM. Drug‐induced liver injury from statins. Clin Liver Dis. 2020;24:107–19. [DOI] [PubMed] [Google Scholar]
- 95.Sawada K, Hayashi H, Nakajima S, Hasebe T, Fujiya M, Okumura T. Non‐alcoholic fatty liver disease is a potential risk factor for liver injury caused by immune checkpoint inhibitor. J Gastroenterol Hepatol. 2020;35:1042–8. [DOI] [PubMed] [Google Scholar]
- 96.Zen Y, Yeh MM. Checkpoint inhibitor‐induced liver injury: a novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol. 2019;36:434–440. [DOI] [PubMed] [Google Scholar]
- 97.Zen Y, Chen YY, Jeng YM, Tsai HW, Yeh MM. Immune‐related adverse reactions in the hepatobiliary system: second‐generation check‐point inhibitors highlight diverse histological changes. Histopathology. 2020;76:470–80. [DOI] [PubMed] [Google Scholar]
- 98.Zhang X, Ouyang J, Thung SN. Histopathologic manifestations of drug‐induced hepatotoxicity. Clin Liver Dis. 2013;17:547–64. [DOI] [PubMed] [Google Scholar]
- 99.Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rabinowich L, Shibolet O. Drug induced steatohepatitis: an uncommon culprit of a common disease. Biomed Res Int. 2015;2015:168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bessone F, Dirchwolf M, Rodil MA, Razori MV, Roma MG. Review article: drug‐induced liver injury in the context of nonalcoholic fatty liver disease—a physiopathological and clinical integrated view. Aliment Pharmacol Ther. 2018;48:892–913. [DOI] [PubMed] [Google Scholar]
- 102.Tsirigotis P, Sella T, Shapira MY, Bitan M, Bloom A, Kiselgoff D, et al. Peliosis hepatis following treatment with androgen‐steroids in patients with bone marrow failure syndromes. Haematologica. 2007;92:e106–10. [DOI] [PubMed] [Google Scholar]
- 103.Lu HC, González IA, Byrnes K. Ground‐glass hepatocellular inclusions are associated with polypharmacy. Ann Diagn Pathol. 2021;52:151740. [DOI] [PubMed] [Google Scholar]
- 104.Kleiner DE. Liver histology in the diagnosis and prognosis of drug‐induced liver injury. Clin Liver Dis (Hoboken). 2014;4:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Björnsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug‐induced liver injury. Aliment Pharmacol Ther. 2007;25:1411–21. [DOI] [PubMed] [Google Scholar]
- 106.Medina‐Caliz I, Robles‐Diaz M, Garcia‐Munoz B, Stephens C, Ortega‐Alonso A, Garcia‐Cortes M, et al. Definition and risk factors for chronicity following acute idiosyncratic drug‐induced liver injury. J Hepatol. 2016;65:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayashi PH, Fontana RJ. Clinical features, diagnosis and natural history of drug‐induced liver injury. Semin Liver Dis. 2014;34:134–44. [DOI] [PubMed] [Google Scholar]
- 108.Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2016;27:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug‐induced hepatitis. Hepatology. 1997;26:664–9. [DOI] [PubMed] [Google Scholar]
- 110.Lucena M, Camargo R, Andrade RJ, Perez‐Sanchez CJ, De LaCuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33:123–30. [DOI] [PubMed] [Google Scholar]
- 111.Takikawa H, Takamori Y, Kumagi T, Onji M, Watanabe M, Shibuya A, et al. Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the international consensus meeting. Hepatol Res. 2003;27:192–5. [DOI] [PubMed] [Google Scholar]
- 112.Hanatani T, Sai K, Tohkin M, Segawa K, Kimura M, Hori K, et al. A detection algorithm for drug‐induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of medical sciences/the Roussel Uclaf causality assessment method scale. Pharmacoepidemiol Drug Saf. 2014;23:984–8. [DOI] [PubMed] [Google Scholar]
- 113.Hayashi PH, Lucena MI, Fontana RJ, Bjornsson E, Aithal GP, Barnhart H, et al. A revised electronic version of RUCAM for the diagnosis of drug induced liver injury. Hepatology. 2022;76:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.The use of the WHO–UMC system for standardised case causality assessment. 2021. [cited 2021 Sep 29]. Available from: http://www.who‐umc.org/graphics/4409.pdf [Google Scholar]
- 115.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. [DOI] [PubMed] [Google Scholar]
- 116.Tillmann HL, Suzuki A, Barnhart HX, Serrano J, Rockey DC. Tools for causality assessment in drug‐induced liver disease. Curr Opin Gastroenterol. 2019;35:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayashi PH, Barnhart HX, Fontana RJ, Chalasani N, Davern TJ, Talwalkar JA, et al. Reliability of causality assessment for drug, herbal and dietary supplement hepatotoxicity in the drug‐induced liver injury network (DILIN). Liver Int. 2015;35:1623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teschke R, Danan G. Worldwide use of RUCAM for causality assessment in 81,856 idiosyncratic DILI and 14,029 HILI cases published 1993–mid 2020: a comprehensive analysis. Medicines (Basel). 2020;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teschke R, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: challenges and pitfalls of causality assessment methods. World J Gastroenterol. 2013;19:2864–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fontana RJ, Avigan MI, Janssen HLA, Regev A, Mishra P, Gaggar A, et al. Liver safety assessment in clinical trials of new agents for chronic hepatitis B. J Viral Hep. 2020;27:96–109. [DOI] [PubMed] [Google Scholar]
- 121.Regev A, Seeff LB, Merz M, Ormarsdottir S, Aithal GP, Gallivan J, et al. Causality assessment for suspected DILI during clinical phases of drug development. Drug Saf. 2014;37(Suppl 1):S47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB, et al. Reliability of the Roussel Uclaf causality assessment method for assessing causality in drug‐induced liver injury. Hepatology. 2008;48:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary supplement use among adults: United States, 2017–2018. NCHS Data Brief. 2021;399:1–8. [PubMed] [Google Scholar]
- 124.Huang YS, Chang TT, Peng CY, Lo GH, Hsu CW, Hu CT, et al. Herbal and dietary supplement‐induced liver injury in Taiwan: comparison with conventional drug‐induced liver injury. Hepatol Int. 2021;15:1456–65. [DOI] [PubMed] [Google Scholar]
- 125.US herbal supplement sales increase by 8.6% in 2019, record‐breaking sales predicted for 2020. 2020. [cited 2021 Sep 19]. Available from: http://herbalgram.org/news/press‐releases/2020/us‐herbal‐supplement‐sales‐2019/ [Google Scholar]
- 126.Roytman MM, Poerzgen P, Navarro V. Botanicals and hepatotoxicity. Clin Pharm Ther. 2018;104:458–69. [DOI] [PubMed] [Google Scholar]
- 127.Food supplements. European Commission website. 2021. [cited 2021 Sep 19]. Available from: https://ec.europa.eu/food/safety/labelling‐and‐nutrition/food‐supplements_en [Google Scholar]
- 128.MedWatch: the FDA safety information and adverse event reporting program. US Food and Drug Administration. [cited 2021 Sep 19]. Available from: https://www.fda.gov/safety/medwatch‐fda‐safety‐information‐and‐adverse‐event‐reporting‐program [Google Scholar]
- 129.Navarro VJ, Lucena MI. Hepatotoxicity induced by herbal and dietary supplements. Semin Liv Dis. 2014;34:172–93. [DOI] [PubMed] [Google Scholar]
- 130.Navarro V, Avula B, Khan I, Verma M, Seeff L, Serrano J, et al. The contents of herbal and dietary supplements implicated in liver injury in the United States are frequently mislabeled. Hepatol Commun. 2019;3:792–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann Intern Med. 2006;144:68–71. [DOI] [PubMed] [Google Scholar]
- 132.Oketch‐Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, Navarro V, et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep. 2020;7:386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kesar V, Channen L, Masood U, Grewal P, Ahmad J, Roth NC, et al. Liver transplantation for acute liver injury in asians is more likely due to herbal and dietary supplements. Liver Transpl. 2022;28:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghabril M, Ma J, Patidar KR, Nephew L, Desai AP, Orman E, et al. Eight fold increase in the dietary supplement related liver failure leading to transplant waitlisting over the last quarter century in the US. Liver Transpl. 2022;28:169–79. [DOI] [PubMed] [Google Scholar]
- 135.Bjornsson HK, Bjornsson ES, Avula B, Khan IA, Jonasson JG, Ghabril M, et al. Ashwagandha‐induced liver injury: a case series from Iceland and the US drug‐induced liver injury network. Liver Int. 2020;40:825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vuppalanchi R, Bonkovsky HL, Ahmad J, Barnhart H, Durazo F, Fontana RJ, et al. Garcinia cambogia, either alone or in combination with green tea, causes moderate to severe liver injury. Clin Gastroenterol Hepatol. 2022;20:e1416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dong H, Slain D, Cheng J, Ma W, Liang W. Eighteen cases of liver injury following ingestion of polygonum multiflorum. Complement Ther Med. 2014;22:70–4. [DOI] [PubMed] [Google Scholar]
- 138.Ahmad J, Odin JA, Hayashi PH, Fontana RJ, Conjeevaram H, Avula B, et al. Liver injury associated with kratom, a popular opioid‐like product: experience from the U.S. drug induced liver injury network and a review of the literature. Drug Alcohol Depend. 2021;218:108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stolz A, Navarro V, Hayashi PH, Fontana RJ, Barnhart HX, Gu J, et al. Severe and protracted cholestasis in 44 young men taking body building supplements: assessment of genetic, clinical, and chemical risk factors. Ali Pharmacol Ther. 2019;49:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abdallah MA, Abdalla A, Ellithi M, Abdalla AO, Cunningham AG, Yeddi A, et al. Turmeric‐associated liver injury. Amer J Ther. 2020;27:e642–4. [DOI] [PubMed] [Google Scholar]
- 141.Lombardi N, Crescioli G, Maggini V, Ippoliti I, Menniti‐Ippolito F, Gallo E, et al. Acute liver injury following turmeric use in Tuscany: an analysis of the Italian phytovigilance database and systematic review of case reports. Br J Clin Pharmacol. 2021;87:741–53. [DOI] [PubMed] [Google Scholar]
- 142.Lukefahr AL, McEvoy S, Alfafara C, Funk JL. Drug‐induced autoimmune hepatitis associated with turmeric dietary supplement use. BMJ Case Rep. 2018;2018:bcr‐2018‐224611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hayashi PH, Rockey DC, Fontana RJ, Tillmann HL, Kaplowitz N, Barnhart HX, et al. Death and liver transplantation within 2 years of onset of drug‐induced liver injury. Hepatology. 2017;66:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Robles‐Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina‐Cáliz I, González‐Jimenez A, et al. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with DILI. Gastroenterology. 2014;147:109–18. [DOI] [PubMed] [Google Scholar]
- 145.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–3. [DOI] [PubMed] [Google Scholar]
- 146.Ghabril M, Gu J, Yoder L, Corbito L, Ringel A, Beyer CD, et al. Development and validation of a model consisting of comorbidity burden to calculate risk of death within 6 months for patients with suspected DILI. Gastroenterology. 2019;157:1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug‐induced liver injury. Am J Gastroenterol. 2015;110:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N‐acetylcysteine improves transplant‐free survival in early stage non‐acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moosa MS, Maartens G, Gunter H, Allie S, Chughlay MF, Setshedi M, et al. A randomized controlled trial of intravenous N‐acetylcysteine in the management of anti‐tuberculosis drug‐induced liver injury. Clin Infect Dis. 2021;73:e3377–83. [DOI] [PubMed] [Google Scholar]
- 150.Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Rodriguez‐Baez N, et al. Intravenous N‐acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo‐controlled clinical trial. Hepatology. 2015;57:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999;21:489–501. [DOI] [PubMed] [Google Scholar]
- 152.Avancini J, Maragno L, Santi CG, Criado PR. Drug reaction with eosinophilia and systemic symptoms/drug‐induced hypersensitivity syndrome: clinical features of 27 patients. Clin Exp Dermatol. 2015;40:851–9. [DOI] [PubMed] [Google Scholar]
- 153.Hu PF, Wang PQ, Chen H, Hu XF, Xie QP, Shi J, et al. Beneficial effect of corticosteroids for patients with severe drug‐induced liver injury. J Dig Dis. 2016;17:618–27. [DOI] [PubMed] [Google Scholar]
- 154.Wree A, Dechêne A, Herzer K, Hilgard P, Syn WK, Gerken G, et al. Steroid and ursodesoxycholic acid combination therapy in severe drug‐induced liver injury. Digestion. 2011;84:54–9. [DOI] [PubMed] [Google Scholar]
- 155.Lang SM, Ortmann J, Rostig S, Schiffl H. Ursodeoxycholic acid attenuates hepatotoxicity of multidrug treatment of mycobacterial infections: a prospective pilot study. Int J Mycobacterol. 2019;8:89–92. [DOI] [PubMed] [Google Scholar]
- 156.Perrott J, Murphy NG, Zed PJ. l‐carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44:1287–93. [DOI] [PubMed] [Google Scholar]
- 157.Stine JG, Lewis JH. Current and future directions in the treatment and prevention of drug induced liver injury: a systematic review. Expert Rev Gastroenterol Hepatol. 2016;10:517–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richardson PG, Riches ML, Kernan NA, Brochstein JA, Minieshi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno‐occlusive disease and muti‐organ failure. Blood. 2016;127:1656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetamal)‐associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. [DOI] [PubMed] [Google Scholar]
- 160.Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med. 2016;164:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40:3–20. [DOI] [PubMed] [Google Scholar]
- 162.Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–7. [DOI] [PubMed] [Google Scholar]
- 163.Craig DGN, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. Overdose pattern and outcome in paracetamol‐induced acute severe hepatotoxicity. Br J Clin Pharm. 2010;71:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA. 1994;272:1845–50. [DOI] [PubMed] [Google Scholar]
- 165.Louvet A, Wandji LCN, Lemaître E, Khaldi M, Lafforgue C, Artru F, et al. Acute liver injury with therapeutic doses of acetaminophen: a prospective study. Hepatology. 2021;73:1634–6. [DOI] [PubMed] [Google Scholar]
- 166.James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, et al. Pharmacokinetics of acetaminophen‐protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Prescott LF, Critchley JA. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23:87–101. [DOI] [PubMed] [Google Scholar]
- 168.Koch DG, Tillman H, Durkalski V, Lee WM, Reuben A. Development of a model to predict transplant‐free survival of patients with acute liver failure. Clin Gastroenterol Hepatol. 2016;14:1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen‐induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. [DOI] [PubMed] [Google Scholar]
- 170.Underhill TJ, Greene MK, Dove AF. A comparison of the efficacy of gastric lavage, ipecacuanha and activated charcoal in the emergency management of paracetamol overdose. Arch Emerg Med. 1990;7:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen M, Suzuki A, Thakkar S, Yu K, Hu C, Tong W. DILIrank: the largest reference drug list ranked by the risk for developing drug‐induced liver injury in humans. Drug Discov Today. 2016;21:648–53. [DOI] [PubMed] [Google Scholar]
- 172.Bjornsson ES, Jacobsen EI, Einarsdottir R, Chalasani N. Discrepancies in liver disease labeling in the package inserts of commonly prescribed medications. Gastroenterology. 2015;148:269–73. [DOI] [PubMed] [Google Scholar]
- 173.Wu Y, Xiao W, TongW BJ, Chen M. A systematic comparison of hepatobiliary adverse drug reactions in FDA and EMA drug labelling reveals discrepancies. Drug Discov Today. 2021;27:337–46. [DOI] [PubMed] [Google Scholar]
- 174.Avigan MI. DILI and drug development: a regulatory perspective. Semin Liver Dis. 2014;34:215–26. [DOI] [PubMed] [Google Scholar]
- 175.Council for International Organizations of Medical Sciences; Drug‐Induced Liver Injury (DILI). Current status and future directions for drug development and the post marketing setting. A consensus by a CIOMS working group [cited 2021 Sep 30]. Geneva: CIOMS; 2020. Available from: https://cioms.ch/wp‐content/uploads/2020/06/CIOMS_DILI_Web_16Jun2020.pdf [Google Scholar]
- 176.Lewis JH, Gelderblom H, van de Sande M, Stacchiotti S, Healey JH, Tap WD, et al. Pexidartinib long‐term hepatic safety profile in patients with tenosynovial giant cell tumors. Oncologist. 2021;26:e863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mitchell JR, Long MW, Thorgeirsson UP, Jollow DJ. Acetylation rates and monthly liver function tests during one year of isoniazid preventative therapy. Chest. 1975;68:181–90. [DOI] [PubMed] [Google Scholar]
- 178.Dara L, Liu ZX, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int. 2016;36:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Talwar A, Tsang CA, Price SF, Pratt RH, Walker WL, Schmit KM, et al. Tuberculosis– United States 2018. MMWR Morb Mortal Wkly Rep. 2019;68:257–62.30897076 [Google Scholar]
- 180.Jiang F, Yan H, Liang L, Du J, Jin S, Yang S, et al. Incidence and risk factors of anti‐tuberculosis drug induced liver injury (DILI): large cohort study involving 4652 Chinese adult tuberculosis patients. Liver Int. 2021;41:1565–75. [DOI] [PubMed] [Google Scholar]
- 181.Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventative therapy. A 7‐year survey from a public health tuberculosis clinic. JAMA. 1999;281:1014–8. [DOI] [PubMed] [Google Scholar]
- 182.Sotgiu G, Matteelli A, Getahun H, Girardi E, Sañé Schepisi M, Centis R, et al. Monitoring toxicity in individuals receiving treatment for latent tuberculosis infection: a systematic review versus expert opinion. Eur Respir J. 2015;45:1170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52. [DOI] [PubMed] [Google Scholar]
- 184.Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax. 1998;53:536–48. [PMC free article] [PubMed] [Google Scholar]
- 185.Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug‐susceptible tuberculosis. Clin Infect Dis. 2016;63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Singanayagam A, Sridhar S, Dhariwal J, Abdel‐Aziz D, Munro K, Connell DW, et al. A comparison between two strategies for monitoring hepatic function during antituberculous therapy. Am J Respir Crit Care Med. 2012;185:653–9. [DOI] [PubMed] [Google Scholar]
- 188.Hayashi PH, Fontana RJ, Chalasani NP, Stolz AA, Talwalkar JA, Navarro VJ, et al. Under‐reporting and poor adherence to monitoring guidelines for severe cases of isoniazid hepatotoxicity. Clin Gastroenterol Hepatol. 2015;13:1676–82.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Senior JR, Sr. Unintended adverse events associated with cancer chemotherapy. Toxicol Pathol. 2010;38:142–7. [DOI] [PubMed] [Google Scholar]
- 190.Zuberi BF, Zuberi FF, Bader N, Alvi H, Salahuddin J. Comparison of British Thoracic Society and American Thoracic Society reintroduction guidelines for anti‐tuberculous therapy induced liver injury. J Pak Med Assoc. 2014;64:896–9. [PubMed] [Google Scholar]
- 191.Schmajuk G, Miao Y, Yazdany J, Boscardin WJ, Daikh DI, Steinman MA. Identification of risk factors for elevated transaminases in methotrexate users through an electronic health record. Arthritis Care Res. 2014;66:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Erikson AR, Reddy V, Vogelgesang SA, West SG. Usefulness of the American College of Rheumatology recommendations for liver biopsy in methotrexate‐treated rheumatoid arthritis patients. Arthritis Rheum. 1995;38:1115–9. [DOI] [PubMed] [Google Scholar]
- 193.Aithal GP. Hepatotoxicity related to methotrexate. In: Kaplowitz N, Deleve LD, editors. Drug‐Induced Liver Disease. 3rd ed. London: Academic Press; 2013. [Google Scholar]
- 194.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 195.Fraenkel L, Bathon JM, England BR, St. Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73:924–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Menter A, Gelfand JM, Connor C, Armstrong AW, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445–86. [DOI] [PubMed] [Google Scholar]
- 197.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pfeffer MA, Keech A, Sacks FM, Cobbe SM, Tonkin A, Byington RP, et al. Safety and tolerability of pravastatin in long term clinical trials. Circulation. 2002;105:2341–6. [DOI] [PubMed] [Google Scholar]
- 199.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, et al. Efficacy and safety of high dose pravastatin in hypercholesterolemic patients with well‐compensated chronic liver disease: results of a prospective, randomized, double‐blind, placebo‐controlled multicenter trial. Hepatology. 2007;46:1453–63. [DOI] [PubMed] [Google Scholar]
- 200.Bays H, Cohen DE, Chalasani N, Harrison SA. The National Lipid Association's statin safety task force. An assessment by the statin liver safety task force: 2014 update. J Clin Lipidol. 2014;8(Suppl 3):S47–57. [DOI] [PubMed] [Google Scholar]
- 201.Athyros VG, Boutari C, Stavropoulos K, Anagnostis P, Imprialos KP, Doumas M, et al. Statins: an under‐appreciated asset for the prevention and treatment of NAFLD and NASH and the related cardiovascular risk. Cur Vasc Pharmacol. 2018;16:246–53. [DOI] [PubMed] [Google Scholar]
- 202.FDA Drug Safety Communication. Important safety label changes to cholesterol‐lowering statin drugs. US Food and Drug Administration; 2012. Available from: http://www.fda.gov/drugs/drugsafety/ucm293101.htm [Google Scholar]
- 203.Haslam A, Prasad V. Estimation of the percentage of us patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Net Open. 2019;2:e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Peeraphatdit T, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72:315–29. [DOI] [PubMed] [Google Scholar]
- 205.Tsung I, Dolan R, Lao CD, Fecher L, Riggenbach K, Yeboah‐Korang A, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther. 2020;50:800–8. [DOI] [PubMed] [Google Scholar]
- 206.Li M, Sack JS, Bell P, Rahma OE, Srivastava A, Grover S, et al. Utility of liver biopsy in diagnosis and management of high‐grade immune checkpoint inhibitor hepatitis in patients with cancer. JAMA Oncol. 2021;7:1711–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–126. [DOI] [PubMed] [Google Scholar]
- 208.Yeboah‐Korang A, Louissant J, Tsung I, Prabhu S, Fontana RJ. Utility of computerized ICD‐10 algorithm to identify idiosyncratic drug‐induced liver injury cases in the electronic medical record. Drug Saf. 2020;43:371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heidemann L, Law J, Fontana RJ. A text searching tool to identify patients with idiosyncratic drug‐induced liver injury. Dig Dis Sci. 2017;62:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Harrill AH, Roach J, Fier I, Eaddy JS, Kurtz CL, Antoine DJ, et al. The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin Pharmacol Ther. 2012;92:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen‐induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Thulin P, Nordahl G, Gry M, Yimer G, Aklillu E, Makonnen E, et al. Keratin‐18 and microRNA‐122 complement alanine aminotransferase as novel safety biomarkers for drug‐induced liver injury in two human cohorts. Liver Int. 2014;34:367–78. [DOI] [PubMed] [Google Scholar]
- 213.Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, et al. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Church RJ, Kullak‐Ublick GA, Aubrechts J, Bonkovsky HL, Chalasani N, Fontana RJ, et al. Candidate biomarkers for the diagnosis and prognosis of drug‐induced liver injury: an international collaborative effort. Hepatology. 2019;69:767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Roth SE, Avigan MI, Bourdet D, Brott D, Church R, Dash A, et al. Next‐generation of DILI biomarkers: prioritization for biomarkers for qualification and best practices for biospecimen collection in drug development. Clin Pharm Ther. 2020;107(2):333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wen Y, Jeong S, Xia Q, Kong Z. Role of osteopontin in liver diseases. Int J Biol Sci. 2015;12:1121–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Whritenour J, Ko M, Zong Q, Wang J, Tartaro K, Schneider P, et al. Development of a modified lymphocyte transformation test for diagnosing drug induced liver injury associated with an adaptive immune response. J Immunotoxicol. 2017;14:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Benesic A, Rotter I, Dragoi D, Weber S, Buchholtz ML, Gerbes AL. Development and validation of a test to identify drugs that cause idiosyncratic drug‐induced liver injury. Clin Gastroenterol Hepatol. 2018;16:1488–94. [DOI] [PubMed] [Google Scholar]
- 219.Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, et al. High‐fidelity drug‐induced liver injury screen using human pluripotent stem cell‐derived organoids. Gastroenterology. 2021;160:831–46.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Foureau DM, Walling TL, Maddukuri V, Anderson W, Culbreath K, Kleiner DE, et al. Comparative analysis of portal hepatic infiltrating leukocytes in acute drug‐induced liver injury, idiopathic autoimmune hepatitis, and viral hepatitis. Clin Exp Immunol. 2015;180:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


