Abstract
Background
/Objectives: A weekly combination of a high volume of moderate-intensity continuous training (MICT) with a low volume of high-intensity interval training (HIIT) provides important improvements in body composition and physical capacities in individuals with obesity. However, previous studies did not determine the weekly proportions of HIIT and MICT a priori. This study aimed to investigate changes in body composition, physical capacities and the fat oxidation rate in obese male adults by comparing a combination of MICT and HIIT, called combined training (COMB), with HIIT for a 12-week period.
Methods
Thirty-four obese male adults (mean age: 39.4 ± 7.0 y; mean body mass index [BMI] 34.0 ± 4.2 kg m−2) participated in this study (n = 18 for COMB, n = 16 HIIT), attending ∼ 36 training sessions. The COMB group performed 3 repetitions of 2 min at 95% of peak oxygen uptake (V’O2 peak) (e.g., HIIT ≤20%), followed by 30 min at 60% of VO2 peak (e.g., MICT ≥80%). The HIIT group performed 5–7 repetitions of 2 min at 95% of VO2 peak. At baseline (PRE) and at the end of the training period (POST), body composition, VO2 peak, and the fat oxidation rate were measured. The two training programs were equivalent in caloric expenditure.
Results
At POST, body mass (BM) and fat mass (FM) decreased by a mean of 3.09 ± 3.21 kg and 3.90 ± 2.40 kg, respectively (P < 0.05), in both groups and V’O2 peak increased in both groups by a mean of 0.47 ± 0.34 L min−1 (P < 0.05). The maximal fat oxidation rate increased similarly in both groups from 0.32 ± 0.05 to 0.36 ± 0.06 g min−1 (P < 0.05).
Conclusion
COMB training represents a viable alternative to HIIT to improve anthropometric characteristics, physical capacities and fat oxidation in obese male adults.
Keywords: Body composition, Peak oxygen uptake, Combined training, High intensity interval training, Obesity
1. Introduction
In the last two decades, the number of obese adults has tripled in developed countries.1 This is mainly due to excess food intake, an increase in sedentary time, and a decrease in physical activity.2,3 Health consequences associated with obesity include hypertension,4 type 2 diabetes, cardiovascular disease (CVD), some types of cancer and psychosocial complications.5
Compared to lean counterparts, obese individuals have lower cardiorespiratory fitness (CRF)6 and impaired capacity to oxidize lipids at rest7 and during physical activity,8 associated with low insulin sensitivity and a higher clustering of metabolic syndrome risk factors.9,10 This condition has worsened in recent years due to the coronavirus disease 2019 (COVID-19) pandemic. Studies have shown that as physical activity decreases, maximal oxygen uptake (V’O2 max) also decreases by approximately 0.3–0.4%/day,11 with negative effects on body weight (increases of ∼1.5 kg per month).12 On the other hand, recent evidence suggests that obese adults with a higher level of CRF (typically expressed as V’O2 max) or peak oxygen uptake (V’O2 peak) have a lower risk of morbidity and mortality than inactive lean individuals13 and a similar fat oxidation rate compared to lean individuals matched for CRF.14 For these reasons, aerobic training is recognized as an important lifestyle intervention in weight management programs for obese individuals as it creates an energy deficit to reduce body mass (BM),15 improves CRF,16 and optimizes fat oxidation capacity.17,18
In this context, moderate-intensity continuous training (MICT) is the most prescribed exercise modality in weight management programs, although high-intensity interval training.
(HIIT) has emerged as an attractive, time-efficient option compared to MICT.19 HIIT typically combines high-intensity bouts (i.e., duration between 1 and 4 min at ≥ 85% of maximum heart rate (HRmax)) separated by recovery periods of low-intensity activity or rest with an average total duration between 4 and 16 min.19,20 In obese individuals, HIIT improves CRF,21,22 body composition23,24 and fat oxidation25,26 to a greater extent and in a shorter period (i.e., between 4 and 12 weeks) than MICT. In contrast, a number of systematic reviews and meta-analyses have reported similar improvements in V’O2 peak16 and body composition27,28 induced by HIIT and MICT in obese adults. However, the heterogeneity22,29 and the lack of equalization among protocols comparing the effects of HIIT and MICT28,30 in most of the studies included in the systematic reviews and meta-analysis may have led to contrasting findings.
Recent studies have shown that the combination of MICT (30–40 min/session at 65–70% of HRmax or 90% of the first ventilatory threshold) and HIIT (6–12 min/session at 80–90% of HRmax or 90% of V’O2 peak)31, 32, 33 performed in the same training session or in a weekly training program induced equal or greater effects on CRF,31, 32, 33 body composition31, 32, 33 and substrate oxidation33,34 in lean and obese sedentary adults compared to MICT or HIIT alone with equal volumes or energy expenditures per session. Analysing data from previous studies, we observed that weekly aerobic exercise included a combination of high exercise volumes at moderate intensity (i.e., 70–94% of weekly exercise) and low exercise volumes at high intensity (i.e., 6–28% of weekly exercise).31, 32, 33 In addition, studies on athletes have suggested that a combination of moderate- (i.e., ∼70–80% of total training volume)35 and high-intensity training (i.e., ∼20–30% of total training volume)35 provides greater improvements in body composition36 and endurance performance-related variables (e.g., V’O2 max and lactate thresholds)37,38 than HIIT or MICT alone. To date, to our knowledge, no studies on individuals with obesity have used a priori percentages of MICT and HIIT during weekly training to optimize cardiorespiratory function and the fat oxidation rate during walking or running.
Thus, the aims of the present study were to determine the effects of 12 weeks of a combination of HIIT and MICT (combined training; COMB)34 and HIIT alone on body composition, V’O2 peak, and the fat oxidation rate in healthy adults with obesity. COMB training in our study involves a combination of high-volume low-intensity exercise (≥80% of overall training volume) and low-volume high-intensity training (≤20%). Our hypothesis was that COMB training would result in greater improvements in the abovementioned parameters than HIIT alone.
2. Material and methods
2.1. Subjects
Thirty-five obese male adults were recruited by researchers from the School of Sport Sciences of the University of Udine. All volunteers provided a full medical history and underwent physical and nutritional examinations. Their BM was stable during the previous two months. The inclusion criteria were as follows: 1) aged between 18 and 50 years, 2) BMI ≥30 kg m−2, and 3) physically inactive (i.e., performing less than 30 min of continuous aerobic activity on most days) based on the International Physical Activity Questionnaire Short Form (IPAQ-SF).39 The exclusion criteria were as follows: 1) previous participation in weight management programs or 2) presence of cardiovascular, respiratory, neurologic, musculoskeletal, metabolic and/or endocrine diseases. None of the volunteers were taking medications regularly or using any medications known to influence energy metabolism. The data reported in the manuscript are not a part of a larger dataset.
2.2. Study protocol
The study was approved by the Ethics Committee of the Friuli-Venezia-Giulia Region (Italy) (protocol number 1764). Before the study began, the purpose and objective of the study were carefully explained to each participant, and written informed consent was obtained.
Participants followed a 12-week weight management program involving one of two types of physical training programs (COMB vs. HIIT). Due to the restrictions implemented in Italy during the study period due to the third wave of the COVID-19 pandemic,40 participants were followed at their own homes (see below for details), ensuring both the ecological validity of the study and participant safety as well as adhering to public health recommendations at that time. Participants were randomly allocated (using sealed envelopes and a 1:1 ratio) into two groups: the COMB (n = 18) and HIIT groups (n = 16). A volunteer in the HIIT group left the study before the start of the study period due to heart disease. Full testing sessions were conducted just before the beginning (PRE) and at completion of the 3-month weight-management period (POST). The testing sessions were conducted during one visit and included assessment of anthropometric characteristics, body composition, and substrate oxidation during graded exercise; V’O2 peak; and physical and dietary habits. In addition, physical capacities were monitored weekly to individualize physical training.
At the beginning of the intervention period, all participants received the same general nutritional advice based on the Italian Guidelines for healthy nutrition41 to avoid confounding effects due to nutritional variables on the outcomes.
3. Measurements
3.1. Anthropometric characteristics and body composition
BM was measured to the nearest 0.1 kg with a manual weighing scale (Seca 709, Hamburg, Germany) with the subject dressed only in light underwear and no shoes.
Height was measured to the nearest 0.5 cm on a standardized wall-mounted height board. BMI was calculated as BM (kg) × height−2 (m). Waist circumference (WC) was measured at the narrowest point between the lower costal border and the top of the iliac crest.42 Hip circumference (HC) was measured at the greatest posterior protuberance.42 Body composition was calculated by bioelectrical impedance analysis (BIA, Human IM Plus; DS Dietosystem, Milan, Italy) according to the method of Lukaski et al.43 The values of fat mass (FM) and fat-free mass (FFM) were obtained with equations derived in obese people of either different ages or BMIs (fat-specific formulae) by utilizing a two-compartment model.44
3.2. Physical capacities and maximal fat oxidation rate
The V’O2 peak values and maximal fat oxidation rate were determined by a graded exercise test on a motorized treadmill (H/P/Cosmos Sports and Medical Gmbh, Germany) under medical supervision. Before the start of the study, individuals were familiarized with the equipment and the procedures. All the participants avoided strenuous exercise and maintained the same eating habits the day before the test and came to the laboratory after a 12-h fast.
Each test was undertaken at the same time of the day in the different periods of the study and comprised a 5-min rest period followed by walking in stages of 4-min duration. When the respiratory exchange ratio (RER) value reached 1, the duration of each step was reduced from 4 min to 1 min until voluntary exhaustion. We modified the protocol proposed by Lazzer et al.45 The starting treadmill speed was set to 3 km h−1. Then, it increased by 1 km h−1 each step, except in the transition from the first to the second stage, in which it increased by 2 km h−1. The incline of the treadmill was kept constant throughout the test at 1%. During the test, ventilatory and gas-exchange responses were measured continuously by indirect calorimetry (CPET, Cosmed, Italy). The flowmeter and gas analysers of the system were calibrated using a 3-L calibration syringe and calibration gas (16.00% O2; 4.00% CO2), respectively. For the duration of the entire test, an electrocardiogram was continuously recorded and displayed online for visual monitoring, and the heart rate (HR) was recorded with a dedicated monitor (Garmin, US). VO2 peak was determined for each subject from the last 30 s of the graded exercise tests.
Fat oxidation rates were obtained from V’O2 and V’CO2 values determined during the last minute of each workload level17 using the following equations46:
Fat oxidation rate (g min−1) = 1.67 × V’O2 (l ⋅ min−1) - 1.67 × V’CO2 (l ⋅ min−1) - 0.307 × Poxi.
Carbohydrate oxidation rate (g min−1) = 4.55 × V’CO2 (l ⋅ min−1) - 3.21 × V’O2 (l ⋅ min−1) - 0.459 × Poxi.where Poxi is the protein oxidation rate. Poxi was estimated by assuming that protein oxidation contributed approximately 12% of resting energy expenditure46:
Protein oxidation rate (g min−1) = [energy expenditure kJ min−1 × 0.12] × 16.74−1 (kJ g−1).
The results of the graded exercise test were used to compute the relationship between the fat oxidation rate as a function of exercise intensity, expressed as %V’O2 peak. The best fit was obtained with a second-order polynomial relationship. Before and after the training program, the graded exercise test was performed following the same protocol.
3.3. Dietary and physical activity habits
Participants were invited to compile a 4-day dietary record (4-dDR), recording food and beverage consumption on 2 weekdays and 2 weekend days, at two time points: PRE and POSTWith the 4-dDR, instructions on how to record the type and portion size of the foods consumed were provided.
The intakes of selected macro- and micronutrients were derived after uploading individual food information from the 4-dDRs to Microdiet V4.4.1 software (Microdiet software–Downlee Systems Ltd., High, Peak, UK), which contains the Italian “Food Composition Database for Epidemiological Studies in Italy” 47, along with information from nutritional labels, when needed.
Physical activity levels were evaluated with the IPAQ-SF.39 The questionnaire records vigorous-intensity activity, moderate-intensity activity, walking and sitting duration during the previous 7 days. The IPAQ-SF scores were converted into metabolic equivalents (MET-min week−1) using the Guidelines for Data Processing and Analysis of the IPAQ.39 Furthermore, health-related quality of life was investigated with the 12-item Short Form Health Survey (SF-12). The questionnaire is composed of 12 items from which physical (SF-12_PI) and mental health (SF-12_MI) indices are obtained.48
3.4. Training program
The training program included three training sessions per week for 12 weeks under otherwise normal living conditions. Subjects ran or walked (or a combination of the two) on flat terrain, on a track or city circuit. Each participant monitored their walking/running speed with the Polar Flow smartphone app (Polar Electro Oy, Finland) and their HR with the optical heart rate sensor Polar Verity Sense (Polar Electro Oy, Finland).
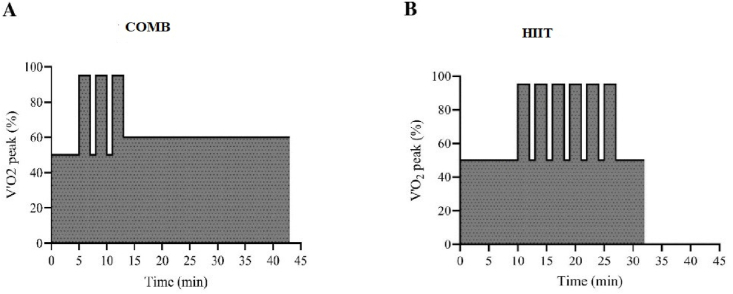
In each training session, the COMB group underwent a combination of high-volume exercise at low intensity (≥80% of overall training volume) with low-volume exercise at high intensity (≤20%),35 as proposed by Borowik et al.34 Each session consisted of 5 min of warm-up (50% of V’O2 peak) followed by 3 repetitions of 2-min bouts at high intensity (95% of V’O2 peak), separated by 1 min of walking at low intensity (50% of V’O2 peak), followed by 30 min of MICT (60% of V’O2 peak)22 (Fig. 1A).
Fig. 1.
Schematic representation of training protocols: Combined training (COMB, panel A) and High Intensity Interval Training (HIIT, panel B).
The HIIT group performed 10 min of warm-up at low intensity (50% of V’O2 peak) followed by 5–7 repetitions of 2-min bouts at high intensity (95% of V’O2 peak),22,24 separated by 1 min of walking at low intensity (50% of V’O2 peak), followed by 5 min of cool down (50% of V’O2 peak) (Fig. 1B). Exercise intensity was manipulated by adjusting the pace (expressed in min·km−1) corresponding to the values of 50, 60 and 95% of V’O2 peak measured during the graded test and then calculating the corresponding HR values.
Both groups repeated these training protocols in each training session. If participants improved their performance capacity, such that their HR tended to decrease, their speed was increased to ensure that the HR reached the specified values. After each training session, all participants reported their rating of perceived exertion (RPE) on the Borg 6–20 Scale,49 their mean HR (bpm) and the distance covered (in km). Research assistants and physical trainers were responsible for verifying that each participant performed the exercises correctly and completed at least 90% of the training sessions through the online platform Polar Coach (Polar Electro Oy, Finland). The amounts of energy expended during the training sessions were similar for both groups: 20 kJ per kg of fat-free mass (FFM), which corresponds to approximately 1.5 MJ per session, as shown by Vaccari et al.25 All volunteers were also advised to practice leisure physical activities during the weekend and holidays.
3.5. Statistical analyses
The data were analysed using GraphPad Prism version 9.1.0 (IBM, Chicago, USA), with a significance threshold of p < 0.05. All the results are expressed as the means and standard deviations (SDs). The normality of data distribution was evaluated using the Shapiro–Wilk test. Sphericity was verified by Mauchly's test; if the sphericity assumption was violated, a Greenhouse–Geisser correction was used. To assess training adherence, energy consumption and the total duration of training, unpaired Student's t tests were used.
Anthropometric characteristics, body composition, V’O2 peak, training characteristics and data derived from questionnaires and food diaries were analysed with a 2-way ANOVA or a general linear mixed model that included the between-subjects factor of group (COMB or HIIT) and the within-subjects factor of time (PRE vs. POST, i.e., repeated-measures analysis). Significant main effects were further analysed by the Šídák post hoc test. The same analyses were applied for the fat oxidation rate during exercise, adding the % of V’O2 peak as a fixed factor to examine differences in fat oxidation rates in response to HIIT or COMB training separately. A three-way ANOVA or a general linear mixed model (2 groups × 2 time points × 9 stage measurements) was conducted to examine differences in fat oxidation rates during the test between the COMB and HIIT groups. Finally, the corrected effect size (ES) was calculated.50 An ES < 0.20 was considered small, <0.50 was considered medium, and >0.50 was considered large, as proposed by Cohen et al.51
To estimate the sample size a priori, power analysis of 12 participants per group with an F test for repeated-measures ANOVA with a statistical power of 0.80, a probability α level of 0.05, and an effect size f of 0.40 (G-Power software, v. 3.1.9.2, Universität Kiel, Kiel, Germany) revealed a predicted improvement of V’O2 peak by 16%.52
4. Results
4.1. Anthropometric characteristics and body composition
Before the intervention, no differences were observed between the groups in anthropometric characteristics or body composition except for FM (%) (+4.0 ± 5.0% COMB group, P = 0.001, Table 1).
Table 1.
Anthropometric characteristic before (PRE), and after 3 months (POST) of weight management program in combined training (COMB) and high-intensity interval training (HIIT) groups.
| COMB (n: 18) |
HIIT (n: 16) |
P |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | G | T | G x T | |||||||||
| Age (y) | 40.3 | ± | 6.9 | 38.3 | ± | 7.1 | 0.434 | ||||||||
| Height (m) | 1.76 | ± | 0.07 | 1.77 | ± | 0.07 | 0.750 | ||||||||
| Body mass (kg) | 106.6 | ± | 16.0 | 104.0 | ± | 16.0a | 103.8 | ± | 9.3 | 100.2 | ± | 9.6a | 0.489 | 0.001 | 0.345 |
| BMI (kg m−2) | 34.5 | ± | 5.3 | 33.6 | ± | 5.2a | 33.2 | ± | 2.3 | 32.1 | ± | 2.6a | 0.352 | 0.001 | 0.389 |
| Waist circumference (cm) | 106.6 | ± | 10.8 | 104.1 | ± | 11.3a | 103.6 | ± | 6.2 | 101.3 | ± | 6.3a | 0.363 | 0.001 | 0.843 |
| Hip circumference (cm) | 112.9 | ± | 7.9 | 112.3 | ± | 7.7 | 111.2 | ± | 4.5 | 110.5 | ± | 3.7 | 0.450 | 0.046 | 0.813 |
| Waist-to-hip ratio | 0.94 | ± | 0.06 | 0.93 | ± | 0.07a | 0.93 | ± | 0.06 | 0.92 | ± | 0.06a | 0.610 | 0.001 | 0.732 |
| Fat-free mass (kg) | 62.9 | ± | 6.5 | 63.9 | ± | 6.7 | 65.7 | ± | 7.8 | 66.0 | ± | 7.3 | 0.322 | 0.110 | 0.383 |
| Fat Mass (kg) | 43.6 | ± | 10.2 | 40.1 | ± | 10.2a | 38.1 | ± | 4.7 | 34.2 | ± | 4.7a | 0.052 | 0.001 | 0.623 |
| Fat Mass (%) | 40.5 | ± | 3.5 | 37.7 | ± | 4.1a | 36.8 | ± | 3.7 | 34.2 | ± | 3.2a | 0.006 | 0.001 | 0.838 |
All values are presented as mean ± standard deviation.
BMI: body mass index.
G: group effect, T: time effect; G × T: groups × time effect.
Significantly different from PRE, P < 0.05.
At POST, the mean weight loss was 2.55 ± 2.26 kg (P = 0.004; ES = 0.16, small) and 3.43 ± 3.97 kg (P < 0.001; ES = 0.37, medium) (Table 1) in the COMB and HIIT groups, respectively. BMI decreased by 0.83 ± 0.75 kg m−2 (P = 0.003; ES = 0.18, small) in the COMB group and by 1.15 ± 1.31 kg m−2 (P < 0.001; ES = 0.37, medium) in the HIIT group. The mean FM loss was 3.84 ± 1.72 kg (P < 0.001; ES = 0.34, medium) and 3.92 ± 3.08 kg (P < 0.001; ES = 0.80, large) in the COMB and HIIT groups, respectively, and FM (%) decreased similarly in the COMB (2.77 ± 1.63%) and HIIT groups (2.63 ± 2.05%) (P < 0.001; ES = 0.73, large), while FFM did not change significantly in the COMB and HIIT groups (Table 1). WC decreased both in the COMB group (-2.53 ± 2.58 cm, P = 0.005; ES = 0.47, medium) and HIIT group (-2.30 ± 3.93 cm, P = 0.020; ES = 0.36, medium), and the waist-to-hip ratio decreased similarly in both groups (-0.02 ± 0.02 cm, main effect of time, P < 0.001; ES = 0.15, small). The HC did not change significantly in either group (Table 1). There was no significant group × time interaction on any anthropometric or body composition variable (0.345< P < 0.843) (Table 1).
4.2. Peak oxygen uptake
At PRE, no significant differences were found between the COMB and HIIT groups in terms of V’O2 peak, V’O2 peak normalized by FFM or HRpeak (Table 2).
Table 2.
Physical capacities and physical activity habits before (PRE) and after 3 months (POST) of weight management program in combined training (COMB) and high-intensity interval training (HIIT) groups.
| COMB (n: 18) |
HIIT (n: 16) |
P |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | G | T | G x T | |||||||||
| V′O2peak (L min−1) | 2.95 | ± | 0.43 | 3.42 | ± | 0.40∗ | 3.12 | ± | 0.45 | 3.58 | ± | 0.52∗ | 0.254 | 0.001 | 0.962 |
| V′O2peak (mL min−1 kg−1 FFM) | 47.0 | ± | 6.4 | 53.8 | ± | 6.7∗ | 47.5 | ± | 5.1 | 54.5 | ± | 6.5∗ | 0.758 | 0.001 | 0.942 |
| HRpeak (bpm) | 176.1 | ± | 15.6 | 174.5 | ± | 11.5 | 175.8 | ± | 11.2 | 172.1 | ± | 13.5 | 0.794 | 0.092 | 0.539 |
| IPAQ_TOT (MET-min week−1) | 1665 | ± | 666 | 2569 | ± | 807∗ | 1673 | ± | 975 | 2247 | ± | 735∗ | 0.727 | 0.040 | 0.633 |
| IPAQ_VIG (MET-min week−1) | 1005 | ± | 529 | 1163 | ± | 442 | 1136 | ± | 579 | 1401 | ± | 932 | 0.361 | 0.238 | 0.796 |
| IPAQ_MOD (MET-min week−1) | 435 | ± | 250 | 842 | ± | 344 | 462 | ± | 313 | 712 | ± | 326 | 0.826 | 0.137 | 0.710 |
| IPAQ_WALK (MET-min week−1) | 710 | ± | 448 | 865 | ± | 363 | 649 | ± | 379 | 635 | ± | 476 | 0.683 | 0.360 | 0.610 |
| SF12_PI (pt) | 49.6 | ± | 7.6 | 52.6 | ± | 3.6 | 50.4 | ± | 7.5 | 51.0 | ± | 6.5 | 0.796 | 0.237 | 0.440 |
| SF12_MI (pt) | 41.5 | ± | 9.3 | 42.0 | ± | 11.9 | 46.6 | ± | 9.9 | 48.2 | ± | 7.7 | 0.024 | 0.650 | 0.825 |
All values are presented as mean ± standard deviation.
V′O2peak: peak oxygen uptake, V′O2peak FFM−1: peak oxygen uptake normalized by fat-free mass, IPAQ_TOT: International Physical Activity Questionnaire.
IPAQ_VIG: vigorous activity, IPAQ_MOD: moderate-intensity activity, IPAQWALK: physical activity derived from walking, SF12_PI: Short-Form 12, questionnaire about health-related quality of life concerning physical index, SF12_MI: Short-Form 12, questionnaire about health-related quality of life concerning mental index.
G group effect, T: time effect; G × T: groups × time effect. ∗Significantly different from PRE, P < 0.05.
At POST, the absolute V’O2 peak increased in the COMB (+16.7 ± 9.6%, P < 0.001; ES = 1.11, large) and HIIT (+16.0 ± 15.9%, P < 0.001; ES = 0.92, large) groups. Additionally, V’O2 peak normalized by FFM increased by 14.4 ± 10.3% (P < 0.001; ES = 1.02, large) in the COMB group and by 15.5 ± 17.2% (P < 0.001; ES = 1.17, large) in the HIIT group. However, HRpeak did not change significantly (main effect of time, P = 0.092) (Table 2). There was no significant group × time interaction effect on HRpeak, V’O2 peak, V’O2 peak normalized by FFM or HRpeak (Table 2).
4.3. Fat oxidation rate
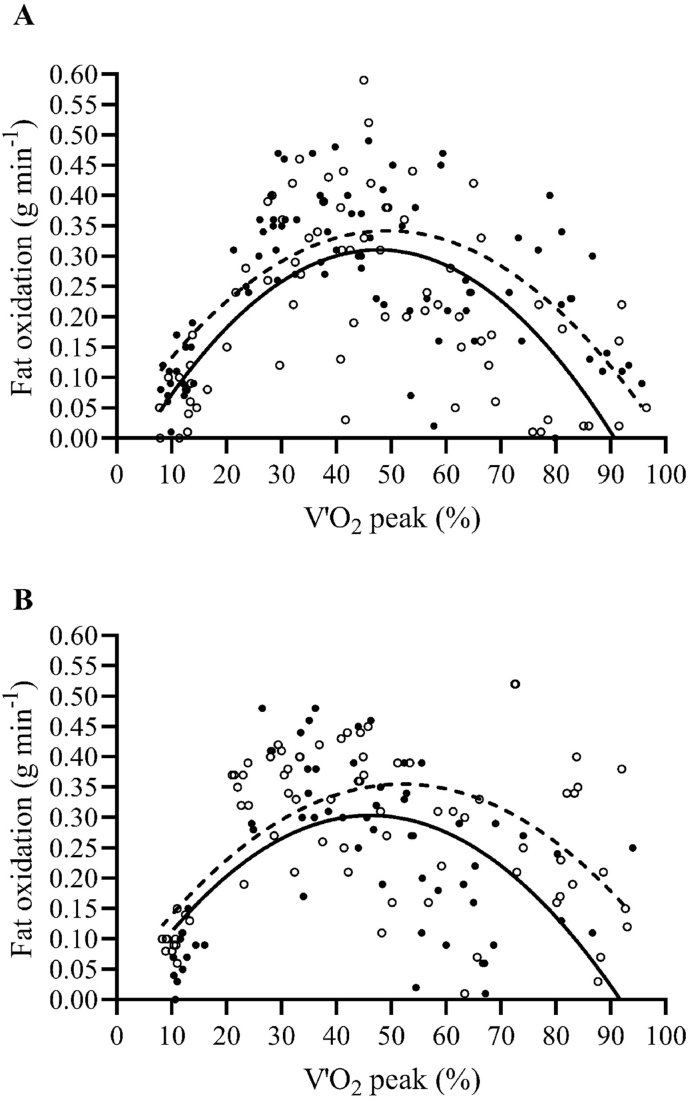
At baseline, fat oxidation rates during the graded test were not significantly different between groups (main effect of group, P = 0.914). The maximal fat oxidation (MFO) rate was observed at 46 ± 6% of VO2 peak in the COMB group (0.31 ± 0.04 g min−1, Fig. 2A) and at 44 ± 8% of V’O2 peak in the HIIT group (0.33 ± 0.07 g min−1, Fig. 2B). On average, at exercise intensities above 60 ± 3% of V’O2 peak, the fat oxidation rate decreased markedly in both groups, and the contribution of fat oxidation to the energy supply became negligible above 74 ± 6% of V’O2 peak.
Fig. 2.
Fat oxidation rate as a function of exercise intensity expressed as percent of peak oxygen uptake (V’O2peak) before (PRE, black points continuous line) and after 3 months (POST, white points dashed line) of weight management program, in combined training (COMB, panel A) and high intensity interval training (HIIT, panel B) groups.
At POST, fat oxidation rates increased in the COMB (main effect of time, P < 0.001) and HIIT groups (main effect of time, P = 0.009) without differences between groups at any exercise intensity (main effect of group, P = 0.984; group × time interaction, P = 0.986) (Fig. 2A and B). The exercise intensity corresponding to the MFO rate, expressed as a percentage of V’O2 peak, increased in a similar manner in both groups (by 6.0 ± 7.0%; main effect of time, P = 0.006). No main effect of group or group × time interaction was found for the MFO rate.
4.4. Training characteristics
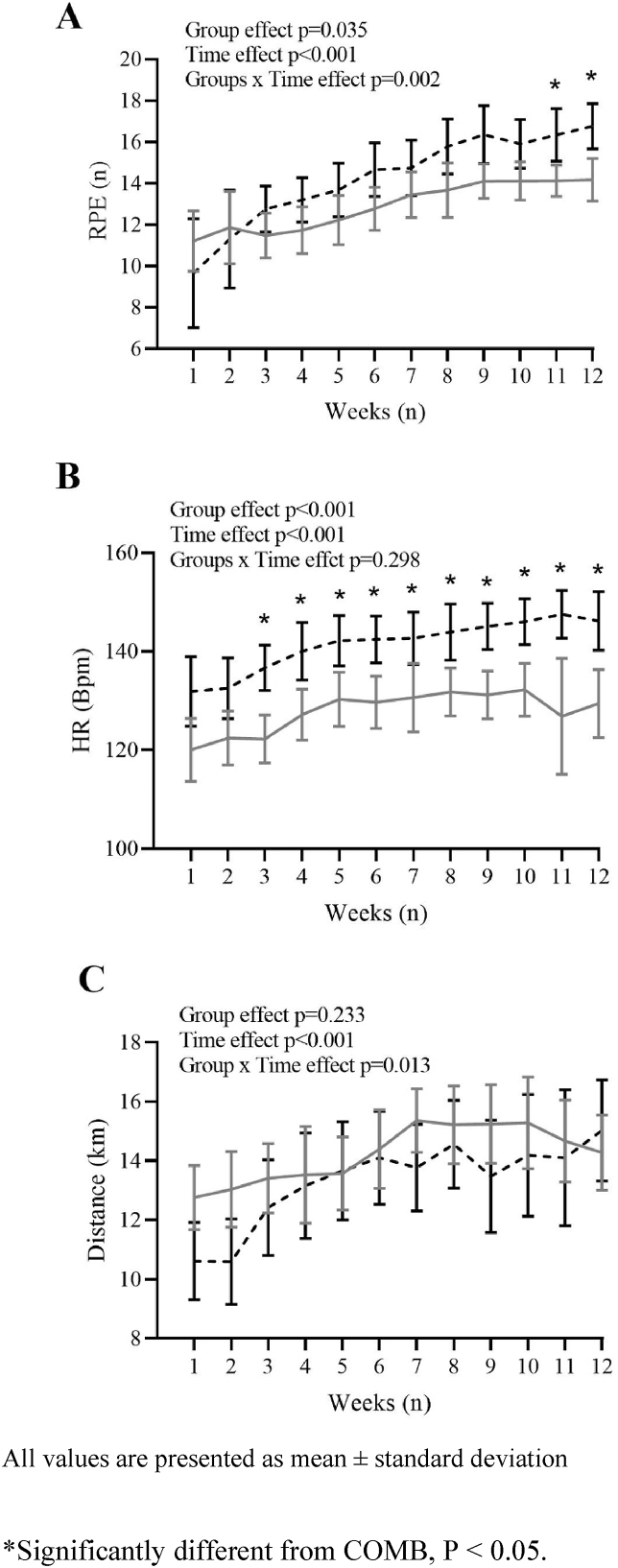
At the end of the training intervention, subjects had performed 34.6 ± 1.2 and 33.7 ± 1.8 training sessions in the COMB and HIIT groups, respectively (P = 0.137), out of 36 total sessions, without adverse events. On average, the mean HR during the training sessions was 128 ± 13 bpm and 141 ± 11 bpm in the COMB and HIIT groups, respectively (P < 0.001). The mean HR increased in both groups during the 12 weeks of the intervention (main effect of time, P < 0.001) (Fig. 3B), but the COMB group exhibited a lower HR (by 10.6 ± 2.2%) than the HIIT group (main effect of group, P < 0.001) (Fig. 3B) without a significant group × time interaction.
Fig. 3.
Mean of rating of perceived exertion (RPE, panel A), Heart Rate (HR, panel B), and Distance covered (km, panel C) during the 12 weeks of training programs in combined training (COMB, grey continuous line) and high intensity interval training (HIIT, black dashed line) groups. All values are presented as mean ± standard deviation. ∗Significantly different from COMB, P < 0.05.
On average, the energy expended during the training sessions was 22.9 ± 2.7 and 21.8 ± 2.6 kJ kg−1 of FFM in the COMB and HIIT groups, respectively (P = 0.208). However, the COMB group exhibited a greater duration of each training session (42.6 ± 3.4 min) than the HIIT group (32.6 ± 2.5 min, P < 0.001). The COMB group performed 86.0 ± 1.0% of the total training volume at MICT and 14.0 ± 1.0% at HIIT, while the HIIT group spent 62.0 ± 2.0% of the total training below MICT and 38.0 ± 2.0% at HIIT; thus, the groups significantly differed (P < 0.001).
During the 12 weeks of the training intervention, the mean RPE values were lower in the COMB group (13.0 ± 2.2) than in the HIIT group (14.3 ± 3.4) (main effect of group, P = 0.035) (Fig. 3A), with an increase in effort perceived over the 12 weeks in both groups (main effect of time, P < 0.001) (Fig. 3A). There was a significant group × time interaction in the RPE (group × time interaction, P = 0.002).
The mean HR (bpm) increased in both groups during the 12 weeks of the intervention (main effect of time, P < 0.001) (Fig. 3B), but the COMB group exhibited a lower HR (by 10.6 ± 2.2%) than the HIIT group (main effect of group, P < 0.001) (Fig. 3B) without a significant group × time interaction.
On average, the distance (km) covered by each participant during the 12 weeks of training was 167.9 ± 23.6 km in the COMB group and 156.8 ± 30.7 km in the HIIT group (main effect of group, P = 0.223) (Fig. 3C).
4.5. Physical activity and nutritional habits
At baseline, physical activity habits, evaluated by the IPAQ, were similar between the two groups (Table 2). After the training period, total (IPAQ_TOT) physical activity increased by 98.0 ± 21.3% and 84.8 ± 15.8% (P = 0.033; ES = 1.19, large and P = 0.044; ES = 0.65, medium, respectively) in the COMB and HIIT groups. Vigorous activity (IPAQ_VIG), moderate activity (IPAQ_MOD) and physical activity derived from walking (IPAQ_WALK) did not change significantly in either group (Table 2). The quality of life assessed by the SF-12, including physical and mental indices, showed no significant differences over time in either group (Table 2).
At PRE, no significant differences were found between the COMB and HIIT groups in terms of energy intake (8667 ± 2183 vs. 8981 ± 2620 kJ day−1; main effect of group, P = 0.721) or macronutrient percentage contribution to total energy intake in terms of carbohydrates (42.6 ± 5.9 vs. 39.7 ± 8.5%; main effect of group, P = 0.436), fat (33.1 ± 5.6 vs. 37.5 ± 7.5%; main effect of group, P = 0.232) or protein (16.7 ± 2.9 vs. 18.0 ± 3.0%; main effect of group, P = 0.807). At POST, the mean energy intake was significantly lower than at PRE in both groups, without differences between groups (group × time interaction, P = 0.981); the mean energy intakes were 7380 ± 1823 kJ day−1 (main effect of time, P < 0.001; ES = 0.62, large) in the COMB group and 7681 ± 2290 kJ day−1 (main effect of time, P < 0.001; ES = 0.51, large) in the HIIT group. The proportions of carbohydrate, lipid, and protein contributions to total energy intake did not change significantly in either group.
5. Discussion
The present study showed that 12 weeks of COMB training and HIIT performed by obese male adults induced (1) significant reductions in BM and FM; (2) significant improvements in V’O2 peak; and (3) similar increases in fat oxidation rates during submaximal exercise. Furthermore, (4) the COMB group exhibited lower values of RPE and HR during training than the HIIT group.
The first main finding was that both COMB training and HIIT produced similar decreases in BM and FM, by ∼3 kg and ∼4 kg, respectively. Although energy intake decreased similarly in both groups after the training intervention, we observed that the combination of moderate energy restriction and aerobic training was effective in improving body composition.52,53 In particular, in the COMB group, a short initial bout of high-intensity training (i.e., 1 × 2 min of walking or running at 130% of V’O2 max, or 5 × 1 min of cycling at 100% power max [Pmax] separated by 1 min of passive recovery) before prolonged moderate-intensity exercise increased fat oxidation (%) during the moderate-intensity exercise and during the initial stage of recovery to a greater extent than MICT alone.34,54 In contrast, HIIT increased excess postexercise oxygen consumption (EPOC) compared to MICT during the slow phase of O2 kinetics in the recovery phase (i.e., from 30 min to 22 h after the end of the training session).55 This occurs due to greater postexercise fat utilization to sustain energy demands while glycogen resynthesis occurs56 and occurs through significant increases in circulating hormones that promote fat oxidation (i.e., catecholamines and growth hormone),56 although the results are conflicting.45 Thus, through different physiological mechanisms, our results demonstrate that combining reduced energy intake with physical exercise (i.e., COMB training or HIIT) is a useful strategy for both weight- and FM-loss programs for obese adults. Although we expected that COMB training would have a greater effect on improving body composition than HIIT in obese male adults, both exercise intensity and a combination of volume and intensity may be effective in the initial stage of weight reduction programs for decreasing BM and FM27,28 due to similar improvements in skeletal muscle capacity for the uptake and oxidation of fatty acids57 and for increasing both glycogen content and utilization,58 which are typically impaired in adults with obesity.59 In addition, FFM was maintained in both exercise groups, which might be helpful for maintaining weight loss.28,30
The second main finding was that both COMB training and HIIT significantly increased V’O2 peak by ∼16% in both groups, confirming previous results observed in people with obesity (i.e., improvements ranging between 15% and 25%)31, 32, 33 and highly trained endurance athletes.60,61 As mentioned in the Introduction, the main features of COMB training reported in previous studies were a combination of MICT (30–40 min/session at 65–70% of HRmax or 90% of the first ventilatory threshold) and HIIT (6–12 min/session at 80–90% of HRmax or 90% of VO2 peak)31, 32, 33 performed in the same training session and 3 days/week during the long-term training period (12–16 weeks); these features elicited equal or superior improvements in V'O2 peak compared to HIIT alone. The present study applied a combination of high-volume low-intensity exercise (≥80% of the overall training volume) and low-volume high-intensity exercise (≤20%) derived from analysis of the above studies,31, 32, 33 as shown in middle- and long-distance runners.35 HIIT is superior to MICT in improving CRF when energy expenditure is held constant.22 To our knowledge, this study is the first to apply an a priori combination of HIIT and MICT in obese individuals, considering primarily the weekly distribution of HIIT and MICT rather than the individual training session itself. Although we expected that COMB training would improve CRF to a greater extent than HIIT, we observed similar improvements in V’O2 peak. It is possible that during the initial stage of a weight reduction program, exercise intensity (rather than volume) is critical in male adults with obesity.20,22 Nonetheless, based on the few available data, we confirmed the positive results obtained in previous studies, expanding upon these findings with an a priori manipulation of HIIT and MICT (i.e., ∼85% MICT and ∼15% HIIT). Further studies are needed to compare various combinations of COMB training over a long period of time (i.e., ≥6 months).
In the present study, HIIT may have enhanced V’O2 peak by increasing stroke volume and maximal cardiac output (central adaptation).62 In contrast, COMB training may have increased V’O2 peak by increasing mitochondrial content (peripheral adaptation).63 Exercise volume (i.e., a combination of HIIT + MICT) is more important than exercise intensity (i.e., HIIT alone) for promoting increases in mitochondrial content.64
Another main finding was the increase in fat oxidation over a wide range of exercise intensity (i.e., 20–80% of VO2 peak), obtained in both the COMB and HIIT groups, without significant differences between the two groups. Previous studies have shown that HIIT increases fat oxidation in individuals with obesity to a greater extent than MICT alone.25,65,66 However, our study showed that COMB training increased fat oxidation in obese individuals in a similar manner to HIIT, as demonstrated earlier in obese young women.33 The key mechanisms underlying these metabolic adaptations induced by HIIT and COMB training appear to be different. Contrary to the theory that above 80% of V’O2 max, the contribution of fat oxidation is near negligible,45,67 recent studies have shown that during HIIT, fat oxidation rates increase (i.e., threefold higher in well-trained athletes than in moderately active people),18 as confirmed by metabolomics analysis.68 In fact, fat oxidation during HIIT, compared to continuous exercise, occurred mainly due to increased levels of fatty acid transport proteins taking up plasmatic free fatty acids69 in type II fibres of human skeletal muscle.70 On the other hand, in COMB training, increased fat oxidation during MICT34 primarily occurs in type I fibres71 that exhibit high rates of intramuscular fatty acid oxidation.72 Thus, in line with a recent meta-analysis,66 our study showed that combining low-volume HIIT with high-volume moderate-intensity training (i.e., COMB training) was effective for improving fat oxidation, metabolic health and body composition in individuals with obesity.66
In contrast to the physiological data, during the 12 weeks of the training intervention, the COMB group exhibited lower HR and RPE values than the HIIT group, in line with previous research in which both MICT and COMB training were perceived to be less strenuous33,73 than HIIT, despite similar improvements in anthropometric and physiological parameters. Nevertheless, the COMB group covered more kilometres on average (i.e., ∼11 km for each participant) with a greater duration of each training session (i.e., ∼30%) than the HIIT group. Although HIIT is an effective, time-efficient exercise protocol for improving body composition and physical capacities in individuals with obesity,21,22 over a long period of training, high-intensity workout programs could increase lower limb injury rates,74 and overweight and obese patients experience fear of joint damage.75 Thus, a weekly training distribution with a low volume of HIIT and the largest volume of MICT could be recommended in obese adults to increase adherence to international physical activity guidelines while minimizing the risk of joint injuries.
The present study has some limitations. First, although we showed that 12 weeks of COMB training or HIIT, administered with the same energy restriction, improved body composition and physical capacities, it remains unclear whether the training program was the determining factor for improving body composition, as we did not have an inactive control group to rule out dietary factors. Second, our study was carried out on healthy individuals with obesity; thus, it is not possible to extend our results to obese people with one or more comorbidities. Third, since we compared only one a priori COMB training with HIIT, it is difficult to conclude whether our selected combination ratio was indeed optimal or whether there are even better combinations.
In conclusion, COMB training and HIIT improved anthropometric and cardiovascular parameters as well as fat oxidation rates to a similar extent. However, COMB training was less intense than HIIT, confirmed by lower values of RPE and HR. Future studies should investigate various combinations of HIIT and MICT (i.e., polarized, pyramidal or threshold) over a long period of time (≥6 months) and compare them to HIIT and MICT alone to identify the optimal combination of HIIT and MICT. Such research would provide more evidence on the use of COMB training in obese adults for improving body composition and optimizing and maintaining aerobic fitness.
Funding
The study was supported by the University of Udine research funds.
Author contributions
MD and SL conceived the overall study; all the authors contributed to design the research. MD, FG, FF, and MM conducted the experiments. MD, FF, MM and SL analysed the data. MD wrote the manuscript with the help of FV, NG, MF, FF, MM, MP and SL who revised the manuscript. All the authors read and approved this manuscript.
Data availability statement
The corresponding author is available to share the primary data to those interested.
Declaration of competing interest
There are no real or potential conflicts of financial or personal interest with the financial sponsors of the scientific project.
Acknowledgements
The authors thank the subjects who agreed to participate to the study. They thank the physicians and nursing staff at the Department of Public Health of—Gemona del Friuli (UD), Azienda Sanitaria Medio Friuli (Italy), for their qualified assistance during the study.
References
- 1.Inoue Y., Qin B., Poti J., Sokol R., Gordon-Larsen P. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7(4):276–288. doi: 10.1007/s13679-018-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthold R., Stevens G.A., Riley L.M., Bull F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Global Health. 2018;6(10):e1077–e1086. doi: 10.1016/s2214-109x(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 3.Swinburn B. Commentary: physical activity as a minor player in the obesity epidemic: what are the deep implications? Int J Epidemiol. 2013;42(6):1838–1840. doi: 10.1093/ije/dyt162. [DOI] [PubMed] [Google Scholar]
- 4.Henry S.L., Barzel B., Wood-Bradley R.J., Burke S.L., Head G.A., Armitage J.A. Developmental origins of obesity-related hypertension. Clin Exp Pharmacol Physiol. 2012;39(9):799–806. doi: 10.1111/j.1440-1681.2011.05579.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;4(3):363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 6.Lin X., Zhang X., Guo J., et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7) doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanzi S., Codecasa F., Cornacchia M., et al. Fat oxidation, hormonal and plasma metabolite kinetics during a submaximal incremental test in lean and obese adults. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berggren J.R., Boyle K.E., Chapman W.H., Houmard J.A. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab. 2008;294(4):E726–E732. doi: 10.1152/ajpendo.00354.2007. [DOI] [PubMed] [Google Scholar]
- 9.Robinson S.L., Hattersley J., Frost G.S., Chambers E.S., Wallis G.A. Maximal fat oxidation during exercise is positively associated with 24-hour fat oxidation and insulin sensitivity in young, healthy men. J Appl Physiol. 2015;118(11):1415–1422. doi: 10.1152/japplphysiol.00058.2015. [DOI] [PubMed] [Google Scholar]
- 10.Rosenkilde M., Nordby P., Nielsen L.B., Stallknecht B.M., Helge J.W. Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. Int J Obes. 2010;34(5):871–877. doi: 10.1038/ijo.2010.11. [DOI] [PubMed] [Google Scholar]
- 11.Narici M., Vito G De, Franchi M., et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur J Sport Sci. 2021;21(4):614–635. doi: 10.1080/17461391.2020.1761076. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini M., Ponzo V., Rosato R., et al. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaesser G.A., Angadi S.S. Obesity treatment: weight loss versus increasing fitness and physical activity for reducing health risks. iScience. 2021;24(10) doi: 10.1016/j.isci.2021.102995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croci I., Hickman I.J., Wood R.E., Borrani F., Macdonald G.A., Byrne N.M. Fat oxidation over a range of exercise intensities: fitness versus fatness. Appl Physiol Nutr Metabol. 2014;39(12):1352–1359. doi: 10.1139/apnm-2014-0144. [DOI] [PubMed] [Google Scholar]
- 15.Petridou A., Siopi A., Mougios V. Exercise in the management of obesity. Metabolism. 2019;92:163–169. doi: 10.1016/j.metabol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Rugbeer N., Constantinou D., Torres G. Comparison of high-intensity training versus moderate-intensity continuous training on cardiorespiratory fitness and body fat percentage in persons with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. J Phys Activ Health. 2021;18(5):610–623. doi: 10.1123/jpah.2020-0335. [DOI] [PubMed] [Google Scholar]
- 17.Achten J., Jeukendrup A.E. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20(7-8):716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Hetlelid K.J., Plews D.J., Herold E., Laursen P.B., Seiler S. Rethinking the role of fat oxidation: substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. BMJ Open Sport Exerc Med. 2015;1(1) doi: 10.1136/bmjsem-2015-000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacInnis M.J., Gibala M.J. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibala M.J., Gillen J.B., Percival M.E. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med. 2014;44(Suppl 2):S127–S137. doi: 10.1007/s40279-014-0259-6. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batacan R.B., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 22.Su L., Fu J., Sun S., et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Türk Y., Theel W., Kasteleyn M.J., et al. High intensity training in obesity: a Meta-analysis. Obes Sci Pract. 2017;3(3):258–271. doi: 10.1002/osp4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreato L.V., Esteves J.V., Coimbra D.R., Moraes A.J.P., de Carvalho T. The influence of high-intensity interval training on anthropometric variables of adults with overweight or obesity: a systematic review and network meta-analysis. Obes Rev. 2019;20(1):142–155. doi: 10.1111/obr.12766. [DOI] [PubMed] [Google Scholar]
- 25.Vaccari F., Passaro A., D'Amuri A., et al. Effects of 3-month high-intensity interval training vs. moderate endurance training and 4-month follow-up on fat metabolism, cardiorespiratory function and mitochondrial respiration in obese adults. Eur J Appl Physiol. 2020;120(8):1787–1803. doi: 10.1007/s00421-020-04409-2. [DOI] [PubMed] [Google Scholar]
- 26.Tsirigkakis S., Mastorakos G., Koutedakis Y., et al. Effects of two workload-matched high-intensity interval training protocols on regional body composition and fat oxidation in obese men. Nutrients. 2021;13(4) doi: 10.3390/nu13041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wewege M., van den Berg R., Ward R.E., Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18(6):635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 28.Keating S.E., Johnson N.A., Mielke G.I., Coombes J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–964. doi: 10.1111/obr.12536. [DOI] [PubMed] [Google Scholar]
- 29.Rugbeer N., Constantinou D., Torres G. Comparison of high-intensity training versus moderate-intensity continuous training on cardiorespiratory fitness and body fat percentage in persons with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. J Phys Activ Health. 2021;18(5):610–623. doi: 10.1123/JPAH.2020-0335. [DOI] [PubMed] [Google Scholar]
- 30.Wewege M., van den Berg R., Ward R.E., Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18(6):635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 31.Poon E.T.C., Siu P.M.F., Wongpipit W., Gibala M., Wong S.H.S. Alternating high-intensity interval training and continuous training is efficacious in improving cardiometabolic health in obese middle-aged men. J Exerc Sci Fit. 2022;20(1):40–47. doi: 10.1016/j.jesf.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roxburgh B.H., Nolan P.B., Weatherwax R.M., Dalleck L.C. Is moderate intensity exercise training combined with high intensity interval training more effective at improving cardiorespiratory fitness than moderate intensity exercise training alone? J Sports Sci Med. 2014;13(3):702–707. [PMC free article] [PubMed] [Google Scholar]
- 33.Zapata-Lamana R., Henríquez-Olguín C., Burgos C., et al. Effects of polarized training on cardiometabolic risk factors in young overweight and obese women: a randomized-controlled trial. Front Physiol. 2018;9:1287. doi: 10.3389/fphys.2018.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borowik A., Chacaroun S., Tessier D., Doutreleau S., Verges S., Flore P. A short bout of high-intensity intermittent exercise before moderate-intensity prolonged exercise as a mean to potentiate fat oxidation. J Sports Sci. 2020;38(9):1046–1052. doi: 10.1080/02640414.2020.1740478. [DOI] [PubMed] [Google Scholar]
- 35.Campos Y., Casado A., Vieira J.G., et al. Training-intensity distribution on middle- and long-distance runners: a systematic review. Int J Sports Med. 2022;43(4):305–316. doi: 10.1055/a-1559-3623. [DOI] [PubMed] [Google Scholar]
- 36.Kim T.H., Han J.K., Lee J.Y., Choi Y.C. The effect of polarized training on the athletic performance of male and female cross-country skiers during the general preparation period. Health Care. 2021;9(7):851. doi: 10.3390/healthcare9070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stöggl T., Sperlich B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front Physiol. 2014;5:33. doi: 10.3389/fphys.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez A., Ramos-Campo D.J., Freitas T.T., Rubio-Arias Já, Marín-Cascales E., Alcaraz P.E. Effect of two different intensity distribution training programmes on aerobic and body composition variables in ultra-endurance runners. Eur J Sport Sci. 2019;19(5):636–644. doi: 10.1080/17461391.2018.1539124. [DOI] [PubMed] [Google Scholar]
- 39.Craig C.L., Marshall A.L., Sjöström M., et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453. (FB) [DOI] [PubMed] [Google Scholar]
- 40.Pelagatti M., Maranzano P. Assessing the effectiveness of the Italian risk-zones policy during the second wave of COVID-19. Health Pol. 2021;125(9):1188–1199. doi: 10.1016/j.healthpol.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linee C.R.E.A. Revisione 2018).; 2019. Guida Per La Sana Alimentazone. [Google Scholar]
- 42.Kagawa M., Byrne N.M., Hills A.P. Comparison of body fat estimation using waist:height ratio using different “waist” measurements in Australian adults. Br J Nutr. 2008;100(5):1135–1141. doi: 10.1017/S0007114508966095. [DOI] [PubMed] [Google Scholar]
- 43.Lukaski H.C., Bolonchuk W.W., Hall C.B., Siders W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol. 1986;60(4):1327–1332. doi: 10.1152/jappl.1986.60.4.1327. 1985. [DOI] [PubMed] [Google Scholar]
- 44.Gray D.S., Bray G.A., Gemayel N., Kaplan K. Effect of obesity on bioelectrical impedance. Am J Clin Nutr. 1989;50(2):255–260. doi: 10.1093/ajcn/50.2.255. [DOI] [PubMed] [Google Scholar]
- 45.Lazzer S., Tringali G., Caccavale M., De Micheli R., Abbruzzese L., Sartorio A. Effects of high-intensity interval training on physical capacities and substrate oxidation rate in obese adolescents. J Endocrinol Invest. 2017;40(2):217–226. doi: 10.1007/s40618-016-0551-4. [DOI] [PubMed] [Google Scholar]
- 46.Frayn K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 47.Gnagnarella Patrizia, Salvini Simonetta, Parpinel M. 2015. Food Composition Database for Epidemiological Studies in Italy. Published online. [DOI] [PubMed] [Google Scholar]
- 48.Ware J.J., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- 50.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4(NOV):1–12. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen J. second ed. Routledge; 1988. Statistical Power Analysis for the Behavioral Sciences. [DOI] [Google Scholar]
- 52.Vaccari F., Passaro A., D'Amuri A., et al. Effects of 3-month high-intensity interval training vs. moderate endurance training and 4-month follow-up on fat metabolism, cardiorespiratory function and mitochondrial respiration in obese adults. Eur J Appl Physiol. 2020;120(8):1787–1803. doi: 10.1007/s00421-020-04409-2. [DOI] [PubMed] [Google Scholar]
- 53.Reljic D., Frenk F., Herrmann H.J., Neurath M.F., Zopf Y. Effects of very low volume high intensity versus moderate intensity interval training in obese metabolic syndrome patients: a randomized controlled study. Sci Rep. 2021;11(1):1–14. doi: 10.1038/s41598-021-82372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mello-Silva B.N., Protzen G.V., Del Vecchio F.B. Inclusion of sprints during moderate-intensity continuous exercise enhances post-exercise fat oxidation in young males. Appl Physiol Nutr Metabol. 2022;47(2):165–172. doi: 10.1139/apnm-2021-0383. [DOI] [PubMed] [Google Scholar]
- 55.Panissa V.L.G., Fukuda D.H., Staibano V., Marques M., Franchini E. Magnitude and duration of excess of post-exercise oxygen consumption between high-intensity interval and moderate-intensity continuous exercise: a systematic review. Obes Rev. 2021;22(1):1–16. doi: 10.1111/obr.13099. [DOI] [PubMed] [Google Scholar]
- 56.Moniz S.C., Islam H., Hazell T.J. Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand J Med Sci Sports. 2020;30(4):638–651. doi: 10.1111/sms.13610. [DOI] [PubMed] [Google Scholar]
- 57.Purdom T., Kravitz L., Dokladny K., Mermier C. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr. 2018;15(1):1–10. doi: 10.1186/s12970-018-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray B., Rosenbloom C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr Rev. 2018;76(4):243–259. doi: 10.1093/NUTRIT/NUY001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Georgiev A., Granata C., Roden M. The role of mitochondria in the pathophysiology and treatment of common metabolic diseases in humans. Am J Physiol Cell Physiol. 2022;322(6):C1248–C1259. doi: 10.1152/ajpcell.00035.2022. [DOI] [PubMed] [Google Scholar]
- 60.Stöggl T.L., Sperlich B. The training intensity distribution among well-trained and elite endurance athletes. Front Physiol. 2015;6:295. doi: 10.3389/fphys.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenblat M.A., Perrotta A.S., Vicenzino B. Polarized vs. Threshold training intensity distribution on endurance Sport performance: a systematic review and meta-analysis of randomized controlled trials. J Strength Condit Res. 2019;33(12):3491–3500. doi: 10.1519/JSC.0000000000002618. [DOI] [PubMed] [Google Scholar]
- 62.Astorino T.A., Edmunds R.M., Clark A., et al. High-intensity interval training increases cardiac output and V˙O2max. Med Sci Sports Exerc. 2017;49(2):265–273. doi: 10.1249/MSS.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 63.Granata C., Jamnick N.A., Bishop D.J. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 2018;48(8):1809–1828. doi: 10.1007/s40279-018-0936-y. [DOI] [PubMed] [Google Scholar]
- 64.Bishop D.J., Botella J., Granata C. CrossTalk opposing view: exercise training volume is more important than training intensity to promote increases in mitochondrial content. J Physiol. 2019;597(16):4115–4118. doi: 10.1113/JP277634. [DOI] [PubMed] [Google Scholar]
- 65.Alkahtani S.A., King N.A., Hills A.P., Byrne N.M. Effect of interval training intensity on fat oxidation, blood lactate and the rate of perceived exertion in obese men. SpringerPlus. 2013;2:532. doi: 10.1186/2193-1801-2-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atakan M.M., Guzel Y., Shrestha N., et al. Effects of high-intensity interval training (HIIT) and sprint interval training (SIT) on fat oxidation during exercise: a systematic review and meta-analysis. Br J Sports Med. 2022;56(17):988–996. doi: 10.1136/bjsports-2021-105181. [DOI] [PubMed] [Google Scholar]
- 67.Achten J., Venables M.C., Jeukendrup A.E. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism. 2003;52(6):747–752. doi: 10.1016/s0026-0495(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 68.Zagatto A.M., Bishop D.J., Antunes B.M., et al. Impacts of high-intensity exercise on the metabolomics profile of human skeletal muscle tissue. Scand J Med Sci Sports. October 2021 doi: 10.1111/sms.14086. Published online. [DOI] [PubMed] [Google Scholar]
- 69.Talanian J.L., Holloway G.P., Snook L.A., Heigenhauser G.J.F., Bonen A., Spriet L.L. Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299(2):E180–E188. doi: 10.1152/ajpendo.00073.2010. [DOI] [PubMed] [Google Scholar]
- 70.Kristensen D.E., Albers P.H., Prats C., Baba O., Birk J.B., Wojtaszewski J.F.P. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol. 2015;593(8):2053–2069. doi: 10.1113/jphysiol.2014.283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skelly L.E., Gillen J.B., Frankish B.P., et al. Human skeletal muscle fiber type-specific responses to sprint interval and moderate-intensity continuous exercise: acute and training-induced changes. J Appl Physiol. 2021;130(4):1001–1014. doi: 10.1152/japplphysiol.00862.2020. [DOI] [PubMed] [Google Scholar]
- 72.Shaw C.S., Swinton C., Morales-Scholz M.G., et al. Impact of exercise training status on the fiber type-specific abundance of proteins regulating intramuscular lipid metabolism. J Appl Physiol. 2020;128(2):379–389. doi: 10.1152/japplphysiol.00797.2019. [DOI] [PubMed] [Google Scholar]
- 73.Sun S., Zhang H., Kong Z., Shi Q., Tong T.K., Nie J. Twelve weeks of low volume sprint interval training improves cardio-metabolic health outcomes in overweight females. J Sports Sci. 2019;37(11):1257–1264. doi: 10.1080/02640414.2018.1554615. [DOI] [PubMed] [Google Scholar]
- 74.Rynecki N.D., Siracuse B.L., Ippolito J.A., Beebe K.S. Injuries sustained during high intensity interval training: are modern fitness trends contributing to increased injury rates? J Sports Med Phys Fit. 2019;59(7):1206–1212. doi: 10.23736/S0022-4707.19.09407-6. [DOI] [PubMed] [Google Scholar]
- 75.Hamer O., Larkin D., Relph N., Dey P. Fear-related barriers to physical activity among adults with overweight and obesity: a narrative synthesis scoping review. Obes Rev. 2021;22(11) doi: 10.1111/obr.13307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author is available to share the primary data to those interested.