Abstract
Background
There have been limited data examining the age‐dependent relationship of wide‐range risk factors with the incidence of each subtype of cardiovascular disease (CVD) event. We assessed age‐related associations between modifiable risk factors and the incidence of CVD.
Methods and Results
We analyzed 3 027 839 participants without a CVD history enrolled in the JMDC Claims Database (mean age, 44.8±11.0 years; 57.6% men). Each participant was categorized as aged 20 to 49 years (n=2 008 559), 50 to 59 years (n=712 273), and 60 to 75 years (n=307 007). Using Cox proportional hazards models and the relative risk reduction, we identified associations between risk factors and incident CVD, consisting of myocardial infarction, angina pectoris, stroke, and heart failure (HF). We assessed whether the association of risk factors for developing CVD would be modified by age category. Over a mean follow‐up of 1133 days, 6315 myocardial infarction, 56 447 angina pectoris, 28 079 stroke, and 56 369 HF events were recorded. The incidence of myocardial infarction, angina pectoris, stroke, and HF increased with age category. Hazard ratios of obesity, hypertension, and diabetes in the multivariable Cox regression analyses for myocardial infarction, angina pectoris, stroke, and HF decreased with age category. The relative risk reduction of obesity, hypertension, and diabetes for CVD events decreased with age category. For example, the relative risk reduction of hypertension for HF decreased from 59.2% in participants aged 20 to 49 years to 38.1% in those aged 60 to 75 years.
Conclusions
The contribution of modifiable risk factor to the development of CVD is greater in younger compared with older individuals. Preventive efforts for risk factor modification may be more effective in younger people.
Keywords: age, cardiovascular disease, prevention, risk factors
Subject Categories: Cardiovascular Disease, Epidemiology, Primary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- AP
angina pectoris
- RRR
relative risk reduction
Clinical Perspective.
What Is New?
Our analysis of ≈3 000 000 people without a history of cardiovascular disease showed that hazard ratios of modifiable risk factors (eg, obesity, hypertension, and diabetes) for myocardial infarction, angina pectoris, stroke, and heart failure decreased with age category.
Similarly, the relative risk reduction of these risk factors for cardiovascular disease events decreased with age category.
The discriminative ability of each prediction model evaluated using the Harrell C statistic, including modifiable risk factors for cardiovascular disease events in participants aged 20 to 49 years, was higher than in those aged 50 to 59 and 60 to 75 years.
What Are the Clinical Implications?
This is the first investigation of age‐dependent association between the full range of risk factors and each cardiovascular event separately using a large‐scale epidemiological data set and showed that the relationship of specific risk factors, including obesity, hypertension, and diabetes, and the risk of developing myocardial infarction, angina pectoris, stroke, and heart failure attenuated with age.
Despite the fact that risk factor modification is crucial throughout the life course to reduce the cardiovascular disease burden, preventive efforts for risk factor optimization may bring younger people a greater benefit.
Cardiovascular disease (CVD) is one of the leading causes of mortality and morbidity. 1 , 2 CVD predominantly occurs in middle‐aged or elderly people. However, recent epidemiological data suggest that the incidence of CVD has remained stable or even increased among young adults, 3 , 4 , 5 , 6 , 7 and, thus, attempts to identify the optimal strategy to prevent CVD in younger people are becoming increasingly essential. Risk factor modification is the most important preventive measure in CVD, and we have reported that modifiable risk factors played a critical role in developing CVD among young adults as well. 8 , 9 , 10 Furthermore, several studies reported that the relative contribution of modifiable risk factors to developing CVD could be greater in younger people. 11 , 12 , 13 , 14 However, preceding studies focused on age‐dependent differences in specific risk factors or specific CVD events, and there has been to date no comprehensive investigation examining the age‐dependent relationship between the full range of risk factors and the subsequent risk of each CVD event. The objective of the present study was, therefore, to identify the age‐dependent relationship between each modifiable risk factor and the risk of developing each CVD event using a nationwide combined data set of health checkup and administrative claims records.
Methods
Data Availability
The JMDC Claims Database is accessible under a contract from JMDC Inc (Tokyo, Japan; https://www.jmdc.co.jp/en/index).
Study Design
We conducted this retrospective observational study through analyses of the JMDC Claims Database (JMDC Inc) between 2005 and 2020. 15 , 16 , 17 This data set includes individual health insurance claims data of >60 insurers as well as employees' annual health checkup data, including data and hospital claims (inpatient and outpatient) according to the International Classification of Diseases, Tenth Revision (ICD‐10), coding.
Study Participants
We extracted 3 918 148 individuals who underwent the health checkup, including physical examination (eg, body mass index and blood pressure) and blood test, >4 months after the enrollment of the insurance. Given that the prescription period is 3 months maximum in the universal health insurance system in Japan, a 4‐month look‐back period was set. We obtained information on the history of CVD, dialysis, and renal transplantation, as well as medication prescription for hypertension, diabetes, and dyslipidemia, from the administrative claims data. From this population, we excluded the following individuals: (1) those aged <20 years (n=13 258), (2) those with a CVD history of myocardial infarction (MI), angina pectoris (AP), stroke, heart failure (HF), dialysis, or renal transplantation (n=149 428), and (3) those with missing data on cigarette smoking (n=261 579) and physical activity (n=466 044). Consequently, the final study population included 3 027 839 participants (Figure 1).
Figure 1. Flowchart.
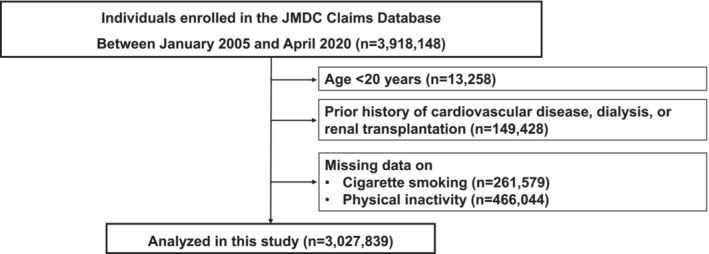
We extracted 3 918 148 individuals who underwent the health checkup, including physical examination and blood test data, >4 months after the insurance enrollment. Given that the maximum prescription period is 3 months in the universal health insurance system in Japan, we set a 4‐month look‐back period. We obtained information on the history of CVD, dialysis, and renal transplantation, as well as medication prescription for hypertension, diabetes, and dyslipidemia, from the administrative claims data. From this population, we excluded the following individuals: (1) those aged <20 years (n=13 258), (2) those with a CVD history of myocardial infarction, angina pectoris, stroke, heart failure, dialysis, or renal transplantation (n=149 428), and (3) those with missing data on cigarette smoking (n=261 579) and physical activity (n=466 044). Consequently, the final study population included 3 027 839 participants. CVD indicates cardiovascular disease.
Ethical Approval
The University of Tokyo's Ethical Committee granted approval for this study (number 2018‐10862), which adhered to the Declaration of Helsinki. Because all of the information included in the JMDC Claims Database was deidentified, the requirement for informed consent was waived.
Variables and Measurement
We collected the following data using standardized protocols at the initial health checkup of each participant: body mass index, blood pressure, and fasting laboratory values. Information for cigarette smoking (current or noncurrent) was self‐reported. We defined obesity as body mass index of ≥25 kg/m2. 18 Hypertension was defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or use of blood pressure–lowering medications. Diabetes was defined as fasting glucose level of ≥126 mg/dL or use of glucose‐lowering medications. Dyslipidemia was defined as low‐density lipoprotein cholesterol level of ≥140 mg/dL, high‐density lipoprotein cholesterol level of <40 mg/dL, triglyceride level of ≥150 mg/dL, or use of lipid‐lowering medications. Physical inactivity was defined as not exercising for 30 minutes at least twice a week or not walking 1 hour each day, as we previously described. 19 The outcomes between January 2005 and April 2020 were collected. The primary outcomes included MI (ICD‐10 codes: I210, I211, I212, I213, I214, and I219), AP (ICD‐10 codes: I200, I201, I208, and I209), stroke (ICD‐10 codes: I630, I631, I632, I633, I634, I635, I636, I638, I639, I600, I601, I602, I603, I604, I605, I606, I607, I608, I609, I610, I611, I613, I614, I615, I616, I619, I629, and G459), and HF (ICD‐10 codes: I500, I501, I509, and I110).
Statistical Analysis
Data are expressed as mean (SD) for continuous variables or number (percentage) for categorical variables. We calculated summary statistics for the characteristics of study participants between 3 categories based on age (20–49, 50–59, and 60–75 years). We performed multivariable Cox regression analyses adjusted for age, sex, obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity, and obtained hazard ratios (HRs) of age (per 1 year), obesity (nonobese as reference), hypertension (nonhypertension as reference), diabetes (nondiabetes as reference), dyslipidemia (nondyslipidemia as reference), cigarette smoking (nonsmoking as reference), and physical inactivity (physical activity as reference) to assess the association between each of these risk factors and incidence of CVD events in each age category. The P values for multiplicative interactions between each disease and the 3 age categories were calculated. The relative risk reduction (RRR) presents the potential proportion of individuals with a disease that could be attributed to each exposure factor (ie, the proportional reduction estimated to occur if the exposure to that particular risk factor [eg, hypertension or diabetes] would be normalized). On the basis of this concept, we estimated the RRR as the proportion of each CVD event that would be preventable if each modifiable risk factor could be normalized based on HRs of each risk factor (ie, RRR=[HR–1]/HR). 20 The discriminative ability of each prediction model, including modifiable risk factors (obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity) with and without age and sex was assessed using C statistics (Harrell C statistics). 21 We performed 8 sensitivity analyses. First, we used multiple imputations for missing data on the assumption of data missing at random, as previously described, 22 , 23 and we imputed the missing data for covariates using the chained equation method with 20 iterations, as described by Aloisio. 24 After that, we calculated HRs and SEs using Rubin rules. 25 Second, we analyzed the population after excluding participants who were receiving medications for hypertension, diabetes, or dyslipidemia. Third, we included body mass index, systolic blood pressure, and low‐density lipoprotein cholesterol as continuous values instead of obesity, hypertension, and dyslipidemia in the multivariable model. Fourth, death could be considered as a competing risk with CVD events, and thus, we conducted the cause‐specific Cox proportional hazard modeling as a competing risks analysis. 26 , 27 Fifth, we added the year of the initial heath checkup of each participant (3‐year interval) into covariates in the multivariable model. Sixth, we categorized the study participants using the tertile of age (20–40, 41–50, and 51–75 years). Seventh, because the general retirement age of employees in Japan is 60 years, we excluded people aged >60 years and compared the association between risk factors and incident CVD between people aged 20 to 44, 45 to 54, and 55 to 60 years. Eighth, we examined age‐stratified relationships of risk factors with all‐cause mortality. The statistical significance level was set at P<0.05, and STATA version 17 was used to conduct statistical analyses (StataCorp LLC, College Station, TX).
Results
Clinical Characteristics
The clinical characteristics of study participants are summarized in Table 1. On the basis of the age at health checkup, we categorized 3 027 839 participants into 3 groups: 20 to 49 years (n=2 008 559), 50 to 59 years (n=712 273), and 60 to 75 years (n=307 007). The prevalence of hypertension, diabetes, and dyslipidemia increased with age, whereas that of cigarette smoking and physical inactivity decreased with age.
Table 1.
Characteristics of Study Population
| Characteristic | Individuals aged 20–49 y | Individuals aged 50–59 y | Individuals aged 60–75 y |
|---|---|---|---|
| No. | 2 008 559 | 712 273 | 307 007 |
| Age, y | 39 (7) | 54 (3) | 64 (3) |
| Men, n (%) | 1 144 560 (57.0) | 416 249 (58.4) | 184 729 (60.2) |
| Obesity, n (%) | 463 787 (23.1) | 194 274 (27.3) | 76 122 (24.8) |
| Body mass index, kg/m2 | 22.7 (3.9) | 23.2 (3.6) | 23.0 (3.3) |
| Hypertension, n (%) | 209 465 (10.4) | 212 735 (29.9) | 135 040 (44.0) |
| Systolic blood pressure, mm Hg | 116 (15) | 123 (17) | 128 (17) |
| Diastolic blood pressure, mm Hg | 71 (11) | 77 (12) | 78 (11) |
| Diabetes, n (%) | 42 803 (2.1) | 51 260 (7.2) | 35 826 (11.7) |
| Fasting plasma glucose, mg/dL | 91 (15) | 98 (20) | 102 (21) |
| Dyslipidemia, n (%) | 642 656 (32.0) | 373 802 (52.5) | 179 372 (58.4) |
| Low‐density lipoprotein cholesterol, mg/dL | 116 (31) | 129 (31) | 128 (31) |
| High‐density lipoprotein cholesterol, mg/dL | 63 (16) | 65 (18) | 65 (17) |
| Triglycerides, mg/dL | 98 (82) | 114 (89) | 113 (76) |
| Cigarette smoking, n (%) | 533 999 (26.6) | 183 858 (25.8) | 64 130 (20.9) |
| Physical inactivity, n (%) | 1 076 534 (53.6) | 381 395 (53.5) | 138 086 (45.0) |
Data are presented as mean (SD) for continuous variables or number (percentage) for categorical variables. Participants were categorized into 3 groups, aged 20 to 49, 50 to 59, and 60 to 75 years.
Incidence of CVDs and HRs of Each Risk Factor for CVD Stratified by Age
The age‐stratified incidence of CVDs and HRs of each risk factor for CVD stratified are summarized in Table S1 and Figure 2. During a mean (SD) follow‐up of 1133 (931) days, 6315 MI, 56 447 AP, 28 079 stroke, and 56 369 HF events were documented. The incidence rates (95% CI) for CVD events, including MI, AP, stroke, and HF, were the lowest in the 20 to 49 years age group (4.1 [3.9–4.3], 41.0 [40.5–41.5], 16.6 [16.3–16.9], and 39.1 [38.6–39.6] per 10 000 person‐years, respectively), followed by those in the 50 to 59 years age group (10.6 [10.2–11.1], 90.9 [89.7–92.2], 47.2 [46.3–48.1], and 89.4 [88.1–90.6] per 10 000 person‐years, respectively) and 60 to 75 years age group (16.3 [15.4–17.2], 134.7 [132.2–137.4], 88.9 [86.8–91.0], and 153.2 [150.5–156.0] per 10 000 person‐years, respectively). The HRs of obesity, hypertension, and diabetes for each CVD event decreased with age. However, this age‐dependent tendency was not clearly observed in cigarette smoking and physical inactivity. P values for age–risk factor interactions were significant for obesity, hypertension, diabetes, and cigarette smoking in MI; hypertension, diabetes, dyslipidemia, and cigarette smoking in AP; obesity, hypertension, and diabetes in stroke; and obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity in HF.
Figure 2. HRs of modifiable risk factors for cardiovascular events.
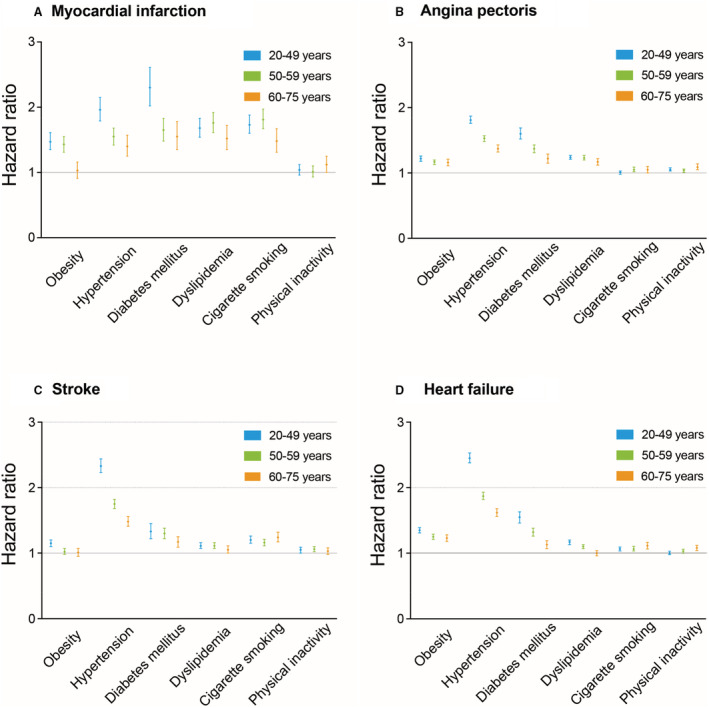
HRs (95% CIs) of obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity for myocardial infarction (A), angina pectoris (B), stroke (C), and heart failure (D) are summarized. HR indicates hazard ratio.
Relative Risk Reduction
The RRR of each risk factor, stratified by age, is summarized in Figure 3 and Table S2. The RRR (95% CI) for each CVD event associated with obesity, hypertension, and diabetes decreased with age categories. Detailed data on RRR are shown in Table S2.
Figure 3. Relative risk reduction of modifiable risk factors for cardiovascular events.
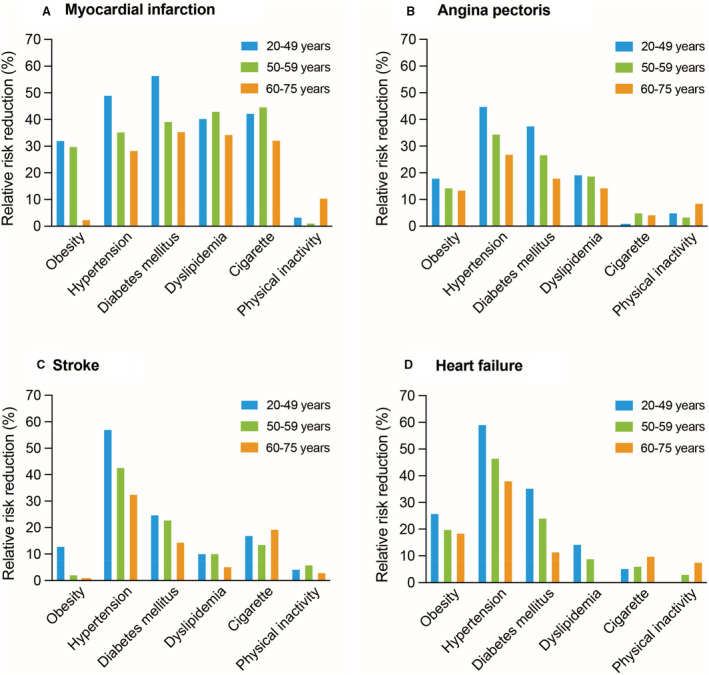
Relative risk reductions of obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity for myocardial infarction (A), angina pectoris (B), stroke (C), and heart failure (D) are summarized. Using HRs of modifiable risk factors in multivariable Cox regression models, we calculated relative risk reduction as following formula: relative risk reduction=(HR–1)/HR. HR indicates hazard ratio.
Harrell C Statistic
The C statistics for models including obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity for each CVD event in participants aged 20 to 49 years were 0.712, 0.614, 0.610, and 0.620, respectively; those in participants aged 50 to 59 years were 0.694, 0.596, 0.598, and 0.604, respectively; and those in participants aged 60 to 75 years were 0.640, 0.577, 0.585, and 0.586, respectively. The C statistics for MI, AP, stroke, and HF were increased after adding age and sex to the model (Table 2).
Table 2.
Harrell C Statistics for CVD Event
| Variable | Individuals aged 20–49 y | Individuals aged 50–59 y | Individuals aged 60–75 y | Overall |
|---|---|---|---|---|
| Model including obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity | ||||
| Myocardial infarction | 0.712 | 0.694 | 0.640 | 0.726 |
| Angina pectoris | 0.614 | 0.596 | 0.577 | 0.639 |
| Stroke | 0.610 | 0.598 | 0.585 | 0.651 |
| Heart failure | 0.620 | 0.604 | 0.586 | 0.655 |
| Model including age, sex, obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity | ||||
| Myocardial infarction | 0.738 | 0.717 | 0.686 | 0.771 |
| Angina pectoris | 0.652 | 0.601 | 0.592 | 0.691 |
| Stroke | 0.630 | 0.615 | 0.612 | 0.707 |
| Heart failure | 0.647 | 0.615 | 0.609 | 0.708 |
The discriminative ability of each prediction model, including modifiable risk factors (obesity, hypertension, diabetes, dyslipidemia, cigarette smoking, and physical inactivity) with and without age and sex, was assessed using C statistics (Harrell C statistic). CVD indicates cardiovascular disease.
Sensitivity Analyses
First, we analyzed 3 755 462 participants after incorporating multiple imputations for missing data. During a mean (SD) follow‐up of 1201 (977) days, 8074 MI, 73 053 AP, 35 765 stroke, and 72 452 HF cases were recorded. The age‐stratified associations between risk factors and incident CVD are summarized in Table S3. The age‐stratified relationship between each risk factor was similar, and incident CVD findings were unchanged after multiple imputations for missing data. Second, after excluding 373 289 participants who were receiving blood pressure–lowering medications, glucose‐lowering medications, or lipid‐lowering medications, we analyzed 2 654 550 participants. During a mean (SD) follow‐up of 1134 (942) days, 4329 MI, 41 039 AP, 20 211 stroke, and 39 949 HF events were recorded. Even in this model, the age‐stratified relationship of risk factors with the incidence of CVD was similar (Table S4). Third, in the multivariable model using body mass index, systolic blood pressure, and low‐density lipoprotein cholesterol level instead of obesity, hypertension, and dyslipidemia, respectively, the results were similar in terms of the age‐dependent relationship between risk factors and incident CVD, as shown in Table S5. Fourth, we calculated cause‐specific HR to account for the competing risk of death, as shown in Table S6. The age‐specified association between risk factors and the risk of developing CVD was comparable in this case scenario. Fifth, the age‐dependent relationship between risk factors and incident CVD did not change after adding the year of the initial health checkup (3‐year interval) in the multivariable model (Table S7). Sixth, when we stratified our study participants using the tertile of age, our primary findings did not change (Table S8). Seventh, the age‐dependent relationship was also present in people aged 20 to 60 years (Table S9). Eighth, the age‐dependent association was observed in hypertension when we defined all‐cause mortality as outcome (Table S10).
Discussion
Our findings based on the analyses using a nationwide claims database, enrolling a general population of >3 million adults without a prevalent CVD history, demonstrated age‐specific differences in the relationship between traditional risk factors and incident CVD, including MI, AP, stroke, and HF. Specifically, modifiable risk factors, such as obesity, hypertension, and diabetes, imposed a higher proportion of risk and a greater RRR on younger individuals than on older individuals.
To the best of our knowledge, the present study is the largest population‐based investigation to describe age‐stratified differences in the strengths of relationship between the full spectrum of modifiable risk factors and the risk of each CVD event separately. Our results were in agreement with those of previous studies, with smaller populations that focused on specific risk factor or composite CVD outcomes.
The WHS (Women's Health Study) analyzed 28 024 women and indicated that the associations between risk factors, such as obesity and hypertension, and coronary heart disease were attenuated with increasing age at onset. 11 The analysis of the US National Inpatient Sample demonstrated that modifiable risk factors, such as obesity, hypertension, and diabetes, were common and that they increased over time in young adults (aged 18–59 years) with the first acute MI. 12 In terms of HF, recent analysis of the FHS (Framingham Heart Study), PREVEND (Prevention of Renal and Vascular Endstage Disease) study, and MESA (Multi‐Ethnic Study of Atherosclerosis) demonstrated that hypertension, diabetes, current smoking, and history of MI conferred a greater relative risk for future HF in younger people than in elderly people. The known risk factors explained a higher proportion of population‐attributable risk for HF in younger people (aged <55 years) than in elderly people (aged ≥75 years) (75% versus 53%, respectively). 13 The CALIBER (Cardiovascular Research Using Linked Bespoke Studies and Electronic Health Records) demonstrated that the relative risk of hypertension for 12 CVDs declined with age. The association between both systolic and diastolic blood pressure and incident CVD decreased with age (eg, the HRs of 20‐mm Hg increase in systolic blood pressure for ischemic stroke were 1.57, 1.37, and 1.16 in patients aged 30–59, 60–79, and ≥80 years, respectively). 28 The importance of risk factor management in young adults is suggested by the analyses from the CARDIA (Coronary Artery Risk Development in Young Adults) study. 29 , 30 , 31 For example, early onset of hypertension (aged <35 years) was related to a higher risk of end‐organ damage. 29 Similar age‐dependent association was observed in diabetes as well. 14 , 32 , 33 An analysis of the Swedish NDR (National Diabetes Registry) demonstrated that mortality risk and cardiovascular risk were attenuated with increasing age of diagnosis for type 2 diabetes. 14 An analysis of the National Diabetes Services Scheme in Australia showed that an earlier diagnosis of type 2 diabetes was associated with a higher relative risk for mortality, mainly attributable to CVDs. A 10‐year earlier diagnosis was associated with a 1.2 to 1.3 times higher mortality and ≈1.6 times increased risk of death attributable to CVD. 32 These findings are in line with patients with type 1 diabetes. 33 These studies amplify the support for preventing lifestyle‐related disease onset in younger people. Our findings demonstrating the possible age‐dependent relationship between modifiable risk factors and the subsequent risk of developing CVDs are in agreement with these previous studies.
Several potential explanations could be suggested for our findings. First, the relative risk associated with risk factors (such as obesity, hypertension, and diabetes) may be enhanced in young people because the baseline absolute risk of CVDs is generally lower in younger individuals than that in older individuals. Second, as previous epidemiological data have demonstrated, 34 , 35 the control status of risk factors could be worse in younger people than that in older people; therefore, the higher severity of risk factors might have more pronounced the effects of risk factors on incident CVD in younger participants. However, this is less likely judging from our data that even after excluding participants who were receiving treatment for hypertension, diabetes, or dyslipidemia, the results were similar. Third, although we included established risk factors for CVD in multivariable Cox regression analyses, unmeasured or unknown factors could affect the results. For example, socioeconomic status could be poor in young people with multiple risk factors, which may have contributed to a higher incidence of CVD. Fourth, people with elevated risk factors at a young age could represent a more “severe phenotype of risk” and likely also experience a longer exposure to the cumulative effects of each risk factor, certainly compared with older people with elevated risk factors who may have recently developed these risk factor abnormalities. 36 , 37 , 38 Fifth, the pathological features of CVD in older people may be complicated by more prolonged interaction between risk factors and environment, as well as development of noncardiovascular comorbidities (eg, cancer), compared with younger people. Most important, physiological or pathological aging process itself could contribute to the development of CVD greater in older population than other risk factors.
The strengths of our investigation include a large general population and high retention of study participants. Our data set includes administrative claims records combined with annual health checkup data from employees' insurance programs. Notably, clinical observational records collected from claims data were included in this data set as well, and thus, we could theoretically track an individual's clinical information even if the individual visits multiple medical providers as long as the individual remains under the same insurance coverage.
We acknowledge several limitations in the present study, and most limitations are attributable to the use of this health checkup and administrative claims data set. For example, data on the socioeconomic status of participants, which could influence the results, are not available. The recorded diagnoses in claims databases are considered less well validated compared with prospective registries. Yet, as for the incidence rate of CVD events, our data from the JMDC Claims Database are comparable to other epidemiological data in Japan. 39 Because this database primarily consists of the working‐age population in Japan, we should consider a possibility of selection bias when we interpret the results. In particular, this data set does not include people who were aged >75 years, and thus, we could not evaluate the relationship of risk factors and CVD in the elderly population. Further investigations using other independent data sets (eg, including elderly population or other races and ethnicities) are required to validate our findings. Detection bias could cause overestimation or underestimation of the link between some risk factors and the subsequent risk of developing CVD. For example, CVD could be more likely to be diagnosed in participants with multiple risk factors, such as obesity, hypertension, and diabetes, because these people use medical services more frequently than those with no risk factors, which may influence the possibility of detection of CVD. In this study, the relative association between risk factors, including obesity, hypertension, and diabetes, and the incidence of CVD attenuated with age. However, this age‐dependent relationship of risk factors with the incidence of CVD appeared less pronounced for other risk factors. Indeed, HRs of cigarette smoking and physical inactivity might even be higher in the older age categories than the younger age categories, particularly for HF. Underlying mechanisms of this reversed interaction are needed to be investigated in further studies. Further investigations, including data on the cumulative exposure and trajectory of each risk factor, are necessary to confirm our findings. In addition, the comparison of biomarkers (eg, carotid artery intima‐media thickness) would be important as well. We excluded individuals with a CVD history, and this criterion would lead to a selection bias, particularly in older individuals. Given that the prevalence of people having a CVD history increases with age, healthier elderly individuals could have been included, according to this enrollment criterion. Data on cigarette smoking and physical activity were obtained from self‐reported questionnaires. This subjective manner should be considered a study limitation. Last, our results presented that the relative association of modifiable risk factors with incident CVD was attenuated with age. On the other hand, given the increasing prevalence of these risk factors with age, we need to consider the absolute risk as well. Further investigations and discussions are required to identify the best use of public health/primordial prevention resources from the public health viewpoint.
In conclusion, these large nationwide data show that, despite the lower incidence of CVDs (such as MI, AP, stroke, and HF) in younger compared with older individuals, the contribution of modifiable risk factors (including obesity, hypertension, and diabetes) to the development of CVD is greater in younger compared with older people. Risk factor optimization is essential throughout the life course to lessen the burden of CVD; preventive efforts for risk factor optimization may be of higher value in younger individuals.
Sources of Funding
This research was funded by the Ministry of Health, Labour and Welfare, Japan (21AA2007), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, and 21K08123). The funding sources possessed no role in this project.
Disclosures
Drs Kaneko and Fujiu have received research funding and scholarship funds from Medtronic Japan CO, LTD; Biotronik Japan; SIMPLEX QUANTUM CO, LTD; Boston Scientific Japan CO, LTD; and Fukuda Denshi, Central Tokyo CO, LTD. The other authors have no competing interest to disclose.
Supporting information
Tables S1–S10
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027684
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, et al. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41:12–85. doi: 10.1093/eurheartj/ehz859 [DOI] [PubMed] [Google Scholar]
- 3. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 4. Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056 [DOI] [PubMed] [Google Scholar]
- 5. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995‐2008. Ann Neurol. 2011;70:713–721. doi: 10.1002/ana.22539 [DOI] [PubMed] [Google Scholar]
- 6. Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. doi: 10.1212/WNL.0b013e318270401d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosengren A, Aberg M, Robertson J, Waern M, Schaufelberger M, Kuhn G, Aberg D, Schioler L, Toren K. Body weight in adolescence and long‐term risk of early heart failure in adulthood among men in Sweden. Eur Heart J. 2017;38:1926–1933. doi: 10.1093/eurheartj/ehw221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaneko H, Itoh H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Morita H, Yasunaga H, Komuro I. Association of cardiovascular health metrics with subsequent cardiovascular disease in young adults. J Am Coll Cardiol. 2020;76:2414–2416. doi: 10.1016/j.jacc.2020.09.545 [DOI] [PubMed] [Google Scholar]
- 9. Kaneko H, Itoh H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N, Morita H, et al. Fasting plasma glucose and subsequent cardiovascular disease among young adults: analysis of a nationwide epidemiological database. Atherosclerosis. 2021;319:35–41. doi: 10.1016/j.atherosclerosis.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 10. Kaneko H, Itoh H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N, Morita H, et al. Lipid profile and subsequent cardiovascular disease among young adults aged <50 years. Am J Cardiol. 2021;142:59–65. doi: 10.1016/j.amjcard.2020.11.038 [DOI] [PubMed] [Google Scholar]
- 11. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh‐Ali AA, Ridker PM, Glynn RJ, Mora S. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. 2021;6:437–447. doi: 10.1001/jamacardio.2020.7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yandrapalli S, Nabors C, Goyal A, Aronow WS, Frishman WH. Modifiable risk factors in young adults with first myocardial infarction. J Am Coll Cardiol. 2019;73:573–584. doi: 10.1016/j.jacc.2018.10.084 [DOI] [PubMed] [Google Scholar]
- 13. Tromp J, Paniagua SMA, Lau ES, Allen NB, Blaha MJ, Gansevoort RT, Hillege HL, Lee DE, Levy D, Vasan RS, et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. doi: 10.1136/bmj.n461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sattar N, Rawshani A, Franzen S, Rawshani A, Svensson AM, Rosengren A, McGuire DK, Eliasson B, Gudbjornsdottir S. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139:2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885 [DOI] [PubMed] [Google Scholar]
- 15. Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, Fujiu K, Michihata N, Jo T, Takeda N, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation. 2021;143:2244–2253. doi: 10.1161/CIRCULATIONAHA.120.052624 [DOI] [PubMed] [Google Scholar]
- 16. Ohbe H, Goto T, Miyamoto Y, Yasunaga H. Risk of cardiovascular events after Spouse's ICU admission. Circulation. 2020;142:1691–1693. doi: 10.1161/CIRCULATIONAHA.120.047873 [DOI] [PubMed] [Google Scholar]
- 17. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N, et al. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association blood pressure guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc. 2020;9:e017963. doi: 10.1161/JAHA.120.017963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Itoh H, Kaneko H, Kiriyama H, Yoshida Y, Nakanishi K, Mizuno Y, Daimon M, Morita H, Yatomi Y, Yamamichi N, et al. Effect of metabolically healthy obesity on the development of carotid plaque in the general population: a community‐based cohort study. J Atheroscler Thromb. 2020;27:155–163. doi: 10.5551/jat.48728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suzuki Y, Kaneko H, Okada A, Itoh H, Morita K, Fujiu K, Michihata N, Jo T, Takeda N, Morita H, et al. Change in Cardiovascular Health Metrics and Risk for Proteinuria Development: Analysis of a Nationwide Population‐Based Database. Am J Nephrol. 2022;53(2‐3):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki Y, Kaneko H, Yano Y, Okada A, Itoh H, Matsuoka S, Fujiu K, Yamaguchi S, Michihata N, Jo T, et al. Age‐dependent relationship of hypertension subtypes with incident heart failure. J Am Heart Assoc. 2022;11:e025406. doi: 10.1161/JAHA.121.025406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yano Y, O'Donnell CJ, Kuller L, Kavousi M, Erbel R, Ning H, D'Agostino R, Newman AB, Nasir K, Hofman A, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population‐based studies. JAMA Cardiol. 2017;2:986–994. doi: 10.1001/jamacardio.2017.2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Morita H, et al. Association of body weight gain with subsequent cardiovascular event in non‐obese general population without overt cardiovascular disease. Atherosclerosis. 2020;308:39–44. doi: 10.1016/j.atherosclerosis.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 23. Yagi M, Yasunaga H, Matsui H, Morita K, Fushimi K, Fujimoto M, Koyama T, Fujitani J. Impact of rehabilitation on outcomes in patients with ischemic stroke: a nationwide retrospective cohort study in Japan. Stroke. 2017;48:740–746. doi: 10.1161/STROKEAHA.116.015147 [DOI] [PubMed] [Google Scholar]
- 24. Aloisio KM, Swanson SA, Micali N, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014;14:863–883. doi: 10.1177/1536867X1401400410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410 [DOI] [PubMed] [Google Scholar]
- 26. Morita K, Ono S, Ishimaru M, Matsui H, Naruse T, Yasunaga H. Factors affecting discharge to home of geriatric intermediate care facility residents in Japan. J Am Geriatr Soc. 2018;66:728–734. doi: 10.1111/jgs.15295 [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suvila K, McCabe EL, Lehtonen A, Ebinger JE, Lima JAC, Cheng S, Niiranen TJ. Early onset hypertension is associated with hypertensive end‐organ damage already by midLife. Hypertension. 2019. HYPERTENSIONAHA11913069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nwabuo CC, Appiah D, Moreira HT, Vasconcellos HD, Yano Y, Reis JP, Shah RV, Murthy VL, Allen NB, Sidney S, et al. Long‐term cumulative blood pressure in young adults and incident heart failure, coronary heart disease, stroke, and cardiovascular disease: the CARDIA study. Eur J Prev Cardiol. 2021;28:1445–1451. doi: 10.1177/2047487320915342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huo L, Magliano DJ, Ranciere F, Harding JL, Nanayakkara N, Shaw JE, Carstensen B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997‐2011. Diabetologia. 2018;61:1055–1063. doi: 10.1007/s00125-018-4544-z [DOI] [PubMed] [Google Scholar]
- 33. Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjornsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register‐based cohort study. Lancet. 2018;392:477–486. doi: 10.1016/S0140-6736(18)31506-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daugherty SL, Masoudi FA, Ellis JL, Ho PM, Schmittdiel JA, Tavel HM, Selby JV, O'Connor PJ, Margolis KL, Magid DJ. Age‐dependent gender differences in hypertension management. J Hypertens. 2011;29:1005–1011. doi: 10.1097/HJH.0b013e3283449512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steinarsson AO, Rawshani A, Gudbjornsdottir S, Franzen S, Svensson AM, Sattar N. Short‐term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: a longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia. 2018;61:599–606. doi: 10.1007/s00125-017-4532-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74:330–341. doi: 10.1016/j.jacc.2019.03.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ference BA, Bhatt DL, Catapano AL, Packard CJ, Graham I, Kaptoge S, Ference TB, Guo Q, Laufs U, Ruff CT, et al. Association of genetic variants related to combined exposure to lower low‐density lipoproteins and lower systolic blood pressure with lifetime risk of cardiovascular disease. JAMA. 2019;322:1381–1391. doi: 10.1001/jama.2019.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Domanski MJ, Tian X, Wu CO, Reis JP, Dey AK, Gu Y, Zhao L, Bae S, Liu K, Hasan AA, et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. doi: 10.1016/j.jacc.2020.07.059 [DOI] [PubMed] [Google Scholar]
- 39. Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S. Association between mortality and incidence rates of coronary heart disease and stroke: the Japan public health center‐based prospective (JPHC) study. Int J Cardiol. 2016;222:281–286. doi: 10.1016/j.ijcard.2016.07.222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S10
Data Availability Statement
The JMDC Claims Database is accessible under a contract from JMDC Inc (Tokyo, Japan; https://www.jmdc.co.jp/en/index).


