Abstract
Protein engineering has contributed to successes in the field of T cell-based immunotherapy, including chimeric antigen receptor (CAR) T cell therapy. CAR T cell therapy has become a pillar of cancer immunotherapy, demonstrating clinical effectiveness against B cell malignancies by targeting the B cell antigen CD19. Current gene editing techniques have limited safety controls over CAR T cell activity, which presents a hurdle for control of CAR T cells in patients. Alternatively, CAR T cell activity can be controlled by engineering CARs to bind soluble adapter molecules that direct the interaction between the CAR T cell and target cell. The flexibility in this adapter-mediated approach overcomes the rigid specificity of traditional CAR T cells to allow targeting of multiple cell types. Here we describe adapter CAR T technologies and how these methods emphasize the growing role of protein engineering in the design of programmable tools for T cell therapies.
Keywords: chimeric antigen receptors, cancer immunotherapy, protein engineering, synthetic biology, adoptive cell therapy
1. Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a genetically engineered cellular immunotherapy that directs cytotoxicity to cancer cells. CAR T cells that are engineered to recognize the B cell lineage antigen CD19 are remarkably effective treatment for patients with relapsed/refractory B-cell leukemias and a range of lymphomas, leading to the FDA approval of Tisagenlecleucel (Kymriah), Axicabtagene Ciloleucel (Yescarta), and Brexucabtagene autoleucel (Tecartus) CAR T cell therapies in the U.S. in 2017 and 2020 respectively [1,2]. The clinical success of CD19-directed CAR T cells is large part because CD19 is a pan-B cell marker expressed by B cells throughout development and it is not found on other cell types [3]. Importantly, cancer patients whose B cells are depleted by anti-CD19 CAR T cell therapy can receive IV-administered immunoglobulin replacement therapy, an effective protection from foreign pathogens until B cell count is restored to normal levels [4]. Despite high rates of response (70–90% in B-cell acute lymphoblastic leukemia (B-ALL), ~63–100% in lymphomas, and 50–70% for chronic lymphocytic leukemia (CLL)) against refractory blood cancers, CD19 CAR T cells are commonly associated with cytokine release syndrome (CRS) and a unique CAR T cell-related encephalopathy syndrome (CRES). Several strategies have been developed to minimize these treatment-mediated toxicities including inducible suicide genes for apoptosis of CAR T cells and transient mRNA transfection of CAR T genes [5–7]. The incidence of CRES neurotoxicity may depend on hematologic disease and CAR design but is not well understood. Moreover, loss of CD19 expression is a prominent mechanism of resistance in CLL and accounts for a significant portion of relapse cases in lymphomas [8]. Finally, translating CAR T therapies to target the bulk of cancers (solid tumors) has been met with sub-optimal results due to limited efficacy, safety and control [9,10]. For CAR T cell therapy to overcome these hurdles, innovative engineering strategies to regulate and instruct CAR T cells continue to evolve rapidly. In this review, we summarize the ongoing efforts of researchers to induce CAR T cell activity using a novel class of modular engineered CAR T cells that render tumor cell killing dependent on co-administration of an adapter molecule, providing more versatility and control of therapeutic CAR T cell activity.
1.1. Anatomy of Traditional Chimeric Antigen Receptors (CARs)
Traditional engineered CARs consist of three primary components: an extracellular single-chain variable fragment (scFv) recognition domain, a transmembrane domain, and one or more intracellular signaling domain [11,12]. First-generation CARs used the CD3ξ signaling domain with three signaling immunoreceptor tyrosine-based activation motifs (ITAMs) and no costimulatory signaling domains (Fig. 1.A). These CAR constructs only exhibited low levels of cytotoxicity and proliferation after antigen exposure [13]. Second- and third-generation CARs contain one or two additional intracellular costimulatory signaling domains (usually CD28, 4–1BB or OX40) for improved expansion, persistence, and anti-tumor activity [14]. The FDA-approved CAR T cell therapies in the US possess second-generation intracellular CAR architecture.
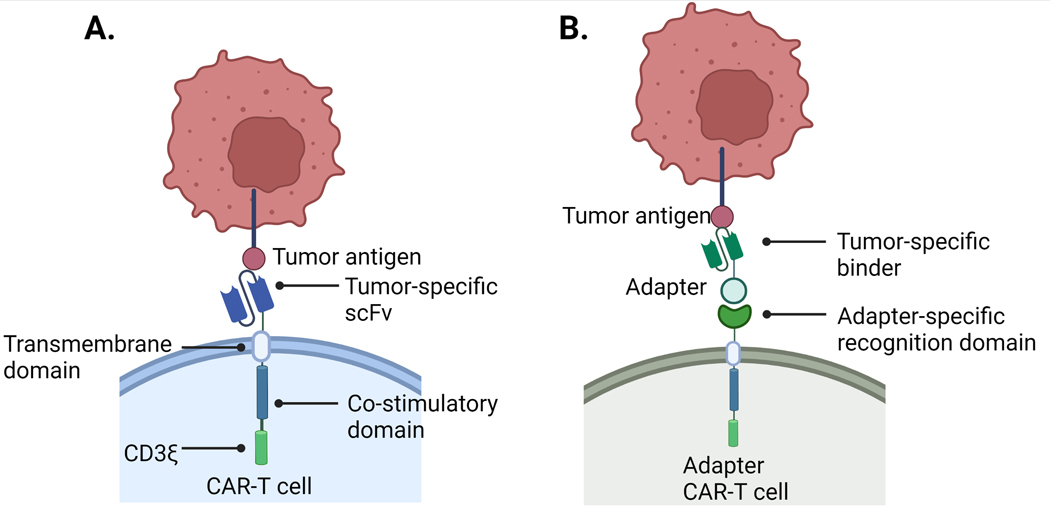
Fig 1. Differences in Conventional CAR and Adapter CAR T Cell Components.
A) Conventional CARs directly bind tumor antigens with a tumor-specific scFv recognition domain. Second-generation conventional CARs contain both a CD3ζ and a costimulatory domain. B) Adapter CARs bind to and direct adapter molecules to recognize tumor antigen. The adapter molecule is required for T cell cytotoxicity.
The recognition domain in a traditional CAR is an antigen-specific scFv that mediates direct binding to cancer cells. CAR T cell activation is dependent on the interaction between cancer antigen and CAR scFv and is independent of the canonical T cell receptor (TCR) activation signaling instigated by major histocompatibility complex presentation (pMHC) [15]. An extracellular hinge region proximal to the T cell membrane connects the recognition and transmembrane domains and often is a CD8-, CD28- or IgG-derived sequence that contains cysteines to induce the CARs to dimerize via disulfide formation. Dimerization results in a higher local concentration of intracellular ITAMs (six ITAMs per dimer), leading to docking of signaling proteins that lead to full T cell activation [16]. The native TCR/CD3 signaling complex contains ten ITAMs – CD3δ, CD3ε (two per complex), and CD3γ contain a single ITAM and CD3ξ has six. The transmembrane domain consists of a hydrophobic alpha helix that spans the T cell plasma membrane and is critical for receptor expression and stability [11,17]. Finally, the intracellular signaling domain(s) are commonly comprised of a CD3ξ subunit containing three ITAMs and one or more costimulatory subunit(s), usually CD28, 4–1BB, OX40 or a combination of these. Different costimulatory molecules have varying implications on CAR T cell persistence, expansion and cytokine production [18]. Upon scFv binding to antigen, a signal is transmitted through the membrane resulting in conformational changes of the intracellular signaling domains that result in potent signaling and in many cases, sustained signaling and persistence of T cells [13,19].
The traditional CAR design is a successful therapy for patients with refractory and relapsed B cell malignancies, specifically with the use of CD19-directed CAR T cells. However, CD19 is a unique cancer target in the sense that on-target cytotoxicity can be tolerated by the body and the damage to healthy B cells is clinically manageable. This is contrary to most cancer targets that are heterogeneously expressed on cancerous as well as vital tissues that cannot tolerate CAR T-cell related cytotoxicity. Treating other types of cancers with CAR T cells will require more sophisticated control to promote both efficacy and safety.
2.0. Overview of Adapter-Binding CARs and Tumor-Targeting Adapter Molecules
As a living drug, once canonical CAR T cells are transfused into patients there are limited approaches for controlling tissue specificity, expansion, activation or persistence [14]. Adapter CAR T platforms hold promise as an approach for addressing some of these limitations. Adapter CARs differ from traditional CARs by replacing the cancer-specific scFv extracellular domain (ECD) with an inert adapter-specific recognition domain that binds to a secondary adapter molecule directed to the cancer-specific target (Fig. 1.B). The adapter molecule is required for CAR T cytotoxicity enabling temporal control over T cell activation, expansion and persistence.
Protein engineering is a critical component for adapter CAR T platform design and can directly impact clinical outcomes. In the following sections, we highlight recent adapter CAR T approaches that have been developed to finely tune T cell activation, control target specificity and direct cytotoxicity towards multiple antigens without the need to reengineer T cells [20].
2.1. Biotin-Binding CARs and Biotinylated Adapter Antibodies
Biotin-binding immune receptor (BBIR) CAR T cells use an avidin ECD linked to intracellular T cell signaling domains to redirect T cell cytotoxicity using cancer-targeted biotinylated antibody adapter molecules [21]. BBIR CARs can recognize cancer cells pre-labeled with specific biotinylated adapters (Fig 2.A). In the absence of biotinylated adapter, BBIR CAR T cells are inert and have no tumor-specific reactivity. Furthermore, the avidin ECD serves as a modular scaffold to allow for a multitude of biotinylated antibodies to direct tumor-targeted T cell activity [21]. This strategy employs the versatility of the well-characterized biotin/streptavidin protein interaction that has been used for many biotechnology applications [22,23].
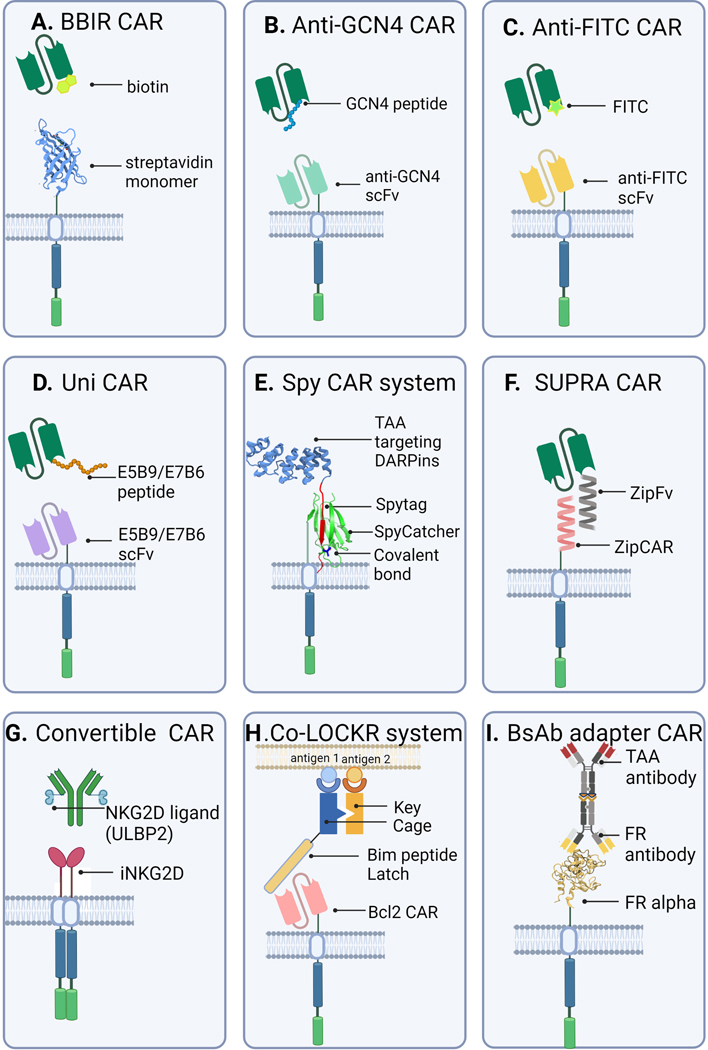
Fig 2. Overview of Adapter CAR T Platforms.
A) Biotin-binding immune receptor (BBIR) CAR; B) Anti-GCN4 CAR; C) Anti-FITC CAR; D) UniCAR; E) SpyCatcher CAR; F) SUPRA CAR; G) ConvertibleCAR; H) Co-LOCKR CAR; and I) bsAb-mediated CAR.
BBIR CARs expressing a single avidin monomer ECD (mc.Av.BBIR) did not bind biotinylated adapter antibodies, instead, binding required a second avidin ECD per BBIR CAR (dc.Av.BBIR). The dimeric avidin CAR T cells (dc.Av.BBIR) release the antitumor cytokine interferon gamma (IFNγ) in an adapter dose-dependent fashion upon encounter with immobilized biotinylated molecules or when cultured with cancer cells that are preincubated with biotinylated antibodies. However, dc.Av.BBIR CARs are unable to stably preload biotinylated adapter antibodies suggesting the affinity (and avidity) of interaction against immobilized biotin allows specific immune recognition that does not occur with biotinylated adapter antibodies in solution. This suggests that sequential antigen targeting is a possible strategy for dc.Av.BBIR CAR-directed cytotoxicity.
Reduction of an A1847 human ovarian cancer subcutaneous tumor was observed with administration of BBIR CARs followed by biotinylated anti-EPCAM antibodies. Physiologic levels or significantly higher biotin concentrations do not activate BBIR CARs nor competitively inhibit biotinylated antibody adapter molecules. This is important because biotin is a B vitamin present endogenously in humans [24]. A xenograft model of A1847 cells in immunodeficient mice showed delayed tumor growth in mice treated with antigen-specific biotinylated antibodies over control non-biotinylated antibodies.
A compact (122 amino acids) monomeric, high-affinity streptavidin monomer mSA2 (Kd biotin = 5.5 × 10−9 M) CAR design has comparable affinity for biotin as dimeric BBIR CARs [25,26]. The mSA2-CAR-T cells are efficiently activated by plate-immobilized biotin and can mediate tumor-specific T cell effector functions after incubation with biotinylated Rituximab (anti-CD20), FMC63 (anti-CD19), or Cetuximab (anti-EGFR) antibodies. Both BBIR-CARs and mSA2-CARs have shown promise but the relative merits of either monomeric streptavidin or dimeric streptavidin CARs are not well established.
Various approaches have been developed for biotinylating proteins, some are site-specific and some are not site-specific [27]. Site-specific biotinylation is advantageous because it allows precise control of biotin placement and valency within a protein while biotinylation using N-hydroxysuccinimide (NHS) chemistry is rapid and low-cost, but not site-specific, meaning a biotin molecule could covalently modify a macromolecule in a functionally important location. The experiments described above direct BBIR-CARs and mSA2-CARs using non-site-specific NHS chemical modification that couples a NHS-activated biotin to primary amines such as lysine side chains or the N-terminus of the antibody. The number of biotin molecules per adapter can also vary using NHS chemistry, an important consideration for CAR signaling that is impacted by changes in avidity. The CAR immune synapse is also sensitive to spatial organization and distance constraints [10]. An adapter molecule will distance the immune synapse formed between a CAR T cell and target cell, therefore, the location of a biotin conjugation site may be functionally relevant. The limitations to this approach are possible immunogenicity of biotinylated adapters and streptavidin CAR ECDs and interference with specificity from endogenous biotinylated proteins [28].
2.2. Anti-Tag CARs and Tumor-Targeting Tag-Adapter Antibodies
2.2.1. sCAR-T (anti-GCN4) System
The switchable CAR (sCAR-T) T cell has a high affinity anti-GCN4 scFv ECD (clone 52SR4) fused to a second generation CAR hinge, transmembrane and intracellular domain [29]. The sCAR-T ECD antigen is a 14-residue linear peptide from the yeast transcription factor GCN4 (Fig 2.B). The sCAR-T design takes advantage of a synthetically evolved high-affinity scFv-peptide interaction to create a highly specific switchable CAR system [30,31]. Importantly, the peptide antigen sequence from GCN4 does not occur in the human proteome and has a low probability of inducing an antibody response, as determined via an in silico immunogenicity analysis [29]. Therefore, the GCN4 peptide and high-affinity scFv binder are most likely at a lower risk for cross-reactivity when incorporated into a therapeutic CAR-T and antibody adapter design.
GCN4 peptide tags were inserted into two therapeutic adapter antibodies FMC63 (anti-CD19) and Ofatumumab (OFA) (anti-CD20). The short peptide tag can be introduced into tumor-targeting antibody fabs at designated sites both mono- and bivalently and either proximal to the antigen-binding site at the N-terminus or distal at the C-terminus. Peptide incorporation had minimal impact on protein stability and antigen-binding, as determined with thermal melt assays and flow cytometry-based binding to CD19+ RS4;11- and CD20+ Raji cells. Insertion site location and valency does impact cytokine production. Notably, the bivalent FMC63 (anti-CD19) antibody adapter with two N-terminal GCN4 peptides induced the greatest interleukin 2 (IL-2) cytokine production against RS4;11 cells. This trend is consistent with accompanying studies of bivalent FITC-grafted adapter antibodies/anti-FITC CAR-T cells that outperform monovalent adapter antibodies (as described in section 2.2.2. below) [32]. These results suggest that increased cytotoxicity of bivalent adapter antibodies results from avidity effects mediated by the adapter for the anti-GCN4/anti-FITC CARs. Furthermore, in situ dimerization of a mutant IgG4 hinge (IgG4m, S228P) improved CAR-T cell activity through enhanced interchain disulfide formation [33,34].
FMC63 (anti-CD19) adapter antibodies with bivalent N-terminal GCN4 peptide fusions exhibited superior cytokine release to bivalent C-terminal peptide fusions, highlighting the importance of optimizing immunological synapse (IS) geometry for sCAR T-cell activity [16]. Interestingly, this result was opposite for OFA (anti-CD20) adapter antibodies such that C-terminal GCN4 peptide fusions allowed for enhanced sCAR T-cell activity when connected to shortened 12 residue IgG4m hinge sCAR hinge. These results underscore the multiple facets – CAR structure, adapter configuration, and the target antigen proximity to membrane – that all spatially influence efficient synapse formation and desired targeted sCAR T cytotoxicity.
sCAR-T cells with FMC63 (anti-CD19) adapter fab with one N-terminal GCN4 peptide tag fused to the fab light chain (LCNT) eliminated Nalm-6 tumor cells in a xenograft model with comparable efficacy to conventional CART-19 T cells. CAR T cytotoxicity, expansion and trafficking was observed only in the presence of GCN4-tagged FMC63 anti-CD19 adapter fab with controlled dosing. Serum levels of inflammatory cytokines IL-2, TNF-α and TNF-γ were tightly regulated by levels of FMC63 adapter fab postulating low amounts (0.05 mg/kg) of adapter Fab as a possible approach to eliminate tumor cells while maintaining tolerable levels of cytokine release [29]. A limitation of anti-GCN4 sCAR T cells is the use of possible immunogenic peptides (GCN4) and activators (FMC63).
2.2.2. Anti-FITC sCAR System
Anti-FITC CARs have an anti-FITC scFv ECD that permits FITC-labeled tumor-specific adapter antibodies, specifically cetuximab (anti-EGFR), trastuzumab (anti-Her2) and rituximab (anti-CD20) to redirect CAR T cell cytotoxicity (Fig 2.C) [28,34]. Fluorescein isothiocyanate (FITC) is a widely-used fluorescent dye in biological research that can be easily and cheaply conjugated to antibodies and other proteins through reactive primary amines on solvent-accessible side chains and has been used in the human body [37,38]. The anti-FITC CAR specifically recognizes proteins tagged with FITC molecules. FITC-labeled antibodies were generated using non-specific N-hydroxysuccinimide (NHS) chemistry (see section 2.1) to generate an average of three FITC molecules per antibody. Anti-FITC CAR T cells undergo expansion and cytokine release in the presence of immobilized FITC dye, and elimination of tumor cells occurs via FITC-labeled antibody-dependent interactions in vitro. In human tumor models with immunodeficient and immunocompetent mice, anti-FITC CAR T cells together with FITC-labeled antitumor antibodies prevented tumor growth and reduced tumor size [35].
Further optimization of the anti-FITC CAR used site-specific FITC conjugation to FMC63 (anti-CD19) and M971(anti-CD22) adapter fabs and showed target cell killing both in vitro and in vivo [32]. Site-specific conjugation was accomplished with genetic integration of noncanonical amino acids (para-azidophenylalanine, pAzF) with bio-orthogonal chemical reactivity at defined positions within the antibody. FITC was then attached in a chemical click reaction at neutral pH 7.4. Both monovalent and bivalent FITC conjugates were made. The molecular weight and purity of FITC-conjugated FMC63, but not M971, were confirmed by SDS-PAGE and mass spectrometry with a conjugation efficiency greater than 95%. These results contrast with FITC conjugation using NHS chemistry that revealed ~58% of the FMC63 conjugates were modified at the heavy chain N-terminal amino group and other modifications occurred at lysine residues within the light chain, including antibody complementarity-determining region CDR1. Modification at these positions with a FITC molecule could detrimentally reduce the affinity of FMC63 or M971 for their target cancer antigens. NHS conjugation was shown to slightly reduce CD19 fab binding affinity to CD19+ target cells (Nalm-6) (EC50 = 4.0 nM) in comparison with the bivalent site-specific FITC antibodies (EC50 = 3.0 nM) while site-specific monovalent FITC antibodies had lower affinity (EC50 = 6–9 nM). The optimal anti-CD19 FITC bivalent adapter antibody is ~20 times more efficacious on Nalm-6 target cells than the optimized anti-CD22 FITC bivalent equivalent, a result of lower CD22 expression on Nalm-6 cells. Importantly, in vivo activity of bivalent and monovalent anti-CD19 adapter antibodies correlate with in vitro data but have more pronounced differences – monovalent adapters showed tumor relapse at day 47 and random/distally-labeled conjugated adapters delayed tumor growth but did not clear tumors. These results reaffirm the importance that conjugation site has on immune synapse geometry and cytotoxicity [39].
Anti-FITC CAR T cells can be directed to multiple targets using different FITC-labeled antitumor antibodies. This offers a potential solution to re-arm CAR T cells when antigen escape occurs [40]. Terminating antibody adapter dosing or injecting FITC-labeled isotype IgG antibodies that competitively block effector T cell function could prevent severe CRS and long-term B-cell depletion by dampening anti-FITC CAR T cell activity. While feasible, this approach is limited therapeutically because FITC is immunogenic, and competitive inhibition requires excess FITC to quench the reactivity of anti-FITC CAR T cells [41].
2.2.3. UniCARs
The adapter-based CAR-T platform UniCAR has two components - an inert peptide-specific scFv (5B9) CAR ECD and a peptide-tagged targeting molecule (TM) adapter that binds to antigens on the cancer cell surface [42]. Neither component individually elicits T cell cytotoxicity, instead, together they form a functional bispecific immune complex mediated by the scFv (5B9) binding its peptide antigen E5B9 (Fig 2.D). UniCAR is built from the framework of a bispecific T cell engager (BiTE) known as UniMAB [43–46]. Briefly, UniMAB is a split BiTE molecule with two components – an effector molecule (EM) and a targeting molecule (TM). The UniMAB system was translated to CAR T cells (UniCAR) using a 5B9 antibody CAR ECD that binds existing antibody TMs optimized from the UniMAB system [47]. UniMAB and other split protein systems may be advantageous therapeutically by enabling better temporal control of T cell cytotoxicity.
UniCARs direct tumor cell killing through 5B9 ECD recognition of E5B9-tagged therapeutic antibody TMs [48]. E5B9 is a 10-residue peptide derived from the human nuclear autoantigen La/SS-B and part of a cryptic epitope that is present in the primary sequence of La/SS-B but importantly is inaccessible physiologically on the surface of human tissue reducing the issue of cross-reactivity [43]. This epitope has been extensively screened and shown to be non-immunogenic at both T and B cell levels [49,50]. Antibody 5B9 can react with synthetic peptides and peptide-tagged fusion proteins but 5B9 binding is not accessible to endogenous human La protein. Consequently, UniCAR T cells are inactive until cross-linked to tumor cells by TMs tagged with the E5B9 peptide. T cell cytotoxicity can be controlled by infusion of antibody TM [51]. A variety of different antibody TMs have demonstrated proof-of-concept retargeting of UniCARs in vitro and in mice to many targets including CD19, CD33, CD123, PSCA, PSMA, GD2, EFGR, MUC1, STn, BMCA and others [47,52–57]. Furthermore, TMs were constructed from human scFvs, murine scFvs and camelid nanobodies with the E5B9 peptide fused to the C-terminus of the scFv or nanobody. The serum half-life and valency of these different TMs were also explored. Bivalent TMs were created by fusing scFvs or nanobodies together using an E5B9 peptide-containing linker. Affibodies, soluble TCRs and even small molecules fused to E5B9 peptide can be used as TMs [51].
TMs that have different pharmacokinetics could be useful for optimizing dosing regimens to prevent CAR T cell-related toxicity. A short half-life TM constant infusion is initially advantageous because the small size of these TMs allows rapid elimination if severe side effects occur. Once tumor burden is reduced, a transition to extended half-life TMs can be used as the risk of CRS is low [58]. Furthermore, simultaneous or subsequent administration of different TMs can reduce the risk of tumor-escape variants [54]. Results are anticipated in 2023 for clinical trials NCT04230265 and NCT04633148 in Germany that enrolled patients using autologous UniCAR T cells (UNICAR02-t) in combination with anti-CD123 or anti-PMSA TMs for the treatment of acute myeloid leukemia (AML) and late-stage solid tumors that are PMSA+, respectively. Final data collection for these trials will be November 2022. Clinical results will reveal if UniCARs are superior to traditional CAR T cell therapy. See table 1 in section 4 below for more details.
Table 1.
In vivo adapter dosing schedule for adapter-mediated CAR T platform tumor killing.
| Ref | CAR/Adapter | Cell line | CAR | Cell # Infused | Adapter | Dose | Timing |
|---|---|---|---|---|---|---|---|
| [21] | Biotin receptor/Streptavidin | A1847 ovarian cancer cells | BBIR CAR | 6 × 106 | Bio-EpCAM Ab | 100 ng | On days 45, 56, 60 |
| [31] | Anti-FITC scFv/FITC | Nalm-6 B-ALL cancer cells | Anti-FITC sCAR | 40 × 106 | Bivalent FITC anti-CD19 fab switch |
0.5 mg/kg | Every other day, six doses total |
| [112] | Anti-GCN4 scFv/GCN4 peptide | Nalm-6 B-ALL cancer cells | Anti-GCN4 sCAR | 40 × 106 | Bivalent GCN4 anti-CD19 fab switch |
0.5 mg/kg | Every day for 10 days |
| [42] | 5B9 scFv/5B9 peptide | A431 EGFR+ cells | UniCAR | 5 × 106 | Anti-EGFR scFv | 100 ug | Single dose |
| [16] | SpyCatcher/SpyTag | SKOV ovarian cancer cells | SpyCatcher CAR (preloaded with 1 uM Herceptin-ST) | 12.5 × 106 | Herceptin-ST | 25 ug | Every 3 days for 40 days |
| [82] | SUPRA CAR | SK-BR-3 Her2+ breast cancer cells | zipCAR | 10 × 106 | Anti-Her2 zipFv | 5 mg/kg | Every 2 days for 2 weeks |
| [82] | SUPRA CAR | Her2+ Jurkat T cells | zipCAR | 10 × 106 | Anti-Her2 zipFv | 3 mg/kg | Every day for 6 days |
| [89] | ConvertibleCAR | Raji CD19+ cancer cells | iNKGD2 CAR | 35 × 106 | Rit-S3 | 60 ug | Single dose |
2.2.4. RevCARs
RevCAR T cells are based on the same 5B9/E5B9 peptide interaction as the UniCAR platform (section 2.2.3) but in the reverse orientation [59]. Therefore, the RevCAR ECD is a linear peptide (E5B9 or E7B6) fused to second-generation CAR hinge transmembrane and intracellular domains. Redirection of RevCARs against tumor targets require RevTMs (reversed targeting molecules) which are bispecific antibodies in the bispecific T cell engager (BiTE) format. RevTMs bind the RevCAR ECD linear peptide with one arm and to tumor antigens with the other. The RevCAR system redirects T cell cytotoxicity to multiple tumor targets (PSCA or PSMA) with no killing induced by RevCAR-T cells alone or in the presence of decoy RevTMs targeting a non-matching peptide epitope. A side-by-side comparison of the cytotoxic potential to kill tumor cells in vitro revealed that redirected UniCAR- and RevCAR-T cells have comparable maximum tumor cell lysis [60,61].
Splitting the CAR activation input into two signals, both of which are required for full CAR T cell activation (AND gate strategy) is a targeted approach that can reduce reactivity and increase tumor specificity [62–65]. This split CAR signaling has previously been accomplished using signaling and costimulatory receptors that are separately encoded (two separate CAR receptors per T cell) [66]. The RevCAR ECD is a small peptide and thus is capable of being genetically encoded into a single bicistronic vector that includes both the primary activation and secondary costimulatory signals. This new AND gate targeting strategy is called Dual-RevCAR T cells. Dual-RevCAR T cells can kill tumor cells in the presence of both signaling- and costimulatory-RevTMs. Importantly, significant cytokine release is triggered with both signals but not either alone [60].
In summary, the RevCAR platform expands on the UniCAR platform and allows more nuanced T cell control. RevCARs are fully humanized and optimized to minimize the risk of cross-reactivity and immunogenicity. RevTM adapters initiate efficient killing of tumor cells in an epitope-specific manner and can easily be re-engineered to recognize a diverse range of antigens with different affinities.
2.2.5. Anti-CARs
The anti-CAR platform works as an emergency cellular switch for elimination of CAR T cells that become life-threatening. The approach is unique because the anti-CAR T cells eliminate other cancer-directed CAR T cells. This contrasts with the approach of eliminating CAR T cells using inducible suicide genes [6,67–69] or antibody-triggered destruction receptors as safety switches [70–73].
An 18-residue peptide from La/SS-B was incorporated as an elimination tag into the extracellular spacer region of the anti-cancer CAR [74]. The direct insertion of the elimination tag into the CAR receptor differs from the destruction receptor that is separate entirely from the CAR and potentially susceptible to escape mutations. Correspondingly, anti-CAR T cells were designed to recognize and eliminate cells containing the elimination tag. Flow cytometry cytotoxicity assays showed that anti-CAR T cells could specifically eliminate tagged CAR T cells while sparing T cells without the tag. High expression levels of the elimination tag are more prone to destruction by anti-CAR T cells than cells with low-level tag expression, as shown by EGFR destruction receptor surface-expression levels and cetuximab- (anti-EGFR) triggered ADCC [71,75]. Soluble drugs and antibody destruction inducers have short half-lives and limited tissue penetration, so anti-CARs may be more comprehensive and specific to eliminate CAR-T cells in case of emergency.
2.2.6. SpyCatcher/SpyTag CARs
The SpyCatcher CAR uses covalent loading of a SpyTagged peptide-modified ligand for redirection of T cells (Fig 2.E) [76]. This technology uses a truncated split protein-peptide from the Streptococcus pyogenes fibronectin-binding protein FbaB to create a rapidly-formed covalent amide bond between proteins tagged with the truncated split protein-peptide domains, known as SpyCatcher and SpyTag respectively [77]. This covalent attachment forms a native isopeptide bond between a Lys and Asp residue. The SpyCatcher and SpyTag split protein technology has been used for many biotechnological applications [77,78].
Therapeutic antibodies trastuzumab (anti-Her2), rituximab (anti-CD20) and cetuximab (anti-EGFR) were modified covalently with the SpyTag peptide at the antibody CH2/CH3 interface of each IgG heavy chain (2:1 SpyTag:antibody) using a light-activated site-specific conjugation method (LASIC) [79]. Additionally, designed ankyrin repeat proteins (DARPins) targeting Her2, EGFR and EpCAM were site-specifically bioconjugated with a C-terminal SpyTag using sortase-tag expressed protein ligation (STEPL) [80]. SpyCatcher CAR constructs contain second-generation components and can covalently load both IgGs and DARPins and demonstrate T cell activation and proliferation upon antigen encounter in vitro. In the absence of tagged ligand, SpyCatcher T cells do not elicit cytotoxicity. Cytotoxicity is titratable based on the amount of SpyTagged-ligand and is highest when target antigen density is high. SpyCatcher CARs can also target multiple ligands simultaneous in vitro. In murine tumor models, control of subcutaneous tumor growth and clearance of intraperitoneal disease was observed after treatment with SpyCatcher CAR T cells loaded with SpyTag target ligands [76].
The covalent linkage between the SpyCatcher CAR and SpyTag targeting antibody adapter ligand is important for potent T cell anti-cancer activity. A mutant SpyTag antibody adapter that transiently interacts with the SpyCatcher CAR (KD = 200 nM) lacks T cell lytic activity. One limitation of covalent linkage of SpyCatcher CARs is that once armed with a SpyTagged target ligand, its target specificity is determined until the receptor is internalized. SpyCatcher CARs can be successfully reloaded with more SpyTag target ligand, but as more CARs are internalized the level of receptors on the cell surface is reduced leading to less potent activity [81]. Another concern is that both SpyCatcher/SpyTag and DARPins are of bacterial origin and potentially immunogenic.
2.3. SUPRA CARs
The split, universal, and programmable (SUPRA) CAR combines multiple engineered tunable elements to target the activity of CAR T cells using a leucine zipper receptor (zipCAR) and a tumor-targeting adapter scFv (zipFv) fragment fused to a cognate leucine zipper (Fig 2.F) [82]. Leucine zipper coiled-coil pairs can participate in specific interactions and thus can be powerful tools for integrating distinct molecular systems [83–85]. Extensive biophysical characterization of SYNZIP synthetic coiled-coil leucine zipper pairs [83,84] enabled the integration of both competing and orthogonal leucine zipper pairs into the zipCAR and zipFv designs to create switchable and logic-gated control of CAR T cell activity [86].
SUPRA CARs release IFN-γ and kill tumor cells in vitro and in vivo in an adapter-dependent zipFv manner that correspond with leucine zipper pair affinity. As an additional safety measure to prevent CRS, zipCAR activation can be reduced through competitive binding of an inhibitory zipFv that sequesters activating zipFv preventing binding to the zipCAR [86]. This competitive approach requires 4-fold higher inhibitory zipFv to dampen T cell activation. ZipCARs and zipFv pairs demonstrate the first integration of leucine zipper coiled-coil pairs into CAR T design. Conventional scFv CAR ECDs are prone to aggregation that can lead to T cell exhaustion but leucine zipper ECDs may be an alternative to mitigate this issue [87]. By exchanging zipper components of varying affinity and zipFv doses, cytokine release and cytotoxicity of SUPRA CARs can be modulated.
Tumor antigen escape can be addressed without further genetic manipulation of the SUPRA CAR system. Co-culturing zipCAR T cells with different zipFv combinations (anti-Axl zipFv, anti-Her2 zipFv, or both) showed effective killing towards either of the two antigens on the cell surface (OR gate strategy) [86]. This OR gate logic expands targeting capabilities to multiple antigens, illustrating the potential for SUPRA CAR to prevent cancer evasion by antigen loss.
Solid and blood tumor models targeting Her2 antigen established the potential of SUPRA CAR technology to decrease tumor burden to the same extent as conventional CARs [86]. With the SUPRA CAR system established, in vivo control of zipCAR T cells cytokine secretion was explored in a zipFv dose-dependent manner. Notably, secretion of IFN-γ increases in a dose-dependent manner with zipFv concentration. IFN-γ levels can further be modulated using zipFvs with different leucine zipper (SYN3, SYN5, and EE) affinities for the zipCAR. The in vivo IFN-γ release correlated with the affinity between zipper pairs. Moreover, in vivo experiments that added a competitive zipFv after the anti-Her2 zipFv injection demonstrated that cytokine levels can be reduced by blocking the adapter/zipCAR interaction. Cytokine levels in vivo are tunable by changing leucine zipper components of the SUPRA CAR design [82].
The number of characterized mutually exclusive SYNZIP leucine zipper pairs make it possible to combinatorially control distinct signaling pathways with multiple zipper pairs in the SUPRA CAR system [84]. Two zipCAR/zipFv pairs were incorporated into a split CAR to make an AND gate where a FOS zipCAR (binds to anti-Her2-SYN9 zipFv) contains the activation signal domain (CD3ζ) and a RR zipCAR (binds to anti-Axl-EE zipFv) contains the co-stimulatory domains (CD28 and 4–1BB). This split CAR requires binding of both Her2 and Axl on the same cell for a functional T cell activation signal. Increasing cytokine levels (IFN-γ, IL-2 and IL-4) that are downstream of CD28 and 4–1BB signaling with increasing anti-Her2-SYN9 zipFv concentration (CD3ζ increased stimulation) demonstrate AND gated antigen-sensing of SUPRA CARs [82].
The orthogonal SUPRA CAR system can also be used to control distinct CD4+ and CD8+ T cell subtypes. By engineering RR zipCAR into CD4+ T cells and FOS zipCAR into CD8+ T cells, each cell type can be triggered independently in response to the addition of anti-Axl-EE or anti-Her2-SYN9 zipFv, respectively. When both zipFvs were added simultaneously, an additive, but not synergistic level of cytokine secretion was observed [86]. The split design SUPRA Car system has been extensively characterized to create a collection of synthetic immune cells [88].
2.4. convertibleCARs
The ConvertibleCAR platform uses an exclusive receptor-ligand pair interaction engineered to interact solely with one another and not with endogenous human proteins to control CAR T cell tumor-specific cytotoxicity and IFN-γ secretion (Fig 2.G) [89]. The engineered interaction is between an inert human NKG2D-based CAR receptor (iNKG2D) and tumor-directed adapter ligands based on the NKG2D ligands (ULBP1 through ULBP6, MICA, and MICB). By design, both components of the ConvertibleCAR system – the NKG2D-based CAR receptor and ULBP2 ligand – are functionally inert on their own.
Human NKG2D is a homodimer membrane protein found on natural killer (NK) cells, some myeloid cells, and select T cells [89]. This receptor can bind a variety of ligands which are expressed on the cell surface in response to various cellular stresses. Two critical tyrosine residues on each NKG2D monomer drive receptor-ligand interactions. To create an exclusive mutant NKG2D receptor, point mutations at these residue locations were explored. The single Y152A mutant (iNKG2D.YA) and Y152A/Y199F double mutant (iNKG2D.AF) were confirmed by biolayer interferometry (BLI) and enzyme-linked immunosorbent assay (ELISA) to have lost all binding to natural NKG2D ligands and selected for expression as a CAR. The ULBP2 ligand was chosen to be engineered, creating a mutually exclusive ligand for iNKG2D receptors. Three variants – U2S1, U2S2 and U2S3 – bound exclusively to the mutant iNKG2D ligands and not wtNKG2D while also retaining iNKG2D specificity when fused to the C-terminus of antibody heavy or light chains. Two U2S3 ligands were genetically fused on the C-terminus of rituximab (anti-CD20) IgG light chains (rituximab.LC-U2S3) [89].
Primary human T cells transduced with iNKGD.YA fused to second-generation CAR components can stably bind to rituximab.LC-U2S3 adapter as evaluated with surface staining via flow cytometry. Activation of ConvertibleCAR T cells and release of IL-2 was specific to plates coated with U2S3 ligands and not wild type ULBP2 ligands, demonstrating their specificity for the mutant ligand. The same ConvertibleCAR T cell behavior was demonstrated with the iNKGD.YF expressing cells and a second ULBP2 mutant - U2R. Thus, there are multiple mutant pairs that can be used to target different cell surface antigens or selectively engage ConvertibleCAR T cells for expansion. ConvertibleCAR T cells are saturated with mutant ligand at 5 nM of rituximab.LC-U2S3 adapter while cell lysis in vitro against Ramos target cells reached a saturation response at only 30 pM, suggesting it may be possible to arm ConvertibleCAR T cells with multiple targeting ligands to reduce the likelihood of antigen escape without compromising the potency of target cell lysis [89].
Two murine models – a Raji CD19+ disseminated and a subcutaneous lymphoma - were used to probe in vivo control of ConvertibleCAR T cells with rituximab.LC-U2S3 (Rit-S3) adapter ligand. Rit-S3 administration every 2 days plus ConvertibleCAR T cells blocked tumor expansion in the disseminated model to the same extent as control CAR T cells that directly kill Raji cells (RITscFv-CAR). Suppression of tumor growth in the subcutaneous lymphoma required higher dosing of both ConvertibleCAR T cells + Rit-S3 (see section 3.5) [89].
2.5. Colocalization-Dependent protein switches (Co-LOCKR) for CAR T Logic Gating
The Co-LOCKR system uses de novo designed proteins that behave in a combinatorial lock and key manner requiring colocalization of the proteins on the cell surface for retargeting CAR T effector functions against tumor cells in vitro (Fig 2.H) [90]. Colocalization-dependent protein switches (Co-LOCKR) can instigate distinct, combinatorial binding interactions - namely, AND, OR and NOT gate logic. Co-LOCKR protein switches are activated through a conformational change that occurs only when all conditions of gated logic are met; essentially a multiple-key locking system designed to avoid off-target effects [91].
The interaction between the protein Bcl1 and its natural peptide substrate, Bim, were the starting point for installing function into Co-LOCKR [91]. For the redirection of CAR T cells, a Bcl2 CAR was designed to bind Co-LOCKR adaptor proteins constructed to control access to the Bim peptide. Co-LOCKR has two separate components: (i) a “cage” protein that contains a sequestered Bim peptide in an inactive conformation that requires binding of (ii) a “key” protein that induces the conformational change necessary to free the Bim peptide and allow the Bcl2 CAR to bind. For Bcl2 CAR binding to occur, both Co-LOCKR (cage and key) components must be localized in close proximity on the surface of the same cell. The Co-LOCKR cage and key proteins was genetically fused to DARPin domains recognizing cancer markers Her2, EGFR and EpCAM markers on the cell surface. ScFvs, rather than DARPins can also be swapped as recognition domains for Co-LOCKR targeting [90].
With Bcl2 CAR T cells, Co-LOCKR proteins are capable of directing CAR T cell cytotoxicity against specific subsets of cells in a mixed cell population, selectively targeting Raji/EpCAM/Her2 cells while sparing EpCAM-only and Her2-only Raji cells (AND gate strategy). Furthermore, Co-LOCKR AND gate works for multiple antigen pairs [Her2/EpCAM] and [Her2/EGFR] with varying antigen densities on tumor target cells. Co-LOCKR induced CAR T cells proliferate and release IFN-γ only upon co-culture with target cells that co-express the correct antigen pairs. The degree of proliferation correlated with antigen density on the target cells [90].
More complex gating combinations of logic gating are also possible with the Co-LOCKR system. CAR T cells co-cultured with AND/OR gate Co-LOCKRs can accomplish binding to antigen 1 AND either antigen 2 OR antigen 3 with respect to IFN-γ production and proliferation. Similar results are possible for CAR T cells co-cultured with AND/NOT gate Co-LOCKRs that can recognize Her2 AND EpCAM but NOT EGFR. For robustness, the NOT antigen expression was higher than the AND antigen because the decoy NOT Co-LOCKR needs to sequester all molecules of the Co-LOCKR key.
2.6: Bispecific Antibody Adapter CARs
Bispecific antibody (bsAb) adapter CARs combine adoptive CAR T cell therapy with bsAb antibody technology [92]. The main objective behind this strategy is to provide CAR-induced co-stimulation that a bispecific antibody alone lacks [93]. The first BsAb-binding immune receptor (BsAb-IR) T cells used a truncated 231-residue portion of folate receptor alpha (FRα) as a CAR ECD. FRα has limited expression on normal tissue and is absent on T cells, making it a distinct CAR receptor for interaction with an anti-FRα/anti-tumor bsAb antibody adapter (Fig 2.I) [92]. BsAb adapters that recognize both FRα and Her2/CD20 receptors were created using chemical conjugation, resulting in low yields of the desired bsAb heterodimers (11% for anti-FRα/anti-Her2 and 21% for anti-FRα/anti-CD20). Subsequent bsAb-mediated T cell activation and anti-tumor activity of the BsAb-IR CAR constructs had reduced overall potency, which may be a result of the low yield of functional bsAb adapters.
An alternate bsAb-binding adapter CAR design consists of an inert extracellular domain restricted to tumors (EGFRvIII or Cripto-1) fused to second-generation transmembrane and intracellular domains [94]. Both trivalent and tetravalent bsAb adapters were tested for mediating on-target toxicity. Soluble trivalent 2:1 bsAb adapters that bind to EpCAM on tumor cells and engage CARs monovalently elicit on-target toxicity, however, a tetravalent 2:2 bsAb adapter crosslinks the CAR ECD and causes activation in the absence of tumor. Furthermore, CAR T cells could reduce but not eliminate established tumors. One possible explanation for this result is that the large size of the trivalent bsAb adapter reduces the strength of the immune synapse formed when bound to the CAR [16]. The construction of a multitude of bsAb adapter formats is possible with standard recombinant DNA technology and the use of antibody heterodimerization techniques like “knob-into-holes” and CrossMab [95–97]. While modular, bispecific adapter CARs are limited by required individual optimization for each adapter as demonstrated by the different results from trivalent and tetravalent bsAb adapters.
3. Engineering Aspects for Next-Generation CARs
3.1. CAR Adapters – Size, Valency and Serum Half-life Impacts T Cell Cytotoxicity, Persistence and Memory
A major distinction between traditional and adapter CAR T cells is the adapter CAR T cell design uncouples tumor antigen recognition and T cell signaling. This uncoupling adds an additional layer of control and specificity over the rigid format of traditional CAR T cell design, but also introduces additional complexities that can be beneficial or detrimental.
Consider the native functional immunological synapse distance of ~15 nm between a T cell and antigen presenting cell [98]. The spatial organization of the immune synapse is important for proper T cell activity against a target antigen as it dictates the appropriate recruitment of costimulatory molecules or inhibitory phosphatases necessary for signal outcome and strength. Therefore, careful examination of adapter size and geometry is important for proper CAR signaling and may need optimization depending on the proximity of a specified tumor target antigen to the cell membrane. For example, in the sCAR T platform, placement of the engrafted peptide proximal or distal to the fab adapter antigen binding site resulted in different outcomes depending on whether CD19 or CD20 was the target antigen. The binding epitope on CD20 is close to the cell membrane and a larger distance was preferred between the fab binding site and the embedded peptide in the adaptor, while the binding epitope on CD19 is distal to the cell membrane and better T cell activity was observed with an adaptor that spanned a shorter distance to CD19 on the surface of T cells. One advantage of adapter CAR T cells relative to traditional CAR cells is the opportunity to readily test adaptors with different sizes and geometries to tune the system for optimal T cell activity.
The specific activation of CAR T cells only in the presence of CAR adapters is a key element of adapter CAR T platforms. T cell activity is primarily determined by the adapter pharmacokinetic properties and their biodistribution, as well as the affinity for the tumor target and CAR ECD. In this review we’ve highlighted protein-based adapters including scFvs (30kDa), Fabs (50kDa), IgGs (150kDa), and DARPins (14kDa) which vary in size and half-life. The therapeutic dosing regimen of a smaller adapter may require higher and more frequent dosing but could provide faster on and off switching of CAR T cell activity compared to a larger adapter with a longer half-life. The importance of on/off-switch rate will be particularly important for targeting CAR T cells to tumors in cases where the risk of CRS and neurotoxicity is high. Because animal studies did not effectively predict CRS for CAR19 CAR T cells and the adapter platform is inherently armed with enhanced controllability, fast on and off rate CAR T switches are valuable until our understanding of the pathophysiology of these toxicities is better understood [99].
An important finding for the engineering of T cell therapies was the observation that the activation threshold for T cells does not require a mature, stable immune synapse [100]. For adapter CARs that require an indirect encounter via adapter molecules to recognize a target antigen, a more transient interaction can still elicit T cell activity. Additionally, there exist distinct thresholds for T cell lytic activity and cytokine release which are required for target destruction and clonal expansion respectively [101]. The ability to readily modulate the interaction strength between the adapter molecule and tumor antigen as well as between the adapter molecule and CAR provides an opportunity to control cytokine secretion and stimulation for T cell lysis. For example, in experiments with the SUPRA-CAR system increasing IFNγ cytokine release was observed in response to increasing adapter zipFv affinity for Her2 antigen. For CAR/adapter systems like SUPRA-CARs and ConvertibleCARs that consist of multiple pairs of orthogonal adapter-CAR interactions with various affinities (leucine zippers and ULBP ligands respectively), cytotoxicity can be tuned specifically for the target, and can also be implemented to target multiple antigens either simultaneously or sequentially [86,89].
Furthermore, adapter CAR T platforms may be leveraged for induction of desired T cell memory phenotypes in CAR T cells that cannot be currently recapitulated by traditional CAR T cell architecture. A central memory (TCM) T cell phenotype correlates with sustained remission for patients with ALL [102]. This is a subpopulation of CAR T cells that persist and proliferate longer in patients and have more robust tumor-killing ability, as distinguished by flow staining of cell surface markers. Adapter CAR T cells can define TCM subsets through distinct ‘rest’ phases where CAR T cells are not stimulated (absence of adapter) allowing apoptosis of short-lived CAR T cell populations and subsequent enrichment of the CAR TCM memory subset [102]. Initial encounter with a target antigen followed by a rest phase in the absence of antigen is required for the formation of a pool of memory T cells that harbor a range of functionalities and phenotypes. Some of these T cells populations, commonly characterized by high expression of IL-7 receptor (CD127) and adhesion markers CD44 and CD62L have increased potential for proliferation and more rapid effector response and are generally known as TCM cells [103].
Classical T cell contraction and expansion behavior was accomplished using sCAR T cells and GCN4-based adapter for CD19 [104]. In a syngeneic mouse model with murine/rat sCAR components, a vaccination/boosting approach that centered adapter dosing around the timing of a natural adaptive immune response resulted in a five-fold increase in the sCAR T cell populations at day 35 than initially detected one week after cell transfer. This contrasts with traditional CAR T cells that steadily decline after an initial expansion upon antigen encounter [18].
Persistence of CAR19 T cells in patients treated for B cell cancer causes the destruction of healthy B cells, known as B cell aplasia. In patients with sustained remission, B cell aplasia can last up to two years [105]. Patients can tolerate B cell destruction with immunoglobulin replacement therapy to protect against infections, but it can potentially be ameliorated entirely with the use of adapter CARs. In a preclinical murine model, sCAR T cell therapy with GCN4-based adapter for CD19 showed that B cell populations rebounded when adaptor dosing was stopped and B cells returned to levels of untreated controls 60 days after last switch dose [104].
3.2. CAR Oligomerization
Each structural component of a CAR has functional implications on CAR T cell lytic activity, cytokine release, persistence, and memory [17,106]. Generally, the domains that comprise traditional second-generation CARs are the same domains as those in adapter CAR T cell designs. Therefore, we briefly discuss the CAR domains below in terms of structural concerns for T cell activity towards cancer.
The hinge and transmembrane regions of adapter CARs are primarily the same components as traditional CARs with either CD8- or CD28-derived domains. Native CD8 and CD28 exist on the cell surface as dimers, therefore, adapter and traditional CARs also form spontaneous homodimer CARs in the absence of stimulation. One exception is the sCAR T cell that uses a native IgG4 hinge that does not dimerize [107]. However, in one rendition of this design the IgG4 hinge contains a serine-to-proline mutation (S228P) that enables interchain sCAR disulfide formation. The S228P substituted hinge is most effective for driving cytokine release in combination with the proximal engrafted GCN4-based adapter for CD19 [29]. The IgG4 hinge lacking the S228P mutation has reduced cytokine production, highlighting the importance of dimerization for potent CAR T cell expansion. Although the hinge and transmembrane regions do not interact directly with the target adapter molecule, they still have the potential to adjust the functional strength of the CAR T cell [108].
A concern with CAR dimerization is the possibility of tonic signaling that results from scFv domain swapping and/or aggregation [109]. Neighboring scFvs that interact can induce CAR T cell stimulation in the absence of antigen, limiting their therapeutic utility and longevity. The length of residues in the spacer region between the transmembrane and recognition domains can also influence the ability of a CAR to aggregate in the absence of antigen [51]. One possible advantage of the SUPRA CAR and ConvertibleCAR platforms are the use of non-scFv ECDs which could reduce the possibility of ECD aggregation at the CAR surface. Domain swapping appears to be specific to the scFv on the surface of the CAR and is not uniform across all constructs. A short linker between the ECD scFv heavy chain and light chain can also induce domain-swapping.
3.3. Addressing CRS and Neurotoxicity
CRS is a unique toxicity associated with CAR T cell therapy that occurs following the release of inflammatory cytokines and is characterized by fever, hypoxia, and hypotension that can lead to multi-organ toxicity and death [87,110]. Acute neurotoxicity is a second dangerous and poorly understood side effect to CAR T therapy [110]. The pathogenesis of neurologic toxicity remains unclear but is defined separately from CRS due to distinct onset and response to treatment [87]. The timing of CRS occurs mostly within 100 days of CAR T cell infusion and neurotoxicity within the first month [19]. Correlative studies during the initial phase of treatment with CAR19 CAR T cells identified a rise in interleukin-6 (IL-6) levels that were associated with CRS, leading to the investigation of IL-6 signaling and receptor blockade to remedy severe CRS. The therapeutic anti-IL-6 antibody tocilizumab and corticosteroids were approved to treat CRS alongside the FDA approval of CD19 CAR T cell products in 2017 [111]. Overall, toxic symptoms of CD19 CAR T cell-induced CRS have been managed successfully for patients with acute care and therapeutic intervention as required until symptoms resolve. Importantly, risk of CRS and neurotoxicity still prevents a broader application of CAR T therapy for other cancers, particularly solid tumors [20].
The production of cytokines are necessary for CAR T cell proliferation and successful therapeutic response, therefore, implementing better control of cytokine release with adapter CAR T cells offers a potential solution to reduce the severity of CRS. In a Nalm-6 xenograft model of CD19+ tumors, sCAR T cells in combination with a low dose of FITC-conjugated anti-CD19 fab adapter reduces tumor burden and releases cytokines at low levels. Re-administration of the anti-CD19 adapter at higher doses once tumor burden is reduced clears tumors and does not elicit high levels of cytokine release [112]. Similarly, the UniCAR design which harbors an anti-peptide CAR can crosslink a tumor-targeting adapter scFv that is rapidly cleared (15 – 45 min) in case side effects occur. Subsequent administration of an adapter with an extended half-life eliminates the need for constant infusion of the adapter once risk of severe CRS is lower. This strategy has successfully been tested in vitro and in mice for a variety of tumor targets including CD19, CD123, CD33 and others [48].
Adapter molecules have extensively been described in this review to mobilize CAR T cell effector functions but adapter strategies are also poised to use adapter interactions to turn CARs off (see also section 3.4). The SUPRA CAR and Co-LOCKR systems implement NOT-gate strategies using decoy adapters with no targeting domains which sequester targeting adapter molecules through high affinity interactions to prevent engagement with the CAR and keep T cell activity minimal [86,90]. These strategies add yet another layer of control of T cell effector function using adapter CAR T cells without destruction of the T cell. Alternatively, traditionally structured CARs have advanced a way to rapidly destroy CAR T cells in cases of severe side effects. Here, an inducible Caspase9 suicide gene causes apoptosis specifically of CAR T cells with addition of a chemical agent [7,69]. This approach works to rapidly clear CAR T cells in life-threatening cases but is not a solution to implement control over activity. In general, all adapter CAR T platforms provide a mechanism to escalate or reduce treatment by adjusting the dose of adapter molecule, thus providing a way to control T cell effector function without the destruction of costly engineered T cells.
3.4. Switches and Combinatorial Antigen Sensing to Reduce On-target Off-tumor Effects
Tumor-associated antigens are usually, if not always, expressed also on healthy cells leading to the targeted destruction of healthy tissues when CAR T cells are administered [62]. As demonstrated by profound B cell aplasia in patients treated with CD19-directed CAR T cell therapy, T cells are very effective at destroying healthy cells in addition to cancerous cells that display the target antigen. With antigen targets other than CD19 that have even low expression on vital tissue, for example, the breast cancer antigen Her2 that is expressed with low density on healthy cardiac and lung tissue, on-target cytotoxicity of CAR-T cells poses a significant toxicity risk [110]. The ability to design therapeutic T cells that can discriminate effectively between target tumor cells expressing antigen and healthy cells expressing antigen remains a safety priority for all present and future CAR T cell therapies.
One technique to prevent CAR T cell activity if necessary is to incorporate an OFF switch into CAR T cells. The SUPRA CAR platform uses leucine zipper pairs that bind competitively to activating zipFvs to sequester them away from zipCARs as an OFF switch mechanism (Fig 3.A) [86]. This competitive binding is accomplished with a tight-binding inhibitory zipFv. The SUPRA CAR system also employs gating strategies (AND, OR) for combinatorial antigen sensing. ZipCARs can redirect T cell cytotoxicity to one or multiple tumor antigens with the use of adapter zipFvs for OR gate implementation. ZipCARs can kill cells expressing Her2 OR Axl antigens by co-culturing with anti-Her2 and anti-Axl zipFvs (Fig 3.B). An OR gate can help prevent antigen escape [86]. Further engineering of SUPRA CARs with split signaling domains demonstrates 2-input AND gate function to spare cells that do not express both antigens. In this design, SUPRA CARs can be transduced with two CAR domains – an activation zipCAR fused to CD3ζ and a costimulatory zipCAR fused to CD28 and 4-BB costimulatory domains. Engagement of both zipCARs by their mutually-exclusive zipFvs is required for full SUPRA CAR T cell stimulation as measured by expression of T cell activation markers and cytokine release in vitro.
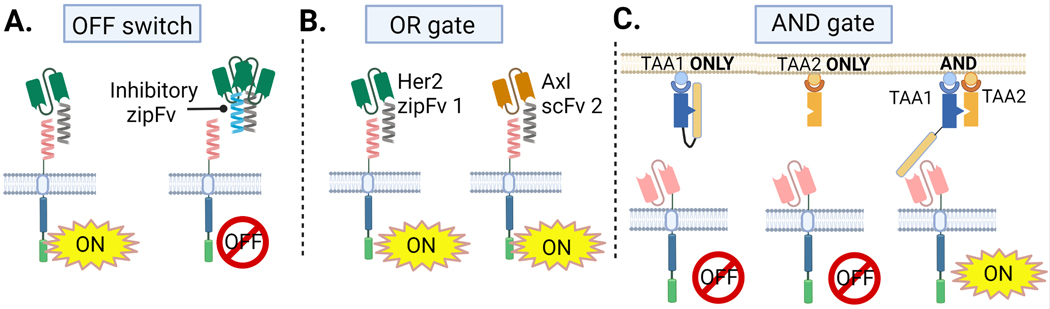
Fig 3. OFF, OR, AND Strategies with Adapter CAR T Cells.
A) An inhibitory scFv works as an OFF switch in the SUPRA CAR design. B) SUPRA CAR T cells can target cytotoxicity to Her+ OR Axl+ tumor cells with different zipFvs. C) Co-LOCKR AND gate requires both antigen A and B on a tumor cell surface for functional CAR T cytotoxicity.
The Co-LOCKR system also performs 2- and 3-input AND/OR gating to discriminate tumor targets within mixed cell populations [90]. Co-LOCKR requires colocalization of two DARPin-based adapter molecules for functional Bcl2-CAR signaling (Fig 3.C). Using Her2, EpCAM and EGFR as model tumor antigens, all combinations to target cells that express two of these antigens [Her2 AND either EGFR OR EpCAM], [EGFR AND either Her2 OR EpCAM] and [EpCAM AND either Her2 OR EGFR] selectively kill the subset of cells expressing the dual combination in vitro [90]. Colocalization works to control biological activity by bringing the cage and key proteins together in close proximity permitting a conformational change in the cage protein that exposes the Bim key peptide antigen that Bcl2-CAR T cells bind (Fig 2.H). In the absence of the colocalized key, the cage remains latched and the Bcl2-CAR T cell cannot become activated.
3.5. Overcoming Antigen Loss
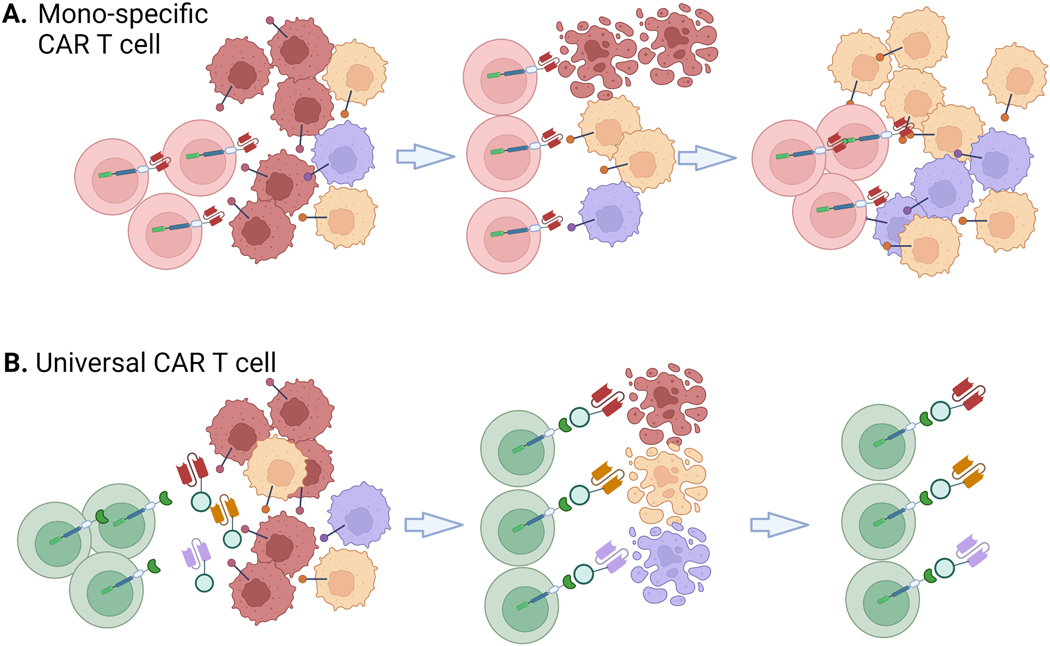
Circumventing tumor antigen escape by targeting multiple tumor antigens either sequentially or simultaneously with adapter CARs could mitigate tumor growth and relapse (Fig 4.A) [40,61,66,113]. About 10–20% of relapsed B-ALL patients after treatment with CD19-directed CARs present with CD19-negative tumors, highlighting an important limitation of traditional CARs and an opportunity for adapter-mediated CARs to redirect activity towards multiple antigens [40]. Adapter-mediated CARs can potentially combat antigen loss and resistance by switching adapter molecules to target different antigens (Fig 4.B). A limitation of this approach is that each adapter targeting a tumor antigen may require optimization. For example, targeting the same sCAR T cells to CD19 and CD22 antigens using Fab adapters conjugated to FITC elicited different cytotoxicity profiles dependent on location of FITC conjugation [32]. Steric hindrance at the surface of the cell when adapter epitopes are close to the cell membrane (as is the case for CD22) could explain these results and emphasizes the challenge of creating a truly universal adapter CAR system.
Fig 4. Targeting Multiple Tumor Antigens is a Major Advantage of Adapter CAR T Cells.
A) Conventional CAR T cells only recognize a single antigen which may enable antigen escape, as depicted. B) Adapter CAR T cells can use multiple adapters to target T cells to different tumor antigens.
3.6. Adapter Dosing
An important clinical consideration for adapter CAR T-based platforms is how much adapter molecule and how often it will have to be administered to be effective at clearing tumors in vivo without severe toxicity. In this section we summarize preclinical in vivo data from the adapter CAR T platforms described in section 2.
Adapter CAR T cell platforms are designed to work with different formats of adapter molecules. For example, the UniCAR platform uses a series of adapter molecules with short and extended half-life and has shown proof-of-concept in vitro and in mice for scFvs, BiTEs, nanobodies, soluble TCRs, affibodies, and even small molecules like PET tracers. In vivo CAR T cell activity and expansion can be manipulated by changing adapter dosage. Moreover, the different half-lives and formats of adapters easily allow for dose escalation to control CAR T cell cytotoxicity [48].
Adapter CARs may also be able to leverage dosing schemes to expand CAR T cell subpopulations with desired T cell central memory (TCM) phenotype [104]. CAR T cells that are defined by this phenotype have been seen in patients with sustained remission from ALL. Controlled dosing of adapter can mimic the expansion and rest phase of naïve T cell pathogen encounter to improve the functionality of adapter CAR T cells. For example, sCAR T cells demonstrated that 1 week of Fab adapter daily followed by 3 weeks rest phase had a five-fold higher increase of sCAR T cells during the second dosing compared to sCAR T cell count at week one [104].
4. Universal CARs and Adapters in the Clinic
The translation of CAR T therapies with adapter molecules into the clinic began in 2016 and continues to expand. The first adapter CAR T cells products (Unum Therapeutics Inc.) ACTR087 and ACTR707 are CD16 (FcγRIII) CARs given in combination with clinical grade mAbs Rituximab, SEA-BCMA and Trastuzumab to treat CD20+ B cell lymphoma, BCMA+ multiple myeloma and Her2+ solid tumors respectively. CARs with CD16-ECDs mediate antibody dependent cellular toxicity (ADCC) when the Fc region of antibodies binds CD16 on CAR T cells. This strategy has been described in thorough detail elsewhere [114]. An initial hold on this trial in February 2018 resulted from serious adverse reactions in CD20+ B cell lymphoma patients observed at high ACTR087 T cells doses [115]. A second hold was placed on the trial in a dose-escalation study when a patient experienced serious neurotoxicity and respiratory distress [116]. Additionally, the FDA partially halted Phase 1 studies of ACTR707 with Rituximab as of March 2020 for the treatment of NHL after a serious adverse event was experienced by one patient. Meanwhile, the Phase 1 trial with ACTR707 in combination with Trastuzumab for Her2+ solid tumors was completed with no dose-limiting toxicities in early 2020 [117]. As of October 2020, Unum Therapeutics has rebranded as Cogent Biosciences and shifted their focus towards development of non-CAR T therapeutics [118].
In early 2020, the FDA accepted Calibr and Abbvie’s Investigational New Drug (IND) filing to initiate clinical trials for treating relapsed and refractory AML using sCAR T cells with a peptide-tagged anti-CD19 Fab adapter (CLBR001 + SWI019) [119]. The first in-human clinical study results were presented in December 2021. Two out of five patients experienced a complete response. There were no adverse effects related to the CAR T cells CLBR001 and only mild CRS that resolved quickly by reducing adapter SWI019 dose, as designed. Treatment in a second cohort of patients is ongoing [120].
In Germany, the first in-person treatment with switchable UniCAR targeting CD123 for AML (Cellex Patient Treatment GmbH) successfully treated three patients. All patients had a clinical response with two patients showing complete remission and one with partial remission. There were no dose-limiting toxicities and mild side effects observed, with myelodepletion recovering after withdrawal of TM123 on day 24 for all patients. Enrollment in a phase IA study is underway. Autologous UniCAR-T cells were given on day 1, dosing starting with 100 × 106 cells and 0.5 mg TM123 per day in patient 1, 250 × 106 cells in patient 2 with 0.5 mg TM123 per day, and 250 × 106 cells and 1.0 mg TM123 per day patient 3 [121].
As of December 2020, the switchable UniCAR platform is being used in a Phase 1A study with PMSA-expressing late-stage malignancies. In July 2021 a new company founded between Blackstone Life Sciences, Intellia and Cellex is combining Intellia’s CRISPR/Cas 9 allogenic platform with Cellex’s switchable, universal CAR-T cells (UniCAR and RevCAR) [122].
5. Conclusions and Future Directions for Adapter CARs
In this review, we described adapter CAR T cell designs that illustrate innovative progress in the field of CAR T engineering to improve its therapeutic window. Adapter CAR T platforms have shown promising in vitro and in vivo preclinical results. They represent a new class of CAR T-based therapies that have engineered components with enhanced safety controls, the ability to target multiple antigens to avoid relapse, and to use combinatorial antigen sensing to discriminate cancer cells in a mixed cell population. There is currently limited clinical trial data, and this will be critical in determining if the adapter strategy can maintain its controllability in a clinical setting and show any clinical advantages over traditional CAR T cell therapy.
Table 2.
Clinical trials information for adapter CAR T cell therapy in the United States and Europe.
| ClinicalTrials.gov identifier | Study Start/Completion Dates | Phase | Disease | CAR ECD, Adapter | Status |
|---|---|---|---|---|---|
| NCT02776813 | 5/18/16 – 2/20/20 | 1 | CD20+ Non-Hodgkin’s lymphoma | CAR ECD – CD16 (ACT087) Adapter – Rituximab |
Completed |
| NCT03266692 | 2/22/18 – 10/1/19 | 1 | Multiple myeloma | CAR ECD – CD16 (ACT087) Adapter – SEA-BCMA |
Terminated |
| NCT03189836 | 10/4/17 – 9/21/20 | 1 | DLBCL, follicular lymphoma | CAR ECD – CD16 (ACT707) Adapter – Rituximab |
Terminated |
| NCT03680560 | 3/3/19 – 3/12/20 | 1 | Solid tumors overexpressing Her2 | CAR ECD – CD16 (ACT707/ACT087) Adapter – Trastuzumab |
Terminated |
| NCT04450069 | 8/14/20 - | 1 | Relapsed/Refractory B-cell lymphomas | CAR ECD – anti-PNE scFv (CLBR001) Adapter – anti-CD19 fab SWI019 |
Recruiting |
| NCT04230265 | 1/28/20 - | 1 | AML/ALL | CAR ECD – UniCAR02-T Adapter – TM123 (anti-CD123) |
Recruiting |
| NCT04633148 | 11/23/20 - | 1 | PMSA+ solid tumors – CRPC, NSCLC, TNBC | CAR ECD – UniCAR02-T Adapter – TMpPMSA (anti-PMSA) |
Recruiting |
Acknowledgements
A.M. and Z.Y. researched and wrote the review. B.K supervised and edited the manuscript. We would like to thank our colleague Ernesto Leon, PhD for his assistance proof-reading this article.
Funding
A.M., Z.Y. and B.K. are supported by NIH grant R35GM131923.
Abbreviations
- TCR
T cell receptor
- CAR
chimeric antigen receptor
- EPCAM
epithelial cell adhesion molecule
- IS
immunological synapse
- CRS
cytokine release syndrome
- BiTE
bispecific T cell engager
- PNE
peptide neo-epitope
- ALL
acute lymphoblastic leukemia
- DLBCL
diffuse large B-cell lymphoma
- AML
acute myeloid lymphoma
- PMSA
prostrate-specific membrane antigen
- CRPC
castrate-resistant prostrate cancer
- NSCLC
non-small cell lung cancer
- TNBC
triple negative breast cancer
References
- [1].Bouchkouj N, Kasamon YL, de Claro RA, George B, Lin X, Lee S, Blumenthal GM, Bryan W, McKee AE, Pazdur R, FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-cell Lymphoma, Clin. Cancer Res. 25 (2019) 1702–1708. 10.1158/1078-0432.CCR-18-2743. [DOI] [PubMed] [Google Scholar]
- [2].MC O, X L, Y H, X L, I M, D P, D G, S L, K L, B G, W B, MR T, R P, FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia, Clin. Cancer Res. 25 (2019) 1142–1146. 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- [3].Wang K, Wei G, Liu D, CD19: a biomarker for B cell development, lymphoma diagnosis and therapy, Exp. Hematol. Oncol 1 (2012) 36. 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH, Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia, Sci. Transl. Med 7 (2015). 10.1126/SCITRANSLMED.AAC5415/SUPPL_FILE/7-303RA139_TABLES_S6_TO_S11.ZIP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kenderian S, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette J, Scholler J, Song D, Porter D, Carroll M, June C, Gill S, CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia, Leukemia. 29 (2015) 1637. 10.1038/LEU.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Diaconu I, Ballard B, Zhang M, Chen Y, West J, Dotti G, Savoldo B, Inducible Caspase-9 Selectively Modulates the Toxicities of CD19-Specific Chimeric Antigen Receptor-Modified T Cells, Mol. Ther. 25 (2017) 580–592. 10.1016/j.ymthe.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].A DS, SK T, G D, Y F, N. A K, C M, K S, E L, AG D, B G, H L, CR C, B S, AP G, J S, RA K, HE H, DM S, CM R, MK B, Inducible apoptosis as a safety switch for adoptive cell therapy, N. Engl. J. Med 365 (2011) 1673–1683. 10.1056/NEJMOA1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ruella M, Maus MV, Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies, Comput. Struct. Biotechnol. J 14 (2016) 357–362. 10.1016/J.CSBJ.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yu S, Li A, Liu Q, Li T, Yuan X, Han X, Wu K, Chimeric antigen receptor T cells: a novel therapy for solid tumors, J. Hematol. Oncol 2017 101. 10 (2017) 1–13. 10.1186/S13045-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Watanabe K, Kuramitsu S, Posey AD, June CH, Expanding the therapeutic window for CAR T cell therapy in solid tumors: The knowns and unknowns of CAR T cell biology, Front. Immunol 9 (2018) 2486. 10.3389/FIMMU.2018.02486/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang C, Liu J, Zhong JF, Zhang X, Engineering CAR-T cells, Biomark. Res. 5 (2017). 10.1186/S40364-017-0102-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M, Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor, Nat. Biotechnol 2002 201. 20 (2002) 70–75. 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- [13].Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G, CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients, J. Clin. Invest 121 (2011) 1822–1826. 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S, Teaching an old dog new tricks: next-generation CAR T cells, Br. J. Cancer 120 (2019) 26. 10.1038/S41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S, Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells, Int. J. Mol. Sci 20 (2019). 10.3390/IJMS20061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Minutolo NG, Hollander EE, Powell DJJ, The emergence of universal immune receptor t cell therapy for cancer, Front. Oncol 9 (2019). 10.3389/fonc.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lim WA, June CH, The Principles of Engineering Immune Cells to Treat Cancer, Cell. 168 (2017) 724–740. 10.1016/J.CELL.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jafarzadeh L, Masoumi E, Fallah-Mehrjardi K, Mirzaei HR, Hadjati J, Prolonged Persistence of Chimeric Antigen Receptor (CAR) T Cell in Adoptive Cancer Immunotherapy: Challenges and Ways Forward, Front. Immunol 11 (2020) 702. 10.3389/FIMMU.2020.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ, The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden, 10.1200/EDBK_238691. (2019) 433–444. 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]
- [20].Choe JH, Williams JZ, Lim WA, Engineering T Cells to Treat Cancer: The Convergence of Immuno-Oncology and Synthetic Biology, Annu. Rev. Cancer Biol 4 (2020) 121–139. 10.1146/annurev-cancerbio-030419-033657. [DOI] [Google Scholar]
- [21].Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell DJ, A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor, Cancer Res. 72 (2012) 1844–1852. 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dundas CM, Demonte D, Park S, Streptavidin-biotin technology: Improvements and innovations in chemical and biological applications, Appl. Microbiol. Biotechnol 97 (2013) 9343–9353. 10.1007/s00253-013-5232-z. [DOI] [PubMed] [Google Scholar]
- [23].Wilchek M, Bayer EA, Livnah O, Essentials of biorecognition: The (strept)avidin-biotin system as a model for protein-protein and protein-ligand interaction, Immunol. Lett 103 (2006) 27–32. 10.1016/j.imlet.2005.10.022. [DOI] [PubMed] [Google Scholar]
- [24].Zempleni J, Mock DM, Biotin biochemistry and human requirements, J. Nutr. Biochem 10 (1999) 128–138. 10.1016/S0955-2863(98)00095-3. [DOI] [PubMed] [Google Scholar]
- [25].Lim KH, Huang H, Pralle A, Park S, Stable, high-affinity streptavidin monomer for protein labeling and monovalent biotin detection, Biotechnol. Bioeng 110 (2013) 57–67. 10.1002/bit.24605. [DOI] [PubMed] [Google Scholar]
- [26].Lohmueller JJ, Ham JD, Kvorjak M, Finn OJ, mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting, Oncoimmunology. 7 (2017). 10.1080/2162402X.2017.1368604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].De Boer E, Rodriguez P, Bonte E, Krijgsveldt J, Katsantoni E, Heckt A, Grosveld F, Strouboulis J, Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 7480–7485. 10.1073/PNAS.1332608100/SUPPL_FILE/2608FIG5NEW.JPG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grant MKO, Shapiro SL, Ashe KH, Liu P, Zahs KR, A Cautionary Tale: Endogenous Biotinylated Proteins and Exogenously-Introduced Protein A Cause Antibody-Independent Artefacts in Western Blot Studies of Brain-Derived Proteins, Biol. Proced. Online 21 (2019) 1–11. 10.1186/S12575-019-0095-Z/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cao Y, Rodgers DT, Du J, Ahmad I, Hampton EN, Ma JSY, Mazagova M, Choi SH, Yun HY, Xiao H, Yang P, Luo X, Lim RKV, Pugh HM, Wang F, Kazane SA, Wright TM, Kim CH, Schultz PG, Young TS, Design of Switchable Chimeric Antigen Receptor T Cells Targeting Breast Cancer, Angew. Chemie - Int. Ed 55 (2016) 7520–7524. 10.1002/anie.201601902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zahnd C, Spinelli S, Luginbühl B, Amstutz P, Cambillau C, Plückthun A, Directed in Vitro Evolution and Crystallographic Analysis of a Peptide-binding Single Chain Antibody Fragment (scFv) with Low Picomolar Affinity, J. Biol. Chem 279 (2004) 18870–18877. 10.1074/jbc.M309169200. [DOI] [PubMed] [Google Scholar]
- [31].Hanes J, Jermutus L, Weber-Bornhauser S, Bosshard HR, Plückthun A, Ribosome display efficiently selects and evolves high-affinity antibodies in vitro from immune libraries, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 14130–14135. 10.1073/pnas.95.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ma JSY, Kim JY, Kazane SA, Choi S-H, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, Fonslow BR, Kochenderfer JN, Wright TM, Schultz PG, Young TS, Kim CH, Cao Y, June CH, Shokat KM, Versatile strategy for controlling the specificity and activity of engineered T cells, (n.d.). 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Classon BJ, Willis AC, Brown MH, Garnett D, Somoza C, Barclay AN, Williams AF, The hinge region of the cd8a chain: Structure, antigenicity, and utility in expression of immunoglobulin superfamily domains, Int. Immunol 4 (1992) 215–225. 10.1093/intimm/4.2.215. [DOI] [PubMed] [Google Scholar]
- [34].Aalberse RC, Schuurman J, IgG4 breaking the rules, Immunology. 105 (2002) 9–19. 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E, Redirecting gene-modified T cells toward various cancer types using tagged antibodies, Clin. Cancer Res 18 (2012) 6436–6445. 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- [36].Ma JSY, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, Fonslow BR, Kochenderfer JN, Wright TM, Schultz PG, Young TS, Kim CH, Cao Y, Versatile strategy for controlling the specificity and activity of engineered T cells, Proc. Natl. Acad. Sci. U. S. A 113 (2016) E450–E458. 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, De Jong JS, Arts HJG, Van Der Zee AGJ, Bart J, Low PS, Ntziachristos V, Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results, Nat. Med 17 (2011) 1315–1319. 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- [38].McKinney R, Thacker L, Hebert GA, Conjugation methods in immunofluorescence, J. Dent. Res 55 (1976). 10.1177/002203457605500117011. [DOI] [PubMed] [Google Scholar]
- [39].Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E, Redirecting Gene-Modified T Cells toward Various Cancer Types Using Tagged Antibodies, Clin. Cancer Res 18 (2012) 6436–6445. 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- [40].Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, Li M, Tu H, Liu Y, Tu S, Li Y, Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies, Front. Immunol 10 (2019) 2664. 10.3389/FIMMU.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sutherland AR, Owens MN, Geyer CR, Modular Chimeric Antigen Receptor Systems for Universal CAR T Cell Retargeting, Int. J. Mol. Sci. 2020, Vol. 21, Page 7222. 21 (2020) 7222. 10.3390/IJMS21197222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cartellieri M, Feldmann A, Koristka S, Arndt C, Loff S, Ehninger A, von Bonin M, Bejestani EP, Ehninger G, Bachmann MP, Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts, Blood Cancer J. 6 (2016) e458. 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koristka S, Cartellieri M, Arndt C, Bippes CC, Feldmann A, Michalk I, Wiefel K, Stamova S, Schmitz M, Ehninger G, Bornhäuser M, Bachmann M, Retargeting of regulatory T cells to surface-inducible autoantigen La/SS-B, J. Autoimmun 42 (2013) 105–116. 10.1016/j.jaut.2013.01.002. [DOI] [PubMed] [Google Scholar]
- [44].Arndt C, Feldmann A, Töpfer K, Koristka S, Cartellieri M, Temme A, Ehninger A, Ehninger G, Bachmann M, Redirection of CD4 + and CD8 + T lymphocytes via a novel antibody-based modular targeting system triggers efficient killing of PSCA + prostate tumor cells, Prostate. 74 (2014) 1347–1358. 10.1002/pros.22851. [DOI] [PubMed] [Google Scholar]
- [45].Arndt C, Feldmann A, Koristka S, Cartellieri M, Dimmel M, Ehninger A, Ehninger G, Bachmann M, Simultaneous targeting of prostate stem cell antigen and prostate-specific membrane antigen improves the killing of prostate cancer cells using a novel modular T cell-retargeting system, Prostate. 74 (2014) 1335–1346. 10.1002/pros.22850. [DOI] [PubMed] [Google Scholar]
- [46].Arndt C, Feldmann A, Von Bonin M, Cartellieri M, Ewen EM, Koristka S, Michalk I, Stamova S, Berndt N, Gocht A, Bornhäuser M, Ehninger G, Schmitz M, Bachmann M, Costimulation improves the killing capability of T cells redirected to tumor cells expressing low levels of CD33: Description of a novel modular targeting system, Leukemia. 28 (2014) 59–69. 10.1038/leu.2013.243. [DOI] [PubMed] [Google Scholar]
- [47].Cartellieri M, Feldmann A, Koristka S, Arndt C, Loff S, Ehninger A, von Bonin M, Bejestani EP, Ehninger G, Bachmann MP, Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts, Blood Cancer J. 6 (2016) e458. 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bachmann M, The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells, Immunol. Lett 211 (2019) 13–22. 10.1016/J.IMLET.2019.05.003. [DOI] [PubMed] [Google Scholar]
- [49].Yiannaki EE, Tzioufas AG, Bachmann M, Hantoumi J, Tsikaris V, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM, The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods, Clin. Exp. Immunol 112 (1998) 152–158. 10.1046/j.1365-2249.1998.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Troüster H, Bartsch H, Klein R, Metzger TE, Pollak G, Semsei I, Schwemmle M, Pruijn GJM, van Venrooij WJ, Bachmann M, Activation of a murine autoreactive B cell by immunization with human recombinant autoantigen La/SS-B: Characterization of the autoepitope, J. Autoimmun 8 (1995) 825–842. 10.1016/S0896-8411(95)80020-4. [DOI] [PubMed] [Google Scholar]
- [51].Bachmann M, The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells, Immunol. Lett 211 (2019) 13–22. 10.1016/j.imlet.2019.05.003. [DOI] [PubMed] [Google Scholar]
- [52].Albert S, Arndt C, Feldmann A, Bergmann R, Bachmann D, Koristka S, Ludwig F, Ziller-Walter P, Kegler A, Gärtner S, Schmitz M, Ehninger A, Cartellieri M, Ehninger G, Pietzsch HJ, Pietzsch J, Steinbach J, Bachmann M, A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform, Oncoimmunology. 6 (2017) e1287246. 10.1080/2162402X.2017.1287246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Albert S, Arndt C, Koristka S, Berndt N, Bergmann R, Feldmann A, Schmitz M, Pietzsch J, Steinbach J, Bachmann M, From mono- to bivalent: Improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo, Oncotarget. 9 (2018) 25597–25616. 10.18632/oncotarget.25390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Feldmann A, Arndt C, Bergmann R, Loff S, Cartellieri M, Bachmann D, Aliperta R, Hetzenecker M, Ludwig F, Albert S, Ziller-Walter P, Kegler A, Koristka S, Gärtner S, Schmitz M, Ehninger A, Ehninger G, Pietzsch J, Steinbach J, Bachmann M, Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR,” Oncotarget. 8 (2017) 31368–31385. 10.18632/oncotarget.15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Loureiro LR, Feldmann A, Bergmann R, Koristka S, Berndt N, Arndt C, Pietzsch J, Novo C, Videira P, Bachmann M, Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells, Blood Cancer J. 8 (2018) 81. 10.1038/s41408-018-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mitwasi N, Feldmann A, Bergmann R, Berndt N, Arndt C, Koristka S, Kegler A, Jureczek J, Hoffmann A, Ehninger A, Cartellieri M, Albert S, Rossig C, Ehninger G, Pietzsch J, Steinbach J, Bachmann M, Development of novel target modules for retargeting of UniCAR T cells to GD2 positive tumor cells, Oncotarget. 8 (2017) 108584–108603. 10.18632/oncotarget.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bachmann D, Aliperta R, Bergmann R, Feldmann A, Koristka S, Arndt C, Loff S, Welzel P, Albert S, Kegler A, Ehninger A, Cartellieri M, Ehninger G, Bornhäuser M, von Bonin M, Werner C, Pietzsch J, Steinbach J, Bachmann M, Retargeting of UniCAR T cells with an in vivo synthesized target module directed against CD19 positive tumor cells, Oncotarget. 9 (2018) 7487–7500. 10.18632/oncotarget.23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Brudno JN, Kochenderfer JN, Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management, Blood Rev. 34 (2019) 45. 10.1016/J.BLRE.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Feldmann A, Hoffmann A, Bergmann R, Koristka S, Berndt N, Arndt C, Rodrigues Loureiro L, Kittel-Boselli E, Mitwasi N, Kegler A, Lamprecht C, González Soto KE, Bachmann M, Versatile chimeric antigen receptor platform for controllable and combinatorial T cell therapy, Oncoimmunology. 9 (2020) 1785608. 10.1080/2162402X.2020.1785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Feldmann A, Hoffmann A, Bergmann R, Koristka S, Berndt N, Arndt C, Rodrigues Loureiro L, Kittel-Boselli E, Mitwasi N, Kegler A, Lamprecht C, González Soto KE, Bachmann M, Versatile chimeric antigen receptor platform for controllable and combinatorial T cell therapy, Oncoimmunology. 9 (2020) 1785608. 10.1080/2162402X.2020.1785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Feldmann A, Hoffmann A, Kittel-Boselli E, Bergmann R, Koristka S, Berndt N, Arndt C, Loureiro LR, Mitwasi N, Jureczek J, Bornhaeuser M, Bachmann M, A Novel Revcar Platform for Switchable and Gated Tumor Targeting, Blood. 134 (2019) 5611–5611. 10.1182/blood-2019-128436. [DOI] [Google Scholar]
- [62].Ebert LM, Yu W, Gargett T, Brown MP, Logic-gated approaches to extend the utility of chimeric antigen receptor T-cell technology, Biochem. Soc. Trans 46 (2018) 391–401. 10.1042/BST20170178. [DOI] [PubMed] [Google Scholar]
- [63].Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M, Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells, Nat. Biotechnol 31 (2013) 71–75. 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wilkie S, Van Schalkwyk MCI, Hobbs S, Davies DM, Van Der Stegen SJC, Pereira ACP, Burbridge SE, Box C, Eccles SA, Maher J, Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling, J. Clin. Immunol 32 (2012) 1059–1070. 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- [65].Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ, Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo, Cancer Immunol. Res 1 (2013) 43–53. 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].King-Peoples TR, Posey AD, Splitting signals drives CARs further, Nat. Cancer 2021 29. 2 (2021) 873–875. 10.1038/s43018-021-00257-x. [DOI] [PubMed] [Google Scholar]
- [67].Larson SM, Truscott LC, Chiou TT, Patel A, Kao R, Tu A, Tyagi T, Lu X, Elashoff D, De Oliveira SN, Pre-clinical development of gene modification of haematopoietic stem cells with chimeric antigen receptors for cancer immunotherapy, Hum. Vaccines Immunother 13 (2017) 1094–1104. 10.1080/21645515.2016.1268745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G, Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety, Leukemia. 24 (2010) 1160–1170. 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, Brouns SA, Spencer DM, Till BG, Jensen MC, Riddell SR, Press OW, Combining a CD20 Chimeric Antigen Receptor and an Inducible Caspase 9 Suicide Switch to Improve the Efficacy and Safety of T Cell Adoptive Immunotherapy for Lymphoma, PLoS One. 8 (2013) e82742. 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang X, Chang WC, Wong CLW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC, A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells, Blood. 118 (2011) 1255–1263. 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Paszkiewicz PJ, Fräßle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, Sadelain M, Liu L, Jensen MC, Riddell SR, Busch DH, Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia, J. Clin. Invest 126 (2016) 4262–4272. 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vogler I, Newrzela S, Hartmann S, Schneider N, Von Laer D, Koehl U, Grez M, An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy, Mol. Ther 18 (2010) 1330–1338. 10.1038/mt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Philip B, Kokalaki E, Mekkaoui L, Thomas S, Straathof K, Flutter B, Marin V, Marafioti T, Chakraverty R, Linch D, Quezada SA, Peggs KS, Pule M, A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy, Blood. 124 (2014) 1277–1287. 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- [74].Koristka S, Ziller-Walter P, Bergmann R, Arndt C, Feldmann A, Kegler A, Cartellieri M, Ehninger A, Ehninger G, Bornhäuser M, Bachmann MP, Anti-CAR-engineered T cells for epitope-based elimination of autologous CAR T cells, Cancer Immunol. Immunother 68 (2019) 1401–1415. 10.1007/s00262-019-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Derer S, Bauer P, Lohse S, Scheel AH, Berger S, Kellner C, Peipp M, Valerius T, Impact of Epidermal Growth Factor Receptor (EGFR) Cell Surface Expression Levels on Effector Mechanisms of EGFR Antibodies, J. Immunol 189 (2012) 5230–5239. 10.4049/jimmunol.1202037. [DOI] [PubMed] [Google Scholar]
- [76].Minutolo NG, Sharma P, Poussin M, Shaw LC, Brown DP, Hollander EE, Smole A, Rodriguez-Garcia A, Hui JZ, Zappala F, Tsourkas A, Powell DJ, Quantitative Control of Gene-Engineered T-Cell Activity through the Covalent Attachment of Targeting Ligands to a Universal Immune Receptor, J. Am. Chem. Soc 142 (2020) 6554–6568. 10.1021/JACS.9B11622/SUPPL_FILE/JA9B11622_SI_007.AVI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M, Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin, Proc. Natl. Acad. Sci. U. S. A 109 (2012) E690–E697. 10.1073/PNAS.1115485109/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li L, Fierer JO, Rapoport TA, Howarth M, Structural analysis and optimization of the covalent association between SpyCatcher and a peptide Tag, J. Mol. Biol 426 (2014) 309–317. 10.1016/J.JMB.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hui JZ, Tamsen S, Song Y, Tsourkas A, LASIC: Light Activated Site-Specific Conjugation of Native IgGs, Bioconjug. Chem 26 (2015) 1456. 10.1021/ACS.BIOCONJCHEM.5B00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Warden-Rothman R, Caturegli I, Popik V, Tsourkas A, Sortase-Tag Expressed Protein Ligation (STEPL): combining protein purification and site-specific bioconjugation into a single step, Anal. Chem 85 (2013) 11090–11097. 10.1021/AC402871K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Eyquem J, Mansilla-Soto J, Giavridis T, Van Der Stegen SJC, Hamieh M, Cunanan KM, Odak A, Gönen M, Sadelain M, Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection, Nat. 2017 5437643. 543 (2017) 113–117. 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cho JH, Collins JJ, Wong WW, Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses, Cell. 173 (2018) 1426–1438.e11. 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reinke AW, Grant RA, Keating AE, A synthetic coiled-coil interactome provides heterospecific modules for molecular engineering, J. Am. Chem. Soc 132 (2010) 6025–6031. 10.1021/ja907617a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Thompson KE, Bashor CJ, Lim WA, Keating AE, Synzip protein interaction toolbox: In vitro and in vivo specifications of heterospecific coiled-coil interaction domains, ACS Synth. Biol 1 (2012) 118–129. 10.1021/sb200015u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Acharya A, Ruvinov SB, Gal J, Moll JR, Vinson C, A heterodimerizing leucine zipper coiled coil system for examining the specificity of a position interactions: Amino acids I, V, L, N, A, and K, Biochemistry. 41 (2002) 14122–14131. 10.1021/bi020486r. [DOI] [PubMed] [Google Scholar]
- [86].Cho JH, Collins JJ, Wong WW, Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses HHS Public Access, Cell. 173 (2018) 1426–1438. 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS, ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells, Biol. Blood Marrow Transplant 25 (2019) 625–638. 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- [88].Cho JH, Okuma A, Sofjan K, Lee S, Collins JJ, Wong WW, Engineering advanced logic and distributed computing in human CAR immune cells, Nat. Commun 12 (2021). 10.1038/S41467-021-21078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Landgraf KE, Williams SR, Steiger D, Gebhart D, Lok S, Martin DW, Roybal KT, Kim KC, convertibleCARs: A chimeric antigen receptor system for flexible control of activity and antigen targeting, Commun. Biol 3 (2020) 1–13. 10.1038/s42003-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lajoie MJ, Boyken SE, Salter AI, Bruffey J, Rajan A, Langan RA, Olshefsky A, Muhunthan V, Bick MJ, Gewe M, Quijano-Rubio A, Johnson J, Lenz G, Nguyen A, Pun S, Correnti CE, Riddell SR, Baker D, Designed protein logic to target cells with precise combinations of surface antigens, Science (80-. ). 369 (2020) eaba6527. 10.1126/science.aba6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ng AH, Nguyen TH, Gómez-Schiavon M, Dods G, Langan RA, Boyken SE, Samson JA, Waldburger LM, Dueber JE, Baker D, El-Samad H, Modular and tunable biological feedback control using a de novo protein switch, Nature. 572 (2019) 265–269. 10.1038/s41586-019-1425-7. [DOI] [PubMed] [Google Scholar]
- [92].Urbanska K, Lynn RC, Stashwick C, Thakur A, Lum LG, Powell DJ, Targeted cancer immunotherapy via combination of designer bispecific antibody and novel gene-engineered T cells, J. Transl. Med 12 (2014). 10.1186/s12967-014-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G, CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients, J. Clin. Invest 121 (2011) 1822–1826. 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Karches CH, Benmebarek MR, Schmidbauer ML, Kurzay M, Klaus R, Geiger M, Rataj F, Cadilha BL, Lesch S, Heise C, Murr R, Vom Berg J, Jastroch M, Lamp D, Ding J, Duewell P, Niederfellner G, Sustmann C, Endres S, Klein C, Kobold S, Bispecific Antibodies Enable Synthetic Agonistic Receptor-Transduced T Cells for Tumor Immunotherapy, Clin. Cancer Res 25 (2019) 5890–5900. 10.1158/1078-0432.CCR-18-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ridgway JBB, Presta LG, Carter P, “Knobs-into-holes” engineering of antibody C H 3 domains for heavy chain heterodimerization, Protein Eng. 9 (1996) 617–621. https://academic.oup.com/peds/article/9/7/617/1527831 (accessed November 19, 2021). [DOI] [PubMed] [Google Scholar]
- [96].Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, Schwaiger M, Stubenrauch KG, Sustmann C, Thomas M, Scheuer W, Klein C, Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 11187–11192. 10.1073/PNAS.1019002108/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, Hansen-Estruch C, Chamberlain AK, Truhlar SM, Conner EM, Atwell S, Kuhlman B, Demarest SJ, Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface, Nat. Biotechnol 2014 322. 32 (2014) 191–198. 10.1038/nbt.2797. [DOI] [PubMed] [Google Scholar]
- [98].Krummel MF, Cahalan MD, The Immunological Synapse: a Dynamic Platform for Local Signaling, J. Clin. Immunol 30 (2010) 364. 10.1007/S10875-010-9393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia, N. Engl. J. Med 371 (2014) 1507–1517. 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Purbhoo MA, Irvine DJ, Huppa JB, Davis MM, T cell killing does not require the formation of a stable mature immunological synapse, Nat. Immunol 2004 55. 5 (2004) 524–530. 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- [101].Faroudi M, Utzny C, Salio M, Cerundolo V, Guiraud M, Müller S, Valitutti S, Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: Manifestation of a dual activation threshold, Proc. Natl. Acad. Sci 100 (2003) 14145–14150. 10.1073/PNAS.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH, T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia, Sci. Transl. Med 3 (2011). 10.1126/SCITRANSLMED.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM, T cell responses: naïve to memory and everything in between, Adv. Physiol. Educ 37 (2013) 273. 10.1152/ADVAN.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Viaud S, Ma JSY, Hardy IR, Hampton EN, Benish B, Sherwood L, Nunez V, Ackerman CJ, Khialeeva E, Weglarz M, Lee SC, Woods AK, Young TS, Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory, Proc. Natl. Acad. Sci. U. S. A 115 (2018) E10898–E10906. 10.1073/pnas.1810060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Halim L, Maher J, CAR T-cell immunotherapy of B-cell malignancy: the story so far., Ther. Adv. Vaccines Immunother 8 (2020) 2515135520927164. 10.1177/2515135520927164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Milone MC, Xu J, Chen S-J, Collins MA, Zhou J, Powell DJ, Melenhorst JJ, Engineering-enhanced CAR T cells for improved cancer therapy, Nat. Cancer 2021 28. 2 (2021) 780–793. 10.1038/s43018-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, Ma J, Nunez V, Shen J, Woods AK, Wright TM, Schultz PG, Kim CH, Young TS, Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies, Proc. Natl. Acad. Sci. U. S. A 113 (2016) E459–E468. 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, Yang S, Kochenderfer JN, Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains, Mol. Ther 25 (2017) 2452–2465. 10.1016/J.YMTHE.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Albert S, Koristka S, Gerbaulet A, Cartellieri M, Arndt C, Feldmann A, Berndt N, Loureiro LR, von Bonin M, Ehninger G, Eugster A, Bonifacio E, Bornhäuser M, Bachmann MP, Ehninger A, Tonic Signaling and Its Effects on Lymphopoiesis of CAR-Armed Hematopoietic Stem and Progenitor Cells, J. Immunol 202 (2019) 1735–1746. 10.4049/JIMMUNOL.1801004. [DOI] [PubMed] [Google Scholar]
- [110].Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, de Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ, Chimeric antigen receptor T-cell therapy — assessment and management of toxicities, Nat. Rev. Clin. Oncol. 2017 151. 15 (2017) 47–62. 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, Pazdur R, FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell‐Induced Severe or Life‐Threatening Cytokine Release Syndrome, Oncologist. 23 (2018) 943. 10.1634/THEONCOLOGIST.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, Ma J, Nunez V, Shen J, Woods AK, Wright TM, Schultz PG, Kim CH, Young TS, Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies, Proc. Natl. Acad. Sci. U. S. A 113 (2016) E459–E468. 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Arndt C, Fasslrinner F, Loureiro LR, Koristka S, Feldmann A, Bachmann M, Adaptor car platforms—next generation of T cell-based cancer immunotherapy, Cancers (Basel). 12 (2020). 10.3390/cancers12051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Caratelli S, Sconocchia T, Arriga R, Coppola A, Lanzilli G, Lauro D, Venditti A, Del Principe MI, Buccisano F, Maurillo L, Ferrone S, Sconocchia G, FCγ chimeric receptor-engineered T cells: Methodology, advantages, limitations, and clinical relevance, Front. Immunol 8 (2017) 457. 10.3389/FIMMU.2017.00457/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Munoz J, Jaglowski S, McKinney MS, Isufi I, Stiff PJ, Sachs J, Ranger A, Harris P, Payumo F, Akard LP, A Phase 1 Study of ACTR087 in Combination with Rituximab, in Subjects with Relapsed or Refractory CD20-Positive B-Cell Lymphoma, Blood. 134 (2019) 244–244. 10.1182/BLOOD-2019-123810. [DOI] [Google Scholar]
- [116].Unum Therapeutics shares nosedive as FDA places hold on cell therapy trial - MedCity News, (n.d.). https://medcitynews.com/2019/07/unum-shares-nosedive-as-fda-places-hold-on-cell-therapy-trial/ (accessed November 22, 2021). [Google Scholar]
- [117].Unum Therapeutics Provides Updates to its Phase 1 Trial of ACTR707 for HER2+ Solid Tumor Cancers | BioSpace, (n.d.). https://www.biospace.com/article/releases/unum-therapeutics-provides-updates-to-its-phase-1-trial-of-actr707-for-her2-solid-tumor-cancers/ (accessed November 22, 2021). [Google Scholar]
- [118].Unum Therapeutics Announces Company Name Change to Cogent Biosciences, Highlights Recent Scientific and Operational Progress, (n.d.). https://www.prnewswire.com/news-releases/unum-therapeutics-announces-company-name-change-to-cogent-biosciences-highlights-recent-scientific-and-operational-progress-301145637.html (accessed November 22, 2021).
- [119].Calibr and AbbVie’s switchable cancer CAR-T begins clinical trials -, (n.d.). https://pharmaphorum.com/news/calibr/ (accessed November 22, 2021). [Google Scholar]
- [120].Calibr Announces Preliminary Clinical Data from First-in-Human Clinical Trial of Switchable CAR-T Cells | Scripps Research, (n.d.). https://www.scripps.edu/news-and-events/press-room/2021/20211208-calibr-announces-clinical-data-car-t-cells.html (accessed January 13, 2022). [Google Scholar]
- [121].Wermke M, Kraus S, Ehninger A, Bargou RC, Goebeler ME, Middeke JM, Kreissig C, von Bonin M, Koedam J, Pehl M, Bornhäuser M, Einsele H, Ehninger G, Cartellieri M, Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML, Blood. 137 (2021) 3145–3148. 10.1182/BLOOD.2020009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Blackstone Life Sciences, Cellex Cell Professionals, and Intellia Therapeutics Launch New CAR T-Cell Company — GEMoaB, (n.d.). https://www.gemoab.com/newsandinsights/gemoab-announces-first-patient-dosed-with-the-affinity-tailored-t-cell-adaptor-gem3psca-in-a-phase-i-study-in-psca-positive-late-stage-solid-tumors-83×7k-d2clp-sbcbr-7cnwe-88p3z-f4jt2-6gxws (accessed November 22, 2021). [Google Scholar]