Abstract
We examined various methods to enhance the accessibility of intracytoplasmic sperm injection (ICSI) technology to more users by making the technique easier, more efficient, and practical. First, the methods for artificially removing the mouse sperm tail were evaluated. Trypsin treatment was found to efficiently remove the sperm tails. The resultant sperm cells had a lower oocyte activation capacity; however, the use of activated oocytes resulted in the same fecundity as that of fresh, untreated sperm. Pre-activated oocytes were more resistant to physical damage, showed higher survival rates, and required less time per injection. Testing this method in rats yielded similar results, although the oocyte activation method was different. Remarkably, this method resulted in higher birth rates of rat progeny than with conventional methods of rat ICSI. Our method thereby streamlines mouse and rat ICSI, making it more accessible to laboratories across many disciplines.
Keywords: Intracytoplasmic sperm injection (ICSI), Mouse, Oocyte activation, Rat, Trypsin treatment
Intracytoplasmic sperm injection (ICSI) has been improved in mice through the usage of piezo-driven micromanipulators [1, 2]. However, it is difficult for inexperienced operators to hold a motile sperm cell with a thin glass pipette, remove the sperm tail with a piezoelectric pulse, and inject only the sperm head into a fragile oocyte. ICSI would be more accessible if the technique were streamlined.
Currently, various mechanical and chemical methods are employed to remove the tail or plasma membrane of spermatozoa to study fertilization. Mechanical treatments, including sonication, freeze-thawing, and freeze-drying, have been reported [3,4,5,6,7,8]. When these sperm are injected, the oocytes are activated. However, studies showed that spermatozoa with their tails removed by heat, lysolecithin, pronase, and NaOH treatments could not activate oocytes after ICSI [9,10,11,12]. Nonetheless, artificial activation of injected oocytes by SrCl2 or electrical stimulation treatments [13, 14] can result in live offspring [9,10,11,12,13, 15]. However, in both cases, the developmental potential of ICSI embryos from these spermatozoa is reduced compared to that of recently generated and untreated spermatozoa [3, 4, 16]. Thus, mechanically or chemically treated spermatozoa may lose their activation capacity as well as their nuclear integrity. To simplify the ICSI technique, a method to remove the sperm tails while retaining the developmental potential of the sperm is crucial.
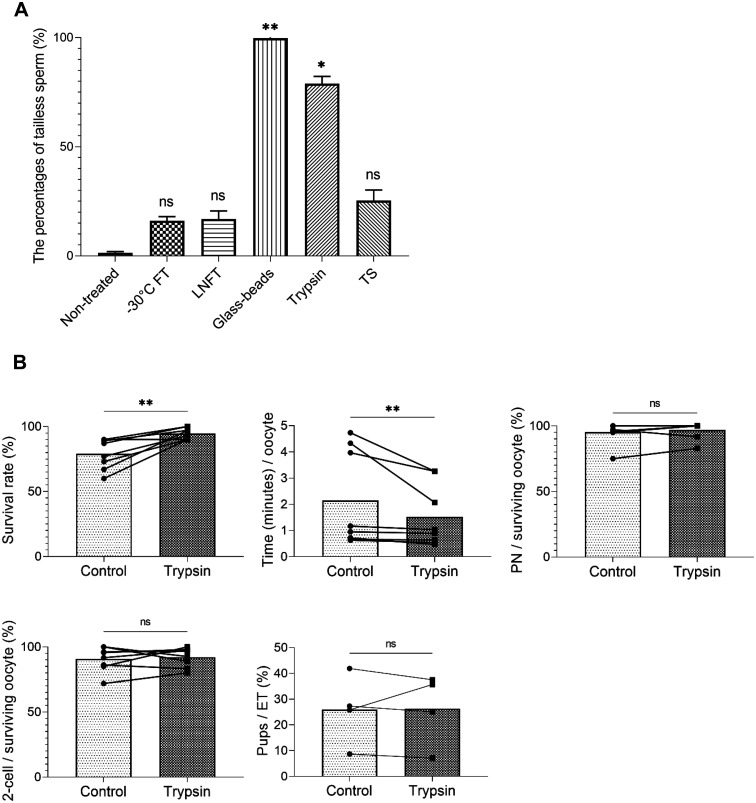
First, we attempted to remove the sperm tails by the following methods: two different freeze-thaw procedures (freezing at –30°C [–30°CFT] and freezing in liquid nitrogen [LNFT]), glass-bead homogenization (glass beads), trypsin treatment (trypsin), and TrypLETM Select (TS) (Thermo Fisher Scientific, Inc., Minato-ku, Tokyo, Japan) treatment. Trypsin is commonly used in cell culture, and its action can be easily halted by serum, whereas TS is widely used as a trypsin substitute. All methods successfully removed the tails with varying efficiency (Fig. 1A, Supplementary Fig. 1). The freeze-thaw methods of –30°CFT and LNFT resulted in 16% and 16.7% tailless sperm, respectively. Treatment with glass beads, trypsin, and TS removed the tails in 99.7%, 79.3%, and 25.4% of the total sperm cells, respectively (Fig. 1A).
Fig. 1.
Comparison of the efficiency of sperm tail removal between treatments, and the comparison of two different ICSI methods, conventional and trypsin treatment with pre-activated oocytes. (A) The percentage of tailless sperm in sperm suspensions after each treatment. All treatments could remove the sperm tail, but the efficiency varied. non-treated control: non-treated, –30°C freeze-thawed: –30°CFT, liquid nitrogen freeze-thawed: LNFT, glass bead homogenized: glass beads, trypsin-treated: trypsin, and TrypLETM Select-treated: TS. * P ≤ 0.05, ** P ≤ 0.01, ns P > 0.05 using Kruskal–Wallis test with Dunn’s multiple comparisons test. Mean ± SD. (B) Comparison of control conventional ICSI and ICSI with trypsin-treated sperm with pre-activated oocytes. Eight operators with different ICSI technical experience performed both ICSI methods. Survival rate (%) refers to the oocyte survival rate after sperm injection. Time (min)/oocyte denotes the required time for sperm injection per one oocyte. PN/surviving oocyte (%) and 2-cell/surviving oocyte (%) indicate the rate of ICSI embryos that developed into PN and 2-cells among the surviving oocytes, respectively. Pups/ET (%) corresponds to the litter size rates from ICSI embryos that were transferred into pseudo-pregnant females. Time (min)/oocyte, survival rate (%), PN/surviving oocyte (%), and 2-cell/surviving oocyte (%) in control and trypsin treatment. n = 8. Pups/ET (%) in control and trypsin treatment. n = 4. Normality was tested with the Shapiro–Wilk test. ** P ≤ 0.01, ns P > 0.05 using Wilcoxon test (Time (min)/oocyte, PN/surviving oocyte (%)) and paired t-test (survival rate (%), 2-cell/surviving oocyte (%), pups/ET (%)). See Supplementary Table 1 for the original data for the graphs of Fig. 1B. All data were analyzed using the Prism 9 software (GraphPad, San Diego, CA).
Secondly, to examine the developmental ability of sperm after each treatment, we injected the sperm heads into mature oocytes. The freeze-thaw methods retained the sperm cells’ oocyte activation ability, while the other treatments did not. Sperm heads from glass beads, trypsin, and TS treatments showed a significantly lower pronucleus formation rate than those from the untreated piezo-cut control (Table 1). However, as mentioned earlier, even sperm cells that are unable to activate oocytes may still produce offspring upon artificially activating the oocytes after injection. Incomplete oocyte activation by the injected sperm head can cause early termination of embryonic development, which complicates developmental analysis. To avoid these complications, we artificially activated the oocytes prior to the injection of glass bead-, trypsin-, and TS-treated sperm. After ICSI, embryos produced from the treated sperm, except those treated with glass beads, showed blastocyst development rates similar to those produced from the untreated control sperm (Supplementary Table 1). Kishigami et al. [17] showed that activated oocytes were more tolerant than non-activated oocytes to the mechanical stress involved in the injection procedure (Table 1), which is very advantageous for inexperienced operators. They also reported that artificially activated oocytes can support full-term development following the injection of sperm or round spermatids for 100 min after activation.
Table 1. In vivo development of mouse ICSI embryos using sperms with their tails artificially removed.
| Animal | Treatment of sperm | Pre-activation of oocyte | No. of oocytes injected | No. of oocytes survived (%) * | No. of embryos developed to |
No. of embryos transferred | No. of offspring (%) *** | |
|---|---|---|---|---|---|---|---|---|
| pn (%) ** | 2-cell (%) ** | |||||||
| Mouse | Piezo-cut | – | 116 | 93 (80) | 91 (98) | 91 (98) | 91 | 49 (54) a |
| Naturally tail less | – | 55 | 53 (96) | 52 (98) | 50 (94) | 50 | 8 (16) b | |
| –30°CFT | – | 119 | 110 (92) a | 98 (89) | 88 (80) | 88 | 9 (10) b | |
| –30°CFT | + | 60 | 60 (100) b | 60 (100) | 57 (95) | 57 | 12 (22) b | |
| LNFT | – | 126 | 105 (83) | 103 (98) | 100 (95) | 100 | 37 (37) b | |
| LNFT | + | 60 | 55 (92) | 55 (100) | 51 (93) | 51 | 10 (20) b | |
| Glass-beads | – | 60 | 52 (87) | 18 (35) | N.D. | N.D. | N.D. | |
| Glass-beads | + | 119 | 111 (93) | 111 (100) | 105 (95) | 105 | 19 (18) b | |
| Trypsin | – | 60 | 41 (68) a | 11 (27) | N.D. | N.D. | N.D. | |
| Trypsin | + | 109 | 96 (88) b | 93 (97) | 93 (97) | 93 | 48 (52) a | |
| TS | – | 60 | 44 (73) a | 11 (25) | N.D. | N.D. | N.D. | |
| TS | + | 109 | 94 (86) b | 93 (99) | 89 (95) | 89 | 23 (26) b | |
* Percentages relative to the number of oocytes injected. ** Percentages relative to the number of oocytes survived. *** Percentages relative to the number of embryos transferred. Each experiment was repeated three times. N.D.: not determined. pn: pronucleus. – indicates not artificially activated oocyte. + indicates artificially activated oocyte. Statistical analysis of oocyte viability was performed between – and + oocytes before activation for each treatment, and that of the number of offspring was performed between piezo-cut control and each treatment. Significant χ2 comparisons a vs. b, P < 0.05.
To evaluate the full-term developmental ability of sperm heads from each treatment group, ICSI embryos were transferred into the oviducts of pseudo-pregnant female mice. As shown in Table 1, ICSI embryos from all treatment groups could develop to full-term; however, the developmental rates of –30°CFT-, LNFT-, glass beads-, and TS-treated sperm (10%, 37%, 18%, and 26%, respectively) were significantly lower than those of the non-treated piezo-cut control (54%). We also examined the combination of pre-activated oocytes injected with –30°CFT or LNFT sperm. Even in this case, the full-term developmental rates of the sperm were significantly lower than those of the control (Table 1), indicating that oocyte activation itself does not affect the in vivo developmental efficiency of mouse ICSI embryos. Conversely, sperm treated with trypsin showed full-term developmental rates equivalent to those of non-treated control sperm (52%, Table 1). TS treatment, but not trypsin treatment, diminished the full-term developmental potential of the sperm. Unlike trypsin treatment, it is not necessary to quench the enzymatic reaction of TS with trypsin inhibitors (e.g., serum) in cell culture. Instead, the enzyme was diluted through several washes. The remaining enzyme, although negligible in cell culture, may have affected the developmental ability of the ICSI embryos.
To investigate why trypsin-treated sperm exhibited higher viability than sperm treated by other means, we analyzed the chromosome dynamics of ICSI embryos using live-cell imaging with monomeric red fluorescent protein 1 fused with histone H2B (H2B-mRFP1, Supplementary Fig. 2A). Normal chromosome segregation during early cleavage of the embryo is necessary for full-term development. Previous studies demonstrated that membrane-damaged sperm, such as those subjected to freeze-thaw treatment, showed chromosomal aberrations after ICSI [18], and such abnormal chromosomal segregation (ACS) during early embryogenesis in ICSI or cloned embryos leads to early pregnancy loss [6, 18]. In our experiment, the percentages of embryos from the –30°CFT-, LNFT-, glass beads-, and TS-treated groups with normal chromosome segregation (NCS) through the morula stage were only 10%–24% (Supplementary Fig. 2B). In contrast, 40% of ICSI embryos from trypsin-treated sperm developed to the morula stage without ACS, and the overall NCS rates were the same as those from the untreated control (Supplementary Fig. 2B). These results applied to the embryo birth rates as well. Thus, trypsin treatment may be gentler on the sperm genome than the other tail-removal methods tested, although it impedes the oocyte activation ability of the sperm heads.
Subsequently, to investigate whether our method could practically facilitate mouse ICSI, eight operators with varying levels of micromanipulation experience were asked to perform both conventional piezo-cut and trypsin-treated sperm ICSI with pre-activated oocytes. Pre-activated oocytes injected with trypsin-treated sperm by seven operators tended to have higher survival rates than those injected conventionally, and the time taken for injection was also shorter in the trypsin/pre-activation method than the conventional method (Supplementary Table 2). Although there were no significant differences in the two-cell and full-term developmental rates between the two methods, statistical analysis using all operator data showed significant differences between the two ICSI methods in oocyte viability and the time required for sperm injection (Fig. 1B). This reduction in time may be effective in reducing the burden on the operators and vulnerability of the tailless sperm during incubation [19]. Survival rates also tend to be higher with the new method, which will be useful in the maintenance of mouse strains, and in reproduction and fertilization studies. Operator proficiency plays a vital role in the developmental rate of ICSI embryos using either method; however, these data suggest that the new ICSI method is simpler than the conventional method, even when performed by operators with suboptimal manipulation experience.
Rat sperm and oocytes are more difficult to handle than those of mice; thus, rat ICSI has been a challenge in many laboratories. Therefore, in this study, we validated the ICSI method using rats. Because tail removal by sonication is commonly employed in rats [20, 21], we compared ICSI using sonicated and trypsin-treated sperm (Supplementary Figs. 1G–I). As expected, trypsin treatment removed the tails of the rat sperm. Conventional rat ICSI is performed with a thin injection needle to prevent oocyte death [20, 22]. However, the thin injection needle cannot aspirate the entire rat sperm head. As only one sperm head can be aspirated at a time, conventional rat ICSI requires more skill and time than that required for mice (Supplementary Fig. 3A–A”). However, as in mice, activated rat oocytes were resistant to physical manipulation and enabled the use of a larger needle (Supplementary Fig. 3B–B”). Despite the thick needle, sperm injection into pre-activated rat oocytes resulted in embryonic survival rates comparable to or better than those of conventional rat ICSI with a thin needle. Furthermore, oocyte pre-activation improved the birth rate in rats, unlike in mice (Table 2). Collected rat oocytes are known to show spontaneous activation [23], and we avoided this by artificially activating oocytes immediately after their collection. These findings indicate that this method not only streamlines ICSI, but also improves the rat oocyte survival rate.
Table 2. In vivo development of rat ICSI embryos using sperms with their tails artificially removed.
| Animal | Treatment of sperm | Type of injection needle * | Pre-activation of oocyte | No. of oocytes injected | No. of oocytes survived (%) ** | No. of embryos developed to pn (%) *** | No. of embryos transferred | No. of offspring (%) **** |
|---|---|---|---|---|---|---|---|---|
| Rat | Sonicated | Thin | – | 89 | 66 (74) | 61 (92) | 61 | 5 (8) |
| Sonicated | Thick | – | 70 | 52 (74) | 52 (100) | 36 | 2 (6) | |
| Sonicated | Thick | + | 141 | 118 (84) | 118 (100) | 90 | 16 (18) | |
| Trypsin | Thick | – | 70 | 50 (71) a | 41 (82) | 31 | 0 | |
| Trypsin | Thick | + | 302 | 252 (83) b | 225 (89) | 167 | 26 (16) |
* The diameters of the thin and thick injection needles are approximately 3 µm and 10 µm, respectively. ** Percentages relative to the number of oocytes injected. *** Percentages relative to the number of oocytes survived. **** Percentages relative to the number of embryos transferred. Each experiment was repeated three times. pn: pronucleus. – indicates not artificially activated oocyte. + indicates artificially activated oocyte. Statistical analysis of oocyte viability was performed between – and + oocytes before activation for each treatment. Significant χ2 comparisons a vs. b, P < 0.05.
In conclusion, we found that trypsin treatment efficiently removed the sperm tail, and pre-activated oocytes injected with such trypsin-treated sperm had higher full-term developmental potential when compared with that used in conventional ICSI, in both mice and rats. Moreover, the time required for the injection procedure can be reduced and the oocyte survival rate can be increased by using trypsin-treated sperm and pre-activated oocytes. Thus, trypsin treatment of sperm and pre-activation of oocyte will make mouse and rat ICSI procedures easier and more readily accessible to laboratories.
Methods
Animals
Female and male C57BL/6 × DBA/2 (B6D2F1) and ICR mice (8–10 weeks of age) were obtained from SLC (Hamamatsu, Japan). Female and male Wistar rats were obtained from Charles River Laboratories (Yokohama, Japan). Four-week-old females, 12–16-week-old males, and eight-week-old female rats were used for oocyte collection, sperm collection, and embryo transfer, respectively. Animals were maintained in pathogen-free conditions. All animal experiments were approved by the Animal Experimentation Committee of Yamanashi University, University of Tokyo, and University of Tsukuba, and performed in accordance with the guidelines of the committee.
Media
HEPES-buffered CZB [1] medium was used for gamete handling and ICSI. TYH medium (LSI Medience Corporation, Tokyo, Japan) [24] was used for rat sperm collection. CZB [25] for mice and mR1ECM [26] or rat KSOM (ARK Resource, Kumamoto, Japan) for rats were used as embryo culture media in an atmosphere of 5% CO2.
Collection of spermatozoa and removal of sperm tail
Mouse sperm cells were collected from the cauda epididymides of male mice. While testing various methods of removing sperm tails, sperm clumps were placed in tubes containing 1 mL of HEPES–CZB, centrifuged at 200 g for 5 min, and incubated at 37°C for 30 min to disperse the intact sperm. After incubation, they were aliquoted into other tubes and subjected to various treatments to remove the tails, and one tube was used as the control for untreated sperm. For freeze-thaw treatment, tubes with sperm suspension were placed directly in a freezer at –30 °C and stored overnight (–30°CFT) or in liquid nitrogen for 5 min (LNFT). For the glass-bead homogenization treatment, approximately half the volume of glass beads was added to the tubes and vortexed for 45 sec, and the supernatant was transferred to a new tube. For tail removal with trypsin, the sperm suspension was centrifuged again at 200 g for 5 min. After discarding the supernatant, 0.25% trypsin–EDTA was added to the tube and was mixed by gentle pipetting, and incubated at 37 °C for 5 min. DMEM with 10% fetal bovine serum was added to inactivate the trypsin and centrifuged at 200 g for 5 min at 4°C. It was washed three more times with DMEM. After washing, approximately 10 µl of HEPES–CZB was added to the tube and stored at 4°C until further use. For the TrypLETM Select treatment, the sperm suspension was centrifuged again at 200 g for 5 min. After the supernatant was discarded, TrypLETM Select was added to the tube. They were mixed by gentle pipetting, and the tube was incubated at 37 °C for 20 min. After incubation, the sperm samples were washed three times with DMEM and resuspended in 10 µL of HEPES–CZB. The suspension was stored at 4°C until further use. The percentage of tailless sperm in each treatment group was calculated using a counting chamber.
Rat sperm clumps were collected from the cauda epididymides of male rats and suspended in 1 ml of TYH medium. After incubation for 30 min, the supernatant of the sperm suspension was transferred to another tube. Sonication was performed for 15–20 sec using VS-25 (VELVO-CLEAR Co., Tokyo, Japan). Trypsin treatment of rat sperm was performed in the same way as for mouse sperm.
Oocyte preparation
To efficiently induce superovulation, mature female mice and rats were injected with equine chorionic gonadotropin (eCG, ASKA Animal Health, Tokyo, Japan) followed by human chorionic gonadotropin (hCG, ASKA) after 48 h. For rats, LHRH and anti-inhibin serum (Central Research, Tokyo, Japan) were administered simultaneously 2 days before eCG administration [27]. Cumulus–oocyte complexes were collected from the oviducts 14–16 h after the injection of hCG, and incubated in HEPES–CZB drops containing 0.1% bovine testicular hyaluronidase (Sigma-Aldrich, MA, USA) for 3 min to disperse the cumulus cells. The denuded oocytes were washed twice and transferred to fresh embryo culture medium.
ICSI and embryo transfer
To inject spermatozoa, 1–2 μL of untreated or treated spermatozoa suspension was added to a drop of HEPES–CZB containing 10% (w/v) polyvinylpyrrolidone and mixed. Sperm heads were then injected into oocytes following the method described by Kimura and Yanagimachi [1]. Mouse oocytes were pre-activated by culturing in Ca2+-free CZB medium containing 10 mM SrCl2 for 20 min and washing thrice in CZB medium, and rat oocytes were cultured in medium containing 5 µM ionomycin for 5 min and washed thrice in a new culture medium [12, 28]. The sperm heads were then injected into the activated oocytes. The oocyte-injected sperm were incubated in culture media at 37 °C and 5% atmospheric CO2. ICSI embryos with two pronuclei at 6 h for mice and 20 h for rats after injection were considered fertilized. Two-cell-stage mouse embryos and pronuclear-stage rat embryos were transferred to a 0.5-day pseudo-pregnant female oviduct that had been mated with a vasectomized male. The offspring were delivered by cesarean section on days 18.5 and 20.5 in mice and rats, respectively.
Live-cell imaging analysis
To observe the chromosome dynamics in ICSI embryos, mRNAs of H2B-mRFP1 and EGFP-tubulin were prepared as previously described [29]. The collected oocytes were injected with a few pL of the mRNA solution before activation or sperm injection. The embryos that formed pronuclei were transferred to drops of CZB medium on a glass-bottomed dish, placed in an incubator with the CV1000 imaging system (Yokogawa Electric Corp., Musashino, Tokyo, Japan), and incubated at 37°C and 5% atmospheric CO2. Time-lapse images were taken over a duration of 70 h at 15 min intervals. At each time point, 51 fluorescence images were captured 2 μm apart along the z-axis for optical sectioning.
Statistical analysis
The oocyte survival rates (Table 1, Table 2, Supplementary Table 1), blastocyst development rates (Supplementary Table 1), and offspring birth rates (Table 1 and Table 2) were analyzed using the chi-squared test. P < 0.05 was considered statistically significant. Fig. 1A and B data were analyzed using Prism 9 software (GraphPad, San Diego, CA, USA).
Conflict of interests
There are no conflicts of interest.
Supplementary
Acknowledgments
The authors thank Miss K. Kishida for providing critical comments and caring for the experimental animals in this study. This work was partially funded by the Japan Society for the Promotion of Science to E. M. (15H04605, 20H03638) and T. W. (16H02593), Naito Foundation to S. W., Asada Science Foundation to T. W., and Takeda Science Foundation to T. W.
References
- 1.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod 1995; 52: 709–720. [DOI] [PubMed] [Google Scholar]
- 2.Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod Biomed Online 2005; 10: 247–288. [DOI] [PubMed] [Google Scholar]
- 3.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol Reprod 1996; 55: 789–795. [DOI] [PubMed] [Google Scholar]
- 4.Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol 1998; 16: 639–641. [DOI] [PubMed] [Google Scholar]
- 5.Wakayama T, Whittingham DG, Yanagimachi R. Production of normal offspring from mouse oocytes injected with spermatozoa cryopreserved with or without cryoprotection. J Reprod Fertil 1998; 112: 11–17. [DOI] [PubMed] [Google Scholar]
- 6.Tateno H, Kamiguchi Y. Evaluation of chromosomal risk following intracytoplasmic sperm injection in the mouse. Biol Reprod 2007; 77: 336–342. [DOI] [PubMed] [Google Scholar]
- 7.Kwon IK, Park KE, Niwa K. Activation, pronuclear formation, and development in vitro of pig oocytes following intracytoplasmic injection of freeze-dried spermatozoa. Biol Reprod 2004; 71: 1430–1436. [DOI] [PubMed] [Google Scholar]
- 8.Liu JL, Kusakabe H, Chang CC, Suzuki H, Schmidt DW, Julian M, Pfeffer R, Bormann CL, Tian XC, Yanagimachi R, Yang X. Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol Reprod 2004; 70: 1776–1781. [DOI] [PubMed] [Google Scholar]
- 9.Cozzi J, Monier-Gavelle F, Lièvre N, Bomsel M, Wolf JP. Mouse offspring after microinjection of heated spermatozoa. Biol Reprod 2001; 65: 1518–1521. [DOI] [PubMed] [Google Scholar]
- 10.Jiang MX, Zhu Y, Zhu ZY, Sun QY, Chen DY. Effects of cooling, cryopreservation and heating on sperm proteins, nuclear DNA, and fertilization capability in mouse. Mol Reprod Dev 2005; 72: 129–134. [DOI] [PubMed] [Google Scholar]
- 11.Yan W, Morozumi K, Zhang J, Ro S, Park C, Yanagimachi R. Birth of mice after intracytoplasmic injection of single purified sperm nuclei and detection of messenger RNAs and MicroRNAs in the sperm nuclei. Biol Reprod 2008; 78: 896–902. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Mizutani E, Ono T, Wakayama T. Production of normal mice from spermatozoa denatured with high alkali treatment before ICSI. Reproduction 2009; 137: 779–792. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development 1995; 121: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 14.Bos-Mikich A, Whittingham DG, Jones KT. Meiotic and mitotic Ca2+ oscillations affect cell composition in resulting blastocysts. Dev Biol 1997; 182: 172–179. [DOI] [PubMed] [Google Scholar]
- 15.Ogura A, Matsuda J, Yanagimachi R. Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc Natl Acad Sci USA 1994; 91: 7460–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward MA, Kaneko T, Kusakabe H, Biggers JD, Whittingham DG, Yanagimachi R. Long-term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection. Biol Reprod 2003; 69: 2100–2108. [DOI] [PubMed] [Google Scholar]
- 17.Kishigami S, Wakayama S, Nguyen VT, Wakayama T. Similar time restriction for intracytoplasmic sperm injection and round spermatid injection into activated oocytes for efficient offspring production. Biol Reprod 2004; 70: 1863–1869. [DOI] [PubMed] [Google Scholar]
- 18.Yamagata K, Suetsugu R, Wakayama T. Assessment of chromosomal integrity using a novel live-cell imaging technique in mouse embryos produced by intracytoplasmic sperm injection. Hum Reprod 2009; 24: 2490–2499. [DOI] [PubMed] [Google Scholar]
- 19.Long H, Lu SS, Kuang YP, Yan ZG, Liang HX, Yu S, Chai WR, Yan Z, Lyu QF. Incubation of sperm heads impairs fertilization and early embryo development following intracytoplasmic sperm injection (ICSI) by decreasing oocyte activation in mice. Biotechnol Lett 2013; 35: 1823–1829. [DOI] [PubMed] [Google Scholar]
- 20.Hirabayash M, Kato M, Aoto T, Sekimoto A, Ueda M, Miyoshi I, Kasai N, Hochi S. Offspring derived from intracytoplasmic injection of transgenic rat sperm. Transgenic Res 2002; 11: 221–228. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Ishikawa A, Kaneko R, Yagi T, Hochi S, Hirabayashi M. Production of transgenic rats by ooplasmic injection of spermatogenic cells exposed to exogenous DNA: a preliminary study. Mol Reprod Dev 2004; 69: 153–158. [DOI] [PubMed] [Google Scholar]
- 22.Hirabayashi M, Kato M, Ito J, Hochi S. Viable rat offspring derived from oocytes intracytoplasmically injected with freeze-dried sperm heads. Zygote 2005; 13: 79–85. [DOI] [PubMed] [Google Scholar]
- 23.Keefer CL, Schuetz AW. Spontaneous activation of ovulated rat oocytes during in vitro culture. J Exp Zool 1982; 224: 371–377. [DOI] [PubMed] [Google Scholar]
- 24.Toyoda Y, Yokoyama M, Hoshi T. Studies on the fertilization of mouse eggs in vitro: I. In vitro fertilization of eggs by fresh epididymal sperm. Jpn J Anim Reprod 1971; 16: 5. (in Japanese). [Google Scholar]
- 25.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- 26.Miyoshi K, Kono T, Niwa K. Stage-dependent development of rat 1-cell embryos in a chemically defined medium after fertilization in vivo and in vitro. Biol Reprod 1997; 56: 180–185. [DOI] [PubMed] [Google Scholar]
- 27.Honda A, Tachibana R, Hamada K, Morita K, Mizuno N, Morita K, Asano M. Efficient derivation of knock-out and knock-in rats using embryos obtained by in vitro fertilization. Sci Rep 2019; 9: 11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizutani E, Jiang JY, Mizuno S, Tomioka I, Shinozawa T, Kobayashi J, Sasada H, Sato E. Determination of optimal conditions for parthenogenetic activation and subsequent development of rat oocytes in vitro. J Reprod Dev 2004; 50: 139–146. [DOI] [PubMed] [Google Scholar]
- 29.Yamagata K, Suetsugu R, Wakayama T. Long-term, six-dimensional live-cell imaging for the mouse preimplantation embryo that does not affect full-term development. J Reprod Dev 2009; 55: 343–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.