Abstract
Acetaminophen (N-acetyl-p-aminophenol (APAP), also known as paracetamol) is one of the most common medications used by the general population, including pregnant people. Although many human observational and animal model studies have shown associations between prenatal and early postnatal APAP exposure and attention deficit hyperactivity disorder, autism spectrum disorders, and altered neurodevelopment, the existing literature is limited. In particular, no mouse studies of prenatal APAP exposure have investigated offspring attention deficits in behavioral tasks specifically designed to measure attention, and no prior rodent studies have utilized ‘omics’ technologies, such as transcriptomics, for an untargeted exploration of potential mechanisms. We randomly assigned pregnant mice (starting embryonic day 4–10) to receive APAP (150 mg/kg/day) or vehicle control through postnatal day 14. We evaluated 111 mouse offspring in a battery of behavioral tests, including pup ultrasonic vocalizations, elevated plus-maze, open field test, CatWalk (gait), pre-pulse inhibition, and the automated 5-choice serial reaction time task. Prefrontal cortex was collected at birth from 24 pups for RNA sequencing. Developmental APAP treatment resulted in increased and hastened separation-induced pup vocalizations between postnatal days 2 and 11, as well as decreased ambulation and vertical rearings in the open field in male but not female adult offspring. APAP treatment was also associated with altered sex-specific prefrontal cortex gene expression relating to glutathione and cytochrome p450 metabolism, DNA damage, and the endocrine and immune systems. This study provides additional evidence for the neurodevelopmental harm of prenatal APAP exposure and generates hypotheses for underlying molecular pathways via RNA sequencing.
Keywords: Acetaminophen, Paracetamol, ADHD, Autism, Neurodevelopment, Behavior, RNA sequencing, Prenatal
1. Introduction
Acetaminophen (N-acetyl-p-aminophenol (APAP), also known as paracetamol) is the most common pharmaceutical ingredient in the United States – it is the active ingredient in over 600 prescription and over-the-counter medications for pain, fever, cough, cold, and allergies (Bauer et al., 2021; Consumer Healthcare Products Association, n.d.). APAP-containing medications are among the only analgesics not contraindicated during pregnancy (FDA has reviewed possible risks of pain medicine use during pregnancy, 2015). Consequently, their use is reported by over half of pregnant women in many populations (Werler et al., 2005; Rebordosa et al., 2009; Bandoli et al., 2020).
Despite such widespread use, evidence from human observational studies suggests that prenatal APAP exposure may be associated with attention deficit hyperactivity disorder (ADHD) (Avella-Garcia et al., 2016; Baker et al., 2020a; Brandlistuen et al., 2013; Chen et al., 2019; Gustavson et al., 2021; Ji et al., 2020; Liew et al., 2019; Liew et al., 2014; Stergiakouli et al., 2016; Streissguth et al., 1987; Thompson et al., 2014; Tovo-Rodrigues et al., 2018; Ystrom et al., 2017), autism spectrum disorders (ASD) (Avella-Garcia et al., 2016; Ji et al., 2020; Liew et al., 2016a), and a multitude of other behavioral and neurodevelopmental abnormalities (Bauer et al., 2021). Although many of these observational studies accounted for confounding by indications (e.g., fever, chronic pain, and headaches), and maternal characteristics (e.g., age at birth, race/ethnicity, body mass index, neuropsychiatric conditions, and tobacco and alcohol use), unobserved and unmeasured confounding remain a possibility. For instance, maternal polygenic risk scores for ADHD are associated with use of APAP during late pregnancy (Leppert et al., 2019), indicating a strong potential for genetic confounding, yet no observational studies of prenatal APAP and child ADHD have explicitly accounted for genetic factors. Furthermore, a sibling study that examined unmeasured familial confounding in the Norwegian national cohort found a substantial family effect, suggesting that unmeasured familial factors, including genetics, may partially explain the association of maternal APAP use with child ADHD (Gustavson et al., 2021). These limitations can be partially addressed via randomized animal studies, in which confounding is impossible.
Supporting the human epidemiological evidence, various randomized experimental studies have found adverse effects of prenatal and early postnatal APAP exposure on rodent behavior and cognition (Hay-Schmidt et al., 2017; Klein et al., 2020; Philippot et al., 2017; Rigobello et al., 2021; Suda et al., 2021; Viberg et al., 2014; Philippot et al., 2018; Blecharz-Klin et al., 2017; Blecharz-Klin et al., 2018). However, studies on neurodevelopmental effects of prenatal APAP exposure in animal models remain limited. First, the results of these studies are not always in agreement. For instance, one study found no behavioral effects of prenatal acetaminophen exposure in mice (Saad et al., 2016). Second, while many observational studies have linked prenatal APAP with child ADHD in humans (Avella-Garcia et al., 2016; Baker et al., 2020a; Brandlistuen et al., 2013; Chen et al., 2019; Gustavson et al., 2021; Ji et al., 2020; Liew et al., 2019; Liew et al., 2014; Stergiakouli et al., 2016; Streissguth et al., 1987; Thompson et al., 2014; Tovo-Rodrigues et al., 2018; Ystrom et al., 2017), no mouse studies of prenatal APAP exposure have investigated offspring attention deficits in behavior tasks specifically designed to measure attention. Finally, the mechanisms linking APAP exposure to abnormal neurodevelopment are unclear. Rodent studies have explored APAP effects on the cannabinoid (Philippot et al., 2018; Gould et al., 2012) and prostaglandin (Dean et al., 2012) pathways, altered neurotransmission (Blecharz-Klin et al., 2017; Blecharz-Klin et al., 2015a; Blecharz-Klin et al., 2015b; Blecharz-Klin et al., 2016; Blecharz-Klin et al., 2019), brain-derived neurotrophic factor (Klein et al., 2020; Viberg et al., 2014; Blecharz-Klin et al., 2018), neuronal number in the sexually dimorphic nucleus of the hypothalamus (Hay-Schmidt et al., 2017), and oxidative stress (Klein et al., 2020; Rigobello et al., 2021). However, no studies have employed ‘omics’ technologies such as RNA sequencing for an untargeted exploration of potential mechanisms. Here, we address these limitations by 1) examining the effect of prenatal and early postnatal APAP exposure on offspring behavior, including attention deficits in the 5-choice serial reaction time task; and 2) exploring mechanistic pathways that may underly the effects of APAP exposure via unbiased RNA sequencing.
2. Methods
2.1. Mice
Two cohorts of timed pregnant C57BL/6 J females were purchased from the Jackson Laboratory (Bar Harbor, ME). Half were randomized to control and half to APAP treatments. The first cohort of 12 dams were ordered at embryonic day 3 (E3) and randomized into control and APAP treatment groups at E4. Only 1 APAP-treated and 3 control mice were pregnant among the first cohort. Owing to this low pregnancy rate (25%), we ordered the second cohort of 28 timed pregnant females at E7 and randomized them at E10. The second cohort had 23 (82%) pregnancies. One litter in the second cohort had only 3 pups. All other litters in the second cohort were reduced to 4 pups per dam, with remaining offspring euthanized for tissue collection. Offspring sex ratios within reduced litters were balanced except for the 3-pup litter (1 male, 2 females), and a litter with 3 males and 1 female. Litter reduction occurred at birth for all litters except 4 control and 4 treatment litters which were tested for Pup Ultrasonic Vocalizations (USV) through P11. These USV-tested litters were reduced to 4 pups per litter after USVs were measured on P11. All procedures were performed within the Columbia University Institute of Comparative Medicine, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All protocols were approved by the Columbia Institutional Animal Care and Use Committee.
2.2. Treatment
Pregnant mice were given 150 mg/kg/day APAP (rounded to the nearest gram of mouse weight) or 0 mg APAP (control) in gelatin tablets. Gelatin tablets consisted of 14% gelatin powder, 5% sucrose, and 0.5% fruit flavor (SodaStream™ drops) in water. Mice received one 0.5 mL tablet each day with the appropriate APAP dose or control. One day prior to the start of treatment, all mice received a control gelatin tablet in their home cage to become habituated. During treatment, pregnant mice were weighed daily to determine dosage, and monitored to ensure gelatin tablets were completely consumed (typically in under 5 min). The dose of 150 mg/kg/day is within the range of human exposure accounting for allometric scaling (Reagan-Shaw et al., 2008), and has previously been shown to result in the highest serum concentrations of acetaminophen without inducing liver toxicity in mice (Yang et al., 2013; Reel et al., 1992; Larrey et al., 1986; Whitehouse et al., 1977; Boyd and Bereczky, 1966). We continued to dose mice through postnatal day 14 (P14) because the peak brain growth that occurs during gestation in humans does not occur until the postnatal period in mice (Semple et al., 2013).
2.3. Behavioral tasks
Mouse behavioral analyses were conducted in the Columbia Mouse NeuroBehavior core. Offspring of control and APAP treated dams from the 1st cohort were tested in the open field test (OFT), elevated plus-maze (EPM), and 5-choice serial reaction time task (5CSRTT). Offspring in the 2nd cohort underwent the same tasks, and were additionally tested for pup ultrasonic vocalizations (USV), CatWalk XT (Noldus), and pre-pulse inhibition (PPI). Behavior tasks tested 21 offspring from the 1st cohort and 90 offspring from the 2nd cohort (n = 111). Because test history can influence animal behavior in future tasks, a deliberate ordering of tasks from least to most invasive has been suggested (McIlwain et al., 2001; Blokland et al., 2012; Schneider et al., 2011). Therefore, the most stressful task – PPI – was completed last. Additionally, at least one week of spacing between tasks was maintained to allow time for effects of prior experience to diminish before moving to subsequent tasks. Female estrous cycle was not monitored.
2.3.1. Pup ultrasonic vocalizations (USV)
Pup ultrasonic vocalizations were measured for 6 pups per dam among 4 control and 4 treatment dams (22 female and 26 male pups) in the second cohort. Repeated testing occurred on postnatal days 2, 5, 8, and 11, and pup paw tattoos were employed to track individual pups. In a random order, pups were removed one at a time from the dam and placed into a 10 × 8 × 8.5 cm plastic container with a 0.5 cm layer of fresh bedding. The container holding the individual pup was immediately placed inside a sound-attenuating chamber (Med Associates, St. Albans, VT, USA) where USVs were recorded for 3 min by an ultrasonic microphone sensitive to frequencies of 10–180 kHz (Avisoft Ultra-SoundGate condenser microphone capsule CM16; Avisoft Bioacoustics, Berlin, Germany). After recording, each pup was marked before being returned to the nest to avoid repeated handling. Calls were recorded in 16-bit resolution with a sampling rate of 250 kHz by Avisoft Recorder software. Calls were manually counted from USV spectrograms displayed in Avisoft SASLab Pro by a trained investigator blind to treatment status.
2.3.2. Open field test (OFT)
Mice (7 weeks old) were allowed to roam freely in a clear Plexiglas open field arena (27.31 × 27.31 × 20.32cm, Med Associates ENV-510) lit with dim light (~5 lx) for 60 min. Activity Monitor Version 7 tracking software (Med Associates Inc.) was used in conjunction with infrared beams along the x, y, and z planes of the arena to automatically measure total distance traveled (ambulatory movement), time spent in the center versus edge zones, and the total number of rearings which were defined as the number of times the mouse disrupted two infrared beams by standing on its hind limbs. After 60 min, the mouse was returned to the home cage and the arena cleaned with 70% ethanol followed by water, and wiped dry.
2.3.3. Elevated plus-maze (EPM)
Mice (6 weeks old) were evaluated in one trial in the elevated plus maze (EPM), an apparatus with two open arms (30 × 5 cm) and two closed arms (30 × 5 cm) extending from a central junction (5×5 cm). Photo beams embedded at arm entrances register movements. Room illumination was approximately 5 lx. Mice were placed in the junction facing a closed arm and allowed to roam the maze for five min, during which the time spent in the open arms, closed arms, and junction was recorded automatically by the MED-PC V 64 bit Software (Med Associates). The maze was cleaned with 70% ethanol and wiped dry between individuals. Due to equipment malfunction, data were not recorded for 7 offspring in the first cohort. The task could not be re-run for these mice because their first trial partially habituated them to the maze.
2.3.4. CatWalk
Mice (8 weeks old) were allowed to ambulate freely across the CatWalk XT apparatus (Noldus Information Technology, Leesburg, VA), an illuminated walled glass walkway (130 × 10 cm) until they completed three compliant runs (full crossings with a speed variation under 80% in 20 s or less). Room illumination was approximately 30 lx. During the task, footprints were captured by a high-speed camera underneath the walkway. CatWalk XT software was used to automatically measure 226 parameters related to mouse gait and locomotion, including paw statistics, intensity measures, stride length, width, base of support, distance between ipsilateral prints, cadence, % limb support, regularity index, speed, and speed variation. A trained experimenter visually inspected all automatically scored runs, and manually classified any prints that were too ambiguous for the software to identify accurately. The walkway was cleaned with a paper towel moistened with 70% ethanol and wiped dry between individuals.
2.3.5. Pre-pulse inhibition (PPI)
Pre-pulse inhibition of acoustic startle was measured using the SR-Laboratory System (San Diego Instruments). Each mouse (29–30 weeks old) was placed inside a plexiglass tube and subjected to a five-minute habituation period with ambient light and a background noise of 68 dB (dB). Following habituation, each mouse was presented with seven trial types across six discrete blocks of trials for a total of 42 trials of acoustic stimuli separated by randomly generated inter-trial intervals ranging between 10 and 20 s. Trials were presented pseudo randomly such that each type occurred once within each block. Mouse startle response was measured as the pressure exerted against the tube, which was translated into a voltage. From the onset of the acoustic startle stimulus, voltage was measured every 1 ms over a 65 ms period, and the maximum amplitude over this sampling period was defined as the startle response. One trial measured the response to 100 ms 68 dB background noise (baseline), and another measured the response to a 40 ms 110 dB sound burst. The remaining five trial types consisted of a 20 ms pre-pulse followed by 100 ms background then a 40 ms, 110 dB burst. Pre-pulses were 74, 78, 82, 86, or 92 dB. PPI % inhibition was calculated separately for each pre-pulse dB level as: (response to 110 dB alone - response to 110 dB with pre-pulse) / response to 110 dB alone. The baseline response was defined as: (response to 110 dB alone - response to 68 dB alone) / response to 110 dB alone.
2.3.6. 5-choice serial reaction time task (5CSRTT)
From each cohort, 4 males and 4 females were randomly selected to participate in this task. Testing started at 14–16 weeks of age and continued through 26–27 weeks of age. Mice were tested in a chamber with five touch screens and a food reward tray (Campden Instruments Ltd., Loughborough, UK), which can deliver food reward of 1:1 dilution of water to strawberry flavored Ensure. The deployment of tasks and measurement of outcomes was managed with Whisker Server and ABET II software (Layfette Instruments, Layfette, Indiana). Prior to pretraining, mice were restricted to 85% free-feeding weight. Body weights were monitored throughout the experiment and diets adjusted accordingly, with each mouse receiving approximately 1.5–4 g of rodent chow per day.
2.3.6.1. Pre-training.
The pre-training consisted of four stages. In stage one (ABET II habituation 2a procedure), mice underwent one 20 min session of habituation in the chamber with freely available reward and no images on the touch screens. Stage two (initial touch procedure) consisted of 30 trials over 60 min. The stimulus (a white square) is presented on one of the five touch screens at a time. The stimulus position is pseudo randomly chosen such that it does not appear in the same position more than three times consecutively. After 30 s, the stimulus is removed and 7 μL reward delivered. If the mouse touches the screen with the stimulus, three-times the reward is dispensed. Food rewards are accompanied by illumination of the tray and a tone. Mouse entry into the tray turns off the light and starts a five-second inter-trial-interval (ITI). All mice advanced to stage three after one day of initial touch training. In stage three (must touch procedure), the stimulus is displayed on one of five screens following the pseudo random procedure outlined above. There is no response if the mouse touches a blank screen. If the mouse touches the stimulus, 7 μL reward is dispensed accompanied by illumination of the tray and a tone. Mouse entry into the tray turns off the light and starts a five-second ITI. Mice were required to complete 30 trials within 60 min on one day of testing before advancing to stage four. In stage four (must initiate procedure), mice are trained to initiate trials by entering and exiting the illuminated food tray. The procedure begins with 7 μL free reward in the illuminated tray. The mouse must nose poke and exit the tray for a trial to begin, in which the stimulus appears on one screen following the pseudo random procedure outlined above. As in stage three, there is no response if the mouse touches a blank screen, but touching the stimulus results in 7 μL reward delivery accompanied by illumination of the tray and a tone. Mouse entry into the tray to collect the reward turns off the light and starts a five-second ITI, but does not automatically start the next trial as in stage three. Instead, after the ITI, the tray light is illuminated and the mouse must nose poke and exit to initiate the next trial. In stage four, mice were required to complete 30 trials within 60 min during two consecutive days of testing to proceed to the 5CSRTT.
2.3.6.2. 5CSRTT training.
All sessions begin with 7 μL free reward in the illuminated tray. The mouse must nose poke and exit the tray for a trial to begin, in which the stimulus appears on one screen. Stimuli are presented in a pseudo random sequence in which the stimulus is presented four times on each of the five screens within a block of 20 trials. As in stage four of pre-training, touching the screen with the stimulus (correct response) triggers a 7 μL food reward paired with the tray light and tone. Mouse entry into the tray to collect the reward turns off the light and starts a five-second ITI. After the ITI, the tray light is illuminated, and the mouse must nose poke and exit to initiate the next trial. Unlike the pre-training procedures, failure to correctly touch the stimulus is punished. Selecting a touch screen during the 5 s delay before a stimulus is presented (premature response), selecting any of the four touchscreens that do not have the white square stimulus (incorrect response), or making no response at all during the stimulus duration (omission) causes a five s time-out paired with illumination of the entire chamber. Just like a correct response, these failures are followed by the five-second ITI and illumination of the tray, prompting the mouse to initiate the next trial.
On the first day of the 5CSRTT procedure, the white square stimulus appears for 32 s (stimulus duration). For each mouse, the stimulus duration is halved upon successfully completing the task. Task completion consists of finishing at least 50 trials in 60 min with >80% accuracy and < 20% omissions on two consecutive testing days. Mice were tested daily until they successfully completed the task with a 2 s stimulus duration. Then, mice were tested with the probes described below.
2.3.6.3. 5CSRTT probes.
In the variable stimulus duration probe, mice were randomly presented stimulus durations of 1.5, 1, 0.8, and 0.6 s (5 of each in 3 blocks of 20 trials) rather than the standard 2 s duration. In the variable delay probe, mice were randomly presented delays (between initiating the trial and the stimulus appearing on screen) of 5, 6, 7 and 8 s (5 of each in each block of 20 trials) rather than the standard 5 s delay. In the distraction probe, mice experienced the standard 2 s stimulus duration procedure with the addition of a 0.5 s burst of noise sounded at a pseudorandom time during the 5 s delay period.
2.4. Statistical analysis
For all behavior tasks, summary outcome variables were modeled with linear mixed effects models, with fixed effects for treatment, sex, and cohort, and random intercepts for dam to account for clustering by litter. Offspring sex was modeled with mixed effects logistic regression, with fixed effects for treatment and cohort, and random intercepts for dam to account for clustering by litter. All 5CSRTT mice came from different litters, so outcomes were modeled with linear regressions not including random intercepts for dam. Repeated measures models additionally included the repeated measures variable (e.g., time-bin for open field, and pre-pulse dB level for PPI) and its interaction with treatment, and random intercepts for individual nested within dam. Treatment by sex interactions were retained in final models when significant. We performed Bonferroni-corrected post-hoc pairwise comparisons for treatment interactions with sex and repeated measures variables. For behavior tasks completed by both cohorts (OFT, EPM, and 5CSRTT), we repeated the above analyses separately within each cohort. CatWalk models additionally controlled for mass and average speed during the CatWalk test, and only included mice with <60% speed variation. CatWalk results were similar in sensitivity analyses 1) excluding mass and speed terms in the model, and 2) not filtering for speed variation. In addition to modeling all 226 CatWalk parameters as separate outcomes with a false-discovery rate correction, Principal Component Analysis (PCA) was employed to identify locomotion patterns across all parameters. We then used linear regression to assess the association of acetaminophen treatment with univariate PCs, retaining only the top variance explaining PCs above the “elbow” of the scree plot.
2.5. RNA sequencing
In the second cohort, 24 pups (equal number per sex and treatment) among those euthanized at birth were randomly selected. Prefrontal cortex was collected, and RNA extracted with the RNeasy Kit (Qiagen, Hilden, Germany). Library preparation and RNA sequencing (TruSeq RNA Library Prep Kit v2 and HiSeq 4000, Illumina, San Diego, CA, USA) were performed by Genewiz (South Plainfield, NJ, USA).
FASTQ files were preprocessed with fastp using the default settings to filter bad reads, trim low quality bases, and cut adaptors (Chen et al., 2018). Reads were mapped to the GRCM38(mm10) mouse genome using BWA-MEM (Li, 2013) and assigned to exons using featureCounts (Liao et al., 2014) before performing differential expression analysis using DESeq2 (Love et al., 2014). Gene expression fold-changes compared APAP treated to control offspring, accounting for sex. Normalized counts from DESeq2 were input into Ensemble of Gene Set Enrichment Analysis (EGSEA) (Alhamdoosh et al., 2017) to determine enrichment for molecular signatures database (MSigDB) hallmark gene sets (Subramanian et al., 2005; Liberzon et al., 2015) and Kyoto encyclopedia of genes and genomes (KEGG) pathways (Kanehisa et al., 2021). We examined the top 20 EGSEA enrichments sorted by median ranking score, and also present enriched pathway-level information using FDR-adjusted P-values. To investigate sex-specific effects, we repeated EGSEA analysis stratified by sex. RNA sequencing data are deposited at Gene Expression Omnibus (GEO) accession number GSE198424.
2.6. Quantitative polymerase chain reaction (qPCR)
We performed qPCR validation for genes (excluding pseudo and predicted genes) that were identified as differentially expressed by DESeq2. From the same RNA samples used in RNA-sequencing, cDNA was generated using the SuperScript IV First-Strand Synthesis System (Invitrogen, Waltham, MA, USA). An RNA-negative control was used in reverse transcription and run in-parallel with experimental samples. Predesigned TaqMan probes (Thermo Fisher Scientific, Waltham, MA, USA) were used in conjunction with the QuantStudio Real-Time PCR (Applied Biosystems, Waltham, MA, USA) system and software to conduct qPCR and generate cycle threshold (Ct) values for three technical replicates per gene per each of the 24 samples. Reverse transcription and qPCR were performed by Genewiz (South Plainfield, NJ, USA).
Technical replicates with Ct values >35 were removed before computing the mean of technical replicates per gene per sample. Relative acetaminophen group versus control gene expression ratios for each gene were calculated using the Pfaffl equation (Pfaffl, 2001). We assumed 100% primer efficiencies (E = 2), normalized to the geometric mean (Hellemans et al., 2007; Vandesompele et al., 2002) of two reference genes (Tbp and Rcc2), and normalized to the average Ct of all control samples (e.g. delta Ct for each gene was computed as the average Ct of all control samples minus the average Ct of each sample). Thus, this equation was evaluated as 2 to the power of delta Ct for the gene of interest divided by the geometric mean of 2 to the power of delta Ct of both reference genes. The resulting relative expression values were logarithm base 2 transformed to facilitate comparison with RNA sequencing results.
3. Results
Mean [SD] mass (grams) did not differ between control (1.30 [0.095]) and APAP-treated (1.30 [0.104]) litters at birth, nor between control (22.6 [3.15]) and APAP-treated (22.9 [3.05]) adult mice at 8 weeks of age. Offspring sex ratios (including mice euthanized at birth) did not differ between control (37 females [46.3%]) and APAP-treated (46 females [49.5%]) groups (P = 0.752).
3.1. Pup ultrasonic vocalizations (USV)
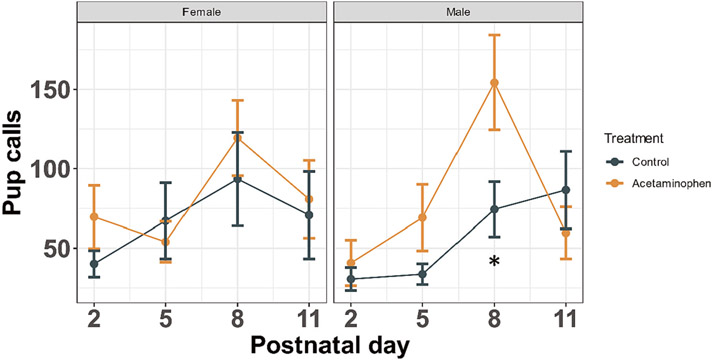
Separation-induced pup vocalizations peaked on postnatal day 8 for control and treated females and for treated males (Fig. 1). In males, control pups peaked later than treated pups, around P11. Treatment by sex by day contrasts revealed increased calls on postnatal day 8 among offspring of dams treated with APAP during pregnancy (Fig. 1, P = 0.047).
Fig. 1.
Pup Ultrasonic Vocalizations (USV). Among 4 control and 4 treatment dams, 6 pups per dam were removed one at a time from the dam and placed into an insulated chamber where USVs were recorded for 3 min by an ultrasonic microphone. Mean ± standard error shown for number of USVs recorded across 4 days of testing, stratified by sex (n = 48). Treatment by sex interaction P = 0.638. Significant post-hoc pairwise treatment by sex by day contrasts shown for repeated measures models (*P < 0.05 following Bonferroni correction).
3.2. Open field test (OFT)
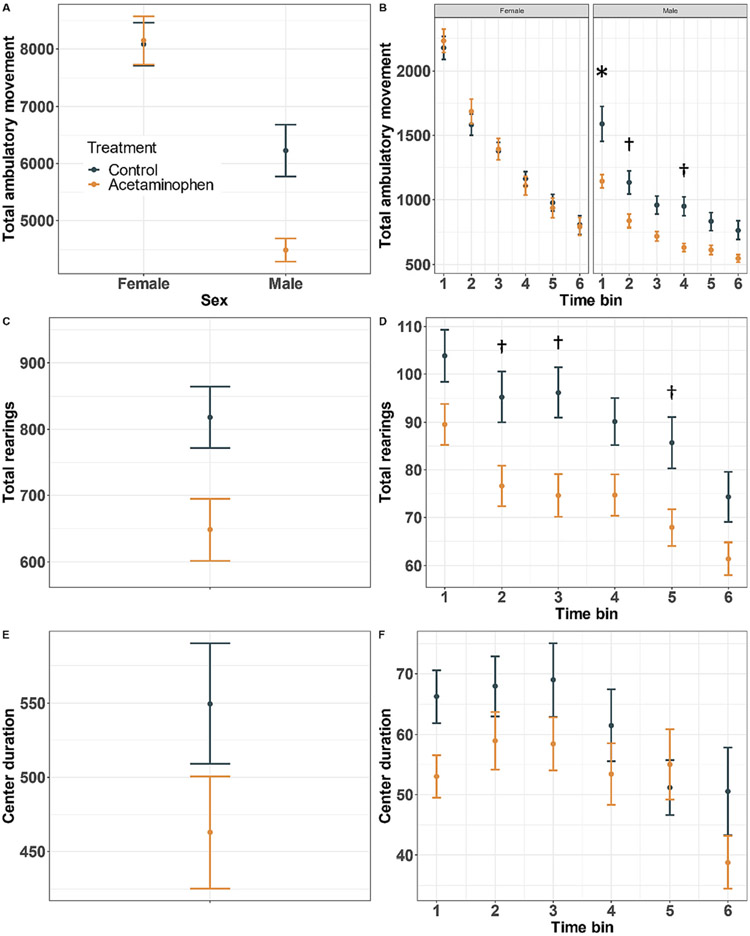
In the open field test, distance traveled was comparable in control and treated females, while control males had higher total ambulation compared to the male offspring of APAP treated dams (Fig. 2A, sex by treatment P = 0.020). There was also a sex by treatment interaction in the repeated measures model (P = 0.001), and post-hoc contrasts revealed elevated ambulatory movement in control males in the first 10-min time-bin (Fig. 2B, P = 0.004). Consistent with this finding, control offspring exhibited more total rearings than APAP-exposed offspring (Fig. 2C, P = 0.033), although there was no interaction with sex. Treatment by time-bin contrasts showed the largest difference in rearings during the third time bin (Fig. 2D, P = 0.053). While control offspring spent more time in the center of the open field chamber, the treatment term was non-significant in the overall and repeated measures models for the center duration outcome (Fig. 2E, F; treatment P = 0.378 and 0.174, respectively).
Fig. 2.
Open Field Test (OFT). Mice were allowed to roam freely in a clear Plexiglas open field arena for 60 min. Mean ± standard error shown for total ambulatory movement stratified by sex (A, B: treatment by sex interaction P = 0.020 and 0.001, respectively), total number of rearings defined as the number of times the mouse disrupted two infrared beams by standing on its hind limbs (C, D; treatment P = 0.033 and 0.127, respectively), and (E, F) time spent in the center zone of the arena (E, F; treatment P = 0.378 and 0.174, respectively) (n = 111). Significant post-hoc pairwise treatment by sex by time bin contrasts shown for repeated measures models (†P < 0.05; *P < 0.05 following Bonferroni correction).
In cohort-stratified analyses, control males and females had higher total ambulation values compared to treated mice in cohort one, but these differences were not statistically significant (Supplemental Fig. 1A, sex by treatment P = 0.932). In cohort two, however, results were consistent with the main analysis above: Distance traveled was comparable in control and treated females, while control males had higher total ambulation compared to the male offspring of APAP treated dams (Supplemental Fig. 1A, sex by treatment P = 0.050). Total ambulatory movement was generally lower for cohort two, especially among male mice (Supplemental Fig. 1A). Differences in rearings were in the same direction as in the main analysis above, with control offspring exhibiting more total rearings than APAP-exposed offspring, although these results were not statistically significant (Supplemental Fig. 1B, treatment P = 0.195 and 0.127 for cohorts one and two, respectively). As in the main analysis above, there were no significant differences among control and treatment mice in the time spent in the center of the chamber (Supplemental Fig. 1C, treatment P = 0.979 and 0.417 for cohorts one and two, respectively).
3.3. CatWalk
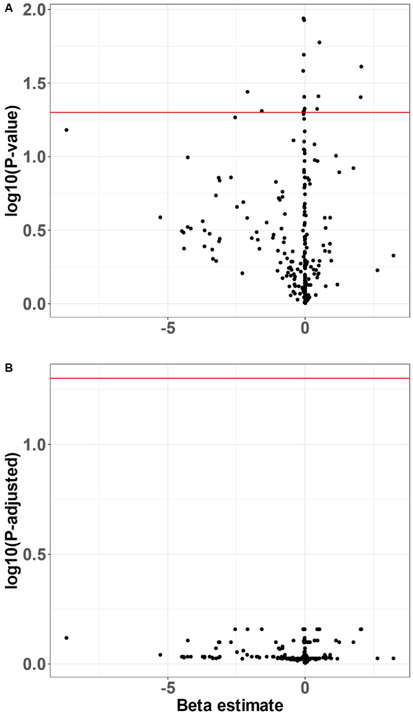
Although a small number of CatWalk parameters appeared to differ between APAP-treated and control mice, none survived adjustment for multiple comparisons (Fig. 3, Supplemental Table 1). The PCA scree plot demonstrated a steep drop off in variance explained beyond the first two PCs, which together accounted for 50.6% of the variance in the CatWalk parameter data (Supplemental Fig. 2). Linear models revealed no effects of APAP treatment on PC1 or PC2 (P = 0.870 and 0.447, respectively).
Fig. 3.
CatWalk gait analysis. Mice completed three full crossings over an illuminated walled glass walkway. CatWalk XT software automatically measured 226 parameters related to mouse gait and locomotion via a high-speed camera underneath the walkway. Raw (A) and FDR-adjusted (B) P-values and beta estimates from separate linear regressions of treatment on each parameter shown. Red horizontal line at significance threshold of P = 0.05 (n = 90).
3.4. Elevated plus-maze (EPM)
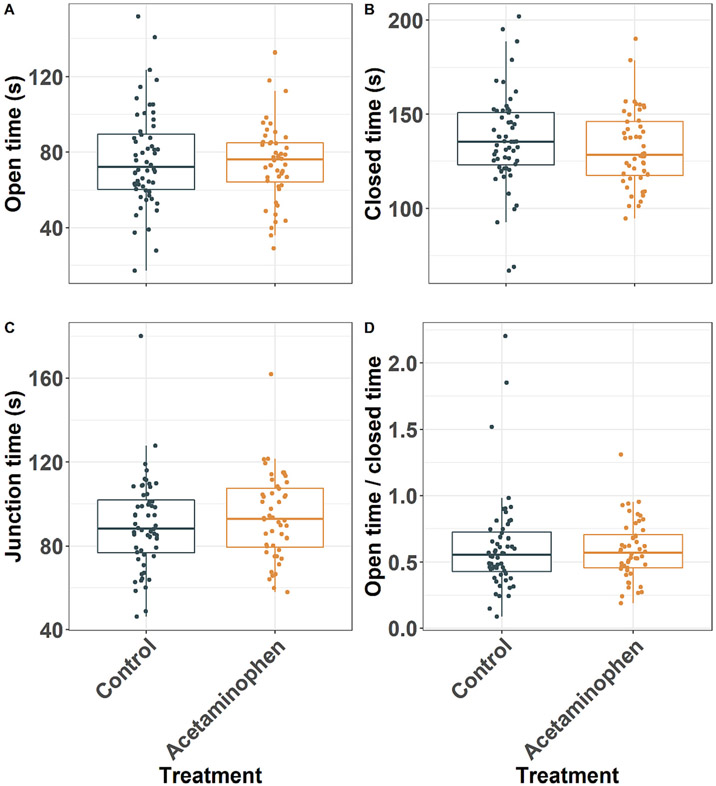
There was no difference among groups in the time mice spent in the open arms, closed arms, or junction of the elevated plus maze, nor in the ratio of time spent in the open arms versus the closed arms (Fig. 4). Results were similar in cohort-stratified analyses (Supplemental Fig. 3).
Fig. 4.
Elevated plus maze (EPM). P values presented for acetaminophen treatment effect from mixed effects models. Mice were placed in the central junction facing a closed arm and allowed to roam the maze for five-min. Individual data points and box and whiskers shown for the time spent in open arms (A, P = 0.958), closed arms (B, P = 0.469), the central junction (C, P = 0.753), and the ratio of time spent in the open versus closed arms (D, P = 0.788) (n = 104).
3.5. Pre-pulse inhibition (PPI)
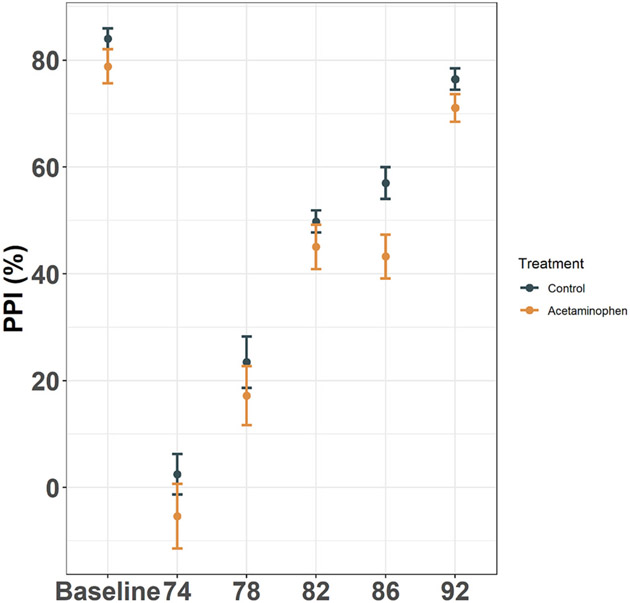
Although the APAP-treated mice had reduced PPI at a pre-pulse of 86 dB, the main effect of treatment was non-significant in the repeated measures model (P = 0.525), and no PPIs were significantly different between groups in post-hoc contrasts (Fig. 5).
Fig. 5.
Pre-pulse inhibition (PPI). Mean ± standard error shown for PPI % inhibition, calculated separately for each pre-pulse dB level as: (response to 110 dB alone - response to 110 dB with pre-pulse) / response to 110 dB alone. The baseline response was defined as: (response to 110 dB alone - response to 68 dB alone) / response to 110 dB alone (n = 90). Treatment P = 0.525; all post-hoc pairwise treatment by pre-pulse dB level contrasts were non-significant.
3.6. 5-choice serial reaction time task (5CSRTT)
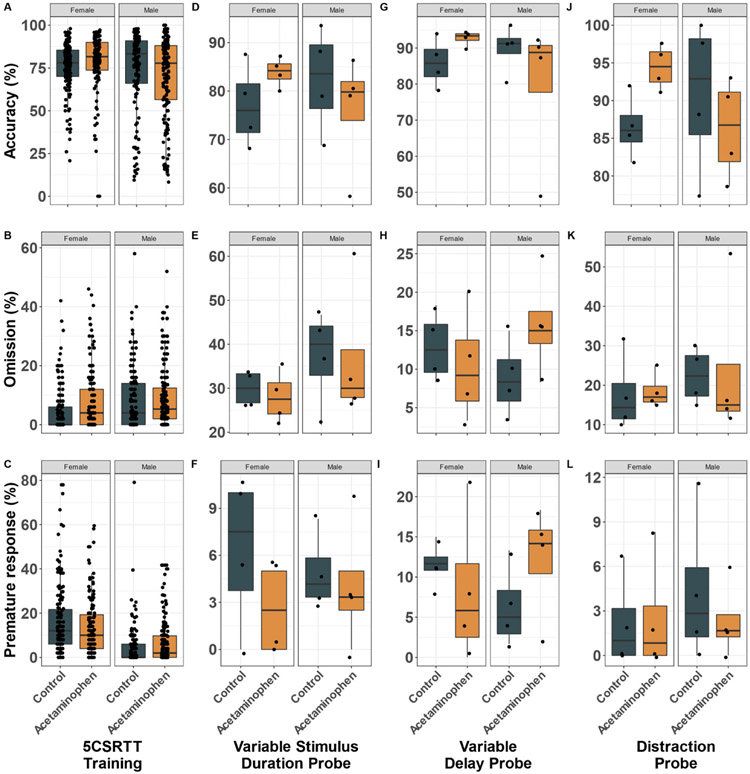
During the 5CSRTT, mean [SD] training days to advance to the 2-s stimulus duration procedure did not significantly differ between control (29.8 [7.7]) and APAP-treated (25.9 [11.6]) mice (P = 0.456). Control and treatment groups shared similar percent accuracy, omissions, and premature responses during 5CSRTT training (Fig. 6 A,B,C), in the variable stimulus duration probe (Fig. 6 D,E,F), the variable delay probe (Fig. 6 G,H,I), and the distraction probe (Fig. 6 J,K,L). Treatment and treatment by sex interactions were non-significant in all 5CSRTT models.
Fig. 6.
5-Choice Serial Reaction Time Task (5CSRTT). Individual data (one point per mouse per 5CSRTT session) and box and whiskers shown for accuracy, omission, and premature response percent for 5CSRTT training (A-C, treatment P = 0.743, 0.738, and 0.910, respectively), variable stimulus duration probe (D-F, treatment P = 0.946, 0.803, and 0.229, respectively), variable delay probe (G-I, treatment P = 0.781, 0.484, and 0.722, respectively) and distraction probe (J-L, treatment P = 0.643, 0.848, and 0.663, respectively) (n = 16 mice, see methods for details).
Results were similarly null in cohort-stratified analyses (Supplemental Figs. 4 and 5). Although there were several borderline significant treatment effects, they were not consistent between cohorts. APAP-treated mice in cohort one showed a tendency toward more omissions during 5CSRTT training (Supplemental Fig. 4B, treatment P = 0.081). In cohort two, control mice showed a tendency for more premature responses on the variable stimulus duration probe (Supplemental Fig. 5F, treatment P = 0.079), while APAP-treated mice showed a tendency for more premature responses on the variable delay probe (Supplemental Fig. 5I, treatment P = 0.096).
3.7. RNA sequencing
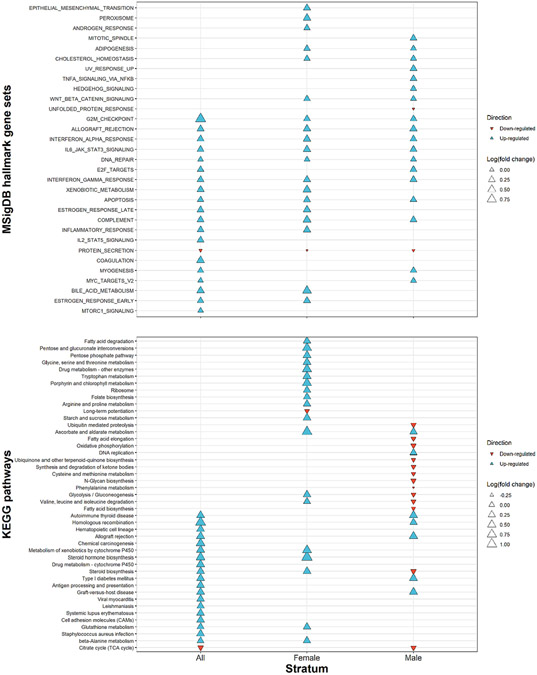
RNA-sequencing revealed eight differentially expressed genes (Rad54l, Car3, Derl3, Pax6os1, Plbd1, H2-Ea, H4c8, and Myc) and two differentially expressed predicted genes (Gm7285 and Gm20762) in prefrontal cortex between control and APAP-exposed pups (Table 1). All ten targets were upregulated by APAP treatment. Consistent with the RNA-sequencing analysis, qPCR validation showed similar APAP over control fold changes, with every gene being upregulated by APAP treatment (Table 1, Supplemental Fig. 6). Note, however, that two genes failed to amplify during qPCR validation. Enriched pathways from EGSEA (Fig. 7, Supplemental Table 2) were related to the known metabolism of APAP by glutathione S-transferase and cytochrome p450 enzymes (XENOBIOTIC_METABOLISM, Metabolism of xenobiotics by cytochrome P450, Drug metabolism - cytochrome P450, Glutathione metabolism), DNA damage (G2M_CHECKPOINT, DNA_REPAIR, E2F_TARGETS, APOPTOSIS, Homologous recombination, Chemical carcinogenesis), the endocrine system (ESTROGEN_RESPONSE_LATE, ESTROGEN_RESPONSE_EARLY, Autoimmune thyroid disease, Steroid hormone biosynthesis, Steroid biosynthesis), and the immune system (ALLOGRAFT_REJECTION, INTERFERON_ALPHA_RESPONSE, IL6_JAK_STAT3_SIGNALING, INTERFERON_GAMMA_RESPONSE, COMPLEMENT, INFLAMMATORY_RESPONSE, IL2_STAT5_SIGNALING, Autoimmune thyroid disease, Antigen processing and presentation, Graft-versus-host disease, Systemic lupus erythematosus).
Table 1.
Acetaminophen treatment over control fold change for significantly differentially expressed genes from RNA sequencing (RNA-seq).
| Gene Symbol |
Base mean |
RNA-seq log2 (Fold Change) |
Standard Error |
P-adj | qPCR log2 (Fold Change) |
|---|---|---|---|---|---|
| Rad541 | 50.502 | 0.575 | 0.091 | 0 | 0.087 |
| Car3 | 30.118 | 0.373 | 0.077 | 0.009 | 0.910 |
| Gm7285 | 38.219 | 0.453 | 0.093 | 0.009 | Untested |
| Gm20762 | 200.379 | 0.442 | 0.093 | 0.009 | Untested |
| Derl3 | 66.597 | 0.404 | 0.087 | 0.013 | 0.206 |
| Pax6os1 | 76.029 | 0.415 | 0.091 | 0.013 | Did not amplify |
| Plbd1 | 75.504 | 0.43 | 0.093 | 0.013 | 0.597 |
| H2-Ea | 70.906 | 0.414 | 0.091 | 0.016 | Did not amplify |
| H4c8 | 63.742 | 0.417 | 0.093 | 0.017 | 0.184 |
Fig. 7.
Ensemble of Gene Set Enrichment Analysis (EGSEA). APAP treatment over control fold changes in top 20 enriched terms for (A) MSigDB hallmark gene sets and (B) KEGG pathways sorted from top to bottom by EGSEA median ranking score. Analysis repeated for all mice and stratified by sex (n = 24).
Sex-stratified EGSEA revealed which pathways were either consistently or differentially enriched depending on sex (Fig. 7, Supplemental Table 2). Both sexes saw enrichment of pathways related to DNA damage (G2M_CHECKPOINT, DNA_REPAIR, APOPTOSIS) and immune activation (ALLOGRAFT_REJECTION, INTERFERON_ALPHA_RESPONSE, IL6_JAK_STAT3_SIGNALING, INTERFERON_GAMMA_RESPONSE, COMPLEMENT). However, developmental APAP effects on hormone regulation and APAP metabolism were sex-specific. APAP treatment caused upregulation of the estrogen response in females but not males, and upregulation of autoimmune thyroid disease in males but not females. In females but not males, APAP treatment upregulated pathways related to APAP metabolism by glutathione S-transferase and cytochrome p450 enzymes (XENOBIOTIC_METABOLISM, Drug metabolism – other enzymes, Metabolism of xenobiotics by cytochrome P450, Glutathione metabolism). Furthermore, some of the DNA damage related pathways were upregulated by APAP-treatment in males but not females (E2F_TARGETS and Homologous recombination).
4. Discussion
In this study of >100 mouse offspring from 27 dams, prenatal and early postnatal APAP treatment was associated with increased pup vocalizations after separation from the litter, as well as decreased ambulation and rearings in the open field task among male offspring. APAP treatment did not affect locomotion patterns in the CatWalk, time spent in the open versus closed arms in the elevated plus maze, precent inhibition in PPI, or accuracy, omissions, and premature responses in the 5CSRTT. In addition to behavioral changes, developmental APAP treatment was associated with altered prefrontal cortex gene expression relating to glutathione and cytochrome p450 metabolism, DNA damage, and the endocrine and immune systems.
Behavioral changes among male offspring in the treatment group may indicate elevated anxiety. First, treated male offspring exhibited decreased total movement and rearings during the open field test. Reduced exploratory behavior in the novel environment of the open field could indicate increased anxiety when confronted with a stressful situation. Although the open field test has certain shortcomings as a model for human anxiety disorders, the test is still sensitive to drugs used for the clinical treatment of anxiety including benzodiazepines and 5-HT1A receptor agonists (Prut and Catherine, 2003). The altered exploratory behaviors observed in the open field were most likely not attributable to motor deficits given the null treatment effect on the CatWalk test. Furthermore, treated male pups exhibited increased peak ultrasonic vocalization calls after being separated from the dam and litter. Vocalizations have commonly been used as a measure of anxiety in rodent adults and infants (Branchi et al., 2001; Hofer, 1996; Sánchez, 2003). Despite concordance of anxiety-like behaviors across two tasks, we still cannot rule out the possibility that changes in pup vocalizations were influenced by some effect of APAP exposure on maternal responsiveness (D’Amato et al., 2005). Furthermore, no differences were observed between control and APAP-treated offspring in the elevated plus-maze.
Based on genetic animal models, our results demonstrating a lack of hyperactivity does not preclude ADHD relevance. Complex human neurodevelopmental conditions such as ADHD are difficult to replicate in animal models. In fact, some of the most common rodent models of ADHD do not exhibit hyperactivity (Regan et al., 2022). Spontaneously hypertensive rats (SHR), which were developed by selective breeding of Wistar-Kyoto (WKY) rats (Okamoto and Aoki, 1963), are less active than WKY rats in running wheel and less active than Sprague Dawley rats in open field tests (Ferguson and Cada, 2003). Nevertheless, SHR rats have been used as a model for ADHD because they exhibit hyperactivity, impulsivity, and inattentiveness in other tasks, and these ADHD-like symptoms can be attenuated with stimulant treatment (Regan et al., 2022).
In agreement with well-known sex differences across a wide range of neurodevelopmental conditions, we found that APAP treatment interacted with sex in its effect on mouse behavior. Regarding associations of prenatal and early life APAP exposure with neurodevelopmental outcomes in humans, there are several reports of sex interactions (Avella-Garcia et al., 2016; Tovo-Rodrigues et al., 2018; Bertoldi et al., 2020; Laue et al., 2019), although some studies explicitly testing sex interactions have found no evidence (Tovo-Rodrigues et al., 2020; Rifas-Shiman et al., 2020; Liew et al., 2016b). These contrasting results could reflect the use of different neurobehavioral outcome measures or a lack of generalizability across study populations. Investigators have also uncovered sex interactions with APAP exposure in animal models. One study found more vertical exploration in the open field in female but not male rats exposed to 350 mg/kg/day APAP during gestation compared to controls (Klein et al., 2020). In another study, compared to control animals, male but not female rats exposed to 350 mg/kg/day APAP during pregnancy and lactation had increased apomorphine-induced stereotyped behavior, while males exposed to 35 mg/kg/day APAP displayed elevated ambulation in the open field (Rigobello et al., 2021).
RNA sequencing uncovered several candidate mechanisms of the prenatal toxicity of APAP. Many of the pathways significantly enriched in the prefrontal cortex of treated offspring have been previously studied in rodents following prenatal and early life APAP treatment. For instance, we found pathway enrichment for DNA damage and glutathione metabolism. In agreement with our findings, one study found that 350 mg/kg/day APAP during pregnancy and lactation resulted in decreased hippocampal glutathione levels and striatal superoxide dismutase activity in rat offspring (Rigobello et al., 2021). However, another rat study found no changes in prefrontal cortex or hippocampus glutathione levels following 350 mg/kg/day APAP from gestational day 6 until delivery (Klein et al., 2020). We also found altered endocrine pathways between control and treated offspring, which may align with prior work showing APAP interference with sexual development in rodents. Prenatal APAP exposure in rodents may cause decreased anogenital distance (Kristensen et al., 2011; Holm et al., 2015; van Den Driesche et al., 2015; Holm et al., 2016), decreased pelvic floor muscle weights (Axelstad et al., 2014), increased nipple retention (Axelstad et al., 2014; Mandrup et al., 2015), increased testosterone (Pereira et al., 2020), and decreased male and female fertility (Dean et al., 2016; Johansson et al., 2016; Axelstad et al., 2018; Rossitto et al., 2019a; Rossitto et al., 2019b).
In addition to pathways previously studied, we uncovered pathways that, to our knowledge, have not been studied as candidate mechanisms for the effects of prenatal APAP in vivo. RNA-sequencing revealed an upregulation of pathways related to thyroid disease in pups prenatally exposed to APAP. Although the effects of prenatal APAP exposure on thyroid hormone regulation have not been previously studied, there is evidence for APAP-associated thyroid dysregulation following adult exposure in humans (Albert et al., 2013) and rats (Motawi et al., 2019). Furthermore, in humans, maternal thyroid hormone dysregulation is associated with a wide range of child neurodevelopmental disorders (Thompson et al., 2018), including ADHD (Ghassabian et al., 2012; Päkkilä et al., 2014; Modesto et al., 2015; Ghassabian et al., 2011; Baker et al., 2020b). Thus, thyroid hormone changes might link APAP exposure with altered neurodevelopment. Similarly, transcriptomics revealed upregulation of immune system pathways in our treatment group compared to controls. The immune response remains understudied in relation to prenatal APAP exposure. Maternal immune adaptation to pregnancy involves suppressing the immune response to the fetus, which is allogeneic to the mother. Prior work indicates that non-hepatotoxic doses of APAP may interfere with fetal immune tolerance (Tiegs et al., 2014), and a recent synthesis of emerging evidence suggests a critical role of the immune system in normal neurodevelopment (Pronovost and Hsiao, 2019). Therefore, immune system alterations might link APAP exposure and neurodevelopment, and should be the subject of future studies.
Sex-stratified EGSEA revealed differential enrichment of hormone and APAP metabolic pathways, allowing us to generate hypotheses to explain the sex-specific effects of APAP on behavior observed here. One possibility is that developmental APAP exposure negatively impacted thyroid hormone signaling in males but not females. In humans, thyroid dysfunction during pregnancy is associated with child intellectual disability (Thompson et al., 2018) and ADHD (Ghassabian et al., 2012; Ghassabian et al., 2012; Päkkilä et al., 2014). Sex-specific effects on behavior could also be explained by differential APAP metabolism by males and females. Females may have been better able to upregulate glutathione and cytochrome p450 metabolism and thereby efficiently detoxify APAP and avoid adverse effects on the developing brain. This deficient metabolism of APAP in males may also explain why some DNA damage related terms were upregulated by APAP treatment in males but not females.
While being the first assessment of the effects of prenatal and early postnatal APAP exposure on gene expression in the brain and attention in the 5CSRTT is a considerable strength of this study, the results should be considered in the context of several limitations. First, despite strong evidence for associations of prenatal APAP with ADHD in humans (Avella-Garcia et al., 2016; Baker et al., 2020a; Brandlistuen et al., 2013; Chen et al., 2019; Gustavson et al., 2021; Ji et al., 2020; Liew et al., 2019; Liew et al., 2014; Stergiakouli et al., 2016; Streissguth et al., 1987; Thompson et al., 2014; Tovo-Rodrigues et al., 2018; Ystrom et al., 2017), developmental APAP exposure was not associated with mouse attention deficits in the 5CSRTT. In humans, not all individuals exposed prenatally to APAP develop ADHD. It is possible that only sub-groups with genetic predispositions or concurrent additional exposures develop ADHD following prenatal APAP exposure. Such synergism could be tested in future mouse work by using genetic models with altered neurodevelopment, or by exposing mice to multiple chemicals in addition to APAP. It is also possible that ADHD is too complex a human disorder to be translated into animal behavior. Second, although the open field and pup ultrasonic vocalizations tests indicated elevated anxiety in male offspring exposed developmentally to APAP, we saw no effect of APAP treatment on another common assay for anxiety-related behavior, the elevated plus-maze (Walf and Frye, 2007; Pellow et al., 1985). Third, gene expression was measured in the brains of offspring euthanized at delivery, and not in the animals that underwent behavior testing. While this design reduced the time-lag between prenatal APAP exposure and RNA-sequencing, it also prevented us from conducting mediation analyses to assess whether specific pathways linked APAP exposure to altered mouse behavior.
In conclusion, 150 mg/kg/day APAP exposure during gestation and lactation was associated with increased separation-induced pup vocalizations and reduced exploratory behavior in the open field. Exposure was also associated with gene expression changes in offspring prefrontal cortex indicative of increased DNA damage and altered endocrine and immune system activity. Future studies are needed to explore whether the potential mechanisms revealed by RNA-sequencing directly link perinatal APAP exposure with behavior changes.
Supplementary Material
Funding
This work was supported by the National Institute of Environmental Health Sciences (P30ES009089). The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Availability of data and materials
RNA sequencing data are deposited at Gene Expression Omnibus (GEO) accession number GSE198424. Mouse behavior data are available upon request.
CRediT authorship contribution statement
Brennan H. Baker: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. Elizabeth E. Rafikian: Methodology, Investigation. Paul B. Hamblin: Methodology, Investigation. Madeleine D. Strait: Methodology, Investigation, Writing – review & editing. Mu Yang: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition, Supervision. Brandon L. Pearson: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2022.105970.
Data availability
RNA sequencing data are deposited at Gene Expression Omnibus (GEO) accession number GSE198424. Mouse behavior data are available upon request.
References
- Albert O, Desdoits-Lethimonier C, Lesné L, et al. , 2013. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro, 28 (7), 1890–1898. [DOI] [PubMed] [Google Scholar]
- Alhamdoosh M, Ng M, Wilson NJ, et al. , 2017. Combining multiple tools outperforms individual methods in gene set enrichment analyses, 33 (3), 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella-Garcia CB, Julvez J, Fortuny J, et al. , 2016. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms, 45 (6), 1987–1996. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Christiansen S, Boberg J, et al. , 2014. Mixtures of endocrine-disrupting contaminants induce adverse developmental effects in preweaning rats, 147 (4), 489–501. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Hass U, Scholze M, Christiansen S, Kortenkamp A, Boberg J, 2018. EDC IMPACT: Reduced sperm counts in rats exposed to human relevant mixtures of endocrine disrupters. Endocrine Connect 7 (1), 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BH, Lugo-Candelas C, Wu H, et al. , 2020a. Association of prenatal acetaminophen exposure measured in meconium with risk of attention-deficit/hyperactivity disorder mediated by frontoparietal network brain connectivity, 174 (11), 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BH, Wu H, Laue HE, et al. , 2020b. Methylparaben in meconium and risk of maternal thyroid dysfunction, adverse birth outcomes, and Attention-Deficit Hyperactivity Disorder (ADHD), 139, 105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoli G, Palmsten K, Chambers CJP, 2020. Epidemiology p. Acetaminophen use in pregnancy: Examining prevalence, timing, and indication of use in a prospective birth cohort, 34 (3), 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AZ, Swan SH, Kriebel D, et al. , 2021. Paracetamol use during pregnancy—a call for precautionary action, 17 (12), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldi AD, Rifas-Shiman SL, Boing AC, et al. , 2020. Associations of acetaminophen use during pregnancy and the first year of life with neurodevelopment in early childhood, 34 (3), 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecharz-Klin K, Joniec-Maciejak I, Jawna K, et al. , 2015a. Developmental exposure to paracetamol causes biochemical alterations in medulla oblongata, 40 (2), 369–374. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Joniec-Maciejak I, Jawna K, et al. , 2015b. Effect of prenatal and early life paracetamol exposure on the level of neurotransmitters in rats—Focus on the spinal cord, 47, 133–139. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Joniec-Maciejak I, Jawna-Zboińska K, et al. , 2016. Cerebellar level of neurotransmitters in rats exposed to paracetamol during development, 68 (6), 1159–1164. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Piechal A, Jawna-Zboińska K, et al. , 2017. Paracetamol– Effect of early exposure on neurotransmission, spatial memory and motor performance in rats, 323, 162–171. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Wawer A, Jawna-Zboińska K, et al. , 2018. Early paracetamol exposure decreases brain-derived neurotrophic factor (BDNF) in striatum and affects social behaviour and exploration in rats, 168, 25–32. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Wawer A, Pyrzanowska J, Piechal A, Jawna-Zboińska K, Widy-Tyszkiewicz E, 2019. ,. Hypothalamus–Response to early paracetamol exposure in male rats offspring. Int. J. Develop. Neurosci 76, 1–5. [DOI] [PubMed] [Google Scholar]
- Blokland A, Ten Oever S, Van Gorp D, et al. , 2012. The use of a test battery assessing affective behavior in rats: order effects, 228 (1), 16–21. [DOI] [PubMed] [Google Scholar]
- Boyd E-M, Bereczky G, 1966. Liver necrosis from paracetamol. Br. J. Pharmacol. Chemother 26 (3), 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E, 2001. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav. Brain Res 125 (1–2), 49–56. [DOI] [PubMed] [Google Scholar]
- Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H, 2013. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int. J. Epidemiol 42 (6), 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu JJB, 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor., Bioinformatics 34 (17), i884–i890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-H, Pan T-L, Wang P-W, et al. , 2019. Prenatal exposure to acetaminophen and the risk of attention-deficit/hyperactivity disorder: a nationwide study in Taiwan, 80 (5), 15264. [DOI] [PubMed] [Google Scholar]
- Consumer Healthcare Products Association. https://www.chpa.org/our-issues/otc-medicines/acetaminophen. Accessed January 14, 2022.
- D’Amato FR, Scalera E, Sarli C, Moles A, 2005. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behavior Genet. 35 (1), 103–112. [DOI] [PubMed] [Google Scholar]
- Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM, 2012. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Europ. J. Neurosci 35 (8), 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A, Van Den Driesche S, Wang Y, et al. , 2016. Analgesic exposure in pregnant rats affects fetal germ cell development with inter-generational reproductive consequences 6 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2015. Accessed October 31, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy.
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, et al. , 2011. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study Pediatr. Res, 69 (7), 454–459. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Cada AM, 2003. A longitudinal study of short-and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav. Neurosci 117 (2), 271. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, De Rijke YB, et al. , 2012. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study Thyroid, 22 (2), 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Seillier A, Weiss G, et al. , 2012. Acetaminophen differentially enhances social behavior and cortical cannabinoid levels in inbred mice, 38 (2), 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson K, Ystrom E, Ask H, et al. , 2021. Acetaminophen use during pregnancy and offspring attention deficit hyperactivity disorder–a longitudinal sibling control study, 1 (2), e12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Schmidt A, Finkielman OTE, Jensen BA, et al. , 2017. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour, 154 (2), 145–152. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J, 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data,. Genome Biol. 8 (2), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MAJP, 1996. Multiple regulators of ultrasonic vocalization in the infant rat, 21 (2), 203–217. [DOI] [PubMed] [Google Scholar]
- Holm JB, Chalmey C, Modick H, et al. , 2015. Aniline is rapidly converted into paracetamol impairing male reproductive development, 148 (1), 288–298. [DOI] [PubMed] [Google Scholar]
- Holm JB, Mazaud-Guittot S, Danneskiold-Samsøe NB, et al. , 2016. Intrauterine exposure to paracetamol and aniline impairs female reproductive development by reducing follicle reserves and fertility, 150 (1), 178–189. [DOI] [PubMed] [Google Scholar]
- Ji Y, Azuine RE, Zhang Y, et al. , 2020. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood, 77 (2), 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson HKL, Jacobsen PR, Hass U, et al. , 2016. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging, 61, 186–194. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M, 2021. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 49 (D1), D545–D551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM, Rigobello C, Vidigal CB, et al. , 2020. Gestational exposure to paracetamol in rats induces neurofunctional alterations in the progeny, 77, 106838. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Hass U, Lesné L, et al. , 2011. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat, 26 (1), 235–244. [DOI] [PubMed] [Google Scholar]
- Larrey D, Letteron P, Foliot A, et al. , 1986. Effects of pregnancy on the toxicity and metabolism of acetaminophen in mice. J. Pharmacol. Exp. Ther. 237 (1), 283–291. [PubMed] [Google Scholar]
- Laue HE, Cassoulet R, Abdelouahab N, et al. , 2019. Association between meconium acetaminophen and childhood neurocognitive development in GESTE, a Canadian cohort study, 167 (1), 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert B, Havdahl A, Riglin L, et al. , 2019. Association of maternal neurodevelopmental risk alleles with early-life exposures, 76 (8), 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, 2013. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM arXiv preprint arXiv, 1303.3997. [Google Scholar]
- Liao Y, Smyth GK, Shi W, 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30 (7), 923–930. [DOI] [PubMed] [Google Scholar]
- Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P, 2015. The molecular signatures database hallmark gene set collection. Cell Syst. 1 (6), 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J, 2014. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediat. 168 (4), 313–320. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Virk J, Olsen J, 2016a. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Research 9 (9), 951–958. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Virk J, Arah OA, Olsen J, 2016b. Prenatal use of acetaminophen and child IQ: a Danish cohort study. Epidemiology 27 (6), 912–918. [DOI] [PubMed] [Google Scholar]
- Liew Z, Kioumourtzoglou M-A, Roberts AL, O’Reilly ÉJ, Ascherio A, Weisskopf MG, 2019. Use of negative control exposure analysis to evaluate confounding: an example of acetaminophen exposure and attention-deficit/hyperactivity disorder in Nurses’ Health Study II. Am. J. Epidemiol 188 (4), 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup KR, Johansson HKL, Boberg J, et al. , 2015. Mixtures of environmentally relevant endocrine disrupting chemicals affect mammary gland development in female and male rats, 54, 47–57. [DOI] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R, 2001. behavior. The use of behavioral test batteries: effects of training history,. Physiol. Behavior 73 (5), 705–717. [DOI] [PubMed] [Google Scholar]
- Modesto T, Tiemeier H, Peeters RP, et al. , 2015. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children, 169 (9), 838–845. [DOI] [PubMed] [Google Scholar]
- Motawi TK, Ahmed SA, El-Boghdady NA, Metwally NS, Nasr NN, 2019. Protective effects of betanin against paracetamol and diclofenac induced neurotoxicity and endocrine disruption in rats. Biomarkers 24 (7), 645–651. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Aoki K, 1963. Development of a strain of spontaneously hypertensive rats. Japan. Circul. J 27 (3), 282–293. [DOI] [PubMed] [Google Scholar]
- Päkkilä F, Männistö T, Pouta A, et al. , 2014. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the childJ. Clin. Endocrinol. Metabol., 99 (1), E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14 (3), 149–167. [DOI] [PubMed] [Google Scholar]
- Pereira MRF, Aleixo JF, de Freitas Cavalcanti L., et al. , 2020. Can maternal exposure to paracetamol impair reproductive parameters of male rat offspring?, 93, 68–74. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic acids Res. 29 (9), e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot G, Gordh T, Fredriksson A, Viberg H, 2017. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): characterization of a critical period. J. Appl. Toxicol 37 (10), 1174–1181. [DOI] [PubMed] [Google Scholar]
- Philippot G, Hallgren S, Gordh T, Fredriksson A, Fredriksson R, Viberg H, 2018. A cannabinoid receptor type 1 (CB1R) agonist enhances the developmental neurotoxicity of acetaminophen (paracetamol). Toxicol. Sci 166 (1), 203–212. [DOI] [PubMed] [Google Scholar]
- Pronovost GN, Hsiao EY, 2019. Perinatal interactions between the microbiome, immunity, and neurodevelopment. Immunity 50 (1), 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung Catherine, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review, 463 (1–3), 3–33. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N, 2008. Dose translation from animal to human studies revisited. The FASEBJ 22 (3), 659–661. [DOI] [PubMed] [Google Scholar]
- Rebordosa C, Kogevinas M, Bech BH, Sørensen HT, Olsen J, 2009. Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes. Int. J. Epidemiol. 38 (3), 706–714. [DOI] [PubMed] [Google Scholar]
- Reel JR, Lawton AD, LAMB JC IV, 1992. Reproductive toxicity evaluation of acetaminophen in Swiss CD-1 mice using a continuous breeding protocol. Toxicol. Sci. 18 (2), 233–239. [DOI] [PubMed] [Google Scholar]
- Regan SL, Williams MT, Vorhees CV, 2022. Reviews B. Review of rodent models of attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 132, 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Cardenas A, Hivert MF, et al. , 2020. Associations of prenatal or infant exposure to acetaminophen or ibuprofen with mid-childhood executive function and behaviour, 34 (3), 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigobello C, Klein RM, Debiasi JD, et al. , 2021. Perinatal exposure to paracetamol: Dose and sex-dependent effects in behaviour and brain’s oxidative stress markers in progeny, 408, 113294. [DOI] [PubMed] [Google Scholar]
- Rossitto M, Ollivier M, Déjardin S, et al. , 2019a. In utero exposure to acetaminophen and ibuprofen leads to intergenerational accelerated reproductive aging in female mice, 2 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossitto M, Marchive C, Pruvost A, et al. , 2019b. Intergenerational effects on mouse sperm quality after in utero exposure to acetaminophen and ibuprofen, 33 (1), 339–357. [DOI] [PubMed] [Google Scholar]
- Saad A, Hegde S, Kechichian T, et al. , 2016. Is there a causal relation between maternal acetaminophen administration and ADHD?, 11 (6), e0157380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C, 2003. Stress-induced vocalisation in adult animals. A valid model of anxiety? Europ. J. Pharmacol. 463 (1–3), 133–143. [DOI] [PubMed] [Google Scholar]
- Schneider P, Ho Y-J, Spanagel R, Pawlak CR, 2011. A novel elevated plus-maze procedure to avoid the one-trial tolerance problem. Front. Behav. Neurosci. 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ, 2013. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progr. Neurobiol. 106, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E, Thapar A, Smith GD, 2016. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. AMA Pediatrics 170 (10), 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Treder RP, Barr HM, et al. , 1987. Aspirin and acetaminophen use by pregnant women and subsequent child IQ and attention decrements, 35 (2), 211–219. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, et al. , 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles, 102 (43), 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda N, Cendejas Hernandez J, Poulton J, et al. , 2021. Therapeutic doses of acetaminophen with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats, 16 (6), e0253543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JM, Waldie KE, Wall CR, Murphy R, Mitchell EA, ABC Study Group, 2014. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PloS one 9 (9), e108210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W, Russell G, Baragwanath G, Matthews J, Vaidya B, Thompson-Coon JJCe, 2018. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysisClin. Endocrinol., 88 (4), 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G, Karimi K, Brune K, Arck P, 2014. New problems arising from old drugs: second-generation effects of acetaminophen. Expert Rev. Clin. Pharmacol 7 (5), 655–662. [DOI] [PubMed] [Google Scholar]
- Tovo-Rodrigues L, Schneider BC, Martins-Silva T, et al. , 2018. Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? Findings from the 2004 Pelotas birth cohort, 18 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovo-Rodrigues L, Carpena MX, Martins-Silva T, et al. , 2020. Low neurodevelopmental performance and behavioural/emotional problems at 24 and 48 months in Brazilian children exposed to acetaminophen during foetal development, 34 (3), 278–286. [DOI] [PubMed] [Google Scholar]
- van Den Driesche S, Macdonald J, Anderson RA, et al. , 2015. Prolonged exposure to acetaminophen reduces testosterone production by the human fetal testis in a xenograft model, 7 (288), 288ra280–288ra280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, et al. , 2002. Accurate normalization of realtime quantitative RT-PCR data by geometric averaging of multiple internal control genes, 3 (7), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Eriksson P, Gordh T, Fredriksson Anders, 2014. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice, 138 (1), 139–147. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols 2 (2), 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA, 2005. Gynecology. Use of over-the-counter medications during pregnancy. Am. J. Obstetr. Gynecol 193 (3), 771–777. [DOI] [PubMed] [Google Scholar]
- Whitehouse L, Paul C, Wong L, Thomas B, 1977. Effect of aspirin on a subtoxic dose of 14C-acetaminophen in mice. J. Pharm. Sci 66 (10), 1399–1403. [DOI] [PubMed] [Google Scholar]
- Yang X, Greenhaw J, Shi Q, et al. , 2013. Mouse liver protein sulfhydryl depletion after acetaminophen exposure. J. Pharmacol. Exp. Ther 344 (1), 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystrom E, Gustavson K, Brandlistuen RE, et al. , 2017. Prenatal exposure to acetaminophen and risk of ADHD, 140, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data are deposited at Gene Expression Omnibus (GEO) accession number GSE198424. Mouse behavior data are available upon request.