Abstract
Introduction
The present study aimed to analyze the clinical features and laboratory markers of patients with Delta variant SARS-CoV-2 and explore the role of platelet in predicting the severity of Delta.
Methods
This retrospective, observational study was conducted on 863 patients laboratory-confirmed Delta variant SARS-CoV-2. These cases were sub-classified based on disease severity into mild (n = 304), moderate (n = 537), and severe (n = 22). A series of laboratory findings and clinical data were collected and analyzed during hospitalization.
Results
Of 863 hospitalized patients with Delta, the median age was 38 years (interquartile range, 30–51 years) and 471 (54.58%) were male. The most common clinical symptoms mainly included cough, fever, pharyngalgia, expectoration, dyspnea, fatigue, and headache, and the commonest comorbidities were hypertension and diabetes. Among the hematological variables, neutrophil count, red blood cell count, and hemoglobin, were found to be statistically significant with regard to subcategories based of disease severity (p < 0.05). Among coagulation parameters, there was a statistically significant difference in D-dimer, fibrinogen, international normalized ratio, and prothrombin time (p < 0.05). Statistically significant differences were observed in platelet markers including platelet count, large platelet count, and plateletcrit (p < 0.05). Additionally, there was strong correlation between platelet and other parameters with disease severity. Logistical regression analysis and ROC curves showed that D-dimer was a single best marker of disease severity (p = 0.005, p < 0.0001); however, platelet (p = 0.009, p = 0.002) and plateletcrit (p = 0.002, p = 0.001) could also predict severe disease. Platelet was identified as an independent risk factor for severe Delta.
Conclusion
Low platelet may be a marker of disease severity in Delta variant SARS-CoV-2 and may contribute to determine the severity of patients infected with Delta.
Keywords: COVID-19, Delta variant SARS-CoV-2, Platelets, Risk indicators, Laboratory characteristics
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) Delta variant (Phylogenetic Assignment of Named Global Outbreak lineage B.1.617.2 and AY) is a major, highly contagious SARS-CoV-2 variant that may be associated with more severe disease [1, 2]. The Delta VOC was initially identified in India at time of December 2020 [3, 4]. An outbreak of SARS-CoV-2 Delta variant infection occurred in Xi'an, China, in December 2021. Therefore, it is crucial to evaluate the possible factors affecting the progression of disease in Delta patients.
The pathogenesis of coronavirus disease 2019 (COVID-19) is complex with multi-system involvement including hematopoietic system, hemostasis, and immune system. COVID-19 is increasingly recognized as a thromboinflammatory disease where alterations of both coagulation and platelets are closely linked to mortality and clinical outcomes [5]. COVID-19 induces a hypercoagulatory state that frequently leads to thromboembolic complications [6]. Platelets, also called thrombocytes, are best known as mediators of thrombus formation and hemostasis [7, 8]. Thrombocytopenia is one of the common hematological findings observed in the COVID-19 cases [9]. COVID-19 patients often have mild thrombocytopenia and appear to have increased platelet consumption, together with a corresponding increase in platelet production [10]. Numerous studies expound that the platelets of COVID-19 patients appear to be over-reactive and may interact with SARS-CoV-2 to promote coagulation dysfunction during COVID-19 infection [10, 11, 12, 13]. Multiple lines of evidence listed platelet counting as economic, ubiquitous, and ephemeral in discriminating the COVID-19 into severe/moderate as thrombocytopenia is linked to severe COVID-19 [12, 14, 15, 16, 17, 18]. Autopsies of COVID-19 patients have shown that platelet-rich thrombi exist in the microcapillaries of multiple organs. The number of megakaryocytes in the heart and lungs is abnormally increased [19, 20]. The objectives of the current study were to elucidate the clinical characteristics of 863 hospitalized patients with Delta variant, elucidate the usefulness of platelet as a diagnostic marker in patients with Delta, and to assess its role in determining the severity of Delta.
Materials and Methods
Study Participants
The current study was a retrospective observational study conducted on patients diagnosed with Delta variant SARS-CoV-2. If the transmission chain of this epidemic was clear, epidemiological investigation and big data technology were used to verify the event details of the cases. Close contacts at the exposure points were traced, and the samples were collected for testing. Serum samples were collected from the key persons for antibody detection. Whole-genome sequencing and alignment analysis were applied on the positive samples collected from reported cases and environment at the early stage of the outbreak. The source of infection and the transmission chain were analyzed. The gene sequencing result indicated that the pathogen was Delta variant of 2019-nCoV. If the source of infection was unclear, whole-genome sequencing were applied on the positive samples to confirm. We collected case data from 863 patients with who were admitted to Xi'an Chest Hospital from December 17, 2021, to February 5, 2022. This study was approved by the local Ethics Committee of the hospital (protocol No: S2022-0002), and written informed consent from the patients was obtained. Classified based on clinical severity, the study cases were categorized into three groups: mild, moderate, and severe.
Clinical Classifications
All diagnoses and clinical classifications of COVID-19 were based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) issued by the Chinese National Health Committee. The disease severity was defined according to the interim guidelines from the World Health Organization and the National Health Commission of China [21]. Clinically, the disease is divided into four types of cases: mild, moderate, severe, and critical.
Mild Cases
The clinical symptoms are mild and no pneumonia manifestation can be found in imaging. Moderate cases: Patients have symptoms like fever and respiratory tract symptoms, etc., and pneumonia manifestation can be seen in imaging.
Severe Cases
Meeting any of the following respiratory distress, respiratory rate ≥30 breaths/min; SpO2 ≤ 93% at rest; and PaO2/FIO2 ≤ 300. Patients with greater than 50% lesion progression within 24–48 h in pulmonary imaging should be treated as severe cases.
Critically Cases
Meeting any of the following respiratory failure occurs and mechanical ventilation is required, shock, and complications from other organ failure that require monitoring and treatment in the ICU.
Sample Collection
Clinic characteristics and laboratory data were retrieved from electronic records of 863 confirmed cases of Delta. Detailed clinical data and laboratory characteristics were obtained with data collection forms from electronic medical records. Information recorded included demographic data, medical history, underlying comorbidities, symptoms, and signs, and laboratory findings of the study subjects were documented.
Vaccinated Defined
Individuals were considered fully vaccinated if they had received both doses of a two-dose vaccine schedule at least 14 days before symptom onset, or one dose of a single-dose vaccine schedule at least 28 days before symptom onset. Participants, who received one dose of a two-dose vaccine at least 14 days before symptom onset, were considered partially vaccinated. All patients who did not receive a COVID-19 vaccine or received a first dose <14 days prior to symptom onset (or <28 days for vaccination with a single-dose vaccine) were considered unvaccinated.
Laboratory Testing
All medical laboratory data for all patients were generated by the clinical laboratory of Xi'an Chest Hospital in Xi'an, China. The venous blood samples were collected on days 1–2 of admission to the isolation ward. The following tests were performed: (1) hematological parameters complete blood counts (CBC) parameters evaluated including neutrophil count (NC), lymphocyte count (LC), red blood cell count (RBC), white blood cell count (WBC), platelet count, hemoglobin (hb), albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, creatinine, and urea. (2) Coagulation markers including D-dimer, fibrinogen, fibrin degradation products, international normalized ratio, thrombin time, prothrombin time (PT), and activated partial thromboplastin time. (3) Platelet parameters including platelet distribution width, mean platelet volume (MPV), large platelet count, platelet large cell ratio, and plateletcrit (PCT).
Statistical Analysis
Categorical variables were expressed as number (n) and percentage (%) in each category. Continuous variables were summarized as the median with interquartile range (IQR; P25-P75) or mean ± standard deviation. ANOVA was used in the comparison of more than two groups, in normal distributions and the Kruskal-Wallis H(K) test for non-normal distributions. Pearson's correlation coefficient was calculated for analyzing association between platelet and laboratory parameters. Stepwise multivariate binary logistic regression was used to select and estimate the association between disease severity and variables that were clinically relevant on grounds of professional knowledge and were statistically significant in preliminary univariate binary logistic regression. Specifically, variables with p < 0.05 in the univariate analysis were entered into multivariate analysis to select the predictors. Results are presented as OR values with 95% confidence intervals (95% CI) and p values. The receiver operating characteristic (ROC) curve was used to evaluate the efficiency of each parameter in predicting disease severity and calculated the area under the curve (AUC) for parameters. Statistical analysis was performed using SPSS 26.0 (IBM SPSS Statistics) and GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). p < 0.05 were considered statistically significant.
Results
Comparison of Clinical and Demographic Characteristics of Hospitalized Patients with Delta
Out of a total of 863 Delta positive patients were enrolled for the study. This study participants were divided into three groups including mild (304), moderate (537), and severe (22) base on based on severity of SARS-CoV-2 Delta variant. The median age was 38 years (IQR, 30–51; range, 19–90 years). Most of the patients (80.2%) belonged to 19–64 years age. Males outnumbered females with a ratio of 1.2:1. The most common clinical symptoms of the patient's at onset of illness were cough (56.55%), fever (44.61%), pharyngalgia (40.79%), expectoration (25.96%), dyspnea (17.96%), and fatigue (10.54%). Less common symptoms were headache (8.23%), and loss of taste (8.23%). Comorbidities, including hypertension (9.27%), diabetes (2.55%), coronary heart disease (1.39%), chronic liver disease (1.97%), chronic respiratory diseases (1.62%), cerebrovascular disease (0.93%), and malignancy (1.04%), were the most common coexisting conditions. The median length of hospital stay (LOS) was 18.0 (IQR, 15.0–22.0) days. Comparisons of the patients in terms of the demographic, clinical presentations, and comorbid conditions at the time of admission were presented in Table 1.
Table 1.
Demographics and clinical characteristics of patients infected with Delta on admission
| Clinical characteristics | Total (n = 863) | Mild (n = 304) | Moderate (n = 537) | Severe (n = 22) | p value |
|---|---|---|---|---|---|
| Age (IQR), years | 38 (30−51) | 34 (26−44) | 40 (32−52) | 71 (55−76) | <0.0001 |
| Age-group, years | |||||
| 19−40 | 480 (55.62) | 202 (66.45) | 276 (51.40) | 2 (9.09) | |
| 41−64 | 315 (36.50) | 93 (30.59) | 217 (40.41) | 5 (22.72) | |
| ≥65 | 68 (7.88) | 9 (2.96) | 44 (8.19) | 15 (68.18) | |
| Gender, n (%) | |||||
| Male | 471 (54.58) | 174 (57.24) | 283 (52.70) | 14 (63.63) | |
| Female | 392 (45.42) | 130 (42.76) | 254 (47.30) | 8 (36.36) | |
| Signs and symptoms, n (%) | |||||
| Cough | 488 (56.55) | 133 (43.75) | 337 (62.76) | 18 (81.82) | |
| Fever | 385 (44.61) | 86 (28.29) | 280 (52.14) | 19 (86.36) | |
| Pharyngalgia | 352 (40.79) | 127 (41.78) | 221 (41.15) | 4 (18.18) | |
| Expectoration | 224 (25.96) | 53 (17.43) | 163 (30.35) | 8 (36.36) | |
| Dyspnea | 155 (17.96) | 50 (16.45) | 96 (17.88) | 9 (40.91) | |
| Fatigue | 91 (10.54) | 24 (7.89) | 60 (11.17) | 7 (31.82) | |
| Headache | 71 (8.23) | 15 (4.93) | 53 (9.87) | 3 (13.64) | |
| Loss of taste | 71 (8.23) | 16 (5.26) | 54 (10.06) | 1 (4.55) | |
| Comorbidities, n (%) | 167 (19.35) | ||||
| Hypertension | 80 (9.27) | 16 (5.26) | 54 (10.06) | 10 (45.45) | |
| Diabetes (Type I or II) | 22 (2.55) | 7 (2.30) | 14 (2.61) | 1 (4.55) | |
| Coronary heart disease | 12 (1.39) | 1 (0.33) | 7 (1.30) | 4 (18.18) | |
| Chronic liver disease | 17 (1.97) | 7 (2.30) | 9 (1.68) | 1 (4.55) | |
| Chronic respiratory diseases | 14 (1.62) | 1 (0.33) | 12 (2.23) | 1 (4.55) | |
| Cerebrovascular diseases | 8 (0.93) | 2 (0.66) | 6 (1.11) | 0 | |
| Chronic renal diseases | 5 (0.58) | 2 (0.66) | 3 (0.56) | 0 | |
| Malignancy | 9 (1.04) | 1 (0.33) | 7 (1.30) | 1 (4.55) | |
| Vaccination status, n (%) | |||||
| Fully vaccinated | 777 (90.03) | 281 (92.43) | 483 (89.94) | 13 (59.09) | |
| Partially vaccinated | 27 (3.13) | 9 (2.96) | 18 (3.35) | 0 | |
| Not vaccinated | 56 (6.49) | 12 (3.95) | 35 (6.52) | 9 (40.91) | |
| Unknown vaccination status | 3 (0.35) | 2 (0.66) | 1 (0.19) | 0 | |
| Low molecular weight heparin | 575 (66.63) | 114 (37.50) | 441 (82.12) | 20 (90.91) | |
| Length of hospital (IQR), d | 18.0 (15.0−22.0) | 17.0 (14.0−22.0) | 19 0.0 (16.0−22.0) | 20.5 (14.8−30.5) | <0.0001 |
Comparative Analysis of Hematological and Coagulation Parameters in Categories of Delta Patients Based on Disease Severity
The main hematological and laboratory results in this study were collected for each patient on admission. There were statistically significant differences in NC, RBC, WBC, hb, albumin, aspartate aminotransferase, creatinine, and urea on admission among the three categories based on disease severity (p < 0.05). Additionally, aspartate aminotransferase was significantly higher in the severe group. Analysis of hematological and biochemical markers for patients on admission was summarized in Table 2. Meanwhile, there was a statistically significant difference in coagulation parameters including D-dimer, fibrinogen, international normalized ratio, and PT on admission among the three subcategories of Delta patients (p < 0.05). Comparison of coagulation parameters among the subcategories based on disease severity is depicted in Table 3. Of note, the level of D-dimer was progressively increased as the disease progressed and clinical status deteriorated.
Table 2.
Comparative analysis of laboratory parameters among the different subcategories of Delta cases based on disease severity
| Laboratory examination | Mild (n = 304) | Moderate (n = 537) | Severe (n = 22) | p value |
|---|---|---|---|---|
| NC, 109/L | 3.53±1.47 | 3.22±1.30 | 4.07±2.58 | 0.010 |
| LC, 109/L | 1.60±0.85 | 1.47±1.47 | 1.93±2.82 | 0.141 |
| RBC, 1012/L | 4.61±0.48 | 4.50±0.54 | 4.19±0.76 | <0.0001 |
| WBC, 109/L | 5.63±1.75 | 5.14±1.97 | 5.59±2.55 | 0.002 |
| hb, g/L | 141.97±17.74 | 137.57±18.88 | 130.41±24.35 | <0.0001 |
| Albumin, g/L | 47.16±3.31 | 45.60±3.47 | 42.13±5.22 | <0.0001 |
| Alkaline phosphatase, U/L | 71.38±20.25 | 70.72±24.56 | 75.67±22.40 | 0.606 |
| Alanine aminotransferase, U/L | 44.38±36.29 | 47.34±45.90 | 47.48±25.00 | 0.631 |
| Aspartate aminotransferase, U/L | 35.76±23.13 | 38.30±26.73 | 53.38±44.47 | 0.009 |
| Creatinine, µmol/L | 62.07±24.00 | 61.70±15.62 | 83.50±55.13 | <0.0001 |
| Urea, mmol/L | 4.09±1.09 | 4.07±2.20 | 7.03±7.45 | <0.0001 |
Table 3.
Comparison of various coagulation parameters among the three subcategories of Delta patients
| Coagulation parameters | Mild (n = 304) | Moderate (n = 537) | Severe (n = 22) | p value |
|---|---|---|---|---|
| D-dimer, µg/L | 0.47±0.37 | 0.54±0.52 | 0.89±0.79 | <0.0001 |
| Fibrinogen, g/L | 3.08±0.65 | 3.45±0.79 | 3.66±0.91 | <0.0001 |
| FDP, µg/L | 2.07±2.44 | 2.22±1.62 | 2.86±1.86 | 0.200 |
| International normalized ratio, % | 1.05±0.15 | 1.03±0.14 | 1.06±0.16 | 0.017 |
| Thrombin time, s | 15.09±1.36 | 14.98±1.38 | 15.26±1.40 | 0.391 |
| Prothrombin time, s | 13.47±1.58 | 13.16±1.54 | 13.58±1.73 | 0.016 |
| Activated partial thromboplastin time, s | 31.93±3.59 | 31.84±3.46 | 31.49±2.59 | 0.822 |
Comparison of Platelet Parameters among the Different Subcategories of Delta Cases Based on Disease Severity
Comparison of platelet parameters among three subcategories of Delta disease is depicted in Table 4. Among the platelet parameters, platelet count, large platelet count, and PCT on admission were found to be significantly different among the three groups (p = 0.01, p = 0.01, and p = 0.01, respectively). It was worth noting that patients with severe Delta had substantially lower platelet count levels than those with mild or moderate Delta. The level of large platelet count and PCT was progressively decreased as the disease progressed and clinical status deteriorated.
Table 4.
Comparative analysis of platelet parameters among the clinical subcategories of Delta disease
| Platelet parameters | Mild (n = 304) | Moderate (n = 537) | Severe (n = 22) | p value |
|---|---|---|---|---|
| Platelet count, 109/L | 226.56±68.97 | 211.49±74.70 | 176.23±87.43 | 0.001 |
| Platelet distribution width, % | 16.23±0.36 | 16.24±0.36 | 16.30±0.41 | 0.727 |
| MPV, fL | 10.56±1.22 | 10.70±1.30 | 10.83±1.65 | 0.258 |
| Large platelet count | 64.44±18.19 | 61.73±20.65 | 49.64±11.77 | 0.001 |
| Platelet large cell ratio, % | 29.65±8.36 | 30.70±9.09 | 31.93±11.19 | 0.182 |
| PCT, % | 0.23±0.06 | 0.22±0.07 | 0.18±0.06 | 0.001 |
Correlation Analysis between Platelets and Various Laboratory Parameters in Delta
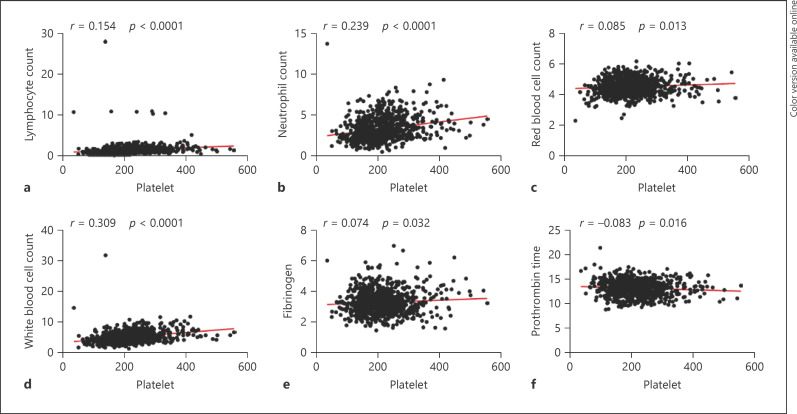
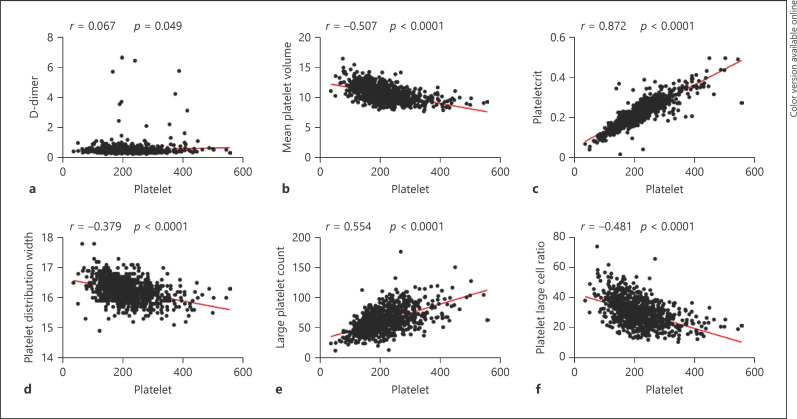
The correlation between platelets and various parameters is summarized in Table 5 and Figures 1, 2. We observed that a common positive correlation between platelet counts and other hematological parameters including LC (p < 0.0001), NC (p < 0.0001), RBC (p = 0.013), WBC (p < 0.0001). Meanwhile, there was a negative relationship between platelets and MPV (p < 0.0001), platelet distribution width (p < 0.0001), platelet large cell ratio (p < 0.0001), and PT (p = 0.016) reached statistical significance. Besides, platelet counts were positively related to PCT (p < 0.0001), large platelet count (p < 0.0001), and fibrinogen (p = 0.032).
Table 5.
Correlation of between platelet and various biomarkers in Delta
| Variable | Platelet |
|
|---|---|---|
| R | p value | |
| hb, g/L | −0.053 | 0.120 |
| LC, 109/L | 0.154** | <0.0001 |
| NC, 109/L | 0.239** | <0.0001 |
| RBC, 1012/L | 0.085* | 0.013 |
| WBC, 10^9/L | 0.309** | <0.0001 |
| Activated partial thromboplastin time, s | −0.058 | 0.094 |
| Fibrinogen, g/L | 0.074* | 0.032 |
| FDP, pg/L | 0.022 | 0.567 |
| International normalized ratio, % | −0.085* | 0.013 |
| PT, s | −0.083* | 0.016 |
| TT, s | −0.035 | 0.312 |
| D-dimer, pg/L | 0.067* | 0.049 |
| Albumin | 0.005 | 0.877 |
| MPV, fL | −0.507** | <0.0001 |
| PCT, % | 0.872** | <0.0001 |
| Platelet distribution width, % | −0.379** | <0.0001 |
| Large platelet count | 0.554** | <0.0001 |
| Platelet large cell ratio, % | −0.481 ** | <0.0001 |
Significant correlation at the level of 0.05.
Significant correlation at the level of 0.01.
Fig. 1.
a–f Correlations between platelet count and lymphocyte count, neutrophils count, red blood cell count, white blood cell count, fibrinogen, and prothrombin time in patients with Delta.
Fig. 2.
a–f Correlations between platelet count and D-dimer, mean platelet volume, plateletcrit, platelet distribution width, large platelet count, and platelet large cell ratio in patients with Delta.
Logistic Regression Analysis of Various Parameters for the Severity of Delta
In order to clarify the potential factors related to the disease severity, we conducted binary logistical regression. The results displayed that NC, D-dimer, and fibrinogen independent risk factors for severe Delta, and their OR values were 1.318, 1.661, and 1.651, respectively (p = 0.017, p = 0.005, and p = 0.040, respectively) (Table 6). In addition, RBC, platelets, large platelet count, and PCT were an independent predictive factors for severe Delta, and their OR values were 0.292, 990, 0.959, and 0.958, respectively (p = 0.002, p = 0.009, p = 0.002, and p = 0.002, respectively) (Table 6). These results indicated that NC, RBC, D-dimer, fibrinogen, platelets, large platelet count, and PCT at hospital admission were independent factors associated with disease severity in patients with the Delta infection.
Table 6.
Binary logistic regression analysis of various parameters associated with severe Delta
| Parameters | B | SE | Wals | p value | OR | 95% CI |
|---|---|---|---|---|---|---|
| NC, 109/L | 0.276 | 0.116 | 5.663 | 0.017 | 1.318 | 1.050−1.656 |
| LC, 109/L | 0.097 | 0.077 | 1.580 | 0.209 | 1.102 | 0.947−1.128 |
| RBC, 1012/L | −1.231 | 0.399 | 9.523 | 0.002 | 0.292 | 0.134−0.638 |
| WBC, 109/L | 0.057 | 0.086 | 0.438 | 0.508 | 1.059 | 0.894−1.254 |
| Fibrinogen, µg/L | 0.501 | 0.244 | 4.219 | 0.040 | 1.651 | 1.023−2.664 |
| PT, s | 0.124 | 0.137 | 0.817 | 0.366 | 1.132 | 0.865−1.481 |
| D-dimer, µg/L | 0.508 | 0.18 | 7.951 | 0.005 | 1.661 | 1.167−2.364 |
| Platelet count, 109/L | −0.010 | 0.004 | 6.74 | 0.009 | 0.990 | 0.983−0.998 |
| Platelet distribution width, % | 0.424 | 0.591 | 0.516 | 0.472 | 1.529 | 0.480−4.864 |
| MPV, fL | 0.105 | 0.162 | 0.421 | 0.517 | 1.111 | 0.809−1.526 |
| Large platelet count | −0.042 | 0.014 | 9.540 | 0.002 | 0.959 | 0.933−0.985 |
| Platelet large cell ratio, % | 0.019 | 0.023 | 0.701 | 0.403 | 1.019 | 0.975−1.066 |
| PCT, % | −11.662 | 3.823 | 9.306 | 0.002 | 0.958 | 0.933−0.984 |
B, regression coefficient; SE, standard error; OR, odds ratio; CI, confidence interval.
ROC Curves for Predicting the Severe of Delta
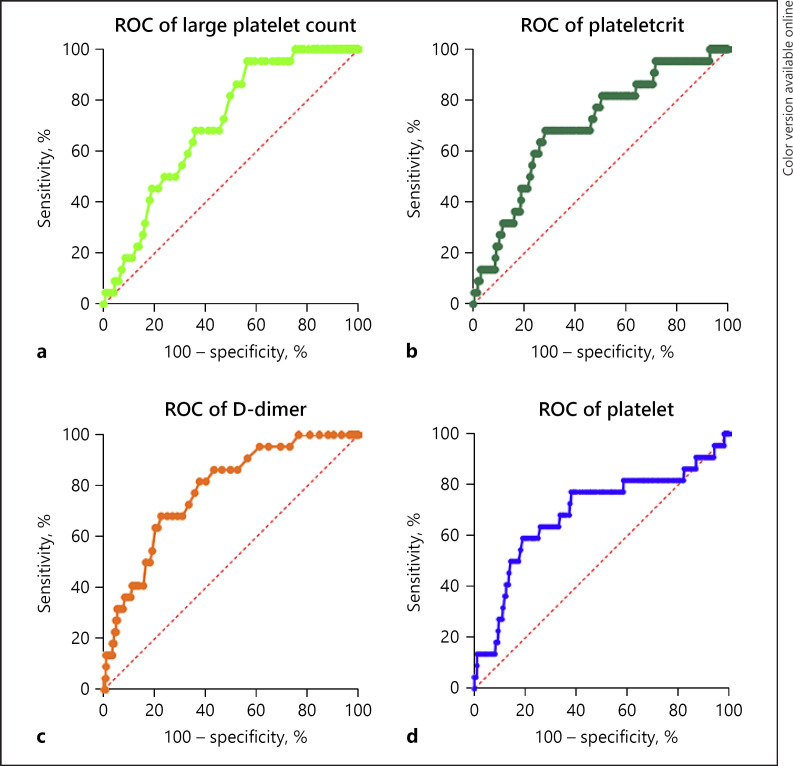
The ROC curve was drawn for severe Delta. ROC curves and the AUC of each independent risk or predictive factors are presented in Figure 3 and Table 7. We observed that D-dimer (AUC: 0.779, p < 0.0001) emerged as the single best parameter as per area under curve of ROC in distinguishing severe cases, followed by large platelet count (AUC: 0.706, p = 0.001), platelets (AUC: 0.702, p = 0.002), and PCT (AUC: 0.699, p = 0.001). These observations indicated that the detection of parameters including D-dimer, platelets, large platelet count, and PCT in patients infected with Delta were all great valuable for predicting the severity of Delta, and can effectively assess the severity of Delta.
Fig. 3.
a–d ROC curves analysis for predicting the severe of Delta. ROC plot of D-dimer, platelet large platelet count, and plateletcrit.
Table 7.
ROC curve for various laboratory biomarkers in the severity of Delta
| Test result variable | Area | SE | p value | 95% confidence interval |
|
|---|---|---|---|---|---|
| lower bound | upper bound | ||||
| Lymphocyte | 0.638 | 0.060 | 0.027 | 0.520 | 0.756 |
| Red blood cell | 0.623 | 0.062 | 0.049 | 0.501 | 0.744 |
| D-dimer | 0.779 | 0.044 | <0.0001 | 0.693 | 0.866 |
| Fibrinogen | 0.604 | 0.061 | 0.094 | 0.486 | 0.723 |
| Platelet | 0.702 | 0.067 | 0.002 | 0.558 | 0.821 |
| Large platelet count | 0.706 | 0.043 | 0.001 | 0.621 | 0.791 |
| PCT | 0.699 | 0.055 | 0.001 | 0.591 | 0.806 |
SE, standard error.
Discussion
Coronaviruses are categorized into four genera: Alpha, Beta, Delta, and Gamma. Recently, the Delta variant of SARS-CoV-2 has emerged and spread globally [22]. A total of 863 Delta variant-infected patients were admitted into the isolation unit of our hospital during the study period. Classified on the basis of disease severity, the study population was divided into mild, moderate, and severe. In the present study, we observed with the highest incidence of Delta infection in the 19–40-year age-group, and 41–64-year age-group was the second most common group, indicating that the onset of the Delta showed a trend of younger age. The median age of Delta cases varies from 30 to 51 years with increased severity in older patients. More men were infected with the Delta when compared with women. Analysis of clinical presentation reveals that cough (56.55%), fever (44.61%), pharyngalgia (40.79%), expectoration (25.96%), and dyspnea (17.96%) were the most common presenting symptoms in our setup. Additionally, about 19.35% of patients had one or more underlying comorbidity, the most common of which were chronic diseases, such as hypertension, and diabetes. We also observed that 66.63% of patients received anticoagulant treatment with low molecular weight heparin. For moderate patients with normal D-dimer, prophylactic dose of low molecular weight heparin can be given without contraindications, 2,500 IU subcutaneously q12h. Patients with no anticoagulant contraindications and higher D-dimer, prophylactic anticoagulant dose of low molecular weight heparin 5000 IU subcutaneously q12h. The median LOS was 18.0 (IQR, 15.0–22.0) days. Patients with COVID-19 in China appeared to remain in hospital for longer than elsewhere. This may be explained by differences in criteria for admission and discharge between countries, and different timing within the pandemic. There are many factors that affect the LOS. Patients who developed complications during hospitalization tended to have longer LOS than those without complications.
Recent preliminary data following the COVID-19 outbreak indicated an association of CBC parameters and coagulation profile with disease progression. Among all CBC parameters, LCs showed the most significant and consistent trend and might reflect the progression of disease. On evaluating the most routine and cost-effective tests like CBC, leucocytosis, neutrophilia, and lymphopenia were characteristic findings observed in COVID-19 patients [23, 24]. In the present study, the basic laboratory parameters measured at the time of admission were examined. According to the laboratory findings, there were statistically significant differences between patients with mild, moderate, and severe Delta in terms of NC, RBC, WBC, hb, albumin, aspartate aminotransferase, creatinine, and urea. Moreover, NCs were significantly higher in the severe group; and red blood cell, hb, and albumin were significantly lower in the severe group.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has demonstrated how the interplay between hemostasis and the immune response is particularly evident [25]. The cytokine storm leads to intravascular coagulopathy, documented by the presence of venous thromboembolism and microthrombi in arterioles and venules in COVID-19 patient autopsies [26, 27, 28]. D-dimer could serve as an early indicator of severity to improve the management of COVID-19 patients. D-dimer and fibrinogen were significantly higher in the severe group. Among different subgroups based on disease severity, significant differences were observed in D-dimer and fibrinogen, which is in concordance with previous report [10, 29, 30, 31]. Thrombocytopenia is a hallmark of severe COVID-19 infections, and associated with a high risk of severe COVID-19 and an increased risk of in-patient death [14, 30, 32]. The excessive formation of thrombi is at least partly due to hyperactivation of platelets caused by the COVID-19 infections [33]. The platelet count monitoring may act as a clinical pointer for the worsening and prognosis of the disease [14, 32]. In COVID-19 patients, the platelet count differs between mild and serious infections [34]. Several reports have demonstrated that platelet was an independent protective factor for severe COVID-19 [35, 36]. MPV is a reflection of the average size of platelets. In the current study, there was a statistically significant difference in platelet count, large platelet count, and PCT with regard to subcategories based of disease severity. We also found a downward trend in platelet counts in Delta variant SARS-CoV-2 patients among the subgroups based on clinical severity, which is in accordance with previous literature [29, 37]. Moreover, we investigate the correlations between platelet count and other various parameters, a common positive correlation was observed between platelet and other hematological parameters increasing along with the severity of disease. Meanwhile, platelet count presented a major negative correlation with platelet parameters. Additionally, binary logistic regression analysis was performed to identify predictors of severe Delta and showed that a lower level of platelets was strongly associated with more severe disease, suggesting that platelet was an independent predictive factor for severe Delta, which was in agreement with a previous published study [37, 38]. The biomarkers including D-dimer, platelet, large platelet count, and PCT were chosen for further ROC analysis because they presented a significant prediction for Delta severity in logistic regression analysis. The above observations demonstrated that D-dimer, platelet, large platelet count, and PCT level were independent predictive markers of the severity of Delta, suggesting a reasonable prediction for the Delta severity. Therefore, platelet level was significant predictors for Delta severity, which may help the clinicians in predicting the disease severity and take effective treatment measures well in advance.
Our study had some limitations. First, our study was a single-center and retrospective study, which may affect the generalization of the results due to the limitation of enrolled patients. Second, most patients included in the study had mild or moderate disease, and the sample size in severe group was relatively small, which may have some impact on the statistical results. Third, the associations observed between various biomarkers and disease severity cannot be considered as causal on account of the cross-sectional nature of the study.
Overall, as a summary of our findings in this study demonstrated that low platelet may be a predictor of disease severity in Delta. Therefore, our results provided important insights into this topic that assessing platelet count at admission has the benefit of enabling doctors with essential information at the right time and helping doctors in providing valuable treatments quickly in the early stages of hospital treatment.
Statement of Ethics
The study protocol was reviewed and approved by Xi'an Chest Hospital, approval number S2022-0002. Written informed consent was obtained from participants in this study.
Conflict of Interest Statement
The authors declare that there is no conflict of interests.
Funding Sources
This work was supported by the Bureau Level Scientific Research Project, Xi'an (2023qn18).
Author Contributions
Yue-e Chen was responsible for data analysis, manuscript writing, and data control. Fu-le Ren performed data collection, acquisition, and revised the manuscript. Yue-e Chen, Xing Gu, Hong-jun Zhang, Wen-jie Li, Han Yang, and Fen-qing Shang performed data collection, analyzed the patient data. Yue-e Chen, Xing Gu, Hong-jun Zhang, Han Yang, and Fen-qing Shang performed validation and statistical analysis. Yue-e Chen and Fen-qing Shang were responsible for the development of study design and project coordination as well as he reviewed and revised manuscript. All authors read and approved the final manuscript.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated during this study are included in this published article.
Funding Statement
This work was supported by the Bureau Level Scientific Research Project, Xi'an (2023qn18).
References
- 1.Li M, Lou F, Fan H. SARS-CoV-2 Variants of Concern Delta: a great challenge to prevention and control of COVID-19. Signal Transduct Target Ther. 2021;6((1)):349. doi: 10.1038/s41392-021-00767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A, et al. Emergence of SARS-CoV-2 B.1.1.7 lineage: United States, December 29, 2020–January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70((3)):95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397((10293)):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torjesen I. Covid-19: Delta variant is now UK's most dominant strain and spreading through schools. BMJ. 2021;373:n1445. doi: 10.1136/bmj.n1445. [DOI] [PubMed] [Google Scholar]
- 5.Mellema RA, Crandell J, Petrey AC. Platelet dysregulation in the pathobiology of COVID-19. Hamostaseologie. 2022;42((4)):221–228. doi: 10.1055/a-1646-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, et al. Platelets can associate with SARS-cov-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;127((11)):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarychanski R, Houston DS. Assessing thrombocytopenia in the intensive care unit: the past, present, and future. Hematology Am Soc Hematol Educ Program. 2017;2017((1)):660–666. doi: 10.1182/asheducation-2017.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Violi F, Cammisotto V, Pignatelli P. Thrombosis in Covid-19 and non-Covid-19 pneumonia: role of platelets. Platelets. 2021;32((8)):1009–1017. doi: 10.1080/09537104.2021.1936478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zeng X, Jiao Y, Li Z, Liu Q, Ye J, et al. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb Res. 2020;193:110–115. doi: 10.1016/j.thromres.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88((1)):15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen S, Zhang J, Fang Y, Lu S, Wu J, Zheng X, et al. SARS-CoV-2 interacts with platelets and megakaryocytes via ACE2-independent mechanism. J Hematol Oncol. 2021;14((1)):72. doi: 10.1186/s13045-021-01082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136((11)):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, et al. Coagulation dysfunction is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2021;93((2)):962–972. doi: 10.1002/jmv.26336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18((6)):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei H, Luo L, Hu Y. Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J Hematol Oncol. 2020;13((1)):161. doi: 10.1186/s13045-020-01003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Khaiat MM, El-Lehlah AM, Kesheita MA, Abdel-Samiee M, Teima AAA. Association between thrombocytopenia and the severity of Covid-19 infection among hospitalized Egyptian patients. Ann Med Surg. 2022;79:103973. doi: 10.1016/j.amsu.2022.103973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esmaeel HM, Ahmed HA, Elbadry MI, Khalaf AR, Mohammed NA, Mahmoud HA, et al. Coagulation parameters abnormalities and their relation to clinical outcomes in hospitalized and severe COVID-19 patients: prospective study. Sci Rep. 2022;12((1)):13155. doi: 10.1038/s41598-022-16915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173((4)):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piva S, Filippini M, Turla F, Cattaneo S, Margola A, De Fulviis S, et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J Crit Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loconsole D, Centrone F, Morcavallo C, Campanella S, Sallustio A, Accogli M, et al. Rapid spread of the SARS-CoV-2 variant of concern 202012/01 in southern Italy (December 2020–March 2021) Int J Environ Res Public Health. 2021;18((9)):4766. doi: 10.3390/ijerph18094766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Jiang L, Li X, Lin F, Wang Y, Li B, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5((12)) doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130((5)):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolla R, Puricelli C, Bertoni A, Boggio E, Gigliotti CL, Chiocchetti A, et al. Platelets: “multiple choice” effectors in the immune response and their implication in COVID-19 thromboinflammatory process. Int J Lab Hematol. 2021;43((5)):895–906. doi: 10.1111/ijlh.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savla SR, Prabhavalkar KS, Bhatt LK. Cytokine storm associated coagulation complications in COVID-19 patients: Pathogenesis and Management. Expert Rev Anti Infect Ther. 2021;19((11)):1397–1413. doi: 10.1080/14787210.2021.1915129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20((10)):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113((1)):45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujani M, Raychaudhuri S, Singh M, Kaur H, Agarwal S, Jain M, et al. An analysis of hematological, coagulation and biochemical markers in COVID-19 disease and their association with clinical severity and mortality: an Indian outlook. Am J Blood Res. 2021;11((6)):580–591. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395((10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozen M, Yilmaz A, Cakmak V, Beyoglu R, Oskay A, Seyit M, et al. D-Dimer as a potential biomarker for disease severity in COVID-19. Am J Emerg Med. 2021;40:55–59. doi: 10.1016/j.ajem.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31((4)):490–496. doi: 10.1080/09537104.2020.1754383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohlfing AK, Rath D, Geisler T, Gawaz M. Platelets and COVID-19. Hamostaseologie. 2021;41((5)):379–385. doi: 10.1055/a-1581-4355. [DOI] [PubMed] [Google Scholar]
- 34.Nigam JS, Kumar A, Sinha R, H H, Kumar N, Surabhi, et al. Association of peripheral blood parameters with outcomes of COVID-19 infection in a tertiary care setting of eastern India: an institute-based study. Cureus. 2021;13((12)):e20745. doi: 10.7759/cureus.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Yang G, Long Y, Li C. Lymphocyte and platelet counts, as well as interleukin-6 levels, predict mortality of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2021;2021:5582908. doi: 10.1155/2021/5582908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. 2020;92((10)):2188–2192. doi: 10.1002/jmv.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao LN, Ran X, Zhong YX, Li SS. Clinical value of blood markers to assess the severity of coronavirus disease 2019. BMC Infect Dis. 2021;21((1)):921. doi: 10.1186/s12879-021-06623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou X, Li S, Fang M, Hu M, Bian Y, Ling J, et al. Acute physiology and chronic Health evaluation II score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48((8)):e657–e665. doi: 10.1097/CCM.0000000000004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated during this study are included in this published article.