ABSTRACT
Despite advancements in critical care and resuscitation, traumatic injuries are one of the leading causes of death around the world and can bring about long-term disabilities in survivors. One of the primary causes of death for trauma patients are secondary phase complications that can develop weeks or months after the initial insult. These secondary complications typically occur because of systemic immune dysfunction that develops in response to injury, which can lead to immunosuppression, coagulopathy, multiple organ failure, unregulated inflammation, and potentially sepsis in patients. Recently, extracellular vesicles (EVs) have been identified as mediators of these processes because their levels are increased in circulation after traumatic injury and they encapsulate cargo that can aggravate these secondary complications. In this review, we will discuss the role of EVs in the posttrauma pathologies that arise after burn injuries, trauma to the central nervous system, and infection. In addition, we will examine the use of EVs as biomarkers for predicting late-stage trauma outcomes and as therapeutics for reversing the pathological processes that develop after trauma. Overall, EVs have emerged as critical mediators of trauma-associated pathology and their use as a therapeutic agent represents an exciting new field of biomedicine.
KEYWORDS: Extracellular vesicles, trauma, sepsis, immunotherapeutics, review
INTRODUCTION
Globally, trauma is the one of the leading causes of death and produces an enormous economic burden with an annual economic cost of 4.2 trillion dollars in the United States (1). Traumatic injuries are severe injuries that happen suddenly and require immediate medical intervention. These types of injuries include a variety of disease mechanisms related to burn injury and mechanical or penetrating trauma. Trauma-related mortality follows a bimodal distribution, with immediate/early, and late peaks of mortality after injury (2). The immediate mortality (at injury) and early mortality are mainly due to severe central nervous system (CNS) injury or exsanguination. Deaths that occur late after injury are due to multiple organ system failure (MOSF) or sepsis, which can occur weeks after the original insult (2). Major contributors to MOSF after trauma include immune dysfunction and hypercoagulation. Improvements in prehospital care have greatly reduced mortality associated with traumatic injuries (3). However, significant room for improvement remains to better survival and outcomes during the later phase of recovery. To establish strategies that improve survival late after trauma, it is imperative to identify mediators of immune dysfunction and hypercoagulability that can serve as therapeutic targets to reduce posttrauma MOSF and/or biomarkers that can improve disease classification, predict outcomes, and guide therapies.
Recently, extracellular vesicles (EVs) have been identified as critical mediators of the complications that occur after traumatic injury. Extracellular vesicles are spherical structures enclosed by a phospholipid bilayer and are secreted by nearly all cell types (4). Several categories of EVs have been established; however, the three main classes include exosomes, microvesicles (MVs), and apoptotic bodies. Exosomes (40–120 nm) and are released from multivesicular bodies directly into extracellular space. Microvesicles bud from the plasma membrane and range from 100 nm to 1 μm in diameter. Apoptotic bodies are the largest EV type (500 nm to 2 μm) that are released by dying cells and can package cellular organelles (4). Figure 1 illustrates the differences in size of these EVs, secretion mechanisms, cargo, and their impact on recipient cells. Extracellular vesicles are the key mediators of cell-to-cell communication through their transportation of a diverse array of biological cargo. This includes DNA, mRNA, miRNA, histones, long noncoding RNAs, proteins, and bioactive lipids (4). While such cargo of EVs can mediate communication between cells, EVs can also contain damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) that can induce responses in immune and nonimmune cell types. After traumatic injuries, the concentration of plasma EVs typically increases and can promote immune dysfunction (6) and enhance coagulation (7). Extracellular vesicles also contain surface markers that reflect their cell of origin, enabling them to serve as high definition and informative biomarkers. Furthermore, exogenous EVs can also be administered therapeutically, opening a new field of biomedicine (8). Therefore, EVs are an attractive area of research for understanding late trauma-associated pathology and mortality.
Fig. 1.
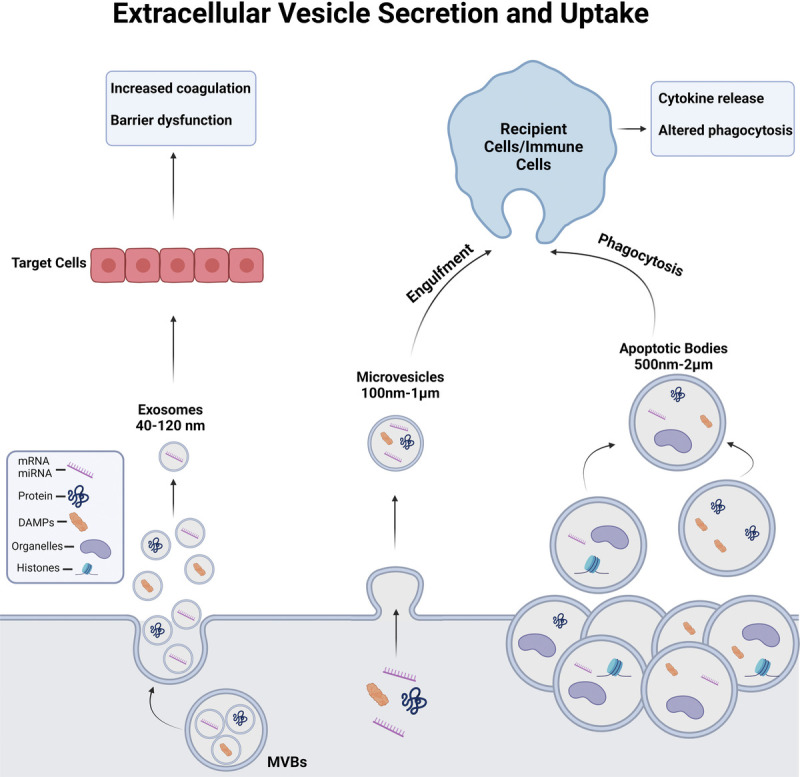
Overview of EV sizes, secretion mechanisms, cargo, and uptake/ effect on recipient cells. Exosomes are a small class of EVs (40–120 nm) released from cells after a multivesicular body (MVB) fuses with the plasma membrane and are enriched in miRNAs, protein, and some DAMPs. Microvesicles are 0.1- to 1-μm diameter EVs that bud from the plasma membrane in a highly regulated fashion containing miRNAs, proteins, DAMPs, and some organelles, such as mitochondria. Apoptotic bodies are large EVs, which are released from cells upon programmed cell death. While all EV classes can contain miRNA, mRNA, DAMPs, lipids, and proteins, apoptotic bodies are large enough that they can also encapsulate larger cellular organelles and histones (5). DAMP, damage-associated molecular pattern; EV, extracellular vesicle.
In this review, we will discuss the emerging field that is focused on the role of EVs after severe forms of trauma, such as severe burn injury, CNS trauma, and posttrauma sepsis. In addition, we assess the current evidence for the use of EVs as biomarkers to predict outcomes after trauma. Finally, we will discuss the potential for EVs as therapeutic targets as well as their possible use as a drug-delivery vehicle for treating traumatic injuries and reprogramming immune cell types late after trauma. Extracellular vesicles show promise as biomarkers for late-stage trauma outcomes, risk of comorbidities, and for informing diagnostic and therapeutic efforts. If understood in their entirety, EVs could serve as therapeutic targets or potentially be harnessed for use as therapeutic agents.
EXTRACELLULAR VESICLES AS MEDIATORS IN TRAUMA
Extracellular vesicles mediate immune dysfunction after severe burn injury
The systemic response to traumatic injuries can result in organ dysfunction or MOSF. Multiple organ system failure involves immune dysregulation and is one of the most difficult clinical scenarios to manage. Severe burn injury is a prime example of how a traumatic injury can lead to immune dysfunction and systemic responses throughout the body. According to the Centers for Disease Control and Prevention, there are approximately 450,000 burns each year in the United States that require medical attention, resulting in close to 3,500 deaths (9). Moreover, burn patients have some of the longest hospital stays compared with other debilitating injuries, highlighting the need for research aimed at improving diagnostic and therapeutic efficacy (10). This may be accomplished by gaining a better understanding into the underlying immune dysfunction after burn injury.
The immune dysfunction after burn injury is complex and involves multiple events related to the initial tissue damage. After a severe burn injury, tissue damage leads to the release of DAMPs, such as hyaluronic acid, dsDNA, and high mobility group box 1 (HMGB1) (11,12). These DAMPs can then activate toll-like receptors to induce the release of inflammatory cytokines that lead to epithelial and immune cell activation to cause a compounding positive feedback loop. In the acute phase of burn injury (<72 hours), patients can develop systemic inflammatory response syndrome (SIRS) due to the release of DAMPs and proinflammatory cytokines, which promote barrier and organ dysfunction (13). After the acute phase, most patients develop compensatory anti-inflammatory response syndrome (CARS). Compensatory anti-inflammatory response syndrome can last for weeks or months, and patients are often left immunosuppressed and susceptible to infection during this time (14). There have been great advancements in critical care, resuscitation, and burn wound management that have led to significant reductions in mortality early after burn injury. Thus, the primary cause of death for burn patients currently is due to hospital-acquired infections that occur during the later CARS phase (13). For this reason, current therapeutic efforts have focused on limiting the intensity or duration of immune dysregulation after severe burn injury. Extracellular vesicles, the main vessels for cell-to-cell communication, have recently been established as important drivers of immune dysregulation after severe burn injuries.
Extracellular vesicles have emerged recently as novel mediators of immune dysfunction that can influence the pathology after burn injury (6). We and others have found that circulating EV levels change after burn injury and participate in burn pathology. After a severe burn injury, there are increased numbers of circulating plasma EVs (6,15,16). Furthermore, there is a positive correlation between the level of circulating EVs and severity of burn injury, with increased EV concentrations corresponding to increases in percentage of total body surface area burn, and these concentrations correlate with postburn sepsis, wound infection, and admission to intensive care units (15,16). Inhalation injury due to smoke or chemical fume inhalation often occurs at the time of burn injury and increases morbidity and mortality for patients (14). Patients with a combined burn and inhalation injury were found to have a substantially higher number of circulating EVs (16). Thus, plasma EV numbers are increased after burn injury and can predict disease course and severity. These studies also suggest that EVs play a functional role in systemic responses after injury.
New studies have identified functional roles for EVs in immune responses after burn injury. Extracellular vesicles isolated during either the SIRS or CARS phase after severe burn injury were found to elicit immune responses that reflect the pathology of these two phases (6). By transferring EVs isolated from plasma early after burn injury to uninjured mice, there was a significant increase in the expression of IL-6, MCP-1, IL-10, interferon- γ (IFN-γ), and IL-8 in recipient mice. Thus, transfer of EVs isolated early after severe burn injury can invoke an immune response in a manner similar to burn injury alone, suggesting that EVs are powerful immunomodulators that drive the immune dysfunction observed after burn injury (6). Furthermore, the in vitro transfer of these EVs onto macrophages resulted in induction of the proinflammatory cytokines MCP-1, IL-12p70, IL-6, and IFN-γ as well as the innate immune genes MyD88, MCP-1, IL-6, and NFκβ1. However, transfer of EVs isolated during the later phases of burn injury onto macrophages resulted in different patterns of cytokine secretion and gene expression (6). In addition, EVs isolated late after burn injury reduced the phagocytic capacity of macrophages and suppressed their proinflammatory response to lipopolysaccharide (LPS). Thus, EVs isolated early after burn recapitulate the immediate/early SIRS response, while EVs isolated late reproduced features of the immunosuppressive CARS phase. In addition to immune responses, EVs promote burn-induced endothelial barrier dysfunction. Extracellular vesicles isolated after burn impaired the barrier function of endothelial cells as measured by a transendothelial electrical resistance assay (16). Based on these studies, the alterations in the cargo of EVs could be an adjuvant or a driver of pathologic responses after severe burn injury.
Extracellular vesicles transfer several immunomodulatory molecules such as HMGB1 after severe burn injury (17). High mobility group box 1 is a nuclear histone binding protein that is often secreted after tissue injury, necrotic cell death, or cellular stress (11). High mobility group box 1 is also capable of forming heterocomplexes with other immune molecules, such as IL-1β, which can lead to more potent immune responses. In a mouse model of severe cutaneous burn injury, HMGB1 and IL-1β were found to be elevated within plasma EVs, but not EV-depleted plasma (17). This was similarly observed in human burn patients who had sustained a more than 20% total body surface area cutaneous burn, with IL-1β reaching peak concentrations 24 to 48 hours after injury. Furthermore, there was an increase in HMGB1 and IL-1β heterocomplexes in plasma EVs after burn injury that enhanced expression of proinflammatory IL-6 and IFN-β in human THP-1 monocytes, two key cytokines that drive the immune response observed after burn injury (17). Specifically, IL-6 correlates with burn mortality (18). These findings suggest that the HMGB1/IL-1β EV heterocomplexes play an important role in the immune response to severe burn injury. Together, these studies implicate EVs in immune and barrier dysfunction after severe burn injury. Future work should further define the role EVs play in postburn pathology and whether modulating EV signaling improves outcomes after burn injury.
Extracellular vesicles as mediators in CNS trauma
Traumatic brain injuries
In the United States, there are approximately 2.8 million emergency department visits and hospitalizations for a traumatic brain injury (TBI) each year, resulting in 56,000 deaths (19). Similar to severe burn injury, TBI is classified by two phases. The primary phase is caused by sudden trauma to the brain that causes tissue injury due to hemorrhaging and physical damage. Neurological damage associated with TBI continues to develop during the secondary phase, which is the phase where most hospital deaths occur (20). The primary cause of brain injury during this phase is due to brain swelling (21). Furthermore, the secondary phase is characterized by neuroinflammation that is activated to repair the site of injury and restore barrier integrity. However, this neuroinflammation can exacerbate the initial injury by increasing oxidative stress and disrupting the blood-brain barrier (BBB), which can persist for years after injury (22). While this response is meant to heal and repair the injured tissue, unregulated neuroinflammation and coagulopathy can cause increases in morbidity and mortality in TBI patients (23). A better understanding of the complex immune responses that occur during the secondary phase of TBI could lead to the identification of new therapeutic targets that may improve health outcomes in TBI patients. Recent evidence has demonstrated that EVs play an important role in exacerbating complications during the later phase of TBI.
Cell-to-cell communication is pivotal for regulating the immune response after TBI and EVs are the critical mediators involved in this communication. During the secondary phase of injury after TBI, a robust inflammatory response occurs that results in the migration and activation of peripheral immune cells and resident glia to the site of injury (24). While this inflammation is important for healing at the injury site, a severe and unregulated neuroinflammatory response following TBI can cause further damage. After TBI, there is a significant increase in circulating EV levels in both human patients and mice (25,26). In humans, this increase in circulating EVs was found at the time of admission, with a decline over the next 72 hours. This trend was consistent for platelet-, endothelial-, and leukocyte-derived EVs (26). In mice, there was a surge in EV concentrations within 24 hours after TBI, with a shift in size of the EVs from MVs to exosomes, that could be caused by barrier breakdown or inflammation in the CNS (25). Thus, EV concentrations in the periphery in both mice and humans increase early after TBI and could implicate EVs as regulators of the second, proinflammatory, and coagulopathic phase of TBI.
To evaluate the changes in EV content after TBI, next-generation sequencing was used to analyze the miRNA content in EVs isolated 7 days after TBI in mice (27). An increase in miR-21, miR-146, miR-7a, and miR-7b was found in the injured cortical hemisphere compared with sham mice. miR-21 showed the largest fold change, and the increase in expression was primarily localized to neurons (27). miR-21 is a strong stimulator of toll-like receptor 7/8 (28) and has been previously identified as being proinflammatory and neurotoxic (29). Exposure of microglia in vitro to neuron-derived EVs that highly express miR-21 induced proinflammatory M1 microglia polarization (30). This proinflammatory polarization of microglia resulted in the release of neuroinflammatory factors and encouraged the apoptosis of neuronal cells (30). Thus, TBI causes changes in EV miRNA content that can result in further aggravation of neuroinflammation and neuronal damage. Besides neurons being a source of EVs after TBI, EVs derived from microglia have also been shown to be elevated early after TBI in mice (31). These EVs contained proinflammatory molecules such as miR-155, IL-1β, and TNF-α. Injection of these EVs into naive mice caused an inflammatory response in the brain (31), suggesting that microglia release EVs that exacerbate neuroinflammation following TBI. Therefore, EVs secreted both from neurons and microglia after TBI promote further tissue injury.
One of the most serious consequences following TBI is coagulopathy (23). Nearly one third of patients hospitalized for a TBI will develop clinically significant coagulopathy (23). One of the major contributors to coagulopathy is platelet dysfunction. Extracellular vesicles, many of which are platelet-derived, have independent thrombotic activity and their release after TBI can influence platelet function. In a mouse model of TBI, it was found that EVs released after TBI cause platelet hypoaggregation (32). These researchers also found that the EVs released after TBI contained the Adenosine diphosphate (ADP) P2Y12 receptor, a key receptor for increasing the sensitivity of platelet aggregation (32). When co-treating these EVs with an ADP P2Y12 receptor antagonist, the post-TBI platelet hypoaggregation was ameliorated. On the contrary, EVs released from the brain into circulation after TBI enriched with tissue factor promote hypercoagulation (33). Thus, EVs can contribute to both platelet dysfunction and hypercoagulation after TBI.
The late phase of TBI is the main period where significant damage to the brain and BBB occurs. Extracellular vesicles can also contribute to increases in BBB permeability after TBI. The BBB is primarily made up of highly specialized brain endothelial cells that are known to respond to mechanical forces. Both the early and late phases of TBI can trigger alterations in the BBB. Extracellular vesicles released by these brain endothelial cells can impact the structure of the BBB or be used as a potential marker for changes in the BBB. Utilizing an in vitro model of rapid tissue deformation in human-brain endothelial cells, a time dependent release of EVs from these cells was observed (34). These EVs contained tight junction proteins (occludin) and endothelial markers (e.g., ICAM-1 and PECAM-1) that were released following the stretch procedure. In a controlled-cortical impact mouse model of TBI, the authors further found that there was an increase in circulating occludin-containing EVs in the plasma of these mice (34).
Though the main problems after TBI are related to the brain or coagulopathy, complications may also occur that impact organ systems throughout the body. One of the most common complications after TBI is secondary acute lung injury (ALI) that develops in 20–25% of patients (35). The mechanism underlying how TBI can induce ALI remains unclear; however, attention has been placed on EVs as being a driver of this secondary complication (36,37). A robust release of DAMPs post-TBI can promote ALI. In a mouse model of TBI, an increase in the AIM2 inflammasome and HMGB1 expression in lungs was observed, with EVs being implicated as a major cause for the increased expression in the lungs (37). Extracellular vesicles isolated from serum after severe TBI increased inflammasome production and induced ALI in naive recipient mice. However, combined treatment of naive mice with TBI EVs and enoxaparin, a heparin that can inhibit EV uptake, reduced inflammasome activation and prevented ALI from occurring (37). To corroborate these findings, EVs were isolated from the serum of human subjects that were diagnosed with a TBI. Human TBI EVs caused pyroptotic cell death through inflammasome signaling in human lung microvascular endothelial cells (38). Another characteristic of ALI is the disruption of the blood-air-barrier (BAB), which results in deficiencies in gas exchange at the alveolar capillaries in the lung. Kerr et al. (2019) found that EVs released from the brain can cause inflammation in the lung as well as disrupting the integrity of BAB (38). Additional studies have also revealed that the brain EVs present after TBI can disrupt this endothelial barrier through a synergistic interaction with platelets (33). Further characterization of these EVs illustrated that they contained mitochondria, which preferentially bind to platelets (39). These platelets bound to EVs containing mitochondria that reduced the expression of tight junction proteins and impact the barrier integrity of endothelial cells, contributing to ALI (39). These studies indicate that EVs released from the brain after TBI can alter the BAB and thus can play a role in the development of ALI. Together, these studies implicate EVs in multiple facets of TBI pathology including neuronal damage, neuroinflammation, coagulopathy, and ALI (Fig. 2).
Fig. 2.
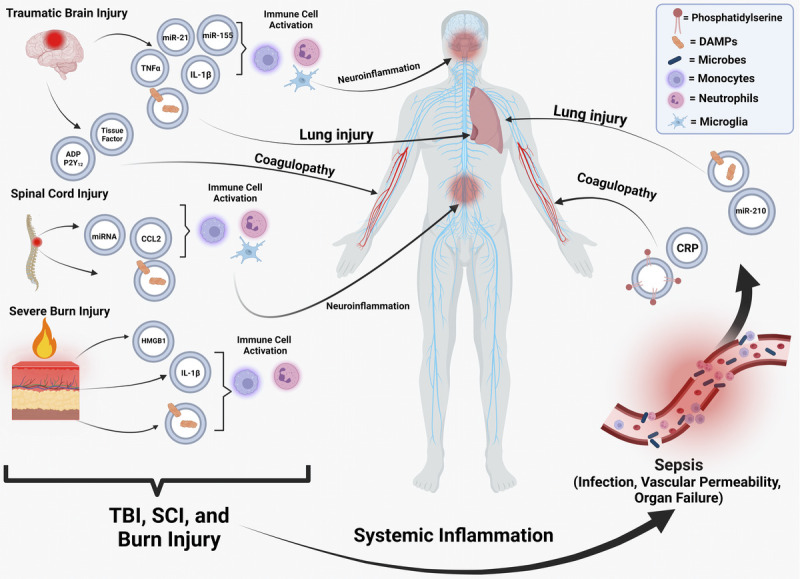
The impact of EVs released after traumatic injuries and sepsis on organ systems throughout the body. After various traumatic injuries, EVs are released that contain a variety of different cargo capable of promoting immune dysfunction, coagulopathy, and inflammation at the site of injury or systemically. This includes DAMPs, miRNAs, cytokines, tissue factor, and P2Y12. After trauma, EVs can predispose a patient to sepsis, which subsequently results in the release of EVs enriched with CRP, phosphatidylserine, DAMPs, and key miRNAs that negatively impact coagulation and lung function (5). CRP, C-reactive protein; DAMP, damage-associated molecular pattern; EV, extracellular vesicle.
Spinal cord injury
Spinal cord injury (SCI) is another severe form of trauma to the CNS. There are approximately 17,000 SCI cases in the United States each year, and there is an estimated 363,000 SCI survivors living in the United States (40,41). While advancements in critical care and symptom management have slightly reduced mortality associated with SCI (40), mortality rates are still high and there is a drop in life expectancy for survivors (42). Currently, there is a paucity of effective treatments to handle the long-term complications associated with SCI. One of the main reasons for this is because there is not a comprehensive understanding of the complex pathology following this form of neurotrauma. Traumatic injury to the spinal cord develops similarly to TBI, with the primary phase occurring because of a mechanical or penetrating force that causes immediate damage to the neural tissue. Subsequently, the secondary phase begins where inflammation and immune dysfunction exacerbate the damage caused by the initial trauma over time (43). The secondary phase is a window wherein therapeutic interventions could improve morbidity and mortality for patients. Similar to burn injury and TBI, EVs participate in the progression of the secondary phase of SCI by delivering cargo that alters the function of cells in the CNS and throughout the body.
To our current knowledge, there have not been any studies to date evaluating the impact of SCI on the circulating EV concentrations in human patients. However, there has been work exploring the impact of SCI on circulating EV concentrations in rodents. After thoracic contusion SCI in adult male mice, total EV numbers were decreased 1 day after injury before recovering to normal physiological levels over time. However, there was a significant increase in plasma tetraspanin CD81+ EVs after SCI before returning to sham levels by day 14 after injury (44). The source of these EVs was thought to originate from astrocytes at the site of injury, because local astrocytes had dramatic decreases in CD81 surface expression. Despite the limited amount of data on the circulating EV concentrations after SCI, there have been numerous studies evaluating the alterations in EV cargo after this form of trauma.
One of the major complications that develops during the secondary phase of SCI is unregulated neuroinflammation that can aggravate the initial injury. Extracellular vesicles also participate in this process. In a rat model of SCI, alterations in the miRNA content of EVs in the serum 6 hours after injury were analyzed. In this study, 217 miRNAs were found to be differentially expressed between sham rats and SCI rats, with 78 being upregulated and 139 downregulated. These miRNAs have postulated functions related to axonal guidance, Wnt signaling, and long-term potentiation (45). These EVs could contribute to the pathology of SCI by influencing these signaling pathways. Furthermore, during the analysis of the miRNA content of these EVs, the miRNA profile reflected those that were previously identified in EVs derived from astrocytes after in vitro stimulation with IL-1β or TNF-α (45,46). To further corroborate these findings, EVs isolated from either SCI mice or sham mice were intracerebroventricularly injected into naive mice. After 24 hours, mice injected with SCI EVs had increased expression of key inflammatory genes as well as increased intracellular IL-1β and IL-1α levels in brain astrocytes (44). In another study, authors found that the C-C motif chemokine ligand 2, a cytokine involved in the regulation of the migration and penetration of leukocytes, was encapsulated and secreted in EVs by astrocytes after SCI (47). The secretion of this cytokine acted on both microglia and neurons, encouraging microglial activation and neuronal apoptosis. The subsequent activation of microglia led to the release of IL-1β, which further stimulated apoptosis in neurons, acting as a positive feedback loop (47). While astrocytes have been primarily studied as a source of EVs after SCI, other cell types might also participate in the secretion of EVs after SCI. Thus, similar to TBI, EVs play a key role in immune responses and pathologic progression after SCI.
Extracellular vesicles promote the progression of sepsis and septic shock
Sepsis is a potentially life-threatening condition that is caused by a dysregulated response to infection, which can cause tissue damage, organ failure, and death (48). Nearly 1.7 million adults are affected by sepsis in the United States each year and 270,000 of these people will die as a result (49). Early diagnosis and prediction of patients at risk for sepsis are difficult, and there are limited therapeutic countermeasures (50). The risk of developing sepsis in patients undergoing treatment for trauma is of particular concern for clinicians. While the mortality rates associated with sepsis has decreased overall (51), this rate has remained unchanged in trauma patients (52). Similar to severe burn injury and CNS injuries, EVs play a role in the progression of sepsis. However, because both pathogens and the host use EVs as signaling vehicles, it is difficult to pinpoint which EVs from the host exacerbate sepsis (50). Here, we will primarily discuss EVs that originate from the host that influence the development of—and pathology associated with sepsis.
Septic patients have substantially higher concentrations of circulating EVs than matched controls, and EV numbers correlate with the severity of sepsis (53). The primary sources of plasma EVs released during sepsis are platelets and circulating immune cells, such as macrophages, dendritic cells, neutrophilic granulocytes, and natural killer cells (54,55). However, recent studies have found that EVs released from the astrocytes and perhaps neurons could possibly contribute to sepsis-related complications (56). Lin et al. measured expression of glial fibrillary acidic protein (GFAP) and neuron-specific enolase in plasma EVs. Because GFAP is localized in hepatic stellate cells and spinal cord astrocytes, and neuron-specific enolase is in platelets and erythrocytes (57,58), this study cannot definitively identify the brain as the source of these EVs. However, the authors did find EVs isolated directly from brain parenchymal tissue using gentle digestion methods did promote hypercoagulation in plasma as well as lung, liver, and kidney injury in naive mice. Further evidence that suggests postsepsis EVs can be harmful. In preclinical and clinical sepsis, lymphocyte apoptosis is common and is promoted by the release of caspase-1 from circulating EVs in septic patients (59). Thus, EVs can promote sepsis-associated pathology.
One of the major complications in septic patients that clinicians must combat is sepsis-induced lung injury. Sepsis is one of the leading causes of ALI and acute respiratory distress syndrome in hospitals, and the lung is considered to be one of the most vulnerable organs during sepsis (60). Extracellular vesicles are a possible contributor to the development of sepsis-induced lung injury. In one study, the intratracheal administration of EVs released from LPS-stimulated alveolar macrophages into naive mice increased lung injury parameters, such as bronchoalveolar fluid protein and neutrophil counts (61). There was also an increase in ICAM-1 expression on both type I and type II epithelial cells, which is indicative of deficiencies in epithelial cell barrier function (61). Furthermore, dendritic cells that were activated by LPS stimulation secreted EVs that, in turn, caused the secretion of chemokines by epithelial cells (62). While the release of cytokines is important for the innate immune response, excess cytokine secretion can intensify septic complications (63). In another study, EVs derived from septic patients caused the secretion of inflammatory factors in THP-1 monocytes (64). Analysis of the EV contents in these septic patient samples found that they were enriched for miR-210, which induced inflammation and apoptosis in bronchial epithelial cells (64). These principal studies provide a link between EVs and lung complications in septic patients.
Another severe and fatal clinical complication of sepsis is disseminated intravascular coagulation. Approximately 35% of septic patients develop this serious condition that involves consumption of coagulation factors and platelets, which can lead to clotting in blood vessels throughout the body. Disseminated intravascular coagulation reduces the blood supply to organs and contributes to organ failure (65). Because of the procoagulant activity of EVs, they could be involved in the development and pathology of disseminated intravascular coagulation (66). Phosphatidylserine on the surface of EVs derived from platelets, leukocytes, and endothelial cells could contribute to sepsis-induced coagulopathy (67). Zhang et al. found that EVs isolated from septic patients had elevated levels of phosphatidylserine on their surface and that these EVs increased thrombin formation in vitro on cultured endothelial cells (67). Furthermore, an additional study found that platelet derived EVs promoted thrombin generation. However, this effect was negated by annexin V, which binds and inactivates phosphatidylserine (68). Furthermore, EVs contain DNA, histones, and other components, such as C-reactive protein (CRP) that cause procoagulant activity (69,70). Thus, EVs secreted during sepsis are most likely contributing to sepsis-induced coagulopathy.
EXTRACELLULAR VESICLES AS BIOMARKERS AND PROGNOSTICS IN TRAUMATIC INJURIES
As detailed previously, EVs carry important signaling molecules that can promote complications after trauma. Therefore, characterization of EV contents could identify potential biomarkers of disease severity and complication risk. As such, EV biomarkers have been identified in the settings of severe burn injury, blast induced TBI, SCI, and sepsis that could predict disease severity and outcomes (12,50,71,72).
In severe burn injury, both plasma EV contents and concentrations could serve as predictive markers. After severe burn injury, EV concentrations were positively correlated with burn size (12,16). liquid chromatography tandem mass spectrometry proteomic assessment of plasma EVs from 50 burn patients found increased levels of both serum amyloid A1 (SAA1) and CRP in EVs 72 hours after injury, which correlated with length of hospital stay in females (12). Importantly, SAA1 and CRP in EV-depleted plasma did not associate with disease outcomes, suggesting SAA1 and CRP in plasma EVs are superior prognostic biomarkers. In another study, 43 miRNAs were found to be differentially expressed in the dermal interstitial fluid of a human ex vivo skin model of a deep partial-thickness burn. miR-497, an miRNA upregulated in hypertrophic scars, was persistently downregulated after burn. The degree of loss of miR-497 could be used an indicator for the regenerative capacity of an individual’s injured tissue a burn injury (73). This becomes important, because there is currently no diagnostic metric to help burn surgeons decide when to surgically restore wounds after burn injury. Because dermal interstitial fluid can be collected safely and noninvasively, the EVs from this biofluid could be used in the future to assist surgical decision making.
Extracellular vesicles have also been investigated as potential biomarkers in TBI. Traumatic brain injury diagnosis and prognosis are determined using neurological and neuroimaging techniques, such as the Glasgow Coma Scale, computed tomography scans, and magnetic resonance imaging. However, these techniques have several limitations, such as the low sensitivity of computed tomography and the inability to perform magnetic resonance imaging if metal fragments are present. Furthermore, these approaches fail to clearly assess other physiological parameters of TBI. To circumvent this, recent work has examined the composition of EVs present in cerebral spinal fluid (CSF) or plasma after TBI as a means of potentially determining injury severity (74). Extracellular vesicles in CSF of patients diagnosed with severe TBI were collected for up to 10 days after injury and found previously identified TBI biomarkers, such as GFAP and αII-spectrin breakdown products (75,76), as well as new candidates such as C1qb (74). Future work should attempt to associate such EV contents with patient outcomes. Other studies have assessed plasma EVs in patients with TBI. Puffer et al. found that after TBI, GFAP was increased in plasma EVs as well as numerous miRNAs related to molecular and cellular functions (77). Collecting CSF or plasma is invasive and not easily accessible; thus, there have been other studies to evaluate the presence of biomarkers indicative of TBI using more readily accessible collection methods. For instance, in plasma EVs isolated after TBI, there were increases in EV p-tau, tau, Aβ42, and IL-10 (78,79). Recently, a positive correlation between plasma EVs containing neurofilament light and TBI severity was recently seen in a military population (80). Within 1 year of injury, plasma EV neurofilament light was found to be superior to GFAP in discriminating between patients of differing TBI severity (80). Extracellular vesicles in the saliva of TBI patients have also been assessed and revealed an injury-dependent upregulation of the CDC2, CSNK1A1, and CTSD transcripts (81). These transcripts all have established roles in neurodegenerative diseases correlating with TBI, suggesting a role for salivary EVs in diagnosing TBI, especially mild cases of TBI that are not severe enough to be detected by traditional methods. However, compared with TBI, there is a paucity of research that has identified EV biomarkers for SCI. These early studies illustrate that EVs are useful tools as biomarkers for CNS trauma.
Sepsis is often caused by an aberrant host response to infection and is a common complication for trauma patients. In sepsis, there is an urgent need to identify biomarkers that could predict risk and assist in guiding diagnosis and treatment. Markers are needed that have low variability between patients and can be quickly measured (50). This is quite challenging given the robust immune and parenchymal cell dysfunction in sepsis. However, prognostic biomarkers are of great interest because the rapid identification of sepsis can greatly improve survival (50,82). Although procalcitonin levels are currently used in some capacity to inform the cessation of antibiotics, it is not always reliable and is not a strongly recommended method for governing the treatment regimens for septic patients (83). Recently, CRP was found to be increased in plasma EVs in septic patients, with CRP+ EVs promoting monocyte inflammatory activation (70). Extracellular vesicle CRP levels were not associated with patient outcomes in this study. However, this should be pursued given the association of EV CRP with outcomes in burn patients (12). When treating septic patients, it is imperative to quickly identify the microbial agent driving sepsis. Typically, this is done using blood cultures; however, evidence suggests that up to 30% of these cultures fail to identify the microbial source (84). A method of solving this problem is to evaluate EV surface markers, content, and concentration to accurately determine the type of microbial infection that is present. Woth et al. found that septic patients with mixed fungal infections had higher levels of plasma EVs with PAC1− and CD42a− surface markers early during infection compared with septic patients who did not have a fungal infection (85). These results suggest that measuring the presence of these surface markers in EVs isolated early after infection could be useful for identifying the type of microbial infection in septic patients. The stability of EVs and their potential to provide more specificity than total plasma raise the need for further studies to determine if EV contents, numbers, or surface markers can be used to predict sepsis risk and outcomes.
EXTRACELLULAR VESICLES AS A TREATMENT VEHICLE FOR TRAUMATIC INJURY COMPLICATIONS
In addition to their roles in disease pathogenesis and biomarker discovery, EVs are also becoming harnessed as therapeutic agents themselves in many clinical pathologies. For instance, EVs from mesenchymal stem cells (MSCs), multipotent cells found in virtually every human organ, have been studied because of their regenerative and immunomodulatory effects (86). With the limited cell engraftment observed with MSCs themselves, MSC-derived EVs have emerged as a more effective alternative (87). Indeed, preclinical data collected across multiple models suggest that a primary mechanism of MSC efficacy is mediated by the release of EVs into the extracellular space (88). In addition, isolation of MSC-derived EVs from in vitro conditioned media has proven to induce similar or even greater immunomodulatory responses than the MSCs themselves (89). Therefore, MSC-EV therapy is perhaps the next generation of cellular therapeutics, with nearly 1,000 clinical trials currently ongoing worldwide. As traumatic injury is associated with immune dysfunction as well as tissue destruction, the anti-inflammatory and regenerative capacity of MSC-EVs make them an attractive treatment strategy. Furthermore, therapies for trauma need to be field deployable in a rapid and universal fashion. Mesenchymal stem cell EVs have the potential to fulfill this need because they are amenable to universal applicability in a rapid, stable, and scalable manner. For instance, MSCs do not possess cell histocompatibility molecules that necessitate donor-recipient matching. As such MSC-EVs can be provided in a universal and allogeneic manner. Extracellular vesicles are also non–self-replicating, are stable at ambient temperatures for long periods, can be cryopreserved, freeze-dried, and/or sterile filtered allowing for development of standardized, off-the-shelf products (90). Mesenchymal stem cells are also a naturally renewable cell type and thus can be scaled up for the mass production of EVs. Together, the intrinsic properties of MSC-EVs make them useful in treatment of individuals, groups of individuals, or even mass casualty use.
Emerging studies have found utility in MSC-EVs in settings of trauma and sepsis. Mesenchymal stem cell EVs mitigated visual deficits after mild TBI in mice (91). In rat and monkey TBI models, MSC-EVs reduce neuroinflammation and might potentially improve recovery (92,93). In SCI, MSC-EVs loaded with the miRNA-22 reduced microglial pyroptosis and improved inflammatory profiles after injury (94). Recently, MSC-EVs given to rats after SCI increased neuronal coverage of the injured area, reduced levels of apoptotic caspase-3, and improved functional scores (95). In models of hemorrhagic shock, MSC-EVs reduced shock-induced vascular permeability in the lungs (96,97). Mesenchymal stem cell EVs were found to improve wound healing (98). In an early life sepsis model, MSC-EVs improved neuroinflammation and brain structural change (99). Nanovesicles similar to EVs from MSCs were also found to improve hypothermia and suppress cytokine stormed in a model of Escherichia coli"–induced sepsis (100). Although this field is still in its early phases, these studies are promising and warrant future investigations into using EVs as biomedicine in trauma and sepsis.
CONCLUSIONS
Secondary complications that develop in trauma patients remain a major challenge for clinicians to combat. Recently, EVs have emerged as key mediators in posttrauma pathology. The release of EVs after traumatic injuries can cause immune dysfunction, hyperinflammation, coagulopathy, and damage to other organ systems. In addition, EVs can also promote the progression of sepsis. While EVs can exacerbate the damage associated with traumatic injuries and sepsis, they could also be harnessed as powerful prognostic biomarkers that could aid in the treatment and diagnosis of patients. Lastly, the use of MSC-EVs as a therapeutic for severe trauma and sepsis represents a promising future for biomedicine.
Footnotes
Robert Maile and Leon G. Coleman contributed equally.
The study was supported by NIH NIGMS R01GM131124 (to R.M./M.L.W.), NIH NIGMS F31GM149109 (to M.L.W.), NIH NIEHS T32ES007126 (to M.L.W./R.F.S.), and NIH K08-AA024829 (to L.G.C.).
The authors report no conflict of interests.
Contributor Information
Roland F. Seim, Email: seimroland@unc.edu.
Micah L. Willis, Email: mlatw@unc.edu.
Shannon M. Wallet, Email: swallet@dental.ufl.edu.
Robert Maile, Email: robert.maile@surgery.ufl.edu.
REFERENCES
- 1.Peterson C, Miller GF, Barnett SBL, Florence C: Economic cost of injury—United States, 2019. Morb Mortal Wkly Rep 70(48):1655–1659, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD: Epidemiology of trauma deaths. Am J Surg 140(1):144–150, 1980. [DOI] [PubMed] [Google Scholar]
- 3.Gunst M, Ghaemmaghami V, Gruszecki A, Urban J, Frankel H, Shafi S: Changing Epidemiology of Trauma Deaths Leads to a Bimodal Distribution. Taylor & Francis, 2010:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaborowski MP, Balaj L, Breakefield XO, Lai CP: Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65(8):783–797, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Created with BioRender.com. 2022.
- 6.Willis ML, Mahung C, Wallet SM, Barnett A, Cairns BA, Coleman LG, Jr., Maile R: Plasma extracellular vesicles released after severe burn injury modulate macrophage phenotype and function. J Leukoc Biol 111(1):33–49, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curry N, Raja A, Beavis J, Stanworth S, Harrison P: Levels of procoagulant microvesicles are elevated after traumatic injury and platelet microvesicles are negatively correlated with mortality. J Extracell Vesicles 3(1):25625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsaadi N, Srinivasan AJ, Seshadri A, Shiel M, Neal MD, Scott MJ: The emerging therapeutic potential of extracellular vesicles in trauma. J Leukoc Biol 111(1):93–111, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe CS, Massenburg BB, Morrison SD, Naghavi M, Pham TN, Gibran NS. Trends of burn injury in the United States: 1990 to 2016. Annals of surgery. 270(6):944–953, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Ghaed Chukamei Z, Mobayen M, Bagheri Toolaroud P, Ghalandari M, Delavari S: The length of stay and cost of burn patients and the affecting factors. Int J Burns Trauma 11(5):397, 2021. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X-L, Sun L, Guo F, Wang F, Liu S, Liang X, Wang R-S, Wang Y-J, Sun Y-X: High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs-dependent signaling pathway after burn injury. PloS one 7(11):e50668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maile R, Willis ML, Herring LE, Prevatte A, Mahung C, Cairns B, Wallet S, Coleman LG: Burn injury induces proinflammatory plasma extracellular vesicles that associate with length of hospital stay in women: CRP and SAA1 as potential prognostic indicators. Int J Mol Sci 22(18):10083, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S: Burn injury. Nat Rev Dis Primers 6(1):1–25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maile R, Jones S, Pan Y, Zhou H, Jaspers I, Peden DB, Cairns BA, Noah TL: Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. Am J Physiol Lung Cell Mol Physiol 308(9):L855–L860, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Dea KP, Porter JR, Tirlapur N, Katbeh U, Singh S, Handy JM, Takata M: Circulating microvesicles are elevated acutely following major burns injury and associated with clinical severity. PLoS One 11(12):e0167801, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Chatterjee V, Zheng E, Reynolds A, Ma Y, Villalba N, Tran T, Jung M, Smith DJ, Wu MH: Burn injury-induced extracellular vesicle production and characteristics. Shock 57(6):228–242, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman LG, Jr., Maile R, Jones SW, Cairns BA, Crews FT: HMGB1/IL-1β complexes in plasma microvesicles modulate immune responses to burn injury. PLoS One 13(3):e0195335, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur J, Yang HT, Chun W, Kim J-H, Shin S-H, Kang HJ, Kim HS: Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann Lab Med 35(1):105–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor CA, Bell JM, Breiding MJ, Xu L: Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ 66(9):1–16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall LF, Gautille T, Klauber MR, Eisenberg HM, Jane JA, Luerssen TG, Marmarou A, Foulkes MA: The outcome of severe closed head injury. J Neurosurg 75(supplement):S28–S36, 1991. [Google Scholar]
- 21.Ghajar J: Traumatic brain injury. Lancet 356(9233):923–929, 2000. [DOI] [PubMed] [Google Scholar]
- 22.van Vliet EA, Ndode-Ekane XE, Lehto LJ, Gorter JA, Andrade P, Aronica E, Gröhn O, Pitkänen A: Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol Dis 145:105080, 2020. [DOI] [PubMed] [Google Scholar]
- 23.Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI: Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 150(2):165–175, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Gyoneva S, Ransohoff RM: Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell–cell communication by chemokines. Trends Pharmacol Sci 36(7):471–480, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazelton I, Yates A, Dale A, Roodselaar J, Akbar N, Ruitenberg MJ, Anthony DC, Couch Y: Exacerbation of acute traumatic brain injury by circulating extracellular vesicles. J Neurotrauma 35(4):639–651, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Nekludov M, Mobarrez F, Gryth D, Bellander B-M, Wallen H: Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J Neurotrauma 31(23):1927–1933, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Harrison EB, Hochfelder CG, Lamberty BG, Meays BM, Morsey BM, Kelso ML, Fox HS, Yelamanchili SV: Traumatic brain injury increases levels of miR-21 in extracellular vesicles: implications for neuroinflammation. FEBS Open Bio 6(8):835–846, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, Levy E, Fox HS: MiR-21 in extracellular vesicles leads to neurotoxicity via TLR7 signaling in SIV neurological disease. PLoS Pathog 11(7):e1005032, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabbri M Paone A Calore F Galli R Gaudio E Santhanam R Lovat F Fadda P Mao C Nuovo GJ, et al.: MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci 109(31):E2110–E2116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S, Wang L, Yu J, Li W, Wang Y: Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun 83:270–282, 2020. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI: Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14(1):1–17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin GE, Pugh AM, Moran R, Veile R, Friend LA, Pritts TA, Makley AT, Caldwell CC, Goodman MD: Microvesicles generated following traumatic brain injury induce platelet dysfunction via adenosine diphosphate receptor. J Trauma Acute Care Surg 86(4):592–600, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, Wu X, Zhang Y, Konkle BA, Thiagarajan P: Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 125(13):2151–2159, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrews AM, Lutton EM, Merkel SF, Razmpour R, Ramirez SH: Mechanical injury induces brain endothelial-derived microvesicle release: implications for cerebral vascular injury during traumatic brain injury. Front Cell Neurosci 10:43, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr N, de Rivero Vaccari JP, Dietrich WD, Keane RW: Neural-respiratory inflammasome axis in traumatic brain injury. Exp Neurol 323:113080, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang CN, Li FJ, Zhao ZL, Zhang JN: The role of extracellular vesicles in traumatic brain injury-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 321(5):L885–L891, 2021. [DOI] [PubMed] [Google Scholar]
- 37.Kerr NA, de Rivero Vaccari JP, Abbassi S, Kaur H, Zambrano R, Wu S, Dietrich WD, Keane RW: Traumatic brain injury-induced acute lung injury: evidence for activation and inhibition of a neural-respiratory-inflammasome axis. J Neurotrauma 35(17):2067–2076, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr NA, de Rivero Vaccari JP, Umland O, Bullock MR, Conner GE, Dietrich WD, Keane RW: Human lung cell pyroptosis following traumatic brain injury. Cell 8(1):69, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z Zhou Y Hilton T Li F Han C Liu L Yuan H Li Y Xu X Wu X, et al.: Extracellular mitochondria released from traumatized brains induced platelet procoagulant activity. Haematologica 105(1):209–217, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, Garshick E: Traumatic spinal cord injury in the United States, 1993–2012. JAMA 313(22):2236–2243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee BB, Cripps RA, Fitzharris M, Wing PC: The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 52(2):110–116, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S: Life expectancy after spinal cord injury: a 50-year study. Spinal Cord 50(11):803–811, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S: Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 10:282, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan NZ, Cao T, He J, Ritzel RM, Li Y, Henry RJ, Colson C, Stoica BA, Faden AI, Wu J: Spinal cord injury alters microRNA and CD81+ exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav Immun 92:165–183, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding S-Q, Chen J, Wang S-N, Duan F-X, Chen Y-Q, Shi Y-J, Hu J-G, Lü H-Z: Identification of serum exosomal microRNAs in acute spinal cord injured rats. Exp Biol Med 244(14):1149–1161, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhuri AD, Dastgheyb RM, Yoo SW, Trout A, Talbot CC, Jr., Hao H, Witwer KW, Haughey NJ: TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis 9(3):363, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rong Y, Ji C, Wang Z, Ge X, Wang J, Ye W, Tang P, Jiang D, Fan J, Yin G: Small extracellular vesicles encapsulating CCL2 from activated astrocytes induce microglial activation and neuronal apoptosis after traumatic spinal cord injury. J Neuroinflammation 18(1):196, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien JM, Jr., Ali NA, Aberegg SK, Abraham E: Sepsis. Am J Med 120(12):1012–1022, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE: Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA 318(13):1241–1249, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raeven P, Zipperle J, Drechsler S: Extracellular vesicles as markers and mediators in sepsis. Theranostics 8(12):3348–3365, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowan KM Angus DC Bailey M Barnato AE Bellomo R Canter RR Coats TJ Delaney A Gimbel E, et al. PRISM Investigators .: Early, goal-directed therapy for septic shock—a patient-level meta-analysis. N Engl J Med 376(23):2223–2234, 2017. [DOI] [PubMed] [Google Scholar]
- 52.Eguia E Bunn C Kulshrestha S Markossian T Durazo-Arvizu R Baker MS Gonzalez R Behzadi F Churpek M Joyce C, et al.: Trends, cost, and mortality from sepsis after trauma in the United States: an evaluation of the national inpatient sample of hospitalizations, 2012–2016. Crit Care Med 48(9):1296–1303, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dakhlallah DA Wisler J Gencheva M Brown CM Leatherman ER Singh K Brundage K Karsies T Dakhlallah A Witwer KW, et al.: Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock. J Extracell Vesicles 8(1):1669881, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H: Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma Acute Care Surg 50(5):801–809, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Pugholm LH, Bæk R, Søndergaard EK, Revenfeld AL, Jørgensen MM, Varming K: Phenotyping of leukocytes and leukocyte-derived extracellular vesicles. J Immunol Res 2016:6391264, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin H, Chen H, Qi B, Jiang Y, Lian N, Zhuang X, Yu Y: Brain-derived extracellular vesicles mediated coagulopathy, inflammation and apoptosis after sepsis. Thromb Res 207:85–95, 2021. [DOI] [PubMed] [Google Scholar]
- 57.Marangos PJ, Campbell IC, Schmechel DE, Murphy DL, Goodwin FK: Blood platelets contain a neuron-specific enolase subunit. J Neurochem 34(5):1254–1258, 1980. [DOI] [PubMed] [Google Scholar]
- 58.Day IN, Thompson RJ: Levels of immunoreactive aldolase C, creatine kinase-BB, neuronal and non-neuronal enolase, and 14-3-3 protein in circulating human blood cells. Clin Chim Acta 136(2–3):219–228, 1984. [DOI] [PubMed] [Google Scholar]
- 59.Exline MC, Justiniano S, Hollyfield JL, Berhe F, Besecker BY, Das S, Wewers MD, Sarkar A: Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis. PLoS One 9(3):e90968, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan E, Brodie D, Slutsky AS: Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA 319(7):698–710, 2018. [DOI] [PubMed] [Google Scholar]
- 61.Soni S, Wilson MR, O'Dea KP, Yoshida M, Katbeh U, Woods SJ, Takata M: Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 71(11):1020–1029, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP: Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-α-mediated pathway. Am J Pathol 175(2):696–705, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blackwell TS, Christman JW: Sepsis and cytokines: current status. Br J Anaesth 77(1):110–117, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Li G, Wang B, Ding X, Zhang X, Tang J, Lin H: Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp Mol Med 53(7):1180–1191, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levi M, Ten Cate H: Disseminated intravascular coagulation. New Engl J Med 341(8):586–592, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Gheldof D, Haguet H, Dogné J-M, Bouvy C, Graux C, George F, Sonet A, Chatelain C, Chatelain B, Mullier F: Procoagulant activity of extracellular vesicles as a potential biomarker for risk of thrombosis and DIC in patients with acute leukaemia. J Thromb Thrombolysis 43(2):224–232, 2017. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y Meng H Ma R He Z Wu X Cao M Yao Z Zhao L Li T Deng R, et al.: Circulating microparticles, blood cells, and endothelium induce procoagulant activity in sepsis through phosphatidylserine exposure. Shock 45(3):299–307, 2016. [DOI] [PubMed] [Google Scholar]
- 68.Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB, Weber V: Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep 7(1):6522, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez MC, Andriantsitohaina R: Circulating microparticles in septic shock. Am J Respir Crit Care Med 180(1):100–101, 2009. [DOI] [PubMed] [Google Scholar]
- 70.Fendl B, Weiss R, Eichhorn T, Linsberger I, Afonyushkin T, Puhm F, Binder CJ, Fischer MB, Weber V: Extracellular vesicles are associated with C-reactive protein in sepsis. Sci Rep 11(1):6996, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balakathiresan N, Bhomia M, Chandran R, Chavko M, McCarron RM, Maheshwari RK: MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J Neurotrauma 29(7):1379–1387, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ko J, Hemphill M, Yang Z, Sewell E, Na YJ, Sandsmark DK, Haber M, Fisher SA, Torre EA, Svane KC: Diagnosis of traumatic brain injury using miRNA signatures in nanomagnetically isolated brain-derived extracellular vesicles. Lab Chip 18(23):3617–3630, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foessl I Haudum CW Vidakovic I Prassl R Franz J Mautner SI Kainz S Hofmann E Obermayer-Pietsch B Birngruber T, et al.: miRNAs as regulators of the early local response to burn injuries. Int J Mol Sci 22(17):9209, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manek R, Moghieb A, Yang Z, Kumar D, Kobessiy F, Sarkis GA, Raghavan V, Wang KKW: Protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol Neurobiol 55(7):6112–6128, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mondello S Robicsek SA Gabrielli A Brophy GM Papa L Tepas J Robertson C Buki A Scharf D Jixiang M, et al.: αII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J Neurotrauma 27(7):1203–1213, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ringger NC, O'Steen BE, Brabham JG, Silver X, Pineda J, Wang KK, Hayes RL, Papa L: A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J Neurotrauma 21(10):1443–1456, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Puffer RC, Cumba Garcia LM, Himes BT, Jung MY, Meyer FB, Okonkwo DO, Parney IF: Plasma extracellular vesicles as a source of biomarkers in traumatic brain injury. J Neurosurg 134(6):1921–1928, 2020. [DOI] [PubMed] [Google Scholar]
- 78.Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, Motamedi V, Osier N, Stern RA, Kapogiannis D: Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj 32(11):1359–1366, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL: Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB J 30(11):3853–3859, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guedes VA, Lange RT, Lippa SM, Lai C, Greer K, Mithani S, Devoto C, A Edwards K, Wagner CL, Martin CA: Extracellular vesicle neurofilament light is elevated within the first 12-months following traumatic brain injury in a US military population. Sci Rep 12(1):1–11, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng Y Pereira M Raukar N Reagan JL Queseneberry M Goldberg L Borgovan T LaFrance WC Jr. Dooner M Deregibus M, et al.: Potential biomarkers to detect traumatic brain injury by the profiling of salivary extracellular vesicles. J Cell Physiol 234(8):14377–14388, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walley KR: Biomarkers in sepsis. Curr Infect Dis Rep 15(5):413–420, 2013. [DOI] [PubMed] [Google Scholar]
- 83.Rhodes A Evans LE Alhazzani W Levy MM Antonelli M Ferrer R Kumar A Sevransky JE Sprung CL Nunnally ME, et al.: Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43(3):304–377, 2017. [DOI] [PubMed] [Google Scholar]
- 84.Annane D, Bellissant E, Cavaillon J-M: Septic shock. Lancet 365(9453):63–78, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Woth G, Tőkés-Füzesi M, Magyarlaki T, Kovács GL, Vermes I, Mühl D: Activated platelet-derived microparticle numbers are elevated in patients with severe fungal (Candida albicans) sepsis. Ann Clin Biochem 49(6):554–560, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Coleman LG, Jr.: The emerging world of subcellular biological medicine: extracellular vesicles as novel biomarkers, targets, and therapeutics. Neural Regen Res 17(5):1020–1022, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rani S, Ryan AE, Griffin MD, Ritter T: Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23(5):812–823, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elahi FM, Farwell DG, Nolta JA, Anderson JD: Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 38(1):15–21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seo Y, Kim HS, Hong IS: Stem cell–derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int 2019:5126156, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nguyen VVT, Witwer KW, Verhaar MC, Strunk D, van Balkom BWM: Functional assays to assess the therapeutic potential of extracellular vesicles. J Extracell Vesicles 10(1):e12033, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jha KA Pentecost M Lenin R Klaic L Elshaer SL Gentry J Russell JM Beland A Reiner A Jotterand V, et al.: Concentrated conditioned media from adipose tissue derived mesenchymal stem cells mitigates visual deficits and retinal inflammation following mild traumatic brain injury. Int J Mol Sci 19(7):2016, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL: Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience 42(1):1–17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B: Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation 16(1):216, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheng Y, Zhou X, Wang J, Shen H, Wu S, Guo W, Yang Y: MSC derived EV loaded with miRNA-22 inhibits the inflammatory response and nerve function recovery after spinal cord injury in rats. J Cell Mol Med 25(21):10268–10278, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han T, Song P, Wu Z, Xiang X, Liu Y, Wang Y, Fang H, Niu Y, Shen C: MSC secreted extracellular vesicles carrying TGF-beta upregulate Smad 6 expression and promote the regrowth of neurons in spinal cord injured rats. Stem Cell Rev Rep 18(3):1078–1096, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Potter DR Miyazawa BY Gibb SL Deng X Togaratti PP Croze RH Srivastava AK Trivedi A Matthay M Holcomb JB, et al.: Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg 84(2):245–256, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barry M, Trivedi A, Pathipati P, Miyazawa BY, Vivona LR, Togarrati PP, Khakoo M, Tanner H, Norris P, Pati S: Mesenchymal stem cell extracellular vesicles mitigate vascular permeability and injury in the small intestine and lung in a mouse model of hemorrhagic shock and trauma. J Trauma Acute Care Surg 92(3):489–498, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pelizzo G Avanzini MA Icaro Cornaglia A De Silvestri A Mantelli M Travaglino P Croce S Romano P Avolio L Iacob G, et al.: Extracellular vesicles derived from mesenchymal cells: perspective treatment for cutaneous wound healing in pediatrics. Regen Med 13(4):385–394, 2018. [DOI] [PubMed] [Google Scholar]
- 99.Drommelschmidt K Serdar M Bendix I Herz J Bertling F Prager S Keller M Ludwig AK Duhan V Radtke S, et al.: Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun 60:220–232, 2017. [DOI] [PubMed] [Google Scholar]
- 100.Park KS, Svennerholm K, Shelke GV, Bandeira E, Lasser C, Jang SC, Chandode R, Gribonika I, Lotvall J: Mesenchymal stromal cell–derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther 10(1):231, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


