Abstract
Background:
Frailty is prevalent in older adults and has adverse effects on multiple health outcomes. Pain, insomnia, and depressive symptoms are commonly seen and treatable symptoms in older adults and are associated with frailty. However, it is unknown whether these symptoms are independently associated with frailty and how they interact with each other creating a greater impact on frailty than individual symptoms. It is important to understand these associations for nurses to provide high-quality patient-centered care for older adults with frailty.
Objectives:
To determine independent associations of pain, insomnia, and depressive symptoms with frailty and examine their synergistic impact on frailty among older adults.
Design:
A cross-sectional analysis of a cohort study.
Setting:
Communities in the United States.
Participants:
Community-dwelling older adults from the National Health and Aging Trend Study (N = 7,609), a nationally representative survey of Medicare Beneficiaries in the United States.
Methods:
Frailty status was determined by five criteria of the Physical Frailty Phenotype: exhaustion, low physical activity, weakness, slowness, and shrinking. Pain was determined by self-reports of bothersome pain in the last month. Insomnia included self-reports of difficulty initiating sleep and difficulty maintaining sleep. Depressive symptom was assessed by the Patient Health Questionnaire-2. Logistic regression models were used adjusting for sociodemographic, health-related and behavioral covariates.
Results:
The sample was mainly under 80 years old (72%), female (57%), and non-Hispanic White (81%). Approximately 53% experienced bothersome pain, 11% had difficulty initiating sleep, 6% had difficulty maintaining sleep, and 15% had depressive symptom; 46% were pre-frail and 14% were frail. Independent associations with pre-frailty and frailty were found in pain (odds ratio [OR]: 1.81, 95% CI: 1.60, 2.04), difficulty initiating sleep (OR: 1.23, 95% CI: 1.04, 1.46) and depressive symptom (OR: 2.29, 95% CI: 1.85, 2.84). Interaction terms between pain and depressive symptom (OR: 1.87, 95% CI: 1.14, 3.07), and between difficulty initiating sleep and depressive symptom (OR: 2.66, 95% CI: 1.15, 6.13) were significant, suggesting a synergistic impact on pre-frailty and frailty.
Conclusions:
Pain, difficulty initiating sleep, and depressive symptoms are independent risk factors of frailty and may have a synergistic impact on frailty. Interventions should be developed to address these symptoms to reduce the adverse effects of frailty.
Keywords: Depression, Frailty, Insomnia, Pain, Symptom
1. Introduction
Frailty is a multifactorial syndrome characterized by diminished physiological reserves in older adults (Deepa et al., 2017; Fried et al., 2001). A range of 18.7%–53.1% community-dwelling older adults are pre-frail, and 4.2%–59.1% are frail worldwide (Fried et al., 2004; Gill et al., 2002; Mitnitski et al., 2005). Frailty is a dynamic process that can be gradually alleviated or worsened, and its prevalence increases with age (Morley et al., 2013). It can also have adverse effects on health, including the decline in physical function and mental health, and can lead to disability and death in older adults (Clegg et al., 2013; Eeles et al., 2012; Fried et al., 2001; Langlois et al., 2012; Song et al., 2010; Walston et al., 2006). Recognizing modifiable risk factors associated with the development or deterioration of frailty is important to develop effective and targeted interventions to prevent or delay the onset and development of frailty (Tian et al., 2018).
Pain and depression are commonly seen, and treatable symptoms in older adults and are associated with frailty. Bothersome pain refers to the severity of pain between mild-chronic pain and high-impact chronic pain (Korff et al., 2020). Among community-dwelling older adults, about 46% to 74% are bothered by pain and 3% to 39% are bothered by depression, respectively (Barcelos-Ferreira et al., 2010; Takai et al., 2010). A systematic review including 23 studies concluded that approximately 45% to 70% of frail patients had chronic pain; chronic pain had a predictive effect for frailty in older adults (Reyes et al., 2019). Another systematic review showed that older adults with depression were associated with four times the risks of developing frailty (Soysal et al., 2017). Insomnia is defined as self-reported difficulty sleeping, including difficulty initiating sleep and maintaining sleep (Roth, 2007). It is another important symptom that usually occurs with pain and depression and is also a risk factor of frailty (Lee et al., 2018). Although current literature indicates an overall positive association between insomnia and frailty, the evidence has been less consistent and robust compared to that of pain and depression. One possible reason is that insomnia includes heterogenous subtypes (e.g., difficulty initiating sleep, difficulty maintaining sleep) that share distinct characteristics (Hohagen et al., 1994).
An increasing body of literature suggests complicated relationships among pain, insomnia and depressive symptoms (Dunietz et al., 2018; Liu et al., 2018, 2019). Supported by the Theory of Unpleasant Symptoms (TOUS), these symptoms may interact with one another to create synergistic effects on health outcomes (Lenz et al., 1995, 1997). This adds another layer of complexity when determining the associations of these symptoms with frailty. However, to our knowledge, few studies had attempted to examine whether the independent association of one symptom with frailty sustains after accounting for other symptoms, and determine whether there are interactions between these symptoms and frailty. For example, one cross-sectional study found that depression was a mediator of the association between pain and frailty and interacted with pain, creating greater risks of frailty (Tian et al., 2018). However, this study did not assess sleep problems. From the practice perspective, older adults with frailty have a high and growing demand for health care (Patterson et al., 2011), but they receive suboptimal care in hospital, such as inadequate care in terms of clinical outcomes (e.g., mortality and morbidity) (Finnbakk et al., 2012), and poor quality of care experience (Tadd et al., 2011). In such cases, when frailty and these symptoms coexist, nurses will face greater challenges in assisting these patients to manage their health (Nicholson et al., 2017). Therefore, it is important for nurses to identify these symptoms to provide quality care for frail older adults.
Hence, the purpose of this study was to determine the independent associations of pain, insomnia, and depressive symptoms with frailty and examine whether these symptoms interact with each other creating greater risks of frailty in older adults.
2. Methods
The study is a cross-sectional analysis using data from the National Health and Aging Trends Study (NHATS), a prospective cohort study. It is a publicly available, nationally representative survey of Medicare Beneficiaries in the United States aged 65 or older. It investigates the late-life disability risks and advances our understanding of trends in functional changes in older adults. The initial sample was first interviewed in 2011 (N = 8245, weighted 71.3% response rate) and then followed up annually to document changes over time. Detailed information was collected on participants’ physical and cognitive capacity, how activities of daily living were carried out, and information on the social and physical environment. Johns Hopkins University Institutional Review Board approved the NHATS protocol. Data of the first round was used in the current study. Participants living in nursing homes who were not expected to return to their previous residence (n = 468) and those who did not complete in-person interviews (n = 168) were excluded from the analysis, resulting in a final sample of 7609 community-dwelling older adults.
2.1. Measures
2.1.1. Frailty
Frailty was measured by the physical frailty phenotype determined by five criteria: 1) exhaustion; 2) low physical activity; 3) weakness; 4) slowness; and 5) shrinking. Participants met criteria for “exhaustion” if they reported recently having low energy or being easily exhausted: enough to limit their activities. They met criteria for “low physical activity” if, recently, they never walked for exercise or engaged in vigorous activities. Participants met criteria for “shrinking” if they had body mass index (BMI) less than 18.5 kg/m 2, based on self-reported height and weight, or reported unintentionally losing 10 or more pounds in the last year. “Low walking speed” was determined using the first of two usual pace walking trials and this criteria was met if the result was at or below the 20th percentile of the weighted population distribution within four sex-by-height categories. “Weakness” was defined, using maximum dominant hand grip strength over two trials, as being at or below the 20th percentile within eight sex-by-BMI categories. For each of the five criteria, participants not tested because of safety concerns, ineligible due to recent surgery or pain, or who attempted but were unable to complete a test, were scored as “0.” This definition of frailty has been shown to be highly predictive of poor health-related outcomes in different populations (Avila-Funes et al., 2008; Fried et al., 2001). Participants were classified into three frailty groups based on these criteria: 1) non-frail – no criteria met (total score of 0); 2) pre-frail – one to two (total score of 1 or 2); and 3) frail – three to five (total score of 3 to 5).
2.1.2. Symptom measures
Pain symptom is a binary variable measured using the verbal descriptor scale (VDS). Participants were asked if they had any bothersome pain in the last month (Hunt et al., 2015). Studies showed that similar single-item verbal descriptor scales had good reliability to self-reported pain in older adults (Closs et al., 2004; Lukas et al., 2013).
Insomnia was measured using the following questions: 1) “How often does it take you more than 30 min to fall asleep at night?” and 2) “How often do you have trouble falling back to sleep on nights after waking up from sleep?” with one representing difficulty initiating sleep and the other difficulty maintaining sleep. Responses to these questions included never (1), rarely (2), some nights (3), most nights (4), and every night (5). We defined participants having difficulty initiating sleep or difficulty maintaining sleep if they reported most nights or every night difficulty initiating sleep. Insomnia variables were coded as having no sleep problems (0), difficulty initiating sleep only (1), difficulty maintaining sleep only (2), and both difficulty initiating sleep and difficulty maintaining sleep (3) (Endeshaw and Yoo, 2016). Difficulty initiating sleep and maintaining sleep are two key components in the diagnosis of insomnia for both the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and International Classification of Sleep Disorders (ICSD) nosologies (Rockville, 2016; Sateia, 2014).
Depressive symptom was assessed by the 2-item Patient Health Questionnaire-2 (PHQ-2), which is a 2- question standardized and validated measure of depression in older adults (Harris et al., 2018; Li et al., 2007). It includes 1) had little interest or pleasure in doing things; and 2) felt down, depressed, or hopeless. Responses and coding are “Not at all” = 0, “Several days” = 1, “More than half the days” = 2, and “Nearly every day” = 3. Scores of these two items were averaged to represent the severity of depression (0 to 6). In accordance with the PHQ-2 criteria, participants were classified as having depressive symptoms if the cumulative score for both items was 3 or more (Kroenke et al., 2003).
2.1.3. Sociodemographic, health-related and behavioral factors
Sociodemographic characteristics were also recorded, including age (65–69, 70–74, 75–79, 80–84, 85–89, 90–94, 95+), sex, race (non-Hispanic White, non-Hispanic Black, Hispanic, other), education (less than high school, high school graduates, some college or vocational school, Bachelor or higher) and living arrangement (alone, with spouse only, with others only, with spouse and others). Health-related factors including self-perceived health status and the number of chronic conditions (e.g., heart disease, arthritis, diabetes) were recorded. Behavioral factors included whether participants currently smoked and whether they ever spent time on vigorous activities in the last month.
2.2. Data analysis
A final sample of 7609 participants was included in this analysis. The percentage of missing data among the main variables ranged from 0.07% (pain) to 2.23% (frailty status). Given the small percentage of missing data and large sample size, we did not conduct multiple imputations of missing data. We used means with standard deviations and proportions to describe the prevalence of pain, insomnia, depressive symptoms, and frailty. Participants’ characteristics and symptoms of pain, insomnia, and depression were compared between non-frail participants, and pre-frail and frail participants using Chi-squared tests.
To better understand the independent associations of each symptom (pain, difficulty initiating sleep, difficulty maintaining sleep, and depression) with frailty, we employed sets of logistic regression models to examine the associations between these symptoms and status of being pre-frail and frail step by step. In the first set of models (Model 1), we included individual symptoms as the main independent variable. In the second set of models (Model 2), we then further added other symptoms one by one to examine whether significant associations diminished or disappeared because of the newly added symptom(s). In the last set of models (Model 3), we included all symptoms and two-way and three-way interaction terms of those symptoms that showed significant association with frailty in Model 3. To confirm the findings of the previous models and provide a more straightforward interpretation, we then created a composite participant category variable, which represented possible combinations of pain, difficulty initiating sleep, difficulty maintaining sleep, and depressive symptoms in older adults. We used it as a main independent variable to examine the odds of being pre-frail and frail using a logistic regression model. All of these models adjusted for sociodemographic, health-related, and behavioral factors. Odds ratios (OR) and 95% confidence interval (CI) were estimated in regression models. All statistical analyses were performed using Stata version 15.0. All tests were two-tailed; statistical significance was defined as a value of p < 0.05. Data analyses were weighted to account for unequal probabilities of selection into the NHATS sample. Weighted analyses were necessary to adjust for different probabilities of age and race selection, as well as different initial response rates and potential biases of attrition over time (Freedman and Kasper, 2019).
3. Results
Table 1 presents the sociodemographic, health-related, and behavioral characteristics of the overall sample and a comparison of these characteristics among the symptoms and frailty status. Approximately 72% of the sample was below 80 years old and 57% were female. The majority was non-Hispanic White (81%), approximately 51% of the sample received some college, vocational school education or higher, and approximately 30% lived alone. The majority had a BMI of less than 30 m/kg 2 (72%) and approximately half had three or more chronic conditions (47%). The majority had a good or better self-perceived health (75%), approximately 9% of the sample currently smoked, and 39% had vigorous physical activity in the last month. Among the weighted sample, approximately half experienced bothersome pain (53%), and 27% had sleep problems, with 11% having difficulty initiating sleep, 6% difficulty maintaining sleep and 10% having both problems. Approximately 15% of participants reported symptoms of depression. More than half of the participants were prone to frailty: 46% pre-frail and 14% frail. The percentages of participants who were non-frail vs. those who were pre-frail and frail differed by participants’ sociodemographic, health-related, and behavioral characteristics (all p values were less than 0.001). In addition, the distribution of participants’ frailty status differed by their pain, insomnia, and depressive symptoms (p values < 0.001). For example, participants who experienced bothersome pain, insomnia, or depressive symptoms were more likely to be pre-frail and frail compared to those who did not have the symptoms.
Table 1.
Comparisons of participants’ characteristics between non-frail participants, and pre-frail and frail participants (N = 7609).
| Variables | All n (weighted %) | Non-frail n (weighted %) | Pre-frail and frail n (weighted %) | P values |
|---|---|---|---|---|
| Age | p < 0.001 | |||
| 65 – 69 | 1409 (27.9) | 683 (36.6) | 705 (22.5) | |
| 70 – 74 | 1579 (24.9) | 668 (28.0) | 885 (23.0) | |
| 75 – 79 | 1513 (19.1) | 520 (18.2) | 958 (19.7) | |
| 80 – 84 | 1505 (14.7) | 418 (11.1) | 1054 (17.0) | |
| 85 – 89 | 953 (9.1) | 183 (4.6) | 742 (11.9) | |
| 90 + | 650 (4.3) | 86 (1.5) | 537 (5.9) | |
| Gender | p < 0.001 | |||
| Female | 4438 (56.5) | 1343 (51.3) | 2977 (59.9) | |
| Male | 3171 (43.5) | 1215 (48.7) | 1904 (40.1) | |
| Race/Ethnicity | p < 0.001 | |||
| Non-Hispanic White | 5186 (80.5) | 1898 (84.6) | 3231 (79.4) | |
| Non-Hispanic Black | 1662 (8.1) | 463 (6.6) | 1178 (9.3) | |
| Indian/Asian/Native/Hawaii | 219 (3.5) | 78 (3.6) | 129 (3.2) | |
| Hispanic | 454 (6.8) | 113 (5.0) | 327 (7.8) | |
| Other | 88 (1.1) | 6 (0.2) | 16 (0.3) | |
| Education | p < 0.001 | |||
| Less than high school | 2047 (21.8) | 440 (13.6) | 1555 (26.7) | |
| High school graduates | 2069 (27.6) | 676 (25.7) | 1365 (28.8) | |
| Some college or vocational school | 1818 (26.2) | 663 (27.2) | 1145 (25.9) | |
| Bachelor or higher | 1579 (24.4) | 773 (33.5) | 798 (18.6) | |
| Living arrangement | p < 0.001 | |||
| Alone | 2474 (29.8) | 760 (26.1) | 1674 (31.8) | |
| With spouse/partner only | 3050 (46.7) | 1290 (56.6) | 1710 (40.7) | |
| With others only | 1366 (13.9) | 289 (9.1) | 1039 (17.0) | |
| With spouse/partner and with others | 688 (9.6) | 206 (8.2) | 469 (10.5) | |
| Body mass index (BMI) | p < 0.001 | |||
| <30 m/KG2 | 5364 (72.2) | 1955 (77.1) | 3407 (68.8) | |
| >=30 m/KG2 | 1968 (27.8) | 579 (22.9) | 1389 (31.2) | |
| Numbers of chronic conditions | p < 0.001 | |||
| 0 – 2 | 3831 (53.3) | 1748 (70.0) | 1977 (42.2) | |
| 3 – 5 | 3345 (41.3) | 766 (28.2) | 2512 (50.1) | |
| >= 6 | 451 (5.4) | 44 (1.8) | 392 (7.7) | |
| Self-perceived health | p < 0.001 | |||
| Excellent | 938 (14.7) | 573 (25.1) | 347 (8.0) | |
| Very good | 2029 (29.5) | 1001 (40.9) | 996 (22.2) | |
| Good | 2426 (30.7) | 767 (27.5) | 1614 (33.0) | |
| Fair | 1607 (18.4) | 194 (5.9) | 1362 (26.2) | |
| Poor | 603 (6.7) | 23 (0.6) | 557 (10.6) | |
| Current smoker | p < 0.001 | |||
| Yes | 588 (8.5) | 172 (6.5) | 410 (9.9) | |
| No | 7020 (91.5) | 2386 (93.5) | 4470 (90.1) | |
| Physical activity | p < 0.001 | |||
| Yes | 3189 (38.8) | 1554 (64.5) | 971 (21.8) | |
| No | 5030 (61.2) | 1004 (35.5) | 3907 (78.2) | |
| Pain | p < 0.001 | |||
| Yes | 4023 (53.1) | 962 (37.6) | 3061 (63.4) | |
| No | 3413 (46.9) | 1596 (62.4) | 1817 (36.6) | |
| Insomnia | p < 0.001 | |||
| No | 5331 (73.0) | 2088 (82.5) | 3243 (66.6) | |
| DIS | 866 (11.4) | 219 (8.4) | 647 (13.5) | |
| DMS | 413 (5.5) | 108 (4.3) | 305 (6.2) | |
| Both | 783 (10.1) | 138 (4.8) | 645 (13.7) | |
| Depressive symptom | p < 0.001 | |||
| Yes | 1179 (14.5) | 140 (5.1) | 1039 (20.7) | |
| No | 6201 (85.5) | 2404 (94.9) | 3797 (79.3) |
Note:
DIS-difficulty initiating sleep; DMS-difficulty maintaining sleep; Chi-squared tests were used to examine whether the distribution of frailty status differed in categories of participants’ characteristics.
Table 2 shows the association between symptoms of pain, difficulty initiating sleep, difficulty maintaining sleep, and depression, and the status of being pre-frail and frail in older adults. All symptoms were associated with being pre-frail and frail after adjusting covariates, with depressive symptom presenting the highest odds ratio (OR: 2.42, 95% CI: 1.96, 2.98), followed by pain (OR: 1.86, 95% CI: 1.65, 2.10), difficulty initiating sleep (OR: 1.43, 95% CI: 1.23, 1.67), and difficulty maintaining sleep (OR: 1.41, 95% CI: 1.18, 1.68). While introducing two symptoms simultaneously into adjusted models, all odds ratios decreased but still remained significant. When three or four symptoms simultaneously were included in the models, independent associations with pre-frailty and frailty remained significant for pain (OR: 1.81, 95% CI: 1.60, 2.04), difficulty initiating sleep (OR: 1.23, 95% CI: 1.04, 1.46) and depressive symptom (OR: 2.29, 95% CI: 1.85, 2.84). However, difficulty maintaining sleep was no longer associated with being pre-frail and frail status. After including two-way and three-way interaction terms, significant interactions between pain and depressive symptom (OR: 1.87, 95% CI: 1.14, 3.07), and difficulty initiating sleep and depressive symptom (OR: 2.66, 95% CI: 1.15, 6.13) were found.
Table 2.
Associations between symptoms of pain, insomnia and depressive symptoms with status of being pre-frail and frail in older adults.
| Frailty | Model 1 (OR) | Model 2 (OR) | Model 3 (OR) |
|---|---|---|---|
| Individual symptom | |||
| Pain (ref: no pain) | 1.86 (1.65, 2.10)*** | ||
| DIS (ref: no DIS) | 1.43 (1.23, 1.67)*** | ||
| DMS (ref: no DMS) | 1.41 (1.18, 1.68)*** | ||
| Depression (ref: no depression) | 2.42 (1.96, 2.98)*** | ||
| Pain & DIS | |||
| Pain | 1.83 (1.62, 2.07)*** | ||
| DIS | 1.35 (1.16, 1.58)*** | ||
| Pain & DMS | |||
| Pain | 1.84 (1.63, 2.08)*** | ||
| DMS | 1.32 (1.11, 1.58)** | ||
| Pain & Depression | |||
| Pain | 1.84 (1.63, 2.08)*** | ||
| Depression | 2.37 (1.92, 2.93)*** | ||
| DIS & Depression | |||
| DIS | 1.37 (1.17, 1.60)*** | ||
| Depression | 2.35 (1.90, 2.90)*** | ||
| DMS & Depression | |||
| DMS | 1.36 (1.13, 1.62)** | ||
| Depression | 2.36 (1.91, 2.91)*** | ||
| Pain, DIS & DMS | |||
| Pain | 1.80 (1.62, 2.06)*** | ||
| DIS | 1.28 (1.08, 1.51)** | ||
| DMS | 1.18 (0.97, 1.43) | ||
| Pain, DIS & Depression | |||
| Pain | 1.81 (1.60, 2.05)*** | ||
| DIS | 1.29 (1.11, 1.51)** | ||
| Depression | 2.31 (1.87, 2.86)*** | ||
| Pain, DMS & Depression | |||
| Pain | 1.82 (1.62, 2.06)*** | ||
| DMS | 1.27 (1.06, 1.53)** | ||
| Depression | 2.32 (1.88, 2.87)*** | ||
| DIS, DMS & Depression | |||
| DIS | 1.28 (1.08, 1.52)** | ||
| DMS | 1.21 (0.99, 1.47) | ||
| Depression | 2.33 (1.88, 2.87)*** | ||
| Pain, DIS, DMS, & Depression | |||
| Pain | 1.81 (1.60, 2.04)*** | 1.74 (1.52, 2.00)*** | |
| DIS | 1.23 (1.04, 1.46)* | 1.16 (0.91, 1.50) | |
| DMS | 1.16 (0.95, 1.41) | 1.16 (0.95, 1.41) | |
| Depression | 2.29 (1.85, 2.84)*** | 1.51 (1.09, 2.08)* | |
| Pain*DIS | 0.99 (0.72, 1.38) | ||
| Pain*Depression | 1.87 (1.14, 3.07)* | ||
| DIS*Depression | 2.66 (1.15, 6.13)* | ||
| Pain*DIS*Depression | 0.45 (0.15, 1.34) |
Note:
OR – odds ratio; CI – confidence interval; ref – reference group; DIS-difficulty initiating sleep; DMS-difficulty maintaining sleep;
p < 0.05;
p < 0.01;
p < 0.001.
Model 1 presents ORs of individual symptom at one time; Model 2 presents odds of two or three symptoms at the same time; Model 3 present odds of all symptoms with interactions; All models adjusted for demographics, behavioral, and health-related factors.
In Fig. 1, the adjusted odds ratios of being pre-frail and frail in older adults with different symptom combinations of pain, difficulty initiating sleep, difficulty maintaining sleep, and depression are shown. Overall, the odds of being pre-frail and frail increased with the numbers of symptoms, but the results also indicated the important role of pain, difficulty initiating sleep, and depressive symptoms. Participants with pain only and depressive symptoms only were 1.71 and 1.47 times more likely to be pre-frail and frail compared to those without symptoms, respectively. Participants with pain, difficulty initiating sleep, and depressive symptoms tended to have the highest odds of being pre-frail and frail compared to those without these symptoms (OR: 10.20, 95% CI: 4.21, 24.77), followed by participants with difficulty initiating sleep and depressive symptoms (OR: 7.33, 95% CI: 2.42, 22.17), and participants with all symptoms (OR: 5.69, 95% CI: 2.81, 11.52) compared to those without these symptoms. Among all the possible symptom combinations, participants with difficulty initiating sleep only, difficulty maintaining sleep only, both difficulty initiating sleep and difficulty maintaining sleep only, and difficulty maintaining sleep and depressive symptom, were not significantly associated with pre-frailty and frailty in older adults.
Fig. 1.
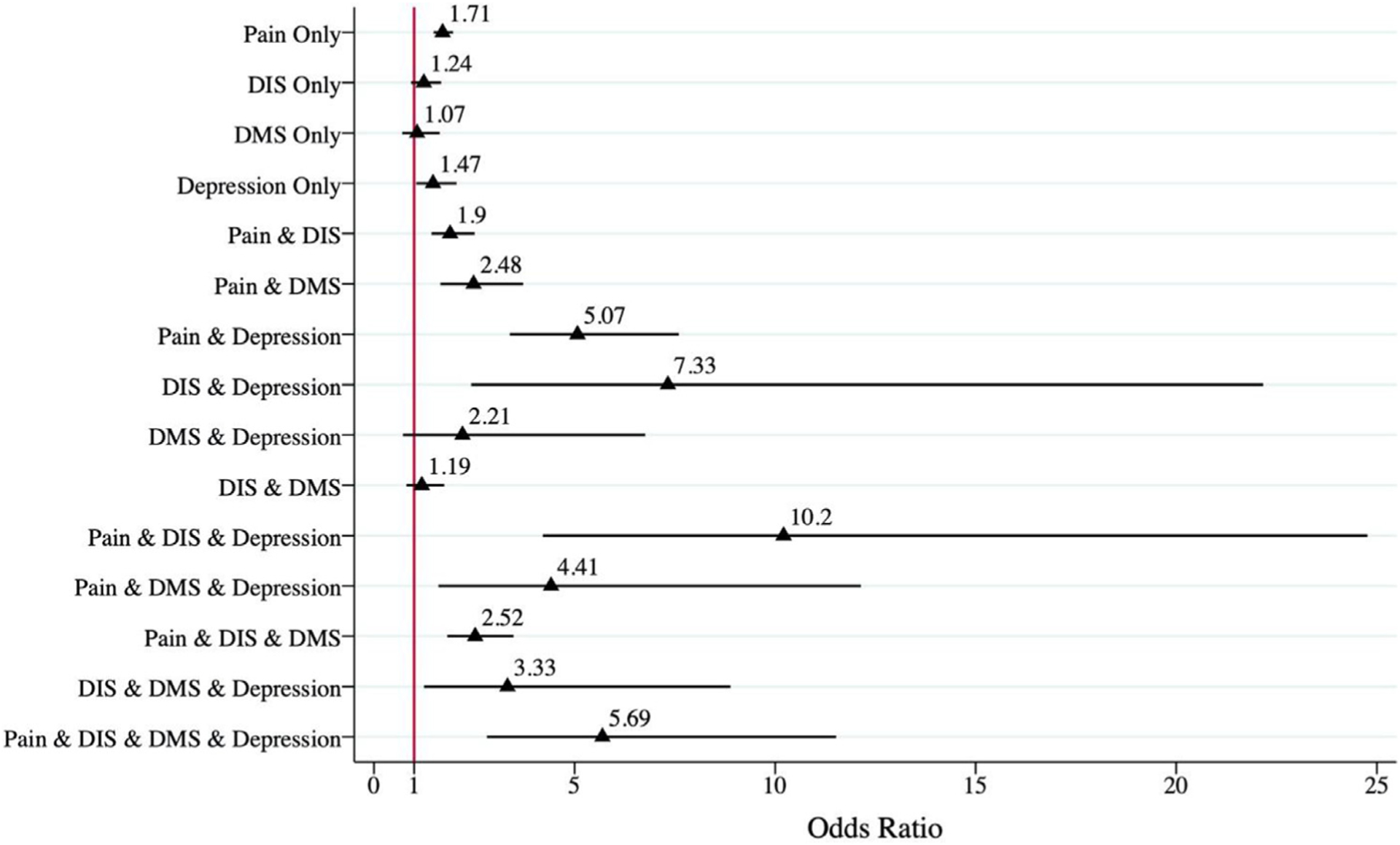
Adjusted odds ratio of being pre-frail and frail in older adults with different concurrent pain, insomnia and depressive symptoms.
4. Discussion
To our knowledge, this is the first study to examine the independent associations between pain, insomnia (difficulty initiating sleep and difficulty maintaining sleep), and depressive symptoms with frailty using a nationally representative sample. The results showed that approximately half of community-dwelling older adults were prone to frailty, and about half of community-dwelling older adults experienced bothersome pain, confirming that frailty and bothersome pain were major health problems for older people. In addition, pain, difficulty initiating sleep, and depressive symptoms were independently associated with pre-frailty and frailty. Both pain and difficulty initiating sleep interacted with depressive symptoms creating a higher risk of being pre-frail and frail.
Consistent with previous studies, our study found that pain, insomnia, and depressive symptoms were prevalent in a nationally representative sample of older adults and were overall associated with frailty (Brown et al., 2020; Ensrud et al., 2009; Wade et al., 2017). In this study, pain and depressive symptoms were associated with being pre-frail and frail regardless of the adjustment for covariates and symptoms. This finding is expected given that several systematic reviews reported relatively robust reciprocal relationships between pain and frailty, and depression and frailty (Reyes et al., 2019; Saraiva et al., 2018; Soysal et al., 2017). In addition, our study found that difficulty initiating sleep was independently associated with being pre-frail and frail, but difficulty maintaining sleep was not. Although previous studies reported an association between insomnia and frailty, these studies rarely examined insomnia subtypes separately and adjusted for pain and depression that are usually correlated with insomnia and frailty (Ensrud et al., 2009; Moreno-Tamayo et al., 2020). However, the cross-sectional analysis of a prospective cohort study did show similar findings that multiple long wake episodes were no longer associated with frailty after adjustment, but prolonged sleep latency was still associated with frailty in male older adults (Ensrud et al., 2009). It should be noted that it was after introducing difficulty initiating sleep into the models with pain and depression, the association between difficulty maintaining sleep and frailty became insignificant. This may suggest that difficulty initiating sleep and maintaining sleep could possess heterogenous characteristics that contribute to these different associations with frailty, and difficulty initiating sleep was more stable over the time compared to difficulty maintaining sleep (Bjorøy et al., 2020; Blanken et al., 2019; Earley, 1997). Another explanation can be the complicated pathophysiological relationship between frailty and sleep disturbances. Insomnia and daytime sleepiness may be mediators of immunological pathways that raise levels of inflammatory molecules associated with frailty risk, such as interleukin 6 and C-reactive protein (Arnardottir et al., 2012; Bollinger et al., 2010; Li et al., 2017; Walston et al., 2002). However, future empirical research is needed to identify the biological mechanisms to provide more clear and solid explanations for our research findings.
Given the significant independent associations of pain, difficulty initiating sleep, and depressive symptoms with frailty, we examined whether they interacted with each other presenting greater associations with frailty. As the Theory of Unpleasant Symptoms theory suggests, symptoms may interact with each other (Lenz et al., 1995, 1997). The results showed that the association of pain and depressive symptom, and the association of difficulty initiating sleep and depressive symptom were multiplicative, which means the combined association of two symptoms is larger than the product of the individual association. This indicates that nurses should be more alert to the synergistic impact of these symptoms on frail older adults, so as to take targeted care measures to improve the quality of care (Collard et al., 2015). Although complicated relationships were found among pain, sleep problems, and depressive symptoms in older adults, few studies have examined their interactions on certain health indicators. Results of one of these studies demonstrated that the combined cross-sectional association of pain and depressive symptoms with frailty was 8.13 (odds ratio), which was greater than the addition of individual association of pain (1.41) and depression (3.63) (Tian et al., 2018). It was documented in the study that further studies should examine other pain associated conditions such as sleep problems. It is important to identify significant interactions among these symptoms, given their high prevalence in older adults and negative impact on frailty. However, further research is needed to evaluate whether these significant interactions remain in longitudinal studies.
We also created a composite variable indicating categories of participants with possible symptom combinations and analyzed its association with pre-frailty and frailty. This analysis confirmed our findings on the interactions between pain and depressive symptoms, and difficulty initiating sleep and depressive symptom. For example, the odds ratio of participants with pain, difficulty initiating sleep and depressive symptom was 10.20, which was greater than the addition of odds ratios of participants with an individual symptom only. The category of participants with difficulty initiating sleep only was not associated with being pre-frail and frail could be because of its small sample size. In addition, the confidence intervals were wide for some categories, probably because of the relatively small number of participants in those categories. Nevertheless, the results of our additional analysis have outlined the association between the symptom combinations and frailty.
This study has important implications for nursing practice and research. Despite the adverse impact of pain, insomnia, and depressive symptoms on frailty, these symptoms are treatable, and frailty is reversible (Woods et al., 2005). By recognizing the independent associations of these symptoms with frailty and their synergistic impact on frailty, nurses can identify people who are at risk of frailty or have frailty more easily by examining their pain, insomnia and depressive symptom profiles in their routine practice. This assessment helps create more comprehensive care plans to achieve the goal of preventing frailty onset or progression in older adults. For example, frail older adults can experience a variety of chronic illnesses and take a variety of medications, which can lead to adverse reactions (Weiss, 2011). Nurses can alleviate frailty and adverse reactions in older adults by identifying pain symptoms of frail older adults, tailoring treatment plans for them, and individually assessing the efficacy of those plans (Booker et al., 2016). In addition, nurses can initiate a collaborative conversation with other health professionals to provide high-quality patient-centered care for people with frailty with the knowledge of the complex interaction between pain, difficulty initiating sleep and depressive symptoms. In addition to practice implications, for nurse researchers, it is important to develop multi-component interventions that particularly address pain, difficulty initiating sleep and depressive symptoms to achieve optimal therapeutic effects for frail older adults (Rnn et al., 2011). Evaluating these interventions will in turn help researchers understand how improved symptoms may reverse frailty status. However, frail older adults are usually at different stages of the process of functional decline, and their need for care varies according to their overall health and functional status. Thus, it is necessary to provide frail older adults with flexible and targeted interventions, which makes the task of nurses more difficult and complex (Gustafsson et al., 2009; Markle-Reid et al., 2013).
Limitations should be noted in this study. First, the measurements of symptoms were not comprehensive. Bothersome pain is only measured by single item, which does not provide information on pain severity, intensity, and duration. Similarly, the depressive symptom was measured by PHQ-2, a tool for initial screening for depression but not for the severity of depression (Xiang and Brooks, 2017). In addition, difficulty initiating and maintaining sleep is not the equivalent of a formal diagnosis of insomnia disorder but rather a best estimate given the information available in the database. However, the questionnaire items for these symptoms used in NHATS have face validity, and previous studies have verified the criterion-validity and screening properties of these questionnaire items (Kroenke et al., 2003; Patel et al., 2013; Spira et al., 2014). Second, although we evaluated the relationship between these symptoms and frailty, the variables investigated were restricted to those collected in the NHATS database. Factors that have been shown to be associated with frailty in previous studies, such as polypharmacy use and sedentary lifestyle, are not available for analysis (Gutiérrez-Valencia et al., 2018; Piovezan et al., 2013). Therefore, future research is needed to investigate whether the independent associations and interactions between these symptoms and frailty remain after controlling for these unmeasured variables. Third, the analysis was a cross-sectional analysis of a cohort study, so causal relationships among the symptoms and frailty could not be established. Thus, longitudinal research is needed to understand how these three common symptoms contribute to frailty, particularly when they correlate with each other. Finally, results from this study were based on a nationally representative sample of community-dwelling older adults; therefore, results may not be generalizable to older people in residential or clinical settings.
5. Conclusions
In conclusion, pain, difficulty initiating sleep, and depressive symptoms were independently associated with pre-frailty and frailty among older adults. In addition, the depressive symptom interacted with pain and difficulty initiating sleep creating a greater synergistic impact on pre-frailty and frailty than the addition of individual impact. Nurses should be aware of this synergistic impact of these symptoms to provide high-quality patient-centered care for older adults with frailty.
What is already known about the topic?
Pain, insomnia, depression, and frailty are prevalent in older adults and have an adverse impact on older adults’ health.
Pain, insomnia, and depressive symptoms are associated with frailty in older adults.
Relationships among pain, insomnia, and depressive symptoms are complicated in older adults. These symptoms may interact with each other presenting a greater impact on frailty than individual symptoms.
What this paper adds
Pain, difficulty initiating sleep, and depressive symptoms were independently associated with being pre-frail and frail in older adults. Difficulty maintaining sleep was not.
There was an interaction between depressive symptoms and pain, and depressive symptoms and difficulty initiating sleep, which had a greater impact on pre-frailty and frailty than these symptoms individually.
Among participants with all possible symptom combinations, participants with pain, difficulty initiating sleep, and depressive symptoms tended to have the highest odds of being pre-frail and frail, followed by those with difficulty initiating sleep and depressive symptoms, and those with all symptoms.
Acknowledgments
We thank Nancy A. Perrin, PhD and Chakra Budhathoki, PhD for their feedback on the analyses. We also thank Peishan Ning, PhD for the feedback on the clarity of Table 1.
Funding sources
The publication of this manuscript was supported by Changsha Municipal Natural Science Foundation in China (Grant No. kq2007080), the Special Funding for the Construction of Innovative Provinces in Hunan (Grant No. 2019SK2141), and the China Oceanwide Holding Group Project Fund (Contract No. H201910150780001).
Footnotes
CRediT authorship contribution statement
Minhui Liu: Conceptualization, Methodology, Data curation, Formal analysis, Validation. Tianxue Hou: Writing - original draft, Writing - review & editing. Manka Nkimbeng: Writing - review & editing. Yuxiao Li: Writing - review & editing. Janiece L. Taylor: Writing - review & editing. Xiaocao Sun: Writing - review & editing. Siyuan Tang: Supervision. Sarah L. Szanton: Writing - review & editing.
Declaration of Competing Interest
None.
References
- Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, Juliusson S, Romer M, Gislason T, Pack AI, 2012. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic sleep apnea cohort. Sleep 35 (7), 921–932. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le Goff M, Ritchie K, Portet F, Carrière I, Tavernier B, Gutiérrez-Robledo LM, Dartigues JF, 2008. Frailty among community-dwelling elderly people in France: the three-city study. J. Gerontol. A Biol. Sci. Med. Sci 63 (10), 1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelos-Ferreira R, Izbicki R, Steffens DC, Bottino CMC, 2010. Depressive morbidity and gender in community-dwelling Brazilian elderly: systematic review and meta-analysis. Int. Psychogeriatr 22 (05), 712–726. doi: 10.1017/S1041610210000463. [DOI] [PubMed] [Google Scholar]
- Bjorøy I, Jørgensen VA, Pallesen S, Bjorvatn B, 2020. The prevalence of insomnia subtypes in relation to demographic characteristics, anxiety, depression, alcohol consumption and use of hypnotics. Front. Psychol 11, 527. doi: 10.3389/fpsyg.2020.00527, −527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken TF, Benjamins JS, Borsboom D, Vermunt JK, Paquola C, Ramautar J, Dekker K, Stoffers D, Wassing R, Wei Y, Van Someren EJW, 2019. Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry 6 (2), 151–163. doi: 10.1016/S2215-0366(18)30464-4. [DOI] [PubMed] [Google Scholar]
- Bollinger T, Bollinger A, Oster H, Solbach W, 2010. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology 56 (6), 574–580. doi: 10.1159/000281827. [DOI] [PubMed] [Google Scholar]
- Booker SS, Bartoszczyk DA, Herr KA, 2016. Managing pain in frail elders. Am. Nurse Today 11 (4), 1–13. https://www.americannursetoday.com/managing-pain-frail-elders/. [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Roose SP, O’Boyle KR, Ciarleglio A, Maas B, Igwe KC, Chung S, Gomez S, Naqvi M, Brickman AM, Rutherford BR, 2020. Frailty and its correlates in adults with late life depression. Am. J. Geriatr. Psychiatry 28 (2), 145–154. doi: 10.1016/j.jagp.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K, 2013. Frailty in elderly people. Lancet 381 (9868), 752–762. doi: 10.1016/S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs SJ, Barr B, Briggs M, Cash K, Seers K, 2004. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J. Pain Symptom Manag 27 (3), 196–205. doi: 10.1016/j.jpainsymman.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Collard RM, Arts M, Comijs HC, Naarding P, Verhaak PF, de Waal MW, Oude Voshaar RC, 2015. The role of frailty in the association between depression and somatic comorbidity: results from baseline data of an ongoing prospective cohort study. Int. J. Nurs. Stud 52 (1), 188–196. doi: 10.1016/j.ijnurstu.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, Mcardle A, Jackson MJ, Van Remmen H, Richardson A, 2017. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience 39 (2), 187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunietz GL, Swanson LM, Jansen EC, Chervin RD, O’Brien LM, Lisabeth LD, Braley TJ, 2018. Key insomnia symptoms and incident pain in older adults: direct and mediated pathways through depression and anxiety. Sleep 41 (9), 1–8. doi: 10.1093/sleep/zsy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley JC, 1997. Disorders associated with difficulty in initiating or maintaining sleep. Neurologist 3 (2), 77–94. doi: 10.1097/00127893-199703000-00003. [DOI] [Google Scholar]
- Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE, 2012. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 41 (3), 412–416. doi: 10.1093/ageing/afs021. [DOI] [PubMed] [Google Scholar]
- Endeshaw YW, Yoo W, 2016. Association between social and physical activities and insomnia symptoms among community-dwelling older adults. J. Aging Health 28 (6), 1073–1089. doi: 10.1177/0898264315618921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell TL, Redline S, Ancoli-Israel S, Paudel ML, Cawthon PM, Dam TT, Barrett-Connor E, Leung PC, Stone KL, 2009. Sleep disturbances and frailty status in older community-dwelling men. J. Am. Geriatr. Soc. 57 (11), 2085–2093. doi: 10.1111/j.1532-5415.2009.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnbakk E, Skovdahl K, Blix ES, Fagerström L, 2012. Top-level managersö and politiciansö worries about future care for older people with complex and acute illnesses – a Nordic study. Int. J. Older People Nurs 7 (2), 163–172. doi: 10.1111/j.1748-3743.2012.00312.x. [DOI] [PubMed] [Google Scholar]
- Freedman VA, Kasper JD, 2019. Cohort profile: the national health and aging trends study (NHATS). Int. J. Epidemiol 48 (4). doi: 10.1093/ije/dyz109, 1044–1045 g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G, 2004. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci 59 (3), 255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, 2001. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci 56 (3), M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A, 2002. A program to prevent functional decline in physically frail, elderly persons who live at home. N. Engl. J. Med 347 (14), 1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- Gustafsson S, Edberg A−K, Johansson B, Dahlin-Ivanoff S, 2009. Multicomponent health promotion and disease prevention for community-dwelling frail elderly persons: a systematic review. Eur. J. Ageing 6 (4), 315. doi: 10.1007/s10433-009-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero Á, Inzitari M, Martínez-Velilla N, 2018. The relationship between frailty and polypharmacy in older people: a systematic review. Br. J. Clin. Pharmacol 84 (7), 1432–1444. doi: 10.1111/bcp.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, McNicoll L, Epstein-Lubow G, Thomas KS, 2018. Association between anxious symptoms and sleeping medication use among US older adults. Int. J. Geriatr. Psychiatry 33 (2), E307–E313. doi: 10.1002/gps.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S, Berger M, 1994. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening–temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep 17 (6), 551–554. doi: 10.1093/sleep/17.6.551, 551–554. [DOI] [PubMed] [Google Scholar]
- Hunt LJ, Covinsky KE, Yaffe K, Stephens CE, Miao YH, Boscardin WJ, Smith AK, 2015. Pain in community-dwelling older adults with dementia: results from the national health and aging trends study. J. Am. Geriatr. Soc 63 (8), 1503–1511. doi: 10.1111/jgs.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff MV, DeBar LL, Krebs EE, Kerns RD, Deyo RA, Keefe FJ, 2020. Graded chronic pain scale revised: mild, bothersome, and high-impact chronic pain. Pain 161 (3), 651–661. doi: 10.1097/j.pain.0000000000001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2003. The patient health questionnaire-2: validity of a two-item depression screener. Med. Care 41 (11), 1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Langlois F, Vu TT, Kergoat MJ, Chasse K, Dupuis G, Bherer L, 2012. The multiple dimensions of frailty: physical capacity, cognition, and quality of life. Int. Psychogeriatr 24 (9), 1429–1436. doi: 10.1017/s1041610212000634. [DOI] [PubMed] [Google Scholar]
- Lee DR, Santo EC, Lo JC, Ritterman Weintraub ML, Patton M, Gordon NP, 2018. Understanding functional and social risk characteristics of frail older adults: a cross-sectional survey study. BMC Fam. Pract 19 (1), 1–12. doi: 10.1186/s12875-018-0851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz ER, Suppe F, Gift AG, Pugh LC, Milligan RA, 1995. Collaborative development of middle-range nursing theories: toward a theory of unpleasant symptoms. ANS Adv. Nurs. Sci 17 (3), 1–13. doi: 10.1097/00012272-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F, 1997. The middle-range theory of unpleasant symptoms: an update. ANS Adv. Nurs. Sci 19 (3), 14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Li CY, Friedman B, Conwell Y, Fiscella K, 2007. Validity of the patient health questionnaire 2 (PHQ-2) in identifying major depression in older people. J. Am. Geriatr. Soc 55 (4), 596–602. doi: 10.1111/j.1532-5415.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- Li K, Wei P, Qin Y, Wei Y, 2017. Is C-reactive protein a marker of obstructive sleep apnea? A meta-analysis. Medicine 96 (19), e6850. doi: 10.1097/MD.0000000000006850, e6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MH, McCurry SM, Belza B, Buchanan DT, Dobra A, Von Korff M, Vi-tiello MV, 2018. Effects of pain, insomnia, and depression on psychoactive medication supply in older adults with osteoarthritis. Med. Care 56 (12), 1024–1031. doi: 10.1097/Mlr.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MH, McCurry SM, Belza B, Dobra A, Buchanan DT, Vitiello MV, Von Korff M, 2019. Effects of osteoarthritis pain and concurrent insomnia and depression on health care use in a primary care population of older adults. Arthritis Care Res 71 (6), 748–757. doi: 10.1002/acr.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas A, Niederecker T, Gunther I, Mayer B, Nikolaus T, 2013. Self- and proxy report for the assessment of pain in patients with and without cognitive impairment Experiences gained in a geriatric hospital. Z. Gerontol. Geriatr 46 (3), 214–221. doi: 10.1007/s00391-013-0475-y. [DOI] [PubMed] [Google Scholar]
- Markle-Reid M, Browne G, Gafni A, 2013. Nurse-led health promotion interventions improve quality of life in frail older home care clients: lessons learned from three randomized trials in Ontario, Canada. J. Eval. Clin. Pract 19 (1), 118–131. doi: 10.1111/j.1365-2753.2011.01782.x. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Song X, Skoog I, Broe GA, Rockwood K, 2005. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J. Am. Geriatr. Soc 53 (12), 2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Tamayo K, Manrique-Espinoza B, Ortiz-Barrios LB, Cárdenas-Bahena Á, Ramírez-García E, Sánchez-García S, 2020. Insomnia, low sleep quality, and sleeping little are associated with frailty in Mexican women. Maturitas 136, 7–12. doi: 10.1016/j.maturitas.2020.03.005. [DOI] [PubMed] [Google Scholar]
- Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J, 2013. Frailty consensus: a call to action. J. Am. Med. Dir. Assoc 14 (6), 392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Morrow EM, Hicks A, Fitzpatrick J, 2017. Supportive care for older people with frailty in hospital: an integrative review. Int. J. Nurs. Stud 66, 60–71. doi: 10.1016/j.ijnurstu.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Patel KV, Guralnik JM, Dansie EJ, Turk DC, 2013. Prevalence and impact of pain among older adults in the United States: findings from the 2011 national health and aging trends study. Pain 154 (12), 2649–2657. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Nolan M, Rick J, Brown J, Adams R, Musson G, 2011. From metricsto meaning: culture change and quality of acute hospital care for older people. NIHR SDO programme project (08/1501/93), pp. 253. [Google Scholar]
- Piovezan RD, Poyares D, Tufik S, 2013. Frailty and sleep disturbances in the elderly: possible connections and clinical implications. Sleep Sci 6 (4), 175–179. doi: 10.1016/j.exger.2018.10.010. [DOI] [Google Scholar]
- Reyes PO, Perea EG, Marcos AP, 2019. Chronic pain and frailty in community-dwelling older adults: a systematic review. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurses 20 (4), 309–315. doi: 10.1016/j.pmn.2019.01.0031524-9042. [DOI] [PubMed] [Google Scholar]
- Rnn MR, Gina Browne RN, Gafni A, 2011. Nurse-led health promotion interventions improve quality of life in frail older home care clients: lessons learned from three randomized trials in Ontario, Canada. J. Eval. Clin. Pract 19 (1), 118–131. doi: 10.1111/j.1365-2753.2011.01782.x. [DOI] [PubMed] [Google Scholar]
- Rockville: Substance Abuse and Mental Health Services Administration (US), 2016. Impact of the DSM-IV to DSM-5 changes on the national survey on drug use and health Available from: https://www.ncbi.nlm.nih.gov/books/NBK519697/ [PubMed]
- Roth T, 2007. Insomnia: definition, prevalence, etiology, and consequences. J. Clin. Sleep Med 3 (5 suppl), S7–S10. doi: 10.5664/jcsm.26929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva MD, Suzuki GS, Lin SM, de Andrade DC, Jacob-Filho W, Suemoto CK, 2018. Persistent pain is a risk factor for frailty: a systematic review and meta-analysis from prospective longitudinal studies. Age Ageing 47 (6), 785–793. doi: 10.1093/ageing/afy104. [DOI] [PubMed] [Google Scholar]
- Sateia MJ, 2014. International classification of sleep disorders-third edition highlights and modifications. Chest 146 (5), 1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K, 2010. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc 58 (4), 681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, Solmi M, Schofield P, Koyanagi A, Tseng PT, Lin PY, Chu CS, Cosco TD, Cesari M, Carvalho AF, Stubbs B, 2017. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res. Rev 36, 78–87. doi: 10.1016/j.arr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Spira AP, Kaufmann CN, Kasper JD, Ohayon MM, Rebok GW, Skidmore E, Parisi JM, Reynolds CF, 2014. Association between insomnia symptoms and functional status in U.S. older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci 1, S35–S41. doi: 10.1093/geronb/gbu116, 69 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadd W, Hillman A, Calnan S, Calnan M, Bayer T, Read S, 2011. Right placewrong person: dignity in the acute care of older people. Qual. Ageing Older Adults 12 (1), 33–43. doi: 10.5042/qiaoa.2011.0143. [DOI] [Google Scholar]
- Takai Y, Yamamoto-Mitani N, Okamoto Y, Koyama K, Honda A, 2010. Literature review of pain prevalence among older residents of nursing homes. Pain Manag. Nurs 11 (4), 209–223. doi: 10.1016/j.pmn.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Tian X, Wang C, Qiao X, Liu N, Dong L, Butler M, Si H, Jin Y, 2018. Association between pain and frailty among Chinese community-dwelling older adults: depression as a mediator and its interaction with pain. Pain 159 (2), 306–313. doi: 10.1097/j.pain.0000000000001105. [DOI] [PubMed] [Google Scholar]
- Wade KF, Marshall A, Vanhoutte B, Wu FC, O’Neill TW, Lee DM, 2017. Does pain predict frailty in older men and women? Findings from the English longitudinal study of ageing (ELSA). J. Gerontol. A Biol. Sci. Med. Sci 72 (3), 403–409. doi: 10.1093/gerona/glw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, 2002. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch. Intern. Med 162 (20), 2333–2341. [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP, 2006. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American geriatrics society/national institute on aging research conference on frailty in older adults. J. Am. Geriatr. Soc 54 (6), 991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Weiss CO, 2011. Frailty and chronic diseases in older adults. Clin. Geriatr. Med 27 (1), 39–52. doi: 10.1016/j.cger.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB, 2005. Frailty: emergence and consequences in women aged 65 and older in the women’s health initiative observational study. J. Am. Geriatr. Soc 53 (8), 1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- Xiang X, Brooks J, 2017. Correlates of depressive symptoms among homebound and semi-homebound older adults. J. Gerontol. Soc. Work 60 (3), 201–214. doi: 10.1080/01634372.2017.1286625. [DOI] [PMC free article] [PubMed] [Google Scholar]


