Abstract
Objectives
We investigated the efficacy of intensive rosuvastatin therapy plus 7-day dual antiplatelet therapy (DAPT) in reducing stroke recurrence for patients with acute ischemic stroke (AIS) and compared subgroups of patients.
Methods
We enrolled patients with AIS whose time of onset to medication was ≤ 72 h, and the baseline scores of NIHSS (bNIHSS) were 0–10. The patients received intensive rosuvastatin therapy plus 7-day DAPT with aspirin and clopidogrel (study group) or rosuvastatin plus single antiplatelet therapy (SAPT, control group). The primary outcomes were recurrence of ischemic stroke, bleeding, statin-induced liver injury, and statin-associated myopathy (SAM) within 90 days. We also performed a subgroup analysis to assess the heterogeneity of the two therapy regimens in reducing recurrent stroke.
Results
Recurrent stroke occurred in 10 patients in the study group and 42 patients in the control group (hazard ratio [HR], 0.373, 95% confidence interval [CI], 0.178–0.780; P = 0.009). Bleeding events occurred in 9 patients in the study group and 14 patients in the control group (HR, 1.019; 95%CI, 0.441–2.353; P = 0.966). Statin-induced liver injury and SAM were not recorded. Intensive rosuvastatin plus 7-day DAPT was generally effective in reducing the risk of recurrent stroke, except in the subgroup with bNIHSS ≤ 2. The therapy was particularly efficient in the elderly, male, high-bNIHSS, and hypertension, diabetes, and hyperlipidemia subgroups, with P < 0.02.
Conclusions
Without increasing bleeding and statin-associated adverse events, intensive rosuvastatin therapy plus 7-day DAPT significantly reduced the risk of recurrent stroke, especially for subgroups with high-risk factors. Clinical trial registration. China Clinical Trial Registration Center (ChiCTR1800017809).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00228-022-03442-8.
Keywords: Intensive rosuvastatin, Dual antiplatelet therapy, Recurrent ischemic stroke, Subgroup analysis
Introduction
Disorder of lipid metabolism, dysfunction of endothelial cells, and aggregation of many inflammatory factors are the external conditions of arterial thrombosis [1, 2], while platelet activation is the internal factor [3]. All these factors interact with and promote each other [4], eventually leading to acute coronary syndrome (ACS) and acute ischemic stroke (AIS).
Intensive statin therapy is commonly combined with dual antiplatelet therapy (DAPT) in patients with ACS after percutaneous coronary intervention, stent implantation [5, 6], and AIS [7, 8]. This combination treatment can significantly reduce the risk of recurrent thrombosis after stent implantation [9] and recurrent ischemic stroke after AIS [10, 11]. However, there are few reports on the efficacy of this regimen among different subgroups of patients with AIS. Thus, we investigated the risks and benefits of intensive rosuvastatin therapy plus 7-day DAPT in reducing recurrent ischemic stroke for patients with mild to moderate AIS and compared subgroups.
Materials and methods
Patients
We recruited patients aged 18 years or older who were admitted to the Emergency Department of our hospital from October 2016 to December 2019. Computed tomography (CT) and magnetic resonance imaging (MRI) of the head was used to confirm the new focal infarction lesions within 72 h after the onset. Their National Institutes of Health Stroke Scale (NIHSS) scores at registration were 0–10. We excluded patients with intravenous thrombolysis/arterial thrombectomy or anticoagulation treatments, patients undergoing menstruation or pregnancy, and patients preparing for pregnancy within 3 months. In total, 331 patients with AIS met the above criteria, including 204 patients in the control group and 127 patients in the study group according to the therapy regimens. The Medical Ethics Committee of the China Rehabilitation Research Center approved this study (Ethics approval number: 2018–022-1). All patients provided written informed consent.
Study design and treatment
We stratified the patients based on their baseline demographic characteristics (Table 1). They received one of the two therapy regimens on a voluntary basis (DAPT + intensive rosuvastatin therapy for the study group and single antiplatelet therapy (SAPT) + rosuvastatin for the control group). The follow-up time was 90 days. The primary outcomes were a new ischemic stroke, bleeding events, and the adverse events statin-induced liver injury or statin-associated myopathy (SAM) within 90 days; the secondary outcome was the heterogeneity of the two therapy regimens in reducing recurrent ischemic stroke among subgroups of patients with AIS.
Table 1.
Stratifying basis for subgroups
| Indicators | Cut-off | Assign value |
|---|---|---|
| Age, yearsa | 68 years old | ≤ 68 years old = 0, > 68 years old = 1 |
| Gender | - | Female = 0, male = 1 |
| OMT, hoursb,d | 48 h | ≤ 48 h = 0, > 48 h = 1 |
| SBP at registratione | 140 mm Hg | ≤ 140 mm Hg = 0, > 140 mm Hg = 1 |
| History of diseases | ||
| Hypertension | - | No = 0, yes = 1 |
| Diabetes mellitus | - | No = 0, yes = 1 |
| Hypercholesterolemia | - | No = 0, yes = 1 |
| Known atrial fibrillation | - | No = 0, yes = 1 |
| Prior-stroke | - | No = 0, yes = 1 |
| Prior antiplatelet | - | No = 0, yes = 1 |
| bNIHSSc,f | 2 points | ≤ 2 points = 0, > 2 points (3–10 points) = 1 |
aBy the end of 2015, the average life expectancy in China reached 76.34 years [12], with an increase of 1 year every 5 years [13]. The average life expectancy in 2016–2019 was 68–69 years. Therefore, the cut-off age was 68 years
bThe Chinese Stroke Guidelines [14] only recommend DAPT for AIS patients within 48 h of symptom onset. Therefore, the cut-off time of onset to medication (OMT) was 48 h
cWhen the NIHSS changes by less than 2 points, the symptoms change of focal neurological deficit is not obvious [15]. In addition, whether intravenous thrombolysis or not did not affect the prognosis of AIS patients with NIHSS scores ≤ 2 [16], so the cut-off value of the baseline scores of NIHSS at recruitment (bNIHSS) was 2
dOMT, time of onset to medication
eSBP, systolic blood pressure
fbNIHSS, the baseline scores of National Institute of Health stroke scale at recruitment
Therapy regimens
Patients in the study group received DAPT + intensive rosuvastatin therapy: aspirin (Bayer, 100 mg per tablet) 100 mg/d with an initial dose of 300 mg for 90 days, clopidogrel (Sanofi, 75 mg per tablet) 75 mg/d with an initial dose of 75–300 mg determined based on the clinical symptoms for 7 days, plus rosuvastatin (Nanjing Chia Tai-Tianqing Pharmaceutical Co., Ltd, 10 mg per tablet), 20 mg/d for 21 days, and then 10 mg/d for 90 days in total. Patients in the control group received SAPT + rosuvastatin: aspirin (Bayer, 100 mg per tablet) 100 mg/d or clopidogrel (Sanofi, 75 mg per tablet) 75 mg/d for 90 days, plus rosuvastatin (Nanjing Chia Tai-Tianqing Pharmaceutical Co., Ltd, 10 mg per tablet) 10 mg/d for 90 days.
According to previous studies, aspirin and clopidogrel consistently reduced recurrent vascular events [17] or recurrent ischemic stroke events [18, 19] for stroke patients within 1 year. For a few patients in the control group who were intolerant to aspirin, we used clopidogrel 75 mg/d instead of aspirin 100 mg/d due to its lower gastric toxicity [17], which had no significant effect on the outcome.
Assessment criteria
We assessed focal neurological deficits by assessing the bNIHSS, which ranges from 0 to 43, with higher scores indicating worse deficits [20]. Because there were few patients with bNIHSS above 10 in our Emergency Department, and the therapy regimens in this study were very poor for them, we only registered patients with bNIHSS ≤ 10 in this study.
Recurrent ischemic stroke—the aggravation of existing clinical symptoms or the emergence of new focal neurological deficit symptoms within 90 days after the first treatment—was confirmed by CT and MRI scans of the head, which showed obviously enlarged original lesions or new ischemic lesions. Bleeding events included intracranial and gastrointestinal mucosal hemorrhage, which were confirmed by head CT and gastric contents analysis or fecal occult blood test, respectively, within 90 days after treatment. According to the global use of streptokinase and tissue plasminogen activator to treat coronary occlusion (GUSTO) [21], the severity of bleeding was classified as mild, moderate, or severe. Statin-induced liver injury and SAM were defined as a more than three-fold increase in the normal upper limit levels of transaminase (alanine transferase [ALT] or aspartate transferase [AST]) [22] and creatine kinase (CK) [23] within 90 days.
Statistical analysis
This study adopted an incomplete randomized controlled trial design, with Type I Error α = 0.05 and power of test (1 − β) = 0.85. The PASS 15.0 software (NCSS, LLC, Kaysville, UT, USA) was used to estimate the sample size. According to a meta-analysis by Kwok et al. [24], SAPT reduced the recurrence rate of ischemic stroke by 52%. In line with the CHANCE trial [25], DAPT actually reduced AIS recurrent stroke by nearly 84%, which is significantly more than SAPT. With these studies in mind, we concluded that we needed a total sample size of 312 cases, with a control group to study group size ratio of around 3:2. We effectively recruited 331 patients, with 204 patients in the control group and 127 in the research group.
SPSS 25.0 statistical software (IBM Corporation, Armonk, NY, USA) was used for data analysis. We expressed measurement data as the median (M) and inter-quartile range (IQR) from the rank-sum test results and expressed count data as % from the χ2 test results. A Cox proportional hazards model was used to evaluate differences in the recurrent ischemic stroke events and bleeding events within 90 days between the two groups. We compared the ALT, AST, lactate dehydrogenase (LDH), and CK levels before and 2 weeks after therapy using a rank sum test. Finally, we assessed the heterogeneity of the two therapy regimens of this study in reducing recurrent ischemic stroke by performing a subgroup analysis. P < 0.05 was considered statistically significant for the first three statistical analyses, and P < 0.02 was considered statistically significant for the subgroup analysis.
Results
Baseline data between the two groups
Baseline demographic characteristics were well balanced between the two groups with all P > 0.05 among age; gender; systolic and diastolic blood pressure at registration; OMT; bNIHSS; previous medical histories; and the levels of ALT, AST, LDH, and CK before and 2 weeks (14 ± 3 days) after therapy (Table 2).
Table 2.
Baseline demographic characteristics of patients
| Characteristic | Control (n = 204) | Study (n = 127) | P value |
|---|---|---|---|
| Median age (IQR), year | 67.00 (59.00–82.00) | 66.00 (58.50–76.00) | 0.117 |
| Female, no. (%) | 60 (66.7) | 30 (33.3) | 0.257 |
| Median SBP (IQR), mm Hga | 154.00 (138.00–173.50) | 154.00 (143.00–172.00) | 0.713 |
| Median DBP (IQR), mm Hgb | 89.00 (78.00–102.00) | 91.00 (81.00–103.00) | 0.056 |
| Medical history, no. (%) | |||
| hypertension | 174 (62.1) | 106 (37.9) | 0.643 |
| Diabetes mellitus | 110 (53.9) | 57 (44.9) | 0.115 |
| Hyperlipemia | 182 (89.2) | 106 (83.4) | 0.134 |
| Known atrial fibrillation | 27 (13.2) | 16 (12.6) | 1.000 |
| Ischemic stroke | 89 (43.6) | 44 (34.6) | 0.108 |
| Pre-antiplatelet | 41 (20.1) | 35 (27.6) | 0.139 |
| Median OMT (IQR), hourc | 12.00 (4.00–24.00) | 18.00 (4.50–38.00) | 0.388 |
| Median bNIHSS (IQR)d | 3.00 (2.00–4.00) | 4.00 (3.00–5.00) | 0.076 |
| Median baseline of various enzymology before medication (IQR), U/L | |||
| ALTe | 16.80 (12.05–22.50) | 18.40 (13.70–23.70) | 0.183 |
| ASTf | 17.05 (12.90–23.10) | 16.50 (13.00–21.60) | 0.560 |
| LDHg | 175.50 (157.00–200.50) | 179.00 (151.00–202.00) | 0.825 |
| CKh | 81.00 (54.50–120.50) | 72.00 (52.00–101.50) | 0.099 |
| Median of various enzymology in 2 weeks after medication(IQR), U/L | |||
| ALTe | 16.75 (11.55–24.55) | 17.10 (13.00–25.25) | 0.466 |
| ASTf | 17.70 (13.30–22.80) | 17.60 (14.00–22.55) | 0.956 |
| LDHg | 180.00 (155.50–214.00) | 172.00 (151.00–204.00) | 0.079 |
| CKh | 69.50 (49.00–101.50) | 68.00 (48.00–101.00) | 0.514 |
aSBP, systolic blood pressure
bDBP, diastolic blood pressure
cOMT, time of onset to medication
dbNIHSS, the baseline scores of National Institute of Health stroke scale before medication
eALT, alanine aminotransferase
fAST, aspartate aminotransferase
gLDH, lactate dehydrogenase
hCK, creatine kinase
Recurrent ischemic stroke
Within 90 days, 52 patients underwent recurrent ischemic stroke: 10 (7.87%) in the study group and 42 (20.60%) in the control group. The study group had a 62% lower risk of recurrent ischemic stroke than the control group (hazard ratio [HR] for the study group vs. control group, 0.373, 95% confidence interval [CI], 0.178–0.780, P = 0.009). This result shows that intensive rosuvastatin therapy plus 7-day DAPT was far superior to rosuvastatin plus SAPT in reducing the risk of recurrent ischemic stroke within 90 days (Table 3 and Fig. 1).
Table 3.
The primary outcome within 90 days
| Outcome | Study (n = 127) | Control (n = 204) |
Hazard ratio (95% CI) |
P value | ||
|---|---|---|---|---|---|---|
| Cases with event (no.) | Event rate (%) | Cases with event (no.) | Event rate (%) | |||
| Stroke | 10 | 7.87 | 42 | 20.6 | 0.373 (0.178–0.780) | 0.009 |
| Bleeding* | 9 | 7.09 | 14 | 6.86 | 1.019 (0.441–2.353) | 0.966 |
*The bleeding was divided into mild, moderate, and severe bleeding according to GUSTO criteria [21], and all bleeding events were minor bleeding from gastrointestinal mucosa
Fig. 1.
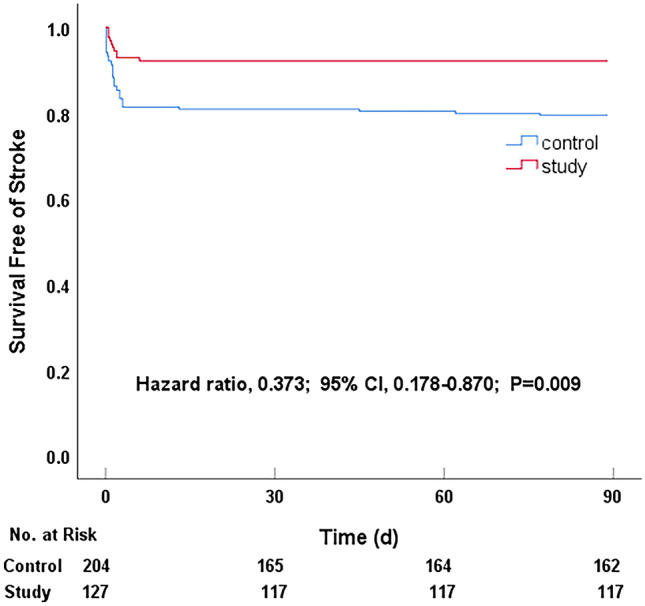
Probability of survival free of recurrent ischemic stroke within 90 days
Bleeding events
A total of 9 patients (7.09%) in the study group and 14 patients (6.86%) in the control group reported bleeding events. A Cox proportional hazards model revealed no significant difference between the two groups (HR, 1.019; 95% CI, 0.441–2.353; P = 0.966), suggesting that intensive rosuvastatin therapy plus 7-day DAPT did not increase the risk of bleeding compared with rosuvastatin plus SAPT (Table 3).
Statin-induced liver injury or SAM
None of the patients showed an increase superior to three-fold in the levels of ALT, AST, or CK. The ALT, AST, LDH, and CK levels remained stable before therapy and after 2 weeks (14 ± 3 days) among the groups (P > 0.05), except for the CK levels in the control group, which were significantly lower after 2 weeks of treatment (P < 0.001) (Table 4). These findings showed that the two regimens did not increase the risk of statin-induced liver injury or SAM.
Table 4.
Comparison of various enzymology before and 2 weeks after medication (comparison of the levels of transaminase and muscle enzymes before and 2 weeks (14 ± 3 days) after treatment intra each group)
| Control (n = 204) | Study (n = 127) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ALT | AST | LDH | CK | ALT | AST | LDH | CK | ||
| Before | 25% | 12.05 | 12.90 | 157.00 | 54.50 | 13.70 | 13.00 | 151.00 | 52.00 |
| Median | 16.80 | 17.05 | 175.50 | 81.00 | 18.40 | 16.50 | 179.00 | 72.00 | |
| 75% | 22.50 | 23.10 | 200.50 | 120.50 | 23.70 | 21.60 | 202.00 | 101.50 | |
| After | 25% | 11.55 | 13.30 | 155.50 | 49.00 | 13.00 | 14.00 | 151.00 | 48.00 |
| Median | 16.75 | 17.70 | 180.00 | 69.50 | 17.10 | 17.60 | 172.00 | 68.00 | |
| 75% | 24.55 | 22.80 | 214.00 | 101.50 | 25.25 | 22.55 | 204.00 | 101.00 | |
| P value (2-tailed) | 0.824 | 0.887 | 0.079 | 0.000 | 0.299 | 0.460 | 0.399 | 0.153 | |
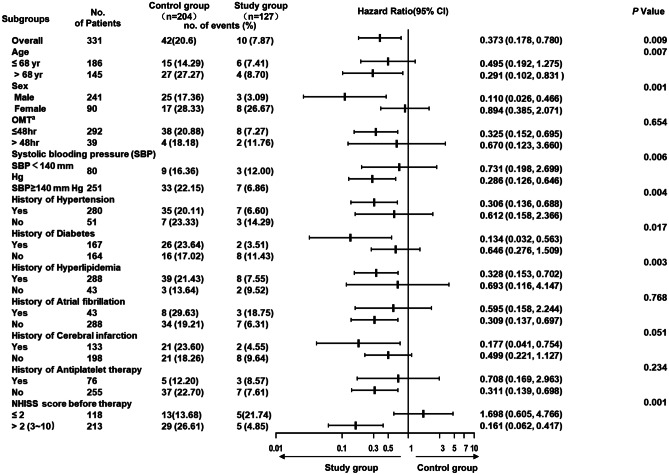
Heterogeneity in reducing recurrent ischemic stroke among subgroups
To explore the heterogeneity of the two different therapy regimens in reducing the risk of recurrent ischemic stroke for AIS patients within 90 days, we performed a subgroup analysis according to the aforementioned stratification. The intensive rosuvastatin therapy plus 7-day DAPT always effectively reduced the risk of recurrent ischemic stroke, with the HR value of each subgroup located on the left side of the invalid line, except the subgroup with bNIHSS ≤ 2. It was particularly efficient in these subgroups with high-risk factors such as the elderly, hypertension, diabetes, hyperlipidemia, or prior-stroke, non-antiplatelet treatment, and high-bNIHSS (3–10 points), while it was less efficient in female, OMT > 48 h, and prior atrial fibrillation subgroups (Fig. 2).
Fig. 2.
Hazard ratio for the recurrent cerebral infarction in subgroups at 90 days
Discussion
In this study, compared with rosuvastatin plus SAPT, the intensive rosuvastatin therapy plus 7-day DAPT significantly reduced the risk of recurrent stroke within 90 days for patients with mild to moderate AIS, without increasing adverse events, such as bleeding, statin-induced liver injury, or SAM. The reduction was particularly significant in the subgroups with high-risk factors (the elderly [> 68 years old], hypertension, diabetes, hyperlipidemia, prior-stroke, non-antiplatelet treatment, and high bNIHSS [3–10 points]), but not significant in female, OMT > 48 h, and prior atrial fibrillation subgroups. These results should be interpreted by considering the characteristics of the therapy regimens in this study, namely, the strong inhibition of platelet aggregation and reduction of thrombosis by DAPT and the multiple effects of intensive statin therapy.
Previous study showed that statins, especially fat-soluble statins, commonly caused statin-induced liver injury and SAM, along with a three-fold or more increase in ALT, AST, or CK levels [26]. However, as a water-soluble statin, rosuvastatin only enters hepatocytes through special channel proteins on the cell membrane, rarely causing rosuvastatin-induced liver injury and SAM [27, 28]. DAPT can increase the risk of bleeding [29–31], although this is most likely to occur after 1 week of treatment [31]. Therefore, intensive rosuvastatin therapy plus 7-day DAPT significantly reduced the risk of ischemic stroke recurrence in patients with mild to moderate AIS, without increasing bleeding, statin-induced liver injury, and SAM.
In the elderly subgroup (> 68 years old), the study therapy reduced the risk of stroke recurrence more than the control therapy did, which may be related to elderly patients’ high risk of stroke [32], low self-healing ability [33], and higher dependence on effective interventions. In the male subgroup, the treatment effect of the study group was significantly better than that of the control group, while. However, there was no statistically significant difference in the female subgroup, which can be ascribed to the older age, higher prevalence of risk factors [34], higher disability severity, and worse prognosis [35–39] for female patients when the ischemic stroke occur, leading to the failure of various interventions. Moreover, the incidence rate of ischemic stroke was significantly higher in men than in women [39], which led to a relatively large sample size for the male subgroup and made it easier to obtain statistically significant results.
At the early stage of cerebral infarction, a large part of the brain tissue is still in the ischemic penumbra due to the incomplete rupture of the lipid plaque and relatively mild inflammatory storm [4]. Therefore, the earlier effective intervention measures are given, the more dormant brain cells are saved, and the more obvious clinical symptoms are relieved, which could explain why the effect of the subgroup of OMT ≤ 48 h surpassed that of the subgroup with OMT > 48 h.
The NIHSS can not only quantify the symptoms and signs of focal neurological deficit [40] but also accurately reflect the volume of cerebral infarction within a certain infarct volume [41]. The lower the score, the lighter the symptoms, and the better the prognosis. Study showed that AIS patients with a bNIHSS < 3 had a good prognosis and were not affected by intravenous thrombolysis [16]. This is consistent with our results in the subgroup with bNHISS ≤ 2, independent of the therapy regimens. However, in the subgroup with bNHISS of 3–10, the study treatment was significantly more efficient than the control treatment, indicating that the intensive rosuvastatin therapy plus 7-day DAPT regimen was more beneficial to the subgroup with high bNIHSS (3–10).
Many studies have shown that AIS patients with high-risk factors [42], such as hypertension [43–45], diabetes [46, 47], hyperlipidemia [48], and prior-stroke [49], have a significantly higher risk of recurrent stroke. The study also showed that statins can reduce the incidence of serious cardiovascular and cerebrovascular events by 20–30% in patients with high-risk factors [50], which is consistent with our results. Moreover, the study treatment reduced the risk of recurrent stroke significantly more than the control treatment did in the subgroups with hypertension, diabetes, hyperlipidemia, and prior-stroke. Additionally, the study treatment did not reduce the risk of recurrent stroke any more than the control treatment did in the atrial fibrillation subgroup, which may be related to the fact that thrombus comes from the heart [51, 52], and the best treatment regimen for these patients is anticoagulation agents [53].
Aspirin and clopidogrel are the most common antiplatelet drugs. Aspirin has an irreversible inhibitory effect on platelet aggregation through acetylated platelet cyclooxygenase, while clopidogrel, as an adenosine diphosphate receptor inhibitor, inhibits platelet aggregation. For patients who took antiplatelet agents regularly and still had AIS, the possible cause was aspirin resistance [54] or clopidogrel resistance [55], which may explain that the study treatment can only reduce the risk of recurrent stroke significantly in patients of the subgroup of non-antiplatelet, but not in patients of the subgroup of prior-antiplatelet.
Limitations
This study was a single-center study with small sample size and incomplete randomized controlled design. These characteristics inevitably led to some weaknesses in the research results, which need to be confirmed by future large sample size and multi-center clinical studies.
Conclusion
Compared with rosuvastatin plus SAPT, the intensive rosuvastatin therapy plus 7-day DAPT with aspirin and clopidogrel significantly reduced the risk of recurrent ischemic stroke within 90 days for patients with mild to moderate AIS, without increasing bleeding, statin-induced liver injury, or SAM. These effects were particularly significant in the subgroups with high-risk factors such as elderly patients (> 68 years old), patients with hypertension, diabetes, hyperlipidemia, prior-stroke, non-antiplatelet treatment, and high bNHISS scores (3–10).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ph.D Yunlei Wang for careful reading of the manuscript.
Author contribution
Study conception or design: TZ and HTL. Acquisition of the data: TD, XML, JMC, XHY. Analysis and interpretation of the data: TD, WH, XML. Drafting and revising the article: TZ and HTL. All authors read and approved the final manuscript.
Funding
This study was funded by Beijing Municipal Commission of Science and Technology (Grand Numbers: Z181100001718066).
Data availability
All the required data about the study are present in the manuscript. We are happy to provide additional data if the reviewer or the editor requires further data.
Declarations
Ethical approval
The Medical Ethics Committee of China Rehabilitation Research Center has approved the study (Ethics approval number: 2018–022-1). Written informed consents were obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ting Deng, Email: dengting318@163.com.
Tong Zhang, Email: tommzhang@163.com.
Haitao Lu, Email: 13051760807@163.com.
Jingmian Chen, Email: cjm20060626@sina.com.
Xiaomeng Liu, Email: liuxiaomeng0323@163.com.
Wei He, Email: hewei13520807222@163.com.
Xiaohua Yao, Email: 13810347072@163.com.
References
- 1.Badimon L, Suades R, Fuentes E, et al. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front Pharmacol. 2016;7:293. doi: 10.3389/fphar.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 3.Koupenova M, Clancy L, Corkrey HA, et al. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ res. 2018;122(2):337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montecucco F, Carbone F, Schindler TH. Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatments. Eur Heart J. 2016;37(16):1268–1283. doi: 10.1093/eurheartj/ehv592. [DOI] [PubMed] [Google Scholar]
- 5.Di Sciascio G, Patti G, Pasceri V, et al. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54(6):558–565. doi: 10.1016/j.jacc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Yun KH, Jeong MH, Oh SK, et al. The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2009;137(3):246–251. doi: 10.1016/j.ijcard.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 7.Yuan HW, Ji RJ, Lin YJ, et al. Intensive versus moderate statin therapy discontinuation in patients with acute ischemic stroke or transient ischemic attack. Clin Ther. 2018;40(12):2041–2049. doi: 10.1016/j.clinthera.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046–e1081. doi: 10.1161/CIR.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 9.Jang HG, Kim K, Park HW et al (2021) Restenosis of a drug eluting stent on the previous bioresorbable vascular scaffold successfully treated with a drug-coated balloon: a case report. World J Clin Cases 9(3). 10.12998/wjcc.v9.i3.758 [DOI] [PMC free article] [PubMed]
- 10.O’Donnell MJ, Chin SL, Rangarajan S et al (2016) Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 388(10046). 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed]
- 11.Ridker PM, Revkin J, Amarenco P et al (2017) Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 376(16). 10.1056/NEJMoa1701488 [DOI] [PubMed]
- 12.Tan YF, Zhu MJ, Zhou K. Life expectancy, healthy working life expectancy, and postponement of retirement age. Popul J. 2016;38(1):26–34. doi: 10.16405/j.cnki.1004-129X.2016.01.003. [DOI] [Google Scholar]
- 13.Hu AG. The core idea of the 13th Five Year Plan is to promote the all-round development of people. Red Flag Manuscript. 2015;23:4–6. [Google Scholar]
- 14.Liu LP, Duan Chen WQ, WY,, et al. Chinese stroke association guidelines for clinical management of cerebrovascular disorder (excerpts) — clinical management of ischemic cerebrovascular disorders. Chin J Stroke. 2019;14(07):709–726. doi: 10.3969/j.issn.1673-5765.2019.07.014. [DOI] [Google Scholar]
- 15.Yoo AJ, Romero J, Hakimelahi R, et al. Predictors of functional outcome vary by the hemisphere of involvement in major ischemic stroke treated with intra-arterial therapy: a retrospective cohort study. BMC Neurol. 2010;10(1):1–11. doi: 10.1186/1471-2377-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romano JG, Gardener H, Campo-Bustillo I, et al. Predictors of outcomes in patients with mild ischemic stroke symptoms: MaRISS. Stroke. 2021;52(6):1995–2004. doi: 10.1161/STROKEAHA.120.032809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CAPRIE steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao WM, Wang SF, Lin XX, et al. Comparison of clinical efficacy of clopidogrel and aspirin in preventing recurrence of non cardiogenic cerebral infarction. Guangdong Med J. 2009;30(2):288–289. doi: 10.13820/j.cnki.gdyx.2009.02.056. [DOI] [Google Scholar]
- 20.Ghandehari K. Challenging comparison of stroke scales. J Res Med Sci. 2013;18(10):906–910. [PMC free article] [PubMed] [Google Scholar]
- 21.The GUSTO Investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Medical Association Guideline for primary care of dyslipidemias: practice version (2019) Chin J Gen Pract. 2018;018(005):417–421. doi: 10.3760/cma,j.issn.1671-7368.2019.05.004. [DOI] [Google Scholar]
- 23.Tournadre A. Statins, myalgia, and rhabdomyolysis. Joint Bone Spine. 2020;87(1):37–42. doi: 10.1016/j.jbspin.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Kwok CS, Shoamanesh A, Copley HC, et al. Efficacy of antiplatelet therapy in secondary prevention following lacunar stroke: pooled analysis of randomized trials. Stroke. 2015;46(4):1014–1023. doi: 10.1161/strokeaha.114.008422. [DOI] [PubMed] [Google Scholar]
- 25.Wang YJ, Wang YL, Zhao XQ, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 26.Kiortsis DN, Filippatos TD, Mikhailidis DP, et al. Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis. 2007;195(1):7–16. doi: 10.1016/j.atherosclerosis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Li YF. Research and development of statins and their fat solubility and water solubility. Chin J Geriatr Heart Brain Vessel Dis. 2018;20(9):1008–1008. doi: 10.3969/j.issn.1009-0126.2018.09.030. [DOI] [Google Scholar]
- 28.Petry NJ, Baye JF, Frear S, et al. Progression of precision statin prescribing for reduction of statin-associated muscle symptoms. Pharmacogenomics. 2022;23(10):585–596. doi: 10.2217/pgs-2022-0055. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Gui L, Dong Y, et al. Dual antiplatelet therapy may increase the risk of non- intracranial haemorrhage in patients with minor strokes: a subgroup analysis of the CHANCE trial. Stroke Vasc Neurol. 2016;1(2):29–36. doi: 10.1136/svn-2016-000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52(6):e217–e223. doi: 10.1161/STROKEAHA.120.033033. [DOI] [PubMed] [Google Scholar]
- 31.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA (POINT) N Engl J Med. 2018;379(3):215–225. doi: 10.1056/NEJMoa1800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisabeth LD, Brown DL, Zahuranec DB, et al. Temporal trends in ischemic stroke rates by ethnicity, sex, and age (2000–2017): the brain attack surveillance in Corpus Christi Project. Neurology. 2021;97(22):e2164–e2172. doi: 10.1212/WNL.0000000000012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao SY, Qin YF, Zhang HL, et al. Correlation between age and 1-year prognosis of patients with acute cerebral infarction. Chin J Stroke. 2021;16(08):810–815. doi: 10.3969/j.issn.1673-5765.2021.08.010. [DOI] [Google Scholar]
- 34.Sarrafzadegan N, Gharipour M, Sadeghi M et al (2017) Metabolic syndrome and the risk of ischemic stroke. J Stroke Cerebrovasc Dis 26(2). 10.1016/j.jstrokecerebrovasdis.2016.09.019 [DOI] [PubMed]
- 35.Phan HT, Reeves MJ, Blizzard CL, et al. Sex differences in severity of stroke in the INSTRUCT study: a meta-analysis of individual participant data. J Am Heart Assoc. 2019;8(1):e010235. doi: 10.1161/JAHA.118.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carcel C, Wang X, Sandset EC, et al. Sex differences in treatment and outcome after stroke: pooled analysis including 19,000 participants. Neurology. 2021;96(23):1106. doi: 10.1212/WNL.0000000000011946. [DOI] [PubMed] [Google Scholar]
- 37.Kremer C, Gdovinova Z, Bejot Y, et al. European stroke organisation guidelines on stroke in women: management of menopause, pregnancy and postpartum. Eur Stroke J. 2022;7(2):I–XIX. doi: 10.1177/23969873221078696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali M, van Os HJA, van der Weerd N, et al. Sex differences in presentation of stroke: a systematic review and meta-analysis. Stroke. 2022;53(2):345–354. doi: 10.1161/STROKEAHA.120.034040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu HQ, Wang CJ, Yang X, et al. Sex differences in vascular risk factors, in-hospital management, and outcomes of patients with acute ischemic stroke in China. Eur J Neurol. 2022;29(1):188–198. doi: 10.1111/ene.15124. [DOI] [PubMed] [Google Scholar]
- 40.Fonarow GC, Saver JL, Smith EF, et al. Relationship of National Institutes of Health Stroke Scale to 30-day mortality in medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. 2012;1(1):42–50. doi: 10.1161/JAHA.111.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra NK, Albers GW, Christensen S, et al. Comparison of magnetic resonance imaging mismatch criteria to select patients for endovascular stroke therapy. Stroke. 2014;45(5):1369–1374. doi: 10.1161/STROKEAHA.114.004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Bai L, Shi M, et al. Trends in age of first-ever stroke following increased incidence and life expectancy in a low-income Chinese population. Stroke. 2016;47(4):929–935. doi: 10.1161/STROKEAHA.115.012466. [DOI] [PubMed] [Google Scholar]
- 43.Robinson TG, Minhas JS, Miller J. Review of major trials of acute blood pressure management in stroke. J Cereb Blood Flow Metab. 2022;42(3):404–410. doi: 10.1177/0271678X211004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jillella DV, Calder CS, Uchino K, et al. Blood pressure and hospital discharge outcomes in acute ischemic stroke patients undergoing reperfusion therapy. J Stroke Cerebrovasc Dis. 2020;29(11):105211. doi: 10.1016/j.jstrokecerebrovasdis.2020.105211. [DOI] [PubMed] [Google Scholar]
- 45.Yannoutsos A, Dreyfuss Tubiana C, Safar ME, et al. Optimal blood pressure target in stroke prevention. Curr Opin Neurol. 2017;30(1):8–14. doi: 10.1097/WCO.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 46.Lau LH, Lew J, Borschmann K, et al. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. 2019;10(3):780–792. doi: 10.1111/jdi.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadini GP, Cosentino F. Diabetes and ischaemic stroke: a deadly association. Eur Heart J. 2018;39(25):2387–2389. doi: 10.1093/eurheartj/ehy033. [DOI] [PubMed] [Google Scholar]
- 48.Jeong W, Joo JH, Kim H, et al. Association between statin adherence and the risk of stroke among South Korean adults with hyperlipidemia. Nutr Metab Cardiovasc Dis. 2022;32(3):560–566. doi: 10.1016/j.numecd.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Report on stroke prevention and treatment in China Writing Group Brief report on stroke prevention and treatment in China, 2020. Chin J Cerebrovasec Dis. 2022;19(2):136–144. doi: 10.3969/j.issn.1672-5921.2022.02.011. [DOI] [Google Scholar]
- 50.Milionis H, Ntaios G, Korompoki E, et al. Statin-based therapy for primary and secondary prevention of ischemic stroke: a meta-analysis and critical overview. Int J Stroke. 2020;15(4):377–384. doi: 10.1177/1747493019873594. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480687 adults. Circulation. 2017;135(8):759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 52.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15(9):913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 53.Kumbhani DJ, Cannon CP, Beavers CJ, et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(5):629–658. doi: 10.1016/j.jacc.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Morton M, Kubiak-Balcerewicz K, Sarnowska A, et al. Biochemical aspirin resistance in acute stroke patients and its association with clinical factors: a prospective pilot study. Folia Neuropathol. 2021;59(3):271–275. doi: 10.5114/fn.2021.109434. [DOI] [PubMed] [Google Scholar]
- 55.Wiśniewski A. Multifactorial background for a low biological response to antiplatelet agents used in stroke prevention. Medicina (Kaunas) 2021;57(1):59. doi: 10.3390/medicina57010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the required data about the study are present in the manuscript. We are happy to provide additional data if the reviewer or the editor requires further data.