Abstract
Aims
Diabetes will lead to serious complications, of which atherosclerosis is the most dangerous. This study aimed to explore the mechanisms of diabetic atherosclerosis.
Methods
ApoE−/− mice were fed with an high-fat diet diet and injected with streptozotocin to establish an in vivo diabetic atherosclerotic model. RAW 264.7 cells were treated with oxidized low-density lipoprotein particles (ox-LDL) and high glucose to produce an in vitro diabetic atherosclerotic model.
Results
In this study, we showed that diabetes promoted the progression of atherosclerosis in ApoE−/− mice and that high glucose potentiates macrophage proinflammatory activation and foam cell formation. Mechanistically, Copper metabolism MURR1 domain-containing 1(COMMD1) deficiency increased proinflammatory activation and foam cell formation, characterized by increased glycolysis, and then accelerated the process of atherosclerosis. Furthermore, 2-Deoxy-D-glucose (2-DG) reversed this effect.
Conclusion
Taken together, we provided evidence that the lack of COMMD1 accelerates diabetic atherosclerosis via mediating the metabolic reprogramming of macrophages. Our study provides evidence of a protective role for COMMD1 and establishes COMMD1 as a potential therapeutic strategy in patients with diabetic atherosclerosis.
Keywords: COMMD1, glycolysis, atherosclerosis, diabetes
Introduction
Diabetes is associated with severe atherosclerosis, and increases the risk of atherosclerosis and the subsequent occurrence of coronary artery disease, stroke, and so on.1,2 Atherosclerosis is a chronic inflammatory disease involving lipid deposition in the vascular wall and the formation of discrete or sheeted atherosclerotic plaques, resulting in the narrowing of the arterial lumen, followed by plaque rupture and hemorrhage, thrombosis.3,4 Macrophages play a central role in the initiation and progression of atherosclerosis.5 Recent studies have shown that macrophages accumulate in atherosclerotic lesions, secrete a large number of pro-inflammatory cytokines, and promote the further evolution of atherosclerosis.6,7 Furthermore, hyperglycemia-induced trained immunity in macrophages may promote the development of atherosclerosis via glycolysis.8
Alterations in energy metabolism have been implicated in atherosclerosis.9,10 The fluorodeoxyglucose positron emission tomography analysis found that local glucose uptake in human and mouse atherosclerotic plaques increased, oxygen consumption also increased, and lactate production increased, but ATP production was low, indicating that glucose fails to generate ATP through the tricarboxylic acid cycle.11,12 Inhibiting the glycolysis of macrophages for energy supply and promoting oxidative phosphorylation for energy supply can not only inhibit the expression of inflammatory cytokines but also inhibit the adhesion of monocytes and endothelial cells, which reduces the migration of monocytes to the arterial intima and extenuate the formation of atherosclerotic lesions.13 These observations indicate that cellular energy metabolic regulation is at the center of atherosclerosis, but the precise regulatory mechanisms remain largely uncertain.
Copper metabolism MURR1 domain-containing 1(COMMD1) is a hypoxia-sensitive 190-amino acid protein involved in diverse cellular functions such as copper homeostasis, cholesterol homeostasis, ion transport, transcriptional regulation, and oxidative stress.14–17 Recent studies have shown that COMMD1 can reduce atherosclerotic lesions by regulating cholesterol homeostasis and low-density lipoprotein receptor membrane expression by stabilizing the CCC complex(COMMD–CCDC22 [coiled-coil domain containing 22]–CCDC93 [coiled-coil domain containing 93].18 However, the role of COMMD1 in atherosclerosis and glycolysis has rarely been reported.
In this study, we showed that diabetes promoted the development of atherosclerosis in ApoE−/− mice, mainly due to increased proinflammatory activation. Moreover, COMMD1 regulates the production of pro-inflammatory cytokines and foam cell formation via glycolysis. Furthermore, the intervention of 2-Deoxy-D-glucose (2-DG) alleviated diabetic atherosclerosis. Thus, our findings provide new insights into the atheroprotective effect of COMMD1.
Methods
Antibodies and reagents
Anti-COMMD1 antibody (Bioss, bs-8034R), anti-HK2 antibody (proteintech, 22,029-1-AP), anti-PFKFB3 antibody (proteintech, 13,763-1-AP), anti-β-actin antibody (proteintech, 20,536-1-AP), anti-TNF‐α antibody (abcam, ab183218) were used in this study.
Reagents: 2-DG (MCE, HY-13,966, china), Oil Red O solution (Solarbio, G1260, China), STZ (Sigma, S0130, USA). AAV-shCOMMD1 (Jikai, Shanghai, China), SiCOMMD1 (Hema Biotechnology, Huzhou, China), Lipofectamine 8000 (Beyotime Biotechnology, Shanghai, China).
Animal procedures
ApoE−/− mice were purchased from Cavens Laboratory Animal Co., Ltd. (China) (SCXK(sun)-0010) and raised by the laboratory animal center of Jiangsu university. All animal experiments in this study were conducted in compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Jiangsu University, China.
ApoE−/− male mice aged 6 weeks were randomly divided into five groups as follows: HFD group (high fat diet) (n = 3), DM group (high fat diet + STZ) (n = 3), AAV-shscramble group (high fat diet + STZ + AAV-shscramble) (n = 3), AAV-shCOMMD1 group (high fat diet + STZ + AAV-shCOMMD1) (n = 3), and AAV-shCOMMD1+2-DG group (high fat diet + STZ + AAV-shCOMMD1+ 2-DG) (n = 3).
STZ-induced diabetic atherosclerosis model. ApoE−/− male mice aged 6 weeks were intraperitoneally injected with streptozotocin (STZ) (40 mg/kg/d) or citrate buffer for five consecutive days and fed a 45% high-fat diet (HFD). After 2 weeks post-final STZ injection, mice with blood glucose levels >300 mg/dL were included in DM group. After 24 weeks, mice were euthanized for subsequent experiments.
Knockdown of COMMD1 in diabetic atherosclerosis mice. AAV-shCOMMD1 was generated using the pAAV-U6-shRNA-CMV bGlobin-eGFP-3Flag (GV390) adeno-associated virus vector and the shRNA sequence targeting COMMD1 was as follows: gaTGAAGTTAAAGTCAAGCAA. In addition, the shRNA sequence targeting scramble was as following: CGCTGAGTACTTCGAAATGTC. DNAse-resistant particles of adeno-associated viral (AAV) vectors consisting of AAV-shscramble or AAV-shCOMMD1 were injected intravenously once into mice at the optimized dose of 1011 PFU. After 24 weeks, mice were euthanized for subsequent experiments.
2-DG in diabetic atherosclerosis mice. The mice were intraperitoneally injected with 2-Deoxy-D-glucose(2-DG) (3 mmol/kg/d) in the morning for 5 weeks. After 24 weeks, mice were euthanized for subsequent experiments.
Cell culture
Primary Peritoneal macrophages were isolated from the ApoE−/− mice. Briefly, ApoE−/− mice were injected intraperitoneally with 1 mL of autoclaved 4% thioglycollate. After 4 days, the peritoneal lavage fluid was aspirated with a sterile syringe and centrifuged. Finally, the cells are seeded on culture plates, and cultured with RPMI 1640 (Gbico) supplemented with 15% fetal bovine serum (FBS, Gbico) and 1% penicillin and streptomycin in a cell culture incubator.
RAW 264.7 cells were treated with oxidized low-density lipoprotein particles (ox-LDL) and high glucose to produce an in vitro model of diabetic atherosclerosis and cultured in a cell culture incubator at 37°C and 5% CO2.
siRNA-mediated knockdown
Three independent sets of siRNAs against COMMD1 were synthesized by Hema Biotechnology. The sequences of the COMMD1 siRNAs were as following: COMMD1-siRNA1 sense: 5′-CGUUGUUGUUGUUGUUGUUGU-3′, antisense: 5′-AACAACAACAACAACAACGAA-3′; COMMD1-siRNA2 sense: 5′-GGAAAUAGUUGUACUGUAACU-3′, antisense: 5′-UUACAGUACAACUAUUUCCUG-3′; COMMD1-siRNA3 sense: 5′-GGACAUUGUUAUUACCUAAAU-3′, antisense: 5′-UUAGGUAAUAACAAUGUCCAG-3′. RAW 264.7 cells transfected with the indicated siRNAs after they reached 75% confluency by using Lipofectamine 8000 according to the manufacturer’s protocol. The efficiency of siRNA transfection was assessed by western blotting.
H&E staining and Masson staining
Paraffin sections were deparaffinized and rehydrated. The steps of H&E staining were followed by the hematoxylin-eosin (H&E) staining kit (Solarbio, China). For Masson staining, Steps were performed according to the Masson trichrome staining Kit (Solarbio. China). Finally, the sections were observed and photographed under a light microscope (Olympus, Japan).
Oil red O staining
Aortas were fixed in 4% paraformaldehyde for over 24 h and washed 3 times in PBS. Then, the aortas were stained for 60 min in Oil Red O working solution (3 parts Oil Red O stock solution: two parts distilled water) and differentiated by 75% alcohol. Finally, the aortas were photographed.
Peritoneal macrophages and RAW264.7 cells were fixed in 4% paraformaldehyde for 20 min and washed 3 times in PBS. Then, the sections were stained for 15 s in an isopropanol working solution (3 parts isopropanol solution: two parts distilled water) and stained for 30 min in an oil red O working solution. Finally, the sections were photographed under a light microscope.
Western blotting
The protein samples of RAW 264.7 cells were extracted by RIPA lysis buffer containing protease and phosphatase inhibitor cocktail. Protein samples were separated by SDS-PAGE and then transferred onto PVDF membranes. Primary antibodies were used to incubate overnight, and secondary antibodies were used to incubate for 1 h. Finally, the bands were detected by a gel imaging system (Amersham Imager 600) by using ECL detection reagents (Bio-Rad, Hercules, CA, USA).
Semi-quantitative RT-PCR analysis
Total RNA was extracted using MolPure® TRIeasy Plus Total RNA Kit (Yishan, China) and reverse transcribed into cDNA. Real-time PCR was performed using primers for mouse TNF-α, IL-6, IL-1β, and GAPDH (Sangon Biotech, China). RNA primer sequences were: TNF-α:5′-GTCTCAGCCTCTTCTCATTC-3′ and 5′-CATAGAACTGATGAGAGGGA-3′; IL-6:5′-GAGTCCTTCAGAGAGATACAG-3′ and 5′-CTGTGACTCCAGCTTATCTG-3′; IL-1β: 5′-AAATACCTGTGGCCTTGGGC-3′ and 5′-CTTGGGATCCACACTCTCCAG-3′; GAPDH:5′-AAGTTCAACGGCA-CAGTCAA-3′ and 5′-TACTCAGCACCAGCAT-CACC-3′. PCR amplification was carried out at 94°C for 1 min, followed by 33 cycles at 94°C for 30 s, 63°C for 30 s, 72°C for 1 min, and a final extension at 72°C for 7 min.
Statistical analysis
Values were expressed as mean ± SEM. Two groups were compared using the Unpaired two-tailed Student’s t-test, and Multiple groups were compared using ANOVA with Bonferroni post-hoc test by Graphpad Prism 7.0 software. p < 0.05 represents statistically significant.
Results
Diabetes accelerates atherosclerosis in ApoE−/− mice
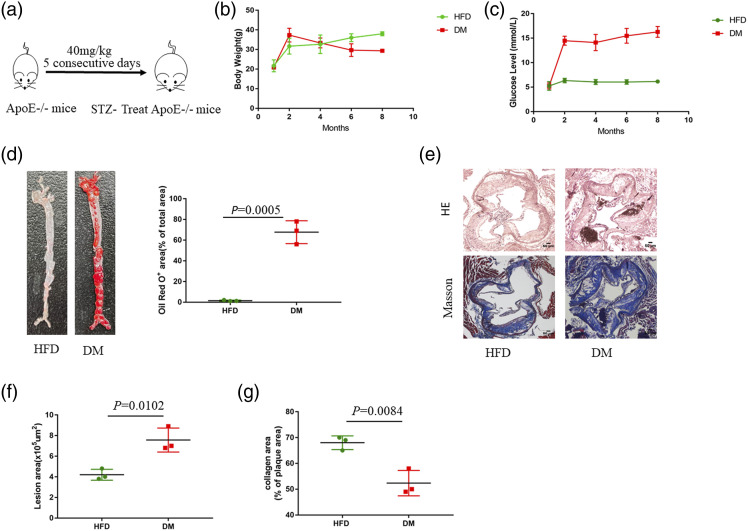
To identify the role of diabetes in the development of atherosclerotic plaque, we established the diabetic atherosclerotic model as described before (Figure 1(a)). During 24 weeks of HFD feeding, the animals gained weight (Figure 1(b)). Diabetic ApoE−/− mice had elevated blood glucose (Figure 1(c)). Oil Red O staining of the aortas revealed that the level of lipid accumulation in the whole aorta of DM group was increased relative to that of HFD group (Figure 1(d)). Furthermore, H&E staining demonstrated that the atherosclerotic lesion area was increased in DM group (Figure 1(e) and (f)). Moreover, collagen content is related to plaque stability.19,20 Previous studies have shown that collagen content in plaques decreases, and the thinner the fibrous cap, the easier the plaque ruptures.21 Notably, Masson staining revealed that the collagen content of atherosclerosis lesions in DM group was reduced compared to that in HFD group, suggesting that the plaque in DM group may be unstable (Figure 1(e) and (g)). These results indicate that diabetes accelerates the process of atherosclerosis.
Figure 1.
Diabetes accelerates the development of atherosclerosis in ApoE−/− mice. (a) ApoE−/− mice were fed an HFD diet and treated with STZ for 5 days to establish a diabetic atherosclerotic model. (b) Body weight (g) (n = 3), (c) Blood glucose (mmol/L) (n = 3). Results are expressed as mean ± SEM (d) Oil Red O staining of aortas from ApoE−/− mice fed an HFD for 24 weeks (n = 3). Data represent the percentage of plaque area/total vessel area. Unpaired two-tailed Student’s t-test (n = 3). (e) H&E staining (the first row) and Masson staining (the second row) of representative aortic root sections (n = 3). Scale bar, 50 μm. (f) Quantitative analysis of the lesion area. Unpaired two-tailed Student’s t-test. (g) Quantitative analysis of plaque collagen area. Unpaired two-tailed Student’s t-test.
High glucose potentiates macrophage proinflammatory activation and foam cell formation
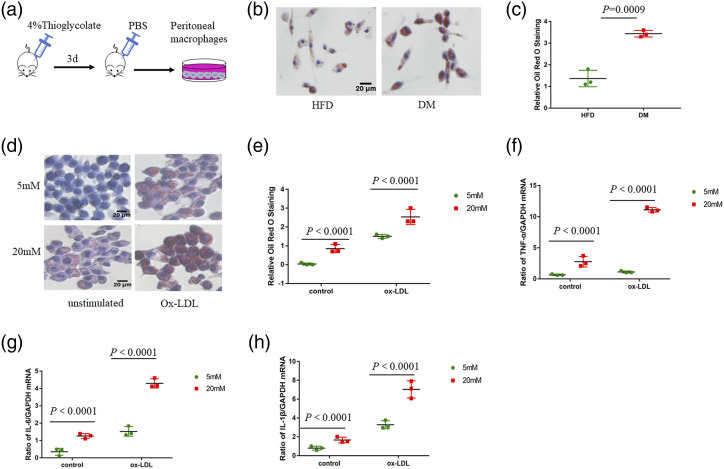
To evaluate the effect of high glucose on macrophage function, we isolated Peritoneal macrophages from ApoE−/− mice (Figure 2(a)). Macrophages in DM group showed more lipid uptake and accumulation compared to that in the HFD group (Figure 2(b) and (c)). A similar trend was observed in RAW 264.7 with 20 mM glucose group compared to the 5 mM glucose group (Figure 2(d) and (e)). Atherosclerosis is a chronic inflammatory process of the vessel wall, which is regulated by macrophages.22,23 RAW 264.7 with 20 mM glucose had increased expression of pro-inflammatory genes, such as tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and interleukin 1β (IL-1β), compared to those from 5 mM glucose group (Figure 2(f)–(h)). These findings suggest that high glucose might potentiate macrophage proinflammatory activation and foam cell formation.
Figure 2.
High glucose induces macrophage proinflammatory activation and foam cell formation. (a) Peritoneal macrophages were isolated from ApoE−/− mice. (b–c) Oil Red O staining of Peritoneal macrophages incubated with ox-LDL (50 μg/mL) for 24 h. Scale bar, 20 μm. (d–e) Oil Red O staining of RAW264.7 cultured with 5 mM glucose or 20 mM glucose and incubated with or without ox-LDL for a further 24 h. Scale bar, 20 μm. (f) Quantitative polymerase chain reaction was performed to detect the mRNA levels of TNF-α. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (g) Quantitative polymerase chain reaction was performed to detect the mRNA levels of IL-6. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (h) Quantitative polymerase chain reaction was performed to detect the mRNA levels of IL-1β. Two-way ANOVA with Bonferroni post-hoc test (n = 3).
COMMD1 deficiency accelerates the development of diabetic atherosclerosis
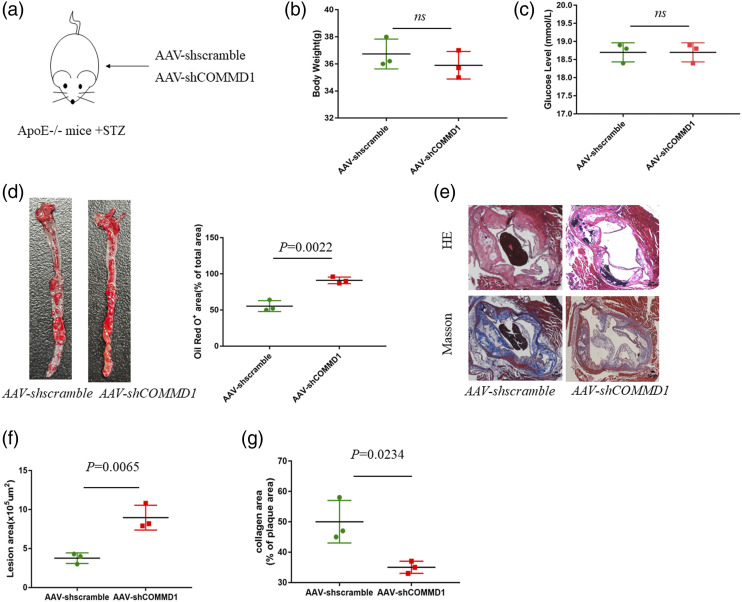
To further explore the role of COMMD1 in diabetic atherosclerosis, we constructed COMMD1-knockdown diabetic ApoE−/− mice (Figure 3(a)). Western blotting confirmed that the infection efficiency of AAV-shCOMMD1 was approximately 75–85% (Figure S1(a), (b)). The difference in body weight and blood glucose have no statistically significant in AAV-shscramble group and AAV-shCOMMD1 group (Figure 3(b) and (c)). Oil Red O staining of aortas revealed that the level of lipid accumulation in the whole aorta of AAV-shCOMMD1 group was increased relative to that of AAV-shscramble group (Figure 3(d)). Furthermore, H&E staining demonstrated that the atherosclerotic lesion area was increased in AAV-shCOMMD1 group. Masson staining revealed that the collagen content of atherosclerosis lesion in AAV-shCOMMD1 group was reduced compared to that in AAV-shscramble group (Figure 3(e)–(g)). These results indicate that the lack of COMMD1 accelerates the development of diabetic atherosclerosis.
Figure 3.
COMMD1 deficiency accelerates the development of atherosclerosis. (a) ApoE−/− mice were fed an HFD diet and treated with AAV-shscramble or AAV-shCOMMD1 for 24 weeks (b) Body weight (g) (n = 3), (c) Blood glucose (mmol/L) (n = 3). Unpaired two-tailed Student’s t-test. (d) Oil Red O staining of aortas from ApoE−/− mice (n = 3). Data represent the percentage of plaque area/total vessel area. Unpaired two-tailed Student’s t-test. (e) H&E staining (the first row) and Masson staining (the second row) of representative aortic root sections (n = 3). Scale bar, 50 μm. (f) Quantitative analysis of the lesion area. Unpaired two-tailed Student’s t-test. (g) Quantitative analysis of plaque collagen area. Unpaired two-tailed Student’s t-test.
Macrophage COMMD1 deficiency promotes glycolysis
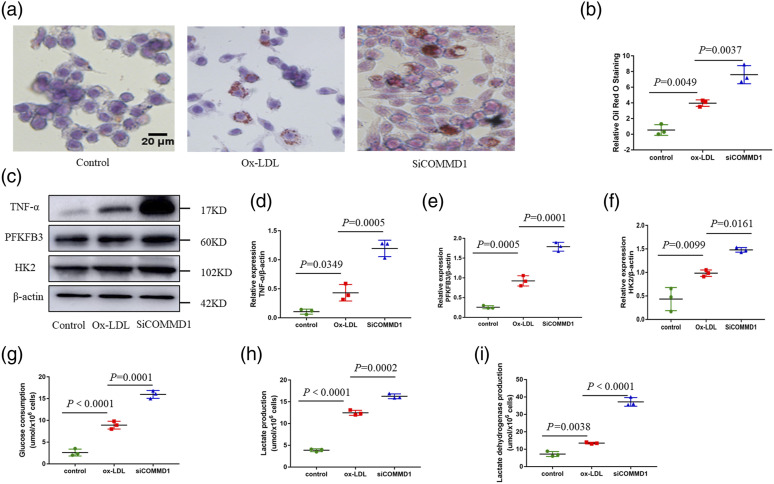
To investigate the functional role of macrophage COMMD1 in proinflammatory activation and foam cell formation, RAW 264.7 was transfected with small interfering RNA (siRNA) targeting COMMD1. We tested three different siRNA, of which we selected the one with the strongest knockdown efficiency evident at the protein level (Figure S1(c), (d)). Oil Red O staining showed that RAW264.7 with siRNA-mediated COMMD1 knockdown showed an increase in the levels of lipid uptake and accumulation compared with that of ox-LDL group (Figure 4(a) and (b)). These results suggest that COMMD1 is involved in the control of foam cell formation. In addition, RAW 264.7 with siRNA-mediated COMMD1 knockdown had increased expression of proinflammatory cytokines (TNF-α) compared to that ox-LDL group (Figure 4(c) and (d)).
Figure 4.
Macrophage COMMD1 deficiency promotes proinflammatory activation and glycolysis. RAW264.7 cells were transfected with siCOMMD1 for 48 h, and incubated with ox-LDL for a further 24 h in the presence of 20 mM glucose. (a–b) Oil Red O staining of RAW264.7 cells. Scale bar, 20 μm (n = 3). (c) Western blot analysis of TNF-α, PFKFB3, HK2, and β-actin expression at the protein level in RAW264.7. (d) Quantification of expression of inflammatory cytokines (TNF-α). Two-way ANOVA with Bonferroni post-hoc test (n = 3). (e) Quantification of expression of PFKFB3. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (f) Quantification of expression of HK2. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (g) Glucose consumption in RAW264.7. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (h) Lactate production in RAW264.7. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (i) lactate dehydrogenase production in RAW264.7. Two-way ANOVA with Bonferroni post-hoc test (n = 3).
We then investigated whether COMMD1 affects Cell metabolism. We detected the expression of several glycolytic genes, including Hexokinase 2 (HK2), 6-phosphofructo-2-kinase 3 (PFKFB3). We observed that the expression of the glycolytic proteins, including HK2 and PFKFB3, was increased upon RAW264.7 with siRNA-mediated COMMD1 knockdown (Figure 4(c), (e) and(f)). Meanwhile, we observed that glucose consumption, lactate production, and lactate dehydrogenase production of RAW264.7 with siRNA-mediated COMMD1 knockdown were significantly increased (Figure 4(g)-(i)). These observations indicate that COMMD1 influences glycolysis under diabetic atherosclerosis conditions.
2-DG decreases COMMD1 deficiency-promoted diabetic atherosclerosis in ApoE−/−mice and Macrophages
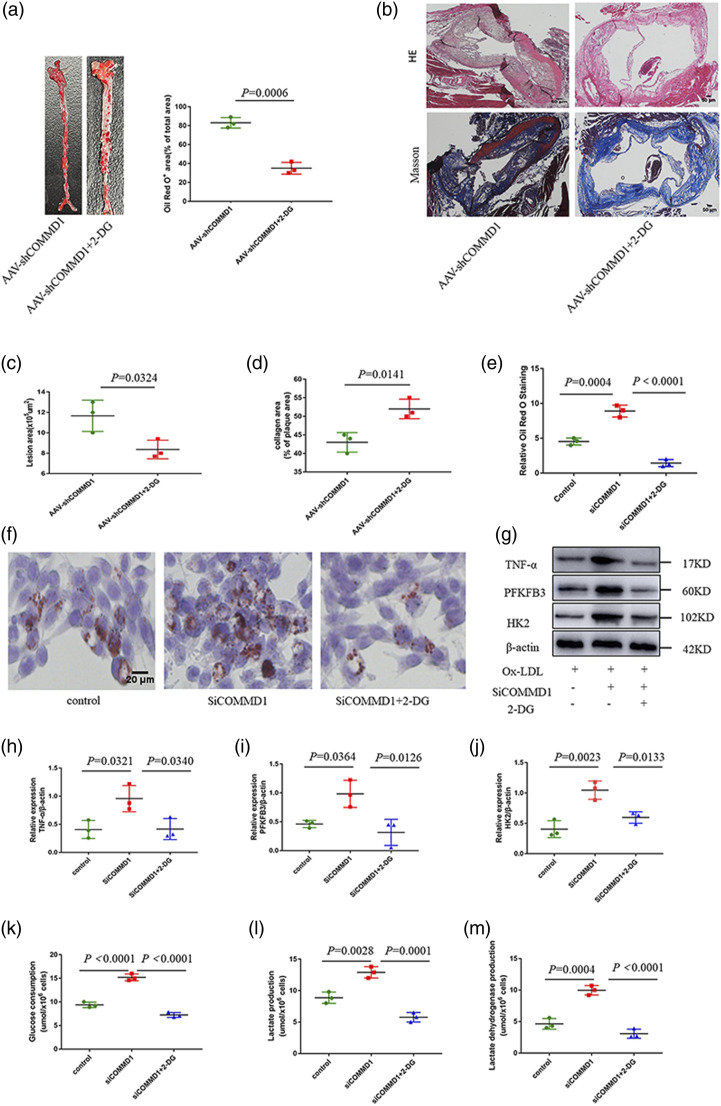
To examine if the glycolysis has a promoting effect in COMMD1 deficiency-promoted atherosclerosis in vivo and in vitro, 2-DG, which is a glycolysis inhibitor,24 was utilized. Oil Red O staining of aortas revealed that the level of lipid accumulation in the whole aorta of AAV-shCOMMD1+2-DG group was reduced relative to that of AAV-shCOMMD1 group (Figure 5(a)). Furthermore, HE staining and Masson staining also revealed that 2-DG treatment attenuated the development of the atherosclerotic lesion (Figure 5(b)–(d)).
Figure 5.
Inhibition of glycolysis reduces COMMD1 deficiency-promoted atherosclerosis. (a) Oil Red O staining of aortas from glycolysis inhibitor administrated ApoE−/− mice in the presence of AAV-shCOMMD1 fed HFD for 24 weeks (n = 3). Data represent the percentage of plaque area/total vessel area. Unpaired two-tailed Student’s t-test. (b) H&E staining (the first row) and Masson staining (the second row) of representative aortic root sections (n = 3). Scale bar, 50 μm. (c) Quantitative analysis of the lesion area. Unpaired two-tailed Student’s t-test. (d) Quantitative analysis of plaque collagen area. Unpaired two-tailed Student’s t-test. (e–f) Oil Red O staining of RAW264.7 cells incubated with 2-DG for 0.5 h, and transfected with siCOMMD1 for 48 h in the presence of 20 mM glucose and ox-LDL. (g) Western blot analysis of TNF-α, PFKFB3, HK2, and β-actin expression at the protein level in RAW264.7. (h) Quantification of expression of inflammatory cytokines (TNF-α). Two-way ANOVA with Bonferroni post-hoc test (n = 3). (i) Quantification of expression of PFKFB3. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (j) Quantification of expression of HK2. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (k) Glucose consumption in RAW264.7. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (l) Lactate production in RAW264.7. Two-way ANOVA with Bonferroni post-hoc test (n = 3). (m) lactate dehydrogenase production in RAW264.7. Two-way ANOVA with Bonferroni post-hoc test (n = 3).
Then, we sought to determine whether COMMD1 modulates glycolysis to mediate proinflammatory activation and foam cell formation in macrophages. RAW 264.7 was intervened with 2-DG. Oil Red O staining showed that the levels of lipid uptake and accumulation in siCOMMD1 + 2-DG group was reduced compared with that of siCOMMD1 group (Figure 5(e) and (f)). In addition, the expression of proinflammatory cytokines (TNF-α) and glycolytic proteins (HK2, PFKFB3) was decreased in siCOMMD1 + 2-DG group (Figure 5(g)–(j)). Moreover, we observed that glucose consumption, lactate production, and lactate dehydrogenase production of RAW264.7 were significantly decreased in siCOMMD1 + 2-DG group (Figure 5(k)–(m)).
Discussion
In the present study, we identified a novel and unexpected role of COMMD1 in diabetic atherosclerosis and attained several interesting findings. First, high glucose promotes macrophage proinflammatory activation and diabetes accelerate atherosclerosis. Second, COMMD1-deficient macrophages were found to enhance inflammation and foam cell formation in response to ox-LDL, and thus, exacerbated atherosclerosis. Third, COMMD1-deficient macrophages increase glycolytic activity, and 2-DG not only reversed the increase in glycolytic activity but also weakened the progression of atherosclerosis. These findings provide novel evidence for the supposition that COMMD1 is a possible therapeutic target for diabetic atherosclerosis intervention.
Atherosclerosis is tightly associated with chronic inflammation and excessive uptake of intracellular lipids.25,26 The mechanism of atherosclerosis is that inflammatory macrophages engulf ox-LDL and subsequently form foam cells.27 Among the known pathological mechanisms connecting diabetes and atherosclerosis is inflammation.28,29 This is consistent with our finding that high glucose caused by diabetes potentiates macrophage proinflammatory activation and foam cell formation. COMMD1 has a role in NF-κB signaling, which is related to inflammation.30,31 We showed that COMMD1 deficiency promoted inflammation and accelerated the development of diabetic atherosclerosis.
Recent studies have indicated that monocytes and macrophages metabolic and functional reprogramming plays a pivotal role in the development of atherosclerosis.32 However, the role and mechanism of macrophage metabolic reprogramming in diabetic atherosclerosis is not clear. In this study, COMMD1-deficient macrophages induced profound glycolytic activation and promoted inflammation, ultimately accelerating the process of diabetic atherosclerosis. Macrophage has two phenotypes traditionally including proinflammatory M1 macrophages and anti-inflammatory M2 macrophages, which are shaped largely by energy requirements.33,34 The possible reasons for the above result under diabetic atherosclerotic conditions may be explained by glycolysis serving as a metabolic adaptation in concert with a proportional upregulation of proinflammatory activity by the lack of COMMD1.
Inhibiting glycolysis recovered the inflammatory potential of macrophages associated with the lack of COMMD1. Our results showed that regulating metabolic states could be used to treat or even prevent diabetic atherosclerosis. Future studies using cell-specific COMMD1 knockout or over-expression mouse models are required to illustrate the role of COMMD1 in diabetic atherosclerosis. COMMD1 is involved in different cellular processes, especially copper metabolism.35 Further studies are needed to elucidate the detailed mechanism by which COMMD1 is regulated in macrophages in diabetic atherosclerosis and confirm the clinical implications of COMMD1 in diabetic atherosclerotic patients.
In summary, this study explored the mechanism by which diabetes enhanced the process of atherosclerosis. COMMD1-deficient macrophages regulated inflammation and glycolysis, thereby facilitating diabetic atherosclerosis. 2-DG reversed this effect. Our study confirms that COMMD1 may be a promising target for the treatment of diabetic atherosclerosis to inhibit early inflammatory and metabolic reprogramming and delay diabetic atherosclerotic progression.
Supplemental Material
Supplemental Material for Disruption of COMMD1 accelerates diabetic atherosclerosis by promoting glycolysis by Lili Zhang, Lihua Li, Yalan Li, Han Jiang, Zhen Sun, Guangyao Zang, Yongjiang Qian, Chen Shao and Zhongqun Wang in Diabetes and Vascular Disease Research
Authors’ contributions: Lili Zhang and Yalan Li designed the study. Han Jiang contributed to the animal data. Lihua Li assisted with getting mice tissues. Yongjiang Qian performed the data analysis. Zhen Sun, Guangyao Zang, Chen Shao wrote the paper. Zhongqun Wang supervised the study. All authors have read and approved final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (82070455); the related Foundation of Jiangsu Province (BK20201225, M2020016); Medical Innovation Team Project of Jiangsu Province (CXTDA2017010); the Open Project Program of Guangxi Key Laboratory of Centre of Diabetic Systems Medicine (GKLCDSM-20210101-02); Postgraduate Research &Practice Innovation Program of Jiangsu Province (KYCX20_3051, SJCX21_1729).
Data availability statement: All data used to support the findings of this study are available from the corresponding author upon request.
Ethical approval: All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committee.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Zhongqun Wang https://orcid.org/0000-0003-3590-9313
References
- 1.Virmani R, Burke AP, Kolodgie F. Morphological characteristics of coronary atherosclerosis in diabetes mellitus. Can J Cardiol 2006; 22(Suppl B): 81b–84b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res 2016; 118: 653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzo C, Delgado P, Busse CE, et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature 2021; 589: 287–292. [DOI] [PubMed] [Google Scholar]
- 5.Groh L, Keating ST, Joosten LAB, et al. Monocyte and macrophage immunometabolism in atherosclerosis. Semin Immunopathol 2018; 40: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edsfeldt A, Swart M, Singh P, et al. Interferon regulatory factor-5-dependent CD11c+ macrophages contribute to the formation of rupture-prone atherosclerotic plaques. Eur Heart J 2022; 9: ehab920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Back M, Yurdagul A,, Jr, Tabas I, et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 2019; 16: 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar L, Akbar N, Braithwaite AT, et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 2021; 144(12): 961–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q, Xu J, Ma Q, et al. PRKAA1/AMPKα1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nat Commun 2018; 9: 4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarrazy V, Viaud M, Westerterp M, et al. Disruption of Glut1 in hematopoietic stem cells prevents myelopoiesis and enhanced glucose flux in atheromatous plaques of ApoE(-/-) mice. Circ Res 2016; 118: 1062–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawakol A, Singh P, Mojena M, et al. HIF-1α and PFKFB3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol 2015; 35(6): 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niyonzima N, Rahman J, Kunz N, et al. Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Sci Immunol 2021; 6(66): eabf2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirai T, Nazarewicz RR, Wallis BB, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med 2016; 213(3): 337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata K, Fang C, Terao C, et al. Hypoxia-Sensitive COMMD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis. Immunity 2017; 47(1): 66–79.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riera‐Romo M. COMMD1: A multifunctional regulatory protein. J Cell Biochem 2018; 119(1): 34–51. [DOI] [PubMed] [Google Scholar]
- 16.Maine GN, Mao X, Komarck CM, et al. COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase. EMBO J 2007; 26(2): 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Sluis B, Groot AJ, Vermeulen J, et al. COMMD1 promotes pVHL and O2-independent proteolysis of HIF-1alpha via HSP90/70. PLoS One 2009; 4(10): e7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedoseienko A, Wijers M, Wolters JC, et al. The COMMD family regulates plasma LDL levels and attenuates atherosclerosis through stabilizing the CCC complex in endosomal LDLR trafficking. Circ Res 2018; 122(12): 1648–1660. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Tang X, Yao L, et al. Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J Clin Invest 2022; 132: e154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez D, Baylis RA, Durgin BG, et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med 2018; 24: 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraga-Silva RA, Seeman H, Montecucco F, et al. Apelin-13 treatment enhances the stability of atherosclerotic plaques. Eur J Clin Invest 2018; 48(3): e12891. [DOI] [PubMed] [Google Scholar]
- 22.Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res 2019; 124(2): 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 2010; 10(1): 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajak B, Siwiak E, Sołtyka M, et al. 2-Deoxy-d-glucose and its analogs: From diagnostic to therapeutic agents. Int J Mol Sci 2019; 21(1): 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S, Celermajer DS, Bao S. Atherosclerosis-underlying inflammatory mechanisms and clinical implications. Int J Biochem Cell Biol 2008; 40(4): 576–580. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Li W, Li X, et al. Inflammation: A novel therapeutic target/direction in atherosclerosis. Curr Pharm Des 2017; 23(8): 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng X, Chen W, Ni X, et al. Metformin, macrophage dysfunction and atherosclerosis. Front Immunol 2021; 12: 682853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poznyak A, Grechko AV, Poggio P, et al. The diabetes mellitus-atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci 2020; 21(5): 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paschou SA, Anagnostis P, Vryonidou A, et al. Diabetes and atherosclerosis: old players in a new field, osteoporosis. Curr Vasc Pharmacol 2018; 16(6): 524–527. [DOI] [PubMed] [Google Scholar]
- 30.Murata K, Fang C, Terao C, et al. Hypoxia-sensitive COMMD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis. Immunity 2017; 47(1): 66–79.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller PAJ, van de Sluis B, Groot AJ, et al. Nuclear-cytosolic transport of COMMD1 regulates NF-kappaB and HIF-1 activity. Traffic 2009; 10(5): 514–527. [DOI] [PubMed] [Google Scholar]
- 32.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res 2016; 118(4): 653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray PJ. Macrophage polarization. Annu Rev Physiol 2017; 79: 541–566. [DOI] [PubMed] [Google Scholar]
- 34.Peled M, Fisher EA. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol 2014; 5: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Wang T, Xiao Y, et al. COMMD1 upregulation is involved in copper efflux from ischemic hearts. Exp Biol Med 2021; 246(5): 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Disruption of COMMD1 accelerates diabetic atherosclerosis by promoting glycolysis by Lili Zhang, Lihua Li, Yalan Li, Han Jiang, Zhen Sun, Guangyao Zang, Yongjiang Qian, Chen Shao and Zhongqun Wang in Diabetes and Vascular Disease Research