Abstract
The impact of aerobic exercise training (AET) on cerebral blood flow (CBF) regulation remains inconclusive. This study investigated the effects of one-year progressive, moderate-to-vigorous AET on CBF, central arterial stiffness, and cognitive performance in cognitively normal older adults. Seventy-three older adults were randomly assigned to AET or stretching-and-toning (SAT, active control) intervention. CBF was measured with 2D duplex ultrasonography. Central arterial stiffness, measured by carotid β-stiffness index, was assessed with the ultrasonography and applanation tonometry. Cerebrovascular resistance (CVR) was calculated as mean arterial pressure divided by CBF. A cognitive battery was administered with a focus on memory and executive function. Cardiorespiratory fitness was measured by peak oxygen consumption ( O2peak). One-year AET increased O2peak and CBF and decreased CVR and carotid β-stiffness index. In the AET group, improved O2peak was correlated with increased CBF (r = 0.621, p = 0.001) and decreased CVR (r = −0.412, p = 0.037) and carotid β-stiffness index (r = −0.478, p = 0.011). Further, increased Woodcock-Johnson recall score was associated with decreased CVR (r = −0.483, p = 0.012) and carotid β-stiffness index (r = −0.498, p = 0.008) in AET group (not in SAT group). In conclusion, one-year progressive, moderate-to-vigorous aerobic exercise training increased CBF and decreased carotid arterial stiffness and CVR which were associated with improved memory function in cognitively normal older adults.
Keywords: Exercise training, central arterial stiffness, cerebral blood flow, neurocognitive function, older adults
Introduction
Cerebral hypoperfusion and central artery stiffening have been recognized as important risk factors for age-related cognitive decline and Alzheimer’s disease and related dementias (ADRD).1–3 Elevated central arterial stiffness exposes the cerebral small blood vessels to the augmented arterial pulsation (i.e., increases in arterial pressure and blood flow pulsatility) due to the reduction of its Windkessel effects4,5 which is referred to as the buffering effects of the large arterial wall to attenuate arterial pulsations generated from the heart. 6 Augmented arterial pulsation can lead to cerebral endothelial dysfunction and vasoconstriction;4,5 thereby, increasing cerebrovascular resistance (CVR) and decreasing cerebral blood flow (CBF) often observed in the aging brain.5,7,8 These changes in cerebral hemodynamics have been linked to brain atrophy, white matter hyperintensity (WMH, a measure of cerebral small blood vessel disease), and cognitive impairment in older adults.9–11 Conversely, pharmacological or non-pharmacological interventions to reduce central arterial stiffness may lead to improvement in cerebral perfusion which may prevent or slow the progression of age-related brain structural changes and cognitive decline and ADRD.4,8,12
Aerobic exercise training reduces central arterial stiffness in older adults. 13 However, at present, the effects of aerobic exercise training on CBF regulation and cognitive performance in older adults are inconclusive.14–18 Recently, we reported that one-year progressive moderate-to-vigorous aerobic exercise training reduces central arterial stiffness and increases global CBF in patients with mild cognitive impairment (MCI), a prodromal stage of ADRD. 19 Differences in the exercise protocol (intensity, frequency, and duration) and the study population (age, sex, pre-existing comorbidities, and cognitive status) may have led to inconsistent observations in previous studies.14,17,18
The purpose of this study was to investigate the effects of a one-year progressive, moderate-to-vigorous aerobic exercise training program on CBF and its associations with changes in central arterial stiffness and cognitive performance in cognitively normal older adults. We hypothesized that aerobic exercise training would increase CBF which is associated with reduction in central arterial stiffness and improvement in cognitive performance in older adults.
Materials and methods
Study design
The present study was a sub-study of a one-year, open-label, parallel randomized control trial (RCT) comparing the effects of progressive moderate-to-vigorous aerobic exercise training with stretching-and-toning (active control) on cognitive performance in cognitively normal older adults. 20 In the parent study, cognitive function and cardiorespiratory fitness were assessed at baseline, midpoint (6-month), and trial completion (12-month). Cardio- and cerebrovascular hemodynamic assessments and magnetic resonance imaging (MRI) were performed at baseline and trial completion (12-month). The detail of randomization procedures and trial design were reported previously. 20 Briefly, a stratified randomization by years of education and sex using a blocking factor of 4 was implemented after participants’ baseline measurements. The flowchart for the trial relevant to the present study is presented in Figure S1. All outcome measures (CBF, central arterial stiffness, and cognitive test scores) were blinded to the investigators who performed measurements and analyzed the data.
The sample size estimate for CBF measurement was not performed in the original parent study which focused on the effects of exercise on cognitive performance in older adults. 20 However, based on our pilot study which showed that aerobic exercise improved CBF in cognitively normal healthy older adults, 21 we anticipated that total CBF would increase by ∼27 ± 10 mL after one year of aerobic exercise compared with the stretching group. We analyzed total CBF using a repeated measures design with 1 between (Group: aerobic exercise vs. stretching) and 1 within effects (Time: baseline and 12 month). Twenty-one participants in 2 groups (42 total) achieves 5.8% power to detect an effect size of 0.041 for group, achieves 93.3% power to detect an effect size of 0.547 for Time, and achieves 97% power to detect an effect size of 0.610 for the interaction of group and time using a Geisser-Greenhouse Correct F test and a 5% significance level.
Participants
Nine-hundred ninety-one men and women aged between 60 and 80 years were recruited from the Dallas-Fort Worth metropolitan area using advertisements. Out of those individuals, 73 participants met the criteria for this study (Figure S1). Exclusion criteria were as follows: (1) clinical diagnosis of psychiatric or neurological disorders or taking medications that have major impacts on cognition, (2) mini-mental status examination (MMSE) score <26 to exclude dementia, (3) history of recurrent epilepsy, stroke, or traumatic brain injury with a loss of consciousness ≥30 minutes, (4) physically active (>90 minutes of moderate-to-vigorous physical activity per week), (5) uncontrolled hypertension (sitting systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg), (6) diagnosed diabetes mellitus (fasting glucose >126 mg/dL or taking antidiabetic medications), (7) history of cigarette smoking over the past 5 years, and (8) body mass index (BMI) ≥35 kg/m2. Screening procedures included a detailed medication history and medication questionnaire, a comprehensive physical examination, 12-lead electrocardiogram (ECG), echocardiography, and carotid artery ultrasonography to exclude severe carotid stenosis (>50%) and overt cardiovascular disease.
This study was approved by the Institutional Review Board of University of Texas Southwestern Medical Center, Texas Health Presbyterian Hospital Dallas, and University of Texas at Dallas in accordance with the guidelines of the Declaration of Helsinki and Belmont Report. All participants gave written informed consent before participation.
Intervention
All participants were randomly assigned to either aerobic exercise training or stretching-and-toning programs. Each participant was closely supervised by an exercise physiologist during the first 4 to 6 weeks of their training programs. Once participants were familiarized with the required exercises and demonstrated understanding of the exercise protocol, they performed the exercises safely by themselves either at a fitness center or home. All participants were instructed to perform the assigned interventions on top of their regular physical activities and record their exercises in written logs. To ensure adherence to aerobic exercise training interventions, participants were required to utilize a heart rate (HR) monitoring during each of the exercise sessions and to meet with the exercise physiologist monthly or as needed to review and resolve training-related issues in addition to keeping a detailed training log. The compliance to exercise training in the aerobic exercise training group was calculated as a ratio of the completed exercise sessions in which participants achieved the target HR over the prescribed exercise sessions. The compliance to stretching-and-toning intervention was calculated as a ratio of the self-reported exercise sessions in exercise logs over the prescribed exercise sessions.
Aerobic exercise training program
The progressive moderate-to-vigorous exercise program was based on each individual’s fitness level evaluated with peak oxygen consumption ( O2peak) using cardiorespiratory fitness testing. Exercise intensity, duration, and frequency were gradually increased as participants adapted to their exercise level. The program began with 3 exercise sessions per week for 25–30 minutes per session at the intensity of 75–85% of maximal HR which was measured during cardiorespiratory fitness testing at baseline. At week 11, three or four aerobic exercise sessions per week for 30–35 minutes per session were performed. In the week participants performed three exercise sessions per week, one high-intensity exercise session was introduced which consisted of 30 minutes of exercise at the intensity of 85–90% of maximal HR (e.g., brisk uphill walking). After week 26, participants performed four or five exercise sessions per week for 30–40 minutes, including two high-intensity sessions. Each exercise session included a 5-minute warm-up and a 5-minute cool-down period. The prescribed exercise program meets the national physical activity guidelines for older adults 22 and has been shown to significantly improve O2peak in sedentary older adults. 23
Stretching-and-toning program
The stretching-and-toning program was used as an active control group to maintain participants’ attention to a similar level as the aerobic exercise training group. The frequency and duration of the stretching-and-toning program were the same as the aerobic exercise training program. This program focused on the upper and lower limb stretching exercises. During each session, participants were asked to keep their HR below 50% of maximal HR from cardiorespiratory fitness testing. At week 19, a more advanced set of whole-body stretching exercises was prescribed. At week 26, a set of low resistance exercises performed with exercise elastic bands focused on strengthening the upper and lower body was prescribed.
Procedures and measurements
All data were collected in a quiet, environmentally controlled laboratory with an ambient temperature of ∼22°C. All participants were instructed to abstain from caffeinated beverages, alcohol, and high-intensity exercise for at least 24 hours prior to the visit of hemodynamics assessment and MRI. The same study protocols were used for baseline and trial completion (12-month).
Cardiorespiratory fitness testing
The O2peak was assessed by a modified Astrand-Saltin protocol on a treadmill. 24 During the cardiorespiratory fitness testing, participants walked or jogged at a fixed speed, which was determined by a submaximal test conducted before O2peak testing. 25 The treadmill grade was subsequently increased by 2% every 2 minutes until volitional exhaustion. Oxygen consumption ( O2) was measured during the 2nd minute of each stage using the Douglas bag method. Gas fractions were analyzed by mass spectrometry (Marquette MGA 1100, Michigan), and ventilatory volume was measured by a Tissot spirometer. HR was monitored continuously via ECG. The O2peak was defined as the highest O2 measured during the last stage of testing from a >40 seconds of Douglas bag collection. 25 The criteria used to suggest that O2peak was achieved included an increase in O2 < 150 mL despite increased work rate of 2% grade, a respiratory exchange ratio >1.1, and HR <5 beats per minute of age-predicted maximal values (220 – age). In all cases, at least two of these criteria were achieved, confirming the identification of O2peak based on the American College of Sports Medicine guideline. 22 Our previous studies have shown that by using these methods, O2peak can be measured reliably in sedentary older adults.23,25
Cardio- and cerebrovascular assessments
To ensure stable hemodynamics throughout cardio- and cerebrovascular assessments, HR using a 3-lead ECG (Hewlett-Packard, Palo Alto, CA, USA), and beat-to-beat arterial pressure were continuously recorded from the middle finger of the left hand using a Finapres (Finapres Medical Systems, Amsterdam, The Netherlands). CBF velocity was measured from the right middle cerebral artery (MCA) using a 2-MHz transcranial Doppler (TCD) probe (Multi-Dop X2, Compumedics/DWL, Singen, Germany) using standard procedures. 26 The probe was securely attached to the temporal bone acoustic window by using either an individually created mold to fit the facial bone structure or a probe holder (Spencer Technologies, Seattle, WA) to keep the position and angle of the probe unchanged. 27 The individually created probe mold ensured the same probe position and insonation angle during repeated CBF velocity measurements. 27 When a probe holder was used, the probe position where the optimal CBF velocity signal was obtained was measured carefully perpendicular to and from the eye and ear tragus line at baseline, and the same position was used for the repeated visit. End-tidal carbon dioxide (CO2) was monitored using capnography (Carpnograd, Novamatrix, Wallingford, CT). All physiological variables were continuously recorded with a sampling frequency of 1000 Hz using data acquisition software (Acknowledge, BIOPAC Systems, Goleta, CA, USA).
After participants were instrumented and rested in supine position for >15 minutes, 6-minutes of baseline hemodynamics was obtained. Then, brachial cuff blood pressure was measured intermittently at least three times using an ECG-gated electronic sphygmomanometer (Suntech, Morrisville, NC, USA) and averaged to obtain SBP and DBP. Beat-by-beat arterial pressure waveforms from the carotid, brachial, and femoral arteries were obtained for >13 seconds using applanation tonometry (SphygmoCor 8.0; AtCO2 Medical, West Ryde, NSW, Australia) at least twice. Mean arterial pressure (MAP) was calculated from the area under the curve of the brachial pressure waveform. The carotid pressure waveform was calibrated to the MAP and DBP to calculate carotid SBP.19,28 Carotid pulse pressure was calculated from the carotid SBP minus DBP. The carotid-femoral pulse wave velocity (cfPWV) was measured between the right carotid and the left femoral arteries at least twice and averaged according to a standard procedure. 29 The right common carotid artery (CCA) images were obtained at least 3 clips with a total of ≥15 complete cardiac cycles using a 3-to-12 MHz linear array transducer with duplex ultrasonography (CX50, Philips Ultrasound, Bothell, WA, USA). The systolic and diastolic CCA diameters were measured from a >10 mm segment of the near-wall media-adventitia layer to the far wall lumen-intima layer at 10–20 mm proximal to the carotid bifurcation using an edge-detection software (Vascular Tool 5; Medical Imaging Applications, Coralville, IA, USA).13,19,28 The carotid β-stiffness index, as a measure of the local carotid arterial stiffness adjusted for the distending pressure, was calculated by the following equation: 30
where Ds and Dd represent the systolic and diastolic diameters at CCA. Carotid intima-media thickness (CIMT) was assessed as a surrogate measure of carotid atherosclerotic severity. Briefly, a >10 mm segment of the far wall at the CCA was measured proximal to the carotid bifurcation at the end-diastolic phase by using the same edge-detection software above. 31
Cerebrovascular hemodynamics in both steady-state and pulsatile components were obtained by duplex ultrasonography (CX50, Philips Ultrasound, Bothell, WA, USA) and TCD (Multi-Dop X2: Compumedics/DWL, Singen, Germany). The former was used to obtain total CBF as a sum of volumetric blood flow measured from the bilateral internal carotid (ICA) and vertebral arteries (VA).19,28,32,33 At least 3 clips of bilateral ICA and VA images with a vessel length of >5 mm were obtained to measure the average arterial diameter over a timespan of >15 cardiac cycles; The diameter was measured using the same edge-detection software above. Further, time-averaged mean velocity (TAMV) of the total ≥15 completed and consecutive cardiac cycles were measured at the same location where the vessel diameters were measured. Volumetric blood flow in each artery was calculated by the following equation:19,28,32,33
Volumetric blood flow of the ICA and VA was summed from both the right and left sides. CVR was calculated as MAP divided by total CBF. To adjust for individual brain size differences, total CBF and CVR were normalized to brain tissue mass which was obtained based on MRI measurement of total brain tissue volume and an estimate of brain tissue density of 1.06 g/mL 34 and were represented as nCBF and nCVR, respectively. CBF velocity at the MCA using TCD was averaged for 6 minutes baseline and CVR index (CVRi) was calculated as MAP divided by CBF velocity. The pulsatility index (PI) was calculated to assess pulsatile components of CBF at the MCA. Briefly, continuous CBF velocity waveforms measured at the MCA by TCD, consisting of >15 cardiac cycles without artifact, were extracted. PI was calculated as systolic minus diastolic CFB velocity divided by mean CBF velocity.19,28
Brain tissue volume
All MRI data were collected by a 3-Tesla scanner (Philips Medical System, Best, The Netherlands) which uses a body coil for radiofrequency transmission and an 8-channel head coil with parallel imaging capability for signal reception. 3D magnetization-prepared rapid acquisition gradient echo (MPRAGE) pulse sequence was acquired to measure brain tissue volume using the following parameters: TE/TR = 3.7/8.1 ms, flip angle = 12°, FOV =256 × 256 mm, number of slices = 160 (no gap), resolution = 1 × 1 × 1 mm3, SENSE factor = 2, and scan duration = 4 min.
The FreeSurfer software (version 6.0, https://surfer.nmr.mgh.harvard.edu) was used for tissue segmentation to measure total gray matter (GM) and white matter (WM) volume. Total brain volume was calculated as a sum of measured cortical and subcortical GM and WM volumes, including brainstem and cerebellum19,28,33 and was used for normalization of CBF and CVR for individual differences in brain size.
Cognitive function assessment
A comprehensive battery of cognitive tests was administered to all participants. The cognitive test took ∼2 hours to complete. The domains of function tested included inductive reasoning, long-term episodic memory, working memory, processing speed, and verbal ability. Reasoning was assessed using the: Educational Testing Service (ETS) Letter Sets test, and the Raven’s Progressive Matrices test. Episodic memory was assessed using the Wechsler Memory Scale (WMS) Logical Memory Immediate and Delayed Recall subtests, and the Woodcock-Johnson (WJ) Memory for Names Immediate and Delayed Recall subtests. Processing speed was measured using the Digit Comparison test. Working memory was tested using the WMS Letter Number Sequencing test, and the Operation Span test. Verbal fluency was measured using the Controlled Oral Word Association – FAS test, and word knowledge was assessed using the ETS Vocabulary subtest. These tests were chosen for administration for credible validity, reliability, and sensitivity to cognitive decline in older adults based on our previous studies.35,36
Statistical analysis approach
The Chi-square test was used when comparing the ratio differences between groups in categorical variables. The Student’s t-test was used when comparing the means differences between groups at baseline and percentage changes from baseline to trial completion in continuous variables. Two-way repeated-measures analysis of variance (ANOVA) was performed on complete outcome data to examine the main effects of time and group as well as the interaction effect of time-by-group. Post-hoc multiple pairwise comparisons were corrected by the Bonferroni method in the case of a significant time-by-group interaction. Partial eta squared (ηp2) was calculated to represent effect sizes of significant terms. Pearson’s product-moment correlation analysis was performed to test linear associations between variables in aerobic exercise training and stretching-and-toning groups separately. Mediation analysis was performed to understand the relationship between aerobic exercise training-related changes in O2peak, carotid arterial stiffness, and cerebral hemodynamics.19,37 We used bootstrapping (5000 samples) to calculate bias-corrected 95% confidence intervals (CI) of the explained associations using the PROCESS statistical package. 37 The indirect effect of the mediation analysis was interpreted as significant if zero was not included in the 95% CI. 37 Data normality was assessed by the Shapiro-Wilk test and the visual inspection of histogram and Q-Q plots. Data are presented as mean ± standard deviation. Statistical significance was set a priori at p < 0.05 for post-hoc pairwise comparisons and correlation analysis. All statistical analyses were performed using SPSS 20.0 (IBM Corporation, Armonk, NY).
Results
Participants’ demographics at study completion
Among 73 participants enrolled in the trial (37 in stretching-and-toning and 36 in aerobic training), 56 participants (28 in stretching-and-toning and 28 in aerobic training) completed the study intervention with complete CBF and central arterial stiffness measurements. Of those who completed the trial, one participant in the stretching-and-toning group was excluded from data analysis because of development of severe atherostenosis in the right ICA at the trial completion. CBF velocity measured in the MCA with TCD was available in 43 participants (22 in stretching-and-toning and 21 in aerobic training) (Figure S1). The demographic characteristics of the participants who completed the cerebral hemodynamic assessments did not significantly differ from those who participated in the parent study (data not shown; p’s > 0.05). 20 There were no significant differences in baseline participant characteristics between groups, including distributions of sex and race, age, years of education, baseline MMSE score, body mass, BMI, the usage rate of antihypertensive drug, systemic hemodynamics, and O2peak measured at baseline (Table 1), demonstrating group equivalence at the study beginning.
Table 1.
Baseline participant demographics by groups.
| Variables | Stretching-and-toning | Aerobic exercise training | p-value |
|---|---|---|---|
| n (men/women) | 27 (8/19) | 28 (7/21) | 0.879 |
| Age (years) | 67.8 ± 4.9 | 68.2 ± 5.3 | 0.773 |
| Race (White/Black) | 26/1 | 28/0 | 0.304 |
| Education (years) | 15.9 ± 1.8 | 16.7 ± 2.2 | 0.153 |
| Mini-Mental State Exam | 29.2 ± 0.8 | 29.3 ± 0.9 | 0.661 |
| Height (cm) | 165.6 ± 8.4 | 165.6 ± 8.1 | 0.991 |
| Body mass (kg) | 73.5 ± 11.0 | 69.6 ± 12.4 | 0.469 |
| Body mass index (kg/m2) | 26.8 ± 3.6 | 25.8 ± 3.9 | 0.311 |
| Hypertension, n (%) | 3 (11) | 6 (21) | 0.301 |
| Systolic BP (mmHg) | 114.1 ± 11.6 | 116.0 ± 11.6 | 0.541 |
| Diastolic BP (mmHg) | 69.5 ± 5.9 | 68.5 ± 7.3 | 0.581 |
| Heart rate (bpm) | 61.5 ± 7.3 | 62.6 ± 7.4 | 0.560 |
| O2peak (mL/kg/min) | 21.7 ± 3.5 | 22.7 ± 3.7 | 0.205 |
Data are mean ± standard deviation unless otherwise noted. BP, blood pressure; bpm, beats per minute; O2peak, peak oxygen consumption.
Intervention effects: Group differences in cardiorespiratory fitness and systemic and carotid hemodynamic measurements
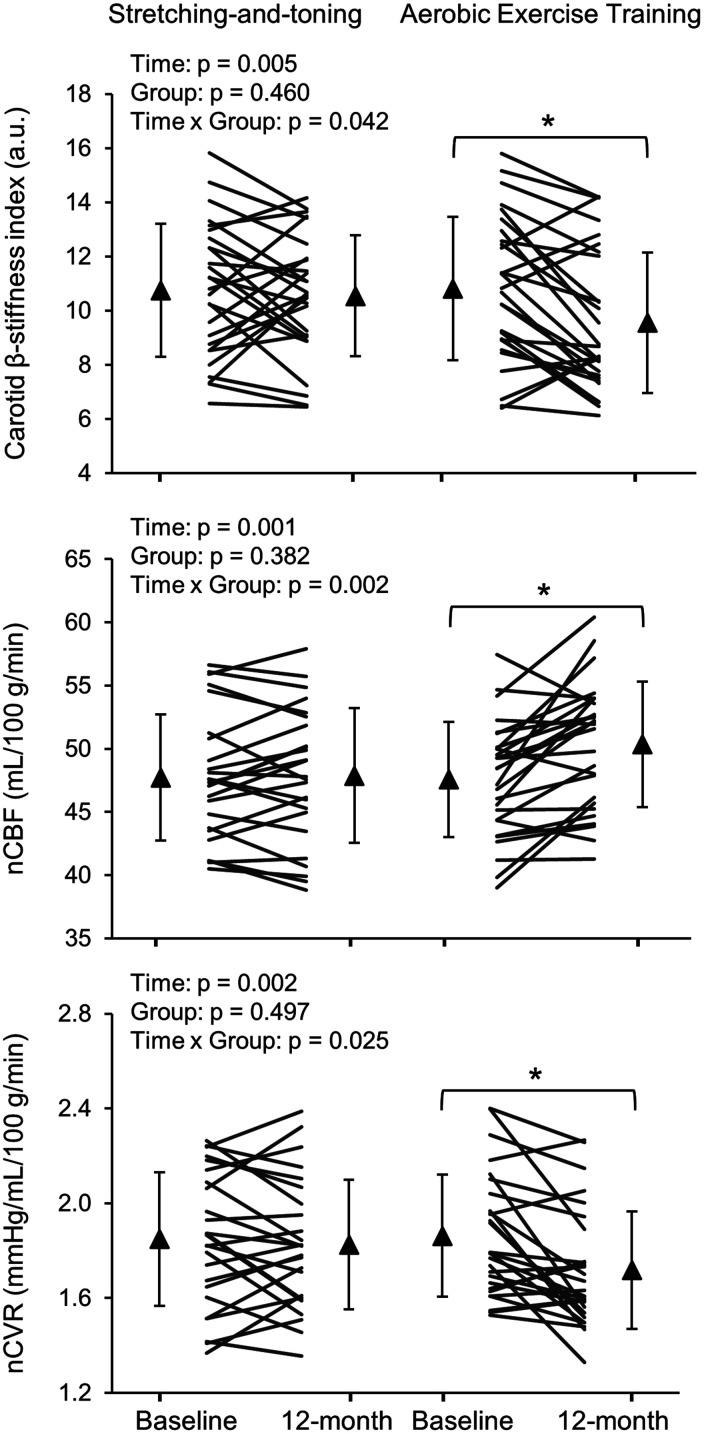
The average compliance to exercise training was 80 ± 14% in the aerobic training group and 77 ± 25% in the stretching-and-toning group, a nonsignificant difference. Repeated measures ANOVA with main effects of Time (baseline and follow-up), Group (aerobic training and stretching-and-toning), and a time-by-group interaction as independent variables predicting the dependent variables of interest were conducted. Specifically, main effect of Time on O2peak showed that this metric improved from baseline to follow-up, regardless of group. O2peak demonstrated main effect of Time, but also a time-by-group interaction, revealing that this metric improved at 12-months for aerobic training more than stretching-and-toning (ηp2 = 0.083) (%change of O2peak (mL/kg/min): 10 ± 12% vs. 4 ± 9%, p = 0.040) (Table 2). Body mass was reduced by ∼1.0% in the SAT and by ∼1.4% in the aerobic training group after one year. No time and group interactions were observed (Table 2). For heart rate, a time-by-group interaction indicated that only for the aerobic training group, heart rate decreased over time. Importantly, a significant time-by-group interaction revealed that the aerobic training group decreased their carotid β-stiffness index over the 12-months significantly more than did the stretching-and-toning group (ηp2 = 0.076) (%change of carotid β-stiffness index: −10 ± 17% vs. 1 ± 20%, p = 0.039) (Table 2 and Figure 1).
Table 2.
Cardiorespiratory fitness and systemic and carotid hemodynamic measurements by groups.
| Stretching-and-toning |
Aerobic exercise training |
Effect p-values |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 12-month | Baseline | 12-month | Time | Group | Time × Group | |
| O2peak | |||||||
| (mL/min) | 1.58 ± 0.27 | 1.62 ± 0.27 | 1.59 ± 0.44 | 1.70 ± 0.44 | 0.001 | 0.625 | 0.130 |
| (mL/kg/min) | 21.7 ± 3.5 | 22.5 ± 3.8 | 22.7 ± 3.7 | 24.9 ± 4.0*† | <0.001 | 0.088 | 0.034 |
| Body mass (kg) | 73.5 ± 11.0 | 72.8 ± 10.9 | 69.6 ± 12.4 | 68.6 ± 13.1 | 0.023 | 0.219 | 0.643 |
| Systemic hemodynamics | |||||||
| Heart rate (bpm) | 61.5 ± 7.3 | 63.2 ± 8.7 | 62.6 ± 7.4 | 60.2 ± 7.5* | 0.670 | 0.644 | 0.005 |
| Systolic BP (mmHg) | 114.1 ± 11.6 | 114.7 ± 12.1 | 116.0 ± 11.6 | 113.8 ± 10.0 | 0.613 | 0.842 | 0.380 |
| MAP (mmHg) | 87.6 ± 7.7 | 86.2 ± 8.2 | 87.3 ± 8.1 | 85.5 ± 6.9 | 0.122 | 0.803 | 0.823 |
| Diastolic BP (mmHg) | 69.5 ± 5.9 | 68.2 ± 6.9 | 68.5 ± 7.3 | 67.9 ± 7.1 | 0.271 | 0.686 | 0.696 |
| Carotid hemodynamics | |||||||
| Systolic BP (mmHg) | 113.0 ± 12.6 | 112.5 ± 11.8 | 113.2 ± 13.0 | 110.0 ± 9.6 | 0.270 | 0.676 | 0.426 |
| Pulse pressure (mmHg) | 43.5 ± 10.0 | 44.2 ± 11.8 | 44.7 ± 10.5 | 42.1 ± 8.0 | 0.489 | 0.843 | 0.213 |
| β-stiffness index (a.u.) | 10.75 ± 2.47 | 10.55 ± 2.23 | 10.81 ± 2.64 | 9.56 ± 2.60* | 0.005 | 0.460 | 0.042 |
| cfPWV (m/sec) | 8.99 ± 1.80 | 8.89 ± 1.41 | 8.99 ± 1.95 | 8.63 ± 1.99 | 0.264 | 0.774 | 0.540 |
| IMT (mm) | 0.71 ± 0.13 | 0.73 ± 0.13 | 0.69 ± 0.09 | 0.68 ± 0.10 | 0.665 | 0.245 | 0.138 |
Data are mean ± standard deviation. Effects are terms reported from individual ANOVA models. BP: blood pressure; bpm: beats per minute; cfPWV: carotid-femoral pulse wave velocity; IMT: intima-media thickness; MAP: mean arterial pressure; O2peak: peak oxygen consumption. Bold values represent p < 0.05 for each of individual ANOVA models. * represents p < 0.05 compared with baseline and † represents p < 0.05 compared with stretching-and-toning after the Bonferroni correction.
Figure 1.
Changes in carotid β-stiffness index (upper panel), normalized cerebral blood flow (nCBF) (middle panel), and normalized cerebrovascular resistance (nCVR) (lower panel) after one-year stretching-and-toning or aerobic exercise training. Thin lines represent individual changes. Triangles show mean values and the error bars represent standard deviations. ∗p < 0.05 compared with baseline after Bonferroni correction.
Intervention effects: Group differences in cerebral hemodynamic measurements and brain tissue volumes
From the repeated measures ANOVAs conducted on cerebral and brain volume variables, Time, Group, and time-by-group terms again served as predictors. Figure S2 shows representative ultrasonography images of blood flow measurements in the ICA and VA at baseline and 12-month from one subject in the aerobic exercise training group. Significant time-by-group interactions revealed that aerobic training increased total CBF (ηp2 = 0.128) (%change of total CBF: 5 ± 7% vs. 0 ± 5%, p = 0.007) and nCBF (ηp2 = 0.172) (%change of nCBF: 6 ± 7% vs. 0.3 ± 5%, p = 0.002) and decreased nCVR (ηp2 = 0.097) (%change of nCVR: −7 ± 10% vs. −1 ± 9%, p = 0.022) at 12-months compared to stretching-and-toning (Table 3 and Figure 1 and S3). Total CVR showed reductions over time in both groups. Of note, volumetric blood flow of the ICA (summed from both the right and left sides) increased more after aerobic training than after stretching-and-toning (ηp2 = 0.115) (% change of volumetric blood flow of the ICA: −7 ± 11% vs. −1 ± 7%, p = 0.007). However, volumetric blood flow, the vessel diameter, and velocity in each of the ICA and VA vessels did not show statistically significant changes after either aerobic training or stretching-and-toning (Table S1). There were time effects of increases in CBF velocity at the MCA and decreases in CVRi for both groups, but no time-by-group interactions after the one-year stretching-and-toning or aerobic training (Table 3). No intervention effect was found for PI. Total brain and GM (but not WM) volumes decreased equally in both groups (Table 3 and Figure S3).
Table 3.
Cerebral hemodynamic measurements and brain tissue volume by groups.
| Stretching-and-toning |
Aerobic exercise training |
Effect p-values |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 12-month | Baseline | 12-month | Time | Group | Time ×Group | |
| Global volumetric CBF and CVR | |||||||
| Total CBF (mL/min) | 600.9 ± 70.1 | 599.5 ± 66.3 | 584.7 ± 61.2 | 611.1 ± 60.2* | 0.015 | 0.889 | 0.007 |
| ICA (mL/min) | 441.0 ± 64.5 | 434.7 ± 66.3 | 431.8 ± 62.1 | 453.2 ± 58.9* | 0.157 | 0.774 | 0.011 |
| VA (mL/min) | 159.9 ± 42.8 | 164.8 ± 41.7 | 152.9 ± 42.5 | 157.8 ± 46.7 | 0.162 | 0.535 | 0.989 |
| nCBF (mL/100 g/min) | 47.7 ± 5.0 | 47.9 ± 5.3 | 47.6 ± 4.6 | 50.4 ± 5.0* | 0.001 | 0.382 | 0.002 |
| CVR (mmHg/mL/min) | 0.148 ± 0.021 | 0.145 ± 0.020 | 0.151 ± 0.020 | 0.141 ± 0.017 | 0.009 | 0.917 | 0.096 |
| nCVR (mmHg/mL/100 g/min) | 1.85 ± 0.28 | 1.83 ± 0.27 | 1.86 ± 0.26 | 1.72 ± 0.25* | 0.002 | 0.497 | 0.025 |
| CBF velocity at the middle cerebral artery | |||||||
| CBF velocity (cm/sec) | 54.4 ± 12.2 | 55.7 ± 11.4 | 50.7 ± 10.9 | 53.1 ± 10.2 | 0.016 | 0.359 | 0.458 |
| CVRi (mmHg/cm/sec) | 1.67 ± 0.41 | 1.62 ± 0.41 | 1.77 ± 0.42 | 1.66 ± 0.35 | 0.022 | 0.594 | 0.343 |
| Pulsatility index (%) | 98.5 ± 12.4 | 102.2 ± 16.3 | 100.9 ± 12.5 | 101.1 ± 13.1 | 0.117 | 0.877 | 0.151 |
| End-tidal CO2 (mmHg) | 37.1± 3.1 | 36.2 ± 3.3 | 36.7 ± 3.9 | 36.5 ± 3.1 | 0.152 | 0.968 | 0.353 |
| Brain tissue volume (mL) | |||||||
| Total brain | 1185 ± 85 | 1179 ± 87 | 1165 ± 106 | 1155 ± 110 | 0.005 | 0.419 | 0.396 |
| Total GM | 691 ± 40 | 683 ± 38 | 679 ± 51 | 676 ± 53 | 0.004 | 0.443 | 0.307 |
| Total WM | 473 ± 54 | 475 ± 57 | 465 ± 59 | 459 ± 60 | 0.455 | 0.462 | 0.081 |
Data are mean ± standard deviation. Effects are terms reported from individual ANOVA models. CBF: cerebral blood flow; CVR: cerebrovascular resistance; CVRi: CVR index; GM: gray matter; ICA: internal carotid artery; nCBF and nCVR: normalized CBF and CVR by total brain tissue mass measured by magnetic resonance imaging; VA: vertebral artery; WM: white matter. Note that CBF velocity at the middle cerebral artery was available for 22 participants in stretching-and-toning and 21 in aerobic exercise training groups. Bold values represent p < 0.05 for each of individual ANOVA models. * represents p < 0.05 compared with baseline after the Bonferroni correction for post hoc comparisons.
Intervention effects: Group differences in cognitive performance
Results from the repeated measures ANOVAs for the cognitive variables were more homogenous. No intervention group effects or time-by-group interactions were found for the cognitive variables. However, most cognitive variables, including tests of reasoning, episodic memory, and verbal abilities showed small but statistically significant increases in cognitive performance over time, regardless of group (Table 4).
Table 4.
Neurocognitive testing scores by groups.
| Stretching-and-toning |
Aerobic exercise training |
Effect p-values |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 12-month | Baseline | 12-month | Time | Group | Time × Group | |
| ETS letter sets | 15.0 ± 5.6 | 15.9 ± 5.5 | 16.5 ± 4.1 | 17.9 ± 4.2 | 0.027 | 0.159 | 0.663 |
| PRM accuracies | 0.78 ± 0.14 | 0.82 ± 0.14 | 0.83 ± 0.11 | 0.85 ± 0.10 | 0.003 | 0.242 | 0.315 |
| LM immediate recalls | 27.6 ± 6.4 | 30.3 ± 5.3 | 27.4 ± 5.7 | 31.9 ± 5.4 | <0.001 | 0.590 | 0.237 |
| LM delayed recalls | 24.9 ± 6.9 | 28.1 ± 6.0 | 24.8 ± 6.4 | 30.5 ± 5.7 | <0.001 | 0.456 | 0.138 |
| WJ immediate recalls | 50.7 ± 8.0 | 58.7 ± 9.3 | 51.0 ± 12.0 | 61.2 ± 9.2 | <0.001 | 0.579 | 0.291 |
| WJ delayed recalls | 21.1 ± 7.6 | 27.5 ± 6.9 | 19.9 ± 9.1 | 27.4 ± 7.7 | <0.001 | 0.753 | 0.532 |
| Digit comparison | 54.8 ± 10.5 | 54.5 ± 10.1 | 57.0 ± 8.6 | 57.6 ± 10.0 | 0.885 | 0.328 | 0.521 |
| Letter number sequencing | 10.5 ± 2.0 | 10.7 ± 1.8 | 10.6 ± 2.2 | 10.4 ± 2.8 | 0.876 | 0.821 | 0.476 |
| Operation span | 14.8 ± 5.4 | 16.2 ± 6.5 | 13.2 ± 6.6 | 16.4 ± 7.4 | 0.014 | 0.685 | 0.324 |
| COWAT-FAS | 37.7 ± 10.6 | 44.6 ± 13.3 | 34.3 ± 8.3 | 39.5 ± 8.4 | <0.001 | 0.125 | 0.361 |
| ETS vocabulary | 21.0 ± 6.9 | 21.6 ± 7.4 | 20.2 ± 4.9 | 21.1 ± 5.9 | 0.019 | 0.719 | 0.735 |
Data are mean ± standard deviation. COWAT-FAS: Controlled Word Association FAS; ETS: educational testing service; LM: logical memory; RPM: Raven’s Progressive Matrices; WJ: Woodcock-Johnson. Higher cognitive test scores indicate better performance. Bold values represent p < 0.05 for each of individual ANOVA models.
Associations among changes in O2peak, Central arterial stiffness, cerebral hemodynamics, and cognitive performance
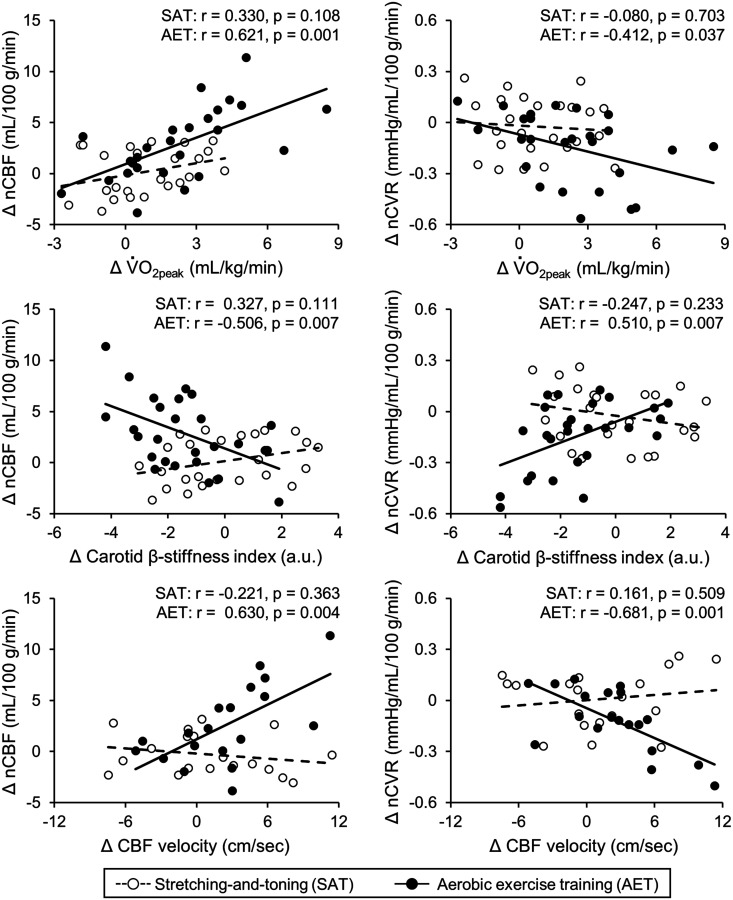
Inspection of zero-order Pearson correlations for each group suggested that increased O2peak was associated with increased nCBF, decreased nCVR (Figure 2), and reduced carotid β-stiffness index (aerobic training: r = −0.478, p = 0.011; stretching-and-toning: r = 0.217, p = 0.277) in the aerobic training group but not in the stretching-and-toning group. Further, decreases in carotid β-stiffness index were associated with increased nCBF and decreased nCVR in the aerobic training group (Figure 2). Based on these observations, we examined whether changes in the carotid β-stiffness index mediate the associations between changes in O2peak and nCBF or nCVR (Figure S4). The carotid β-stiffness index in the mediation path attenuated the negative association between O2peak and nCVR. This result was further confirmed by a bootstrapping assessment, which demonstrated significant indirect effects of the carotid β-stiffness index on nCVR (95% CI: −0.005 to -0.037). Furthermore, decreased carotid β-stiffness index was associated with decreased PI in both groups (Table S2), and increased CBF velocity at the MCA and decreased PI were associated with increased nCBF and decreased nCVR in the aerobic training group (Table S2 and Figure 2).
Figure 2.
Correlations of changes in peak oxygen consumption (V̇O2peak: upper panel), carotid β-stiffness index (middle panel), and mean cerebral blood flow (CBF) velocity measured at the middle cerebral artery (MCA, lower panel) with normalized CBF (nCBF: left side panels) and normalized cerebrovascular resistance (nCVR: right side panels) in the stretching-and-toning and aerobic exercise training groups. Note that the mean CBF velocity at the MCA was available 22 in stretching-and-toning group and 21 in aerobic exercise training group.
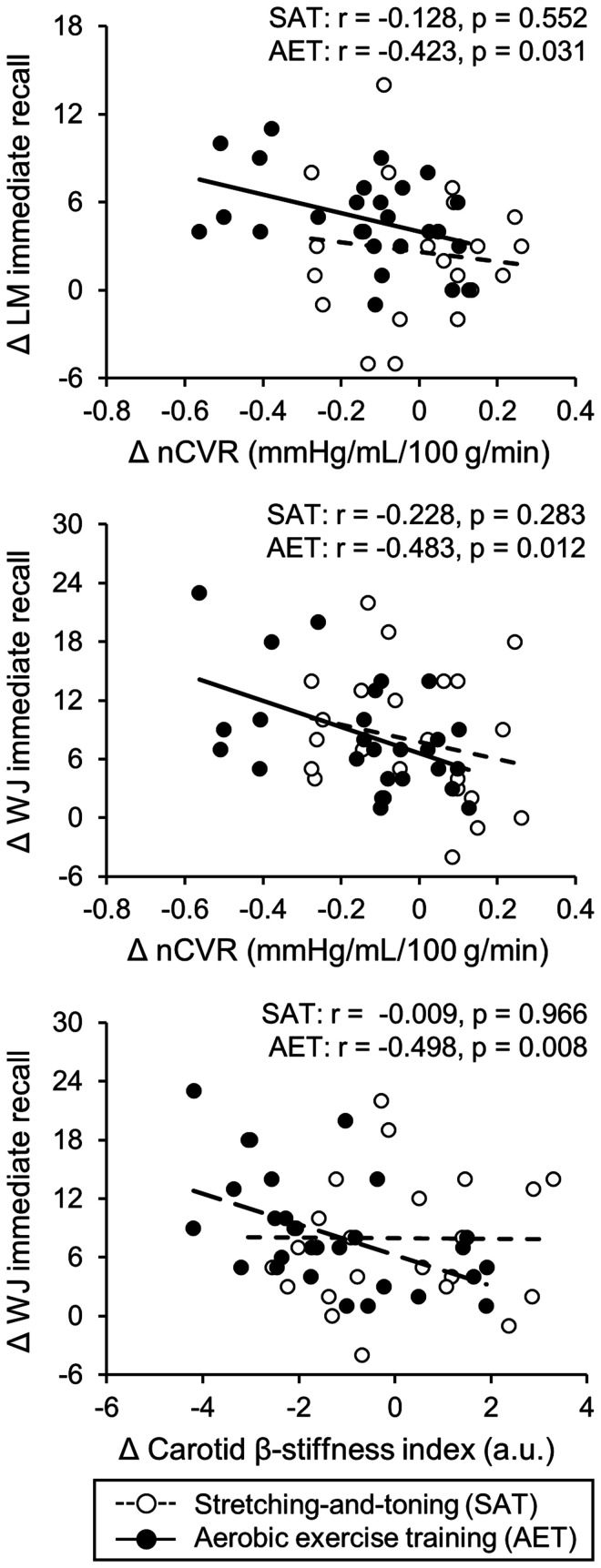
Further correlation analysis by groups revealed that individual changes in carotid and cerebral hemodynamics after the one-year intervention were associated with the changes in cognitive test scores (Table S3 and Figure 3). Notably, decreased nCVR and carotid β-stiffness index were associated with improvements in the Logical Memory and Woodcock-Johnson immediate recall test scores in the aerobic training group but not in the stretching-and-toning group (Figure 3).
Figure 3.
Correlations of changes in normalized cerebrovascular resistance (nCVR) with logical memory (LM) immediate recall (upper panel) and Woodcock-Johnson (WJ) immediate recall (middle panel) and changes in carotid β-stiffness index with WJ immediate recall (lower panel) in the stretching-and-toning and aerobic exercise training groups.
Discussion
This study revealed several interesting findings. First, one-year progressive moderate-to-vigorous aerobic exercise training increased global CBF while decreasing CVR and carotid arterial stiffness in cognitively normal older adults. Second, improved cardiorespiratory fitness assessed by O2peak was associated with increased CBF, decreased CVR, and reduced carotid arterial stiffness in the aerobic exercise training group. This association suggests the presence of a potential “dose-response” relationship between changes in cardiorespiratory fitness, CBF, and arterial stiffness. Furthermore, reduced carotid arterial stiffness was associated with increased CBF and decreased CVR, and mediation analysis showed that the negative association between changes in O2peak and CVR was mediated by the reduction of carotid arterial stiffness. Third, there were small but statistically significant improvements in cognitive performance (mainly memory function) in both groups after one-year intervention, and the improvements in the memory function (Logical Memory and Woodcock-Johnson immediate recall) were associated with reductions in carotid arterial stiffness and CVR in the aerobic exercise training group but not in the stretching-and-toning group. Collectively, these findings demonstrated the beneficial effects of one-year aerobic exercise training on increasing CBF and reducing CVR in cognitively normal older adults which are likely mediated by reduction in carotid arterial stiffness and may improve cognitive performance. We must acknowledge that a causal relationship cannot be determined with either an association or mediation analysis. With this caution in mind, we discuss the potential underlying mechanisms and clinical implications of these findings below.
Effects of aerobic exercise training on cerebral blood flow
At present, the beneficial effects of aerobic exercise training on CBF are inconclusive.14,17 The methodological changes in CBF measurement may partially explain the inconsistent findings.14,17 A recent systematic review and meta-analysis of the effects of cardiorespiratory fitness and aerobic exercise training on CBF reported that higher cardiorespiratory fitness was associated with higher CBF velocity measured at the MCA using TCD among older adults in cross-sectional studies. However, interventional studies with moderate intensity of aerobic exercise training for a duration of 2–12 months had little effect on the MCA CBF velocity and global cerebral perfusion measured using MRI arterial spin labeling (ASL). 17 One major limitation in measuring changes in CBF using TCD is that changes in CBF velocity do not necessarily equal changes in volumetric CBF. 38 In addition, measurements of global cerebral perfusion using ASL are limited by a low signal/noise ratio in the white matter and some arbitrary model parameter assumptions which may be altered by aerobic exercise training or stretching-and-toning. 39 In this study, we did not observe time-by-group interactions of changes in CBF velocity at the MCA consistent with previous findings. 17 However, compared with stretching-and-toning, aerobic exercise training increased global CBF measured as the sum of volumetric blood flow from both the ICA and VA using 2D color-coded duplex ultrasonography which may overcome the limitations of measurement of global CBF using TCD and ASL.32,33
The differences in the study design (e.g., single-arm vs. two-arm trial), exercise protocol (e.g., mode, frequency, intensity, and duration), or study population characteristics (e.g., healthy adults, older adults, middle-aged, and patients with MCI) may also influence the aerobic exercise training effects on CBF.14,17 In this regard, vigorous-intensity aerobic exercise training has been recommended to improve CBF because it was argued that the benefit of aerobic exercise training on CBF may only be manifested when O2peak is improved.17,40 For example, a single-arm, 6-month progressive moderate-to-vigorous aerobic exercise training increased maximal O2 by ∼8% and slightly but significantly increased CBF velocity measured at the MCA in cognitively normal older adults. 41 Further, a 3-month progressive moderate-to-vigorous aerobic exercise training increased O2peak by ∼6% and increased regional cerebral perfusion measured with ASL in the anterior cingulate cortex compared with control group in healthy older adults. 42 We also observed that one-year progressive moderate-to-vigorous aerobic exercise training increased O2peak by ∼9% and increased global CBF measured with ultrasonography 19 and regional cerebral perfusion in the anterior cingulate cortex measured with ASL in patients with MCI. 43
In this study, aerobic exercise training increased O2peak by ∼10%, global CBF by ∼5%, and decreased CVR by ∼7% relative to baseline. Importantly, increases in O2peak were associated with increases in CBF and reductions in CVR. These observations suggest the presence of a “dose-response” relationship between changes in O2peak, CBF, and CVR. Moreover, the findings that aerobic exercise training increased global CBF can be attributed mainly to the increase in the ICA blood flow and are consistent with the observations of increased regional CBF in the anterior and middle parts of the brain in previous studies.42–45
Associations between carotid arterial stiffness and CBF
Regular physical activities ameliorate age-related arterial stiffening in healthy older adults.13,46,47 We can only speculate on possible mechanisms. Arterial stiffening is determined mainly by the elastin and collagen contents as well as the smooth muscle tone of the arterial wall. 47 The elastin-collagen composition of the arterial wall represents a major component of arterial stiffness and is unlikely to be altered with short-term aerobic exercise training. 47 In this regard, several previous studies of 3 - 4 months of aerobic exercise training suggested that reduced vascular smooth muscle tone related to improved endothelial function, decreased sympathetic neural activity, or enhanced sympatholysis mediated by nitric oxide may be the underlying mechanism of exercise-related reduction of central arterial stiffness in older adults.47,48 Thus, if aerobic exercise training reduced carotid artery and large cerebral artery smooth muscle tone which then would lead to reduction in CVR and increases in global CBF observed in the present study.16,48
The findings that aerobic exercise training increased CBF and decreased CVR were associated with reduced carotid arterial stiffness also support the Windkessel effect hypothesis of CBF regulation.4,5,12,48 The wall of large central elastic arteries (e.g., aorta and carotid artery) expands and recoils within each cardiac cycle to attenuate arterial pulsations from the heart in order to maintain continuous blood flow into the peripheral vascular beds, which is referred to as the Windkessel effects. 6 It has been proposed that impaired Windkessel effects and elevated arterial pulsation may cause cerebral endothelial dysfunction, increase cerebrovascular tone (i.e., increased CVR), and reduced CBF.5,7,8 Thus, aerobic exercise training decreased-carotid arterial stiffness may lead to reductions in arterial pulsation as reflected by the attenuated CBF PI in the aerobic exercise training groups and improvement in endothelial function and CBF (Table S2 and Figure 2). Further mediation analysis also shows that aerobic exercise training-induced reduction of CVR, reflecting improved endothelial function, is related to the reduction in carotid arterial stiffness (Figure S4). Finally, we observed that changes in CBF velocity in the MCA were associated positively with changes in nCBF and negatively with changes nCVR in the aerobic exercise training group, suggesting that increases in the cerebral blood vessel wall shear stress may lead to nitric oxide mediated vasodilation and improvement in CBF (Table S2 and Figure 2). In addition, exercise-induced increases in the capillary density 49 and/or increases in cerebral metabolic rate of oxygen may also contribute to increased CBF through neurovascular coupling. 14 Future studies are needed to understand the underlying molecular and cellular mechanisms of increased CBF after aerobic exercise training.
Cognitive performance and brain volume
There is a significant knowledge gap in our understanding of the potential influence of aerobic exercise training-related changes in central arterial stiffness and CBF on cognitive decline or dementia prevention.14,48 Increases in central arterial stiffness,8–10 brain hypoperfusion, 2 and elevated CVR 3 have been recognized as strong predictors of cognitive decline and development of ADRD. Population-based studies reported that lower CBF and higher CVR and central arterial stiffness in older adults are associated with accelerated cognitive decline and increased the risk of dementia.2,3,11 These findings suggest that improving cardio- and cerebrovascular function may prevent and slow cognitive decline and reduces the risk of dementia. There is an increasing recognition that aerobic exercise training may improve neurocognitive function in middle-aged and older adults in addition to the amelioration of cardio- and cerebrovascular function.14,15 In this study, we observed that cognitive performance, mainly memory function, was improved slightly but significantly in both groups. In addition, aerobic exercise training-induced reductions in carotid β-stiffness index and CVR were associated with improved Woodcock-Johnson immediate recall scores in the aerobic exercise training group. These findings are consistent with previous observational studies which suggested that exercise-related reductions in central arterial stiffness and CBF regulations may improve memory function.45,50 However, aerobic exercise training did not prevent brain gray matter reduction in the present study. We speculate but cannot prove that aerobic exercise training-induced improvements in cerebrovascular function may precede changes in brain structure and that it is also possible that it may take a longer time of aerobic exercise training to have cumulative effects on brain structure.
Clinical perspectives
At present, there are no effective treatment strategies to prevent or slow age-related cognitive decline or ADRD. 51 Brain pathophysiology, in particular vascular dysfunction, can begin 10–20 years before the onset of cognitive impairment.51,52 Therefore, lifestyle modification to improve vascular function during the latent phase of ADRD (e.g., the cognitively normal phase) is critical to maintaining brain health.4,7,8,12 Mounting evidence indicates that elevated central arterial stiffness and CVR and reduced CBF may accelerate age-related cognitive decline and increase the risk of ADRD.2,3,8,10,11,53 In this study of cognitively normal older adults, we showed one-year progressive moderate-to-vigorous aerobic exercise training increased physical fitness as measured by O2peak, reduced carotid arterial stiffness, decreased CVR, and improved global CBF, while cognitive performance may be improved in both groups. These findings collectively support the benefits of aerobic exercise training on reducing vascular risk factors in older adults in order to maintain brain health.
Study strengths and limitations
There are several strengths of our study. First, this is the first RCT study in cognitively normal older adults that investigated the effects of one-year aerobic exercise training on central arterial stiffness and cerebral hemodynamics and their associations with neurocognitive performance. The objective measurement of O2peak, the gold standard index of cardiorespiratory fitness, also allows us to investigate the relationship between cardiorespiratory fitness, systemic and cerebral hemodynamics. Lastly, the test-retest reproducibility of our carotid and cerebral hemodynamic measurements exhibited strong intra-class correlations, which indicates the reliability of the methodologies used for these outcome measures (Table S4).
The findings from this study should be interpreted in the context of the following limitations. First, the sample size was relatively small and the sample included more women than men, and more than 90% of participants were well-educated Caucasians, which reduces the generalizability of our findings. Also, the attrition rate was relatively high (∼23%) which may bias our results. Therefore, future studies with larger sample sizes, along with racially and ethnically diversified populations are needed to confirm the findings of this study. This is particularly important in ADRD research due to the existing health care disparities in ADRD. 51 Second, although we observed that both aerobic exercise training and stretching-and-toning improved cognitive scores, this change was minimal and likely related to a practice effect in the repeated measures of cognitive performance,54,55 which may limit the clinical relevance of our findings. In addition, for post hoc comparisons of the main outcomes of the study, that is, changes in CBF and carotid arterial stiffness revealed with ANOVA, we used Bonferroni corrections to reduce type I errors. However, given the exploratory nature of other modeling and data analysis, we did not control the overall study statistical error <5%. We must also acknowledge that the associations observed between changes in CVR, carotid arterial stiffness, and memory performance with aerobic exercise training do not indicate the presence of a causal relationship. A potential neurovascular coupling relationship with aerobic exercise needs to be elucidated in further studies. Third, CVR was calculated based on MAP and CBF measured in the supine position under resting condition; thus, CVR does not take into account possible fluctuations in venous or intracranial pressure (ICP). ICP in the supine position is ∼15 mmHg which is relatively stable under resting conditions. 56 Theoretically, cerebral perfusion pressure (CPP) = MAP-ICP and CVR = CPP/CBF. Since it is difficult to measure ICP invasively in healthy human subjects, CVR, in most studies, was estimated as CVR = MAP/CBF.3,16,17,19 Lastly, the one-year trial duration may still be too short to reveal the cumulative effect of aerobic exercise training on brain structure and function relative to stretching-and-toning given that these effects are likely to be accumulated over years for a significant impact or may occur at microstructural level which cannot be detected with the current resolution of MRI.
Conclusion
This study demonstrates that in cognitively normal older adults, 1) one-year progressive moderate-to-vigorous aerobic exercise training increased global CBF, decreased CVR, and reduced carotid arterial stiffness, 2) aerobic exercise training-induced increase of O2peak was associated with increase in CBF and reductions in CVR and carotid arterial stiffness, and 3) cognitive performance, mainly memory function, was improved in both groups and the improvements in cognitive performance were associated with reduction in carotid arterial stiffness and CVR in the aerobic exercise training group. These findings provide strong evidence that aerobic exercise improves CBF regulation in older adults which may benefit brain health.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221133861 for One-year aerobic exercise increases cerebral blood flow in cognitively normal older adults by Tsubasa Tomoto, Aryan Verma, Kayla Kostroske, Takashi Tarumi, Neena R Patel, Evan P Pasha, Jonathan Riley, Cynthia D Tinajero, Linda S Hynan, Karen M Rodrigue, Kristen M Kennedy, Denise C Park and Rong Zhang in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank all our study participants for their willingness, time, and effort devoted to this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the National Institutes of Health (R01HL102457) and the Josephine Hughes Sterling Foundation.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: T Tomoto wrote the manuscript; DP and RZ designed research; T Tarumi, JR, CT, KR, and KMK performed data collection; T Tomoto, AV, KK, T Tarumi, NP, EP, and LH analyzed data. AV, T Tarumi, JR, KMK, KMR, DP, and RZ edited the manuscript. All of the authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Tsubasa Tomoto https://orcid.org/0000-0001-5936-0332
References
- 1.Araghi M, Shipley MJ, Wilkinson IB, et al. Association of aortic stiffness with cognitive decline: Whitehall II longitudinal cohort study. Eur J Epidemiol 2020; 35: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017; 136: 719–728. [DOI] [PubMed] [Google Scholar]
- 3.Yew B, Nation DA. Alzheimer's disease neuroimaging I. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 2017; 140: 1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorin-Trescases N, de Montgolfier O, Pincon A, et al. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 2018; 314: H1214–H1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toth P, Tarantini S, Csiszar A, et al. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 2017; 312: H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belz GG. Elastic properties and windkessel function of the human aorta. Cardiovasc Drugs Ther 1995; 9: 73–83. [DOI] [PubMed] [Google Scholar]
- 7.Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis 2012; 32: 541–549. [DOI] [PubMed] [Google Scholar]
- 8.Zeki Al Hazzouri A, Yaffe K. Arterial stiffness and cognitive function in the elderly. J Alzheimers Dis 2014; 42 Suppl 4: S503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer J, Trollor JN, Baune BT, et al. Arterial stiffness, the brain and cognition: a systematic review. Ageing Res Rev 2014; 15: 16–27. [DOI] [PubMed] [Google Scholar]
- 10.van Sloten TT, Protogerou AD, Henry RM, et al. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 2015; 53: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zonneveld HI, Loehrer EA, Hofman A, et al. The bidirectional association between reduced cerebral blood flow and brain atrophy in the general population. J Cereb Blood Flow Metab 2015; 35: 1882–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Torre JC. Vascular risk factor detection and control may prevent Alzheimer's disease. Ageing Res Rev 2010; 9: 218–225. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000; 102: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 14.Bliss ES, Wong RH, Howe PR, et al. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J Cereb Blood Flow Metab 2021; 41: 447–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabral DF, Rice J, Morris TP, et al. Exercise for brain health: an investigation into the underlying mechanisms guided by dose. Neurotherapeutics 2019; 16: 580–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claassen J, Thijssen DHJ, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith EC, Pizzey FK, Askew CD, et al. Effects of cardiorespiratory fitness and exercise training on cerebrovascular blood flow and reactivity: a systematic review with meta-analyses. Am J Physiol Heart Circ Physiol 2021; 321: H59–H76. [DOI] [PubMed] [Google Scholar]
- 18.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 2010; 72: 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomoto T, Liu J, Tseng BY, et al. One-year aerobic exercise reduced carotid arterial stiffness and increased cerebral blood flow in amnestic mild cognitive impairment. J Alzheimers Dis 2021; [DOI] [PubMed] [Google Scholar]
- 20.Tarumi T, Patel NR, Tomoto T, et al. Aerobic exercise training and neurocognitive function in cognitively normal older adults: a one-year randomized controlled trial. J Intern Med 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu YS, Parker R, Tseng BY, et al. Exercise training decreases arterial stiffness and improves brain perfusion in sedentary elderly women. Circulation 2011; 124: A16151. [Google Scholar]
- 22.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 11th edn. Philadelphia: Lippincott Williams & Wilkins, 2020. [Google Scholar]
- 23.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 2010; 122: 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA 1965; 194: 646–649. [PubMed] [Google Scholar]
- 25.Okazaki K, Iwasaki K, Prasad A, et al. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol (1985) 2005; 99: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol 1998; 85: 1113–1122. [DOI] [PubMed] [Google Scholar]
- 27.Giller CA, Giller AM. A new method for fixation of probes for transcranial doppler ultrasound. J Neuroimaging 1997; 7: 103–105. [DOI] [PubMed] [Google Scholar]
- 28.Tomoto T, Sugawara J, Tarumi T, et al. Carotid arterial stiffness and cerebral blood flow in amnestic mild cognitive impairment. Curr Alzheimer Res 2021; 17: 1115–1125. [DOI] [PubMed] [Google Scholar]
- 29.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30: 445–448. [DOI] [PubMed] [Google Scholar]
- 30.Hirai T, Sasayama S, Kawasaki T, et al. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 1989; 80: 78–86. [DOI] [PubMed] [Google Scholar]
- 31.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr 2008; 21: 93–111. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 32.Khan MA, Liu J, Tarumi T, et al. Measurement of cerebral blood flow using phase contrast magnetic resonance imaging and duplex ultrasonography. J Cereb Blood Flow Metab 2017; 37: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Zhu YS, Khan MA, et al. Global brain hypoperfusion and oxygenation in amnestic mild cognitive impairment. Alzheimers Dement 2014; 10: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab 1985; 5: 65–69. [DOI] [PubMed] [Google Scholar]
- 35.Park DC, Lautenschlager G, Hedden T, et al. Models of visuospatial and verbal memory across the adult life span. Psychol Aging 2002; 17: 299–320. [PubMed] [Google Scholar]
- 36.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 2009; 60: 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Press, 2013. [Google Scholar]
- 38.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 39.Jezzard P, Chappell MA, Okell TW. Arterial spin labeling for the measurement of cerebral perfusion and angiography. J Cereb Blood Flow Metab 2018; 38: 603–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calverley TA, Ogoh S, Marley CJ, et al. HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol 2020; 598: 2513–2530. [DOI] [PubMed] [Google Scholar]
- 41.Guadagni V, Drogos LL, Tyndall AV, et al. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 2020; 94: e2245–e2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 2013; 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas BP, Tarumi T, Sheng M, et al. Brain perfusion change in patients with mild cognitive impairment after 12 months of aerobic exercise training. J Alzheimers Dis 2020; 75: 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haeger A, Costa AS, Schulz JB, et al. Cerebral changes improved by physical activity during cognitive decline: a systematic review on MRI studies. Neuroimage Clin 2019; 23: 101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarumi T, Gonzales MM, Fallow B, et al. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens 2013; 31: 2400–2409. [DOI] [PubMed] [Google Scholar]
- 46.Shibata S, Fujimoto N, Hastings JL, et al. The effect of lifelong exercise frequency on arterial stiffness. J Physiol 2018; 596: 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka H. Antiaging effects of aerobic exercise on systemic arteries. Hypertension 2019; 74: 237–243. [DOI] [PubMed] [Google Scholar]
- 48.Tarumi T, Zhang R. Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol 2014; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morland C, Andersson KA, Haugen OP, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun 2017; 8: 15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarumi T, Dunsky DI, Khan MA, et al. Dynamic cerebral autoregulation and tissue oxygenation in amnestic mild cognitive impairment. J Alzheimers Dis 2014; 41: 765–778. [DOI] [PubMed] [Google Scholar]
- 51.2021 Alzheimer's disease facts and figures. Alzheimers Dement 2021; 17: 327–406. [DOI] [PubMed]
- 52.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 53.Leijenaar JF, Maurik IS, Kuijer JPA, et al. Lower cerebral blood flow in subjects with Alzheimer's dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimer's Dement 2017; 9: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calamia M, Markon K, Tranel D. Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol 2012; 26: 543–570. [DOI] [PubMed] [Google Scholar]
- 55.Machulda MM, Pankratz VS, Christianson TJ, et al. Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo clinic study of aging. Clin Neuropsychol 2013; 27: 1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol 2017; 595: 2115–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221133861 for One-year aerobic exercise increases cerebral blood flow in cognitively normal older adults by Tsubasa Tomoto, Aryan Verma, Kayla Kostroske, Takashi Tarumi, Neena R Patel, Evan P Pasha, Jonathan Riley, Cynthia D Tinajero, Linda S Hynan, Karen M Rodrigue, Kristen M Kennedy, Denise C Park and Rong Zhang in Journal of Cerebral Blood Flow & Metabolism