Abstract
Neovascular age-related macular degeneration (nAMD) is a major cause of visual impairment and blindness. Anti-vascular endothelial growth factor (VEGF) agents, such as ranibizumab, bevacizumab, aflibercept, brolucizumab and faricimab have revolutionized the clinical management of nAMD. However, there remains an unmet clinical need for new and improved therapies for nAMD, since many patients do not respond optimally, may lose response over time or exhibit sub-optimal durability, impacting on real world effectiveness. Evidence is emerging that targeting VEGF-A alone, as most agents have done until recently, may be insufficient and agents that target multiple pathways (e.g., aflibercept, faricimab and others in development) may be more efficacious. This article reviews issues and limitations that have arisen from the use of existing anti-VEGF agents, and argues that the future may lie in multi-targeted therapies including alternative agents and modalities that target both the VEGF ligand/receptor system as well as other pathways.
Keywords: Neovascular age-related macular degeneration, Vascular endothelial growth factor, VEGF receptors, Anti-VEGF therapy, Aflibercept, Ranibizumab, Bevacizumab, Brolucizumab, Faricimab
Age-related macular degeneration (AMD) is the main cause of severe visual impairment and irreversible blindness in the industrialised world [1]. Late AMD, in the form of either neovascular (n) or atrophic AMD, is responsible for most vision loss. Although the prevalence of nAMD is lower than that of atrophy AMD, nonetheless it is responsible for most cases of severe vision loss [2]. Major advances in the treatment of nAMD over the past decade have occurred with the use of vascular endothelial growth factor (VEGF) inhibitors, most of which target VEGF-A. AMD has a prevalence of around 170 million, which is projected to increase to 288 million by 2040 [2]. Global prevalence in adults 45 years and over of any, early and late AMD is 8.7%, 8.0% and 0.4%, respectively, with early AMD being more common in people with European ancestry (11.2%) than Asians (6.8%) and no significant difference in the prevalence of late AMD [3]. AMD of any type is less frequent in people with African ancestry [3]. Approximately 1 in 10 persons with any AMD signs have nAMD [4]. In the US, about 200,000 new cases of nAMD are diagnosed each year [5].
Pathophysiology and treatment concepts
The pathogenesis of nAMD involves aberrent angiogenesis and macular neovascularization (MNV, also known as choroidal neovascularization (CNV)), vascular leakage, haemorrhage and scarring, which can lead to permanent vision loss [6–8]. A range of mediators have been implicated in this complex process including kinases [9], cytokines [10] and growth factors [11]; the most prominent is VEGF and its receptors (VEGFRs) [12] (Fig. 1). Most therapeutic attention on AMD and diabetic retinopathy (DR) has focused on VEGF-A and its receptors because of its dominant capacity to promote angiogenesis and vascular permeability, and its receptors [12].
Fig. 1.
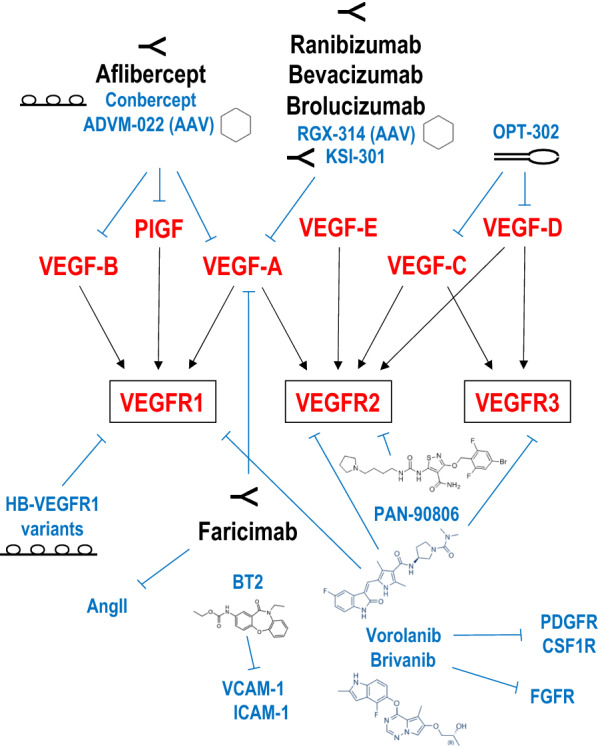
VEGF ligands and receptors. The VEGF ligand family (comprising VEGF-A, VEGF-B, VEGF-C, VEGF-D, virally encoded VEGF-E and placental growth factor (PlGF)) interacts selectively with 3 cell surface receptors (VEGFR1, VEGFR2, VEGFR3). Currently approved therapies are indicated in black font. Examples of emerging or experimental therapies are shown in blue font. Refer to text for detail
AMD is classified clinically as early, intermediate or late. Early AMD is defined as the presence of medium-sized drusen (63–125 µm) without typical retinal pigmentary changes (hyper- or hypo-pigmentation) at the macula [13]. Intermediate AMD is defined as the presence of at least one large druse (> 125 µm) or extensive medium drusen and typical pigmentary changes. Late AMD refers to the presence of either nAMD (also termed “wet”) or atrophic AMD (also termed geographic atrophy). Early and intermediate AMD stages are often asymptomatic. There may be mild central distortion, particularly for near vision with low luminance. Late AMD is frequently symptomatic and reduces central vision for near and distance tasks. Neovascular AMD can progress rapidly over weeks to months, while atrophic AMD progresses more slowly over years to decades. Many patients report that the earliest symptoms of late AMD are distorted central vision, a dark patch (scotoma), which may be measured as a subtle visual field defect on microperimetry, and difficulty recognising faces [14]. Over 60% of AMD patients will develop the same stage of disease in both eyes [15]. In asymmetrically affected patients, the second eye then becomes affected in 19–28% of cases within 5 years of initial diagnosis [16].
The underlying nAMD lesion is the MNV (also known as CNV) complex which can be classified in terms of location—type 1 is restricted to the sub-retinal pigment epithelium (RPE) space; type 2 grows through the RPE and into the sub-retinal space; and type 3 is believed to originate within the retina [8]. A mixture of type 1 and 2 where neovascularisation in both the sub-retinal and sub-RPE spaces can also occur. In addition, a particular subtype known as polypoidal choroidal vasculopathy (PCV) [17, 18], similar to a type 1 CNV with dilated vascular elements has also been described as part of the spectrum of nAMD [8] and may be more prevalent among Asians [17, 18]. CNV is associated with other signs: retinal haemorrhage, intra- or sub-retinal fluid, pigment epithelial detachment (PED), exudate and subretinal fibrosis. AMD is diagnosed through a combination of retinal assessment through dilated pupils, and multimodal imaging, using colour fundus photography, OCT imaging, fundus fluorescein angiography (FFA), fundus autofluorescence (FAF) and more recently, OCT-angiography (OCTa) [14].
Atrophic AMD refers to the presence of geographic atrophy (GA) lesions that may be unifocal or multifocal [19]. Average progression rates of GA lesions is ~ 2 mm2/year with considerable variation [20]. Multimodal imaging is also used to diagnose and monitor GA progression. Reticular pseudodrusen (RPD), also known as subretinal drusenoid deposits, may be associated with more rapid progression of GA, and to a lesser extent, nAMD [21, 22].
AMD risk is influenced by genetic and non-genetic factors. Discovery of AMD associated genetic loci was the first major success of genome wide association study (GWAS) approaches [23]. Large AMD GWAS have since discovered 52 common and rare variants at 34 genetic loci independently associated with late AMD [24–26]. Association of very rare coding variants (frequency < 0.1%) in complement factor H (CFH), complement factor I (CFI) and tissue inhibitor of metalloproteinases-3 (TIMP3) suggest causal roles for these genes in AMD pathogenesis [24]. The alternative complement pathway (CFH, CFI, C2/complement factor B (CFB), C3) is primarily implicated in AMD risk [27] followed by the age-related maculopathy susceptibility2 (ARMS2) locus for which the gene product has not yet been identified [28]. TIMP3 codes for a matrix metalloproteinase inhibitor that regulates degradation of extracellular matrix, and is also implicated in Sorsby fundus dystrophy, a retinal degenerative disorder similar to AMD [29]. These 52 genetic variants together explain 27% of disease variability, which is over half the genomic heritability of AMD [24].
Smoking is the strongest modifiable risk factor for AMD and is associated with a doubling of late AMD risk [30, 31]. Current smoking is also associated with 10-year earlier age at onset of late AMD [30, 31]. Current smoking [32], and polymorphisms in CFH [33] and ARMS2 [34] together account for up to 45% of risk for developing AMD [35]. Higher body mass index, an indicator of obesity, is also consistently associated with increased risk and earlier onset of developing AMD [36–38].
Dietary factors, especially intake of antioxidants, is also consistently associated with risk of developing AMD. Population studies such as the Blue Mountains Eye Study and others have shown a high dietary intake of lutein, zeaxanthin (carotenoids found in leafy green vegetables) and fish are associated with reduced risk of developing AMD [39–41]. Overall, Mediterranean and Oriental dietary patterns appeared to be protective against developing AMD, when compared to Western diets high in animal fats and red/processed meat [42]. The Age-Related Eye Disease Studies I and II were landmark clinical trials that confirmed nutrient supplements containing high doses of zinc and antioxidants (vitamin C, vitamin E, carotenoids, copper) can slow AMD progression in some people [43, 44].
Other factors with less consistent associations with AMD risk include: iris colour and sun exposure [30], alcohol intake [45], inflammatory markers such as C-reactive protein and white cell count [46, 47]. Cardiovascular risk factors, such as hypertension and dyslipidaemia are inconsistently linked with AMD risk [48], with elevated serum lipids associated with increased risk of intermediate AMD in some studies [49] but not others [50]. Long term aspirin use may be linked with a small increased risk of late AMD in a few studies [51, 52] but this has not been confirmed [53–55]. The risk of cataract surgery worsening early AMD is controversial. The latest Cochrane review to study the issue [56] found insufficient evidence to support cataract surgery as a risk factor for late AMD. Risk scores that include age, gender, smoking and drusen type perform well at discriminating persons who go on to develop AMD, with area under the receiver-operating curve of 0.85–0.91 [57–59].
Current management
The present management of late AMD is focused on treating nAMD, as there are no proven treatments to date for atrophic AMD/GA.
Emerging approaches to treating atrophic AMD (GA)
Recently pegcetacoplan, a C3 inhibitor delivered intravitreally, has been shown in two phase 3 trials (DERBY and OAKS) to significantly reduce the rate of grown of atrophic AMD/GA lesions when delivered monthly or every other month. [60–62]. This represents the first potential treatment to delay growth of GA, although visual acuity, reading vision and other functional parameters remained similar in treatment and sham groups. Approval from the US Food and Drug Administration (FDA) is pending and likely to occur in the near future. A number of other agents to treat GA are currently in late phase trials [60, 63–65].
GA is typically largely asymptomatic until the fovea is involved. Visual acuity does not correlate well with GA severity as the fovea may be spared despite extensive GA elsewhere [66], which may account for the lack of efficacy of pegcetacoplan on visual acuity. Clinical trials of GA using traditional visual acuity as an endpoint would need to be prohibitively long to detect differences due to slow growth of GA lesions. Alternative clinical endpoints are being explored, such as reading indices [67], and either single morphologic endpoints (enlargement of fundus autofluorescence (FAF) defects) or composite morphologic endpoints based on multimodal imaging, that may improve power to detect efficacy of interventions [68]. Complement inhibition is the most studied potential therapeutic intervention [69]. Drugs other than pegcetacoplan, such as eculizumab [70], lampalizumab [71] and tandospirone [72] have been investigated in clinical trials that have so far yielded disappointing results. Management of atrophic AMD/GA is not the focus of the present review.
Current anti-VEGF therapies for nAMD
Intravitreal (IVT) anti-VEGF therapy is the standard of care for the treatment of nAMD (Table 1). Therapies include aflibercept [73], ranibizumab [74, 75], bevacizumab [76] which is currently used as off-label therapy but due to be registered, and brolucizumab [77]. Landmark registration trials for VEGF inhibitors demonstrate excellent visual outcomes when treated with monthly ranibizumab. The MARINA [78] and ANCHOR [79] trials showed gains of about 2 lines over 24 months. Similarly, VIEW [80] demonstrated non-inferiority of 2 monthly aflibercept compared to monthly ranibizumab. The Comparison of Age-Related Macular Degeneration Treatment Trials (CATT) found that bevacizumab had similar effectiveness to ranibizumab in treating nAMD [81], while the HAWK and HARRIER trials showed both 2 monthly and 3 monthly brolucizumab was non-inferior to 2 monthly aflibercept in treating nAMD [77]. Comparison between aflibercept, ranibizumab and bevacizumab for retinal disease suggest that aflibercept may have a slight benefit over ranibizumab and bevacizumab [82]. Ongoing CNV activity is associated with poorer visual outcomes [83, 84].
Table 1.
Current anti-VEGF therapies for nAMD
| Currently used therapies | Company | Type of therapeutic | Delivery route | FDA approval for nAMD |
|---|---|---|---|---|
| Ranibizumab/Lucentis | Lucentis, Genentech/Novartis | Antibody | IVT | 2006 |
| Aflibercept/Eylea | Regeneron/Bayer | Fusion protein “trap” | IVT | 2019 |
| Brolucizumab/Beovu | Novartis | Antibody | IVT | 2019 |
| Port delivery systems* | Genentech | Antibody | Implant | 2021 |
| Faricimab/ Vabysmo | Roche/Genetech | Bi-specific antibody | IVT | 2022 |
| Bevacizumab/Avastin | Roche/Genetech | Antibody | IVT | Used off label |
IVT denotes intravitreal
*An already approved anti-VEGF agent (ie ranibizumab) not the port itself
Faricimab (Genentech/Roche) is a bispecific (dual targeted) antibody that simultaneously targets VEGF-A and angiopoietin-2 (Ang II), the latter being involved in distinct pathways that promote vascular permeability and inflammation [85]. In early 2022, two global Phase III studies (TENAYA (NCT03823287) and LUCERNE (NCT03823300) involving 1329 patients, reported that faricimab met its primary endpoint and showed potential to extend time between treatments up to 4 months for people with nAMD and was generally well tolerated [86]. Similar results were obtained in patients with diabetic macular edema (DME) in the YOSEMITE (NCT03622580) and RHINE (NCT03622593) Phase III trials involving 1891 individuals [87]. This follows the Phase II STAIRWAY trial (NCT03038880) which showed that monthly IVT faricimab was not superior to monthly ranibizumab [88], and Phase II AVENUE trial (NCT02484690) which showed that IVT faricimab given every 12 or 16 weeks was not clinically inferior to monthly ranibizumab for the treatment of nAMD [89]. With injections every 4 months rather than each month, faricimab could substantially reduce treatment burden and costs for patients and health care providers. Faricimab was approved by the FDA in January 2022 for the treatment of nAMD and DME.
New therapies with longer durability and better efficacy in early trials that target VEGF-A alone have not been as successful as the initial generation of anti-VEGF agents, largely due to unanticipated side effects. Brolucizumab has been associated with intraocular inflammation (~ 4%) [90, 91], retinal vasculitis and retinal artery occlusion [77]. Intraocular inflammation also occurs with abicipar pegol [92], a designed ankyrin repeat proteins (DARPin)-based drug that binds VEGF-A, exceeding 15% in Phase III trials (CEDAR and SEQUOIA, NCT02462928 and NCT02462486, respectively) as compared with less than 1% with ranibizumab [92–94]. Abicipar was rejected by FDA in June 2020 over risk/benefit concerns [94].
Treatment regimens
Clinical trial treatment dosing regimens are often not reflective of real-world practice and high frequency of therapeutic interventions in registration trial design can result in a high treatment burden. In most clinical practice, non-monthly regimens such as the pro re nata (PRN) approach and the treat and extend (T&E) regimen have gained popularity with many favoring the later. The general principle behind non-monthly regimens is assessment of disease activity to determine the next management step taking into account personalised response to therapy. Briefly, in a PRN approach, disease status is assessed monthly, and treatment administered if disease is deemed active. In the T&E approach, treatment is administered at every visit, but treatment intervals are varied according to disease status [95]. These non-monthly regimens can reduce treatment burden to once every 3 to 4 months in some patients, while maintaining favourable visual outcomes [96, 97]. T&E is now the dominant treatment regime worldwide and future research directions are focused on further extending the speed and extent of increased intervals between injections. This points to the need for more durable agents and the different strategies taken to achieve this.
Pharmacogenomics and personalised medicine
There is emerging evidence that a patient’s underlying genetic predisposition may affect response to existing anti-VEGF therapies. Lower risk genotypes of the VEGFA, CFH, ARMS2 and HTRA1 genes may be associated with better visual outcomes and potentially fewer injections [98–101]. Some of these genotypes were associated with poor response to one, but better response to a different anti-VEGF agent [102]. The effect of high risk alleles on anatomical and visual outcomes has been reported to be detectable even after long term treatment of up to 10 years [103]. The results raise the possibility that multi-targeted therapy could be personalised to individuals based on genetic risk scores, which could guide choice of therapy. Nonetheless, such pharmacogenetic scenarios remain in their infancy as a number of other studies have found very limited (to rare variants) [104] to no effect of high risk alleles on response to therapy [105, 106].
Unmet need from use of current anti-VEGF therapies impacting quality of life
While anti-VEGF therapy has proven to be efficacious, several shortcomings highlight unmet need of this approach, thereby impacting the quality of life of patients suffering from and receiving treatment for nAMD. Table 2 summarises patient, therapeutic and healthcare system factors where there is unmet meet with current anti-VEGF therapies for nAMD. These factors are discussed further below.
Table 2.
Unmet need
| Factors | Unresolved issues | Comments |
|---|---|---|
| Patient factors | Non-adherence and/or non-compliance | Patient education and better understanding of disease and therapy |
| Cost of therapy/visits | Individual healthcare jurisdiction cost and reimbursement policies | |
| High individual treatment burden | Need for more durable agents | |
| Poor prognosis | Need for more efficacious agents or regenerative therapies to reverse damage by nAMD | |
| Therapeutic factors | Relatively short duration of action resulting in repeated treatments | Need for more durable agents |
| Poor efficacy resulting in persistently active disease | Need for more efficacious agents | |
| Safety profile | Need for better preclinical safety models that can provide early safety signals before entry into clinical practice | |
| Healthcare system factors | High treatment burden to society | Combination of more durable and efficacious agents may help address this unmet need by preventing/reversing blindness from nAMD |
| Reimbursement and subsidies | Individual healthcare jurisdiction cost and reimbursement policies | |
| Increasing patient load | Need for more durable agents and/or more precise therapies to minimise unnecessary monitoring visits |
Suboptimal response or the response is not sustained
Despite favorable outcomes in most patients, 25–35% of nAMD patients either fail to respond optimally to current anti-VEGF therapies, exhibit late failure to therapy, or require intensive, frequent IVT treatment [107, 108]. Of the 35% who fail to respond optimally to therapy, over 10% worsen despite treatment, while another 25% show no improvement [83, 84]. Despite maximal intensive anti-VEGF therapy, over 60% of eyes have persistently active disease, which can result in poor long-term outcomes [84, 109]. Around 1 in 5 patients become “injection-intensive” needing treatment at least every 4 to 6 weeks [1]. This rigorous schedule can lead to high non-adherence/dropout over time, which further exacerbates the condition compromising the efficacy of anti-VEGF therapy [110–112]. This may be why poorer visual acuity outcomes are achieved in real world settings than in clinical trials [113–115].
In patients that achieve disease quiescence, suspending therapy may be detrimental and treatment often needs to be continued at regular intervals to maintain vision. Nguyen et al. reported a recurrence rate of > 40% in nAMD patients after treatment cessation following a 12-week interval [116]. Recent studies indicate that pre-treatment VEGF levels in the aqueous humor of nAMD patients correlated significantly with the likelihood of disease recurrence [117]. Moreover, in some nAMD patients with aggressive disease, continuation of anti-VEGF therapy even after achieving stability does not prevent disease recurrence [118–120]. The impact of suboptimal response and poor sustainability with resultant poor vision has significant impacts on patient reported outcomes. Patients were found to be willing to tolerate other inconveniences of receiving repeated anti-VEGF treatments if resultant vision was better [121–123]. CATT showed that 1 in 4 patients who needed aggressive anti-VEGF therapy developed some subretinal fibrosis within 2 years [124] and a greater risk of geographic atrophy in nAMD patients 2 to 5 years after start of therapy [125].
Healthcare-related costs
Recurrent treatments for nAMD impose a high financial burden on health care systems (e.g. [126],). Ross et al. examined compared the cost-effectiveness of aflibercept, bevacizumab and ranibizumab for treatment of DME and found that aflibercept and ranibizumab, respectively, were 31 and 20 times more expensive than bevacizumab [127]. Aflibercept and ranibizumab were not cost-effective relative to bevacizumab [128]. Off label use of bevacizumab for ophthalmic disease however, has helped mitigate cost for eye conditions in jurisdictions throughout Europe [129] and the US [130]. Anti-VEGF costs to patients are hugely variable in Asia; from comparative high cost in Indonesia with no government subsidies, to fully subsidized through public health insurance in South Korea and Japan [131–133]. In Australia, the regulatory framework disincentivizes use of bevacizumab for nAMD since the drug is unlisted and attracts no government subsidy [134]. There are also continuing issues regarding the safety of compounding bevacizumab for off-label intraocular use [135]. There is also increasing interest in biosimilars. Biosimilar medicines are close, but not identical versions of the original. For example, SB11 [136] and razumab [137] are ranibizumab biosimilars and may be available at lower cost. However, pharmacological issues of limited efficacy and durability persist with biosimilars. In addition to the cost effectiveness of therapy itself, there is significant burden of treatment to patients and care givers.
Undesirable sequelae of anti-VEGF use
Existing anti-VEGFs are linked with some adverse events. These, while rare, can significantly impact vision. The major short term adverse effect of anti-VEGF injections is the risk of endophthalmitis, a serious outcome that occurs in approximately 1/3500 injections [138]. Early treatment with either IVT antibiotics, early vitrectomy, or both, is essential and can result in significant improvement in vision post-endophthalmitis [139]. Another major potential complication of anti-VEGF therapy is the risk of intraocular inflammation, which can also lead to irreversible vision loss if severe. The risk of this appears to be highest for the anti-VEGF agent brolucizumab, which is associated with a sixfold higher risk of intraocular inflammation compare to aflibercept [140]. Finally, a transient rise in intraocular pressure is often observed immediately post IVT injection of all anti-VEGF agents. This can sometimes be associated with “black outs” or sudden loss of vision as intraocular perfusion is compromised. In virtually all cases vision recovers over the next few minutes either spontaneously, or after anterior chamber paracentesis to relieve the intraocular pressure rise.
Long term use of anti-VEGF can be associated with adverse effects as well, though the causal relationship of long term anti-VEGF therapy and these adverse effects, as opposed to the natural history of nAMD, remains unclear and is subject of much investigation. Macular atrophy, an end stage phenotype of nAMD that can result in irreversible vision loss, has been reported to be associated with long term anti-VEGF use [141]. Ranibizumab treatment for nAMD over 2 years is associated with macular atrophy in about 30% of eyes [142] and longer term data has shown some detectable macular atrophy in 48% of eyes treated with anti-VEGF for 9 years [141]. Causality remains unclear and macular atrophy may represent the natural history of treated CNV [141–143]. Subretinal fibrosis is another end stage phenotype of nAMD associated with irreversible vision loss. Outcomes from CATT indicate that 1 in 4 patients on aggressive anti-VEGF therapy developed fibrosis within 24 months [124] with greater risk of geographic atrophy in nAMD patients 2 to 5 years after start of therapy [125] which again, could reflect the natural history of treated nAMD [141]. It should also be noted that untreated nAMD itself can result in subretinal fibrosis, which further confounds any potential relationship with long term anti-VEGF use.
There also remain some concerns about potential adverse effects stemming from systemic suppression of VEGF following long term anti-VEGF treatment [144]. While oral therapy in particular circumstances may be desirable, there is concern that systemic and chronic administration of agents that inhibit VEGF may lead to adverse events including kidney damage and hypertension [145] secondary to VEGF acting as a trophic factor in the retina and kidney. Though rare, repeated IVT injection of aflibercept and bevacizumab can result in serum drug levels rising above IC50 concentrations and reduced plasma free VEGF levels [146]. Serial injections of anti-VEGF in nAMD patients can elevate intraocular pressure which in some cases may require glaucoma therapy [147], although the incidence of this is low [148]. Long term IVT bevacizumab may increase risk of developing hypertension [149]. There is also some evidence that IVT anti-VEGFs may carry risk for systemic adverse thromboembolic events [150], however other studies have not found a link [151]. Further, systematic reviews have generally found low, if any, increased risk of thromboembolic events, which are acceptable when balanced against the clear efficacy in preventing vision loss [152].
There is evidence of cross regulation between VEGF ligands when a VEGF ligand is suppressed. Inhibition of one VEGF ligand by an approved anti-VEGF can induce expression of other VEGF ligands. Studies on mechanisms of tumour resistance indicate that resistance to aflibercept coincides with increased levels of VEGF-C [153], a lymphangiogenic growth factor [154] and that resistance to bevacizumab coincides with increased levels of PlGF and VEGF-D [155]. VEGF-C and VEGF-D expression is increased in human brain and tumour derived endothelial cells exposed to bevacizumab [156]. VEGF-D has been implicated in lymphangiogenesis, tumor growth and metastatic spread [157]. In nAMD patients, while IVT injection of bevacizumab reduces VEGF-A levels in the aqueous humor, this elevates levels of VEGF-C and a range of other pro-angiogenic and pro-inflammatory factors [158].
Overall, these shortcomings can result in unfavourable outcomes and potentially visually significant complications in some patients. Longer lasting, alternative agents that achieve the same or better efficacy with fewer injections, could help to alleviate many of these shortcomings.
Emerging therapies
Mono-targeted (anti VEGF) therapies in development for nAMD
Targeting the VEGF/R system has undoubtedly prevented blindness in millions of nAMD patients and improved quality of life and workforce productivity. However, given the shortcomings of current anti-VEGF therapy, there remains a need to identify other types of agents and modalities exploiting this pathway, several of which are summarized in Table 3 and depicted in Fig. 1.
Table 3.
Emerging or attempted therapies for nAMD
| Drug or modality | Company or Institution | Type of agent | Delivery route | Phase | Clinical trials.gov |
|---|---|---|---|---|---|
| Abicipar pegol | Allergan | DARPin | IVT | III, terminated | |
| Conbercept/Lumitin | Chengdu Kang Hong Biotech | Fusion protein “trap” | IVT | III, terminated | NCT03577899, NCT03630952 |
| PAN-90806 | PanOptica | Small molecule | Eye drops | I/II | NCT03479372 |
| RGX-314 | RegenxBio | AAV8 gene therapy delivering anti-VEGF Fab | Subretinal | II | NCT04832724 |
| ADVM-022 | Adverum | AAV7m8 gene therapy delivering aflibercept | IVT | I | NCT03748784, NCT04645212 |
| KSI-301 | Kodiak | Antibody biopolymer conjugate | IVT | IIb/III | NCT04049266 |
| OPT-302 | Opthea | Fusion protein “trap” | IVT | III | NCT04757610, NCT04757636 |
| EYP-1901 | Eyepoint | Small molecule in Durasert | IVT | I | NCT04747197 |
| Pluripotent stem-cells | London Project to Cure Blindness | hESC-derived RPE monolayer | Transplantation | I | NCT01691261 |
| Pluripotent stem-cells | Highway Program for Realization of Regenerative Medicine | Autologous iPSC-derived RPE cell sheet | Transplantation | UMIN000011929 | |
| Heparin-binding VEGFR1 variants | University of California San Diego | Fc fusion proteins | IVT | N/A | |
| OXU-005 | Oxular | Small molecule in Oxuspheres | IVT | N/A | |
| Brivanib | Nantong University | Small molecule | IVT, gavage | N/A | |
| AGX51 | Memorial Sloan Kettering Cancer Center | Small molecule | IVT | N/A | |
| THR-687 | Oxurion | integrin receptor antagonist | IVT | N/A | |
| Vasotide | Beth Israel Deaconess Medical Center | peptidomimetic | Eye drops | N/A | |
| BT2 | UNSW | Small molecule | IVT | N/A | |
| Sunitinib | Johns Hopkins University | Small molecule | IVT | N/A | |
| APX2009, APX2014 | Indiana University | Small molecule | Systemic | N/A | |
N/A denotes not (yet) available, IVT denotes intravitreal, iPSC denotes induced pluripotent stem cells, hESC denotes human embryonic stem cell, RPE denotes retinal pigment epithelium, AAV denotes adeno-associated virus, DARPins denote designed ankyrin repeat proteins
*an already approved anti-VEGF agent (ie ranibizumab) not the port itself
Protein-based agents
Inspired by the need for longer acting VEGF inhibitors, and exploiting the heparin affinity of VEGFRs, Ferrara and colleagues recently developed heparin-binding variants of VEGFR1 that compare favourably with aflibercept in rodent models of retinal neovascularization [159]. Fc-containing proteins with the D3 (Ig-like) domain of VEGFR1 (e.g., V1233, V233) bound to bovine vitreous in vitro and suppressed retinal angiogenesis following IVT injection and laser-induced CNV in mice. These Fc fusion proteins were detectable in serum after IVT delivery albeit at levels less than aflibercept [159].
Conbercept (Lumitin; Chengdu Kang Hong Biotech) is a fusion protein comprising the extracellular domain 2 of VEGFR1 and extracellular domains 3 and 4 of VEGFR2 combined with the Fc portion of human IgG1. Like aflibercept, conbercept is designed to bind VEGF-A, VEGF-B and PlGF [160]. Monthly IVT conbercept appears to improve visual acuity in nAMD patients with no serious adverse reactions or complications [161]. However potential concerns have been raised about the extent of CNV, prior patient treatment and unresolved macular edema secondary to insufficient macular deturgescence suggesting active disease requiring further treatment [162]. The PANDA-1 and PANDA-2 Phase III trials for nAMD involving 1157 participants were terminated in 2021 on the basis that the desired primary endpoint, notably conbercept non-inferiority compared with aflibercept was not met (NCT03577899 and NCT03630952).
OPT-302 is a novel “trap” molecule comprising Ig-like domains 1 to 3 of the extracellular domain of human VEGFR3 fused to the Fc fragment of human IgG1 that binds to VEGF-C and VEGF-D, blocking their activation of VEGFR2 and VEGFR3 [163]. In NCT02543229, Dugel et al. found that IVT OPT-302 was well tolerated with or without ranibizumab (0.5 mg) up to the highest dose of 2 mg given as 3 injections once every 4 weeks [163]. Although VEGF ligand levels were not measured in the aqueous humour, conceptually at least, combining OPT-302 with anti-VEGF-A therapies may prevent mechanistic escape following VEGF-A suppression. OPT-302 is recruiting for Phase III trials with and without either ranibizumab (ShORe trial, NCT04757610) or aflibercept (COAST trial, NCT04757636), each with 990 nAMD patients.
Small molecule-based therapy
Small molecules offer a range of potential advantages over antibody based drugs such as lower costs of manufacture, longer shelf life and reduced need for cold chain transport, oral administration and drugability [164]. The tyrosine kinase inhibitor sunitinib, a small molecule commonly used to treat renal cell carcinoma, has recently been repurposed as an experimental therapy for nAMD [165]. Single IVT or subconjunctival injection of sunitinib, in a non-inflammatory biodegradable polymer-based microparticle formulation (polylactic-co-glycolic acid (PLGA) and PLGA conjugated to polyethylene glycol (PEG)), provided sustained suppression of choroidal neovascularisation in mice over several months. In separate experiments, mice given IVT VEGF into each eye following injection of sunitinib microparticles (as compared with microparticles alone) showed significant reduction in the number of adherent intravascular leukocytes, indicating sunitinib suppression of VEGF-induced leukostasis. Delivery IVT of sunitinib microparticles in rabbits caused self-aggregation which remained localised and efficacious over several months with no intraocular inflammation or apparent retinal toxicity [165].
Eye drops are being developed as potential monotherapy or to facilitate an increased interval duration between IVT injections of standard therapy or for use after IVT injections to further stabilise active disease. Drops would provide convenience, increasing adherence and compliance by patients and caregivers through fewer clinic visits and reduce risk of infection from IVT injections. PAN-90806 (PanOptica), a small molecule that binds VEGFR2 inhibiting its tyrosine kinase activity, is being developed as a once-daily eye drop suspension for nAMD (NCT03479372) [166]. An earlier trial with a different formulation showed punctate keratopathy due to off-target suppression of corneal epithelial EGFR [167].
Gene therapy
Adverum is developing a gene therapeutic approach for nAMD using ADVM-022 in which a proprietary vector capsid (AAV.7m8) delivers an aflibercept coding sequence. ADVM-022, administered by IVT injection was granted fast track designation by FDA in late 2018. Thirty patients completing 2 years in the Phase I OPTIC trial (NCT03748784) are being enrolled into an extension trial (NCT04645212) which will run for up to 5 years. Gelfman et al. reported the efficacy of ADVM-022-derived aflibercept in a CNV model involving non-human primates. ADVM-022 given 13 months prior to laser-induced CNV, prevented CNV lesions to the same extent as aflibercept delivered at the time of lasering [168, 169] demonstrating that one IVT delivery of ADVM-022 is safe and could provide a potential long-term treatment option for nAMD. Ding et al. performed suprachoroidal injections of RGX-314, an adeno-associated virus serotype 8 (AAV8) vector expressing an anti-VEGF-A Fab in rats and suppressed VEGF-inducible vasodilation and vascular leakage [170], building on earlier studies also in rats injecting RGX-314 subretinally [171]. RegenxBio is conducting a Phase II study in 60 subjects with nAMD in which RGX-314 is delivered by subretinal administration (NCT04832724). While gene therapy provides an alternative means of therapeutic intervention, it brings risk of immunogenicity in relation to adenoviral vectors, and risk of transgene integration in relation to retroviral and lentiviral vectors, or inability to carry large transgenes by AAV vectors.
Other antibody-based strategies
Kodiak is developing KSI-301, an anti-VEGF antibody biopolymer conjugate (ABC) platform comprising a humanized IgG1 antibody binding all isoforms of VEGF-A [172] with an inert immune effector function and a biopolymer designed to increase intraocular durability. In the Phase IIb/III DAZZLE trial (NCT04049266) involving 550 nAMD patients, KSI-301 will be administered IVT at 12, 16 and 20 week intervals and compared against aflibercept once every 4 weeks for 3 consecutive months, followed by once every 8 weeks. The Phase Ib study (NCT03790852) showed 66% of nAMD patients achieved a 6 month or longer treatment-free interval and 78% had a 4 month or longer interval after 12 months [173].
Port delivery systems (PDS), enabling surgically implantable reservoirs, have been developed to facilitate continuous delivery of anti-VEGF agents such as ranibizumab inside the eye by passive diffusion [174]. PDS could potentially be used to deliver other therapeutics through sustained release and refilled months later. The Phase III ARCHWAY trial (NCT03677934) in 418 subjects with fixed 24 week refills revealed that 98.4% of PDS patients went 6 months without intervention and achieved vision outcomes equivalent to patients receiving monthly IVT ranibizumab. However, there was a significantly higher rate of ocular adverse effects, particularly endophthalmitis and vitreous haemorrhage in the PDS arm compared to monthly ranibizumab arm [175][175] prompting further virtual reality training strategies on implantation to mitigate risk [177]. The Phase IIIb VELODROME trial (NCT04657289) will evaluate PDS and ranibizumab refill (100 mg/ml) delivered every 36 weeks as compared with every 24 weeks. FDA approved PDS with ranibizumab for the treatment of nAMD in October 2021.
Multi-targeted therapies in development for nAMD
Despite the promise of reduced treatment burden in nAMD patients brought about by brolucizumab, the future may lie with multi-target interventions. This is because considerable research suggests that factors beyond VEGF, such as other growth factors, chemokines and cytokines, also mediate the pathogenesis of nAMD [12]. Angiogenesis and inflammation underpinning nAMD involves signalling and transcriptional regulation mediated by extracellular signal-regulated kinase-1/2, p-ERK) [9], monocyte chemoattractant protein-1 (MCP-1/CCL2) [178], intercellular adhesion molecule-1 (ICAM-1) [178], vascular cell adhesion molecule-1 (VCAM-1) [178], interleukin-1β (IL-1β) [179] and IL-6 [180]. This may account for the inadequacy of strategies solely targeting the VEGF system [181, 182] and points to the therapeutic potential for strategies that also target other mediators of nAMD. For example, faricimab targets two distinct pathways, VEGF-A and Ang-2. Lessons emerging from cancer therapy suggest that simultaneous blockade of multiple pathways can make it harder for tumours to bypass therapy [183]. Indeed, resistance that develops to kinase inhibitors at least in melanoma patients may arise from reactivation of signalling pathways (or activation of parallel pathways) or immune system modulation [184]. There is also major need for agents with greater efficacy (to improve response) and durability (to reduce frequency of injection) for nAMD that may be achieved through multi targeting. For example, while aflibercept, a soluble decoy VEGF receptor, inhibits VEGF-A and VEGF-B it also binds placental growth factor (PlGF), which may account for its prolonged efficacy compared to the mono-targeted anti-VEGF-A antibodies ranibizumab and bevacizumab [82–84]. Several such strategies in development (Table 3) and are described below. It is unclear at this stage as to whether multi-targeted therapies are prone to more unpredictable side effects in the long-term.
Small molecule-based therapy
A range of small molecules have been tested as multi-target therapeutics in preclinical models of nAMD. For example, brivanib, a pyrrolotriazine-based dual receptor tyrosine kinase inhibitor of FGFR1/R2 and VEGFR1/R2/R3 [185, 186] delivered IVT or by oral gavage blocked reduced CNV leakage and area following laser-induced CNV in mice [187]. Wojnarowicz et al. identified a first-in-class small molecule, AGX51, from an in silico screen, that caused ubiquitin-mediated Id protein degradation, G0/G1 arrest and reduced endothelial cell viability. IVT administration of AGX51 reduced CNV following laser injury in mice, and the AGX51/aflibercept combination had greater efficacy than aflibercept alone [188]. Hu et al. demonstrated that HR-687, a pan RGD (arginylglycylaspartic acid) integrin receptor antagonist that blocks the principal RGD integrins αvβ3, αvβ5 and α5β1, inhibits VEGF-induced leakage in the mouse retina and retinal leakage in cynomolgus monkeys following laser-induced CNV as effectively as ranibizumab [189]. Additionally, Sidman et al. found that vasotide, a small cyclic retro-inverted peptidomimetic, D(Cys-Leu-Pro-Arg-Cys) which binds neuropilin-1 (NRP-1) and VEGFR1 can inhibit retinal CNV in a laser-induced African Green monkey model after eye drop delivery [190].
We recently identified BT2, a dibenzoxazepinone that can suppress not only VEGF-A, but also p-ERK, MCP-1, ICAM-1, VCAM-1, IL-1β and IL-6 among a range of other pro-angiogenic and pro-inflammatory mediators relevant to nAMD [191, 192]. This includes transcription factors (e.g., Egr-1, c-Rel/NF-κB, KLF) and pro-angiogenic chemokines (e.g., CXCL1, CXCL3, CXCL8, CCL20). BT2 inhibits endothelial cell proliferation, migration, tubule formation and angiogenesis in mice bearing Matrigel plugs [191]. IVT BT2 reduced retinal permeability in rats as effectively as aflibercept at the same dose but needed threefold fewer injections than aflibercept [191]. BT2 also reduced retinal vascular permeability in rabbits induced by VEGF-A [191]. BT2 suppressed laser injury-induced CD31, pERK, VEGF-A and FosB/AP-1 (a family of transcription factors that regulates VEGF-A [193, 194]) expression in the retina [191]. The catalytic oligonucleotide, Dz13, provides another example of a molecular approach that inhibits VEGF-A and retinal neovascularization by targeting transcription factor (c-Jun/AP-1) controlling its expression [195, 196]. Thus, strategies that suppress regulatory factors other than merely VEGF, could potentially assist patients resistant to standard anti-VEGF therapy, or may permit a longer duration of action, as suggested by faricimab.
Ocular reservoirs
Biodegradable reservoirs implanted in the vitreous provide an alternative approach. Eyepoint Pharmaceuticals is developing EYP-1901, an indolinone-based small molecule tyrosine kinase inhibitor (vorolanib/CM082/X-82) [197] in Phase I trials for nAMD (DAVIO, NCT04747197). EYP-1901 blocks VEGFR1, R2 and R3 but also targets the platelet-derived growth factor receptor (PDGFR and ß) and colony stimulating factor 1 receptor (CSF1R), and is delivered IVT in a bioerodible (Durasert) platform for potential twice-yearly sustained delivery [198].
OXU-005 (Oxular) is developing an alternate sustained release strategy of a proprietary narrow-spectrum kinase inhibitor using a biodegradable polymer system (Oxuspheres) which seeks to provide up to 12 months’ treatment after single administration into the suprachoroidal space [199].
Systemic delivery
Agents that demonstrate efficacy in the retina following systemic delivery have been developed. This could potentially avoid the potential damaging effects of IVT injection and improve patient non-compliance. This includes small molecule inhibitors of reduction–oxidation factor 1–apurinic/apyrimidinic endonuclease 1 (APE/REF-1). APE/REF-1 redox activity regulates retinal endothelial cell growth, migration and tubule formation [200]. Intraperitoneal administration of APX2009 and APX2014 (50 mg/kg, twice daily, 5 days on/2 days off), blocked REF-1 redox signaling and attenuated laser-induced CNV in mice [200]. The likely increased risk of side effects from systemic medication administration should be balanced against benefits of systemic administration.
Stem cell-based therapy
Stem cell-based experimental therapies, while in their infancy, have been tested in AMD patients. IVT administration of adipose tissue–derived “stem cells” in those with non-neovascular AMD caused severe vision loss (NCT02024269) [201]. This was associated with a range of pathologic effects including hemorrhagic retinopathy, vitreous haemorrhage, ocular hypertension, retinal detachment or lens dislocation. Transplantation of an autologous induced pluripotent stem-cells (iPSC)-derived RPE cell sheet in a patient with nAMD did not improve or worsen BCVA after 1 year and while cystoid macular edema was present, did not cause serious adverse events after 25 months (UMIN000011929) [202]. In a Phase I study (NCT01691261), da Cruz and colleagues delivered a synthetic basement membrane-based patch made of RPE that had been differentiated from human embryonic stem cells into the subretinal space of 2 patients with neovascular AMD. Patch transplantation was achieved using biomicroscopy and optical coherence tomography. This resulted in visual acuity gain of 29 and 21 letters in each patient, respectively, over a year [203], suggesting the safety and feasibility of stem cell-based RPE regenerative therapy for AMD.
Future directions and conclusions
Since their first use as IVT drugs with nAMD patients 15 years ago [79], anti-VEGF therapies have transformed the treatment of macular degeneration and largely replaced less-effective treatments, such as photodynamic therapy [204]. Anti-VEGF agents have reduced incident legal blindness and visual impairment caused by nAMD, decreased economic and societal costs [205] and improved vision-related quality of life [206]. However, there remains unmet clinical need for improved therapies for nAMD since many patients do not respond optimally, lose response over time, or exhibit sub-optimal durability. Many patients in real-life clinical settings receive fewer anti-VEGF injections than those in clinical trial settings, and this can result in poor visual outcomes. There is a need for longer acting agents to reduce injection frequency, treatment burden, and for agents that do not leak into the systemic circulation from the vitreous.
Expansion of targets beyond VEGF-A is a promising strategy to address the contribution of non-VEGF mediated pathways to the pathogenesis and clinical manifestation of nAMD. Other agents and modalities exploiting the VEGF system and alternate pathways include heparin-binding variants of VEGF receptor 1, conbercept and iPSC-derived cells. Ultimately, a combination of approaches targeting the VEGF system concurrently with other key processes may be needed to satisfy unmet need in the treatment of nAMD. This could also allow for personalisation of treatment. Heterogeneity in clinical response to current VEGF-based therapies in nAMD suggest that different pathways predominate between individual patients. Targeting multiple pathways could improve response, prevent resistance and underpin future tailored treatments for nAMD and other neovascular/exudative retinal disorders.
Acknowledgements
The authors regret that space constraints limited the number and scope of references cited.
Author contributions
LMK wrote the first draft. All authors provided intellectual input and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from NSW Health, the National Heart Foundation of Australia, and the National Health and Medical Research Council of Australia (LMK).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
LMK has IP interests in BT2. GL has consulted for Bayer and Novartis and has received travel grants and research funding from Bayer. KYCT reports consultancy fees, honorarium, travel support and speaker fees from Topcon, Roche, Bayer and Novartis outside the submitted work. TYW is a consultant for Bayer, Boehringer-Ingelheim, Eden Ophthalmic, Genentech, Iveric Bio, Merck, Novartis, Oxurion, Roche, Samsung, Shanghai Henlius and Zhaoke Pharmaceutical. He is an inventor, holds patents and is a co-founder of start-up companies (Plano and EyRiS), which have interests in, and develop digital solutions for eye diseases. All potential conflicts of interests for consultancy, advisory boards and positions in the start-up companies, and financial renumeration, if any, are managed by institutional policies under SingHealth and Duke-NUS Medical School. PM has consulted for Bayer, Novartis, and Allergan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6:493–497. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–126. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Photodynamic Therapy, http://www.macular.org/archives/photodt.html
- 6.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7:31. doi: 10.1038/s41572-021-00265-2. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyosseva SV. Targeting MAPK signaling in AMD. Ophthalmol Eye Dis. 2016;8:23–30. doi: 10.4137/OED.S32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wooff Y, Man SM, Aggio-Bruce R, Natoli R, Fernando N. IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front Immunol. 2019;10:1618. doi: 10.3389/fimmu.2019.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Ma W, Han S, Meng Z, Zhao L, Yin Y, Wang Y, Li J. TGF-beta participates choroid neovascularization through Smad2/3-VEGF/TNF-alpha signaling in mice with Laser-induced wet age-related macular degeneration. Sci Rep. 2017;7:9672. doi: 10.1038/s41598-017-10124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR, Beckman Initiative for Macular Research Classification Committee Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apte RS. Age-related macular degeneration. N Engl J Med. 2021;385:539–547. doi: 10.1056/NEJMcp2102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilde C, Poostchi A, Mehta RL, MacNab HK, Hillman JG, Vernon SA, Amoaku WM. Prevalence of age-related macular degeneration in an elderly UK Caucasian population-The Bridlington Eye Assessment Project: a cross-sectional study. Eye (Lond) 2017;31:1042–1050. doi: 10.1038/eye.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joachim N, Colijn JM, Kifley A, Lee KE, Buitendijk GHS, Klein BEK, Myers CE, Meuer SM, Tan AG, Holliday EG, et al. Five-year progression of unilateral age-related macular degeneration to bilateral involvement: the Three Continent AMD Consortium report. Br J Ophthalmol. 2017;101:1185–1192. doi: 10.1136/bjophthalmol-2016-309729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teo KYC, Cheung GCM. New concepts in polypoidal choroidal vasculopathy imaging: a focus on optical coherence tomography and optical coherence tomography angiography. Asia Pac J Ophthalmol (Phila) 2019;8:165–171. doi: 10.22608/APO.201909. [DOI] [PubMed] [Google Scholar]
- 18.Liew G, Hyun-Jin HD, Hooper C, Chia EM, Mitchell P, Ong S, Ho IV. Prevalence of polypoidal choroidal vasculopathy in Caucasian patients as estimated from optical coherence tomography signs. Eye (Lond) 2021;35:1011–1012. doi: 10.1038/s41433-020-0834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner M, Boker A, Mauschitz MM, Gobel AP, Fimmers R, Brinkmann CK, Schmitz-Valckenberg S, Schmid M, Holz FG, Fleckenstein M, Fundus Autofluorescence in Age-Related Macular Degeneration Study Group Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122:1356–1365. doi: 10.1016/j.ophtha.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology. 2013;120:2042–2050. doi: 10.1016/j.ophtha.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Q, Daniel E, Maguire MG, Grunwald JE, Martin ER, Martin DF, Ying GS, Comparison of Age-Related Macular Degeneration Treatments Trials Research Group Pseudodrusen and incidence of late age-related macular degeneration in fellow eyes in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1530–1540. doi: 10.1016/j.ophtha.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finger RP, Wu Z, Luu CD, Kearney F, Ayton LN, Lucci LM, Hubbard WC, Hageman JL, Hageman GS, Guymer RH. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121:1252–1256. doi: 10.1016/j.ophtha.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipriani V, Leung HT, Plagnol V, Bunce C, Khan JC, Shahid H, Moore AT, Harding SP, Bishop PN, Hayward C, et al. Genome-wide association study of age-related macular degeneration identifies associated variants in the TNXB-FKBPL-NOTCH4 region of chromosome 6p21.3. Hum Mol Genet. 2012;21:4138–4150. doi: 10.1093/hmg/dds225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holliday EG, Smith AV, Cornes BK, Buitendijk GH, Jensen RA, Sim X, Aspelund T, Aung T, Baird PN, Boerwinkle E, et al. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PLoS ONE. 2013;8:e53830. doi: 10.1371/journal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, Mullins RF. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SanGiovanni JP, Chew EY. Clinical applications of age-related macular degeneration genetics. Cold Spring Harb Perspect Med. 2014;4:a017228. doi: 10.1101/cshperspect.a017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gliem M, Muller PL, Mangold E, Holz FG, Bolz HJ, Stohr H, Weber BH, Charbel Issa P. Sorsby fundus dystrophy: novel mutations, novel phenotypic characteristics, and treatment outcomes. Invest Ophthalmol Vis Sci. 2015;56:2664–2676. doi: 10.1167/iovs.14-15733. [DOI] [PubMed] [Google Scholar]
- 30.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/S0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5-year incidence of age-related maculopathy: the blue mountains eye study. Arch Ophthalmol. 2002;120:1357–1363. doi: 10.1001/archopht.120.10.1357. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarthy U, Augood C, Bentham GC, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology. 2007;114:1157–1163. doi: 10.1016/j.ophtha.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson J, Cree A, Collins A, Lotery A, Ennis S. Determination of a gene and environment risk model for age-related macular degeneration. Br J Ophthalmol. 2010;94:1382–1387. doi: 10.1136/bjo.2010.182568. [DOI] [PubMed] [Google Scholar]
- 36.Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:583–587. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddon JM, Widjajahakim R, Rosner B. Rare and common genetic variants, smoking, and body mass index: progression and earlier age of developing advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61:32. doi: 10.1167/iovs.61.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenhauer B, Natoli S, Liew G, Flood VM. Lutein and zeaxanthin-food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients. 2017;9:120. doi: 10.3390/nu9020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gopinath B, Flood VM, Kifley A, Liew G, Mitchell P. Smoking, antioxidant supplementation and dietary intakes among older adults with age-related macular degeneration over 10 years. PLoS ONE. 2015;10:e0122548. doi: 10.1371/journal.pone.0122548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu W, Wu Y, Meng YF, Xing Q, Tao JJ, Lu J. Fish consumption and age-related macular degeneration incidence: a meta-analysis and systematic review of prospective cohort studies. Nutrients. 2016;8:743. doi: 10.3390/nu8110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman NA, Jacobs RJ, Braakhuis AJ. Role of diet and food intake in age-related macular degeneration: a systematic review. Clin Exp Ophthalmol. 2019;47:106–127. doi: 10.1111/ceo.13343. [DOI] [PubMed] [Google Scholar]
- 43.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Age-Related Eye Disease Study 2 Research Group et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014;132:142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams MK, Chong EW, Williamson E, Aung KZ, Makeyeva GA, Giles GG, English DR, Hopper J, Guymer RH, Baird PN, et al. 20/20–Alcohol and age-related macular degeneration: the Melbourne Collaborative Cohort Study. Am J Epidemiol. 2012;176:289–298. doi: 10.1093/aje/kws004. [DOI] [PubMed] [Google Scholar]
- 46.Shankar A, Mitchell P, Rochtchina E, Tan J, Wang JJ. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am J Epidemiol. 2007;165:375–382. doi: 10.1093/aje/kwk022. [DOI] [PubMed] [Google Scholar]
- 47.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung CM, Wong TY. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J Intern Med. 2014;276:140–153. doi: 10.1111/joim.12227. [DOI] [PubMed] [Google Scholar]
- 49.Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 2016;3:34. doi: 10.1186/s40662-016-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kabasawa S, Mori K, Horie-Inoue K, Gehlbach PL, Inoue S, Awata T, Katayama S, Yoneya S. Associations of cigarette smoking but not serum fatty acids with age-related macular degeneration in a Japanese population. Ophthalmology. 2011;118:1082–1088. doi: 10.1016/j.ophtha.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Liew G, Mitchell P, Wong TY, Rochtchina E, Wang JJ. The association of aspirin use with age-related macular degeneration. JAMA Intern Med. 2013;173:258–264. doi: 10.1001/jamainternmed.2013.1583. [DOI] [PubMed] [Google Scholar]
- 52.Klein BE, Howard KP, Gangnon RE, Dreyer JO, Lee KE, Klein R. Long-term use of aspirin and age-related macular degeneration. JAMA. 2012;308:2469–2478. doi: 10.1001/jama.2012.65406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song D, Hua P, VanderBeek BL, Dunaief JL, Grunwald JE, Daniel E, Maguire MG, Martin DF, Ying GS, CATT Research Group Systemic medication use and the incidence and growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Retina. 2021;41:1455–1462. doi: 10.1097/IAE.0000000000003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keenan TD, Wiley HE, Agron E, Aronow ME, Christen WG, Clemons TE, Chew EY, Age-Related Eye Disease Study, Age-Related Eye Disease Study 2 Research Group The association of aspirin use with age-related macular degeneration progression in the age-related eye disease studies: age-related eye disease study 2 report no. 20. Ophthalmology. 2019;126:1647–1656. doi: 10.1016/j.ophtha.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rim TH, Yoo TK, Kwak J, Lee JS, Kim SH, Kim DW, Kim SS. Long-term regular use of low-dose aspirin and neovascular age-related macular degeneration: national sample cohort 2010–2015. Ophthalmology. 2019;126:274–282. doi: 10.1016/j.ophtha.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Casparis H, Lindsley K, Kuo IC, Sikder S, Bressler NM. Surgery for cataracts in people with age-related macular degeneration. Cochrane Database Syst Rev. 2017;2:CD006757. doi: 10.1002/14651858.CD006757.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu CJ, Mitchell P, Klein R, Klein BE, Chang ML, Gensler G, Taylor A. A risk score for the prediction of advanced age-related macular degeneration: development and validation in 2 prospective cohorts. Ophthalmology. 2014;121:1421–1427. doi: 10.1016/j.ophtha.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seddon JM, Reynolds R, Yu Y, Daly MJ, Rosner B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011;118:2203–2211. doi: 10.1016/j.ophtha.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buitendijk GH, Rochtchina E, Myers C, van Duijn CM, Lee KE, Klein BE, Meuer SM, de Jong PT, Holliday EG, Tan AG, et al. Prediction of age-related macular degeneration in the general population: the Three Continent AMD Consortium. Ophthalmology. 2013;120:2644–2655. doi: 10.1016/j.ophtha.2013.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, Wykoff CC, Singerman LJ, Abraham P, Grassmann F, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, Staurenghi G, Mones J, Baumal CR, Saroj N, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology. 2021;128:1325–1336. doi: 10.1016/j.ophtha.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 62.Pegcetacoplan reduces geographic atrophy lesion growth, https://www.practiceupdate.com/content/aao-2022-pegcetacoplan-reduces-geographic-atrophy-lesion-growth/143197/45
- 63.Holz FG, Sadda SR, Busbee B, Chew EY, Mitchell P, Tufail A, Brittain C, Ferrara D, Gray S, Honigberg L, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136:666–677. doi: 10.1001/jamaophthalmol.2018.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuppermann BD, Patel SS, Boyer DS, Augustin AJ, Freeman WR, Kerr KJ, Guo Q, Schneider S, Lopez FJ, et al. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (Brimo Dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina. 2021;41:144–155. doi: 10.1097/IAE.0000000000002789. [DOI] [PubMed] [Google Scholar]
- 65.Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, Lad EM, Silverman D, Henry EC, Anderesi M, et al. Visual function decline resulting from geographic atrophy: results from the chroma and spectri phase 3 trials. Ophthalmol Retina. 2020;4:673–688. doi: 10.1016/j.oret.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 66.Lindner M, Boker A, Mauschitz MM, Gobel AP, Fimmers R, Brinkmann CK, Schmitz-Valckenberg S, Schmid M, Holz FG, Fleckenstein M. Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122:1356–1365. doi: 10.1016/j.ophtha.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 67.Kimel M, Leidy NK, Tschosik E, Dolan C, Souied EH, Varma R, Bressler NM. Functional reading independence (FRI) index: a new patient-reported outcome measure for patients with geographic atrophy. Invest Ophthalmol Vis Sci. 2016;57:6298–6304. doi: 10.1167/iovs.16-20361. [DOI] [PubMed] [Google Scholar]
- 68.Schaal KB, Rosenfeld PJ, Gregori G, Yehoshua Z, Feuer WJ. Anatomic clinical trial endpoints for nonexudative age-related macular degeneration. Ophthalmology. 2016;123:1060–1079. doi: 10.1016/j.ophtha.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 69.Boyer DS, Schmidt-Erfurth U, van Lookeren CM, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:819–835. doi: 10.1097/IAE.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, Zhang K, Sadda S, Feuer W, Rosenfeld PJ. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, van Lookeren CM, Le KN, Graham RR, Beres T, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9:395. doi: 10.1126/scitranslmed.aaf1443. [DOI] [PubMed] [Google Scholar]
- 72.Jaffe GJ, Schmitz-Valckenberg S, Boyer D, Heier J, Wolf-Schnurrbusch U, Staurenghi G, Schmidt-Erfurth U, Holz FG. Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. Am J Ophthalmol. 2015;160:1226–1234. doi: 10.1016/j.ajo.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 73.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, et al. Intravitreal aflibercept for DME. Ophthalmology. 2014;121:2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 74.Folk JC, Stone EM. Ranibizumab therapy for neovascular age-related macular degeneration. N Engl J Med. 2010;363:1648–1655. doi: 10.1056/NEJMct1000495. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 76.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372.e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 78.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 79.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 80.Thomas M, Mousa SS, Mousa SA. Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2013;7:495–501. doi: 10.2147/OPTH.S29974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, CATT_Research_Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pham B, Thomas SM, Lillie E, Lee T, Hamid J, Richter T, Janoudi G, Agarwal A, Sharpe JP, Scott A, et al. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open. 2019;9:e022031. doi: 10.1136/bmjopen-2018-022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Comparison of Age-related Macular Degeneration Treatments Trials Research Group Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Heier JS, Singh RP, Wykoff CC, Csaky KG, Lai TYY, Loewenstein A, Schlottmann PG, Paris LP, Westenskow PD, Quezada-Ruiz C. The angiopoietin/tie pathway in retinal vascular diseases: a review. Retina. 2021;41:1–19. doi: 10.1097/IAE.0000000000003003. [DOI] [PubMed] [Google Scholar]
- 86.Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–740. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 87.New phase III data show Roche’s faricimab is the first investigational injectable eye medicine to extend time between treatments up to four months in two leading causes of vision loss, potentially reducing treatment burden for patients, https://www.roche.com/media/releases/med-cor-2021-02-12.htm
- 88.Sahni J, Dugel PU, Patel SS, Chittum ME, Berger B, Del Valle RM, Sadikhov S, Szczesny P, Schwab D, Nogoceke E, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the AVENUE phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:955–963. doi: 10.1001/jamaophthalmol.2020.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khanani AM, Patel SS, Ferrone PJ, Osborne A, Sahni J, Grzeschik S, Basu K, Ehrlich JS, Haskova Z, Dugel PU. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:964–972. doi: 10.1001/jamaophthalmol.2020.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Novartis provides update on use and safety of Beovu® in patients with wet AMD, https://www.novartis.com/news/novartis-provides-update-use-and-safety-beovu-patients-wet-amd
- 91.Baumal CR, Spaide RF, Vajzovic L, Freund KB, Walter SD, John V, Rich R, Chaudhry N, Lakhanpal RR, Oellers PR, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127:1345–1359. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Callanan D, Kunimoto D, Maturi RK, Patel SS, Staurenghi G, Wolf S, Cheetham JK, Hohman TC, Kim K, Lopez FJ, Schneider S. Double-masked, randomized, phase 2 evaluation of abicipar pegol (an anti-VEGF DARPin therapeutic) in neovascular age-related macular degeneration. J Ocul Pharmacol Ther. 2018;34:700–709. doi: 10.1089/jop.2018.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kunimoto D, Yoon YH, Wykoff CC, Chang A, Khurana RN, Maturi RK, Agostini H, Souied E, Chow DR, Lotery AJ, et al. Efficacy and safety of abicipar in neovascular age-related macular degeneration: 52-week results of phase 3 randomized controlled study. Ophthalmology. 2020;127:1331–1344. doi: 10.1016/j.ophtha.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 94.Mullard A. FDA rejects first DARPin. Nat Rev Drug Discov. 2020;19:501. doi: 10.1038/d41573-020-00127-8. [DOI] [PubMed] [Google Scholar]
- 95.Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, Clark WL, Abdelfattah NS, Sadda SR, TREX-AMD Study Group Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1:314–321. doi: 10.1016/j.oret.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, Lambrou G, Schmelter T, Wolf S. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the aries study: a randomized clinical trial. Retina. 2021;41:1911–1920. doi: 10.1097/IAE.0000000000003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, Investigators A. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37:1173–1187. doi: 10.1007/s12325-020-01236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59:1–18. doi: 10.1016/j.survophthal.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Dedania VS, Grob S, Zhang K, Bakri SJ. Pharmacogenomics of response to anti-VEGF therapy in exudative age-related macular degeneration. Retina. 2015;35:381–391. doi: 10.1097/IAE.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 100.Abedi F, Wickremasinghe S, Richardson AJ, Makalic E, Schmidt DF, Sandhu SS, Baird PN, Guymer RH. Variants in the VEGFA gene and treatment outcome after anti-VEGF treatment for neovascular age-related macular degeneration. Ophthalmology. 2013;120:115–121. doi: 10.1016/j.ophtha.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 101.Kozhevnikova OS, Fursova AZ, Derbeneva AS, Nikulich IF, Tarasov MS, Devyatkin VA, Rumyantseva YV, Telegina DV, Kolosova NG. Association between polymorphisms in CFH, ARMS2, CFI, and C3 genes and response to anti-VEGF treatment in neovascular age-related macular degeneration. Biomedicines. 2022;10:1658. doi: 10.3390/biomedicines10071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kubicka-Trzaska A, Zuber-Laskawiec K, Dziedzina S, Sanak M, Romanowska-Dixon B, Karska-Basta I. Genetic variants of complement factor H Y402H (rs1061170), C2 R102G (rs2230199), and C3 E318D (rs9332739) and response to intravitreal anti-VEGF treatment in patients with exudative age-related macular degeneration. Medicina (Kaunas) 2022;58:658. doi: 10.3390/medicina58050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vofo BN, Beykin G, Levy J, Chowers I. Long-term outcome of neovascular age-related macular degeneration: association between treatment outcome and major risk alleles. Br J Ophthalmol. 2021;122:1837–1845. doi: 10.1136/bjophthalmol-2021-319054. [DOI] [PubMed] [Google Scholar]
- 104.Lores-Motta L, Riaz M, Grunin M, Corominas J, van Asten F, Pauper M, Leenders M, Richardson AJ, Muether P, Cree AJ, et al. Association of genetic variants with response to anti-vascular endothelial growth factor therapy in age-related macular degeneration. JAMA Ophthalmol. 2018;136:875–884. doi: 10.1001/jamaophthalmol.2018.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hagstrom SA, Ying GS, Pauer GJT, Sturgill-Short GM, Huang J, Callanan DG, Kim IK, Klein ML, Maguire MG, Martin DF, Comparison of AMD Treatments Trials Research Group Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT) Ophthalmology. 2013;120:593–599. doi: 10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, Maguire MG, Martin DF, Comparison of Age-Related Macular Degeneration Treatments Trials Research Group VEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy: comparison of age-related macular degeneration treatments trials (CATT) JAMA Ophthalmol. 2014;132:521–527. doi: 10.1001/jamaophthalmol.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spooner K, Hong T, Wijeyakumar W, Chang AA. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017;11:161–177. doi: 10.2147/OPTH.S125676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Broadhead GK, Hong T, Chang AA. Treating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014;92:713–723. doi: 10.1111/aos.12463. [DOI] [PubMed] [Google Scholar]
- 109.Teo CKY, Nguyen V, Cheung GCM, Arnold JJ, Chen FK, Barthelmes D, Gillies MC. The impact of disease activity on 5 year outcomes in patients undergoing treatment for neovascular age related macular degeneration. Retina. 2022;42:95–106. doi: 10.1097/IAE.0000000000003267. [DOI] [PubMed] [Google Scholar]
- 110.Okada M, Mitchell P, Finger RP, Eldem B, Talks SJ, Hirst C, Paladini L, Barratt J, Wong TY, Loewenstein A. Non-adherence or non-persistence to intravitreal injection therapy for neovascular ARMD. Ophthalmology. 2021;128:234–247. doi: 10.1016/j.ophtha.2020.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, Hoyng CB, Hykin P, Staurenghi G, Heldner S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teo KY, Nguyen V, O’Toole L, Daien V, Sanchez-Monroy J, Ricci F, Ponsioen TL, Morros HB, Cheung CMG, Arnold JJ. Longer treatment intervals are associated with reduced treatment persistence in neovascular age related macular degeneration. Eye (Lond). 2023;37:467–473. doi: 10.1038/s41433-022-01957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Horner F, Lip PL, Clark H, Chavan R, Sarmad A, Mushtaq B. Real-world visual and clinical outcomes for patients with neovascular age-related macular degeneration treated with intravitreal ranibizumab: an 8-year observational cohort (AMD8) Clin Ophthalmol. 2019;13:2461–2467. doi: 10.2147/OPTH.S218378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehta H, Tufail A, Daien V, Lee AY, Nguyen V, Ozturk M, Barthelmes D, Gillies MC. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146. doi: 10.1016/j.preteyeres.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Teo KY, Saxena N, Gan A, Wong TY, Gillies MC, Chakravarthy U, Gemmy Cheung CM. Detrimental effect of delayed re-treatment of active disease on outcomes in neovascular age-related macular degeneration: the RAMPS study. Ophthalmol Retina. 2020;4:871–880. doi: 10.1016/j.oret.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen CL, Gillies MC, Nguyen V, Daien V, Cohn A, Banerjee G, Arnold J, Fight Retinal Blindness! Study Group Characterization of poor visual outcomes of neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor agents. Ophthalmology. 2019;126:735–742. doi: 10.1016/j.ophtha.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 117.Ichiyama Y, Sawada T, Sawada O, Ito Y, Kakinoki M, Obata S, Saishin Y, Ohji M. The correlation between aqueous vascular endothelial growth factor level and clinical activity in neovascular age-related macular degeneration. Retina. 2021;41:111–117. doi: 10.1097/IAE.0000000000002790. [DOI] [PubMed] [Google Scholar]
- 118.Garweg JG, Traine PG, Garweg RA, Wons J, Gerhardt C, Pfister IB. Continued anti-VEGF treatment does not prevent recurrences in eyes with stable neovascular age-related macular degeneration using a treat-and-extend regimen: a retrospective case series. Eye (Lond) 2022;36:862–868. doi: 10.1038/s41433-021-01562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khurana RN. Long-term management of neovascular age-related macular degeneration: to suspend or not to suspend? Ophthalmol Retina. 2019;3:621–622. doi: 10.1016/j.oret.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 120.Teo ZL, Tham YC, Yan Yu MC, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–1591. doi: 10.1016/j.ophtha.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 121.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Health Med. 2015;20:296–310. doi: 10.1080/13548506.2014.936886. [DOI] [PubMed] [Google Scholar]
- 122.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23:127–140. doi: 10.1080/13548506.2016.1274040. [DOI] [PubMed] [Google Scholar]
- 123.Droege KM, Muether PS, Hermann MM, Caramoy A, Viebahn U, Kirchhof B, Fauser S. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251:1281–1284. doi: 10.1007/s00417-012-2177-3. [DOI] [PubMed] [Google Scholar]
- 124.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, Huang J, Ying GS, Hagstrom SA, Winter K, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:656–666. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, Toth CA, Hagstrom SA, Maguire MG, Martin DF, Comparison of Age-Related Macular Degeneration Treatments Trials Research Group Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124:97–104. doi: 10.1016/j.ophtha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khachigian LM. Pharmaceutical patents: reconciling the human right to health with the incentive to invent. Drug Discov Today. 2020;25:1135–1141. doi: 10.1016/j.drudis.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR, Diabetic Retinopathy Clinical Research Network : Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134:888–896. doi: 10.1001/jamaophthalmol.2016.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR, Jampol LM, Stone TW, Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375–381. doi: 10.1016/j.ophtha.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bro T, Derebecka M, Jorstad OK, Grzybowski A. Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol. 2020;258:503–511. doi: 10.1007/s00417-019-04569-8. [DOI] [PubMed] [Google Scholar]
- 130.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. doi: 10.3928/1542-8877-20050701-14. [DOI] [PubMed] [Google Scholar]
- 131.Parikh R, Ross JS, Sangaralingham LR, Adelman RA, Shah ND, Barkmeier AJ. Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and medicare advantage patients. Ophthalmology. 2017;124:352–358. doi: 10.1016/j.ophtha.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 132.Lai TYY, Cheung CMG, Mieler WF. Ophthalmic application of anti-VEGF therapy. Asia Pac J Ophthalmol (Phila) 2017;6:479–480. doi: 10.22608/APO.2017559. [DOI] [PubMed] [Google Scholar]
- 133.Yanagi Y, Fukuda A, Barzey V, Adachi K. Cost-effectiveness of intravitreal aflibercept versus other treatments for wet age-related macular degeneration in Japan. J Med Econ. 2017;20:204–212. doi: 10.1080/13696998.2016.1245196. [DOI] [PubMed] [Google Scholar]
- 134.Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology. 2003;49:1–11. doi: 10.1159/000066507. [DOI] [PubMed] [Google Scholar]
- 135.Shehab N, Brown MN, Kallen AJ, Perz JF. U.S. Compounding pharmacy-related outbreaks, 2001–2013: public health and patient safety lessons learned. J Patient Saf. 2018;14:164–173. doi: 10.1097/PTS.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woo SJ, Veith M, Hamouz J, Ernest J, Zalewski D, Studnicka J, Vajas A, Papp A, Gabor V, Luu J, et al. Efficacy and safety of a proposed ranibizumab biosimilar product vs a reference ranibizumab product for patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2021;139:68–76. doi: 10.1001/jamaophthalmol.2020.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharma A, Kumar N, Parachuri N, Bandello F, Kuppermann BD, Loewenstein A. Ranibizumab biosimilar (Razumab) vs innovator ranibizumab (Lucentis) in neovascular age-related macular degeneration (n-AMD)- efficacy and safety (BIRA study) Eye (Lond) 2021;36:1106–1107. doi: 10.1038/s41433-021-01616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Merani R, Hunyor AP. Endophthalmitis following intravitreal anti-vascular endothelial growth factor (VEGF) injection: a comprehensive review. Int J Retina Vitreous. 2015;1:9. doi: 10.1186/s40942-015-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ho IV, Fernandez-Sanz G, Levasseur S, Ting E, Liew G, Playfair J, Downie J, Gorbatov M, Hunyor AP, Chang AA. Early pars plana vitrectomy for treatment of acute infective endophthalmitis. Asia Pac J Ophthalmol (Phila) 2019;8:3–7. doi: 10.22608/APO.2018414. [DOI] [PubMed] [Google Scholar]
- 140.Patil NS, Dhoot AS, Popovic MM, Kertes PJ, Muni RH. Risk of intraocular inflammation after injection of antivascular endothelial growth factor agents: a meta-analysis. Retina. 2022;42:2134–2142. doi: 10.1097/IAE.0000000000003582. [DOI] [PubMed] [Google Scholar]
- 141.Daien V, Nguyen V, Essex RW, Guymer R, Arnold JJ, Munk M, Ceklic L, Gillies MC, Barthelmes D, Fight Retinal Blindness! investigators Prevalence and characteristics of macular atrophy in eyes with neovascular age-related macular degeneration. A study from a long-term observational dataset: the Fight Retinal Blindness project. Br J Ophthalmol. 2020;104:1064–1069. doi: 10.1136/bjophthalmol-2019-315055. [DOI] [PubMed] [Google Scholar]
- 142.Sadda SR, Tuomi LL, Ding B, Fung AE, Hopkins JJ. Macular Atrophy in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2018;125:878–886. doi: 10.1016/j.ophtha.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 143.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Group S-US: Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 144.Avery RL. What is the evidence for systemic effects of intravitreal anti-VEGF agents, and should we be concerned? Br J Opthalmol. 2014;98(Suppl):1. doi: 10.1136/bjophthalmol-2013-303844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Usui-Ouchi A, Friedlander M. Anti-VEGF therapy: higher potency and long-lasting antagonism are not necessarily better. J Clin Invest. 2019;129:3032–3034. doi: 10.1172/JCI129862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Maia M, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37:1847–1858. doi: 10.1097/IAE.0000000000001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tseng JJ, Vance SK, Della Torre KE, Mendonca LS, Cooney MJ, Klancnik JM, Sorenson JA, Freund KB. Sustained increased intraocular pressure related to intravitreal antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. J Glaucoma. 2012;21:241–247. doi: 10.1097/IJG.0b013e31820d7d19. [DOI] [PubMed] [Google Scholar]
- 148.Dedania VS, Bakri SJ. Sustained elevation of intraocular pressure after intravitreal anti-vegf agents: what is the evidence? Retina. 2015;35:841–858. doi: 10.1097/IAE.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 149.Sengul A, Rasier R, Ciftci C, Artunay O, Kockar A, Bahcecioglu H, Yuzbasioglu E. Short-term effects of intravitreal ranibizumab and bevacizumab administration on 24-h ambulatory blood pressure monitoring recordings in normotensive patients with age-related macular degeneration. Eye (Lond) 2017;31:677–683. doi: 10.1038/eye.2016.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Costagliola C, Morescalchi F, Duse S, Romano D, Mazza G, Parmeggiani F, Bartollino S, Semeraro F. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: an update. Expert Opin Drug Saf. 2019;18:803–815. doi: 10.1080/14740338.2019.1643838. [DOI] [PubMed] [Google Scholar]
- 151.Dalvin LA, Starr MR, AbouChehade JE, Damento GM, Garcia M, Shah SM, Hodge DO, Meissner I, Bakri SJ, Iezzi R. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophthalmol. 2019;137:483–490. doi: 10.1001/jamaophthalmol.2018.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Semeraro F, Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: an overview. Expert Opin Drug Saf. 2014;13:785–802. doi: 10.1517/14740338.2014.911284. [DOI] [PubMed] [Google Scholar]
- 153.Li D, Xie K, Ding G, Li J, Chen K, Li H, Qian J, Jiang C, Fang J. Tumor resistance to anti-VEGF therapy through up-regulation of VEGF-C expression. Cancer Lett. 2014;346:45–52. doi: 10.1016/j.canlet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 154.Rauniyar K, Jha SK, Jeltsch M. Biology of vascular endothelial growth factor C in the morphogenesis of lymphatic vessels. Front Bioeng Biotechnol. 2018;6:7. doi: 10.3389/fbioe.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, Eng C, Morris J, Ellis L, Heymach JV, Kopetz S. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS ONE. 2013;8:e77117. doi: 10.1371/journal.pone.0077117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grau S, Thorsteinsdottir J, von Baumgarten L, Winkler F, Tonn JC, Schichor C. Bevacizumab can induce reactivity to VEGF-C and -D in human brain and tumour derived endothelial cells. J Neurooncol. 2011;104:103–112. doi: 10.1007/s11060-010-0480-6. [DOI] [PubMed] [Google Scholar]
- 157.Stacker SA, Achen MG. Emerging roles for VEGF-D in human disease. Biomolecules. 2018;8:1. doi: 10.3390/biom8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cabral T, Lima LH, Mello LGM, Polido J, Correa EP, Oshima A, Duong J, Serracarbassa P, Regatieri CV, Mahajan VB, Belfort R., Jr Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmol Retina. 2018;2:31–37. doi: 10.1016/j.oret.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xin H, Biswas N, Li P, Zhong C, Chan TC, Nudleman E, Ferrara N. Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders. Proc Natl Acad Sci U S A. 2021;118:e1921252118. doi: 10.1073/pnas.1921252118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Q, Li T, Wu Z, Wu Q, Ke X, Luo D, Wang H. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS ONE. 2013;8:e70544. doi: 10.1371/journal.pone.0070544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu BH, Wang B, Wu HQ, Chang Q, Lu HQ. Intravitreal conbercept injection for neovascular age-related macular degeneration. Int J Ophthalmol. 2019;12:252–257. doi: 10.18240/ijo.2019.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calugaru D, Calugaru M. Comment on "Intravitreal conbercept injection for neovascular age-related macular degeneration". Int J Ophthalmol. 2020;13:362–364. doi: 10.18240/ijo.2020.02.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dugel PU, Boyer DS, Antoszyk AN, Steinle NC, Varenhorst MP, Pearlman JA, Gillies MC, Finger RP, Baldwin ME, Leitch IM. Phase 1 study of OPT-302 inhibition of vascular endothelial growth factors C and D for neovascular age-related macular degeneration. Ophthalmol Retina. 2020;4:250–263. doi: 10.1016/j.oret.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 164.Li K, Tian H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J Drug Target. 2019;27:244–256. doi: 10.1080/1061186X.2018.1440400. [DOI] [PubMed] [Google Scholar]
- 165.Tsujinaka H, Fu J, Shen J, Yu Y, Hafiz Z, Kays J, McKenzie D, Cardona D, Culp D, Peterson W, et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat Commun. 2020;11:694. doi: 10.1038/s41467-020-14340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Samanta A, Aziz AA, Jhingan M, Singh SR, Khanani AM, Chhablani J. Emerging therapies in neovascular age-related macular degeneration in 2020. Asia Pac J Ophthalmol (Phila) 2020;9:250–259. doi: 10.1097/APO.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rush JS, Bingaman DP, Chaney PG, Wax MB, Ceresa BP. Administration of menadione, vitamin K3, ameliorates off-target effects on corneal epithelial wound healing due to receptor tyrosine kinase inhibition. Invest Ophthalmol Vis Sci. 2016;57:5864–5871. doi: 10.1167/iovs.16-19952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gelfman CM, Grishanin R, Bender KO, Nguyen A, Greengard J, Sharma P, Nieves J, Kiss S, Gasmi M. Comprehensive preclinical assessment of ADVM-022, an intravitreal anti-VEGF gene therapy for the treatment of neovascular AMD and diabetic macular edema. J Ocul Pharmacol Ther. 2021;37:181–190. doi: 10.1089/jop.2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grishanin R, Vuillemenot B, Sharma P, Keravala A, Greengard J, Gelfman C, Blumenkrantz M, Lawrence M, Hu W, Kiss S, Gasmi M. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol Ther. 2019;27:118–129. doi: 10.1016/j.ymthe.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ding K, Shen J, Hafiz Z, Hackett SF, Silva RLE, Khan M, Lorenc VE, Chen D, Chadha R, Zhang M, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019;129:4901–4911. doi: 10.1172/JCI129085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y, Fortmann SD, Shen J, Wielechowski E, Tretiakova A, Yoo S, Kozarsky K, Wang J, Wilson JM, Campochiaro PA. AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol Ther. 2018;26:542–549. doi: 10.1016/j.ymthe.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chandrasekaran PR, Madanagopalan VG. KSI-301: antibody biopolymer conjugate in retinal disorders. Ther Adv Ophthalmol. 2021;13:25158414211027708. doi: 10.1177/25158414211027708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kodiak: Kodiak Announces 1-Year Durability, Efficacy and Safety Data from Ongoing Phase 1b Study of KSI-301 in Patients with Wet AMD, DME and RVO at Angiogenesis 2021. https://kodiak.com/press-releases/kodiakannounces-1-year-durability-efficacy-and-safety-data-from-ongoing-phase-1b-study-of-ksi-301-in-patients-with-wet-amd-dme-and-rvo-at-angiogenesis-2021/
- 174.Chen ER, Kaiser PK. Therapeutic potential of the ranibizumab port delivery system in the treatment of AMD: evidence to date. Clin Ophthalmol. 2020;14:1349–1355. doi: 10.2147/OPTH.S194234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Phase III data show Roche’s Port Delivery System with ranibizumab enabled over 98% of patients to go six months between treatments for neovascular age-related macular degeneration. https://www.roche.com/media/releases/med-cor-2020-07-22b.htm
- 176.Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, Chiang Y, Ehrlich JS, Erickson S, Hanley WD, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmology. 2019;126:1141–1154. doi: 10.1016/j.ophtha.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 177.Pieramici DJ, Heimann F, Brassard R, Barteselli G, Ranade S. Virtual reality becomes a reality for ophthalmologic surgical clinical trials. Transl Vis Sci Technol. 2020;9:1. doi: 10.1167/tvst.9.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jonas JB, Tao Y, Neumaier M, Findeisen P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch Ophthalmol. 2010;128:1281–1286. doi: 10.1001/archophthalmol.2010.227. [DOI] [PubMed] [Google Scholar]
- 179.Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, Jonet L, Behar-Cohen F, Chemtob S, Guillonneau X, et al. Interleukin-1beta inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol. 2011;178:2416–2423. doi: 10.1016/j.ajpath.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Izumi-Nagai K, Nagai N, Ozawa Y, Mihara M, Ohsugi Y, Kurihara T, Koto T, Satofuka S, Inoue M, Tsubota K, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170:2149–2158. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cabral T, Mello LGM, Lima LH, Polido J, Regatieri CV, Belfort R, Jr, Mahajan VB. Retinal and choroidal angiogenesis. Int J Retina Vitreous. 2017;3:31. doi: 10.1186/s40942-017-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Prasad PS, Schwartz SD, Hubschman JP. AMD: current and novel therapies. Maturitas. 2010;66:46–50. doi: 10.1016/j.maturitas.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 183.Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Janne PA, Engelman JA. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. 2011;71:1081–1091. doi: 10.1158/0008-5472.CAN-10-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Welsh SJ, Rizos H, Scolyer RA, Long GV. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur J Cancer. 2016;62:76–85. doi: 10.1016/j.ejca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 185.Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, Lombardo LJ, Borzilleri RM, Zheng X, Wu LI, et al. Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem. 2006;49:2143–2146. doi: 10.1021/jm051106d. [DOI] [PubMed] [Google Scholar]
- 186.Cai ZW, Zhang Y, Borzilleri RM, Qian L, Barbosa S, Wei D, Zheng X, Wu L, Fan J, Shi Z, et al. Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4] triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215) J Med Chem. 2008;51:1976–1980. doi: 10.1021/jm7013309. [DOI] [PubMed] [Google Scholar]
- 187.Li L, Zhu M, Wu W, Qin B, Gu J, Tu Y, Chen J, Liu D, Shi Y, Liu X, et al. Brivanib, a multitargeted small-molecule tyrosine kinase inhibitor, suppresses laser-induced CNV in a mouse model of neovascular AMD. J Cell Physiol. 2020;235:1259–1273. doi: 10.1002/jcp.29041. [DOI] [PubMed] [Google Scholar]
- 188.Wojnarowicz PM, Lima ESR, Ohnaka M, Lee SB, Chin Y, Kulukian A, Chang SH, Desai B, Garcia Escolano M, Shah R, et al. A small-molecule pan-id antagonist inhibits pathologic ocular neovascularization. Cell Rep. 2019;29(62–75):e67. doi: 10.1016/j.celrep.2019.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu TT, Vanhove M, Porcu M, Van Hove I, Van Bergen T, Jonckx B, Barbeaux P, Vermassen E, Feyen JHM. The potent small molecule integrin antagonist THR-687 is a promising next-generation therapy for retinal vascular disorders. Exp Eye Res. 2019;180:43–52. doi: 10.1016/j.exer.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 190.Sidman RL, Li J, Lawrence M, Hu W, Musso GF, Giordano RJ, Cardo-Vila M, Pasqualini R, Arap W. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci Transl Med. 2015;7:309ra165. doi: 10.1126/scitranslmed.aac4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li Y, Alhendi MN, Yeh MC, Elahy M, Santiago FS, Deshpande N, Wu B, Chan E, Inam S, Prado-Lourenco L, et al. Thermostable small molecule inhibitor of angiogenesis and vascular permeability that suppresses a pERK-FosB/∆FosB-VCAM-1 axis. Sci Adv. 2020 doi: 10.1126/sciadv.aaz7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yeh MC, Wu B, Li Y, Elahy M, Prado-Lourenco L, Sockler J, Lau H, Day RO, Khachigian LM. BT2 suppresses human monocytic-endothelial cell adhesion, bone erosion and inflammation. J Inflamm Res. 2021;14:1019–1028. doi: 10.2147/JIR.S296676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jia J, Ye T, Cui P, Hua Q, Zeng H, Zhao D. AP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation. Microvasc Res. 2016;105:103–108. doi: 10.1016/j.mvr.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Damert A, Risau W. Activator protein-1 binding potentiates the hypoxia-inducible factor-mediated hypoxia-induced transcriptional activation of vascular -endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327:419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fahmy R, Waldman A, Zhang G, Mitchell A, Tedla N, Cai H, Chesterman CN, Geczy CR, Perry MA, Khachigian LM. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol. 2006;24:856–863. doi: 10.1038/nbt1225. [DOI] [PubMed] [Google Scholar]
- 196.Chan CWS, Kaplan W, Parish CR, Khachigian LM. Regression of retinal neovascularization, improvement in forepaw reach, comparative microarray and gene set enrichment analysis with c-jun targeting DNA enzyme. PLoS ONE. 2012;7:e39160. doi: 10.1371/journal.pone.0039160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sheng X, Yan X, Chi Z, Cui C, Si L, Tang B, Li S, Mao L, Lian B, Wang X, et al. Phase 1 trial of vorolanib (CM082) in combination with everolimus in patients with advanced clear-cell renal cell carcinoma. EBioMedicine. 2020;55:102755. doi: 10.1016/j.ebiom.2020.102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pipeline, https://eyepointpharma.com/pipeline/
- 199.Pipeline, https://oxular.com/pipeline/
- 200.Sardar Pasha SPB, Sishtla K, Sulaiman RS, Park B, Shetty T, Shah F, Fishel ML, Wikel JH, Kelley MR, Corson TW. Ref-1/APE1 inhibition with novel small molecules blocks ocular neovascularization. J Pharmacol Exp Ther. 2018;367:108–118. doi: 10.1124/jpet.118.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE, 2nd, Parrott MB, Rosenfeld PJ, Flynn HW, Jr, Goldberg JL. Vision loss after intravitreal injection of autologous "Stem Cells" for AMD. N Engl J Med. 2017;376:1047–1053. doi: 10.1056/NEJMoa1609583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 203.da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 204.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 205.Hodgson N, Wu F, Zhu J, Wang W, Ferreyra H, Zhang K, Wang J. Economic and Quality of Life Benefits of Anti-VEGF Therapy. Mol Pharm. 2016;13:2877–2880. doi: 10.1021/acs.molpharmaceut.5b00775. [DOI] [PubMed] [Google Scholar]
- 206.Finger RP, Guymer RH, Gillies MC, Keeffe JE. The impact of anti-vascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology. 2014;121:1246–1251. doi: 10.1016/j.ophtha.2013.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


