Keywords: hormones, mechanisms of taste, SARS-CoV-2, stem cell, taste
Abstract
The tongue is a complex multifunctional organ that interacts and senses both interoceptively and exteroceptively. Although it is easily visible to almost all of us, it is relatively understudied and what is in the literature is often contradictory or is not comprehensively reported. The tongue is both a motor and a sensory organ: motor in that it is required for speech and mastication, and sensory in that it receives information to be relayed to the central nervous system pertaining to the safety and quality of the contents of the oral cavity. Additionally, the tongue and its taste apparatus form part of an innate immune surveillance system. For example, loss or alteration in taste perception can be an early indication of infection as became evident during the present global SARS-CoV-2 pandemic. Here, we particularly emphasize the latest updates in the mechanisms of taste perception, taste bud formation and adult taste bud renewal, and the presence and effects of hormones on taste perception, review the understudied lingual immune system with specific reference to SARS-CoV-2, discuss nascent work on tongue microbiome, as well as address the effect of systemic disease on tongue structure and function, especially in relation to taste.
CLINICAL HIGHLIGHTS.
The tongue is a multifunctional organ, and the teaching of its physiology has been, at best, perfunctory. It would be best to consider it in the context of digestion and metabolism. The present SARS-CoV-2 pandemic has illuminated the deficits in clinicians’ (dentists, oral surgeons, nurses, physiotherapists, occupational therapists, psychologists, physicians, etc.) understanding of how taste is perceived.
In this review, we give an overview of tongue physiology and anatomy, with emphasis on taste papillae, taste buds, and taste receptor cells within the buds. We hope that by outlining the elegance and complexity of signaling required for taste perception we will stimulate basic and clinical researchers and all health care providers to appreciate the tongue as an organ and teach it as part of a complete history and physical examination (H&P).
We devote space to discussing the mechanism as to how SARS-CoV-2 infection of taste receptor cells results in taste loss. The symptoms due to this virus highlight the importance of a complete H&P in every patient, especially those with viral infections.
We add information concerning the local production of insulin in taste receptors cells and what function that insulin might have.
We summarize ongoing research on stem cells in taste papillae and the various in vitro mechanisms investigators use to study stem cell turnover and taste receptor cell renewal: this has implications for other stem cell niches in the body.
1. INTRODUCTION
“But when from a long-distant past nothing subsists, after the people are dead, after the things are broken and scattered, taste and smell alone, more fragile but more enduring, more unsubstantial, more persistent, more faithful, remain poised a long time”, “and bear, unflinchingly, in the tiny and almost impalpable drop of their essence.” (Proust; Remembrance of Things Past)
Most of what we know about tongue development, taste bud formation and renewal, and the molecular mechanisms of taste perception is derived from work on mouse gene-specific knockout models. We review here the more recent developments that have solidified in the past 7 years since our previous review (1). The functions of the primary taste bud cell subtypes in taste transduction have been more clearly defined in the past 7 years. Perception of the prototypic tastes is now refined to understand not only the specific tastant moieties engaging with the channels and receptors but also how the tastant is processed as either an attractive or aversive sensation. The involvement of neurotransmitters in the transmission of taste information from the taste bud cells to the afferent gustatory neurons is now more comprehensively understood. Yet, some mysteries remain: specific downstream signaling mechanisms within taste buds are not fully elucidated, the mode of transmission of gustatory information from the periphery to the brain is in debate, and the nature of the taste stem cell is not clear and has not been described in humans.
While the mechanisms of taste perception in the human tongue have come under greater scrutiny in the past few years, much of our information is derived from fungiform papillae (FP), as these can be readily biopsied and are known to regrow. One laboratory, in particular, has propagated human fungiform papillae-derived taste cells in culture and has used them to examine some specific physiological questions that we discuss. These topics cover some aspects that are not yet solidified in rodent taste cells such as the roles of adenylyl cyclase, the neurotransmitters acetylcholine (ACh), and gamma-aminobutyric acid (GABA) in taste perception. Since the development of intestinal organoid cultures by Sato and colleagues in 2009 (2), organoids have shown their utility in the study of tongue epithelium development and renewal in an in vitro model, and we discuss the recent work on rodent taste organoids. Apart from the diseases of the tongue, age, modulation of the composition of the tongue microbiome, and SARS-CoV-2 infection can all affect taste perception, and we review the recent developments on these topics.
While we acknowledge that taste and smell are linked and important for flavor perception, this review will focus primarily on taste and not flavor. Flavor perception is a complex and highly individualized concept deriving from several sensory cues including, but not limited to, smell, specific texture or “mouth feel,” temperature and appearance of a particular food, as well as a combination of individual taste signals (3–6). A prime example of how important the coordination of these senses is to our perception of food is found in human space flight. The decreased perception of flavor combined with a restricted selection of food types leads to calorie deficits and has adverse effects on the overall health of astronauts who spend prolonged periods of time in space (7).
2. OVERVIEW OF THE TONGUE: ANATOMY IS THE GATEWAY INTO BIOLOGY
2.1. Gross Anatomical Structure: Corpus Linguae and Radix Linguae, Anterior Versus Posterior Tongue
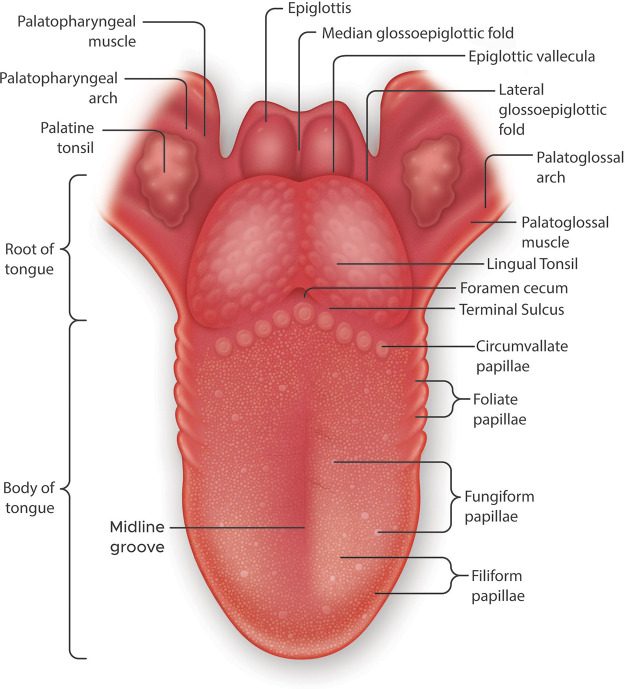
A fully developed human tongue consists of two parts: the anterior two-thirds referred to as the corpus linguae or body of the tongue and the posterior third known as the radix linguae or root of the tongue (FIGURE 1). The radix linguae and corpus linguae are structurally and developmentally distinct having discrete innervation and are supplied with different vasculature and different lymphatic drainage. The radix linguae consists of the lingual tonsils and also serves to anchor the tongue to the mandible and hyoid bone. The posterior tongue is separated from the anterior tongue by a V-shaped groove called the terminal sulcus. The corpus linguae is where the taste papillae are located in three different specialized structural types (FIGURE 1); circumvallate papillae (CVP), which run parallel to the terminal sulcus; the foliate papillae (FLP), which are found embedded in the epithelium on both sides of the tongue; and the FP, which are located throughout the anterior surface of the lingual epithelium. A recent very comprehensive review discusses the anatomy and neuroanatomical structures of the human tongue to which we refer the reader (8). Here, we provide a brief overview that can be put in the context of the tongue function.
FIGURE 1.
Gross anatomy of the human tongue emphasizing the locations of taste papillae. The tongue is divided transversely into the root or radix lingue (the posterior third) and the body of the tongue or corpus lingue (the anterior two-thirds). The root of the tongue is a papilla-free mucosa, covered with mucous glands and lymphatic tissue, referred to as the lingual tonsil. The 3 types of taste papillae are located in the body of the tongue. Circumvallate papillae are in a V-shaped arrangement at the back of the tongue. The foliate papillae consist of some ridges and slits generally arranged irregularly along the sides of the tongue. The fungiform papillae are the most numerous of the taste papillae and are located on the anterior surface of the tongue interspersed with filiform papillae. Both types of papillae are sparse along the lingual margin and abundant in the middle regions.
The bulk of the human tongue is constituted of muscle that is covered with a nonkeratinized stratified squamous epithelial layer in which reside lingual papillae. As their name implies, CVPs are mushroom-shaped prominences surrounded by a deep circular valley in which tastants come into contact with the taste buds. While the number of CVPs varies between mammalian species, it is often fixed for that species (9). Given the amount of research that is performed on tongue and taste bud development in laboratory rodents, it is worth noting that mice and rats have one CVP (10). Domestic pigs (11), guinea pigs (12), wild boar (13), raccoon dogs and foxes (14), and rabbits (15) have two CVP, the American beaver reportedly has three (16), tigers have four (17), jaguars are reported to have seven (18), while cows have been shown to have as many as 30 (19), and the number in nonhuman primates can vary from 3 to 9 (9, 20). Unlike the mammals described above the number of CVP in humans varies between 4 and 18 with an average of 9 ± 8 (21). Information on the number of FLP is confounded by the fact that they are deeply embedded in epithelial folds on the sides of the tongue in the posterior half (9) and thus more likely to quickly undergo degradation postmortem; they are even regarded by some anatomic pathologists as vestigial organs even though they do contain functioning taste buds (22). One report states that there are between 4 and 12 FLP in humans (9). Filiform papillae are nonaste papillae that function primarily for mechanosensation and cover the tongue from the sulcus terminalis to the anterior tip of the tongue. FP can be visually distinguished from filiform as they have a greater height, a thinner epithelial layer, a flatter surface, and a larger surface area than filiform. Recently, we quantified the number of FP in female and male tongues (16.14 ± 9.54 papillae/cm2 vs. 13.77 ± 8.61 papillae/cm2; Ref. 23). The fibrous connective tissue in the center of papillae is referred to as the lamina propria, which provides structural support, nutrition, nerves, arteries and veins, and immune surveillance in addition to binding the papillae to the musculature.
The complex articulation of speech is facilitated by the combination of a broad attachment base of the tongue and the complex coordination of both the intrinsic and extrinsic muscles of the body of the tongue (8, 22, 24–27). The intrinsic muscles of the tongue are those that connect to other parts of the tongue and are not attached to bones. They are the superior and inferior longitudinal, transverse, and vertical muscles. These are bilateral and contract across the midline septum to modify the shape of the tongue dorsum in three dimensions. The superior and inferior longitudinal muscles shorten and curl the tongue upward (superior) and downward (inferior). The transverse muscle elongates and narrows the tongue while the vertical lingual muscle flattens it. There are four sets of paired extrinsic muscles; the hyoglossus (HG), genioglossus (GG), styloglossus (SG), and palatoglossus, which are attached to the hyoid bone, mandible, base of the skull, and soft palate, respectively. The SG muscle acts to lift the lateral edges and retract the tongue. The HG muscle causes retraction as well as depression of the tongue. The GG muscle protrudes the tongue and is the main force generator in bolus propulsion in swallowing. The palatoglossus muscles elevate the posterior part of the tongue. The tongue is anchored to the mandible by the lingual frenulum (28). For a full overview of tongue musculature, see Refs. 27, 29, 30.
The serous glands of the tongue referred to as Ebner glands are found distributed throughout the intrinsic muscles and at the base of both CVP and FLP. Given the location of the taste buds in the clefts of these papillae, the salivary secretions of the Ebner glands provide a means of moving the tastants through these cavities. These salivary secretions contain enzymes that have been implicated in the modulation of oral fat perception both in rodents and in humans (31–34).
2.2. Neuronal Architecture of the Tongue
Human tongue innervation is extremely complex and possesses specializations not found elsewhere in the mammalian kingdom (35).
Given the multifunctional nature of the tongue, three different innervation types need to be considered 1) somatosensory neurons that encompass taste perception, nociception (noxious stimuli), proprioception (positional awareness), and mechanoreception; 2) motor control of the tongue that is critical for mastication, swallowing, speech, and breathing; and 3) autonomic innervation that provides salivatory stimuli for the lingual salivary glands and regulates blood flow to sites of need.
2.2.1. Somatosensory.
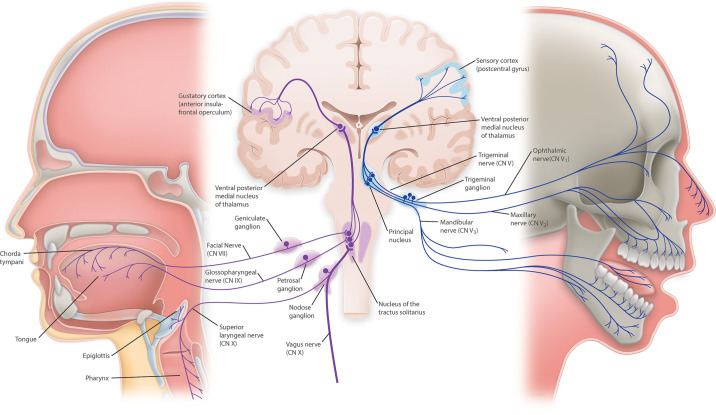
Gustatory nerve fibers represent a specialized sensory neuron. Branches from three cranial nerves (CNs) relay taste information centrally; facial nerve (CN VII) consisting of the chorda tympani and greater superficial petrosal (GSP) branches, the lingual branch of the glossopharyngeal nerve (CN IX), and the superior laryngeal branch (SLN) of the vagal nerve (CN X) (8, 36–38). CNs VII, IX, and X have cell bodies located in the geniculate ganglion (GG), inferior petrosal ganglion (iPG), and nodose ganglion (NG), respectively. The CVP and posterior part of the FLP are innervated by the lingual branch of the CN IX with the CVP having a larger nerve plexus relative to the other gustatory papillae (8). The anterior portion of the FLP and the FP receive gustatory nerve fibers from the chorda tympani branch of CN VII. The soft palate is innervated by the GSP while the root of the tongue, epiglottis, and larynx are innervated by the SLN. Afferent nerve fibers from taste buds converge in the nucleus of the tractus solitarius (NTS) within the medulla of the brain stem, and the information is then routed through the thalamus, limbic system, and finally to the insula in the gustatory cortex (FIGURE 2). It is important to note that most of the work on the mechanisms of peripheral taste perception has been carried out using rodent taste buds/taste pathways to the brain and primates do have differences from rodents. In humans, for example, there appear to be direct projections from the NTS to the ventral-posteromedial (VPM) nucleus of the thalamus, while in mice taste perception is relayed through the parabrachial nucleus on the way to the thalamus (1).
FIGURE 2.
Taste sensation and sensory information are transmitted by cranial nerves to gustatory and sensory cortices though nuclei in the brainstem and thalamus. Gustatory information is relayed to the cemtral nervous system through gustatory nerve fibers from branches of 3 cranial nerves: chorda tympani branch of the facial (CN VII), glossopharyngeal (CN IX), and the superior laryngeal branch of the vagal nerve (CN X). Afferent gustatory nerve fibers converge in the gustatory area of the nucleus of the tractus solitarius, within the medulla of the brainstem. From there, second order neurons transmit information to the ventral posterior medial nucleus (VPM) of the thalamus and, from there, afferent neurons relay gustatory signals to the gustatory cortex. In the brainstem, above the pons, decussation of some second-order neurons occurs, and from there gustatory pathways ascend bilaterally. Touch-position and pain-temperature sensory information from the mouth is carried by branches of the trigeminal cranial nerve (CN V). Afferent neurons enter the brainstem and innervate the principal nucleus. From there, neurons project to VPM, and, finally, from the thalamus, sensory information is transmitted to the sensory cortex in the postcentral gyrus.
In addition to the aforementioned cranial nerves relaying taste information to the central nervous system (CNS), somatosensory innervation of the oral mucosa is also performed by the trigeminal nerve (CN V). CN V consists of three main branches, named after the three different zones of the face they innervate: the ophthalmic, maxillary, and mandibular. The lingual nerve, itself a branch from the mandibular nerve, carries sensory information, but not taste sensation, from the tongue. Neurons from all three main branches of the CN V have their cell bodies in the trigeminal ganglion. From there, axonal projections from sensory neurons enter the brainstem and terminate on neurons in the principal nucleus (sometimes referred to as the pontine nucleus or main sensory nucleus). Ascending neurons from the principal nucleus project to the VPM, the same thalamic nucleus relaying taste information to the gustatory cortex. Finally, from the thalamus, sensory information is transmitted to the sensory cortex in the postcentral gyrus, where information about touch-position and pain-temperature sensations from the mouth are processed.
Circuitry carrying taste information spread from subcortical regions to forebrain structures. Of note, the lateral hypothalamus (LH), the central nucleus of the amygdala (CeA) (39), and the ventral striatum (40) all receive taste-related input. These structures play an important role in modulating food intake and energy homeostasis, as well as reward perception. fMRI studies conducted by van Opstal and colleagues (41) show blood oxygen level-dependent (BOLD) responses in the hypothalamus (energy homeostasis) and the ventral tegmental area (VTA) of the ventral striatum (reward) in response to glucose and/or a flavoring agent. While hypothalamic activity is seen in response to glucose alone, activation of the VTA is observed only when a flavoring agent is added to the glucose solution, suggesting how taste, but not caloric intake alone, is crucial for hedonic eating. For a more in-depth discussion on how taste information is relayed to regions related to feeding and reward, please see Yamamoto (42).
The lingual nerve is responsible for dense sensory innervation of the tongue. Wu and colleagues (43) identified and characterized sensory neurons from the trigeminal ganglions that innervate the tongue using reporter animal models for a variety of markers and retrograde labeling of neurons, as well as single-cell PCR of retrogradely labeled lingual neurons in mice. They were able to label subtypes of sensory neurons according to their size, myelination status, and expression of different markers and found that tongue-innervating sensory neurons primarily expressed calcitonin gene-related peptide (CGRP), TRPV1, tropomyosin receptor kinase C (TrkC), the 5-HT3A receptor, and parvalbumin. These are for C-nociceptors (both peptidergic and nonpeptidergic), peptidergic A nociceptors, proprioceptors, and myelinated low-threshold mechanoreceptors (LTMRs), making the trigeminal innervation of the tongue a complex source of detailed sensory information.
Sensory acuity in the lingual mucosa is due to its innervation by multiple classes of specialized mechanosensitive neurons recently described in detail by Moayedi and colleagues (44, 45). Human filiform papillae are innervated by multiple end bulbs of Krause (1–5 total end bulbs per 25-µm section), which are bulbous capsules containing myelinated mechanosensory neurons. Mechanoreceptors are myelinated neurons that are detected by the expression of the protein neurofilament heavy. Filiform papillae also contain additional clusters of Merkel cells, tactile epithelial cells essential for the perception of light touch sensation. Unmyelinated free nerve endings sense nociceptive, thermal, and chemical stimuli such as the sensations induced by chilies and menthol (chemesthesis) in the tongue. Small bundles of unmyelinated neurons traverse into the apical lamina propria invaginations. These are likely the nociceptors that detect temperature and chemical agonists. Moayedi and coworkers (45) also describe two types of myelinated afferents; one terminating just below the epithelium, which they refer to as subepithelial neuronal densities, and a second type innervating the basal epithelium of the filiform papillae. Mechanosensory nerves located at the base of the filiform papilla epithelium are optimally positioned to detect deflections in the papillae. Human FP have a dense network of innervation in the lamina propria below the taste bud. Both myelinated and unmyelinated nerve fibers extend into taste buds, and the bud itself is surrounded by mechanosensory nerve fibers. Meissner-like corpuscles are also present in human FP. Meissner corpuscles are sensitive to low-frequency vibrations and in this context could be instrumental in speech.
2.2.2. Motor control.
The tongue proper is almost exclusively composed of groups of skeletal muscles, with adipose tissue interspersed among the muscle fibers, especially in the posterior half (26). These muscles control the shape and size of the tongue and facilitate speech, mastication, and swallowing. Motor nerve fibers travel within the hypoglossal nerve (CN XII) to innervate these muscles.
2.2.3. Autonomic innervation.
On initiation of mastication, parasympathetic nerve fibers traveling with CNs VII and IX release synaptically stored acetylcholine (ACh) onto muscarinic (M3) receptors in buccal and lingual salivary glands. This results in an increased volume of saliva and release of amylase from the acinar cells of the salivary glands into salivary ducts, so complex carbohydratein the food gets broken down. Blood supply also increases via vasodilation of the local arterioles and this leads to reduced viscosity of the saliva due to increased ductal cell bicarbonate secretion, which protects the villi projecting from taste receptor cells (TRCs) from damage. Sympathetic innervation of all salivary glands is through postganglionic fibers from the superior cervical ganglion, which travels with the blood supply. Its activation results in the release of norepinephrine onto adrenergic receptors in salivary glands. This also results in stimulating salivary gland secretion, but the effects are of much shorter duration and weaker than those of cholinergic stimulation. During acute anxiety and the consequent norepinephrine surge from adrenal glands, vasoconstriction of the blood supply occurs so that salivary secretions are inhibited causing, for example, dry mouth before giving lectures.
2.3. Vascular Architecture of the Tongue
The lingual artery, a branch of the external carotid, is responsible for most of the arterial supply, but there is a branch from the facial artery, called the tonsillar artery, that provides some collateral circulation. Drainage is by the lingual vein to the external jugular vein.
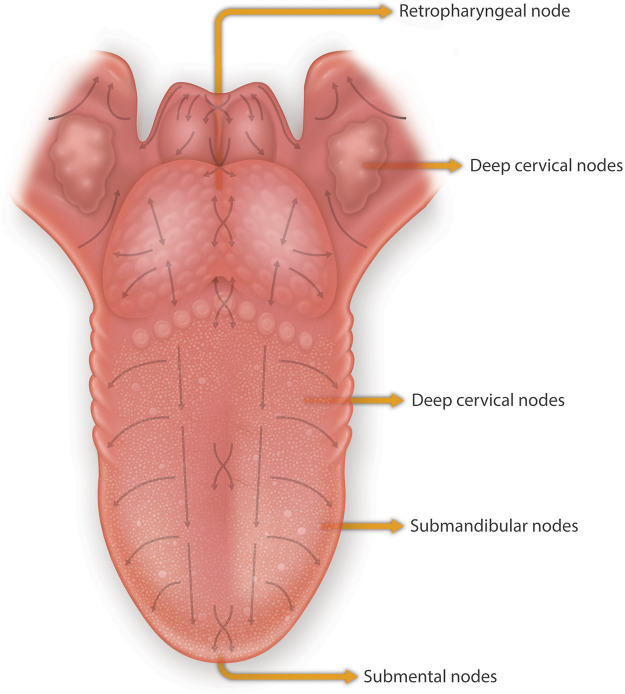
The mucosa of the tongue has a dense lymphatic network with a denser network of small lymphatic capillaries in the mucosal layer than in the musculature of the tongue (46, 47). The number and diameter of larger precollecting and collecting lymphatic vessels increase from the mucosa into the tongue musculature. The lymphatic architecture of the anterior of the tongue can be divided into 1) the central group of vessels situated at the tip of the tongue and then proceed through the hyoglossus muscle to drain into submental nodes and onwards to deep cervical nodes, and 2) the marginal vessels that drain the middle of the tongue through the genioglossus muscle into submandibular nodes, which then empty also into the deep cervical lymph nodes. The posterior third of the tongue where the CVP and FLP are located drains directly into the deep cervical lymph nodes. The deep cervical lymph nodes drain into the thoracic duct on the left side and the right lymphatic duct on the right side. Regional draining nodes are located throughout the tongue musculature. The clinical significance of the structural lymphatics of the tongue resides in understanding the patterns of metastatic spread of tongue carcinomas, and the collection of lymphatic fluid and peptide products from taste buds and lingual epithelium (FIGURE 3 illustrates the draining lymphatics of the tongue).
FIGURE 3.
Lymphatic drainage of the dorsum and the base of the tongue. The mapped arrows show the direction of drainage of lymph fluid from the base of the tongue predominantly to the lymph nodes of levels II and III. Lymph fluid of the mucosa located around the midline flows vertically to the submandibular (anterior tongue) and upper jugular lymph nodes (posterior tongue) by way of 5 to 7 collectors located between the genioglossal muscles. Modified from Ref. 46, with permission from John Wiley & Sons.
2.4. Taste Papillae and Taste Bud Structure
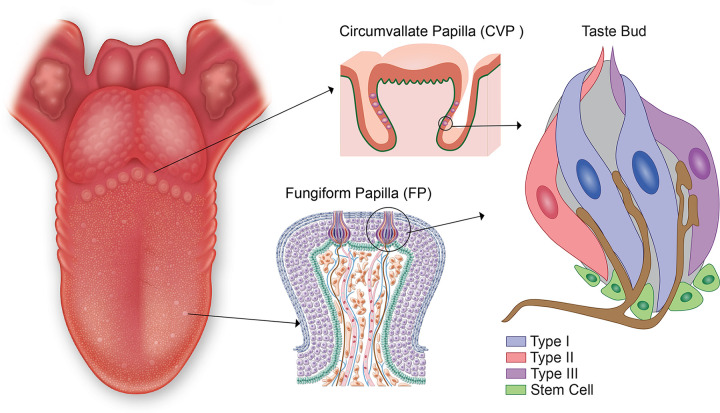
The cells that detect tastants are found as collections of taste receptor cells (TRCs) packaged together in taste buds that are embedded in taste papillae (FIGURE 4). Based on a recent meta-analysis of the literature on human taste papillae density, a total of roughly 4,600 taste buds are distributed across the three different types of taste papillae: 48% in CVP, 28% in FLP, and 24% in FP (8). Taste buds are compact assemblies of heterogeneous epithelial cell types that possess some neuronal cell-like properties. The immunohistochemical study of human taste buds is in its infancy, but so far what has been shown in rodent taste bud structure and physiology does seem to be conserved in human taste bud architecture (48–51). Historically, TRCs have been classified broadly into four morphologically distinct types in taste buds (50, 52). The original characterization was based on staining rodent taste buds in electron microscopy (EM), dark, type I; light, type II; and light, type III cells. Type I TRCs resemble astrocytes, having fine processes that wrap around other cells types, and are defined by the presence of the glial glutamate transporter (GLAST) and the enzyme ectonucleoside triphosphate diphosphohydrolase 2 (NTPDase 2), which degrades adenosine triphosphate (ATP), which is a primary neurotransmitter of the type II TRCs (53). They have small voltage-gated outward potassium (K+) and inward sodium ion (Na+) currents but no voltage-gated calcium currents (54, 55). Type II TRCs are long spindle-shaped cells, and they have specialized G protein-coupled (GPCR) taste receptors to detect bitter, sweet, and umami tastants. They were originally described as having a more lucent cytoplasm under EM, sparse heterochromatin, large round nuclei, and a single short, thick, apical microvillus (51, 56). However, a more recent report shows more diversity in microvilli structure on type II TRCs in mice taste buds with ∼14% exhibiting a bushy top that ends before the taste pore and an irregular assembly of short microvilli (57). It is suggested that these more diverse appearing cells may represent a more immature type II cell. As we will discuss below in sect. 6.2, type II TRCs also express most of the hormones (1) found in the taste buds, including insulin (58). They are also the cells directly infected by SARS-CoV-2 (59) as they express angiotensin-converting enzyme-2 (ACE2), which provides a portal for viral entry (53). An important fact when considering how type II TRCs transfer tastant signals to afferent neuron fibers is that they do not possess synaptic vesicles (56, 57, 60) or synaptic molecules (56). This begs the question of how do they transmit taste sensory information to the primary gustatory neurons? We discuss the recent research on this in the section below on how tastant information is processed. An important structural observation in relation to this is the large “atypical” appearing mitochondria (large mitochondria with tubular cristae) located next to the plasma membrane of type II cells adjacent to the areas where innervating afferent fibers are located, which we will discuss in detail in sect. 3.3.2 (61).
FIGURE 4.
Taste is first discriminated in taste receptor cells (TRCs) within taste buds located in circumvallate papillae (CVP), foliate papillae (FLP), and fungiform papillae. By convention, 4 subtypes of taste bud cells are present in taste buds. Of the 4 subtypes, only type III TRCs form synapses with the afferent nerve fibers, represented by the footplates shown in brown.
Type III morphologically resemble type II TRCs in that are also elongated, spindle-shaped cells, but unlike type 1 and II TRCs, they have direct synaptic connections with afferent nerve fibers that are present in taste buds, and, again unlike type 1 and II TRCs, they contain numerous dense-core, secretory-type vesicles (50, 52, 62). As our understanding of taste perception continues to evolve, it is becoming increasingly clear that morphological-based classification belies the true complexity of the subtypes of TRCs because there are even subtypes within subtypes. Therefore, maybe the time has come to retire the numbering of the TRCs and refer to them by function.
All three types of TRCs are found in the taste buds of mammals regardless of location; however, type I TRCs are sparse in taste buds within FP of mice (58) and humans (59). In the taste buds of the CVP, type I TRCs comprise ∼50% of the total number of TRCs, and type II and type III cells each comprise ∼25% of the remaining TRCs (57). There is no regional selectivity of taste within the human tongue as all taste buds contain the three different TRC types. However, while type II cells have a common second messenger signal transduction pathway, the evidence in mice is that each subtype is specific to one of the three tastes detected by type II TRCs (63). The existence of the type IV cell is controversial (1, 51). Originally referred to as a basal cell it is thought to be an immature cell that may still be cycling and has just entered the taste bud from the intergemmal space hence is located at the base of the taste bud (64, 65). We discuss this more fully in the sect. 5.4.
An interesting structural feature of murine taste buds is the taste bud/blood barrier, which is composed of glycosaminoglycans encapsulating the individual taste buds and separating them from the surrounding epithelium (66). This has implications for how tastants, hormones, and pharmaceuticals might enter taste buds either systemically or by direct application to the lingual epithelium and for the ability of taste buds to function as immune privilege sites, both of which we elaborate on further below. It is not clear whether this barrier or a similar one exists in human taste papillae. There are several methodological issues we should note when studying the architecture of human taste buds: the only papillae we can safely biopsy are the FP as they have been demonstrated to regenerate (59); CVP have not been shown to regenerate; the number of taste buds embedded in a FP even in young people is just one to three and sometimes none; and the taste bud is not visible to the naked human eye and requires serial section identification by hematoxylin and eosin staining to determine the region where a particular taste bud is located. Therefore, each study requiring a human taste bud necessities time-consuming histological work before staining for a protein of interest can be performed (59).
3. TONGUE FUNCTIONS: MECHANICAL AND SENSORY
3.1. Mastication, Speech, and Swallowing
The tongue functions as both a motor and sensory organ: motor because we require it for talking, mastication, and swallowing, and sensory because we require it for chemosensation and to coordinate with the motor functions. Free tongue movement is also required for the movement and mastication of food in the oral cavity. Upon initial entry of solid food into the oral cavity, the cupped tongue initiates a pull-back movement to propel the food into the area behind the canines. Transport of chewed food through the oropharyngeal surface of the tongue to the molars occurs intermittently during jaw motion cycles. Ultrasonographic imaging of healthy human tongues while chewing demonstrates that the tongue turns the food, mixes it with saliva, sorts out unsuitable particles, and aids in bolus formation (67). Masticatory performance is measured by the average size distribution of food particles of a comminuted test food (68). The comminution index is decreased when tongue movement is restricted toward the lingual side of the bilateral mandibular premolar regions (69). The comminuted food is triturated into a cohesive bolus of a size suitable for swallowing. The tongue moves the bolus to the midline of the tongue and propels it to the oropharynx for swallowing. Using quantitative kinematics from ultrasound imaging, Genna and colleagues (70) recently demonstrated that when an adult swallows a liquid bolus, the anterior of the tongue moves to the palate to seal the oral cavity and enclose the bolus. It is then directed toward the depressed posterior portion of the tongue, which then elevates the bolus toward the oropharynx using peristaltic-like movements in the anterior and posterior segments (70). Understanding the mechanics of the tongue during mastication and swallowing is important for the treatment of dysphagia.
Similarly, ultrasound imaging has been employed to monitor tongue movement during speech (71). A key function of the adult human tongue muscles is to perform shape changes to facilitate tongue articulation for speech. Mechanosensory feedback from the tip of the tongue to the rough or hard palate is essential for the articulation of vowels and sibilants (72). The high degree of complex innervation observed in the filiform papillae (FP) and hard palate facilitates the accurate positioning of the tongue during speech articulation. It is worth noting that half of adult human tongue muscle fibers are slow-twitch muscle fibers, which is twice the amount observed in nonhuman primates and the largest number seen in any mammal studied so far. This has significance for motor control of the tongue during human speech (30). Slow tonic muscle fibers do not contract with a single twitch-like skeletal muscle fibers, rather they have prolonged tonic contraction that can be finely controlled. This allows for precise control of localized shape changes in the tongue that contribute to speech articulation. Therefore, the stretch receptors or muscle spindles are the most important tongue muscle proprioceptors and are more numerous in adult human tongue muscles than in nonhuman primate tongues (30, 35).
3.2. Taste Sensation: How It Works
“No sooner had the warm liquid mixed with the crumbs touched my palate than a shudder ran through me and I stopped, intent upon the extraordinary thing that was happening to me.” “The sight of the little madeleine had recalled nothing to my mind before I tasted it.” (Proust; In Search of Lost Time)
After tasting a delicate madeleine dipped in tea, Proust’s narrator recalled from his childhood, a memory of his gustatory responses. Sight alone was not sufficient to evoke recollections of times past and the love his aunt Leonie displayed by the simple act of offering him a madeleine, having first immersed it in her own cup of tea. It beautifully illustrates the ineffable connection between our gustatory sense, our memories of tastants, and the entrainment that occurs when we have tastants presented to us: the oral-gut-brain-emotional connection was laid out for us by Proust. We might also recall Charles Dickens’s description of Miss Havisham’s decaying wedding cake in Great Expectations: “a torrid, insect-infested, decaying mass that mirrors the rotting soul of its bride-to-be.” This is another, albeit unpleasant, connection between food (or the decaying mass) and Miss Havisham’s state of mind. We shall now, in prosaic terms alas, describe the biology of taste transduction.
Mammalian physiological systems have developed such that an animal’s drive to get calories and feel good is paramount. In general, d-glucose is the preferred fuel for brain cells, and therefore, humans are highly motivated to obtain it. Additionally, animals are primed to eat some salt and avoid bitter-tasting compounds, one purpose of which is to prevent ingestion of chemicals that can cause bodily harm. Strychnine for example, which is found in all Strychnos species but is especially abundant in Strychnos nux vomita, is a deadly neurotoxin that elicits a bitter taste. The common name for Strychnos nux vomita is the Asian vomit button tree because hopefully if you ingested it you would vomit up the poison. Bitter receptors are indeed present in the stomach and small intestine and are likely the culprit for this autonomic reaction (73). One way to hide nefarious intentions for strychnine was to combine it with something that would be expected to be bitter, such as beer (bitters to the Brits), or mix it into bromine powder used by rich old British ladies as a sleeping aid, at least in Agatha Christie novels (74). We should point out, in the interests of fairness, that Agatha’s novels are fiction, and well-bred British scions never have had nefarious intentions. Strychnine activates T2R10 and T2R46 bitter receptors. T2R46 is activated at concentrations as low as 0.1 μM strychnine (75), and all other bitter compounds, such as quinine, were once graded for bitterness against strychnine.
The study of the molecular basis of taste perception is a relatively young field with some false starts and misinterpretations of how certain tastes are perceived. For an in-depth understanding of the studies underlying the conclusions on how tastant signals are currently conveyed, we refer the reader to the original publications and reviews cited throughout. Here we present an overview of the recent advances and what is currently understood to be the primary molecular mechanisms of taste perception. In general, animals have five primary tastes: salt, sweet, umami (savory), bitter, and sour, although fat has recently been added to this as a sixth taste, oleogustus. However, not all animals can taste all five. Giant pandas cannot taste umami (76) and are the only known nonmeat eaters within the bear family. Cats, both domestic and wild, cannot taste sweet (77). Whereas in the hummingbird, a specialized nectar feeder, the umami receptor has been repurposed to detect carbohydrates (78). Cetaceans (whales and dolphins) have reduced gustatory range reportedly only detecting salt and a limited range for bitter (79). However, aquatic chemosensation is also still poorly understood but is equally if not more complex than that of mammalian tongue perception with the chemotactile sensation of the octopus as a prime example of that complexity (80).
3.2.1. Salt.
“Our shells cracked on the plates.
My tongue was filled with estuary, my palate hung with starlight:
As I tasted the salty Pleiades, Orion dipped his foot in the water.” (Seamus Heaney; Oysters)
Regulation of internal sodium chloride levels requires tight control to protect against hypernatremia and dehydration. Therefore, the perception of salt is a fundamental taste sensation present in just about the whole of the animal kingdom. However, the mechanism by which salt is perceived is probably the least understood of all the tastes, and a series of papers has attempted to outline how this complex taste detection system works in mice (81–84). Salt elicits a bimodal response being attractive, or appetitive, at low concentrations (<100–150 mM) and aversive at higher concentrations involving what appears to be at least two different molecular mechanisms. The low-salt attractive taste is specific to sodium ions (Na+) and thus is referred to as a “sodium taste,” is inhibited by the diuretic amiloride as it involves the epithelial sodium channel (ENaC) composed of α-, β-, and ɣ-subunits located on the apical side of the TRC that is blocked by amiloride. This sensitivity is also lost in P2X2/P2X3 double knockout mice, implying ATP must be necessary (or at least permissive) to transduce “sodium taste” (85) (see discussion of ATP and purinergic receptors below in sect. 3.3.1).
Entry of Na+ into the cell leads to action potential generation, driving voltage-activated ATP release via the calcium homeostasis modulator channels 1 and 3 (CALHM1/3) to the adjacent afferent taste fibers (83) (FIGURE 5A). CALHM1 and its homolog, CALHM3, hetero-hexamerize to form a large-pore (15–18 nm), nonselective fast-activating voltage-gated channel, CALHM1/3 that is permeable to large molecules including ATP (86, 89, 90). Therefore, CALHM1/3 channels accommodate the outrush of ATP molecules and the influx of numerous ionic species. Previously, as ENaC expression was not found to be coexpressed with markers for type II or III cells, the default position was that the type I cell must be the detector cell for sodium taste (81). Moreover, taste cells detecting the sodium taste are thought to be present on the FP and absent from mouse CVP (83, 91, 92) and are not defined by the canonical characteristics or markers of the classical taste cell subtypes. Additionally, Nomura and colleagues (83) were not able to find evidence of coexpression of the type I TRC marker NTPDase2 with both the ENaC and CALHM1/3, leading them to speculate that the sodium taste detecting cells represent a separate cell subtype (which by default would appear to be a type II TRC subtype since ATP transduction is needed). Going one step further, they nullified CALHM1/3 in just ENaC-containing cells and found absent chorda tympani responses to NaCl while maintaining intact responses to KCl, NH4Cl, and other tastants. They also found a few cells (∼4%) in CVP that contained both ENaC and CALHM1/3, while approximately one-third of the TRCs in FP contained both (83).
FIGURE 5.
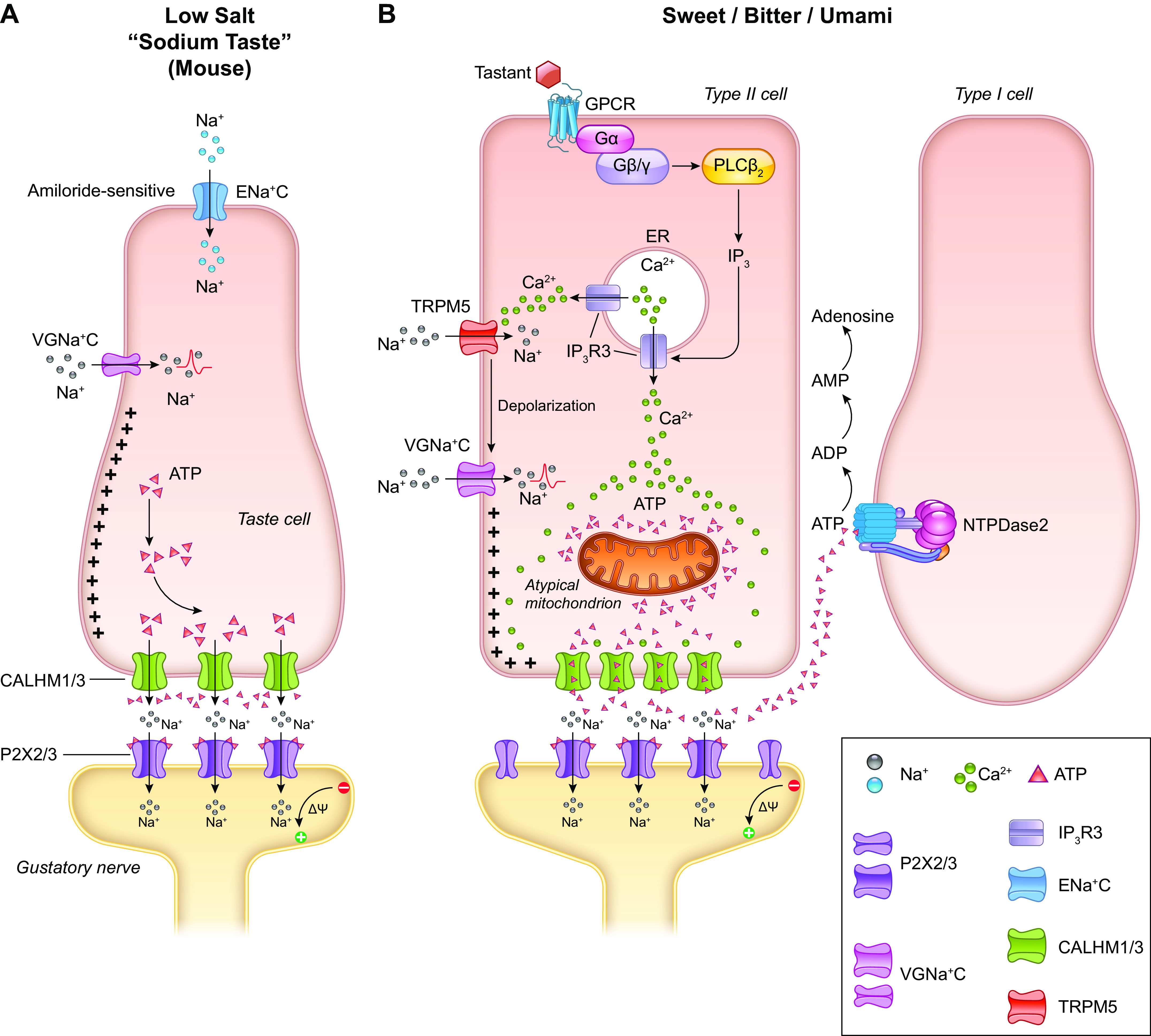
Signal transduction mechanism for sodium taste perception (A). The low-sodium-sensing cell is depolarized by the influx of Na+ via amiloride-sensitive epithelial sodium channel (ENaC). Additional influx of Na+ through voltage-gated sodium channel (VGNaC ) creates an action potential that ultimately leads to the release of ATP through CALHM1/3, without the involvement of intracellular calcium (Ca2+: 86,100). Mechanistic overview of signal transduction in sweet, bitter, and umami taste perception (B). Tastants bind to cell surface G protein-coupled receptors (GPCRs) and initiate a signaling cascade through phospholipase Cβ2 (PLCβ2) and inositol 1,4,5-triphosphate (IP3) that mobilizes Ca2+ from the endoplasmic reticulum (ER) by activating the IP3 receptor type 3 (IP3R3), thus increasing intracellular Ca2+ concentration. The spike in intracellular calcium activates transient receptor potential cation channel subfamily M member 5 (TRPM5) channels that depolarize the plasma membrane and create action potentials via voltage-gated Na+ channels (VGNa + C). These changes in the membrane potential and the increased presence of intracellular Ca2+ trigger the release of ATP into the channel synapse through the CALHM1/3s. This stimulates the gustatory neuron, thus completing transduction of the signal from the taste cell to the afferent (86–88). Image created with BioRender.com, with permission.
Perception of low-salt concentrations is also operative in humans; however, the signaling molecules and responsive pathways are even less clear than in mice. In humans, the sodium taste is not obviously found to be amiloride sensitive. However, this could be confounded by cross activation of other ion channels and transporters. The ENaC components are not expressed on the apical side but on the basolateral side of taste buds, suggesting a role for the channel downstream of Na+ entry (93).
The high-salt taste is less specific in mice. It includes various salts (e.g., sodium and potassium chloride) and may necessitate the negative chloride ion (84). It is amiloride insensitive and is mediated by the type II (bitter sensing) and type III (sour sensing) TRCs. The molecular sensor for salt and the intermediate steps in this pathway are not known, but they precipitate the release of the neurotransmitters ATP or 5-HT (or both) in the case of the bitter or sour detecting cells, respectively. It must also be noted that high-salt detection in the lingual epithelial is probably not limited to taste buds as the transient receptor potential vanilloid 1 (TRPV1) receptor on free trigeminal nerve endings in the oral mucosa could be activated by high-salt concentrations (94). For now, more comprehensive evidence is needed to establish the paradigm of salty taste, especially in humans, and more especially in relation to the control of blood pressure to which salt intake is a major contributor.
3.2.2. Sweet, umami, and bitter.
“Loins of pork and chicken thighs and standing rib, so prime,
Pork chops brown and fresh ground round (I crave them all the time).
Irish stews and boiled corned beef and hot dogs by the scores,
or any place that saves a space for smoking carnivores.” (Maya Angelou; The Health-Food Diner)
In general, three members of the T1R class of taste-specific G protein-coupled receptors (GPCRs; T1R1, T1R2, and T1R3) function in combination as heterodimeric sweet (glucose, fructose, artificial sweeteners; T1R2 +T1R3) and umami (T1R1 + T1R3; broths, mushrooms, l-amino acids, in particular l-glutamate) taste receptors: The foods craved by Maya Angelou all contain glutamate. Bitter tastants [such as strychnine, quinine, absinth, caffeine, denatonium benzoate, phenylthiocarbamide (PTC), and 6-propyl-2-thiouracil (PROP)] are sensed by an unrelated family of GPCRs, the T2Rs, consisting of ∼33 members in humans (95). As stated above, the current evidence indicates that each taste (bitter, sweet, or umami) is recognized by a type II subtype expressing only the receptors for that one taste (63) [although this is sometimes questioned for sweet and umami (96)], while many T2Rs are coexpressed in the same subset of type II cells (97). Studies examining electrical responses of the receptors to bitter substances suggest that they can either be broadly or specifically tuned (98).
The T1Rs and T2Rs converge on the same molecular pathways to transduce their signals (FIGURE 5B). Both are coupled to the taste-specific Gα protein gustducin (Gαgust), Gβ3, Gɣ13, Gα14, and Gαi (63, 99–101). The Gβɣ complex dissociates from the taste receptor upon engagement of the tastant with the receptor to activate phospholipase Cβ2 (PLCβ2), hydrolyzing phosphatidylinositol 4,5-biphosphate into inositol 1,4,5-triphosphate (IP3) and diacyglycerol. IP3 opens the IP3R3 channel on the endoplasmic reticulum membrane allowing for increased cytosolic calcium levels and activation of the calcium-responsive sodium channel transient receptor potential M5 (TRPM5) and TRPM4 (99), thereby causing the cell to depolarize and generate an action potential (86, 88). Gαgust is thought to play a longer acting, modulatory role through the activation of phosphodiesterase 1 A (PDE1A), which facilitates activation of CALHM1/3 that also serves to both depolarize the cell and allows for ATP release (90). The amount of ATP released is directly proportional to the number of action potentials produced in the type II TRC. The released ATP is then degraded to ADP and AMP by NTPDase2 on plasma membranes of type I TRCs (see sect. 3.3.2).
The umami receptor primarily detects the amino acid glutamate that is found in meat and the food additive monosodium glutamate (MSG). It acts through the mechanism utilizing Gαgust and also has a secondary pathway through the related G protein rod α-transducin (102). T1R1 + T1R3 double knockout mice lack umami taste preference except at the highest dose (0.6 M) of l-glutamate tested (103). It is possible that at the highest dose, l-glutamate activates mGluR1 and 4, both of which are found in taste buds, or a receptor complex of T1Rs and mGluRs (103, 104). Maya Angelou’s poem extols the delight of sinking one’s teeth into umami-tasting foods, while slyly having fun at the expense of vegetarians.
In addition to the GPCR-dependent mechanism described above, sweet is likely to be transduced by the sodium-glucose cotransporter I (sGLT1) both in mice and in humans (105, 106), independent of T1Rs. While T1R2 mediates taste responses from both sugars and nonnutritive sweeteners, the sGLT1 is very likely to be glucose exclusive (107). Recent experiments showing residual neural responses to monosaccharides in the T1R null mouse (108) would suggest this but the exact mechanism by which this pathway operates is still a subject of study (109). However, the release of ATP itself may not be the neurotransmitter responsible for the residual responses to glucose in this case. Glucagon-like peptide-1 (GLP-1) is secreted from enteroendocrine L cells in the small bowel in response to food ingestion. It is generally described in physiology as inhibiting short-term food intake by slowing gastric emptying, dampening the CNS effects on feeding behavior, and potentiating glucose-mediated insulin release from islets of Langerhans (for review of nontaste-related GLP-1 physiology, see Refs 1, 85, 110).
The enzyme proconvertase 1/3 (PC1/3) is necessary for posttranslational processing of proglucagon to generate GLP-1. We detected GLP-1 and PC1/3 in a subset of type II and III cells (111), and we found its specific receptor (GLP-1R) to be expressed on intragemmal nerve fibers in mouse CVP (112). GLP-1 release from TRCs is selectively sweet and lipid dependent, and we found that it potentiates sweet tastant attraction (112). Analogous to TRCs, L cells contain sGLT1s that are necessary for glucose-mediated GLP-1 secretion in the small bowel (FIGURE 6) (113). We propose that GLP-1 is a candidate for signal transmission from taste bud cells to gustatory nerves and is responsible for the residual sweet sensing remaining in T1R null mice (see TABLE 1 for effects of other hormones on sweet, bitter, and umami perception).
FIGURE 6.
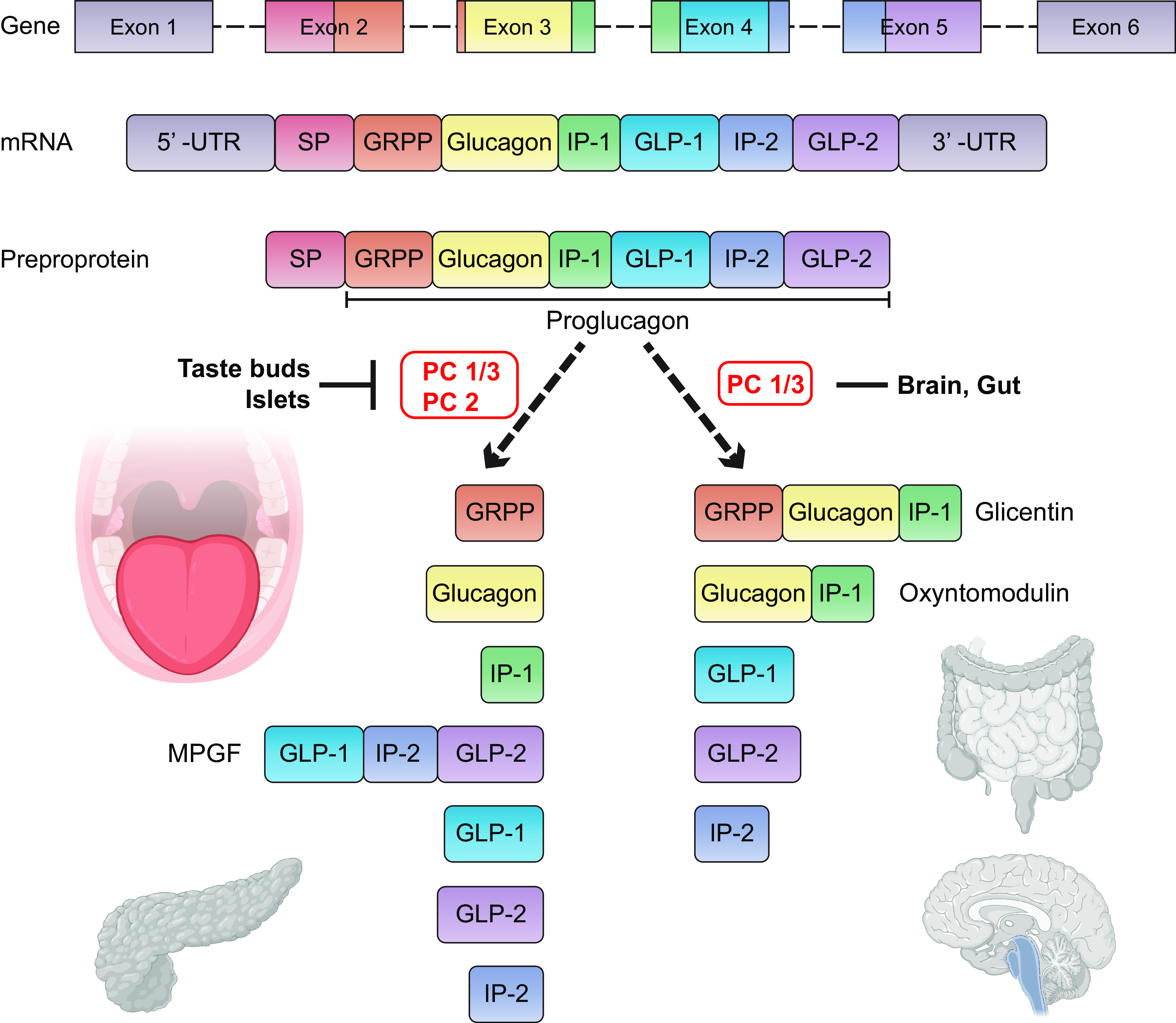
Tissue-specific proglucagon processing by different proconvertases yields different mature peptides in gut, brain, pancreas, and tongue. The proglucagon gene is composed of 6 exons and is transcribed and translated into a preproprotein containing a signal peptide (SP). Cleavage of the signal peptide results in the release of proglucagon, a 158 amino acid precursor protein that is found in the gut, brain, pancreas, and tongue. Posttranslational processing by different proconvertases results in multiple peptides. In enteroendocrine L cells of the ileum and colon, and in Glucagon-like peptide-1 (GLP-1)-producing (PPG) neurons in the nucleus of the tractus solitarius of the brainstem, where there is predominant expression of proconvertase 1/3 (PC 1/3), proglucagon is cleaved into glicentin, oxyntomodulin, GLP-1, GLP-2, and intervening peptide-2 (IP-2). In type II and type III taste receptor cells, and in α-cells in pancreatic islets, where both PC 1/3 and PC 2 are expressed, proglucagon is cleaved into glicentin-related pancreatic polypeptide (GRPP), glucagon, intervening peptide-1 (IP-1), major proglucagon fragment (MPGF), GLP-1, GLP-2, and IP-2. GLP-1 and glucagon are enhancers of sweet taste and are produced in type II cells (85, 111).
Table 1.
Hormones and their receptors in TRCs
| Ligand | Ligand Type | Ligand Cell Type | Receptor | Receptor Type | Primary Signaling Mechanisms |
|---|---|---|---|---|---|
| Cholecystokinin | Polypeptide 33 aa | Type II | CCKA CCKB | Class A “rhodopsin-like” | CCKA(l): Gαq PLC activation + Gαs AC activation CCKB (2): Gαq PLC activation (114, 115) |
| Neuropeptide Y | Polypeptide 36 aa | Type II | NPY1, 2, 3, 4, 5 | Class A “rhodopsin-like” | NPY1R: Gαi AC inhibition NPY2R: Gαq PLC activation NPY4R: Gαi AC inhibition + Gαq PLC activation NPY5R: Gαi AC inhibition (114, 116, 117) |
| Vasoactive intestinal peptide | Polypeptide 28 aa | Type II | VPAC1, VPAC2 | Class B “secretin-like” | VPAC1: Gαs AC activation VPAC2: Gαs AC activation + PLC/PLD (118, 119) |
| Glucagon-like peptide-1 | Polypeptide 30 aa | Type II and III | GLP-1 | Class B “secretin-like” | Gαs AC activation (85, 120) |
| Glucagon | Polypeptide 29 aa | Type II | Glucagon | Class B “secretin-like” | Protein kinase A activation via increased AC activity and cAMP production (110, 121) |
| Ghrelin | Polypeptide 28 aa | Type I, II, and III | Ghrelin | Class A GPCR | Increased intracellular calcium release due to Gαq/11 activation and induced IP3 production, protein kinase C (PKC) activation, high basal IP3 induced PLC-PKC-dependent calcium mobilization (122–124) |
| Galanin | Neuropeptide 29–30 aa | Type II and III | Galanin type 2 | Rhodopsin-like G protein-coupled receptor (GPCR) | Phospholipase C/protein kinase C pathway activation (125) |
| Insulin | Polypeptide 51 aa | Type II | Insulin | Tyrosine kinase | Insulin receptor substrate (IRS) phosphorylation (58) |
| Oxytocin | Neuropeptide 9 aa | Not produced in taste cells | Oxytocin | Rhodopsin-like Class I GPCR | Intracellular calcium signaling via stimulated phospholipase C (126) calcium |
| Leptin | Polypeptide 167 aa | Not produced in taste cells | Leptin | Cytokine receptor | Jak-STAT activation (127) |
| Medium- to long-chain free fatty acids | Fatty acids | Not produced in taste cells | GPR120 (free fatty acid) | Rhodopsin-like GPCR | Linoleic acid-induced calcium signaling (128) |
| Long-chain fatty acids | Fatty acids | Not produced in taste cells | CD36 | Class B scavenger receptor | Linoleic acid-induced calcium signaling (128) |
A recent paper describes a broadly responsive subset of type III cells (BR type III cells) that can respond to bitter, sweet, and umami identifiable when type II cells have been electrically deactivated by the deletion of IP3R3 in mice (129). While none of these BR type III cells respond to all five stimuli, unlike type II cells however very few were exclusive to one of sweet, bitter, or umami (5.5, 7, and 5.5% respectively); 39% of the cells responded to two stimuli while 43% responded to all three. The authors present evidence that PLCβ3 (an isoform of PLCβ they find in a subset of type III cells only and not in type I or II cells) and IP3R1 may transduce this signal in the BR type III cells.
3.2.3. Sour.
“Things sweet to taste prove in digestion sour.” (Shakespeare; Richard II)
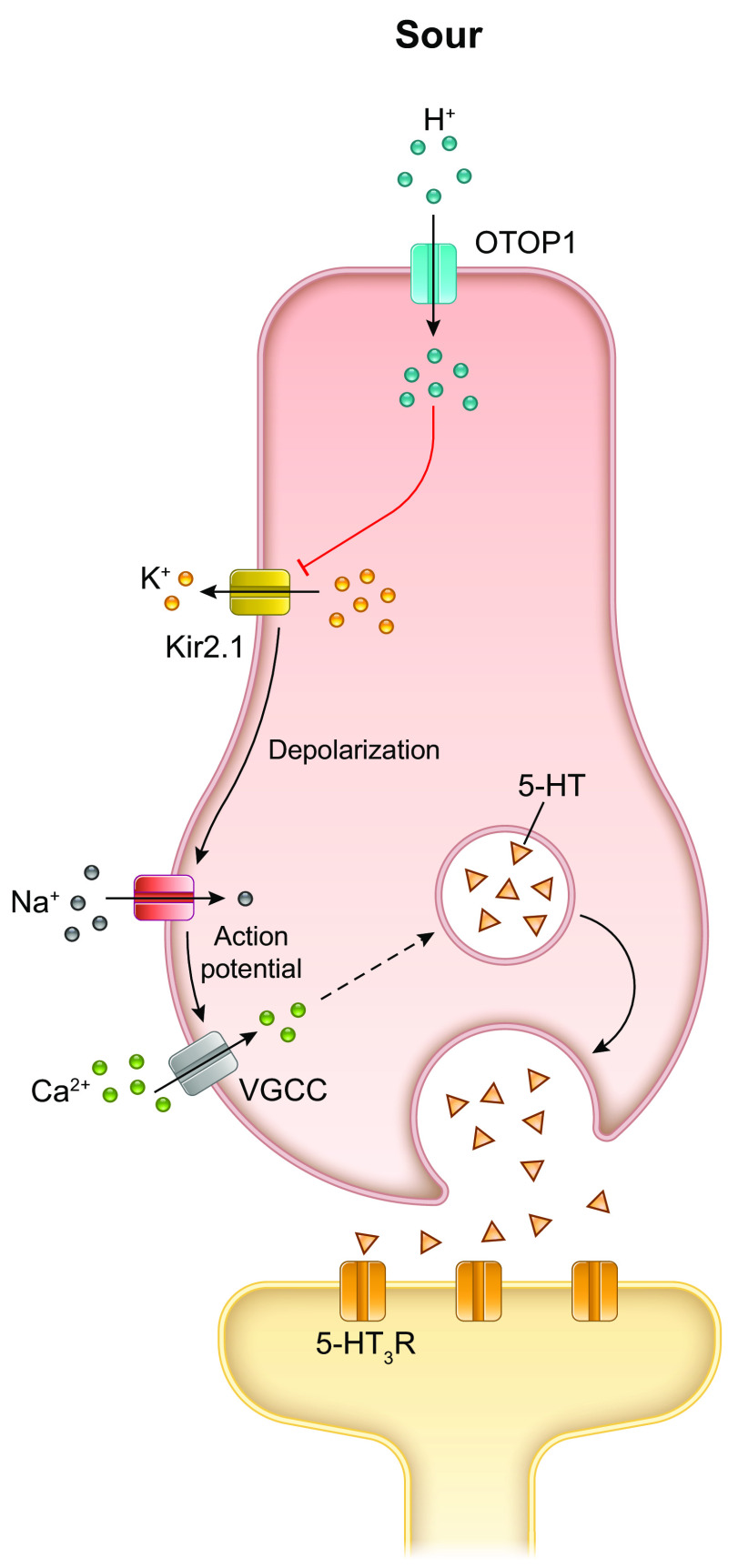
Sour taste is evoked by the hydrogen ions (protons) of both organic acids, including lactic, citric, malic, and acetic acid, and inorganic acids, such as hydrochloric, nitric, and sulfuric acid. The transient potential cation channel, polycystic kidney disease 2-like 1 (PKD2L1) was previously shown to be a marker specific to the type III cells (130) and is known to be required for sour taste perception (23, 131). Therefore, this was considered a promising candidate as the primary locus for peripheral sour taste detection (131). However, the evidence for the absolute requirement of PKD2L1 was not overwhelmingly convincing because there was only a minimal reduction in response to sour stimulation of type III cells in the PKD2L1 null mice (132, 133). Liman and colleagues showed recently that the proton channel otopetrin 1 (OTOP1), a highly selective proton channel located on the apical side of the type III cells (134, 135), is essential for cellular response to acids (134, 136) (FIGURE 7). The consequent intracellular acidification depolarizes the cells and blocks the inwardly rectifying K+ channels (Kir2.1), which serves to amplify the depolarization (137). This depolarization in turn triggers voltage-gated sodium ion channels (SCN2A) to generate action potentials that activate voltage-gated calcium channels, increase intracellular calcium, and finally, release of 5-HT-containing synaptic vesicles. As mentioned above (sect. 2.4), type III cells have conventional synapses and synaptic vesicles cluster adjacent to the presynaptic membrane which abuts the postsynaptic nerve process (57, 138, 139). The most established neurotransmitter for type III TRCs is vesicular-derived 5-HT. 5-HT is synthesized, stored in vesicles, and secreted by type III TRCs upon stimulation, and gustatory nerve fibers expressing the 5-HT3 receptors within taste buds show preferential connectivity with type III cells (140). Currently, it is understood that type III cells transduce sour sensation by two mechanisms: 1) mobilization of intracellular calcium stores consequent to ATP activation, and ii) a calcium influx through voltage-gated calcium channels initiated by the detection of sour tastants.
FIGURE 7.
Mechanism of transduction of sour taste. OTOP1 is recognized as the sour receptor and conducts the H+ ions (protons) from acids into the cell cytosol. The influx of cations causes the membrane potential to change direction, and the change in intracellular pH blocks KIR2.1 K+ channels, which further depolarizes the membrane potential. With sufficient depolarization, voltage-gated Na+ channels open causing a train of action potentials that open voltage-gated calcium channels and lead to release of the neurotransmitter 5-HT. 5-HT released at the synapses activates the afferent nerve fibers via excitatory 5-HT3 receptors (R). Image created with BioRender.com, with permission.
Of note, mice in which tetanus toxin is expressed in tandem with PKD2L1 do not have type III cells, yet they still display an aversion, although blunted, to sour tastants (141). This indicates that type III cells may not in fact mediate the aversive effects of sour or at least not be the sole arbiters of sour sensation. One possibility is that aversion to acids is mediated by acid-sensitive nociceptive afferents (mandibular branch of CN V) innervating the oral cavity and larynx (131, 136) and candidate receptors have been proposed for this effect, but none have been established (142). Given the basic nature of this physiological response in terms of the perception of sour tastant and the aversion to regurgitated gastric acid, it is probable that there are many mechanisms to elicit aversion to acids in the oral cavity (131, 136).
In a related observation, the proton detection machinery in type III cells may sense dissolved carbon dioxide (CO2) in carbonated drinks, whereby the enzyme carbonic anhydrase catalyzes the hydration of CO2 to form bicarbonate and free protons (143).
3.2.4. Fat (oleogustus).
Fat detection is not via the five prototypic taste mechanisms outlined above. The exact receptor that detects fat and the ligand component(s) of fat are still subjects of much debate within the chemosensory research community (144–146). Putative candidate receptors are CD36 and GPR120 (FFAR4), both of which are expressed in human FP (147). Activating GPR120 in isolated, cultured TRCs by both a synthetic agonist and linoleic acid (LA) leads to increased intracellular calcium and release of GLP-1 into the culture medium (148). Moreover, Yasumatsu and colleagues (149) recently provided electrophysiological evidence for transmission of fat signals from GPR120 along the F-type fibers to the mouse chorda tympani branch of CN VII.
3.3. Overview of Neurotransmitters in TRCs
In general, classical neurotransmission occurs through chemical neurotransmitters that are stored in synaptic vesicles and released into the synaptic cleft by calcium-activated exocytosis. As described above, this is the primary mechanism involved in the transmission of proton (sour) taste information from type III cells to the afferent neurons (150). TRCs also use nonvesicular transmitters.
3.3.1. 5-HT.
5-HT was the first neurotransmitter to be identified in TRCs. It is stored in classical synaptic vesicles and is released directly from type III TRCs in a depolarization-dependent and calcium-dependent manner, such as in response to sour stimulants, as described above. Type III TRCs are the only TRCs that form traditional synapses with afferent nerve fibers, and they store 5-HT at those synapses (138, 151). 5-HT is also secreted indirectly in response to ATP released from type II TRCs due to sweet, umami, and bitter tastants that activate P2Y4 receptors on type III cells. This is analogous to enterochromaffin cells in the gut where ATP induces 5-HT release (152). 5-HT released at the synapses activates the afferent nerve fibers via excitatory, ligand-gated 5-HT3 receptors (153). We have found that insulin release from choroid plexus epithelial cells is regulated not by glucose or its metabolism (as it is in β-cells in islets of Langerhans) but by 5-HT (154). It is therefore likely that 5-HT not only serves as a neurotransmitter, but when present in the intergemmal spaces of taste buds, it regulates insulin synthesis and secretion in type II TRCs (see sect. 6.2) as 5-HT receptors are also present on type II cells (155). However, this has not been proven.
3.3.2. ATP.
While we are familiar with ATP providing energy to drive processes such as muscle contraction, ATP is also a signal transduction molecule, distinct from its consumption during metabolic processes. ATP is a purine, and thus signal transduction that requires ATP as the signaling molecule is called purinergic. ATP signals by way of GPCR-coupled P2Y receptors and ligand-gated P2X receptors/ion channels. There are three ways ATP may activate these receptors. First, ATP is a regulated neurotransmitter in CNS and peripheral and enteric nervous systems (156). Second, ATP release is unregulated in that it is released from damaged tissue, and activates sensory P2X receptors on nerve terminals, and nociceptive and pain signals are then transmitted to the CNS (157). Finally, the regulated release of ATP from nonneuronal cells activates sensory nerve terminals and thereby transduces sensory stimuli. An example of this latter mechanism is in the carotid bodies, where ACh and ATP are cosecreted from glomus cells and transmit low blood Po2 (hypoxic) signal (158). Another example is regulated ATP release from type II TRCs. There is no evidence however that type II cells contain synaptic vesicles or synaptosomal-associated protein 25 (SNAP25), a key protein required for calcium-mediated merger of the synaptic vesicles with the plasma membrane, such as is typical for neurons and type III TRCs.
How type II cells affect neurotransmission of tastant signals is extremely interesting. They appear to do this by generation of ATP on demand to sweet-, bitter-, or umami-type ligand activation of T1Rs and T2Rs. As alluded to above, rodent type II TRCs form mitochondrial “synapses” with CALHM1/3 located close to the plasma membrane in areas of the cells where the purinergic receptors (P2X2/P2X3) on afferent fibers pass in apposition (see FIGURE 5B) (61). P2X2/P2X3 double knockout mice were found to be unresponsive to all five taste qualities as monitored via electrophysiological responses in both the chorda tympani branch of CNs VII and IX (159). Therefore, purinergic signaling must be required for sour sensing, which is the prevue of the type III TRC (160). As mentioned earlier (see sect. 3.2.3), type III TRCs form conventional synapses with afferent fibers and release 5-HT from storage in vesicles to activate 5-HT3 receptors on the fibers (153) (FIGURE 7). However, type III cells do not contain the ATP vesicular nucleotide transporter (VNUT) that seemingly would be required for the transport of ATP into the serotonergic vesicles for corelease (160). To add to the mystery, another recent study suggests that type II cells are surely not the source of ATP required for sour transmission because mice lacking the transcription factor Pou2f3 do not have type II cells, and yet they have normal responses to sour stimuli (161). Therefore, if type II cells are not the source of ATP required for sour transmission, where is it coming from? Clearly, this is a mystery wrapped up in an enigma that has yet to be solved. Perhaps small amounts of ATP are actually packaged with 5-HT in secretory vesicles, and methodologies so far are simply not sensitive enough to detect its release. In addition, in the case of the Pou2f3 knockout mice, more type III cells than usual are present in taste buds, and perhaps ATP is generated in those cells as a compensatory mechanism.
There is a very important contribution by the type I TRCs to the mechanisms of taste perception: the hydrolysis of extracellular ATP and ADP via NTPDase2 located on their surface (162, 163). Mice lacking this enzyme have decreased electrophysiological responses to all five taste stimuli, similar to the P2X double knockout mice. This is attributed to the excess extracellular ATP present at the afferent neurons that downregulates the purinergic receptors due to the lack of an “off” signal, that is, no hydrolysis of ATP to ADP (163). One interesting feature of SARS-CoV-2 infection is that it specifically infects the PLCβ2-containing type II TRCs. This is because they contain ACE2, the receptor for the spike protein of the virus (see sect. 11.2). Consequently, taste is either lost or distorted. As TRCs get damaged, there is likely to be unregulated release of large amounts of ATP that will result in desensitization of the purinergic-containing receptors on the afferent nerve fibers. Added to this, if type 1 TRCs were damaged from the local infection, NTPDase2 activity would also be compromised. This would result in decreased, disordered, or even absent electrophysiological responses until such time as new TRCs and/or new afferent fibers enter taste buds. It could also transmit nociceptive and pain signals (102). Moreover, ATP amplifies its own release by activating P2X receptors on adjacent type II cells (164) and, in a paracrine fashion, stimulates 5-HT secretion from type III cells. Moreover, Rodriguez et al. (165) demonstrated that type I cells have increases in intracellular calcium in response to ATP, but not 5-HT, via P2Y receptors responding secondarily, presumably, to tastant-triggered ATP released from type II cells.
3.3.3. GABA.
The inhibitory neurotransmitter GABA is synthesized, stored, and released from type III cells upon acid (sour) stimulation (166). Type II TRCs express GABAA and GABAB receptors by which they seem to inhibit ATP release when presumably activated by locally released GABA. GABA is also synthesized in β-cells where it is stored in synaptic-like microvesicles, although not in the same large-dense vesicles as insulin. This partitioning therefore allows for differential regulation of insulin and GABA release. GABA release by rat and human β-cells was shown to reflect net GABA production that varies with the metabolic state of the cells because it is regulated by the amount of available glutamine and glucose. Glucose inhibits glutamine-driven GABA formation and inhibits its release by shunting it to mitochondrial metabolism (167). Furthermore, GABA may play a role within islets to inhibit glucagon release from α-cells by hyperpolarizing their cell membranes via GABAA receptors (168). It is possible, based on the research done in β-cells, that GABA within TRCs is a source of energy for type I and III TRCs, especially at the time when they are in an active state of responding to tastants. Since type II cells also contain proglucagon-derived peptides, it is therefore also plausible that their release is regulated by GABA in the same manner. Additionally, sensory afferent neurons and their peripheral, taste bud-innervating processes express GABAA receptors (150) as well as P2X receptors necessary for ATP-mediated purinergic signaling. However, while GABA inhibits the activation of gustatory ganglion neurons in vitro, we do not know if GABA does so in vivo. In addition to its synthesis in type III TRCs, GABA is synthesized in type I cells (132). However, the membranal signal, if any, for its release is unknown; while increased intracellular calcium does occur in type 1 TRCs on ATP stimulation via P2Y receptors, it has not been shown that this results in GABA release. Furthermore, we do not know if the GABA present in type I cells is vesicular or nonvesicular (165). In the CNS, GABA is stored in vesicles in cortical interneurons, and in neurons in the dorsal raphe that also have 5-HT2C receptors (169). It regulates the proliferation of progenitor cells and migration of said cells to their final resting niche. Additionally, it regulates the elongation of neurites and formation of synapses, and its release has an inhibitory role in the firing of target neurons through GABAA receptors. It is therefore plausible that locally derived GABA released from either type I and III TRCs is involved in intragemmal neuronal fiber migration and stem cell differentiation, and it may only be released in an unregulated manner when type I cells, the most abundant and shortest living of the TRCs, undergo apoptosis.
3.3.4. Acetylcholine.
Type II cells, in addition to having ATP as a neurotransmitter, contain ACh, analogous to glomus cells in carotid bodies, and taste buds are rich in acetylcholinesterase (170), the enzyme necessary for its breakdown. ACh release, in turn, results in an increase in the release of ATP from type II TRCs, either from the same cell or by the spread of ACh in the intragemmal space from adjacent cells: M3 muscarinic receptors are expressed on type II cells (171). Interestingly, cholecystokinin (CCK), present in type II cells (1), is also found in neurons of the thalamic, limbic, and cortical areas of the brain and regulates ACh secretion in the striatum (172). TRC-derived CCK, ACh, and ATP fine tune the afferent transmission of information from the subsets of type II cells expressing either bitter (T2Rs) or sweet and umami (T1Rs) receptors (1).
3.4. Sensory Coding from TRCs to the Gustatory Cortex
How the gustatory neurons convey information on the specific tastant type from the taste bud along the sensory afferent neurons to the gustatory cortex is a fascinating topic (173, 174). Labeled line mechanism (as opposed to cross-fiber pattern or combinatorial coding) is the most favored and posits that one sensory neuron receives input from only one TRC that has one receptor type, and when activated, specific information from that receptor gets directly sent to the brain. Calcium imaging in rodents in response to tastants indicates brain areas in the insula dedicated to each taste (175–177). Zhang and colleagues (136) identified specific markers for each taste: Spondin1 for sweet; cadherin 4 (Cdh4) for umami; cadherin 13 (Cdh13) for bitter; Penk for sour; and early growth response 2 (Egr2) for salty. They then generated transgenic mice labeled with Cre for each specific marker and observed behavioral defects and selective responses of the labeled ganglia, respectively. Furthermore, single-cell RNA sequencing (scRNAseq) classifies the gustatory neurons of the geniculate ganglion into five distinct types matching the five prototypic tastes (174, 178). The labeled line mechanism therefore proposes a gustotopic map with spatial segregation of neurons selectively responsive to individual taste qualities (179). This seems the most parsimonious explanation, at least for type II TRC signaling of basic bitter, sweet, and umami taste information to the brain. However, the information relay from type I and III TRCs to the brain is not quite so simple. As outlined above, type III TRCs possess neuronal characteristics, and in addition to responding to sour stimuli, they process hormonal and neurotransmitter signals from type II cells. Isolated mouse type I cells have robust increases in intracellular calcium in response to ATP activation of P2Y membrane receptors but do not have such increases in response to tastants, including sodium chloride. They also have such responses in lingual slices that have been stimulated with bitter tastants, presumably due to ATP release from type II TRCs. Therefore, type I cells participate in shaping the sensory output of taste buds (165). While they do contain GABA, it has not been shown that it is released in response to the rise in intracellular calcium, as stated above, so it is not conclusive that type I cells actually transmit information directly to afferent fibers. Therefore, whether they directly transmit sensory information to the brain is yet not proven.
Furthermore, an increase in tastant concentration increases the number of neurons that become broadly responsive ones (177). Until recently, conventional thinking was that most taste fibers innervate only one taste bud. Using mouse FP as their model, Huang and colleagues (180) have shown that terminal branching of peripheral taste fibers is far more complex than originally thought and branching patterns of nerves in FP showed that 28% of fibers contact both type II and type III cells. It it likely that some nerve fibers respond to stimuli by transducing the different taste information from the two cell types, indicating that wiring specificity in CVP is not consistent with the labeled-line model (57). This has implications for the composition of the taste signal as it is relayed to the gustatory cortex in the brain. Sensory coding of taste in the brain has been reviewed in other articles and reviews before (150, 181–183). Additionally, elegant behavioral research in awake mice [Chandrashekar and colleagues (143) performed their experiments on sedated mice] is now adding to the complexity of signaling, so all is not as straightforward as it once seemed (142, 144). Ohla and colleagues (173), for example, found that in the NTS cells classified as taste responsive constitute a small minority of the cells that convey information solely about the type of tastant. Most of the cells contribute information not just concerning what the tastant actually is (i.e., which of the 5 prototypic tastes is being presented to their respective receptor on the TRCs) but how much is being consumed, what is being consumed, the number and types of licking patterns, and taste quality. This implies that there must be sensorimotor coordination occurring in the NTS such that information from both taste and food quality and environmental and behavior cues are integrated.
Also, a subject of active investigation is the aversive and avoidance qualities of taste. We have briefly alluded to the peripheral aspect of aversion to salt and sour above. It is evident that these “bad taste” responses originate from both within the taste bud, the lingual epithelium, and the oral mucosa and are linked to complex behavioral response circuits. This topic is covered in depth in another review recently in this journal (184).
4. TONGUE DEVELOPMENT
Our understanding of the molecular underpinnings of tongue development comes from extensive lineage tracing, molecular profiling, and gene-targeting studies in mice, and we refer the reader to the comprehensive recent reviews on this topic (8, 25, 185, 186). At week 4 of gestation in human development [embryonic day 10.5 (E10.5) in mice] a small pit-like structure called the stomodeum forms between the frontal prominence (which eventually become the brain) and the developing cardiac bulge (185, 186) see FIGURE 8A. The stomodeum will eventually become the oral cavity. The tongue itself begins to develop around week 5 of human gestation with the anterior two-thirds developing from the first pharyngeal arch and the posterior third from the second, third, and fourth pharyngeal arches. They come together to form an initial swelling or primordium in the center of the oral cavity. This primordium is initially populated by cranial neural crest cells [CNCC (187)] covered by an epithelium. By E11.5, the myogenic progenitors have begun to migrate into this area. Given that the mature tongue consists of a variety compartmentalized tissue types, it is not surprising that it is derived from several different cell populations within the embryo. The lingual muscles are of mixed origin deriving from myoblasts originating in the most anterior set of somites, the occipital somites, and are labeled by the transcription factors mesoderm posterior BHLH transcription factor 1 (MesP1) and the paired box transcription factors Pax3 and Pax7 (188–194) and from the cranial paraxial mesoderm (195). Pax3 is essential for the expansion of the pool of lingual myogenic progenitors at around embryonic day 10 (E10) (196). The tongue undergoes rapid enlargement due to the expansion of the musculature such that by E13.5, a prototype tongue structure is established, with a symmetrically arranged intrinsic musculature and extrinsic muscles and their connections in place. The posterior third of the tongue is derived from endoderm, whereas the anterior two-thirds of the tongue is derived from stomodeal ectoderm (197). This has implications for lingual innervation (see sect. 2.2), the composition and function of the taste buds in the papillae of the two regions (see sect. 3.2), and TRC renewal (see sect. 5.2). The completion of tongue development requires interaction and molecular signaling between the overlying epithelial layer, CNCCs, and the myogenic progenitors all in a coordinated effort to regulate cellular proliferation, differentiation, and survival. This involves coordination between the Wnt/β-catenin, FGF, TGF-β, and SHH signaling pathways (the intricacies of which have been reviewed previously (8, 25, 185, 186, 193, 198).
FIGURE 8.
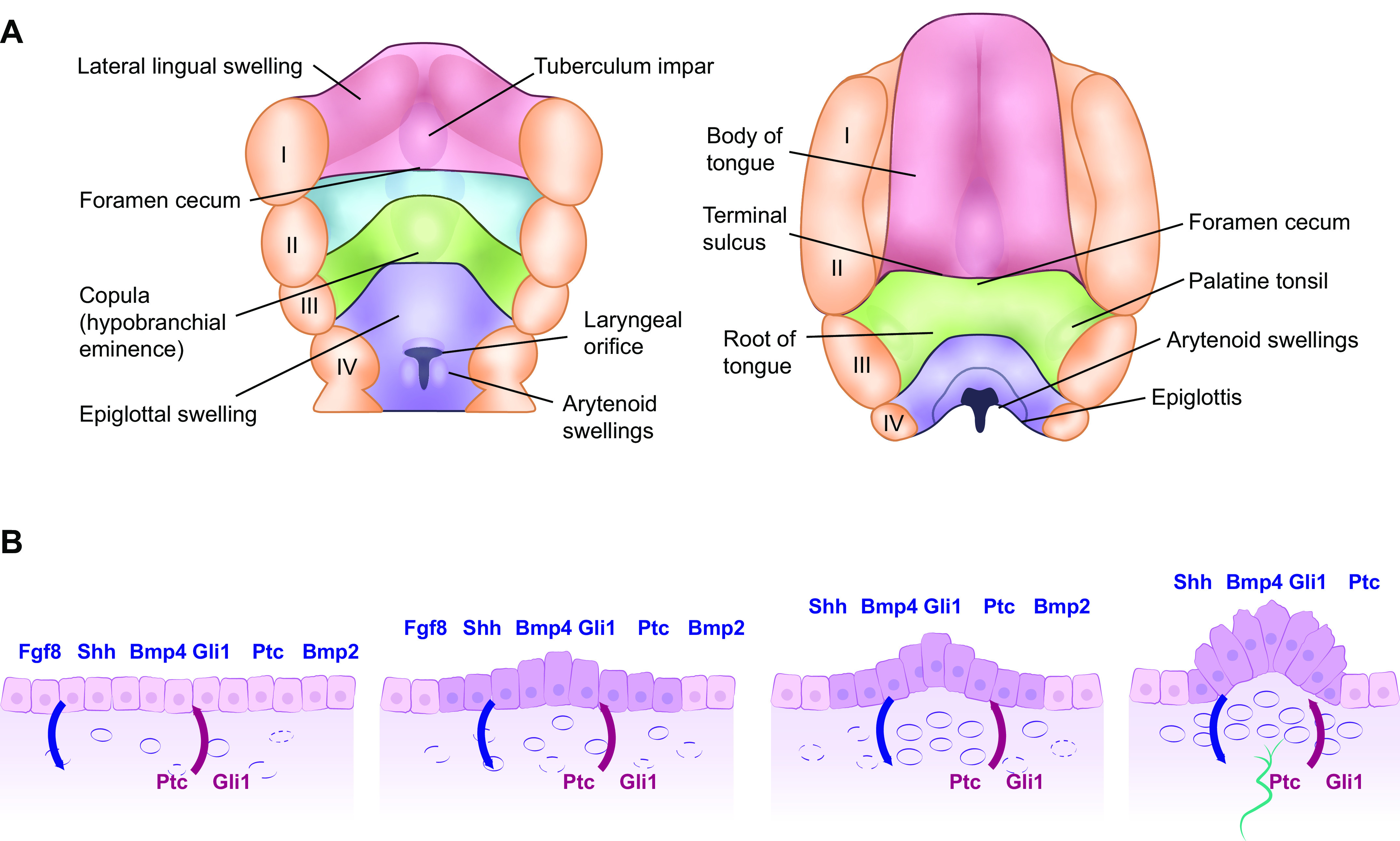
Mammalian tongue (A) and FP formation from the lingual epithelia layer (B). A: development of the mammalian tongue: fourth postovulatory week of human development is shown on the left and the 5-month stage is shown on the right-hand side. The anterior two-thirds of the tongue (shown in red) emerges from the lateral lingual swellings and tuberculum impar while the posterior third (shown in green) emerges from the copula. I–IV: position of the 5 pharyngeal arches. The posterior third of the tongue is derived from endoderm, whereas the anterior two-thirds of the tongue is derived from ectoderm, a fact that resonates through the many differences observed in innervation, taste bud composition, nature of taste receptor cells (TRC), and TRC renewal. B: schema showing early morphogenesis of fungiform papillae (FP) from taste placodes during mouse tongue development. 1: Morphogenesis of the taste placode begins at E12.5 through the epithelial-mesenchymal interaction signals [fibroblast growth factor 8 (Fgf8) and sonic hedgehog (shh) and its ligands Gli1 and Patched (Ptc) and bone morphogenic protein 2 and 4 (Bmp2 and 4)] from the epithelium are shown above in blue and those from the mesenchyme are shown below in red. 2: At E13, epithelial thickening occurs with the epithelial cells at the basal epithelium on the anterior part of the tongue increased in height. 3: At embryonic day (E) 13.5, the columnar epithelial cells begin to form an arch to which expression of the epithelial signaling molecules is restricted. 4: At E14, the FP commit to the next developmental phase with nerve innervations shown in green: neuronal innervation is required for taste bud development within the FP.
The taste papillae emerge as taste placodes which are focal thickenings in the lingual epithelium around embryonic day 12 (199) or week 7 of human gestation (8), a process that involves epithelium-mesenchymal interaction (FIGURE 8B). This is then followed by nerve innervation, which is a requirement for taste bud development. The development of the FP is regulated by the Wnt/B-catenin, bone morphogenetic proteins (BMPs), SHH, EGF, and FGF pathways, all of which have been reviewed previously (200–202). An understanding of how tongue development occurs aids in the management and treatment of congenital disorders of the tongue. A comprehensive review of the congenital abnormalities of tongue formation in humans is presented in two recent reviews (25, 203). The unique origin of the lingual muscle cells is significant when considering lingual muscle repair following injury as we discuss in sect. 5.
5. TONGUE RENEWAL
5.1. Tongue Muscle Renewal and Injury Response
Unlike other muscles in the body, the cellular and molecular responses of craniofacial muscles have not been extensively studied (204). Muscle regeneration is mainly orchestrated by a population of muscle stem cells, termed satellite cells (MuSCs). As tongue MuSCs are of the Mesp1/Pax3/Pax7 lineage, they share more in common with limb muscles (193, 194, 205, 206) than do the other craniofacial muscles, which are of different lineages. A recent report compared the regenerative potential of tongue muscle and tibialis anterior (TA) limb muscle in response to acute injury by intramuscular injection of cardiotoxin, a cytolytic agent (194). Tongue muscle displayed an efficient regenerative response similar to TA but with slightly faster kinetics. In vitro, tongue-derived satellite cells differentiated robustly into mature myotubes with spontaneous contractile behavior and myogenic marker expression. The authors noted the expression of a number of transcripts of the homeobox transcription factors (HOX) genes (associated with positional guidance) in TA muscle cultures which were mainly absent in the tongue MuSC cultures. The absence of the HOX genes in the tongue MuSCs could be functionally relevant to cellular behavior and responses. As with skeletal limb muscle, tongue muscle is also thought to undergo degenerative changes with aging including loss of MuSCs alterations in myosin heavy chain (MyHC) isoform and myofiber type composition, atrophy and death of myofibers and myo-nuclei, increased regions of muscle fibrosis, and fragmentation of the neuromuscular junction as monitored in rodents (207–212). A recent study in rats (213) indicates loss of Pax7 expression and an increase in levels of the senescence marker p16INK4a in the genioglossus (GG) with age and increased protein expression of p16INK4a in isolated, aged Pax7 expressing MuSCs from the GG. Due to the high demand placed on the GG during swallowing and speech, the authors postulated that it is likely more susceptible to changes with age. Following tongue-strengthening exercises, where to obtain water the rat had to press its tongue against a force-incremented disk, the authors only observed increases in Pax7+ MuSCs in the SG and HG muscles and not in the GG. Given the importance of tongue muscles for speech and eating it is surprising that more studies of the regeneration of the lingual muscles and their response to exercise injury or aging have not been performed. Tongue muscle renewal is important in the context of recovery of tongue cancer patients and in the maintenance of tongue muscle integrity and strength in aging to preserve the masticatory capacity to preserve nutritional intake (213, 214).
5.2. The Identity of the Taste Stem Cells
Unlike sensory cells in the inner ear and retina but similar to olfactory sensory neurons, TRCs are in a constant state of apoptosis and renewal (201, 215). In mice, leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) marks progenitor cells in the posterior part of the tongue (216, 217) while Lgr6 marks progenitor cells in both anterior and posterior tongue (218). These cells can entirely replenish both the taste buds and lingual epithelium (216–218). These progenitor cells are the characteristic hyperproliferating organ-specific stem cells found in multiple organ types, the parenchyma of which is composed of self-renewing tissues (2). Additionally, lineage tracing in mice shows that cytokeratin 5 (Krt5) (219) and SRY-box-2 (SOX2) (219, 220) label taste stem cells. The SHH pathway is instrumental in the regulation of taste bud renewal as illustrated by the taste disturbances described in chemotherapy using SHH inhibitors such as vismodigeb (221, 222). In mice, SHH is expressed by the type IV cells located within the base of the taste bud and they relay their signal to the SHH receptor-expressing cells, that is, the Gli1- and Ptch1-positive taste progenitor cells, outside the base of the taste bud (223). Prior to our work (59), nobody had examined taste stem cell proliferation in situ in human FP. Here in FIGURE 9, we show, for the first time, that the basal layer that contains the proliferating cells also expresses dipeptidyl peptidase 4 (DPP4). We also show a proliferating cell within the taste bud, indicating the possible presence of a type IV cell in human taste buds.
FIGURE 9.
Human fungiform papillae (FP) showing taste bud and basal stem cell layer. Immunofluorescence stainings are of Ki67 (green, a marker of proliferating cells) and dipeptidyl peptidase (DDP; red). DPP4, also known as CD26 and adenine deaminase complexing protein 2, is a multifunctional membrane-bound and soluble protein that is best known for its activity as an exopeptidase. It is also a viral receptor (for MERS-CoV) and is involved in the regulation of intracellular signal transduction of T lymphocytes and apoptosis. DPP4 staining of individual cells, mostly likely T lymphocytes, can be seen in the lamina propria of the fungiform papillae and in all the stem cells of the basal layer, including the few cells directly underneath the taste bud. This is the first time that DPP4 has been shown to be present on membranes of this stem cell layer. No DPP4 is present in taste buds, and therefore mature taste receptor cells lose this cell surface marker. The arrow indicates the position of a taste bud (outlined in black), which can be seen in the hematoxylin and eosin staining image. FP biopsy was obtained from a young male in the fasted state. Scale bar = 50 µm.
There are a number of questions that we do not have the answers to yet in our understanding of taste stem cell dynamics. How different are the CVP and FLP stem cells from the FP stem cells given the differences in innervation and taste bud architecture and cellular expression patterns we have discussed above? What cell surface markers could be used to identify and isolate the taste stem cells? Lgr5 and Lgr6 are very lowly expressed, and when not genetically tagged with a fluorescent protein (as in the transgenic mice that have been used) are not useful for the isolation of human Lgr5+/Lgr6+ cells (224, 225). What is the repertoire of markers of human taste stem cells in addition to DPP4? The recent development of taste organoid cultures allows researchers a promising empirical avenue to address some of these questions (see sect. 11.1.2). More information on the regulation of taste stem cell dynamics and the molecular pathways influencing them can be obtained from in vitro work on taste organoids, which include the stem cells, and the mature cells into which they differentiate in culture, namely the epithelial cells and the taste bud cells. This would be complementary to work using transgenic mouse models as one drawback of using organoids is the absence of the trophic factors from the underlying lamina propria. The advantage of an organoid culture system is the potential to study human taste stem cell dynamics in vitro.
5.3. Taste Bud Cell Death and Renewal
Extensive studies in mouse taste bud renewal have established that taste stem cells require signals from the taste buds, gustatory nerves, and mesenchyme to both proliferate and be directed toward differentiation into new TRCs. It has been well established in mice and humans that gustatory innervation provides trophic support to taste buds, with the CVP more sensitive to denervation than FP (226). In turn, the gustatory nerves require taste bud-derived brain-derived neurotrophic factor (BDNF) (227) to maintain innervation of taste buds in adulthood (228). Recently Jiang and colleagues (148, 229) have shown that the peptide R Spondin can substitute for the neuronal input that signals maintenance of taste buds. Type II bitter and sweet TRCs instruct neurons via the guidance molecules Sema3A and Sema7A (174) providing some insight into how taste cell specificity and balance in the numbers of the different types of cells is preserved during TRC renewal. Signaling pathways known to support taste bud renewal are Wnt/β-Catenin, SHH, and FGF. Very elegant reviews describing in detail these mechanisms in mice have been published recently, and we refer the reader to these comprehensive publications (200–202, 215).
One critical element is neglected in all but one of the recent studies on taste bud renewal in rodents. Cell cycling is routinely examined in the light phase when rodents are not active and consequently eat very little. This is problematic as Sullivan and colleagues (64) have demonstrated that taste stem cells in rodents follow a circadian rhythm with two main periods of heightened proliferation during the dark/active phase. One smaller peak in proliferation was observed at the beginning of the dark phase and there is a larger peak toward the end. They also demonstrated the presence of proliferating cells in the synthesis phase both at the base of the taste bud (the putative type IV cell) and within the core of the taste bud itself. To our knowledge, this is the only time that proliferating cells have been detected in the core of rodent taste buds. This paper highlights a deficiency of many studies that rely on rodents to examine taste stem cell proliferation; namely, that they are performed at a time that is convenient for the researcher during the light phase. The circadian dependence of taste stem cell proliferation and the possibility that mammalian taste progenitor cell proliferation increases postprandially add a new aspect to consider when timing taste stem cell labeling and cell cycle analysis.
By examining cellular retention of the thymidine analog 5-ethynyl-2′-deoxyuridine (EdU) in combination with markers for the mature TRCs, Perea-Martinez and colleagues (65) were able to determine TRC longevity mice. Type III cells appear to differentiate over 3 days and and live the longest with a half-life of 22 days with type I and II cells and have a shorter half-life of ∼8 days. The older TRCs are removed by apoptotic cell death with between 8 and 11% of TRCs in taste buds from young mice expressing markers of apoptosis at any one time (230, 231). As the taste stem cells require signals such as β-catenin from the taste buds to proliferate and differentiate, the loss of taste buds can in turn lead to the loss of taste stem cells (201, 232). As discussed in sect. 10.1, chemotherapeutics and radiation adversely affect taste bud renewal. The effect of radiation in cancer has been modeled in mice. A single dose of radiation reduced taste stem cell proliferation and increased stem cell apoptosis, disrupting the replenishment of TRCs, and leading to reduced numbers of type II and III cells in the taste buds (233). The repeated low-dose radiation protocol preferentially caused cell death in TRCs, but not taste stem cells (232). In addition, a more prolonged effect was observed in FP over CVP. Repeated dosing also led to a reduction in Wnt/β-catenin signaling upon which FP TRC renewal is more dependent than CVP TRC renewal (234). It is possible that Wnt pathway activators (that do not enhance tumor growth) could be applied locally to mitigate against the loss of TRCs and taste sensation in irradiation of head and neck cancers.
6. HORMONES IN TASTE BUDS
We summarize the hormones and hormonal receptors expressed on taste bud cells per our previous review (Ref. 1; TABLE 1).
Ligands and their receptors found in taste receptor cells and afferent fibers mediate important signaling mechanisms involved in taste perception and metabolism. Ligands bind to their corresponding receptor molecule(s), thus initiating their primary signaling mechanism.
6.1. Function of Hormones in Taste Buds
An overview of the hormones in taste buds can be found in TABLE 1. Since our last review in which we discussed the role of the hormones in modulating the TRC signaling (1), with regards to CCK, Y, proglucagon, and VIP families of peptides, galanin, proghrelin and ghrelin, oxytocin, and leptin, very little new information has been added. It is likely that the present COVID epidemic has slowed basic research in this area. Hormones produced in taste buds have autocrine, paracrine (1), endocrine (see sect. 6.2), and neurotransmitter functions because it is possible that GPCR-independent perception of sweet (see discussion above) is mediated by GLP-1 acting directly on GLP-1 receptors on afferent fibers (112). For a comprehensive review of the role of GLP-1 and taste, see the recent reference 235. We here now add new information on another endocrine hormone that is synthesized in TRCs, namely insulin (236).
6.2. Insulin
We have found that mature, biologically active insulin is synthesized in type II TRCs in taste buds in CVP and FLP. Type II cells contain all the necessary molecular machinery for the synthesis and secretion of insulin (1, 124, 237). However, we could only detect insulin mRNA in FP definitively. Our inability to detect insulin protein is due to the high endogenous background fluorescence in FP (58). Per gram of tissue, insulin levels in the taste tissue of mice were much lower than in pancreatic tissue, and therefore, TRC-derived insulin is unlikely to contribute to the insulin that is in circulation (58). The only other tissue where mature, biologically active insulin is produced and secreted, besides in β-cells, is in the epithelial cells of the choroid plexus where it is regulated by 5-HT, in a calcium-dependent manner, through activation of GPCR 5-HT2C receptors (154). The role of locally produced insulin in taste buds has not yet been established, but it is present in many species that we tested, such as rodents, pigs, monkeys, and humans. Tyrosine kinase growth factors such as insulin regulate growth, differentiation, and final organ size in the body, and tyrosine kinases are more highly expressed in taste bud tissue than in the surrounding lingual tissue (238). Furthermore, IGF-1 receptors have been found in TRCs (239). Therefore, it is plausible that IGF-1 could be the major trophic factor for taste buds as it is in skeletal muscle, including the tongue skeletal muscle. However, this does not appear to be the case because the genetic elimination of IGF-1 receptors in TRCs did not alter the number or types of TRCs or the size of taste buds in mice (240). Therefore, locally produced insulin could be a trophic factor influencing taste bud cell size, stem cell proliferation, and/or differentiation. Recent information has lent credence to that possibility, because Takai and coworkers (241) have reported on the presence of insulin receptors (IR) on type II and III TRCs, as well as on the LGR5+ cell, which is the stem cell for TRC renewal. The data acquired with reference to insulin’s effects in lingual-derived organoids would also support this speculation (see below for description in sect. 11.1.3. Insulin is also a potent vasodilator via nitric oxide (NO) dependent pathways. Therefore, TB100-derived insulin may be involved in increasing blood flow to taste buds and thereby increasing blood supply to stem cells, in addition to acting as a signal to differentiation and replacement of TBCs lost from mechanical injury. Vasoactive intestinal peptide (VIP), which is present in type II cells (TABLE 1), also causes vasodilation in an NO-dependent manner (242), and therefore, insulin and VIP should have synergistic effects on blood flow to taste buds and papillae. Parenthetically, VIP, on a molar basis, is 50–100 times more potent than ACh as a vasodilator (243).
Another possible role for locally produced insulin is involvement in the regulation of white adipose fat deposition in the tongue. Tongue fat accumulation causes an increase in tongue volume/size, it correlates with whole body adiposity based on magnetic resonance imaging measurements, and lingual fat, in turn, correlates with the degree of obstructive sleep apnea (244). Furthermore, fat in the tongue mainly accumulates in the posterior half of the tongue (245). Schwab et al. (245) propose that increased tongue fat impairs the functioning of the muscles that attach the tongue to bone, preventing these muscles from positioning the tongue away from the airway, especially during sleep (see FIGURE 10A for MRI of tongue fat).
FIGURE 10.
Tongue fat and lymphatic drainage. A: representative 3-dimensional volumetric reconstruction of tongue and fat within tongue (yellow) from a series of 3-mm contiguous axial MR images superimposed on a midsagittal image. There is substantially more fat at the base of the tongue. RG, retroglossal; RP, retropalatal. Image from Ref. 246, with permission from Oxford University Press. B: transverse section through the oral cavity illustrating lymphatic drainage of the tongue.
Additionally, fat can infiltrate the muscle bundles thereby decreasing muscle contractile ability, and there are inverse relationships between muscle lipid content and muscle force, velocity, and power (247). As locally produced insulin in CVP and FLP would be expected to drain through lingual lymphatics through the GG muscle to deep cervical nodes (see sect. 2.3), it seems logical that insulin derived from taste TRCs would be the main metabolic driver for controlling adipose size and triglyceride deposition, as it is in all white adipose tissue, within tongue muscle fibers as excess energy intake gets stored as fat. We have already published that insulin expression is increased in CVP TRCs in obese Sprague-Dawley rats (58).
7. LINGUAL EPITHELIUM AND THE MUCOSAL IMMUNE SYSTEM
The lingual epithelium is exposed on a constant basis to potentially infectious agents and toxins, both in the air inhaled and the food consumed. Like the rest of the mucosal epithelium, the dorsal surface of the tongue relies heavily on the innate immune system to mount a swift response for the defense of the local tissues hence the body as a whole. Again, most of the work that has been done in characterizing the surveilling immune cells in lingual papillae has been done in mouse FP, with some characterization also being done in human FP. Taste buds (based on work in mice) are considered to be an immune-privileged site, as no immune cells, such as resident macrophages, are found within them. It is understood at least in mouse taste buds that part of this immune privilege is conferred by the taste bud blood barrier (66) similar to the concept of immune privilege conferred by the blood-brain barrier (248). However, taste buds do possess an innate immune defense system that has been described in the literature. Many elements of the innate immune system, cytokines and their receptors, chemokines and their receptors, components of the complement system, and the Toll-like receptors (TLRs) are enriched in taste buds relative to the intergemmal epithelium (249–253). Inflammation is initiated when TLRs are activated by inflammatory stimuli, and many have been found on TRCs. Transcripts for 10 of 12 of the known mouse TLRs (1–7, 9, 12, 13) are found in mouse gustatory epithelium, and TLR 2, 3, and 4 are coexpressed with the type II marker Gαgust (251). Innate interferon (IFN) induction is the first line of defense against infection (254). IFN-gamma (γ) is produced in mouse taste buds (255), and exogenous IFN infusion alters taste perception in humans (256). Evidence from studies in mice shows that TRCs express functional interferon IFN I and II signaling pathways that are activated during systemic inflammation (252). Two IFN-α receptor subunits, IFNAR1 and IFNAR2; their downstream JAK kinases JAK1 and TYK2; and the transcription factors STAT1, STAT2, and IRF-9 are all expressed in normal mice TBCs. Systemic inflammation and an associated IFN response increase cleaved caspase levels and cause TRC death within taste buds (252). Feng and colleagues (249) have shown that the bitter subset of type II cells in mouse taste buds produce the anti-inflammatory cytokine interleukin-10 (IL-10). IL-10 plays a critical role in limiting host immune responses to pathogens by suppressing the production of IFN-gamma and TNF-α. A separate subset of type II cells (T1R3 positive) makes the proinflammatory cytokine TNF-α (249, 250), and there is evidence that it is involved in mouse perception of bitter substances (257).
Bitter taste receptors are potentially significant contributors to the innate immune defense both within the taste buds and in solitary chemosensory cells throughout the respiratory tract (258). Tas2R38, the specific human bitter receptor for the ligand phenylthiocarbamide (PTC), is the most studied of the bitter receptors. Perception of PTC is primarily genetically determined by three nucleotide polymorphisms resulting in five haplotypes (259). These correlate with the functional allele of the receptor containing proline, alanine, and valine (PAV/PAV) and a nonfunctional allele containing alanine, valine, and isoleucine (AVI/AVI) with an intermediate allele (PAV/AVI) categorized into three groups as supertasters, nontasters, and tasters, respectively. This categorization while accurate for activation of T2R38 by PTC is not accurate phenotypically as nontasters can detect higher concentrations of PTC, suggesting that other receptors in oral mucosa may bind compound less avidly (98, 260). Significantly, T2R38 is activated by bacterial quorum-sensing molecules, resulting in calcium-dependent activation of nitric oxide synthase (NOS). This drives robust NO production, thus increasing cilia motility, both of which are innate immune responses to infection (261). Whether this also occurs in the type II TRCs of the taste buds has not yet been determined. Interestingly, tasters have also been shown to have higher TNF-α levels in their saliva than nontasters (262).
Cumulatively this implies the existence of intragemmal cell-to-cell communication through cytokine release that is responsive to environmental insults and perception of different tastes and provides a mechanism(s) for how bacterial and viral infection can directly affect taste and cause apoptosis of TRCs. It is not currently known how much of the observations on TRC cytokine production extend to human taste buds, but the mouse data certainly serve as a guide to future work.
The surveilling immune cells are found mainly in the lamina propria and nongustatory epithelium of the FP (263). In FP from healthy individuals, there are many MHC class II positive antigen-presenting cells in the lamina propria but also found to some extent intraepithelially. Additionally, there are four categories of dendritic cells (DCs) based on their cell surface markers; a set positive for the characteristic DC marker cluster of differentiation 11c (CD11c); immature DC cells positive for DC-specific intercellular adhesion molecule (ICAM)-grabbing nonintegrin (DC-SIGN), which functions as cell adhesion receptor mediating both DC migration and T-cell activation; CD83-positive mature DCs, and CD1a-positive DCs more commonly referred to as Langerhans cells.
However, B lymphocytes were rarely found in the population studied (263, 264). In conclusion, although taste buds are a site of immune privilege, they play an active role in protection from pathogens by means of their self-contained innate immune signaling system, and the lamina propria of FP are equipped with antigen-presenting cells and T lymphocytes.
8. EFFECT OF OBESITY AND SUGAR ON TASTE
“Late August, given heavy rain and sun
For a full week, the blackberries would ripen.
At first, just one, a glossy purple clot
Among others, red, green, hard as a knot.
You ate that first one and its flesh was sweet
Like thickened wine: summer's blood was in it
Leaving stains upon the tongue and lust for
Picking.” (Seamus Heaney; Blackberry Picking)
The study of taste and its perception in human obesity is a nascent field. In a mouse model of obesity, taste epithelial proliferation and taste bud number in the CVP were significantly reduced compared to normal-weight mice (265). Type III TRCs are longer lived than type II and they, in turn, are longer lived than type I TRCs in mice (65, 266) (see sect. 5.3). Therefore, the renewal process must consider differences in life span because it may be influenced by environmental effects, such as more calorie intake than is required for homeostasis. In another study, obesity led to reduced numbers of type II TRCs with a concomitant reduction in nerve and behavioral responses to sweet and bitter but not potassium chloride stimuli (267). This is suggestive of a selective impact of obesity on type II TRC maintenance and potentially implies that the number and proportions of TRC types produced are indeed subject to environmental cues. However, this may be a result of inflammation in the oral cavity and/or tongue itself due to the composition of the diets given to the mice and alterations in the microbiome. Simply put, mice cannot clean their teeth and perform oral hygiene (265).
The potential role of taste and its perception in the pathogenesis of human obesity is unclear. Whether taste is a primary cause of obesity or whether taste disturbances secondary to systemic inflammation further aggravate the obesogenic condition is not deciphered. Obese subjects have reduced sweet perception. However, this may be due to an attitude of “wanting” more sweet stimuli, rather than increased “liking” of sweet stimuli (268, 269) as in the child wanting more sweet blackberries. The increased liking could result from decreased numbers of type II TRCs such that an increase in the ligand is required to achieve the desired taste perception. In addition, this could be a primary cause or secondary to the obese environment. Eating excess calories will lead to their storage as triglycerides in adipose tissue, even in tongue muscle (FIGURE 10, A and B), and whether excess calorie intake has any effect on the numbers and ratios of types of TRCs is not yet known. Sugar has a bittersweet reputation when it comes to health, especially cardiovascular health. For example, in a 2014 study, an association between a high-sugar diet and a greater risk of dying from cardiovascular disease was found. Over the course of the 15-yr study, men and women who ingested 17–21% of their calories from added sugar had a 38% higher risk of dying from cardiovascular disease compared with those who consumed 8% of their calories as added sugar. Basically, the higher the intake of added sugar, the higher the risk for heart disease, and this was independent of body mass index (270). The authors concluded that most U.S. adults consume sugar to such an excess as to be detrimental to health, over and beyond its role in causing obesity due to unneeded calories. Moreover, excess sugar and/or fat in foods are a cause of bacterial overgrowth, dysbiosis, and gum disease (271) that could have environmental effects on the renewal of TRCs and taste buds, as is the case even more so for rodents and be a source of proinflammatory, proatherosclerotic cytokines.
To lose weight a net negative energy balance is required. This can be achieved in three ways: reducing calorie intake, increasing calorie expenditure, and reducing calorie absorption. The first two are difficult, take time, and often fail. The most effective approach to weight loss presently is bariatric surgery of which there are now several techniques. These operations reduce obesity because of profound loss of white adipose tissue and in the process major metabolic improvements occur that result in mitigation of type 2 diabetes and hypertension (272). Current studies are ongoing to determine the long-term adverse effects and the durability of the various surgical interventions. A recent study of 48 females (ages 38–40 yr, body mass index of >40) investigated changes in taste preferences after both Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG). Both surgeries reduced body weight as early as 2 weeks, which was sustained for 6 months after surgery; however, RYGB resulted in greater weight loss than VSG at 6 months. Both resulted in decreases in liking sucrose mixtures. Results also suggested that preoperative taste preference could function as a predictor of weight loss success because those with the strongest liking for sweet mixtures had the greatest weight loss success (273).
For those reluctant to undergo major abdominal surgery and to alter their intestinal anatomy and/or fear long-term complications from said alterations, there is now new hope with pharmaceuticals. Over the last 2 years, treatment of obese patients with GLP-1 receptor agonists (GLP-1Rs) is being reported to be achieving weight loss figures on a par with bariatric surgery. Weight loss through surgery results in a rapid loss of fat mass, but some weight loss of lean muscle mass also occurs. In contrast, treatment with GLP-1Rs results in a gradual but steady weight loss that seems mostly fat loss. It has been known for several years that continuous administration of GLP-1Rs results in weight loss in rodents (274), and this information is finally now being operationalized for human obesity. Two synthetic GLP-1Rs, liraglutide and semaglutide, are now available for reducing body weight in humans. These compounds engage the GLP-1R in the stomach and small intestine to activate mechanoreceptors resulting in a perception of fullness. They also engage GLP-1Rs in the area postrema of the brain that leads to aversive sensations, and they diminish nonhunger-related (hedonic) feeding by activating GLP-1Rs in brain areas involved in reward, such as the nucleus accumbens and ventral tegmentum areas (275, 276). Liraglutide is administered subcutaneously once daily while semaglutide, originally also subcutaneous (but once weekly), is now available orally. Adults with obesity (or overweight with one or more weight-related coexisting conditions) receiving once weekly subcutaneous semaglutide, for example, had a mean weight loss of 14.9% from baseline over 68 weeks of treatment (277). This percentage is similar to that achieved with sleeve gastrectomy. It is likely that the GLP-1Rs also engage the receptors on the sensory fibers in taste buds (as discussed above in 3.2.2.) and decrease the “liking” for sweet taste in TRCs, thereby adding to the overall decrease in hedonic feeding that is a hallmark of these compounds: However, taste perception was not studied during the liraglutide or semaglutide obesity trials.
Given the taste disturbances observed in obesity, researchers have already begun probing the lingual epithelium for changes in expression levels of transcripts and proteins to decipher the mechanisms underlying these taste changes. Complementary to the work of Sullivan and coworkers (64) demonstrating a circadian rhythm to taste stem cell proliferation, Bernard and coworkers (278) show that mouse CVP gene expression also demonstrates a circadian rhythm. Diet-induced obesity reduced the oscillation of key genes involved both in the circadian clock and lipid detection/signaling. Previously Dutt and colleagues (279) had shown that CD36 was increased in extracts of whole mouse tongue. In one study on human FP (280), the authors found a decrease in transcript levels of several type II TRC proteins (PLCβ2, GNAT3, T1R2, GNG13, TRPM5, T2R31, PKD2L1, and T1R1) and SHH transcript levels in FP from obese versus lean women suggesting loss of SHH signaling could lead to reduced type II cell function in obesity. With the recent surge of studies on the molecular underpinnings of taste loss perhaps we will see more studies on human FP and see a more comprehensive rendering of protein expression in the different tongue tissues in the Human Protein Atlas (281).
9. EFFECTS OF SMOKING, ALCOHOL CONSUMPTION, AND CANNABINOIDS ON TASTE
“It was the best day of Jimmy Sr’s life. The people he served that night got far more chips than they were entitled to. And they still made a small fortune, sold everything. They hadn’t even a Mars bar left to sell. They closed up at ten, lovely and early, and had a few quiet pints.” (Roddy Doyle; The Van)
Tobacco smoking and alcohol may have direct effects on taste papillae numbers. Moreover, smoking is linked directly to microvascular occlusion thereby diminishing vascularization and causing occlusion of blood supply that can easily be appreciated to result in loss of taste papillae. Although there is an inconsistent relationship between the use of nicotine products and taste dysfunctions based on published reports, we know that tobacco smoking and smoke inhalation cause an increased propensity to develop upper respiratory infections and dental and salivary gland problems, which would gradually impact the ability to properly taste food (282). Cigarettes and other tobacco products such as E-cigarettes contain nicotine, which could contribute to the chemosensory properties of tobacco (through ACh receptors present on type II TRCs), and bitter taste receptor gene variants have been shown to be variably associated with smoking status, depending on the populations studied (283). Flavors play a role in compensating or masking consumers’ perceptions by introducing chemosensory sensations such as heat, burning, tingling, or cooling. Menthol is a usual flavoring additive to cigarettes and tobacco products to reduce and even mask the harshness of smoke because of its cooling and anesthetic properties and is especially used among African Americans (284). Of course, increasing the use of these products raises the likelihood of nicotine addiction and profits for tobacco companies. The profligate marketing of flavored e-cigarettes, particularly targeted toward adolescents, is especially egregious (285).
In general, drinking alcohol beverages, such as with a meal, can serve as flavor enhancers. Excessive alcohol consumption, and what is considered excessive, vary considerably between cultures. However, persistent heavy drinking is associated with taste dysfunction and a deterioration in overall quality of life (286). Furthermore, excessive and persistent alcohol intake can influence taste perception by leading to deficiency in micronutrients such as vitamins A and B (thiamine for example), and zinc. Alcoholics have, in fact, been reported to show less sensitivity to sweet taste compared to control groups, suggesting that drinking habits may influence the choice of foods, with a greater preference for foods with higher sucrose and salt concentrations (287). Consumers and food providers are intuitively aware of this, which is why “chippers” serving well-salted fish and chips and kebab shops are commonly located close to pubs in Ireland and Britain and are open past the mandated closing times of alcohol-serving establishments.
Endocannabinoids (ECs) are lipid-based mediators synthesized from lipid precursors in plasma membranes (288). There are two EC mediators: N-arachidonoylethanolamide [anandamide (AEA)] and 2-arachidonoylglycerol (2-AG), and two EC receptors, CB1R and CB2R, both of which are GPCRs. CB1Rs are most abundant in the CNS and are responsible for the central actions of the exogenous cannabinoid, Δ9-tetrahydrocannabinol (THC), found in cannabis, while CB2 receptors are largely restricted to immune and hematopoietic cells, although they are expressed in some specific regions of the brain and cells such as brain stem neurons and glial cells. CB1Rs are involved in both hedonic and homeostatic energy regulation in the CNS, and it is well documented that ECs and THC are powerful orexigenic drivers of food-seeking behavior. CB1Rs are highly expressed in hypothalamus and limbic system and when activated in those sites there is a marked increase in food intake, especially for sweetened food (289). Cannabis products are employed to stimulate food intake in patients with cancer cachexia and when taking chemotherapy (290). As regards taste buds, CB1Rs are present on type II TRCs and intergemmal nerve fibers and cannabinoids increase electrophysiological responses to sweeteners without seemingly affecting responses to salty, sour, bitter, and umami compounds (291). Leptin administration selectively inhibits behavioral, taste nerve, and taste cell responses to sweet compounds [Refs. 292, 293; and see TABLE 1]. In contrast, db/db mice that have impaired leptin (Ob-Rb) receptors have increased preferences for sweet substances (293). It therefore seems the case that CB1R activation serves as a counter to leptin action as regards sweet preferences.
10. DISEASES OF THE TONGUE AND SYSTEMIC DISEASES THAT MAY BE REFLECTED IN TASTE CHANGES
10.1. Tumors
Tongue squamous cell carcinoma is one of the most prevalent malignant cancers of the head and neck region. It was commonly associated with alcohol and tobacco use, especially the chewing of tobacco, but now a large proportion is due to infection by the human papilloma virus (HPV). The prognosis for tongue and head and neck cancers due to HPV is excellent (294). Treatments however most commonly involve a combination of surgery, chemotherapy, and radiation: these lead to taste dysfunction that may get worse over time. Many patients have decreased sensitivity to low concentrations of bitter, sweet, and salty tastants. Patients are thought to have long-term damage to the corda tympani branch of CN VII (295). However, there may be effects, especially from radiation, on the taste buds themselves due to alterations in stem cell numbers or their differentiation into mature TRCs (232). A recent overview of the mechanisms involved in TRC disturbances is presented by Gaillard and Barlow (222). Our group has previously shown that a chemotherapeutic agent had significant effects on stem cell turnover in CVP of mice, leading to taste alterations (221).
10.2. Systemic Diseases
10.2.1. How taste dysfunction is measured in the clinical setting.
In systemic diseases with taste dysfunction involvement, there is commonly a misconception that this symptom is secondary to other complications of the disease such as oral dryness, microbiome dysbiosis, or decline of central processing. However, as knowledge of how taste signals are processed and more accurate tests to test the same have developed, the nature of taste loss in various systemic diseases has come under renewed interest (296–298). Two tests being utilized to decipher distinct involvement of the peripheral gustatory apparatus are the taste strip tests (TST) to measure taste responses in different regions of the tongue (299) and the electrogustometer (EGM) to measure peripheral neuronal activation (300). The format of the TST is to use commercially available spoonshaped filter paper strips impregnated with each of the four prototypic tastes (sweet, sour, salty, and bitter), sometimes referred to as Burghart taste strips, although a version was also developed by Tomita and Ikeda (301). Each tastant is presented at four defined concentrations. These strips are placed on the left and right sides of the anterior third of the extended tongue, resulting in a total of 32 trials per test. Before each test, the mouth is washed with water and the strips are presented with sequentially increasing concentrations. Tastants are generally applied in a randomized fashion at each of the four levels of concentration and alternating on the side of the tongue. The number of correctly identified tastes per side is added up to a “taste score” with scores of the left and right sides yielding the total number identified tastants. The EGM (301) is an apparatus that delivers an electrical stimulus by means of a sterilized stainless steel, circular electrode, 5-mm diameter applied to the upper tongue surface for between 1 and 1.5 s. It gives a quantitative evaluation of taste neurotransmission in decibels. When applied together with TST (to diagnose the nature of a taste disorder), the EGM can be used first to survey taste over the tongue to determine whether the lesion is in an area innervated by either the chorda tympani or glossopharyngeal gustatory nerve. This can then be followed by the application of the TST to determine the threshold concentration for the taste quality detection in the area where nerve function is impaired (301). A more recent review written since the start of the SARS-CoV-2 pandemic outlines updated versions of the TST that have been employed in research settings (302).
More commonly paper strips impregnated with a single concentration of PTC or PROP are used to categorize participants based on the functionality of their variant of the T2R38 receptor. However, this test is limited to one prototypic taste and suffers from the ambiguity described above in sect. 7.
10.2.2. Immune-mediated systemic disease.
10.2.2.1. SARCOIDOSIS.
This multisystem granulomatous disease can affect any organ. In a few instances, sarcoidosis was diagnosed based on the presence of granulomas on biopsy of tongue lesions (303, 304). Its histological hallmark is a noncaseating epithelioid-cell granuloma. While sarcoidosis classically affects salivary glands, most especially the parotid glands, and cervical lymph nodes, reported sites of disease also include the tongue. Erosive and nonerosive nodules, some extending into the skeletal muscle part of the tongue, may be manifestations of sarcoidosis. These small nodules may look like taste papillae, to a novice, and not be recognized for what they are. Sarcoidosis of the tongue only, that is, affecting no other organ, has not been described and should not be assumed to be confined to the tongue: lingual granulomas are therefore a harbinger of systemic disease (305–307). While dry mouth is a feature of sarcoid involvement of salivary glands that makes mastication and taste perception difficult, granulomas in taste buds per se have not been reported in sarcoidosis. In a few instances, sarcoidosis was diagnosed on the basis of a noncaseating granulomas on biopsy of tongue lesions (303, 304). Tongue lesions are reported to appear as indurations on the submucosa. In contrast, palate lesions, where sarcoidosis is also rare, appear as nodules (305, 308). It is conceivable that granulomas due to sarcoidosis would impinge on afferent signaling due to granulomas in the lamina propria itself and inflammatory responses locally in the taste papillae.
10.2.2.2. primary SJöGREN’S SYNDROME.
Primary Sjögren’s Syndrome (pSS) is a systemic autoimmune disease characterized by leukocytic infiltrations of the salivary (309) and lacrimal glands (310), occasionally of the kidney and lung, and accompanying central and peripheral neuronal complications (311). Loss of the salivary exocrine function results in dry mouth; thus loss of taste in pSS patients has been routinely attributed to the pathology accompanying loss of saliva (312). However, a recent study addressed the possibly that neurosensory dysfunction underlies taste impairment in pSS (313). Applying both TST and EGM, the authors showed that oral dryness did not correlate with impaired taste function but there was an association between taste acuity and neurosensory threshold. Therefore, pSS is another example of a disease in which impairment in the gustatory afferents could be a direct cause of taste dysfunction that had previously been overlooked.
10.2.3. Neurological diseases.
Olfactory dysfunction in Parkinson’s Disease (PD) has been well documented and has a prevalence of up to 90%. It is thought to be a consequence of the deposition of abnormal protein aggregates such as alpha-synuclein in olfactory bulbs (314). Taste impairment in PD has not been as well documented as taste disturbances are often considered secondary to hyposmia (296, 315). In one longitudinal study, taste impairment, though stable, was a persistent finding based on TST. In this study, sweet tastant seemed to be primarily affected in PD patients (316). However, when cognitive performance was diminished in PD, salty and sour tastant identification, but not sweet, was diminished. In this case, cortical involvement in PD is likely the culprit rather than lesions at the periphery (317).
Frequently researchers question whether T2R38 genotypes are associated with disease prevalence. As discussed above in sect. 7, the ability to detect PROP is mediated by the T2R38 receptor. Cossu and colleagues (318) recruited 109 PD patients and 131 non-PD controls. They found that ∼60% of PD patients detected PROP as compared to ∼90% of the control subjects. They found an association between PD and the nontasting haplotype of T2R38 (AVI/AVI). Only 5% of the PD patients were homozygous for the PROP haplotype (PAV/PAV) as compared to 25% of the non-PD controls. A connection may lie between PD, T2R38 and bacteria, and bacterial products. When the T2Rs are activated, such as by bacterial products, cilia activation is more robust, and bacterial killing is increased as NO is produced (as discussed above in sect. 7.1). Less robust T2R activation may be permissive for increased inflammation in the gut and play a role in the initiation of the misfolding of alpha-synuclein.
Similarly there does appear to be a mild and slowly progressive disorder of taste in Alzheimer’s Disease as reviewed by Doty and Hawkes (319).
10.2.4. Multiple sclerosis.
Taste defects due to multiple sclerosis (MS) were previously thought to be rare, as in-depth studies probing responses to specific tastants had not been conducted until recently. A very comprehensive taste study using the TST testing was carried out on 73 MS patients and 73 age-and sex-matched controls, on both the front (CN VII) and back (CN IX) of the tongue. Subjects also had brain MRIs performed with gadolinium contrast. The percentage of MS patients with difficulty identifying sweet was 25%, bitter was 15%, sour was 22%, and NaCl was 32%. The scores correlated with the number of MS lesions throughout the brain and not just in the sensory cortex (320). As a primary pathology of MS is demyelination, it is possible that a decrease in taste sensation could be due to demyelination of the myelinated somatosensory fibers and lesions/plaques anywhere along the route of transmission of sensory signaling from tastes buds to the gustatory cortex (FIGURE 2).
10.2.5. Guillain-Barre Syndrome and Miller Fisher Syndrome.
In general, d-glucose is the preferred fuel for brain cells, and therefore, humans are highly motivated to obtain it. Involvement of cranial nerves subserving taste is common in Guillain-Barre Syndrome (GBS), an autoimmune condition in which acute demyelination of large, myelinated nerve fibers results in acute flaccid paralytic neuropathy with absent reflexes. Antiganglioside antibodies are strongly associated with acute motor axonal neuropathy subtype more than acute inflammatory demyelinating polyneuropathy (321). MFS is another autoimmune disease sometimes classified as a subset within GBS and is characterized by the presence of anti-GQ1b ganglioside antibodies in circulation (322). The cardinal features of MFS are a triad composed of ataxia, ophthalmoplegia, and absent reflexes. Both GBS and MFS are usually preceded by infection. The frequency of taste impairment in patients with GBS has been reported to be 0.6–2% while a handful of MFS patients have taste impairments (323–325). Taste abnormalities in GBS are likely very underreported because of the overall overwhelming seriousness of the neurological manifestations and have not been specifically characterized with regards to the 5 prototypic tastes (326). MFS sufferers have reported dysgeusia and diminished salt and sweet taste (327). Therefore, should taste symptoms be reported in the presence of acute-onset ataxia, this provides an important clue to aid in diagnosis of MFS.
10.2.6. Burning mouth syndrome.
Burning mouth syndrome (BMS) is characterized by burning pain and dysesthesia in the mouth, in conjunction with dysgeusia or even total ageusia, in the setting of oral mucosa that to all appearances is normal. It has a preponderance in females, especially with increasing age (328). Sour tastants in particular can be very distressing in BMS and patients have a very low tolerance for capsaicin (noxious heat) (329). For a recent review with a focus on taste dysfunction in this condition, see Ref. 330. BMS is thought to be due to misprocessing of tastants such as might occur due to small fiber neuropathy involving the sensory afferents of CN V in the tongue epithelium and lamina propria (331). Burning pain sensation and taste dysfunction do not completely follow the anatomical distribution of the peripheral sensory nerves, and in most cases, they occur spontaneously without an obvious precipitating oral, or indeed systemic, factor although approximately one-third of sufferers have an oral habit such as tooth grinding and/or jaw clenching. Tongue biopsies have pointed to increased expression of transient receptor potential cation channels subfamily V member 1 (TRPV1) channels (332). These channels are involved in temperature perception and nociception, and not taste sensation, making it possible that there is a central origin to the syndrome, especially as it is intractable with no known treatments other than the use of drugs commonly used to treat peripheral neuropathy, such as gabapentin and clonazepam that at best have a marginal benefit (330, 332), which would be in keeping with the strong burning, painful sensation. As in most cases, the sufferers are postmenopausal this would seem to suggest that lack of estrogen plays a role. Estrogen has been shown to downregulate TRPV1 channel activity and ATP signaling via reducing P2X signaling, specifically on CN V (333). It is therefore also possible that there is cross talk from unregulated ATP release from tissues in response to maladapted TRPV1 channels in the lamina propria (see discussion on ATP neurotransmission above), P2X receptors on the afferents from CNs, and the transmission of nociceptive signals to the CNS: In short, the problem may arise in the transmission of the signals from the tongue and not in the gustatory cortex itself.
10.3. Deposition Diseases
10.3.1. Amyloidosis.
In this condition, there is extracellular deposition of fibrillar protein in organs and tissues. The most common proteins that get deposited in the tongue derive from immunoglobulin/light chains and the protein transthyretin (TTR) (334). The tongue gradually enlarges (macroglossia) and thickens and may have nodules that can cause difficulty with swallowing and speech (335). Taste abnormalities due to deposition around afferent fibers can occur. Diagnoses are based on biopsy and a positive Congo red stain and/or apple-green birefringence being visible on light microscopy (336).
10.3.2. Hypothyroidism.
Hypothyroidism is a consequence of low plasma levels of thyroid hormone and can be either congenital or acquired. Tongue manifestations, which occur with long-standing hypothyroidism, are dysgeusia and enlarged tongue (macroglossia). If acquired, there is a decrease in the regulation of the amounts of glycosaminoglycans, such as hyaluronic acid, and other connective tissue components (337). These are hydrophilic and disrupt and diminish the rate of neuronal signaling along the sensory pathways to the gustatory cortex. The depositions occur also in skin, particularly around the eyes and hands, and therefore provide corroborative evidence of the cause of the enlarged tongue. The collagenous depositions potentially can interfere with signaling transmission from taste buds through the lamina propria of taste papillae. Defects in taste, such as hypogeusia more especially to bitter, and even dysgeusia, were reported in 83% of hypothyroid patients in one series. Thyroid replacement essentially reversed these defects (338), as was also reported in a more recent study (339). The outlook for recovery and eventual shrinkage of tongue size is excellent once thyroid replacement is stabilized. In rare cases and especially if severe obstruction to airways and food swallowing, surgical reduction in the tongue may be required (340).
10.3.3. Acromegaly.
This is usually caused by excessive growth hormone (GH) levels mostly commonly due to a GH-secreting tumor in the pituitary, which causes progressive somatic enlargement because of excessive IGF-1 production in the liver in response to increased circulating GH levels. Similar to enlarged tongue due to hypothyroidism, obstruction of airways is the biggest problem, exacerbated by also having an enlarged uvula and soft palate. Diminished perception of all tastants has been reported, again possibly due to connective tissue deposition and diminution of signal transmission in lamina propria. It is also commonly associated with prognathism. Treatment of the cause of elevated growth hormone is required. However, the tongue and palate size and prognathism may not recede and may require surgical intervention (341).
11. RESEARCH IN PROGRESS
11.1. In Vitro Model Systems to Examine Taste Development Function and Renewal
11.1.1. Adult human FP taste bud cultures.
As discussed above sect. 1, most of our knowledge of how human taste perception operates derives from studies on the FP, and in particular from the work of Ozdener and colleagues (342–344), who have propagated cells derived from human FP (HBO cells) in in vitro cultures. These cells can be maintained for up to seven passages and retain the molecular and biochemical properties of isolated TRCs. Thus, they have been used to examine responses of human TRCs to tastants. Ozdener and colleagues (345) have demonstrated empirical evidence for GPR120 as a fat-responsive receptor on HBO cells, for example. Applying either the specific synthetic GPR120 agonist TUG891 or linoleic acid-induced a rapid increase in intracellular calcium followed by GLP-1 secretion into the culture medium; they also induced ERK1/2 phosphorylation in HBO cells. Both actions were decreased in HBO cells when calcium homeostasis modulator 1 (CALMH1) was genetically nullified (148). Interestingly, inhibition of ERK1/2 on the tongue of healthy human volunteers decreased orogustatory sensitivity to linoleic acid (346). This could imply an indirect effect of CD120 activation on fat taste preference in which calcium and ERK1/2 MAP kinase signaling lead to gustatory nerves relaying the message of fat intake to the brain that then secondarily triggers the release of satiety and other gut hormones, rather than a direct effect to the insula relaying taste information per se via a local release neurotransmitter from TRCs.
Arginine amino acid and the arginyl dipeptides Ala-Arg (AR), Arg-Ala (RA), and Arg-Pro (RP) had previously been shown to enhance the human perception of salt taste in both aqueous and model broth solutions in human sensory evaluations (347). While arginine or the peptides by themselves did not elicit electrical responses in HBOs, AR did significantly increase the responses of amiloride-sensitive but not amiloride-insensitive cells (348). This work could be functionally important in determining ways to enhance salt perception without increasing salt concentrations in food.
Rodent type II TRCs secrete the neurotransmitter ACh and tastebuds express acetylcholinesterase, as discussed above (sect. 3.3.4) (170, 171, 349, 350). Again, using HBO cells, Ozdener and colleagues (351) showed that T2R-expressing cells express nicotinic receptors and respond directly to nicotine and acetylcholine, producing transient elevations in intracellular calcium. The significance of this opus is that it may provide a mechanism for the taste disturbances that are a side effect of certain anticholinergic drugs.
A large component of taste perception is olfaction with retronasal olfaction affecting the taste and flavor of foods (352, 353). There are over 350, seven-transmembrane, GPCR olfactory receptors that transduce different smells by activating the Gα s/olf, which in turn activates adenylyl cyclase stimulating cAMP production (354). Cultured HBO cells express three olfactory signal transduction molecules; the G-protein Gα s/olf, adenylyl cyclase, and olfactory marker protein (OMP) (355). HBO cells functionally respond to the odorants by increasing intracellular calcium concentrations, an action that was blocked by mRNA and pharmacological inhibition of adenylyl cyclase. While this work potentially moves the initial point of integration of gustatory and olfactory information on food and flavor from the retro-nasal area to the taste buds of the tongue, it also has fundamental implications for the role of adenylyl cyclase and cAMP production in taste cells. Gαgust is akin to Gαi protein in that it inhibits adenylyl cyclase. However, a function for cAMP in TRCs has not been clearly defined (356, 357). This is an example of the need for more basic research to determine the function of cAMP in TRCs.
As the popularity of three-dimensional (3-D) cell culturing for taste studies grows, the inherent difficulties it presents in terms of live cell imaging also need to be also overcome. These challenges include stabilization of cell cultures during perfusion, high-resolution imaging of the many z-planes, and complex data analysis spanning from single cells to entire specimens (358). In 2020, von Molitor and colleagues (358) developed a system that overcame these challenges and worked under light-sheet and confocal microscopy. Using this system, they conducted a time-series analysis of calcium signaling in human HTC-8 cell spheroids during gustatory substance perfusion. They found that extracellular calcium is required for a dose-dependent response to saccharin while the signals induced would be delayed and diminish in amplitude from the periphery toward the center of the spheroid (358). Similar calcium transient kinetics were observed also for bitter and sweet compounds (358).
This body of work continues to demonstrate the importance of integrating the investigation of functional activity of taste signaling pathways with observational studies on human taste perception and with electrophysiological and molecular signaling studies on human primary taste cells so as to gain a more comprehensive picture of taste transduction in humans; research in rodents can only take one so far when it comes to understanding human taste mechanisms.
11.1.2. Taste organoids.
Epithelial-derived taste organoids have been used to better understand taste stem cell dynamics and taste bud renewal. Hedgehog signaling, Notch signaling, and the Wnt/β-catenin pathway that plays an essential role in the rapid turnover of TRCs and hence the maintenance of overall taste reception are also major developmental pathways that are often targeted by certain therapeutics such as chemotherapy and radiotherapy in the treatment of diseases such as cancer (222, 359). Hence, studying the effect of such therapeutics on the regulation of taste cell proliferation and differentiation is of paramount importance in understanding and mitigating taste disfunction as a side effect. Consequently, the taste system in mice has been used for this purpose due to its similarity to humans (360). However, high-throughput in vivo taste studies in mice are not considered feasible due to the cost and rigor involved (359). As an alternative, generic primary cell cultures of taste cells have been attempted with an emphasis on modeling taste cell function. Due to the intrinsic properties of the system where taste cells have a limited life span and are terminally differentiated, the success of these primary cultures in terms of studying the proliferation and differentiation of taste cells is not recognized (361). This has been done by a few laboratories that have used both mouse and rat taste epithelium to generate taste organoid cultures.
To overcome these issues, stem cell research has recently been focused on establishing in vitro culture methods to develop organoids, which are three-dimensional structures derived from Lgr5+/Lgr6+ adult taste stem cells or pluripotent stem cells (218, 359, 361, 362). These organoid structures would contain both proliferating and differentiated TRCs that are functionally similar to mature TRCs (218). Taste organoids capable of self-rejuvenation have also been developed from the native CVP tissue of mice in primary 3-D culture. These organoids would carry phenotypic characteristics similar to the native CVP tissue that they are derived from, comprising multiple outer layers made up of stem/progenitor cells and differentiated taste cells filling up inner layers (361). Sato and coworkers (2) published groundbreaking research in 2009, describing how intestinal adult stem cells (ASCs) could be used to grow intestinal organoids. In 2011, a group led by Yoshiki Sasai (363) described how pluripotent stem cell (PSC) aggregates of mouse embryonic origin could be manipulated to develop retinal-primordium organoids. Their work pioneered a cardinal concept in developmental biology laying down the foundation for future organoid work, i.e., that stem cells possess the inherent ability to form 3-D structures that are structurally and functionally similar to their in vivo origins (364). In their work published in 2014, Ren and colleagues (218) first described how Lgr5+ cells isolated using fluorescence-activated cell sorting can be cultured in Lgr5+ promoting media defined in the studies from the Clevers laboratory, to grow taste organoids. These organoids could be passaged at least six times and maintained in culture for 2 months. Immunohistochemistry and RT-PCR done at various stages of organoid development ranging from days 1–3 to day 30–32 revealed the presence of proliferating taste stem cells and differentiated type I, II, and III TRCs. These taste cells were found to be responsive to tastants in a dose-dependent manner. Through generic lineage tracing and RT-PCR, the authors were able to confirm that taste cells originated from LGR6+ cells and that both LGR5 and LGR6 marked the same subset of taste progenitor cells (218). Aihara and colleagues (361) were able to identify distinct cell cycles and populations within the taste stem cells of CVP organoids derived from these transgenic mice in which various phases of the cell cycle were labeled. Their work suggests progenitor cell re-positioning during in vivo maintenance of taste bud cells suggesting, as did Sullivan et al. (64) and Perea-Martinez et al. (65), that there are potentially two pools of taste cell progenitors, a slow cycling and a faster cycling pool. They further observed that exogenous Wnt3a may play an important role in budding and may regulate the differentiation of stem cells in taste organoids (361).
In their study conducted in 2017 based on bulk RNA-Seq data, Ren and coworkers (362) ascertained the temporal expression of TRC marker genes. Transcripts of mature taste cell markers could only be detected in the later stage of organoid development, and organoids in the early stages of their development would only demonstrate upregulation of cell signaling elements and relevant transcription factors (362). The authors were also able to observe a higher number of type I, II, and III TRCs with increasing concentrations of Wnt3a. Similarly, at higher concentrations of Noggin, an increase in the number of type II and III TRCs was observed. The overall organoid size was also observed to be comparatively higher with increasing concentrations of Noggin (362). Furthermore, when the organoids were treated with dibenzazepine (DBZ), a γ-secretase inhibitor that blocks Notch signaling, an acceleration in taste cell differentiation or cell fate determination was seen. Expression of the type II cell marker Gαgust and the type III cell marker SNAP25 were significantly increased, while proliferation, as measured by Ki67 expression, remained the same (362). Later, Lin and colleagues (229) (in their work published in 2021) found that R-spondin-2 is required for taste cell differentiation in mouse CVP organoids. In their previous work, they observed that some differentiated TRCs could still be found in taste organoids even in the absence of exogenous Wnt3a, Noggin and Shh (229). However, in the absence of exogenous R-spondin-2, no mature TRCs were observed in the mouse CVP organoids. Hence, Lin and coworkers (189) proposed that R-spondin-2 or at least a combination of R-spondin-1 to 4 are the neuronal inputs required for TRC development, perhaps in addition to the previously identified SHH (365).
The growth efficiency of organoids and percentage of mature TRCs (type II and III) was shown to be higher in organoids developed from a population of single cells enriched for LGR5+ expression (366). This was commonly observed in all three types of mouse organoid systems i.e., CVP, FP, and FLP organoids. Furthermore, Ren and colleagues (366) were also able to identify that LGR5+ cells from CVP of neonatal mice had a higher ability to generate TRCs in comparison to adult mice, with vast differences in gene expression profiles and signaling pathways (Wnt, Notch, Shh, BMP, MAPK, etc.) in their respective organoids. In their work published 2020, Aihara and coworkers (361) found that mouse CVP organoids express TLR4 (a Toll-like receptor) involved in LPS-induced expression/secretion of cytokines such as tumor necrosis factor (TNF) and IL-6 interleukin-6 (IL-6). Consequently, they observed that similar to the native tissue epithelia, the taste organoids efficiently induce the expression of TNF and IL-6 with similar induction kinetics. Bacterial lipopolysaccharides (LPS) or other proinflammatory cytokines can induce the expression of inducible nitric oxide synthase (iNOS) in a cell. iNOS is expressed downstream of the NF-κB signaling pathway (361). When mouse taste organoids were stimulated with LPS they exhibited iNOS gene expression patterns that are very similar to that of the native taste tissue. Taken together, these findings indicate that taste organoids may prove to be a useful tool in studying the underlying mechanisms of inflammatory response in taste cells.
Following on from our observation of the expression of insulin in type II cells (58), Takai and colleagues (241) found that the insulin receptors (IR) are widely expressed in type II and III TRCs of both mouse CVP and FP taste buds; they also detected the presence of IR in Lgr5+ cells. When mouse CVP organoid cultures were grown in increasingly higher concentrations of insulin, a decrease in the expression of taste cell markers (NTPDase2, T1R3, gustducin, carbonic anhydrase 4, GLUT8, and sGLT1) was observed (241). Furthermore, when the mammalian target of rapamycin (mTOR) pathway was inhibited by rapamycin, the effect of insulin on taste cell differentiation was negated (241). This would indicate that insulin is acting as a regulator of TRC generation through the activation of the mTOR pathway, which is a central regulator of mammalian metabolism and physiology (see sect. 6.2 above).
Human epithelial organoids grown in matrigel (3-D culture) do exhibit apicobasal polarity; however in contrast to the natural situation, the outer layer consists of basal cells, i.e., the proliferative stem cells with the differentiated cells enclosed inside the organoid (367). In the context of taste organoids, this makes it difficult for mature taste cells to be presented with tastants in in vitro experiments. However, the work published by Adpaikar and colleagues (368) revealed that transferring organoids grown in the basement membrane, into suspension culture could reverse this apicobasal polarity, thus producing taste organoids with mature TRCs localized on its apical surface. Furthermore, they also observed that attempts to grow single cell-derived mouse taste organoids directly in suspension culture were not successful. The mouse taste organoids first cultured in the basement membrane and then in suspension culture showed cell differentiation and renewal rates that are more similar to the native tissue, had TRCs that are more accessible to calcium imaging (thus enabling evaluation of taste transduction), and could subsequently be integrated into the host lingual epithelium without impeding its functional capacity (368).
So far, these reports only demonstrate a low passage number with no indication of the unlimited expansion typical of other organoid cultures. It is possible that this could be due to less-than-optimal culture conditions that still need to be determined empirically. Furthermore, no cell surface markers have been identified for the Lgr5/Lgr6 taste stem cells to facilitate the isolation of stem cells from higher order animals. All these issues require further work to obtain organoids that can address the questions on the true nature of the taste stem cell. More critically from a clinical perspective, there are no reports of human taste organoids in culture at the time of writing this review.
11.2. Taste Loss in Sars-Cov-2 Infection
Taste dysfunction following SARS-CoV-2 infection falls into approximately four different categories; complete loss of taste (ageusia), reduced taste intensity (hypogeusia), distorted taste (dysgeusia), and a sickening or foul taste (paraguesia) (369–371). Taste loss is a feature of the illness and often precedes other symptoms (371, 372). A meta-analysis of the SARS-CoV-2 literature indicates that ∼24% of patients report taste loss (370). Viral-induced changes in the lingual epithelial environment, loss of taste perception in the taste bud cells per se, dysfunction in the gustatory neurons (371, 373), and loss or impairment of the neurons in the gustatory cortex (374) have been proposed as probable mechanisms for taste dysfunction in the context of active SARS-CoV-2. Using both immunofluorescence and in situ hybridization, we demonstrated the presence of ACE2 on the PLCβ2 expressing type II TRCs and we found an actively dividing virus in those same cells. Therefore, we can attribute the acute loss of taste sensation to direct infection of TBCs (59). It is interesting that these cells are also the cells that possess the bitter receptors, which are key components of the taste bud immune surveillance system and potentially key to the innate immune interferon response. The production of interferon and TNF-α (as outlined in sect. 7.1) could lead to altered taste perception and the consequent upregulation of caspase 3 and subsequent TB100 death. The close proximity of type I and III TRCs also means the latter are potentially subject to infection by a mechanism by which SARS-CoV-2 promotes casein kinase II (CK2) activation in the infected cell that, in turn, stimulates the formation of filopodial protrusions with budding viral particles that then infect neighboring cells (375). Furthermore, previous literature shows that infected and damaged cells secrete ATP in an unregulated manner, which could also be a cause of taste abnormalities, including parageusia experienced by some patients (376). We also showed that the basal stem cell layer in infected individuals both during and immediately postinfection was disrupted and the number of proliferating stem cells was reduced (59). Recovery of taste perception was associated with a restoration of the basal stem cell layer integrity and stem cell proliferation. Interestingly, we did not observe any apoptotic taste stem cells as is the observation in normal mouse lingual epithelium (230). Thus, loss of TRC replacement because of reduced precursor numbers is one possible mechanism for long-term taste dysfunction.
A growing number of people previously infected with SARS-CoV-2 have reported persistent symptoms, or the onset of long-term symptoms ≥4 wk after acute infection. These postacute sequelae of COVID-19 (PASC) are colloquially referred to as post-COVID conditions, or long COVID, and often include dysfunction in smell and/or taste (377). A meta-analysis of 18 studies encompassing the experience of 3,699 patients indicates that while most people recover their lost senses ∼5% of adult patients develop long-lasting changes to their sense of smell or taste after the initial SARS-CoV-2 infection (378). At the time of writing this review, the World Health Organization has reported over 572 million SARS-CoV-2 infections worldwide. If 24% of those had taste alteration during the acute phase of their illness and if 5% of those go on to experience long-lasting taste disturbances, then an estimated 7 million people worldwide will be expected to have long-term sequelae related to chemosensation: this is quite a substantial number.
The pathogenesis of SARS-CoV-2 postacute taste alterations is far from clear (379). One hypothesis is as we outline above: a depletion of taste stem cells through reduced replication rather than death. Inflammation and/or clotting occurring during the acute phase of infection can cause tissue damage within the lamina propria of taste papillae and thereby interrupt afferent signaling and supply of nutrients to tissues. Additionally, the innate immune response within the infected taste buds would lead to the production of cytokines including the immunosuppressive IL-10, which we know is produced by a subset of mouse bitter TRCs (see sect. 7). In an Italian study from early in the pandemic, full recovery of gustatory function in 119 patients hospitalized with SARS-CoV-2 was significantly associated with high serum levels of IL-10 at admission (380). Then, inflammation in situ can cause malfunction of the gustatory system and gustatory dysfunction (381). Finally, in addition to the local effects of the virus itself within taste epithelium, the neuroinvasive properties of SARS-CoV-2 could be an underlying cause of long-term taste disturbances. Making use of their repository of health data collected on individuals before mild SARS-CoV-2 infection (not requiring hospitalization), the UK biobank researchers were able to compare the health metrics of individuals postinfection. Comparing data from brain MRI imaging on the same people pre- and postinfection, they observed both a loss of gray matter and tissue damage in the insula, where the gustatory cortex is, when their MRI images from postinfection were compared with those who had never tested positive for SARS-CoV-2 and compared to preinfection (374).
Our published study (59) was performed on patients who contracted SARS-CoV-2 during the first year of the pandemic and therefore infected with the original untyped variant. As new variants emerge, it is becoming evident that they have different effects on chemosensation. Patients infected with the Omicron variant are significantly less likely to develop smell and taste loss than those infected with Delta and earlier COVID-19 variants (382). Coelho and colleagues (382) studied more than 3.6 million COVID-19 cases from the U.S. National COVID Cohort Collaborative database with and without smell and taste loss within 2 weeks of COVID-19 diagnosis since the start of the pandemic. When they set the initial/untyped variant (June 22–August 3, 2020) as the baseline then the mean rates of smell and taste loss were 50% for Alpha (April 19–May 31, 2021) (381), 44% for Delta (September 20–November 1, 2021), and 17% for Omicron (December 27–February 7, 2022) (382, 383).
While SARS-CoV-2 in now the best-known virus, the virus-of-the-moment, that results in alterations in taste, this phenomenon has been reported to occur with influenza. Influenza A virus subtype H3N2 that caused the pandemic of 1968–1969 resulted in long-term alternations of taste and smell in many patients. Some of those patients had biopsies performed that showed disrupted taste bud architecture and decreased numbers of TRCs that were lacking cilia in the pore region of the taste bud (384). This points to pathology in the primary sensory organ as a cause of sensory dysfunction, and not a central (CNS) effect. Moreover, dengue fever had also been reported to result in taste alterations (385): there is no information in the medical literature on whether Dengue results in long-term taste abnormalities. As stated in clinical highlights, a thorough H&P examination retaining to taste (and smell) should be carried out on anyone who has postviral syndromes because abnormalities in those senses are more likely than has been previously appreciated.
11.3. The Microbiome of the Tongue
It is well established that the host-microbiome interactions play an important role in determining human health (386). The microbiome of the mouth is now getting attention not only because of local effects in the mouth (caries and periodontal disease, taste changes, malodor) but because it also plays a role in systemic diseases such as inflammation and endocarditis. Synergistic relationships via participation in various metabolic and physiological processes are essential in maintaining the ecological balance of the specific organs they inhabit (386, 387). The human gut microbiome has been shown to have implications for obesity, hypertension, thromboembolism, glucose metabolism, Alzheimer’s Disease, and even in human behavior (388–392). “It’s not my fault, my gut bacteria made me do it” might yet be a defense in a court of law. These implications are due to mechanistic ties between gut microbial metabolites, host receptors, and the host phenotypical response (387). The oral cavity is heavily colonized by resident microbiota comprised of several different heterogeneous microbial communities (393, 394). This is regardless of the fact that it can be a difficult environment for microbes to survive, due to being constantly challenged by mastication, hygiene practices, and major fluctuations in other physicochemical parameters (pH, temperature, and nutrient supply) (395). The tongue, lip, cheek, gingiva, gingival sulcus, and teeth are all known to harbor microbial communities that are significantly different from each other (394–396).
The tongue dorsum is home to a multilayered, diverse, and structurally complex microbial community that comprises free bacteria, bacteria attached to its squamous epithelium, and organized bacterial consortia (multilayered bacterial biofilms with epithelial cells at its core) (386, 397). A study on the spatial ecology of the tongue dorsum microbiome by Wilbert and coworkers in 2020 (397), revealed 17 bacterial genera most abundant in at least 80% of the individuals tested (n = 77), that may form the core-microbiome of a healthy human tongue. Streptococcus, Veillonella, Actinomyces, Neisseria, and Prevotella/Alloprevotella were the top five genera inhabiting the normal microbiome of the tongue dorsum with Rothia, Veillonella, or Actinomyces dominating the free bacteria and squamous epithelium bound niches. The organized consortia were found to be more homogenous in nature (397). At the phylum level, Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria were found to be the dominant taxa in the tongue-coating microbiome, based on 16S rRNA amplicon sequence data analysis (386, 398, 399). The presence of bacteria has been documented on the papillae, in the valleys between them, and on the gustatory villi of FP and CVP alike (397, 400). A study conducted by Cattaneo and coworkers in 2018 (401) found that certain microbial genera in the tongue microbiome were associated with bitter taste perception. This was assessed by the test subject’s sensitivity to paper strips impregnated with PROP and the microbiome was analyzed using 16S rRNA V3-V4 amplicon sequencing data from tongue coating microbial community. Their findings suggest that the tongues of individuals who possess high sensitivity to bitter taste compounds (the proverbial supertasters) had their tongue microbiomes enriched with bacterial genera such as Actinomyces, Campylobacter, and Oribacterium (401). Certain microbial metabolic pathways may increase the concentration of certain tastants in close proximity to gustotary papillae, thus modulating taste perception. For example, Actenobacteria (higher order phylum for Actinomyces) are known to produce secondary metabolites such as phenols that can have an enhancing effect on astringency and bitter taste perception near taste buds (401). However, further research is required to draw causal relationships between an altered tongue microbiome and bitter taste perception.
A variance in the composition of the microbial community surrounding the CVP has been found to be associated with the perception of linoleic-acid (LA), the ligand for GPR120/FFA4 thought to be responsible for lipid taste discussed above (sect. 3.2.4) (402). The CVP microenvironment of LA tasters is enriched with anti-inflammatory bacteria while that of the LA nontasters is enriched with proinflammatory bacteria. The bacterial families Bacteroidaceae, Sphingomonadaceae, Prevotellaceae, and Sutterellaceae, while the bacterial family TM7 and Lactobacillaceae were found to be enriched in LA tasters (402). When bacterial metabolic pathway prediction was conducted on 16S rRNA V3-V4 amplicon sequencing data, the authors found that sterol and bile acid synthesis was enriched in nontasters while toluene and xylene degradation was enriched in tasters (402). The candidate phylum TM7 or Saccharibacteria are known to contain hydrocarbon (including toluene)-degrading bacteria (403, 404), although its effect on the physiology of the oral cavity is not yet established. Furthermore, when the same study was conducted on male diabetes patients, the authors discovered a significant difference in the alpha diversity of CVP-associated microbiomes of diabetic low linoleic-acid tasters (DLLT) and diabetic high linoleic-acid tasters (DHLT) at the genus level. The microbial signature of DLLT group was characterized by an increased relative abundance of Bacteroides and Helicobacter and decreased abundance of Barnesiella (400).
Apart from taste perception, the dysbiosis of the tongue microbiome has been associated with various other conditions, such as intraoral halitosis (IOH), smoking, and gastritis (405–408). In a study conducted using 26 test subjects (16 IOH and 10 healthy), based on 16S rRNA sequence data from V3 to V4 region of the tongue-coating microbiome, the authors found that bacterial genera such as Aggregatibacter, Campylobacter, Dialister, Capnocytophaga, Clostridiales, Leptotrichia, Peptostreptococcus, Parvimonas, Tannerella, Peptococcus, Saccharibacteria, Prevotella, and Selenomonas were significantly associated with IOH patients. However, there was no significant difference observed in the overall species diversity or abundance (405). The tongue microbiome has also been shown to be susceptible to smoking, where certain bacterial genera such as Dialister and Atopobium have been found to be enriched in the tongue-coating microbiome of smokers. The Brinkman index (BI) values, used to measure lifetime smoking, were found to be negatively corelated with the genus Granulicatella in the tongue, whereas Cryptobacterium, Megasphaera, Dialister, Mitsuokella, and Bifidobacterium were found to be positively corelated (406). A study based on metagenomic sequencing data (shotgun sequencing of whole genome DNA extractions from tongue scrapings) involving 78 patients (with confirmed diagnosis of gastritis) and 50 controls was able to identify a microbial signature comprising 21 species as associated with gastritis that could differentiate gastritis patients from healthy control subjects. In particular, Campylobacter concisus was suggested by the authors as a biomarker for gastric precancerous cascade (407). The overall bacterial diversity of the tongue-coating microbiome also differed both in species richness and evenness, depicting a less diverse tongue microbiome in gastritis patients (407). Twenty-eight bacterial metabolic pathways including fatty acid metabolism, tyrosine metabolism, nicotine and nicotinamide metabolism, methane metabolism, pertussis, bacterial chemotaxis, nucleotide oligomerization domain (NOD)-like receptor (NLR) signaling (a receptor that recognizes nonself components), and oxidative phosphorylation were found to be significantly upregulated in gastritis patients (407).
Dysbiosis of the tongue microbiome has recently been correlated with SARS-CoV-2 infection. In a study conducted by Haran and colleagues (408), they analyzed the tongue microbiome of 84 such patients of which 37% had on-going symptoms >4 weeks. Shotgun metagenome sequencing was used to analyze whole genomic DNA extracted from tongue swabs, and random forest classification (RFC) was used to identify differentially abundant species (biomarkers) associated with ongoing symptomatic patients. Bacterial species belonging to genera Veillonella and Prevotella, both well known to produce lipopolysaccharides (LPS), were the top two biomarkers highly associated with on-going symptoms (FIGURE 11). Consequently, microbial metabolic pathways pertaining to LPS biosynthesis and proinflammatory molecule synthesis were also enriched in patients (408).
FIGURE 11.
The tongue microbiome and its implications in human health. The healthy human tongue-coating microbiome is dominated by the genera Streptococcus, Veillonella, Actinomyces, Neisseria, and Prevotella; it is dysbiosis being associated with many other systemic diseases. The onset of COVID19 due to SARS-CoV2 infection and its prolonged symptoms are associated with enriched Veillonella and Prevotella species in the tongue-coating microbiome. Tongue microbiome dysbiosis is associated with local and systemic diseases. Image created with BioRender.com, with permission.
In summary, Atopobium, Aggregatibacter, Campylobacter, Veillonella, and Prevotella appear to be the combination of bacterial genera that can be used as biomarkers of tongue microbiome dysbiosis due to external factors. However, a vast majority of studies concerning the tongue microbiome are based on 16S rRNA gene amplicon sequencing data that carry inherent limitations such as low taxonomic resolution and the inability to provide direct functional insight into metabolic frameworks operating within the microbiome. Such data can only help in predicting bacterial gene functions and cannot reliably classify bacterial taxa beyond the genus level. Therefore, the elucidation of true mechanistic underpinnings that helps to materialize these relationships between dysbiosis of the tongue microbiome and human disease, remains a research gap that needs to be filled in future research.
11.4. Tongue Function and Aging
Quality of food ingestion is a critical lifestyle factor that has a primordial impact on health and quality of life and there is a huge interindividual variation in the perception of the five basic tastes. Initial findings in humans and in animal models suggest that lower taste perception, especially to sweet tastants, may be associated with higher obesity risk, and as being obese is associated with a reduced life span, lack of obesity gives a survival advantage and therefore dropout of epidemiological studies; however, this is confounded by many things (loss of weight with age, for example, especially in the eighth and ninth decades, and obesity itself may downregulate sweet perception). Because of the large interindividual variation large numbers of subjects would be required for cross-sectional investigation. However, it seems likely that aging is associated with a decrease in all senses, as is certainly the case for sight, hearing, and touch. Barragán and colleagues (409) found that all five primary taste perceptions decrease with age with the biggest decrement in bitter and sour. Taste sensation may decline with age because there are fewer taste papillae, fewer taste buds, fewer TRCs in taste buds, changes in the distribution of the TRC types, and alterations in afferent nerve signaling to the brain. There may additionally be age-related changes in saliva production, tactile and mechanosensation, and the ability to masticate and swallow (410). The Beaver Dam Offspring Study (BOSS) undertaken in Wisconsin on a large sample of participants (n = 2,371) did find a decrease in FP density within ages ranging from 21 to 84 years old (411). They also measured four tastants in their population (sweet, salty, bitter, and sour) but only used one concentration for each tastant: The authors found a statistically significant association between age and sweet taste perception. It is possible that because the study population was predominantly middle-aged, there was insufficient power to observe significant aging effects occurring in later life. In the BOSS study, the tastant used for bitter taste perception was quinine (412). Hansen and coworkers (413) studying an Australian population reported an inverse association between age and the bitter taste of caffeine as well as of PTC.
We also examined the cross-sectional and longitudinal trajectories of FP density in 1,084 participants from the Baltimore Longitudinal Study of Aging using linear regression and mixed effects models (414). At baseline, the mean age was 68 years, with a mean follow-up time among those with repeat visits of 4 years. Women (53%) were younger (67 versus 69 years) and had a higher FP density than men (P < .001). FP density decreased linearly and very significantly with follow-up (P < .001). The rate of decline was not affected by sex, race, BMI, waist-hip ratio, smoking, or alcohol use. Our future research will involve examining if each FP has fewer taste buds embedded in them and if there are fewer TRCs and/or changes in the distribution of the TRC types within taste buds over time.
Tactile sensitivity in the tongue is difficult to study and any local problems on the tongue surface could potentially confound results. Notwithstanding the difficulties, 48 subjects were tested for lingual threshold sensitivity via a modified capitalized letter identification task, whereby the participant is required to distinguish capitalized letters of varying sizes (415). In addition, taste bud density on the anterior tip of each adult's tongue was estimated by counting the number of FP in a 0.317-cm2 circumscribed area. Lingual tactile thresholds were significantly impacted by age group as subjects 40 years or older had higher thresholds than those in their 20s. Interestingly, threshold sensitivity increased with increasing FP count (415). However, there are too few subjects and not enough elderly people to make definitive conclusions. Other studies report age-related reductions in two-point discrimination abilities in the tongue, cheeks, and lips (416); touch threshold detection in the cheeks, tongue, and anterior palate (417); and tongue vibrotactile sensitivity (418).
Taste buds (and filiform papillae) are supplied by both myelinated and demyelinated nerve fibers through the lamina propria, and demyelinated fibers, at least in mice, extend into taste buds and into filiform papillae (see sect. 2.2 above). Therefore, the afferent fibers have two roles; transduction of information concerning tastants and information related to the mechanical properties of food, such as temperature, pressure, and consistency that are also coming from filiform papillae and around taste buds. During aging, the ability to detect the mechanical properties of foodstuffs declines. Impaired mechanosensitivity has serious implications for quality of life, as is well illustrated when the lingual branch of the trigeminal nerve is damaged.
Finally, zinc deficiency is a not uncommon case of taste disorders in the elderly. In one analysis of the literature of the topic up to 2012, zinc deficiency accounted for 14.5% of taste complaints (419).
12. SUMMARY
To conclude, taste perception is finally getting the attention it deserves from the medical community. Thanks to SARS-CoV-2, this vital sense has now become much more prominent in general and research literature. Unlike sight and sound, before 2020 clinicians, even ear nose and throat specialists, rarely tested the sense of taste. This is a shame because it is an endlessly fascinating sense and still a fertile field for research. Moreover, most of the research on taste has been carried out in rodents, while humans were neglected, even though taste papillae can be visualized and are easily accessible for biopsy. The taste system has multiple receptors and multiple neurotransmitters in taste buds housed within taste papillae that detect energy sources such as sugars, starches, and amino acids. It can also detect the correct amount of salt intake up to a point, give information on the types of sour-tasting acids in foods, and sense bitter-tasting alkaloids that can be spit out before ingesting and causing bodily harm. Processing of information on the content of food is essentially a qualitative (what kind) function of the sense of taste. The mechanisms by which taste information is transmitted to the gustatory cortex in humans are subject to increasing research activity, some of it fueled by the SARS-CoV-2 epidemic and some of it fueled by the obesity and diabetes epidemics.
The taste receptor cells containing the receptors and neurotransmitters reside within the taste buds. Due to their location, they are subject to massive amounts of trauma from many environmental insults and therefore must be continuously renewed. The renewal occurs from stem cells present in taste papillae, and some of those migrate into taste buds and differentiate into the specific taste receptor cell type to be replaced. This process is also subject to increasing research in particular bioinformatics studies on both mouse lingual epithelium and human FP. It should prove fruitful in understanding what controls stem cell turnover, the signals needed to replace taste receptor cells that undergo apoptosis, and what happens to those cells with aging and under disease conditions such as local infection and systemic inflammation.
GRANTS
This research was supported by the Intramural Research Program of the National Institute on Aging (NIA/NIH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.D. and J.M.E. conceived and designed research; M.E.D., Q.Y., and J.M.E. performed experiments; M.E.D., Q.Y., and J.M.E. analyzed data; M.E.D. and J.M.E. interpreted results of experiments; M.E.D., H.U.P., Q.Y., C.H.M., and J.M.E. prepared figures; M.E.D., C.H.M., and J.M.E. drafted manuscript; M.E.D., H.U.P., C.H.M., and J.M.E. edited and revised manuscript; M.E.D., H.U.P., Q.Y., C.H.M., and J.M.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lauren Brick and Marc Riley in Visual Media NIH for help with the Graphical Abstract and for help with constructing FIGURES 1–4, 8, and 10. We also thank Nandita Vegesna and Vernon Kennedy for editorial assistance. FIGURES 5, 7, and 11 were generated using Biorender, with permission.
GLOSSARY
- 2-AG
2-Arachidonoylglycerol
- 3-D
Three-dimensional
- 5-HT
5-Hydroxytryptamine or serotonin
- AC
Adenylyl cyclase
- ACE2
Angiotensin-converting enzyme 2
- ACh
Acetylcholine
- ADP
Adenosine monophosphate
- AEA
N-arachidonoylethanolamine or anandamide
- Anti-GQ1b
IgG antibody against ganglioside GQ1
- APCs
Antigen-presenting cells
- AR
Ala-Arg, alanine arginyl dipeptide
- ATP
Adenosine triphosphate
- AVI
Alanine, valine, and isoleucine, representing the nonfunctional (for PTC and PROP taste) allele of the T2R38 gene
- AVI/AVI
Haplotype of the nontaster phenotype for the T2R38 gene; they do not sense PTC or PROP or at least it is barely perceptible to them
- β-Catenin
Beta-catenin
- BDNF
Brain-derived neurotrophic factor
- BHLH
Basic helix loop heli, a type of transcription factor
- BMI
Body mass index
- BMS
Burning mouth syndrome
- BOLD
Blood oxygen level dependent
- BOSS
Beaver Dam Offspring Study: research program focused on the epidemiology of aging, with an emphasis on sensory and cognitive impairments
- BR type III cells
Broadly responsive type III cells, reported to respond to bitter, sweet, and umami
- Ca+
Calcium ion
- CA4
Carbonic anhydrase 4, a marker of the type III taste cell
- CALHM1/3
Calcium homeostasis modulator 1 (CALHM1) and its homolog, CALHM3, hetero-hexamerize to form a nonselective fast-activating voltage-gated channel
- cAMP
Cyclic adenosine monophosphate
- CB1R
Cannabinoid receptor 1
- CB2R
Cannabinoid receptor 2
- CCK
Cholecystokinin
- CCKA
Cholecystokinin receptor subtype A
- CCKB
Cholecystokinin receptor subtype B
- CD11c
Cluster of differentiation 11c, characteristic cell surface marker of dendritic cells
- CD36
Cluster of differentiation 36, also known as platelet glycoprotein 4, fatty acid translocase (FAT)
- Cdh13
Cadherin 13, a label for neurons transmitting the bitter signal to the insula of mouse brain
- Cdh4
Cadherin 4, a label for neurons transmitting the umami signal to the insula of mouse brain
- CeA
Central amygdala
- CGRP
Calcitonin gene-related peptide
- CHRN
Nicotinic acetylcholine receptor
- CK2
Casein kinase II
- Cl−
Chloride ion
- CN IX
Cranial nerve IX or glossopharyngeal nerve
- CN VII
Cranial nerve VII or facial nerve
- CN X
Cranial nerve X or vagal nerve
- CN
Cranial nerve
- CNCC
Cranial neural crest cells
- CNS
Central nervous system
- CO2
Carbon dioxide
- COVID-19
Coronavirus disease 2019
- CVP
Circumvallate papilla(e)
- DCs
Dentritic cells
- DC-SIGN
Dendritic cell-specific ICAM-grabbing nonintegrin, where ICAM is intercellular adhesion molecule), a recently described mannose-specific C-type lectin expressed by dendritic cells
- DHLT
Diabetic high linoleic-acid tasters
- DLLT
Diabetic low linoleic-acid tasters
- DPP4
Dipeptidyl peptidase 4
- E10.5
Mouse embryonic day 10.5
- E11.5
Mouse embryonic day 11.5
- E12
Mouse embryonic day 12
- EC
Endocannabinoid
- EdU
5-Ethynyl-2′-deoxyuridine, a thymidine analog used to study cellular proliferation
- EGF
Epidermal growth factor
- EGM
Electrogustometer
- Egr2
Early growth response 2, transcription factor that labels neurons that transmit salt taste to mouse insula
- EM
Electron microscopy
- ENaC
Epithelial sodium channel, an amiloride-sensitive sodium channel implicated in the transduction of salt taste
- ER
Endoplasmic reticulum
- ERK1/2
Extracellular signal-regulated protein kinase 1/2
- FFAR4
Free fatty acid receptor 4, implicated in fat taste reception
- FGF
Fibroblast growth factor
- FLP
Foliate papilla(e)
- fMRI
Functional magnetic resonance imaging
- FP
Fungiform papilla(e)
- F-type
Nerve fibers in mouse chorda tympani that appear particularly responsive to fatty acid taste stimuli
- GABA
Gamma-aminobutyric acid
- GABAa
Ionotropic receptor and ligand-gated ion channel for gamma-aminobutyric acid
- GABAb
Metabotropic receptors for gamma-aminobutyric acid
- GBS
Guillain-Barre Syndrome
- GG
Genioglossus, muscle of the tongue
- Gɣ13
G-protein gamma 13 subunit
- GH
Growth hormone
- GLAST
Glial glutamate transporter
- GLP-1
Glucagon like peptide-1
- GLP-1R
Glucagon like peptide-1 receptor
- GLUT8
Also known as SLC2A8 is the eighth member of glucose transporter superfamily
- GNAT3
G-protein subunit alpha transducin 3
- GNG13
G-protein subunit gamma 13
- GPCR
G protein-coupled receptor
- GPR120
G protein-coupled receptor 120 also known as FFAR4, implicated in the ability to taste fats.
- GSP
Greater superficial petrosal
- Gα14
G-protein alpha subunit 14
- Gαgust
Alpha gustducin, a G-protein alpha subunit
- Gαi
G-protein alpha i subunit
- Gβ3
G-protein beta 3 subunit
- HBO
Cultured adult human fungiform taste cells
- HG
Hyoglossus, muscle of the tongue
- HOX
Homeobox transcription factors
- HPV
Human papilloma virus
- H&P
History and physical
- HTC-8
[HRT-18] cells are isolated from the large intestine of a 67-yr-old, male, adenocarcinoma patient
- ICAM
Intercellular adhesion molecule
- IFN1
Interferon 1
- IFNAR1
Interferon alpha- and beta-receptor subunit 1
- IFNAR2
Interferon alpha- and beta-receptor subunit 2
- IFN-γ
Interferon gamma
- IFNII
Interferon type II
- IGF-1
Insulin-like growth factor 1
- IL-10
Interleukin 10
- IL-6
Interleukin 6
- IOH
Intraoral halitosis
- IP3
Inositol 1,4,5-triphosphate
- IP3R3
Inositol 1,4,5-trisphosphate receptor, type 3
- iPG
Inferior petrosal ganglion
- JAK1
Janus kinase 1
- K+
potassium ion
- KCL
Potassium chloride
- Ki67
Ki67 protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent in resting (quiescent) cells (G0)
- Krt5
Cytokeratin 5
- LA
Linoleic acid
- Lgr5
Leucine-rich repeat-containing G protein-coupled receptor 5
- Lgr6
Leucine-rich repeat-containing G protein-coupled receptor 6
- LH
Lateral hypothalamus
- LPS
Lipopolysaccharide
- LTMR
Myelinated low-threshold mechanoreceptor
- M3
Muscarinic receptor 3
- MAP kinase
Mitogen-activated protein kinase
- Mesp1
Mesoderm posterior BHLH transcription factor 1
- MFS
Miller Fisher Syndrome
- mGLuR1
Glutamate metabotropic receptor 1
- mGluRs
Glutamate metabotropic receptors
- MyHC
Myosin heavy chain
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- MSG
Monosodium glutamate
- mTOR
Mechanistic target of rapamycin
- MuSCs
Muscle stem cells
- Na+
Sodium ion
- NG
Nodose ganglion
- NH4Cl
Ammonium chloride
- NO
Nitric oxide
- NOD
Nucleotide-binding oligomerization domain
- NLR
NOD-like receptor
- NOS
Nitric oxide synthase
- NTPDase 2
Nucleoside triphosphate diphosphohydrolase 2
- NTS
Nucleus tractus solitarius
- OMP
Olfactory marker protein
- OTOP1
Otopetrin 1
- p16INK4a
Cyclin-dependent kinase inhibitor 2A, CDKN2A, multiple tumor suppressor 1
- P2X, P1Y, P2Y
Purinergic (P) receptors: 2Xs, ligand-gated, ionotropic channels; P1Y and P2Ys, G protein-coupled, metabotropic receptors
- PASC
Postacute sequelae of COVID-19
- PAV
Proline, alanine, valine, representing the nonfunctional (for PTC and PROP taste) allele of the T2R38 gene
- PAV/AVI
Haplotype of the taster phenotype of the T2R38 gene; they sense PTC and PROP
- PAV/PAV
haplotype of the supertaster phenotype of the T2R38 gene; they sense PTC and PROP intensely
- Pax3
Paired box gene 3
- Pax7
Paired box gene 7
- PC1/3
Prohormone convertase 1/3
- PD
Parkinson’s Disease
- PDE1A
Phosphodiesterase1A
- Penk
Proenkephalin
- PKC
Protein kinase C
- PKD2L1
Polycystic kidney disease 2-like 1 protein
- PLC
Phospholipase C
- PLCβ2
Phospholipase C beta2
- PLD
Phospholipase D
- Po2
Partial pressure of oxygen, reflects the amount of oxygen dissolved in the blood
- Pou2f3
Pituitary-specific Pit-1, octamer transcription factor, neural Unc-86 transcription factor, POU class 2 homeobox 3
- PROP
6-n-Propylthiouracil
- pSS
Primary Sjögren’s Syndrome
- PTC
Phenylthiocarbamide
- RNA
Ribonucleic acid
- RA
Arg-Ala (arginine alanine), an arginyl dipeptide
- RNA-Seq
Ribonucleic acid sequencing
- RP
Arg-Pro arginine proline, an arginyl dipeptide
- rRNA
Ribosomal ribonucleic acid
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- RYGB
Roux en-Y gastric bypass
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SCN2A
Sodium voltage-gated channel alpha subunit 2
- scRNAseq
Single-cell ribonucleic acid sequencing
- Sema3A
Semaphorin 3A
- Sema7A
Semaphorin 7A
- SG
Styloglossus
- sGLT1
Sodium-dependent glucose cotransporter 1
- SHH
Sonic hedgehog
- SLN
Superior laryngeal branch, a branch of CN X or vagal nerve
- SNAP25
Synaptosome-associated protein 25
- SOX2
Sex-determining region Y-box 2
- STAT1
Signal transducer and activator of transcription 1
- STAT2
Signal transducer and activator of transcription 2
- T1R1
Taste receptor, type 1, member 1
- T1R2
Taste receptor, type 1, member 2, bitter receptors
- T1R3
Taste receptor, type 1, member 3
- T2R
Taste 2 receptor
- T2R10
Taste 2 receptor member 10
- T2R31
Taste 2 receptor member 31
- T2R38
taste 2 receptor member 38
- T2R46
Taste 2 receptor member 46
- TA
Tibialis anterior
- TBC
Taste bud cell
- TGF-β
Transforming growth factor-beta
- THC
Δ9-Tetrahydrocannabinol
- TLRs
Toll-like receptors
- TNF
Tumor necrosis factor
- TNF-α
Tumor necrosis factor-alpha
- TRC
Taste receptor cell
- TrkC
Tropomyosin receptor kinase C
- TRPM4
Transient receptor potential cation channel subfamily M (melastatin) member 4
- TRPM5
Transient receptor potential cation channel subfamily M member 5
- TRPV1
Transient receptor potential cation channel subfamily vanilloid member 1
- TST
Taste strip tests
- TTR
Transthyretin
- TUG891
3-(4-((4-Fluoro-4'-methyl-[1,1'-biphenyl]-2-yl)methoxy)phenyl)propanoic acid
- TYK2
Tyrosine kinase 2
- V3–V4
Variable region 3–variable region 4
- VIP
Vasoactive intestinal peptide
- VNUT
Vesicular nucleotide transporter
- VPAC1
Vasoactive intestinal peptide receptor type 1
- VPAC2
Vasoactive intestinal peptide receptor type 1
- VPM
Ventral-posteromedial
- VSG
Vertical sleeve gastrectomy
- VTA
Ventral tegmental area
- Wnt3a
Wingless-type MMTV integration site family, member 3A
REFERENCES
- 1. Calvo SS, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol 11: 213–227, 2015. doi: 10.1038/nrendo.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 3. Goldberg EM, Wang K, Goldberg J, Aliani M. Factors affecting the ortho- and retronasal perception of flavors: a review. Crit Rev Food Sci Nutr 58: 913–923, 2018. doi: 10.1080/10408398.2016.1231167. [DOI] [PubMed] [Google Scholar]
- 4. Prescott J. Flavour as a psychological construct: implications for perceiving and measuring the sensory qualities of foods. Food Quality Pref 10: 349–356, 1999. doi: 10.1016/S0950-3293(98)00048-2. [DOI] [Google Scholar]
- 5. Delwiche J. The impact of perceptual interactions on perceived flavor. Food Quality Prefer 15: 137–146, 2004. doi: 10.1016/S0950-3293(03)00041-7. [DOI] [Google Scholar]
- 6. Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. Flavor processing: more than the sum of its parts. Neuroreport 8: 3913–3917, 1997. doi: 10.1097/00001756-199712220-00014. [DOI] [PubMed] [Google Scholar]
- 7. Taylor AJ, Beauchamp JD, Briand L, Heer M, Hummel T, Margot C, McGrane S, Pieters S, Pittia P, Spence C. Factors affecting flavor perception in space: does the spacecraft environment influence food intake by astronauts? Compr Rev Food Sci Food Saf 19: 3439–3475, 2020. doi: 10.1111/1541-4337.12633. [DOI] [PubMed] [Google Scholar]
- 8. Witt M. Anatomy and development of the human taste system. Handb Clin Neurol 164: 147–171, 2019. doi: 10.1016/B978-0-444-63855-7.00010-1. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi K, Kumakura M, Yoshimura K, Takahashi M, Zeng JH, Kageyama I, Kobayashi K, Hama N. Comparative morphological studies on the stereo structure of the lingual papillae of selected primates using scanning electron microscopy. Ann Anat 186: 525–530, 2004. doi: 10.1016/S0940-9602(04)80101-8. [DOI] [PubMed] [Google Scholar]
- 10. Davydova L, Tkach G, Tymoshenko A, Moskalenko A, Sikora V, Kyptenko L, Lyndin M, Muravskyi D, Maksymova O, Suchonos O. Anatomical and morphological aspects of papillae, epithelium, muscles, and glands of rats’ tongue: Light, scanning, and transmission electron microscopic study. Interv Med Appl Sci 9: 168–177, 2017. doi: 10.1556/1646.9.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar S, Bate LA. Scanning electron microscopy of the tongue papillae in the pig (Sus scrofa). Microsc Res Tech 63: 253–258, 2004. doi: 10.1002/jemt.20036. [DOI] [PubMed] [Google Scholar]
- 12. Ciena AP, Santos AC, Vasconcelos BG, Rici RE, de Assis Neto AC, de Almeida SR, Miglino MA, Watanabe IS. Morphological characteristics of the papillae and lingual epithelium of guinea pig (Cavia porcellus). Acta Zool 100: 53–60, 2019. doi: 10.1111/azo.12230. [DOI] [Google Scholar]
- 13. Reginato GS, Barbosa GK, Ferreira AO, Vasconcelos BG, Rici RE, Watanabe IS, Ciena AP. Morphological and ultrastructural characteristics of the tongue of wild boar. Eur J Histochem 64: 3128, 2020. doi: 10.4081/ejh.2020.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emura S, Okumura T, Chen H, Shoumura S. Morphology of the lingual papillae in the raccoon dog and fox. Okajimas Folia Anat Jpn 83: 73–76, 2006. doi: 10.2535/ofaj.83.73. [DOI] [PubMed] [Google Scholar]
- 15. Nonaka K, Zheng JH, Kobayashi K. Comparative morphological study on the lingual papillae and their connective tissue cores in rabbits. Okajimas Folia Anat Jpn 85: 57–66, 2008. doi: 10.2535/ofaj.85.57. [DOI] [PubMed] [Google Scholar]
- 16. Shindo J, Yoshimura K, Kobayashi K. Comparative morphological study on the stereo-structure of the lingual papillae and their connective tissue cores of the American beaver (Castor canadensis). Okajimas Folia Anat Jpn 82: 127–137, 2006. doi: 10.2535/ofaj.82.127. [DOI] [PubMed] [Google Scholar]
- 17. Emura S, Hayakawa D, Chen H, Shoumura S. Morphology of the lingual papillae in the tiger. Okajimas Folia Anat Jpn 81: 39–43, 2004. doi: 10.2535/ofaj.81.39. [DOI] [PubMed] [Google Scholar]
- 18. Emura S, Okumura T, Chen H. Morphology of the lingual papillae in the jaguar. Okajimas Folia Anat Jpn 89: 93–97, 2013. doi: 10.2535/ofaj.89.93. [DOI] [PubMed] [Google Scholar]
- 19. Davies RO, Kare MR, Cagan RH. Distribution of taste buds on fungiform and circumvallate papillae of bovine tongue. Anat Rec 195: 443–446, 1979. doi: 10.1002/ar.1091950304. [DOI] [PubMed] [Google Scholar]
- 20. Iwasaki SI, Yoshimura K, Shindo J, Kageyama I. Comparative morphology of the primate tongue. Ann Anat 223: 19–31, 2019. doi: 10.1016/j.aanat.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 21. Münch F. Die topographie der papillen der Zunge des Menschen und der Säugethiere. Morphologische Arbeiten 6: 605–690, 1896. [Google Scholar]
- 22. Dotiwala AK, Samra NS. Anatomy, head and neck, tongue. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 23. Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (type III) cells in mouse taste buds sense sour (acid) taste. J Physiol 586: 2903–2912, 2008. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berkovitz BK, Holland GR, Moxham BJ. A Color Atlas and Text of Oral Anatomy, Histology and Embryology. Prescott, AZ: Wolfe, 1992. [Google Scholar]
- 25. Cobourne MT, Iseki S, Birjandi AA, Adel Al-Lami H, Thauvin-Robinet C, Xavier GM, Liu KJ. How to make a tongue: cellular and molecular regulation of muscle and connective tissue formation during mammalian tongue development. Semin Cell Dev Biol 91: 45–54, 2019. doi: 10.1016/j.semcdb.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 26. Dubner R, Sessle BJ, Storey AT. Jaw, facial, and tongue reflexes. In: The Neural Basis of Oral and Facial Function. Boston, MA: Springer, 1978, p. 246–310. [Google Scholar]
- 27. Abd-El-Malek S. Observations on the morphology of the human tongue. J Anat 73: 201–210.203, 1939. [PMC free article] [PubMed] [Google Scholar]
- 28. Mills N, Keough N, Geddes DT, Pransky SM, Mirjalili SA. Defining the anatomy of the neonatal lingual frenulum. Clin Anat 32: 824–835, 2019. doi: 10.1002/ca.23410. [DOI] [PubMed] [Google Scholar]
- 29. Stone M, Woo J, Lee J, Poole T, Seagraves A, Chung M, Kim E, Murano EZ, Prince JL, Blemker SS. Structure and variability in human tongue muscle anatomy. Comput Methods Biomech Biomed Eng Imaging Vis 6: 499–507, 2018. doi: 10.1080/21681163.2016.1162752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanders I, Mu L, Amirali A, Su H, Sobotka S. The human tongue slows down to speak: muscle fibers of the human tongue. Anat Rec (Hoboken) 296: 1615–1627, 2013. doi: 10.1002/ar.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gurkan S, Bradley RM. Secretions of von Ebner's glands influence responses from taste buds in rat circumvallate papilla. Chemical Senses 13: 655–661, 1988. doi: 10.1093/chemse/13.4.655. [DOI] [Google Scholar]
- 32. Liao ML, Kung HN, Lu KS, Shen JH, Peng WH. Alterations in the von Ebner's gland secretion and implications for taste sensation in diabetic (db/db) mice. Histol Histopathol 37: 69–79, 2021. doi: 10.14670/hh-18-379. [DOI] [PubMed] [Google Scholar]
- 33. Vors C, Drai J, Gabert L, Pineau G, Laville M, Vidal H, Guichard E, Michalski MC, Feron G. Salivary composition in obese vs normal-weight subjects: towards a role in postprandial lipid metabolism? Int J Obes (Lond) 39: 1425–1428, 2015. doi: 10.1038/ijo.2015.71. [DOI] [PubMed] [Google Scholar]
- 34. Feron G, Poette J. In-mouth mechanism leading to the perception of fat in humans: from detection to preferences. The particular role of saliva. OCL 20: 102–107, 2013. doi: 10.1051/ocl.2012.0496. [DOI] [Google Scholar]
- 35. Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat 23: 777–791, 2010. doi: 10.1002/ca.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rolls ET. The texture and taste of food in the brain. J Texture Stud 51: 23–44, 2020. doi: 10.1111/jtxs.12488. [DOI] [PubMed] [Google Scholar]
- 37. Rolls ET. Chapter 7–taste and smell processing in the brain. In: Handbook of Clinical Neurology, edited by Doty RL. Amsterdam, The Netherlands: Elsevier, 2019, p. 97–118. [DOI] [PubMed] [Google Scholar]
- 38. Gutierrez R, Fonseca E, Simon SA. The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity. Cell Mol Life Sci 77: 3469–3502, 2020. doi: 10.1007/s00018-020-03458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tokita K, Inoue T, Boughter JD. Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience 171: 351–365, 2010. doi: 10.1016/j.neuroscience.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boughter JD Jr, Lu L, Saites LN, Tokita K. Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus. Brain Res 1714: 99–110, 2019. doi: 10.1016/j.brainres.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Opstal AM, van den Berg-Huysmans AA, Hoeksma M, Blonk C, Pijl H, Rombouts S, van der Grond J. Effect of flavor on neuronal responses of the hypothalamus and ventral tegmental area. Sci Rep 9: 11250, 2019. doi: 10.1038/s41598-019-47771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol 69: 243–255, 2006. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- 43. Wu P, Arris D, Grayson M, Hung CN, Ruparel S. Characterization of sensory neuronal subtypes innervating mouse tongue. PLoS One 13: e0207069, 2018. doi: 10.1371/journal.pone.0207069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moayedi Y, Duenas-Bianchi LF, Lumpkin EA. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci Rep 8: 9975, 2018. doi: 10.1038/s41598-018-28195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moayedi Y, Michlig S, Park M, Koch A, Lumpkin EA. Somatosensory innervation of healthy human oral tissues. J Comp Neurol 529: 3046–3061, 2021. doi: 10.1002/cne.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Werner JA, Dünne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck 25: 322–332, 2003. doi: 10.1002/hed.10257. [DOI] [PubMed] [Google Scholar]
- 47. Gvetadze SR, Ilkaev KD. Lingual lymph nodes: anatomy, clinical considerations, and oncological significance. World J Clin Oncol 11: 337–347, 2020. doi: 10.5306/wjco.v11.i6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tizzano M, Grigereit L, Shultz N, Clary MS, Finger TE. Immunohistochemical analysis of human vallate taste buds. Chem Senses 40: 655–660, 2015. doi: 10.1093/chemse/bjv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Behrens M, Born S, Redel U, Voigt N, Schuh V, Raguse JD, Meyerhof W. Immunohistochemical detection of TAS2R38 protein in human taste cells. PLoS One 7: e40304, 2012. doi: 10.1371/journal.pone.0040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Azzali G. Ultrastructure and immunocytochemistry of gustatory cells in man. Ann Anat 179: 37–44, 1997. doi: 10.1016/S0940-9602(97)80133-1. [DOI] [PubMed] [Google Scholar]
- 51. Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, Lasher RS, Kinnamon JC, Finger TE. Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud. J Comp Neurol 528: 756–771, 2020. doi: 10.1002/cne.24779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murray RG, Murray A, Fujimoto S. Fine structure of gustatory cells in rabbit taste buds. J Ultrastruct Res 27: 444–461, 1969. doi: 10.1016/s0022-5320(69)80043-2. [DOI] [PubMed] [Google Scholar]
- 53. Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci 12: 3163–3171, 2000. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 54. Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci 23: 2608–2617, 2003. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol 517: 1–14, 2009. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol 468: 311–321, 2004. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 57. Wilson CE, Lasher RS, Yang R, Dzowo Y, Kinnamon JC, Finger TE. Taste bud connectome: implications for taste information processing. J Neurosci 42: 804–816, 2022. doi: 10.1523/JNEUROSCI.0838-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Doyle ME, Fiori JL, Gonzalez Mariscal I, Liu QR, Goodstein E, Yang H, Shin YK, Santa-Cruz Calvo S, Indig FE, Egan JM. Insulin is transcribed and translated in mammalian taste bud cells. Endocrinology 159: 3331–3339, 2018. doi: 10.1210/en.2018-00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Doyle ME, Appleton A, Liu QR, Yao Q, Mazucanti CH, Egan JM. Human type II taste cells express angiotensin-converting enzyme 2 and are infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J Pathol 191: 1511–1519, 2021. doi: 10.1016/j.ajpath.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds: III. J Comp Neurol 270: 1–10, 1988. doi: 10.1002/cne.902700102. [DOI] [PubMed] [Google Scholar]
- 61. Yang R, Montoya A, Bond A, Walton J, Kinnamon JC. Immunocytochemical analysis of P2X2 in rat circumvallate taste buds. BMC Neurosci 13: 51, 2012. doi: 10.1186/1471-2202-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Farbman AI. Fine structure of the taste bud. J Ultrastruct Res 12: 328–350, 1965. doi: 10.1016/S0022-5320(65)80103-4. [DOI] [PubMed] [Google Scholar]
- 63. Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 64. Sullivan JM, Borecki AA, Oleskevich S. Stem and progenitor cell compartments within adult mouse taste buds. Eur J Neurosci 31: 1549–1560, 2010. doi: 10.1111/j.1460-9568.2010.07184.x. [DOI] [PubMed] [Google Scholar]
- 65. Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLoS One 8: e53399, 2013. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dando R, Pereira E, Kurian M, Barro-Soria R, Chaudhari N, Roper SD. A permeability barrier surrounds taste buds in lingual epithelia. Am J Physiol Cell Physiol 308: C21–C32, 2015. doi: 10.1152/ajpcell.00157.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Imai A, Tanaka M, Tatsuta M, Kawazoe T. Ultrasonographic images of tongue movement during mastication. J Osaka Dent Univ 29: 61–69, 1995. [PubMed] [Google Scholar]
- 68. Olthoff LW, van der Bilt A, Bosman F, Kleizen HH. Distribution of particle sizes in food comminuted by human mastication. Arch Oral Biol 29: 899–903, 1984. doi: 10.1016/0003-9969(84)90089-x. [DOI] [PubMed] [Google Scholar]
- 69. Nakasima A. An analysis of several physiological factors influencing the masticatory function. J Kyushu Dent Soc 30: 20–36, 1976. doi: 10.2504/kds.30.20. [DOI] [Google Scholar]
- 70. Genna CW, Saperstein Y, Siegel SA, Laine AF, Elad D. Quantitative imaging of tongue kinematics during infant feeding and adult swallowing reveals highly conserved patterns. Physiol Rep 9: e14685, 2021. doi: 10.14814/phy2.14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sugden E, Lloyd S, Lam J, Cleland J. Systematic review of ultrasound visual biofeedback in intervention for speech sound disorders. Int J Lang Commun Disord 54: 705–728, 2019. doi: 10.1111/1460-6984.12478. [DOI] [PubMed] [Google Scholar]
- 72. Niemi M, Laaksonen JP, Vähätalo K, Tuomainen J, Aaltonen O, Happonen RP. Effects of transitory lingual nerve impairment on speech: an acoustic study of vowel sounds. J Oral Maxillofac Surg 60: 647–652, 2002. doi: 10.1053/joms.2002.33113. [DOI] [PubMed] [Google Scholar]
- 73. Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A 99: 2392–2397, 2002. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Southward RE, Hollis WG, Thompson DW. Precipitation of a murder: a creative use of strychnine chemistry in Agatha Christie’s The Mysterious Affair at Styles. J Chem Educ 69: 536, 1992. doi: 10.1021/ed069p536. [DOI] [Google Scholar]
- 75. Xue AY, Di Pizio A, Levit A, Yarnitzky T, Penn O, Pupko T, Niv MY. Independent evolution of strychnine recognition by bitter taste receptor subtypes. Front Mol Biosci 5: 9, 2018. doi: 10.3389/fmolb.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao H, Yang JR, Xu H, Zhang J. Pseudogenization of the umami taste receptor gene Tas1r1 in the giant panda coincided with its dietary switch to bamboo. Mol Biol Evol 27: 2669–2673, 2010. doi: 10.1093/molbev/msq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, Brand JG. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS Genet 1: 27–35, 2005. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baldwin MW, Toda Y, Nakagita T, O'Connell MJ, Klasing KC, Misaka T, Edwards SV, Liberles SD. Sensory biology. Evolution of sweet taste perception in hummingbirds by transformation of the ancestral umami receptor. Science 345: 929–933, 2014. doi: 10.1126/science.1255097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouchard B, Barnagaud JY, Verborgh P, Gauffier P, Campagna S, Célérier A. A field study of chemical senses in bottlenose dolphins and pilot whales. Anat Rec (Hoboken) 305: 668–679, 2022. doi: 10.1002/ar.24703. [DOI] [PubMed] [Google Scholar]
- 80. van Giesen L, Kilian PB, Allard CA, Bellono NW. Molecular basis of chemotactile sensation in octopus. Cell 183: 594–604.e14, 2020. doi: 10.1016/j.cell.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature 494: 472–475, 2013. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nomura K, Nakanishi M, Ishidate F, Iwata K, Taruno A. All-electrical Ca(2+)-independent signal transduction mediates attractive sodium taste in taste buds. Neuron 106: 816–829.e6, 2020. doi: 10.1016/j.neuron.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 84. Roebber JK, Roper SD, Chaudhari N. The role of the anion in salt (NaCl) detection by mouse taste buds. J Neurosci 39: 6224–6232, 2019. doi: 10.1523/JNEUROSCI.2367-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim KS, Egan JM, Jang HJ. Denatonium induces secretion of glucagon-like peptide-1 through activation of bitter taste receptor pathways. Diabetologia 57: 2117–2125, 2014. doi: 10.1007/s00125-014-3326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Taruno A, Nomura K, Kusakizako T, Ma Z, Nureki O, Foskett JK. Taste transduction and channel synapses in taste buds. Pflugers Arch 473: 3–13, 2021. doi: 10.1007/s00424-020-02464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dutta Banik D, Martin LE, Freichel M, Torregrossa AM, Medler KF. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci U S A 115: E772–E781, 2018. doi: 10.1073/pnas.1718802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jang JH, Kwon O, Moon SJ, Jeong YT. Recent advances in understanding peripheral taste decoding I: 2010 to 2020. Endocrinol Metab (Seoul) 36: 469–477, 2021. doi: 10.3803/EnM.2021.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, Payne R, Demuro A, Parker I, Mitchell CH, Henao-Mejia J, Tanis JE, Matsumoto I, Tordoff MG, Foskett JK. CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98: 547–561.e10, 2018. doi: 10.1016/j.neuron.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226, 2013. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47: 51–64, 1999. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- 92. Ninomiya Y. Reinnervation of cross-regenerated gustatory nerve fibers into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci U S A 95: 5347–5350, 1998. doi: 10.1073/pnas.95.9.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bigiani A. Does ENaC work as sodium taste receptor in humans? Nutrients 12: 1195, 2020. doi: 10.3390/nu12041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Simon SA, Gutierrez R. Frontiers in neuroscience TRP channels at the periphery of the taste and trigeminal systems. In: Neurobiology of TRP Channels, edited by Emir TL. Boca Raton, FL: CRC Press/Taylor & Francis, 2017, p. 113–124. [PubMed] [Google Scholar]
- 95. Wooding SP, Ramirez VA, Behrens M. Bitter taste receptors: genes, evolution and health. Evol Med Public Health 9: 431–447, 2021. doi: 10.1093/emph/eoab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yoshida R, Ninomiya Y. Taste information derived from T1R-expressing taste cells in mice. Biochem J 473: 525–536, 2016. doi: 10.1042/BJ20151015. [DOI] [PubMed] [Google Scholar]
- 97. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell 100: 693–702, 2000. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 98. Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35: 157–170, 2010. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 99. Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature 376: 80–85, 1995. doi: 10.1038/376080a0. [DOI] [PubMed] [Google Scholar]
- 100. McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357: 563–569, 1992. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 101. Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2: 1055–1062, 1999. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 102. He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24: 7674–7680, 2004. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Blonde GD, Travers SP, Spector AC. Taste sensitivity to a mixture of monosodium glutamate and inosine 5'-monophosphate by mice lacking both subunits of the T1R1+T1R3 amino acid receptor. Am J Physiol Regul Integr Comp Physiol 314: R802–R810, 2018. doi: 10.1152/ajpregu.00352.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Choudhuri S P, Delay RJ, Delay ER. L-amino acids elicit diverse response patterns in taste sensory cells: a role for multiple receptors. PLoS One 10: e0130088, 2015. doi: 10.1371/journal.pone.0130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yasumatsu K, Ohkuri T, Yoshida R, Iwata S, Margolskee RF, Ninomiya Y. Sodium-glucose cotransporter 1 as a sugar taste sensor in mouse tongue. Acta Physiol (Oxf) 230: e13529, 2020. doi: 10.1111/apha.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Breslin PA, Izumi A, Tharp A, Ohkuri T, Yokoo Y, Flammer LJ, Rawson NE, Margolskee RF. Evidence that human oral glucose detection involves a sweet taste pathway and a glucose transporter pathway. PLoS One 16: e0256989, 2021. doi: 10.1371/journal.pone.0256989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kinnamon S, Finger T. Recent advances in taste transduction and signaling. F1000Res 8: F1000 Faculty Rev-2117, 2019. doi: 10.12688/f1000research.21099.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kalyanasundar B, Blonde GD, Spector AC, Travers SP. Electrophysiological responses to sugars and amino acids in the nucleus of the solitary tract of type 1 taste receptor double-knockout mice. J Neurophysiol 123: 843–859, 2020. doi: 10.1152/jn.00584.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. von Molitor E, Riedel K, Krohn M, Hafner M, Rudolf R, Cesetti T. Sweet taste is complex: signaling cascades and circuits involved in sweet sensation. Front Hum Neurosci 15: 667709, 2021. doi: 10.3389/fnhum.2021.667709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Elson AE, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J 24: 3960–3969, 2010. doi: 10.1096/fj.10-158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shin YK, Egan JM. Roles of hormones in taste signaling. Results Probl Cell Differ 52: 115–137, 2010. doi: 10.1007/978-3-642-14426-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106: 455–463, 2008. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sun EW, de Fontgalland D, Rabbitt P, Hollington P, Sposato L, Due SL, Wattchow DA, Rayner CK, Deane AM, Young RL, Keating DJ. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes 66: 2144–2149, 2017. doi: 10.2337/db17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martin B, Maudsley S, White CM, Egan JM. Hormones in the naso-oropharynx: endocrine modulation of taste and smell. Trends Endocrinol Metab 20: 163–170, 2009. doi: 10.1016/j.tem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sekiguchi T. Cholecystokinin. In: Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research, edited by Ando H. Amsterdam, The Netherlands: Elsevier, 2015, p. 309–311. [Google Scholar]
- 116. Pons J, Kitlinska J, Jacques D, Perreault C, Nader M, Everhart L, Zhang Y, Zukowska Z. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can J Physiol Pharmacol 86: 438–448, 2008. doi: 10.1139/y08-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hurtado MD, Acosta A, Riveros PP, Baum BJ, Ukhanov K, Brown AR, Dotson CD, Herzog H, Zolotukhin S. Distribution of Y-receptors in murine lingual epithelia. PLoS One 7: e46358, 2012. doi: 10.1371/journal.pone.0046358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Langer I. Mechanisms involved in VPAC receptors activation and regulation: lessons from pharmacological and mutagenesis studies. Front Endocrinol (Lausanne) 3: 129, 2012. doi: 10.3389/fendo.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, Egan JM. Vasoactive intestinal peptide–null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes 59: 1143–1152, 2010. doi: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Saltiel M, Kuhre R, Christiansen C, Eliasen R, Conde-Frieboes K, Rosenkilde M, Holst J. Sweet taste receptor activation in the gut is of limited importance for glucose-stimulated GLP-1 and GIP secretion. Nutrients 9: 418, 2017. doi: 10.3390/nu9040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol 10: 413, 2019. doi: 10.3389/fphys.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kaiya H. Ghrelin. Amsterdam, The Netherlands: Elsevier, 2021, p. 321–324. doi: 10.1016/B978-0-12-820649-2.00083-8. [DOI] [Google Scholar]
- 123. Camina JP. Cell biology of the ghrelin receptor. J Neuroendocrinol 18: 65–76, 2006. doi: 10.1111/j.1365-2826.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- 124. Shin YK, Martin B, Kim W, White CM, Ji S, Sun Y, Smith RG, Sévigny J, Tschöp MH, Maudsley S, Egan JM. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One 5: e12729, 2010. doi: 10.1371/journal.pone.0012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen D, Kodama Y, Kulseng B, Johannessen H, Zhao CM. Galanin. Amsterdam, The Netherlands: Elsevier, 2013, p. 1210–1218. [Google Scholar]
- 126. Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, Chaudhari N. Oxytocin signaling in mouse taste buds. PLoS One 5: e11980, 2010. doi: 10.1371/journal.pone.0011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ladyman SR, Grattan DR. JAK-STAT and feeding. JAK-STAT 2: e23675, 2013. doi: 10.4161/jkst.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36- and GPR120-mediated Ca(2)(+) signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 146: 995–1005, 2014. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dutta Banik D, Benfey ED, Martin LE, Kay KE, Loney GC, Nelson AR, Ahart ZC, Kemp BT, Kemp BR, Torregrossa AM, Medler KF. A subset of broadly responsive type III taste cells contribute to the detection of bitter, sweet and umami stimuli. PLoS Genet 16: e1008925, 2020. doi: 10.1371/journal.pgen.1008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses 33: 243–254, 2008. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Turner HN, Liman ER. The cellular and molecular basis of sour taste. Annu Rev Physiol 84: 41–58, 2022. doi: 10.1146/annurev-physiol-060121-041637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Nelson TM, Lopezjimenez ND, Tessarollo L, Inoue M, Bachmanov AA, Sullivan SL. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem Senses 35: 565–577, 2010. doi: 10.1093/chemse/bjq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y. Sour taste responses in mice lacking PKD channels. PLoS One 6: e20007, 2011. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Teng B, Wilson CE, Tu YH, Joshi NR, Kinnamon SC, Liman ER. Cellular and neural responses to sour stimuli require the proton channel Otop1. Curr Biol 29: 3647–3656.e5, 2019. doi: 10.1016/j.cub.2019.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, Mulhall EM, Ye W, Smith AD, Liman ER. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 359: 1047–1050, 2018. doi: 10.1126/science.aao3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhang J, Jin H, Zhang W, Ding C, O'Keeffe S, Ye M, Zuker CS. Sour sensing from the tongue to the brain. Cell 179: 392–402.e15, 2019. doi: 10.1016/j.cell.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 137. Ye W, Chang RB, Bushman JD, Tu YH, Mulhall EM, Wilson CE, Cooper AJ, Chick WS, Hill-Eubanks DC, Nelson MT, Kinnamon SC, Liman ER. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci U S A 113: E229–E238, 2016. doi: 10.1073/pnas.1514282112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol 235: 48–60, 1985. doi: 10.1002/cne.902350105. [DOI] [PubMed] [Google Scholar]
- 139. Royer SM, Kinnamon JC. HVEM serial-section analysis of rabbit foliate taste buds: I. Type III cells and their synapses. J Comp Neurol 306: 49–72, 1991. doi: 10.1002/cne.903060105. [DOI] [PubMed] [Google Scholar]
- 140. Stratford JM, Larson ED, Yang R, Salcedo E, Finger TE. 5-HT(3A)-driven green fluorescent protein delineates gustatory fibers innervating sour-responsive taste cells: a labeled line for sour taste? J Comp Neurol 525: 2358–2375, 2017. doi: 10.1002/cne.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zocchi D, Wennemuth G, Oka Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci 20: 927–933, 2017. doi: 10.1038/nn.4575. [DOI] [PubMed] [Google Scholar]
- 142. Liman ER, Kinnamon SC. Sour taste: receptors, cells and circuits. Curr Opin Physiol 20: 8–15, 2021. doi: 10.1016/j.cophys.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science 326: 443–445, 2009. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mattes RD. Taste, teleology and macronutrient intake. Curr Opin Physiol 19: 162–167, 2021. doi: 10.1016/j.cophys.2020.11.003. [DOI] [Google Scholar]
- 145. Running CA, Craig BA, Mattes RD. Oleogustus: the unique taste of fat. Chem Senses 40: 507–516, 2015. doi: 10.1093/chemse/bjv036. [DOI] [PubMed] [Google Scholar]
- 146. Brondel L, Quilliot D, Mouillot T, Khan NA, Bastable P, Boggio V, Leloup C, Pénicaud L. Taste of fat and obesity: different hypotheses and our point of view. Nutrients 14: 555, 2022. doi: 10.3390/nu14030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liu D, Costanzo A, Evans MD, Archer NS, Nowson C, Duesing K, Keast R. Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br J Nutr 120: 64–73, 2018. doi: 10.1017/S0007114518001265. [DOI] [PubMed] [Google Scholar]
- 148. Lu C, Lin X, Yamashita J, Xi R, Zhou M, Zhang YV, Wang H, Margolskee RF, Koo BK, Clevers H, Matsumoto I, Jiang P. RNF43/ZNRF3 negatively regulates taste tissue homeostasis and positively regulates dorsal lingual epithelial tissue homeostasis. Stem Cell Reports 17: 369–383, 2022. doi: 10.1016/j.stemcr.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yasumatsu K, Iwata S, Inoue M, Ninomiya Y. Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol (Oxf) 226: e13215, 2019. doi: 10.1111/apha.13215. [DOI] [PubMed] [Google Scholar]
- 150. Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 18: 485–497, 2017. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26: 3971–3980, 2006. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schwörer H, Reimann A, Ramadori G, Racké K. Characterization of histamine H3 receptors inhibiting 5-HT release from porcine enterochromaffin cells: further evidence for H3 receptor heterogeneity. Naunyn Schmiedebergs Arch Pharmacol 350: 375–379, 1994. doi: 10.1007/BF00178954. [DOI] [PubMed] [Google Scholar]
- 153. Larson ED, Vandenbeuch A, Voigt A, Meyerhof W, Kinnamon SC, Finger TE. The role of 5-HT3 receptors in signaling from taste buds to nerves. J Neurosci 35: 15984–15995, 2015. doi: 10.1523/JNEUROSCI.1868-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mazucanti CH, Liu QR, Lang D, Huang N, O’Connell JF, Camandola S, Egan JM. Release of insulin produced by the choroid plexis is regulated by serotonergic signaling. JCI Insight 4: e131682, 2019. doi: 10.1172/jci.insight.131682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jaber L, Zhao FL, Kolli T, Herness S. A physiologic role for serotonergic transmission in adult rat taste buds. PLoS One 9: e112152, 2014. doi: 10.1371/journal.pone.0112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Galligan JJ. Nicotinic acetylcholine and P2X receptors in the enteric nervous system. Proc West Pharmacol Soc 45: 231–234, 2002. [PubMed] [Google Scholar]
- 157. Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol 6: 526–532, 1996. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 158. Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol 525: 143–158, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 160. Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun 388: 1–5, 2009. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 161. Larson ED, Vandenbeuch A, Anderson CB, Kinnamon SC. Function, innervation, and neurotransmitter signaling in mice lacking type-II taste cells. eNeuro 7: ENEURO.0339-19.2020, 2020. doi: 10.1523/ENEURO.0339-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol 497: 1–12, 2006. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci U S A 110: 14789–14794, 2013. doi: 10.1073/pnas.1309468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC, Roper SD. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci 31: 13654–13661, 2011. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rodriguez YA, Roebber JK, Dvoryanchikov G, Makhoul V, Roper SD, Chaudhari N. “Tripartite synapses” in taste buds: a role for type I glial-like taste cells. J Neurosci 41: 9860–9871, 2021. doi: 10.1523/JNEUROSCI.1444-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One 6: e25471, 2011. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wang C, Kerckhofs K, Van de Casteele M, Smolders I, Pipeleers D, Ling Z. Glucose inhibits GABA release by pancreatic β-cells through an increase in GABA shunt activity. Am J Physiol Endocrinol Metab 290: E494–E499, 2006. doi: 10.1152/ajpendo.00304.2005. [DOI] [PubMed] [Google Scholar]
- 168. Bansal P, Wang S, Liu S, Xiang YY, Lu WY, Wang Q. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 β-cells. PLoS One 6: e26225, 2011. doi: 10.1371/journal.pone.0026225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Craige CP, Lewandowski S, Kirby LG, Unterwald EM. Dorsal raphe 5-HT2C receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology 93: 41–51, 2015. doi: 10.1016/j.neuropharm.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Paran N, Mattern CF. The distribution of acetylcholinesterase in buds of the rat vallate papilla as determined by electron microscope histochemistry. J Comp Neurol 159: 29–44, 1975. doi: 10.1002/cne.901590104. [DOI] [PubMed] [Google Scholar]
- 171. Dando R, Roper SD. Acetylcholine is released from taste cells, enhancing taste signalling. J Physiol 590: 3009–3017, 2012. doi: 10.1113/jphysiol.2012.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Petkova-Kirova P, Giovannini MG, Kalfin R, Rakovska A. Modulation of acetylcholine release by cholecystokinin in striatum: receptor specificity; role of dopaminergic neuronal activity. Brain Res Bull 89: 177–184, 2012. doi: 10.1016/j.brainresbull.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 173. Ohla K, Yoshida R, Roper SD, Di Lorenzo PM, Victor JD, Boughter JD, Fletcher M, Katz DB, Chaudhari N. Recognizing taste: coding patterns along the neural axis in mammals. Chem Senses 44: 237–247, 2019. doi: 10.1093/chemse/bjz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lee H, Macpherson LJ, Parada CA, Zuker CS, Ryba NJ. Rewiring the taste system. Nature 548: 330–333, 2017. doi: 10.1038/nature23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ninomiya Y, Tonosaki K, Funakoshi M. Gustatory neural response in the mouse. Brain Res 244: 370–373, 1982. doi: 10.1016/0006-8993(82)90100-7. [DOI] [PubMed] [Google Scholar]
- 176. Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, Zuker CS. The neural representation of taste quality at the periphery. Nature 517: 373–376, 2015. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun 6: 8171, 2015. doi: 10.1038/ncomms9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Dvoryanchikov G, Hernandez D, Roebber JK, Hill DL, Roper SD, Chaudhari N. Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat Commun 8: 760, 2017. doi: 10.1038/s41467-017-01095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science 333: 1262–1266, 2011. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Huang T, Ohman LC, Clements AV, Whiddon ZD, Krimm RF. Variable branching characteristics of peripheral taste neurons indicates differential convergence. J Neurosci 41: 4850–4866, 2021. doi: 10.1523/JNEUROSCI.1935-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 182. Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7: 890–901, 2006. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 183. Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci 33: 326–334, 2010. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Schier LA, Spector AC. The functional and neurobiological properties of bad taste. Physiol Rev 99: 605–663, 2019. doi: 10.1152/physrev.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Parada C, Chai Y. Mandible and tongue development. Curr Top Dev Biol 115: 31–58, 2015. doi: 10.1016/bs.ctdb.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Parada C, Han D, Chai Y. Molecular and cellular regulatory mechanisms of tongue myogenesis. J Dent Res 91: 528–535, 2012. doi: 10.1177/0022034511434055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Han D, Zhao H, Parada C, Hacia JG, Bringas P Jr, Chai Y. A TGFβ-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 139: 1640–1650, 2012. doi: 10.1242/dev.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Dong F, Sun X, Liu W, Ai D, Klysik E, Lu MF, Hadley J, Antoni L, Chen L, Baldini A, Francis-West P, Martin JF. pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development 133: 4891–4891, 2006. doi: 10.1242/dev.02693. [DOI] [PubMed] [Google Scholar]
- 189. Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 127: 3215–3226, 2000. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- 190. Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development 126: 3437–3447, 1999. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 191. Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimarães-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell 16: 822–832, 2009. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Saga Y, Hata N, Kobayashi S, Magnuson T, Seldin MF, Taketo MM. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122: 2769–2778, 1996. doi: 10.1242/dev.122.9.2769. [DOI] [PubMed] [Google Scholar]
- 193. Zhu XJ, Yuan X, Wang M, Fang Y, Liu Y, Zhang X, Yang X, Li Y, Li J, Li F, Dai ZM, Qiu M, Zhang Z, Zhang Z. A Wnt/Notch/Pax7 signaling network supports tissue integrity in tongue development. J Biol Chem 292: 9409–9419, 2017. doi: 10.1074/jbc.M117.789438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Goto A, Kokabu S, Dusadeemeelap C, Kawaue H, Matsubara T, Tominaga K, Addison WN. Tongue muscle for the analysis of head muscle regeneration dynamics. J Dent Res 101: 962–971, 2022. doi: 10.1177/00220345221075966. [DOI] [PubMed] [Google Scholar]
- 195. Czajkowski MT, Rassek C, Lenhard DC, Bröhl D, Birchmeier C. Divergent and conserved roles of Dll1 signaling in development of craniofacial and trunk muscle. Dev Biol 395: 307–316, 2014. doi: 10.1016/j.ydbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 196. Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89: 127–138, 1997. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 197. Rothova M, Thompson H, Lickert H, Tucker AS. Lineage tracing of the endoderm during oral development. Dev Dyn 241: 1183–1191, 2012. doi: 10.1002/dvdy.23804. [DOI] [PubMed] [Google Scholar]
- 198. Okuhara S, Birjandi AA, Adel Al-Lami H, Sagai T, Amano T, Shiroishi T, Xavier GM, Liu KJ, Cobourne MT, Iseki S. Temporospatial sonic hedgehog signalling is essential for neural crest-dependent patterning of the intrinsic tongue musculature. Development 146: dev180075, 2019. doi: 10.1242/dev.180075. [DOI] [PubMed] [Google Scholar]
- 199. Mistretta CM, Liu HX. Development of fungiform papillae: patterned lingual gustatory organs. Arch Histol Cytol 69: 199–208, 2006. doi: 10.1679/aohc.69.199. [DOI] [PubMed] [Google Scholar]
- 200. Barlow LA, Klein OD. Developing and regenerating a sense of taste. Curr Top Dev Biol 111: 401–419, 2015. doi: 10.1016/bs.ctdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Barlow LA. The sense of taste: development, regeneration, and dysfunction. WIREs Mech Dis 14: e1547, 2021. doi: 10.1002/wsbm.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mistretta CM, Kumari A. Tongue and taste organ biology and function: homeostasis maintained by hedgehog signaling. Annu Rev Physiol 79: 335–356, 2017. doi: 10.1146/annurev-physiol-022516-034202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Maynard TM, Zohn IE, Moody SA, LaMantia AS. Feeding, and swallowing: behaviors, circuits, and targets for neurodevelopmental pathology. Ann Rev Neurosci 43: 315–336, 2020. doi: 10.1146/annurev-neuro-100419-100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Cheng X, Shi B, Li J. Distinct embryonic origin and injury response of resident stem cells in craniofacial muscles. Front Physiol 12: 690248, 2021. doi: 10.3389/fphys.2021.690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Buckingham M. Gene regulatory networks and cell lineages that underlie the formation of skeletal muscle. Proc Natl Acad Sci U S A 114: 5830–5837, 2017. doi: 10.1073/pnas.1610605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development 138: 2401–2415, 2011. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- 207. Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res 52: 732–744, 2009. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Cullins MJ, Connor NP. Alterations of intrinsic tongue muscle properties with aging. Muscle Nerve 56: E119–E125, 2017. doi: 10.1002/mus.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Johnson AM, Connor NP. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve 43: 203–211, 2011. doi: 10.1002/mus.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Kletzien H, Hare AJ, Leverson G, Connor NP. Age-related effect of cell death on fiber morphology and number in tongue muscle. Muscle Nerve 57: E29–E37, 2018. doi: 10.1002/mus.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia 26: 256–263, 2011. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Russell JA, Connor NP. Effects of age and radiation treatment on function of extrinsic tongue muscles. Radiat Oncol 9: 254, 2014. doi: 10.1186/s13014-014-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kletzien H, Kelm-Nelson CA, Wang S, Suzuki M, Connor NP. Myogenic marker expression as a function of age and exercise-based therapy in the tongue. Exp Gerontol 142: 111104, 2020. doi: 10.1016/j.exger.2020.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Rogus-Pulia N, Connor NP. Muscle strengthening approaches to dysphagia rehabilitation. Curr Phys Med Rehab Rep 4: 277–286, 2016. doi: 10.1007/s40141-016-0136-3. [DOI] [Google Scholar]
- 215. Feng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chem Senses 39: 3–16, 2014. doi: 10.1093/chemse/bjt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Takeda N, Jain R, Li D, Li L, Lu MM, Epstein JA. Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One 8: e66314, 2013. doi: 10.1371/journal.pone.0066314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells 31: 992–1000, 2013. doi: 10.1002/stem.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci U S A 111: 16401–16406, 2014. doi: 10.1073/pnas.1409064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ohmoto M, Lei W, Yamashita J, Hirota J, Jiang P, Matsumoto I. SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue. PLoS One 15: e0240848, 2020. doi: 10.1371/journal.pone.0240848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ohmoto M, Ren W, Nishiguchi Y, Hirota J, Jiang P, Matsumoto I. Genetic lineage tracing in taste tissues using Sox2-CreERT2 strain. Chem Senses 42: 547–552, 2017. doi: 10.1093/chemse/bjx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Yang H, Cong WN, Yoon JS, Egan JM. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med 4: 245–252, 2015. doi: 10.1002/cam4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gaillard D, Barlow LA. A mechanistic overview of taste bud maintenance and impairment in cancer therapies. Chem Senses 46: bjab011, 2021. doi: 10.1093/chemse/bjab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Golden EJ, Larson ED, Shechtman LA, Trahan GD, Gaillard D, Fellin TJ, Scott JK, Jones KL, Barlow LA. Onset of taste bud cell renewal starts at birth and coincides with a shift in SHH function. Elife 10: e64013, 2021. doi: 10.7554/eLife.64013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JC, Martin MG, Magness ST. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 31: 2024–2030, 2013. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Smith NR, Swain JR, Davies PS, Gallagher AC, Parappilly MS, Beach CZ, Streeter PR, Williamson IA, Magness ST, Wong MH. Monoclonal antibodies reveal dynamic plasticity between Lgr5- and Bmi1-expressing intestinal cell populations. Cell Mol Gastroenterol Hepatol 6: 79–96, 2018. doi: 10.1016/j.jcmgh.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol 504: 206–216, 2007. doi: 10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol 459: 15–24, 2003. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
- 228. Meng L, Ohman-Gault L, Ma L, Krimm RF. Taste bud-derived BDNF is required to maintain normal amounts of innervation to adult taste buds. eNeuro 2: ENEURO.0097-15.2015, 2015. doi: 10.1523/ENEURO.0097-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Lin X, Lu C, Ohmoto M, Choma K, Margolskee RF, Matsumoto I, Jiang P. R-spondin substitutes for neuronal input for taste cell regeneration in adult mice. Proc Natl Acad Sci U S A 118: e2001833118, 2021. doi: 10.1073/pnas.2001833118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zeng Q, Oakley B. p53 and Bax: putative death factors in taste cell turnover. J Comp Neurol 413: 168–180, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 231. Zeng Q, Kwan A, Oakley B. Gustatory innervation and bax-dependent caspase-2: participants in the life and death pathways of mouse taste receptor cells. J Comp Neurol 424: 640–650, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 232. Gaillard D, Shechtman LA, Millar SE, Barlow LA. Fractionated head and neck irradiation impacts taste progenitors, differentiated taste cells, and Wnt/β-catenin signaling in adult mice. Sci Rep 9: 17934, 2019. doi: 10.1038/s41598-019-54216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci 32: 3474–3484, 2012. doi: 10.1523/JNEUROSCI.4167-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Gaillard D, Bowles SG, Salcedo E, Xu M, Millar SE, Barlow LA. β-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice. PLoS Genet 13: e1006990, 2017. doi: 10.1371/journal.pgen.1006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Jensterle M, DeVries JH, Battelino T, Battelino S, Yildiz B, Janez A. Glucagon-like peptide-1, a matter of taste? Rev Endocr Metab Disord 22: 763–775, 2021. doi: 10.1007/s11154-020-09609-x. [DOI] [PubMed] [Google Scholar]
- 236. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60: 470–512, 2008. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kokrashvili Z, Yee KK, Ilegems E, Iwatsuki K, Li Y, Mosinger B, Margolskee RF. Endocrine taste cells. Br J Nutr 111 (Suppl 1): S23–S29, 2014. doi: 10.1017/S0007114513002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. McLaughlin SK. Erb and c-Kit receptors have distinctive patterns of expression in adult and developing taste papillae and taste buds. J Neurosci 20: 5679–5688, 2000. doi: 10.1523/JNEUROSCI.20-15-05679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Suzuki Y, Takeda M, Sakakura Y, Suzuki N. Distinct expression pattern of insulin-like growth factor family in rodent taste buds. J Comp Neurol 482: 74–84, 2005. doi: 10.1002/cne.20379. [DOI] [PubMed] [Google Scholar]
- 240. Biggs BT, Tang T, Krimm RF. Insulin-like growth factors are expressed in the taste system, but do not maintain adult taste buds. PLoS One 11: e0148315, 2016. doi: 10.1371/journal.pone.0148315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Takai S, Watanabe Y, Sanematsu K, Yoshida R, Margolskee RF, Jiang P, Atsuta I, Koyano K, Ninomiya Y, Shigemura N. Effects of insulin signaling on mouse taste cell proliferation. PLoS One 14: e0225190, 2019. doi: 10.1371/journal.pone.0225190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol (1985) 97: 1291–1298, 2004. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- 243. Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res 49: 27–37, 2001. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 244. Godoy IR, Martinez-Salazar EL, Eajazi A, Genta PR, Bredella MA, Torriani M. Fat accumulation in the tongue is associated with male gender, abnormal upper airway patency and whole-body adiposity. Metabolism 65: 1657–1663, 2016. doi: 10.1016/j.metabol.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Schwab RJ, Kim C, Siegel L, Keenan BT, Black J, Farid-Moayer M, Podmore J, Vaska M. Examining the mechanism of action of a new device using oral pressure therapy for the treatment of obstructive sleep apnea. Sleep 37: 1237–1247, 2014. doi: 10.5665/sleep.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep 37: 1639–1648, 2014. doi: 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Wang SH, Keenan BT, Wiemken A, Zang Y, Staley B, Sarwer DB, Torigian DA, Williams N, Pack AI, Schwab RJ. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat. Am J Respir Crit Care Med 201: 718–727, 2020. doi: 10.1164/rccm.201903-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol 62: 593–604, 2003. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 249. Feng P, Chai J, Zhou M, Simon N, Huang L, Wang H. Interleukin-10 is produced by a specific subset of taste receptor cells and critical for maintaining structural integrity of mouse taste buds. J Neurosci 34: 2689–2701, 2014. doi: 10.1523/JNEUROSCI.3074-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Feng P, Zhao H, Chai J, Huang L, Wang H. Expression and secretion of TNF-α in mouse taste buds: a novel function of a specific subset of type II taste cells. PLoS One 7: e43140, 2012. doi: 10.1371/journal.pone.0043140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci 1170: 596–603, 2009. doi: 10.1111/j.1749-6632.2009.04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Wang H, Zhou M, Brand J, Huang L. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci 27: 10703–10713, 2007. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Hevezi P, Moyer BD, Lu M, Gao N, White E, Echeverri F, Kalabat D, Soto H, Laita B, Li C, Yeh SA, Zoller M, Zlotnik A. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One 4: e6395, 2009. doi: 10.1371/journal.pone.0006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Lee AJ, Ashkar AA. The dual nature of type I and type II interferons. Front Immunol 9: 2061, 2018. doi: 10.3389/fimmu.2018.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Cohn ZJ, Kim A, Huang L, Brand J, Wang H. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci 11: 72, 2010. doi: 10.1186/1471-2202-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Kellokumpu-Lehtinen P, Nordman E, Toivanen A. Combined interferon and vinblastine treatment of advanced melanoma: evaluation of the treatment results and the effects of the treatment on immunological functions. Cancer Immunol Immunother 28: 213–217, 1989. doi: 10.1007/BF00204991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Feng P, Jyotaki M, Kim A, Chai J, Simon N, Zhou M, Bachmanov AA, Huang L, Wang H. Regulation of bitter taste responses by tumor necrosis factor. Brain Behav Immun 49: 32–42, 2015. doi: 10.1016/j.bbi.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Martens K, Steelant B, Bullens DM. Taste receptors: the gatekeepers of the airway epithelium. Cells 10: 2889, 2021. doi: 10.3390/cells10112889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 299: 1221–1225, 2003. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 260. Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med (Berl) 92: 1235–1244, 2014. doi: 10.1007/s00109-014-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias PT, Ischiropoulos H, Kreindler JL, Reed DR, Cohen NA. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122: 4145–4159, 2012. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Wang R, van Keeken NM, Siddiqui S, Dijksman LM, Maudsley S, Derval D, van Dam PS, Martin B. Higher TNF-α, IGF-1, and leptin levels are found in tasters than non-tasters. Front Endocrinol (Lausanne) 5: 125, 2014. doi: 10.3389/fendo.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Feng P, Yee KK, Rawson NE, Feldman LM, Feldman RS, Breslin PA. Immune cells of the human peripheral taste system: dominant dendritic cells and CD4 T cells. Brain Behav Immun 23: 760–766, 2009. doi: 10.1016/j.bbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Feng P, Wang H, Feldman RS, Pribitkin EA, Breslin PA. The T cells in peripheral taste tissue of healthy human adults: predominant memory T cells and Th-1 cells. Chem Senses 35: 501–509, 2010. doi: 10.1093/chemse/bjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Kaufman A, Kim J, Noel C, Dando R. Taste loss with obesity in mice and men. Int J Obes (Lond) 44: 739–743, 2020. doi: 10.1038/s41366-019-0429-6. [DOI] [PubMed] [Google Scholar]
- 266. Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience 141: 2129–2138, 2006. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 267. Ahart ZC, Martin LE, Kemp BR, Dutta Banik D, Roberts SG, Torregrossa AM, Medler KF. Differential effects of diet and weight on taste responses in diet-induced obese mice. Obesity (Silver Spring) 28: 284–292, 2020. doi: 10.1002/oby.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci 361: 1137–1148, 2006. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, Kubis HP. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite 57: 237–246, 2011. doi: 10.1016/j.appet.2011.05.107. [DOI] [PubMed] [Google Scholar]
- 270. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 174: 516–524, 2014. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Hujoel PP, Lingström P. Nutrition, dental caries and periodontal disease: a narrative review. J Clin Periodontol 44: S79–S84, 2017. doi: 10.1111/jcpe.12672. [DOI] [PubMed] [Google Scholar]
- 272. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 376: 1492, 2017. doi: 10.1056/NEJMc1701944. [DOI] [PubMed] [Google Scholar]
- 273. Smith KR, Papantoni A, Veldhuizen MG, Kamath V, Harris C, Moran TH, Carnell S, Steele KE. Taste-related reward is associated with weight loss following bariatric surgery. J Clin Invest 130: 4370–4381, 2020. doi: 10.1172/JCI137772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141: 1936–1941, 2000. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- 275. Krieger JP. Intestinal glucagon-like peptide-1 effects on food intake: physiological relevance and emerging mechanisms. Peptides 131: 170342, 2020. doi: 10.1016/j.peptides.2020.170342. [DOI] [PubMed] [Google Scholar]
- 276. Trapp S, Brierley DI. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br J Pharmacol 179: 557–570, 2022. doi: 10.1111/bph.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MT, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF, STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 384: 989–1002, 2021. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 278. Bernard A, Dastugue A, Maquart G, Delhaye S, Duez H, Besnard P. Diet-induced obesity alters the circadian expression of clock genes in mouse gustatory papillae. Front Physiol 11: 726, 2020. doi: 10.3389/fphys.2020.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Dutt M, Ng YK, Molendijk J, Karimkhanloo H, Liao L, Blazev R, Montgomery MK, Watt MJ, Parker BL. Western diet induced remodelling of the tongue proteome. Proteomes 9: 22, 2021. doi: 10.3390/proteomes9020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Archer N, Shaw J, Cochet-Broch M, Bunch R, Poelman A, Barendse W, Duesing K. Obesity is associated with altered gene expression in human tastebuds. Int J Obes (Lond) 43: 1475–1484, 2019. doi: 10.1038/s41366-018-0303-y. [DOI] [PubMed] [Google Scholar]
- 281. Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 282. Schneller LM, McIntosh S, Li D, Rahman I, Ossip D, Goniewicz M, O'Connor RJ. Tobacco use and chemosensory impairments among current adult tobacco users in the US: data from NHANES 2013–2014. Tobacco Induced Diseases 16: 43, 2018. doi: 10.18332/tid/94202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Risso DS, Kozlitina J, Sainz E, Gutierrez J, Wooding S, Getachew B, Luiselli D, Berg CJ, Drayna D. Genetic variation in the TAS2R38 bitter taste receptor and smoking behaviors. PLoS One 11: e0164157, 2016.doi: 10.1371/journal.pone.0164157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Kozlitina J, Risso D, Lansu K, Olsen RH, Sainz E, Luiselli D, Barik A, Frigerio-Domingues C, Pagani L, Wooding S, Kirchner T, Niaura R, Roth B, Drayna D. An African-specific haplotype in MRGPRX4 is associated with menthol cigarette smoking. PLoS Genet 15: e1007916, 2019. doi: 10.1371/journal.pgen.1007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Johnson NL, Patten T, Ma M, De Biasi M, Wesson DW. chemosensory contributions of e-cigarette additives on nicotine use. Front Neurosci 16: 893587, 2022. doi: 10.3389/fnins.2022.893587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Agarwal K, Luk JW, Manza P, McDuffie C, To L, Jaime-Lara RB, Stangl BL, Schwandt ML, Momenan R, Goldman D, Diazgranados N, Ramchandani VA, Joseph PV. Chemosensory alterations and impact on quality of life in persistent alcohol drinkers. Alcohol Alcohol 58: 84–92 2022. doi: 10.1093/alcalc/agac047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Silva CS, Dias VR, Almeida JA, Brazil JM, Santos RA, Milagres MP. Effect of heavy consumption of alcoholic beverages on the perception of sweet and salty taste. Alcohol Alcohol 51: 302–306, 2016. doi: 10.1093/alcalc/agv116. [DOI] [PubMed] [Google Scholar]
- 288. Pertwee RG. Endocannabinoids and their pharmacological actions. Handb Exp Pharmacol 231: 1–37, 2015. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- 289. Jarrett MM, Limebeer CL, Parker LA. Effect of Delta9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav 86: 475–479, 2005. doi: 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 290. Mattes RD, Engelman K, Shaw LM, Elsohly MA. Cannabinoids and appetite stimulation. Pharmacol Biochem Behav 49: 187–195, 1994. doi: 10.1016/0091-3057(94)90475-8. [DOI] [PubMed] [Google Scholar]
- 291. Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K, Sanematsu K, Shigemura N, Yamamoto T, Margolskee RF, Ninomiya Y. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci U S A 107: 935–939, 2010. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 145: 839–847, 2004. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 293. Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A 97: 11044–11049, 2000. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Tahtali A, Hey C, Geissler C, Filman N, Diensthuber M, Leinung M, Stöver T, Wagenblast J. HPV status and overall survival of patients with oropharyngeal squamous cell carcinoma–a retrospective study of a German head and neck cancer center. Anticancer Res 33: 3481–3485, 2013. [PubMed] [Google Scholar]
- 295. Alfaro R, Crowder S, Sarma KP, Arthur AE, Pepino MY. Taste and smell function in head and neck cancer survivors. Chem Senses 46: bjab026, 2021. doi: 10.1093/chemse/bjab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Bogdanov V, Reinhard J, McGlone F, Haehner A, Simons CT, Hummel T. Oral somatosensory sensitivity in patients with taste disturbance. Laryngoscope 131: 2572–2577, 2021. doi: 10.1002/lary.29843. [DOI] [PubMed] [Google Scholar]
- 297. Welge-Lüssen A, Dörig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol 258: 386–392, 2011. doi: 10.1007/s00415-010-5763-5. [DOI] [PubMed] [Google Scholar]
- 298. Payne T, Kronenbuerger M, Wong G. Gustatory testing. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 299. Landis BN, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T. “Taste Strips”–a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol 256: 242–248, 2009. doi: 10.1007/s00415-009-0088-y. [DOI] [PubMed] [Google Scholar]
- 300. Schuster B, Iannilli E, Gudziol V, Landis BN. Gustatory testing for clinicians. B-ENT 5: 109–113, 2009. [PubMed] [Google Scholar]
- 301. Tomita H, Ikeda M. Clinical use of electrogustometry: strengths and limitations. Acta Otolaryngol 122: 27–38, 2002. doi: 10.1080/00016480260046391. [DOI] [PubMed] [Google Scholar]
- 302. Cao AC, Nimmo ZM, Mirza N, Cohen NA, Brody RM, Doty RL. Objective screening for olfactory and gustatory dysfunction during the COVID-19 pandemic: a prospective study in healthcare workers using self-administered testing. World J Otorhinolaryngol Head Neck Surg 8: 249–256, 2021. doi: 10.1016/j.wjorl.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Marie I, Proux A, Levesque H, Bony-Rerolle S, Chenal P. Tongue involvement revealing sarcoidosis. QJM 101: 909–911, 2008. doi: 10.1093/qjmed/hcn117. [DOI] [PubMed] [Google Scholar]
- 304. Nagata Y, Kanekura T, Kawabata H, Shimomai K, Higashi Y, Setoyama M, Kanzaki T. A case of sarcoidosis involving the tongue. J Dermatol 26: 666–670, 1999. doi: 10.1111/j.1346-8138.1999.tb02069.x. [DOI] [PubMed] [Google Scholar]
- 305. Mendelsohn S, Field E, Woolgar J. Sarcoidosis of the tongue. Clin Exp Dermatol 17: 47–48, 1992. doi: 10.1111/j.1365-2230.1992.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 306. Tillman HH, Taylor RG, Carchidi JE. Sarcoidosis of the tongue. Plastic Reconstruct Surg 38: 278, 1966. [DOI] [PubMed] [Google Scholar]
- 307. Van Maarsseveen AC, Van der Waal I, Stam J, Veldhuizen RW, Van der Kwast WA. Oral involvement in sarcoidosis. Int J Oral Surg 11: 21–29, 1982. doi: 10.1016/S0300-9785(82)80044-6. [DOI] [PubMed] [Google Scholar]
- 308. Macleod RI, Snow MH, Hawkesford JE. Sarcoidosis of the tongue–a case report. Br J Oral Maxillofac Surg 23: 243–246, 1985. doi: 10.1016/0266-4356(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 309. Vivino FB, Bunya VY, Massaro-Giordano G, Johr CR, Giattino SL, Schorpion A, Shafer B, Peck A, Sivils K, Rasmussen A, Chiorini JA, He J, Ambrus JL Jr.. Sjogren’s syndrome: an update on disease pathogenesis, clinical manifestations and treatment. Clin Immunol 203: 81–121, 2019. doi: 10.1016/j.clim.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 310. Doyle ME, Boggs L, Attia R, Cooper LR, Saban DR, Nguyen CQ, Peck AB. Autoimmune dacryoadenitis of NOD/LtJ mice and its subsequent effects on tear protein composition. Am J Pathol 171: 1224–1236, 2007. doi: 10.2353/ajpath.2007.070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Fauchais AL, Magy L, Vidal E. Central and peripheral neurological complications of primary Sjögren's syndrome. Presse Med 41: e485–e493, 2012. doi: 10.1016/j.lpm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 312. Stewart CM, Berg KM, Cha S, Reeves WH. Salivary dysfunction and quality of life in Sjögren syndrome: a critical oral-systemic connection. J Am Dent Assoc 139: 291–299, 2008. doi: 10.14219/jada.archive.2008.0158. [DOI] [PubMed] [Google Scholar]
- 313. Al-Ezzi M, Khan K, Tappuni AR. Is the taste acuity affected by oral dryness in primary Sjögren's syndrome patients? Oral Dis 26: 688–695, 2020. doi: 10.1111/odi.13259. [DOI] [PubMed] [Google Scholar]
- 314. Haugen J, Müller ML, Kotagal V, Albin RL, Koeppe RA, Scott PJ, Frey KA, Bohnen NI. Prevalence of impaired odor identification in Parkinson disease with imaging evidence of nigrostriatal denervation. J Neural Transm (Vienna) 123: 421–424, 2016. doi: 10.1007/s00702-016-1524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. De Rosa A, Nettore IC, Cantone E, Maione L, Desiderio S, Peluso S, Saccà F, Manganelli F, Bruzzese D, Colao A, De Michele G, Macchia PE. The flavor test is a sensitive tool in identifying the flavor sensorineural dysfunction in Parkinson's disease. Neurol Sci 40: 1351–1356, 2019. doi: 10.1007/s10072-019-03842-2. [DOI] [PubMed] [Google Scholar]
- 316. Ricatti MJ, Ottaviani S, Boschi F, Fasano A, Tinazzi M, Cecchini MP. A prospective evaluation of taste in Parkinson’s disease. J Neural Transm (Vienna) 124: 347–352, 2017. doi: 10.1007/s00702-016-1638-y. [DOI] [PubMed] [Google Scholar]
- 317. Cecchini MP, Federico A, Zanini A, Mantovani E, Masala C, Tinazzi M, Tamburin S. Olfaction and taste in Parkinson's disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J Neural Transm (Vienna) 126: 585–595, 2019. doi: 10.1007/s00702-019-01996-z. [DOI] [PubMed] [Google Scholar]
- 318. Cossu G, Melis M, Sarchioto M, Melis M, Melis M, Morelli M, Tomassini Barbarossa I. 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson's disease. Mov Disord 33: 1331–1339, 2018. doi: 10.1002/mds.27391. [DOI] [PubMed] [Google Scholar]
- 319. Doty RL, Hawkes CH. Chapter 20–Chemosensory dysfunction in neurodegenerative diseases. In: Handbook of Clinical Neurology, edited by Doty RL. Amsterdam, The Netherlands: Elsevier, 2019, p. 325–360. [DOI] [PubMed] [Google Scholar]
- 320. Doty RL, Tourbier IA, Pham DL, Cuzzocreo JL, Udupa JK, Karacali B, Beals E, Fabius L, Leon-Sarmiento FE, Moonis G, Kim T, Mihama T, Geckle RJ, Yousem DM. Taste dysfunction in multiple sclerosis. J Neurol 263: 677–688, 2016. doi: 10.1007/s00415-016-8030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Cutillo G, Saariaho AH, Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol 17: 313–322, 2020. doi: 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Paparounas K. Anti-GQ1b ganglioside antibody in peripheral nervous system disorders: pathophysiologic role and clinical relevance. Arch Neurol 61: 1013–1016, 2004. doi: 10.1001/archneur.61.7.1013. [DOI] [PubMed] [Google Scholar]
- 323. Winer JB, Hughes RA, Osmond C. A prospective study of acute idiopathic neuropathy. I. Clinical features and their prognostic value. J Neurol Neurosurg Psychiatry 51: 605–612, 1988. doi: 10.1136/jnnp.51.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Odaka M, Yuki N, Nishimoto Y, Hirata K. Guillain-Barré syndrome presenting with loss of taste. Neurology 58: 1437–1438, 2002. doi: 10.1212/wnl.58.9.1437. [DOI] [PubMed] [Google Scholar]
- 325. Ohe Y, Shintani D, Kato Y, Tanahashi N. Fisher syndrome with taste impairment. Intern Med 51: 2977–2979, 2012. doi: 10.2169/internalmedicine.51.7769. [DOI] [PubMed] [Google Scholar]
- 326. Nishijima H, Tomiyama M, Suzuki C, Kon T, Funamizu Y, Ueno T, Haga R, Miki Y, Arai A, Baba M, Kimura T. Taste impairment in Guillain-Barré syndrome: more frequent than thought? J Peripher Nerv Syst 16: 270–271, 2011. doi: 10.1111/j.1529-8027.2011.00347.x. [DOI] [PubMed] [Google Scholar]
- 327. Ueno T, Kimura R, Kon T, Haga R, Nishijima H, Nunomura JI, Tomiyama M. The differential diagnosis of acute onset truncal ataxia: the importance of dysgeusia in Miller Fisher syndrome. Intern Med 57: 2057–2060, 2018. doi: 10.2169/internalmedicine.0313-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: clinical features. J Orofac Pain 13: 172–184, 1999. [PubMed] [Google Scholar]
- 329. Imamura Y, Shinozaki T, Okada-Ogawa A, Noma N, Shinoda M, Iwata K, Wada A, Abe O, Wang K, Svensson P. An updated review on pathophysiology and management of burning mouth syndrome with endocrinological, psychological and neuropathic perspectives. J Oral Rehabil 46: 574–587, 2019. doi: 10.1111/joor.12795. [DOI] [PubMed] [Google Scholar]
- 330. Kolkka-Palomaa M, Jääskeläinen SK, Laine MA, Teerijoki-Oksa T, Sandell M, Forssell H. Pathophysiology of primary burning mouth syndrome with special focus on taste dysfunction: a review. Oral Dis 21: 937–948, 2015. doi: 10.1111/odi.12345. [DOI] [PubMed] [Google Scholar]
- 331. Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A, Sapelli P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 115: 332–337, 2005. doi: 10.1016/j.pain.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 332. Yilmaz Z, Renton T, Yiangou Y, Zakrzewska J, Chessell IP, Bountra C, Anand P. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci 14: 864–871, 2007. doi: 10.1016/j.jocn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 333. Seol SH, Chung G. Estrogen-dependent regulation of transient receptor potential vanilloid 1 (TRPV1) and P2X purinoceptor 3 (P2X3): Implication in burning mouth syndrome. J Dent Sci 17: 8–13, 2022. doi: 10.1016/j.jds.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Gillmore JD, Hawkins PN. Pathophysiology and treatment of systemic amyloidosis. Nat Rev Nephrol 9: 574–586, 2013. doi: 10.1038/nrneph.2013.171. [DOI] [PubMed] [Google Scholar]
- 335. Picken MM, Herrera GA, Dogan A. Amyloid and Related Disorders: Surgical Pathology and Clinical Correlations. Totowa, NJ: Humana Press, 2015. [Google Scholar]
- 336. Xavier SD, Bussoloti IF, Müller H. Macroglossia secondary to systemic amyloidosis: case report and literature review. Ear Nose Throat J 84: 358–361, 2005. doi: 10.1177/014556130508400615. [DOI] [PubMed] [Google Scholar]
- 337. Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases the thyroid. Endocr Rev 10: 366–391, 1989. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 338. McConnell RJ, Menendez CE, Smith FR, Henkin RI, Rivlin RS. Defects of taste and smell in patients with hypothyroidism. Am J Med 59: 354–364, 1975. doi: 10.1016/0002-9343(75)90394-0. [DOI] [PubMed] [Google Scholar]
- 339. Baskoy K, Ay SA, Altundag A, Kurt O, Salihoglu M, Deniz F, Tekeli H, Yonem A, Hummel T. Is there any effect on smell and taste functions with levothyroxine treatment in subclinical hypothyroidism? PLoS One 11: e0149979, 2016. doi: 10.1371/journal.pone.0149979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Melville JC, Menegotto KD, Woernley TC, Maida BD, Alava I 3rd.. Unusual case of a massive macroglossia secondary to myxedema: a case report and literature review. J Oral Maxillofac Surg 76: 119–127, 2018. doi: 10.1016/j.joms.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 341. Smith CB, Waite PD. Surgical management of obstructive sleep apnea in acromegaly with mandibular prognathism and macroglossia: a treatment dilemma. J Oral Maxillofac Surg 70: 207–210, 2012. doi: 10.1016/j.joms.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 342. Ozdener MH, Rawson NE. Primary culture of mammalian taste epithelium. Methods Mol Biol 945: 95–107, 2013. doi: 10.1007/978-1-62703-125-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Ozdener H, Spielman AI, Rawson NE. Isolation and culture of human fungiform taste papillae cells. J Vis Exp 63: e3730, 2012. doi: 10.3791/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Ozdener MH, Brand JG, Spielman AI, Lischka FW, Teeter JH, Breslin PA, Rawson NE. Characterization of human fungiform papillae cells in culture. Chem Senses 36: 601–612, 2011. doi: 10.1093/chemse/bjr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Murtaza B, Hichami A, Khan AS, Shimpukade B, Ulven T, Ozdener MH, Khan NA. Novel GPR120 agonist TUG891 modulates fat taste perception and preference and activates tongue-brain-gut axis in mice. J Lipid Res 61: 133–142, 2020. doi: 10.1194/jlr.RA119000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Subramaniam S, Ozdener MH, Abdoul-Azize S, Saito K, Malik B, Maquart G, Hashimoto T, Marambaud P, Aribi M, Tordoff MG, Besnard P, Khan NA. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans. Faseb J 30: 3489–3500, 2016. doi: 10.1096/fj.201600422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Schindler A, Dunkel A, Stähler F, Backes M, Ley J, Meyerhof W, Hofmann T. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J Agric Food Chem 59: 12578–12588, 2011. doi: 10.1021/jf2041593. [DOI] [PubMed] [Google Scholar]
- 348. Xu JJ, Elkaddi N, Garcia-Blanco A, Spielman AI, Bachmanov AA, Chung HY, Ozdener MH. Arginyl dipeptides increase the frequency of NaCl-elicited responses via epithelial sodium channel alpha and delta subunits in cultured human fungiform taste papillae cells. Sci Rep 7: 7483, 2017. doi: 10.1038/s41598-017-07756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol 505: 302–313, 2007. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- 350. Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci 28: 13088–13093, 2008. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Qian J, Mummalaneni S, Larsen J, Grider JR, Spielman AI, Özdener MH, Lyall V. Nicotinic acetylcholine receptor (CHRN) expression and function in cultured human adult fungiform (HBO) taste cells. PLoS One 13: e0194089, 2018. doi: 10.1371/journal.pone.0194089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Prescott J, Johnstone V, Francis J. Odor-taste interactions: effects of attentional strategies during exposure. Chem Senses 29: 331–340, 2004. doi: 10.1093/chemse/bjh036. [DOI] [PubMed] [Google Scholar]
- 353. Dalton P, Doolittle N, Nagata H, Breslin PA. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci 3: 431–432, 2000. doi: 10.1038/74797. [DOI] [PubMed] [Google Scholar]
- 354. Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev 62: S184–188, 2004. doi: 10.1111/j.1753-4887.2004.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 355. Malik B, Elkaddi N, Turkistani J, Spielman AI, Ozdener MH. Mammalian taste cells express functional olfactory receptors. Chem Senses 44: 289–301, 2019. doi: 10.1093/chemse/bjz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Abaffy T, Trubey KR, Chaudhari N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol Cell Physiol 284: C1420–C1428, 2003. doi: 10.1152/ajpcell.00556.2002. [DOI] [PubMed] [Google Scholar]
- 357. Trubey KR, Culpepper S, Maruyama Y, Kinnamon SC, Chaudhari N. Tastants evoke cAMP signal in taste buds that is independent of calcium signaling. Am J Physiol Cell Physiol 291: C237–C244, 2006. doi: 10.1152/ajpcell.00303.2005. [DOI] [PubMed] [Google Scholar]
- 358. von Molitor E, Nürnberg E, Ertongur-Fauth T, Scholz P, Riedel K, Hafner M, Rudolf R, Cesetti T. Analysis of calcium signaling in live human tongue cell 3D-cultures upon tastant perfusion. Cell Calcium 87: 102164, 2020. doi: 10.1016/j.ceca.2020.102164. [DOI] [PubMed] [Google Scholar]
- 359. Shechtman LA, Piarowski CM, Scott JK, Golden EJ, Gaillard D, Barlow LA. Generation and culture of lingual organoids derived from adult mouse taste stem cells. J Vis Exp 17: 10.3791/62300, 2021. doi: 10.3791/62300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 190: 285–296, 2010. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Aihara E, Mahe MM, Schumacher MA, Matthis AL, Feng R, Ren W, Noah TK, Matsu-Ura T, Moore SR, Hong CI, Zavros Y, Herness S, Shroyer NF, Iwatsuki K, Jiang P, Helmrath MA, Montrose MH. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci Rep 5: 17185, 2015.doi: 10.1038/srep17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Ren W, Aihara E, Lei W, Gheewala N, Uchiyama H, Margolskee RF, Iwatsuki K, Jiang P. Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci Rep 7: 4004, 2017. doi: 10.1038/s41598-017-04099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472: 51–56, 2011. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 364. Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet 19: 671–687, 2018. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 365. Lu WJ, Mann RK, Nguyen A, Bi T, Silverstein M, Tang JY, Chen X, Beachy PA. Neuronal delivery of Hedgehog directs spatial patterning of taste organ regeneration. Proc Natl Acad Sci U S A 115: E200–E209, 2018. doi: 10.1073/pnas.1719109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Ren W, Liu Q, Zhang X, Yu Y. Age-related taste cell generation in circumvallate papillae organoids via regulation of multiple signaling pathways. Exp Cell Res 394: 112150, 2020. doi: 10.1016/j.yexcr.2020.112150. [DOI] [PubMed] [Google Scholar]
- 367. Co JY, Margalef-Català M, Monack DM, Amieva MR. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat Protoc 16: 5171–5192, 2021. doi: 10.1038/s41596-021-00607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Adpaikar AA, Zhang S, Kim HY, Kim KW, Moon SJ, Lee JM, Jung HS. Fine-tuning of epithelial taste bud organoid to promote functional recapitulation of taste reactivity. Cell Mol Life Sci 79, 2022. doi: 10.1007/s00018-022-04242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Fjaeldstad AW. Prolonged complaints of chemosensory loss after COVID-19. Dan Med J 67: A05200340, 2020. [PubMed] [Google Scholar]
- 370. Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, Lin C, Joseph PV, Reed DR. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chem Senses 47: bjac001, 2022. doi: 10.1093/chemse/bjac001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 371. Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, Albers MW, Barlow LA, Datta SR, Di Pizio A. COVID-19 and the chemical senses: supporting players take center stage. Neuron 107: 219–233, 2020. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Le Bon SD, Payen L, Prunier L, Steffens Y, Horoi M, Vaira LA, Hopkins C, Lechien JR, Saussez S. Making scents of loss of taste in COVID-19: Is self-reported loss of taste due to olfactory dysfunction? A prospective study using psychophysical testing. Int Forum Allergy Rhinol 11: 1504–1507, 2021. doi: 10.1002/alr.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Vitale-Cross L, Szalayova I, Scoggins A, Palkovits M, Mezey E. SARS-CoV-2 entry sites are present in all structural elements of the human glossopharyngeal and vagal nerves: clinical implications. EBioMedicine 78: 103981, 2022. doi: 10.1016/j.ebiom.2022.103981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JL, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604: 697–707, 2022. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Correa Marrero M, et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell 182: 685–712.e19, 2020. doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Alves VS, Leite-Aguiar R, Silva JP, Coutinho-Silva R, Savio LE. Purinergic signaling in infectious diseases of the central nervous system. Brain Behav Immun 89: 480–490, 2020. doi: 10.1016/j.bbi.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html [2022].
- 378. Tan BK, Han R, Zhao JJ, Tan NK, Quah ES, Tan CJ, Chan YH, Teo NW, Charn TC, See A, Xu S, Chapurin N, Chandra RK, Chowdhury N, Butowt R, von Bartheld CS, Kumar BN, Hopkins C, Toh ST. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ 378: e069503, 2022. doi: 10.1136/bmj-2021-069503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12: 698169, 2021. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Locatello LG, Bruno C, Maggiore G, Cilona M, Orlando P, Fancello G, Piccica M, Vellere I, Lagi F, Trotta M, Gallo O. The prognostic role of IL-10 in non-severe COVID-19 with chemosensory dysfunction. Cytokine 141: 155456, 2021. doi: 10.1016/j.cyto.2021.155456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Paul P, France AM, Aoki Y, Batra D, Biggerstaff M, Dugan V, Galloway S, Hall AJ, Johansson MA, Kondor RJ, Halpin AL, Lee B, Lee JS, Limbago B, MacNeil A, MacCannell D, Paden CR, Queen K, Reese HE, Retchless AC, Slayton RB, Steele M, Tong S, Walters MS, Wentworth DE, and Silk BJ. Genomic surveillance for SARS-CoV-2 variants circulating in the United States, December 2020–May 2021. MMWR Morb Mortal Wkly Rep 70: 846–850, 2021. doi: 10.15585/mmwr.mm7023a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Coelho DH, Reiter ER, French E, Costanzo RM. Decreasing incidence of chemosensory changes by COVID-19 variant. Otolaryngol Head Neck Surg 019459982210976, 2022. doi: 10.1177/01945998221097656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Katella K. Omicron, delta, alpha, and more: what to know about the coronavirus variants. Yale Medicine. https://www.yalemedicine.org/news/covid-19-variants-of-concern-omicron [2022 Sept]. [Google Scholar]
- 384. Henkin RI, Larson AL, Powell RD. Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann Otol Rhinol Laryngol 84: 672–682, 1975. doi: 10.1177/000348947508400519. [DOI] [PubMed] [Google Scholar]
- 385. Schiffman SS. Taste and smell in disease. N Engl J Med 308: 1337–1343, 1983. doi: 10.1056/NEJM198306023082207. [DOI] [PubMed] [Google Scholar]
- 386. Li Y, Cui J, Liu Y, Chen K, Huang L, Oral LY. Tongue-coating microbiota, and metabolic disorders: a novel area of interactive research. Front Cardiovasc Med 8: 730203, 2021. doi: 10.3389/fcvm.2021.730203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut 68: 1108–1114, 2019. doi: 10.1136/gutjnl-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Utzschneider KM, Kratz M, Damman CJ, Hullar M. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab 101: 1445–1454, 2016. doi: 10.1210/jc.2015-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens 24: 403–409, 2015. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Hasan RA, Koh AY, Zia A. The gut microbiome and thromboembolism. Thromb Res 189: 77–87, 2020. doi: 10.1016/j.thromres.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res 63: 1–9, 2015. doi: 10.1016/j.jpsychires.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 392. Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S, Colao A, on behalf of the Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Gut microbiota: a new path to treat obesity. Int J Obes Suppl 9: 10–19, 2019. doi: 10.1038/s41367-019-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E. The oral microbiome–an update for oral healthcare professionals. Br Dent J 221: 657–666, 2016. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 394. Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A 113: E791–E800, 2016. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput Struct Biotechnol J 19: 1335–1360, 2021. doi: 10.1016/j.csbj.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Xu X, He J, Xue J, Wang Y, Li K, Zhang K, Guo Q, Liu X, Zhou Y, Cheng L, Li M, Li Y, Li Y, Shi W, Zhou X. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol 17: 699–710, 2015. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 397. Wilbert SA, Mark Welch JL, Borisy GG. Spatial ecology of the human tongue dorsum microbiome. Cell Rep 30: 4003–4015.e3, 2020. doi: 10.1016/j.celrep.2020.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Jiang B, Liang X, Chen Y, Ma T, Liu L, Li J, Jiang R, Chen T, Zhang X, Li S. Integrating next-generation sequencing and traditional tongue diagnosis to determine tongue coating microbiome. Sci Rep 2: 936, 2012. doi: 10.1038/srep00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Sun B, Zhou D, Tu J, Lu Z. Evaluation of the bacterial diversity in the human tongue coating based on genus-specific primers for 16S rRNA sequencing. BioMed Res Int 2017: 1–12, 2017. doi: 10.1155/2017/8184160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Besnard P, Christensen JE, Bernard A, Simoneau-Robin I, Collet X, Verges B, Burcelin R. Identification of an oral microbiota signature associated with an impaired orosensory perception of lipids in insulin-resistant patients. Acta Diabetol 57: 1445–1451, 2020. doi: 10.1007/s00592-020-01567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Cattaneo C, Gargari G, Koirala R, Laureati M, Riso P, Guglielmetti S, Pagliarini E. New insights into the relationship between taste perception and oral microbiota composition. Sci Rep 9: 3549, 2019. doi: 10.1038/s41598-019-40374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Besnard P, Christensen JE, Brignot H, Bernard A, Passilly-Degrace P, Nicklaus S, Pais De Barros JP, Collet X, Lelouvier B, Servant F, Blasco-Baque V, Verges B, Lagrost L, Feron G, Burcelin R. Obese subjects with specific gustatory papillae microbiota and salivary cues display an impairment to sense lipids. Sci Rep 8: 6742, 2018. doi: 10.1038/s41598-018-24619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Luo C, Xie S, Sun W, Li X, Cupples AM. Identification of a novel toluene-degrading bacterium from the candidate phylum TM7, as determined by DNA stable isotope probing. Appl Environ Microbiol 75: 4644–4647, 2009. doi: 10.1128/AEM.00283-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Figueroa-Gonzalez PA, Bornemann TL, Adam PS, Plewka J, Revesz F, von Hagen CA, Tancsics A, Probst AJ. Saccharibacteria as organic carbon sinks in hydrocarbon-fueled communities. Front Microbiol 11: 587782, 2020. doi: 10.3389/fmicb.2020.587782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Seerangaiyan K, van Winkelhoff AJ, Harmsen HJM, Rossen JWA, Winkel EG. The tongue microbiome in healthy subjects and patients with intra-oral halitosis. J Breath Res 11: 036010, 2017. doi: 10.1088/1752-7163/aa7c24. [DOI] [PubMed] [Google Scholar]
- 406. Suzuki N, Nakano Y, Yoneda M, Hirofuji T, Hanioka T. The effects of cigarette smoking on the salivary and tongue microbiome. Clin Exp Dent Res 8: 449–456, 2022. doi: 10.1002/cre2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Cui J, Cui H, Yang M, Du S, Li J, Li Y, Liu L, Zhang X, Li S. Tongue coating microbiome as a potential biomarker for gastritis including precancerous cascade. Protein Cell 10: 496–509, 2019. doi: 10.1007/s13238-018-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Haran JP, Bradley E, Zeamer AL, Cincotta L, Salive MC, Dutta P, Mutaawe S, Anya O, Meza-Segura M, Moormann AM, Ward DV, McCormick BA, Bucci V. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight 6, 2021. doi: 10.1172/jci.insight.152346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Barragán R, Coltell O, Portolés O, Asensio EM, Sorlí JV, Ortega-Azorín C, González JI, Sáiz C, Fernández-Carrión R, Ordovas JM, Corella D. Bitter, sweet, salty, sour and umami taste perception decreases with age: sex-specific analysis, modulation by genetic variants and taste-preference associations in 18 to 80 year-old subjects. Nutrients 10: 1539, 2018. doi: 10.3390/nu10101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Gervis JE, Fernández-Carrión R, Chui KK, Ma J, Coltell O, Sorli JV, Asensio EM, Ortega-Azorín C, Pérez-Fidalgo JA, Portolés O, Lichtenstein AH, Corella D. associations between taste perception profiles and empirically derived dietary patterns: an exploratory analysis among older adults with metabolic syndrome. Nutrients 14: 142, 2021. doi: 10.3390/nu14010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein R, Pankratz N, Pankow JS, Huang GH. Factors related to fungiform papillae density: the beaver dam offspring study. Chem Senses 38: 669–677, 2013. doi: 10.1093/chemse/bjt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein BE, Klein R, Nieto FJ, Pankow JS, Huang GH, Snyder DJ. Taste intensity in the Beaver Dam Offspring Study. Laryngoscope 123: 1399–1404, 2013. doi: 10.1002/lary.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Hansen JL, Reed DR, Wright MJ, Martin NG, Breslin PA. Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem Senses 31: 403–413, 2006. doi: 10.1093/chemse/bjj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Karikkineth AC, Tang EY, Kuo PL, Ferrucci L, Egan JM, Chia CW. Longitudinal trajectories and determinants of human fungiform papillae density. Aging (Albany NY) 13: 24989–25003, 2021. doi: 10.18632/aging.203741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Bangcuyo RG, Simons CT. Lingual tactile sensitivity: effect of age group, sex, and fungiform papillae density. Exp Brain Res 235: 2679–2688, 2017. doi: 10.1007/s00221-017-5003-7. [DOI] [PubMed] [Google Scholar]
- 416. Brill N, Tryde G, Edwards C, Thomas H. Age changes in the two-point discrimination threshold in human oral mucosa. J Oral Rehabil 1: 323–333, 1974. doi: 10.1111/j.1365-2842.1974.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 417. Teranaka S, Shibaji T, Minakuchi S, Uematsu H. Age-related changes in oral mechanosensitivity of symptom-free subjects. J Med Dent Sci 55: 61–69, 2008. [PubMed] [Google Scholar]
- 418. Fucci D, Petrosino L. Lingual vibrotactile sensation magnitudes: comparison of suprathreshold responses for three different age ranges. Percept Mot Skills 57: 31–38, 1983. doi: 10.2466/pms.1983.57.1.31. [DOI] [PubMed] [Google Scholar]
- 419. Imoscopi A, Inelmen EM, Sergi G, Miotto F, Manzato E. Taste loss in the elderly: epidemiology, causes and consequences. Aging Clin Exp Res 24: 570–579, 2012. doi: 10.3275/8520. [DOI] [PubMed] [Google Scholar]