Abstract
Purpose
The Utrecht Cardiovascular Cohort–Second Manifestations of Arterial Disease (UCC-SMART) Study is an ongoing prospective single-centre cohort study with the aim to assess important determinants and the prognosis of cardiovascular disease progression. This article provides an update of the rationale, design, included patients, measurements and findings from the start in 1996 to date.
Participants
The UCC-SMART Study includes patients aged 18–90 years referred to the University Medical Center Utrecht, the Netherlands, for management of cardiovascular disease (CVD) or severe cardiovascular risk factors. Since September 1996, a total of 14 830 patients have been included. Upon inclusion, patients undergo a standardised screening programme, including questionnaires, vital signs, laboratory measurements, an ECG, vascular ultrasound of carotid arteries and aorta, ankle-brachial index and ultrasound measurements of adipose tissue, kidney size and intima–media thickness. Outcomes of interest are collected through annual questionnaires and adjudicated by an endpoint committee.
Findings to date
By May 2022, the included patients contributed to a total follow-up time of over 134 000 person-years. During follow-up, 2259 patients suffered a vascular endpoint (including non-fatal myocardial infarction, non-fatal stroke and vascular death) and 2794 all-cause deaths, 943 incident cases of diabetes and 2139 incident cases of cancer were observed up until January 2020. The UCC-SMART cohort contributed to over 350 articles published in peer-reviewed journals, including prediction models recommended by the 2021 European Society of Cardiology CVD prevention guidelines.
Future plans
The UCC-SMART Study guarantees an infrastructure for research in patients at high cardiovascular risk. The cohort will continue to include about 600 patients yearly and follow-up will be ongoing to ensure an up-to-date cohort in accordance with current healthcare and scientific knowledge. In the near future, UCC-SMART will be enriched by echocardiography, and a food frequency questionnaire at baseline enabling the assessment of associations between nutrition and CVD and diabetes.
Keywords: vascular medicine, epidemiology, preventive medicine, general diabetes
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The Utrecht Cardiovascular Cohort–Second Manifestations of Arterial disease (UCC-SMART) Study is an ongoing cohort of almost 15 000 patients with various manifestations of cardiovascular disease and cardiovascular risk factors.
The UCC-SMART Study covers long follow-up duration and prospectively captures extensive outcome data in a high cardiovascular risk population.
The use of a standardised screening programme that includes baseline characteristics, physical examination, laboratory testing and non-invasive imaging provides an extended resource of data for research on cardiovascular disease epidemiology.
Limitations of the cohort include measurement of the determinants only at baseline for the majority of patients, and the sparse information on socioeconomic status.
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide, causing around one-third of all deaths globally in 2019.1 Atherosclerosis, the dominant cause of CVD, is fuelled by multiple mutually reinforcing and coexisting risk factors. Because of the progressive nature of atherosclerosis, patients with established CVD are at high risk of recurrent CVD and mortality.2 3 Treatment of cardiovascular risk factors is known to markedly reduce the risk of new cardiovascular events.4 5 Slowing down the process of atherosclerosis by timely identification and treatment of cardiovascular risk factors is therefore of utmost importance.
In 1996, the Second Manifestations of Arterial Disease (SMART) cohort study was set up enrolling patients newly referred to the University Medical Center (UMC) Utrecht with clinically manifest CVD or marked risk factors for atherosclerosis. The study was designed with the aim of determining the prevalence of concomitant atherosclerotic disease and risk factors, as well as studying the incidence of future cardiovascular events and its predictors. Furthermore, the SMART Study contributes to the complete and protocolised multidisciplinary care of these high-risk patients by integrating a standardised set of measurements into usual patient care. The rationale and design of the study were previously published in 1999,6 with the study containing around 600 patients at that time. In 2018, the name of SMART was changed to Utrecht Cardiovascular Cohort (UCC)-SMART. By now, 26 years after enrolment of the first patient, many baseline measurements have been added, substudies have been initiated, the study has been linked to national registries and the data have been used in several large (inter)national collaborations. At the same time, demographic and guideline changes have led to differences in the baseline characteristics and absolute risk of the patients included in the cohort. The aim of the current article is to provide an update on the rationale, design, included patients, baseline measurements and follow-up to date.
Cohort description
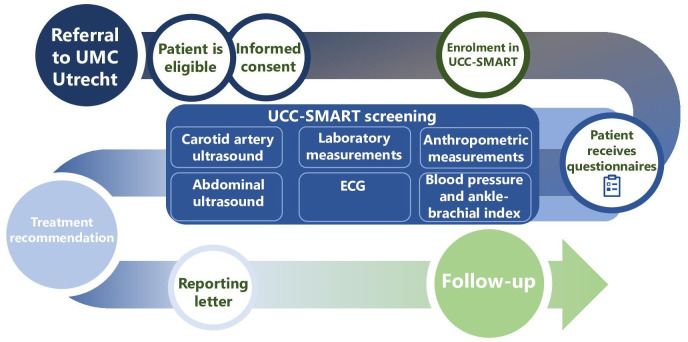
The UCC-SMART Study is a single-centre prospective cohort study, ongoing in both inclusion and follow-up, in which patient care and scientific research concerning cardiovascular risk factors and disease are integrated. This is depicted in figure 1 and discussed in more detail in the sections below.
Figure 1.
Course of the UCC-SMART Study. UCC-SMART, Utrecht Cardiovascular Cohort–Second Manifestations of Arterial Disease; UMC Utrecht, University Medical Center Utrecht.
Study population
Starting from September 1996, patients aged 18–80 years referred to the UMC Utrecht, the Netherlands, for management of CVD or severe risk factors for CVD, have been recruited. Patients with cerebrovascular disease (CeVD), coronary artery disease (CAD), abdominal aortic aneurysm (AAA), peripheral artery disease (PAD), renal artery stenosis or one or more of the following cardiovascular risk factors, if rated as severe, are eligible to be included: hypertension, hyperlipidaemia, diabetes mellitus, renal insufficiency and a positive family medical history. Patients with a chronic HIV infection as a cardiovascular risk-increasing condition or with hypertensive pregnancy disorders have been included since 2007 and 2012, respectively. Definitions of the inclusion criteria are listed in online supplemental table 1. If patients have a history of multiple vascular events or risk factors, the referral reason (usually the most recent event) is listed as the qualifying inclusion diagnosis and any comorbidities are also registered. Pregnant women, patients with a short life expectancy and those insufficiently fluent in Dutch are not eligible.
bmjopen-2022-066952supp001.pdf (884.1KB, pdf)
Qualifying patients with CVD and/or risk factors listed above are recruited upon their first visit to the outpatient clinics and hospital wards of the departments of vascular medicine, internal medicine, nephrology, neurology, cardiology, cardiac surgery, obstetrics and vascular surgery. From 2021 onwards, the outpatient clinic of the department of geriatric medicine has been added to this list and the maximum age to be eligible for inclusion has been raised from 80 to 90 years old. In case of a recent cardiovascular event or intervention as the reason for inclusion, patients are invited after discharge from the hospital. In such cases, baseline measurements are generally performed more than 30 days after the acute event. All qualifying patients receive written and oral information about study goals and methods and are included only after written informed consent to use their data for study goals, the reporting of incidental findings to their treating physician, indefinite period storage of blood samples for future research and follow-up through annual questionnaires. In addition, participants can opt in or out to the following items: retrieval of data from regional and national registries, use of their data in research collaborations with for-profit organisations, use of coded data and laboratory samples for research outside the European Union and possible future requests to participate in follow-up studies of UCC-SMART. When patients do not consent to any of these additional items, they can still partake in the UCC-SMART Study.
Baseline data collection
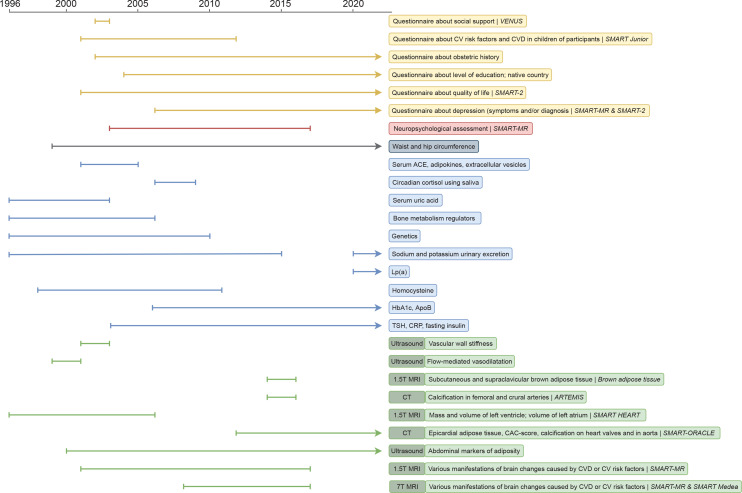
The screening programme consists of questionnaires, physical examination, an ECG, blood, urine and radiology testing. Except for the questionnaires, to be filled out before the hospital visit, the diagnostic components of the programme take place during a 1-day visit. An overview of all the variables available in UCC-SMART is provided in online supplemental table 2. Some measurements have only been collected for or starting from a certain time period (figure 2 and online supplemental table 3).
Figure 2.
Timeline of measurements collected for or starting from a certain period. apoB, apolipoprotein B; CAC, coronary artery calcium; CRP, C reactive protein; CV, cardiovascular; CVD, cardiovascular disease; HbA1c, glycated haemoglobin; Lp(a), lipoprotein a; T, Tesla; TSH, thyroid-stimulating hormone.
Health questionnaires
The questionnaires collect data on medical history including established CVD (CeVD, CAD, AAA and PAD as described in online supplemental table 4), cardiovascular risk factors, symptoms of CVD (based on the Rose Angina Questionnaire7), medication use, family history and lifestyle. For women, a question on the age at menopause (if applicable) is included as well. From 2002 onwards, information on obstetric history has been collected including the number of full-term pregnancies, miscarriages (<14 weeks of gestation), preterm deliveries (14–32 weeks of gestation), birth weight and pregnancy complications. As of August 2022, a 160-item food frequency questionnaire, validated in the Dutch population, has been added to the questionnaires.8 Recently, these questionnaires have also been sent to people who were included in the UCC-SMART Study before August 2022. The results of the questionnaires will follow in 2023.
Physical examination
Anthropometric measurements are taken by trained (research) nurses and include body height in centimetres, weight in kilograms, and waist and hip circumference in centimetres with patients wearing light clothing and no shoes. Weight and length are used to calculate body mass index in kg/m2. Waist circumference is measured horizontally at the midpoint between the iliac crest and lower costal margin, and hip circumference is taken at the maximum horizontal circumference around the gluteal muscles. The mean of two measurements is calculated. If the two measurements differ by >2 cm, a third is taken and the mean of the closest two is calculated.
From 1996 up until 1999, office blood pressure was measured using a semiautomatic oscillometric device (Omega 1400; Invivo Research Laboratories, Broken Arrow, Oklahoma, USA) every 4 min for a total of 25 min at the right brachial artery in supine position, and the mean systolic (SBP) and diastolic blood pressure (DBP) were calculated. From April 1999 until 2015, using a non-random sphygmomanometer (Iso-Stabil 5; Speidel & Keller, Jungingen, Germany), three simultaneous measurements with an interval of 30 s were taken at both upper arms in upright position, and the SBP and DBP of the last two measurements were calculated from the arm yielding the highest values. From 2015 onwards, office blood pressure has been measured using an automatic oscillometric device (Microlife WatchBP Office AFIB; Microlife Corp, Widnau, Switzerland). The measurement is performed unattended, in triplicate with an interval of 30 s, at both upper arms in supine position after the patient has rested for 30 s. The measurements on the arm with the highest blood pressure are recorded and the mean SBP and DBP are calculated.
In order to calculate the ankle-brachial index (ABI), blood pressure measurements are taken at rest at both upper arms every 2 min while the blood pressure is measured at both lower legs. For this, a Falcon Quad 8 MHz Doppler probe (Viasonix, Ra’anana, Israel) is used at a 60° angle at the dorsal pedal and posterior tibial arteries. The ABI is defined for each leg as the highest SBP at the ankle divided by the highest brachial SBP.
Laboratory testing
On the day of screening, a venous blood sample is drawn after at least 8 hours of fasting to measure glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, creatinine and haemoglobin. Laboratory measurements of fasting insulin, C reactive protein (CRP) and thyroid-stimulating hormone (TSH) were added in 2003 and glycated haemoglobin and apolipoprotein B (apoB) were added in 2006. Lastly, measurement of lipoprotein(a) was added in June 2020.
Glucose is measured using an enzymatic colorimetric assay (Beckman Coulter, Brea, California, USA). Total cholesterol and triglycerides are measured using a commercial enzymatic dry chemistry kit (Johnson & Johnson, New Brunswick, New Jersey, USA) and HDL-C with a commercial enzymatic kit (Boehringer, Mannheim, Germany). Low-density lipoprotein cholesterol (LDL-C) is calculated using the Friedewald formula up to a plasma triglyceride level of 9 mmol/L.9 Estimated glomerular filtration rate is calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.10 Spectrophotometry (Abbott Diagnostics, Santa Clara, California, USA) is used to determine haemoglobin levels. CRP in plasma was initially determined using immunonephelometry (Nephelometer Analyzer BN II, Siemens, The Hague, The Netherlands) and from 2013 in heparin plasma on an AU5811 routine chemistry analyser using turbidimetry (Beckman Coulter, Brea, California, USA). These types of measurements are strongly correlated (r=0.99) and can therefore be pooled for analyses.11 Before November 2006, TSH was quantified using a third-generation assay on a Centaur analyser (Bayer, Germany). Since December 2006, TSH has been measured by a third-generation assay on a DXi analyser (Beckman Coulter, Woerden, The Netherlands). Correlation between the two analysers is r=0.9991 (n=69), with an intercept of –0.05 mU/L (95% CI −0.22 to 0.12) and a slope of 1.04 (95% CI 1.029 to 1.052) (range 0–95 mU/L). ApoB and lipoprotein(a) are measured using nephelometry (Atellica Neph 630, Siemens, The Hague, The Netherlands). A morning-void urine sample is collected to determine urine albumin, creatinine, sodium and potassium levels. Urine albumin is measured using immunoturbidimetric assays. Ion selective electrode (Beckman Coulter, Brea, California, USA) is used to determine urine sodium and potassium levels. DNA can be isolated from 10 mL of EDTA-augmented blood stored at −80° for genotyping.
Radiology testing
Non-invasive vascular imaging testing is performed by specially trained ultrasound technicians. Duplex examination of the carotid arteries is conducted to assess possible stenosis using peak systolic velocity measurements at the brachiocephalic trunk, carotid arteries (mid and distal common, external and proximal and distal internal) and vertebral arteries (proximal and distal). Measurements are performed using an EPIQ-7 ultrasound machine (Philips Medical Systems, Eindhoven, The Netherlands). In case of abnormal signals and/or retrograde flow in the vertebral arteries, the proximal subclavian arteries are evaluated in search of severe stenosis or occlusion. For research purposes, intima–media thickness of the carotid arteries is measured using a linear array transducer. With the patient lying down and the head turned 45° away from the side investigated, the ultrasound frame yielding an optimal longitudinal picture of the common carotid arterial wall is frozen at the time of the R-peak of ECG recording. Over a length of 1 cm starting from the carotid bulb towards proximal direction, the arterial wall thickness is measured from the lumen–intima interface to the media–adventitia interface. The mean of measurements in anterolateral, lateral and posterolateral direction is calculated.
Abdominal ultrasound examination is performed using the same ultrasound machine to obtain the maximal anterior–posterior diameter of the juxtarenal and infrarenal abdominal aorta and kidney length and volume. As of January 2000, visceral and subcutaneous adipose tissue measurements were added. The amount of subcutaneous fat is estimated as the distance from the linea alba to the skin. Visceral adipose tissue thickness is measured as the distance between the lumbar spine and the peritoneum. Measurements are taken at the end of a quiet expiration on a frozen ultrasound frame at three points on the imaginary transversal line halfway between the iliac crest and lower costal margin: at the midsternal line and 10 cm to the left and right on the transversal line. Each measurement is taken three times and then the mean of the measurements is recorded as the actual thickness. Ultrasonography has been proven a suitable technique to measure intra-abdominal adipose tissue with good reproducibility.12 13 Moreover, from September 1998 onwards, a protocolised 12-lead resting ECG has been recorded.
In the near future, echocardiography will be added to the UCC-SMART Programme to facilitate research on the presence of heart failure at baseline. Echocardiography will be performed using a Philips Affiniti 70 ultrasound machine (Philips Medical Systems, Andover, Massachusetts, USA) by using a specific protocol involving two-dimensional (2D), M-mode, Doppler, tissue Doppler and 2D speckle-tracking (STE) imaging in accordance with the European Association of Cardiovascular Imaging 2016 recommendations for chamber quantification.14 In particular, left ventricular dimensions will be measured in order to calculate the left ventricular mass index.15 Left ventricular ejection fraction will be assessed quantitatively, preferably with automated three-dimensional imaging or alternatively with the Simpsons biplane method. Left atrial maximal volume and right ventricular dimensions and function will be measured as recommended.14 Multiple parameters of left ventricular diastolic function will be assessed, including pulsed-wave Doppler of the mitral inflow and tissue Doppler imaging of the mitral annulus motion. Left ventricular diastolic function will be evaluated according to current diagnostic algorithms.16 A minimal of three sequential complexes will be recorded. Standard image analysis will then be performed off-line in accordance with clinical guidelines using Philips IntelliSpace Cardiovascular software and will include 2D STE analysis of the left ventricle and left atrium.
Treatment recommendation
After completion of the screening, the findings are assessed by a multidisciplinary team of two medical specialists (internist, cardiologist, neurologist or vascular surgeon). A treatment recommendation is formulated based on current applicable guidelines, according to which patients are already treated by their general practitioner or medical specialist. The screening results and treatment recommendation are reported in a medical letter, which is sent to the treating specialist and general practitioner. Patients receive a summary of relevant findings and recommendations.
Incidental medical findings during the screening are reported to one of the study physicians and if needed, discussed with specialists from the multidisciplinary team. The findings are added to the medical record and sent to the treating specialist or general practitioner for further action.
Follow-up
Patients receive annual questionnaires with questions on hospital admissions and outpatient clinic visits, regardless of whether they are still under the care of the UMC Utrecht. In case patients no longer wish to complete the questionnaires, they are asked if they consent to collection of information from their general practitioner. When the replies indicate possible outcome events, additional information is collected through hospital discharge letters and relevant laboratory and radiology examinations. Clinical events of interest include stroke, myocardial infarction, heart failure, AAA rupture, renal insufficiency, vascular interventions, bleeding, diabetes and vascular and non-vascular mortality as defined in online supplemental table 5. Incident type 2 diabetes has been assessed since July 2006. To assess incident diabetes between 1996 and 2006, a questionnaire was sent to all patients without diabetes at baseline who were included before July 2006. Incident heart failure has been assessed since October 2011. Three members from the endpoint committee independently judge reported events. The endpoint committee consists of medical specialists from the recruiting departments. If all three physicians judge differently, the event is discussed with two other physicians from the committee to reach a consensus. Secondary outcomes are adjudicated by trained research nurses. As of 2021, diagnoses of dementia and mild cognitive impairment have been added to the annual questionnaire as self-reported diagnoses.
Data quality and management
Data collected in the UCC-SMART Programme are stored in the electronic medical record of the UMC Utrecht. Blood samples (serum, citrate plasma, EDTA plasma and erythrocytes concentrate aliquots) are stored at −80°C according to the Biobanks Regulations to be found at the UMC Utrecht website (https://www.umcutrecht.nl/nl/centrale-biobank). The central biobank of the UMC Utrecht is ISO9001 certified (certificate number 2175592). Release of material for future research is reviewed by the UMC Utrecht Biobanks Review Committee.
Recorded data are downloaded from the electronic medical record and pseudonymised by the data manager who holds the encryption key, only to be accessed after permission of the principal investigator. The UCC-SMART Study group periodically performs quality checks for missing values and inconsistencies compared with source documents, or values outside of the range deemed likely.
Patient and public involvement
Patients were not involved in the study design. Their experiences of burden and required time are considered in the implementation of new components in the programme. Relevant findings of the UCC-SMART screening programme and corresponding recommendations are sent to the patients. In addition, patients regularly receive a newsletter containing up-to-date facts and figures of the UCC-SMART Study and substudies and findings of publications using UCC-SMART data. The UMC Utrecht policies are in line with open science, for opening up the research agenda to societal stakeholders, open research data and open-access publications.
Linkage to other registries
Data in the UCC-SMART Study can be enriched by collecting data from various registries and organisations, for example, to obtain additional information on outcomes and medication use. Some examples of these linkages are described below.
Netherlands Cancer Registry
CVD and cancer share many risk factors and pathophysiological mechanisms, including body fat distribution, diet, physical inactivity, smoking, chronic inflammation burden and oxidative stress.17 To evaluate the relation between several cardiovascular risk factors and the risk of cancer, the UCC-SMART cohort has been linked to the Netherlands Comprehensive Cancer Organisation (IKNL), a nationwide registry receiving notifications of all new cancer diagnoses. By linking the cohort to the national cancer registry repeatedly, with the most recent linkage taking place in 2022, information on cancer incidence and details of cancer types and histopathology were obtained.
Central Agency for Statistics Netherlands
The UCC-SMART cohort can be linked to the Central Agency for Statistics (CBS), also known as Statistic Netherlands, which contains data on International Classification of Diseases 10th Revision (ICD-10)-coded diagnoses and hospital admissions since 1996. This allows for, among others, collection of endpoints that are not regularly collected in UCC-SMART or have been collected from a later time point, such as heart failure diagnoses. The CBS collects data from all hospitals in the Netherlands and from general practitioner practices affiliated with ‘Nivel’ healthcare registration, which are a good reflection of the Dutch population.18 19
Utrecht Patient Oriented Database
The UCC-SMART cohort can be linked to Utrecht Patient Oriented Database,20 a database containing electronic patient data from routine clinical care in the UMC Utrecht. This database has been collecting patient characteristics, medication orders, laboratory test results, hospital discharge diagnoses and medical procedures since 2000, enabling the addition of baseline and follow-up information to the UCC-SMART Study.
Consortia
The data collected in UCC-SMART are added to several consortia such as a genetics consortium (GENIUS-CHD21 on genetics of subsequent coronary heart disease), the Netherlands consortium of dementia cohorts and the Chronic Kidney Disease Prognosis Consortium.22
Dutch Foundation for Pharmaceutical Statistics
A future plan is to obtain information on medication use during follow-up by linking the UCC-SMART cohort to the Dutch Foundation for Pharmaceutical Statistics (Stichting Farmaceutische Kengetallen). This foundation obtains data from over 97% of the community pharmacies in the Netherlands.23
Substudies
SMART-2
Patients with a history of CVD or diabetes are invited to participate in the SMART-2 substudy. In this study, the baseline measurements of UCC-SMART are repeated in order to investigate the course of atherosclerosis and vascular risk factors over time, and to evaluate the effects of treatment. Until May 2022, 2313 patients have participated in SMART-2 after a median of 9.9 years (IQR 9.2–10.8) since their inclusion in UCC-SMART. As with UCC-SMART, the findings of SMART-2 with an accompanying treatment recommendation are communicated to the patient, his or her treating medical specialist and general practitioner.
SMART-ORACLE
SMART-ORACLE aims to determine the additional value of contrast-enhanced CT of the coronary and carotid arteries on top of traditional cardiovascular risk factors in patients with a history of CVD, diabetes or hypertension.24 The study is still ongoing and has currently been conducted in 1252 patients.
SMART-MR and SMART Medea
SMART-MR and SMART Medea target the investigation of brain changes in patients with CVD using 1.5 T MRI (and 7 T MRI in a subset of patients).25 26 This study was conducted in 1309 patients. Among others, measurements of the total cerebral blood flow have been performed and characteristics of white matter lesions and microbleeds have been mapped.
Athero-Express
In May 2022, the Athero-Express biobank and study cohort have been incorporated into the UCC-SMART Study.27 The objective of Athero-Express is to investigate the value of plaque characteristics in relation to long-term cardiovascular events. This ongoing prospective study, initiated in April 2002, includes patients undergoing femoral or carotid endarterectomy. During surgery, the atherosclerotic plaque is harvested and immunohistochemically stained in order to asses fat, collagen, macrophages and smooth muscle cells.
Other substudies
Several other substudies have been carried out within the UCC-SMART cohort, providing additional information and parameters for subsets of patients (online supplemental table 6). As part of SMART-Junior, additional questionnaires have been sent to 4270 patients in order to investigate the presence of cardiovascular risk factors and CVD in their offspring.28 In DISH, diffuse idiopathic skeletal hyperostosis was scored on chest X-rays of 4791 patients, performed in the context of healthcare, using the Resnick criteria.29 30 SMART-HEART aimed to detect patient characteristics related to the development of left ventricle hypertrophy using 1.5 T cardiac MRI in 536 patients with hypertension, but free of known coronary or valvular disease.31 In order to determine whether intima and media calcification differ in their associated CVD risks and to elucidate which risk factors lead to the development of those types of calcification, CT scans of the femoral head to the feet have been performed in 520 patients as part of ARTEMIS.32 The aim of the small aneurysms trial was to estimate the overall rupture rates of small AAAs and to investigate demographic characteristics and cardiovascular risk factors for association with AAA growth using ultrasound scanning of the aorta in 230 patients with an initial AAA diameter of 30–55 mm.33 In Brown adipose tissue, supraclavicular and subcutaneous adipose tissue fat signal fractions were assessed in 50 patients with CVD using 1.5 T water-fat MRI.34 SPAIN evaluated the feasibility of a web-based coaching programme for vascular risk factor treatment, described the patterns of use of this programme and measured changes in risk factors in 50 patients with CVD.35 RULE investigated the impact of the UCC-SMART Study compared with usual care on cardiovascular risk factors in 604 patients with CVD or type 2 diabetes.36
A few clinical trials have been conducted within the UCC-SMART Study. TEMPUS was a randomised crossover trial in 78 patients that investigated the effects of a cardiovascular polypill on LDL-C, ambulatory blood pressure and adherence as compared with the administration of the individual, identically dosed components of the polypill.37 SMART-Inform was a three-armed randomised controlled trial (RCT) in 303 patients using a statin with CVD.38 The aim was to determine whether communicating personalised statin therapy effects leads to lower decisional conflicts associated with statin use compared with standardised (non-personalised) therapy effects. BEST was an RCT investigating whether a clearly written agreement on risk factor management between general practitioners and hospitals improved the vascular risk profile of 197 patients compared with usual care.39 Another RCT was VENUS, which included 236 patients with ≥2 modifiable risk factors, investigating whether risk factor management in the hospital improved with nurse practitioner care on top of usual care compared with usual care alone.40 Lastly, IRIS was an RCT that evaluated whether an internet-based vascular risk factor management programme promoting self-efficacy on top of usual care is more effective than usual care alone in reducing vascular risk factors in 330 patients with CVD.41 A timeline showing the different substudies is presented in online supplemental figure 1.
Characteristics of the study population
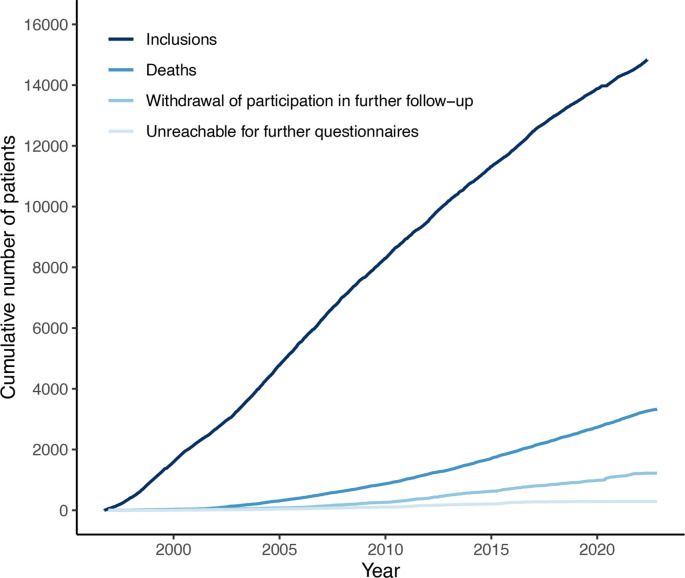
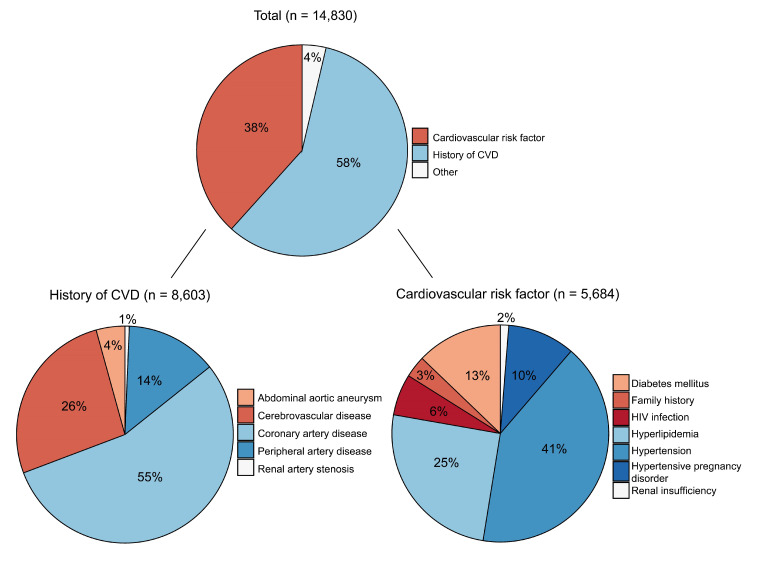
By May 2022, a total of 14 830 patients have been included (figure 3). Of those, 3294 patients died and 89% (n=10 219) of the surviving patients are still being followed up. Reasons for follow-up to end in surviving patients include withdrawal of participation in further follow-up (80%) or being unreachable for further questionnaires (20%). The median follow-up time of these patients without complete follow-up data is 7.4 years (IQR 3.9–11.4). Figure 4 shows the numbers and distribution of the reasons for inclusion. The most common inclusion diagnosis was CAD (n=4729), followed by hypertension (n=2344) and CeVD (n=2276). PAD was the enrolment diagnosis in 1173 patients and AAA in 369 patients. Hyperlipidaemia was the inclusion diagnosis in 1433 patients and diabetes mellitus in 730 patients.
Figure 3.
Cumulative number of patients over time. Inclusion in the UCC-SMART Study started in September 1996. UCC-SMART, Utrecht Cardiovascular Cohort–Second Manifestations of Arterial Disease.
Figure 4.
Distribution of inclusion diagnoses. CVD, cardiovascular disease.
Patient characteristics, medication use and measurements at baseline are listed in table 1. This table is stratified for medical history at baseline, with the items of medical history either being the inclusion diagnosis or a comorbidity. This means that patients may fall within more than one category as listed in table 1. The majority of patients included in the cohort are male (65%), especially among the subgroup of patients with established CVD (73% male). The mean age of the total population is 56.8±12.5 years. In total, 2608 individuals (18%) had diabetes and 9633 individuals (65%) had established CVD at baseline. Of these patients with CVD, 1399 (15%) had polyvascular disease, that is, multiple vascular beds (cerebral, coronary, abdominal aorta or lower extremity) being affected. The proportion of missing variables is less than 3% for all variables, except for adipose tissue measurements on ultrasound (3.6%), albuminuria (4.7%) and CRP level (9.0%). Vascular screening indicated significant carotid artery stenosis (>50% stenosis) in 526 (4%) patients, AAA in 188 (1%) patients and low ABI (≤0.9) in 829 (6%) patients who were not previously diagnosed with CeVD, AAA or PAD, respectively. Of the 3095 patients with established CVD included between 2012 and 2022 (to account for applicable guidelines), 2075 (67%) had an SBP <140 mm Hg, 753 (25%) had an LDL-C ≤1.8 mmol/L and 2737 patients (88%) were using antithrombotic agents at baseline. Baseline characteristics of patients with complete follow-up data available were comparable with the characteristics of patients who withdrew from or were unreachable for further follow-up (online supplemental table 7).
Table 1.
Baseline characteristics stratified for medical history
| History of CVD | Cardiovascular risk factors | |||||||
| Cerebrovascular disease | Coronary artery disease | Abdominal aortic aneurysm | Peripheral artery disease | Hypertension | Hyperlipidaemia | Diabetes mellitus (type 1+2) | Renal insufficiency | |
| Number of patients | 2801 | 5999 | 767 | 1646 | 8228 | 12 972 | 2608 | 1118 |
| Medical history* | ||||||||
| Cerebrovascular disease | 2801 (100) | 553 (9) | 117 (15) | 209 (13) | 1655 (20) | 2492 (19) | 442 (17) | 248 (22) |
| Coronary artery disease | 553 (20) | 5999 (100) | 322 (42) | 433 (26) | 3192 (39) | 5762 (44) | 1131 (43) | 497 (44) |
| Abdominal aortic aneurysm | 117 (4) | 322 (5) | 767 (100) | 134 (8) | 466 (6) | 693 (5) | 114 (4) | 151 (14) |
| Peripheral artery disease | 209 (7) | 433 (7) | 134 (17) | 1646 (100) | 906 (11) | 1492 (12) | 328 (13) | 205 (18) |
| Hypertension | 1655 (60) | 3192 (54) | 466 (62) | 906 (57) | 8228 (100) | 7285 (57) | 1736 (68) | 902 (82) |
| Hyperlipidaemia | 2492 (90) | 5762 (96) | 693 (91) | 1492 (92) | 7285 (90) | 12 972 (100) | 2275 (88) | 1016 (92) |
| Diabetes mellitus | 442 (16) | 1131 (19) | 114 (15) | 328 (20) | 1736 (21) | 2275 (18) | 2608 (100) | 365 (33) |
| Health questionnaire | ||||||||
| Age (years) | 60±11 | 62±10 | 65±9 | 60±11 | 59±12 | 58±12 | 59±12 | 63±11 |
| Male sex | 1744 (62) | 4849 (81) | 636 (83) | 1100 (67) | 5174 (63) | 8699 (67) | 1815 (70) | 911 (82) |
| Previous or current smoking | 2106 (76) | 4511 (75) | 661 (86) | 1473 (90) | 5697 (69) | 9265 (72) | 1865 (72) | 847 (76) |
| Pack-years in (former) smokers | 20.2 (9.4–35.1) | 20.7 (9.4–33.6) | 28.0 (13.8–42.3) | 27.9 (14.6–40.6) | 18.9 (8.3–33.3) | 18.9 (8.8–32.5) | 21.0 (9.5–36.2) | 22.8 (10.5–37.8) |
| Current alcohol use | 1484 (53) | 3641 (61) | 368 (48) | 770 (47) | 4787 (58) | 7584 (59) | 1229 (47) | 511 (46) |
| Highest level of education | ||||||||
| Primary/secondary school | 554 (31) | 1248 (29) | 128 (34) | 315 (40) | 1764 (31) | 2569 (29) | 553 (35) | 210 (32) |
| Vocational school | 631 (35) | 1466 (35) | 117 (31) | 236 (30) | 1824 (32) | 2891 (33) | 519 (33) | 223 (34) |
| University (of applied science) | 560 (31) | 1415 (33) | 125 (33) | 196 (25) | 1914 (34) | 3031 (35) | 422 (27) | 194 (30) |
| Exercise (METhour/week) | 0.0 (0.0–10.5) | 0.0 (0.0–12.0) | 0 (0.0–6.0) | 0 (0.0–5.5) | 0 (0.0–11.0) | 0 (0.0–12.0) | 0 (0.0–6.0) | 0 (0.0–5.5) |
| Medication use | ||||||||
| Lipid-lowering therapy | 1682 (60) | 4995 (83) | 417 (54) | 849 (52) | 4720 (57) | 8253 (64) | 1664 (64) | 678 (61) |
| Antihypertensive therapy | 1724 (62) | 5409 (90) | 545 (71) | 912 (55) | 7130 (87) | 9080 (70) | 1980 (76) | 965 (86) |
| Platelet inhibitors | 2062 (74) | 5263 (88) | 450 (59) | 987 (60) | 4532 (55) | 7694 (59) | 1453 (56) | 640 (57) |
| Oral anticoagulant therapy | 311 (11) | 821 (14) | 123 (16) | 234 (14) | 743 (9) | 1188 (9) | 271 (10) | 182 (16) |
| Glucose-lowering therapy | 287 (10) | 757 (13) | 67 (9) | 189 (11) | 1176 (14) | 1475 (11) | 1621 (62) | 216 (19) |
| Anthropometric measurements | ||||||||
| Systolic blood pressure (mm Hg) | 141±22 | 137±20 | 142±20 | 144±21 | 150±23 | 140±22 | 144±21 | 150±24 |
| Diastolic blood pressure (mm Hg) | 82±12 | 80±11 | 83±12 | 81±11 | 87±14 | 83±13 | 82±12 | 85±14 |
| Ankle-brachial index ≤0.9 | 398 (14) | 680 (11) | 165 (22) | 1063 (66) | 1195 (15) | 1751 (14) | 434 (17) | 283 (26) |
| Body mass index (kg/m2) | 26.6±4.2 | 27.3±4.0 | 26.4±3.8 | 26.3±4.3 | 27.6±4.6 | 27.0±4.3 | 28.7±5.0 | 27±4 |
| Waist circumference (cm) | 93.7±12.9 | 97.4±11.6 | 97.6±12.1 | 95.0±12.5 | 96.4±13.3 | 95.1±12.7 | 100.7±13.7 | 98.9±12.5 |
| Hip circumference (cm) | 103.6±8.7 | 104.2±7.6 | 103.8±7.8 | 103.0±8.7 | 105.1±9.2 | 104.1±8.5 | 106.3±9.8 | 104.4±8.4 |
| Visceral fat (cm) | 8.6±2.6 | 9.3±2.6 | 9.5±2.6 | 9.2±2.7 | 9.0±2.8 | 8.8±2.7 | 10.1±2.9 | 9.9±2.8 |
| Subcutaneous fat (cm) | 2.5±1.2 | 2.4±1.2 | 2.2±1.1 | 2.4±1.5 | 2.6±1.4 | 2.5±1.3 | 2.4±1.4 | 2.2±1.4 |
| Carotid artery stenosis | 652 (24) | 443 (8) | 84 (11) | 255 (16) | 77 (10) | 1104 (9) | 283 (11) | 181 (16) |
| cIMT (mm) | 0.9 (0.7–1.0) | 0.9 (0.7–1.0) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | 0.9 (0.7–1.0) | 0.9 (0.8–1.1) |
| Aortic aneurysm | 81 (3) | 244 (4) | 307 (41) | 72 (4) | 289 (4) | 458 (4) | 61 (2) | 108 (10) |
| Kidney size (cm) | 11.1±1.0 | 11.3±1.0 | 11.3±1.0 | 11.2±1.1 | 11.2±1.0 | 11.2±1.0 | 11.5±1.0 | 10.9±1.3 |
| Laboratory measurements | ||||||||
| Haemoglobin (mmol/L) | 8.9±0.8 | 8.9±0.8 | 8.8±0.9 | 8.9±0.9 | 8.9±0.8 | 8.9±0.8 | 8.8±0.9 | 8.5±1.0 |
| Total cholesterol (mmol/L) | 4.9±1.2 | 4.5±1.1 | 5.1±1.3 | 5.3±1.3 | 5.0±1.3 | 5.1±1.4 | 4.7±1.3 | 5.0±1.4 |
| LDL-C (mmol/L) | 2.9±1.1 | 2.6±0.9 | 3.1±1.1 | 3.2±1.1 | 2.9±1.1 | 3.1±1.2 | 2.7± 1.0 | 2.9±1.1 |
| HDL-C (mmol/L) | 1.3±0.4 | 1.2±0.3 | 1.2±0.4 | 1.2±0.4 | 1.3±0.4 | 1.3±0.4 | 1.2±0.3 | 1.2±0.4 |
| Apolipoprotein B (g/L) | 0.8±0.3 | 0.8±0.2 | 0.9±0.2 | 0.9±0.3 | 0.9±0.3 | 0.9±0.3 | 0.9±0.3 | 0.9±0.3 |
| Triglycerides (mmol/L) | 1.3 (0.9–1.9) | 1.4 (1.0–2.0) | 1.5 (1.1–2.1) | 1.5 (1.1–2.3) | 1.4 (1.0–2.1) | 1.4 (1.0–2.1) | 1.6 (1.1–2.4) | 1.7 (1.2–2.5) |
| HbA1c (mmol/mol) | 38 (36–42) | 39 (36–43) | 39 (36–43) | 40 (37–48) | 39 (36–44) | 38 (36–43) | 52 (45–62) | 41 (37–52) |
| Fasting glucose (mmol/L) | 5.7 (5.3–6.3) | 5.9 (5.4–6.6) | 5.8 (5.4–6.5) | 5.8 (5.3–6.7) | 5.8 (5.4–6.6) | 5.8 (5.3–6.4) | 8.1 (6.9–10.0) | 6.0 (5.5–7.2) |
| eGFR (mL/min/1.73 m2) | 48±40 | 63±34 | 58±32 | 51±40 | 49±40 | 54±40 | 55±41 | 40±26 |
| Albuminuria (mg/L) | 10.0 (6.0–24.1) | 9.0 (6.0–20.0) | 12.9 (8.0–39.9) | 11.0 (7.0–32.0) | 11.0 (7.0–29.0) | 9.0 (6.0–22.0) | 14.0 (8.0–41.0) | 82.0 (16.0–257.6) |
| CRP (mg/L) | 2.1 (1.0–4.5) | 1.9 (1.0–4.0) | 3.3 (1.6–6.9) | 3.1 (1.4–6.3) | 2.2 (1.0–4.7) | 2.0 (1.0–4.2) | 2.4 (1.1–5.1) | 3.2 (1.5–7.2) |
| TSH (mU/L) | 1.7 (1.2–2.5) | 1.7 (1.2–2.5) | 1.7 (1.2–2.5) | 1.7 (1.2–2.5) | 1.8 (1.2–2.6) | 1.8 (1.2–2.5) | 1.9 (1.3–2.7) | 1.8 (1.3–2.7) |
Data are presented as number (percentage), mean±SD or median (IQR).
*Based on inclusion diagnosis, items of the health questionnaire and/or measurements at baseline: cerebrovascular disease: history of stroke, carotid surgery or percutaneous transluminal angioplasty; coronary artery disease: history of myocardial infarction, cardiac arrest, coronary bypass surgery or percutaneous transluminal coronary angioplasty; abdominal aortic aneurysm: history of abdominal aortic aneurysm, transluminal or surgical treatment of abdominal aortic aneurysm; peripheral artery disease: history of amputation of (part of) lower limb, lower limb peripheral artery surgery or percutaneous transluminal angioplasty; hypertension: treatment with antihypertensive drugs or blood pressure ≥160/95 mm Hg at baseline measurement; hyperlipidaemia: treatment with lipid-lowering agents, total cholesterol ≥5 mmol/L or LDL-C ≥3.2 mmol/L at baseline measurement; diabetes mellitus: treatment with antidiabetic agents, fasting glucose ≥7.0 mmol/L or non-fasting glucose ≥11.1 mmol/L at baseline measurement; renal insufficiency: creatinine >120 mmol/L and/or microprotein/creatinine ratio in urine >20. Cut-off values applied at the start of UCC-SMART Study; please note target values have changed over time and continuous variables are available.
cIMT, carotid intima–media thickness; CRP, C reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MET, metabolic equivalent of task; TSH, thyroid-stimulating hormone; UCC-SMART, Utrecht Cardiovascular Cohort–Second Manifestations of Arterial Disease.
Findings to date
The findings of this section are reported for patients included up to January 2020 (n=13 898), because the collection and processing of outcome events have been completed up until this date. These patients contributed to a total follow-up time of 134 439 person-years. Median follow-up time was 9.2 years (IQR 4.8–14.1 years). During follow-up, 2259 (16%) patients suffered a first combined major cardiovascular endpoint (including non-fatal myocardial infarction, non-fatal stroke or cardiovascular death). Furthermore, there were 943 (7%) cases of incident diabetes, 105 (1%) cases of end-stage kidney disease, 161 (1%) cases of heart failure and 434 (3%) cases of major bleeding. A total of 3264 (23%) patients underwent a vascular intervention during follow-up. Of patients with established CVD, 1906 patients (21%) suffered the combined vascular endpoint mentioned above as subsequent event, whereas 353 patients (7%) with severe risk factors without prior CVD experienced this combined outcome as their first ever event. Of the 2450 individuals with diabetes at baseline, 568 (23%) individuals suffered the combined vascular endpoint. Corresponding incidence rates are 21.2 per 1000 person-years for patients with established CVD and 8.2 per 1000 person-years for patients without a history of CVD. Numbers and observed incidence rates of all specific outcome events of interest are listed in table 2. Through linkage with the Dutch National Cancer Registry, a total of 2139 patients (15%) were diagnosed with cancer during follow-up. This includes 414 diagnoses of lung cancer, 354 of prostate cancer, 294 of intestinal cancer and 163 of breast cancer as most common diagnoses.
Table 2.
Number and incidence rates of outcome events from 1996 to 2020
| Outcome event | Number of first events | Person-years of follow-up | Incidence rate per 1000 person-years |
| Non-fatal stroke | 613 | 131 684 | 4.66 |
| Ischaemic stroke | 502 | 132 042 | 3.8 |
| Haemorrhagic infarction | 20 | 134 362 | 0.15 |
| Intracerebral haemorrhage | 66 | 134 285 | 0.49 |
| Subarachnoid haemorrhage | 17 | 134 322 | 0.13 |
| Type not determined | 8 | 134 430 | 0.06 |
| Retinal syndromes | 16 | 134 338 | 0.12 |
| Infarction | 13 | 134 353 | 0.1 |
| Haemorrhage | 3 | 134 424 | 0.02 |
| Non-fatal myocardial infarction | 793 | 130 065 | 6.1 |
| Heart failure | 161 | 134 075 | 1.2 |
| Systolic heart failure, due to | 115 | 134 203 | 0.86 |
| Coronary disease | 85 | 134 266 | 0.63 |
| Valve disorders | 11 | 134 425 | 0.08 |
| Other causes | 19 | 134 390 | 0.14 |
| HFpEF, due to | 46 | 134 311 | 0.34 |
| Coronary disease | 15 | 134 390 | 0.11 |
| Valve disorders | 8 | 134 418 | 0.06 |
| Other causes | 23 | 134 381 | 0.17 |
| Non-fatal rupture AAA | 5 | 139 895 | 0.04 |
| End-stage kidney disease | 105 | 134 118 | 0.78 |
| Vascular intervention | 3264 | 110 154 | 29.6 |
| Heart | 1606 | 121 936 | 13.2 |
| Carotid or intracranial arteries | 240 | 132 611 | 1.81 |
| Aorta | 439 | 131 553 | 3.34 |
| Peripheral arteries | 953 | 127 914 | 7.45 |
| Renal artery | 62 | 133 970 | 0.46 |
| Major bleeding | |||
| ISTH major bleeding | 434 | 129 804 | 3.34 |
| BARC 3 or 5 bleeding | 457 | 132 497 | 3.45 |
| Incident diabetes | 943 | 124 310 | 7.59 |
| Type 1 diabetes | 1 | 131 417 | 0.01 |
| Type 2 diabetes | 942 | 124 330 | 7.58 |
| Vascular mortality | 1267 | 134 439 | 9.42 |
| Fatal cerebral infarction | 85 | 0.63 | |
| Fatal cerebral haemorrhage | 65 | 0.48 | |
| Fatal stroke—type not determined | 21 | 0.16 | |
| Fatal myocardial infarction | 63 | 0.47 | |
| Fatal heart failure | 198 | 1.47 | |
| Fatal rupture AAA | 29 | 0.22 | |
| Sudden death | 401 | 2.98 | |
| Other | 405 | 3.01 | |
| Non-vascular mortality | 1317 | 134 439 | 9.8 |
| Fatal malignancy | 800 | 5.95 | |
| Fatal infection | 169 | 1.26 | |
| Unnatural death | 58 | 0.43 | |
| Other | 290 | 2.16 | |
| All-cause mortality | 2794 | 134 439 | 20.78 |
| Malignancy* | 2139 | 127 514 | 16.77 |
| Lung | 414 | 3.25 | |
| Prostate | 354 | 2.78 | |
| Breast | 163 | 1.28 | |
| Intestinal | 294 | 2.31 | |
| Other | 914 | 7.17 |
*Other subtypes of cancer in the dataset include cancer of the lip, oral cavity or pharynx; oesophagus; stomach; liver, intrahepatic bile ducts or gallbladder; pancreas; respiratory tract; thymus; bone or articular cartilage of limb; melanoma; mesothelial or soft tissue; vulva or vagina; cervix uteri or corpus uteri; ovary; penis or testes; kidney, renal pelvis or ureter; bladder; eye, brain and other parts of the central nervous system; thyroid gland; lymphatic/haematopoietic.
AAA, abdominal aortic aneurysm; BARC, Bleeding Academic Research Consortium; HFpEF, heart failure with preserved ejection fraction; ISTH, International Society on Thrombosis and Haemostasis.
The large database of observational data has been used for over 350 aetiological and prognostic studies so far, and the coverage of a wide age range and long follow-up provides opportunity to develop and validate prediction models. This has been done with the SMART risk score,42 43 the SMART-REACH lifetime model for patients with previous CVD3 and the DIAL lifetime model44 for patients with type 2 diabetes (to be found at https://u-prevent.com and the European Society of Cardiology ‘CVD risk calculation’ app). These estimates serve clinical practice by providing insight into risk and thus supporting patient education and shared decision-making. Moreover, routine collection of patient data allows for embedding clinical trials within the cohort, as has been done with, among others, TEMPUS37 and SMART-Inform.38
The vascular screening in the UCC-SMART Study is a structured uniform programme to detect risk factors and asymptomatic atherosclerosis and provides a basis for optimising treatment of high-risk patients. In a previous study comparing the UCC-SMART screening programme with usual care in another university hospital in the Netherlands, a beneficial effect of the screening programme on SBP and LDL-C was seen.36 Previous research on screening programmes in the general population shows improvement of cardiovascular risk factors and detection of patients at risk, but conflicting results are found on mortality and cardiovascular events.2 45 In a population at risk (eg, with hypertension or diabetes), the beneficial effect of cardiovascular screening is more pronounced.2 46 In addition, a higher baseline achievement of secondary prevention targets is associated with improved cardiovascular health outcomes in patients with established CVD and type 2 diabetes.47
Strengths and limitations
The UCC-SMART Study is a unique ongoing prospective cohort study in over 14 000 patients with a history of various manifestations of CVD or severe cardiovascular risk factors, providing a large up-to-date cohort of a population at high cardiovascular risk. Collecting diverse outcome events in this population allows for research on risk factors for different manifestations of CVD and incident diabetes. Linkage to multiple registries facilitates the investigation of relationships between cardiovascular risk factors and diseases and other conditions such as cancer and dementia. By the integration of healthcare and scientific research, patient care becomes more complete and data already to be collected for patient care are used to increase knowledge of CVD, while the additive burden for participating patients is limited.
The main strengths of the UCC-SMART cohort include the large size, its capture of a high-risk population with various CVD manifestations and risk factors with few exclusion criteria, the use of a standardised diagnostic protocol, the long follow-up duration and the comprehensive capture of a wide range of data. Because inclusion of patients is still ongoing, the UCC-SMART cohort provides a good representation of the past and current population of patients at high cardiovascular risk. Due to the high-risk study population, the prevalence and incidence of the main outcome variables are higher than in the general population, thereby increasing the power to study these outcomes. Furthermore, all outcome events are adjudicated independently by three physicians of the endpoint committee, reducing the risk of misclassification. The proportion of missing data is small, possibly explained by the protocolised screening programme taking place in 1 day. The substudies provide additional information on specific cardiovascular risk factors (eg, parental history of CVD,48 characteristics related to left ventricle hypertrophia31 and the presence of diffuse idiopathic skeletal hyperostosis49), manifestations of atherosclerosis (eg, brain changes on MRI25 and cognitive decline26) and other important aspects in cardiovascular risk management (eg, the effect of a cardiovascular polypill50).
Limitations also need to be considered. Due to the prospective observational design, for the majority of the patients, risk factors and medication use are only recorded at baseline and may have changed during follow-up. This could be reflected by the finding of this article that not all patients with CVD meet treatment goals for modifiable risk factors at baseline. Since patients are included several weeks to months after an index CVD event, risk factors are likely to be further optimised during this period after baseline examination. For a subset of patients with CVD or diabetes, a repeat of the baseline measurements after a median of 9.9 years is indeed available, allowing for investigating the course of atherosclerosis over time. Furthermore, in 10.6% of the included patients, follow-up ended due to either withdrawal of participation in further follow-up (8.5%) or being unreachable for further questionnaires (2.1%). Yet, the median follow-up time for these patients is 7.4 years, so those patients still contribute to a fair amount of patient-years. In addition, because UCC-SMART is a single-centre study in a university hospital, it can be disputed whether it represents the general high-risk population and patients with established CVD. The UMC Utrecht provides care to nationwide patients referred for complex and specialised care, but also to patients referred by general practitioners from the region. Patients included in UCC-SMART correspond to patients with severe cardiovascular risk factors or established CVD from the general population. As reflected by the inclusion criteria, the UCC-SMART Study does not include patients requiring highly specialised care (including heart transplantation and rare causes of vascular disease). Lastly, except for information on education level, the database does not contain extensive information on socioeconomic status.
In conclusion, we have provided an updated extensive overview of the design of the UCC-SMART Study as well as an overview of the findings to date. This underlines the value of the UCC-SMART Study as a basis for contemporary and future epidemiological research in CVD using a well-characterised high-risk cardiovascular population with long-term follow-up. A future goal is to make the UCC-SMART data Findable, Accessible, Interoperable and Reusable.51
Collaboration
The UCC-SMART Study group directs the academic focus of research using the UCC-SMART data and consists of staff members from both epidemiological and clinical departments. All data presented in this manuscript will be available upon reasonable request, and specific datasets will be compiled based on the research proposal. The data are to be used only for the purposes as described in the research proposal. Datasets are provided to interested researchers after approval of request by the UCC-SMART Study group. Access to the data request module can be applied for via ucc-smart@umcutrecht.nl. We encourage collaborations within overarching cardiovascular topics in which datasets are combined.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contribution of the participants, the research nurses, RBvP (data manager), AV (study manager), the founders of the SMART Study in 1996: A Algra, J D Banga, B C Eikelboom and Y van der Graaf, and the members of the UCC-SMART Study group: MJMC, HMN and MGvdM (co-PI), Department of Cardiology; GJdB and MT (co-PI), Department of Vascular Surgery; MLB and MvS, Julius Center for Health Sciences and Primary Care; MHE-V, Department of Geriatrics; PAdJ, Department of Radiology; ATL, Department of Gynaecology and Obstetrics; NPvdK, Department of Cardiothoracic Surgery; LJK and YMR, Department of Neurology; MCV, Department of Nephrology and Hypertension; JAND (co-PI) and FLJV (PI), Department of Vascular Medicine, UMC Utrecht.
Footnotes
MCC and MAGH contributed equally.
Contributors: FLJV, JW, SHJH, MCC and MAGH contributed to the conception and design of the work. MAGH and MCC drafted the manuscript and contributed equally to this paper. MCC, MAGH, SHJH, FWA, GJdB, MLB, MJC, JAND, MHE-V, MIG, PAdJ, NPvdK, LJK, ATL, MGvdM, BMM, HMN, NCO-M, RBvP, YMR, MvS, MT, AV, MCV, JW and FLJV contributed to the interpretation of data and critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. FLJV is the guarantor of this work.
Funding: The UCC-SMART Study was financially supported by the UMC Utrecht, the Netherlands.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The UCC-SMART Study group directs the academic focus of research using the UCC-SMART data and consists of members from both epidemiological and clinical cardiovascular research. Datasets are provided to interested researchers after approval of request by the UCC-SMART Study group. Access to the data request module can be applied for via ucc-smart@umcutrecht.nl.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Medical Research Ethics Committee (MREC) NedMec of the UMC Utrecht (reference number 22-088). Participants gave informed consent to participate in the study before taking part.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol 2022;29:5–115. 10.1093/eurjpc/zwab154 [DOI] [PubMed] [Google Scholar]
- 3.Kaasenbrood L, Bhatt DL, Dorresteijn JAN, et al. Estimated life expectancy without recurrent cardiovascular events in patients with vascular disease: the SMART-REACH model. J Am Heart Assoc 2018;7:e009217. 10.1161/JAHA.118.009217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler A, Agodoa L, Algra A. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 2021;397:1625–36. 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–61. 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 6.Simons PC, Algra A, van de Laak MF, et al. Second manifestations of arterial disease (smart) study: rationale and design. Eur J Epidemiol 1999;15:773–81. 10.1023/a:1007621514757 [DOI] [PubMed] [Google Scholar]
- 7.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1–188. [PubMed] [Google Scholar]
- 8.Sluik D, Geelen A, de Vries JHM, et al. A national FFQ for the Netherlands (the FFQ-NL 1.0): validation of a comprehensive FFQ for adults. Br J Nutr 2016;116:913–23. 10.1017/S0007114516002749 [DOI] [PubMed] [Google Scholar]
- 9.Tremblay AJ, Morrissette H, Gagné JM, et al. Validation of the friedewald formula for the determination of low-density lipoprotein cholesterol compared with beta-quantification in a large population. Clin Biochem 2004;37:785–90. 10.1016/j.clinbiochem.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusnierz-Cabala B, Gernand W, Zabek-Adamska A, et al. Comparison of high-sensitivity C-reactive protein serum assay results obtained using dade-behring BNII nephelometer and ortho VITROS FS 5.1 clinical analyzer in respect of CRP-related risk assessment of chronic metabolic diseases. Clin Lab 2008;54:341–6. [PubMed] [Google Scholar]
- 12.Stolk RP, Wink O, Zelissen PM, et al. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord 2001;25:1346–51. 10.1038/sj.ijo.0801734 [DOI] [PubMed] [Google Scholar]
- 13.Bazzocchi A, Filonzi G, Ponti F, et al. Ultrasound: which role in body composition? Eur J Radiol 2016;85:1469–80. 10.1016/j.ejrad.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Journal of the American Society of Echocardiography 2015;28:1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. 10.1016/0002-9149(86)90771-x [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 2016;29:277–314. 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DHD . Ontdek de mogelijkheden van de LBZ [internet]. 2022. Available: https://www.dhd.nl/producten-diensten/registratie-data/ontdek-de-mogelijkheden-van-de-lbz
- 19.Nivel . Methode vaststellen cijfers zorgverlening huisartsen [internet]. 2022. Available: https://www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/methoden/methoden-vaststellen-cijfers-zorgverlening/methode-vaststellen-cijfers-zorgverlening-huisartsen
- 20.ten Berg MJ, Huisman A, van den Bemt PMLA, et al. Linking laboratory and medication data: new opportunities for pharmacoepidemiological research. Clin Chem Lab Med 2007;45:13–9. 10.1515/CCLM.2007.009 [DOI] [PubMed] [Google Scholar]
- 21.Patel RS, Asselbergs FW. The GENIUS-CHD Consortium. Eur Heart J 2015;36:2674–6. [PubMed] [Google Scholar]
- 22.Matsushita K, Ballew SH, Astor BC, et al. Cohort profile: the chronic kidney disease prognosis Consortium. Int J Epidemiol 2013;42:1660–8. 10.1093/ije/dys173 [DOI] [PubMed] [Google Scholar]
- 23.SFK . Foundation for pharmaceutical statistics [internet]. 2022. Available: https://www.sfk.nl/english
- 24.Franssens BT, Nathoe HM, Leiner T, et al. Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events. Eur J Prev Cardiol 2017;24:660–70. 10.1177/2047487316679524 [DOI] [PubMed] [Google Scholar]
- 25.Geerlings MI, Appelman APA, Vincken KL, et al. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis 2010;210:130–6. 10.1016/j.atherosclerosis.2009.10.039 [DOI] [PubMed] [Google Scholar]
- 26.Grool AM, van der Graaf Y, Mali WPTM, et al. Location of cerebrovascular and degenerative changes, depressive symptoms and cognitive functioning in later life: the SMART-medea study. J Neurol Neurosurg Psychiatry 2011;82:1093–100. 10.1136/jnnp.2010.232413 [DOI] [PubMed] [Google Scholar]
- 27.Verhoeven BAN, Velema E, Schoneveld AH, et al. Athero-express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. rationale and design. Eur J Epidemiol 2004;19:1127–33. 10.1007/s10564-004-2304-6 [DOI] [PubMed] [Google Scholar]
- 28.Weijmans M, van der Graaf Y, de Borst GJ, et al. Prevalence and risk of cardiovascular risk factors and events in offspring of patients at high vascular risk and effect of location of parental vascular disease. Int J Cardiol 2015;195:195–202. 10.1016/j.ijcard.2015.05.059 [DOI] [PubMed] [Google Scholar]
- 29.Harlianto NI, Oosterhof N, Foppen W, et al. Diffuse idiopathic skeletal hyperostosis is associated with incident stroke in patients with increased cardiovascular risk. Rheumatology (Oxford) 2022;61:2867–74. 10.1093/rheumatology/keab835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976;119:559–68. 10.1148/119.3.559 [DOI] [PubMed] [Google Scholar]
- 31.Meijs MFL, Bots ML, Vonken JA, et al. Rationale and design of the smart heart study. NHJL 2007;15:295–8. 10.1007/BF03086003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwakenberg SR, de Jong PA, Hendriks EJ, et al. Intimal and medial calcification in relation to cardiovascular risk factors. PLoS One 2020;15:e0235228. 10.1371/journal.pone.0235228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlösser FJV, Tangelder MJD, Verhagen HJM, et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg 2008;47:1127–33. 10.1016/j.jvs.2008.01.041 [DOI] [PubMed] [Google Scholar]
- 34.Franssens BT, Eikendal AL, Leiner T, et al. Reliability and agreement of adipose tissue fat fraction measurements with water-fat MRI in patients with manifest cardiovascular disease. NMR Biomed 2016;29:48–56. 10.1002/nbm.3444 [DOI] [PubMed] [Google Scholar]
- 35.Goessens BMB, Visseren FLJ, de Nooijer J, et al. A pilot-study to identify the feasibility of an Internet-based coaching programme for changing the vascular risk profile of high-risk patients. Patient Educ Couns 2008;73:67–72. 10.1016/j.pec.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 36.Brouwer BG, Visseren FLJ, Algra A, et al. Effectiveness of a hospital-based vascular screening programme (smart) for risk factor management in patients with established vascular disease or type 2 diabetes: a parallel-group comparative study. J Intern Med 2010;268:83–93. 10.1111/j.1365-2796.2010.02229.x [DOI] [PubMed] [Google Scholar]
- 37.Lafeber M, Grobbee DE, Bots ML, et al. The evening versus morning polypill utilization study: the TEMPUS rationale and design. Eur J Prev Cardiol 2014;21:425–33. 10.1177/2047487313476961 [DOI] [PubMed] [Google Scholar]
- 38.Jaspers NEM, Visseren FLJ, van der Graaf Y, et al. Communicating personalised statin therapy-effects as 10-year CVD-risk or CVD-free life-expectancy: does it improve decisional conflict? three-armed, blinded, randomised controlled trial. BMJ Open 2021;11:e041673. 10.1136/bmjopen-2020-041673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouwer BG. SMART risk factor screening in patients at high vascular risk [dissertation]. Utrecht University, 2008 [Google Scholar]
- 40.Goessens BMB, Visseren FLJ, Sol BGM, et al. A randomized, controlled trial for risk factor reduction in patients with symptomatic vascular disease: the multidisciplinary vascular prevention by nurses study (Venus). Eur J Cardiovasc Prev Rehabil 2006;13:996–1003. 10.1097/01.hjr.0000216549.92184.40 [DOI] [PubMed] [Google Scholar]
- 41.Vernooij JWP, Kaasjager HAH, van der Graaf Y, et al. Internet based vascular risk factor management for patients with clinically manifest vascular disease: randomised controlled trial. BMJ 2012;344:e3750. 10.1136/bmj.e3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dorresteijn JAN, Visseren FLJ, Wassink AMJ, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the smart risk score. Heart 2013;99:866–72. 10.1136/heartjnl-2013-303640 [DOI] [PubMed] [Google Scholar]
- 43.Hageman SHJ, McKay AJ, Ueda P, et al. Estimation of recurrent atherosclerotic cardiovascular event risk in patients with established cardiovascular disease: the updated SMART2 algorithm. Eur Heart J 2022;43:1715–27. 10.1093/eurheartj/ehac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkelmans GFN, Gudbjörnsdottir S, Visseren FLJ, et al. Prediction of individual life-years gained without cardiovascular events from lipid, blood pressure, glucose, and aspirin treatment based on data of more than 500 000 patients with type 2 diabetes mellitus. Eur Heart J 2019;40:2899–906. 10.1093/eurheartj/ehy839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho JH, Han KD, Jung H-Y, et al. National health screening may reduce cardiovascular morbidity and mortality among the elderly. Public Health 2020;187:172–6. 10.1016/j.puhe.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 46.Antoku Y, Takemoto M, Mito T, et al. Impact of annual cardiovascular screening tests in patients with type 2 diabetes mellitus without previous histories of cardiovascular disease: four-year clinical outcomes. Intern Med 2021;60:2725–32. 10.2169/internalmedicine.6893-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagidipati NJ, Navar AM, Pieper KS, et al. Secondary prevention of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 2017;136:1193–203. 10.1161/CIRCULATIONAHA.117.027252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weijmans M, van der Graaf Y, de Borst GJ, et al. Parental history and the risk of subsequent vascular events in patients with clinically manifest vascular disease: the effects of sex of the parent and vascular disease location. Atherosclerosis 2014;234:129–35. 10.1016/j.atherosclerosis.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 49.Harlianto NI, Westerink J, Foppen W, et al. Visceral adipose tissue and different measures of adiposity in different severities of diffuse idiopathic skeletal hyperostosis. J Pers Med 2021;11:663. 10.3390/jpm11070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lafeber M, Grobbee DE, Schrover IM, et al. Comparison of a morning polypill, evening polypill and individual pills on LDL-cholesterol, ambulatory blood pressure and adherence in high-risk patients; a randomized crossover trial. Int J Cardiol 2015;181:193–9. 10.1016/j.ijcard.2014.11.176 [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson MD, Dumontier M, Aalbersberg IJJ, et al. The fair guiding principles for scientific data management and stewardship. Sci Data 2016;3:160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066952supp001.pdf (884.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The UCC-SMART Study group directs the academic focus of research using the UCC-SMART data and consists of members from both epidemiological and clinical cardiovascular research. Datasets are provided to interested researchers after approval of request by the UCC-SMART Study group. Access to the data request module can be applied for via ucc-smart@umcutrecht.nl.