Abstract
Background
Recent advances in telecommunications technology have raised the possibility of telehealth intervention delivering cardiac telerehabilitation, which may provide the efficacy of health services in patients after percutaneous coronary intervention (PCI). This study aimed to investigate the effects of home-based cardiac telerehabilitation (HBCTR) in patients undergoing PCI.
Methods
We performed a comprehensive search of the following electronic databases: PubMed, Cochrane Central, Web of Science, Embase, CNKI, and WANFANG. For the prespecified outcomes, the primary outcomes were results of physical function (the six-minute walking test, 6MWT) and quality of life (QoL) of the participants. The secondary outcomes were results of (1) blood pressure; (2) full lipid profile (3) reliable assessment of anxiety and depression in patients.
Results
All studies were conducted between 2013 and 2022, and a total of 5 articles could be included in the quantitative meta-analysis. The results showed that there was a statistically significant difference between the HBCTR intervention group and the control group in 6WMT (MD 16.59, 95%CI 7.13 to 26.06, P = 0.0006), but there was no difference in QoL (SMD − 0.25, 95%CI − 1.63 to 1.13, P = 0.73). According to the fixed effects model, there was a statistically significant difference between the HBCTR group versus the control group (MD − 2.88, 95%CI − 5.19 to − 0.57, P = 0.01), but not in diastolic blood pressure. Likewise, significant improvements of triglycerides and in low-density lipoprotein cholesterol were observed in HBTCR groups, but no significant differences were observed regarding total cholesterol and high-density lipoprotein cholesterol.
Conclusion
This systematic review and meta-analysis have proven that the HBCTR is one of the promisingly effective cardiac rehabilitation strategies that improve cardiorespiratory fitness and reduce cardiovascular disease risk factors. With the continuous improvement of the telerehabilitation network, it is expected to serve in clinical.
Keywords: Home-based cardiac telerehabilitation, Coronary artery disease, Percutaneous coronary intervention, Meta-analysis
Introduction
Cardiovascular disease is the most predominant cause of death globally, with about estimated over 17 million people who died of cardiovascular disease in 2016, representing 31% of all deaths worldwide [1], among which coronary artery disease (CAD) remains one of the top killers [2]. CAD refers to the condition of vascular lumen stenosis or occlusion and vascular spasm based on coronary artery atherosclerosis, leading to myocardial ischemia, hypoxia, or necrosis [3]. CAD has become one of the causes of high morbidity and mortality and the leading cause of severe long-term disability in developed and some developing countries.
Percutaneous coronary intervention (PCI) is the primary way to obtain revascularization in patients with CAD [4] due to advances in PCI technology and technique [5]. After PCI, knowledge of cardiac rehabilitation (CR) and timely management of complications [6] are essential health services for patients, associated with decreasing the rate of vascular restenosis and recurrent ischemia, to improve quality of life [7]. As such, CR was recommended for secondary prevention, established by the American Heart Association and American College of Cardiology after PCI [4]. CR is a complete, full-cycle, and effective medical management strategy. However, the development of CR also faces many opportunities and challenges, such as the continuity of CR throughout the life cycle of patients [8, 9]. In the in-hospital rehabilitation period, the care team supervises the patient's daily life and motor ability to recover. But many patients do not transition to outpatient CR centers and receive recommended prescriptions in time after discharge [10]. Therefore, there are still gaps in this continuous medical behavior, which may eventually lead to unsatisfactory treatment effects and prognosis for patients. Despite the obvious evidence-based benefits, the participation rate of CR remains poor [11]. The reasons why people have low adherence to the traditional facility-based CR are multi-faceted [12], such as private insurance, the travel distance to a healthcare site and possibly affiliated CR facility, demographic and clinical factors, and existing comorbidity [13]. Therefore, it is reported that the center-based CR programs were challenged by low participant rates, insufficient attendance, and high drop-out rates. As a result, there is an urgent need for effective strategies to increase patient engagement, and home-based cardiac rehabilitation (HBCR) is one of the most potent strategies [14]. It also confirmed that the benefits of HBCR in terms of exercise capacity, control of risk factors, quality of life, and cost-effectiveness is similar to center-based CR [15, 16].But how to adequately assess the patient's situation and get timely feedback is also a major issue.
Recent advances in telecommunications technology have raised the possibility of telehealth interventions delivered by CR, which is able to overcome barriers of time and distance [17], and increase the rate of utilization mainly due to avoidance of expensive medical costs [18]. Therefore, we pay attention to the fact that home-based cardiac telerehabilitation (HBCTR) for patients in the home environment can link doctors and patients, better continue in-hospital rehabilitation, and also provide rehabilitation guarantee for out-hospital rehabilitation. Previous research has shown that the sooner CR begins in patients with CAD, the greater the benefit for patients [19]. CR for patients with CAD is divided into three stages, including stage I (in-hospital rehabilitation), stage II (out-of-hospital early rehabilitation or outpatient rehabilitation), and stage III (long-term community/family rehabilitation) [20]. Each stage of rehabilitation should follow the principle of safety. Therefore, most patients eligible for HBCTR are at low to intermediate risk, or in the transition from acute to convalescent phase and convalescent phase [14]. Telehealth can be defined as providing health management through emerging mobile devices such as mobile computing, medical sensor, and communications technologies [21]. The use of telehealth has grown tremendously and covers a wide range of content, such as digital information collection, precision medicine, virtual diagnosis, and treatment. Compared to other telehealth interventions, HBCTR focuses on the rehabilitation and prognosis of heart disease patients, and the core components of management include exercise training, risk factor control, psychological counseling, drug guidance, and nutritional prescription [22, 23]. The based model established by HBCTR is: the doctors formulate the CR prescription and send it remotely, and the patients execute the prescription, report data and conduct follow-up feedback, after that doctors make the personalized modification of the rehabilitation prescription in the standardized medical behavior. The closed-loop mechanism improves the patient's self-efficacy and enhances cardiac rehabilitation compliance. Meanwhile, HBCTR appears to be a more feasible and effective innovative rehabilitation model than conventional in-hospital CR [24]. Moreover, Stefanakis et al. showed patients received HBCTR with a low rate of adverse events after being fully evaluated before receiving the intervention [25].
It has been reported that telerehabilitation has proved beneficial effect for many patients, such as stroke survivors [26], patients with knee osteoarthritis [27], and patients after total knee arthroplasty [28, 29] and cardiovascular disease [30]. However, no systematic review of the effectiveness of HBCTR for patients after PCI has been substantially published. Therefore, this systematic review and meta-analysis aimed to investigate the benefits of telemedicine.
Methods
This analysis was performed as following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [31]. The review was registered in the International Prospective Register of Systematic Review (PROSPERO) Registry: CRD42021291148. All analyses involved were based on previously published studies. Thus, no ethical approval and patient consent were required.
Literature search
We performed a comprehensive search of the following electronic databases for the potentially eligible studies until January 2023: PubMed, Cochrane Central, Web of Science, Embase, CNKI, and WANFANG. The keywords were performed in the following combinations: "percutaneous coronary intervention" AND "telemedicine or telerehabilitation or remote rehabilitation or e-health or telehealth or internet-based," with no time limit or language restrictions. A manual screening was performed for the reference lists of retrieved studies and relevant reviews to include additional eligible articles until no further report was identified.
Study selection
The studies were included while they reached the specific criteria for this review were: (a) population: all participants hospitalized for documented coronary heart disease and treated with a successful PCI were included; (b) intervention: any form of the following technology conducted HBCTR, such as mobile phones, tablet computers, computers, television or video-conferencing; (c) control: the control group included usual care or active outpatient CR; (d)outcome: one of the following outcomes had to be reported: the six-minute walking test (6MWT), quality of life (QoL), blood pressure, total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), assessment of anxiety and depression; and (e) study design: randomized controlled trial design. We excluded articles that only focused on telerehabilitation systems development or patients assigned to HBCTR without systematic and regular rehabilitation treatment, which is just medical staff unilaterally reminding patients to pay attention to rehabilitation therapies by using social media such as emails, text messages, etc. And duplicate reports of the same team's study were not considered. Two reviewers first screened all titles and abstracts based on all searched results to identify all potentially relevant articles following the above criteria and then performed full-text filtering.
Data extraction and outcomes
Two reviewers extracted data independently by using a developed Excel sheet. Information extracted from the relevant randomized controlled trials (RCTs) included: essential patient characteristics, data on sample size, study design, the intervention of telerehabilitation and control group, and outcome measures. For the outcomes, the primary outcomes were results of physical function (6MWT) and QoL of the participants. The secondary outcomes were results of (1) blood pressure, (2) full lipid profile (mmol/L) (3) reliable assessment of anxiety and depression in patients.
Risk of bias
According to the Cochrane risk of bias tool, we conducted a quality assessment of the included studies by evaluating the risk of different forms of bias, such as selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias. The PEDro scale also assessed the quality of the studies. All included trial reports were checked, and each item was rated as 'yes' or 'no.' Trials with higher scores are valid and reliable. If the judgments of two reviewers were uncertain, a third reviewer settled the discrepancy.
Statistical analysis and outcome interpretation
All meta-analytic statistical analyses were performed with RevMan software for Windows (Version 5.3, The Cochrane Collaboration, Software Update, Oxford, UK) in this study. Mean differences (MDs) with 95% confidence intervals (CIs) for continuous outcome variables after therapy were used to estimate the total effects. The standardized mean difference (SMD) was calculated when studies used different scales to measure the same outcome, such as QoL. Statistical heterogeneity was assessed using the chi-square test, and P < 0.10 was considered statistically significant. The extent was measured using I2 tests, and I2 > 50% was regarded as high heterogeneity. Therefore, we used the random effects model meta-analysis when it had high heterogeneity and basically used a fixed effect model for meta-analysis.
Results
Study selection
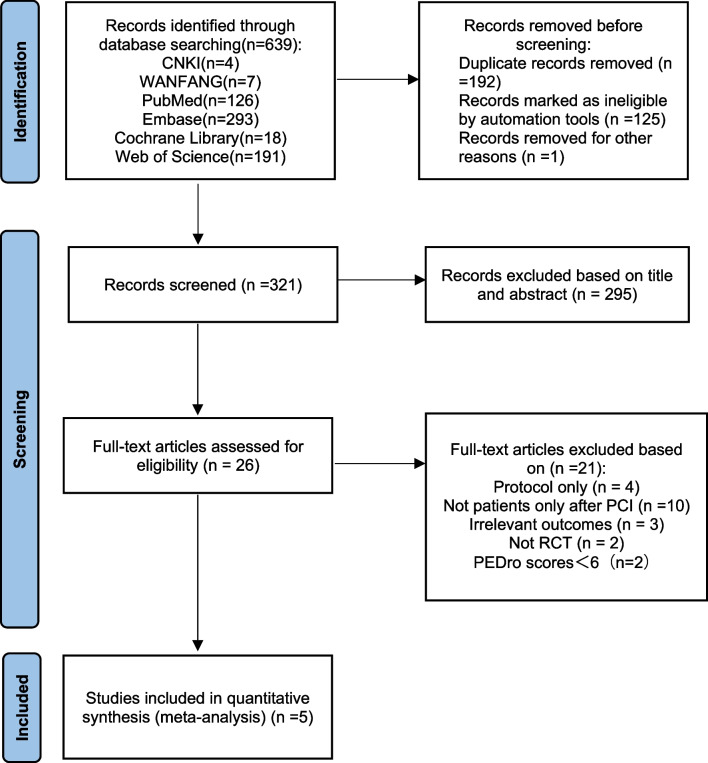
In all, we retrieved a total of 639 records to be potentially relevant through electronic searching from the six electronic databases, of which 192 duplicated studies and 126 ineligible studies marked by automation tools or for other reasons were removed. Then, 295 studies were excluded for not meeting the inclusion criteria. Only 26 records were identified as eligible after the initial screening of the title and abstract of 321 documents from 26 full-text papers screened. At the same time, only five articles fulfilled the absolute eligibility criteria, and all five articles [32–36] could be included in the quantitative meta-analysis. Figure 1 shows the details of our screening process.
Fig. 1.
The PRISMA flow diagram for the study selection process
Study characteristics
The main forms of HBCTR are mobile device-based applications, wearables, and social media management platforms. The first form is to use applications to build HBCTR, mainly through remote assessment, electronic prescriptions, related health education, real-time doctor-patient online communication, and other ways. For example, Widmer et al. [36]and Dorje et al. [32] developed digital health interventions with smartphones as a carrier. The app provides patients with appropriate medical care and related information on CAD knowledge and awareness, as well as facilitates the monitoring and supervision of the physical activity. Patients also upload self-monitor information such as nutrition diaries, exercise records, and weight records through the software platform to guarantee the quality of HBTCR. The second form is the use of wearable devices such as heart rate belts and wristbands directly worn on the patient for CR, which can monitor heart rate and electrocardiogram. Wearable devices can not only urge patients to exercise, but also record exercise data, which is essential for the CR of patients with coronary heart disease. For example, Lee et al. [34] provided remote health education and exercise training for patients, who wore the provided wireless monitoring device (HeartCallTM, U-Heart, Korea) to monitor heart rate during exercise. At the same time, the researchers compared the patient's heart rate real-time heart rate with the target heart rate (target heart rate = (maximum heart rate—resting heart rate) × percentage + resting heart rate) and designed the patient's exercise intensity to increase from 40 to 80% in stages. Furthermore, Fang et al. [33]combined smartphones and wearable sensors used in HBCTR. This integrated telerehabilitation system has functions such as remote real-time exercise monitoring and guidance, and uses sensors to record the type of movement, duration of exercise, intensity, and frequency of daily activities of patients after being connected to a smartphone via Bluetooth, which is more accurate than self-reported physical activity. The third form is the formation of close doctor-patient connections through commonly used social media, with regular prompts and reminders, without the need for app development and algorithm optimization of wearable devices. For instance, Li et al. [35] used the WeChat platform, and they were divided into six groups according to the attending doctors, and 6 WeChat groups were established. One doctor and one nurse in each group followed the implementation of the exercise program. The baseline characteristics were summarized in Table 1.
Table 1.
Characteristics of Included Studies
| Author (Year) Country |
Population | Intervention a. Telemonitoring and telecoaching design b. Exercise prescription (intensity, duration of exercise, modality of exercise) |
Comparison | Outcome: a. Primary b. Secondary |
Follow-up (months) | ||
|---|---|---|---|---|---|---|---|
| Number(n) | Age (Mean ± SD) | Female, n (%) | |||||
|
Widmer et al (2017) United States |
IG: n = 37 CG: n = 34 |
IG: 62.5 ± 10.7 CG: 63.6 ± 10.9 |
IG: 8 (22%) CG: 5 (15%) |
a. DHI + CR: patients receive the online and smartphone-based CR program and report their dietary and exercise habits to the DHI b. Standard phase II CR program (NA, 36 sessions/12 weeks, NA) |
standard CR |
a. CV-related ED visits and rehospitalizations b. Weight, blood pressure, heart rate, glucose/HbA1c, lipids, physical activity, diet, QoL, mood, compliance |
3 |
|
Dorje et al (2019) China |
IG: n = 156 CG: n = 156 |
IG: 59.1 ± 9.4 CG: 61.9 ± 8.7 |
IG: 28 (18%) CG: 19 (30%) |
a. SMART-CR/SP: participants received educational modules by smartphone app delivered via WeChat, and patient-centered coronary heart disease risk factor monitoring and management support via WeChat-interfaced monitor b. The regular exercise protocol with daily step count supervision (NA, 1 per week/8 weeks and biweekly /24 weeks, NA) |
Usual care |
a. 6MWT b. Participants’ knowledge and awareness of coronary heart disease; resting heart rate; systolic blood pressure; cardiac rehabilitation and secondary prevention needs; full lipid profile; adherence to cardioprotective medications; smoking status; obesity (presented as BMI and waist-to-hip ratio); psychosocial wellbeing; and quality of life |
12 |
|
Lee et al (2013) Korea |
IG: n = 26 CG: n = 29 |
IG: 54.3 ± 8.9 CG: 57.8 ± 7.5 |
IG: 4(15%) CG: 7 (24%) |
a. HBTCR: participants received a standard CR program, which consisted of educational rehabilitation and exercise training with wearing a wireless heart rate monitoring device during exercise b. The tailored exercise program (40% to 80% heart rate reserve, five times weekly/10 weeks, flexibility exercise for warm-up exercise for 10 min and cool-down exercise for 10 min, and main exercise of gait for 30 min) |
Usual care |
a. Exercise capacity (submaximal rate pressure product, submaximal rate of perceived exertion, metabolic equivalent of the tasks, and maximal exercise time other outcomes) b. Quality of life |
3 |
|
Fang (2019) China |
IG: n = 33 CG: n = 34 |
IG: 60.24 ± 9.35 CG: 61.41 ± 10.17 |
IG: 21(63.6%) CG: 21(61.8%) |
a. HBCTR: participants received a real-time physiological monitoring system consisting of a belt strap with a sensor, a smartphone with an application, computer servers, and a web portal b. Individualized outdoor exercise (NA, no less than thrice per week/6 weeks, walking or jogging) |
Usual care |
a. 6MWT b. Clinical status (systolic blood pressure, diastolic blood pressure); anxiety and depression (CDS score); risk factors (FTND score); quality of life (SF-36 (PCS), SF-36 (MCS)) |
1.5 |
|
Li (2022) China |
IG: n = 40 CG: n = 40 |
IG: 55.4 ± 8.9 CG: 55.6 ± 8.3 |
IG: 13(32.5%) CG: 15(37.5%) |
a. HBCTR: received supervision intervention by a medical rehabilitation team, divided into six WeChat groups according to the doctor in charge, and they used an exercise bracelet to monitor heart rate b. The walking program (NA, NA, NA) |
Outpatient rehabilitation treatment |
a. Compliance, satisfaction evaluation, incidence of cardiac events b. Heart rate, quality of life score, and 6-min walking test |
6 |
IG Intervention group; CG control group; DHI Digital health interventions; CR cardiac rehabilitation; NA Not Available; CV cardiovascular; ED emergency department; QoL quality of life; SMART-CR/SP a system involved smartphone-based home cardiac rehabilitation and secondary prevention program; 6MWT the six-minute walking test; BMI body mass index; HBTCR home-based cardiac telerehabilitation; CDS the Cardiac Depression Scale; FTND the Fagerstrom Test for Nicotine Dependence questionnaire; SF-36 (MCS) SF-36 Health Survey (mental component summary scale); SF-36 (PCS) SF-36 Health Survey (physical component summary scale)
Risk of bias assessment results
Quality assessment of the risk of bias was undertaken for included studies conducted by two authors independently. Since the participants could not be blinded to allocation due to the intervention's characteristics, there were high risks for performance bias in all included trials. The other risk of bias assessments of the included studies is summarized in Fig. 2. Furthermore, all five studies were assessed by the PEDro scale (Table 2) to evaluate the methodological quality of the included literature. Among them did not have inadequate blinding of participants and therapists, three studies did not reveal concealed allocation, and four did not perform an intention-to-treat analysis.
Fig. 2.
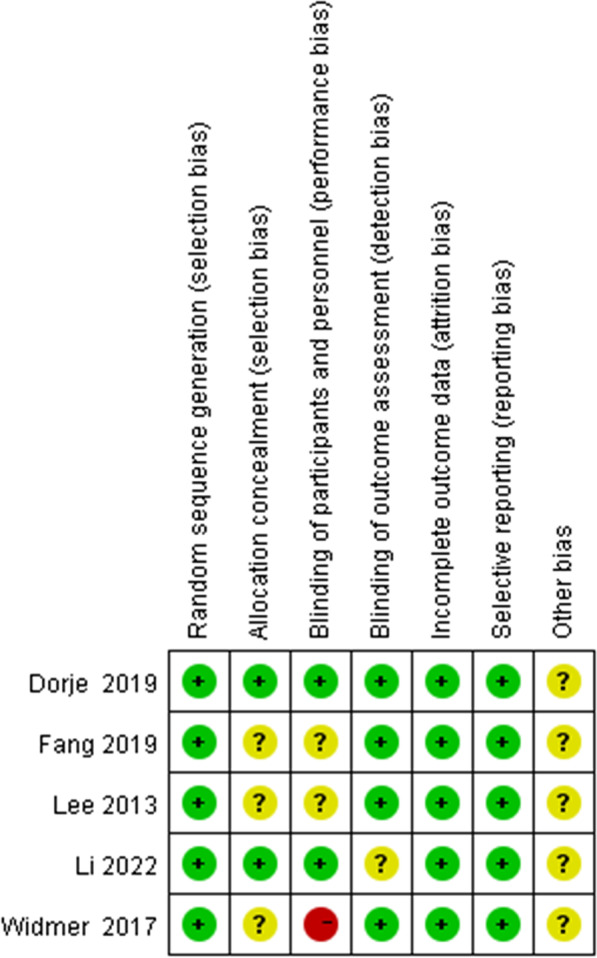
Risk of bias assessment summary according to the Cochrane risk of bias tool: red, green, and yellow colors indicate high, low, and unclear risk of bias, respectively
Table 2.
The PEDro quality assessment of the included studies. Eligibility criteria did not contribute to the total score: 1 = yes (reported in study), 0 = no (not met)
| Quality metric | Widmer et al. | Dorje et al. | Lee et al. | Fang et al. | Li et al. |
|---|---|---|---|---|---|
| Eligibility criteria | Yes | Yes | Yes | Yes | Yes |
| Random allocation | Yes | Yes | Yes | Yes | Yes |
| Concealed allocation | No | Yes | No | No | Yes |
| Baseline comparability | Yes | Yes | Yes | Yes | Yes |
| Blinded subjects | No | No | No | No | No |
| Blinded therapists | No | No | No | No | No |
| Blinded assessors | Yes | Yes | Yes | Yes | Yes |
| Adequate follow up | Yes | Yes | Yes | Yes | Yes |
| Intention-to-treat analysis | No | Yes | No | No | No |
| Between-group comparisons | Yes | Yes | Yes | Yes | Yes |
| Point estimates and variability | Yes | Yes | Yes | Yes | Yes |
| Total score | 6 | 8 | 6 | 6 | 7 |
Assessment of outcomes
Physical function
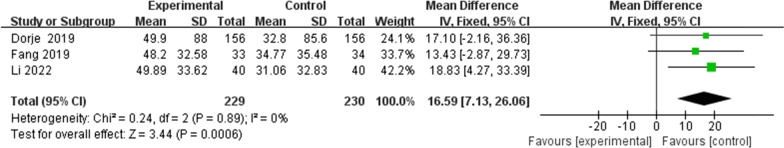
Three studies [32, 33, 35] were included in the review to evaluate the change in the 6MWT. According to the fixed-effect model, there was statistically a significant difference between two groups (MD 16.59, 95%CI 7.13 to 26.06, P = 0.0006) with no heterogeneity among these studies (P = 0.89, I2 = 0%) (Fig. 3). Although Lee et al. did not include the 6MWT, studies have shown a significant increase in metabolic equivalent of the tasks (METs) (+ 34.6%) in the HBCTR group.
Fig. 3.
The six-minute walking test (6MWT)
QoL
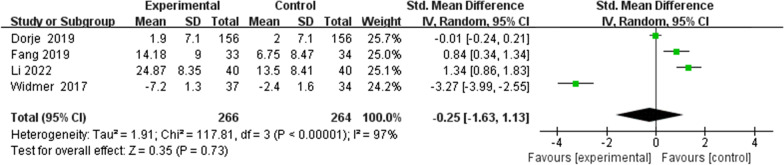
Four studies [32, 33, 35, 36] reported on the QoL of patients. Due to the different scales used for measurements, such as Dartmouth quality of life, the 12-item short form health survey (SF-12) and the 36-item short form health survey (SF-36), the standardized mean difference was used as the effective index. However, there was no significant difference between the HBCTR group versus the control group (SMD − 0.25, 95%CI − 1.63 to 1.13, P = 0.73) with statistical heterogeneity among these studies (P < 0.00001, I2 = 97%) (Fig. 4).
Fig. 4.
Quality of Life (QoL)
Blood pressure
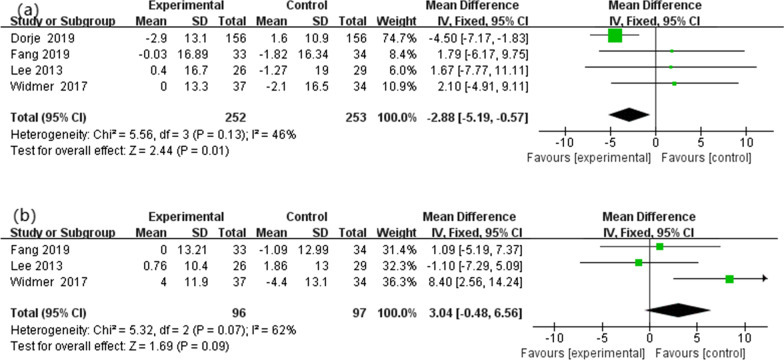
Four studies [32–34, 36] were included in the review to evaluate the change in systolic blood pressure. According fixed effects model, there was statistically significant difference between the HBCTR group versus the control group (MD − 2.88, 95%CI − 5.19 to − 0.57, P = 0.01) with no high heterogeneity among these studies (P = 0.13, I2 = 46%). Nevertheless, only three articles [33, 34, 36] have reported on diastolic blood pressure. According to the fixed-effect model, there was no significant difference between the two groups (MD 3.04, 95%CI − 0.48 to 6.56, P = 0.09) (Fig. 5).
Fig. 5.
Blood pressure: a systolic blood pressure and b diastolic blood pressure
Blood lipid concentrations
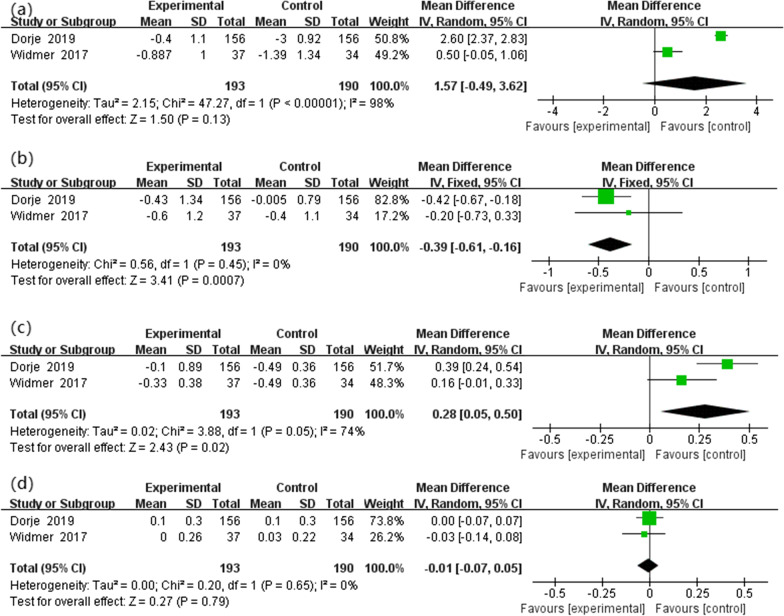
Two studies reported data on blood lipid concentrations. All were included in the review to evaluate the change in TC, TGs, LDL-C and HDL-C. According to the fixed effects model, there was a statistically significant difference between the two groups in TG (MD − 0.39, 95%CI − 0.61 to − 0.16, P = 0.0007). Meta-analysis of the included trials also shows significant differences in LDL-C (MD 0.28, 95%CI 0.05 to 0.50, P = 0.02). However, there was no statistically significant difference in TC and (MD 1.57, 95%CI − 0.49 to 3.62, P = 0.13) and HDL-C (MD − 0.01, 95%CI − 0.07 to 0.05, P = 0.79) (Fig. 6).
Fig. 6.
Blood lipid concentrations: a the change of total cholesterol (TC), b triglyceride (TG), c low-density lipoprotein cholesterol (LDL-C) and d high-density lipoprotein cholesterol (HDL-C)
Discussion
This systematic review and meta-analysis explored the effects of telehealth interventions delivered to cardiac telerehabilitation patients after PCI. The primary outcomes we focus on are exercise capacity and QoL. The findings of this meta-analysis show that patients undergoing HBRCT can significantly improve physical exercise capacity (6WMT) but do not observe a statistically significant improvement in QoL. Secondary outcomes focused on blood pressure and lipids, and HBCTR significantly improved systolic blood pressure but not diastolic blood pressure. Similarly, there were statistically significant differences in TGs and LDL-C between the two groups but no significant differences in TC and HDL-C.
Exercise capacity is a vital measure of the effectiveness of CR in patients with coronary heart disease and is associated with all-cause and cardiovascular mortality. There is also evidence that increased physical activity, exercise training, and overall cardiorespiratory fitness are protective in preventing coronary heart disease [37]. The Peak oxygen consumption (VO2 peak) in the cardiopulmonary exercise test (CPET) is the gold standard for reflecting the patient's cardiorespiratory fitness and exercise capacity [38]. During the test, the patient was set up with a gradual increase in load power. When the patient exercises to a certain extent, cell uptake of oxygen appears a plateau, that is, even if the test power is increased, oxygen uptake does not increase, which is VO2 peak at this time. A recent systematic review and meta-analysis [39] showed that HBTCR assisted by wearable sensors improved cardiorespiratory fitness in people with CVD. Similarly, Duscha et al. [40] showed that the HBCTR sustain the gains in VO2 peak compared to the usual care group. In addition, indicators such as 6MWT, the daily activity time, and daily walking steps can also reflect the patient's cardiorespiratory fitness. Because finally included article only reflected the results of the 6MWT and did not measure by CPET, our meta-analysis focused on 6MWT as the primary outcome. The 6MWT results lack accuracy compared to VO2 peak, but it is also the basis for prescribing exercise for patients as a submaximal exercise capacity test. For the determination of exercise intensity, as described in the study by Luo et al. [41], the average velocity of 6MWT correlated well with the anaerobic threshold of CPET. It is an easy and efficient way to correlate closely with METs values at the anaerobic threshold [42]. At the same time, the 6MWT can be used to judge the degree of disease progression in patients with heart disease, and it also has prognostic value related to cardiorespiratory health.
In our results, telerehabilitation improved 6WMT, which is consistent with previous studies. Schopfer et al. [43] found that patients allocated in-home rehabilitation group achieved a more remarkable 3-month improvement in 6MWT distance (+ 95 vs. + 41 m; P < 0.001). Moreover, a recent meta-analysis [44] showed a significant improvement in the 6-min walk test distance of functional ability participating in HBCTR in 14 randomized controlled trials. HBCTR improves exercise capacity and cardiorespiratory fitness primarily through exercise prescription. Exercise training CR can reduce oxidative stress, improve endothelial progenitor cell function, improve ventricular remodeling and regulate inflammation [45]. These mechanisms dilate the coronary arteries and establish collateral circulation, as mentioned in the study by Mj et al. [46], and exercise also increases blood flow and myocardial energy supply by enlarging the luminal area of collateral vessels and increasing myocardial capillary density [47]. Therefore, telerehabilitation can improve the cardiopulmonary function of patients, thereby increasing the patient's exercise ability and physical activity, which is beneficial to the QoL (MD 25.58 m, 95%CI 14.74 to 36.42).
Another interesting finding of our review is that the improvement of HBCTR in QoL was comparable to that of the control group, with no statistically significant difference. Although our results show that telerehabilitation improves patients' physical capacity, it is closely related to patients' QoL after PCI, including physical and psychological functions. And anxiety and depression in patients with coronary heart disease will seriously affect the QoL [48]. Therefore, we performed a meta-analysis of anxiety or depression scores in the included articles. The research results of included articles show that telerehabilitation training has no obvious advantages in improving negative anxiety and depression (anxiety: MD − 0.03, 95%CI − 0.16 to 0.21, P = 0.0006; depression: MD − 0.43, 95%CI − 1.41 to 0.55, P = 0.0006). The reason for this may be that fewer studies were included and that the duration or intensity of the exercise intervention was insufficient to detect the effect of the intervention. Therefore, confirming the value of telemedicine in this regard should be the focus of future research.
At the same time, some modifiable risk factors, such as blood pressure and blood lipid level, were also focused on in our review. Blood pressure management for Patients Undergoing PCI should be aimed at reducing cardiovascular events [49]. Our findings show that telerehabilitation can effectively improve systolic blood pressure, which may be enhanced by exercise and lifestyle. It reduces sympathetic nerve activity [50], antagonizes the renin–angiotensin–aldosterone system [51], attenuates inflammatory responses and oxidative stress, and improves endothelial dysfunction and vascular remodeling [52]. Then, our results showed that telerehabilitation did not significantly improve diastolic blood pressure in patients, which may be related to the fluctuation of blood pressure for patients after PCI [53]. Studies have shown that the blood vessel wall cannot be significantly contracted or relaxed in patients with coronary heart disease due to atherosclerosis, which may lead to high diastolic blood pressure and difficulty controlling [54]. Therefore, further research is needed to improve the diastolic blood pressure of patients with coronary heart disease by telerehabilitation. In terms of blood lipids, the results showed that HBCTR improved TGs and LDL-C, with no statistically significant improvement in TC and HDL-C. Similar to previous findings, such as in the randomized controlled trial of Pfaeffli et al. [55], patients with CAD in the experimental group received 6 months of personalized telehealth intervention with online support and found that the LDL-C levels of the experimental group were lower than those in the control group. In the trial of Dalli-Peydró et al. [56], who used a smartphone app to instruct participants through exercise schedules and communicate with patients via text message, the results showed that prevented deterioration of the TG/HDL ratio. And one study [57] has shown that if patients with acute myocardial infarction who received PCI and standard medical therapy after 1 year follow-up of LDL-C still ≥ 1.8 mmol/L, more than 65% of patients had an increased risk of long-term death by 42% ~ 45%. Therefore, HBCTR is crucial for the control of risk factors such as blood lipids, which can not only supervise the standardized use of lipid-lowering drugs in patients after PCI surgery, but also further control blood lipids to the ideal level through exercise and lifestyle improvement.
PCI can quickly restore the blood circulation of coronary arteries, improve myocardial ischemia and save heart function. It's been mentioned in many articles that patients after PCI need to pay attention to improving exercise capacity, lower cardiovascular risk profile, and increasing physical functioning, which is associated with an increased incidence of late cardiovascular events [7, 58]. So, CR is a valuable treatment for patients After PCI [59]. Patients after PCI in CR are associated with improved quality of life, reduced readmission rates, and cardiovascular mortality. Hospital-based or center-based CR programs were reportedly challenged by low participant uptake, insufficient attendance, and high drop-out rates. And under telehealth intervention, the benefits are significantly greater because it is more convenient, flexible, and easier to access [60]. Telehealth intervention delivered cardiac telerehabilitation is defined as a telemedicine program that implements telemedicine rehabilitation services for patients by using remote leading media technology. HBCTR includes many aspects, such as remote follow-up, exercise training, health education guidance, remote treatment, monitoring, etc. It mainly sends personalized rehabilitation programs to patients through the network system to improve their conditions for rehabilitation, enhance patients' compliance and persistence in rehabilitation, and save time and cost. At the same time, the patient's self-monitoring and self-evaluation are also crucial for HBCTR. Karen et al. [61] found in the study of 172 patients with acute myocardial infarction that the HBCTR group significantly improved the patient's self-management ability, which was also confirmed by Maddison et al. [62]. And in a descriptive qualitative study of 20 patients in the intervention group using the eHealth CR website, the results showed that telerehabilitation provides social support for changes in patients' cognitive determinants during the intervention [63].
As a result, our goal was to investigate the effect of the HBCTR for patients undergoing PCI. These results are comparable with the results of other meta-analyses. Clark et al. [17] and Neubeck et al. [64] showed that significant favorable changes in TC and systolic blood pressure with telehealth interventions were observed in a meta-analysis. Avila et al. [65] also showed that exercise capacity remained stable during one year following phase II cardiac rehabilitation by home-based exercise with telemonitoring guidance but also using wrist HR monitors. In Batalik et al.'s study [66], each patient received feedback, motivation, and education through telehealth, and the researchers used the Global positioning system to supervise the patient's training site. Helping to self-regulate lifestyle through motivational coaching strategies and objective feedback on training data is an important part of cardiac rehabilitation execution. And the primary findings include evidence that HBTCR is more effective than center-based CR at maintaining long-term cardiorespiratory fitness levels. However, our review is not exactly consistent with previously published systematic reviews, like the FIT@Home study [67], a heart rate monitor with a chest strap and a web application uploaded recorded heart rate data via the Internet were used to guide the exercise process of home telerehabilitation. However, the results showed that there was no significant difference in physical fitness between home exercise training and central exercise training guided by remote monitoring. And our systematic review has some new strengths. We investigated the participants who were restricted after PCI. Post-PCI patients urgently need self-management to improve clinical outcomes, such as reducing depression and anxiety, reducing mortality and morbidity, and improving health-related quality of life (HRQoL) [68]. Compared with a former systematic review, exercise training is a core component in previous studies, but we performed this including some multidisciplinary interventions and multifaceted care, such as physical exercise, nutritional advice, and target-driven pharmacological therapies.
Limitations
There are some limitations to this study. The first limitation is the great variability and complexity of intervention models, such as different frequency and intensity forms. Moreover, the included studies used various models of telerehabilitation (different duration, frequency, length, and intensity). For example, there were a wide of telehealth intervention models, such as smartphone-based CR platforms, remote monitoring systems, wireless monitoring, and sports band with a smartphone. Therefore, future research needs to explore which model is best for these patients. Second, some results could not be quantitatively analyzed due to the relatively small sample size of the included studies. Third, we only focused on treatment efficacy and need to pay attention to operability and cost of services, which should be included in future studies. Therefore, more extensive randomized controlled trials are required in order to confirm the current evidence.
Conclusions
This systematic review and meta-analysis have proved that HBCTR can effectively improve patients' physical function after PCI. These results justify that the home-based telehealth intervention is one of the promisingly effective CR strategies that reduce cardiovascular disease risk factors. In order to further confirm HBCTR increasing uptake and make CR available, the sample size needs to be increased, and future research needs to explore which model is best for these patients.
Acknowledgements
No
Abbreviations
- CAD
Coronary artery disease
- PCI
Percutaneous coronary intervention
- CR
Cardiac rehabilitation
- HBCR
Home-based cardiac rehabilitation
- HBCTR
Home-based cardiac telerehabilitation
- 6MWT
The six-minute walking test
- QoL
Quality of life
- TC
Total cholesterol
- TGs
Triglycerides
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- RCTs
Relevant randomized controlled trials
- VO2 peak
Peak oxygen consumption
- CPET
Cardiopulmonary exercise test
Author contributions
Conceptualization: WZ and QW; Methodology: QW, CF and LX; Formal analysis and investigation: XS, CF and QW; Writing of original draft preparation: WZ; Writing of review and editing: QW, CF, LX, XS and SW; Funding acquisition: QW; Resources: CH; Supervision: QW. All authors read and approved the final manuscirpt.
Funding
This work was supported by National Key R&D Program of China (Grand No. 2020YFC2008500 and 2020YFC2008502) and National Natural Science Foundation of China (Grant No. 81572231 and 82172534) and 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC21038).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author(s) has/have no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen Zhong and Chenying Fu have contributed equally to this work
References
- 1.Kang EH, Park EH, Shin A, Song JS, Kim SC. Cardiovascular risk associated with allopurinol vs. benzbromarone in patients with gout. Eur Heart J. 2021;42(44):4578–4588. doi: 10.1093/eurheartj/ehab619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen RH, Wedell-Neergaard AS, Lehrskov LL, Legaard GE, Dorph E, Larsen MK, Launbo N, Fagerlind SR, Seide SK, Nymand S, et al. Effect of aerobic and resistance exercise on cardiac adipose tissues: secondary analyses from a randomized clinical trial. JAMA Cardiol. 2019;4(8):778–787. doi: 10.1001/jamacardio.2019.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi S, Kim K, Kim SM, Lee G, Jeong SM, Park SY, Kim YY, Son JS, Yun JM, Park SM. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med. 2018;178(8):1060–1068. doi: 10.1001/jamainternmed.2018.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the american college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124(23):e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL. Percutaneous coronary intervention in 2018. JAMA. 2018;319(20):2127–2128. doi: 10.1001/jama.2018.5281. [DOI] [PubMed] [Google Scholar]
- 6.Giannini F, Candilio L, Mitomo S, Ruparelia N, Chieffo A, Baldetti L, Ponticelli F, Latib A, Colombo A. A practical approach to the management of complications during percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(18):1797–1810. doi: 10.1016/j.jcin.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;1:Cd001800. doi: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keib CN, Reynolds NR, Ahijevych KL. Poor use of cardiac rehabilitation among older adults: a self-regulatory model for tailored interventions. Heart Lung. 2010;39(6):504–511. doi: 10.1016/j.hrtlng.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas RJ, King M, Lui K, Oldridge N, Piña IL, Spertus J. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services endorsed by the American college of chest physicians, the American college of sports medicine, the American physical therapy association, the Canadian association of cardiac rehabilitation, the clinical exercise physiology association, the European association for cardiovascular prevention and rehabilitation, the inter-American heart foundation, the national association of clinical nurse specialists, the preventive cardiovascular nurses association, and the society of thoracic surgeons. J Am Coll Cardiol. 2010;56(14):1159–1167. doi: 10.1016/j.jacc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American college of cardiology/American heart association task force on performance measures. Circ Cardiovasc Qual Outcomes. 2018;11(4):e000037. doi: 10.1161/HCQ.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 11.Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the behavioral risk factor surveillance system. J Am Heart Assoc. 2017;7(1). [DOI] [PMC free article] [PubMed]
- 12.Neubeck L, Freedman SB, Briffa T, Bauman A, Redfern J. Four-year follow-up of the choice of health options in prevention of cardiovascular events randomized controlled trial. Eur J Cardiovasc Prev Rehabil. 2011;18(2):278–286. doi: 10.1097/HJR.0b013e32833cca66. [DOI] [PubMed] [Google Scholar]
- 13.Sukul D, Seth M, Barnes GD, Dupree JM, Syrjamaki JD, Dixon SR, Madder RD, Lee D, Gurm HS. Cardiac rehabilitation use after percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(24):3148–3152. doi: 10.1016/j.jacc.2019.03.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home-based cardiac rehabilitation: a scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American heart association, and the American college of cardiology. Circulation. 2019;140(1):e69–e89. doi: 10.1161/CIR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2010;(1):Cd007130. [DOI] [PMC free article] [PubMed]
- 16.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol. 2015;22(1):35–74. doi: 10.1177/2047487313501093. [DOI] [PubMed] [Google Scholar]
- 18.Thaker DA, Monypenny R, Olver I, Sabesan S. Cost savings from a telemedicine model of care in northern Queensland, Australia. Med J Aust. 2013;199(6):414–417. doi: 10.5694/mja12.11781. [DOI] [PubMed] [Google Scholar]
- 19.Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, Warburton D, Jones L, Clark AM. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials. 2011;12:92. doi: 10.1186/1745-6215-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King M, Bittner V, Josephson R, Lui K, Thomas RJ, Williams MA. Medical director responsibilities for outpatient cardiac rehabilitation/secondary prevention programs: 2012 update: a statement for health care professionals from the American Association of Cardiovascular and Pulmonary Rehabilitation and the American Heart Association. Circulation. 2012;126(21):2535–2543. doi: 10.1161/CIR.0b013e318277728c. [DOI] [PubMed] [Google Scholar]
- 21.Istepanian R, Jovanov E, Zhang YT. Introduction to the special section on M-Health: beyond seamless mobility and global wireless health-care connectivity. IEEE Trans Inf Technol Biomed. 2004;8(4):405–414. doi: 10.1109/TITB.2004.840019. [DOI] [PubMed] [Google Scholar]
- 22.Wongvibulsin S, Habeos EE, Huynh PP, Xun H, Shan R, Porosnicu Rodriguez KA, Wang J, Gandapur YK, Osuji N, Shah LM, et al. Digital health interventions for cardiac rehabilitation: systematic literature review. J Med Internet Res. 2021;23(2):e18773. doi: 10.2196/18773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Luo Z, Yang M, Huang W, Yu P. Efficacy and safety of digital therapeutics-based cardiac rehabilitation in heart failure patients: a systematic review. ESC Heart Fail. 2022;9(6):3751–3760. doi: 10.1002/ehf2.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21(1):45–53. doi: 10.1177/1357633X14562732. [DOI] [PubMed] [Google Scholar]
- 25.Stefanakis M, Batalik L, Antoniou V, Pepera G. Safety of home-based cardiac rehabilitation: a systematic review. Heart Lung. 2022;55:117–126. doi: 10.1016/j.hrtlng.2022.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Jin W, Zhang XX, Xu W, Liu XN, Ren CC. Telerehabilitation Approaches for stroke patients: systematic review and meta-analysis of randomized controlled trials. J Stroke Cerebrovasc Dis. 2015;24(12):2660–2668. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Xie SH, Wang Q, Wang LQ, Wang L, Song KP, He CQ. Effect of internet-based rehabilitation programs on improvement of pain and physical function in patients with knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2021;23(1):e21542. doi: 10.2196/21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang S, Xiang J, Gao X, Guo K, Liu B. The comparison of telerehabilitation and face-to-face rehabilitation after total knee arthroplasty: a systematic review and meta-analysis. J Telemed Telecare. 2018;24(4):257–262. doi: 10.1177/1357633X16686748. [DOI] [PubMed] [Google Scholar]
- 29.Russell TG, Buttrum P, Wootton R, Jull GA. Internet-based outpatient telerehabilitation for patients following total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93(2):113–120. doi: 10.2106/JBJS.I.01375. [DOI] [PubMed] [Google Scholar]
- 30.Brouwers RWM, Kraal JJ, Regis M, Spee RF, Kemps HMC. Effectiveness of cardiac telerehabilitation with relapse prevention: smartcare-CAD randomized controlled trial. J Am Coll Cardiol. 2021;77(21):2754–2756. doi: 10.1016/j.jacc.2021.03.328. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorje T, Zhao G, Tso K, Wang J, Chen Y, Tsokey L, Tan BK, Scheer A, Jacques A, Li Z, et al. Smartphone and social media-based cardiac rehabilitation and secondary prevention in China (SMART-CR/SP): a parallel-group, single-blind, randomised controlled trial. Lancet Digit Health. 2019;1(7):e363–e374. doi: 10.1016/S2589-7500(19)30151-7. [DOI] [PubMed] [Google Scholar]
- 33.Fang J, Huang B, Xu D, Li J, Au WW. Innovative application of a home-based and remote sensing cardiac rehabilitation protocol in chinese patients after percutaneous coronary intervention. Telemed J E Health. 2019;25(4):288–293. doi: 10.1089/tmj.2018.0064. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Hur SH, Sohn J, Lee HM, Park NH, Cho YK, Park HS, Yoon HJ, Kim H, Nam CW, et al. Impact of home-based exercise training with wireless monitoring on patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Korean Med Sci. 2013;28(4):564–568. doi: 10.3346/jkms.2013.28.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Hui Z, Zheng Y, Yu J, Zhang J. Efficacy of phase II remote home rehabilitation in patients with acute myocardial infarction after percutaneous coronary intervention. Contrast Media Mol Imaging. 2022;2022:4634769. doi: 10.1155/2022/4634769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widmer RJ, Allison TG, Lennon R, Lopez-Jimenez F, Lerman LO, Lerman A. Digital health intervention during cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2017;188:65–72. doi: 10.1016/j.ahj.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training in secondary coronary heart disease prevention. Prog Cardiovasc Dis. 2011;53(6):397–403. doi: 10.1016/j.pcad.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 39.Antoniou V, Davos CH, Kapreli E, Batalik L, Panagiotakos DB, Pepera G: Effectiveness of home-based cardiac rehabilitation, using wearable sensors, as a multicomponent, cutting-edge intervention: a systematic review and meta-analysis. J Clin Med. 2022, 11(13). [DOI] [PMC free article] [PubMed]
- 40.Duscha BD, Piner LW, Patel MP, Craig KP, Brady M, McGarrah RW, 3rd, Chen C, Kraus WE. Effects of a 12-week mHealth program on peak VO(2) and physical activity patterns after completing cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2018;199:105–114. doi: 10.1016/j.ahj.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Luo Q, Li C, Zhuang B, Li G, Luo L, Ni Y, Huang Z, Wang L, Song H, Yan W, et al. Establishment of exercise intensity for patients with chronic heart failure equivalent to anaerobic threshold based on 6-minute walking test. Ann Palliat Med. 2020;9(5):2766–2775. doi: 10.21037/apm-20-265. [DOI] [PubMed] [Google Scholar]
- 42.Forman DE, Fleg JL, Kitzman DW, Brawner CA, Swank AM, McKelvie RS, Clare RM, Ellis SJ, Dunlap ME, Bittner V. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60(25):2653–2661. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schopfer DW, Whooley MA, Allsup K, Pabst M, Shen H, Tarasovsky G, Duvernoy CS, Forman DE. Effects of home-based cardiac rehabilitation on time to enrollment and functional status in patients with ischemic heart disease. J Am Heart Assoc. 2020;9(19):e016456. doi: 10.1161/JAHA.120.016456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran HJ, Jiang Y, Tam WWS, Yeo TJ, Wang W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(7):1017–1043. doi: 10.1093/eurjpc/zwab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15(12):731–743. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong MJ, Sigal RJ, Arena R, Hauer TL, Austford LD, Aggarwal S, Stone JA, Martin BJ. Cardiac rehabilitation completion is associated with reduced mortality in patients with diabetes and coronary artery disease. Diabetologia. 2015;58(4):691–698. doi: 10.1007/s00125-015-3491-1. [DOI] [PubMed] [Google Scholar]
- 47.Marzolini S, Blanchard C, Alter DA, Grace SL, Oh PI. Delays in referral and enrolment are associated with mitigated benefits of cardiac rehabilitation after coronary artery bypass surgery. Circ Cardiovasc Qual Outcomes. 2015;8(6):608–620. doi: 10.1161/CIRCOUTCOMES.115.001751. [DOI] [PubMed] [Google Scholar]
- 48.Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14(3):145–155. doi: 10.1038/nrcardio.2016.181. [DOI] [PubMed] [Google Scholar]
- 49.Boersma E, Keil U, De Bacquer D, De Backer G, Pyörälä K, Poldermans D, Leprotti C, Pilotto L, de Swart E, Deckers JW, et al. Blood pressure is insufficiently controlled in European patients with established coronary heart disease. J Hypertens. 2003;21(10):1831–1840. doi: 10.1097/00004872-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Yoo JK, Fu Q. Impact of sex and age on metabolism, sympathetic activity, and hypertension. Faseb J. 2020;34(9):11337–11346. doi: 10.1096/fj.202001006RR. [DOI] [PubMed] [Google Scholar]
- 51.Shim CY, Ha JW, Park S, Choi EY, Choi D, Rim SJ, Chung N. Exaggerated blood pressure response to exercise is associated with augmented rise of angiotensin II during exercise. J Am Coll Cardiol. 2008;52(4):287–292. doi: 10.1016/j.jacc.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 52.Ikizler TA, Robinson-Cohen C, Ellis C, Headley SAE, Tuttle K, Wood RJ, Evans EE, Milch CM, Moody KA, Germain M, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29(1):250–259. doi: 10.1681/ASN.2017010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54(20):1827–1834. doi: 10.1016/j.jacc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, et al. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;130(6):466–474. doi: 10.1161/CIRCULATIONAHA.113.004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaeffli Dale L, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text message and internet support for coronary heart disease self-management: results from the text4heart randomized controlled trial. J Med Internet Res. 2015;17(10):e237. doi: 10.2196/jmir.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalli-Peydró E, Gisbert-Criado R, Amigó N, Sanz-Sevilla N, Cosín-Sales J. Cardiac telerehabilitation with long-term follow-up reduces GlycA and improves lipoprotein particle profile: a randomised controlled trial. Int J Cardiol. 2022;369:60–64. doi: 10.1016/j.ijcard.2022.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sud M, Han L, Koh M, Abdel-Qadir H, Austin PC, Farkouh ME, Godoy LC, Lawler PR, Udell JA, Wijeysundera HC, et al. Low-density lipoprotein cholesterol and adverse cardiovascular events after percutaneous coronary intervention. J Am Coll Cardiol. 2020;76(12):1440–1450. doi: 10.1016/j.jacc.2020.07.033. [DOI] [PubMed] [Google Scholar]
- 59.McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. 2017;27(6):420–425. doi: 10.1016/j.tcm.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subedi N, Rawstorn JC, Gao L, Koorts H, Maddison R. Implementation of telerehabilitation interventions for the self-management of cardiovascular disease: systematic review. JMIR Mhealth Uhealth. 2020;8(11):e17957. doi: 10.2196/17957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh KW, Wang W, Richards AM, Chan MY, Cheng KK. Effectiveness of advanced practice nurse-led telehealth on readmissions and health-related outcomes among patients with post-acute myocardial infarction: ALTRA Study Protocol. J Adv Nurs. 2016;72(6):1357–1367. doi: 10.1111/jan.12933. [DOI] [PubMed] [Google Scholar]
- 62.Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, Jiang Y, Gao L, Moodie M, Warren I, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105(2):122–129. doi: 10.1136/heartjnl-2018-313189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su JJ, Paguio J, Baratedi WM, Abu-Odah H, Batalik L. Experience of coronary heart disease patients with a nurse-led eHealth cardiac rehabilitation: qualitative process evaluation of a randomized controlled trial. Heart Lung. 2023;57:214–221. doi: 10.1016/j.hrtlng.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2009;16(3):281–289. doi: 10.1097/HJR.0b013e32832a4e7a. [DOI] [PubMed] [Google Scholar]
- 65.Avila A, Claes J, Buys R, Azzawi M, Vanhees L, Cornelissen V. Home-based exercise with telemonitoring guidance in patients with coronary artery disease: Does it improve long-term physical fitness? Eur J Prev Cardiol. 2020;27(4):367–377. doi: 10.1177/2047487319892201. [DOI] [PubMed] [Google Scholar]
- 66.Batalik L, Dosbaba F, Hartman M, Konecny V, Batalikova K, Spinar J. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur J Phys Rehabil Med. 2021;57(5):807–814. doi: 10.23736/S1973-9087.21.06653-3. [DOI] [PubMed] [Google Scholar]
- 67.Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, Stut W, Peek N, Kemps HM. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: results of the FIT@Home study. Eur J Prev Cardiol. 2017;24(12):1260–1273. doi: 10.1177/2047487317710803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sukul D, Seth M, Thompson MP, Keteyian SJ, Boyden TF, Syrjamaki JD, Yaser J, Likosky DS, Gurm HS. Hospital and operator variation in cardiac rehabilitation referral and participation after percutaneous coronary intervention: insights from blue cross blue shield of michigan cardiovascular consortium. Circ Cardiovasc Qual Outcomes. 2021;14(11):e008242. doi: 10.1161/CIRCOUTCOMES.121.008242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.