Abstract
Introduction
The process of cell product changeover poses a high risk of cross-contamination. Hence, it is essential to minimize cross-contamination while processing cell products. Following its use, the surface of a biosafety cabinet is commonly disinfected by ethanol spray and manual wiping methods. However, the effectiveness of this protocol and the optimal disinfectant have not yet been evaluated. Here, we assessed the effect of various disinfectants and manual wiping methods on bacterial removal during cell processing.
Methods
The hard surface carrier test was performed to evaluate the disinfectant efficacy of benzalkonium chloride with a corrosion inhibitor (BKC + I), ethanol (ETH), peracetic acid (PAA), and wiping against Bacillus subtilis endospores. Distilled water (DW) was used as the control. A pressure sensor was employed to investigate the differences in loading under dry and wet conditions. The pre-spray for wiping was monitored by eight operators using a paper that turns black when wet. Chemical properties, including residual floating proteins, and mechanical properties, such as viscosity and coefficient of friction, were examined.
Results
In total, 2.02 ± 0.21-Log and 3.00 ± 0.46-Log reductions from 6-Log CFU of B. subtilis endospores were observed for BKC + I and PAA, respectively, following treatment for 5 min. Meanwhile, wiping resulted in a 0.70 ± 0.12-Log reduction under dry conditions. Under wet conditions, DW and BKC + I showed 3.20 ± 0.17-Log and 3.92 ± 0.46-Log reductions, whereas ETH caused a 1.59 ± 0.26-Log reduction. Analysis of the pressure sensor suggested that the force was not transmitted under dry conditions. Evaluation of the amount of spray by eight operators showed differences and bias in the spraying area. While ETH had the lowest ratio in the protein floating and collection assays, it exhibited the highest viscosity. BKC + I had the highest friction coefficient under 4.0–6.3 mm/s; however, that of BKC + I decreased and became similar to the friction coefficient of ETH under 39.8–63.1 mm/s.
Conclusions
DW and BKC + I are effective for inducing a 3-Log reduction in bacterial abundance. Moreover, the combination of optimal wet conditions and disinfectants is essential for effective wiping in specific environments containing high-protein human sera and tissues. Given that some raw materials processed in cell products contain high protein levels, our findings suggest that a complete changeover of biosafety cabinets is necessary in terms of both cleaning and disinfection.
Keywords: Changeover, Wiping, Cross-contamination, Biosafety cabinet, Cell-product processing
Abbreviations: BSC, biosafety cabinet; CFU, colony forming unit; FBS, fetal bovine serum; DW, distilled water; BKC + I, benzalkonium chloride with corrosion inhibitor; ETH, ethanol; PAA, peracetic acid; SUS, stainless steel
Highlights
-
•
Cell product changeover poses the risk of cross-contamination in cell processing.
-
•
Ethanol spraying and wiping are commonly used for cleaning during changeover.
-
•
We evaluated disinfectant spraying and wiping using a hard surface career test.
-
•
Wet condition and appropriate disinfectant are necessary for effective wiping.
-
•
Spraying can vary based on the operator; thus, proper protocols should be devised.
1. Introduction
While processing cell products that cannot be sterilized, is important to ensure sterility of the working space. In fact, the cell products must be protected against environmental bacteria present in cell processing facilities [[1], [2], [3], [4]] and bacteria and fungi in raw materials [5,6]. These microorganisms may remain in biosafety cabinets—for example, in culture media droplets—potentially contributing to the risk of cell product contamination [7,8]. Hence, minimizing the risk of contamination during cell processing may ensure a higher degree of cell sterility. For this purpose, it is necessary to carry out an appropriate changeover process to ensure a sterile cell processing environment. Commonly used cleaning methods like fogging with hydrogen peroxide have raised concerns over their effects on cells due to their leftover residues after treatment [9]. Additionally, ethanol spray followed by wiping and UV irradiation is commonly applied as another standard biosafety cabinet cleaning method after sampling environmental bacteria. However, these protocols can require more time to confirm sterility prior to initializing subsequent cell processing. In fact, the effectiveness of this standard cleaning method has not been fully evaluated, and in particular, the effectiveness of wiping remains unclear.
The current study sought to assess the efficacy of wiping in eliminating bacteria during cell processing, and to establish an optimal disinfectant protocol for biosafety cabinets. More specifically, we evaluated the efficacy of disinfectants and wiping of biosafety cabinets using the hard surface carrier test as per the guidelines for the evaluation of disinfection methods specified in the Japanese Pharmacopoeia. We then evaluated the factors that contribute to the variability in wiping between different operators by using pressure sensors and fact-finding surveys. Finally, we investigated the chemical properties of disinfectants using protein floating and collection assays, as well as the mechanical properties in terms of viscosity and coefficient of friction.
2. Materials and methods
2.1. Hard surface carrier test for spraying and wiping
Bacteria that are highly resistant to disinfectants and frequently detected in cell-processing facilities were used [8]. More specifically, Bacillus subtilis endospores (1 × 108 CFU, NBRC 13722, Bioball Multishot 10E8; bioMérieux, Marcy l'Etoile, France) dissolved in rehydration fluid (bioMérieux) were diluted to 1 × 106 CFU in physiological saline (Otsuka Pharmaceutical Co. Ltd., Tokushima, Japan) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, MA, USA). To simulate spraying, 100 μL of the bacterial dilutions were added dropwise onto a SUS304 stainless steel plate (5 × 5 cm; AS ONE Co., Osaka, Japan) using a micropipette and subsequently air-dried.
Disinfectant treatment included distilled water (DW, Otsuka Pharmaceutical Co. Ltd.) as a control group, benzalkonium chloride with corrosion inhibitor (BKC + I, Zalkonin N solution; 0.1% w/v benzalkonium chloride containing 0.5% w/v dicyclohexylamine nitrite as an anticorrosive and 8% w/v ethanol as a preservative, Kenei Pharmaceutical Co., Ltd., Osaka, Japan), disinfectant ethanol (ETH, ethanol for disinfection; 76.9–81.4% w/v ethanol and 3.7% w/v isopropyl alcohol, Yamazen Pharm Co., Osaka, Japan), and peracetic acid (PAA, Acecide; 0.3% w/v peracetic acid, Saraya Co., Ltd, Osaka, Japan) for 1 or 5 min of static treatment. Wiping treatment was performed using a 7 × 7 cm cutting BEMCOT (Asahi Kasei Co., Tokyo, Japan) with or without the relevant solution immediately after applying 1.5 kg of one-way force at 50 mm/s.
After disinfectant or wiping treatment, bacteria were collected by the swab method using 300 μL physiological saline and appropriate amounts of neutralizer (Saraya Co.) during the PAA collection. The collected solution was diluted, seeded in soybean-casein digest agar medium (Nissui-seiyaku Ltd., Tokyo, Japan), and incubated at 37 °C. The bacterial collection and seeding were performed in triplicates.
2.2. Wiping pressure and contact area
A pressure sensor (Tekscan, Inc., MA, USA) was used to record the peak contact load distribution, contact load, contact area, and maximum contact pressure. The data were analyzed using MATLAB (MathWorks, Inc., MA, USA). To simulate wiping, the wiper was dipped in 3 mL of ETH, and wrapped around a 20-mm diameter stainless steel tube to ensure application of even force, followed by manual wiping with a 3 × 3 cm sensor at 5 mm/s using 1.5 kg of force. This simulation was performed six times under dry and wet conditions.
2.3. Survey on pre-spraying for wiping
To determine the amount of spray applied following the regular use of a biosafety cabinet, a study on pre-spraying for wiping was performed by eight operators using distilled water on paper (60 cm × 90 cm; Mizukakigoo, Tokyo, Japan) that becomes black when wet. The amount of water was measured before and after spraying, and each spray weighed approximately 1 mg. Blackened papers were photographed using a digital camera (EOS 60D; Canon, Tokyo, Japan), and specific central regions (50 cm × 40 cm) in the image were extracted and quantified using ImageJ software version 1.53a (National Institutes of Health, MD, USA). The area of the image was divided in the middle to denote left and right panel differences. The difference between the coverage areas were presented as an absolute value.
2.4. Protein floating and collection assay for analyzing chemical properties
A total of 100 μL of FBS with a known protein concentration was air-dried on an SUS plate and collected in 300 μL of each solution. The protein concentration was measured at 280 nm using a NanoDrop spectrophotometer (Thermo Scientific).
2.5. Mechanical properties of disinfectants
A dynamic viscoelasticity apparatus (MCR302, AntonPaar, Graz, Austria) was used to measure the viscosity. In brief, a cone-plate sensor (diameter, 75 mm; cone angle, 1°) was used as the measurement sensor under the following conditions: temperature of 25 °C, and shear rate of 10 s−1 to 1000 s−1. After adding approximately 2.5 mL of the sample to the lower Peltier temperature-controlled plate, the measurement was initiated when the sensor reached the measuring gap. Values of 51.8 and 72 s−1 were selected from three independent measurements.
A ball-on-3-pin type tribological cell, included in the dynamic viscoelasticity apparatus, was used to measure the friction coefficient. The ball was made of glass with a diameter of 12.7 mm, and the pin, with a diameter of 6 mm and length of 6 mm, was made of dimethylpolysiloxane. Measurement conditions were, a temperature of 25 °C, slip velocity of 0.01–100 mm/s, and load of 1 N. After adding approximately 0.5 mL sample to the lower holder, the measurement was initiated when the measuring sensor reached the set load. From three independent measurements, values of 4.0 and 6.3 mm/s, which were considered practical condition, and of 39.8 and 63.1 mm/s, which were used for analysis condition, were compared, respectively.
2.6. Statistical analysis
GraphPad Prism version 9.0.0 (GraphPad Software, La Jolla, CA, USA) was employed for all statistical analyses. Data were presented as the mean ± standard deviation (SD). Two-group analysis was performed by the Mann–Whitney test. Multiple comparisons were performed by the Kruskal–Wallis test, followed by Dunn's multiple comparison test and correlation analysis by Spearman's correlation. Statistical significance was set at P < 0.05.
3. Results
3.1. Hard surface carrier test for spraying and wiping
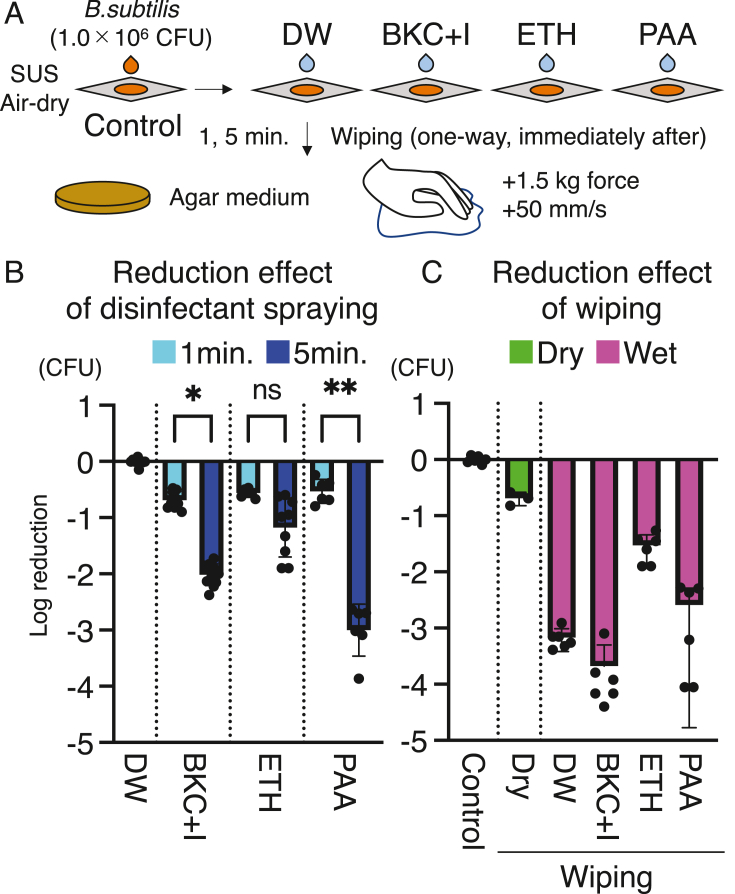
We evaluated the effect of disinfectant spraying and manual wiping against 6-Log CFU of B. subtilis endospores using a hard surface carrier test (Fig. 1A). No disinfectant showed more than a 1-Log reduction effect after 1 min of treatment (Fig. 1B). In total, 2.02 ± 0.21-Log reduction and 3.00 ± 0.46-Log reduction were observed for BKC + I and PAA, respectively, after 5 min treatment. Meanwhile, ETH induced a 1.18 ± 0.52-Log reduction after 5 min, with no significant difference from the 0.56 ± 0.08-Log reduction observed after 1 min of treatment. Wiping did not result in a more than 0.70 ± 0.12-Log reduction under dry conditions (Fig. 1C). Additionally, under wet conditions, DW and BKC + I induced a 3.20 ± 0.17-Log and 3.92 ± 0.46-Log reduction, respectively, whereas ETH showed 1.59 ± 0.26-Log reduction, and PAA resulted in a 3.04 ± 0.86-Log reduction with large dispersion (Fig. 1C).
Fig. 1.
Hard surface carrier test with wiping. (A) Experimental design of hard surface tests to evaluate the effects of disinfectants and wiping. CFU: colony forming unit. DW: distilled water. BKC + I: benzalkonium chloride with corrosion inhibitor. ETH: ethanol. PAA: peracetic acid. SUS: stainless steel. (B) Reduction effect of disinfectants. Log reduction of CFU from 1.0 × 106 CFU B. subtilis in the collection solution after disinfectant treatment of SUS plates. ∗P < 0.05, and ∗∗P < 0.01. P values were calculated using Kruskal–Wallis test followed by Dunn's multiple comparison test. (C) Reduction effect of wiping. Log reduction of CFU from 1.0 × 106 CFU B. subtilis in the collection solution immediately after wiping as the disinfectant treatment of SUS plates.
3.2. Wiping pressure between dry and wet
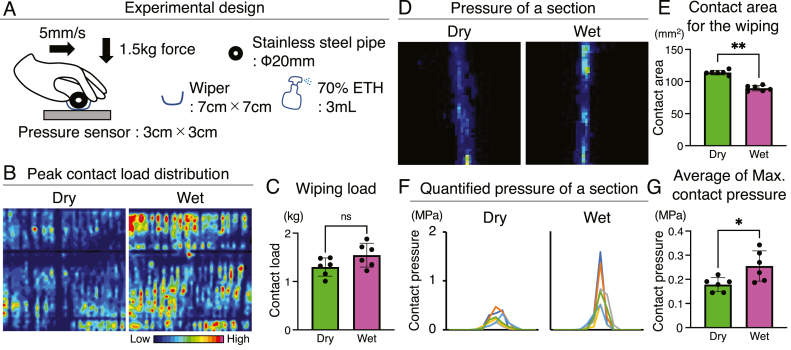
To investigate the differences between wiping methods, we performed evaluations using a pressure sensor (Fig. 2A). Under these examinations, the wiping contact load was 1.30 ± 0.19 kg for dry and 1.55 ± 0.25 kg for wet, with no significant differences (Fig. 2B and C), indicating that the experimental conditions were comparable. However, the contact area for the wiping differed between dry and wet condition, with 114.0 ± 3.56 mm2 for dry and 89.57 ± 4.23 mm2 for wet (Fig. 2D and E). Therefore, the maximum load applied was 0.26 ± 0.06 MPa in wet, which was significantly higher than the 0.18 ± 0.03 MPa in dry (Fig. 2F and G).
Fig. 2.
Wiping pressure between dry and wet. (A) Experimental design of wiping between dry and wet using a pressure sensor. The pressure was evaluated independently six times. (B) The representative peak contact load distribution of each sensor cell recorded by an indenter moving in one direction. (C) Wiping contact load per sensor. P value was calculated using Mann–Whitney test. (D) The representative pressure of a section between dry and wet. (E) Contact area for the wiping to pressure sensor. (F) The representative quantified pressure of a section. (G) Average of maximum contact pressure per cell of the sensor. Data are presented as mean ± SD. ∗P < 0.05, and ∗∗P < 0.01. Data are presented as mean ± SD. P values were calculated using Mann–Whitney test.
3.3. Survey on pre-spraying for wiping
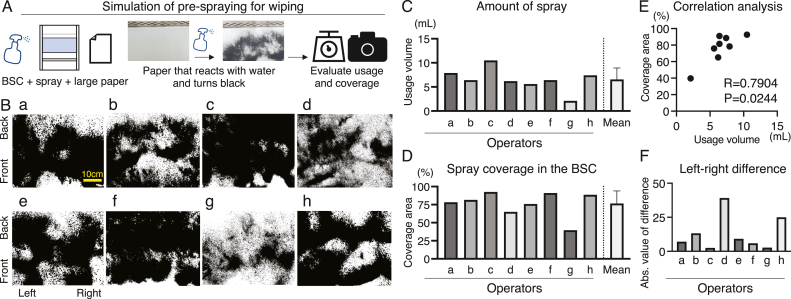
A survey on pre-spraying for wiping was conducted to analyze the differences in wiping between operators (Fig. 3A). A survey of eight operators revealed significant differences (Fig. 3B). The average amount of spray was 6.56 ± 2.36 g (Fig. 3C). The average coverage area during the use of these sprays was 76.59 ± 17.46 (Fig. 3D). Spray usage and coverage area were correlated (R = 0.79, P = 0.02; Fig. 3E). Left–right differences in coverage area were observed in some cases, exceeding 25 (Fig. 3F).
Fig. 3.
Survey on pre-spraying for wiping. (A) Experimental design of pre-spraying simulation for wiping. BSC: biosafety cabinet. (B) Results of a survey of eight operators. Scale bar: 10 cm. (C) Amount of spray used in the BSC by each operator. (D) Spray coverage in the BSC was evaluated for each operator. (E) Correlation analysis between usage volume and coverage area. R and P values were calculated using Spearman's correlation analysis. (F) Left-right difference of spray coverage area for each operator. The difference between the coverage areas were presented as an absolute value. Abs.: absolute.
3.4. Wiping force, chemical and mechanical properties of disinfectant
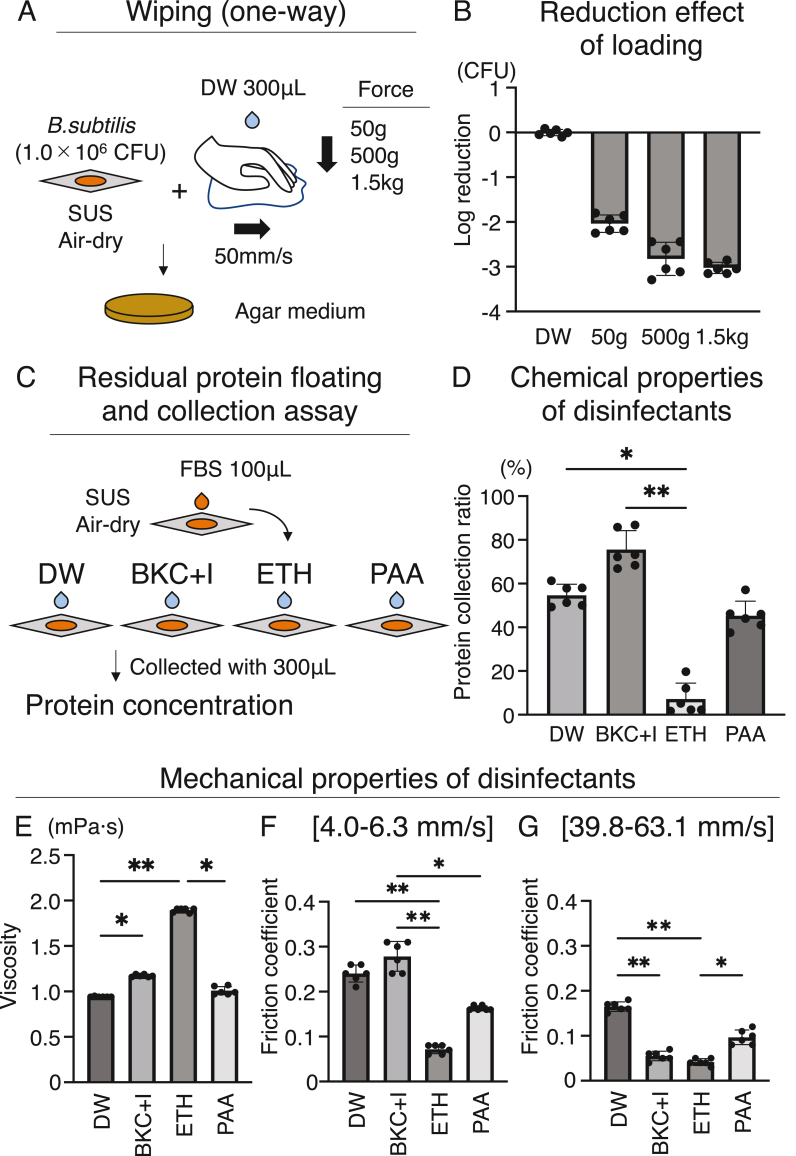
Analysis of differences caused by mechanical loading of wiping showed 2.03 ± 0.19-Log reduction for 50 g loading and 3.02 ± 0.12-Log reduction for 1.5 kg loading (Fig. 4A and B).
Fig. 4.
Wiping force, chemical, and mechanical properties of disinfectant. (A) Experimental design of wiping between force difference. FBS: fetal bovine serum. (B) Log reduction effect of wiping between force difference. (C) Experimental design of residual protein floating and collection assay. (D) Protein collection ratios were used to determine the chemical properties of the disinfectants. (E) Viscosity. (F) Friction coefficient by sliding velocity condition under 4.0–6.3 mm/s. (G) Friction coefficient by sliding velocity condition under 39.8–63.1 mm/s. (E) Viscosity and (F) Friction coefficient were used to determine the mechanical properties of the disinfectants. Data are presented as mean ± SD. ∗P < 0.05, and ∗∗P < 0.01. P values were calculated by Kruskal–Wallis test followed with Dunn's multiple comparison test.
The chemical properties of the disinfectant were assessed using a floating protein collection assay, which reproduced contamination by a cell culture with serum, revealed that the protein collection rate was 54.63 ± 5.09%, 74.55 ± 8.66%, 7.22 ± 7.25%, and 45.3 ± 6.66% for DW, BKC + I, ETH, and PAA, respectively (Fig. 4C and D). ETH had a significantly lower collection rate than DW and BKC + I.
The mechanical properties of the disinfectants were analyzed in terms of their viscosity and friction coefficient. ETH exhibited the highest viscosity, i.e., 1.90 ± 0.02 mPa s (Fig. 4E), and it was suggested to have the poorest diffusion when sprayed into the biosafety cabinet. The friction coefficient, which indicates the ease of wiping off, under 4.0–6.3 mm/s and 39.8–63.1 mm/s showed that the ETH had the lowest value, i.e., 0.07 ± 0.01 and 0.04 ± 0.01 (Fig. 4F and G). BKC + I had the highest friction coefficient, 0.28 ± 0.03 under 4.0–6.3 mm/s, while BKC + I decreased to 0.06 ± 0.01 and became similar to the value of ETH under 39.8–63.1 mm/s (Fig. 4F and G).
4. Discussion
Manual cell product processing in biosafety cabinets poses a serious risk of cross-contamination from culture media. Therefore, cleanup should be performed by disinfectant spraying and wiping for the cell product changeover. Here, we evaluated the effect of disinfectant spraying and wiping using a hard surface carrier test and subsequently analyzed the pressure sensor, operator survey, and properties of disinfectants to investigate the differences in wiping methods. Disinfectants are frequently utilized in the medical field, and their effectiveness on their own is well established; however, their combination with wiping can enhance the disinfection process. This effect may depend on the chemical and mechanical properties of the disinfectant, such as its ability to float proteins and its coefficient of friction. The selection of these disinfectants may be an important factor in cell processing for regenerative therapy, where serum and human tissues are handled with care due to various contamination factors.
In our study, disinfectant spraying and wiping were evaluated using the hard surface carrier test, which is listed as an evaluation method for disinfection in the Japanese Pharmacopoeia. Although there is some disagreement over the efficacy of the hard surface carrier test due to its manual procedure, it is deemed beneficial for determining the log 10 reduction in the number of active bacteria [[10], [11], [12]]. The analysis of the effect of disinfectants on Bacillus subtilis endospores in this study did not differ from the known effect of disinfectants alone [[13], [14], [15]]. Although a 5-min treatment with PAA showed a high reduction effect, its practical implementation is low owing to its toxicity and odor. Indeed, the odor of disinfectants is a source of stress to operators [16], and a simple and less burdensome method that allows treatment within a short time must be established. Wiping is one of the candidate methods for operators; however, its effectiveness in cleaning biosafety cabinets remains unclear. A quantitative evaluation of the reduction effect of wiping showed that wiping with DW also had a reduction effect of 10−3. The results obtained in the current study indicated that conventional simple wiping was effective in eliminating bacteria.
Although wiping after ethanol spraying is a standard environmental initialization approach, wiping in dry conditions may be the results if spraying is not performed properly. However, wiping under dry conditions exerts only a 1-Log reduction effect, which lower than the 3-Log reduction effect achieved under wet conditions when using DW. Analysis of the pressure sensor suggested that a sufficient force was not transmitted under dry conditions. In addition, considering that wiping in dry conditions does not float dirt, it may not be completely effective. Hence, such dry conditions may not achieve the objective of environmental initialization. In similar cases of manual operation, mechanical removal by manual endoscope cleaning enhances the effectiveness of subsequent mechanical cleaning; however, this step is most prone to human error [17,18]. Thus, the usefulness and limitations of manual operation in various situations have been discussed and it is necessary to establish a method to maximize the effect of firm wiping and improve reproducibility.
Unfortunately, wiping is not reproducible and has been challenging in various medical situations [[19], [20], [21], [22]]. The examination of this study showed differences in the amount of spray by operators and left-right differences in the spraying area. These differences lead to wiping due to dry conditions, resulting in low wiping effectiveness. Since a large area may be covered by spraying many times, the repeatability of operator training and standard operating procedures (SOPs) might be enhanced by mandating spraying over a larger area, for instance, by recording pre- and post-weights. It would be desirable to further develop quantitative evaluation techniques for wiping coverage and automate the wiping process to achieve more precise and reproducible methods.
While studying wiping strength, a 2-log reduction was observed even at a strength of 50 g. The most crucial factor may be ensuring that the wiping process is performed accurately in a contaminated area. Further technological development may be required for techniques that utilize the recording of wiping operations.
The differences in the effects of different types of disinfectants on wiping were examined in this study. A low bacterial reduction effect of ethanol, with a low protein collection rate, may be attributed to its protein-fixing action. Indeed, ethanol has been shown to increase the difficulty of cleaning reused surgical instruments owing to the presence of binding proteins such as blood on stainless steel [23,24], the same material as the floor surface of the biosafety cabinet. In the present analysis, BKC + I with a high reduction effect on bacteria showed an increased protein collection rate and a lower friction coefficient under sliding speed conditions that approximated the experimental wiping conditions. This indicates that BKC + I floats a speck of dirt containing proteins in a biosafety cabinet, which is slippery and easy to wipe. Despite the poor disinfection strength, these characteristics suggest the improved efficacy of BKC + I due to its high detergency. Although ethanol is commonly used to clean biosafety cabinets, other combinations should be considered for cell processing while employing serum and human tissue. For example, ethanol is volatile and residues are not a problem; however, BKC + I is nonvolatile and residues in a biosafety cabinet may be a problem. Therefore, a reliable method for the removal of the disinfectant should be considered. A more thorough examination of the changeover of biosafety cabinets will be required, not only from the aspect of disinfection but also from the standpoint of cleaning.
In Japan, new clinical trials on cell products are underway [[25], [26], [27]], and various new cell products are also being approved. Of the 16 cell products approved in Japan as of December 2022, 14 are autologous tissue- or cell-derived products. The processing methods of each cell product vary widely from type to type, and the risks associated with each cell product also differ. As the number of processing steps increases, challenges related to changeover will become more apparent. It will be necessary to dispel these concerns by taking a scientific approach and aiming to develop new technologies from the current stage.
4.1. Limitations
This study had three main limitations. First, we did not evaluate the effects of the different risks. The raw materials used in regenerative medicine may contain residual bacteria and viruses [28]. However, other risks have been adequately considered in the present analysis, as we have verified the use of proteins that are considered most likely to remain and the Bacillus subtilis endospores, which are highly resistant to disinfection.
Second, we were unable to investigate the reproducibility of wiping. In our experimental design, the visible dirt was manually wiped. In actual cell processing, there is no visible dirt. Therefore, it is necessary to establish a technique for accurately wiping from one end to another and a technique for their evaluation. For instance, it is necessary to develop new procedures, such as image analysis, and design robots that can track the precise wiping process.
Third, although we demonstrated the usefulness of wiping, the actual operational issues require further discussion. For example, soaked wipers have the risk of microbial transmission due to bacterial contamination and storage problems [29,30]. Further research is required to establish how to manage the system to achieve effective wiping.
5. Conclusions
The effect of wiping on bacterial removal during cell processing was examined and DW and BKC + I were found to be effective at reducing the bacterial cell count by 3-Log. Moreover, the combination of adequate wet conditions and appropriate disinfectant selection are necessary for proper wiping in specific environments where high-protein human serum and tissues are processed. The establishment of highly reliable wiping methods requires the development of quantitative evaluation methods for wiping coverage and robotization of the process to achieve more accurate and reproducible methods.
Authors' contributions
MM; Data acquisition: MM and JM; Data analysis and interpretation: MM, JM, KW, NS, and IS; Manuscript drafting: MM; Manuscript revision for important intellectual content: MM, JM, KW, NS, and IS. All authors have read and approved the final manuscript.
Funding
This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP22bk0304003 to IS.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgements
We thank Kimiko Takanashi and Mayumi Tsukamoto for managing our laboratory. We would also like to thank Airi Minamikawa, Ayako Tsuji, Yuri Kohno, Yukari Usui, Kanako Nagano, and Eriko Sawada for their cooperation with the survey and experiments.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Raval J.S., Koch E., Donnenberg A.D. Real-time monitoring of non-viable airborne particles correlates with airborne colonies and represents an acceptable surrogate for daily assessment of cell-processing cleanroom performance. Cytotherapy. 2012;14:1144–1150. doi: 10.3109/14653249.2012.698728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negre H., Pinte L., Manduke R., Cunningham A., Anderson H., Richard S., et al. Personnel environmental monitoring during manufacture of manipulated cell therapy products. Cytotherapy. 2018;20 [Google Scholar]
- 3.Mizuno M., Endo K., Katano H., Tsuji A., Kojima N., Watanabe K., et al. The environmental risk assessment of cell-processing facilities for cell therapy in a Japanese academic institution. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin P.G., Gonzalez M.B., Martinez A.R., Lara V.G., Naveros B.C. Isolation and characterization of the environmental bacterial and fungi contamination in a pharmaceutical unit of mesenchymal stem cell for clinical use. Biologicals. 2012;40:330–337. doi: 10.1016/j.biologicals.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Mizutani M., Samejima H., Terunuma H., Kino-Oka M. Experience of contamination during autologous cell manufacturing in cell processing facility under the Japanese Medical Practitioners Act and the Medical Care Act. Regen Ther. 2016;5:25–30. doi: 10.1016/j.reth.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T., et al. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regen Ther. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa Y., Mizutani M., Okamoto R., Kitajima H., Ezoe S., Kino-Oka M. Understanding the formation and behaviors of droplets toward consideration of changeover during cell manufacturing. Regen Ther. 2019;12:36–42. doi: 10.1016/j.reth.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno M., Yori K., Takeuchi T., Yamaguchi T., Watanabe K., Tomaru Y., et al. Cross-contamination risk and decontamination during changeover after cell-product processing. Regen Ther. 2023;22:30–38. doi: 10.1016/j.reth.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chihara R., Kitajima H., Ogawa Y., Nakamura H., Tsutsui S., Mizutani M., et al. Effects of residual H2O2 on the growth of MSCs after decontamination. Regen Ther. 2018;9:111–115. doi: 10.1016/j.reth.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton H.G., Porter F.C., Woodward B.B., Clarke P.H., Bauer J.M., Rubino J.R. Hard surface carrier test for efficacy testing of disinfectants: collaborative study. J AOAC Int. 1992;75:635–645. [Google Scholar]
- 11.Hamilton M.A., Devries T.A., Rubino J.R. Hard surface carrier test as a quantitative test of disinfection: a collaborative study. J AOAC Int. 1995;78:1102–1108. [PubMed] [Google Scholar]
- 12.Parker A.E., Hamilton M.A., Goeres D.M. Reproducibility of antimicrobial test methods. Sci Rep. 2018;8 doi: 10.1038/s41598-018-30282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merchel Piovesan Pereira B., Tagkopoulos I., Vieille C. Benzalkonium chlorides: uses, regulatory status, and microbial resistance. Appl Environ Microbiol. 2019;85 doi: 10.1128/AEM.00377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybka A., Gavel A., Kroupa T., Meloun J., Prazak P., Draessler J., et al. Peracetic acid-based disinfectant is the most appropriate solution for a biological decontamination procedure of responders and healthcare workers in the field environment. J Appl Microbiol. 2021;131:1240–1248. doi: 10.1111/jam.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno M., Sugahara Y., Iwayama D., Miyashita N., Katano H., Sekiya I. Stress and motivation of cell processing operators: a pilot study of an online questionnaire survey. Regen Ther. 2022;21:547–552. doi: 10.1016/j.reth.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deva A.K., Vickery K., Zou J., West R.H., Selby W., Benn R.A.V., et al. Detection of persistent vegetative bacteria and amplified viral nucleic acid from in-use testing of gastrointestinal endoscopes. J Hosp Infect. 1998;39:149–157. doi: 10.1016/s0195-6701(98)90329-2. [DOI] [PubMed] [Google Scholar]
- 18.Moses F.M., Lee J. Surveillance cultures to monitor quality of gastrointestinal endoscope reprocessing. Am J Gastroenterol. 2003;98:77–81. doi: 10.1111/j.1572-0241.2003.07165.x. [DOI] [PubMed] [Google Scholar]
- 19.Tyan K., Zuckerman J.M., Cutler C., Modupe K., Ray D., Marmolejo L., et al. A multiphase intervention of novel color additive for bleach disinfectant wipes improves thoroughness of cleaning in an academic medical center. Am J Infect Control. 2022;50:469–472. doi: 10.1016/j.ajic.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Rutala W.A., Weber D.J. Best practices for disinfection of noncritical environmental surfaces and equipment in health care facilities: a bundle approach. Am J Infect Control. 2019;47:A96–A105. doi: 10.1016/j.ajic.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald T., Sholtz L.A., Marion N., Turner P., Carling P.C., Rupp M.E. Maintenance of environmental services cleaning and disinfection in the ICU after a performance improvement project. Am J Infect Control. 2012;40:E159. [Google Scholar]
- 22.Frota O.P., Ferreira A.M., Koch R., de Andrade D., Rigotti M.A., Borges N.M.A., et al. Surface cleaning effectiveness in a walk-in emergency care unit: influence of a multifaceted intervention. Am J Infect Control. 2016;44:1572–1577. doi: 10.1016/j.ajic.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Costa D.M., Lopes L.K.O., Hu H., Tipple A.F.V., Vickery K. Alcohol fixation of bacteria to surgical instruments increases cleaning difficulty and may contribute to sterilization inefficacy. Am J Infect Control. 2017;45:e81–e86. doi: 10.1016/j.ajic.2017.04.286. [DOI] [PubMed] [Google Scholar]
- 24.Prior F., Fernie K., Renfrew A., Heneaghan G. Alcoholic fixation of blood to surgical instruments-a possible factor in the surgical transmission of CJD? J Hosp Infect. 2004;58:78–80. doi: 10.1016/j.jhin.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno M., Endo K., Katano H., Amano N., Nomura M., Hasegawa Y., et al. Transplantation of human autologous synovial mesenchymal stem cells with trisomy 7 into the knee joint and 5 years of follow-up. Stem Cells Transl Med. 2021;10:1530–1543. doi: 10.1002/sctm.20-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekiya I., Koga H., Otabe K., Nakagawa Y., Katano H., Ozeki N., et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant. 2019;28:1445–1454. doi: 10.1177/0963689719863793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekiya I., Katano H., Mizuno M., Koga H., Masumoto J., Tomita M., et al. Alterations in cartilage quantification before and after injections of mesenchymal stem cells into osteoarthritic knees. Sci Rep. 2021;11 doi: 10.1038/s41598-021-93462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K., Otabe K., Shimizu N., Komori K., Mizuno M., Katano H., et al. High-sensitivity virus and mycoplasma screening test reveals high prevalence of parvovirus B19 infection in human synovial tissues and bone marrow. Stem Cell Res Ther. 2018;9:80. doi: 10.1186/s13287-018-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutala W.A. APIC guideline for selection and use of disinfectants. Am J Infect Control. 1996;24:313–342. doi: 10.1016/s0196-6553(96)90066-8. [DOI] [PubMed] [Google Scholar]
- 30.Gebel J., Exner M., French G., Chartier Y., Christiansen B., Gemein S., et al. The role of surface disinfection in infection prevention. GMS Hyg Infect Control. 2013;8 doi: 10.3205/dgkh000210. Doc10. [DOI] [PMC free article] [PubMed] [Google Scholar]