Maternal and Infant Vulnerability and Resilience to Infectious Diseases
Infectious diseases remain a major contributor of infant morbidity and mortality globally, especially in low- and middle-income countries. Newborns, in particular, bear a disproportionate burden, estimated at 7 million cases and 700,000 deaths per year (1). Routine immunization, which prevents many infectious diseases during childhood, has had limited impact preventing illness during the first months of life, partly due to newborns’ reduced ability to rapidly mount an effective response to these vaccines (2). On the other hand, strengthening of maternal health and immune fitness holds tremendous potential to reduce vulnerability to infection and promote well-being of both mothers and infants. Infants born to healthy mothers are more resilient to infection and likely to grow and develop to their full potential (ideal trajectory of steady health, Figure 1), whereas those born to unhealthy mothers are more likely to fall into a cycle of increased susceptibility to disease and death (the vulnerable trajectory, Figure 1). Repeated bouts of illness reduce quality of life, and the resulting cumulative disadvantages can perpetuate for generations. Although the path of vulnerability involves multiple economic and social aspects, initiatives aimed at improving maternal health through disease prevention and treatment can increase infant survival, reduce vulnerability, and promote childhood health and lifelong wellness.
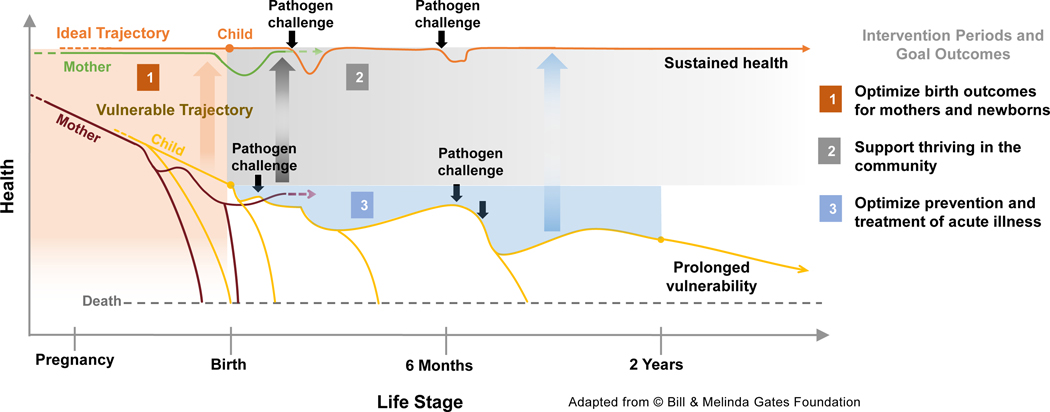
Figure 1. Infant susceptibility to pathogens is influenced by maternal health.
Infants born to healthy mothers are more resilient to pathogen exposure and likely to grow and develop to their full potential (ideal trajectory of steady health), whereas those born to unhealthy mothers (pre-term or with low birth weight) fall into a cycle of increased susceptibility to repeat infections, growth faltering, malnutrition and death (the vulnerable trajectory). Repeated illness dramatically reduces child’s potential for physical growth, development, and quality of life. Mothers who become pregnant again (dashed arrow) initiate a repeat cycle of sustained health or vulnerability, which can perpetuate for generations.
To this end, vaccination during pregnancy seeks to protect mothers and infants from infectious threats by boosting maternal cellular and humoral immunity. The offspring benefit from the placental sieve of systemic antibodies and from mucosal antibodies, cells, and immunomodulatory and regulatory molecules, which constitute the “immune system” in human milk (3). Success achieved by maternal immunization against tetanus, pertussis, and influenza has increased global interest in this approach and stimulated the development and evaluation of vaccines against other pathogens that cause life-threatening infections during the first months of life, such as respiratory syncytial virus and group B streptococcus (4). However, given the many known (and unknown) immunological adaptations intrinsic to pregnancy, much remains to be learned about the principles underpinning responses to vaccination during this period. Accordingly, maternal immunization strategies could be refined and improved by better understanding the mechanisms underlying maternal and infant immunity to pathogens, especially the elements that influence responses to vaccines in pregnant women, maternal antibody transfer, and adaptive immune responses to vaccine antigens in infants. A holistic approach focused on the mother-infant dyad as one entity from conception through 2 years of age, in-depth interrogation and integrative analysis of potential health determinants such as microbial communities; transcriptional, proteomic, and cellular states, and their interactions, is necessary to define the rich interactions within the maternal-child unit, refine clinical practices, and uncover novel actionable targets for intervention. The ultimate goal is to reduce the number of mothers and infants on the vulnerable trajectory and to promote the path of sustained health. The systems biology approaches and computational tools that have become available in recent years, and the impetus for collaborative and interdisciplinary science provide an unprecedented opportunity to bring about new knowledge and harness the benefits of vaccination to improve maternal-child health. Key proposed research areas to bolster integrated maternal and infant immunization and reduce vulnerability to infections [reviewed also in (4)] are listed in Table 1.
Table.
Key research gaps to improve maternal and infant immunization strategies.
| Vaccine responses during Pregnancy | Pregnancy-associated changes in the magnitude and quality of T and B cell immunity to pathogens and responses to vaccines |
| Impact of repeated immunization with each pregnancy | |
| Potential to improve/modulate vaccine responses through formulation/dose/route | |
| Influence of co-morbidities, including chronic maternal infections, on vaccine responses | |
| Maternal antibody transfer | Mechanism of selective transfer of maternal antibodies across the placenta |
| Quality and function of maternal antibodies transferred through breast-feeding | |
| Impact of co-morbidities (including chronic maternal infections) on antibody transfer | |
| Interactions of maternal antibodies with the infant immune system | Interactions between maternal antibodies and infant innate immune system for pathogen control |
| Impact of maternal antibodies on magnitude and quality of infant T and B cell responses to vaccines | |
| Systems biology of maternal and infant immunity | Cellular and molecular determinants of vaccine responses during pregnancy and infancy |
| Maternal determinants of infant vaccine responses, beyond antibodies | |
| Role of the microbiome in regulating vaccine responses in pregnancy and infancy |
Vaccine Responses During Pregnancy
Vaccines currently administered during pregnancy have not been specifically developed or approved for pregnant women. Their use in this population has been recommended based on the evidence of increased antibody levels in their newborns and of reduced risk of infection in mothers and infants. Women’s immune system undergoes major changes during pregnancy and whether and how these adaptations impact responses to vaccine remains largely unknown. Some studies have reported decreased levels of antibody in response to seasonal influenza and pertussis immunization in pregnant women compared to non-pregnant controls, while others have observed similar responses in the two groups (5,6). There is also evidence that higher levels of maternal antibodies are transferred to the newborn following immunization during the second as compared to the third trimester of pregnancy, which likely reflects an extended period of placental transfer (7). Given also the precise temporal shift of immunological events during pregnancy (8), timing of vaccination is likely to influence immune state outcome. Studies of vaccine immunogenicity in pregnant women have focused primarily on the magnitude of antibody response while important functional and qualitative aspects of these responses have been overlooked. For example, the impact of pregnancy on vaccine-induced antibody avidity, isotype and subclass selection, glycosylation, and effector functions remains largely unknown (5). Recent work from our group indicates that pregnancy modifies the glycosylation profile of antibodies induced by seasonal influenza immunization and their function, which may have relevance for protection (9). Given its wide-reaching implications, understanding the basis of effective maternal immunization is essential to inform vaccine development and implementation to ensure its safety and to leverage this strategy to strengthen maternal-infant health.
Maternal Antibody Transfer
Host immunity against pathogens in the newborn stage depends on the efficiency of maternal antibody transfer across the placenta. The placenta is a complex transient organ consisting of multiple cell layers that facilitate the exchange of essential molecules, while separating maternal and fetal circulation. Maternal IgG is known to cross the syncytiotrophoblast in a process that involves uptake by micropinocytosis and binding to the neonatal Fc receptor (FcRn) expressed endogenously in acidified vesicles. Details of the subsequent steps, namely, how the IgG reaches fetal circulation and is maintained and recycled, and the elements driving and regulating transplacental IgG transfer are less clear. Recent studies demonstrated that maternal antibodies with specific biophysical and functional properties are preferentially transferred across the placenta, and that this process potentially involves Fcγ receptors, including FcγRIII, working in cooperation with FcRn (10,11). Defining the determinants of maternal antibody transfer has translational relevance to enhancing newborn health in the context of natural infection, maternal vaccination, and antibody therapies, each of which can contribute to prevent perinatal infection. Further, such information would enable the engineering of monoclonal antibody therapies with restricted transfer to the fetus, which would allow for safe use in pregnant women while avoiding negative effects on infants, such as the potential risk of fatal Bacillus Calmette-Guérin (BCG) dissemination following administration of anti-TNF antibodies during gestation (12).
In addition to systemic, placentally-acquired antibodies, maternal antibodies (mainly IgA and IgG), immune cells, and a variety of cytokines, growth factors, anti-microbials (e.g. lactoferrin), oligosaccharides, and immunomodulatory molecules are available to the infant via maternal milk (4). The near- and long-term benefits of breast-feeding in preventing infection have been documented in multiple epidemiological studies [Reviewed in (13)]. There is an abundance of descriptive information on the composition of human breast milk, and yet a dearth of mechanistic understanding of the protracted immune enhancing effects and health benefits of maternal milk. The full and large-scale potential of maternal vaccination can only be appreciated by a combination of efficacy/effectiveness studies with longitudinal analyses of maternal immunity and immunological components conveyed via placenta and breast milk.
Interactions of Maternal Antibodies with the Infant Immune System
Maternal antibodies provide protection against infectious agents but can also influence infant vaccine responses. The mechanisms underlying antibody-dependent immunity against pathogens and the regulation of vaccine responses in early life remain poorly understood. Maternal IgG molecules transferred to the newborn have biophysical attributes, including distinct subclass and glycosylation profiles, which are relevant to their effector functions (10). The biophysical features of infant’s antibodies are therefore expected to change during the first year of life as maternal antibodies decline and infants start producing their own. This shift would predictably affect the antibody interactions with other immune components (e.g. B cells, innate cells, and complement) and function during the first years of life and onwards (14). This notion is consistent with our current understanding of the programming of the immune system in early life, which adapts to meet environmental demands during fetal life and after birth (2). Interestingly, maternal antibodies can reduce the magnitude of antibody responses to routine vaccines in infants but do not appear to impact T cell responses (4,15). The impact of maternal antibodies on the quality of infant B and T cell responses and the mechanisms involved remain largely unknown. Defining the principles underlying antibody-dependent immunity in early life and the influence of maternal antibodies on infant immune responses to vaccine antigens is essential to harness integrated maternal and infant immunization strategies.
Toward a Systems Immunology Approach to Maternal Immunization
Beyond antibodies, little is known about other immune correlates and determinants of effective immunization in pregnant women and infants, as well as about how other maternal immune-related components (e.g. cells, cytokines, and other molecules shared via placenta and/or breast milk) may interact and shape the immune status and health of the infant. Vaccination triggers complex innate and adaptive molecular and cellular processes that lead to T and B cell activation and antibody production. Systems approaches combining large-scale measurements of immune states and computational modeling and analyses have been successfully applied to discern underlying immune components and how their interactions shape response behavior, including the quality and quantity of known correlates of protection against infection (e.g., antibody levels) (16). Studies conducted in healthy adults have revealed unexpected predictors and potential determinants of antibody and T cell responses to vaccines, including (1) temporally stable (within individuals) but highly variable (across individuals) cell frequency and transcriptional signatures before vaccination, likely reflecting the homeostatic immune state of the individual; and (2) the level of increase in certain immune components, such as monocytes and plasma cells, in the first days after vaccination, reflecting the early response to a specific vaccine formulation. The transfer of maternal antibodies is the most well-known and well-studied immune connection between mother and infant. However, myriad molecular and cellular maternal factors, including those affected by maternal vaccination, can influence both infant immune states and vaccine responses. Mapping the network of interactions among maternal and infant immune components could expand current paradigms of maternal determinants of infant immunity. Recent studies demonstrate the feasibility of this approach in pediatric populations (17). The ensuing novel associative insights can then be reinforced by interrogation of specific immune functions and pathways in vivo using genetically modified animals or ex vivo using human tissue models. Defining vaccine responses and connections in the mother-infant immunological dyad can provide a comprehensive view of maternal and infant immune system states. The resulting understanding of the maternal factors shaping infant vaccine responses and immunity could lead to new targets for preventing infant infections and predicting protective outcome.
Closing Remarks
Maternal health is a key determinant of infant heath. Maternal immunity is also intimately associated with infants’ immune status and their responses to vaccines and capacity to resist infection. Maternal-infant immunity can set the future course of infant health and quality of life. The principles and mechanisms underlying the complex and dynamic maternal-infant interactions that collectively determine resilience or vulnerability to infections remain to be defined. A holistic approach focused on the maternal-child unit (from early stages of pregnancy and through the first second year life) and going beyond the analysis of antibodies is needed to better delineate elements and temporal dimension of these interactions. Systems immunology approaches (i.e. measuring and computational modeling of diverse immune components) applied to the analysis of the immune system and vaccine responses in pregnant women and infants have the potential to identify novel biomarkers and predictors of vaccine responses in both mothers and babies. Furthermore, the pursuit of multi-faceted, interdisciplinary, and team-based research is needed to dissect the rich immunobiology of the maternal-infant dyad. The combination of technology, expertise, and exploration of new and unconventional ideas will be important to refine and bring to fruition integrated maternal and infant immunization strategies and discover novel interventional targets to improve maternal and infant health.
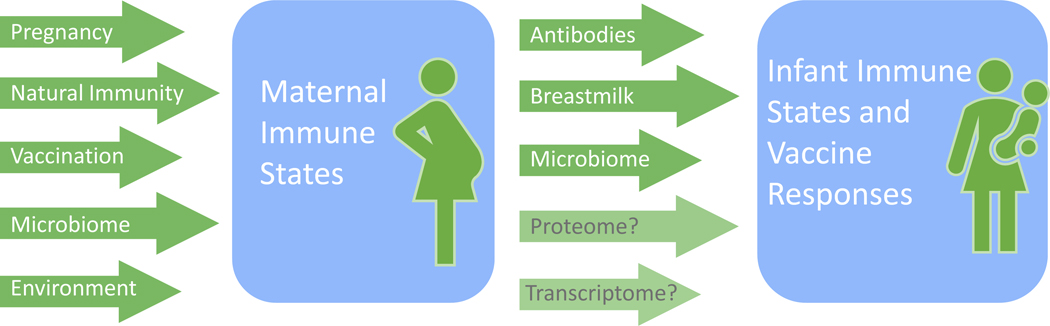
Figure 2. Determinants of maternal and infant immune states and vaccine responses.
Maternal immune states and vaccine responses can be influenced by the immunomodulation that occurs during pregnancy, by changes in microbiome composition, by exposure to pathogens which may result in maternal disease or naturally-acquired immunity, and by multiple other environmental factors. Maternal determinant such as placentally acquired antibodies, immune components of breastmilk, microbiome, as well as complex interactions at the proteomic and transcriptomic levels can also influence infant immune states and vaccine responses.
References
- 1.Bhutta ZA, Black RE. Global maternal, newborn, and child health--so near and yet so far. N Engl J Med. 2013;369(23):2226–35. [DOI] [PubMed] [Google Scholar]
- 2.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity. 2017;46(3):350–63. [DOI] [PubMed] [Google Scholar]
- 3.Goldman AS. The immune system in human milk and the developing infant. Breastfeed Med Off J Acad Breastfeed Med. 2007;2(4):195–204. [DOI] [PubMed] [Google Scholar]
- 4.Marchant A, Sadarangani M, Garand M, Dauby N, Verhasselt V, Pereira L, et al. Maternal immunisation: collaborating with mother nature. Lancet Infect Dis. 2017;17(7):e197–208. [DOI] [PubMed] [Google Scholar]
- 5.Schlaudecker EP, Ambroggio L, McNeal MM, Finkelman FD, Way SS. Declining responsiveness to influenza vaccination with progression of human pregnancy. Vaccine. 2018;36(31):4734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortner KB, Swamy GK, Broder KR, Jimenez-Truque N, Zhu Y, Moro PL, et al. Reactogenicity and immunogenicity of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant and nonpregnant women. Vaccine. 2018;36(42):6354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox CR, Holder B, Jones CE. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front Immunol. 2017;8:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghaeepour N, Ganio EA, Mcilwain D, Tsai AS, Tingle M, Van Gassen S, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasetti Marcela. Pregnancy-induced changes in antibody glycosylation and non-neutralizing functions. International Neonatal and Maternal Immunization Symposium; 2019. Sep 15; Vancouver. [Google Scholar]
- 10.Jennewein MF, Goldfarb I, Dolatshahi S, Cosgrove C, Noelette FJ, Krykbaeva M, et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell. 2019;178(1):202–215.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez DR, Fong Y, Li SH, Yang F, Jennewein MF, Weiner JA, et al. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell. 2019;178(1):190–201.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling J, Koren G. Challenges in vaccinating infants born to mothers taking immunoglobulin biologicals during pregnancy. Expert Rev Vaccines. 2016;15(2):239–56. [DOI] [PubMed] [Google Scholar]
- 13.Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet Lond Engl. 2016;387(10017):475–90. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HD, Tirosh I, de Haan N, Stöckmann H, Adamczyk B, McManus CA, et al. IgG Fc glycosylation as an axis of humoral immunity in childhood. J Allergy Clin Immunol. 2019; pii: S0091–6749(19)31364–8. doi: 10.1016/j.jaci.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orije MRP, Maertens K, Corbière V, Wanlapakorn N, Van Damme P, Leuridan E, et al. The effect of maternal antibodies on the cellular immune response after infant vaccination: A review. Vaccine. 2020; 38(1):20–28. [DOI] [PubMed] [Google Scholar]
- 16.Tsang JS. Utilizing population variation, vaccination, and systems biology to study human immunology. Trends Immunol. 2015;36(8):479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AH, Shannon CP, Amenyogbe N, Bennike TB, Diray-Arce J, Idoko OT, et al. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nat Commun. 2019;10(1):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]