Abstract
The gut microbiome has coevolved with its hosts over the years, forming a complex and symbiotic relationship. It is formed by what we do, what we eat, where we live, and with whom we live. The microbiome is known to influence our health by training our immune system and providing nutrients for the human body. However, when the microbiome becomes out of balance and dysbiosis occurs, the microorganisms within can cause or contribute to diseases. This major influencer on our health is studied intensively, but it is unfortunately often overlooked by the surgeon and in surgical practice. Because of that, there is not much literature about the microbiome and its influence on surgical patients or procedures. However, there is evidence that it plays a major role, showing that it needs to be a topic of interest for the surgeon. This review is written to show the surgeon the importance of the microbiome and why it should be taken into consideration when preparing or treating patients.
Keywords: bacteria, microbiome, surgery, symbiosis, dysbiosis
What is the microbiome? If a surgeon (or any clinician) is asked to answer this question, they will probably say that it is the term for all bacteria in the gastrointestinal tract. That answer is not wrong, but it is not completely correct either. The microbiome is defined as “a characteristic microbial community occupying a reasonable well-defined habitat which has distinct physio-chemical properties,” 1 including its activity and influences on its surroundings. The microbial community in this definition, however, consist of not only bacteria but also all microorganisms present in that habitat, meaning that the microbiome also includes viruses, protozoa, fungi, etc. In addition, the habitat is not defined, and the human microbiome therefore also includes the microbial communities of the skin, ear, mouth, and so forth. Recent studies estimate the ratio of human-to-bacterial cells is close to 1:1, although there are also studies that estimate that there is a 10-fold of microorganisms inhabiting the human gastrointestinal tract. 2 The estimated densities of bacterial populations in the gastrointestinal tract are ∼10 2–4 in proximal ileum and jejunum, ∼10 7–11 in the distal ileum, and ∼10 11–14 colony forming units (cfu) per gram within the colon. 3 4 5 Because the gastrointestinal bacteria form the majority of the human microbiome, the term microbiome is most practically used for all bacteria present in the gastrointestinal tract. The term gut microbiota is often used as a synonym for the gut microbiome and for gut bacteria.
The microbiome has coevolved with its hosts over the years, forming a complex and symbiotic relationship. 6 Our microbiome is influenced by what we eat, where we are, with whom we are, and how we live. In turn, the microbiome also influences us. The gut microbiome is associated with diseases, such as inflammatory bowel diseases (IBD), 7 obesity, 8 9 diabetes mellitus, 10 a diversity of cancers, 11 psychological disorders such as depression, 12 and even neurological disorders such as Alzheimer's disease and Parkinson's disease. 13 14 15 Although the mechanisms behind these associations are mostly still to be elucidated, there is consensus that the interaction between the bacteria within the microbiome and the interaction with the human body (the habitat) is important in health and, with that, disease in humans. Therefore, it is not surprising that with the development of novel (culture-independent) techniques this area of research has become an upcoming research topic in the last decennia. In this review, we will try to provide an insight into the complex network that we maintain with our gut microbiome and how it influences our health, disease, and (surgical) treatment.
Composition and Function of the Microbiome: A Social Network
From all sites of the human body, the gut microbiome has been studied the most intensively and is seen as the microbiome with the most influence on the physiological processes of the human body. Culturing methods had already shown a great diversity of the gut microbiota. With the use of high-throughput sequencing methods such as 16S ribosomal RNA (rRNA) gene sequencing and the more extensive shotgun metagenomic sequencing, mapping of the microbial composition has become more detailed. An analysis of large databases of isolated species showed that over 2,100 species were isolated in human beings. 16 New techniques suggest that more than twice as many species are to be found in the gut microbiome. 17 The gut microbiome is composed of both aerobic and anaerobic microorganisms. As the luminal content of the bowel is in principle anaerobic, aerobic bacteria probably find a habitat close to the mucosal cells that “leak” oxygen toward the lumen.
A study in twins and a large cohort study have suggested that only around 5 to 8% of the bacterial composition is heritable. 18 19 In addition, almost half of the microbiome is determined by who we live with and the rest by other factors like environment, intake, hygiene, and drug use. 18 These (external) influences determine the diversity and composition of the microbiome. A microbiome with a high diversity is linked with human health and with living a long and healthy life. 20 Centenarians, for example, have a higher diversity of the gut microbiome than people who live shorter. 21 Therefore in general, a healthy gut microbiome is considered diverse although the interindividual differences in composition can be high. 22 Therefore, one typical healthy gut microbiome with a determined microbial composition probably does not exist. In addition, not all beneficial bacteria can live together, as some known beneficial bacterial genera and families are negatively correlated with one another. 23
The symbiotic equilibrium that the microbiome has reached in a healthy state of the human is known to consist of certain types of bacteria. For its symbiotic function, the microbiome should harbor bacteria that are important for the breakdown of dietary substrates, producing energy and micronutrients, and in the synthesis of vitamins, essential amino acids, and short-chain fatty acids (SCFAs). The balance within the gut's microbial composition determines the function and resilience of the microbiomes. They do that by communicating with each other (e.g., by quorum sensing, see below), fighting each other (e.g., by bacteriocins), and interacting with the host. They hereby form a social network of interactions that protects the symbiotic relationship.
The Human Factor
As the presence of the microorganisms in the gastrointestinal lumen is a natural phenomenon, it is important for the host to keep the microbiome to the lumen. Various defense mechanisms have been established for this containment. The intestinal epithelial cells and the tight junctions between them compose the most important physical intestinal barrier limiting interaction between luminal contents and the human body. 24 The tight junctions are a mechanical link between epithelial cells that are able to move with the peristaltic movements of the gastrointestinal tract. These junctions maintain epithelium polarity, allowing transport for nutrients, secretion of enzymes, and antimicrobial peptides into the lumen. 24 It also regulates the passage of ions, water, and various macromolecules from the lumen to the internal system. A dysfunction of these tight junctions, often induced by enteric pathogens, increases intestinal permeability and allows for microbial and other potential harmful components to enter the systemic circulation. 25
The intestinal epithelial cells also provide the secretion of mucus. This mucus forms a layer on top of the cells, creating a barrier to minimize contact between the epithelial cell surface and the microorganisms. This is essential to limit tissue inflammation and microbial translocation. 26 27 This layer of mucus contains immunoglobulins, antimicrobial peptides, and immune cells to keep the microorganisms from invading the mucus toward the epithelial cells. 28 29 However, it also contains nutrients, excreted by the gastrointestinal tract, providing food for various microorganisms regulating thereby the normal composition of the microbiome. 30 Furthermore, bacteria produce among others lactate and SCFAs, which provide an additional intestinal barrier function, for example, by reducing the pH, making it inhabitable for some bacteria, 31 or act on the host causing it to lower the oxygen concentrations in the lumen, making the environment less favorable for certain pathogens. 32 These SCFAs also promote the differentiation of T-cells, providing the immune system with a large pool of immune cells that protect against pathogens. 33 34 The SCFAs, such as propionate, acetate, and butyrate, are produced by the digestion of dietary fibers by bacteria, a process that is impossible for intestinal cells. 35 This digestion of dietary fibers is a good example of a beneficial social interaction with bacteria, because butyrate is the primary energy source for colonic cells. 36 A lack of butyrate can cause colitis (e.g., deviation colitis in the rectum). 37 38
The Microbiome Factor
The interaction between the microorganisms to regulate their social network indirectly via the host is not the only way of communication. They also have direct contact by “quorum sensing,” which is the ability to detect and respond to cell population density by producing signals that activate gene expression in their co-inhabitants, for example, to induce growth. 39 This signaling system helps maintain the balance of the present bacteria, preventing pathogens to thrive and become an actual threat for the gastrointestinal cells. To compete with each other, bacteria may use bacteriocins (bacterially derived antimicrobial proteins), which kill or inhibit other bacteria when secreted. 40 In addition, the microbiome interacts with the host immune system by triggering pattern recognition receptors at the mucosa. 41 This causes epithelial cells to secrete antimicrobial peptides (RegIII), immunoglobulins, interleukins, and T-cell responses to the gastrointestinal lumen, helping the microbiome to keep its balance. 42 Recent research shows that there is evidence that the bacteria also use their quorum sensing to interact with the epithelial cells and the human cells mimic quorum sensing signals to communicate back. 39 This crosstalk is essential for humans because it triggers the mucosal immune system and maintains the epithelial barrier integrity, for example, by increasing epithelial cell tight junction resistance. 43 44 This defense is important, not only against the pathogens that inhabit the gastrointestinal tract, which are continuously trying to reach the mucosal cells, but also for the daily contact with an abundance of microorganisms from the outside world by our oral intake. This introduction of microorganisms also shapes part of our microbiome and the diversity of our diet determines the diversity of our microbiome (and its function). 18 45 But as long as this social network is beneficial for the bacteria within the microbiome and for the host, it functions ( Fig. 1 ).
Fig. 1.
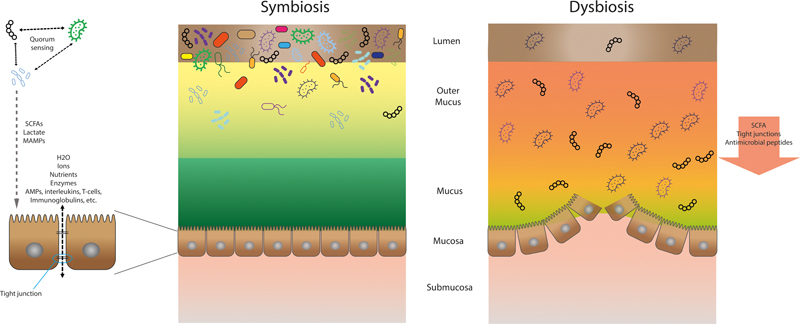
Description of symbiosis and dysbiosis. In symbiosis bacterial quorum sensing and interactions with host immune cells maintains a dense and diverse microbiome and the epithelial barrier integrity. In dysbiosis, microbial community breakdown results in decreased antimicrobial peptides and disruption of the epithelial barrier.
Dysbiosis, When a Social Network Becomes a Fight Club
When the microbial composition gets severely altered, known as dysbiosis, the network can become fragile and its influence on the host's physiology can change. As said, there are multiple factors that influence the microbiota. Examples are age; lifestyle factors like diet, smoking, drugs, activity level, and stress; and environmental factors such as geographic location, hygiene, and exposure to animals and other humans. 46
A dysbiosis of the microbiome is associated with various diseases, such as the prevalence of obesity, 8 9 type 2 diabetes, 10 IBD, 7 and even neurological disorders such as Alzheimer's disease and Parkinson's disease. 13 14 15 Interestingly, these pathologies have shown to have a reduced diversity of the gut microbial composition. 47 48 49 This dysbiosis of the microbial composition is often induced by the above-mentioned environmental factors. This leads to the loss of the symbiotic network and creates the circumstances for bacteria to outcompete others and to thrive: it becomes a fight club.
The Relevance for Surgeons
Related to surgery, preoperative fasting, the use of perioperative antibiotics, mechanical bowel preparation, and the surgery itself have been shown to disrupt the existing microbiome, thereby possibly influencing the postoperative outcomes. 46 The use of antibiotics has been associated with changes to the composition of the gut microbiome. 50 Antibiotics aim to kill or stop pathogenic bacterial species that may cause infectious problems for the host. However, the current generation of antibiotics are broad spectrum and have a bacteriostatic or bacteriocidic effect on “normal” microbiota as well. 51 A short course of commonly used (broad-spectrum) antibiotics, such as ciprofloxacin, causes a significant decrease in species richness (the number of species disregarding the diversity within a defined region) and microbial diversity (the number of different species within a define region). 52 53 54 55 While some individuals changed back to their preantibiotic microbial composition, these changes were persistent in some individuals, as some of their microbiota did not reinstate the pretreatment population levels. 52 53 These effects are persistent up to years after antibiotic treatment. 53 56
The use of prophylactic antibiotics is the standard care and should be given within 2 hours before surgery. If administered before or after this time window, the wound infection rate is higher. 57 Many surgeons continue the antibiotic prophylactic regime for one or more days, despite no evidence that this yields any benefit. 58 The long-term effects of this continuation of antibiotics is not studied well but might have a large impact on the microbiome and antimicrobial resistant patterns of the patients' gut microbiota.
Mechanical bowel preparation influences the microbiome as well, but not as long lasting as the antibiotic treatment. The duration of the effect of bowel preparation on the composition and diversity differs between studies performed on fecal samples, but are mostly a few days up to a month, after which the composition and diversity is restored. 59 60 61 62 Similar changes in composition and diversity of mucosal-adherent microbiota are seen in colonoscopic biopsies. 63 These effects on the microbiome may have consequences for (surgical) procedures. They are, for example, associated with postoperative delirium, 64 but, when combined with oral antibiotics, they are associated with a reduction in surgical site infections, ileus, and anastomotic leaks after colorectal surgery. 65
Another influencer of the microbiome network is (gastrointestinal) surgery, which can profoundly alter the microbiome. There are multiple studies performed on how the microbiome changes after gastrointestinal surgery, but there is a lack of consistency in results due to, among others, the absence of standardized methods. 66 In addition to an altered microbiome, gastrointestinal surgery damages the epithelial cell wall integrity, giving microorganisms the opportunity to reach the intestinal cells. 67 This extends the inflammatory response, influencing wound healing processes, but also gives opportunistic pathogens a chance to adhere to, invade, or attack these cells. Pathogens are able to do that by expression virulence factors, which are characteristics to help the bacteria survive but reduce host health. Such factors are species and even strain specific, including structural features like flagella that facilitate attachment to host cells 68 69 and toxins and enzymes that degrade host tissue. 70 71 An example is the expression of the genes Ace (adhesin) and GelE (gelatinase E) by Enterococcus faecalis , which, respectively, make the bacteria adhere to host tissue and degrade the collagen. 72 73 E. faecalis has been shown to adhere to the anastomosis after colorectal surgery and is associated with subsequent anastomotic leakage. 73 Additionally, research by our group has shown that a low microbial diversity is strongly associated with the development of anastomotic leakage after colorectal surgery. 74 75 The trophic network of species in intestinal microbiota with a low diversity may be more easily disturbed by the mentioned factors than in microbiota with a high diversity.
The Future Potential of the Microbiome in Surgery
As recent research on the microbiome and its influence on (gastrointestinal) surgery has gained interest, the next step would be to expand this research, subsequently integrate microbiome analysis methods in daily practice and influence the microbiome to change negative influences. Screening methods could be developed to assess whether the patient is fit for surgery. For example, a patient's microbial composition might be analyzed to evaluate the frailty of the patient. 76 Or the microbial diversity might be determined to see whether it is diverse enough to make an anastomosis that is not likely to leak. 74 In addition, it could be proposed to screen patients for the presence of highly virulent species. 73 These views on the microbiome can hypothetically be used to decide whether it is better to wait to operate on the patient or prepare the patient for surgery.
The microbiome should also be considered more often when patients are prepared for (surgical) procedures. Although conventional therapy and prophylaxis have been proven to be successful in preventing or overcoming bacterial infections, they are mostly antibiotic, aimed to eliminate or reduce pathogens from the gut. These therapies have been implemented globally, but are also often used improperly and cause many antimicrobial resistance microorganisms. Therefore, the search to more sustainable methods to prevent or overcome infections is needed taking the microbiome into account.
An example might be the use of probiotics or prebiotics. Probiotics, which are defined by the World Health Organization as “live microorganisms, which when consumed in adequate amounts, confer a health benefit on the host,” have been suggested as beneficial for health for over a century and have been marketed in various forms over the last decennia. 77 Prebiotics are food ingredients that stimulate the growth or activity of selective bacterial genera in the colon. 78 A recent systematic review has suggested that the combination of both pre- and probiotics, called synbiotics, reduce postoperative infections after elective abdominal surgery. 79 There is, however, a lack of consistency in the (bacterial) products used and the potency of these microbiome altering products depends among other on the metabolic effects of specific strains used. 80 The perfect formula has not been established yet, but they have the potential to reinforce the intestinal barrier, reduce inflammation, and improve immune status. 80
Fecal microbiota transplantation (FMT) has also been suggested as a solution for gastrointestinal-related problems. It can be highly effective against Clostridium difficile infections of the intestine 81 and has shown promise in IBD and related diseases. 82 FMT might restore the diversity of a disturbed gut flora and may improve the intestinal epithelial barrier. 83 In addition, it has been shown to restore systemic immunity responses in mice, protecting them from sepsis induced by pathogens. 84 However, with this treatment strategy, there are also many uncertainties on the long-term effects for the microbial composition and many studies show (serious) adverse events. 85 For example, one study shows the transfer of drug-resistant pathogens by FMT leading to bacteremia. 86 Therefore, this method needs optimization for further clinical implementation.
Another approach to change the microbiome is to make lifestyle changes. There is much to gain in changing the microbiome with exercise and diet. This should be mostly considered in patients who live a western lifestyle with not enough physical activity and too much (processed) food full of fat and sugars and with a low amount fiber. 87 88 Exercise leads to an increased diversity of the gut microbiome. 89 In addition, exercise leads to an increase in SCFA production and butyrate-producing bacteria. 90 The intake of processed foods and animal-derived foods is associated with higher abundances of known proinflammatory microbiota. In contrast, a diet with plants and fish is positively associated with SCFA-producing commensals and pathways of nutrient metabolism. 91 Relating this to surgery, a study in mice showed that when chronically fed a high-fat and low-fiber diet, and subsequently fed a 2-day course of low-fat and high-fiber diet, the microbial composition changed and reduced the incidence of anastomotic leakage compared with continuation of a high-fat diet. 92 Although translation to a human situation is difficult, there is evidence that the change of the human gut microbial composition can be reached within a considerable time. In a study with human subjects, groups received after a 2-week western diet of either 6 weeks of whole grain diet or 6 weeks of refined grain diet. The whole grain group showed an increase in gut microbial diversity and SCFA-producing bacteria and a decrease in proinflammatory bacterial families. 93 The intake of fiber is even seen to have a positive correlation within 1 day, showing next-day abundant changes among 15% of gut microbiota members. 94 Dietary nutrients have been shown to have a profound effect on both the microbial composition and its function, mediating in the social network between our environment and our genes. 48
Conclusion
Although it is not even visible to the surgical eye, the gut microbiome is of great importance for the surgeon and its patients. Unfortunately, microbiome research in surgery and surgical complications is still scarce. With the high-throughput techniques that have become readily available in many centers, we hope that awareness will rise and more research on this topic will be performed. This is important because the effects of surgery on the microbiome are significant, but the effects of the (changed) microbiome on the outcome of surgery might be even more considerable for patients.
Footnotes
Conflict of Interest None declared.
References
- 1.Berg G, Rybakova D, Fischer D. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8(01):103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. Biorxiv. 2016:36103. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckburg P B, Bik E M, Bernstein C N.Diversity of the human intestinal microbial flora Science 2005308(5728):1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(03):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 5.de Vos W M, Tilg H, Van Hul M, Cani P D. Gut microbiome and health: mechanistic insights. Gut. 2022;71(05):1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bäckhed F, Ley R E, Sonnenburg J L, Peterson D A, Gordon J I.Host-bacterial mutualism in the human intestine Science 2005307(5717):1915–1920. [DOI] [PubMed] [Google Scholar]
- 7.Frank D N, St Amand A L, Feldman R A, Boedeker E C, Harpaz N, Pace N R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley R E, Turnbaugh P J, Klein S, Gordon J I.Microbial ecology: human gut microbes associated with obesity Nature 2006444(7122):1022–1023. [DOI] [PubMed] [Google Scholar]
- 9.Ley R E, Bäckhed F, Turnbaugh P, Lozupone C A, Knight R D, Gordon J I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurung M, Li Z, You H. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain T, Sharma P, Are A C, Vickers S M, Dudeja V. New insights into the cancer-microbiome-immune axis: decrypting a decade of discoveries. Front Immunol. 2021;12:622064. doi: 10.3389/fimmu.2021.622064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Y, Havulinna A S, Liu Y. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54(02):134–142. doi: 10.1038/s41588-021-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez A, Stombaugh J, Lozupone C, Turnbaugh P J, Gordon J I, Knight R. The mind-body-microbial continuum. Dialogues Clin Neurosci. 2011;13(01):55–62. doi: 10.31887/DCNS.2011.13.1/agonzalez. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson T R, Debelius J W, Thron T. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(06):1469–1.48E15. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 2016;74(10):624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 16.Hugon P, Dufour J C, Colson P, Fournier P E, Sallah K, Raoult D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect Dis. 2015;15(10):1211–1219. doi: 10.1016/S1473-3099(15)00293-5. [DOI] [PubMed] [Google Scholar]
- 17.Almeida A, Nayfach S, Boland M. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat Biotechnol. 2021;39(01):105–114. doi: 10.1038/s41587-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gacesa R, Kurilshikov A, Vich Vila A.Environmental factors shaping the gut microbiome in a Dutch population Nature 2022604(7907):732–739. [DOI] [PubMed] [Google Scholar]
- 19.Goodrich J K, Davenport E R, Beaumont M. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19(05):731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong F, Deng F, Li Y, Zhao J. Identification of gut microbiome signatures associated with longevity provides a promising modulation target for healthy aging. Gut Microbes. 2019;10(02):210–215. doi: 10.1080/19490976.2018.1494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoro A, Ostan R, Candela M. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018;75(01):129–148. doi: 10.1007/s00018-017-2674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milieu Intérieur Consortium . Scepanovic P, Hodel F, Mondot S. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome. 2019;7(01):130. doi: 10.1186/s40168-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manor O, Dai C L, Kornilov S A. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. 2020;11(01):5206. doi: 10.1038/s41467-020-18871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odenwald M A, Turner J R. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(01):9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sears C L. Molecular physiology and pathophysiology of tight junctions V. assault of the tight junction by enteric pathogens. Am J Physiol Gastrointest Liver Physiol. 2000;279(06):G1129–G1134. doi: 10.1152/ajpgi.2000.279.6.G1129. [DOI] [PubMed] [Google Scholar]
- 26.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(06):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Sluis M, De Koning B A, De Bruijn A C. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(01):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Belkaid Y, Hand T W. Role of the microbiota in immunity and inflammation. Cell. 2014;157(01):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macpherson A J, Slack E, Geuking M B, McCoy K D. The mucosal firewalls against commensal intestinal microbes. Semin Immunopathol. 2009;31(02):145–149. doi: 10.1007/s00281-009-0174-3. [DOI] [PubMed] [Google Scholar]
- 30.Arike L, Hansson G C. The densely o-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. J Mol Biol. 2016;428(16):3221–3229. doi: 10.1016/j.jmb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherrington C A, Hinton M, Pearson G R, Chopra I. Short-chain organic acids at ph 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J Appl Bacteriol. 1991;70(02):161–165. doi: 10.1111/j.1365-2672.1991.tb04442.x. [DOI] [PubMed] [Google Scholar]
- 32.Rivera-Chávez F, Zhang L F, Faber F. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host Microbe. 2016;19(04):443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oxford IBD Cohort Investigators . Hegazy A N, West N R, Stubbington M JT. Circulating and tissue-resident CD4 + T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation . Gastroenterology. 2017;153(05):1320–1.337E19. doi: 10.1053/j.gastro.2017.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith P M, Howitt M R, Panikov N.The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis Science 2013341(6145):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holscher H D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(02):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong J MW, de Souza R, Kendall C WC, Emam A, Jenkins D JA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(03):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Roediger W EW. The starved colon: diminished mucosal nutrition, diminished absorption, and colitis. Dis Colon Rectum. 1990;33(10):858–862. doi: 10.1007/BF02051922. [DOI] [PubMed] [Google Scholar]
- 38.Harig J M, Soergel K H, Komorowski R A, Wood C M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320(01):23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Luo Y. Bacterial quorum-sensing systems and their role in intestinal bacteria-host crosstalk. Front Microbiol. 2021;12:611413. doi: 10.3389/fmicb.2021.611413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton J T, Freed S D, Lee S W, Friedberg I. A large scale prediction of bacteriocin gene blocks suggests a wide functional spectrum for bacteriocins. BMC Bioinformatics. 2015;16(01):381. doi: 10.1186/s12859-015-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(04):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Hooper L V, Littman D R, Macpherson A J.Interactions between the microbiota and the immune system Science 2012336(6086):1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev Immunol. 2020;38(01):23–48. doi: 10.1146/annurev-immunol-070119-115104. [DOI] [PubMed] [Google Scholar]
- 44.Bansal T, Alaniz R C, Wood T K, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010;107(01):228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heiman M L, Greenway F L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab. 2016;5(05):317–320. doi: 10.1016/j.molmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guyton K, Alverdy J C. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2017;14(01):43–54. doi: 10.1038/nrgastro.2016.139. [DOI] [PubMed] [Google Scholar]
- 47.Larsen N, Vogensen F K, van den Berg F WJ. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(02):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbaugh P J, Hamady M, Yatsunenko T.A core gut microbiome in obese and lean twins Nature 2009457(7228):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott S J, Musfeldt M, Wenderoth D F. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(05):685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elvers K T, Wilson V J, Hammond A. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10(09):e035677. doi: 10.1136/bmjopen-2019-035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaser M.Antibiotic overuse: stop the killing of beneficial bacteria Nature 2011476(7361):393–394. [DOI] [PubMed] [Google Scholar]
- 52.Dethlefsen L, Huse S, Sogin M L, Relman D A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dethlefsen L, Relman D A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108 01:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubourg G, Lagier J C, Robert C. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. Int J Antimicrob Agents. 2014;44(02):117–124. doi: 10.1016/j.ijantimicag.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 55.Anthony W E, Wang B, Sukhum K V. Acute and persistent effects of commonly used antibiotics on the gut microbiome and resistome in healthy adults. Cell Rep. 2022;39(02):110649. doi: 10.1016/j.celrep.2022.110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jernberg C, Löfmark S, Edlund C, Jansson J K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1(01):56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 57.Classen D C, Evans R S, Pestotnik S L, Horn S D, Menlove R L, Burke J P. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(05):281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 58.de Jonge S W, Boldingh Q JJ, Solomkin J S. Effect of postoperative continuation of antibiotic prophylaxis on the incidence of surgical site infection: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(10):1182–1192. doi: 10.1016/S1473-3099(20)30084-0. [DOI] [PubMed] [Google Scholar]
- 59.O'Brien C L, Allison G E, Grimpen F, Pavli P. Impact of colonoscopy bowel preparation on intestinal microbiota. PLoS One. 2013;8(05):e62815. doi: 10.1371/journal.pone.0062815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jalanka J, Salonen A, Salojärvi J. Effects of bowel cleansing on the intestinal microbiota. Gut. 2015;64(10):1562–1568. doi: 10.1136/gutjnl-2014-307240. [DOI] [PubMed] [Google Scholar]
- 61.Drago L, Toscano M, De Grandi R, Casini V, Pace F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur J Gastroenterol Hepatol. 2016;28(05):532–537. doi: 10.1097/MEG.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 62.Nagata N, Tohya M, Fukuda S. Effects of bowel preparation on the human gut microbiome and metabolome. Sci Rep. 2019;9(01):4042. doi: 10.1038/s41598-019-40182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrell L, Wang Y, Antonopoulos D. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One. 2012;7(02):e32545. doi: 10.1371/journal.pone.0032545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Z, Tong C, Qian X, Wang H, Wang Y. Mechanical bowel preparation is a risk factor for postoperative delirium as it alters the gut microbiota composition: a prospective randomized single-center study. Front Aging Neurosci. 2022;14:847610. doi: 10.3389/fnagi.2022.847610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiran R P, Murray A CA, Chiuzan C, Estrada D, Forde K.Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery Ann Surg 201526203416–425., discussion 423–425 [DOI] [PubMed] [Google Scholar]
- 66.Ferrie S, Webster A, Wu B, Tan C, Carey S. Gastrointestinal surgery and the gut microbiome: a systematic literature review. Eur J Clin Nutr. 2021;75(01):12–25. doi: 10.1038/s41430-020-0681-9. [DOI] [PubMed] [Google Scholar]
- 67.Tarazi M, Jamel S, Mullish B H, Markar S R, Hanna G B. Impact of gastrointestinal surgery upon the gut microbiome: a systematic review. Surgery. 2022;171(05):1331–1340. doi: 10.1016/j.surg.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Kazmierczak B I, Schniederberend M, Jain R. Cross-regulation of pseudomonas motility systems: the intimate relationship between flagella, pili and virulence. Curr Opin Microbiol. 2015;28:78–82. doi: 10.1016/j.mib.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. Int J Med Microbiol. 2002;291(08):605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 70.Vasil M L, Chamberlain C, Grant C C. Molecular studies of pseudomonas exotoxin A gene. Infect Immun. 1986;52(02):538–548. doi: 10.1128/iai.52.2.538-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lebrun I, Marques-Porto R, Pereira A S, Pereira A, Perpetuo E A. Bacterial toxins: an overview on bacterial proteases and their action as virulence factors. Mini Rev Med Chem. 2009;9(07):820–828. doi: 10.2174/138955709788452603. [DOI] [PubMed] [Google Scholar]
- 72.Nallapareddy S R, Qin X, Weinstock G M, Höök M, Murray B E. Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun. 2000;68(09):5218–5224. doi: 10.1128/iai.68.9.5218-5224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shogan B D, Belogortseva N, Luong P M. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7(286):286ra68. doi: 10.1126/scitranslmed.3010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Praagh J B, de Goffau M C, Bakker I S. Mucus microbiome of anastomotic tissue during surgery has predictive value for colorectal anastomotic leakage. Ann Surg. 2019;269(05):911–916. doi: 10.1097/SLA.0000000000002651. [DOI] [PubMed] [Google Scholar]
- 75.van Praagh J B, de Goffau M C, Bakker I S, Harmsen H JM, Olinga P, Havenga K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: a pilot study. Surg Endosc. 2016;30(06):2259–2265. doi: 10.1007/s00464-015-4508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson M A, Jeffery I B, Beaumont M. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(01):8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown A C, Valiere A. Probiotics and medical nutrition therapy. Nutr Clin Care. 2004;7(02):56–68. [PMC free article] [PubMed] [Google Scholar]
- 78.Roberfroid M, Gibson G R, Hoyles L. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104 02:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 79.Chowdhury A H, Adiamah A, Kushairi A. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2020;271(06):1036–1047. doi: 10.1097/SLA.0000000000003581. [DOI] [PubMed] [Google Scholar]
- 80.Wieërs G, Belkhir L, Enaud R. How probiotics affect the microbiota. Front Cell Infect Microbiol. 2020;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y-T, Cai H-F, Wang Z-H, Xu J, Fang J-Y. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection . Aliment Pharmacol Ther. 2016;43(04):445–457. doi: 10.1111/apt.13492. [DOI] [PubMed] [Google Scholar]
- 82.König J, Siebenhaar A, Högenauer C. Consensus report: faecal microbiota transfer: clinical applications and procedures. Aliment Pharmacol Ther. 2017;45(02):222–239. doi: 10.1111/apt.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gai X, Wang H, Li Y. Fecal microbiota transplantation protects the intestinal mucosal barrier by reconstructing the gut microbiota in a murine model of sepsis. Front Cell Infect Microbiol. 2021;11:736204. doi: 10.3389/fcimb.2021.736204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S M, DeFazio J R, Hyoju S K. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat Commun. 2020;11(01):2354. doi: 10.1038/s41467-020-15545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang S, Xu M, Wang W. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11(08):e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeFilipp Z, Bloom P P, Torres Soto M. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant . N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 87.Doubeni C A, Major J M, Laiyemo A O. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104(18):1353–1362. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doubeni C A, Laiyemo A O, Major J M. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118(14):3636–3644. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clarke S F, Murphy E F, O'Sullivan O. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 90.Allen J M, Mailing L J, Niemiro G M. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(04):747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 91.Bolte L A, Vich Vila A, Imhann F. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70(07):1287–1298. doi: 10.1136/gutjnl-2020-322670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hyoju S K, Adriaansens C, Wienholts K. Low-fat/high-fibre diet prehabilitation improves anastomotic healing via the microbiome: an experimental model. Br J Surg. 2020;107(06):743–755. doi: 10.1002/bjs.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanegas S M, Meydani M, Barnett J B. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. 2017;105(03):635–650. doi: 10.3945/ajcn.116.146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.David L A, Materna A C, Friedman J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(07):R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]


