Abstract
Background:
Major depressive disorder (MDD) is often accompanied by changes in appetite and weight. Prior task-based functional magnetic resonance imaging (fMRI) findings suggest these MDD phenotypes are associated with altered reward and interoceptive processing.
Methods:
Using resting state fMRI data, we compared fractional amplitude of low-frequency fluctuations (fALFF) and seed-based connectivity (SBC) among hyperphagic (n = 77), hypophagic (n = 66), and euphagic (n = 42) MDD groups and a healthy comparison group (n = 38). We examined fALFF and SBC in a mask restricted to reward (nucleus accumbens (NAcc), putamen, caudate, ventral pallidum, and orbitofrontal cortex (OFC)) and interoceptive (anterior insula and hypothalamus) regions and also performed exploratory whole-brain analyses. SBC analyses included as seeds the NAcc and also regions demonstrating group differences in fALFF (i.e., right lateral OFC and right anterior insula). All analyses used threshold-free cluster enhancement.
Results:
Mask-restricted analyses revealed stronger fALFF in the right lateral OFC, and weaker fALFF in the right anterior insula, for hyperphagic MDD versus healthy comparison. We also found weaker SBC between the right lateral OFC and left anterior insula for hyperphagic MDD versus healthy comparison. Whole-brain analyses revealed weaker fALFF in the right anterior insula, and stronger SBC between the right lateral OFC and left precentral gyrus, for hyperphagic MDD versus healthy comparison. Findings were no longer significant after controlling for body mass index, which was higher for hyperphagic MDD.
Conclusions:
Our results suggest hyperphagic MDD may be associated with altered activity in and connectivity between interoceptive and reward regions.
Keywords: Major Depressive Disorder, Appetite, Weight Change, Eating, fMRI
Introduction
Marked decreases or increases in appetite or weight are a common symptom included in the diagnostic criteria for major depressive disorder (MDD) (American Psychiatric Association, 2013). Research suggests that almost one half of adults with MDD present with hypophagic behaviors, whereas approximately one quarter present with hyperphagic behaviors attributable to the current MDD episode (Husain et al., 2005). These phenotypes differ not only behaviorally, but also in terms of brain activity and metabolism (Simmons et al., 2020). Further elucidating the neurobiological substrates of these differing MDD presentations may help develop more effective treatments.
Few studies have focused on neurobiological differences between appetite/weight phenotypes in MDD. One recent study used functional magnetic resonance imaging (fMRI) to evaluate differences in unmedicated participants with MDD who had an increase versus a decrease in appetite (Simmons et al., 2016). Participants viewed food and non-food stimuli during fMRI and rated how pleasant it would be to eat foods from a second set of visual stimuli. When presented with food versus non-food stimuli, participants with an increase in appetite (n=16) showed significantly stronger activation in the orbitofrontal cortex (OFC), ventral striatum (including the nucleus accumbens (NAcc)), ventral pallidum, and putamen, relative to individuals with a decrease in appetite (n=16). Additionally, participants in the decreased appetite group had lower activation in the caudal anterior and dorsal mid-insula, compared to participants in the increased appetite group. Although the authors did not examine resting-state functional connectivity (RSFC) group differences, food pleasantness ratings were positively correlated with functional connectivity between the right dorsal mid-insula and two other regions showing group differences in activation during the food stimuli task (the left medial ventral striatum and the left ventral medial prefrontal cortex); additionally, pleasantness ratings were higher in the hyperphagic MDD group than the controls and hypophagic MDD group. Moreover, another study investigated differences in fMRI responsivity to food versus non-food stimuli in participants with remitted MDD who had experienced MDD-attributed appetite changes (Cerit et al., 2019). For high-calorie food (versus non-food) stimuli, the increased appetite group had greater activation in the right putamen and right anterior insula compared to the decreased appetite group. For high- (versus low-) calorie food stimuli, the increased appetite group showed greater activation than healthy controls in the right and left caudate and the left pallidum, and participants in the decreased appetite group were characterized by greater activation than healthy controls in the left hypothalamus and the right caudate (Cerit et al., 2019). Collectively, these findings indicate that different appetite phenotypes in MDD are associated with abnormalities within regions critically implicated in reward processing (e.g., NAcc, orbitofrontal cortex, putamen, caudate, ventral pallidum) and interoceptive perception (e.g., anterior insula, hypothalamus).
Differences in brain activation to food stimuli have also been investigated in conjunction with metabolic markers (Simmons et al., 2020). Specifically, in the hyperphagic MDD group, individuals with greater insulin resistance exhibited higher activation to food cues in the insular cortex. In combination with other findings, such as increased levels of immune markers in the increased appetite group, Simmons et al. (2020) suggested that hyperphagic MDD is associated with metabolic and immune dysfunction, independent of body mass index (BMI).
Existing studies have examined differences in how MDD appetite phenotypes respond to food cues (Cerit et al., 2019; Simmons et al., 2016; Simmons et al., 2020). Because brain activity and connectivity are less constrained by specific task-based demands during rest, analyses of resting-state fMRI for hyperphagic and hypophagic MDD can provide important, complementary insights into the neural underpinnings of these presentations, revealing potential differences that exist independent of exposure to food cues. Besides using RSFC to investigate connectivity between regions, fractional amplitude of low-frequency fluctuations (fALFF) analyses may be helpful to assess spontaneous activity in specific brain regions (Zou et al., 2008), since disorder-related abnormalities may be due to local neuronal activity rather than connectivity between different locations (Egorova, Veldsman, Cumming, & Brodtmann, 2017). These methods have been used to elucidate the neural correlates of MDD (Mulders, van Eijndhoven, Schene, Beckmann, & Tendolkar, 2015; Zhou et al., 2017) and conditions associated with abnormal food intake (Parsons, Steward, Clohesy, Almgren, & Duehlmeyer, 2021). However, no investigations have compared resting state activity or connectivity between different appetite phenotypes in MDD, despite the potential insights that may be gleaned from such investigations. In particular, the aforementioned resting state fMRI measures may prove sensitive to detecting the appetitive, metabolic, and reward-related differences that differentiate MDD with hyperphagia from MDD with hypophagia or euphagia, given prior evidence suggesting that resting state measures can index individual’s metabolic state and perceived food pleasantness (Al-Zubaidi, Heldmann, Mertins, Jauch-Chara, & Munte, 2018; Simmons et al., 2016).
In the present study, we seek to establish a more comprehensive view of the neural underpinnings of MDD appetite and/or weight phenotypes by examining resting state activity and connectivity. This is important in order to understand whether there are group differences in spontaneous activity and connectivity that exist independent of the response to external food cues. To achieve this, we examined differences in fALFF and RSFC in hyperphagic, hypophagic and euphagic (no change in appetite/weight attributable to the MDD episode) MDD in comparison to a healthy comparison group. To focus on the regions specified in our a priori hypotheses, we first restricted fALFF and RSFC analyses to a mask containing the brain regions reported to show differences across MDD eating phenotypes in prior research, i.e., reward and interoceptive regions. For RSFC analyses, regions exhibiting fALFF group differences were used as seeds, as was the NAcc given its role in reward processing and involvement in food-related disorders and depression (Demos, Heatherton, & Kelley, 2012; Domingo-Rodriguez et al., 2020; Lawrence, Hinton, Parkinson, & Lawrence, 2012). Second, we explored fALFF and RSFC (using the same regions as seeds) in the whole brain. Given that RSFC to reward regions (e.g., NAcc) was greater in conditions associated with increased food intake (Parsons et al., 2021) and reduced in conditions associated with decreased food intake (Haynos et al., 2019), we expected the connectivity between the NAcc and other reward and interoceptive regions to vary according the MDD appetite/weight phenotype, with hyperphagic MDD showing higher connectivity than the hypophagic MDD, and the euphagic and healthy comparison groups being in an intermediate position between the other two groups. We expected fALFF in reward and interoceptive regions to vary with respect to appetite/weight phenotype but did not have specific hypotheses regarding directionality given the limited existing literature.
Methods
Participants
Participants (n = 223) were drawn from the multi-site clinical trial Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care for Depression (EMBARC) (Trivedi et al., 2016). Data were collected at Columbia University, Massachusetts General Hospital, the University of Texas Southwestern Medical Center, and the University of Michigan. For the MDD group, exclusion criteria included lifetime psychotic depressive disorder, bipolar disorder, and any psychotic disorder, as well as current primary anxiety disorder, substance dependence (previous six months), and substance abuse (previous two months). Further, to increase homogeneity, individuals were excluded from MDD groups if they did not score 14 or above on the Quick Inventory of Depressive Symptomatology (QIDS) (Rush et al., 2003). For the healthy comparison group, exclusion criteria included any lifetime Axis I disorder. For all groups, further exclusion criteria included a history of neurological disorders, head injury, major medical illnesses, or current pregnancy. For the present analyses, we additionally excluded individuals reporting current diabetes or whose resting state fMRI data did not pass quality control (Figure 1). No participants were on anti-depressant medication in the present analyses, based on data collected at the screening and baseline visits.
Figure 1.
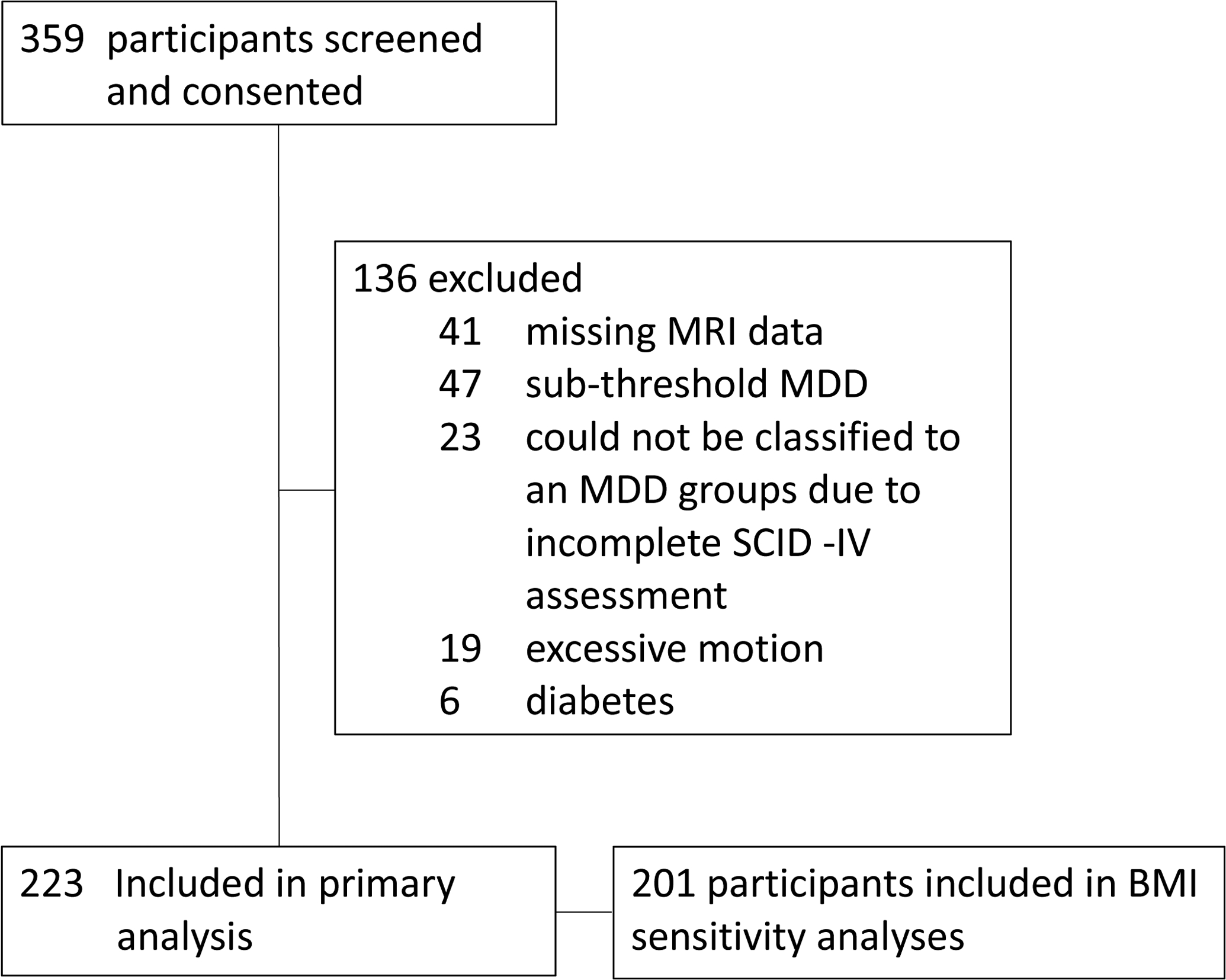
Flow chart of participants’ inclusion.
For the present analyses, participants with MDD were divided into groups categorized as hyperphagic MDD (i.e., individuals with MDD who experienced an increase in appetite and/or weight during the current MDD episode; n = 77), hypophagic MDD (i.e., individuals with MDD who experienced a decrease in appetite and/or weight during the current MDD episode; n = 66), and euphagic MDD (i.e., individuals with MDD who experienced no significant or detectable changes in appetite and/or weight during the current MDD episode; n = 42); in addition, healthy comparison participants without MDD (n = 38) were included. MDD participants were allocated to groups based on their responses to items assessing appetite and/or weight changes from the MDD section of the mood disorders module of the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-IV) (Kübler, 2013). Specifically, individuals with scores of 3 (i.e., threshold or true) on the appetite and/or weight change item were assigned to either the hyperphagic or hypophagic groups, depending on whether the additional items indicated weight gain / increased appetite or weight loss / decreased appetite, respectively. Individuals with scores of 1 on the appetite and/or weight change item were assigned to the euphagic MDD group. Individuals with a score of 2 (i.e., subthreshold) on the appetite and/or weight change item (n = 23) were excluded from the analyses, given that the additional items indicating directionality of the change were not consistently completed for those participants and therefore sub-threshold hyperphagia/hypophagia groups would have been extremely small.
Procedures
During screening, all participants signed the consent and completed the SCID-IV, demographics, as well as the Mood and Anxiety Symptoms Questionnaire (MASQ) (Watson et al., 1995), the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995), and the QIDS. Following screening (range = 1–70 days; mean = 13.1 days), participants presented to the fMRI scan used in the present analyses.
Imaging acquisition and preprocessing of fMRI data
See Supplemental Information for details on data acquisition, preprocessing, and region of interest (ROI) definition.
Statistical analyses
Demographic and clinical measures
To more fully characterize the groups, we compared them on various demographic and clinical measures. See Supplemental Material for more information on analyses of demographic variables.
Clinical symptoms were assessed using the MASQ, SHAPS, and QIDS. As in Simmons et al. (2016), SHAPS items assessing loss of pleasure to food and drink were excluded when calculating the score since previous analyses of food pleasantness showed differences between hyperphagic vs hypophagic MDD (Simmons et al., 2016). Similarly, QIDS items assessing appetite and weight change were excluded from calculation of the overall QIDS score. However, for participants with MDD, we used responses to the QIDS appetite and weight change items to investigate whether group differences in fALFF and RSFC analyses were driven by MDD-related changes in appetite and/or weight. Specifically, for MDD participants who correctly followed instructions for completing the QIDS appetite and weight change items, we multiplied the item assessing decreased appetite by −1 and added it to the item assessing increased appetite, resulting in a composite appetite change score that ranged from −3 to 3; the same procedure was used to create a composite weight change score.
Functional activity and connectivity
The Functional Connectivity Toolbox v19.c (CONN) (Whitfield-Gabrieli & Nieto-Castanon, 2012) was used for all fMRI analyses. All analyses included Sex and Site as regressors, since data were collected at different locations and groups significantly differed with respect to sex frequencies. To address multiple comparisons, we used threshold-free cluster enhancement (TFCE) (Smith & Nichols, 2009) with 1,000 permutations (statistical threshold set at p < 0.05, FWE-corrected). Finally, Cohen’s effect sizes (d) were calculated for significant findings.
Fractional amplitude of low-frequency fluctuations (fALFF).
We first performed mask-restricted analyses that examined group differences in fALFF in regions of interest (the NAcc, lateral OFC, hypothalamus, putamen, caudate, ventral pallidum, and anterior insula). Specifically, we investigated significant clusters within the mask including these regions. Secondly, we performed exploratory analyses examining fALFF group differences across the whole brain. In both analyses, the timeseries of each voxel was transformed to the frequency domain using a Discrete Cosine Transform, and power spectra were calculated. Voxelwise fALFF analysis was conducted by calculating the average square root of power with the 0.008–0.09 Hz frequency band for each voxel and dividing by the total power spectra (Hallquist, Hwang, & Luna, 2013). fALFF maps were standardized into subject-level Z-score maps.
Seed-based connectivity analyses.
We first performed mask-restricted analyses that examined group differences in seed-based connectivity from three seed regions to voxels within regions of interest. Seed regions included the NAcc, as well as the clusters in the lateral OFC and anterior insula that showed significant fALFF differences between groups. Secondly, we performed exploratory whole-brain seed-based connectivity analyses using the same three regions as seeds to evaluate group differences in connectivity involving the NAcc, lateral OFC, and anterior insula with the rest of the brain.
Sensitivity analyses.
Given evidence that hyperphagic MDD is associated with metabolic dysfunction (Simmons et al., 2020), the analyses described above were also performed with adjustment for BMI (n = 201 participants with BMI available) as an additional covariate. Additionally, to explore moderation by sex, we examined whether group differences in fALFF and RSFC differed for males and females. Finally, we used Pearson’s correlations and multiple regression to examine the relationship between beta values from significant fALFF and RSFC findings and the QIDS appetite and weight change scores (n = 147 participants with change scores available), as well as BMI (see Supplemental Materials for more information).
Preregistration
Hypotheses and data analysis plans were pre-registered on Open Science Framework (see https://osf.io/fxqdp). See Supplemental Material for details.
Results
Analyses of demographic and clinical measures
Sample characteristics can be found in Table 1. See Supplemental Material for further description of demographics and clinical measures.
Table 1.
Demographic and clinical characteristics of participants included in the imaging analyses (N=223)
| Variable | Healthy Comparison (HC) | MDD | Omnibus Test | Post hoc comparison p values | ||
|---|---|---|---|---|---|---|
| Euphagic (Eu) | Hypophagic (Hypo) | Hyperphagic (Hyper) | ||||
| N | 38 | 42 | 66 | 77 | - | - |
| Age in years, mean (SD) | 38.1 (14.9) | 35.8 (12.5) | 35.3 (11.6) | 38.4 (13.5) |
F = 0.84 p = 0.486a |
- |
| Women, number (%) | 24 (63.2) | 25 (59.5) | 42 (63.6) | 62 (80.5) | p = 0.038b | = 0.017 Hyper > Eu = 0.037 Hyper > Hypo = 0.066 Hyper > HC = 0.819 HC – Eu = 1.000 HC – Hypo = 0.688 Hypo – Eu |
| Body mass index in kg/m2, mean (SD) | 25.0 (3.8) | 26.8 (6.6) | 25.9 (5.5) | 31.4 (6.8) |
F = 12.88 p < 0.001a |
= 0.001 Hyper > Eu < 0.001 Hyper > Hypo < 0.001 Hyper > HC = 0.598 HC – Eu = 0.897 HC – Hypo = 0.895 Hypo – Eu |
| Education in years, mean (SD) | 15.2 (2.2) | 15.6 (2.3) | 15.0 (2.8) | 15.1 (2.4) | F = 0.11 p = 0.952a |
- |
| Other | 2 (5.3) | 2 (4.7) | 6 (9.0) | 5 (6.5) | ||
| Hispanic or Latino origin, number (%) | 3 (7.9) | 7 (16.6) | 12 (18.1) | 21 (27.2) | p = 0.088b | - |
| Mood congruent psychotic features | 0 (0) | 1 (1.5) | 0 (0) | |||
| Recurrent | 29 (69.0) | 57 (86.3) | 69 (89.7) | |||
| Age at MDD onset in years, mean (SD) | - | 15.7 (5.4) | 16.2 (5.9) | 17.4 (6.2) | F = 1.30 p = 0.274a |
- |
| Length of current major depressive episode in months, median | - | 24.0 | 12.5 | 12.0 | X2 = 3.662 p = 0.160c |
- |
| Number of prior major depressive episodes, median | - | 3 | 4 | 4 | X2 = 5.811 p = 0.054c |
- |
| QIDS weight change score, mean (SD) | - | 0 (1.1) | −1.1 (1.3) | 1.6 (1.4) | F = 60.23a p < 0.001a |
< 0.001 Hyper > Eu > Hypo |
| Other current diagnosese, No. (%) | ||||||
| Mood and anxiety symptoms questionnaire | ||||||
Note: p-values are comparisons between groups via one-way ANOVA (and Tukey’s Test for post hoc comparisons when applicable),
Fisher’s Exact tests, or
Kruskal-Wallis tests.
Quick Inventory of Depressive Symptomatology (QIDS) overall scores did not include the items measuring appetite/weight change. QIDS appetite change scores were calculated by multiplying the item assessing decreased appetite by −1 and adding it to the item assessing increased appetite, resulting in a composite appetite change score that ranged from −3 to 3; the same procedure was used to create a composite weight change score.
Using the Structured Clinical Interview for DSM-IV Axis I disorders (Kübler, 2013).
There was no significant difference between MDD groups for the other clinical measures unless stated otherwise.
fMRI analyses
Unthresholded maps for fMRI analyses can be viewed at https://neurovault.org/collections/11836/.
Fractional amplitude of low-frequency fluctuations (fALFF)
Mask-restricted analyses.
Relative to the healthy comparison group, the hyperphagic MDD group was characterized by significantly lower fALFF in the right anterior insula (TFCE = 146.73, p-FWE = 0.018, d(cluster level) = −0.942; Fig. 2), but higher fALFF in the right lateral OFC (TFCE = 125.40, p-FWE = 0.036, d(cluster level) = 0.918; Fig. 2). See Table 2 for further details. No other group differences emerged.
Figure 2. Resting state fractional amplitude of low-frequency fluctuations (fALFF) in the right anterior insula and right lateral orbitofrontal cortex (OFC).
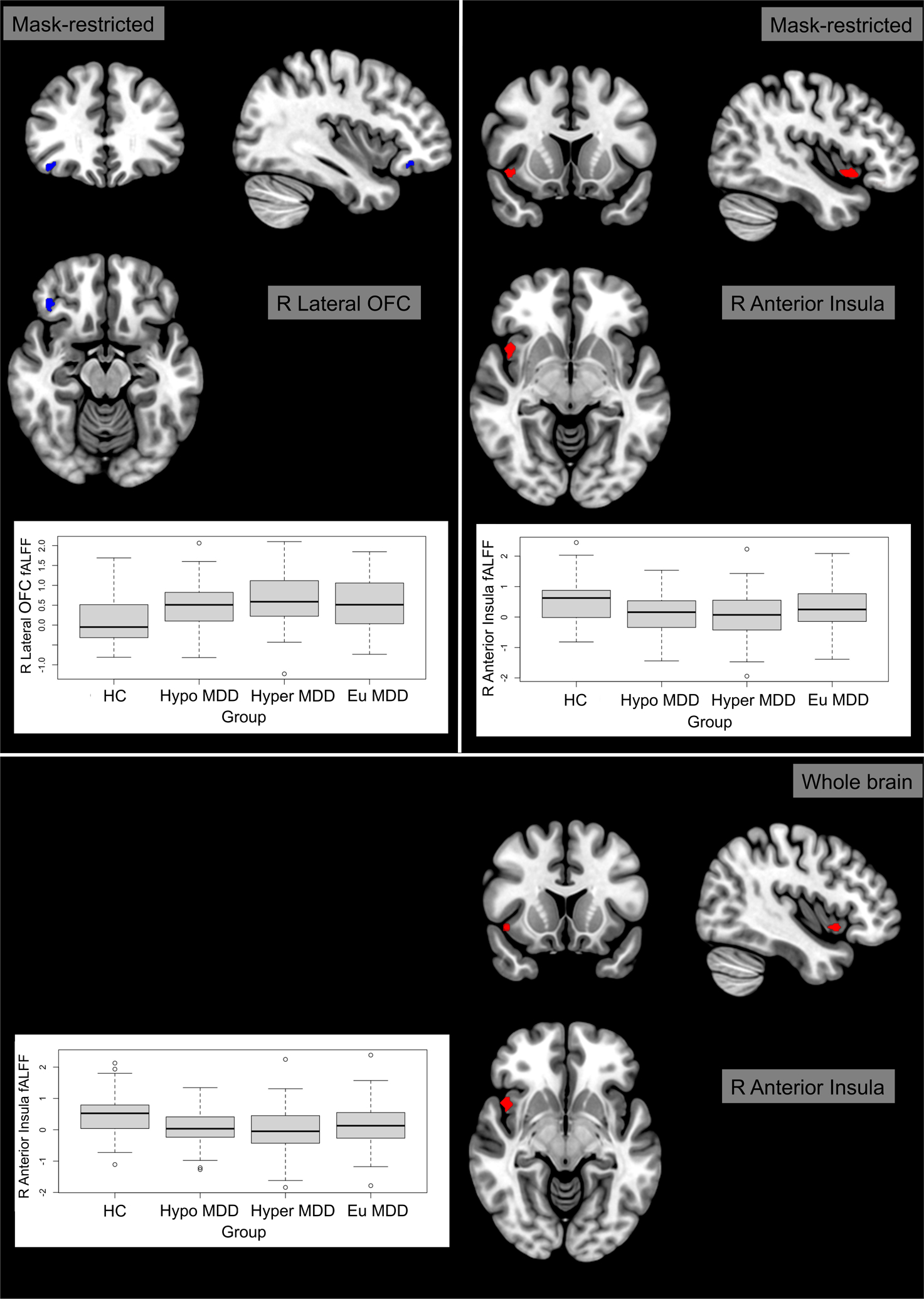
Compared to the healthy comparison group, the hyperphagic major depressive disorder group showed increased fALFF in the right lateral OFC and decreased fALFF in the right anterior insula. Brain slices are presented in accordance with radiological convention (i.e., right hemisphere presented in left side of the image). Models included adjustment for sex and site.
(Abbreviations: Eu = euphagic; fALFF = fractional amplitude of low-frequency fluctuations; HC = healthy comparisons; Hyper = hyperphagic; Hypo = hypophagic; MDD = major depressive disorder; OFC = orbitofrontal cortex; R = right)
Table 2.
Clusters exhibiting group differences in mask-restricted or whole brain analyses of fractional amplitude of low-frequency fluctuations. Models included adjustment for sex and site.
| Regiona | Clusterb (x, y, z)c | Size | Peaks | TFCE | Peak p-FWE | Peak p-unc | Analyses; Comparison | ||
|---|---|---|---|---|---|---|---|---|---|
| R Anterior Insula | +44 | +08 | −10 | 42 | 1 | 146.73 | 0.017667 | 0.000069 | MR; HC > Hyper |
| R Lateral OFC | +40 | +32 | −14 | 30 | 3 | 125.40 | 0.035667 | 0.000191 | MR; Hyper > HC |
| R Anterior Insula | +44 | +10 | −10 | 32 | 1 | 411.98 | 0.037000 | 0.000031 | WB; HC > Hyper |
Abbreviations: FWE = Family-wise error; HC = Healthy comparison group; Hyper = Hyperphagic major depressive disorder group; MR = Mask-restricted; OFC = Orbitofrontal cortex; R = Right; TFCE = Threshold-free cluster enhancement; unc = uncorrected; WB = Whole brain
Region with the most voxels within the relevant cluster
Only clusters with more than 20 voxels are reported
Montreal Neurological Institute (MNI)
Whole-brain analysis.
Whole brain analyses confirmed lower fALFF for hyperphagic MDD relative to the healthy comparison group in the right anterior insula (TFCE = 411.98, p-FWE = 0.037, d(cluster level) = −0.958; Fig. 2). See Table 2 for further details. No other significant differences were found.
Seed-based connectivity
Mask-restricted analyses.
The connectivity between the right lateral OFC and the left anterior insula was significantly lower in hyperphagic MDD compared to the healthy comparison group (TFCE = 173.71, p-FWE = 0.045, d(cluster level) = −0.730; Fig 3). See Table 3 for further details. No significant differences emerged when the NAcc or anterior insula were used as seeds.
Figure 3. Resting state functional connectivity analyses with the right lateral orbitofrontal cortex (OFC) as seed.
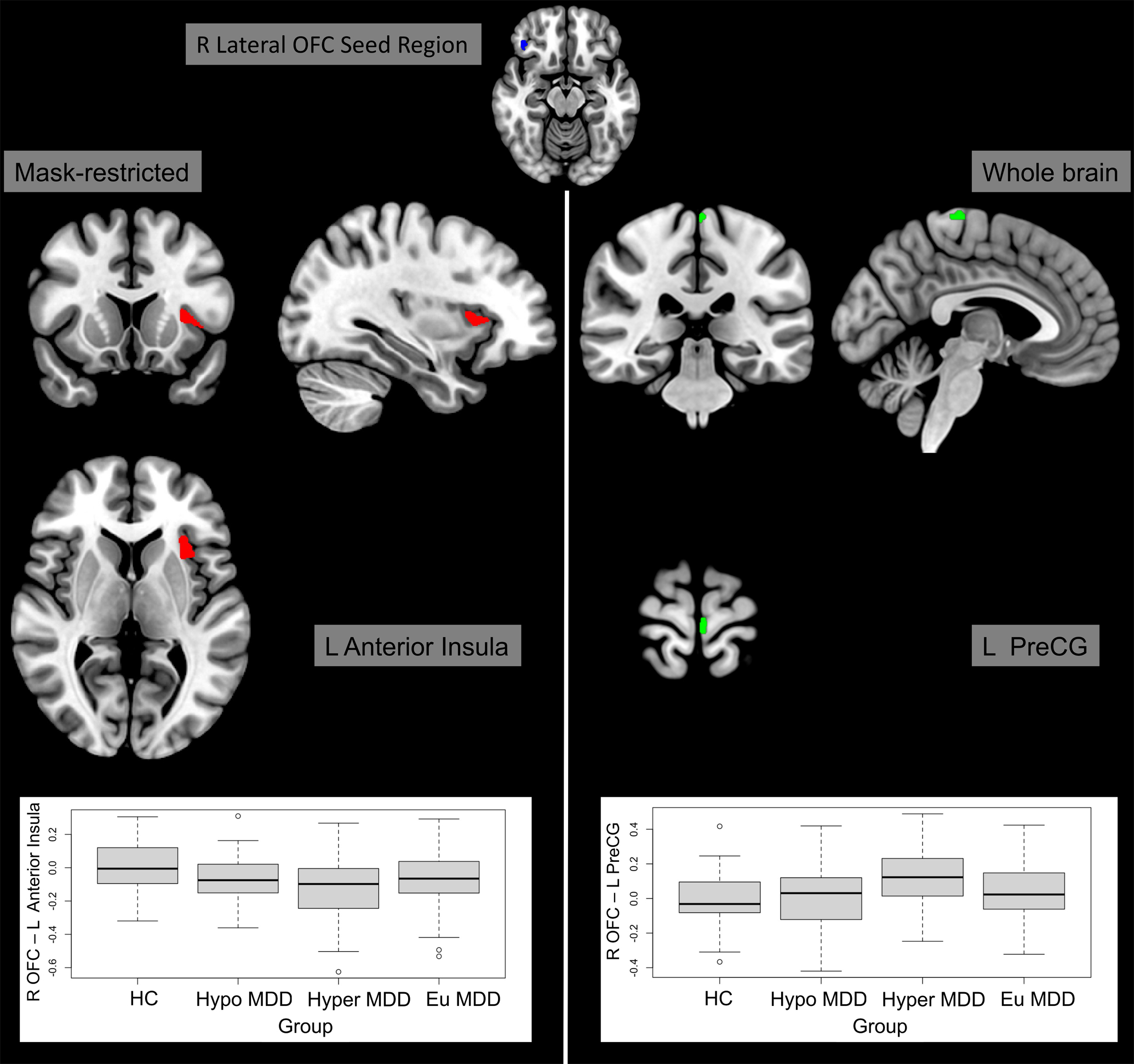
Compared to the healthy comparison group, the hyperphagic major depressive disorder group showed decreased resting-state functional connectivity to the left anterior insula and increased connectivity to the left precentral gyrus. Brain slices are presented in accordance with radiological convention (i.e., right hemisphere presented in left side of the image). Models included adjustment for sex and site.
(Abbreviations: Eu = euphagic; HC = healthy comparisons; Hyper = hyperphagic; Hypo = hypophagic; L = left; MDD = major depressive disorder; OFC = orbitofrontal cortex; PreCG = precentral gyrus; R = right)
Table 3.
Clusters exhibiting group differences in mask-restricted or whole brain analyses of seed-based connectivity (with the right lateral OFC as the seed). Models included adjustment for sex and site.
| Regiona | Clusterb (x, y, z)c | Size | Peaks | TFCE | Peak p-FWE | Peak p-unc | Analyses; Comparison | ||
|---|---|---|---|---|---|---|---|---|---|
| L Anterior Insula | −42 | +14 | +00 | 89 | 1 | 173.71 | 0.045000 | 0.001030 | MR; HC > Hyper |
| L Precentral Gyrus | −04 | −28 | +72 | 24 | 3 | 593.07 | 0.047000 | 0.000282 | WB; Hyper > HC |
Abbreviations: FWE = Family-wise error; HC = Healthy comparison group; Hyper = Hyperphagic major depressive disorder group; L = Left; MR = Mask-restricted; OFC = Orbitofrontal cortex; TFCE = Threshold-free cluster enhancement; unc = uncorrected; WB = Whole brain
Region with the most voxels within the relevant cluster
Only clusters with more than 20 voxels are reported
Montreal Neurological Institute (MNI)
Whole-brain analysis.
We found significantly higher connectivity between the right lateral OFC and the left precentral gyrus in hyperphagic MDD compared to the healthy comparison group (TFCE = 593.07, p-FWE = 0.047, d(cluster level) = 0.862; Fig. 3). See Table 3 for further details. No significant differences were found for connectivity of the NAcc, or the anterior insula, to the whole brain.
Sensitivity analysis
When BMI was included as a covariate in the analyses above, no significant differences were found among groups for fALFF or RSFC. Also, in the sex moderation analyses, the effect of group did not differ significantly between males and females for fALFF or RSFC (all p values > 0.05).
Finally, beta values were significantly correlated (all p values < 0.05), in the expected direction, with both appetite and weight changes scores, with two exceptions (Supplemental Figure 2). Beta values for the anterior insula finding from the fALFF mask-restricted analyses were not significantly correlated with weight change scores (p = 0.15), and beta values for the R lateral OFC – L anterior insula finding from SBC mask-restricted analyses were not significantly correlated with appetite change scores (p = 0.077). Correlations between beta values and BMI were in the same direction as the appetite- and weight- change items, but generally smaller and non-significant (Supplemental Figure 2). In multiple regressions for appetite (or weight) change scores as a function of the beta values for the various significant fALFF and RSFC findings (see Supplemental Materials), connectivity between right lateral OFC and left precentral gyrus was the most reliable predictor of appetite changes scores (p < 0.05) and weight change scores (p < 0.01).
Discussion
Investigating different appetite/weight change phenotypes in MDD may reveal important neurobiological differences between groups and help guide more targeted treatments. Specifically, individuals with MDD may be more likely to benefit from interventions that normalize the distinct patterns of resting state activity and connectivity found to be aberrant for that individual’s particular MDD phenotype. We investigated resting-state fractional amplitude of low-frequency fluctuations (fALFF) and seed-based resting-state functional connectivity in individuals with MDD presenting with different appetite/weight phenotypes. In comparison with the healthy comparison group, individuals with hyperphagic MDD showed: 1) lower fALFF in the right anterior insula and higher fALFF in the right lateral OFC; 2) lower connectivity between the right lateral OFC and the left anterior insula; and 3) higher connectivity between the right lateral OFC and the left precentral gyrus. Further, examination of correlations between the beta values for these significant findings and responses to the appetite and weight change items from the Quick Inventory of Depressive Symptomatology suggests that these differences in fALFF and connectivity are generally correlated with both appetite and weight changes; exceptions were fALFF in the right anterior insula, which was significantly correlated with appetite changes but not weight changes, and connectivity between the right lateral OFC and left anterior insula, which was significantly correlated with weight changes but not appetite changes.
The anterior insula is one of two regions containing the primary gustatory cortex; it is also implicated in interoceptive awareness (Craig, 2009), and may play a role in communicating interoceptive changes (Barrett & Simmons, 2015). Prior research in current (Simmons et al., 2016) and remitted (Cerit et al., 2019) MDD suggests higher activity in response to presentation of visual food stimuli in the right anterior insula for individuals with increased (vs. decreased) appetite in the context of MDD. Our fALFF finding for the anterior insula extends these prior data, showing that independent of food presentation, individuals with hyperphagic MDD present with decreased spontaneous activity in the anterior insula and that these alterations are correlated with appetite changes. Notably, the anterior insula has been implicated in the pathophysiology of MDD (Sliz & Hayley, 2012), and some meta-analyses of resting state activity suggest that amplitude of low-frequency fluctuations (ALFF) is elevated in the anterior insula in individuals with MDD (Li et al., 2017; Zhou et al., 2017). Our findings suggest that the nature of alterations in spontaneous activity in the anterior insula may partly depend on the particular appetite phenotype of MDD.
In addition to lower fALFF in the right anterior insula, hyperphagic MDD also had higher fALFF in the right lateral OFC, compared to the healthy comparison group. The OFC is involved in valuation and decision-making. This includes the representation of stimuli (e.g., food) as rewards, with activation in the OFC encoding, for example, food pleasantness (Simmons et al., 2014) and reward value (Rudebeck & Murray, 2014), as well as tastiness (Londeree & Wagner, 2020). Research has also evidenced differences in reward processing between the medial and lateral parts of the OFC (Rolls, Cheng, & Feng, 2020). An early study in primates suggested that neurons in the lateral portion of OFC were activated in response to a food-reward extinction procedure, i.e., when food reward was no longer delivered as expected (Rolls, 2021; Thorpe, Rolls, & Maddison, 1983). Additionally, evidence suggests that the lateral OFC may represent unpleasant stimuli, such as unpleasant odors (Rolls, Kringelbach, & de Araujo, 2003). Relevant to the present study, Simmons et al. (2016) found increased activation in response to food (vs. non-food) stimuli in the right OFC for the increased appetite MDD group in comparison to healthy controls. Also notably, some meta-analyses have found increased ALFF in more medial parts of the OFC for individuals with MDD (Li et al., 2017). Our results for the lateral OFC extend these prior findings, by suggesting that hyperphagic MDD is associated with increased spontaneous activity in the lateral OFC independent of food-related stimuli.
Besides differences in fALFF, the hyperphagic MDD group exhibited differences in resting-state functional connectivity to the right lateral OFC seed region, including lower connectivity with the left anterior insula and greater connectivity with the left precentral gyrus. Until recently, few studies have examined seed-based resting-state functional connectivity to the lateral OFC in MDD (Mulders et al., 2015), but recent research suggests that MDD is generally associated with hyperconnectivity between the lateral OFC and diverse brain regions (Cheng et al., 2016). Our finding of hypoconnectivity between the lateral OFC and anterior insula suggests that the generally heterogeneous findings on seed-based resting-state functional connectivity for the insula (Mulders et al., 2015; Yin et al., 2018) may be clarified by accounting for different appetite/weight phenotypes of MDD.
It is important to note that when BMI was added as a covariate, the aforementioned differences between hyperphagic MDD and healthy comparison were no longer significant. Given that hyperphagic MDD is associated with current, as well as past (Lamers et al., 2012), weight gain in the context of MDD, it is not surprising that the hyperphagic MDD group had higher BMI than the other MDD groups and healthy comparison participants, especially since many participants with MDD in the present sample have had a substantial history of depression. In fact, since hyperphagic MDD may be one cause of higher BMI in the hyperphagic MDD group, it would be expected that findings for hyperphagic MDD would overlap to some extent with those for BMI. Further, given that hyperphagic MDD may be one cause of higher BMI, adjustment for BMI or matching on BMI status could introduce bias into investigations of group differences, in addition to or instead of reducing bias (Diemer, Hudson, & Javaras, 2021). The partial overlap of findings for BMI and for MDD-related appetite/weight change is supported by the pattern of correlations for beta values from significant group differences: correlations with BMI were in the same direction, but generally smaller and less significant for BMI than for MDD-related appetite and weight changes.
Indeed, several of the regions (e.g., the insula and OFC) exhibiting differences for the hyperphagic MDD group, which had a mean BMI of 31.4, have been implicated in resting state studies of obesity (Parsons et al., 2021). Further, our functional connectivity findings are generally in the same direction as those for obesity. For instance, in a recent review of resting-state studies in obesity, Parsons et al. (2021) reported that obesity is associated with reduced insular and increased OFC functional connectivity, consistent with our respective findings of decreased connectivity of the OFC with the anterior insula and increased connectivity of the OFC with the precentral gyrus, in hyperphagic MDD relative to controls. In contrast, findings regarding resting state activity in obesity, though few (Parsons et al., 2021), are in the opposite direction as our fALFF findings (i.e., lower anterior insula and higher OFC) for hyperphagic MDD. In our sample, the pattern of results for BMI (see Supplemental Materials) differed from the findings reported here (i.e., for hyperphagic MDD versus healthy comparison), making it unlikely that findings for hyperphagic MDD versus healthy comparison are attributable solely to BMI differences between those groups. However, more research is needed to disentangle the effects of higher BMI and hyperphagic MDD on resting state activity and connectivity.
It is also important to note that, despite our relatively large sample size, our analyses did not find significant differences in fALFF between the healthy comparison group and other MDD groups, or between MDD groups, in contrast to studies comparing fALFF between MDD (all subtypes combined) and healthy controls (Li et al., 2017; Zhong, Pu, & Yao, 2016; Zhou et al., 2017) and to studies comparing task-based fMRI activity between MDD eating phenotypes (Cerit et al., 2019; Simmons et al., 2016), respectively. The absence of differences in fALFF between MDD groups may indicate that measures of spontaneous activity are less sensitive to the appetitive and metabolic differences that distinguish these groups, in contrast to activity relative to food stimuli (Cerit et al., 2019; Simmons et al., 2016). It is also important to note that our analyses did not find any significant differences between groups in functional connectivity to the accumbens seed region, in contrast to studies comparing striatal connectivity between MDD (all subtypes combined) and healthy controls (Furman, Hamilton, & Gotlib, 2011; Mulders et al., 2015).
Although our study had a relatively large sample and used threshold-free cluster enhancement, a method of analysis that enhances sensitivity (Smith & Nichols, 2009), and analyses were pre-registered, it also has some limitations. First, the healthy comparison group was relatively small. Second, with respect to the distribution of BMI, the healthy comparison group was not well matched to the MDD groups, especially the hyperphagic MDD group, reducing the precision of adjustment for BMI. Third, our hyperphagic and hypophagic MDD groups were defined based on changes in appetite or weight, which may have introduced heterogeneity into these groups if MDD-related changes in appetite and weight involve partially differing neurobiological alterations. Fourth, groups included two individuals with bulimia nervosa, and they may have included individuals with binge-eating disorder or an eating disorder not otherwise specified, which were not assessed and could have confounded our results. Fifth, the interval between assessment of MDD symptoms and actual scanning was as high as 70 days for some participants. Although research suggests that eating phenotypes in MDD are relatively stable across episodes and multi-year time frames (Lamers et al., 2012; Nierenberg, Pava, Clancy, Rosenbaum, & Fava, 1996), this does not preclude some temporal variability in appetite and weight symptoms between the screening and baseline imaging visits, and future studies should aim for smaller intervals between screening and scanning. Sixth, participants were not standardized in terms of fasting/eating, and no information was available as to the timing or content of participant’s food consumption before the scanning session, which would be important to control in future studies since satiety has been shown to modulate resting-state activity and connectivity (Al-Zubaidi et al., 2018). Seventh, due to signal dropout, we were unable to examine fALFF or connectivity for more medial portions of the OFC, although these regions have been implicated in both eating behavior (Rolls, 2021) and MDD (Li et al., 2017). Eighth, although the exact reliability of the fMRI measures used in our analyses is not known, most of these measures likely do not have high test-retest reliability (Noble, Scheinost, & Constable, 2019; Zuo & Xing, 2014). Low reliability of fMRI measures may have reduced our power to detect differences between groups. For example, larger samples may be needed to detect group differences in NAcc seed-based connectivity if reliability for this measure is low. Ninth, sensitivity analyses involving QIDS appetite and weight changes scores should be treated as exploratory since these analyses are not corrected for multiple comparisons. Finally, to our knowledge, the reliability and validity of the appetite and weight change scores is not known, and these scores may not be correlated with more objective measures of appetite and weight change in the context of MDD.
Conclusion
Task-based fMRI studies have shown that appetite phenotypes in MDD are associated with altered activation in response to food stimuli in regions involved in processing interoceptive and reward-related information (Cerit et al., 2019; Simmons et al., 2016; Simmons et al., 2020). However, little is known about differences in spontaneous activity and connectivity for these phenotypes. Our study adds to prior research by suggesting that, independent of the presentation of food stimuli, hyperphagic MDD is associated with alterations in spontaneous activity and connectivity in the anterior insula and lateral orbitofrontal cortex which are generally correlated with changes in both appetite and weight. Although no differences were found for the other appetite/weight change groups, our results suggest that alterations in processing interoceptive and reward-related information may contribute to hyperphagic MDD.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Lisa Nickerson and Dr. Thomas Carmody for providing helpful advice and information throughout the development of this study.
Financial Support
The EMBARC study was supported by the National Institute of Mental Health under award numbers U01MH092221 (Trivedi, M.H.) and U01MH092250 (McGrath, P.J., Parsey, R.V., Weissman, M.M.). DAP was partially supported by R37 MH068376. KNJ’s work on the present study was supported by K23 DK120517. MP’s work on the present study was supported by a DocMobility Fellowship from the Swiss National Science Foundation (P1FRP1 188057). LMH’s work on the present study was supported by R01 DK104772. ELB was supported by K23 MH122668 and the Klingenstein Third Generation Foundation. This work was supported by the EMBARC National Coordinating Center at UT Southwestern Medical Center, Madhukar H. Trivedi, M.D., Coordinating PI, and the Data Center at Columbia and Stony Brook Universities. The funder has no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
In the last three years, the authors report the following financial disclosures, for activities unrelated to the current research:
Dr. Weissman: In the last 3 years has received research funding from NIMH, Brain and Behavior Foundation, Templeton Foundation and has received book royalties from Perseus Press, Oxford Press, and APA Publishing and receive royalties on the social adjustment scale from Multihealth Systems for work that she is not involved in.
Dr. Trivedi: Dr. Trivedi reports the following lifetime disclosures: research support from the Agency for Healthcare Research and Quality, Cyberonics Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Diabetes and Digestive and Kidney Diseases, Johnson & Johnson, and consulting and speaker fees from Abbott Laboratories Inc., Akzo (Organon Pharmaceuticals Inc.), Allergan Sales LLC, Alkermes, AstraZeneca, Axon Advisors, Brintellix, Bristol-Myers Squibb Company, Cephalon Inc., Cerecor, Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals Inc., Forest Pharmaceuticals, GlaxoSmithKline, Health Research Associates, Johnson & Johnson, Lundbeck, MedAvante Medscape, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America Inc., MSI Methylation Sciences Inc., Nestle Health Science-PamLab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories.
Dr. Pizzagalli: funding from NIMH, Brain and Behavior Research Foundation, the Dana Foundation, and Millennium Pharmaceuticals; consulting fees from Albright Stonebridge Group, Boehreinger Ingelheim, Compass Pathways, Concert Pharmaceuticals, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; honoraria from the Psychonomic Society (for editorial work) and Alkermes; stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), and Neuroscience Software.
All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
This work was previously presented as a poster at the Society of Biological Psychiatry 2021 Virtual Meeting, and the abstract was published in the proceedings for the conference (Piccolo, Belleau, Holsen, Pizzagalli, & Javaras, 2021).
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Al-Zubaidi A, Heldmann M, Mertins A, Jauch-Chara K, & Munte TF (2018). Influences of hunger, satiety and oral glucose on functional brain connectivity: a multimethod resting-state fMRI study. Neuroscience, 382, 80–92. doi: 10.1016/j.neuroscience.2018.04.029 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition ed.): American Psychiatric Association. [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16(7), 419–429. doi: 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerit H, Christensen K, Moondra P, Klibanski A, Goldstein JM, & Holsen LM (2019). Divergent associations between ghrelin and neural responsivity to palatable food in hyperphagic and hypophagic depression. Journal of Affective Disorders, 242, 29–38. doi: 10.1016/j.jad.2018.07.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, … Feng J (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, 139(Pt 12), 3296–3309. doi: 10.1093/brain/aww255 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, & Kelley WM (2012). Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience, 32(16), 5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer EW, Hudson JI, & Javaras KN (2021). More (adjustment) is not always better: how directed acyclic graphs can help researchers decide which covariates to include in models for the causal relationship between an exposure and an outcome in observational research. Psychotherapy and Psychosomatics, 1–10. doi: 10.1159/000517104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Rodriguez L, Ruiz de Azua I, Dominguez E, Senabre E, Serra I, Kummer S, … Maldonado R (2020). A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nature Communications, 11(1), 782. doi: 10.1038/s41467-020-14458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N, Veldsman M, Cumming T, & Brodtmann A (2017). Fractional amplitude of low-frequency fluctuations (fALFF) in post-stroke depression. Neuroimage: Clinical, 16, 116–124. doi: 10.1016/j.nicl.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, & Gotlib IH (2011). Frontostriatal functional connectivity in major depressive disorder. Biology of Mood & Anxiety Disorders, 1(1), 11. doi: 10.1186/2045-5380-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, & Luna B (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 82, 208–225. doi: 10.1016/j.neuroimage.2013.05.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynos AF, Hall LMJ, Lavender JM, Peterson CB, Crow SJ, Klimes-Dougan B, … Camchong J (2019). Resting state functional connectivity of networks associated with reward and habit in anorexia nervosa. Hum Brain Mapp, 40(2), 652–662. doi: 10.1002/hbm.24402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MM, Rush AJ, Sackeim HA, Wisniewski SR, McClintock SM, Craven N, … Hauger R (2005). Age-related characteristics of depression: a preliminary STAR*D report. The American Journal of Geriatric Psychiatry, 13(10), 852–860. doi: 10.1176/appi.ajgp.13.10.852 [DOI] [PubMed] [Google Scholar]
- Kübler U (2013). Structured Clinical Interview for DSM-IV (SCID). In Gellman MD & Turner JR (Eds.), Encyclopedia of Behavioral Medicine (pp. 1919–1920). New York, NY: Springer New York. [Google Scholar]
- Lamers F, Rhebergen D, Merikangas KR, de Jonge P, Beekman AT, & Penninx BW (2012). Stability and transitions of depressive subtypes over a 2-year follow-up. Psychological Medicine, 42(10), 2083–2093. doi: 10.1017/S0033291712000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Hinton EC, Parkinson JA, & Lawrence AD (2012). Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage, 63(1), 415–422. doi: 10.1016/j.neuroimage.2012.06.070 [DOI] [PubMed] [Google Scholar]
- Li W, Chen Z, Wu M, Zhu H, Gu L, Zhao Y, … Gong Q (2017). Characterization of brain blood flow and the amplitude of low-frequency fluctuations in major depressive disorder: A multimodal meta-analysis. Journal of Affective Disorders, 210, 303–311. doi: 10.1016/j.jad.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Londeree AM, & Wagner DD (2020). The orbitofrontal cortex spontaneously encodes food health and contains more distinct representations for foods highest in tastiness. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, & Tendolkar I (2015). Resting-state functional connectivity in major depressive disorder: A review. Neuroscience and biobehavioral reviews, 56, 330–344. doi: 10.1016/j.neubiorev.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Pava JA, Clancy K, Rosenbaum JF, & Fava M (1996). Are neurovegetative symptoms stable in relapsing or recurrent atypical depressive episodes? Biological Psychiatry, 40(8), 691–696. doi: 10.1016/0006-3223(96)00029-7 [DOI] [PubMed] [Google Scholar]
- Noble S, Scheinost D, & Constable RT (2019). A decade of test-retest reliability of functional connectivity: A systematic review and meta-analysis. NeuroImage, 203, 116157. doi: 10.1016/j.neuroimage.2019.116157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons N, Steward T, Clohesy R, Almgren H, & Duehlmeyer L (2021). A systematic review of resting-state functional connectivity in obesity: Refining current neurobiological frameworks and methodological considerations moving forward. Rev Endocr Metab Disord. doi: 10.1007/s11154-021-09665-x [DOI] [PubMed] [Google Scholar]
- Piccolo M, Belleau E, Holsen LM, Pizzagalli D, & Javaras K (2021). Alterations in resting-state functional activity and connectivity for major depressive disorder eating phenotypes. Biological Psychiatry, 89(9). doi: 10.1016/j.biopsych.2021.02.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2021). The orbitofrontal cortex, food reward, body weight, and obesity. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsab044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, & Feng J (2020). The orbitofrontal cortex: reward, emotion and depression. Brain Communications, 2(2), fcaa196. doi: 10.1093/braincomms/fcaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, & de Araujo IE (2003). Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience, 18(3), 695–703. doi: 10.1046/j.1460-9568.2003.02779.x [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, & Murray EA (2014). The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron, 84(6), 1143–1156. doi: 10.1016/j.neuron.2014.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, … Keller MB (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. doi: 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, & Drevets WC (2016). Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. American Journal of Psychiatry, 173(4), 418–428. doi: 10.1176/appi.ajp.2015.15020162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J, … Drevets WC (2020). Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Molecular Psychiatry, 25(7), 1457–1468. doi: 10.1038/s41380-018-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Ingeholm JE, Avery J, Kallman S, Hall KD, & Martin A (2014). The ventral pallidum and orbitofrontal cortex support food pleasantness inferences. Brain Structure and Function, 219(2), 473–483. doi: 10.1007/s00429-013-0511-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliz D, & Hayley S (2012). Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Frontiers in Human Neuroscience, 6, 323. doi: 10.3389/fnhum.2012.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. doi: 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British Journal of Psychiatry, 167(1), 99–103. doi: 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, & Maddison S (1983). The orbitofrontal cortex: neuronal activity in the behaving monkey. Experimental Brain Research, 49(1), 93–115. doi: 10.1007/BF00235545 [DOI] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, … Weissman MM (2016). Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. Journal of Psychiatric Research, 78, 11–23. doi: 10.1016/j.jpsychires.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, & McCormick RA (1995). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104(1), 3–14. doi: 10.1037//0021-843x.104.1.3 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn : A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Yin Z, Chang M, Wei S, Jiang X, Zhou Y, Cui L, … Tang Y (2018). Decreased functional connectivity in insular subregions in depressive episodes of bipolar disorder and major depressive disorder. Frontiers in Neuroscience, 12, 842. doi: 10.3389/fnins.2018.00842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Pu W, & Yao S (2016). Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naive patients with major depressive disorder: A meta-analysis of resting-state fMRI data. Journal of Affective Disorders, 206, 280–286. doi: 10.1016/j.jad.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Zhou M, Hu X, Lu L, Zhang L, Chen L, Gong Q, & Huang X (2017). Intrinsic cerebral activity at resting state in adults with major depressive disorder: A meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 75, 157–164. doi: 10.1016/j.pnpbp.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, … Zang YF (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–141. doi: 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, & Xing XX (2014). Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience and biobehavioral reviews, 45, 100–118. doi: 10.1016/j.neubiorev.2014.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


