Abstract
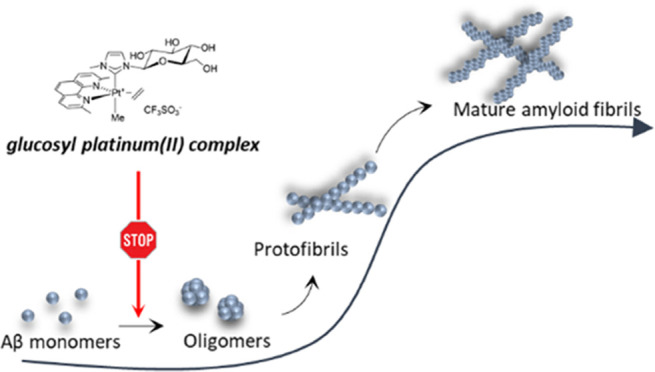
Neurodegenerative diseases are often caused by uncontrolled amyloid aggregation. Hence, many drug discovery processes are oriented to evaluate new compounds that are able to modulate self-recognition mechanisms. Herein, two related glycoconjugate pentacoordinate Pt(II) complexes were analyzed in their capacity to affect the self-aggregation processes of two amyloidogenic fragments, Aβ21–40 and Aβ25–35, of the C-terminal region of the β-amyloid (Aβ) peptide, the major component of Alzheimerʼs disease (AD) neuronal plaques. The most water-soluble complex, 1Ptdep, is able to bind both fragments and to deeply influence the morphology of peptide aggregates. Thioflavin T (ThT) binding assays, electrospray ionization mass spectrometry (ESI-MS), and ultraviolet–visible (UV–vis) absorption spectroscopy indicated that 1Ptdep shows different kinetics and mechanisms of inhibition toward the two sequences and demonstrated that the peptide aggregation inhibition is associated with a direct coordinative bond of the compound metal center to the peptides. These data support the in vitro ability of pentacoordinate Pt(II) complexes to inhibit the formation of amyloid aggregates and pave the way for the application of this class of compounds as potential neurotherapeutics.
Short abstract
Two related glycoconjugate pentacoordinate Pt(II) complexes are able to modulate the self-aggregation mechanism of two amyloidogenic fragments, Aβ21−40 and Aβ25−35, of the β-amyloid peptide, the major component of neuronal plaques in Alzheimerʼs disease, corroborating the potential application of transition-metal complexes as therapeutics in neurodegenerative diseases.
Introduction
In Alzheimerʼs disease (AD) pathophysiology, the β-amyloid (Aβ) peptide represents the prevalent component of senile plaques1 even if it is present at the early phases of life, and in its monomeric form, it can act as a positive regulator of the presynaptic release in hippocampal neurons.2 Aβ is a polypeptide spanning 1–40 or 42 residues that is mostly intrinsically disordered.3 Through a self-assembly process that is typical of amyloid aggregation, it forms different Aβ oligomers, endowed with diverse levels of order that represent key pathogenic species in AD.4 Amyloid assemblies induce synaptic dysfunction and neuronal death5 and oxidative damage and inflammation, which further corroborate the progression of the disease.6 The presence of metals like Zn, Fe, Cu, and Al inside amyloid plaques enhances Aβ-induced oxidative damage and its aggregation level; thus, a chelation approach to directly target metals in the brain can be conceived as a way to reduce harmful consequences of metal/fibril accumulation.7 However, the most powerful therapeutic approach for AD is based on the inhibition of the aggregation of the Aβ peptide8 and great efforts have been devoted to the identification of molecules capable of inhibiting its self-recognition. It has been shown that these compounds can have different origins (synthetic, natural) and chemical nature (phenols, peptide, antibodies, small molecules, etc.).9−12 Even though many trials are actually ongoing, especially on natural compounds,13 no drugs have entered into clinical use yet: this can be mainly due to the inability of targeting protein interfaces without regular secondary structures that could be assumed as templates to design inhibitors.14,15 Recently, multivalent systems (as dendrimers) were investigated to gain access to different protein subregions.16 Indeed, different Aβ regions contribute to amyloid aggregation: the N-terminus,17 hydrophobic core,18 so-called hinge and turn regions,19 and C-terminus.20,21 Experimental data indicated that the C-terminal region of Aβ can be addressed by the cyclohexanehexol scaffold: indeed, the scyllo-inositol compound interferes with the fibrillization process and competes with endogenous phosphatidylinositol for binding to the Aβ polypeptide, appearing as a promising therapeutic agent, currently in Phase II trials.22
The unique properties exhibited by transition-metal complexes as drugs, including their tunability in the oxidation and the coordination states, allow them to enter into many pharmaceutical applications.23−25 In the amyloid context, they can be considered as good starting compounds for the development of novel neuroprotective agents.26,27 The stability and inertness of Pt(II) and Ru(III)28 complexes allowed a wide range of investigations with different amyloid systems: phenanthroline (phen)–Pt(II) complexes with two monodentate ligands (e.g., chlorides) inhibit the aggregation of Aβ1–40 and its N-terminal region29−31 as well as of prion protein (PrP) fragments.32,33 Polyoxometalate derivatives of Pt34 and V35 suppress amyloid aggregation in a mechanism involving multiple interactions: (i) the coordination of the metal, (ii) electrostatic, (iii) hydrogen, and (iv) van der Waals forces. Octahedral Co compounds,36 as well as square-planar Pt complexes bearing polyaromatic ligands, interact with the Aβ peptide via π–π stacking.37 Furthermore, hetero-multinuclear Pt–Ru complexes are able to revert amyloidosis: they regulate amyloid-induced cytotoxicity in insulinoma β-cells and significantly increase cell viability.38 Traditionally, due to the presence of His residues in its sequence, the N-terminal region of the Aβ peptide was considered the main target for inhibition studies of Aβ aggregation by metal compounds.39 Conversely, with the aim to deepen the druggability of C-terminus by transition-metal complexes, in our recent investigations we assumed as amyloid model the fragment spanning residues 21–40 of Aβ (Aβ21–40, Table 1),40−42 which is also deeply involved in the mechanism of aggregation of the entire Aβ peptide. Herein, we also studied the behavior of a shorter Aβ fragment spanning residues 25–35 (Aβ25–35, Table 1). It has been shown that this peptide is the most cytotoxic among the known Aβ peptides.43
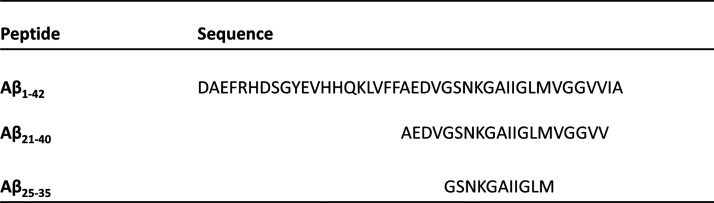
Table 1. Sequences of the Aβ1-42 Peptide and Peptides Investigated in this Study and Derived from its C-Terminal Domain.
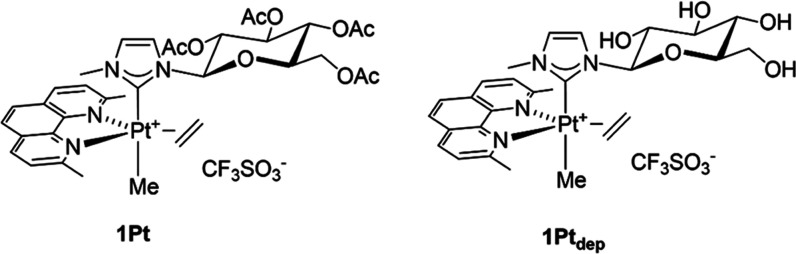
Very recently, we have investigated the ability of a series of square-planar Pt(II) complexes to inhibit the aggregation of amyloid peptides.40−42 Among the investigated systems, the pyridine-based platinum(II) complex, called Pt-terpy, exhibited good inhibitory effects of amyloid aggregation, also allowing, for its better water solubility when compared to other investigated complexes, to get insights into the mechanism of action of these types of molecules: the presence of the Pt complex stabilized soluble β-structures of the Aβ21–40 peptide.44 Some of us also designed new pentacoordinate glycoconjugate platinum(II) complexes that can act as anticancer compounds.45 Sugar ligands were introduced aiming to enhance their biocompatibility, aqueous solubility, and recognition by cancer cells through the “Warburg effect”.46,47 The coordinative saturation is also an important stereoelectronic requisite that improves their general stability. In particular, the peracetylated NHC complex 1Pt in Figure 1, prepared along with its deprotected counterpart 1Ptdep, showed high activity and selectivity toward a panel of cell lines.48
Figure 1.
Pentacoordinate platinum(II) complexes 1Pt and 1Ptdep.
Here, we present investigations focused on the ability of the two complexes to act as effective inhibitors of aggregation of Aβ peptides reported in Table 1. The ability of 1Ptdep to inhibit the aggregation of Aβ peptides was confirmed via a range of spectroscopic and biophysical techniques.
Results and Discussion
Effects of Pt Complexes on the Thioflavin T (ThT) Assay of Aβ21–40 and Aβ25–35
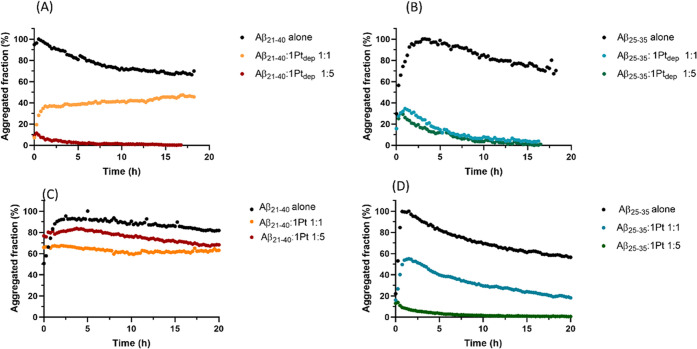
Spectroscopic investigations provided evidence of the ability of both 1Pt and 1Ptdep to modulate the amyloid aggregation of Aβ-derived peptides. Initially, ThT was employed as a typical amyloid dye, which is able to bind to amyloid prefibrils, inducing a strong fluorescence signal at ∼482 nm when excited at 440 nm.49,50 The overlays of ThT fluorescence emission profiles of the investigated peptides in the absence and presence of 1Pt and 1Ptdep over time are reported in Figure 2.
Figure 2.
Time course of ThT fluorescence emission intensity of (A, C) Aβ21–40 and (B, D) Aβ25–35, alone and upon incubation with (A, B) 1Ptdep or (C, D) 1Pt at indicated peptide-to-metal molar ratios. Values are the average of two measurements.
Aβ peptide aggregation was investigated in the presence of metal complexes at peptide-to-metal molar ratios of 1:1 and 1:5. A reduction of aggregation in the presence of metal compounds is observed for almost all samples; fluorescence quenching is more evident in the case of Aβ25–35. Comparing the effects of Pt complexes on the Aβ21–40 aggregation, 1Ptdep exhibits greater suppressive effects (Figure 2A) with respect to 1Pt (Figure 2C). 1Ptdep decreases aggregation of 60 and 90% at 1:1 and 1:5 peptide-to-metal molar ratios, respectively. Under similar experimental conditions (with the addition of dimethyl sulfoxide (DMSO), 2% (v/v), required for dissolution of the compound), the reduction of aggregation is less evident when the peptide is treated with 1Pt: it is about 35 and 20% at 1:1 and 1:5 peptide-to-metal compound molar ratios, respectively (Figure 2C). The differences between the behavior of the two Pt complexes are more evident when the ThT profiles of Aβ25–35 are compared: when the peptide is incubated with 1Ptdep, the reduction of the aggregated fraction (70%) is essentially independent of the equivalents of the complex that were used (Figure 2B); on the contrary, when the peptide is treated with 1Pt, the behavior is similar to that observed in the experiments carried out with Aβ21–40. The suppressive effect of Aβ25–35 aggregation exerted by 1Pt is less significant than that of 1Ptdep: the level of aggregation inhibition is comparable to that exhibited by 1Ptdep only when the peptide-to-metal compound molar ratio is 1:5 (Figure 2D). Noticeably, a preliminary experiment employing the entire Aβ1–42 sequence as aggregating polypeptide,51 reported in Figure S1, confirmed the ability of 1Ptdep to inhibit amyloid aggregation, at a 1:5 Aβ1–42/1Ptdep molar ratio. Future experiments will detail the different involvements of N- and C-terminal regions in this inhibitory process.
Electrospray Ionization Mass Spectrometry (ESI-MS) Analysis of Adducts between Aβ21–40, Aβ25–35, and Pt(II) Complexes
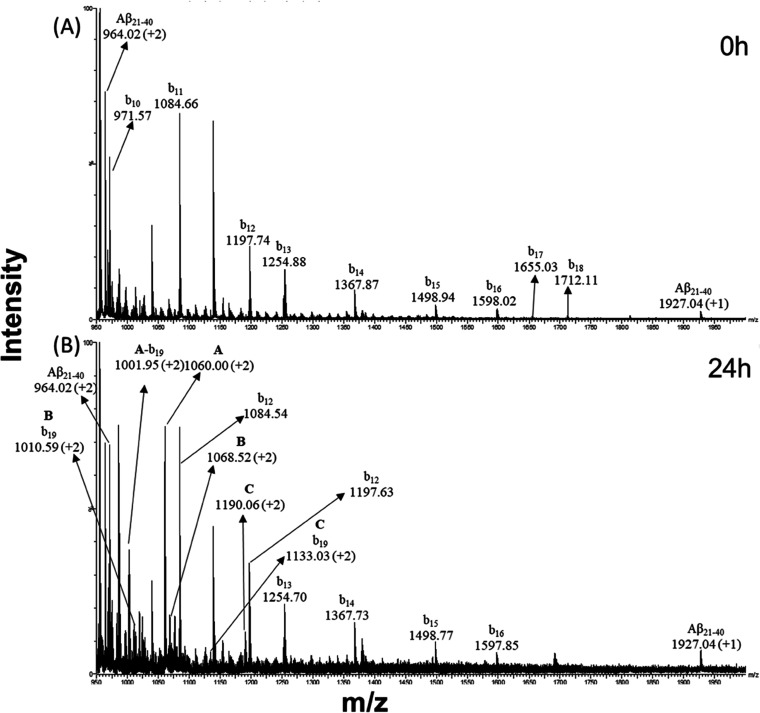
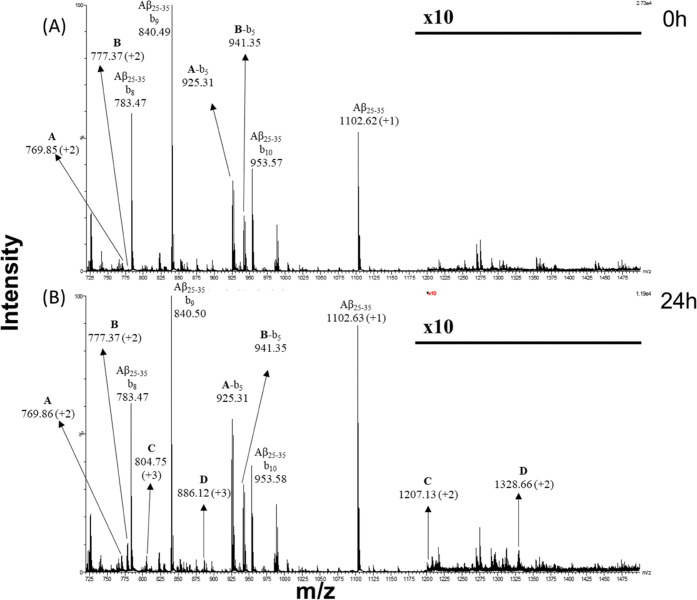
Aβ21–40 and Aβ25–35 were incubated with Pt compounds (at 1:5 peptide-to-metal complex molar ratio) and the samples were analyzed by electrospray ionization mass spectrometry (ESI-MS); the peptides alone were analyzed as references. Signals of the b series for both peptides were generated from spontaneous in-source fragmentation events (Figure S2A,B). The spectra (Figures 3 and 4) of samples containing metal complexes were registered at two times of incubation, t = 0 (Figures 3A and 4A) and t = 24 h (Figures 3B and 4B). The species recorded in Aβ21–40 and Aβ25–35 spectra are summarized in Tables 2 and 3, respectively.
Figure 3.
ESI-MS spectra of the Aβ21–40 peptide incubated with 1Ptdep for (A) 0 h and (B) 24 h. Signals from ion fragmentation are also reported.
Figure 4.
ESI-MS spectra of the Aβ25–35 peptide incubated with 1Ptdep for (A) 0 h and (B) 24 h. Signals from ion fragmentation are also reported. The m/z range between 1200 and 1500 is 10 times magnified.
Table 2. Results of ESI-MS of the Aβ21–40 Peptide Incubated for 0 and 24 h with 1Ptdepa.
| component | experimental m/z, (charge state) | experimental monoisotopic mass (Da) | theoretical monoisotopic mass (Da) | Aβ21–40-Pt adducts | time (h) |
|---|---|---|---|---|---|
| 964.02 (+2) | 1926.03 ± 0.01 | 1926.01 | Aβ21–40 | ||
| 1927.04 (+1) | |||||
| A | 1060.00 (+2) | 2118.00 | 2121.09 | Aβ21–40 + Pt(II) | 24 |
| B | 1068.01 (+2) | 2134.02 | 2136.12 | Aβ21–40 + (Pt + Me) | 24 |
| C | 1190.06 (+2) | 2378.12 | 2379.36 | Aβ21–40 + (Pt + sugar + Me) | 24 |
Table 3. Results of ESI-MS of the Aβ25–35 Peptide Incubated for 0 and 24 h with 1Ptdepa.
| component | experimental m/z, (charge state) | experimental monoisotopic mass (Da) | theoretical monoisotopic mass (Da) | Aβ25–35-Pt adducts | time (h) |
|---|---|---|---|---|---|
| 1102.63 (+1) | 1101.63 | 1100.58 | Aβ25–35 | ||
| A | 769.86 (+2) | 1537.72 | 1538.91 | Aβ25–35 + (Pt + sugar) | 0, 24 |
| B | 777.37 (+2) | 1552.75 | 1553.94 | Aβ25–35 + (Pt + sugar + Me) | 0, 24 |
| C | 804.75 (+3) | 2411.73 ± 0.49 | 2411.28 | 2·(Aβ25–35) + (Pt + Me) | 24 |
| 1207.13 (+2) | |||||
| D | 886.12 (+3), | 2655.49 ± 0.25 | 2654.52 | 2·(Aβ25–35) + (Pt + sugar + Me) | 24 |
| 1328.66 (+2) |
1Ptdep appears to be able to bind to both peptides by substituting the equatorial 2,9-dimethyl-1,10-phenanthroline (Dmphen) and ethylene ligands, as demonstrated by the presence of the peak at m/z 1190.06 (Table 2, C component MW = 2378.12 Da) for Aβ21–40 (Figure 3B) and the peak at m/z 777.37 (Table 3, B component MW = 1552.75 Da) for Aβ25–35 (Figure 4). Moreover, each peptide also showed the ability to form adducts where the metal complex lacks the glucosyl axial ligand, as revealed by the presence of the species showing molecular weights of 2134.02 Da (component B in Table 2) and 2411.73 Da (component C in Table 3) and corresponding to the adducts generated by Aβ21–40 and Aβ25–35. In addition to these common features, several relevant differences emerge from the analysis of the spectra: the first difference is regarding the kinetics of adduct formation. Indeed, contrary to that found for Aβ21–40, Aβ25–35 peptide spectra (Figure 4A) show the formation of adducts at the beginning of incubation (t = 0). Peaks due to these adducts were assigned to Aβ25–35 bound to the 1Ptdep fragment carrying solely the glucosyl (component A, m/z = 769.86) or both the axial ligands (component B, m/z = 777.37). Moreover, the amount of both adducts increases over time. Aβ21–40 showed the formation of the adduct containing glucosyl and methyl ligands only after 24 h (component C, m/z = 1190.06, Figure 3B, Table 2).
At the longest time, an additional distinguishing feature of Aβ25–35 samples was further detected: the occurrence of species with MWs 2411.73 ± 0.49 and 2655.49 ± 0.25 Da (components C and D, respectively, Table 3), which were both attributed to the formation of adducts with a 2:1 peptide/metal complex stoichiometry. Component D (2655.49 Da) was attributed to the adduct containing 1Ptdep lacking the equatorial ligands and carrying two Aβ25–35 chains bound in a monodentate mode, while component C, showing 2411.73 Da as MW, consisted of the same species lacking also the axial sugar moiety. The simultaneous binding of two Aβ25–35 sequences is not encountered in Aβ21–40 samples. Conversely, only Aβ21–40 exhibited an adduct with naked Pt(II) ions as indicated by the presence of component A (m/z = 1060.00, Figure 3B, Table 2).
The analysis of the b series in the spectra of the peptide with 1Ptdep provided insights into the peptide fragments mainly involved in the adduct formation. In the spectra of Aβ25–35 with 1Ptdep (Figure 4), the monocharged signals at m/z 925.31 and 941.35 were due to the b5 element obtained by Aβ25–35 + (Pt + sugar) and Aβ25–35 + (Pt + sugar + Me) fragmentation, respectively.
The presence of the b series fragments suggested that the N-terminal stretch of the peptide is mainly responsible for the binding. Similarly, the b series signals were encountered also in the spectra of Aβ21–40 adducts. The doubly charged signals at m/z 1001.95, 1010.59, and 1133.03 (Figure 3B) are in accordance with the b19 signal of Aβ21–40 bound to naked Pt(II), Pt + Me, and Pt + sugar + Me fragments, as previously reported.44
ESI-MS analysis was also carried out by incubating β-peptides with the 1Pt complex. In this case, no peaks deriving from adducts were detected over the time for Aβ21–40 (Figure S3). A peak was found only for Aβ25–35, in the spectral background, consistent with a 2:1 stoichiometry and compatible with an adduct of the Pt(II) complex with both the axial ligands (Figure S4). These findings confirm the results already described by Annunziata et al.:48 small variations in terms of the ligand structure, such as the presence of protecting groups, are able to substantially modify the binding capacity of a metal compound toward the same biomolecule. On the basis of these results, we further investigated only the ability of 1Ptdep to modulate the amyloid aggregation of Aβ peptides.
Spectroscopic Investigations of Adducts with 1Ptdep
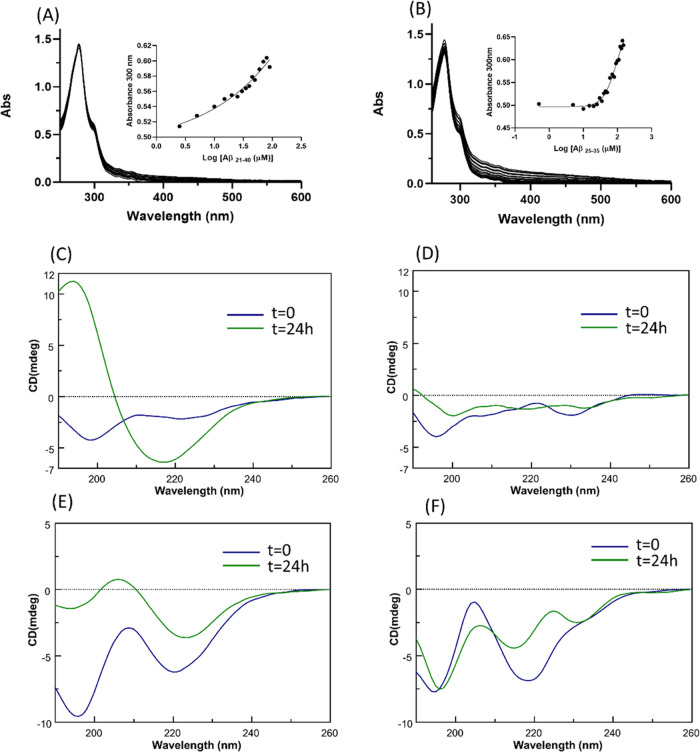
Ultraviolet–visible (UV–vis) absorption spectroscopy was employed to detect potential variations of the ligand field of 1Ptdep induced by the presence of amyloid peptides. In agreement with literature studies on Pt(II)–diimine complexes,52,53 the spectra are characterized by the presence of the π → π* intraligand and Pt(5d) → π* metal-to-ligand charge transfer (MLCT) transitions in the 200–400 nm region. As reported in Figure 5 upper panel, an enhancement of absorbances upon increasing the amounts of both Aβ25–35 (Figure 5A) and Aβ21–40 (Figure 5B) is observable: this suggests that a mechanism of substitution of ligands around the Pt center occurred. This titration, in the case of Aβ25–35, allowed us to estimate EC50 = 93.7 ± 0.2 μM, through the fitting of absorbance values at 330 nm (inset of Figure 5A). This value is comparable to that observed in other studies of metal complex/amyloid peptide systems.41,44 Data fitting did not converge in the case of Aβ21–40, hampering the evaluation of the EC50 value for this peptide (inset of Figure 5B).
Figure 5.
Spectroscopic investigations of the adducts of Aβ21–40 and Aβ25–35 with 1Ptdep. Upper panel: absorption spectra of 1Ptdep upon the addition of increasing amounts of (A) Aβ25–35 and (B) Aβ21–40. As insets, UV intensities at indicated wavelengths vs log of Aβ peptide concentrations. Lower panel: overlay of circular dichroism (CD) spectra of Aβ21–40 (C) alone and (D) incubated with 1Ptdep at the 1:5 peptide/Pt(II) molar ratio and Aβ25–35 (E) alone and (F) incubated with 1Ptdep at a 1:5 peptide/Pt(II) molar ratio.
To evaluate if the presence of 1Ptdep could have effects on the conformation of Aβ peptides, we registered CD spectra of freshly prepared samples and of the peptides incubated for 24 h with the metal compound. Spectra are reported in Figure 5. At t = 0, Aβ21–40 presented a substantial random coil profile, while Aβ25–35 presented a mixture of random coil and β-sheet signals;54 after 24 h, for both sequences, a clear conformational transition toward β-structures occurred (Figure 5C,E), as often observed during amyloid aggregation.44,55 Spectra registered in the presence of 1Ptdep, which exhibits a nonnull Cotton effect (Figure S5), at two different times, indicate the absence of the minimum at 220 nm typical of β-sheet structures (Figure 5D,F). This finding suggests that the presence of the Pt complex causes the inhibition of the conformational transition that preludes fibrillization.
NMR Investigations
To gain further structural insights on the interaction between 1Ptdep and Aβ peptides, NMR studies were carried out. We focused on Aβ25–35 since its sequence is more influenced by the presence of the 1Ptdep complex than Aβ21–40.
We first run one-dimensional (1D) [1H] spectra, reported in Figure 6, of Aβ25–35 alone at t = 0 and 4 h from a freshly prepared sample. Spectra at t = 0 and 4 h present rather sharp signals, except for the solvent-exposed HN peaks (Figure 6 upper panel), indicating the presence of species with small molecular weights and a disordered organization. This is not surprising as large oligomers and protofibrils cannot be observed by solution NMR due to fast relaxation, while disaggregated and/or small oligomers are NMR visible.56,57 After 4 h, a slight decrease of signal intensity is observed (Figure 6). This suggests that large aggregates are formed, although most of the peptide remains in the disaggregated and/or in small oligomer forms (Figure 6).
Figure 6.
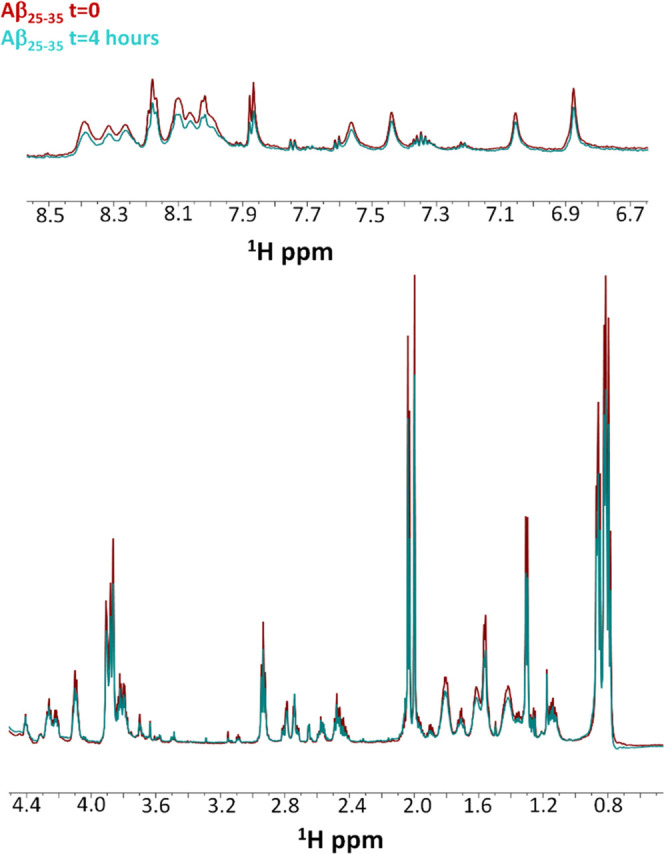
Comparison of 1D [1H] spectra of Aβ25–35 alone at t = 0 (red) and 4 h (cyan). The HN region is shown in the upper panel, the Hα and side chain proton region is reported in the lower panel.
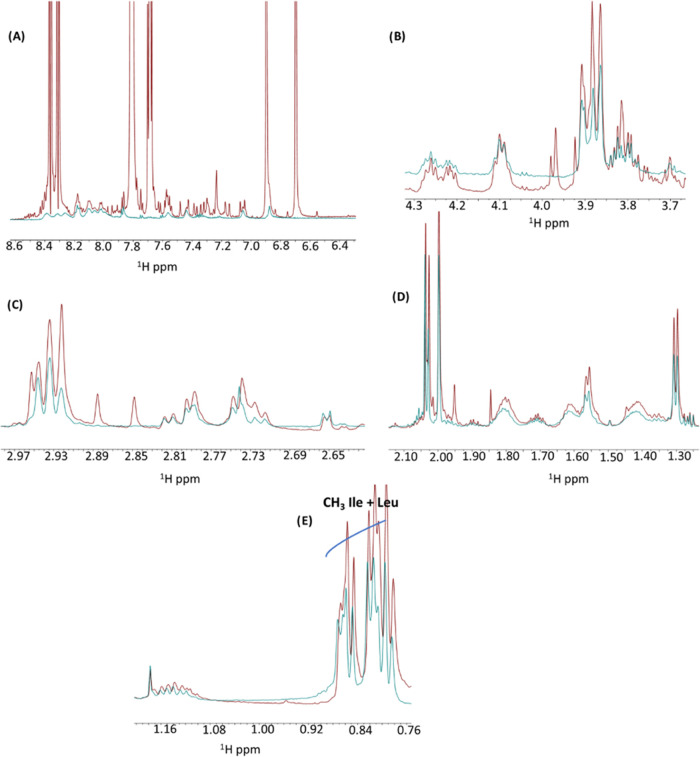
A two-dimensional (2D) [1H, 1H] total correlation spectroscopy (TOCSY) spectrum reported in Figure S6 allows us to distinguish side chain protons of different residues of Aβ25–35. 1D [1H] NMR spectra were also recorded for 1Ptdep alone at t = 0 and 4 h and are reported in Figure 7.
Figure 7.
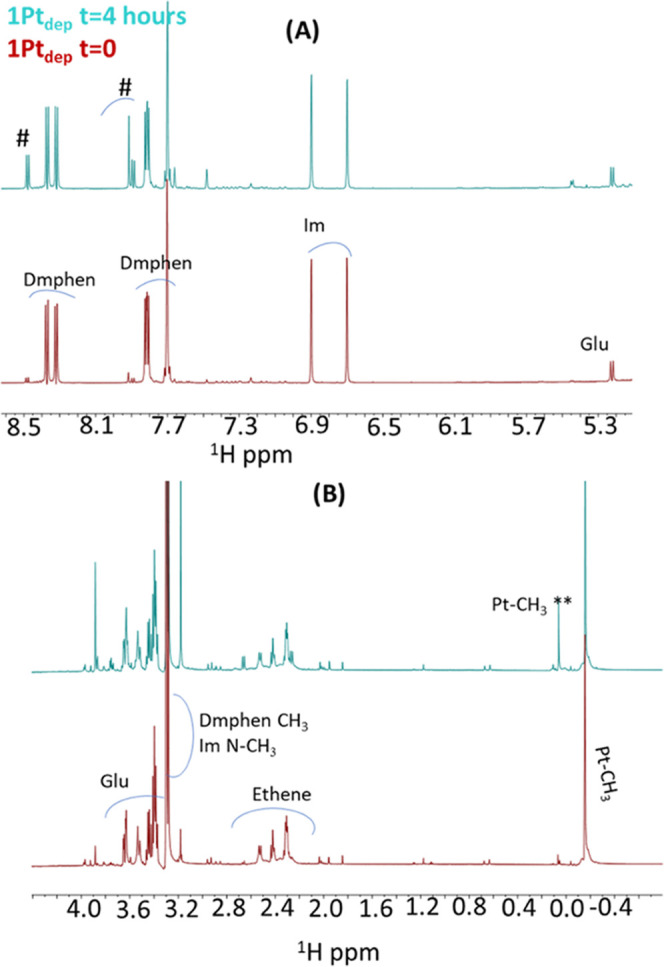
Comparison of 1D [1H] spectra of 1Ptdep at t = 0 (red) and 4 h (cyan). Intervals of chemical shift: (A) 5.3–8.5 and (B) −0.4 to 4.0 ppm. Assignment of Pt ligands is reported at t = 0. Dmphen: 2,9-dimethyl-1,10-phenanthroline, Im: imidazole derivative, Glu: glucosyl unit. On the top cyan spectrum, peaks arising from free Dmphen are indicated by #, while ** refers to Pt-CH3 in a square-planar complex.48
After 4 h, many changes occur in the spectrum: additional signals appear, and the peaks of the main compound decrease in intensity. New peaks are assigned to free ligands that are released from the metal coordination sphere. Indeed, previous 1H NMR studies of the 1Pt analogue compound conducted in DMSO-d6 indicated that both Dmphen and ethylene could be displaced by solvent molecules leading, over time, to square-planar species.48 In detail, the signal close to 0.0 ppm is due to Pt-CH3 in a square-planar geometry, supporting the coexistence of square-planar along with the bipyramidal trigonal geometry that, however, is still predominant after 4 h48 (Figure 7B).
1D [1H] spectra were also acquired for Aβ25–35 in the presence of 1Ptdep (1:5 peptide/metal molar ratio). Spectra recorded at t = 0 and 4 h appear quite similar, indicating that no time effects are observable under NMR conditions (Figure S7). Interestingly, in the comparison of 1D [1H] spectra of 1Ptdep at t = 0 and of 1Ptdep + Aβ25–35 at either t = 0 or 4 h, no relevant changes can be observed, clearly indicating a mutual influence between the complex and the peptide. Interestingly, it can be noted that the presence of Aβ25–35 stabilizes the bipyramidal trigonal geometry of the metal compound since 1H signals from the 1Ptdep spectrum recorded at t = 0 can be overlayed with those present in the spectrum of 1Ptdep + Aβ25–35 at t = 4 h if one excludes minor chemical shift changes (Figure S8). By comparing 1D [1H] spectra of Aβ25–35 alone and 1Ptdep + Aβ25–35 at t = 4 h, a few chemical shift changes could be noticed (Figure 8): these variations do not affect HN (Figure 8A) and Hα (Figure 8B) protons but concern mainly with CH2 (Figure 8C,D) and CH3 (Figure 8E) side chain protons, except for the serine residue that seems unaffected (Figure 8B). The chemical shift changes indicate some conformational variations induced in the Aβ25–35 peptide by the presence of 1Ptdep.
Figure 8.
Overlay of 1D [1H] spectra of 1Ptdep + Aβ25–35 (red) and Aβ25–35 alone (cyan) at t = 4 h. Different chemical shift regions are reported in each panel.
Structural Analysis of Amyloid Fibers of Aβ-Derived Peptides in the Presence of 1Ptdep
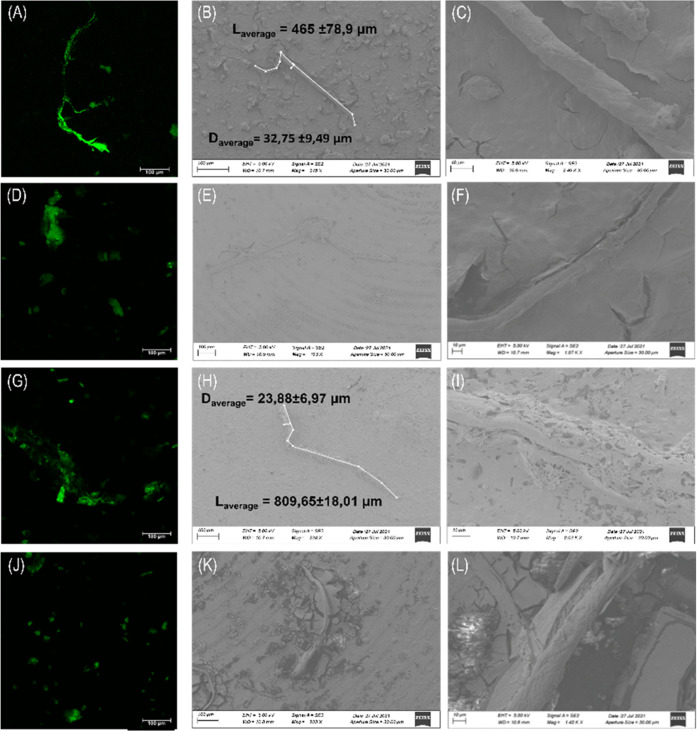
To get insights into the morphology of the aggregates derived from Aβ peptides in the presence and absence of 1Ptdep, confocal and scanning electron microscopies (SEMs) were used. Confocal analysis of Aβ peptides alone presents typical amyloid fiber shapes (Figure 9A,G), while the presence of the metal complex at a 1:5 metal complex-to-peptide molar ratio determines the suppression of the fiber, favoring the formation of amorphous aggregates (Figure 9D,J). SEM experiments corroborate these results: micrographs registered at different magnifications (reported in Figure 9) delight the presence of well-structured fibers with an average diameter of 3.3 ± 1.0 × 10 μm and a length of 4.7 ± 0.8 × 102 μm for Aβ21–40 (Figure 9B,C) and a diameter of 24 ± 7 μm and a length of 8.1 ± 0.2 × 102 μm for Aβ25–35 (Figure 9H,I). The presence of 1Ptdep perturbs microstructure formation in this case as well: the microstructures appear immersed in the stub matrix, not a well defined and faintly visible event at high magnification values (Figure 9E,F,K,L).
Figure 9.
Microscopic studies of Aβ peptides in the absence and presence of 1Ptdep. Confocal microscopy of ThT incubated systems: (A, G) Aβ21–40 and Aβ25–35 alone, (D) Aβ21–40:1Ptdep, (J) Aβ25–35:1Ptdep both at a 1:5 ratio (λexc = 440 nm and λemiss between 460 and 600 nm). SEM micrographs of (B, C) Aβ21–40 and (E, F) Aβ21–40:1Ptdep. (H, I) Aβ25–35 and (K, L) Aβ25–35:1Ptdep at 1:5, at (B, E, H, K) 100 μm and (C, F, I, L) 10 μm.
Experimental Section
Reagent Syntheses
1Pt and 1Ptdep(48) and Aβ21–40 and Aβ25–35 peptides58 were synthesized as already reported, synthetic Aβ1–42 was purchased from Abcam, and related sequences are reported in Table 1. After purification, they were treated with 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and then stored at −20 °C until use.
Fluorescence Assays
ThT fluorescence assays were performed at 25 °C, employing a peptide concentration of 100 μM for Aβ21–40 and 200 μM for Aβ25–35 in 10 mM phosphate buffer at pH 7.4, using a ThT final concentration of 50 μM, at different ratios with Pt(II) complexes (stock solutions 1 mM in water for 1Ptdep and in 100% DMSO for both complexes). ThT experiments that have been carried out with 1Pt were acquired in solutions containing DMSO at 2% (v/v). In this solvent, 1Pt is stable, as suggested by UV–vis absorption spectra collected as a function of time and reported in Figure S9. To correctly compare the time course fluorescence intensities of the peptides in the presence of 1Pt and 1Ptdep, percentages of aggregated fractions are reported. Fluorescence was measured using a Jasco FP 8300 fluorescence spectrofluorometer in a 1 cm cuvette under magnetic stirring. The excitation wavelength was 440 nm and the emission wavelength was between 450 and 600 nm. A scanning speed of 100 nm/min was used. Spectra were recorded every 15 min at the indicated times and assays were performed in duplicates. The fluorescence intensity peak was determined at 482 nm.
Circular Dichroism
CD spectra of Aβ21–40 (50 μM) and Aβ25–35 (100 μM) in 10 mM phosphate buffer, alone or at a 1:2.5 peptide-to-metal complex molar ratio with 1Ptdep, were registered on a Jasco J-815 spectropolarimeter (JASCO, Tokyo, Japan), in a 0.1 cm cuvette.
UV–Vis Absorption Spectroscopy
UV–vis spectra of 1Ptdep at increasing amounts of Aβ peptides were registered on a Nanodrop 2000c spectrophotometer (Thermo Scientific, Milan). The complex concentration was fixed at 50 μM. Peptides were added through the incorporation of 2.0 μL of peptide stock solution (500 μM) in water, kept at 0 °C. Spectra were recorded in the range of 260–600 nm after the peptide addition and 2 min under stirring. Upon addition, the metal complex-to-peptide molar ratio was 1:3 for both peptides. The estimation of the EC50 value was obtained from the nonlinear regression using the “dose–response stimulation equation” of GraphPad program and employing log [inhibitor] vs absorbance intensities as experimental data.59
ESI-MS Analysis
Solutions of Aβ21–40 and Aβ25–35 at a concentration of 50 μM in 15 mM ammonium acetate (pH = 6.8), at a 1:5 molar ratio with Pt(II) complexes, were incubated for two different time durations (0 and 24 h). The reaction mixtures were diluted 10 times with 15 mM ammonium acetate (pH = 6.8) and then analyzed using a Q-ToF Premier (Waters, Milliford, MA) mass spectrometer. For 1Ptdep, the m/z acquisition range spanned from 900 to 2500 m/z and from 500 to 1500 m/z for Aβ21–40 and Aβ25–35. Differently, for 1Pt, the Aβ25–35m/z acquisition range was changed to 500–2000 m/z.
Scanning Electron Microscopy
SEM analysis was performed with an Ultra Plus FESEM scanning electron microscope (Zeiss, Germany). Samples (100 μL) containing Aβ21–40 (100 μM) and Aβ25–35 (200 μM), alone or mixed with 1Ptdep at a 1:5 ratio (10 mM phosphate buffer), were mounted on stub and gold-sputtered at 20 nm thickness using a HR208 Cressington sputter coater and analyzed at 10–15 kV with an SE2 detector, as already reported.60,61 Fiber diameters and length were determined by ImageJ software.
Confocal Microscopy
Confocal microscopy analysis was performed by a Leica SP5 microscope using a HCX IRAPO L 40×/0.95 water objective, as previously reported.62 Aβ21–40 (100 μM) and Aβ25–35 (200 μM) alone or with 1Ptdep at a 1:5 ratio incubated with ThT (50 μM) (50 mM phosphate buffer) were analyzed using an excitation of 440 nm and an emission between 460 and 600 nm.
NMR Assays
Analyzed NMR samples were (1) Aβ25–35 (400 μM), (2) 1Ptdep (2 mM), and (3) Aβ25–35+ 1Ptdep (400 μM:2 mM nominal concentration) at t = 0 and 4 h of aggregation. Final volumes were of 540 μL (500 μL of sodium phosphate buffer pH 7.2 and 40 μL of D2O (deuterium oxide, 98% D, Sigma-Aldrich, Milan, Italy). NMR spectra were recorded on a Bruker Avance 700 MHz spectrometer at 294 K. 1D [1H] spectra were recorded with 64 scans. In addition, a 2D [1H, 1H] TOCSY63 spectrum was recorded for Aβ25–35 with mixing time equal to 70 ms after 4 h. The 2D [1H, 1H] TOCSY spectrum was acquired with 58 scans, 128 free induction decays (FIDs) in t1, and 1024 data points in t2. Water suppression was achieved by presaturation. Spectra were processed and analyzed with TopSpin4.1.1 (Bruker, Italy). The 2D TOCSY spectrum was analyzed with NEASY64 included in the Cara (computer-aided resonance assignment, http://www.nmr.ch/). Chemical shifts were referenced to the residual water peak at 4.75 ppm.
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl Tetrazolium Bromide (MTT) Assay
Human SH-SY5Y neuroblastoma cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO, Paisley, U.K.) containing 10% heat-inactivated fetal bovine serum (FBS) (GIBCO), supplemented with 2 mM l-glutamine, 50 ng/mL streptomycin, and 50 units/mL penicillin and maintained in a humidified atmosphere (5% CO2 at 37 °C). Once at 70–80% of confluence, cells were harvested with 0.25% trypsin (Sigma-Aldrich, St. Louis, MO). Aβ21–40 and Aβ25–35 alone or with 1Ptdep or 1Pt at a 1:5 molar ratio, respectively, were incubated in 50 mM sodium phosphate buffer, pH 7.2, under stirring, and samples were taken at three different times: 0, 2, and 24 h. Peptides were added to the cells in culture media in 96-well plates at 100 μM and then incubated for 24 h at 37 °C. Cell viability was then assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay as previously described.55,65
Conclusions
The present study well reflects our recent focus on the applicability of metal-based anticancer drugs in the field of neurodegeneration: by assuming different amyloidogenic peptides as model systems of neurodegenerative proteins, we assessed the ability of several metal complexes with different metal ions (such as Pt, Ru, and Au) and diverse geometries (square-planar and octahedral) to act as inhibitors of self-aggregation processes.40−42,44
Herein, we have carried out several biophysical investigations to deepen, at the molecular level, the ability of two glycoconjugate Pt(II) bipyramidal complexes (Figure 1) to modulate amyloid aggregation. For its crucial involvement in AD, we have assumed as amyloid systems two fragments of the Aβ polypeptide: Aβ21–40 and Aβ25–35.
In the first assay, ThT fluorescence of the peptides in the absence and presence of metal compounds over time has been registered. Data indicated a clear suppression of the aggregation process of both the peptides, mostly by water-soluble 1Ptdep, with a greater effect on the inhibition of aggregation of Aβ25–35. ESI-MS and UV–vis absorption spectroscopy experiments outline that the inhibition of aggregation occurs through the formation of adducts between the Pt(II) bipyramidal complexes and Aβ peptides, through the insertion of peptides into the coordination sphere of the Pt center that implies the preferential release of the axial ligands, even if other exchanges can occur. ESI-MS analysis also indicated that in the case of the Aβ25–35/1Ptdep system, the metal complex can coordinate two peptide molecules at the same time, forming an adduct with 1:2 metal/peptide stoichiometry. This adduct cannot be formed in the case of Aβ21–40, probably because of its longer sequence. Conversely, Aβ25–35 was the only sequence to provide an adduct, although of minimum intensity, with 1Pt. This complex has a lower intrinsic ability to form adducts with the peptides, probably because of its minor capacity, when compared to its deprotected analogue, to form hydrogen bonds that could be important in the early stage of the peptide/metal complex recognition process that precedes the formation of the coordinative bond. Because of limited ability of 1Pt to form adducts with the investigated peptides, we focused on 1Ptdep in further studies. From a conformational perspective, both CD and NMR experiments pointed out a deep mutual influence between the amyloidogenic peptide and 1Ptdep; indeed, the presence of the metal complex stabilizes monomeric forms/small aggregate forms of the peptide that do not evolve, during time, toward large aggregated species. In NMR assays, we observed that also the bipyramidal geometry of 1Ptdep is stabilized by the presence of Aβ25–35. Finally, microscopy investigations confirmed all spectroscopic data showing that 1Ptdep suppresses the formation of amyloid fibers for both Aβ sequences. Unfortunately, the Pt complexes investigated in this study are not able to rescue the cytotoxicity induced by amyloid peptides, as reported in Figure S10. Thus, these compounds cannot be directly translated as neurodrugs, but, instead, they can be assumed as valid templates to develop more specific drugs, preferentially able to cross the brain barrier.
In conclusion, this study represents an important example of how biophysical characterization of the adducts formed upon the reaction of metallodrugs with amyloid peptides can highlight on their mechanism of aggregation inhibition and is promising for the application of analogous glycoconjugate Pt(II) bipyramidal derivatives as novel therapeutics in neurodegenerative diseases.
Acknowledgments
S.L.M. was supported by the AIRC fellowship for Italy.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.1c03540.
Time course of ThT fluorescence emission intensity of Aβ1–42, alone and upon incubation with 1Ptdep at the indicated peptide-to-metal molar ratio: the values are the average of two measurements (Figure S1); ESI-MS spectra of Aβ21–40 and Aβ25–35 alone (Figure S2); ESI-MS spectra of the Aβ21–40 peptide incubated with 1Pt for 0 h and 24 h (Figure S3); ESI-MS spectra of the Aβ25–35 peptide incubated with 1Pt for 0 h and 24 h (Figure S4); overlay of CD spectra at 0 and 24 h of 1Ptdep (Figure S5); 2D [1H, 1H] TOCSY spectrum of Aβ25–35 recorded at t = 4 h (Figure S6); overlay of 1D [1H] NMR spectra of 1Ptdep plus Aβ25–35 (Figure S7); comparison between 1D [1H] NMR spectra of 1Ptdep alone at t = 0 and 1Ptdep in the presence of Aβ25–35 at t = 4 h (Figure S8); time course UV–vis spectra of 1Pt at 50 μM in 2% DMSO phosphate buffer, 10 mM, at pH 7.4 (Figure S9); survival of SH-SY5Y cells treated with Aβ21–40 and Aβ25–35 (100 μM) alone or with 1Ptdep or 1Pt at the 1:5 molar ratio at three time points: 0, 2, and 24 h; cells with peptides were incubated for 24 h and then processed for the MTT test (*p < 0.05, **p < 0.005, #p < 0.001, and &p < 0.0001 at statistical analysis of one experiment performed in triplicates) (Figure S10) (PDF)
Author Contributions
S.L.M. synthesized peptides and performed fluorescence, UV, and CD studies, I.I. and M.M. performed ESI experiments. M.L. performed NMR studies, E.L. and C.D.N. conducted SEM assays, A.A. and F.R. synthesized and characterized the metal complexes. D.M., A.M., and M.M. designed the concept and supervised the experiments. M.M., A.M., and D.M. wrote the manuscript. All authors have read and approved the final version of the manuscript
The authors declare no competing financial interest.
Supplementary Material
References
- Nie Q.; Du X.-g.; Geng M.-y. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimerʼs disease. Acta Pharmacol. Sin. 2011, 32, 545–551. 10.1038/aps.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida M. L.; Caraci F.; Pignataro B.; Cataldo S.; De Bona P.; Bruno V.; Molinaro G.; Pappalardo G.; Messina A.; Palmigiano A.; et al. β-amyloid monomers are neuroprotective. J. Neurosci. 2009, 29, 10582–10587. 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco D.; Scognamiglio P. L. Identification of inhibitors of biological interactions involving intrinsically disordered proteins. Int. J. Mol. Sci. 2015, 16, 7394–7412. 10.3390/ijms16047394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. H.; Ramamoorthy A.; Sahoo B. R.; Zheng J.; Faller P.; Straub J. E.; Dominguez L.; Shea J. E.; Dokholyan N. V.; De Simone A.; Ma B.; Nussinov R.; Najafi S.; Ngo S. T.; Loquet A.; Chiricotto M.; Ganguly P.; McCarty J.; Li M. S.; Hall C.; Wang Y.; Miller Y.; Melchionna S.; Habenstein B.; Timr S.; Chen J.; Hnath B.; Strodel B.; Kayed R.; Lesne S.; Wei G.; Sterpone F.; Doig A. J.; Derreumaux P. Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimerʼs Disease, Parkinsonʼs Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem. Rev. 2021, 121, 2545–2647. 10.1021/acs.chemrev.0c01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. H.; Beal M. F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimerʼs disease. Trends Mol. Med. 2008, 14, 45–53. 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan L.; Kang Z.; Pei G.; Le Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimerʼs disease. Curr. Alzheimer Res. 2009, 6, 531–540. 10.2174/156720509790147070. [DOI] [PubMed] [Google Scholar]
- Jokar S.; Khazaei S.; Behnammanesh H.; Shamloo A.; Erfani M.; Beiki D.; Bavi O. Recent advances in the design and applications of amyloid-beta peptide aggregation inhibitors for Alzheimerʼs disease therapy. Biophys. Rev. 2019, 11, 901–925. 10.1007/s12551-019-00606-2. [DOI] [PubMed] [Google Scholar]
- Estrada L.; Soto C. Disrupting β-amyloid aggregation for Alzheimer disease treatment. Curr. Top. Med. Chem. 2007, 7, 115–126. 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- Pagano K.; Tomaselli S.; Molinari H.; Ragona L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667 10.3389/fnins.2020.619667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N.; Kundu L. M. Breaker peptides against amyloid-β aggregation: a potential therapeutic strategy for Alzheimerʼs disease. Future Med. Chem. 2021, 13, 1767–1794. 10.4155/fmc-2021-0184. [DOI] [PubMed] [Google Scholar]
- Salahuddin P.; Khan R. H.; Furkan M.; Uversky V. N.; Islam Z.; Fatima M. T. Mechanisms of amyloid proteins aggregation and their inhibition by antibodies, small molecule inhibitors, nano-particles, and nano-bodies. Int. J. Biol. Macromol. 2021, 186, 580–590. 10.1016/j.ijbiomac.2021.07.056. [DOI] [PubMed] [Google Scholar]
- Marasco D.; Vicidomini C.; Krupa P.; Cioffi F.; Huy P. D. Q.; Li M. S.; Florio D.; Broersen K.; De Pandis M. F.; Roviello G. N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine-based compounds on beta-amyloid aggregation. Chem.-Biol. Interact. 2021, 334, 109300 10.1016/j.cbi.2020.109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade S.; Ramalho M. J.; Loureiro J. A.; Pereira M. dC. Natural compounds for Alzheimerʼs disease therapy: a systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019, 20, 2313 10.3390/ijms20092313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scognamiglio P. L.; Di Natale C.; Perretta G.; Marasco D. From peptides to small molecules: an intriguing but intricated way to new drugs. Curr. Med. Chem. 2013, 20, 3803–3817. 10.2174/09298673113209990184. [DOI] [PubMed] [Google Scholar]
- Russo A.; Aiello C.; Grieco P.; Marasco D. Targeting ″Undruggable″ Proteins: Design of Synthetic Cyclopeptides. Curr. Med. Chem. 2016, 23, 748–762. 10.2174/0929867323666160112122540. [DOI] [PubMed] [Google Scholar]
- Bartus É.; Olajos G.; Schuster I.; Bozso Z.; Deli M. A.; Veszelka S.; Walter F. R.; Datki Z.; Szakonyi Z.; Martinek T. A.; Fulop L. Structural Optimization of Foldamer-Dendrimer Conjugates as Multivalent Agents against the Toxic Effects of Amyloid Beta Oligomers. Molecules 2018, 23, 2523 10.3390/molecules23102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J.; Cecal R.; Kierstead M. E.; Tian X.; Phinney A. L.; Manea M.; French J.; Lambermon M. H.; Darabie A. A.; Brown M. E.; et al. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat. Med. 2002, 8, 1263–1269. 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Permanne B.; Adessi C.; Saborio G. P.; Fraga S.; Frossard M. J.; Van Dorpe J.; Dewachter I.; Banks W. A.; Van Leuven F.; Soto C. Reduction of amyloid load and cerebral damage in transgenic mouse model of Alzheimerʼs disease by treatment with a β-sheet breaker peptide. FASEB J. 2002, 16, 860–862. 10.1096/fj.01-0841fje. [DOI] [PubMed] [Google Scholar]
- Fawzi N. L.; Phillips A. H.; Ruscio J. Z.; Doucleff M.; Wemmer D. E.; Head-Gordon T. Structure and dynamics of the Aβ21–30 peptide from the interplay of NMR experiments and molecular simulations. J. Am. Chem. Soc. 2008, 130, 6145–6158. 10.1021/ja710366c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo N. D.; Grant M. A.; Condron M. C.; Rigby A. C.; Teplow D. B. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005, 14, 1581–1596. 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-H.; Ke S.-C.; Lin T.-H.; Huang H.-B.; Chen Y.-C. Effect of C-terminal residues of Aβ on copper binding affinity, structural conversion and aggregation. PLoS One 2014, 9, e90385 10.1371/journal.pone.0090385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J.; Kierstead M. E.; Brown M. E.; Hawkes C. A.; Lambermon M. H.; Phinney A. L.; Darabie A. A.; Cousins J. E.; French J. E.; Lan M. F.; et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006, 12, 801–808. 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- Son G.; Lee B. I.; Chung Y. J.; Park C. B. Light-triggered dissociation of self-assembled beta-amyloid aggregates into small, nontoxic fragments by ruthenium (II) complex. Acta Biomater. 2018, 67, 147–155. 10.1016/j.actbio.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Suh J. M.; Kim G.; Kang J.; Lim M. H. Strategies Employing Transition Metal Complexes To Modulate Amyloid-beta Aggregation. Inorg. Chem. 2019, 58, 8–17. 10.1021/acs.inorgchem.8b02813. [DOI] [PubMed] [Google Scholar]
- Wan P. K.; Tong K. C.; Lok C. N.; Zhang C.; Chang X. Y.; Sze K. H.; Wong A. S. T.; Che C. M. Platinum(II) N-heterocyclic carbene complexes arrest metastatic tumor growth. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2025806118 10.1073/pnas.2025806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjos K. D.; Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. 10.1021/cr400460s. [DOI] [PubMed] [Google Scholar]
- Derrick J. S.; Lee J.; Lee S. J.; Kim Y.; Nam E.; Tak H.; Kang J.; Lee M.; Kim S. H.; Park K.; Cho J.; Lim M. H. Mechanistic Insights into Tunable Metal-Mediated Hydrolysis of Amyloid-beta Peptides. J. Am. Chem. Soc. 2017, 139, 2234–2244. 10.1021/jacs.6b09681. [DOI] [PubMed] [Google Scholar]
- Storr T.; Gomes L. M.; Bataglioli J. C.; Jussila A. J.; Smith J. R.; Walsby C. J. Modification of AB Peptide Aggregation via Covalent Binding of a Series of Ru (III) Complexes. Front. Chem. 2019, 7, 838 10.3389/fchem.2019.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham K. J.; Kenche V. B.; Ciccotosto G. D.; Smith D. P.; Tew D. J.; Liu X.; Perez K.; Cranston G. A.; Johanssen T. J.; Volitakis I.; Bush A. I.; Masters C. L.; White A. R.; Smith J. P.; Cherny R. A.; Cappai R. Platinum-based inhibitors of amyloid-beta as therapeutic agents for Alzheimerʼs disease. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 6813–6818. 10.1073/pnas.0800712105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I.; Bijani C.; Ladeira S.; Bourdon V.; Faller P.; Hureau C. Interference of a new cyclometallated Pt compound with Cu binding to amyloid-beta peptide. Dalton Trans. 2012, 41, 6404–6407. 10.1039/c2dt12177h. [DOI] [PubMed] [Google Scholar]
- Collin F.; Sasaki I.; Eury H.; Faller P.; Hureau C. Pt (II) compounds interplay with Cu (II) and Zn (II) coordination to the amyloid-β peptide has metal specific consequences on deleterious processes associated to Alzheimerʼs disease. Chem. Commun. 2013, 49, 2130–2132. 10.1039/c3cc38537j. [DOI] [PubMed] [Google Scholar]
- Wang X.; Cui M.; Zhao C.; He L.; Zhu D.; Wang B.; Du W. Regulation of aggregation behavior and neurotoxicity of prion neuropeptides by platinum complexes. Inorg. Chem. 2014, 53, 5044–5054. 10.1021/ic500092t. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Feng L.; Zhang B.; Wang X.; Huang C.; Li Y.; Du W. Palladium complexes affect the aggregation of human prion protein PrP106-126. Inorg. Chem. 2011, 50, 4340–4348. 10.1021/ic102331x. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Li K.; Wan K.; Sun T.; Zheng N.; Zhu F.; Ma J.; Jiao J.; Li T.; Ni J.; Shi X.; Wang H.; Peng Q.; Ai J.; Xu W.; Liu S. Organoplatinum-Substituted Polyoxometalate Inhibits beta-amyloid Aggregation for Alzheimerʼs Therapy. Angew. Chem., Int. Ed. 2019, 58, 18032–18039. 10.1002/anie.201910521. [DOI] [PubMed] [Google Scholar]
- Xu J.; Gong G.; Huang X.; Du W. Schiff base oxovanadium complexes resist the assembly behavior of human islet amyloid polypeptide. J. Inorg. Biochem. 2018, 186, 60–69. 10.1016/j.jinorgbio.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Chan T. G.; Ruehl C. L.; Morse S. V.; Simon M.; Rakers V.; Watts H.; Aprile F. A.; Choi J. J.; Vilar R. Modulation of amyloid-beta aggregation by metal complexes with a dual binding mode and their delivery across the blood-brain barrier using focused ultrasound. Chem. Sci. 2021, 12, 9485–9493. 10.1039/D1SC02273C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G.; Xu J.; Huang X.; Du W. Influence of methionine-ruthenium complex on the fibril formation of human islet amyloid polypeptide. J. Biol. Inorg. Chem. 2019, 24, 179–189. 10.1007/s00775-019-01637-6. [DOI] [PubMed] [Google Scholar]
- Gong G.; Du W.; Xu J.; Huang X.; Yin G. Regulation of heteronuclear Pt-Ru complexes on the fibril formation and cytotoxicity of human islet amyloid polypeptide. J. Inorg. Biochem. 2018, 189, 7–16. 10.1016/j.jinorgbio.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Józsa É.; Osz K.; Kallay C.; de Bona P.; Damante C. A.; Pappalardo G.; Rizzarelli E.; Sovago I. Nickel(II) and mixed metal complexes of amyloid-beta N-terminus. Dalton Trans. 2010, 39, 7046–7053. 10.1039/c0dt00189a. [DOI] [PubMed] [Google Scholar]
- Florio D.; Iacobucci I.; Ferraro G.; Mansour A. M.; Morelli G.; Monti M.; Merlino A.; Marasco D. Role of the Metal Center in the Modulation of the Aggregation Process of Amyloid Model Systems by Square Planar Complexes Bearing 2-(2’-pyridyl)benzimidazole Ligands. Pharmaceuticals 2019, 12, 154 10.3390/ph12040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio D.; Malfitano A. M.; Di Somma S.; Mugge C.; Weigand W.; Ferraro G.; Iacobucci I.; Monti M.; Morelli G.; Merlino A.; Marasco D. Platinum(II) O,S Complexes Inhibit the Aggregation of Amyloid Model Systems. Int. J. Mol. Sci. 2019, 20, 829 10.3390/ijms20040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio D.; Cuomo M.; Iacobucci I.; Ferraro G.; Mansour A. M.; Monti M.; Merlino A.; Marasco D. Modulation of Amyloidogenic Peptide Aggregation by Photoactivatable CO-Releasing Ruthenium(II) Complexes. Pharmaceuticals 2020, 13, 171 10.3390/ph13080171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldi M.; Fiori J.; Pistolozzi M.; Drake A. F.; Bertucci C.; Wu R.; Mlynarczyk K.; Filipek S.; De Simone A.; Andrisano V. , Amyloid beta-peptide 25-35 self-assembly and its inhibition: a model undecapeptide system to gain atomistic and secondary structure details of the Alzheimerʼs disease process and treatment. ACS Chem. Neurosci. 2012, 3, 952–962. 10.1021/cn3000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manna S.; Florio D.; Iacobucci I.; Napolitano F.; Benedictis I.; Malfitano A. M.; Monti M.; Ravera M.; Gabano E.; Marasco D. A Comparative Study of the Effects of Platinum (II) Complexes on beta-Amyloid Aggregation: Potential Neurodrug Applications. Int. J. Mol. Sci. 2021, 22, 3015 10.3390/ijms22063015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata A.; Cucciolito M. E.; Esposito R.; Ferraro G.; Monti D. M.; Merlino A.; Ruffo F. Five-Coordinate Platinum (II) Compounds as Potential Anticancer Agents. Eur. J. Inorg. Chem. 2020, 2020, 918–929. 10.1002/ejic.201900771. [DOI] [Google Scholar]
- Pettenuzzo A.; Pigot R.; Ronconi L. Metal-based glycoconjugates and their potential in targeted anticancer chemotherapy. Metallodrugs 2016, 1, 36–61. 10.1515/medr-2015-0002. [DOI] [Google Scholar]
- Annunziata A.; Liberti D.; Bedini E.; Cucciolito M. E.; Loreto D.; Monti D. M.; Merlino A.; Ruffo F. Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 8704 10.3390/ijms22168704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata A.; Cucciolito M. E.; Esposito R.; Imbimbo P.; Petruk G.; Ferraro G.; Pinto V.; Tuzi A.; Monti D. M.; Merlino A.; et al. A highly efficient and selective antitumor agent based on a glucoconjugated carbene platinum (ii) complex. Dalton Trans. 2019, 48, 7794–7800. 10.1039/C9DT01614G. [DOI] [PubMed] [Google Scholar]
- Biancalana M.; Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C.; Lin T. Y.; Chang D.; Guo Z. Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696 10.1098/rsos.160696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Loschwitz J.; Strodel B.; Nagel-Steger L.; Willbold D. Interference with Amyloid-beta Nucleation by Transient Ligand Interaction. Molecules 2019, 24, 2129 10.3390/molecules24112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungey K. E.; Thompson B. D.; Kane-Maguire N. A.; Wright L. L. Photobehavior of (α-Diimine) dimesitylplatinum (II) Complexes. Inorg. Chem. 2000, 39, 5192–5196. 10.1021/ic000268r. [DOI] [PubMed] [Google Scholar]
- Esposito R.; Calvanese L.; Cucciolito M. E.; D’Auria G.; Falcigno L.; Fiorini V.; Pezzella P.; Roviello G.; Stagni S.; Talarico G.; Ruffo F. Oxidative Coupling of Imino, Amide Platinum (II) Complexes Yields Highly Conjugated Blue Dimers. Organometallics 2017, 36, 384–390. 10.1021/acs.organomet.6b00798. [DOI] [Google Scholar]
- D’Ursi A. M.; Armenante M. R.; Guerrini R.; Salvadori S.; Sorrentino G.; Picone D. Solution structure of amyloid beta-peptide (25-35) in different media. J. Med. Chem. 2004, 47, 4231–4238. 10.1021/jm040773o. [DOI] [PubMed] [Google Scholar]
- Di Natale C.; Scognamiglio P. L.; Cascella R.; Cecchi C.; Russo A.; Leone M.; Penco A.; Relini A.; Federici L.; Di Matteo A.; Chiti F.; Vitagliano L.; Marasco D. Nucleophosmin contains amyloidogenic regions that are able to form toxic aggregates under physiological conditions. FASEB J. 2015, 29, 3689–3701. 10.1096/fj.14-269522. [DOI] [PubMed] [Google Scholar]
- Pagano K.; Tomaselli S.; Molinari H.; Ragona L. Natural Compounds as Inhibitors of Abeta Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667 10.3389/fnins.2020.619667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli A.; Giuffrida A.; Tomasello M. F.; Giuffrida M. L.; Leone M.; Attanasio F.; Caraci F.; De Bona P.; Naletova I.; Saviano M.; Copani A.; Pappalardo G.; Rizzarelli E. Ac-LPFFD-Th: A Trehalose-Conjugated Peptidomimetic as a Strong Suppressor of Amyloid-beta Oligomer Formation and Cytotoxicity. ChemBioChem 2016, 17, 1541–1549. 10.1002/cbic.201600243. [DOI] [PubMed] [Google Scholar]
- Giuffrida M. L.; Grasso G.; Ruvo M.; Pedone C.; Saporito A.; Marasco D.; Pignataro B.; Cascio C.; Copani A.; Rizzarelli E. Abeta(25-35) and its C- and/or N-blocked derivatives: copper driven structural features and neurotoxicity. J. Neurosci. Res. 2007, 85, 623–633. 10.1002/jnr.21135. [DOI] [PubMed] [Google Scholar]
- Doti N.; Scognamiglio P. L.; Madonna S.; Scarponi C.; Ruvo M.; Perretta G.; Albanesi C.; Marasco D. New mimetic peptides of the kinase-inhibitory region (KIR) of SOCS1 through focused peptide libraries. Biochem. J. 2012, 443, 231–240. 10.1042/BJ20111647. [DOI] [PubMed] [Google Scholar]
- Florio D.; Di Natale C.; Scognamiglio P. L.; Leone M.; La Manna S.; Di Somma S.; Netti P. A.; Malfitano A. M.; Marasco D. Self-assembly of bio-inspired heterochiral peptides. Bioorg. Chem. 2021, 114, 105047 10.1016/j.bioorg.2021.105047. [DOI] [PubMed] [Google Scholar]
- Di Natale C.; Natale C. F.; Florio D.; Netti P. A.; Morelli G.; Ventre M.; Marasco D. Effects of surface nanopatterning on internalization and amyloid aggregation of the fragment 264-277 of Nucleophosmin 1. Colloids Surf., B 2021, 197, 111439 10.1016/j.colsurfb.2020.111439. [DOI] [PubMed] [Google Scholar]
- Di Natale C.; La Manna S.; Avitabile C.; Florio D.; Morelli G.; Netti P. A.; Marasco D. Engineered beta-hairpin scaffolds from human prion protein regions: Structural and functional investigations of aggregates. Bioorg. Chem. 2020, 96, 103594 10.1016/j.bioorg.2020.103594. [DOI] [PubMed] [Google Scholar]
- Griesinger C.; Otting G.; Wuthrich K.; Ernst R. R. Clean TOCSY for proton spin system identification in macromolecules. J. Am. Chem. Soc. 1988, 110, 7870–7872. 10.1021/ja00231a044. [DOI] [Google Scholar]
- Bartels C.; Xia T.; Billeter M.; Güntert P.; Wüthrich K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 1995, 6, 1–10. 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- Di Natale C.; La Manna S.; Malfitano A. M.; Di Somma S.; Florio D.; Scognamiglio P. L.; Novellino E.; Netti P. A.; Marasco D. Structural insights into amyloid structures of the C-terminal region of nucleophosmin 1 in type A mutation of acute myeloid leukemia. Biochim. Biophys. Acta, Proteins Proteomics 2019, 1867, 637–644. 10.1016/j.bbapap.2019.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.