Abstract
Aims/Introduction
Glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are important incretin hormones. They are released from the gut after meal ingestion and potentiate glucose‐stimulated insulin secretion. Their release after meal ingestion and oral glucose are well established and have been characterized previously. During recent years, knowledge of other regulatory aspects that potentially may affect GIP and GLP‐1 secretion after meal ingestion have also begun to emerge. Here, the results of human studies on these novel aspects of meal‐ and nutrient‐stimulated incretin hormone secretion are reviewed.
Materials and Methods
The human literature was revisited by identifying articles in PubMed using key words GIP, GLP‐1, secretion, meal, and nutrients.
Results
The results show that all macronutrients individually stimulate GIP and GLP‐1 secretion. However, there was no synergistic action when given in combination. A pre‐load 30 min before a meal augments the GIP and GLP‐1 response. GIP and GLP‐1 secretion have a diurnal variation with a higher response to an identical meal in the morning than in the afternoon. There is no difference in GIP and GLP‐1 secretion whether a meal is ingested slowly or rapidly. GIP and GLP‐1 secretion after dinner are the same whether or not breakfast and lunch have been ingested. The temperature of the food may be of importance for the incretin hormone response.
Conclusions
These novel findings have increased our knowledge on the regulation of the complexity of the incretin system and are also important knowledge when designing future studies.
Keywords: Glucagon‐like peptide‐1, Glucose‐dependent insulinotropic polypeptide, Meal
Several aspects of the regulation of GIP and GLP‐1 secretion after meal ingestion in humans are reviewed in this article. The Figure shows the GIP and GLP‐1 secretion over the day; the pie chart the relative contribution of breakfast, lunch and dinner to the 24 h GIP and GLP‐1 secretion.
INTRODUCTION
Glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are produced in enteroendocrine cells, released into the blood after meal ingestion and regulate islet function through the important incretin action, as reviewed recently 1 , 2 , 3 , 4 . Some aspects of the secretion of GIP and GLP‐1 after meal ingestion have been established after results in several studies, including the effects and mechanisms of individual nutrients in a meal 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , the reduced GIP and GLP‐1 secretion in insulin resistance 10 , and the largely preserved GIP and GLP‐1 secretion in type 2 diabetes 1 , 2 , 3 , 11 , 12 . However, in recent years, knowledge of other regulatory aspects that also may potentially affect GIP and GLP‐1 secretion after meal ingestion have begun to emerge, such as potential diurnal regulation 13 , dependency on the length of the preceding fast 14 , 15 , the rapidity of meal ingestion 16 and the meal size 17 . Here we have reviewed these novel aspects of meal‐ and nutrient‐stimulated incretin hormone secretion in humans. We have undertaken a survey of the literature by identifying articles in PubMed using key words GIP, GLP‐1, secretion, meal, and nutrients, and we have revisited some of our own earlier studies on these aspects.
GIP AND DLP‐1 SECRETION AFTER A MIXED MEAL
Glucose‐dependent insulinotropic polypeptide was discovered in 1969 18 and GLP‐1 was discovered in 1983 19 . Very early after their discoveries it was shown that their plasma levels increased after meal ingestion 20 , 21 . This has subsequently been repeated in many studies and summarized in excellent reviews 1 , 2 , 3 , 4 . Since the intact forms of GIP and GLP‐1 that are released are rapidly inactivated by dipeptidyl peptidase‐4 21 , the half‐lives of the intact forms of the two hormones are only ≈7 and 2 min, respectively 22 , 23 . It is therefore important to measure both the intact and inactivated forms (i.e., the ‘total’ forms) of the hormones for an estimation of the total secretion after a meal 24 , 25 . In the following review, it is the total GIP and total GLP‐1 that have been measured when we discuss incretin hormone secretion, unless otherwise stated.
It has been demonstrated repeatedly that GIP and GLP‐1 secretion increase within 10 min after a mixed meal ingestion and reach their maxima after 30–45 min 1 , 2 , 13 , 14 , 15 , 16 , 17 , 20 , 21 , 26 . To characterize the pattern of GIP and GLP‐1 secretion, we have taken advantage of a cohort of 12 healthy subjects who ingested the same breakfast on three different occasions 13 . We measured total GIP and total GLP‐1 levels after the meal ingestion, which allowed the characterization of GIP and GLP‐1 secretion with a minimized intraindividual variation. The results of the mean GIP and GLP‐1 levels are shown in Figure 1. It is seen that both GIP and GLP‐1 levels increase after meal ingestion. The increase in total GIP and GLP‐1 levels was rapid for both, as there was already a significant increase after 5 min. This result shows first that the GIP and GLP‐1 producing cells are stimulated within minutes after a meal ingestion, and, second, that the two incretin hormones are released in parallel after meal ingestion, although GIP levels achieve approximately 3 times higher values than GLP‐1 levels. The rapid stimulation of GIP and GLP‐1 secretion is thought to be achieved by nutrients rapidly reaching the enteroendocrine cells potentially in combination with an activation of the enteric nervous system 27 .
Figure 1.
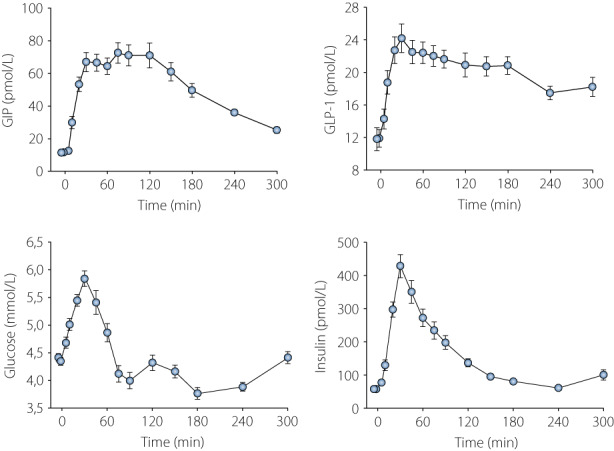
GIP, GLP‐1, glucose, and insulin levels after ingestion of a standardized breakfast consisting of 524 kcal (63% from carbohydrates, 19% from proteins, 18% from fat) as rye and wheat bread (60 g), margarine (10 g), ham (15 g), cheese (15 g), orange juice (150 g), green pepper (40 g), light sour milk, fat 0.5% (200 g), and muesli with fruit (40 g) in 12 healthy non‐diabetic men (mean age 22 years, mean BMI 22.7 kg/m2). Data are mean ± SEM for 36 tests in the 12 subjects, three tests in each subject. Original data from reference 13 and previously unpublished from the same project.
We also estimated the 30 min area under the curve (AUC) for GIP and GLP‐1 and estimated the coefficiency of variation (CV) for these measures, since the test was repeated three times in all subjects. The CV values were 18.2% for AUCGIP and 19.9% for AUCGLP‐1. Also the glucose and insulin levels increased after meal ingestion (Figure 1). GIP, GLP‐1, glucose, and insulin levels all increased linearly during the first 30 min. The linear relation between the concentrations and time for the parameters during the first 30 min after meal ingestion was 2.0t + 8.4 pmol/L for GIP, 0.4t + 12.9 pmol/L for GLP‐1, 0.5t + 4.4 mmol/L for glucose, and 13t + 28 pmol/L for insulin, where t is minutes. This means that for each minute during the first 30 min after breakfast, there was an increase in the GIP levels of 2.0 pmol/L, in GLP‐1 levels of 0.4 pmol/L, in glucose levels of 0.5 mmol/L, and in insulin levels of 13 pmol/L.
GIP AND GLP‐1 SECRETION OVER THE DAY
Incretin hormones are released not only after breakfast, but after all meals throughout the day 28 , 29 . This was confirmed in one study, when the 24 h secretion of GIP and GLP‐1 after ingestion of standardized breakfast, lunch, and dinner were examined in 24 subjects with well‐controlled metformin‐treated type 2 diabetes 30 . Figure 2 shows that after all meals, there was a typical pattern of increase in the GIP and GLP‐1 levels. However, the relative magnitude of the responses after breakfast, lunch, and dinner was different between GIP and GLP‐1. Thus, GIP levels increased to their highest values after the lunch meal, whereas GLP‐1 levels increased to their maximum after breakfast. This is also illustrated in Figure 2 where the relative contribution of 3 h AUC for GIP and GLP‐1 after each of the three meals to the total post meal AUC over the day is displayed in a pie chart. It is seen that the contribution to the 24 h postprandial GIP secretion was larger after lunch (42%) than after breakfast (24%) and dinner (34%), whereas the contribution to the 24 h postprandial GLP‐1 secretion was larger after breakfast (38%) than after lunch (31%) and dinner (31%). A similar finding with the highest GIP levels after lunch has been demonstrated before, whereas in that study, the peak GLP‐1 levels after each meal were similar 28 . A difference between the meals over the day is the composition which may explain the different release patterns over the day for GIP and GLP‐1 and may, perhaps, indicate that the relative importance of GIP vs GLP‐1 varies over the day.
Figure 2.
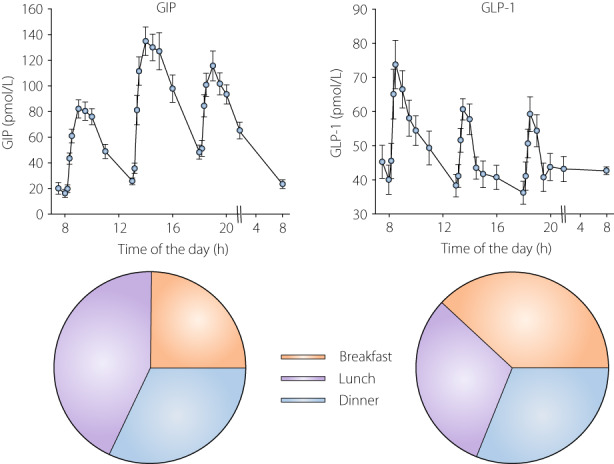
GIP and GLP‐1 levels after ingestion of standardized breakfast (525 kcal; 60% from carbohydrate, 20% from protein and 20% from fat), lunch (780 kcal; 40% from carbohydrate, 25% from protein, 35% from fat), and dinner (560 kcal; 40% from carbohydrate, 25% from protein, 35% from fat) in 24 subjects with metformin‐treated type 2 diabetes (mean age 63 years, BMI 31 kg/m2, HbA1c 45 mmol/mol). Mean ± SEM are shown. Pie chart shows the numerical proportion of 3 h postmeal area under the curves for GIP and GLP‐1 after breakfast, lunch, and dinner. Original data from reference 30 .
GIP AND GLP‐1 SECRETION AFTER INDIVIDUAL MACRONUTRIENTS
Several studies have now demonstrated that individual macronutrients in a meal stimulate both GIP and GLP‐1 secretion 8 , 9 , 15 , 31 . The secretion of GIP and GLP‐1 after oral glucose administration was demonstrated early after their discoveries 32 , 33 and has been repeated in many studies over the years 1 , 2 , 3 , 4 , 5 , 7 . Commonly, a 75 g glucose test has been used, but also other glucose doses have been used 9 and as little as 25 g glucose also results in a robust stimulation of GIP and GLP‐1 secretion 34 . The response to oral glucose is rapid and proportional to the emptying of the stomach 2 . Also fat ingestion stimulates GIP and GLP‐1 secretion as has been demonstrated after oral intake of corn oil 31 , 35 , oleic acid 36 , or double cream 29 . Furthermore, protein administration in terms of beef extract 35 , milk and egg protein 36 and purified whey proteins 34 stimulate GIP and GLP‐1 secretion, whereas ingestion of a grilled lean turkey, while increasing GLP‐1 levels, did not increase the GIP levels 29 . Oral ingestion of an amino acid mixture increased plasma levels of GIP but not GLP‐1 37 . This suggests that for protein ingestion, the resulting effect on incretin hormone secretion may depend on the degradation of the protein to smaller peptides and amino acids, which may differ between protein sources, as reviewed recently 38 , and also differently impact GIP and GLP‐1 secretion.
Nutrients use different mechanisms to stimulate insulin secretion as reviewed by Gribble and Reimann 6 . Thus, glucose is absorbed through sodium glucose transporter 1 (SGLT‐1) followed by intracellular metabolism, long‐chain fatty acids activate free fatty acid receptors 1 (FFAR1), monoglycerides active the G protein coupled receptor 119 (GPR119), and amino acids and small peptides stimulate enteric hormone secretion through the calcium sensing receptor (CASR). Therefore, several meal components, when given alone, stimulate GIP and GLP‐1 secretion through different signaling mechanisms.
To compare the relative effects of macronutrients on GIP and GLP‐1 secretion requires that they are administered in the same study. We performed such a study in which the GIP and GLP‐1 levels were compared after ingestion of glucose (83 g), whey protein (30 g), and a mixture of long chain triglycerides (12 g), alone and together, in 18 healthy subjects with a mean age of 62 years, and a mean BMI of 25 kg/m2 39 . The design of the study allowed conclusions on both the relative potency of the individual macronutrients as well as whether there is a synergistic effect when they are given together. We have here revisited this study and Figure 3 shows the data after giving the individual macronutrients. It is seen that GIP and GLP‐1 levels increased after ingestion of all three macronutrients with a peak level after 30 min. After glucose and fat ingestion, suprabasal AUCGIP during the first 30 min was significantly higher than AUCGLP‐1, whereas AUCGIP and AUCGLP‐1 did not differ significantly after protein ingestion. It can therefore be concluded that carbohydrate, protein, and fat all stimulate GIP and GLP‐1 secretion, that GIP secretion is stimulated more potently than GLP‐1 secretion by fat and glucose ingestion, and that GIP and GLP‐1 secretion are stimulated to a similar potency after protein ingestion. Furthermore, the study also explored the effect of giving all macronutrients together, and it was found that GIP and GLP‐1 levels increased even further than when the individual nutrients had been ingested. However, there was no synergistic action of nutrients when given together.
Figure 3.
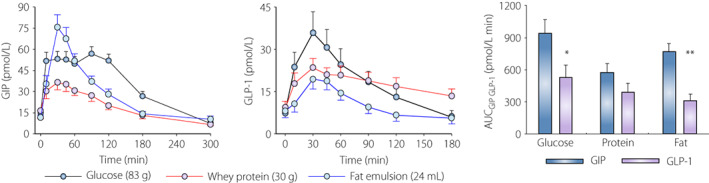
GIP and GLP‐1 levels after oral ingestion of glucose (83 g), whey protein (30 g), and fat emulsion with 50% long‐chain triglycerides (24 mL) in 18 healthy non‐diabetic subjects, mean age 62 years, mean BMI 25 kg/m2, after an overnight fast. Right figure shows area under the GIP and GLP‐1 curves (AUC) for the first 30 min after nutrient ingestion. Mean ± SEM are shown. Asterisks indicate probability level of random difference between AUCGIP and AUCGLP‐1 after each nutrient ingestion; *P < 0.05, **P < 0.01. Original data reported in reference 39 .
INFLUENCE OF THE TIME OF THE DAY, RAPIDITY OF MEAL INGESTION, CALORIC LOAD, AND TEMPERATURE ON GIP AND GLP‐1 SECRETION
Most studies on meal‐related GIP and GLP‐1 secretion have examined the responses to breakfast ingested in the morning after an overnight fast. However, since the incretin hormones are secreted after each meal, it is of interest to also explore the regulation of GIP and GLP‐1 secretion at times of the day other than the morning. This is of particular importance since we have shown that there is a diurnal variation in the metabolic responses to the same meal ingested at different times over the day. We thus compared the GIP and GLP‐1 responses to a standardized breakfast (524 kcal) when ingested at 8 am vs at 5 pm in healthy subjects 13 . When the breakfast was ingested at 5 pm, no lunch was ingested to allow a similar length of fast before meal ingestion in the two conditions. It was found that GIP secretion was 85% higher and GLP‐1 secretion was 25% higher when the breakfast was ingested in the morning vs in the afternoon (Figure 4). This result therefore suggests that there is a diurnal pattern of incretin hormone secretion. This coincided with the glucose response to the breakfast which was lower in the morning than in the afternoon.
Figure 4.
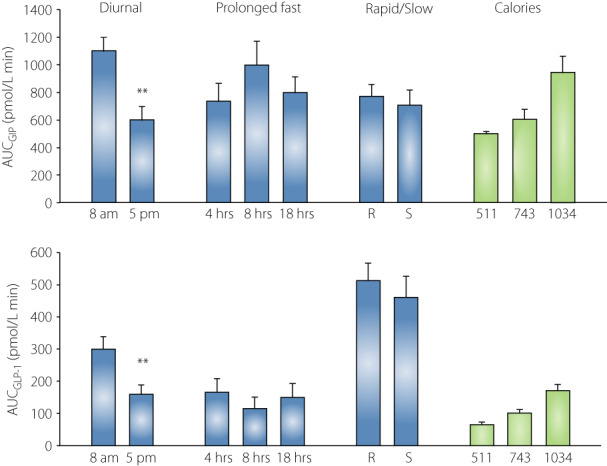
Area under the curve (AUC) for concentrations of total GIP and total GLP‐1 (blue bars) or intact GIP and intact GLP‐1 levels (green bars) during the first 30 min after meal ingestion. In the ‘diurnal’ study, standardized breakfast (524 kcal, 60% from carbohydrates, 20% from proteins, 20% from fat) was ingested at 8 am or 5 pm in 12 young healthy subjects (mean age 22 years, original data from reference 13 ). In the ‘prolonged fast’ study, standardized dinner (690 kcal, 40% from carbohydrates, 25% from proteins, 35% from fat) was ingested at 5 pm following 4, 8, or 18 h fast in 12 healthy non‐diabetic subjects (mean age 22 years; original data from reference 15 and previously unpublished). In the ‘rapid/slow’ study, standardized breakfast (524 kcal, 60% from carbohydrates, 20% from protein, 20% from fat) was ingested at 8 am after an overnight fast, either rapidly (R, within 5 min) or slowly (S, linear intake during 12 min) in 24 healthy middle‐aged non‐diabetic subjects (mean age mean 62 years, original data from reference 16 ). In the ‘calories’ study, a lunch with standardized composition (carbohydrate 50%, protein 18%, fat 32%) in three different sizes (511, 743, and 1,034 kcal) was ingested at 1 pm after a standardized breakfast had been given in 24 young, healthy, non‐diabetic subjects (mean age 24 years; original data from reference 17 ). Mean ± SEM are shown. Asterisks indicate probability level of random difference between the columns; **P < 0.01.
Another study examined whether the rapidity of a meal ingestion would affect the GIP and GLP‐1 responses. However, this was not the case since a rapid ingestion of a breakfast within 5 min had the same incretin hormone response as a more slow ingestion over 12 min 16 (Figure 4). We also examined the influence of caloric load on GIP and GLP‐1 responses by serving a lunch meal with the same composition but with a different caloric content (511, 743, and 1,034 kcal with 50% from carbohydrates, 18% from protein, and 32% from fat) 17 . A limitation in this study is, however, that we only measured the intact (active) forms of the hormones, and therefore this study does not compare GIP and GLP‐1 secretion, but instead the active GIP and GLP‐1 responses. In any case, as expected, the intact GIP and GLP‐1 responses increased by increasing the meal size, as shown in Figure 4 17 . By calculating how much the increased caloric load increased GIP and GLP‐1 responses, the 30 min AUC for GIP increased by 85 ± 8 pmol/L min per 100 kcal, and the corresponding factor for AUCGLP‐1 was 21 ± 3 pmol/L min per 100 kcal. This confirms a previous study that the caloric delivery of a meal to the gut is a regulatory factor for incretin hormone responses 40 .
It was also recently examined whether the temperature of food ingredients affects the incretin hormone responses 41 . In the study, 19 healthy subjects and 22 subjects with diabetes were served a 75 g glucose solution which was either hot (59°C) or cold (8°C). It was found that the glucose responses were higher after the hot glucose load in both groups. In contrast, GIP levels were not different between the hot and cold challenge, whereas the GLP‐1 levels were lower after hot than after cold glucose in healthy subjects but not in subjects with type 2 diabetes. The mechanisms for these effects are not known but may be associated with effects on gastric emptying or on glucose absorption. In any case, the study shows that food temperature may be important for the incretin hormone responses to food ingestion.
EFFECT OF PRE‐LOAD AND PRE‐MEAL FASTING ON GIP AND GLP‐1 SECRETION
It has been demonstrated that the incretin hormone response to a mixed meal is augmented if the meal had been preceded by ingestion of a protein pre‐load before the meal. This was first demonstrated in 2009 in eight subjects with type 2 diabetes. A 55 g whey protein pre‐load 30 min before a mixed 300 kcal breakfast augmented the GIP and GLP‐1 responses to the breakfast 42 . A similar study was performed in 2014 in 15 subjects with type 2 diabetes 43 . In that study, the subjects were served a mixed 330 kcal breakfast with or without a preceding 50 g whey protein load 30 min before. It was found that the GLP‐1 response was more than two‐fold higher if the breakfast had been preceded by a pre‐load; the 30 min AUCGLP‐1 after the breakfast was 642 ± 64 pmol/L in the control situation and 1,631 ± 114 pmol/L min when the pre‐load was added 43 . Similarly, a study in 2016 reported that a 25 g whey protein pre‐load 60 min before a breakfast consisting of 65 g powdered potato, 20 g glucose, and one egg yolk augmented both GIP and GLP‐1 responses to the breakfast 44 . Hence, following a 30 min pre‐load the GIP and GLP‐1 response to breakfast was augmented. The reason is probably that amino acids and small bioactive peptides released by the protein preload have stimulated GLP‐1 secretion 45 .
Whether the length of the preceding fast period before a meal affects the incretin hormone responses has also been examined. The interest in this topic was initiated by a study in subjects with type 2 diabetes which showed that the GLP‐1 response to a lunch was reduced if breakfast had not been ingested, and this was associated with higher glucose levels 14 . This was later confirmed in healthy subjects 46 . This would suggest that a prolonged fasting reduces a subsequent GLP‐1 response which may be caused by metabolic perturbations by the fasting, such as increased levels of free fatty acids and/or glucagon, or the results might be explained by changes in the circadian rhythm by omitting breakfast. A limitation in that study, however, was that it was only the intact form of GLP‐1 that was measured, and, therefore, the results could also be explained by regulation of GLP‐1 inactivation rather than by GLP‐1 secretion. To examine whether it is the secretion that is perturbed, whether this difference is seen also for GIP secretion, and to examine whether the importance of the length of the fast is of relevance also for the incretin hormone responses to dinner ingestion, we performed a study in healthy subjects in which the total forms of both GIP and GLP‐1 were measured after dinner when a preceding lunch was omitted. We found, however, that prolonging the fast from 4 to 9 h by omitting lunch did not affect the GIP or GLP‐1 response to dinner 15 . Also, in the same subjects, we further prolonged the fasting period by omitting also breakfast, i.e., the subjects ingested dinner at 5 pm with a fast for 18 h (previously unpublished). Figure 4 shows the GIP and GLP‐1 responses to a dinner under these three different conditions: a regular dinner with preceding intake of breakfast and lunch (4 h fast), a dinner with a preceding breakfast but no lunch (8 h fast), or a dinner without a preceding breakfast or lunch (18 h fast). The responses were very similar under these three conditions with no significant difference at any of the individual time points. From these results, we therefore conclude that at least up to 24 h, a preceding fast does not affect the incretin hormone response to a dinner meal in healthy subjects.
CONCLUSIONS
While the stimulation of GIP and GLP‐1 secretion after meal ingestion and oral glucose have been known and characterized for a long time, and, in fact, is the basis for the incretin effect, many novel aspects of the regulation of the secretion have emerged only during the recent decade. This review has summarized some of these novel aspects and the main conclusions are:
GIP and GLP‐1 secretion after meal ingestion are rapid and increased levels are already seen 5 min after the start of ingestion with maximum levels after 30–45 min,
All macronutrients individually stimulate GIP and GLP‐1 secretion but there is no synergistic addition of their effects when they are given in combination,
A protein ingestion as a pre‐load 30 min before a meal augments the GIP and GLP‐1 response to the meal,
GIP and GLP‐1 secretion have diurnal variations with a higher response to an identical meal in the morning than in the afternoon,
There is no difference in GIP and GLP‐1 secretion whether a meal is ingested slowly or rapidly,
GIP and GLP‐1 secretion after dinner are the same whether or not breakfast and lunch had been ingested on the same day,
The temperature may be important for incretin hormone secretion.
The knowledge presented and summarized here therefore underscores the complexity of the regulation of GIP and GLP‐1 secretion and is important not only for understanding the regulation of incretin hormone secretion but also for the design of standardized studies.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
This research was funded by the Lund University Medical Faculty, Region Skåne, and the Swedish Research Council (to B. Ahrén). We acknowledge research nurses Gustav Dahl and Bertil Nilsson, and laboratory technician Tina Ovlund, Region Skåne and Lund University for expert assistance in own research.
REFERENCES
- 1. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab 2018; 20: 5–21. [DOI] [PubMed] [Google Scholar]
- 2. Holst JJ. The incretin system in healthy humans: the role of GIP and GLP‐1. Metabolism 2019; 96: 46–55. [DOI] [PubMed] [Google Scholar]
- 3. Müller TD, Finan B, Bloom SR, et al. Glucagon‐like peptide 1 (GLP‐1). Mol Metab 2019; 30: 70–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guccio N, Gribble M, Reimann F. Glucose‐dependent insulinotropic polypeptide – a postprandial hormone with unharnessed metabolic potential. Annu Rev Nutr 2022; 42: 21–44. [DOI] [PubMed] [Google Scholar]
- 5. Bodnaruc A, Prud'homme D, Blanchet R, et al. Nutritional modulation of endogenous glucagon‐like peptide‐1 secretion: a review. Nutr Metab 2016; 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gribble FM, Reimann F. Metabolic messengers: glucagon‐like peptide‐1. Nat Metab 2021; 3: 142–148. [DOI] [PubMed] [Google Scholar]
- 7. Calanna S, Christensen M, Holst JJ, et al. Secretion of glucagon‐like peptide‐1 in patients with type 2 diabetes mellitus: systematic review and meta‐analyses of clinical studies. Diabetologia 2013; 56: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept 2005; 128: 117–124. [DOI] [PubMed] [Google Scholar]
- 9. Ahrén B. GIP secretion after oral macronutrient ingestion – the human literature revisited and a systematic study in model experiments in mice. J Diabetes Investig 2022; 13: 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rask E, Olsson T, Söderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001; 24: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 11. Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687. [DOI] [PubMed] [Google Scholar]
- 12. Nauck MA, Vardarli I, Deacon CF, et al. Secretion of glucagon‐like peptide‐1 (GLP‐1) in type 2 diabetes: what is up, what is down? Diabetologia 2011; 34: 10–18. [DOI] [PubMed] [Google Scholar]
- 13. Lindgren O, Mari A, Deacon CF, et al. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab 2009; 94: 2887–2892. [DOI] [PubMed] [Google Scholar]
- 14. Jakubowicz D, Wainstein J, Ahrén B, et al. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care 2015; 38: 1820–1826. [DOI] [PubMed] [Google Scholar]
- 15. Lindgren O, Ahrén B. Consequences on islet and incretin hormone responses to dinner by omission of lunch in healthy men. Diabetes Obes Metab 2020; 3: e00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alsalim W, Ahrén B. Insulin and incretin hormone responses to rapid versus slow ingestion of a standardized solid breakfast in healthy subjects. Endocrinol Diabetes Metab 2019; 2: e00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alsalim W, Omar B, Pacini G, et al. Incretin and islet hormone adaptation to meals of increasing size in healthy subjects. J Clin Endocrinol Metab 2015; 100: 561–568. [DOI] [PubMed] [Google Scholar]
- 18. Brown JC, Pederson RA, Jorpes E, et al. Preparation of highly active enterogastrone. Can J Physiol Pharmacol 1969; 47: 113–114. [DOI] [PubMed] [Google Scholar]
- 19. Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983; 302: 716–718. [DOI] [PubMed] [Google Scholar]
- 20. Kuzio M, Dryburgh JR, Malloy KM, et al. Radioimmunoassay for gastric inhibitory polypeptide. Gastroenterology 1974; 66: 357–364. [PubMed] [Google Scholar]
- 21. Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon‐like peptide‐1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 1993; 214: 829–835. [DOI] [PubMed] [Google Scholar]
- 22. Deacon CF, Nauck MA, Meier J, et al. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000; 85: 3575–3581. [DOI] [PubMed] [Google Scholar]
- 23. Vilsboll T, Agerso H, Krarup T, et al. Similar elimination rates of glucagon‐like peptide‐1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 2003; 88: 220–224. [DOI] [PubMed] [Google Scholar]
- 24. Deacon CF, Holst JJ. Immunoassay for the incretin hormones GIP and GLP‐1. Best Pract Res Clin Endocrinol Metab 2009; 23: 425–432. [DOI] [PubMed] [Google Scholar]
- 25. Meek CL, Lewis HB, Burling K, et al. Expected values for gastrointestinal and pancreatic hormone concentrations in healthy volunteers in the fasting and postprandial state. Ann Clin Biochem 2021; 58: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veedfald S, Rehfeld J, van Hall G, et al. Entero‐pancreatic hormone secretion, gastric emptying, and glucose absorption after frequently sampled meal tests. J Clin Endocrinol Metab 2022; 107: e188–e204. [DOI] [PubMed] [Google Scholar]
- 27. Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient‐induced glucagon‐liker peptide‐1 secretion. Endocrinology 1999; 140: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 28. Ørskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon‐like peptide‐1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol 1996; 31: 665–670. [DOI] [PubMed] [Google Scholar]
- 29. Elliott RM, Morgan LM, Tredger JA, et al. Glucagon‐like peptide‐1(7–36)amide and glucose‐dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post‐prandial and 24‐h secretion patterns. J Endocrinol 1993; 138: 159–166. [DOI] [PubMed] [Google Scholar]
- 30. Alsalim W, Göransson O, Tura A, et al. Persistent whole day meal effects of three dipeptidyl peptidase 4 inhibitors on glycaemia and hormonal responses in metformin‐treated type 2 diabetes. Diabetes Obes Metab 2020; 22: 590–598. [DOI] [PubMed] [Google Scholar]
- 31. Herrmann C, Göke R, Richter G, et al. Glucagon‐like peptide‐1 and glucose‐dependent insulin‐releasing polypeptide plasma levels in response to nutrients. Digestion 1995; 56: 117–126. [DOI] [PubMed] [Google Scholar]
- 32. Dupré J, Ross SA, Watson D, et al. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol 1973; 37: 826–828. [DOI] [PubMed] [Google Scholar]
- 33. Kreymann B, Williams G, Ghatei MA, et al. Glucagon‐like peptide‐1 7‐36: a physiological incretin in man. Lancet 1987; 2: 1300–1304. [DOI] [PubMed] [Google Scholar]
- 34. Nilsson M, Holst JJ, Björck IME. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose equivalent drinks. Am J Clin Nutr 2007; 85: 996–1004. [DOI] [PubMed] [Google Scholar]
- 35. Cleator IG, Gourlay RH. Release of immunoreactive gastric inhibitory polypeptide (IR‐GIP) by oral ingestion of food substances. Am J Surg 1975; 130: 128–135. [DOI] [PubMed] [Google Scholar]
- 36. Carr RD, Larsen MO, Sörhede Winzell M, et al. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol 2008; 295: E779–E784. [DOI] [PubMed] [Google Scholar]
- 37. Lindgren O, Pacini G, Tura A, et al. Incretin effect after oral amino acid ingestion in humans. J Clin Endocrinol Metab 2015; 100: 1172–1176. [DOI] [PubMed] [Google Scholar]
- 38. Watkins JD, Koumanov F, Gonzalez JT. Protein‐ and calcium‐mediated GLP‐1 secretion: a narrative review. Adv Nutr 2021; 12: 2540–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alsalim W, Tura A, Pacini G, et al. Mixed meal ingestion diminishes glucose excursion in comparison with glucose ingestion via several adaptive mechanisms in people with and without type 2 diabetes. Diabetes Obes Metab 2016; 18: 24–33. [DOI] [PubMed] [Google Scholar]
- 40. Bagger JI, Knop FL, Lund A, et al. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 737–745. [DOI] [PubMed] [Google Scholar]
- 41. Hu Y, Zhang P, Ding B, et al. Response of blood glucose and GLP‐1 to different food temperature in normal subject and patients with type 2 diabetes. Nutr Diabetes 2022; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet‐controlled type 2 diabetes. Diabetes Care 2009; 32: 1600–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jakubowicz D, Froy O, Ahrén B, et al. Incretin, insulinotropic and glucose‐lowering effects of whey protein pre‐load in type 2 diabetes: a randomized clinical trial. Diabetologia 2014; 57: 1807–1811. [DOI] [PubMed] [Google Scholar]
- 44. Wu T, Little TJ, Bound MJ, et al. A protein preload enhances the glucose‐lowering efficacy of vildagliptin in type 2 diabetes. Diabetes Care 2016; 39: 511–517. [DOI] [PubMed] [Google Scholar]
- 45. Nouri M, Purghassem B, Tajfar GP, et al. A systematic review of whey protein supplementation effects on human glycemic control: a mechanistic insight. Diabetes Metab Syndr 2022; 16: 102540. [DOI] [PubMed] [Google Scholar]
- 46. Jakubowicz D, Wainstein J, Landau Z, et al. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: a randomized clinical trial. Diabetes Care 2017; 40: 1573–1579. [DOI] [PubMed] [Google Scholar]


