Abstract
In the causative agent of syphilis, Treponema pallidum, the gene encoding 3-phosphoglycerate mutase, gpm, is part of a six-gene operon (tro operon) that is regulated by the Mn-dependent repressor TroR. Since substrate-level phosphorylation via the Embden-Meyerhof pathway is the principal way to generate ATP in T. pallidum and Gpm is a key enzyme in this pathway, Mn could exert a regulatory effect on central metabolism in this bacterium. To study this, T. pallidum gpm was cloned, Gpm was purified from Escherichia coli, and antiserum against the recombinant protein was raised. Immunoblots indicated that Gpm was expressed in freshly extracted infective T. pallidum. Enzyme assays indicated that Gpm did not require Mn2+ while 2,3-diphosphoglycerate (DPG) was required for maximum activity. Consistent with these observations, Mn did not copurify with Gpm. The purified Gpm was stable for more than 4 h at 25°C, retained only 50% activity after incubation for 20 min at 34°C or 10 min at 37°C, and was completely inactive after 10 min at 42°C. The temperature effect was attenuated when 1 mM DPG was added to the assay mixture. The recombinant Gpm from pSLB2 complemented E. coli strain PL225 (gpm) and restored growth on minimal glucose medium in a temperature-dependent manner. Increasing the temperature of cultures of E. coli PL225 harboring pSLB2 from 34 to 42°C resulted in a 7- to 11-h period in which no growth occurred (compared to wild-type E. coli). These data suggest that biochemical properties of Gpm could be one contributing factor to the heat sensitivity of T. pallidum.
Syphilis, a sexually transmitted disease caused by the spirochete Treponema pallidum, remains a major public health problem in the world. T. pallidum cannot be cultivated in vitro, making it difficult to assess the role of genes in physiology, survival in the host, and pathogenesis. One approach to studying the functions of T. pallidum genes is to clone and overexpress these genes in Escherichia coli and then characterize the recombinant proteins in vitro. This approach was taken recently to characterize the TroR regulatory protein from T. pallidum (28). In the presence of Mn2+, TroR binds the operator of the transport-related operon (tro) and represses transcription. The tro operon contains six genes (14). The first four genes encode a putative ABC metal transport system (troA to -D), the fifth gene encodes TroR (troR), and the last gene encodes a glycolytic enzyme, 3-phosphoglycerate mutase (gpm, referred to as pgm in the T. pallidum genome database), which converts 3-phosphoglycerate (3-PGA) to 2-phosphoglycerate (2-PGA) (8, 11). Since T. pallidum can only generate ATP via glycolysis, 3-phosphoglycerate mutase is a key enzyme for the spirochete.
Bacterial phosphoglycerate mutases are divided into two classes, based on their requirement for the cofactor 2,3-diphosphoglycerate (DPG) (10). Phosphoglycerate mutases from spore-forming Bacillus species, such as Bacillus megaterium, Bacillus subtilis, and Bacillus stearothermophilus, are DPG independent but require Mn2+ for activity (7, 32, 38). E. coli possesses both DPG-dependent and DPG-independent Gpm (13). Given that T. pallidum gpm is located within an operon that includes a metal transport system and a Mn-dependent repressor, we hypothesized that the enzyme would have a Mn2+ requirement similar to that of the B. stearothermophilus enzyme. Thus, Mn would exert effects on both the regulation and activity of the enzyme, thereby affecting the central metabolism and growth of T. pallidum. Therefore, we examined the metal requirement of the T. pallidum phosphoglycerate mutase by cloning, expressing, and purifying a recombinant enzyme from E. coli. However, this enzyme did not require a metal ion for its activity but rather used DPG as a cofactor. The most interesting characteristic of the T. pallidum phosphoglycerate mutase was its extreme heat lability. We found this very intriguing, since it has been long known that syphilis is heat sensitive and it has been shown that there is no heat shock response in T. pallidum (33). Therefore, our results suggest that phosphoglycerate mutase may be one factor contributing to the heat sensitivity of T. pallidum.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemicals.
E. coli strains used in this study were TOP10 (Invitrogen, Carlsbad, Calif.); DH5α (Gibco BRL, Grand Island, N.Y.), and PL225 (gpm): Δ(nadA-galE)35 λ− recA1 relA1 rpsL180 (Strr) spoT1 thi-1 (24). Bacteria were cultivated in Luria-Bertani (LB) medium or M63 medium (31), and growth was monitored at 600 nm. Ampicillin (20 or 100 μg/ml), streptomycin (10 μg/ml), and isopropyl-β-d-thiogalactopyranoside (IPTG) (1 mM) were added as needed. For complementation assays, strains DH5α, PL225, and PL225 harboring pSLB2 were grown overnight in LB medium at 30°C, harvested by centrifugation (5,000 × g, 10 min), and washed twice in M63, and 30 μl was used to inoculate 30 ml of M63 with 0.2% glucose. IPTG (1 mM) was added to induce Gpm synthesis from plasmid pSLB2. Cultures were grown in duplicate at 34 or 42°C. Lactate dehydrogenase was purchased from Boehringer Mannheim (Indianapolis, Ind.). Rabbit 3-phosphoglycerate mutase, pyruvate kinase, enolase, and all other chemicals were obtained from Sigma Chemicals (Saint Louis, Mo.).
DNA manipulations.
Chromosomal DNA from T. pallidum subsp. pallidum strain Nichols was provided by Steve Norris (Department of Pathology and Laboratory Medicine, University of Texas, Houston) or Lola Stamm (Department of Epidemiology, University of North Carolina, Chapel Hill). Oligonucleotide primers were synthesized by the Molecular Genetics Instrumentation Facility, University of Georgia, Athens. Primers TPGpm1 (5′-CGTGAATTCCATGAAGCTTGTGTTGATCCGT-3′) and TPGpm2 (5′-ACTGAATTCATACATACGACCAGAGGATACGA-3′) were designed to incorporate EcoRI sites and used to amplify gpm from 10 ng of T. pallidum chromosomal DNA by PCR using Pfu polymerase (Stratagene, La Jolla, Calif.) in a PTC-100 thermal cycler (MJ Research, Watertown, Mass.) (1 cycle for 2 min at 94°C and 40 cycles of 40 s at 94°C [denaturation], 30 s at 50°C [annealing], and 1 min at 72°C [elongation]). The resulting 0.8-kb PCR product was digested with EcoRI and ligated into the EcoRI site of the expression vector pTrcHisC (Invitrogen), generating pSLB1. This PCR product was also ligated into the EcoRI site of the expression vector pKK223-3 (Amersham-Pharmacia, Piscataway, N.J.), generating pSLB2. Constructs were sequenced at the Molecular Genetics Instrumentation Facility, University of Georgia, and compared to The Institute for Genomic Research DNA database to ensure that no errors had been introduced by PCR. All DNA manipulations were performed as described by Maniatis et al. (21). Qiaprepspin and Qiaquick gel extraction kits (Qiagen, Chatsworth, Calif.) were used for all the DNA purification procedures.
Purification of hexahistidine-tagged Gpm and recombinant Gpm.
A hexahistidine-tagged Gpm (His-Gpm) fusion was expressed in E. coli TOP10 harboring pSLB1 by growing the cells in 600 ml of LB medium at 30°C with vigorous shaking. When the cells reached an A600 of 0.6, IPTG was added to the culture to a final concentration of 1 mM. Cells were grown for an additional 4 h and harvested by centrifugation (5,000 × g, 20 min, 4°C), suspended in 20 ml of 50 mM sodium phosphate buffer–0.3 M NaCl (pH 7.8), and lysed by three passages through a cold French pressure cell at 12,000 lb/in2. Following centrifugation (20,000 × g, 20 min, 4°C), the His-Gpm fusion was mainly found in the soluble fraction. The supernatant was applied to a nickel-nitrilotriacetic acid (Ni-NTA) affinity column (Qiagen), and proteins were washed with 25 mM imidazole and eluted with 250 mM imidazole. These steps were performed at 4°C. The fractions were analyzed by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis (SDS-PAGE) and assayed for Gpm activity. Protein concentration was determined using the Sigma protein assay kit. Purified His-Gpm was used to raise polyclonal antiserum in a New Zealand White rabbit at Cocalico Biologicals, Reamstown, Pa. The antiserum was cross-adsorbed with cell lysate from E. coli PL225 as previously described (5).
Purified Gpm was obtained from His-Gpm as follows. Two milligrams of his-Gpm was dialyzed against 1 liter of 50 mM sodium phosphate–20 mM NaCl (pH 7.6) for 12 h at 4°C. The dialyzed fusion protein was digested with 30 U of enterokinase (Sigma) for 18 h at room temperature, and enterokinase was removed by affinity capture using Ekapture agarose (Novagen, Madison, Wis.). The reaction mixture was applied to a nickel affinity column to remove the hexahistidine peptide and undigested fusion protein. The resulting Gpm was analyzed by SDS-PAGE, and the protein concentration was determined. Metal content of purified Gpm was determined using inductively coupled plasma spectroscopy (ICP-MS) at the Chemical Analysis Laboratory (University of Georgia) as previously described (20).
Electrophoresis and immunoblotting.
Proteins were separated by SDS-PAGE as described previously (18) using an SE600 gel apparatus (Hoefer Scientific, San Francisco, Calif.) and visualized with Coomassie brilliant blue R-250. Molecular weight standards were purchased from Bio-Rad Laboratories (Hercules, Calif.). For immunoblotting, proteins were transferred to nitrocellulose (0.45-μm-pore-size Protran membrane; Schleicher & Schuell, Keene, N.H.) as described by Towbin et al. using a Bio-Rad Trans Blot Cell (35). After transfer, proteins were visualized with Ponceau red (0.1% Ponceau red dye in 1.0% acetic acid), and the standards were marked. Immunoblotting was performed by the Amersham enhanced chemiluminescence method according to the manufacturer's instructions (Amersham Pharmacia). Antisera were used at the following dilutions: cross-adsorbed anti-Gpm rabbit antiserum, 1/1,000; goat anti-rabbit immunoglobulin G–peroxidase, 1/5,000.
Gpm assays.
Gpm activity was assayed as described previously, with minor modifications (17). In the first stage of the assay, a 100-μl reaction mixture contained 20 μl of the preincubated and appropriately diluted Gpm, with excess 3-PGA (10 mM), DPG (100 μM), in 50 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid]-NaOH, pH 7.0. The reaction was stopped after 2 min at 34°C (the assay was linear for 5 min) by addition of trichloroacetic acid (70 mM final concentration) and N-ethylmaleimide (10 mM final concentration). The amount of 2-PGA produced in the original reaction mixture was measured by adding enolase, pyruvate kinase, and lactate dehydrogenase and monitoring the oxidation of NADH at 340 nm as previously described (17). The amount of NAD produced was proportional to the amount of 2-PGA added to the assay. One unit of enzyme activity is defined as 1 μmol of 3-PGA converted to 2-PGA/min.
To determine the pH optimum of the purified Gpm, MES (morpholineethanesulfonic acid) (pH 6.0 to 6.5), TES (pH 7.0), HEPES (pH 7.5 to 8.0), and Tris (pH 8.0 to 9.0) were used for both the preincubation (30 min) and the first stage of the assay. The cofactor requirements were determined by (i) preincubating purified Gpm with ethylenediamine-N,N′-diacetic acid (EDDA), EDTA, ethylenediamine di(o-hydroxyphenylacetic acid) (EDDHA), or deferoxamine mesylate (maximum final concentration, 2 mM) at 25°C for 60 to 240 min, (ii) preincubating enzyme with MnSO4, MnCl2, NiSO4, CaCl2, or MgSO4 (2 mM final concentration) at 25°C for 60 to 240 min, or (iii) adding 0, 0.01, 0.1, 1, 5, or 10 mM DPG to the first stage of the assay. To inhibit DPG-dependent Gpm activity, 10 or 100 μM sodium metavanadate was added to purified Gpm 10 min prior to the first stage of the assay. The temperature stability of Gpm was determined by preincubating the enzyme at 4, 25, 30, 34, 37, or 42°C for various times up to 300 min, in the absence or in presence of 1 mM DPG, prior to the assay. Oryctolagus cuniculus (rabbit) Gpm (a DPG-dependent enzyme) (4) and B. stearothermophilus Gpm (a Mn2+-dependent enzyme provided by Peter Setlow, Department of Biochemistry, University of Connecticut Health Center, Farmington) (7) were used as controls and assayed at 25 and 65°C, respectively. All samples were run in duplicate in three independent experiments.
RESULTS
Purification of T. pallidum Gpm.
Analysis of the deduced amino acid sequences of the open reading frames in the T. pallidum tro operon indicated that one open reading frame encodes a protein with significant identity to phosphoglycerate mutases from various bacteria (e.g., 57.9% identity to E. coli DPG-dependent Gpm, 48.8% identity to Streptomyces coelicolor Gpm, and 48.6% identity to Mycobacterium tuberculosis Gpm, but only 11% identity to E. coli Mn-dependent Gpm) (12, 14). Because T. pallidum cannot be cultured in vitro, a recombinant Gpm was expressed and purified from E. coli. Since E. coli harbors the genes encoding two different Gpms, one of which has properties very similar to those of T. pallidum Gpm (13), a hexahistidine tag was introduced at the amino-terminal end of the protein to simplify the purification. The gene encoding Gpm was amplified by PCR from T. pallidum chromosomal DNA by using primers TPGpm1 and TPGpm2, and the resulting product was introduced into the EcoRI site of expression vector pTrcHisC, generating plasmid pSLB1. Following overexpression in E. coli, the His-Gpm localized to the soluble fraction of the cell and was purified using Ni-NTA affinity chromatography (Fig. 1, lane 2). Enzyme assays indicated that the purified His-Gpm was active (data not shown), demonstrating that the hexahistidine motif did not significantly interfere with Gpm activity. The 0.8-kb PCR product was also introduced into the EcoRI site of expression vector pKK223-3, generating plasmid pSLB2 for complementation experiments.
FIG. 1.
SDS-polyacrylamide gel of the purification steps of T. pallidum Gpm. Lane 1, soluble fraction containing the overexpressed His-Gpm fusion (15 μg of total protein); lane 2, His-Gpm fusion after nickel affinity chromatography (12.5 μg); lane 3, protein fraction obtained after digestion of His-Gpm with enterokinase (12.5 μg); lane 4, protein after enterokinase affinity capture (2 μg); lane 5, Gpm enzyme recovered after second nickel affinity chromatography (2 μg). Molecular mass standards are on the left.
As previously mentioned, TroR represses the tro operon in a Mn2+-dependent manner in T. pallidum, and Mn2+ reactivates a catalytically inactive form of B. megaterium Gpm (17). Therefore, it was possible that the T. pallidum Gpm required Mn2+ for activity. Since the metal-binding hexahistidine motif could interfere with the metal analysis of Gpm, it was removed using enterokinase. After treatment of His-Gpm with enterokinase, a product with an estimated molecular mass of 31 kDa was detected by SDS-PAGE (Fig. 1, lane 3). This corresponded to the predicted molecular mass of the native protein (28.3 kDa) (12) with 16 additional amino acids remaining after enterokinase digestion (30.4 kDa). Enterokinase was removed using an affinity capture system (Fig. 1, lane 4) and minor protein products, generated by the enterokinase, were removed using Ni-NTA affinity chromatography. The Gpm recovered from last purification step (Fig. 1, lane 5) was enzymatically active and was used for subsequent Gpm assays.
The effect of Mn2+ on Gpm activity.
The metal content and/or requirement of the T. pallidum Gpm was determined. The purified enzyme and rabbit Gpm (a Mn-independent enzyme) was incubated at 25°C for 60 to 240 min in the presence of various chelators (EDDA, EDTA, EDDHA, and deferoxamine) at concentrations ranging from 100 μM to 2 mM, and samples were assayed for Gpm activity. None of the chelators affected these enzyme activities at the concentrations tested (Table 1 and data not shown). In contrast, the addition of 1 mM EDTA completely inhibited B. stearothermophilus Gpm (a Mn-dependent enzyme) (Table 1) (7). In addition, the purified recombinant Gpm (0.5 mg) was assayed for bound metals using ICP-MS. No metals were detectable in these preparations (data not shown). Therefore, the initial Gpm activity detected following protein purification appeared to be metal independent. However, it was still possible that this initial enzyme activity represented only a portion of the total Gpm activity and a metal could restore or activate Gpm activity. To investigate this possibility, the purified enzyme was incubated at 25°C for 60 to 240 min in the presence of divalent metal ion (Mn2+, Mg2+, Fe2+, Ni2+, or Ca2+) at concentrations ranging from 100 μM to 2 mM. These treatments had no effect on the T. pallidum Gpm activity (Table 1 and data not shown). Therefore, the T. pallidum phosphoglycerate mutase does not require a metal ion for activity.
TABLE 1.
DPG and Mn dependence of various Gpm enzymes
| Species | Relative Gpm activitya
|
||||
|---|---|---|---|---|---|
| DPG dependence
|
Mn dependence
|
||||
| −DPG | +DPG | +DPG +vanadate | +EDTA | +Mn2+ | |
| O. cuniculus | 7 | 100b | 5 | 100c | 100c |
| T. pallidum | 17 | 100b | 20 | 100c | 100c |
| B. stearothermophilus | 100d | 100d | 100d | <1 | 100b |
Gpm activity was determined as described in Materials and Methods. The first step of the assay was performed with 10 mM 3-PGA in the presence or absence of 100 μM DPG, 10 μM sodium metavanadate, 1 mM EDTA, or 1 mM MnCl2. Assays were done in duplicate, and data are averages of three different experiments. Standard deviations were <10% for each point.
This value was set as 100% (200, 400, and 1,000 U/mg of protein for T. pallidum, rabbit, and B. stearothermophilus Gpm, respectively).
In the presence of 100 μM DPG.
In the presence of 1 mM MnCl2.
Effect of DPG and pH on Gpm activity.
The other class of phosphoglycerate mutases requires DPG as a cofactor (10). Since the T. pallidum Gpm appeared to be metal independent, the effect of DPG on the Gpm activity was determined. When 100 μM DPG was added to the first stage of the assay, the activities from T. pallidum Gpm and DPG-dependent rabbit Gpm were enhanced 5- and 14-fold, respectively (Table 1). In contrast, the addition of DPG had no effect on the activity of B. stearothermophilus Gpm (Table 1). Another diagnostic test for DPG-dependent Gpm activity is inhibition by sodium metavanadate (3). Therefore, 10 μM sodium metavanadate was added to the purified Gpm 10 min prior to the first stage of the assay. This treatment resulted in 80 and 95% inhibition of the T. pallidum and rabbit Gpm activities, respectively (Table 1). In contrast, addition of vanadate had no effect on the activity of B. stearothermophilus Gpm.
Another distinguishing characteristic of DPG-dependent and Mn-dependent Gpms is that they differ in their optimum pH (4). For example, the Mn-dependent enzymes have higher activity at pH ∼8.5 (6, 7, 17), while DPG-dependent enzymes are more active at pH ∼7.0 (4). The pH profile of the T. pallidum Gpm was comparable to that of the DPG-dependent rabbit Gpm, with maximum activity around pH 7.0 (Fig. 2). As predicted, the activity of the DPG-independent B. stearothermophilus Gpm increased with the pH as previously described (7). Taken together, the results from metal, DPG dependence, vanadate sensitivity, and pH profiles clearly showed that T. pallidum enzyme belongs to the DPG-dependent class of Gpms.
FIG. 2.
pH optimum of purified T. pallidum Gpm. T. pallidum Gpm (▴) and rabbit muscle Gpm (a DPG-dependent enzyme) (□) were incubated for 15 min at 25°C at various pHs with 1 mM DPG prior to the enzyme assay. B. stearothermophilus Gpm (○) was incubated 60 min at 37°C with 1 mM MnCl2. Each enzyme was assayed at various pHs at the temperature optimum for that enzyme. Gpm activity is reported as a percentage of the maximum activity obtained at the optimal pH for each enzyme: 200 U/mg of protein for T. pallidum, 400 U/mg for rabbit, and 1,000 U/mg for B. stearothermophilus. Standard deviations were <10% for each time point.
Heat lability of Gpm activity in vitro.
When the purified Gpm was preincubated with inhibitors before the first stage of the assay at 37°C, a significant loss of enzyme activity was observed. Subsequent experiments suggested that this unusual effect was due to temperature of the reaction mixture. To determine the temperature stability of the purified Gpm, the enzyme was incubated at temperatures ranging from 4 to 42°C for various times (0 to 300 min), aliquots were taken, and Gpm activity was assayed (Fig. 3). The enzyme activity was stable at 4°C for several months (data not shown) and retained 90% activity at 25°C for 300 min (Fig. 3A). Fifty percent of the Gpm activity was lost after the purified enzyme was incubated at 30°C for 180 min, at 34°C for 20 min, or at 37°C for 10 min (Fig. 3A). The enzyme lost all activity after 10 min at 42°C (Fig. 3A). Incubating the purified enzyme with Mn or bovine serum albumin prior to the first stage of the assay did not stabilize the enzyme (incubation time ≥120 min) (data not shown), while the addition of DPG partially protected the protein from denaturation. For example, when 1 mM DPG was incubated with the enzyme for 150 min at 42°C, 50% of the activity was retained indicating that DPG could stabilize Gpm to a limited degree (Fig. 3B). Incubating the enzyme with DPG also dramatically increased the stability at 30 and 37°C (Fig. 3B). However, the temperature-dependent loss of Gpm activity was irreversible. The addition of DPG after incubation of the enzyme at 34, 37, or 42°C for 120 min did not restore Gpm activity. These results showed that the T. pallidum Gpm is highly heat sensitive in vitro.
FIG. 3.
Effect of temperature on Gpm activity. Gpm was preincubated for different times at the indicated temperatures. Enzyme was incubated without (A) or with (B) DPG prior to the Gpm assay. Aliquots of reaction mixture were removed at various time points and assayed for enzyme activity. Activity is reported as a percentage of the initial activity (200 U/mg of protein). Standard deviations were <10% for each time point.
Expression of Gpm in T. pallidum.
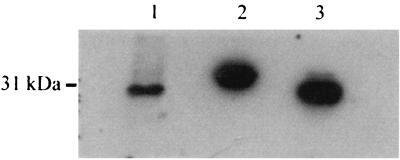
To determine if Gpm was expressed in T. pallidum harvested from infected rabbits, cell lysate isolated from freshly extracted treponemes was probed with anti-Gpm serum. Total cell protein from gradient-purified T. pallidum Nichols cells, purified His-Gpm, and Gpm were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-Gpm serum that had been extensively cross-adsorbed with cell lysate-isolated E. coli strain PL225 (Δgpm) (24) (Fig. 4). A 31-kDa protein band was detected in the T. pallidum cell lysate (Fig. 4, lane 1). This was the same size as the purified Gpm and slightly smaller than His-Gpm (Fig. 4, lanes 2 and 3). These data indicated that Gpm was being expressed in vivo and that this glycolytic pathway was probably functioning during growth of T. pallidum in animals.
FIG. 4.
Immunoblot of Gpm expressed in T. pallidum and E. coli. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was probed with an antiserum to recombinant His-Gpm cross-adsorbed with cell lysate from E. coli gpm mutant PL225. Lane 1, 15 μg of protein from approximately 6.4 × 107 density gradient-purified T. pallidum cells; lane 2, 500 ng of affinity-purified His-Gpm from E. coli; lane 3, 500 ng of purified Gpm obtained after enterokinase digestion.
Complementation of the E. coli gpm mutant with recombinant Gpm.
Because T. pallidum can be grown only in experimental animals, there was too little cell protein isolated from freshly extracted T. pallidum cells to verify the biochemical properties observed in the Gpm assays using recombinant enzyme (e.g., temperature stability). Therefore, we expressed Gpm from pSLB2 in the E. coli gpm mutant PL225 (24) to examine the biochemical properties of the enzyme in vivo. The gene encoding the DPG-dependent Gpm has been deleted in this strain, but it retains a functional gene encoding DPG-independent Gpm (13). Despite the presence of the second enzyme, PL225 does not grow on minimal medium with glucose as the sole carbon source, indicating that the activity of the DPG-independent enzyme alone is not sufficient to restore growth. Strain PL225 harboring pSLB2 was able to grow on glucose minimal medium, while PL225 harboring pKK223-3 did not grow (data not shown). Interestingly, temperature had a dramatic effect on the growth of strain PL225(pSLB2). When PL225(pSLB2) cells were grown in glucose minimal medium (with 1 mM IPTG to induce Gpm expression) at either 34 or 42°C (Fig. 5), a 7- to 11-h period in which no growth occurred was observed, in contrast to wild-type E. coli. (Fig. 5). Without pSLB2, PL225 was unable to grow (Fig. 5). Immunoblots indicated that the recombinant protein was expressed at detectable levels after induction with IPTG in the soluble cell fraction and was not localizing to inclusion bodies (data not shown). This indicated that the subcellular location of the recombinant protein was not responsible for the observed lag in growth. Thus, temperature appears to affect the recombinant Gpm activity in E. coli and may have a similar effect in T. pallidum.
FIG. 5.
The effect of temperature on the complementation of E. coli strain PL225 (gpm). Strains DH5α, PL225, and PL225 harboring pSLB2 were grown overnight, harvested by centrifugation, washed twice in M63, and used to inoculate the fresh M63 medium. Gpm synthesis was induced from plasmid pSLB2 with 1 mM IPTG. Cultures were grown at 34 or 42°C, and cell density was monitored at 600 nm. Samples were run in duplicate, and data represent three independent experiments.
DISCUSSION
It has been long known that temperature has a dramatic effect on the growth and survival of T. pallidum, the etiologic agent of syphilis. As early as 1539, Rodrigo Ruy de Isla observed the beneficial effects of fever on the course of the disease among the sailors of Columbus's crew (37). Furthermore, fever therapy induced by iatrogenic infection with Borrelia or Plasmodium species or by hypertherm cabinets was used as a treatment for human syphilis before the advent of antibiotics (1, 2, 27). It has also been shown that an increase in body temperature of infected humans or experimentally infected rabbits could result in the amelioration of syphilitic infection (30, 36). Fieldsteel and coworkers (9) found that limited multiplication of T. pallidum in an in vitro tissue culture system was achieved only when culture temperatures were maintained within a narrow range (32 to 36°C).
One possible explanation for this temperature sensitivity might be the lack of an efficient heat shock response in T. pallidum (33). Although T. pallidum harbors the genes encoding heat shock proteins, such as GroESL and DnaK, they do not appear to be thermoinducible (33). Additionally, no homolog to positive heat stress regulatory proteins, such as RpoH, was detected in the T. pallidum genome sequence, suggesting that the genes encoding GroESL and DnaK are not regulated by ς32 in this spirochete (12, 19). Likewise, no negative regulators, such as HrcA (for “heat regulation at CIRCE”) from B. subtilis or HspR (for “heat shock protein repressor”) from Streptomyces albus (25) were detected indicating that T. pallidum (12) lacks an efficient, inducible heat-shock response. Clearly, this explains the poor survivability of T. pallidum at temperatures higher than 37°C but may not completely explain the low growth rates estimated for T. pallidum in the later stages of syphilis or those observed for cells grown in tissue culture.
The poor stability of the 3-phosphoglycerate mutase at 37 and 42°C could represent another system by which temperature affects growth rate in T. pallidum. This could happen only because T. pallidum has very limited metabolic capacity. With no tricarboxylic acid cycle, cytochromes, or respiratory electron transport chain, the cells must hydrolyze ATP to generate a proton motive force to drive transport and motility (12). Substrate-level phosphorylation via the Embden-Meyerhof pathway seems to be the only way for T. pallidum to generate ATP. In T. pallidum, glyceraldehyde-3-phosphate is converted to phosphoenolpyruvate in four steps, generating one ATP. Phosphoenol pyruvate is then converted to pyruvate by pyruvate kinase, generating a second ATP (8). Since 3-phosphoglycerate mutase is a key enzyme in this pathway, any factor that affects the activity of this enzyme might influence the overall rate of ATP synthesis. As we have demonstrated in vitro, by enzyme assay, and in vivo, by complementation of strain PL225 (a gpm mutant), T. pallidum Gpm was extremely temperature sensitive and caused a growth defect in E. coli. These data suggest that temperature could affect the enzymatic activity of Gpm in T. pallidum and influence growth of the spirochete. In addition, it is possible that other glycolytic enzymes or DNA polymerases could also be temperature labile, therefore affecting in the same way the growth of T. pallidum.
As T. pallidum colonizes different sites within its human host, it encounters different environmental conditions that could affect enzymatic activity and regulation of Gpm. First, sites within the human body are not at the same temperature. Skin temperature near the groin region, the initial site of infection for T. pallidum, ranges from 30.7 to 34.7°C. In contrast, temperature in the central nervous system (CNS), a secondary site of infection, remains a constant 37°C under normal conditions (15). Therefore, Gpm activity would be higher at initial infection sites than at secondary sites. Second, measured levels of Mn2+ are 3 orders of magnitude higher in the CNS than in the skin (16, 29, 34). Since expression of gpm is dependent on TroR, a Mn2+-dependent repressor, transcription of the tro operon should decrease as T. pallidum moves from the skin to the CNS. Thus, differences in Mn2+ concentration and temperature in the human body could exert effects on the transcription of gpm and stability of Gpm in T. pallidum. These regulatory effects would allow T. pallidum to grow more rapidly in the skin, promoting effective colonization, and more slowly in the CNS (an immunoprivileged site), prolonging survival in the host (22, 23, 26).
ACKNOWLEDGMENTS
We thank Steve Norris (University of Texas, Houston) and Lola Stamm (University of North Carolina, Chapel Hill) for the gift of T. pallidum DNA and proteins. We are also grateful to Monica Chander and Peter Setlow (University of Connecticut Health Center, Farmington), who kindly provided 3-phosphoglycerate mutase purified from B. stearothermophilus. We thank Tim Hoover and Jorge Garcia-Lara for critical reading of the manuscript.
This work was supported by National Institutes of Health grant 10-21-RR-182243.
REFERENCES
- 1.Barbour A G. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol. 1990;44:155–171. doi: 10.1146/annurev.mi.44.100190.001103. [DOI] [PubMed] [Google Scholar]
- 2.Boak R A, Carpenter C M, Jones N, Kampmeier R H, McCann W S, Warren S L, Williams J., Jr The inadequacy of a single prolonged fever for the treatment of early acute syphilis. Am J Syph Gonor Vener Dis. 1942;26:291–298. [Google Scholar]
- 3.Carreras J, Bartrons R, Grisolia S. Vanadate inhibits 2,3-bisphosphoglycerate dependent phosphoglycerate mutases but does not affect the 2,3-bisphosphoglycerate independent phosphoglycerate mutases. Biochem Biophys Res Commun. 1980;96:1267–1273. doi: 10.1016/0006-291x(80)90088-1. [DOI] [PubMed] [Google Scholar]
- 4.Carreras J, Mezquita J, Bosch J, Bartrons R, Pons G. Phylogeny and ontogeny of the phosphoglycerate mutases IV. Distribution of glycerate-2,3-P2 dependent and independent phosphoglycerate mutases in algae, fungi, plants and animals. Comp Biochem Physiol. 1982;71B:591–597. doi: 10.1016/0305-0491(82)90467-9. [DOI] [PubMed] [Google Scholar]
- 5.Carroll J A, Gherardini F C. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect Immun. 1996;64:392–398. doi: 10.1128/iai.64.2.392-398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chander M, Setlow B, Setlow P. The enzymatic activity of phosphoglycerate mutase from gram-positive endospore-forming bacteria requires Mn2+ and is pH sensitive. Can J Microbiol. 1998;44:759–767. doi: 10.1139/cjm-44-8-759. [DOI] [PubMed] [Google Scholar]
- 7.Chander M, Setlow P, Lamani E, Jedrzejas M J. Structural studies on a 2,3-diphosphoglycerate independent phosphoglycerate mutase from Bacillus stearothermophilus. J Struct Biol. 1999;126:156–165. doi: 10.1006/jsbi.1999.4112. [DOI] [PubMed] [Google Scholar]
- 8.Das R, Hegyi H, Gerstein M. Genome analysis of spirochetes: a study of the protein structures, functions and metabolic pathways in Treponema pallidum and Borrelia burgdorferi. J Mol Microbiol Biotechnol. 2000;2:387–392. [PubMed] [Google Scholar]
- 9.Fieldsteel A H, Cox D L, Moeckli R A. Further studies on replication of virulent Treponema pallidum in tissue cultures of Sf1Ep cells. Infect Immun. 1982;35:449–455. doi: 10.1128/iai.35.2.449-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fothergill-Gilmore L A, Watson H C. The phosphoglycerate mutases. Adv Enzymol Relat Areas Mol Biol. 1989;62:227–313. doi: 10.1002/9780470123089.ch6. [DOI] [PubMed] [Google Scholar]
- 11.Fraenkel D G. Glycolysis. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 189–198. [Google Scholar]
- 12.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McCleod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith H O, Venter C J. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–378. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 13.Fraser H I, Kvaratskhelia M, White M F. The two analogous phosphoglycerate mutases of Escherichia coli. FEBS Lett. 1999;455:344–348. doi: 10.1016/s0014-5793(99)00910-2. [DOI] [PubMed] [Google Scholar]
- 14.Hardham J M, Stamm L V, Porcella S F, Frye J G, Barnes N Y, Howell J K, Mueller S L, Radolf J D, Weinstock G M, Norris S J. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene. 1997;197:47–64. doi: 10.1016/s0378-1119(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 15.Houdas Y, Ring E F J. Human body temperature. New York, N.Y: Plenum Press; 1982. [Google Scholar]
- 16.Keen C L, Lonnerdal B, Hurley L S. Manganese. In: Frieden I, editor. Biochemistry of the essential ultratrace elements. New York, N.Y: Plenum Press; 1984. pp. 89–132. [Google Scholar]
- 17.Kuhn N J, Setlow B, Setlow P. Manganese(II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: pH-sensitive interconversion of active and inactive forms. Arch Biochem Biophys. 1993;306:342–349. doi: 10.1006/abbi.1993.1521. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist S, Craig E A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 20.Ma J F, Ochsner U A, Klotz M G, Nanayakkara V K, Howell M L, Johnson Z, Posey J E, Vasil M L, Monaco J J, Hassett D J. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3730–3742. doi: 10.1128/jb.181.12.3730-3742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 22.Miller J N. Potential for vaccines for venereal diseases. Bull N Y Acad Med. 1976;52:986–1004. [Google Scholar]
- 23.Miller J N, Whang S J, Fazzan F P. Studies on immunity in experimental syphilis. Br J Vener Dis. 1963;39:195–198. doi: 10.1136/sti.39.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuuchi K, Fukasawa T. Chromosome mobilization in rec-merodiploids of Escherichia coli K12 following infection with bacteriophage lambda. Virology. 1969;39:467–481. doi: 10.1016/0042-6822(69)90095-6. [DOI] [PubMed] [Google Scholar]
- 25.Narberhaus F. Negative regulation of bacterial heat shock genes. Mol Microbiol. 1999;31:1–8. doi: 10.1046/j.1365-2958.1999.01166.x. [DOI] [PubMed] [Google Scholar]
- 26.Norris S J. Syphilis. In: Wright D J, editor. Immunology of sexually transmitted diseases. Boston, Mass: Kluwer Academic; 1988. [Google Scholar]
- 27.O'Leary P, Bruetsch W L, Ebaugh F G, Simpson W M, Solomon H C, Warren S L, Vonderlehr R A, Usilton L J, Sollins I V. Malaria and artificial fever in the treatment of paresis. JAMA. 1940;115:677–681. [Google Scholar]
- 28.Posey J E, Hardham J M, Norris S J, Gherardini F C. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci USA. 1999;96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabin O, Hegedus L, Bourre J M, Smith Q. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61:509–517. doi: 10.1111/j.1471-4159.1993.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 30.Sell S, Norris S J. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- 31.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 32.Singh R P, Setlow P. Purification and properties of phosphoglycerate phosphomutase from spores and cells of Bacillus megaterium. J Bacteriol. 1979;137:1024–1027. doi: 10.1128/jb.137.2.1024-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamm L V, Gherardini F C, Parrish E A, Moomaw C R. Heat shock response of spirochetes. Infect Immun. 1991;59:1572–1575. doi: 10.1128/iai.59.4.1572-1575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tayarani I, Cloez I, Clement M, Bourre J M. Antioxidant enzymes and related trace elements in aging brain capillaries and choroid plexus. J Neurochem. 1989;53:817–824. doi: 10.1111/j.1471-4159.1989.tb11778.x. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner T B, Hollander D H. Biology of treponematoses. WHO Monogr Ser. 1957;35:73–82. [PubMed] [Google Scholar]
- 37.U.S. Department of Health, Education and Welfare. Syphilis. A synopsis. U.S. Washington, D.C.: Department of Health, Education, and Welfare; 1968. [Google Scholar]
- 38.Watabe K, Freese E. Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis. J Bacteriol. 1979;137:773–778. doi: 10.1128/jb.137.2.773-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]