Abstract
Under starvation conditions, Myxococcus xanthus undergoes a complex developmental process which includes cellular aggregation and sporulation. A transposon insertion mutant (the Tn5-Ω280 mutant) with defects in both aggregation and sporulation was analyzed in this study. The Tn5-Ω280 mutant was found to have a disrupted NtrC-like response regulator designated Myxococcus regulatory protein B (mrpB). Further sequencing analyses revealed a histidine kinase homolog (mrpA) immediately upstream of mrpB and a cyclic AMP receptor protein-like transcriptional regulator (mrpC) downstream of mrpB. In-frame deletion analyses revealed that both the mrpB and mrpC genes were required for cellular aggregation and sporulation but that only mrpA was required for sporulation only. Site-specific mutagenesis of the putative phosphorylation site of MrpB, D58, showed that a D58A mutation caused defects in both aggregation and sporulation but that a D58E mutation resulted in only a sporulation defect. Further genetic and molecular analyses with reporter genes and reverse transcription-PCR indicated that mrpA and mrpB are cotranscribed but that mrpC is transcribed independently and that all of these genes are developmentally regulated. In addition, MrpB is essential for transcription of mrpC and MrpC regulates its own transcription. These data indicate that Mrp proteins are important components required for M. xanthus development. The complicated interaction between Mrp proteins may play an important role in regulating developmental gene expression in M. xanthus.
Myxococcus xanthus is a unique gram-negative bacterium with a complex life cycle. It has a conventional unicellular lifestyle during vegetative growth; however, when nutrients are depleted, it undergoes a complicated developmental program. In response to initial starvation, approximately 100,000 cells aggregate to form a multicellular structure called the fruiting body (for reviews, see references 11 and 38). If starvation continues, cells inside the fruiting bodies develop into spores that are resistant to prolonged periods of starvation, desiccation, and high temperature. Spores germinate when nutrients become available again (12).
This developmental process triggered by starvation involves a complex program of gene regulation. A survey using the promoter probe Tn5lac has identified 36 genetic loci that specifically increase β-galactosidase expression at a particular time during development. The expression times range from minutes after starvation to 24 h, when sporulation begins (25), indicating the complexity of gene expression during development.
Understanding how these developmental genes are regulated has been one of the central themes for the biology of myxobacteria. Through extensive genetic and biochemical studies, a number of regulatory proteins thought to be involved in cellular aggregation and sporulation, including two-component proteins, such as histidine kinases and response regulators, serine threonine kinases, sigma factors and their associated transcriptional factors, and other proteins with no homology to known proteins, have been identified (2, 7, 8, 9, 10, 13, 14, 33, 42, 47). In this study we have focused on a putative ς54 activator that may play an important role in M. xanthus developmental gene regulation.
Bacteria typically use more than one sigma factor. ς70 is the housekeeping sigma factor that transcribes most genes. Alternative sigma factors are often used for specialized cellular functions. For example, in Escherichia coli, Caulobacter crescentus, and Pseudomonas aeruginosa, the ς54 family of sigma factors is involved in transcribing genes related to nitrogen utilization and flagellum or pilus biosynthesis (27, 29). Unlike the ς70 family of sigma factors, the initiation of ς54-dependent transcription requires activator proteins to open the sigma factor-promoter complex. These activator proteins are often connected to a sensory circuit, and ς54-dependent transcription is often positively regulated in response to signals from the environment (39). ς54 has been found in M. xanthus (23). Unlike all other organisms, ς54 appears to be essential for growth of M. xanthus. Nevertheless, ς54 has been suggested to play an important role in the development of M. xanthus, as several developmental genes of M. xanthus are under the control of ς54 (14, 22, 36, 46). Furthermore, 13 putative ς54 activator genes have been isolated from M. xanthus using degenerate PCR probes (21). Targeted mutagenesis of these putative ς54 activator proteins has demonstrated that at least three of them are required for normal development (16). In this study, we report a new putative ς54 activator gene, mrpB, that is required for M. xanthus development. Our genetic data suggest that phosphorylation of MrpB is essential for cellular aggregation and that dephosphorylation is required for sporulation. We have also identified a histidine kinase, MrpA, and a cyclic AMP receptor protein (CRP) family transcription activator, MrpC, that may interact with MrpB and play important roles in the development of M. xanthus.
MATERIALS AND METHODS
Bacterial strains, phage, plasmids, and culture conditions.
The M. xanthus strains and plasmids used in this study are listed in Table 1. E. coli XL1-Blue (Stratagene) was used for DNA manipulation. Myxophage Mx4 was used for generalized transduction as described previously (4). M. xanthus strains were grown vegetatively in Casitone yeast extract (CYE) medium containing 1% (wt/vol) Casitone, 0.5% yeast extract, 10 mM MOPS (morpholinepropanesulfonic acid; pH 7.6), and 8 mM MgSO4 (4). Kanamycin (100 μg/ml) was added when needed. Development of M. xanthus was initiated by placing 20 μl of a suspension of 5 × 109 cells/ml (optical density at 600 nm, 10) on MOPS plates (1.5% agar plates containing 10 mM MOPS [pH 7.6] and 8 mM MgSO4), or CF plates (1.5% agar plates containing 10 mM MOPS [pH 7.6], 8 mM MgSO4, 0.015% Casitone, 1 mM KH2PO4, 2% sodium citrate, and 1% pyruvate) (17). Liquid cultures were incubated at 32°C with shaking at 250 rpm. Agar plates were incubated at 32°C. Development of M. xanthus in submerged cultures was carried out as described previously (26).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Reference or source |
|---|---|---|
| Strains | ||
| M. xanthus | ||
| DK1622 | Wild type | 19 |
| SW280 | mrpB::Tn5-Ω280 pilQ | 15 |
| SW2800 | mrpB::Tn5-Ω280 | This study |
| SW2801 | mrpB-lacZ201 MrpB+ | This study |
| SW2802 | ΔmrpB | This study |
| SW2803 | mrpC-lacZ202 mrpC+ | This study |
| SW2804 | mrpC-lacZ202 mrpC+ ΔmrpB | This study |
| SW2806 | mrpB-lacZ203 mrpB | This study |
| SW2807 | ΔmrpA | This study |
| SW2808 | ΔmrpC | This study |
| SW2811 | mrpB-lac201 MrpB+ ΔmrpC | This study |
| SW2841 | mrpB D58E | This study |
| SW2844 | mrpB D58A | This study |
| E. coli XL1-Blue | Host for cloning | Stratagene |
| Plasmids | ||
| pUC18 | Cloning vector, Ampr | Gibco BRL |
| pBlueScript KS | Cloning vector, Ampr | Stratagene |
| pBJ113 | In-frame deletion vector, Kanr Gals | B. Julien and D. Kaiser |
| pKY481 | lacZ fusion vector, Kanr | 7 |
| pSH112 | pUC18 carrying a 1.66-kb wild-type mrpB, template for site-directed mutagenesis | This study |
| pSH113 | pBJ113 carrying an mrpB D58E mutation | This study |
| pSH114 | pBJ113 carrying an mrpB D58A mutation | This study |
| pSH201 | pKY481 carrying an mrpB-lac-201 translation fusion | This study |
| pSH202 | pKY481 carrying an mrpC-lac-202 translation fusion | This study |
| pSH203 | pKY481 carrying a mrpB-lac-203 translation fusion | This study |
| pSH280 | pUC18 carrying a 26-kb DNA from SW2800 | This study |
| pSH401 | pBJ113 carrying the deleted mrpB | This study |
| pSH402 | pBlueScript carrying wild-type mrpA | This study |
| pSH403 | pBJ113 carrying the deleted mrpA | This study |
| pSH404 | pUC18 carrying wild-type mrpC | This study |
| pSH405 | pBJ113 carrying the deleted mrpC | This study |
DNA and RNA manipulations and sequence analysis.
DNA manipulations were performed using standard protocols (37). Oligonucleotides were purchased from Gibco BRL Life Technologies. Taq DNA polymerase (Promega) or Pfu polymerase (Stratagene) was used in PCRs. All restriction enzymes used were purchased from Promega. Total RNA was isolated using a Qiagen RNeasy kit. Reverse transcription (RT) was performed using SuperScript II RNase H reverse transcriptase (Gibco BRL Life Technologies). DNA sequencing was carried out at the sequencing facility of the University of California, Los Angeles. Sequence analysis was performed and presented with the BLAST (1), DNAman (Lynnon BioSoft), and Boxshade programs.
Plasmid construction.
The Tn5 insertion in SW2800 was used as a selectable marker (kanamycin resistance) for mrp cloning. Because Tn5 does not have an internal EcoRI site, SW2800 genomic DNA was digested with EcoRI and cloned into pUC18, generating pSH280.
The lacZ fusion plasmids were constructed using the vector pKY481, courtesy of Kunyung Cho and David Zusman (8). pSH201 is a derivative of pKY481 carrying a translational fusion between the last codon of mrpB and codon 8 of lacZ. The fusion was first created in vitro by cloning a 1.5-kb PCR fragment, containing mrpB from the first codon to the last sense codon, into the EcoRI and BamHI sites of pKY481. The PCR fragment was amplified using two oligonucleotides, 5′-AGAATTCATGGAGACCCTTCTCATCG-3′ and 5′-ATGGATCCAACGCATCCTTCACAGG-3′, as primers and pSH280 as the template. pSH203 is a second mrpB-lacZ fusion construct in which lacZ is fused after codon 7 of mrpB. As with pSH201, the fusion was first created in vitro by cloning a 657-bp PCR fragment, containing the region between 637 bp upstream and 20 bp downstream from the mrpC translation start codon, into the EcoRI and BamHI sites of pKY481. The PCR fragment was amplified using two oligonucleotides, 5′-AGAATTCCTCCTCGCTCAGCCA-3′ and 5′-ATGGATCCACGATGAGAAGGGTCTC-3′, as primers and pSH280 as the template. pSH202 is an mrpC-lacZ construction in which lacZ is fused after codon 97 of mrpC. Similarly, the fusion was first created in vitro by cloning a 666-bp PCR fragment, containing a region between 364 bp upstream and 303 bp downstream from the mrpC translation start codon, into the EcoRI and BamHI sites of pKY481. The PCR fragment was amplified using two oligonucleotides, 5′-AGAATCCCCTGGAGCGCAAGCTCC-3′ and 5′-ATGGATCCAGCTCGCCGAAGAGGTC-3′, as primers and pSH280 as the template.
Construction of mrp in-frame deletion mutants.
The mrp in-frame deletion mutants were constructed by gene replacement using the positive-negative KG cassettes described previously (43). The vector containing the kanamycin-galactose (KG) cassettes, pBJ113, was provided through the courtesy of Bryan Julien and Dale Kaiser at Stanford University.
All three mrp in-frame deletion mutants were constructed using internal restriction sites that gave in-frame deletions (Fig. 1A). For the mrpA in-frame deletion, a 1,345-bp PCR fragment containing the mrpA open reading frame (ORF), including 141 bp upstream and 179 bp downstream, was amplified using two oligonucleotides, 5′-TGAATTCGAGCACCACGGCATG-3′ and 5′-TTGGATCCGAGGATGACCACGCTG-3′ (corresponding to nucleotides 622 to 1967). The PCR product was digested with EcoRI and BamHI and cloned into pBluescript KS to generate pSH402. pSH402 was digested with NarI and religated, with an internal 543-bp fragment of mrpA being deleted. The EcoRI and BamHI fragment containing the mrpA gene with the deletion was cloned into pBJ113, generating pSH403. For the mrpB in-frame deletion, a 1,854-bp PCR fragment containing the mrpB partial ORF, from 824 bp upstream to 1,031 bp into the ORF, was amplified using two oligonucleotides: 5′-TGAATTCCGTGAGCTGGACGCCC-3′ and 5′-TGGATCCGCTTGTGGACCTTCTCG-3′ (corresponding to nucleotides 972 to 2826). The PCR fragment was digested with SalI, removing an internal 606-bp region within the mrpB ORF, religated, digested with EcoRI and BamHI, and cloned into pBJ113, generating pSH401. For the mrpC in-frame deletion, a 1,573-bp PCR fragment containing the mrpC ORF, including 336 bp upstream and 492 bp downstream, was amplified using two oligonucleotides: 5′-TGAATTCTTGCACAGAGCCAGAG-3′ and 5′-TGGATCCGCTGTACTGGAAGGGGA-3′ (corresponding to nucleotides 3214 to 4787). The PCR fragment was cloned into pUC18 using EcoRI and BamHI, generating pSH404. pSH404 was digested with EagI and religated, deleting an internal 468-bp fragment of mrpC. The EcoRI and BamHI fragment, containing the mrpC gene with the deletion, was cloned into pBJ113, generating pSH405.
FIG. 1.
Gene structure of the mrp locus. (A) Physical map of the mrpABC locus and deletion mutant construction. (B) Predicted ribosome binding sites (underlined) and termination codons (asterisks under and lines above the sequence) of the mrpA, mrpB, and mrpC genes. The predicted initiation codons are highlighted in boldface. (C) Detection of the mrpAB transcript by RT-PCR. Two primers between mrpA and mrpB were used to amplify the PCR product, with cDNA (lane 2) or total RNA that was used for the cDNA synthesis (lane 1) as templates. The cDNA was reverse transcribed from the random oligohexamers, using total RNA as the template. Lane L, 100-bp DNA ladder (New England Biolabs).
The in-frame deletion constructs, pSH403, pSH401 and pSH405, were transferred by electroporation into M. xanthus as previously described (20). Chromosomal integration was selected for by plating the cells onto CYE plates containing 100 μg of kanamycin per ml (positive selection). None of the plasmids used in this study could replicate in M. xanthus; thus, all transformants that were resistant to kanamycin were the result of recombination of the plasmid into the chromosome. Individual Kanr colonies were diluted and plated onto CYE plates containing 1% galactose for negative selection. Southern blot analysis was used to screen the kanamycin-sensitive, galactose-resistant colonies for proper excision of the wild-type copy.
Construction of mrpB point mutants.
The mrpB point mutants were created by gene replacement using the positive-negative KG cassettes (43), i.e., a copy of the mrpB gene with a nucleotide change was used to replace the wild-type copy. For the D58E mutation, the codon GAC for aspartic acid was replaced with GAG, a codon for glutamic acid. This change also created a new XhoI restriction site, CTCGAG. The two complementary primers for D58E mutagenesis were 5′-C AGC GTG GTC ATC CTC GAG ATG ATG CTC CCG GAC CGC-3′ and 5′-GCG GTC CGG GAG CAT CAT CTC GAG GAT GAC CAC GCT G-3′. For the D58A mutation, the codon GAC for aspartic acid was replaced with GCC, a codon for alanine. In addition, a silent mutation was also introduced to L57 by changing CTC into CTG. This way, a new MscI restriction site was created: TG GCC A. The two complementary primers for D58A mutagenesis were 5′-C AGC GTG GTC ATC CTG GCC ATG ATG CTC CCG GAC CGC-3′ and 5′-GCG GTC CGG GAG CAT CAT GGC CAG GAT GAC CAC GCT G-3′.
A 1.66-kb PCR fragment containing the target site in the middle was amplified using two oligonucleotides, 5′-AGAATTCCTCCTCGCTCAGCCA-3′ and 5′-TGGATCCGCTTGTGGACCTTCTCG-3′. The PCR product was digested with EcoRI and BamHI and cloned into pUC18 to generate pSH112. Klenow fragment extension was then performed (30 cycles in a thermocycler) using pSH112 as the template and a pair of mutagenesis oligonucleotides. After DpnI digestion to remove the template, the extension product was used to transform E. coli. The transformed colonies were screened for the newly created restriction site, and the mutated version of the gene was then cloned into pBJ113, generating pSH113 for the D58E mutation and pSH114 for the D58A mutation.
The site-directed mutation constructs, pSH113 and pSH114, were transferred by electroporation into M. xanthus as previously described (20). Chromosomal integration was determined by kanamycin resistance (positive selection), and removal of the vector backbone was determined by negative selection on a CYE plate containing 1% galactose. Southern blot analyses using XhoI digestion were used to screen for the D58E mutation, and MscI digestion was used to screen for the D58E mutation.
Mutant characterization.
Fruiting bodies and individual cells were observed with a Leica microscope (model DMLS). Images were captured with a SPOT digital camera. At different time points, cells were scraped off the plate for further analysis. β-Galactosidase activity was assayed as described previously (25). Sonication-resistant spores were visually counted using a hemocytometer.
Nucleotide sequence accession number.
The mrpA, mrpB, and mrpC sequences have been deposited in the GenBank DNA sequence database (accession no. AF285263).
RESULTS
A new genetic locus required for cellular aggregation and sporulation.
SW280 is a Tn5 insertion mutant in the DZF1 background (31). The mutant does not aggregate or sporulate on CF plates. In this study, the Tn5 insertion was transduced via the Mx4 phage into wild-type DK1622 to generate SW2800. Like its parent strain, SW280, SW2800 failed to form cellular aggregates (data not shown) and produced no spores after 2 days on MOPS starvation agar. More than 30 isogenic transductants were obtained, and all of them had the same phenotype as SW2800, suggesting that the Tn5 insertion was responsible for the mutant phenotype. When mixed with an equal number of wild-type cells, the mutants did not form any kanamycin-resistant spores (data not shown), indicating that this mutant phenotype was not caused by lack of production of extracellular signals.
The region containing the Tn5 insertion was cloned into pUC18 based on the kanamycin resistance gene that Tn5 carries. The resulting plasmid, pSH280, contained a 28-kb fragment of SW2800 genomic DNA. pSH280 was further subcloned, and the DNA flanking Tn5 was sequenced. The DNA sequence revealed that the transposon insertion was located in an ORF designated mrpB (Myxococcus regulatory protein B) (Fig. 1A). We also sequenced 2 kb upstream and 4 kb downstream of mrpB. We identified one ORF immediately upstream of mrpB and named it mrpA and another ORF downstream of mrpB and named it mrpC. The mrpA and mrpB genes appear to be in an operon since there are only 7 nucleotides between the stop codon of mrpA and the start codon of mrpB (Fig. 1B). A stem-loop structure and an A/T-rich region were found downstream of the mrpB stop codon, suggesting that mrpB may be the last gene of this operon. mrpC appears to be in a single-gene operon, as the ORF downstream of mrpC (a protein homologous to phenylalanine dehydrogenase) is predicted to be transcribed convergently. The phenylalanine dehydrogenase homolog is not discussed in this paper.
We performed RT-PCR to investigate the proposed transcriptional organization of the mrp locus. Total RNA was isolated from M. xanthus cells that had developed in submerged cultures for 20 h. cDNA was prepared using random hexamers. We then determined by PCR whether this cDNA encoded the mrpA and mrpB sequences using two oligonucleotides: 5′-AGGATAACGGTTCGCTC-3′, which is complementary to mrpA, and 5′-ACGATGAGAAGGGTCTC-3′, which is complementary to mrpB. The two oligonucleotides were able to produce a 191-bp PCR product spanning the two ORFs (nucleotides 1625 to 1815) (Fig. 1A and C). This indicates that mrpA and mrpB are indeed cotranscribed, consistent with the results of sequence analysis. We also examined whether a PCR product spanning the mrpB and mrpC ORFs could be obtained from the cDNA using two oligonucleotides, 5′-TCTTGCACAGAGCCAGAG-3′ and 5′-GACGTTGGAACCGATGG-3′ (corresponding to nucleotides 3214 to 3231 and nucleotides 3578 to 3594, respectively) (Fig. 1A). They were unable to produce any PCR product from the same cDNA (data not shown). This indicates that mrpB and mrpC are not cotranscribed, a finding also consistent with the results of sequence analysis.
Sequence analyses of mrpA, mrpB, and mrpC.
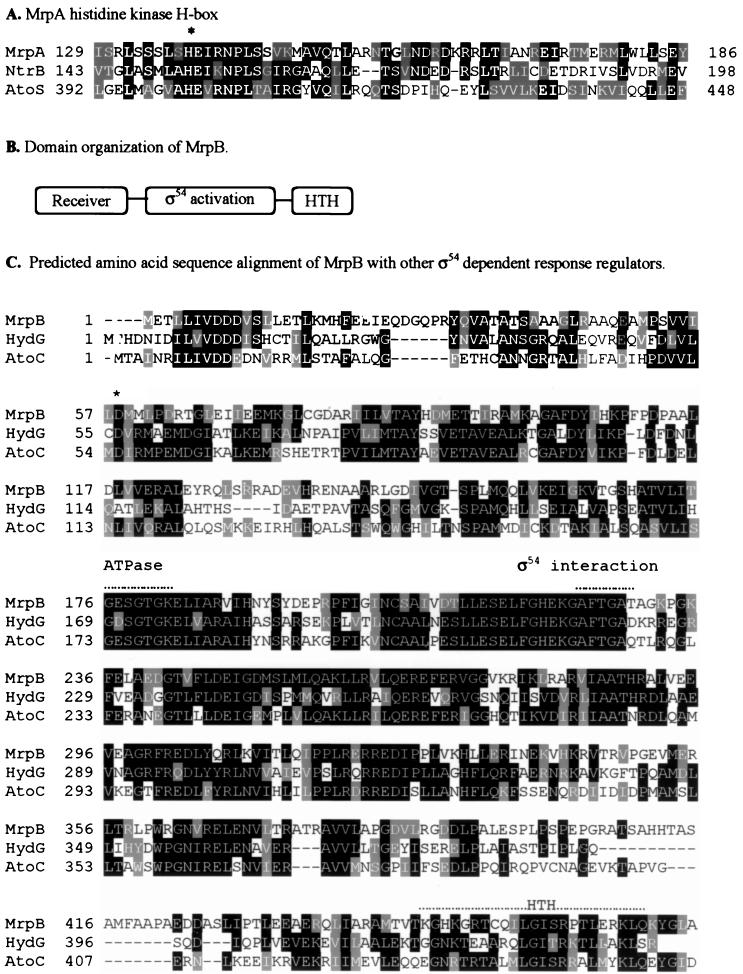
The mrp genes are predicted to encode regulatory proteins based on sequence analysis. The deduced mrpA gene product, MrpA, contains 341 amino acids. The deduced amino acid sequence of MrpA was subjected to a BLAST search (1). The MrpA C-terminal kinase domain (amino acids 129 to 261) is about 25% identical to a group of histidine protein kinases (Fig. 2A) (5, 41). These histidine protein kinases are the sensors of the two-component signal transduction systems. The kinase domain of MrpA contains a conserved N-terminal H box that contains the potential phosphorylation site H138. A similar histidine residue has been determined to be the phosphorylation site in the histidine protein kinase family (18, 32, 35). The MrpA C-terminal kinase domain (amino acids 245 to 321) also contains the nucleotide binding regions, termed the N, F, and G boxes (34), which are highly conserved in all the histidine protein kinases (data not shown). The deduced amino acid sequence in the N-terminal portion of MrpA yielded no significant similarities to protein sequences in the GenBank database. However, previous analyses have shown that the N-terminal regions of histidine kinases are not conserved (34). This lack of conservation reflects the diverse function of the N-terminal domain as input domains that change the activity of each protein in response to a specific signal. Further analysis of the N-terminal region using the TMpred program revealed no significant transmembrane domains, suggesting that MrpA may localize in the cytoplasmic compartment of M. xanthus cells.
FIG. 2.
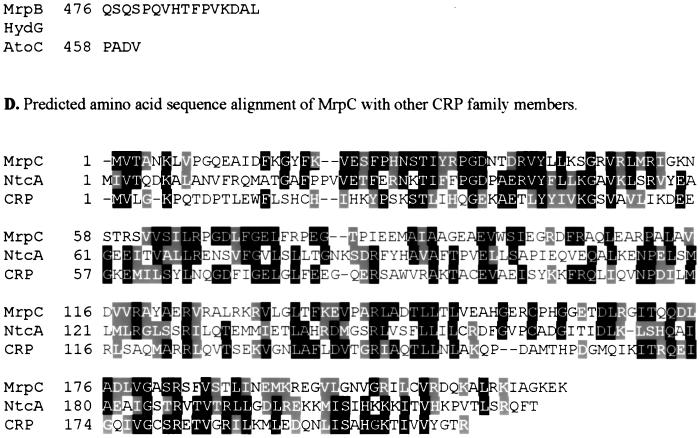
Sequence analyses of the mrp genes. Identical residues have a black background, and homologous residues have a gray background. (A) Sequence alignment of MrpA (∼126 to ∼256 amino acids) with the histidine kinase domains of NtrB from Sinorhizobium meliloti (accession no. Q52977) and AtoS from E. coli (accession no. Q06067). ∗, putative autophosphorylation site. (B) Domain organization of the deduced product of mrpB. MrpB consists of a receiver domain (amino acid residues 3 to 119), a ς54 activation domain (amino acid residues 148 to 375), and an HTH DNA binding domain (amino acid residues 443 to 460). (C) Predicted amino acid sequence alignment of MrpB with HydG (accession no. P14375) and AtoC (accession no. Q06065), both from E. coli. ∗ indicates the putative conserved phosphorylation site. The ATPase motif, the ς54 interaction motif, and the HTH DNA binding motif are indicated by dotted lines above the sequence. (D) Predicted amino acid sequence alignment of MrpC with NtcA from Anabaena variabilis (accession no. Q05061) and CRP from E. coli (accession no. P03020).
The predicted mrpB product has 491 amino acids. The deduced amino acid sequence of mrpB was subjected to a BLAST search, and good correlation was found with ς54 activator sequences. Over 470 residues, MrpB is 40% identical to the E. coli acetoacetate metabolism regulatory protein AtoC (5), 39% identical to the E. coli hydrogenase G transcriptional regulatory protein HydG (3), and 38% identical to the nitrogen regulatory protein NtrC from Azospirillum brasilense (28) (Fig. 2C). MrpB possesses all important conserved domains and amino acid residues present in other ς54 transcriptional activators (Fig. 2B) (30), including an aspartate at position 58 that is analogous to D54, which is phosporylated in NtrC. MrpB has a receiver domain at the N terminus that is made up of alternating alpha helices and beta sheets. In this domain there are also three aspartic acid residues (D8 to D10) and a lysine (K108) that may constitute the regulatory site of the response regulator protein family (Fig. 2C) (40). The central domain has the proposed ATP-binding motif (GESGTGK) found in several ATP-binding proteins and the conserved motif GAFTGA, which has been proposed as the site of interaction with ς54 (45). The C-terminal region contains a helix-turn-helix (HTH) DNA binding motif. In the mutant SW2800, Tn5 was inserted between the ATPase domain and the HTH DNA binding domain. Notably, MrpB does not identify with any of the putative M. xanthus ς54 activator proteins that have been previously reported (6, 21).
There are three possible translation start sites for the mrpC gene, at nucleotides 3550, 3625, and 3628, corresponding to products of 248, 223, and 222 amino acids, respectively (GenBank accession no. AF285263). The most likely translation start site appears to be at nucleotide 3550, for it is preceded (8 nucleotides upstream) by the Shine-Dalgarno-like sequence 5′-AGGAG-3′ (Fig. 1B). The deduced amino acid sequence of MrpC was subjected to a BLAST search. High sequence similarity was found between MrpC and members of the CRP/FNR (the fumarate and nitrate reduction regulator) family of regulators. Strongest homologies were found with NtcA from Synechococcus sp. (PCC 7942, 26% identical) (44) and CRP from E. coli (23% identical) (3) over 184 residues (Fig. 2D).
In-frame deletion analysis of the mrp locus.
Since MrpA and MrpB are encoded by adjacent genes, it is likely that these proteins interact and are components of the same two-component signal transduction pathway. MrpC, as a homolog of a global transcriptional regulator, may also be an important regulator in M. xanthus. To study the role of each gene in the development of Myxococcus, in-frame deletion mutants for each of the mrp genes were created (Fig. 1A). The in-frame deletion mutants were constructed using a markerless deletion method, as described in Materials and Methods. Briefly, plasmids containing the version of the target gene with the deletion were introduced into the Myxococcus genome through electroporation and selection for kanamycin resistance. The kanamycin-resistant cells were then subjected to galactose counterselection for looping out of the vector backbone containing the galK gene. Southern blot analysis was then used to screen for the colonies that had the version of the target gene with the deletion (data not shown).
In SW2807 (also herein called the ΔmrpA mutant), nucleotides 1130 to 1673 (codons 105 to 305) of mrpA were removed (Fig. 1A). This region contains the putative conserved histidine residue (H138) of the autophosphorylation site. In SW2802 (also herein called the ΔmrpB mutant), nucleotides 1814 to 2420 (codons 9 to 210) in mrpB, including the predicted conserved aspartate residue (D58) in the receiver domain and part of the conserved ς54-interacting domain that is essential for transcriptional-enhancer activity (30), were deleted (Fig. 1A). In SW2808 (also herein called the ΔmrpC mutant), nucleotides 3765 to 4233 (codons 74 to 229) of mrpC were deleted, removing 63% of the MrpC protein (Fig. 1A).
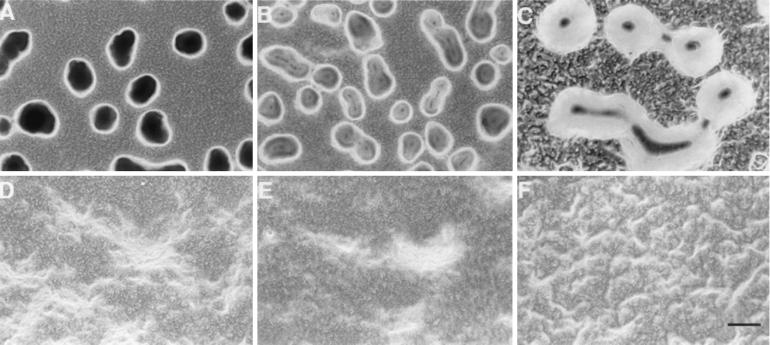
These deletion mutants were plated on development plates to study their developmental phenotypes (Fig. 3). The ΔmrpB mutant (SW2802) and the ΔmrpC mutant (SW2808), like the original transposon mutant, failed to form cellular aggregates or to sporulate (Fig. 3B and D), even after 2 weeks of incubation on MOPS starvation agar or CF agar that contains a small amount of nutrients. The nonaggregating, nonfruiting phenotype remained the same for the mutant cells that were spotted at different cell densities: 109 and 1010 cells/ml. The mutant cells were completely blocked at the very early stages of aggregation, demonstrating that mrpB and mrpC are required for both cellular aggregation and sporulation. Interestingly, the ΔmrpA mutant (SW2807) was able to form translucent mounds (Fig. 3C), which is a typical phenotype of cells known to engage in normal cellular aggregation but which are defective in sporulation (31). Spore counting confirmed that the ΔmrpA mutant was delayed in sporulation. After 3 days on MOPS starvation plates, SW2807 formed only 10% of the number of spores formed by the wild-type strain. After 7 days, SW2807 formed 30% of the number of spores formed by the wild type. After 13 days, SW2807 formed 75% of the number of spores formed by the wild type. These results suggest that mrpB and mrpC are essential for M. xanthus aggregation and sporulation but that mrpA is required only for sporulation. The discrepancy between the phenotype of the ΔmrpA mutant and the ΔmrpB mutant indicates that MrpA and MrpB may not constitute a simple two-component system, although these two-component homologs are located adjacent to each other.
FIG. 3.
Developmental phenotypes of the mrp mutants. Cells were deposited on MOPS plates for 2 days. Fruiting body development was examined under a light microscope with a 10× objective lens and photographed using a digital camera. (A) DK1622 (wild type); (B) SW2802 (ΔmrpB mutant); (C) SW2807 (ΔmrpA mutant); (D) SW2808 (ΔmrpC mutant); (E) SW2841 (mrpB D58E mutant); (F) SW2844 (mrpB D58A mutant). Bar, 50 μm.
Analysis of an MrpB putative phosphorylation site by site-directed mutagenesis.
To further understand the physiological function of Mrp proteins and the possible interactions between them, we performed more-detailed genetic analyses of MrpB. As described above, D58 of MrpB is most likely the phosphorylation site for the response regulator. Based on a previous study of NtrC, a D-to-A mutation in that site would abolish the function of a response regulator whereas a D-to-E mutation may mimic the phosphorylated status of a response regulator for constitutive activity (24). We carried out site-specific mutagenesis to investigate the function of MrpB. Two mrpB point mutants were constructed in this study (Materials and Methods). SW2844 contains a D58A mutation and SW2841 contains a D58E mutation. As shown in Fig. 3, SW2844 shows the same phenotype as the ΔmrpB mutant, i.e., it is completely blocked at a very early stage of aggregation, indicating that the phosphorylation of MrpB is essential for these cellular functions. Interestingly, SW2841 is able to aggregate but is defective in sporulation. After 7 days on MOPS starvation plates, SW2841 formed only 0.5% of the number of spores formed by the wild-type strain. It is noteworthy that SW2841 formed cellular aggregates that were larger than aggregates of the wild-type cells (Fig. 3).
Expression of mrpAB and mrpC during development.
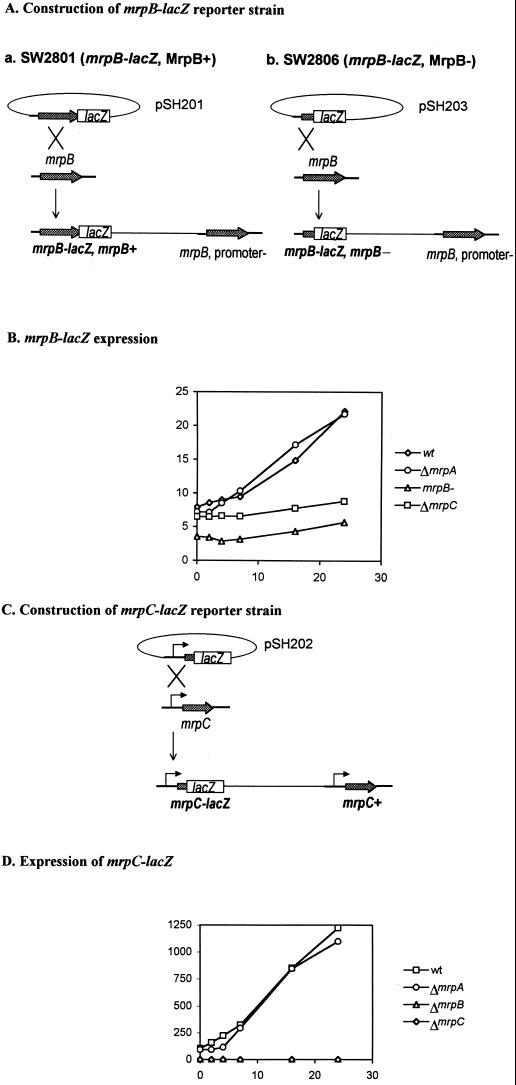
To study the expression of mrpAB and mrpC during development, we constructed the lacZ reporter strains SW2801 and SW2803, respectively. The lacZ gene was fused to mrp genes in vitro as plasmids as described in Materials and Methods. The plasmids were then introduced into M. xanthus (DK1622) through electroporation, and positive clones were selected for by kanamycin resistance and Southern blot analysis. pSH201 contains an mrpB-lacZ translational fusion in which lacZ is fused at the very end of the mrpB C terminus (Fig. 4Aa). After recombination, the resulting strain, SW2801, is able to develop normally (data not shown), indicating that the full-length MrpB protein remains functional although it is a fusion protein. Because mrpA and mrpB are cotranscribed, mrpB-lacZ expression stands for the expression level of this mrpAB operon. pSH202 contains an mrpC-lacZ translational fusion. It includes 364 bp upstream of the mrpC ORF, most likely containing the mrpC promoter region. After recombination into wild-type M. xanthus, the resulting strain, SW2803, retained a wild-type copy of mrpC with its own promoter in addition to the lacZ reporter fusion in mrpC (Fig. 4C). As predicted, SW2803 showed no defects in development (data not shown).
FIG. 4.
Expression of mrp-lacZ during development. (A) Construction of the mrpB–lac-201 and mrpB–lac-203 fusions in M. xanthus. (B) Expression of the mrpB-lacZ fusion under developmental conditions in different strain backgrounds. The cells were placed on MOPS starvation agar and incubated at 32°C, harvested at different time points, and frozen. β-Galactosidase activity was measured after all samples were collected. Each point represents the average of values from two experiments, with a standard error of less than 10%. Results with SW2801 (diamonds; MrpB+; wt, wild type), SW2810 (circles), SW2806 (triangles), and SW2811 (squares) are shown. (C) Construction of the mrpC-lacZ fusion in M. xanthus. (D) Expression of the mrpC-lacZ fusion under developmental conditions in different strain backgrounds. The cells were placed on MOPS starvation agar and incubated at 32°C, harvested at different time points, and frozen. β-Galactosidase activity was measured after all samples were collected. Each point represents the average of values from two experiments, with a standard error of less than 10%. Results with SW2803 (diamonds; mrpC+), SW2812 (circles), SW2804 (triangles), and SW2818 (squares) are shown.
Following starvation of the various recombination strains, a β-galactosidase assay was used to determine the expression of mrpAB and mrpC. We found that both mrpB and mrpC were upregulated during development (Fig. 4B and D). The expression of mrpB-lacZ increased steadily after starvation began, increasing over fourfold after 24 h. Similarly, the expression of mrpC-lacZ increased during development, increasing over sixfold after 24 h. These results were confirmed using RNA dot blot and RT-PCR methods (data not shown).
To study the interaction between the Mrp proteins, we examined mrpB-lacZ and mrpC-lacZ expression in each mrp mutation background. pSH201 was introduced into the ΔmrpA mutant (SW2807) and the ΔmrpC mutant (SW2808) through electroporation, generating SW2810 and SW2811. pSH202 was introduced into the ΔmrpA (SW2807), ΔmrpB (SW2802), and ΔmrpC (SW2808) mutants through electroporation, generating SW2812, SW2804, and SW2818. To study whether mrpB is self-regulated, a second mrpB-lacZ (mrpB-lacZ203) fusion was made in which lacZ was fused after the seventh amino acid of MrpB. After recombination, mrpB transcription was disrupted, generating the mrpB mutant SW2806 (Fig. 4A).
The level of mrpB-lacZ expression under starvation conditions was reduced in the ΔmrpC mutant background and even more so in the mrpB-minus background but was unaffected in the ΔmrpA mutant background (Fig. 4B). The level of mrpC-lacZ expression under starvation conditions was abolished in the ΔmrpB and ΔmrpC mutants but was unaffected by the mutation in mrpA (Fig. 4D). These results were also confirmed by RT-PCR analysis (data not shown). Our findings indicate that MrpB self-regulates the expression of mrpB and that it also controls mrpC expression. In addition, MrpC also self-regulates the expression of mrpC and affects mrpB expression.
DISCUSSION
Development of M. xanthus involves a complex gene expression program. Identification of the key regulators is essential for deciphering the developmental process. We report herein the identification of three M. xanthus proteins that are essential for M. xanthus development. They all contain important regulatory motifs. mrpA encodes a histidine kinase, mrpB encodes an NtrC-like response regulator, and mrpC encodes a CRP family transcription activator. They all control functions important for M. xanthus development: both mrpB and mrpC are essential for aggregation and sporulation of M. xanthus, whereas mrpA is required for sporulation. A single amino acid substitution in the MrpB receiver domain dramatically altered the fruiting body formation of M. xanthus. Furthermore, the mrp genes themselves are developmentally regulated and they interact with each other in their gene expression. All these data demonstrated strongly that this locus, mrp, plays an important role in M. xanthus development, even though at this point the exact molecular functions of the three genes during development remain to be further investigated.
Only 7 nucleotides separate the mrpA and mrpB ORFs, the histidine kinase homolog and the response regulator homolog. Based on sequence analysis and gene arrangement, it seems a logical assumption that mrpA and mrpB might be sensor kinase response regulator partners of a two-component system. MrpA may be the histidine kinase that transfers its phosphate group to MrpB. However, whereas the ΔmrpB and the mrpB D58A mutants are defective in both aggregation and sporulation, the ΔmrpA mutant is defective only in sporulation (Fig. 3). Therefore, MrpA cannot be the only kinase that phosphorylates D58 of MrpB. Either there are other histidine kinases or small phosphate donors such as acetylphosphate and phosphoramidine (34) that can phosphorylate MrpB in the ΔmrpA mutant or MrpA has functions other than that of a kinase. Because the mrpB D58E mutant formed fruiting bodies but was defective in sporulation, a phenotype similar to that of the ΔmrpA mutant, and because the D58E replacement mimics the phosphorylated state of the receiver domain, it is possible that MrpA may serve as the phosphatase for MrpB.
mrpC, like mrpB, is also absolutely required for fruiting body formation and sporulation. The ΔmrpC mutant has the same phenotype as the ΔmrpB mutant and the mrpB D58A mutant (Fig. 3). Moreover, mrpC expression is absolutely dependent on mrpB. Based on these data, we speculate that mrpB controls mrpC expression. In the promoter region of mrpC, there is a stretch of sequence, TGG−24CACGnnnnTTG−12G (where n is any base), whose nucleotide sequence, composition, and positioning are characteristic of those of the ς54 promoter −24/−12 box (14, 22, 36, 46), suggesting that mrpC may be transcribed by ς54. Thus, we propose that mrpB may be the ς54 activator for mrpC expression. Since the mrpC deletion has the same phenotype as the mrpB deletion, mrpC may be the major, if not the only, downstream gene that is activated by mrpB. The multilevel control may enable M. xanthus to finely regulate its gene expression.
As discussed above, MrpA may function as a phosphatase for MrpB, and MrpB activates MrpC synthesis. Such an interaction may serve as a mechanism for sensing different levels of starvation and result in the induction of different developmental genes. For instance, initial starvation triggers phosphorylation of MrpB by an unknown histidine kinase. This modification activates MrpB, which promotes cellular aggregation and activates the expression of downstream genes, the most important of which is mrpC. The putative positive feedback loops of MrpB and MrpC would further enhance the expression of mrpB and mrpC (Fig. 4). MrpC, as a transcriptional activator itself, turns on the expression of more developmentally regulated genes. During this process, new evaluation of the environmental stress is somehow channeled in and MrpC becomes activated only when starvation continues. Those genes that are dependent on MrpB and MrpC are required for fruiting body formation. In the absence of either gene, no cellular aggregation occurs. MrpA functions after the fruiting body is formed. If starvation still persists, MrpA, the histidine kinase, may sense the worsening condition and become phosphorylated at the histidine residue. This may enhance its phosphatase activity, which is required for dephosphorylation of MrpB, allowing the cells to proceed to the sporulation stage.
Thus far, our data on the mrpA, mrpB, and mrpC regulators support this hypothetical scenario. The mrp regulator circuit may be the unique mechanism by which M. xanthus cells respond to various levels of starvation during the course of development. Obviously, there are missing links in this scenario, and our future investigations will be aimed toward identifying them. First, protein-DNA binding assays are needed to confirm that MrpB activates mrpC expression. Second, biochemical assays will be performed for testing the functional association between MrpA and MrpB. Third, the putative sensor kinase or some other protein that activates MrpB early in development will be identified through mutagenesis using mrpC-lacZ as a marker for MrpB activity. This may also provide clues to identify the signal(s) that triggers mrp expression. Fourth, the developmental genes that are under the control of mrpB and mrpC will be identified through genetic studies, ideally through gene arrays, and the interaction between mrp genes and other known development regulators or signals will be examined. Finally, mrpB and mrpC seem to be very important regulators of development. If we can control the expression of the mrp genes with inducible promoters, we might be able to manipulate the developmental process. By undertaking the above-described genetic and biochemical studies, we hope to provide further molecular insights into M. xanthus development.
ACKNOWLEDGMENTS
We thank D. R. Zusman, Z. Yang, R. Lux, M. Kempf, Li Chen, and J. Tsai for helpful discussions. We also thank D. R. Zusman, K. Cho, D. Kaiser, B. Julien, and L. Kroos for kindly providing experimental materials. We are grateful to L. Tong for providing excellent technical support. We also thank S. Hunt Gerardo for careful editing of the manuscript.
This work is supported by NIH grant GM54666 to W. Shi.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Apelian D, Inouye S. Development-specific sigma-factor essential for late-stage differentiation of Myxococcus xanthus. Genes Dev. 1990;4:1396–1403. doi: 10.1101/gad.4.8.1396. [DOI] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage Mx4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 5.Canellakis E S, Paterakis A A, Huang S C, Panagiotidis C A, Kyriakidis D A. Identification, cloning, and nucleotide sequencing of the ornithine decarboxylase antizyme gene of Escherichia coli. Proc Natl Acad Sci USA. 1993;90:7129–7133. doi: 10.1073/pnas.90.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho K, Treuner-Lange A, O'Connor K A, Zusman D R. Developmental aggregation of Myxococcus xanthus requires frgA, an frz-related gene. J Bacteriol. 2000;182:6614–6621. doi: 10.1128/jb.182.23.6614-6621.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho K, Zusman D R. AsgD, a new two-component regulator required for A-signalling and nutrient sensing during early development of Myxococcus xanthus. Mol Microbiol. 1999;34:268–281. doi: 10.1046/j.1365-2958.1999.01594.x. [DOI] [PubMed] [Google Scholar]
- 8.Cho K, Zusman D R. Sporulation timing in Myxococcus xanthus is controlled by the espAB locus. Mol Microbiol. 1999;34:714–725. doi: 10.1046/j.1365-2958.1999.01633.x. [DOI] [PubMed] [Google Scholar]
- 9.Crawford E W, Jr, Shimkets L J. The stringent response in Myxococcus xanthus is regulated by SocE and the CsgA C-signaling protein. Genes Dev. 2000;14:483–492. [PMC free article] [PubMed] [Google Scholar]
- 10.Davis J M, Mayor J, Plamann L. A missense mutation in rpoD results in an A-signalling defect in Myxococcus xanthus. Mol Microbiol. 1995;18:943–952. doi: 10.1111/j.1365-2958.1995.18050943.x. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin M, Kaiser D, editors. Myxobacteria II. Washington, D.C.: ASM Press; 1993. [Google Scholar]
- 13.Garza A G, Harris B Z, Pollack J S, Singer M. The asgE locus is required for cell-cell signalling during Myxococcus xanthus development. Mol Microbiol. 2000;35:812–824. doi: 10.1046/j.1365-2958.2000.01753.x. [DOI] [PubMed] [Google Scholar]
- 14.Garza A G, Pollack J S, Harris B Z, Lee A, Keseler I M, Licking E F, Singer M. SdeK is required for early fruiting body development in Myxococcus xanthus. J Bacteriol. 1998;180:4628–4637. doi: 10.1128/jb.180.17.4628-4637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng Y, Yang Z, Downard J, Zusman D, Shi W. Methylation of FrzCD defines a discrete step in the developmental program of Myxococcus xanthus. J Bacteriol. 1998;180:5765–5768. doi: 10.1128/jb.180.21.5765-5768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorski L, Kaiser D. Targeted mutagenesis of ς54 activator proteins in Myxococcus xanthus. J Bacteriol. 1998;180:5896–5905. doi: 10.1128/jb.180.22.5896-5905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagen D C, Bretscher A P, Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978;64:284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- 18.Hess J F, Bourret R B, Simon M I. Histidine phosphorylation and phosphoryl group transfer in bacterial chemotaxis. Nature. 1988;336:139–143. doi: 10.1038/336139a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman R I, Nixon B T. Use of PCR to isolate genes encoding ς54-dependent activators from diverse bacteria. J Bacteriol. 1996;178:3967–3970. doi: 10.1128/jb.178.13.3967-3970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keseler I M, Kaiser D. An early A-signal-dependent gene in Myxococcus xanthus has a sigma 54-like promoter. J Bacteriol. 1995;177:4638–4644. doi: 10.1128/jb.177.16.4638-4644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keseler I M, Kaiser D. Sigma54, a vital protein for Myxococcus xanthus. Proc Natl Acad Sci USA. 1997;94:1979–1984. doi: 10.1073/pnas.94.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klose K E, Weiss D S, Kustu S. Glutamate at the site of phosphorylation of nitrogen-regulatory protein NTRC mimics aspartyl-phosphate and activates the protein. J Mol Biol. 1993;232:67–78. doi: 10.1006/jmbi.1993.1370. [DOI] [PubMed] [Google Scholar]
- 25.Kroos L, Kuspa A, Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986;117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 26.Kuner J M, Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982;151:458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Y Y, Arsene F, Elmerich C. Characterization of the ntrBC genes of Azospirillum brasilense Sp7: their involvement in the regulation of nitrogenase synthesis and activity. Mol Gen Genet. 1993;240:188–196. doi: 10.1007/BF00277056. [DOI] [PubMed] [Google Scholar]
- 29.Merrick M J. In a class of its own—the RNA polymerase sigma factor sigma 54 (sigma N) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 30.Morett E, Segovia L. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993;175:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison C E, Zusman D R. Myxococcus xanthus mutants with temperature-sensitive, stage-specific defects: evidence for independent pathways in development. J Bacteriol. 1979;140:1036–1042. doi: 10.1128/jb.140.3.1036-1042.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ninfa A J, Bennett R L. Identification of the site of autophosphorylation of the bacterial protein kinase/phosphatase NRII. J Biol Chem. 1991;266:6888–6893. [PubMed] [Google Scholar]
- 33.Ogawa M, Fujitani S, Mao X, Inouye S, Komano T. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol Microbiol. 1996;22:757–767. doi: 10.1046/j.1365-2958.1996.d01-1725.x. [DOI] [PubMed] [Google Scholar]
- 34.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 35.Roberts D L, Bennett D W, Forst S A. Identification of the site of phosphorylation on the osmosensor, EnvZ, of Escherichia coli. J Biol Chem. 1994;269:8728–8733. [PubMed] [Google Scholar]
- 36.Romeo J M, Zusman D R. Transcription of the myxobacterial hemagglutinin gene is mediated by a sigma 54-like promoter and a cis-acting upstream regulatory region of DNA. J Bacteriol. 1991;173:2969–2976. doi: 10.1128/jb.173.9.2969-2976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Shimkets L J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shingler V. Signal sensing by sigma 54-dependent regulators: derepression as a control mechanism. Mol Microbiol. 1996;19:409–416. doi: 10.1046/j.1365-2958.1996.388920.x. [DOI] [PubMed] [Google Scholar]
- 40.Stock J B, Stock A M, Mottonen J M. Signal transduction in bacteria. Nature. 1990;344:395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- 41.Szeto W W, Nixon B T, Ronson C W, Ausubel F M. Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activation of nitrogen fixation genes in free-living and symbiotic cells. J Bacteriol. 1987;169:1423–1432. doi: 10.1128/jb.169.4.1423-1432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thony-Meyer L, Kaiser D. devRs, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus. J Bacteriol. 1993;175:7450–7462. doi: 10.1128/jb.175.22.7450-7462.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueki T, Inouye S, Inouye M. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene. 1996;183:153–157. doi: 10.1016/s0378-1119(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 44.Vega-Palas M A, Flores E, Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the CRP family of bacterial regulators. Mol Microbiol. 1992;6:1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 45.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- 46.Wu S S, Kaiser D. Regulation of expression of the pilA gene in Myxococcus xanthus. J Bacteriol. 1997;179:7748–7758. doi: 10.1128/jb.179.24.7748-7758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C, Kaplan H B. Myxococcus xanthus sasS encodes a sensor histidine kinase required for early developmental gene expression. J Bacteriol. 1997;179:7759–7767. doi: 10.1128/jb.179.24.7759-7767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]