Abstract
The uptake of Mn2+, a cofactor for several enzymes in Escherichia coli, is mediated by MntH, a proton-dependent metal transporter, which also recognizes Fe2+ with lower affinity. MntH belongs to the NRAMP family of eukaryotic Fe2+ and Mn2+ transporters. In E. coli strains with chromosomal mntH-lacZ fusions, mntH was partially repressed by both Mn2+ and Fe2+. Inactivation of fur resulted in the loss of Fe2+-dependent repression of mntH transcription, demonstrating that Fe2+ repression depends on the global iron regulator Fur. However, these fur mutants still showed Mn2+-dependent repression of mntH. The Mn2+-responsive transcriptional regulator of mntH was identified as the gene product of o155 (renamed MntR). mntR mutants were impaired in Mn2+ but not Fe2+ repression of mntH transcription. Binding of purified MntR to the mntH operator was manganese dependent. The binding region was localized by DNase I footprinting analysis and covers a nearly perfect palindrome. The Fur binding site, localized within 22 nucleotides of the mntH operator by in vivo operator titration assays, resembles the Fur-box consensus sequence.
In eukaryotes, Fe2+ and Mn2+ are transported by the NRAMP family of transporters. Recently, NRAMP homologues from both gram-negative and gram-positive bacteria have been biochemically characterized as pH-dependent secondary transporters of divalent metal ions with a preference for Mn2+, and to a lesser extent, for Fe2+ (1, 15, 18, 27), hence the designation MntH, for H+-dependent manganese transporter.
Iron and manganese are both toxic at high intracellular concentrations. Therefore, their uptake into bacteria is tightly regulated. In gram-negative bacteria and in gram-positive bacteria with a low GC content, the genes encoding iron uptake proteins are negatively regulated by Fur (ferric uptake regulator). In the presence of Fe2+, this protein binds to palindromic sequences within Fur-regulated promoters and represses transcription of the genes. A decreased intracellular iron concentration leads to a lower DNA binding activity of the regulatory protein and concomitantly to derepression of the regulated genes. In gram-positive species with a high GC content, iron regulation is accomplished by the diphtheria toxin repressor (DtxR)-like proteins. Fur and DtxR are representatives of two distinct repressor families. In addition to the iron regulator Fur, the Fur family includes the regulator of Zn2+ transport, Zur (8, 11, 21), and the regulator of the peroxide stress response, PerR (4). Recent DNA binding studies have suggested that TroR, a member of the DtxR family, from Treponema pallidum is a Mn2+ sensor (25), but it has not been possible to test the role of TroR in vivo. The distantly related MntR from Bacillus subtilis has been described as a bifunctional regulator of two Mn2+ transporters. Under low-Mn2+ conditions, MntR activates transcription of an ABC transporter, whereas under high-Mn2+ conditions, MntR acts as a transcriptional repressor of mntH, an NRAMP homologue (27). Under high-Mn2+ conditions, the DtxR homologue ScaR from Streptococcus gordonii represses transcription of the scaABC operon, which encodes the components of an ABC-type transporter for Mn2+ (13).
In this work, the regulation of mntH expression in Escherichia coli by divalent metal ions was studied. We report the identification of the gene product of o155, which we renamed MntR, a new Mn2+ regulator in E. coli, and the repression of mntH transcription by Mn2+-MntR and Fe2+-Fur. The MntR protein was purified and shown to bind the mntH operator in vitro.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
M9 minimal medium (19) contained 0.2% glucose as a carbon source. For MA medium, M9 was supplemented with tryptophan, tyrosine, and phenylalanine (0.1 mg ml−1 [each]) and 4-aminobenzoic acid (40 μM) and 4-hydroxybenzoic acid (40 μM). MacConkey plates contained 40 g of MacConkey agar base (Difco, Detroit, Mich.) and 10 g of d-lactose per liter. Mutagenesis with 1-methyl-3-nitro-1-nitrosoguanidine (MNNG), P1 transductions, and β-galactosidase assays were performed as described previously (19).
The E. coli strains and plasmids used in this work are listed in Table 1 or are described in Results and Fig. 3. All nucleotide positions and section numbers are those of the E. coli K-12 strain MG1655 genome sequence reported by Blattner et al. (3).
TABLE 1.
E. coli strains and plasmids
| Strain or plasmid | Relevant genotype and/or propertiesa | Source or reference |
|---|---|---|
| Strains | ||
| BL21(DE3) | hsdS(rB− mB−) gal dcm ompT λ(DE3); T7 RNA polymerase gene under control of the lacUV5 promoter | 31 |
| H1443 | MC4100 aroB | 30 |
| H1717 | H1443 fhuF::λplacMu53 | 30 and 12 |
| LCB272 | lacY gaIU pyrD trp::Tn5 rpsL mal valr F′ 106 zbh-272::Tn10 | 20 |
| MAL103 | araD139 Δ(proAB lacIZYA) rpsL150 araB::Mu(cts) zzz::Mud1 (Amprlac cts) | 6 |
| MC4100 | araD139 Δ(lacIZYA-argF)U169 rpsL150 relA1 flhD5301 deoC1 fruA25 rbsR22 | 5 |
| SIP744, -879 | MC4100 mntH::Mud1 aroB | This study |
| SIP882 | MC4100 mntH::Mud1 | This study |
| SIP890 | MC4100 mntH::Mud1 fur-28 zbf-15::Tn10 aroB | This study |
| SIP891 | MC4100 mntH::Mud1 fur+ zbf-15::Tn10 aroB | This study |
| SIP894 | MC4100 mntH::Mud1 fur-28 zbf-15::Tn10 | This study |
| SIP895 | MC4100 mntH::Mud1 fur+ zbf-15::Tn10 | This study |
| SIP924, -931, -932, -933, -943, -944, -949 | SIP879 but mntR; see Table 2 | This study |
| Plasmids | ||
| pACYC184 | p15A ori, 4.2 kb, Camr Tetr, medium copy number | 7 |
| pBC-SK+ | ColE1 ori, 3.4 kb, Camr, phage T7 φ 10 promoter, expression vector, high copy number | Stratagene |
| pHSG575 | pSC101 ori, 3.6 kb, Camr, low copy number | 33 |
| pSP61/18 | pHSG575 fur (nt 2073–2847, 62) in SmaI site | This study |
| pSP115/25 | pACYC184 f527′ o86 o155 o372′ (nt 10296, 73, to nt 1924, 74) in BamHI site | This study |
| pSP116/1 | pHSG575 mntR (nt 360–1067, 74) in SmaI site | This study |
| pSP116/10 | pACYC184 mntR (nt 360–1067, 74) in EcoRV site | This study |
| pSP116/25 | pT7-7 mntR (nt 580–1067, 74) in NdeI/EcoRI site | This study |
| pSP117/11 | pBC-SK+mntH operator (170 nt of the Mud1 end, nt 3610–4764, 217) in EcoRV site | This study |
| pSP118/14 | pBC-SK+ with corrupt MntR box (nt 4433–4455, 217, with a deletion of nt 4452) in EcoRV site | This study |
| pSP118/18 | pBC-SK+ with MntR box (nt 4433–4454, 217) in EcoRV site | This study |
| pT7-7 | ColE1 ori, 2.5 kb, Ampr, phage T7 φ 10 promoter, expression vector, optimal Shine-Dalgarno sequence | 32 |
For plasmids, the positions and section numbers of the insert in the E. coli K-12 strain MG1655 genome sequence according to Blattner et al. (3) are given in parentheses.
FIG. 3.
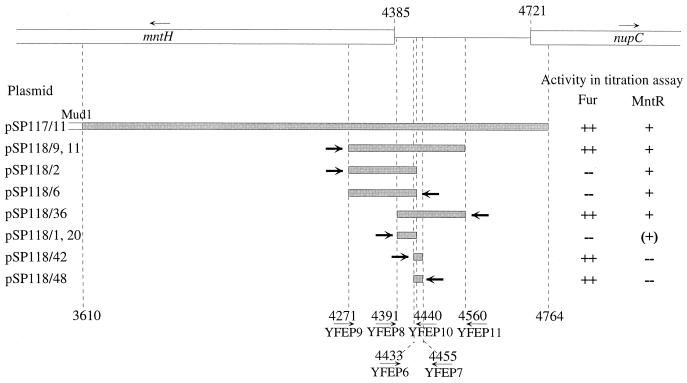
Mapping of the Fur box and of the potential MntR binding site on the mntH operator region. The DNA inserts (shaded boxes) were obtained with the primers specified in Materials and Methods and were then ligated into the EcoRV site of pBC-SK+. Thick arrows show the position and orientation of the lacZ promoter in each construct. The numbers reflect the positions in section 217 of the E. coli genome (3). Activity in the Fur titration assay signifies derepression of fhuF′-lacZ expression in E. coli H1717 carrying the corresponding plasmid. This results in red colonies on MacConkey plates containing 40 μM (NH4)2Fe(SO4)2. E. coli H1717(pBC-SK+) cells grew as pale colonies on these plates. For the MntR titration assay, E. coli SIP879 was transformed with the plasmids and analyzed on MacConkey plates with 80 μM MnCl2. E. coli SIP879(pBC-SK+) formed white colonies; colonies of transformants with activity were reddish. Parentheses indicate partially reduced activity. Plus and minus signs indicate the relative amount of activity, with ++ being the highest relative amount.
Strains with chromosomal lacZ operon fusions were obtained by infection of E. coli H1443 (E. coli MC4100, but aroB; 30) with a Mud1 (Ampr lac cts) lysate prepared from strain MAL103 as described previously (6). SIP744 was isolated as a strain carrying a Mud1 fusion that was repressed by iron and induced by 2,2′-dipyridyl (an Fe2+ chelator) as judged by cross-streaking on MacConkey plates (21). To separate the iron-regulated Mud1 phage from other possible Mud1 phage insertions, a P1 lysate from E. coli SIP744 was used to transduce strains H1443 and MC4100 (5). Selection for Ampr resulted in strains SIP879 and SIP882, respectively. The Mud1 insertion site was localized by the method described in reference 21 and was determined to be at bp 3609 to 3610 of section 217 in the open reading frame f412 (3), which has recently been identified as mntH (15, 18).
E. coli H1717 with the Fur-regulated fhuF-lacZ fusion was utilized for the Fur titration assay as described previously (30).
In the fur-28 allele, nucleotides (nt) 2284 to 2215 of section 62 are deleted, giving rise to an inactive Fur. This mutation was generated by MNNG treatment, fur-28 was introduced into strains SIP879 and SIP882 by cotransduction with zbf-15::Tn10, resulting in strains SIP890 (MC4100, but mntH::Mud1 fur-28 zbf-15::Tn10 aroB) and SIP894 (MC4100, but mntH::Mud1 fur-28 zbf-15::Tn10), respectively. The isogenic fur+ strains are designated SIP891 (MC4100, but mntH::Mud1 zbf-15::Tn10 aroB) and SIP895 (MC4100, but mntH::Mud1 zbf-15::Tn10), respectively. Strain LCB272 carries zbh-272::Tn10 on F′ 106 (20).
Plasmids pSP116/1 and pSP116/10 comprise the PCR product obtained with primers MNTR1 (TAAACACGCGCATACACCTCTTG [nt 360 to 382, section 74]) and MNTR2 (GCGTGCGTAAAAAAGGCAGGCTC [nt 1067 to 1045, section 74]) in the SmaI site of pHSG575 and the EcoRV site of pACYC184, respectively. The PCR product obtained with primers MNTR2 and MNTR3 (TGAGTCGTCGCGCAGGTACGCC [nt 580 to 601, section 74]) was bluntly cloned into the NdeI/EcoRI sites of pT7-7, which were previously end filled, resulting in pSP116/25. The sequence of the PCR-amplified mntR was verified.
Plasmid pSP61/18 carries the fur gene on a 0.78-kb PCR product obtained with primers FUR1 (GTAACTTTTGCTGTTGTACCTGTAC [nt 2847 to 2823, section 62]) and FUR2 (GGCAGGAAATACGCAGTAATAACAA [nt 2073 to 2097 section 62]) inserted in the SmaI site of pHSG575. pSP117/11 contains the PCR product obtained from E. coli SIP879 with primers MUD1 (CACGTACATGCCGCCAAACTCACCA) and YFEP3 (GCAACAACGGCAAGTGCCAGTAC [nt 4764 to 4742, section 217]) inserted in the EcoRV site of pBC-SK+. For subcloning of the mntH operator, PCR was carried out with primers YFEP8 (GCCTCTAAAACATAGCCTTTGCT [nt 4391 to 4413, section 217]), YFEP9 (CAAAGTTACCGGGATCGATATAA [nt 4271 to 4293, section 217]), YFEP10 (ATTCTCGTTTGGCATAGCATGAA [nt 4440 to 4418, section 217]), and YFEP11 (GTTATGTAAATGTGCTAACATTA [nt 4560 to 4538, section 217]), or primers YFEP6 (ACGAGAATGATTATCAAATTCAT [nt 4433 to 4455, section 217]) and YFEP7 (ATGAATTTGATAATCATTCTCGT [nt 4455 to 4433, section 217]) were annealed, and the fragments were then inserted into the EcoRV site of pBC-SK+ as depicted in Fig. 3.
Recombinant DNA techniques.
Standard procedures (28) or those recommended by the manufacturer were followed for isolation of chromosomal and plasmid DNA, DNA modification, ligation, transformation, PCR, and agarose gel electrophoresis. DNA was sequenced by the dideoxy chain termination method using an ALFexpress DNA sequencer (Pharmacia Biotech, Freiburg, Germany). Mutations in the chromosomal mntR allele were localized by cycle sequencing of two independently generated PCR-amplified fragments using the primers MNTR1 and MNTR2 and a Thermo Sequenase Cy5 dye terminator kit (Pharmacia Biotech). Oligonucleotides were synthesized by Eurogentec (Seraing, Belgium).
DNase I footprinting.
DNase I protection experiments were done as described previously (22). For metal ion binding studies, lower concentrations of MgCl2 (50 μM) and CaCl2 (10 μM) were applied. The labeled target DNA was generated by PCR with the primers YFEP9 and carbocyamine dye (Cy5)-labeled YFEP5C (TGTTGTGTATGGAAGCTGAAAG [nt 4581 to 4560, section 217]) for the MntH-coding strand and YFEP3 and fluorescent Cy5-labeled YFEP4C (GCAATGAACGCAGGTCCCATTA [nt 4303 to 4324, section 217]) for the noncoding strand. Standard sequencing reactions were carried out with the Cy5-labeled primer and pSP117/11 as DNA template.
Overproduction and purification of MntR.
MntR was overproduced from pSP116/25 using E. coli BL21 (DE3) (31). Bacterial cells were disrupted in a French pressure cell, and the supernatant was chromatographed on a fast protein liquid chromatography MonoQ HR 5/5 column (Pharmacia) with 50 mM Tris-HCl, pH 7.4, and a linear gradient of NaCl (0 to 0.3 M).
Computer analyses.
Nucleic acid and amino acid sequences were analyzed by the PC/GENE 6.85 program package (IntelliGenetics, Mountain View, Calif.). For sequence similarity searches, the BLAST facilities of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) were used.
RESULTS
Regulation of mntH.
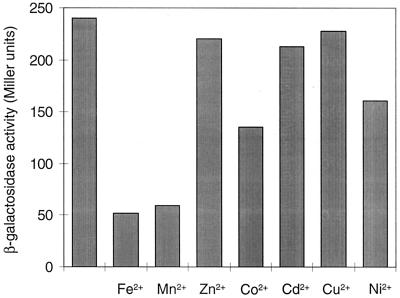
To identify new genes regulated by fur in E. coli, random Mud1 phage insertion mutants that were repressed by iron and derepressed by the iron chelator 2,2′-dipyridyl were selected as described in Materials and Methods. The Mud1 insertion site of one mutant (SIP879) was localized in the mntH gene by sequencing the insertion site as described in Materials and Methods. On MacConkey plates, mutant SIP879 formed red colonies, indicating acid production from lactose and high expression of the operon fusion with the lacZ reporter gene. The mntH-lacZ fusion was repressed in cells in the diffusion zone of a filter paper strip impregnated with 100 μM (NH4)2Fe(SO4)2 or 20 μM MnCl2, as shown by the formation of white colonies. In cells on MacConkey plates containing 40 μM (NH4)2Fe(So4)2, the fusion was derepressed by the chelators 2,2′-dipyridyl, desferri-ferrioxamine B, and EDTA (each 10 mM). The β-galactosidase activity of mutant SIP879 (mntH-lacZ) was measured in the presence of various divalent metal ions (Fig. 1). Of the metal ions tested, only Fe2+ and Mn2+ repressed the mntH-lacZ operon fusion fivefold.
FIG. 1.
Regulation of mntH-lacZ by divalent metal ions. E. coli SIP879 (mntH-lacZ) was grown aerobically for 6 h in MA medium supplemented with a 10 μM concentration of the indicated metal ion, and β-galactosidase activities were then measured. Values are averages of experiments carried out in triplicate.
The influence of iron was also observed in the different behaviors of aroB+ and aroB strains. aroB is one of the genes necessary for the biosynthesis of the E. coli siderophore enterochelin, which mediates ferric iron transport. The mntH-lacZ fusion in strain SIP882 (mntH-lacZ aroB+) was repressed more strongly than in the isogenic aroB mutant SIP879 (data not shown). This is probably due to the better supply of iron, which exerts a repressing effect, for aroB+ strains.
Iron repression of mntH is mediated by Fur.
To determine whether iron regulation is mediated by Fur, the fur gene on the chromosome of strain SIP879 (mntH-lacZ) was inactivated. In the fur mutant SIP890, the mntH-lacZ fusion was no longer repressed by iron, whereas regulation by manganese was unimpaired (Fig. 2A). The isogenic fur+ strain SIP891 showed the same regulation as strain SIP879. The derepression in the presence of iron in the fur mutant was complemented by the fur-carrying plasmid pSP61/18 (Fig. 2A). This indicates that Fur alone is responsible for the iron repression. The same results were obtained for the aroB+ strains SIP894 (mntH-lacZ fur) and SIP895 (mntH-lacZ) (data not shown).
FIG. 2.
Response of mntH-lacZ in wild-type, mntR mutant, and fur mutant cells to various concentrations of (NH4)2Fe(SO4)2 (A) and MnCl2 (B). The mntR mutant SIP933 was complemented with pSP116/1 (plasmid carrying mntR), and the fur mutant SIP890 was complemented with pSP61/18 (plasmid carrying fur). Cells were grown aerobically for 6 h in MA medium (white bars) or in MA medium amended with 5 μM (black bars) or 20 μM (gray bars) metal ions, and β-galactosidase activities were then determined. Values are averages of experiments carried out in triplicate.
Localization of the Fur binding site in vivo.
To localize the DNA region within the mntH operator responsible for Fur binding, an in vivo titration assay (30) was employed. Introduction of a Fur binding site on a high-copy-number plasmid titrates Fur and hence derepressed the Fur-regulated fhuF′-lacZ expression in E. coli H1717, resulting in red colonies on MacConkey plates containing 40 μM (NH4)2Fe(SO4)2. In contrast, E. coli H1717 harboring the vector forms white colonies. E. coli H1717 transformed with the plasmid pSP117/11 carrying the mntH operator region (nt 3610 to 4764, section 217) cloned on pBC-SK+ resulted in red colonies (Fig. 3), indicating Fur binding, whereas the colonies of the vector control were white. The region responsible for the Fur regulation was narrowed down to nt 4433 to 4455 of section 217 by subcloning as depicted in Fig. 3. Deletion of nt 4452 from this region (pSP118/14) resulted in lower activity, whereas pSP118/18, comprising nt 4433 to 4454, was fully active in the in vivo titration assay (data not shown). The binding site GAgAATGATtATCAaatTC matches the Fur-box consensus sequence GATAATGATAATCATTATC (9) in 14 of 19 nt (mismatches are shown as lowercase letters).
Identification of the Mn2+ regulator MntR.
To find the regulator responsible for the Mn2+ regulation, strain SIP879 (mntH-lacZ) was mutagenized with MNNG and screened for derepressed red colonies on MacConkey plates containing 10 μM Mn2+. The impaired manganese repression of the selected mutants is summarized in Table 2. Mutant SIP932 was transformed with an E. coli gene library (21) and screened for white colonies on MacConkey manganese plates. One plasmid (pSP115/25) complemented strain SIP932 as well as mutants SIP924, SIP931, SIP933, SIP943, and SIP949. The plasmid comprises nt 10296 of section 73 to nt 1924 of section 74 of the E. coli genome. Subclones were constructed, of which plasmids pSP116/1 and pSP116/10 contained only o155 and restored repression by manganese (see Fig. 2B for pSP116/1). The mutations were cotransducible with the tetracycline resistance marker zbh-272::Tn10 at 18.4 min on the genetic map of E. coli. This confirmed that the Mn2+-deregulated mutants are mutated in the region of o155. By sequencing, the mutations were all localized in o155 (Table 2). The amino acid sequence of O155 reveals 16% identity to MntR from B. subtilis and shows that it belongs to the DtxR family of bacterial metalloregulators. Hence o155 was renamed mntR (Mn2+ transporter regulation).
TABLE 2.
Deregulation in mutant mntR strainsa
| E. coli strain | Repressionb | Mutated nucleotide | Amino acid substitution |
|---|---|---|---|
| MC4100/SIP879 | 3.4 | Unchanged | Unchanged |
| SIP924 | 1.3 | 954 G→A | 126 Ala→Thr |
| SIP931 | 1.2 | 845 G→A | 89 Trp→stop |
| SIP932 | 1.1 | 882 G→A | 102 Ala→Thr |
| SIP933 | 1.15 | 871 G→A | 98 Gly→Glu |
| SIP943 | 1.2 | 845 G→A | 89 Trp→stop |
| SIP944 | 1.3 | 744 C→T | 56 Arg→Cys |
| SIP949 | 1.3 | 772 G→A | 65 Gly→Glu |
The numbering of the nucleotides reflects the position in the E. coli K-12 strain MG1655 genome, section 74 (3) (MntR is encoded by nt 579 to 1046), and the numbering of the amino acid residues specifies the position in the E. coli wild-type MntR protein.
Ratio of β-galactosidase activity of the mntH-lacZ fusion without and with 5 μM Mn2+.
MNNG mutagenesis of SIP882 (mntH-lacZ aroB+) did not result in derepressed red colonies on MacConkey manganese plates. P1 transduction of the mntR mutant SIP932 with aroB+ revealed that in an aroB+ strain the mntH-lacZ fusion is fully repressed by iron provided by the ferric enterochelin transport system.
Subcloning of the mntH operator region affording manganese regulation in vivo.
When cloned on the high-copy-number vector pBC-SK+, the region nt 3610 to 4764 of section 217 covering the mntH operator (pSP117/11) titrated MntR from the chromosomal mntH-lacZ fusion of strain SIP879; colonies of SIP879 transformed with pSP117/11 were reddish on MacConkey plates containing 80 μM MnCl2, whereas SIP879 transformed with the vector pBC-SK+ formed white colonies. This indicates that the insert binds to MntR. By subcloning, this MntR binding site was narrowed down to nt 4391 to 4440 of section 217 (Fig. 3), which contains a nearly perfect palindrome (see Fig. 5C).
FIG. 5.
DNase I footprinting analysis of the MntH-coding (A) and noncoding (B) strands of the mntH operator with MntR protein. The 311-bp DNA fragment comprising nt 4581 to 4271 of section 217, labeled on the MntH-coding strand with the fluorescence-labeled YFEP5C primer (A), and the 462-bp fragment encompassing nt 4303 to 4764 of section 217, labeled on the noncoding strand with the Cy5-labeled YFEP4C primer (B), were incubated without (row 1) or with (row 2) purified MntR protein as described in Materials and Methods. The nucleotide sequence obtained from the dideoxynucleotide sequencing reaction with the same end-labeled primer is given at the bottom of each panel; the protected region is expanded for clarity. (C) Nucleotide sequence of the mntH operator region with the translational start site. The boxed region indicates the nucleotides protected from DNase I by MntR. The area active in the in vivo Fur titration assay is shaded. Palindromic sequences are denoted by convergent arrows, with complementary bases shown in bold. Nucleotides are numbered according to E. coli genome section 217.
Interaction of MntR with the mntH operator region.
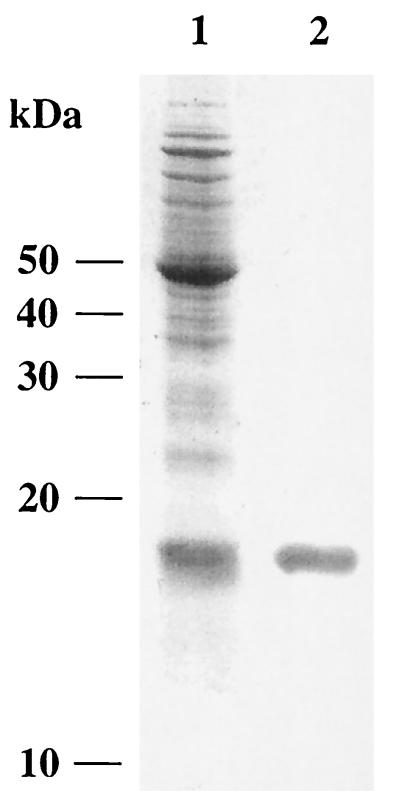
After overproduction, MntR was purified by anion-exchange chromatography to electrophoretic homogeneity. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified MntR revealed a single protein with a molecular mass of ≈17 kDa, consistent with the predicted size of 17.6 kDa (Fig. 4). The DNA binding properties of MntR were investigated by DNase I footprint assays. The target DNA fragment encompassed at least 195 nt upstream of the translational start of mntH and was labeled on each strand. Footprinting experiments in the presence of Mn2+ showed a core region of protection that is depicted in Fig. 5. At higher MntR concentrations, partial protection adjacent to the core region occurred. Even without added Mn2+ the DNA was protected, probably due to the high Mg2+ concentrations (5 mM) in the DNase I buffer. Therefore, lower Mg2+ concentrations at which DNase I was still active were used for metal ion specificity studies (see Materials and Methods). The low-specificity ion chelator EDTA (100 μM) impaired DNA protection. Addition of 150 μM Mn2+ restored DNA binding, whereas no protection was observed in the presence of 150 μM Co2+, Cu2+, Fe2+, Ni2+, or Zn2+. Zn2+ did not protect but altered the DNase I fragmentation pattern.
FIG. 4.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the purified MntR. mntR was overexpressed using the T7 expression system on pSP116/25 carried by strain BL21(DE3). Lane 1, soluble fraction of cell lysate after induction; lane 2, MntR purified by MonoQ anion-exchange chromatography. Proteins were stained with Coomassie blue. The positions and molecular masses of the standard proteins are shown on the left.
DISCUSSION
This study identifies a new metalloregulatory protein in E. coli, MntR, that senses Mn2+ and belongs to the DtxR family. The crystal structures of two iron regulatory proteins of the DtxR family, DtxR from Corynebacterium diphtheriae and IdeR from Mycobacterium tuberculosis, have been solved (24, 26). The proteins comprise three distinct domains: an amino-terminal DNA binding domain containing a helix-turn-helix motif, a central dimerization domain including the key residues for metal ion coordination, and a carboxy-terminal α-spectrin SH3-like domain proposed to regulate repressor activity and also to contribute to metal ion binding (23). In contrast to the Mn2+-responsive metalloregulator ScaR (13), MntR from E. coli, the Mn2+-sensing TroR from T. pallidum (25), and MntR from B. subtilis (27) lack the C-terminal third (SH3-like) domain of DtxR. Therefore, the third domain is not correlated with the metal ion specificity for Mn2+ or Fe2+.
Regulation of the transporter gene mntH by Mn2+ is only accomplished by MntR and not by Fur, although the E. coli Fur protein also functions in vitro with Mn2+ as a corepressor. This might also occur in vivo under certain conditions. At high, toxic Mn2+ concentrations, iron uptake is repressed and growth ceases. Mutants selected for Mn2+ resistance are often mutated in fur, which allows the cells to synthesize specific iron uptake systems (12).
It seems reasonable that in E. coli the NRAMP homologue transporter MntH, which takes up Mn2+ as well as Fe2+, is regulated by both ions via specific regulators. In contrast to mntH from E. coli, no iron regulation has been reported for mntH from B. subtilis (27). It is possible that in B. subtilis the repression by Fe2+ is only found at iron concentrations higher than the ones tested (up to 1 μM). Besides the NRAMP homologous proton-coupled Mn2+ transporter MntH, B. subtilis contains the ABC-type Mn2+ transporter MntABCD, which is specific for Mn2+ and which is activated by MntR under low-Mn2+ conditions (27). ScaR from S. gordonii and TroR from T. pallidum have been described as repressors of ABC-type transporters, probably for Mn2+ uptake (13, 25). The ScaABC transporter from S. gordonii is not controlled by iron (13). It is unlikely that E. coli also possesses an ABC transporter for Mn2+ uptake since Mn2+ uptake is reported to be solely dependent on the membrane potential rather than on ATP (2, 29).
MntR protected a core region of 25 nt on the coding strand and on the noncoding strand of the mntH operator with a 3′ stagger. This is in accordance with other classic helix-turn-helix repressors that bind to DNA as a dimer, e.g., λ phage repressor CI (14, 34). Higher concentrations of MntR extended the protection zone in DNase I footprinting assays. Similarly, for ScaR and TroR, which occupy 22 nt with a palindromic sequence, at least two distinct DNA-protein complexes have been observed in mobility-shift DNA binding assays, which indicates multiple binding interactions between the regulator and the operator (13, 25). Likewise, polymerization of the Fur repressor has been observed in footprinting experiments (e.g., see reference 9) and by electron and atomic force microscopy (10, 16); however, the crystal structure of the DNA complex is not yet known. The structure of the DtxR-DNA complex revealed that DNA surprisingly interacts with two dimeric repressor proteins bound to opposite sides of the operator (35). Similar to ScaR, TroR, and DtxR, MntR-DNA binding seems to be more complex than that of a classic repressor. Consistent with this complexity, MntR from B. subtilis acts not only as a repressor but also as an activator (27).
The sequence within the promoter recognition region of mntH shows similarity to the sequences bound by the MntR regulator of B. subtilis (27) (Fig. 6A) and low similarity to the Mn2+-responsive metalloregulators, TroR from T. pallidum and ScaR (13) (Fig. 6B), to the DtxR consensus sequence (17), and to the Fur-box consensus sequence (9). The operator region of E. coli mntR contains no recognizable MntR box, giving no hint for autoregulation, which has not been examined further.
FIG. 6.
Analysis of the manganese repressor binding site sequences. (A) Alignment of the MntR box from E. coli with the putative MntR recognition site from the mntH and mntABCD control regions of B. subtilis (27). (B) Comparison of the MntR box from E. coli with the binding site for TroR from T. pallidum (25) and ScaR from S. gordonii (13). Bases that are identical in at least two sequences are shaded; bases common to all three sequences are marked by asterisks.
In many enzymes, Mn2+ can be replaced by Mg2+ and vice versa. For example, in the DNase I footprinting assays in this study, DNase I activity was strongly enhanced by Mn2+ in the absence of Mg2+. Thus, the physiological relevance of manganese for enzyme activity is often not known. However, in E. coli, a number of metalloenzymes that require Mn2+ are known, e.g., Mn2+-containing superoxide dismutase (MnSOD), protein phosphatases PrpA and -B, cofactor-independent 3-phosphoglycerate mutase (iPGM), agmatinase, phosphoenolpyruvate carboxylase, and exonuclease SbcCD. Possibly because there are enzymes that certainly need Mn2+ as a cofactor, the MntH uptake system is not completely repressed by Fe2+-Fur (Fig. 1 and 2). Derepression of the Mn2+ transport system via the Fur system may indicate that Mn2+ or Mn2+-containing enzymes substitute for iron or iron-containing enzymes under iron limitation.
ACKNOWLEDGMENTS
We thank Volkmar Braun (Universität Tübingen) for discussions and Karen A. Brune (Konstanz) for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (grant HA 1186/3-1) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Agranoff D, Monahan I M, Mangan J A, Butcher P D, Krishna S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J Exp Med. 1999;190:717–724. doi: 10.1084/jem.190.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya P. Active transport of manganese in isolated membranes of Escherichia coli. J Bacteriol. 1970;104:1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett G I, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 5.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 6.Casadaban M J, Cohen S N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vitro probe for transcriptional control sequences. Proc Natl Acad Sci USA. 1979;76:4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalet K, Gouin E, Cenatiempo Y, Cossart P, Héchard Y. Characterisation of a new operon encoding a Zur-like protein and an associated ABC zinc permease in Listeria monocytogenes. FEMS Microbiol Lett. 1999;174:111–116. doi: 10.1111/j.1574-6968.1999.tb13556.x. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo V, Wee S, Herrero M, Neilands J B. Operator sequences of the aerobactin operon of plasmid CoIV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169:2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fréchon D, Le Cam E. Fur (ferric uptake regulation) protein interaction with target DNA: comparison of gel retardation, footprinting and electron microscopy analyses. Biochem Biophys Res Commun. 1994;201:346–355. doi: 10.1006/bbrc.1994.1708. [DOI] [PubMed] [Google Scholar]
- 11.Gaballa A, Helmann J D. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K-12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 13.Jakubovics N S, Smith A W, Jenkinson H F. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol Microbiol. 2000;38:140–153. doi: 10.1046/j.1365-2958.2000.02122.x. [DOI] [PubMed] [Google Scholar]
- 14.Jordan S R, Pabo C O. Structure of the lambda complex at 2.5 A resolution: details of the repressor-operator interactions. Science. 1988;242:893–899. doi: 10.1126/science.3187530. [DOI] [PubMed] [Google Scholar]
- 15.Kehres D G, Zaharik M L, Finlay B B, Maguire M E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 16.Le Cam E, Fréchon D, Barray M, Fourcade A, Delain E. Observation of binding and polymerization of Fur repressor onto operator-containing DNA with electron and atomic force microscopes. Proc Natl Acad Sci USA. 1994;91:11816–11820. doi: 10.1073/pnas.91.25.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J H, Wang T, Ault K, Liu J, Schmitt M P, Holmes R K. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect Immun. 1997;65:4273–4280. doi: 10.1128/iai.65.10.4273-4280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makui H, Roig E, Cole S T, Helmann J D, Gros P, Cellier M F M. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 20.Pascal M C, Burini J F, Ratouchniak J, Chippaux M. Regulation of the nitrate reductase operon: effect of mutations in chlA, B, D and E genes. Mol Gen Genet. 1982;188:103–106. doi: 10.1007/BF00333001. [DOI] [PubMed] [Google Scholar]
- 21.Patzer S I, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 22.Patzer S I, Hantke K. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem. 2000;275:24321–24332. doi: 10.1074/jbc.M001775200. [DOI] [PubMed] [Google Scholar]
- 23.Pohl E, Holmes R K, Hol W G. Crystal structure of a cobalt-activated diphtheria toxin repressor-DNA complex reveals a metal-binding SH3-like domain. J Mol Biol. 1999;292:653–667. doi: 10.1006/jmbi.1999.3073. [DOI] [PubMed] [Google Scholar]
- 24.Pohl E, Holmes R K, Hol W G J. Crystal structure of the iron-dependent regulator (IdeR) from Mycobacterium tuberculosis shows both metal binding sites fully occupied. J Mol Biol. 1999;285:1145–1156. doi: 10.1006/jmbi.1998.2339. [DOI] [PubMed] [Google Scholar]
- 25.Posey J E, Hardham J M, Norris S J, Gherardini F C. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci USA. 1999;96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu X, Pohl E, Holmes R K, Hol W G. High-resolution structure of the diphtheria toxin repressor complexed with cobalt and manganese reveals an SH3-like third domain and suggests a possible role of phosphate as co-corepressor. Biochemistry. 1996;35:12292–12302. doi: 10.1021/bi960861d. [DOI] [PubMed] [Google Scholar]
- 27.Que Q, Helmann J D. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Silver S, Johnseine P, King K. Manganese active transport in Escherichia coli. J Bacteriol. 1970;104:1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stojiljkovic I, Bäumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. . (Erratum, 240:276) [DOI] [PubMed] [Google Scholar]
- 31.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 32.Tabor S. Unit 16.2: expression using the T7 RNA polymerase/promoter system. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 33.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 34.Tullius T D, Dombroski B A. Hydroxyl radical “footprinting”: high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc Natl Acad Sci USA. 1986;83:5469–5473. doi: 10.1073/pnas.83.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White A, Ding X, van der Spek J C, Murphy J R, Ringe D. Structure of the metal-ion-activated diphtheria toxin repressor/tox operator complex. Nature. 1998;394:502–506. doi: 10.1038/28893. [DOI] [PubMed] [Google Scholar]