Abstract
Production of type IV bundle-forming pili by enteropathogenic Escherichia coli (EPEC) requires BfpB, an outer-membrane lipoprotein and member of the secretin protein superfamily. BfpB was found to compose a ring-shaped, high-molecular-weight outer-membrane complex that is stable in 4% sodium dodecyl sulfate at temperatures of ≤65°C. Chemical cross-linking and immunoprecipitation experiments disclosed that the BfpB multimeric complex interacts with BfpG, and mutational studies showed that BfpG is required for the formation and/or stability of the multimer but not for the outer-membrane localization of BfpB. Formation of the BfpB multimer also does not require BfpA, the repeating subunit of the pilus filament. Functional studies of the BfpB-BfpG complex revealed that its presence confers vancomycin sensitivity, indicating that it may form an incompletely gated channel through the outer membrane. BfpB expression is also associated with accumulation of EPEC proteins in growth medium, suggesting that it may support both pilus biogenesis and protein secretion.
Enteropathogenic Escherichia coli (EPEC) is an important cause of protracted diarrheal illness among children living in developing countries (11). Endoscopically directed biopsies of tissue from naturally infected children show that EPEC mainly infects the small intestine, adhering to epithelial cell surfaces where it induces actin condensation and effacement of microvilli (22, 46). Studies of EPEC-infected epithelial cell monolayers revealed similar cytopathic changes and led to the identification of two distinct but coordinated processes: the formation of adherent microcolonies—the localized adherence (LA) phenotype (12, 51)—and characteristic changes of the cytoskeleton beneath attached bacteria—the attaching and effacing phenotype (31).
The genes coding for the attaching and effacing phenotype are located on the chromosome within a large pathogenicity island, termed the locus of enterocyte effacement (LEE) (37). Among the proteins specified by LEE are intimin, required for close adherence between bacteria and the epithelial cell (25); Tir, which serves as an intimin receptor that is inserted into the epithelial cell plasma membrane (29); EspB, a protein required for the attaching and effacing effect (30); and components of the cognate type III secretion system, including SepC, a member of the secretin protein superfamily (24). Proof that LEE-encoded functions are required for virulence comes from human challenge studies showing that intimin or EspB mutants are significantly attenuated (13, 55).
Genes required for the LA phenotype are found on the 69-kb EPEC adherence factor (EAF) plasmid (38, 53, 54) in a region that specifies the bundle-forming pilus (BFP) of the organism (18). Disruption of this locus abrogates the LA phenotype (45) and significantly reduces the virulence of the mutant in orally challenged human volunteers (3). Examination of this mutant revealed that bundle-forming pili also mediate the formation of transient bacterial aggregates during growth in tissue culture medium (2, 3). Time course, phase-contrast microscopy of this phenomenon, termed the autoaggregation (AA) phenotype, showed that after an overnight culture of dispersed, individual EPEC bacteria was diluted into tissue culture media, the cells reenter the exponential phase of growth. Forty-five to 60 min later, the bacteria begin to coalesce into dynamic, spherical assemblies. These autoaggregates continue to form and enlarge until late exponential phase and then, over an ∼20-min interval, they disaggregate, yielding a suspension of individual bacteria (3).
BFP biogenesis and function are encoded by an operon containing 14 genes, designated bfpA to bfpL (53, 54). All but bfpH are required for BFP filament production and for the LA and AA phenotypes (S. W. Ramer, unpublished data) (1, 45), but the functions of only 3 of the 14 open reading frames (ORFs) have been reported. bfpA encodes the principal repeating subunit of the pilus filament; amino acid sequence analysis showed it to be a member of the type IV family of pilus proteins (53). bfpP codes for the pre-pilin signal peptidase (59), proven to be required for the maturation of BfpA and likely to be required for the processing of three other pilin-like proteins encoded by the operon and denoted BfpI, -J, and -K (53). bfpF is required for the AA phenotype, and bfpF mutants are hyperpiliated, form aggregates that do not disperse, and are less virulent for human volunteers (2, 3).
The study reported here focuses on the biochemical characterization and functional roles of bfpG and bfpB, two genes previously shown to be required for pilus biogenesis (1, 45). bfpG and bfpB are the second and third ORFs of the operon (53, 54), respectively, and RNase protection assays showed that the 3′ end of bfpG and the 5′ end of bfpB are located on a continuous RNA transcript (45). BfpB is an outer-membrane lipoprotein that shares sequence similarity with the E. coli bacteriophage f1 morphogenic protein pIV (45), a member of the secretin protein superfamily (17, 49). Studies of pIV and several other secretin family members show them to be essential outer-membrane components of the main terminal branch of the general secretory pathway (GSP; also termed the type II secretion pathway) (4, 20, 44). The GSP is required in diverse species for pilus and phage biogenesis, DNA uptake, and the secretion of enzymes (43). The type III secretion system, required for the delivery of virulence determinants into host cells, also employs members of the secretin family (5, 8, 32).
Biochemical and ultrastructural studies of several secretins show that they form outer-membrane multimers with ring-like structures (4, 6–8, 32, 34, 39). These findings, in combination with voltage dependence studies of conductance (35, 39), have led to the idea that secretins compose gated channels. The location and dimensions of these structures have naturally raised the possibility that they serve as outer-membrane conduits for the cognate secreted molecule (16, 39, 47, 50, 58), although this notion remains to be proven. The evidence that BfpB may be a secretin arises from its C-terminal region sequence similarity with the phage pIV protein (27, 49), with 21% identity over 282 amino acids (56). In several other respects, it differs significantly from other proven members of the secretin family. First, each of the 15 biochemically characterized members of the secretin family contains a common motif (V/IPXLG/SXIPXXGXLF) (17, 57). By contrast, BfpB lacks this feature of primary structure. Second, BfpB is a proven lipoprotein, while all other biochemically characterized secretins, with the exception of XpsD of Xanthomonas campestris (23), are not modified by the acylation of an N-terminal cysteine. Third, most other secretins are “piloted” to the outer membrane and protected from degradation by small lipoproteins (8, 9, 15, 19, 39, 52). In the BfpB secretin system, however, no such protein has been identified. Instead, as reported here, BfpB physically interacts with BfpG, a small outer-membrane protein required for BfpB multimerization but not for outer-membrane localization. Moreover, BfpG could not be shown to be a lipoprotein. These and other differences in function that are described in this report suggest that BfpB defines a distinctive subclass of outer-membrane proteins that intersect with the secretin protein superfamily.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in these experiments are described in Table 1. Optimal growth conditions for BFP induction were as previously described (42). Briefly, cells from an overnight Luria-Bertani (LB) broth culture were suspended in phosphate-buffered saline (PBS) to an optical density of 1.8 at 600 nm (the standard overnight culture). The bacteria suspension was diluted 1:100 in Dulbecco's modified Eagle's medium (DMEM), and the cultures were grown with shaking at 37°C.
TABLE 1.
Strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| B171-8 | Wild-type EPEC 0111:NM; carries the 80-kb EAF plasmid | 18 |
| B171-8ΔAcm | B171-8 EAF plasmid mutant with an in-frame deletion of the bfpA gene | 3 |
| B171-8ΔB | B171-8 EAF plasmid mutant with base pair substitutions in the bfpB sequence resulting in translational stop codons at amino acids 40, 41, and 43 | 45 |
| B171-8ΔB2 | B171-8 EAF plasmid mutant with an in-frame deletion of the bfpB gene | Ramer, unpublished |
| B171-8ΔG | B171-8 EAF plasmid mutant with an in-frame deletion of the bfpG gene | Ramer, unpublished |
Preparation of membrane fractions.
Cell membranes were prepared from bacteria grown under optimal bfp-inducing conditions, i.e., 3.5 h of growth in DMEM at 37°C from a 1:100 dilution of a standard overnight culture. Cells from 5 liters of culture were harvested by centrifugation at 2,000 × g at 4°C for 20 min, washed once in cold 10 mM HEPES (pH 7.4), and then recentrifuged at 2,000 × g for 20 min. The cell pellets were resuspended in a total of 60 ml of 10 mM HEPES (pH 7.4), and two Complete Protease Inhibitor Cocktail tablets (Boehringer Mannheim) were crushed and added. The bacterial suspension was passed through a French press at 14,000 lb/in2. The suspension was examined with phase-contrast microscopy to determine whether the majority of cells were successfully broken and then was centrifuged at 1,000 × g for 15 min to remove any unbroken cells. The supernatant was applied to sucrose step gradients, with 5 ml of 55% sucrose, 17 ml of 42% sucrose, and 13 ml of 24% sucrose. All sucrose solutions were measured by weight in 10 mM HEPES (pH 7.4). The gradients were centrifuged in a Beckman ultracentrifuge with a SW 28 rotor at 26,000 rpm for 72 h. After centrifugation, outer membranes could be visualized at the interfaces of the 42 and 55% sucrose solutions, and inner membranes could be visualized at the interfaces of the 24 and 42% sucrose solutions. The centrifugation tubes were punctured with a syringe needle to collect the inner- and outer-membrane fractions. Identical fractions were pooled and diluted to less than 5% sucrose with 10 mM HEPES (pH 7.4), and membranes were collected by centrifugation at 100,000 × g for 2 h. Membranes were stored in HEPES buffer at either 4 or −80°C. The degree of cross-contamination between membrane fractions was assessed by quantification of a representative outer-membrane enzyme marker, 2-keto-3-deoxyoctonic acid, as described by Keleti (28). In addition, the localization patterns of two proteins encoded by the bfp operon, BfpA and BfpB, were examined by Western immunoblotting with BfpB and BfpA antisera (described below) and compared to previously published results, which showed that BfpA localizes to the inner membrane whereas BfpB localizes to the outer membrane (45).
Purification of BfpB complexes.
One milligram of EPEC outer membranes was suspended in a buffer containing 100 mM NaCl, 10 mM EDTA, and 10 mM Tris (pH 7.5) (buffer A) and centrifuged at 100,000 × g for 2 h. Membrane pellets were gently resuspended in 500 μl of buffer A containing 20% glycerol, 4% sodium dodecyl sulfate (SDS), and 0.4 M β-mercaptoethanol (2-ME) and incubated at 37°C for 30 min. Solubilized membranes were dialyzed overnight at room temperature against buffer A containing 0.1% SDS. The dialyzed material was concentrated with a Centricon-100 unit (Amicon) and applied to a 15 to 35% (wt/wt) linear sucrose gradient in buffer A. Gradients were centrifuged with a Beckman ultracentrifuge with a SW41 rotor for 24 h at 35,000 rpm. The bottom of the centrifuge tube was punctured with a needle, and 250-μl aliquots were collected from the bottom of the gradient. Aliquots containing BfpB high-molecular-weight (HMW) complexes were identified by polyacrylamide gel electrophoresis (PAGE) and Western blot analysis with BfpB antiserum (described below).
Immunoblotting and gel electrophoresis.
Whole-cell lysates or purified membrane fractions were mixed with standard SDS-PAGE sample buffer (33) containing 1% SDS and 0.1% 2-ME and heated to either 100°C or the indicated temperature for 7 min. Proteins were separated by PAGE (with a 12% acrylamide gel unless otherwise indicated) and then electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked with 5% dry milk in PBS containing 1% Tween 20 (polyoxyethylene-sorbitan monolaurate) and then incubated with a 1:25,000 dilution of a BfpB antiserum (45) or a 1:1,000 dilution of a BfpG affinity-purified antibody (Ramer, unpublished). Membranes were incubated with secondary horseradish peroxidase-labeled anti-rabbit antibodies at a dilution of 1:5,000 and developed by enhanced chemiluminescence (Amersham).
Homology searching.
All protein homology searches were done in pairwise combinations using the BLAST 2 Sequences program, a BLAST-based tool for aligning two protein sequences available at the National Center for Biotechonology Information website, www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html (56).
Electron microscopy (EM)
Five microliters (approximately 0.3 μg of protein/μl) of sucrose gradient fractions identified as containing BfpB complexes was adsorbed to carbon-coated nickel grids for 5 min at room temperature. The proteins were then fixed with 2% formaldehyde–0.2% glutaraldehyde for 5 min, washed once with water, and stained for 2 min with 1% uranyl acetate in water. The excess stain was removed, and the grids were allowed to air dry.
Radiolabeling of lipoproteins.
Standard overnight cultures were diluted 1:100 in DMEM, and the cells were grown with shaking at 37°C to an optical density of 0.5 at 600 nm. Radiolabeling was then performed as described previously (45). Briefly, [3H]palmitic acid was added to a final concentration of 50 μCi/ml, and the cells were then incubated at 37°C for 2 h. Proteins were precipitated with trichloroacetic acid, pelleted by microcentrifugation, and then subjected to SDS-PAGE analysis on a 12% acrylamide gel. Radiolabeled proteins in the dried gel were detected by fluorography after exposure to XAR-5 X-ray film for 2 weeks.
Immunoprecipitation.
Standard overnight cultures were diluted 1:100 in 15 ml of DMEM and grown at 37°C with shaking. After 3.5 h of growth, the bacteria were pelleted by centrifugation. The cell pellets were resuspended in 1 ml of PBS and sonicated on ice using a Branson sonifier until the opacity cleared. Samples (each, 100 μl) of the sonicates were combined with 100 μl of buffer 1 (20 mM Tris [pH 8], 100 mM NaCl, 0.5% deoxycholate, 0.1% SDS) and either 10 μl of a BfpB antiserum (45) or 20 μl of a BfpG antiserum (Ramer, unpublished). The reaction mixtures were then incubated, with rotation, for 2 h at 4°C. Two hundred microliters of a 50% slurry of GammaBind protein G-Sepharose beads (Pharmacia Biotech) in buffer 2 (10 mM Tris [pH 8], 50 mM NaCl, 0.25% deoxycholate, 0.05% SDS) was added to each of the reaction mixtures, which then were incubated overnight, with rotation, at 4°C. The reaction mixtures were centrifuged, the supernatants were removed, and the protein G-Sepharose beads were washed three times with buffer 2. Following the last wash, the beads were resuspended in 100 μl of SDS-PAGE sample buffer and heated to 80°C for 30 min. Forty microliters of the supernatant was then analyzed by PAGE and Western blotting with BfpB and BfpG antibodies.
Cross-linking.
Dithiobis-(sulfosuccinimidyl) propionate (DTSSP) (Pierce) was added to 1.0 ml of 1.0-mg/ml purified outer membranes suspended in PBS to make a final reaction concentration of 0.2 mM. After a 10-min incubation period at room temperature, the reaction mixtures were quenched by the addition of Tris-HCl (pH 7.5) to a concentration of 200 mM. After a 10-min incubation period at room temperature, the membranes were used for the purification of BfpB complexes as described above.
Vancomycin assay
Standard overnight cultures were diluted 1:100 in 30 ml of DMEM and grown at 37°C with shaking. After 1.5 h of growth, 15 μl of a 16-mg/ml vancomycin stock solution (prepared in water) (Sigma) was added to the shaking cultures. At 6.5, 8.5, and 10.5 h after the addition of the antibiotic, 10-μl aliquots (approximately 107 cells) were collected from each of the antibiotic-treated cultures and untreated control cultures. Viable plate counts on LB agar were obtained using 10-fold serial dilutions.
Secretion assay.
Standard overnight cultures were diluted 1:100 in 250 ml of DMEM and grown at 37°C with shaking. After 3, 5, or 8 h of growth, 60-ml aliquots were collected from the shaken cultures. The cells were pelleted by centrifugation at 2,000 × g. The supernatants were collected, filter sterilized, and concentrated to a final volume of 200 μl with Centricon Plus-80 centrifugal filter devices with a molecular mass cutoff of 3 kDa. The concentrated supernatants were ultracentrifuged at 100,000 × g for 2 h to remove any remaining membrane particles. Twenty-five microliters of the concentrated supernatants was analyzed by SDS-PAGE and Coomassie blue staining.
RESULTS
BfpB forms a HMW complex in the outer membrane.
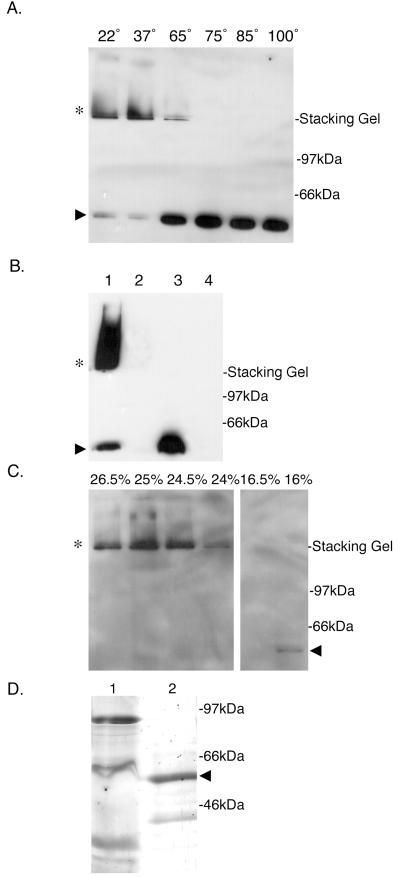
The E. coli bacteriophage f1 protein pIV, a member of the secretin superfamily, exhibits C-terminal region homology with BfpB and exists as an outer-membrane multimer that does not dissociate when boiled in protein gel sample buffer containing 4% SDS (27). To determine if BfpB, which also localizes to the outer membrane (45), forms an SDS-stable multimer, purified outer membranes from wild-type EPEC strain B171-8 were incubated in Laemmli sample buffer containing 4% SDS at temperatures of 22, 37, 65, 75, 85, and 100°C. The molecular mass of BfpB was then determined by SDS-PAGE and Western blotting with a BfpB antiserum. BfpB was found to migrate as a HMW complex that remains in the stacking gel when incubated in sample buffer up to temperatures of 65°C (Fig. 1A). Incubation temperatures of 75, 85, and 100°C caused BfpB to migrate at its monomeric molecular mass of 58.2 kDa. Whole-cell extracts of a BfpB deletion strain, B171-8ΔB2, were also examined for the presence of BfpB antibody-reactive HMW complexes. As expected, no such complexes were detected (Fig. 1B).
FIG. 1.
BfpB forms a HMW outer-membrane complex. (A) Temperature-dependent stability of the BfpB multimer in SDS. Purified outer membranes (3 μg of protein), prepared from the wild-type strain B171-8, were incubated in SDS-PAGE sample buffer at the indicated temperatures (in centrograde). Following SDS-PAGE, Western blot analysis with anti-BfpB antiserum was performed. When incubated in PAGE sample buffer at temperatures of 22 and 37°C, BfpB migrates principally as an HMW complex (∗) with some monomeric species (▸). At temperatures greater than 65°C, the predominant species is the monomer of BfpB (▸). (B) HMW complexes reactive with BfpB antisera (∗) are not detected in the bfpB deletion strain, B171-8ΔB2. Whole cells were sonicated and incubated in SDS-PAGE sample buffer for 7 min at the indicated temperature, and equal protein amounts were loaded onto a 12% polyacrylamide gel. Western blot analysis with anti-BfpB antiserum was performed. Lane 1, B171-8 whole-cell sonicate incubated at room temperature; lane 2, B171-8ΔB2 whole-cell sonicate incubated at room temperature; lane 3, B171-8 whole-cell sonicate incubated at 100°C; lane 4, B171-8ΔB2 whole-cell sonicate incubated at 100°C. (C) Isolation of the BfpB multimer by sucrose gradient centrifugation. Outer membranes isolated from B171-8 bacteria were solubilized at 37°C in a buffer containing 4% SDS and loaded onto a linear sucrose gradient. Following centrifugation, 20-μl aliquots from individual sucrose gradient fractions were mixed with an equal volume of SDS-PAGE sample buffer and loaded onto a 12% polyacrylamide gel without prior heating of the sample. Immunoblotting with anti-BfpB antiserum was used to detect BfpB. The BfpB HMW complex (∗) localized to 25% sucrose, while the BfpB monomer localized to 16% sucrose (◂). (D) Characterization of the isolated BfpB multimer. Sucrose gradient fractions containing the BfpB multimer were boiled in sample buffer and separated on a 12% polyacrylamide gel, and the proteins were visualized by silver staining. Lane 1, 30 μg of the outer-membrane starting material, heated for 7 min at 100°C in SDS-PAGE sample buffer; lane 2, 30 μg of sucrose gradient fractions containing the BfpB HMW complex, heated for 7 min at 100°C in SDS-PAGE sample buffer. The primary protein identified by silver staining corresponds to the size of the BfpB monomer (◂). Immunoblotting with anti-BfpB antiserum was used to confirm the identity of the two silver-stained bands and to conclude that the smaller 39-kDa band is a BfpB breakdown product. A band corresponding to BfpB cannot be detected in the crude outer-membrane fraction, most likely because it is a minor component of the outer membrane.
The stability of the wild-type BfpB complex in 4% SDS at temperatures below 65°C allowed it to be separated from SDS-treated outer membranes by sucrose gradient centrifugation. The BfpB complex localized at 24 to 26% (wt/wt) sucrose, whereas the BfpB monomer localized at 16% (wt/wt) sucrose (Fig. 1C). In addition to the type II secretion system encoded by the bfp operon, EPEC also possesses a type III secretion system, including a secretin homologue, SepC, with a predicted molecular mass of 56 kDa (24). To determine if the latter secretin homologue might have copurified with the BfpB complex, the protein content of sucrose gradient fractions containing BfpB complexes was further characterized by SDS-PAGE analysis and silver staining. Sucrose gradient fractions containing BfpB complexes were heated in SDS sample buffer to 100°C to dissociate BfpB multimers prior to loading the gel. The resulting silver-stained gel showed that the HMW BfpB antibody-reactive complex is composed mainly of a protein that migrates with the same molecular mass as the Western blot-reactive BfpB monomer (Fig. 1D). A minor, lower-molecular-weight protein with an estimated molecular mass of 39 kDa was also detected by silver staining. Because it too reacted with the BfpB antibody, it was presumed to be a BfpB breakdown and/or truncated product. Thus, the HMW complex that was extracted from the outer membrane with 4% SDS and refined by sucrose gradient centrifugation comprises mainly or exclusively BfpB, within the limits of detection of the silver-staining method.
Ultrastructural character of the BfpB complex.
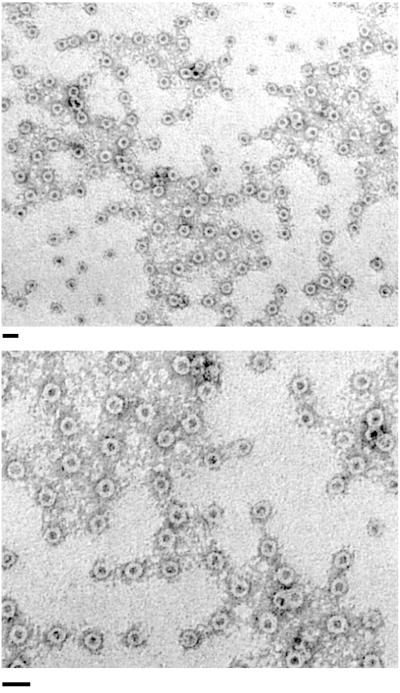
Transmission EM of negatively stained samples of the purified BfpB complex revealed that BfpB multimerizes to form a ring-like structure with a stain-filled center (Fig. 2). The structures have an internal diameter of approximately 7 nm and a total diameter of approximately 20 nm. No other structures were evident—neither membrane vesicles nor pilus filaments.
FIG. 2.
The isolated BfpB HMW complex adopts a ring-like configuration. Negatively stained BfpB complexes, prepared by SDS treatment at 37°C and sucrose gradient centrifugation, were visualized with transmission EM. Bar, 30 nm.
To further confirm the relationship between BfpB and the complexes visualized by EM, a second method of purification was undertaken. A polyhistidine epitope was added to the C terminus of the BfpB amino acid sequence. Gel electrophoresis and Western blot analysis revealed that the His-tagged BfpB protein still forms HMW complexes that copurify with the outer membrane. The His-tagged BfpB multimer was then purified from detergent-solubilized outer membranes by nickel affinity column chromatography. When the affinity-purified, His-tagged BfpB complexes were examined by EM, ring-shaped structures, identical in morphology and size to those depicted in Fig. 2, were observed (data not shown). SepC, a second secretin homologue that is found in EPEC, contains only two histidine residues and these are widely separated in the sequence. Thus, it is unlikely that SepC would have been retained by the nickel column and copurified with BfpB. Taken together, the ultrastructural appearance of the affinity-purified, His-tagged BfpB complexes and the molecular mass of the principal silver-stained species shown in Fig. 1D provide compelling evidence that BfpB forms the structures depicted in Fig. 2.
The BfpB multimer interacts with BfpG.
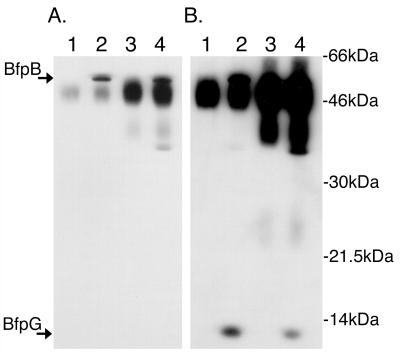
Cell fractionation experiments have revealed that BfpB and BfpG, a protein encoded by a gene located immediately upstream of bfpB, are the only two proteins encoded by the bfp operon that consistently associate with the outer membrane, regardless of the fractionation method used or how those fractions were separated (Ramer, unpublished) (41, 45). To determine if BfpG interacts directly with BfpB, outer membranes were prepared from wild-type strain B171-8 and from two B171-8 mutants, carrying in-frame targeted disruptions of either bfpB or of bfpG. Proteins and protein complexes solubilized from these membranes with an ionic detergent were incubated with protein G-Sepharose beads and either a BfpB- or a BfpG-specific antiserum. The constituents of the resulting immunoprecipitates were then identified by Western blotting. Examination of Fig. 3 shows that antibody to BfpB coprecipitates BfpG and, conversely, that antibody to BfpG coprecipitates BfpB. These data suggest that BfpB is associated with BfpG within the outer membrane.
FIG. 3.
BfpB and BfpG coimmunoprecipitate. (A) An antiserum to BfpG coimmunoprecipitates BfpB. Immunoprecipitates were heated to 80°C in SDS-PAGE sample buffer, separated by PAGE, transferred to a nylon membrane for Western analysis, and probed with anti-BfpB antiserum. Lane 1, B171-8ΔG immunoprecipitated with anti-BfpG; lane 2, B171-8 immunoprecipitated with anti-BfpG; lane 3, B171-8ΔB immunoprecipitated with anti-BfpB; lane 4, B171-8 immunoprecipitated with anti-BfpB. Both the anti-BfpB and the anti-BfpG antibodies were able to immunoprecipitate BfpB protein. The dark abundant bands between BfpB and BfpG are rabbit immunoglobulins, Western reactive with the HRP-labeled secondary donkey anti-rabbit immunoglobulin G antibody used in Western detection. (B) An antiserum to BfpB coimmunoprecipitates BfpG. The membrane shown in panel A was sequentially probed with anti-BfpG antiserum without prior stripping of the BfpB antibodies. Both the anti-BfpB and anti-BfpG antibodies were able to immunoprecipitate BfpG protein.
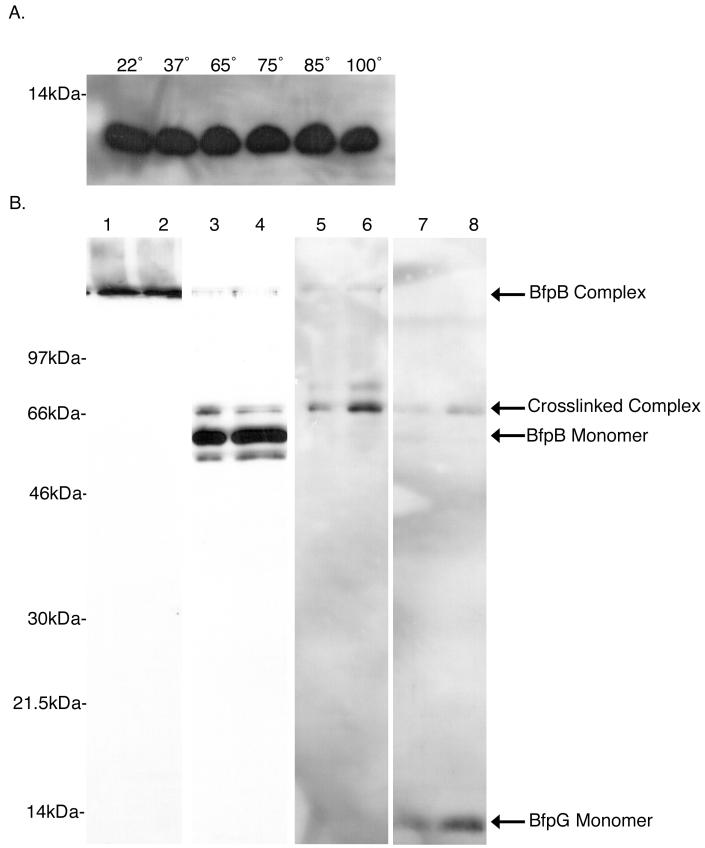
To learn more about the potential association of BfpG with the BfpB complex, outer membranes were prepared from the wild-type strain B171-8, incubated in SDS sample buffer at temperatures ranging from 22 to 100°C, and then subjected to SDS-PAGE and Western blotting with a BfpG antiserum. Unlike BfpB, which migrates as a multimer when incubated in SDS sample buffer at temperatures below 65°C (Fig. 1A), BfpG runs as a monomer at every incubation temperature tested (Fig. 4A). Thus, if BfpG interacts with the BfpB multimer, the association is not SDS stable under the conditions tested.
FIG. 4.
The BfpB complex can be chemically cross-linked with BfpG. (A) BfpG is a monomer under conditions that BfpB is a multimer. Three micrograms of outer-membrane protein from the B171-8 wild-type strain was incubated in sample buffer containing 4% SDS for 7 min at various temperatures. Following SDS-PAGE, Western blot analysis using αBfpG antiserum was performed. BfpG runs as a monomer at every incubation temperature. (B) DTSSP cross-links BfpG to the BfpB multimer. One milligram of outer membranes from the wild-type B171-8 strain was treated with a 0.2 mM solution of the reducible cross-linker DTSSP. After cross-linking, BfpB complexes were purified. The same two adjacent sucrose gradient fractions, containing BfpB complexes, were used in each of the following immunoblots. Lanes 1 and 2, anti-BfpB immunoblot of purified complexes incubated at 22°C in sample buffer with no 2-ME; lanes 3 and 4, anti-BfpB immunoblot of purified complexes incubated at 100°C in sample buffer with no 2-ME; lanes 5 and 6, anti-BfpG immunoblot of purified complexes incubated at 100°C in sample buffer with no 2-ME; lanes 7 and 8, anti-BfpG immunoblot of purified complexes incubated at 100°C in sample buffer with 2-ME. The intensity of the cross-linked complex decreases and BfpG monomer appears.
In light of this information, chemical cross-linking experiments were performed to determine if the HMW BfpB complex shown in Fig. 1 interacts with BfpG when both are present in the outer membrane. For this purpose, purified outer membranes from wild-type strain B171-8 were treated with the bifunctional cross-linker DTSSP. When molecules cross-linked by DTSSP are exposed to reducing agents, including 2-ME in PAGE sample buffer, they dissociate into their constituent parts, permitting their identification by SDS-PAGE analysis and Western blotting. The DTSSP cross-linked outer membranes were solubilized by SDS treatment at room temperature. Then, the cross-linked BfpB complex was isolated by sucrose gradient centrifugation and analyzed by SDS-PAGE and Western blotting. Western blot analysis of the purified, cross-linked BfpB complex that had been boiled in sample buffer without 2-ME was performed with the BfpB antibody and revealed three BfpB-reactive bands: one corresponds to BfpB monomer; a second band corresponds to a lower-molecular-weight species that most likely represents a BfpB breakdown product; and a third corresponds to an higher-molecular-weight band that was hypothesized to comprise cross-linked species (Fig. 4B). The higher-molecular-weight band was also found to react with a BfpG antiserum, and thus the same band reacts with two antisera, one specific for BfpG and the other for BfpB. When the cross-linker was dissociated with 2-ME, Western blot analysis with the BfpG antiserum revealed a decrease in the intensity of the higher-molecular-weight band and the appearance of BfpG monomer. Thus, after purified outer membranes were subjected to a cross-linking reaction, BfpG could be copurified with the BfpB complex, demonstrating that BfpG interacts with the BfpB multimer.
The BfpB multimer requires BfpG for stability and/or formation.
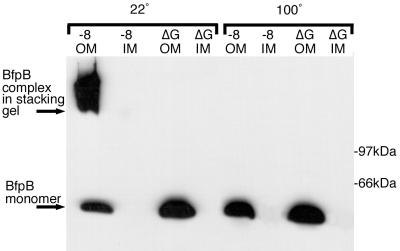
To examine the possibility that BfpB-BfpG interaction is required for the formation and/or stability of the HMW BfpB complex shown in Fig. 1A, a BfpG deletion strain (B171-8ΔG) was examined for the presence of the BfpB multimer. Western blot analysis of purified inner and outer membranes with the BfpB antiserum showed that B171-8ΔG did not produce the HMW BfpB complex produced by the wild-type parent. Instead, when outer membranes from B171-8ΔG were incubated in sample buffer at 22°C prior to SDS-PAGE, the principal BfpB species migrated as a monomer (Fig. 5). Thus, in the absence of BfpG, BfpB migrates as a monomer and assembly of BfpB into a higher-order structure appears to require BfpG. Alternatively, in the absence of BfpG, BfpB might still form an outer-membrane HMW complex that is unstable when incubated in 4% SDS at room temperature.
FIG. 5.
BfpB requires BfpG for multimerization but not localization to the outer membrane. Three micrograms of outer- and inner-membrane proteins from B171-8 and B171-8ΔG was loaded onto a 15% polyacrylamide gel. Western blot analysis with anti-BfpB antiserum was performed. Samples on the left half were incubated at 100°C in sample buffer for 7 min, while samples on the right were incubated at 22°C for 7 min.
To further assess how BfpB depends on BfpG, the localization of BfpB in the BfpG mutant was determined using outer and inner membranes and Western blotting with BfpB antiserum. BfpB was found to localize to the outer membrane of B171-8ΔG (Fig. 5), just as it does in the wild-type parent strain. Thus, BfpG is required for the formation or stability of BfpB multimers but not for the localization of BfpB to the outer membrane.
BfpG cannot be demonstrated to be a lipoprotein.
Several small lipoproteins have been identified that are required to correctly localize their cognate secretin to the outer membrane (8, 9, 19, 39). BfpG was originally predicted to be a lipoprotein (53) because the deduced N-terminal amino acid sequence shows a cysteine residue at amino acid position 14 that might potentially be acylated during processing by signal peptidase II. However, the residues preceding this Cys that would correspond to a signal peptidase II processing site (Leu-Val-Ser-Cys) deviate slightly from the conventional consensus sequence (Leu-[Ala,Ser]-[Gly,Ala]-Cys) (21). Therefore, to determine if BfpG is in fact a lipoprotein, the wild-type strain was metabolically labeled with [3H]palmitic acid and the lysate was analyzed by SDS-PAGE and autoradiography. The BfpG deletion mutant was labeled and analyzed as well to identify BfpG among other labeled proteins of similar molecular mass. Additionally, to increase the sensitivity of the assay, a BfpG antiserum was used to immunoprecipitate BfpG from a lysate of [3H]palmitate-labeled bacteria. While BfpG was successfully recovered within the immunoprecipitate as determined by Western blotting, neither of these methods led to the identification of 3H-labeled BfpG (data not shown) despite the fact that these conditions had successfully labeled BfpB in this strain (45) and several of the small piloting lipoproteins in other species (9, 10, 19, 52).
The BfpB multimer forms in the absence of pilus production.
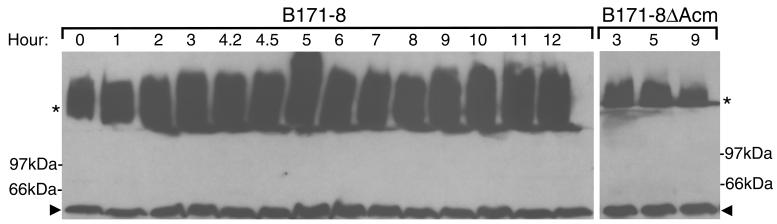
Previous experiments have demonstrated that BFP expression is regulated by environmental and growth phase signals, with maximal BfpA abundance (the principal structural subunit of the pilus filament) being detected during the exponential phase of growth (42). Although BfpA and BfpB are both products of the same operon, it is possible that production of the BfpB multimer and BfpA is cotemporaneous, due to different turnover rates. To address this question, wild-type EPEC cells were collected and examined for the presence of HMW BfpB multimers during both the exponential phase (when BfpA abundance is maximal) and the stationary phase of growth (when BfpA abundance has significantly declined) (39). Whole cells were sonicated and incubated in sample buffer at 22°C, and equal amounts of protein were subjected to SDS-PAGE. Western blot analysis with a BfpB antiserum revealed that BfpB exists as a HMW complex during all phases of growth (Fig. 6), including late stationary phase, at which time Western and Northern blot-detectable levels of BfpA and the corresponding mRNA are greatly reduced (39). Thus, the BfpB complex persists after bfp operon transcriptional expression has declined to basal levels. This finding also pointed to the possibility that BfpB complex formation and/or stability does not require BfpA. To address this issue, B171-8ΔAcm was examined; this mutant does not make the BfpA pilus subunit, but it produces all of the remaining components of the bfp operon, including BfpB and BfpG. Western blot analysis of this mutant with a BfpB antiserum showed that the HMW BfpB complex is present during all growth phases Fig (6B), just as is the case for the wild-type parent (Fig. 6A). Therefore, BfpB multimerization does not require BfpA.
FIG. 6.
BfpB forms a HMW complex in the absence of BfpA expression. Cells were collected from cultures of either the wild-type strain B171-8 or the BfpA knockout mutant, B171-8ΔAcm, at the indicated times and sonicated, and equal amounts of protein were loaded onto 12% polyarcylamide gels. Western analysis with anti-BfpB antiserum was performed with B171-8 (left) and B171-8ΔAcm (right). Arrowheads, BfpB monomers; stars, BfpB complexes.
The BfpB multimer is associated with sensitivity to vancomycin.
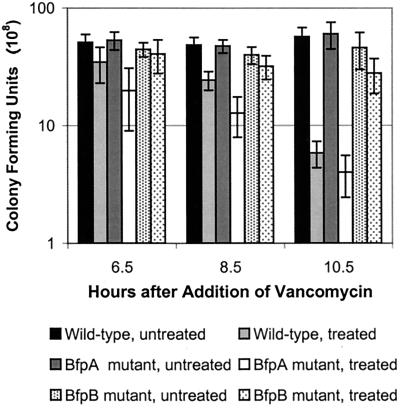
Vancomycin inhibits cell wall synthesis by gram-positive bacteria and is bactericidal at concentrations of 0.5 to 10 μg/ml (26). Gram-negative bacteria, however, are normally resistant to vancomycin, as this hydrophobic molecule (molecular weight, 1,486) cannot penetrate the outer membrane of the cell (26). Because of these properties, vancomycin has previously been used to investigate if secretins form channels through the outer membrane that are normally gated (39, 40, 48, 50). If the BfpB complex shown in Fig. 2 contains a central channel, then the ∼7-nm internal diameter that is predicted from the image would be large enough to allow passage of vancomycin through the outer membrane, rendering the cell susceptible to the drug. Alternatively, if the BfpB complex does not comprise a transmembrane channel or if the channel is completely gated, as is the case for the pIV and PilQ secretins of E. coli f1 phage and Myxococcus xanthus (50, 57), respectively, then the wild-type EPEC strain should be resistant to the antibiotic. To test these possibilities, the bactericidal activity of vancomycin for three related strains was compared: B171-8 (the wild-type parent), B171-8ΔAcm (the mutant which does not produce the BfpA structural pilus subunit, but which continues to produce all other components of the BFP assembly complex, including the BfpB multimer), and B171-8ΔB (the mutant which does not produce either the BfpB monomer or multimer). The bactericidal effect of vancomycin at a concentration of 8 μg/ml for these EPEC strains was determined by adding vancomycin 1.5 h after the 1:100 dilution of a standard overnight culture in DMEM. Antibiotic-treated cultures and untreated control cultures were diluted and plated onto antibiotic-free LB agar 6.5, 8.5, and 10.5 h after the addition of the drug. Surviving colonies were counted the following day.
The B171-8ΔAcm mutant and the wild-type strain were significantly more susceptible to the bactericidal action of vancomycin than the isogenic BfpB mutant strain (Fig. 7). Thus, the presence of the BfpB outer-membrane complex, whether in the BFP filament-producing wild-type strain or in the pilus filament-negative mutant, is associated with susceptibility to vancomycin. While it is possible that the insertion of BfpB complexes in the outer membrane causes nonspecific changes in outer-membrane stability, the observed increased sensitivity to vancomycin and the ultrastructural appearance of the BfpB multimer are consistent with the idea that the BfpB multimer forms an incompletely gated transmembrane pore.
FIG. 7.
The BfpB complex renders cells susceptible to vancomycin. Vancomycin at a final concentration of 8 μg/ml was added to shaking DMEM cultures at 37°C, 1.5 h after inoculation from an overnight culture. Bacteria were collected from vancomycin-treated cultures and untreated control cultures, diluted, and plated 6.5, 8.5, and 10.5 h after addition of the antibiotic. Surviving colonies were counted the following day. Each data point on the graph is the average of at least six separate experiments, and error bars represent the standard deviations. The wild-type strain is B171-8, the BfpA mutant is B171-8ΔAcm, and the BfpB mutant is B171-8ΔB. B171-8 and B171-8ΔAcm, both of which express the outer-membrane BfpB multimer, were significantly killed by vancomycin 10.5 h after the addition of the antibiotic. By contrast, the viability of B171-8ΔB, which does not express the outer-membrane BfpB multimer, was not significantly affected by antibiotic addition.
BfpB is associated with the secretion of nonpilus proteins.
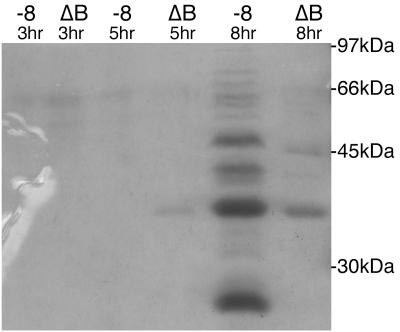
EPEC has been shown previously to secrete several proteins into spent culture media, including EspA, -B, and -D (24, 30). Secretion of these proteins has been shown to be dependent on SepB, a putative inner-membrane protein and one of four proteins identified as a component of the type III secretion system of EPEC (24). To test whether the secretion of proteins during AA in EPEC occurs and if it is dependent on the expression of the BfpB secretin, the wild type-strain B171-8 and the BfpB deletion strain B171-8ΔB were grown in DMEM tissue culture medium, and the supernatants were collected during the early stages of AA of the wild-type strain (at hour 3), during the height of AA (at hour 5), and long after the dispersal of the autoaggregates (at hour 8). Samples of B171-8ΔB supernatants were obtained at the same time points as the wild-type strain, recognizing that the BfpB deletion strain does not exhibit the AA phenotype. Each pair of concentrated and filter-sterilized supernatants was compared by SDS-PAGE, and the separated proteins were visualized by Coomassie blue staining. At the end of 3 and 5 h of growth, little protein was detected in the culture supernatants of either strain. However, at the end of 8 h of growth, approximately 2.5 h after dispersal of the wild-type strain from the autoaggregates, significant differences between the wild-type and BfpB deletion strain were apparent, both with respect to the amount of protein in the medium and the kinds of protein, as judged by estimated molecular mass. In particular, the supernatant from the wild-type strain contained at least 11 proteins that were not detected in the supernatant of the BfpB deletion strain (Fig. 8). To determine if the presence of proteins in the spent culture medium of the wild-type strain might be due to cell lysis, the wild-type and BfpB deletion strains were grown in DMEM containing lactose to induce the expression of endogenous β-galactosidase, a cytoplasmic enzyme. Enzyme activity was then determined in the supernatant of the growth medium and in the cell pellet (after lysis) at 1, 3, 5, and 8 h of growth. Increased levels of β-galactosidase activity were not detected in the spent culture medium of the wild-type strain, compared to the BfpB deletion strain (data not shown). Therefore, we conclude that the presence of proteins in the spent culture medium of strains carrying the BfpB outer-membrane multimer is not due to lysis.
FIG. 8.
Comparative analysis of proteins accumulating in tissue culture medium during growth of the wild-type strain, B171-8, and the BfpB deletion strain, B171-8ΔB. Tissue culture medium was collected 3, 5, and 8 h after a 1:100 dilution of an overnight culture into DMEM, and the cells were pelleted by centrifugation. The supernatants were filter sterilized, concentrated 300-fold by ultrafiltration, and analyzed by SDS-PAGE and Coomassie blue staining. In the absence of the BfpB HMW complex, the secretion of proteins into the media is reduced at the 8-h time point. Each lane contains 25 μl of concentrated supernatant from either B171-8 or B171-8ΔB culture media collected at the indicated time point.
DISCUSSION
All secretins studied thus far form outer-membrane multimers (4, 7, 8, 32, 34, 39), possess significant sequence homology, including a conserved C-terminal secretin motif (17), and, except for XpsD, have an acylated N-terminal cysteine (23). BfpB differs from other secretins by having an acylated N-terminal cysteine (45) and by lacking the secretin signature motif. Further analysis of BfpB primary structure reveals that it does not share significant homology with 15 of 16 previously characterized secretins. By contrast, each of the 16 analyzed secretins shares significant homology with at least 13 other secretins. Despite these distinctive characteristics of BfpB, three features qualify it as a member of the secretin protein family. First, the C-terminal half of the BfpB amino acid sequence is related (21% identical residues over a 282-amino-acid span) (56) to the pIV phage protein, a prototypical member of the secretin family (27). A BLAST search of BfpB also revealed that its sequence is significantly homologous to several probable secretins in Salmonella enterica serovar Typhi, Vibrio cholerae, and Campylobacter jejuni, although these proteins have not yet been isolated and characterized. Second, the pore-like structures depicted in Fig. 2 show that the BfpB multimer resembles, in shape and in size, the five other secretins whose ultrastructural features have been characterized by EM (4, 8, 32, 34, 39). Third, BfpB either forms or is required for the formation of a channel through the outer membrane, based on the sensitivity of BfpB-expressing strains to vancomycin (Fig. 7). Thus, with very little sequence conservation, BfpB seems to have achieved many analogous structural and functional features of other members of the secretin family.
Cell fractionation studies have disclosed that both BfpB and BfpG are outer-membrane proteins, and chemical cross-linking and immunoprecipitation experiments provide compelling evidence that they physically interact in situ. While neither BfpB nor BfpG can be extracted from the outer membrane by Triton X-100 or Sarkosyl (Ramer, unpublished), BfpG readily dissociates from the BfpB multimer upon exposure of outer-membrane fractions to 4.0% SDS at room temperature. Therefore, the stable pore-like structures shown in Fig. 2 do not contain BfpG. In this respect, BfpG resembles small lipoproteins which dissociate from the secretins PilQ, InvG, and YscC during purification (8, 14, 16, 32). Nevertheless, BfpG is required for the formation of stable multimers because in an in-frame bfpG deletion mutant, BfpB does not form a HMW complex and instead migrates as a monomer. Thus, BfpG is not required for the SDS stability of the BfpB multimer once the multimer has formed but rather is required for the multimerization process itself.
Cell fractionation experiments with the wild-type strain and the bfpG deletion mutant also show that BfpG is not required for the outer-membrane localization of BfpB. This is evident in Fig. 5, where the intensity of the Western blot BfpB monomer signal in purified outer membranes from the bfpG deletion mutant is shown to be the same as the intensity of the BfpB multimer signal in outer membranes prepared from the isogenic wild-type parent. Futhermore, the ratio of the outer-membrane BfpB Western signal to the inner-membrane BfpB signal is the same in the wild-type and bfpG deletion strains (Fig. 5). Figure 5 also demonstrates that BfpG is not required for BfpB monomer stability, because nearly all of the Western blot-reactive BfpB protein produced by the bfpG deletion mutant migrates at the molecular mass predicted from the size of the bfpB ORF. Based on these data, BfpG likely plays an essential role after the BfpB monomer associates with the outer membrane but before BfpB exists as an SDS-resistant, HMW complex that no longer requires BfpG for stability.
Several features of the BfpG-BfpB interaction reported here differ significantly from more typical members of the secretin family. First, most biochemically characterized secretins require a small (∼12- to ∼20-kDa) outer-membrane lipoprotein for the outer-membrane localization and/or stability of their cognate secretin—the piloting chaperone function first described by Pugsley and colleagues for the PulS protein of the Klebsiella oxytoca pullulanase secretion system (8, 9, 15, 19, 39, 52). However, BfpG, the main candidate encoded by the bfp operon that might serve an analogous function, could not be shown to be a lipoprotein by metabolic labeling with [3H]palmitate. However, the negative results described here (see Results) do not entirely exclude the possibility that BfpG is a lipoprotein; future structure-function studies of this protein could include intrinsic labeling of overexpressed BfpG, the use of globomycin to determine if this compound causes a change in the SDS-PAGE migration behavior of BfpG, and the engineering of BfpG to effect N-terminal region sequence changes that replace the native sequence with type I and type II signal peptide consensus sequences.
A second feature that distinguishes the BfpG-BfpB interaction from other secretins comes from a comparative analysis of the functional roles of BfpG and the roles of the small lipoprotein pilots discussed above, though such a survey lacks precision because of differences in methodology and genetic backgrounds. Interestingly, while many of the small lipoprotein pilots described thus far share significant functional similarity, the sequences of these proteins are not well conserved. Nonetheless, the functions of BfpG in the biogenesis of the BfpB secretin described here differ fundamentally from the function of the class of small lipoprotein pilots and specifically for the role of PulS in the biogenesis of the PulD secretin (19, 20). Most significantly, PulS is not required for the multimerization of PulD, whereas BfpG is required for the multimerization of BfpB (19). Additionally, PulS is required for the correct insertion of the PulD multimer into the outer membrane (19), whereas BfpG is not required for the outer-membrane localization of BfpB. Finally, PulS protects PulD monomers from degradation (19), whereas BfpB monomers are not degraded in the absence of BfpG. These differences are also apparent in the analogous Erwinia chrysanthemi GSP Out secretion system where OutS, like PulS, was found to be an outer-membrane lipoprotein that stabilizes its cognate OutD secretin and facilitates its insertion into the outer membrane (52). The differences between the BfpG-BfpB secretin system and the other secretins discussed above extend to secretins of the type III secretion apparatus of S. enterica serotype Typhimurium. Here, the InvG secretin forms multimers in the absence of the small lipoprotein InvH; InvH is required for the outer-membrane localization of InvG (9). Taken together, these differences suggest that BfpG is required for different steps in secretin biogenesis than PulS, OutS, and InvH.
The closest parallel between BfpG-BfpB and other secretin systems might be expected for the type IV pilus biogenesis machinery of different species. The PilQ secretin required for Neisseria gonorrhoeae pilus biogenesis requires the small (∼20-kDa) lipoprotein PilP for efficient multimerization of PilQ (15). This PilP function may be comparable to be the role of BfpG in the multimerization of BfpB. However, studies of the PilQ-PilP system in N. gonorrhoeae were conducted using whole-cell lysates, and thus it was not determined if PilP was dispensable for the outer-membrane localization of PilQ or if PilQ and PilP physically interact (15). The PilQ secretin for Pseudomonas aeruginosa type IV pilus biogenesis has also been studied; like BfpB, it forms an outer-membrane pore-like structure that resembles the structures shown in Fig. 2 (4). Moreover, a five-gene cluster required for type IV pilus biogenesis in this species was found to contain an ORF, designated pilP, that is predicted to encode a 19.0-kDa lipoprotein (36). However, the role of this lipoprotein in secretin biogenesis has not been reported. In view of the possible similarities in function between BfpG and the PilQ proteins of N. gonorrhoeae and P. aeruginosa, the three corresponding amino acid sequences were compared. Both PilQ sequences were significantly similar to each other (32% identity and 48% similarity over the entire amino acid sequence), but neither was significantly similar to BfpG (unpublished observation) (56). Thus, even within the type IV pilus secretin subgroup, BfpG differentiates itself with respect to sequence and possibly function.
Ultrastructural studies of BfpB (Fig. 2) and of five previously studied secretins (PilQ and XcpQ of P. aeruginosa [4], pIV [34], InvG [8], and YscC [32]) and cryo-EM studies of PulD (39, 40) suggest that the secretin multimer contains a central channel. The estimated dimensions of these putative channels appear to correspond closely to the diameter of the cognate secreted moiety—in the case of BfpB, the diameter of the BFP filament—and thus it has been proposed that the central pore constitutes a secretion pathway. Since these dimensions greatly exceed the diameter of outer-membrane porins, the channel would need to be gated when not occupied by the secreted compound to prevent loss of periplasmic proteins and provide resistance to osmotic stress and perhaps penetration of antimicrobial compounds. None of these assumptions has been proven conclusively, although the PulD secretin has been reported to exhibit voltage-dependent conductance (39).
To begin to characterize the functional properties of the BfpB secretin, the presence of the BfpB multimer before, during, and after the AA phenotype was determined by Western blotting (Fig. 6). Surprisingly, BfpB multimers were detected 6 h after autoaggregates had dispersed—a period when the transcriptional activity of the bfp operon has declined and BFP filaments are no longer discernible by EM (42). Thus, if the BfpB multimer in fact forms a transmembrane pore, then it is not likely to be gated exclusively by protrusion of a mature pilus filament, at least during the dispersal phase of the AA phenotype.
To evaluate the possibility that BfpB forms an incompletely gated channel during some phase of the AA-disaggregation cycle, permeability studies with vancomycin were performed. Normally, the outer membrane of gram-negative bacteria functions as a permeability barrier for vancomycin, rendering it inactive. Precedence for the use of vancomycin as a test of secretin pore formation and gating function comes from studies by Russel and colleagues with the pIV phage protein (48, 50) and by Wall et al. with PilQ of M. xanthus (57). Both groups demonstrated that the presence of the wild-type secretin did not confer vancomycin sensitivity. However, missense mutations causing amino acid substitutions in the carboxyl halves of PilQ and pIV resulted in vancomycin sensitivity. Wild-type BfpB confers vancomycin sensitivity when compared to the BfpB deletion mutant. Thus, by this criterion, the wild-type BfpB secretin is incompletely gated. Further, any gating of the wild-type secretin does not seem to be due to the BFP filament per se, since the bfpA deletion mutant, which does not produce pilus filaments, is no more or less susceptible to vancomycin than the wild-type strain.
Incomplete gating of the BfpB secretin does not seem to render the bacteria less fit to occupy its normal ecological niche because the wild-type strain used for these studies was recently given to human volunteers and found to be virulent (3). This raises intriguing questions about how EPEC survives in the small intestine where bile salt concentrations are high, since mutations of pIV that conferred vancomycin sensitivity also caused hypersensitivity to deoxycholate. One possibility is that the O111 capsule of this EPEC serotype serves as a permeability barrier for deoxycholate.
The persistence of the BfpB multimer in the outer membrane after active pilus biogenesis has ceased—during the dispersal phase of the AA phenomenon—naturally raises the possibility that it assumes a role secondary to BFP elaboration. One such possibility is to support secretion of a nonpilus molecule. Indeed, evidence in support of this has been sought by Wall et al. (57) in their study of the PilQ secretin of M. xanthus, which is required for type IV pilus biogenesis by this organism. Myxococcus, unlike E. coli, is a prodigious secretor of proteins, which accumulate in spent culture medium. When protein secretion patterns were compared between the wild-type strain and a PilQ deletion mutant, significant differences were evident indicating that, in addition to pilus biogenesis, the PilQ secretin might also effect the secretion of other proteins. To test if this might be true for the BfpB secretin, the wild type and BfpB deletion mutants were grown in tissue culture medium—conditions that normally induce the AA phenotype. Before and after the dispersal phase, the bacteria were removed by centrifugation and the supernatant was concentrated by ultrafiltration. The resulting Coomassie blue-stained SDS-PAGE pattern (Fig. 8) revealed that in the absence of the BfpB HMW complex, the amount and profile of the secreted proteins that accumulate in the growth medium are significantly different from the wild-type protein profile. Specifically, the complexity and amount of accumulated proteins are less in medium containing the BfpB deletion mutant, and these differences are apparent only after the dispersal of the autoaggregates in the wild-type strain. One explanation for these finding is that the BfpB secretin has at least two functions, pilus biogenesis and protein secretion. Since pathogenesis is frequently conceptualized as a temporal sequence of distinct but coordinated steps, it is possible that the BfpB secretin is first required for pilus-mediated bacterial aggregation and epithelial cell adherence and subsequently for secretion of a bioactive molecule that contributes to intestinal survival or pathogenesis. Further work with EPEC and the proteins encoded in the bfp operon might lead to greater understanding of type IV pilus biogenesis as well as the discovery of new virulence factors whose secretion is dependent on BfpB expression.
ACKNOWLEDGMENTS
This work was supported by grant AI39521 from the National Institute of Health. S. A. Schmidt was supported in part by a grant from the Stanford Medical Scholars Program.
We thank M. Koster for advice regarding the visualization of secretins with EM.
REFERENCES
- 1.Anantha R P, Stone K D, Donnenberg M S. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J Bacteriol. 2000;182:2498–2506. doi: 10.1128/jb.182.9.2498-2506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantha R P, Stone K D, Donnenberg M S. Role of BfpF, a member of the PilT family of putative nucleotide-binding proteins, in type IV pilus biogenesis and in interactions between enteropathogenic Escherichia coli and host cells. Infect Immun. 1998;66:122–131. doi: 10.1128/iai.66.1.122-131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 4.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 5.Bitter W, Tommassen J. Ushers and other doorkeepers. Trends Microbiol. 1999;7:4–6. doi: 10.1016/s0966-842x(98)01398-5. , 6–7. [DOI] [PubMed] [Google Scholar]
- 6.Brok R, Van Gelder P, Winterhalter M, Ziese U, Koster A J, de Cock H, Koster M, Tommassen J, Bitter W. The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J Mol Biol. 1999;294:1169–1179. doi: 10.1006/jmbi.1999.3340. [DOI] [PubMed] [Google Scholar]
- 7.Chen L Y, Chen D Y, Miaw J, Hu N T. XpsD, an outer membrane protein required for protein secretion by Xanthomonas campestris pv. campestris, forms a multimer. J Biol Chem. 1996;271:2703–2708. doi: 10.1074/jbc.271.5.2703. [DOI] [PubMed] [Google Scholar]
- 8.Crago A M, Koronakis V. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol. 1998;30:47–56. doi: 10.1046/j.1365-2958.1998.01036.x. [DOI] [PubMed] [Google Scholar]
- 9.Daefler S, Russel M. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol. 1998;28:1367–1380. doi: 10.1046/j.1365-2958.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Enfert C, Pugsley A P. Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J Bacteriol. 1989;171:3673–3679. doi: 10.1128/jb.171.7.3673-3679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg M S. Enteropathogenic Eschericia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 709–726. [Google Scholar]
- 12.Donnenberg M S, Giron J A, Nataro J P, Kaper J B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992;6:3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake S L, Koomey M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol. 1995;18:975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 15.Drake S L, Sandstedt S A, Koomey M. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol. 1997;23:657–668. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 16.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 17.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 18.Giron J A, Ho A S, Schoolnik G K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 19.Hardie K R, Lory S, Pugsley A P. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie K R, Seydel A, Guilvout I, Pugsley A P. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 22.Hill S M, Phillips A D, Walker S J. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut. 1991;32:154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu N T, Hung M N, Liao C T, Lin M H. Subcellular location of XpsD, a protein required for extracellular protein secretion by Xanthomonas campestris pv. campestris. Microbiology. 1995;141:1395–1406. doi: 10.1099/13500872-141-6-1395. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzung B. Basic and clinical pharmacology. 6th ed. Norwalk, Conn: Appleton & Lange; 1995. [Google Scholar]
- 27.Kazmierczak B I, Mielke D L, Russel M, Model P. pIV, a filamentous phage protein that mediates phage export across the bacterial cell envelope, forms a multimer. J Mol Biol. 1994;238:187–198. doi: 10.1006/jmbi.1994.1280. [DOI] [PubMed] [Google Scholar]
- 28.Keleti G, Lederer W H. Handbook of micromethods for the biological sciences. New York, N.Y: Van Nostrand Reinhold; 1973. [Google Scholar]
- 29.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 30.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Linderoth N A, Simon M N, Russel M. The filamentous phage pIV multimer visualized by scanning transmission electron microscopy. Science. 1997;278:1635–1638. doi: 10.1126/science.278.5343.1635. [DOI] [PubMed] [Google Scholar]
- 35.Marciano D K, Russel M, Simon S M. An aqueous channel for filamentous phage export. Science. 1999;284:1516–1519. doi: 10.1126/science.284.5419.1516. [DOI] [PubMed] [Google Scholar]
- 36.Martin P R, Watson A A, McCaul T F, Mattick J S. Characterization of a five-gene cluster required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1995;16:497–508. doi: 10.1111/j.1365-2958.1995.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 37.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nataro J P, Scaletsky I, Kaper J, Levine M, Trabulsi L. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985;48:378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nouwen N, Stahlberg H, Pugsley A P, Engel A. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J. 2000;19:2229–2236. doi: 10.1093/emboj/19.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Possot O M, Vignon G, Bomchil N, Ebel F, Pugsley A P. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J Bacteriol. 2000;182:2142–2152. doi: 10.1128/jb.182.8.2142-2152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puente J L, Bieber D, Ramer S W, Murray W, Schoolnik G K. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol Microbiol. 1996;20:87–100. doi: 10.1111/j.1365-2958.1996.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 43.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugsley A P, Francetic O, Possot O M, Sauvonnet N, Hardie K R. Recent progress and future directions in studies of the main terminal branch of the general secretory pathway in gram-negative bacteria—a review. Gene. 1997;192:13–19. doi: 10.1016/s0378-1119(96)00803-7. [DOI] [PubMed] [Google Scholar]
- 45.Ramer S W, Bieber D, Schoolnik G K. BfpB, an outer membrane lipoprotein required for the biogenesis of bundle-forming pili in enteropathogenic Escherichia coli. J Bacteriol. 1996;178:6555–6563. doi: 10.1128/jb.178.22.6555-6563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothbaum R, McAdams A, Giannella R, Partin J. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982;83:441–454. [PubMed] [Google Scholar]
- 47.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 48.Russel M. Mutants at conserved positions in gene IV, a gene required for assembly and secretion of filamentous phages. Mol Microbiol. 1994;14:357–369. doi: 10.1111/j.1365-2958.1994.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 49.Russel M, Kazmierczak B. Analysis of the structure and subcellular location of filamentous phage pIV. J Bacteriol. 1993;175:3998–4007. doi: 10.1128/jb.175.13.3998-4007.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russel M, Linderoth N A, Sali A. Filamentous phage assembly: variation on a protein export theme. Gene. 1997;192:23–32. doi: 10.1016/s0378-1119(96)00801-3. [DOI] [PubMed] [Google Scholar]
- 51.Scaletsky I C, Silva M, Trabulsi L. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shevchik V E, Condemine G. Functional characterization of the Erwinia chrysanthemi OutS protein, an element of a type II secretion system. Microbiology. 1998;144:3219–3228. doi: 10.1099/00221287-144-11-3219. [DOI] [PubMed] [Google Scholar]
- 53.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C-Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 55.Tacket C O, Sztein M B, Losonsky G, Abe A, Finlay B B, McNamara B P, Fantry G T, James S P, Nataro J P, Levine M M, Donnenberg M S. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect Immun. 2000;68:3689–3695. doi: 10.1128/iai.68.6.3689-3695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatusova T A, Madden T L. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences . FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. . (Erratum, 177:187–188, 1999.) [DOI] [PubMed] [Google Scholar]
- 57.Wall D, Kolenbrander P E, Kaiser D. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J Bacteriol. 1999;181:24–33. doi: 10.1128/jb.181.1.24-33.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolfgang M, van Putten J P, Hayes S F, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;23:6408–6418. doi: 10.1093/emboj/19.23.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H Z, Lory S, Donnenberg M S. A plasmid-encoded prepilin peptidase gene from enteropathogenic Escherichia coli. J Bacteriol. 1994;176:6885–6891. doi: 10.1128/jb.176.22.6885-6891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]