Abstract
Simple Summary
Currently, methods including endoscopy, radiology, and carcinoembryonic antigen levels allow for the detection of colorectal cancer (CRC) at an early stage and the ability to follow the evolution of the disease during treatment. However, these are not always sensitive and specific enough for timely intervention. This leads, amongst other consequences, to delays in treatment or even to overtreatment. Circulating tumor DNA (ctDNA) has shown promise in filling this gap, allowing treatment to be personalized at each stage of the disease and, thus, tailored to each patient’s needs. This review article focuses on the current clinical use and future direction of ctDNA for CRC management.
Abstract
Colorectal cancer (CRC) is the third most common cancer type worldwide, with over 1.9 million new cases and 935,000 related deaths in 2020. Within the next decade, the incidence of CRC is estimated to increase by 60% and the mortality by 80%. One of the underlying causes of poor prognosis is late detection, with 60 to 70% of the diagnoses occurring at advanced stages. Circulating cell-free DNA (ccfDNA) is probably the most promising tool for screening, diagnosis, prediction of therapeutic response, and prognosis. More specifically, the analysis of the tumor fraction within the ccfDNA (circulating tumor DNA, ctDNA) has great potential to improve the management of CRC. The present review provides an up-to-date and comprehensive overview of the various aspects related to ctDNA detection in CRC.
Keywords: colorectal cancer, circulating tumor DNA, treatment, management
1. Circulating Tumor DNA: Detection
Circulating cell-free DNA (ccfDNA) was first described in human plasma by Mandel and Métais in 1948 [1] and has been found to originate from various cell types, including cancer cells (ctDNA). Increased ccfDNA concentrations have been observed in situations of cell lysis and turnover, including pregnancy, intensive exercise, inflammation, infection, autoimmune diseases, diabetes, or soft-tissue injury [2,3,4,5]. The first connection between ccfDNA and cancer was made in 1977 by Leon et al. through the observation that ccfDNA concentrations were also increased in various types of cancers [6].
The above finding is also reflected in colorectal cancer (CRC). The rate of circulating tumor DNA (ctDNA) detection is highly variable across studies and ranges between 40 and 100% for localized tumors to nearly 100% in metastatic CRC [7,8]. ctDNA comprises a fraction of the total DNA circulating freely in the bloodstream and its proportion within ccfDNA depends on the cancer stage and ranges from 0.01 to 0.0001% to more than 50% [9]. Because of the absence of guidelines regarding ctDNA analysis, the interpretation and comparison of clinical studies first require a complete understanding of the detection process and potential issues.
The release of ctDNA depends on tumor burden, vascularity, and location, however, the detection of ctDNA is impaired at early stages. In patients with non-metastatic CRC, the ctDNA concentration is low, and its dilution among total ccfDNA may hinder its detection. Ideally, using the most sensitive and specific technique for ctDNA detection is preferable. Nevertheless, the choice of the detection method is also pragmatic, and the cost of ctDNA analysis needs to be acceptable enough to be translated into clinical practice. Another parameter is the processing time, specifically when the goal is to drive treatment decisions based on the ctDNA detection results. Moreover, there remain considerable differences in the (pre)-analytical conditions between published studies, which limits proper comparisons of ctDNA among individuals.
1.1. Pre-Analytical Conditions
The pre-analytical conditions refer to any variable encountered prior to sample analysis, such as the type of blood collection tubes, the centrifugation delay, the centrifugation protocols, the DNA isolation methods, and the storage conditions. These pre-analytical conditions are of particular importance to protect the integrity of the ccfDNA before downstream analysis [10,11,12]. A general finding is that delaying centrifugation from blood collection increases ccfDNA levels [13]. For example, the rapid lysis of white blood cells (WBCs) in the lavender top EDTA tubes results in genomic DNA (gDNA) release, which dilutes both the ccfDNA and its ctDNA fraction and alters the relative proportion. It is therefore necessary to have a fast plasma separation process (within 4 h). In contrast, other options are “blood preservative tubes” which have been developed to maintain blood cell integrity under various storage and shipping conditions [14].
Hence, controlling the pre-analytical variables and defining the best practices is of the utmost importance for reliable ctDNA analyses and a prerequisite for implementation into routine practice [15]. To the best of our knowledge, no official guidelines exist for the pre-analytical procedures of liquid biopsies, although efforts have been made to standardize these procedures [16]. Moreover, pre-analytical procedures are not routinely documented in published studies, which makes comparisons challenging.
1.2. Analytical Conditions
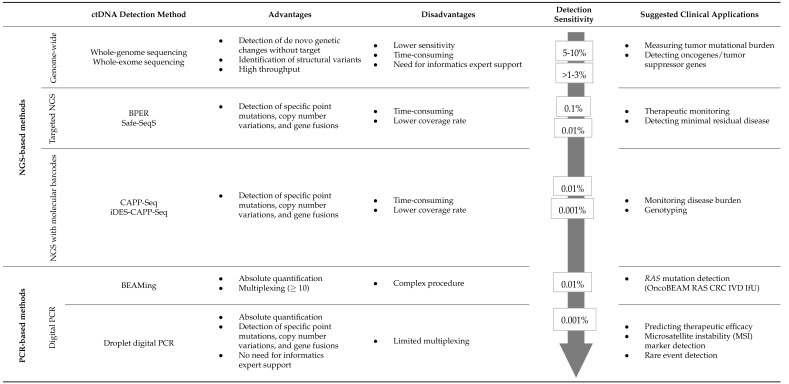
An overview of the main analytical methods is presented in Table 1. The current techniques can be broadly divided into two groups. The first approach consists of the targeted detection of tumor-specific alterations within ccfDNA. The analysis and detection of alterations within the primary tumor are an absolute prerequisite. Sensitivity and specificity are high, and the number of genes to be assessed simultaneously is limited. One of the advantages of this approach is that false positive results are less likely. These targeted approaches can be easily performed by PCR, real-time quantitative PCR (qPCR), digital PCR (dPCR), and droplet digital PCR (ddPCR). A classic example is the detection of RAS mutations that are present in about 50% of CRC patients and localized in hotspot regions that are easily targeted with a limited set of probes [17,18].
Table 1.
Comparison of ctDNA detection and analysis methods.
|
SeqS: Safe-Sequencing System; BPER: Base-Position Error Rate analysis, CAPP-Seq: Cancer Personalized Profiling by deep Sequencing; iDES: integrated Digital Error Suppression; and BEAMing: beads, emulsion, amplification, magnetics.
An alternative option consists of non-targeted (agnostic) methods. Indeed, it is possible to fish for alterations that frequently occur in CRC without baseline tumor tissue analysis. A non-targeted approach implies the use of optimized methods able to screen a large number of alterations concomitantly. Recent dPCR techniques (including droplet-based and microfabricated compartment-based platforms) can also be used, allowing high multiplexing through two to six-color detection [19,20,21]). For example, this technique can be used for methylation assays [22]. Optimized NGS methods including Safe-Sequencing System (Safe-SeqS), CAncer Personalized Profiling by deep Sequencing (CAPP-Seq), integrated Digital Error Suppression-enhanced CAPP-seq (iDES-enhanced CAPP-seq), and Base-Position Error Rate (BPER) have a high sensitivity. Nevertheless, they are more expensive and time-consuming than dPCR [23,24,25,26]. Non-targeted approaches have also been developed using Whole Exome Sequencing (WES) [27] and Whole Genome Sequencing (WGS) [28], allowing a complete genotyping and detection of de novo mutations but with a lower sensitivity and higher cost. In non-targeted approaches, the absence of detection could mean that the alterations were either absent in the blood or in the tumor itself [3].
Overall, detection methods must be selected according to the sampling conditions and the purpose of the studies [29]. For clinical applications, the most favorable test is time- and cost-effective with acceptable sensitivity and specificity.
1.3. Delay of Sampling
Delay between Surgery and ctDNA Sampling
The best timing for ctDNA sampling is controversial, and only a few publications have been dedicated to this topic. In non-metastatic CRC, a drop in ccfDNA concentration immediately after surgical treatment has been reported [30]. The ccfDNA rises beginning at 24 h after surgery and can be used to discriminate patients with recurrence after 48 h [30]. However, early blood collection (before week 4) may theoretically reduce the sensitivity of ctDNA detection because of ccfDNA release as a consequence of the surgical trauma [31]. In the study by Scholer et al., blood samples were collected on day 8, day 30, and every month. Interestingly, out of 26 operated patients, 2 were ctDNA+ eight days after surgery, and 2 others became ctDNA+ one month after surgery [32]. Overall, collecting blood early after surgery might be more relevant for immediate clinical application but must be weighed against a higher rate of false negatives.
2. ctDNA: Clinical Applications
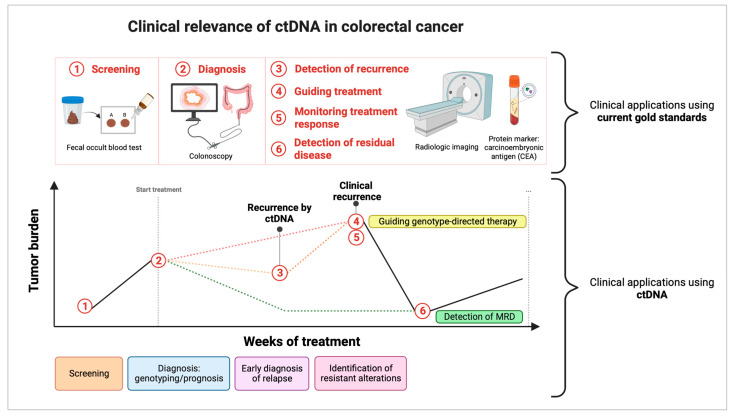
Figure 1 illustrates some applications of ctDNA monitoring before and after treatment in various settings. This section will describe the significance of ctDNA as a screening, diagnostic, prognostic, predictive, minimal residual disease, and recurrence marker.
Figure 1.
Clinical relevance of ctDNA in colorectal cancer. This figure depicts the critical applications in the clinical setting using both the current clinical gold standards and ctDNA. These include tumor genotyping in cancer diagnosis, assessing treatment response, tracking minimal residual disease and relapse, and monitoring clonal evolution. (1) Screening is routinely performed to detect CRC at an early stage using a fecal occult blood test (FOBT), but replacement by ctDNA has yet to be encouraged. (2) Diagnosis is performed before therapy to confirm the tumor’s presence. When using ctDNA, genotyping could determine the tumor profile and identify patients with a high tumor burden. Determination of tumor burden has the potential to help guide neo-adjuvant or adjuvant therapy and monitor response. (3/6) Detection of residual disease and recurrence is performed using radiologic imaging and carcinoembryonic antigen (CEA) detection. However, the former suffers from a delay in detection and the latter suffers from a lack of sensitivity. Assessment of ctDNA after therapy facilitates the detection of both emerging resistance mutations and minimal residual disease (MRD) before progression, with the potential for the non-invasive prediction of recurrence. (4/5) Guiding treatment and monitoring treatment response occurs based on the presence or absence of tumor lesions. On the other hand, ctDNA can guide genotype-directed therapy and allows for the monitoring of the response to treatment based on tumor burden. When acquired resistance to targeted therapies occurs, ctDNA can detect specific mechanisms or resistance, considering the different clones present within the primary tumor and all metastatic sites, and can guide treatment adjustments. In contrast, imaging and the CEA marker can detect the emergence of resistance without knowing the mechanisms of resistance. Created with BioRender.com.
2.1. ctDNA for Early Cancer Detection: Screening
Screening
Because survival is highly affected by the stage at diagnosis, early detection of CRC is critical [33]. Most screening programs for CRC are currently based on a non-invasive stool-based test, either the guaiac-based fecal occult blood test (gFOBT) or the immunological fecal occult blood test (iFOBT), also referred to as the fecal immunochemical test (FIT). These tests are not specific and present a low sensitivity for the detection of CRC. When positive, CRC must be confirmed by a complete colonoscopy, which is invasive, expensive, and often requires sedation [33,34,35,36].
To circumvent these limitations, ctDNA has been explored as a potential screening tool for CRC. Table 2 shows a comparison of the different CRC screening methods. Taking advantage of the fact that aberrant DNA methylation is generally one of the first steps in CRC carcinogenesis, several methylation signatures have been explored [37] using one [38] or multiple gene methylation profiles [39,40]. Amongst various methods, the best sensitivity (75–81%) and specificity (96–99%) so far have been provided by the Epi ProColon® 2.0 test (Epigenomics AG, Berlin, Germany) which is based on the detection of hypermethylation on the SEPT9 promoter [38]. To date, this test is the only blood-based qualitative screening test accepted by the FDA for CRC. In a population of 7941 asymptomatic individuals (PRESEPT), the SEPT9 detected CRC with sensitivities of 35.0%, 63.0%, 46.0%, and 77.4%, in stages I to IV, respectively. In this population, the sensitivity for advanced adenomas was only 11.2% [41] which remains insufficient to replace standard colonoscopy screening. However, one of the main advantages of the SEPT9 test is the increased patient compliance compared to the colonoscopy [42,43]. The Epi ProColon® 2.0 test aims explicitly at detecting CRC, but early detection tests are being developed that target a variety of cancers, including CRC. GRAIL Inc. recently published a novel test (Galleri® test) for the early detection of more than 50 types of cancer simultaneously, including CRC. This test analyses specific methylation patterns in ccfDNA that have been associated with many cancer entities. The tissue of origin can be predicted with 96% specificity and 93% accuracy. Moreover, the sensitivity in all cancer types was 18% (stage I), 43% (stage II), 81% (stage III), and 93% in stage IV. This corresponds to the sensitivity in detecting CRC, approximately 28% (stage I), 70% (stage II), 78% (stage III), and 97% (stage IV). These results indicate an important potential benefit to using methylated ccfDNA for cancer detection [44].
Table 2.
Summary of test accuracy results and corresponding (dis)advantages.
| Sensitivity * | Specificity * | Advantages | Limitations | |||
|---|---|---|---|---|---|---|
| Adenoma | CRC | Adenoma | CRC | |||
| Colonoscopy | 75–95% | 18–100% | 89–94% | 100% |
|
|
| gFOBT | 6–17% | 50–75% | 96–99% | 96–98% |
|
|
| iFOBT/FIT | 23% | 74% | 96% | 94% |
|
|
| ctDNA (Epi proColon) | 22% | 68% | 79% | 79% |
|
|
* Values for sensitivity and specificity were retrieved from the systematic review of Lin et al., containing all relevant studies conducted in asymptomatic populations at general risk of CRC between January 2015 and December 2019 [45].
In general, the concentration of ctDNA in patients’ blood at early stages is low or nonexistent, making its detection challenging for screening purposes. The American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) has concluded that there is still little evidence for the clinical validity of ctDNA detection for cancer screening [46]. However, ctDNA detection could be combined with traditional screening methods to improve the diagnosis of CRC at an early stage [47,48,49].
2.2. The Value of ctDNA Detection at Diagnosis
At diagnosis, the frequency of ctDNA detection is around 10–50% for stage I, 20–89% for stage II, 30–90% for stage III, and 60–100% for stage IV [8,32,50,51,52,53,54,55] (see Table 3).
Table 3.
Clinical relevance of ctDNA detection at baseline.
| Study | Tumor Stage | Rate of ctDNA Detection before Surgery | ctDNA Detection Method | Outcome | |
|---|---|---|---|---|---|
| Tumor burden at diagnosis | [52] | I II III |
50% 89% 90% |
TEC-Seq | Patients with increased pre-operative ctDNA had a shorter PFS and OS compared to patients with a lower ctDNA (HR 1.13, 95% CI: 1.03–1.24) |
| [32] | 60% 56% 86% |
ddPCR | 8/10 ctDNA+ patients relapsed 6/11 ctDNA+ patients did not relapse |
||
| [54] | 64% | ddPCR | No relation between baseline ctDNA and DFS (HR 0.93, 95% CI: 0.33–2.69) | ||
| [8] | 60% 92% 90% |
Multiplex PCR-based NGS | No significant association between ctDNA and the outcome | ||
| [55] | 30% | ddPCR | Pre-operative ctDNA was associated with inferior RFS (HR 2.18, 95% CI: 1.02–4.61) | ||
| [30] | NM | Spectrophotometry (NanoDrop) | Significantly higher cfDNA levels were observed in patients, with early recurrence compared to non-recurrent patients | ||
| [56] | II III |
64% | NGS | Pre-operative ctDNA+ patients had reduced RFS compared with pre-operative ctDNA− patients (HR 5.66; 95% CI: 1.72–18.57) | |
| [57] | 42% | ddPCR | Baseline ctDNA was an independent prognostic factor of DFS (HR 3.35, 95% CI: 1.15–9.77) | ||
| [51] | 25% 30% |
ddPCR | The rate of recurrence was 32.7% in ctDNA+ patients and 11.6% in ctDNA− patients (p = 0.001) | ||
| [53] | 64% 74% |
Real-time multiplex PCR assay | 12/47 (25.5%) ctDNA+ patients relapsed |
NGS: Next-Generation Sequencing, TEC-Seq: Targeted Error Correction Sequencing, and NM: not mentioned.
In the non-metastatic setting, the prognostic value of ctDNA detection at baseline (i.e., before surgery) is unclear. In the study of Reinert et al., almost all patients (stage I-III) presented with ctDNA+ detection before surgery, which was not associated with the risk of recurrence [8]. In the ALGECOLS (Presence of Circulating Tumour DNA in Colorectal Cancer) study (NCT01198743), 27.5% of the patients were ctDNA+ before surgery. These ctDNA+ patients showed a higher rate of recurrence (32.7% versus 11.6% in ctDNA− patients, p = 0.001). In addition, the time to recurrence (TTR) was significantly shorter in ctDNA+ patients compared to ctDNA− patients (adjusted HR = 3.58, 95% CI 1.71–7.47) [51]. These observations were similar in a large Chinese cohort of 276 patients with stage II/III tumors. Pre-operative ctDNA+ patients showed a reduced RFS compared to pre-operative ctDNA− patients (HR 5.66; 95% CI 1.72–18.57; p = 0.004) [56]. Those discrepancies suggest that survival is probably associated with ctDNA concentration rather than a simple ‘yes or no’ detection.
In patients with metastatic CRC, the tumor load before treatment is an important prognostic factor [58,59,60]. The ctDNA concentration before chemotherapy administration can be considered a continuous variable, with the highest concentrations being associated with the shortest survival [61].
2.3. ctDNA as a Prognostic Biomarker in CRC Stage I–III: Detection of Minimal Residual Disease
After curative-intent surgery, adjuvant chemotherapy (ACT) is routinely delivered to patients with high-risk stage II or stage III CRC. However, >50% of stage III and >80% of stage II patients are exposed to unnecessary chemotherapy. In fact, the 5-year DFS rate of stage II and low-risk stage III patients who underwent surgery alone has been reported as 78–91% and 78%, respectively [62]. Since the treatment is associated with lifetime side effects (e.g., chemotherapy-induced neuropathy), one aims to reduce the incidence of ACT when proven unnecessary [63].
2.3.1. Detection of MRD/Recurrence after Surgery
In a pioneering study of 230 patients with stage II colon cancer, Tie et al. investigated the ability to identify patients at high risk of recurrence by detecting post-operative ctDNA [64]. The delay for post-operative plasma withdrawal was 4 to 10 weeks. The rate of ctDNA-positive detection was 8.7% for the whole cohort. In patients not treated with ACT (n = 178), ctDNA was detected post-operatively in 7.9% and was associated with a 79% recurrence at a median follow-up time of 27 months. The recurrence occurred in only 9.8% of patients with negative ctDNA (HR 18; 95% CI, 7.9 to 40; p < 0.001). In this study, 52 patients with histological high-risk stage II CRC were treated with ACT. Among them, six were positive for ctDNA detection after surgery, and three recurred despite the adjuvant treatment [64].
Using a tumor-informed Safe-SeqS platform, Tie et al. further analyzed the ctDNA status in a cohort of patients with stage III CRC [50]. The ctDNA was detectable in 21% of the cohort 4 to 10 weeks after surgery. A recurrence was observed in 42% of the patients with post-operative ctDNA+.
In a longitudinal cohort study, ctDNA was used to monitor tumor burden in 21 CRC patients (stages I-III) who underwent ctDNA analysis three months after complete surgery. In all six patients with detectable ctDNA, the recurrence occurred within three years. In patients without detectable ctDNA, the recurrence rate was 27% (4/15) {HR, 37.7; 95% CI, 4.2–335.5; p < 0.001} [32].
Mixing a population of patients with stage II and III, Li et al. found 27.8% disease progression for ctDNA-positive patients after surgery (ctDNA sampling within one week after surgery and follow-up of 6 months) compared to 4.4% for those who were ctDNA-negative (Fisher test, OR 7.9, p = 0.0169, 95% CI) [65].
Another study, including 125 patients with stages I-III, similarly showed that ctDNA-positive patients were seven times more likely to relapse after surgery than ctDNA-negative patients (HR, 7.2; 95% CI, 2.7–19.0; p < 0.001). Shortly after ACT, ctDNA-positive patients were 17 times more likely to relapse (HR, 17.5; 95% CI, 5.4–56.5; p < 0.001), and all seven patients who were ctDNA positive after ACT experienced relapse [8].
Taieb et al. also worked on the prognostic value of post-operative ctDNA in the IDEA-FRANCE trial (NCT00958737). Overall, 1017 patients were included, of which ctDNA samples were available post-surgery and pre-chemotherapy. Among them, 877 were ctDNA-negative (86.2%) and 140 ctDNA-positive (13.8%) after surgery. With a median follow-up of 6.6 years, the 3-year disease-free-survival (DFS) rate for ctDNA-positive and -negative patients was 66.39% and 76.71%, respectively (p = 0.015) [66].
Similarly, Benhaim et al. evaluated the pertinence of longitudinal detection and quantification of ctDNA prospectively as a prognostic marker of recurrence in the ALGECOLS (Presence of Circulating Tumor DNA in Colorectal Cancer) study (NCT01198743). The ctDNA analysis was performed before and after surgery in 184 patients (stage II-III) during 3–4 years of follow-up using ddPCR. After surgery, 18/171 (10.5%) patients were ctDNA+. Positive ctDNA levels after surgery were associated with a 44.4% recurrence rate versus 13.7% in ctDNA− patients (p = 0.003) [51].
Chen et al. also found that post-operative serial ctDNA detection predicted a high risk for recurrence. Low recurrence risk was observed in ctDNA− patients, with a 2-year RFS rate of 89.4% {95% CI 85.1–93.9%}, while ctDNA+ patients had an extremely high recurrence risk compared to ctDNA− patients {HR 10.98; 95% CI 5.31–22.72}, with a 2-year RFS rate of 39.3% {95% CI 21.5–71.8%}.
The abovementioned studies that have shown the relevance of ctDNA as a marker for the detection of MRD are listed below in Table 4.
Table 4.
Clinical relevance of ctDNA as a prognostic marker for the detection of minimal residual disease and recurrence after surgery.
| Study Design | Sample Size | Study Population | ctDNA Detection Method | Timepoint of ctDNA Sampling | Post-Operative ctDNA Detection Rate | Post-Operative Recurrence for ctDNA+ Patients after Surgery | Post-Operative Recurrence for ctDNA− Patients after Surgery | |
|---|---|---|---|---|---|---|---|---|
| Detection of MRD/recurrence after surgery | Prospective cohort study [64] |
230 | Stage II CC | Safe-SeqS | 4–10 weeks after surgery | 8.7% | 79% | 9.8% |
| Prospective cohort study [50] |
96 | Stage II-III CC | Safe-SeqS | 4–10 weeks after surgery | 21% | 42% | NM | |
| Prospective cohort study [32] | 21 | Stage I-III CRC | ddPCR | 1–4 weeks after surgery | 28.5% | 100% | 27% | |
| Cohort study [65] |
63 | Stage II-III CRC | NGS | Within 1 week after surgery | 28.6% | 27.8% | 4.4% | |
| Prospective, multi-center cohort study [8] |
94 | Stage I-III CRC | Multiplex PCR-based NGS (Signatera™) | 4 weeks after surgery | 10.6% | 70% | 11.9% | |
| Prospective study [66] |
1017 | Stage III CC | ddPCR | 35–50 days after surgery | 13.8% | After 2 years: 31.4% | After 2 years: 17.2% | |
| Prospective, multi-center cohort study [51] |
184 | Stage II-III CRC | ddPCR | 1–6 months after surgery | 10.5% | 44.4% | 10.4% | |
| Prospective, cohort study [56] |
240 | Stage II-III CRC | 425-gene NGS panel-based | 3–7 days after surgery | 8.3% | 60% | NM |
CC: colon cancer, Safe-SeqS: Safe-Sequencing System, NM: not mentioned, and NGS: Next-Generation Sequencing.
2.3.2. ctDNA Clearance after Treatment
At each stage of treatment, variations in ctDNA concentration and ctDNA clearance might reflect treatment efficacy. However, the rate of ctDNA clearance has not been evaluated because of the lack of extensive studies with longitudinal sampling. The main results are summarized in Table 5.
Table 5.
Clinical relevance of ctDNA clearance at each stage of treatment.
| Study Design | Sample Size | Study Population | ctDNA Detection Method | Timepoint of ctDNA Sampling | ctDNA Clearance Rate | Outcome | |
|---|---|---|---|---|---|---|---|
| After surgery | Prospective, multi-center cohort study [51] |
49 | Stage II CRC Stage III CRC |
Droplet Digital PCR | Day 5 after surgery | 75% | Recurrence rate ctDNA+: 44.4% ctDNA−: 13.7% |
| Prospective, multi-center study [56] | 240 | Stage II CRC Stage III CRC |
NGS | Days 3–7 after surgery | 92% | 2-year RFS: ctDNA+: 39.3% ctDNA−: 89.4% |
|
| Prospective, multi-center cohort study [8] |
94 | Stage I CRC Stage II CRC Stage III CRC |
Multiplex PCR-based NGS | Day 30 after surgery | 89% | Recurrence rate: ctDNA+: 70% ctDNA−: 11.9% |
|
| After adjuvant chemotherapy | Prospective, multi-center cohort study [8] |
10 | Stage I CRC Stage II CRC Stage III CRC |
Multiplex PCR-based NGS | After completion of chemotherapy | 30% | NM |
| Prospective cohort study [64] |
6 | Stage II CC | Safe-SeqS | After completion of chemotherapy | 50% | 2-year RFS: ctDNA+: 27% ctDNA−: 82% |
|
| Multi-center, cohort study [50] |
95 | Stage III CC | Safe-SeqS | After completion of chemotherapy | 68% | 3-year RFI: ctDNA+: 30% ctDNA−: 77% |
CC: colon cancer, Safe-SeqS: Safe-Sequencing System, RFS: Regression-Free Survival, NM: Not Mentioned, and RFI: Regression-Free Interval.
Rate of ctDNA clearance after surgery: Within patients with pre-operative ctDNA+, around 75–92% have ctDNA clearance after surgery.
In stages I–III CRC, Reinert et al. observed that 84/94 (89.4%) patients became ctDNA negative and 10/94 (10.6%) patients became ctDNA positive after surgery.
In stages II–III CRC, the ctDNA status changed from positive to negative in 75–92% of the patients after surgery [51,56].
Rate of ctDNA clearance after adjuvant chemotherapy: In stages I–III CRC, the rate of ctDNA clearance observed under chemotherapy was between 50% and 68%. This rate is approximately the same for stage II and stage III CRC.
In stages I–III, Reinert et al. observed 30% of patients who cleared ctDNA after ACT and stayed disease free throughout the study [8].
In stage II, Tie et al. observed that post-operative ctDNA+ remained negative after ACT in three out of six patients [64].
In stage III, the ctDNA status changed from positive to negative in 50–68% of the patients after completion of chemotherapy treatment [50,51,67].
ctDNA clearance is associated with a superior RFS in most series: In stages II and III, superior RFS was observed when ctDNA became undetectable after chemotherapy (HR 5.11; p = 0.02) [64]. The absence of ctDNA clearance after chemotherapy is associated with a rate of 30% RFI at three years (HR, 6.8; 95% CI, 11.0–157.0; p < 0.001) [50].
2.3.3. Value of ctDNA in the Prediction of Relapse before Conventional Imaging Techniques
Predicting relapse before radiologic recurrence is necessary. ctDNA detection can anticipate radiological recurrence with a lead time of 3 to 12 months, as described in recent publications (see Table 6). These results are clearly subject to bias, as the usual interval between plasma sampling is three months, whereas, for imaging assessments, it is six months. Although this anticipation is crucial, we should be aware that false-positive results exist and overall survival has not been shown to increase with the earlier treatment of relapses. The French trial CIRCULATE-MRD has recently been funded and will soon open for inclusion to address this question. In addition, the sensitivity and specificity of ctDNA detection during follow-up should be further studied to determine whether longitudinal sampling could be a way to avoid or delay (reduce) imaging follow-up.
Table 6.
ctDNA in the biological anticipation of radiological recurrence.
| Study Design | Sample Size | Study Population | ctDNA Detection Method | ctDNA Positivity vs. Recurrence Rate | Frequency of Sampling | Delay of Anticipation | |
|---|---|---|---|---|---|---|---|
| The biological anticipation of radiological recurrence | Cohort study [68] | 58 | Stage I–III CRC | Safe-SeqS | ctDNA+ and recurrence: 100% ctDNA− and recurrence: 0% |
Post-surgery: 1 month Follow-up: Every 3–6 months |
3 months |
| Prospective cohort study [32] | 27 | Stage I–III CRC | ddPCR | ctDNA+ and recurrence: 100% ctDNA− and recurrence: 0% |
Post-surgery: Days 8 and 30 Follow-up: Every 3 months |
9 months | |
| Prospective cohort study [64] |
178 | Stage II CC | Safe-SeqS | ctDNA+ and recurrence: 78.6% ctDNA− and recurrence: 9.8% |
Follow-up: Every 3 months |
167 days (5 months) (IQR, 81–279 days) | |
| Prospective study [69] |
11 | Stage I–IV CRC | ddPCR | ctDNA+ and recurrence: 100% ctDNA− and recurrence: 0% |
Post-surgery: Days 8 and 30 Follow-up: Every 3 months |
2–15 months (mean of 10 months) | |
| Prospective, multicenter cohort study [51] |
139 | Stage II–III CRC | ddPCR | ctDNA+ and recurrence: 32.7% ctDNA− and recurrence: 11.6% |
Post-surgery: Day 5 Follow-up: Every 3–6 months |
13.1 weeks (IQR, 28 weeks) | |
| Prospective, multicenter study [70] |
160 | Stage III CRC | Multiplex PCR-based NGS | ctDNA+ and recurrence: 96% ctDNA− and recurrence: 3% |
Follow-up: Every 3 months |
9.8 months (IQR, 5–12 months) | |
| Prospective, multicenter study [56] | 276 | Stage II–III CRC | NGS | ctDNA+ and recurrence: 76% ctDNA− and recurrence: 4% |
Post-surgery: Days 5–8 Follow-up: 6 months after surgery, and then every 3 months |
5.01 months |
CC: colon cancer, Safe-SeqS: Safe-Sequencing System, ddPCR: droplet digital PCR, and IQR: interquartile range.
2.4. ctDNA as a Quantitative Monitoring Tool in Predicting Response to Treatment (Stage IV)
2.4.1. ctDNA Concentration during Treatment
The ctDNA concentration varies throughout the treatment duration; it reflects a response to treatment and allows for the selection of non-responding patients. Table 7 summarizes the main studies that have addressed this knowledge area.
Table 7.
ctDNA as a quantitative monitoring tool in predicting response to treatment.
| Study Design | Sample Size | Study Population | ctDNA Detection Method | PFS | |
|---|---|---|---|---|---|
| Predicting response to treatment | Prospective, multi-center study [71] |
28 | Stage IV CRC | ddPCR NGS * (Ion AmpliSeq Cancer Hotspot Panel) |
ctDNA−: 4.0 months ctDNA+: 1.9 months Hazard ratio, 0.44; 95% CI, 0.18–0.98; p = 0.03) |
| Clinical trial [72] |
29 | Stage IV CRC | Guardant 360 ** assay | ApCN ≥ 25.82: 22.5 weeks ApCN ≤ 25.82: 14.8 weeks Mantel Cox, p = 0.0347 |
|
| Prospective study [73] |
467 | Stage IV CRC | Methy-Light | No effect on PFS | |
| Prospective study [74] |
53 | Stage IV CRC | Safe-SeqS | ≥10-fold reduction in ctDNA: 14.7 months ≤10-fold reduction in ctDNA: 8.1 months Hazard ratio, 1.87; 95% CI, 0.62–5.61; p = 0.266 |
|
| Prospective (PLACOL) study [61] |
82 | Stage IV CRC | ddPCR | “good ctDNA responder” = ctDNA concentration < 0.1 ng/mL and SlopeΔctDNA ≥ 80%: 8.5 months “bad ctDNA responder” = ctDNA concentration > 0.1 ng/mL and SlopeΔctDNA < 80%: 2.4 months Hazard ratio, 0.19; 95% CI, 0.09–0.40; p < 0.0001 |
|
| Prospective study [75] |
45 | Stage IV CRC | dPCR (Methyl-BEAMing) |
A negative change in ctDNA is associated with improved PFS |
Safe-SeqS: Safe-Sequencing System, ddPCR: droplet digital PCR, and ApCN: adjusted plasma copy number. * Ion Torrent S5 XL, Thermo Fisher Scientific; ** Guardant Health, Inc. Redwood City, CA, USA.
Early changes in ctDNA concentration during the treatment course predict subsequent radiologic responses, suggesting that ctDNA is a marker of therapeutic efficacy [61,74,75]. Moreover, in some studies, changes in ctDNA also affected PFS and OS, where patients with relatively low ctDNA concentrations showed longer PFS and OS compared to patients with higher ctDNA concentrations [61].
Overall, the longitudinal surveillance of ctDNA allows for the early detection of relapse and response to intervention [32,76]. The detection of ctDNA could participate in the individual management of patients based on their tumor’s genetic profile, as the behavior of cancer in response to therapy can be predicted by determining ctDNA concentrations [29].
2.4.2. ctDNA Predicts Response to Targeted Therapy
A prime example concerns the eligibility for anti-EGFR treatment, where the RAS mutation status of tumor tissue must be determined prior to formulating a treatment plan [17,18]. Since the concordance level in KRAS mutational status between tumor tissue and ctDNA is high (∼92%) [77], the detection of KRAS mutations in ctDNA has been proposed as a rapid and minimally-invasive alternative method to tissue biopsy for predicting the response to anti-EGFR treatment [78]. Interestingly, KRAS mutations have also been detected in ctDNA, although the primary tumor was considered wild-type. These circulating mutations may reflect the existence of minor cell subclones in the primary tumor or its related metastases [5]. Knebel et al. described the monitoring of a patient with KRAS wild-type mCRC treated with chemotherapy combined with anti-EGFR therapy [79]. Surprisingly, KRAS mutations in ctDNA were detected after the first exposure to anti-EGFR therapy but before clinical progression. Subsequently, the evolution of the disease went along with increasing concentrations of KRAS-mutated ctDNA. These results support the importance of the longitudinal monitoring of KRAS mutations in ctDNA before and during anti-EGFR therapy for the early detection of increasing cell clones that could be associated with drug resistance [79].
The emergence of RAS mutations in initially RAS wild-type tumors is a well-known mechanism of acquired resistance to anti-EGFR therapy. Nevertheless, whether these mutations are acquired de novo or whether initially undetectable mutant subclones proliferate through clonal selection and evolution remains unclear [80,81]. A subsequent treatment involving the withdrawal of EGFR blockade may be followed by an increase in the proportion of wild-type (sensitive) clones and a decrease in resistant (RAS mutant) clones, even to undetectable levels [81]. This work laid the foundation for the activity of anti-EGFR rechallenge. The CRICKET (Cetuximab Rechallenge in Irinotecan-Pre-treated mCRC, KRAS, NRAS, and BRAF wild-type Treated in 1st line With Anti-EGFR Therapy) trial (NCT02296203) demonstrated that a rechallenging strategy (in a third-line setting) with cetuximab and irinotecan can be effective, whereby evaluating the RAS mutation status on ctDNA might help select candidate patients and guide therapeutic decisions [71]. Patients with RAS wild-type ctDNA had a significantly longer PFS than those with RAS mutated ctDNA (median PFS: 4.0 vs. 1.9 months; hazard ratio: 0.44; 95% CI, 0.18–0.95; p = 0.03) [71].
Several preclinical studies have suggested that ERBB2 (HER2) copy number gain is a negative predictor of response to anti-EGFR therapy [82]. Investigators of the HERACLES A study, a phase II trial of trastuzumab and lapatinib in chemotherapy and EGFR antibody-refractory HER2-positive mCRC patients, reported that ctDNA precisely predicted the response to anti-HER2 therapy in HER2-positive CRC [72]. In total, 47 of 48 samples from 29 patients had detectable ctDNA, and 46 out of 47 samples were HER2-positive {2.55–122 copies; 97.9% sensitivity (95% CI, 87.2–99.8%)}. These results support the use of adequately validated ctDNA testing as an alternative to tissue biopsy to identify individuals who may benefit from anti-HER2 therapy [72].
Herbst et al. suggested that detecting HPP1 methylation in ctDNA could be used as an early marker to identify patients likely to benefit from a combination of chemotherapy and bevacizumab [73]. Before starting treatment, 337 of 467 patients had detectable methylated HPP1 ctDNA. Two to three weeks after starting treatment, methylated HPP1 ctDNA levels decreased to undetectable levels in 167 of 337 patients. These patients showed improved OS compared to patients with continued detection of methylated HPP1 ctDNA. In addition, methylated HPP1 ctDNA is predictive for combination therapy as early as 3 weeks after the start of treatment, whereas radiological imaging cannot do so until 12 or 24 weeks [73].
2.5. ctDNA as a Tool for Guiding Treatment
Table 8 lists several ongoing trials using ctDNA to guide treatment. In the non-metastatic setting, ways to better select adjuvant treatment are actively sought. For example, the ongoing ctDNA-guided single-arm phase II CHRONOS (Rechallenge With Panitumumab Driven by RAS Clonal-Mediated Dynamic of Resistance) trial (NCT03227926) aims to determine which patients are eligible for anti-EGFR rechallenge. This study uses the ctDNA analysis of RAS, BRAF, and EGFR mutations to drive anti-EGFR rechallenge therapy in mCRC [83]. Based on the same model, second-line rechallenge with cetuximab is under evaluation in the CRICKET trial [71].
In the metastatic setting, several studies have assessed the accuracy of ctDNA-based genotyping in selecting patients for mutation-directed therapy [84,85,86]. Currently, the ongoing prospective, multicentric interventional study (Following Therapy Response Through Liquid Biopsy in Metastatic Colorectal Cancer Patients, FOLICOLOR) in Belgium is evaluating the utility of ctDNA genotyping to monitor clinical response and guide therapeutic decision-making. In patients with unresectable metastatic disease, progressive disease is identified by NPY methylation in ctDNA. Two primary endpoints are (1) investigating whether ctDNA can detect progressive disease earlier than conventional monitoring based on CT imaging and (2) whether adapting treatment based on ctDNA could improve progression-free survival and overall survival.
Table 8.
Ongoing clinical trials in CRC patients evaluating the use of ctDNA.
| Name of Study and Country | Recruitment Status | Patient Population | Sample Size | ctDNA Detection Method | Intervention | Primary Objective |
|---|---|---|---|---|---|---|
| Cetuximab Rechallenge in irinotecan-pre-treated mCRC, KRAS, NRAS, and BRAF Wild-type Treated in 1st Line With Anti-EGFR Therapy (CRICKET) (NCT02296203) Italy [71] |
Active, not recruiting | KRAS, NRAS, and BRAF wild-type, irinotecan-resistant mCRC patients who have progressed after an initial response to a first-line cetuximab-containing therapy | 28 | ddPCR | Cetuximab and irinotecan (single-arm trial) | Overall response rate |
| Rechallenge With Panitumumab Driven by RAS Dynamic of Resistance (CHRONOS) (NCT03227926) Italy [83] |
Active, not recruiting | RAS wild-type mCRC patients who have progressed on first-line anti-EGFR therapy and whose RAS mutation load has decreased over 50% compared to the mutation load at the time of progression on first-line anti-EGFR therapy | 129 | ddPCR | Panitumumab monotherapy (single-arm trial) | Overall response rate |
| Circulating Tumor DNA Based Decision for Adjuvant Treatment in Stage II Colon Cancer based on ctDNA (CIRCULATE-PRODIGE 70-trial) (NCT04120701) France [87] |
Recruiting | Stage II colon cancer patients who underwent curative-intent surgery | 1980 | ddPCR | ctDNA-positive randomized into two arms (1) Control arm: observation (2) Experimental arm: adjuvant mFOLFOX6 ctDNA-negative: surveillance |
3-year disease-free survival |
|
Following Therapy Response Through Liquid Biopsy in Metastatic Colorectal Cancer Patients (FOLICOLOR) Belgium |
Recruiting | Unresectable, metastatic colorectal cancer patients receiving first-line treatment (pembrolizumab, panitumumab, or FOLFOX/FOLFIRI with(out) targeted therapy) | 336 | ddPCR |
Control arm: Treatment decisions are guided by radiographic evaluation Experimental arm: Treatment decisions are guided by ctDNA |
Primary: To determine the proportion of patients in which PD can be detected earlier in ctDNA than with conventional CT imaging Secondary: - PFS - 3-year overall survival |
| Circulating Tumour DNA Analysis Informing Adjuvant Chemotherapy in Stage II Colon Cancer (DYNAMIC) (ACTRN12615000381583) Australia [88] |
Closed | Stage II colon cancer patients who underwent curative-intent surgery | 455 | Safe-SeqS |
Control arm: All decisions were based on conventional clinicopathological criteria) Experimental arm: ctDNA informed (ctDNA positive: adjuvant chemotherapy; ctDNA negative: no adjuvant chemotherapy) |
2-year recurrence-free survival |
| Circulating Tumor DNA Analysis Informing Adjuvant Chemotherapy in Stage III Colon Cancer (DYNAMIC-III) (ACTRN12617001566325) Australia [89] |
Recruiting | Stage III colon cancer patients who underwent curative-intent surgery | 1000 | Safe-SeqS |
Control arm: standard of care treatment Experimental arm: ctDNA informed (ctDNA negative: therapy de-escalation; ctDNA positive: therapy escalation) |
3-year recurrence-free survival |
| Tracking Mutations in Cell Free Tumor DNA to Predict Relapse in Early Colorectal Cancer (TRACC) (NCT04050345), United Kingdom [90] |
Recruiting | High-risk stage II and III patients with CRC who have measurable ctDNA pre-operatively and underwent R0 resection | 1000 | ddPCR |
Control arm: standard of care ACT after surgery Experimental arm: ctDNA-guided ACT (ctDNA-negative: therapy de-escalation of ACT for 3 months with single Cape, or no chemotherapy; ctDNA positive: 3 months CapOx) |
1. The incidence of pre-operatively detectable ctDNA in stage II and III CRC patients 2. The correlation between post-operatively detectable ctDNA and DFS |
| Circulating Tumor DNA Analysis to Optimize the Operative and Postoperative Treatment for Patients With Colorectal Cancer—Intervention Tial 2 (IMPROVE-IT2) (NCT04084249) Denmark [91] |
Recruiting | Stage I and II patients with colon cancer who underwent surgery | 254 | ddPCR Targeted error correction sequencing (TEC-Seq) [52] |
Control arm: surveillance according to current Danish Guidelines with CT-scans at 12- and 36-months post-operative and colonoscopy every 5 years until age 75 Experimental arm: ctDNA-guided surveillance every 4 months postoperatively. (1) ctDNA-positive: patients undergo a whole-body FDG-PET/CT scan and colonoscopy. (2) ctDNA-negative: high-intensive radiological surveillance with FDG-PET/CT-scan every 3 months until recurrence detection or 21 months have passed |
Fraction of patients with relapse receiving curative-intended resection or local treatment |
| Circulating Tumor DNA Testing in Predicting Treatment for Patients With Stage IIA Colon Cancer After Surgery (COBRA) (NCT0406810 US [92] |
Recruiting | Patients whose stage II colon cancer has been resected and who have no traditional high-risk features | 1408 | LUNAR™ (Guardant Health Inc.) |
Control arm: Standard of care, observation Experimental arm: Prospective testing for ctDNA. (1) ctDNA-positive: treatment with 6 months of adjuvant (FOLFOX) chemotherapy (2) ctDNA-negative: active surveillance |
1. Clearance of ctDNA with adjuvant chemotherapy 2. Recurrence-free survival for ctDNA-positive patients treated with or without adjuvant chemotherapy |
| Circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for CRC (CIRCULATE-Japan, consists of the 3 trials (GALAXY, VEGA & ALTAIR)), Japan [93] | ||||||
|
Genetic Alterations and clinical record in radically resected colorectal cancer revealed by Liquid biopsy and whole eXome analYsis (GALAXY) (UMIN000039205) |
Recruiting | Stage II high-risk and stage III low-risk CRC patients who have recurrence after initial registration and are eligible for radical surgical resection | 2500 | Signatera™ (Natera Inc.) |
Observational study Based on ctDNA results in this study, patients can be enrolled in investigator-initiated phase III trials, either the VEGA (if ctDNA-negative) or the ALTAIL (if ctDNA-positive) trial (see below). |
1. Disease-free survival 2. Sensitivity and specificity of ctDNA for the presence of lymph node metastases in additional colorectal resections |
|
Study to Compare CAPOX Therapy as Post-operative Adjuvant Chemotherapy with Surgery Alone in Patients with Completely Resected Circulating Tumor DNA-negative High-risk Stage II and Low-risk Stage III Colon Cancer (VEGA) (jRCT1031200006) |
Recruiting | Colon cancer patients that have a negative ctDNA status at week 4 after surgery in the GALAXY study | 1240 | Signatera™ (Natera Inc.) |
Control arm: Observation Experimental arm: CapOx therapy for 3 months |
Disease-free survival |
|
Initial Attack on Latent Metastasis Using TAS-102 for ctDNA Identified Colorectal Cancer Patients After Curative Resection (ALTAIR) (NCT04457297) |
Recruiting | CRC patients that have a positive ctDNA status within the previous 3 months at any time after surgery in the GALAXY study, and no obvious relapse on CT-scan | 240 | Signatera™ (Natera Inc.) |
Control arm: Placebo Experimental arm: 6 months of oral trifluridine/tipiracil (FTD/TPI) |
Disease-free survival |
3. Rectal Cancer: The Current State of Management
Approximately one-third of all newly diagnosed CRC is composed of rectal cancer. Currently, the standard treatment for advanced rectal cancer consists of neoadjuvant radiotherapy, with or without sensitizing chemotherapy, followed by surgery with total mesorectal excision (TME) [94,95]. Locally advanced rectal cancer (LARC) is exceptionally challenging to manage, given the structural constrictions of the pelvis. Due to the anatomical challenges encountered during TME, there is an increased risk of operative morbidity and mortality and sexual, urinary, and bowel dysfunction [96].
For smaller tumor lesions and under specific conditions (mostly <4 cm, ≤T3), the neoadjuvant treatment allows avoiding TME for 20–30% of LARC patients who achieve a clinical complete response (cCR) [97,98]. However, the risk of local recurrence and distant metastases remains present within this patient population [99]. Despite improvements in pre-operative care and surgical techniques, the quality of life and survival rates remain subpar among rectal cancer patients. Selecting patients who may most benefit from conservative treatment is crucial.
The clinical application of ctDNA has primarily been evaluated in LARC. At baseline, ctDNA detection could be associated with survival and distant recurrence [100,101,102]. Moreover, ctDNA levels can help monitor the response to radiochemotherapy (RCT) [103]. The detection of ctDNA after radiotherapy [104,105,106,107] or surgery [104,105,107,108,109] is significantly associated with shorter survival (Table 9).
Table 9.
Studies evaluating the association between ctDNA detection and tumor response in LARC patients.
| Study Design | Sample Size | Study Population | ctDNA Detection Method | ctDNA Positivity | Outcome |
|---|---|---|---|---|---|
| Cohort study [110] | 67 | LARC | Real-time PCR |
|
Baseline levels of cfDNA are not associated with tumor response. Post-RCT integrity index is associated with tumor response |
| Prospective cohort study [103] | 4 | LARC | NGS |
|
ctDNA concentration can help monitor response to RCT |
| Prospective cohort study [104] | 159 | LARC T3/T4 and/or N+ | Safe-SeqS |
|
ctDNA+ after RCT and/or surgery is associated with lower 3-year-RFS (33% vs. 87%) |
| Prospective cohort study [101] | 36 | LARC | BEAMing |
|
ctDNA+ at baseline reduced post-operative DFS and OS |
| Prospective cohort study [105] |
47 | LARC | ddPCR |
|
Patients with ctDNA+ during RCT, after RCT, and after surgery have lower RFS |
| Cohort study [100] | 104 | Rectal cancer T4 or N1b-3 | NGS |
|
|
| Prospective cohort [106] | 119 | LARC | NGS |
|
ctDNA clearance is associated with tumor regression grade |
| Prospective study (phase II trial) [102] | 71 | LARC | NGS |
|
Pre-operative ctDNA+ is significantly associated with shorter DFS and OS |
| Prospective cohort study [108] | 29 | LARC | NGS |
|
Patients with post-operative ctDNA+ experience poor RFS compared to ctDNA− patients |
| Prospective cohort study [107] | 60 | LARC | Agnostic and tumor-informed assays |
|
ctDNA+ after RCT is associated with lower RFS |
| Cohort study [109] | 51 | LARC | NGS |
|
Patients with post-neoadjuvant treatment and post-operative ctDNA+ experienced poorer RFS than ctDNA− patients |
LARC: locally advanced rectal cancer, RCT: radiochemotherapy, DFS: Disease-Free Survival, OS: Overall Survival, and RFS: Recurrence-Free Survival.
Other studies are currently investigating the significance of ctDNA in directing non-operative management approaches for LARC patients, as shown in Table 10.
Table 10.
Summary of studies evaluating the utility of ctDNA in LARC patients.
| Name of Study and Country | Recruitment Status | Patient Population | Sample Size | ctDNA Detection Method | Intervention | Primary Objective |
|---|---|---|---|---|---|---|
| Circulating Tumor DNA-guided Neoadjuvant Treatment Strategy for Locally Advanced Rectal Cancer (CINTS-R) (NCT05601505) China |
Recruiting | Patients with rectal adenocarcinoma who have not received any treatment yet | 465 | NGS |
Control arm: Traditional neoadjuvant chemoradiotherapy Experimental arm: Randomization based on ctDNA results:
|
Disease-related treatment failure (DrTF) |
| Establishing a ctDNA Biomarker to Improve Organ Preserving Strategies in Patients With Rectal Cancer (ctTRAC) (NCT05081024) USA |
Recruiting | Patients with stage II–III rectal adenocarcinoma | 50 | Signatera™ (Natera Inc., Austin, TX, USA) |
Observational | Complete clinical response (cCR) |
| Application of Circulating Tumor DNA Test in the Diagnosis and Treatment of Patients with Advanced Rectal Cancer (NCT03615170) China |
Recruiting | Patients with locally advanced rectal cancer, who need to receive neoadjuvant radiotherapy and radical operation | 200 | Not mentioned | Observational | Disease-free survival |
| Systemic Neoadjuvant and Adjuvant Control by Precision Medicine in Rectal Cancer (SYNCOPE) (NCT04842006) Finland |
Recruiting | Patients with rectal adenocarcinoma that require either radiotherapy or long chemoradiotherapy | 93 | Not mentioned | Randomization based on ctDNA results:
|
RFS |
4. Conclusions and Perspectives
This review provides an up-to-date and comprehensive summary of the various areas of ctDNA research in CRC management. As this review points out, numerous studies have shown the association of ctDNA with tumor burden and its usefulness in detecting and monitoring tumor dynamics, drug response, and resistance to therapy with increasing sensitivities and specificities.
The experimental detection methods are numerous and of great importance in the interpretation of study results. Further improvement in the standardization of methods leading to the preanalytical variability of liquid biopsies is imperative to obtain optimal sensitivity and specificity for the reliable use of ctDNA in daily practice.
At this point, ctDNA detection has yet to be accepted as a worthwhile CRC screening tool. The low concentration available in the early stages imposes the use of highly-sensitive tests, which are currently cost-prohibitive for routine use. The cost of a ctDNA detection assay ranges from EUR 168 to EUR 1423 per sample in a maximum-testing condition, as is expected in future standard practice [111]. Despite these current high costs, active research to improve the methodology and reduce costs is underway. These efforts are driven by the marked advantages of ctDNA, including its accuracy, ease of collection, and minimal invasiveness. It is precisely for this reason that large-scale clinical trials are underway to explore how to optimize ctDNA detection alone or in combination with conventional screening methods.
After surgery, ctDNA can clearly identify patients at low and high risk of relapse, which has direct implications for adjuvant therapy decisions. In the French multicenter adjuvant trial, CIRCULATE-PRODIGE 70, the administration of adjuvant therapy in patients with stage II CRC is based on post-operative ctDNA detection [87]. The ongoing Tracking Mutation in Cell Free Tumor DNA to Predict Relapse in Early Colorectal Cancer (TRACC) study aims to compare ctDNA versus standard of care in predicting relapse in patients with stage II and III CRC undergoing ACT after surgery [90]. In this setting, highly-sensitive tests are critical to avoid both over- and undertreatment.
In patients with metastatic CRC, serial ctDNA testing provides early indications of the clinical efficacy of therapy. In this setting, the variation in ctDNA concentration is related to the response to systemic treatments. Future clinical trials incorporating ctDNA concentration into the study design may allow for the real-time measurement of therapeutic efficacy. Serial testing is also used to validate ctDNA as a detection method of recurrence. One example is the IMPROVE-IT2 (Implementing Noninvasive Circulating Tumor DNA analysis to Optimize the Operative and Post-operative Treatment for Patients with Colorectal Cancer–Intervention Trial 2) trial. This randomized controlled trial investigates the benefit of ctDNA-guided post-operative surveillance compared to the current standard-of-care CT imaging-based surveillance. The main objective is to investigate whether ctDNA-guided surveillance increases the proportion of patients with recurrence receiving curative-intended resection or local metastasis-directed treatment [91].
As data from the many ongoing clinical trials of ctDNA in CRC emerge, better guidelines will arise on incorporating ctDNA into clinical decision-making. Nevertheless, given the heterogeneous nature of colorectal tumors, a single biomarker might be insufficient for managing CRC. Biomarkers could be combined in composite panels such as protein biomarkers, circulating tumor cells, micro RNAs, and ctDNA.
Altogether, evidence strongly indicates that ctDNA should be considered a key tool in the implementation of a personalized medicine approach; it is only a matter of time before ctDNA becomes a crucial part of clinical medicine.
Author Contributions
Conceptualization, L.B. and V.T.; writing—original draft preparation, A.R.d.A. and L.B.; writing—review and editing, L.B., K.O.d.B. and V.T.; visualization, A.R.d.A. and K.O.d.B.; supervision, L.B., V.T. and K.O.d.B.; project administration, V.T., K.O.d.B. and P.L.-P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Research performed by Ken Op de Beeck is supported by a research grant awarded by the University of Antwerp (Methusalem grant; FFB190208). Ana Regina de Abreu is supported by a Ph.D. fellowship of the Research Foundation—Flanders (FWO; 1SD3722N).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mandel P., Metais P. Les acides nucléiques du plasma sanguin chez l’Homme. C. R. Seances Soc. Biol. Fil. 1948;142:241–243. [PubMed] [Google Scholar]
- 2.Fettke H., Kwan E.M., Azad A.A. Cell-free DNA in cancer: Current insights. Cell. Oncol. 2019;42:13–28. doi: 10.1007/s13402-018-0413-5. [DOI] [PubMed] [Google Scholar]
- 3.Moati E., Taly V., Didelot A., Perkins G., Blons H., Taieb J., Laurent-Puig P., Zaanan A. Role of circulating tumor DNA in the management of patients with colorectal cancer. Clin. Res. Hepatol. Gastroenterol. 2018;42:396–402. doi: 10.1016/j.clinre.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Ershova E., Sergeeva V., Klimenko M., Avetisova K., Klimenko P., Kostyuk E., Veiko N., Veiko R., Izevskaya V., Kutsev S., et al. Circulating cell-free DNA concentration and DNase I activity of peripheral blood plasma change in case of pregnancy with intrauterine growth restriction compared to normal pregnancy. Biomed. Rep. 2017;7:319–324. doi: 10.3892/br.2017.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alix-Panabieres C.T.V., Pantel K. Circulating Tumor Cells and Circulating nucleic acids in oncology. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 7th ed. in progress .
- 6.Leon S.A., Shapiro B., Sklaroff D.M., Yaros M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 7.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y.X., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M., et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014;6:224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinert T., Henriksen T.V., Christensen E., Sharma S., Salari R., Sethi H., Knudsen M., Nordentoft I., Wu H.T., Tin A.S., et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients with Stages I to III Colorectal Cancer. JAMA Oncol. 2019;5:1124–1131. doi: 10.1001/jamaoncol.2019.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elazezy M., Joosse S.A. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput. Struct. Biotechnol. 2018;16:370–378. doi: 10.1016/j.csbj.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barták B.K., Kalmár A., Galamb O., Wichmann B., Nagy Z.B., Tulassay Z., Dank M., Igaz P., Molnár B. Blood Collection and Cell-Free DNA Isolation Methods Influence the Sensitivity of Liquid Biopsy Analysis for Colorectal Cancer Detection. Pathol. Oncol. Res. 2019;25:915–923. doi: 10.1007/s12253-018-0382-z. [DOI] [PubMed] [Google Scholar]
- 11.Van Ginkel J.H., van den Broek D.A., van Kuik J., Linders D., de Weger R., Willems S.M., Huibers M.M.H. Preanalytical blood sample workup for cell-free DNA analysis using Droplet Digital PCR for future molecular cancer diagnostics. Cancer Med. 2017;6:2297–2307. doi: 10.1002/cam4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markus H., Contente-Cuomo T., Farooq M., Liang W.S., Borad M.J., Sivakumar S., Gollins S., Tran N.L., Dhruv H.D., Berens M.E., et al. Evaluation of pre-analytical factors affecting plasma DNA analysis. Sci. Rep. 2018;8:7375. doi: 10.1038/s41598-018-25810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber T., Taschner-Mandl S., Saloberger-Sindhöringer L., Popitsch N., Heitzer E., Witt V., Geyeregger R., Hutter C., Schwentner R., Ambros I.M., et al. Assessment of Pre-Analytical Sample Handling Conditions for Comprehensive Liquid Biopsy Analysis. J. Mol. Diagn. 2020;22:1070–1086. doi: 10.1016/j.jmoldx.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Poulet G. Université de Paris, UMR-S1138, CNRS SNC5096, Équipe labélisée Ligue Nationale Contre le Cancer. Centre de Recherche des Cordeliers; Paris, France: 2022. to be submitted . [Google Scholar]
- 15.Lianidou E. Detection and relevance of epigenetic markers on ctDNA: Recent advances and future outlook. Mol. Oncol. 2021;15:1683–1700. doi: 10.1002/1878-0261.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connors D., Allen J., Alvarez J.D., Boyle J., Cristofanilli M., Hiller C., Keating S., Kelloff G., Leiman L., McCormack R., et al. International liquid biopsy standardization alliance white paper. Crit. Rev. Oncol. Hematol. 2020;156:103112. doi: 10.1016/j.critrevonc.2020.103112. [DOI] [PubMed] [Google Scholar]
- 17.Wojas-Krawczyk K., Kalinka-Warzocha E., Reszka K., Nicos M., Szumilo J., Mandziuk S., Szczepaniak K., Kupnicka D., Lewandowski R., Milanowski J., et al. Analysis of KRAS, NRAS, BRAF, and PIK3CA mutations could predict metastases in colorectal cancer: A preliminary study. Adv. Clin. Exp. Med. 2019;28:67–73. doi: 10.17219/acem/76162. [DOI] [PubMed] [Google Scholar]
- 18.Vidal J., Muinelo L., Dalmases A., Jones F., Edelstein D., Iglesias M., Orrillo M., Abalo A., Rodriguez C., Brozos E., et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017;28:1325–1332. doi: 10.1093/annonc/mdx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madic J., Jovelet C., Lopez J., André B., Fatien J., Miran I., Honoré A., Mezquita L., Besse B., Lacroix L., et al. EGFR C797S, EGFR T790M and EGFR sensitizing mutations in non-small cell lung cancer revealed by six-color crystal digital PCR. Oncotarget. 2018;9:37393–37406. doi: 10.18632/oncotarget.26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madic J., Jovelet C., Dehri I., Mallory A.C. 6-Color Crystal Digital PCR(TM) for the High-Plex Detection of EGFR Mutations in Non-Small Cell Lung Cancer. Methods Mol. Biol. 2021;2279:127–144. doi: 10.1007/978-1-0716-1278-1_10. [DOI] [PubMed] [Google Scholar]
- 21.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hiddessen A.L., Legler T.C., et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boeckx N., Op de Beeck K., Beyens M., Deschoolmeester V., Hermans C., De Clercq P., Garrigou S., Normand C., Monsaert E., Papadimitriou K., et al. Mutation and Methylation Analysis of Circulating Tumor DNA Can Be Used for Follow-up of Metastatic Colorectal Cancer Patients. Clin. Color. Cancer. 2018;17:e369–e379. doi: 10.1016/j.clcc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Kinde I., Wu J., Papadopoulos N., Kinzler K.W., Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. USA. 2011;108:9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman A.M., Bratman S.V., To J., Wynne J.F., Eclov N.C.W., Modlin L.A., Liu C.L., Neal J.W., Wakelee H.A., Merritt R.E., et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 2014;20:552–558. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman A.M., Lovejoy A.F., Klass D.M., Kurtz D.M., Chabon J.J., Scherer F., Stehr H., Liu C.L., Bratman S.V., Say C., et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat. Biotechnol. 2016;34:547–555. doi: 10.1038/nbt.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecuchet N., Rozenholc Y., Zonta E., Pietrasz D., Didelot A., Combe P., Gibault L., Bachet J.B., Taly V., Fabre E., et al. Analysis of Base-Position Error Rate of Next-Generation Sequencing to Detect Tumor Mutations in Circulating DNA. Clin. Chem. 2016;62:1492–1503. doi: 10.1373/clinchem.2016.258236. [DOI] [PubMed] [Google Scholar]
- 27.Manier S., Park J., Capelletti M., Bustoros M., Freeman S.S., Ha G., Rhoades J., Liu C.J., Huynh D., Reed S.C., et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018;9:1691. doi: 10.1038/s41467-018-04001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heitzer E., Ulz P., Belic J., Gutschi S., Quehenberger F., Fischereder K., Benezeder T., Auer M., Pischler C., Mannweiler S., et al. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med. 2013;5:30. doi: 10.1186/gm434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vymetalkova V., Cervena K., Bartu L., Vodicka P. Circulating Cell-Free DNA and Colorectal Cancer: A Systematic Review. Int. J. Mol. Sci. 2018;19:3356. doi: 10.3390/ijms19113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming C.A., O’Leary D.P., Wang J., Redmond H.P. Association of Observed Perioperative Cell-Free DNA Dynamics with Early Recurrence in Patients with Colon Cancer. JAMA Surg. 2020;155:168–170. doi: 10.1001/jamasurg.2019.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriksen T.V., Reinert T., Christensen E., Sethi H., Birkenkamp-Demtröder K., Gögenur M., Gögenur I., Zimmermann B.G., Dyrskjøt L., Andersen C.L. The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol. Oncol. 2020;14:1670–1679. doi: 10.1002/1878-0261.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholer L.V., Reinert T., Orntoft M.B.W., Kassentoft C.G., Arnadottir S.S., Vang S., Nordentoft I., Knudsen M., Lamy P., Andreasen D., et al. Clinical Implications of Monitoring Circulating Tumor DNA in Patients with Colorectal Cancer. Clin. Cancer Res. 2017;23:5437–5445. doi: 10.1158/1078-0432.CCR-17-0510. [DOI] [PubMed] [Google Scholar]
- 33.Vatandoost N., Ghanbari J., Mojaver M., Avan A., Ghayour-Mobarhan M., Nedaeinia R., Salehi R. Early detection of colorectal cancer: From conventional methods to novel biomarkers. J. Cancer Res. Clin. Oncol. 2016;142:341–351. doi: 10.1007/s00432-015-1928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 35.Bretthauer M. Colorectal cancer screening. J. Intern. Med. 2011;270:87–98. doi: 10.1111/j.1365-2796.2011.02399.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuipers E.J., Grady W.M., Lieberman D., Seufferlein T., Sung J.J., Boelens P.G., van de Velde C.J., Watanabe T. Colorectal cancer. Nat. Rev. Dis. Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petit J., Carroll G., Gould T., Pockney P., Dun M., Scott R.J. Cell-Free DNA as a Diagnostic Blood-Based Biomarker for Colorectal Cancer: A Systematic Review. J. Surg. Res. 2019;236:184–197. doi: 10.1016/j.jss.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Lamb Y.N., Dhillon S. Epi proColon((R)) 2.0 CE: A Blood-Based Screening Test for Colorectal Cancer. Mol. Diagn. Ther. 2017;21:225–232. doi: 10.1007/s40291-017-0259-y. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen S.L., Krarup H.B., Sunesen K.G., Johansen M.B., Stender M.T., Pedersen I.S., Madsen P.H., Thorlacius-Ussing O. Hypermethylated DNA, a circulating biomarker for colorectal cancer detection. PLoS ONE. 2017;12:e0180809. doi: 10.1371/journal.pone.0180809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahier J.F., Druez A., Faugeras L., Martinet J.P., Gehenot M., Josseaux E., Herzog M., Micallef J., George F., Delos M., et al. Circulating nucleosomes as new blood-based biomarkers for detection of colorectal cancer. Clin. Epigenetics. 2017;9:1–7. doi: 10.1186/s13148-017-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Church T.R., Wandell M., Lofton-Day C., Mongin S.J., Burger M., Payne S.R., Castaños-Vélez E., Blumenstein B.A., Rösch T., Osborn N., et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun G.P., Meng J., Duan H., Zhang D.W., Tang Y.X. Diagnostic Assessment of septin9 DNA Methylation for Colorectal Cancer Using Blood Detection: A Meta-Analysis. Pathol. Oncol. Res. 2019;25:1525–1534. doi: 10.1007/s12253-018-0559-5. [DOI] [PubMed] [Google Scholar]
- 43.Adler A., Geiger S., Keil A., Bias H., Schatz P., deVos T., Dhein J., Zimmermann M., Tauber R., Wiedenmann B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14:183. doi: 10.1186/1471-230X-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu M.C., Oxnard G.R., Klein E.A., Swanton C., Seiden M.V. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020;31:745–759. doi: 10.1016/j.annonc.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin J.S., Perdue L.A., Henrikson N.B., Bean S.I., Blasi P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325:1978–1998. doi: 10.1001/jama.2021.4417. [DOI] [PubMed] [Google Scholar]
- 46.Merker J.D., Oxnard G.R., Compton C., Diehn M., Hurley P., Lazar A.J., Lindeman N., Lockwood C.M., Rai A.J., Schilsky R.L., et al. Circulating Tumor DNA Analysis in Patients with Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 2018;36:1631. doi: 10.1200/JCO.2017.76.8671. [DOI] [PubMed] [Google Scholar]
- 47.Bi F., Wang Q., Dong Q., Wang Y., Zhang L., Zhang J. Circulating tumor DNA in colorectal cancer: Opportunities and challenges. Am. J. Transl. Res. 2020;12:1044–1055. [PMC free article] [PubMed] [Google Scholar]
- 48.Marcuello M., Vymetalkova V., Neves R.P.L., Duran-Sanchon S., Vedeld H.M., Tham E., van Dalum G., Flugen G., Garcia-Barberan V., Fijneman R.J., et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol. Asp. Med. 2019;69:107–122. doi: 10.1016/j.mam.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Shi X.Q., Zeng P.W., Mo F.M., Chen Z.H. Circulating cell free DNA as the diagnostic marker for colorectal cancer: A systematic review and meta-analysis. Oncotarget. 2018;9:24514–24524. doi: 10.18632/oncotarget.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tie J., Cohen J.D., Wang Y., Christie M., Simons K., Lee M., Wong R., Kosmider S., Ananda S., McKendrick J., et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019;5:1710–1717. doi: 10.1001/jamaoncol.2019.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benhaim L., Bouché O., Normand C., Didelot A., Mulot C., Le Corre D., Garrigou S., Djadi-Prat J., Wang-Renault S.-F., Perez-Toralla K., et al. Circulating tumor DNA is a prognostic marker of tumor recurrence in stage II and III colorectal cancer: Multicentric, prospective cohort study (ALGECOLS) Eur. J. Cancer. 2021;159:24–33. doi: 10.1016/j.ejca.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Phallen J., Sausen M., Adleff V., Leal A., Hruban C., White J., Anagnostou V., Fiksel J., Cristiano S., Papp E., et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017;9:eaan2415. doi: 10.1126/scitranslmed.aan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Symonds E.L., Pedersen S.K., Murray D.H., Jedi M., Byrne S.E., Rabbitt P., Baker R.T., Bastin D., Young G.P. Circulating tumour DNA for monitoring colorectal cancer—A prospective cohort study to assess relationship to tissue methylation, cancer characteristics and surgical resection. Clin. Epigenetics. 2018;10:63. doi: 10.1186/s13148-018-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarazona N., Gimeno-Valiente F., Gambardella V., Zuñiga S., Rentero-Garrido P., Huerta M., Roselló S., Martinez-Ciarpaglini C., Carbonell-Asins J.A., Carrasco F., et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019;30:1804–1812. doi: 10.1093/annonc/mdz390. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura Y., Yokoyama S., Matsuda K., Tamura K., Mitani Y., Iwamoto H., Mizumoto Y., Murakami D., Kitahata Y., Yamaue H. Preoperative detection of KRAS mutated circulating tumor DNA is an independent risk factor for recurrence in colorectal cancer. Sci. Rep. 2021;11:441. doi: 10.1038/s41598-020-79909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G., Peng J., Xiao Q., Wu H.X., Wu X., Wang F., Li L., Ding P., Zhao Q., Li Y., et al. Postoperative circulating tumor DNA as markers of recurrence risk in stages II to III colorectal cancer. J. Hematol. Oncol. 2021;14:80. doi: 10.1186/s13045-021-01089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bregni G., Pretta A., Senti C., Acedo Reina E., Vandeputte C., Trevisi E., Gkolfakis P., Kehagias P., Deleporte A., Van Laethem J.L., et al. Circulating DNA in the neoadjuvant setting of early stage colon cancer. Acta Oncol. 2022;61:1223–1229. doi: 10.1080/0284186X.2022.2101023. [DOI] [PubMed] [Google Scholar]
- 58.Norcic G. Liquid Biopsy in Colorectal Cancer—Current Status and Potential Clinical Applications. Micromachines. 2018;9:300. doi: 10.3390/mi9060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basnet S., Zhang Z.-y., Liao W.-q., Li S.-h., Li P.-s., Ge H.-y. The Prognostic Value of Circulating Cell-Free DNA in Colorectal Cancer: A Meta-Analysis. J. Cancer. 2016;7:1105–1113. doi: 10.7150/jca.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manca P., Corallo S., Lonardi S., Fucà G., Busico A., Leone A.G., Corti F., Antoniotti C., Procaccio L., Smiroldo V., et al. Variant allele frequency in baseline circulating tumour DNA to measure tumour burden and to stratify outcomes in patients with RAS wild-type metastatic colorectal cancer: A translational objective of the Valentino study. Br. J. Cancer. 2021;126:449–455. doi: 10.1038/s41416-021-01591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garlan F., Laurent-Puig P., Sefrioui D., Siauve N., Didelot A., Sarafan-Vasseur N., Michel P., Perkins G., Mulot C., Blons H., et al. Early Evaluation of Circulating Tumor DNA as Marker of Therapeutic Efficacy in Metastatic Colorectal Cancer Patients (PLACOL Study) Clin. Cancer Res. 2017;23:5416–5425. doi: 10.1158/1078-0432.CCR-16-3155. [DOI] [PubMed] [Google Scholar]
- 62.Osterman E., Glimelius B. Recurrence Risk After Up-to-Date Colon Cancer Staging, Surgery, and Pathology: Analysis of the Entire Swedish Population. Dis. Colon Rectum. 2018;61:1016–1025. doi: 10.1097/DCR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 63.Pachman D.R., Qin R., Seisler D.K., Smith E.M.L., Beutler A.S., Ta L.E., Lafky J.M., Wagner-Johnston N.D., Ruddy K.J., Dakhil S., et al. Clinical Course of Oxaliplatin-Induced Neuropathy: Results from the Randomized Phase III Trial N08CB (Alliance) J. Clin. Oncol. 2015;33:3416–3422. doi: 10.1200/JCO.2014.58.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tie J., Wang Y., Tomasetti C., Li L., Springer S., Kinde I., Silliman N., Tacey M., Wong H.L., Christie M., et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016;8:346ra392. doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L.R., Zhou W.H., Li C., Li P.S., Gong Y.H., Guan Y.F. Analysis of circulating tumor DNA to monitor disease status in colorectal cancer after surgery. J. Clin. Oncol. 2018;36:e15583. doi: 10.1200/JCO.2018.36.15_suppl.e15583. [DOI] [Google Scholar]
- 66.Taieb J., Taly V., Henriques J., Bourreau C., Mineur L., Bennouna J., Desrame J., Louvet C., Lepere C., Mabro M., et al. Prognostic Value and Relation with Adjuvant Treatment Duration of ctDNA in Stage III Colon Cancer: A Post Hoc Analysis of the PRODIGE-GERCOR IDEA-France Trial. Clin. Cancer Res. 2021;27:5638. doi: 10.1158/1078-0432.CCR-21-0271. [DOI] [PubMed] [Google Scholar]
- 67.Tie J., Cohen J., Wang Y.X., Lee M., Wong R., Kosmider S., Ananda S., Cho J.H., Faragher I., McKendrick J.J., et al. Serial circulating tumor DNA (ctDNA) analysis as a prognostic marker and a real-time indicator of adjuvant chemotherapy (CT) efficacy in stage III colon cancer (CC) J. Clin. Oncol. 2018;36:3516. doi: 10.1200/JCO.2018.36.15_suppl.3516. [DOI] [Google Scholar]
- 68.Wang Y., Li L., Cohen J.D., Kinde I., Ptak J., Popoli M., Schaefer J., Silliman N., Dobbyn L., Tie J., et al. Prognostic Potential of Circulating Tumor DNA Measurement in Postoperative Surveillance of Nonmetastatic Colorectal Cancer. JAMA Oncol. 2019;5:1118–1123. doi: 10.1001/jamaoncol.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reinert T., Scholer L.V., Thomsen R., Tobiasen H., Vang S.R., Nordentoft I., Lamy P., Kannerup A.S., Mortensen F.V., Stribolt K., et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 2016;65:625–634. doi: 10.1136/gutjnl-2014-308859. [DOI] [PubMed] [Google Scholar]
- 70.Henriksen T.V., Tarazona N., Frydendahl A., Reinert T., Gimeno-Valiente F., Carbonell-Asins J.A., Sharma S., Renner D., Hafez D., Roda D., et al. Circulating Tumor DNA in Stage III Colorectal Cancer, beyond Minimal Residual Disease Detection, toward Assessment of Adjuvant Therapy Efficacy and Clinical Behavior of Recurrences. Clin. Cancer Res. 2021;28:507–517. doi: 10.1158/1078-0432.CCR-21-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cremolini C., Rossini D., Dell’Aquila E., Lonardi S., Conca E., Del Re M., Busico A., Pietrantonio F., Danesi R., Aprile G., et al. Rechallenge for Patients with RAS and BRAF Wild-Type Metastatic Colorectal Cancer with Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019;5:343–350. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siravegna G., Sartore-Bianchi A., Nagy R.J., Raghav K., Odegaard J.I., Lanman R.B., Trusolino L., Marsoni S., Siena S., Bardelli A. Plasma HER2 (ERBB2) Copy Number Predicts Response to HER2-targeted Therapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019;25:3046–3053. doi: 10.1158/1078-0432.CCR-18-3389. [DOI] [PubMed] [Google Scholar]
- 73.Herbst A., Vdovin N., Gacesa S., Philipp A., Nagel D., Holdt L.M., op den Winkel M., Heinemann V., Stieber P., Graeven U., et al. Methylated free-circulating HPP1 DNA is an early response marker in patients with metastatic colorectal cancer. Int. J. Cancer. 2017;140:2134–2144. doi: 10.1002/ijc.30625. [DOI] [PubMed] [Google Scholar]
- 74.Tie J., Kinde I., Wang Y., Wong H.L., Roebert J., Christie M., Tacey M., Wong R., Singh M., Karapetis C.S., et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 2015;26:1715–1722. doi: 10.1093/annonc/mdv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barault L., Amatu A., Siravegna G., Ponzetti A., Moran S., Cassingena A., Mussolin B., Falcomata C., Binder A.M., Cristiano C., et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut. 2018;67:1995–2005. doi: 10.1136/gutjnl-2016-313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murray D., Symonds E.L., Pedersen S.K., Young G.P. Detection of ctDNA biomarkers post resection and relationship to recurrent disease in colorectal cancer patients. J. Clin. Oncol. 2018;36:e15616. doi: 10.1200/JCO.2018.36.15_suppl.e15616. [DOI] [Google Scholar]
- 77.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 78.Thierry A.R., El Messaoudi S., Mollevi C., Raoul J.L., Guimbaud R., Pezet D., Artru P., Assenat E., Borg C., Mathonnet M., et al. Clinical utility of circulating DNA analysis for rapid detection of actionable mutations to select metastatic colorectal patients for anti-EGFR treatment. Ann. Oncol. 2017;28:2149–2159. doi: 10.1093/annonc/mdx330. [DOI] [PubMed] [Google Scholar]
- 79.Knebel F.H., Bettoni F., da Fonseca L.G., Camargo A.A., Sabbaga J., Jardim D.L. Circulating Tumor DNA Detection in the Management of Anti-EGFR Therapy for Advanced Colorectal Cancer. Front. Oncol. 2019;9:170. doi: 10.3389/fonc.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Emburgh B.O., Arena S., Siravegna G., Lazzari L., Crisafulli G., Corti G., Mussolin B., Baldi F., Buscarino M., Bartolini A., et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat. Commun. 2016;7:13665. doi: 10.1038/ncomms13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siravegna G., Mussolin B., Buscarino M., Corti G., Cassingena A., Crisafulli G., Ponzetti A., Cremolini C., Amatu A., Lauricella C., et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raghav K.P.S., Overman M.J., Yu R., Meric-Bernstam F., Menter D., Kee B.K., Muranyi A., Singh S., Routbort M., Chen K., et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J. Clin. Oncol. 2016;34:3517. doi: 10.1200/JCO.2016.34.15_suppl.3517. [DOI] [PubMed] [Google Scholar]
- 83.Sartore-Bianchi A., Pietrantonio F., Lonardi S., Mussolin B., Rua F., Fenocchio E., Amatu A., Corallo S., Manai C., Tosi F., et al. Phase II study of anti-EGFR rechallenge therapy with panitumumab driven by circulating tumor DNA molecular selection in metastatic colorectal cancer: The CHRONOS trial. J. Clin. Oncol. 2021;39:3506. doi: 10.1200/JCO.2021.39.15_suppl.3506. [DOI] [Google Scholar]
- 84.Turner N.C., Kingston B., Kilburn L.S., Kernaghan S., Wardley A.M., Macpherson I.R., Baird R.D., Roylance R., Stephens P., Oikonomidou O., et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21:1296–1308. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Leo A., Johnston S., Lee K.S., Ciruelos E., Lønning P.E., Janni W., O’Regan R., Mouret-Reynier M.-A., Kalev D., Egle D., et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 86.Paik P.K., Felip E., Veillon R., Sakai H., Cortot A.B., Garassino M.C., Mazieres J., Viteri S., Senellart H., Van Meerbeeck J., et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taïeb J., Benhaim L., Laurent Puig P., Le Malicot K., Emile J.F., Geillon F., Tougeron D., Manfredi S., Chauvenet M., Taly V., et al. Decision for adjuvant treatment in stage II colon cancer based on circulating tumor DNA:The CIRCULATE-PRODIGE 70 trial. Dig. Liver Dis. 2020;52:730–733. doi: 10.1016/j.dld.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 88.Tie J., Cohen J.D., Lahouel K., Lo S.N., Wang Y., Kosmider S., Wong R., Shapiro J., Lee M., Harris S., et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022;386:2261–2272. doi: 10.1056/NEJMoa2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tie J., Lo S.N., Cohen J.D., Wang Y., Wong R., Shapiro J., Harris S., Khattak A., Burge M., Vogelstein B., et al. Circulating tumour DNA analysis informing adjuvant chemotherapy in stage III colon cancer: A multicentre phase II/III randomised controlled study (DYNAMIC-III) Aust. N. Z. Clin. Regist. 2017 [Google Scholar]
- 90.Anandappa G., Starling N., Peckitt C., Bryant A., Begum R., Carter P., Hatt S., Khakoo S.S., Turner A., Kidd S., et al. TRACC: Tracking mutations in cell-free DNA to predict relapse in early colorectal cancer—A randomized study of circulating tumour DNA (ctDNA) guided adjuvant chemotherapy versus standard of care chemotherapy after curative surgery in patients with high risk stage II or stage III colorectal cancer (CRC) J. Clin. Oncol. 2020;38:TPS4120. doi: 10.1200/JCO.2020.38.15_suppl.TPS4120. [DOI] [Google Scholar]
- 91.Nors J., Henriksen T.V., Gotschalck K.A., Juul T., Søgaard J., Iversen L.H., Andersen C.L. IMPROVE-IT2: Implementing noninvasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer—Intervention trial 2. Study protocol. Acta Oncol. 2020;59:336–341. doi: 10.1080/0284186X.2019.1711170. [DOI] [PubMed] [Google Scholar]
- 92.Morris V.K., Yothers G., Kopetz S., Jacobs S.A., Lucas P.C., Iqbal A., Boland P.M., Deming D.A., Scott A.J., Lim H.J., et al. NRG-GI005 (COBRA): Phase II/III study of circulating tumor DNA as a predictive biomarker in adjuvant chemotherapy in patients with stage II colon cancer. J. Clin. Oncol. 2020;38:TPS261. doi: 10.1200/JCO.2020.38.4_suppl.TPS261. [DOI] [Google Scholar]
- 93.Taniguchi H., Nakamura Y., Kotani D., Yukami H., Mishima S., Sawada K., Shirasu H., Ebi H., Yamanaka T., Aleshin A., et al. CIRCULATE-Japan: Circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112:2915–2920. doi: 10.1111/cas.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sauer R., Becker H., Hohenberger W., Rödel C., Wittekind C., Fietkau R., Martus P., Tschmelitsch J., Hager E., Hess C.F., et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 95.Delibegovic S. Introduction to Total Mesorectal Excision. Med. Arch. 2017;71:434–438. doi: 10.5455/medarh.2017.71.434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith J.J., Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J. Clin. Oncol. 2015;33:1797–1808. doi: 10.1200/JCO.2014.60.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dattani M., Heald R.J., Goussous G., Broadhurst J., Sao Juliao G.P., Habr-Gama A., Perez R.O., Moran B.J. Oncological and Survival Outcomes in Watch and Wait Patients with a Clinical Complete Response after Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann. Surg. 2018;268:955–967. doi: 10.1097/SLA.0000000000002761. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Aguilar J., Patil S., Gollub M.J., Kim J.K., Yuval J.B., Thompson H.M., Verheij F.S., Omer D.M., Lee M., Dunne R.F., et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022;40:2546–2556. doi: 10.1200/JCO.22.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van der Valk M.J.M., Hilling D.E., Bastiaannet E., Meershoek-Klein Kranenbarg E., Beets G.L., Figueiredo N.L., Habr-Gama A., Perez R.O., Renehan A.G., van de Velde C.J.H. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet. 2018;391:2537–2545. doi: 10.1016/s0140-6736(18)31078-x. [DOI] [PubMed] [Google Scholar]
- 100.Zhou J., Wang C., Lin G., Xiao Y., Jia W., Xiao G., Liu Q., Wu B., Wu A., Qiu H., et al. Serial Circulating Tumor DNA in Predicting and Monitoring the Effect of Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer: A Prospective Multicenter Study. Clin. Cancer Res. 2021;27:301–310. doi: 10.1158/1078-0432.CCR-20-2299. [DOI] [PubMed] [Google Scholar]
- 101.Pazdirek F., Minarik M., Benesova L., Halkova T., Belsanova B., Macek M., Stepanek L., Hoch J. Monitoring of Early Changes of Circulating Tumor DNA in the Plasma of Rectal Cancer Patients Receiving Neoadjuvant Concomitant Chemoradiotherapy: Evaluation for Prognosis and Prediction of Therapeutic Response. Front. Oncol. 2020;10:1028. doi: 10.3389/fonc.2020.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vidal J., Casadevall D., Bellosillo B., Pericay C., Garcia-Carbonero R., Losa F., Layos L., Alonso V., Capdevila J., Gallego J., et al. Clinical Impact of Presurgery Circulating Tumor DNA after Total Neoadjuvant Treatment in Locally Advanced Rectal Cancer: A Biomarker Study from the GEMCAD 1402 Trial. Clin. Cancer Res. 2021;27:2890–2898. doi: 10.1158/1078-0432.CCR-20-4769. [DOI] [PubMed] [Google Scholar]
- 103.Carpinetti P., Donnard E., Bettoni F., Asprino P., Koyama F., Rozanski A., Sabbaga J., Habr-Gama A., Parmigiani R.B., Galante P.A.F., et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget. 2015;6:38360–38371. doi: 10.18632/oncotarget.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tie J., Cohen J.D., Wang Y., Li L., Christie M., Simons K., Elsaleh H., Kosmider S., Wong R., Yip D., et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: A prospective biomarker study. Gut. 2019;68:663–671. doi: 10.1136/gutjnl-2017-315852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khakoo S., Carter P.D., Brown G., Valeri N., Picchia S., Bali M.A., Shaikh R., Jones T., Begum R., Rana I., et al. MRI Tumor Regression Grade and Circulating Tumor DNA as Complementary Tools to Assess Response and Guide Therapy Adaptation in Rectal Cancer. Clin. Cancer Res. 2020;26:183–192. doi: 10.1158/1078-0432.CCR-19-1996. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y., Yang L., Bao H., Fan X., Xia F., Wan J., Shen L., Guan Y., Bao H., Wu X., et al. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med. 2021;18:e1003741. doi: 10.1371/journal.pmed.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu W.Y., Li Y.F., Tang Y., Song Q.Q., Wang J.J., Li N., Chen S.L., Shi J.M., Wang S.L., Li Y.X., et al. Response prediction and risk stratification of patients with rectal cancer after neoadjuvant therapy through an analysis of circulating tumour DNA. Ebiomedicine. 2022;78:103945. doi: 10.1016/j.ebiom.2022.103945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McDuff S.G.R., Hardiman K.M., Ulintz P.J., Parikh A.R., Zheng H., Kim D.W., Lennerz J.K., Hazar-Rethinam M., Van Seventer E.E., Fetter I.J., et al. Circulating Tumor DNA Predicts Pathologic and Clinical Outcomes Following Neoadjuvant Chemoradiation and Surgery for Patients with Locally Advanced Rectal Cancer. JCO Precis. Oncol. 2021;5 doi: 10.1200/PO.20.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hofste L.S.M., Geerlings M.J., von Rhein D., Rutten H., Westenberg A.H., Weiss M.M., Gilissen C., Hofste T., van der Post R.S., Klarenbeek B.R., et al. Circulating tumor DNA detection after neoadjuvant treatment and surgery predicts recurrence in patients with early-stage and locally advanced rectal cancer. Eur. J. Surg. Oncol. 2023 doi: 10.1016/j.ejso.2023.01.026. [DOI] [PubMed] [Google Scholar]
- 110.Agostini M., Pucciarelli S., Enzo M.V., Del Bianco P., Briarava M., Bedin C., Maretto I., Friso M.L., Lonardi S., Mescoli C., et al. Circulating cell-free DNA: A promising marker of pathologic tumor response in rectal cancer patients receiving preoperative chemoradiotherapy. Ann. Surg. Oncol. 2011;18:2461–2468. doi: 10.1245/s10434-011-1638-y. [DOI] [PubMed] [Google Scholar]
- 111.Kramer A., Schuuring E., Vessies D.C.L., van der Leest P., Geerlings M.J., Rozendal P., Lanfermeijer M., Linders T.C., van Kempen L.C., Fijneman R.J.A., et al. A Micro-Costing Framework for Circulating Tumor DNA Testing in Dutch Clinical Practice. J. Mol. Diagn. 2023;25:36–45. doi: 10.1016/j.jmoldx.2022.10.004. [DOI] [PubMed] [Google Scholar]