Abstract
Risk-reducing bilateral salpingo-oophorectomy (RRSO) is an effective prophylactic surgery provided to premenopausal women carrying BRCA1 or BRCA2 mutations and presenting an increased risk of developing breast or ovarian cancer. This procedure is related to physiological, sexual, and psychosocial distress, which altogether increase uncertainty and complexity in the clinical decision-making process and post-surgery adaptation. Physician-patient communication (PPC) has been pointed out as a determinant factor in the decision-making to undergo RRSO, and the subsequent adjustment of women. However, studies examining the psychosocial impact of the decision-making process have been scarce and often lack clear theoretical frameworks. While the role of PPC in such processes has been highlighted in a few qualitative studies, there is a paucity of quantitative research addressing this question. Therefore, this narrative review, conducted using a multidisciplinary approach, was planned to: (1) present an updated medical background for RRSO; (2) analyze the psychosocial impact of the decision-making process within a theoretical framework of the Health Belief Model; and (3) discuss the role of PPC in such a decision-making process and in post-surgery. The collected research also enabled the recommendation of some additions to the existing clinical guidelines and the outlining of future research directions.
Keywords: risk-reducing salpingo-oophorectomy (RRSO), BRCA mutation, ovarian cancer, breast cancer, shared decision-making, physician-patient communication (PPC), psychosocial adjustment
1. Introduction
Hereditary breast and/or ovarian cancer are associated with specific genetic variants that greatly increase the lifetime risk of developing these conditions [1,2]. For instance, some rare gene mutations correlate with breast and/or ovarian cancer predisposition syndromes [2,3,4,5]. However, the most common and well-known examples are the pathogenic BRCA1 and BRCA2 genomic variants, present in 1 to 300–500 women and responsible for 5–10% of breast cancers, and 10–15% of ovarian cancers [3,4,6,7,8,9].
As long as BRCA1 and BRCA2 mutations are involved, risk-reducing strategies should be implemented to prevent the risk of cancer, such as risk-reducing salpingo-oophorectomy (RRSO). RRSO is an effective risk-reducing surgery recommended to high-risk premenopausal healthy women carrying these mutations, ideally after the age of 35 or immediately after childbearing is complete [10,11]. It includes the surgical removal of the fallopian tubes and ovaries bilaterally and has proved its effectiveness in preventing ovarian cancer in BRCA1/2 carriers [1,6]. Nevertheless, the removal of apparently healthy organs may have serious physiological, sexual, and psychological consequences and does not ensure the elimination of future cancer risk.
The decision-making process to undergo RRSO is highly challenging for women and depends on multiple variables, such as personal appraisals, medical history, and psychological functioning [12]. Several qualitative studies have analyzed how these variables interact and contribute to the decision-making process (e.g., [13,14]). Even though a solid theoretical framework suggests the synthesis of potential interrelations between determinants, processes, and outcomes, ultimately guiding research and clinical intervention in shared decision-making processes [15], this has been systematically lacking in the aforementioned research.
Additionally, the decision to undergo RRSO is expected to be shared between the woman and her physician/oncologist. In this sense, physician-patient communication (PPC) has been acknowledged as a crucial factor in patient-centered care and associated with positive effects over healthcare outcomes (e.g., [16,17,18]). PPC also appears to be distinctively linked to women’s adaptation outcomes after RRSO [13,19,20,21].
In this context, a narrative review [22,23] was conducted employing a multidisciplinary approach (i.e., medicine and psychology) in order to analyze and integrate (i) the psychosocial impact of the decision-making process to undergo RRSO in women carrying BRCA mutations, and (ii) the role of PPC during this process and in post-surgery. Specifically, this narrative review was planned to: (1) present an updated medical background for RRSO, namely the genomic framework that leads its medical recommendation, among other possible alternative prophylactic measures, and its physiological consequences; (2) analyze the psychosocial impact of the decision-making process, within a theoretical framework of the Health Belief Model; and (3) discuss the role of PPC in women’s decision-making processes and post-surgery adaptation outcomes.
2. Clinical Background for the Recommendation of RRSO
2.1. Genomic Susceptibility to Ovarian/Breast Cancer
Some rare moderately penetrant genes confer an increased risk of developing hereditary breast and/or ovarian cancer, such as MLH1, MSH2 or STK11 [2,3,4,5]. Table 1 summarizes the risk rates for developing breast and/or ovarian cancer for each genomic pathogenic variant [1,3,4,5,6,8,9,24,25,26,27,28,29,30,31,32].
Table 1.
Hereditary genomic mutation risk rates for developing breast and/or ovarian cancer.
| Gene | Ovarian Cancer Cumulative Risk (by Age ≥ 70) | Breast Cancer Cumulative Risk (by Age ≥ 70) |
|---|---|---|
| MLH1 | 4–20% | Unknown |
| MSH2 | 8–38% | Unknown |
| MSH6 | 1–13% | Unknown |
| PALB2 | Unknown | 35% |
| ATM | Unknown | 33% |
| STK11 | 18–21% | 45–50% |
| BRIP1 | 7–10% | Unknown |
| RAD51C/D | 5–12% | Unknown |
| BRCA1 | 45–60% | 65–80% |
| BRCA2 | 11–35% | 50–70% |
Despite the risk conferred by these rare pathogenic variants, research is still scarce on this topic, preventing the establishment of clinical management protocols for most of them [5].
It is noteworthy that most cases of hereditary breast and/or ovarian cancers are associated with germline mutations in BRCA1 and BRCA2 genes [8]. BRCA1 and BRCA2 mutations are responsible for 5–10% of breast cancers and 10–15% of ovarian cancers [3,4,6,9]. Besides the high cumulative risk at the age of 70–80, women carrying these mutations may already present an increased risk up to 10% for developing ovarian cancer by the age of 50 [4]. The 5-, 10-, and 15-year cumulative risk for BRCA1 carriers is 13.7%, 23.8%, and 36.1%, respectively; and for BRCA2 carriers is 12.0%, 18.7% and 28.5%, respectively [33].
Given the lifetime and cumulative risks of cancer in BRCA germline mutation carriers, after genetic testing disclosure, risk-reducing strategies are usually recommended by clinical practice guidelines [2,7,10,30].
2.2. Risk-Reducing Measures and RRSO
Various strategies can be used to reduce cancer risk, morbidity, and mortality in women with an increased risk of hereditary breast and ovarian cancer [5], within a multidisciplinary approach [3]. Table 2 represents a summary of risk-reducing measures recommended by clinical guidelines for BRCA mutation carriers [1,2,3,6,7,8,10,27].
Table 2.
Risk-reducing measures recommended by clinical guidelines for BRCA mutation carriers.
| Risk-Reducing Measure | Recommended Implementation |
|---|---|
| Screening | |
| Breast examinations (mammography, MRI) | >age 25 |
| Pelvic ultrasound testing + serum biomarkers | >age 35 |
| Risk-reducing agents | |
| Oral contraceptives | More data needed |
| Chemoprevention | More data needed |
| Risk-reducing surgeries | |
| Mastectomy (RRM) | No age defined |
| Salpingo-oophorectomy (RRSO) | >age 35–40 (BRCA1) >age 40–45 (BRCA2) |
Regarding the screening methods, mammography seems to be the only effective imaging strategy in reducing breast cancer mortality. However, it presents lower detection sensitivity in BRCA mutation carriers in comparison to the general population, particularly in women under 40 years old and carrying a BRCA1 variant. It is noteworthy that breast MRI is reported as the most sensitive screening exam for BRCA mutation carriers. Still, data are missing about the effectiveness of this strategy in reducing long-term mortality in these women [34]. On the other hand, compared to breast cancer screening, ovarian cancer screening methods are largely ineffective, with no reported benefit in reducing ovarian cancer mortality [3,35]. Specifically, in a 2007 observational, follow-up study in the general population, annual screening with combined CA125 and transvaginal ultrasound failed to detect early-stage cancer. In fact, there were women diagnosed with stage III/IV cancers, while having a normal screening 3 to 10 months before diagnosis [6]. Moreover, the large multicenter, prospective phase II, UK Familial Ovarian Cancer Screening Study screened 348 high-risk women using an algorithm that combined CA125 results every 4 months and annual transvaginal ultrasound. Similarly, the MODENA study prospectively screened 661 women with pathogenic BRCA1/2 variants using CA125 and transvaginal ultrasound every 6 months. Even though screening in both studies was associated with diagnosis of early-stage disease (stage I or II), it failed to reduce ovarian cancer mortality [1,7,8]. Therefore, clinical guidelines recommend that high-risk women should be aware of the limitations of solely using ovarian cancer screening as a prevention strategy, and possibly consider other more effective prophylactic measures [1,7,8,30].
The evidence of the effect of oral contraception on breast cancer risk among BRCA1/2 mutation carriers is still controversial. Although a few case-control studies have reported a modest increase in breast cancer risk [8], at least two meta-analyses showed no increased risk of breast cancer in women with BRCA1/2 mutation taking oral contraception [1,3,6,27]. Additionally, oral contraceptives can reduce the risk of ovarian cancer by 40–50% [1,2,3,6,27] in BRCA carriers, and the benefit is greater the longer the treatment [1,2,8]. A meta-analysis published in 2013 included 14 studies and showed a 42% reduction in the risk of ovarian cancer associated with combined hormone contraceptive use in women with BRCA1/2 variants [1]. However, there is a lack of both randomized and controlled trials to determine the effectiveness of this prophylactic measure [27].
The use of tamoxifen and aromatase inhibitors (AI) as chemoprevention strategies has shown efficacy in reducing the risk of invasive breast cancer in high-risk postmenopausal women. However, there are no prospective studies evaluating the risk-reduction effect of tamoxifen in women with BRCA mutations. Available data suggest a benefit for individuals carrying a BRCA2 mutation but possibly not in BRCA1 mutation carriers. Available retrospective data suggest that AI can have a real benefit in reducing breast cancer in BRCA1/2 carriers [2,10].
Women carrying BRCA1 and 2 mutations have a breast cancer risk reduction of at least 90% when undergoing risk-reducing mastectomy (RRM) [2,3,8,27,32,35,36]. This risk reduction also translates in a 90% decrease in cancer-related mortality [35]. In addition, RRM is associated with low rates of postoperative complications and reduced rates of surgery-related mortality, even though the risk of developing breast cancer is not fully eliminated [8]. Nonetheless, NCCN guidelines recommend the discussion of this option with women carrying BRCA1 and 2 mutations [6,7], since RRM has a negative impact on body image and sexuality and is frequently associated with long-term physical symptoms [10]. It is worth noting that risk-reducing mastectomy has no prophylactic effect regarding the risk of developing ovarian cancer, thus highlighting the need to consider a more effective alternative targeting both types of cancer.
As for RRSO, surgical removal of both ovaries and fallopian tubes in premenopausal women significantly reduces the levels of circulating hormones, which may lead to a reduced risk of developing estrogen-dependent breast cancer. Some authors continue to argue that RRSO leads to a 40–70% risk reduction of developing breast cancer [1,2,3,4,6,24,32,33,36,37]. However, hereditary breast cancer is often triple-negative, so hormonal mechanisms alone are not enough to cause breast cancer [38]. In addition, a prospective multicenter cohort study reported that RRSO significantly reduced the risk of breast cancer for BRCA2 pathogenic variant carriers but not for BRCA1 carriers [36]. In turn, when performed in premenopausal women carrying BRCA1/2 mutations, RRSO reduced the risk of ovarian cancer by 80–96% [1,2,4,6,7,8,24,26,27], as well as the mortality rate associated with ovarian cancer by 94% [24]. Specifically, a 2018 Cochrane review compared 2936 women with pathogenic BRCA1/2 variants undergoing RRSO with 5151 women not undergoing this surgery but following other surveillance measures. This study demonstrated that undergoing RRSO decreased both ovarian cancer (HR 0.06; 95% CI (0.02–0.17)) and breast cancer mortality (HR 0.58; 95% CI (0.39–0.88)) [1]. Moreover, in a large 2016 prospective study, RRSO was effective in breast cancer prevention in BRCA2 mutation carriers (age-adjusted HR 0.18; 95% CI (0.05–0.63)). However, in the same study, an effective breast cancer prevention through RRSO for BRCA1 mutation carriers was not evident (age-adjusted HR 0.79; 95% CI (0.55–1.13)) [7].
Despite the evidence for the effectiveness of RRSO, the risk of developing peritoneal cancer after undergoing the surgery remains at approximately 4%, especially in BRCA1 mutation carriers [6,8,24,35]. The incidence of occult cancer in BRCA1/2 mutation carriers at the time of risk-reducing surgery is 2.5 to 3.6% [4,6].
Given the high level of effectiveness in primary prevention of ovarian cancer, the potential efficacy in preventing breast cancer, and an increased overall reduction in mortality, RRSO is highly recommended for women carrying the BRCA1/2 mutations, according to the recommendations of the NCCN and a joint position statement of the Society of Gynecologic Oncology and the American College of Obstetricians and Gynecologists [1,2,3,4,6,7,8,24,25,26,27,29,32]. However, the removal of apparently healthy organs has physiological implications, with concomitant sexual and psychological impacts.
2.3. Physiological Implications of RRSO
Despite being considered a safe surgical procedure [6], RRSO has several physiological implications, mainly related to the surgical removal of the ovaries. Indeed, RRSO will lead to a surgical menopause, associated with a sudden decrease in estrogen levels and, consequently, with the onset of menopausal distress, including vasomotor symptoms, genitourinary syndrome, sleep disturbances, mood swings, and sexual dysfunction (e.g., decreased libido, vaginal dryness, and dyspareunia). These symptoms are generally more severe than in natural, gradual menopause [1,3,7,25].
Bilateral RRSO has also been associated with an increased non-cancer-related morbidity, such as increased risk of osteoporosis, cardiovascular disease, and metabolic syndrome, even though further studies are warranted [1,4,6,25]. First, bone mineral density decreases by up to 6.7% in premenopausal women at 12 months after an oophorectomy, which is much higher than the rate observed in natural menopause [39]. Second, despite the absence of prospective data, cohort studies show a slightly increased risk of cardiovascular disease in premenopausal women undergoing RRSO [40]. Third, these women may experience negative changes in lipid profile, with subsequent development of atherosclerosis [41].
Additionally, the recommendation to offer a RRSO from the age of 35 also limits the fertility window and could represent a major concern, especially in developed countries where the mean age of the first pregnancy is postponed [42].
Recently, and considering the existing evidence that many ovarian cancers originate in the fallopian tubes, it has been suggested that a risk-reducing salpingectomy alone, or followed by an oophorectomy close to the age of natural menopause, might postpone the onset of early menopause symptoms and allow an extended fertility window. However, the level of risk reduction achieved through this strategy is unknown, and data regarding the efficacy of this approach are lacking [1,6,7,8,27,36].
Hormone replacement therapy (HRT) (i.e., the exogenous administration of estrogens) has been recommended to women without a personal history of breast cancer, in the absence of counterindications, and until the natural age of menopause, in order to reduce menopausal distress following RRSO [1,2,8,25,29]. According to a systematic review evaluating the risks and benefits of HRT, this therapy was associated with improved quality of life, better sexual functioning and bone health, less menopausal distress, and reduced risk of cardiovascular disease after RRSO [1,2,3,6,7,25]. Although concerns have been raised about a possible increase in risk of breast cancer with the use of HRT, a large meta-analysis of 1100 women with pathogenic BRCA1/2 variants undergoing RRSO found no increased risk of breast cancer with short-term use of HRT (HR 0.98; 95% CI (0.63–1.52)) [1]. Nevertheless, large, controlled trials are urgently needed, and women should be informed that the existing data are limited [7,8,25].
3. The Psychological Process of Deciding to Undergo RRSO
The decision-making process to undergo RRSO is fraught with uncertainty and complexity for women carrying BRCA1/2 mutations [13,21].
Several factors have been described in the literature as affecting the woman’s decision to undergo, delay, or avoid this surgical intervention [12]. Having a family or personal history of cancer, or having witnessed a close relative dying of cancer, are commonly reported reasons for women favorably choosing RRSO (e.g., [14,43,44]). Demographic factors, such as women’s age and parity, have also been examined in relation to the choice to undergo risk-reducing interventions, suggesting that women closer to menopausal age and with children are more likely to opt for this prophylactic measure [43,45].
It is worth noting that psychological factors have often been reported by women as variables that may hinder the decision-making process, including worries about getting cancer [46], or about the physiological, emotional, and sexual consequences of ovary removal surgery, and surgical menopause [44]. In addition, the availability of internal (e.g., coping skills, self-efficacy) and external (e.g., social support) resources to deal with post-surgery physical and emotional consequences has been commented upon as a key psychological determinant underlying the decision to undergo RRSO [13]. However, most of these studies relied on small samples and qualitative designs, often lacking a solid theoretical framework to guide the research into this complex decision-making process. Besides the methodological deficiencies, the absence of a consistent theoretical model is particularly noteworthy. First, a theoretical model might be used to guide research in a theory-driven manner, permitting, for instance, consistency of results, conceptual coherence, or comparability between results. Second, it might also impact clinical practice by facilitating the physician’s case conceptualization in clinical shared decision-making.
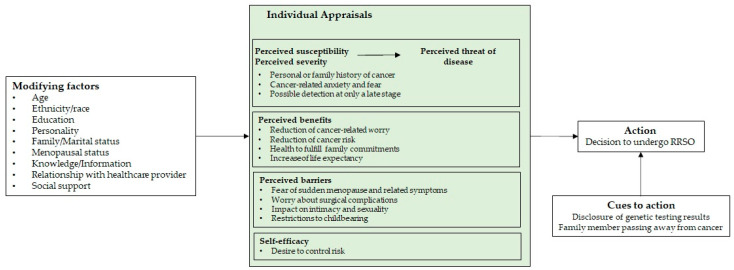
Recently, some researchers applied the Health Belief Model (HBM) [47,48] to examine the decision-making process of women who choose a RRSO. The HBM was developed to explain health behavior change, and portrays a complex interplay of intrapersonal factors, while taking in consideration the context in which health behaviors take place [49,50]. According to this model, when confronted with environmental health-related cues to action, a person’s behavior is guided by their internal appraisals of the perceived threat of a disease (e.g., beliefs about getting a disease and its seriousness); the perceived benefits and barriers of adopting a given health behavior; and the perceived self-efficacy in maintaining such behavior. Additionally, these individual perceptions may vary according to individual attributes, such as age, education, or knowledge [49]. The adaptation of the HBM to women’s decision to undergo RRSO is depicted in Figure 1, based upon currently existing models [51,52].
Figure 1.
The adapted application of the HBM to women’s decision to undergo RRSO. Key concepts: Perceived susceptibility—belief about the likelihood of getting a disease or condition; Perceived severity—belief about the seriousness of contracting an illness or of leaving it untreated; Perceived threat of disease—combination of perceived susceptibility and severity; Perceived benefits—belief in efficacy of advised action to reduce risk; Perceived barriers—belief about the tangible and psychological costs of the advised action; Self-efficacy—confidence in one’s ability to take action. How to read the model: when confronted with contextual cues that demand a decision as to whether to undergo RRSO, women are more likely to decide favorably if they regard themselves as susceptible to cancer, believe that getting cancer would have potentially serious consequences, believe that the benefits of undergoing RRSO outweigh its costs (barriers), and believe they are capable of making that decision. All these appraisals may also be influenced by women’s sociodemographic, psychological, structural, and relational factors (adapted from [49,50]).
3.1. The Psychosocial Impact of Undergoing RRSO
As for the psychological impact of having the surgery per se, the available studies report mixed results. On the one hand, some researchers have found the levels of overall post-surgery quality of life to be comparable to those of the general population, along with a significant decrease in cancer-related worry (e.g., [53,54,55]). On the other hand, there is also evidence for impaired sexual functioning, persistent physiological symptoms derived from surgical menopause and hormonal changes (e.g., vaginal dryness, hot flashes, decreased libido), and reduced body image satisfaction in women who underwent RRSO [20,31,56]. Moreover, it has been reported that around 20% of these women continue to present cancer-specific distress after surgery [53,57,58], and increased depressive and anxiety symptoms that tend to persist during one-year post-surgery [59]. Considering the holistic nature of potential consequences of RRSO (i.e., physical, psychological, relational, social), a multidisciplinary approach would be desirable to manage the deleterious effects of the surgery and support BRCA carriers in their decision-making process and post-surgery adaptation. However, even though clinical guidelines often recommend this approach, it is not always reflected in clinical practice, due to economic and organizational factors, which might contribute to the pervasiveness of the negative consequences of surgical menopause [6,8,26,27,28].
Undergoing RRSO surgery tends to be emotionally challenging, with serious physical and psychosocial consequences that may conflict with its primary purpose, and thus impair the decision-making process for women who carry a BRCA mutation. However, little is known about the role of modifiable psychosocial mechanisms that seem to shape the decision-making process, and women’s subsequent adaptation to its outcomes [14,45]. Notably, physician-patient communication has been increasingly acknowledged as a crucial modifiable variable that appears to be uniquely linked to women’s adaptation outcomes, following their decision to (not) undergo RRSO (e.g., [13,19,20,21]).
3.2. The Role of Physician-Patient Communication in the Decision-Making Process and in Post-Surgery Adaptation
Shared decision-making (SDM) consists of joint participation from both physician and patient in making a health decision, through the discussion of the available options and their benefits and harms, while considering the patient’s values, preferences, and circumstances [60]. There are several SDM models used in healthcare practice [61], with some of them specifically developed for oncology care (e.g., [62,63]). Even though no specific model has yet been examined in the context of the decision to undergo prophylactic surgery in mutation carriers, it is possible to establish a connection between the SDM model suggested by Shay and Lafata (2014) and the previously described HBM model. According to this SDM model, heavily drawn from the patients’ perspective, making a health decision depends on the interaction of several factors, such as: patient self-advocacy; open-mindedness and mutual respect between physician and patient; a physician’s personalized recommendation; and mutual information exchange. These factors occur in the context of the relationship and communication between physician and patient [64]. Considering the aforementioned HBM model, physician-patient communication may, therefore, be considered one of the modifying factors that uniquely shapes the women’s subjective appraisals that lead them to make the decision for RRSO.
Physician-patient communication (PPC), and particularly physicians’ empathic communication skills, are at the core of patient-centered healthcare and of medical practice [65,66]. Research has highlighted the positive effects of PPC on patients’ physical and emotional health improvements (e.g., [16,67]), treatment adherence [17,68], and overall well-being [18,69] across several healthcare settings, including cancer care [70].
In the context of decision-making for RRSO in women carrying a BRCA mutation, there are a few studies where the impact of PPC on women’s adjustment was examined. Some qualitative studies commented that even though women were frequently satisfied with their surgery decision, there was also dissatisfaction with the information provided by their physicians before the surgery, which could ultimately worsen the women’s ability to cope with deleterious post-surgery effects. Typically, these clinical information topics relate to possible treatment alternatives, negative impacts of menopausal symptoms, or the potential changes on sexuality and sexual functioning [21,56,71,72]. In a recent qualitative study describing the psychological experiences of women who underwent RRSO, it was found that, in the majority of cases, physicians failed to provide complete information, showed low levels of empathy and respect for the women’s values, preferences, and individuality, and adopted a disease-focused posture (i.e., focusing on organ health or exclusive disease prevention, and disregarding organism-context interactions and health promotion). Following these clinical attitudes, women experienced an increased psychological burden in the decision-making process, sought other professionals, or unwillingly delayed the surgery [14].
Moreover, some researchers have recently begun to analyze, using a quantitative approach, the impact of PPC on the psychological adjustment outcomes of having a RRSO. For instance, Zarbo and colleagues (2022) suggested that a greater satisfaction with medical communication could be associated with the provision of accurate and complete information about risk, prevention strategies, and post-surgery effects, while using an empathic and patient-centered style. In turn, satisfaction with medical communication may enhance patients’ self-efficacy, cultivate positive perceptions of agency, and contribute to decreased cancer-related anxiety. Furthermore, women who underwent RRSO and experienced greater satisfaction with medical communication reported reduced levels of cancer anxiety and experienced greater levels of post-surgery psychological quality of life [73].
4. Recommendations to Improve Clinical Practice
This review has shown that premenopausal women carrying a BRCA1/2 mutation, with an increased risk of developing ovarian or breast cancer, face a challenging process when deciding to undergo a RRSO. The available studies indicate that the physicians’ ability to sensitively inform, guide, and support women in that process is related to improved post-surgery health outcomes. Even if not abundant and marked by several methodological limitations, the existing research enables the outlining of some recommendations for physicians to improve their practice in this healthcare context, in addition to the already existing clinical guidelines.
First, some harmful impacts of the physiological and psychological consequences of undergoing an RSSO may be reduced, or even prevented, through sensitive physician-patient communication. More often than not, research has shown that physicians’ poor-quality communication competencies negatively impact women’s psychological adaptation during the decision-making process and after RRSO surgery. Therefore, training and developing physicians’ communication skills should be emphasized in medical curricula and continuing professional development [21,73]. Some communication skills training programs for physicians have shown promising results in the improvement of empathy, active listening, non-verbal communication, and emotion regulation (e.g., Empathetics) [74,75]. In addition, recently, mindfulness-based approaches have shown their feasibility within communication skills training for physicians, possibly enhancing adaptive emotion regulation (in self and others), psychological flexibility, and compassion [65].
Second, a constant update of evidence-based clinical guidelines is vital in order to provide the best care possible, while remaining mindful of the potentially enduring negative consequences of this procedure and providing the best information and recommendations to every woman. The combination of advanced technical competence and advanced interpersonal skills is consistent with the values underlying shared decision-making and patient-centered care, leading to patients’ higher satisfaction with healthcare and improved adaptation outcomes.
Third, given the inherent physical and psychosocial impacts of RRSO, it has been suggested that counselling in this context should be interdisciplinary [6,14,71]. A multidisciplinary team encompassing physicians/oncologists, nurses, clinical psychologists, physical therapists, and social workers is likely to enable the comprehensive assessment and management of these women’s complex healthcare needs. Additionally, due to the potentially negative impact of RRSO on intimate relationships, it has been suggested that counselling should be extended to family relatives, particularly the spouse/partner [19]. Specifically, women have frequently reported that sexuality and intimacy tend to be overlooked by health professionals in the discussion of the impact of RRSO [13]. Bearing in mind that sexual health is part of overall well-being and quality of life [76], physicians should be cognizant of the importance of addressing that health dimension in the context of the shared decision-making process related to RRSO [19,20,71].
5. Future Research Directions
Hereditary risk for the development of breast or ovarian cancer, due to BRCA1 or 2 mutations, along with the respective prophylactic measures, require further investigation, in order to improve the understanding of best clinical practices.
There is a reiterated need to deepen the research into breast and ovarian cancer and, particularly, into technological means and imaging techniques for early screening and detection [77,78]. Currently, risk-reducing interventions, such as RRSO, call for more efficacy studies to increase physicians’ and patients’ confidence in this procedure. In this case, a call for research is particularly directed to prophylactic interventions, namely early salpingectomy with delayed oophorectomy, which may postpone the onset of early menopause symptoms and allow an extended fertility window, thus buffering the deleterious effects of RRSO on psychosocial outcomes and increasing the chances for women’s improved quality of life [11]. Additionally, considering the broad scope of hereditary genomic mutations, less prevalent than BRCA but still conferring an increased risk to develop breast and/or ovarian cancer, future research should also focus on the psychological challenges faced by non-BRCA predisposing mutation carriers to better inform the establishment of clinical management protocols.
This review also revealed the need for further research about the psychosocial effects and modifiable mechanisms involved in women’s decision-making process and post-surgery adjustment, while adopting multi-method and longitudinal designs. Specifically, an investment in quantitative research into the role and impact of PPC is necessary, given its unique links to women’s decision-making processes and adjustment outcomes. Moreover, quantitative studies, complementary to qualitative research, should provide more robust and less biased evidence about the mechanisms through which PPC impacts decision-making and adjustment outcomes, thus allowing for a more comprehensive empirical framework for clinical intervention and the improvement of clinical guidelines.
Acknowledgments
A.C.A.-N. and C.C. are grateful to Cristina Canavarro, for her continuous encouragement and support; C.C. is also thankful to Liliana DePaulo, for the many insightful discussions regarding the supportive care needs of women with breast cancer. The authors are especially grateful to Keith Harvey, for his generous assistance in the English language editing of this manuscript.
Author Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. All authors have made an intellectual contribution to this research study. A.C.A.-N.: Conceptualization, Resources, Writing—Original Draft; D.M.: Conceptualization, Resources, Writing—Original Draft; C.C.: Conceptualization, Writing—Original Draft, Writing—Review and Editing, Validation; M.F.-D.: Conceptualization, Writing—Review and Editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was co-funded by the Center for Research in Neuropsychology and Cognitive and Behavioral Intervention (CINEICC)—University of Coimbra (UIDB/PSI/00730/2020). A.C.A.-N. is supported with a PhD grant awarded by the Portuguese Foundation for Science and Technology (2020.06779.BD).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Walker M., Jacobson M., Sobel M. Management of ovarian cancer risk in women with BRCA1/2 pathogenic variants. Can. Med. Assoc. J. 2019;191:E886–E893. doi: 10.1503/cmaj.190281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Santiago S., The SEOM Hereditary Cancer Working Group. Cajal T.R.Y., Aguirre E., Alés-Martínez J.E., Andrés R., Balmaña J., Graña B., Herrero A., Llort G., et al. SEOM clinical guidelines in hereditary breast and ovarian cancer. Clin. Transl. Oncol. 2019;22:193–200. doi: 10.1007/s12094-019-02262-0. [DOI] [PubMed] [Google Scholar]
- 3.Samadder N.J., Giridhar K.V., Baffy N., Riegert-Johnson D., Couch F.J. Hereditary Cancer Syndromes—A Primer on Diagnosis and Management: Part 1: Breast-ovarian cancer syndromes. Mayo Clin. Proc. 2019;94:1084–1098. doi: 10.1016/j.mayocp.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Andrews L., Mutch D.G. Hereditary Ovarian Cancer and Risk Reduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017;41:31–48. doi: 10.1016/j.bpobgyn.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Piombino C., Cortesi L., Lambertini M., Punie K., Grandi G., Toss A. Secondary Prevention in Hereditary Breast and/or Ovarian Cancer Syndromes Other Than BRCA. J. Oncol. 2020;2020:e6384190. doi: 10.1155/2020/6384190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabiani L., Barrou J., Mathis J., Eisinger F., Bannier M., Lambaudie E., Houvenaeghel G. How to manage BRCA mutation carriers? Horm. Mol. Biol. Clin. Investig. 2019;41:1–7. doi: 10.1515/hmbci-2019-0065. [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists Practice Bulletin No. 182 Summary: Hereditary Breast and Ovarian Cancer Syndrome. Obstet. Gynecol. 2017;130:657–659. doi: 10.1097/aog.0000000000002285. [DOI] [PubMed] [Google Scholar]
- 8.AlHilli M.M., Al-Hilli Z. Perioperative Management of Women Undergoing Risk-reducing Surgery for Hereditary Breast and Ovarian Cancer. J. Minim. Invasive Gynecol. 2018;26:253–265. doi: 10.1016/j.jmig.2018.09.767. [DOI] [PubMed] [Google Scholar]
- 9.Kostov S., Watrowski R., Kornovski Y., Dzhenkov D., Slavchev S., Ivanova Y., Yordanov A. Hereditary Gynecologic Cancer Syndromes – A Narrative Review. OncoTargets Ther. 2022;15:381–405. doi: 10.2147/OTT.S353054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly M.B., Pal T., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Goggins M., Hutton M.L., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 11.Gaba F., Goyal S., Marks D., Chandrasekaran D., Evans O., Robbani S., Tyson C., Legood R., Saridogan E., McCluggage W.G., et al. Surgical decision making in premenopausal BRCA carriers considering risk-reducing early salpingectomy or salpingo-oophorectomy: A qualitative study. J. Med. Genet. 2021;59:122–132. doi: 10.1136/jmedgenet-2020-107501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard A.F., Balneaves L.G., Bottorff J.L. Women’s Decision Making about Risk-Reducing Strategies in the Context of Hereditary Breast and Ovarian Cancer: A Systematic Review. J. Genet. Couns. 2009;18:578–597. doi: 10.1007/s10897-009-9245-9. [DOI] [PubMed] [Google Scholar]
- 13.Cherry C., Ropka M., Lyle J., Napolitano L., Daly M.B. Understanding the Needs of Women Considering Risk-Reducing Salpingo-oophorectomy. Cancer Nurs. 2013;36:E33–E38. doi: 10.1097/NCC.0b013e3182642cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meadows R., Padamsee T.J., Paskett E.D. Distinctive psychological and social experiences of women choosing prophylactic oophorectomy for cancer prevention. Health Care Women Int. 2018;39:595–616. doi: 10.1080/07399332.2018.1424855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang L., Members of the Guidelines International Network Implementation Working Group. Bernhardsson S., Vernooij R.W.M., Armstrong M.J., Bussières A., Brouwers M.C., Gagliardi A.R. Use of theory to plan or evaluate guideline implementation among physicians: A scoping review. Implement. Sci. 2017;12:26. doi: 10.1186/s13012-017-0557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang S. Pathways Linking Patient-Centered Communication to Health Improvement: A Longitudinal Study in China. J. Health Commun. 2019;24:156–164. doi: 10.1080/10810730.2019.1587110. [DOI] [PubMed] [Google Scholar]
- 17.Lu X., Zhang R. Impact of Physician-Patient Communication in Online Health Communities on Patient Compliance: Cross-Sectional Questionnaire Study. J. Med. Internet Res. 2019;21:e12891. doi: 10.2196/12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang S., Hong Y.A. Patient-centered communication and emotional well-being in the era of medical violence in China. Health Promot. Int. 2020;36:313–320. doi: 10.1093/heapro/daaa064. [DOI] [PubMed] [Google Scholar]
- 19.Alexandre M., Black J., Whicker M., Minkin M.J., Ratner E. The management of sexuality, intimacy, and menopause symptoms (SIMS) after prophylactic bilateral salpingo-oophorectomy: How to maintain sexual health in “previvors”. Maturitas. 2017;105:46–51. doi: 10.1016/j.maturitas.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Carter J., Stabile C., Gunn A., Sonoda Y. The Physical Consequences of Gynecologic Cancer Surgery and Their Impact on Sexual, Emotional, and Quality of Life Issues. J. Sex. Med. 2013;10:21–34. doi: 10.1111/jsm.12002. [DOI] [PubMed] [Google Scholar]
- 21.Klitzman R., Chung W. The process of deciding about prophylactic surgery for breast and ovarian cancer: Patient questions, uncertainties, and communication. Am. J. Med. Genet. Part A. 2009;152A:52–66. doi: 10.1002/ajmg.a.33068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari R. Writing narrative style literature reviews. Med. Writ. 2015;24:230–235. doi: 10.1179/2047480615Z.000000000329. [DOI] [Google Scholar]
- 23.Green B.N., Johnson C.D., Adams A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006;5:101–117. doi: 10.1016/S0899-3467(07)60142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y.L., Breen K., Catchings A., Ranganathan M., Latham A., Goldfrank D.J., Grisham R.N., Roche K.L., Frey M.K., Chi D.S., et al. Risk-Reducing Bilateral Salpingo-Oophorectomy for Ovarian Cancer: A Review and Clinical Guide for Hereditary Predisposition Genes. JCO Oncol. Pract. 2022;18:201–209. doi: 10.1200/OP.21.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeulen R.F.M., Van Beurden M., Korse C.M., Kenter G.G. Impact of risk-reducing salpingo-oophorectomy in premenopausal women. Climacteric. 2017;20:212–221. doi: 10.1080/13697137.2017.1285879. [DOI] [PubMed] [Google Scholar]
- 26.Altman A.M., Hui J.Y., Tuttle T. Quality-of-life implications of risk-reducing cancer surgery. Br. J. Surg. 2018;105:e121–e130. doi: 10.1002/bjs.10725. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann L.C., Lindor N.M. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. N. Engl. J. Med. 2016;374:454–468. doi: 10.1056/NEJMra1503523. [DOI] [PubMed] [Google Scholar]
- 28.Huber D., Seitz S., Kast K., Emons G., Ortmann O. Hormone replacement therapy in BRCA mutation carriers and risk of ovarian, endometrial, and breast cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2021;147:2035–2045. doi: 10.1007/s00432-021-03629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson M., Coakley N., Bernardini M., Branco K.-A., Elit L., Ferguson S., Kim R. Risk reduction strategies for BRCA1/2 hereditary ovarian cancer syndromes: A clinical practice guideline. Hered. Cancer Clin. Pract. 2021;19:1–7. doi: 10.1186/s13053-021-00196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mau C., Untch M. Prophylactic Surgery: For Whom, When and How. Breast Care. 2017;12:379–384. doi: 10.1159/000485830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer C.F. Non-surgical prevention strategies in women with hereditary breast and ovarian cancer syndromes. Horm. Mol. Biol. Clin. Investig. 2020;41:20190057. doi: 10.1515/hmbci-2019-0057. [DOI] [PubMed] [Google Scholar]
- 32.Peters M.L., Garber J.E., Tung N. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol. Oncol. 2017;146:205–214. doi: 10.1016/j.ygyno.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer. 2020;28:1167–1180. doi: 10.1007/s12282-020-01148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elezaby M., Lees B., Maturen K.E., Barroilhet L., Wisinski K.B., Schrager S., Wilke L.G., Sadowski E. BRCA Mutation Carriers: Breast and Ovarian Cancer Screening Guidelines and Imaging Considerations. Radiology. 2019;291:554–569. doi: 10.1148/radiol.2019181814. [DOI] [PubMed] [Google Scholar]
- 35.Berger E.R., Golshan M. Surgical Management of Hereditary Breast Cancer. Genes. 2021;12:1371. doi: 10.3390/genes12091371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekine M., Nishino K., Enomoto T. BRCA Genetic Test and Risk-Reducing Salpingo-Oophorectomy for Hereditary Breast and Ovarian Cancer: State-of-the-Art. Cancers. 2021;13:2562. doi: 10.3390/cancers13112562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchetti C., De Felice F., Boccia S., Sassu C., Di Donato V., Perniola G., Palaia I., Monti M., Muzii L., Tombolini V., et al. Hormone replacement therapy after prophylactic risk-reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: A meta-analysis. Crit. Rev. Oncol. 2018;132:111–115. doi: 10.1016/j.critrevonc.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Heemskerk-Gerritsen B.A., Seynaeve C., van Asperen C.J., Ausems M.G., Collee J.M., van Doorn H.C., Gomez Garcia E.B., Kets C.M., van Leeuwen F.E., Meijers-Heijboer H.E., et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J. Natl. Cancer Inst. 2015;107:djv033. doi: 10.1093/jnci/djv033. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J.V., Chiel L., Boghossian L., Jones M., Stopfer J.E., Powers J., Rebbeck T.R., Nathanson K., Domchek S.M. Non-cancer endpoints in BRCA1/2 carriers after risk-reducing salpingo-oophorectomy. Fam. Cancer. 2011;11:69–75. doi: 10.1007/s10689-011-9480-8. [DOI] [PubMed] [Google Scholar]
- 40.Michelsen T.M., Tonstad S., Pripp A.H., Tropé C.G., Dørum A. Coronary Heart Disease Risk Profile in Women Who Underwent Salpingo-Oophorectomy to Prevent Hereditary Breast Ovarian Cancer. Int. J. Gynecol. Cancer. 2010;20:233–239. doi: 10.1111/IGC.0b013e3181ca5ff4. [DOI] [PubMed] [Google Scholar]
- 41.Michelsen T.M., Pripp A.H., Tonstad S., Tropé C.G., Dørum A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: A controlled observational study. Eur. J. Cancer. 2009;45:82–89. doi: 10.1016/j.ejca.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 42.Grynberg M., Raad J., Comtet M., Vinolas C., Cédrin-Durnerin I., Sonigo C. Fertility preservation in BRCA-mutated women: When and how? Futur. Oncol. 2018;14:483–490. doi: 10.2217/fon-2017-0415. [DOI] [PubMed] [Google Scholar]
- 43.Bradbury A.R., Ibe C.N., Dignam J.J., Cummings S.A., Verp M., White M.A., Artioli G., Dudlicek L., Olopade O.I. Uptake and timing of bilateral prophylactic salpingo-oophorectomy among BRCA1 and BRCA2 mutation carriers. Anesth. Analg. 2008;10:161–166. doi: 10.1097/GIM.0b013e318163487d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mai P.L., Piedmonte M., Han P.K., Moser R.P., Walker J.L., Rodriguez G., Boggess J., Rutherford T.J., Zivanovic O., Cohn D.E., et al. Factors associated with deciding between risk-reducing salpingo-oophorectomy and ovarian cancer screening among high-risk women enrolled in GOG-0199: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;145:122–129. doi: 10.1016/j.ygyno.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padamsee T.J., Wills C.E., Yee L.D., Paskett E.D. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res. 2017;19:34. doi: 10.1186/s13058-017-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurley K.E., Miller S.M., Costalas J.W., Gillespie D., Daly M.B. Anxiety/Uncertainty Reduction as a Motivation for Interest in Prophylactic Oophorectomy in Women with a Family History of Ovarian Cancer. J. Women’s Health Gend.-Based Med. 2001;10:189–199. doi: 10.1089/152460901300039566. [DOI] [PubMed] [Google Scholar]
- 47.Hochbaum G., Rosenstock I., Kegels S. Health Belief Model. United States Public Health Service; Washington, DC, USA: 1952. [Google Scholar]
- 48.Jones C.L., Jensen J.D., Scherr C.L., Brown N.R., Christy K., Weaver J. The Health Belief Model as an Explanatory Framework in Communication Research: Exploring Parallel, Serial, and Moderated Mediation. Health Commun. 2015;30:566–576. doi: 10.1080/10410236.2013.873363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Champion V.L., Skinner C.S. The health belief model. In: Glanz K., Rimer B.K., Viswanath K., editors. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; San Francisco, CA, USA: 2008. pp. 45–65. [Google Scholar]
- 50.Green E.C., Murphy E.M., Gryboski K. The Health Belief Model. In: Paul R., Cohen L.M., editors. The Wiley Encyclopedia of Health Psychology. Volume 2. Wiley-Blackwell; Hoboken, NJ, USA: 2021. pp. 211–214. [Google Scholar]
- 51.Herrmann A., Hall A., Proietto A. Using the Health Belief Model to explore why women decide for or against the removal of their ovaries to reduce their risk of developing cancer. BMC Women’s Health. 2018;18:184. doi: 10.1186/s12905-018-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gellman C., Ezratty C., Schwarz J., Kolev V., Blank S.V. “It was a no-brainer”: A qualitative study of factors driving previvors’ decision-making when considering risk-reducing salpingectomy with delayed oophorectomy. Gynecol. Oncol. Rep. 2022;40:100948. doi: 10.1016/j.gore.2022.100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finch A., Metcalfe K.A., Chiang J., Elit L., McLaughlin J., Springate C., Esplen M.J., Demsky R., Murphy J., Rosen B., et al. The impact of prophylactic salpingo-oophorectomy on quality of life and psychological distress in women with a BRCA mutation. Psycho-Oncology. 2011;22:212–219. doi: 10.1002/pon.2041. [DOI] [PubMed] [Google Scholar]
- 54.Madalinska J.B., Hollenstein J., Bleiker E., van Beurden M., Valdimarsdottir H.B., Massuger L.F., Gaarenstroom K.N., Mourits M.J., Verheijen R.H., van Dorst E.B., et al. Quality-of-Life Effects of Prophylactic Salpingo-Oophorectomy Versus Gynecologic Screening Among Women at Increased Risk of Hereditary Ovarian Cancer. J. Clin. Oncol. 2005;23:6890–6898. doi: 10.1200/JCO.2005.02.626. [DOI] [PubMed] [Google Scholar]
- 55.Shigehiro M., Kita M., Takeuchi S., Ashihara Y., Arai M., Okamura H. Study on the psychosocial aspects of risk-reducing salpingo-oophorectomy (RRSO) in BRCA1/2 mutation carriers in Japan: A preliminary report. Jpn. J. Clin. Oncol. 2015;46:254–259. doi: 10.1093/jjco/hyv190. [DOI] [PubMed] [Google Scholar]
- 56.Hickey I., Jha S., Wyld L. The psychosexual effects of risk-reducing bilateral salpingo-oophorectomy in female BRCA1/2 mutation carriers: A systematic review of qualitative studies. Gynecol. Oncol. 2020;160:763–770. doi: 10.1016/j.ygyno.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Robson M., Hensley M., Barakat R., Brown C., Chi D., Poynor E., Offit K. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol. Oncol. 2003;89:281–287. doi: 10.1016/S0090-8258(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 58.Bresser P., Seynaeve C., Van Gool A., Niermeijer M., Duivenvoorden H., van Dooren S., van Geel A., Menke-Pluijmers M., Klijn J., Tibben A. The course of distress in women at increased risk of breast and ovarian cancer due to an (identified) genetic susceptibility who opt for prophylactic mastectomy and/or salpingo-oophorectomy. Eur. J. Cancer. 2007;43:95–103. doi: 10.1016/j.ejca.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Hickey M., Moss K.M., Brand A., Wrede C.D., Domchek S.M., Meiser B., Mishra G.D., Joffe H. What happens after menopause? (WHAM): A prospective controlled study of depression and anxiety up to 12 months after premenopausal risk-reducing bilateral salpingo-oophorectomy. Gynecol. Oncol. 2021;161:527–534. doi: 10.1016/j.ygyno.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 60.McFarland D.C., Blackler L., Banerjee S., Holland J. Communicating About Precision Oncology. JCO Precis. Oncol. 2017;1:1–9. doi: 10.1200/PO.17.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bomhof-Roordink H., Gärtner F.R., Stiggelbout A.M., Pieterse A.H. Key components of shared decision making models: A systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bomhof-Roordink H., Fischer M.J., Van Duijn-Bakker N., Baas-Thijssen M.C., Van Der Weijden T., Stiggelbout A.M., Pieterse A.H. Shared decision making in oncology: A model based on patients’, health care professionals’, and researchers’ views. Psycho-Oncology. 2018;28:139–146. doi: 10.1002/pon.4923. [DOI] [PubMed] [Google Scholar]
- 63.Kane H.L., Halpern M.T., Squiers L.B., Treiman K.A., McCormack L.A. Implementing and evaluating shared decision making in oncology practice. CA Cancer J. Clin. 2014;64:377–388. doi: 10.3322/caac.21245. [DOI] [PubMed] [Google Scholar]
- 64.Shay L.A., Lafata J.E. Understanding patient perceptions of shared decision making. Patient Educ. Couns. 2014;96:295–301. doi: 10.1016/j.pec.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amutio-Kareaga A., García-Campayo J., Delgado L.C., Hermosilla D., Martínez-Taboada C. Improving Communication between Physicians and Their Patients through Mindfulness and Compassion-Based Strategies: A Narrative Review. J. Clin. Med. 2017;6:33. doi: 10.3390/jcm6030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baig L.A., Violato C., Crutcher R.A. Assessing clinical communication skills in physicians: Are the skills context specific or generalizable. BMC Med. Educ. 2009;9:22. doi: 10.1186/1472-6920-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maly R.C., Liu Y., Leake B., Thind A., Diamant A.L. Treatment-related symptoms among underserved women with breast cancer: The impact of physician–patient communication. Breast Cancer Res. Treat. 2009;119:707–716. doi: 10.1007/s10549-009-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Świątoniowska-Lonc N., Polański J., Tański W., Jankowska-Polańska B. Impact of satisfaction with physician–patient communication on self-care and adherence in patients with hypertension: Cross-sectional study. BMC Health Serv. Res. 2020;20:1046. doi: 10.1186/s12913-020-05912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang S. Pathway Linking Patient-Centered Communication to Emotional Well-Being: Taking into Account Patient Satisfaction and Emotion Management. J. Health Commun. 2017;22:234–242. doi: 10.1080/10810730.2016.1276986. [DOI] [PubMed] [Google Scholar]
- 70.Venetis M., Robinson J.D., Turkiewicz K.L., Allen M. An evidence base for patient-centered cancer care: A meta-analysis of studies of observed communication between cancer specialists and their patients. Patient Educ. Couns. 2009;77:379–383. doi: 10.1016/j.pec.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Hallowell N., kConFab Psychosocial Group on behalf of the kConFab Investigators. Baylock B., Heiniger L., Butow P.N., Patel D., Meiser B., Saunders C., Price M.A. Looking different, feeling different: Women’s reactions to risk-reducing breast and ovarian surgery. Fam. Cancer. 2011;11:215–224. doi: 10.1007/s10689-011-9504-4. [DOI] [PubMed] [Google Scholar]
- 72.Trister R., Jacobson M., Nguyen P., Sobel M., Allen L., Narod S.A., Kotsopoulos J. Patient reported experiences following laparoscopic prophylactic bilateral salpingo-oophorectomy or salpingectomy in an ambulatory care hospital. Fam. Cancer. 2020;20:103–110. doi: 10.1007/s10689-020-00208-y. [DOI] [PubMed] [Google Scholar]
- 73.Zarbo C., Brugnera A., Frigerio L., Celi C., Compare A., Dessì V., Giordano R., Malandrino C., Sina F.P., Strepparava M.G., et al. Cancer Anxiety Mediates the Association Between Satisfaction with Medical Communication and Psychological Quality of Life After Prophylactic Bilateral Salpingo-Oophorectomy. Front. Psychol. 2022;13:840931. doi: 10.3389/fpsyg.2022.840931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riess H., Kelley J.M., Bailey R., Konowitz P.M., Gray S.T. Improving Empathy and Relational Skills in Otolaryngology Residents: A pilot study. Otolaryngol. Neck Surg. 2010;144:120–122. doi: 10.1177/0194599810390897. [DOI] [PubMed] [Google Scholar]
- 75.Riess H., Kelley J.M., Bailey R.W., Dunn E., Phillips M. Empathy Training for Resident Physicians: A Randomized Controlled Trial of a Neuroscience-Informed Curriculum. J. Gen. Intern. Med. 2012;27:1280–1286. doi: 10.1007/s11606-012-2063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organization (WHO) Defining Sexual Health: Report of a Technical Consultation on Sexual Health, 28–31 January 2002, Geneva. World Health Organization; Geneva, Switzerland: 2006. (Sexual Health Document Series). [Google Scholar]
- 77.Nash Z., Menon U. Ovarian cancer screening: Current status and future directions. Best Pract. Res. Clin. Obstet. Gynaecol. 2020;65:32–45. doi: 10.1016/j.bpobgyn.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Gupta K.K., Gupta V.K., Naumann R.W. Ovarian cancer: Screening and future directions. Int. J. Gynecol. Cancer. 2019;29:195–200. doi: 10.1136/ijgc-2018-000016. [DOI] [PubMed] [Google Scholar]