Abstract
Background & Aims
Liver transplantation (LT) is the only available treatment for end-stage non-alcoholic fatty liver disease (NAFLD) (related decompensated cirrhosis and/or hepatocellular carcinoma). The aim of our study was to evaluate the risk of disease recurrence after LT and the factors influencing it.
Method
This retrospective multicenter study included adults transplanted for NAFLD cirrhosis between 2000 and 2019 in 20 participating French-speaking centers. Disease recurrence (steatosis, steatohepatitis and fibrosis) was diagnosed from liver graft biopsies.
Results
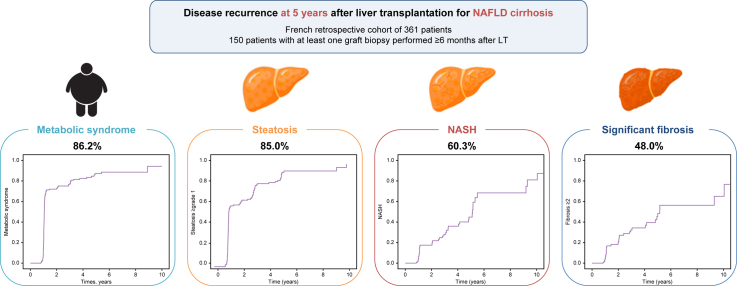
We analyzed 150 patients with at least one graft liver biopsy available ≥6 months after transplantation, among 361 patients transplanted for NAFLD. The median (IQR) age at LT was 61.3 (54.4-64.6) years. The median follow-up after LT was 4.7 (2.8-8.1) years. The cumulative recurrence rates of steatosis and steatohepatitis at 5 years were 80.0% and 60.3%, respectively. Significant risk factors for steatohepatitis recurrence in multivariate analysis were recipient age at LT <65 years (odds ratio [OR] 4.214; p = 0.044), high-density lipoprotein-cholesterol <1.15 mmol/L after LT (OR 3.463; p = 0.013) and grade ≥2 steatosis on the graft at 1 year after LT (OR 10.196; p = 0.001). The cumulative incidence of advanced fibrosis (F3–F4) was 20.0% at 5 years after LT and significant risk factors from multivariate analysis were metabolic syndrome before LT (OR 8.550; p = 0.038), long-term use of cyclosporine (OR 11.388; p = 0.031) and grade ≥2 steatosis at 1 year after LT (OR 10.720; p = 0.049). No re-LT was performed for NAFLD cirrhosis recurrence.
Conclusion
Our results strongly suggest that recurrence of initial disease after LT for NAFLD is inevitable and progressive in a large proportion of patients; the means to prevent it remain to be further evaluated.
Impact and implications
Non-alcoholic fatty liver disease (NAFLD) is a growing indication for liver transplantation, but the analysis of disease recurrence, based on graft liver biopsies, has been poorly studied. Cumulative incidences of steatosis, steatohepatitis and NAFLD-related significant fibrosis recurrence at 5 years were 85.0%, 60.3% and 48.0%, respectively. Grade ≥2 steatosis on graft biopsy at 1 year (present in 25% of patients) is highly predictive of recurrence of steatohepatitis and advanced fibrosis: bariatric surgery should be discussed in these patients specifically.
Keywords: liver transplantation, NASH, metabolic syndrome, NAFLD recurrence, Bariatric surgery
Abbreviations: ABM, Agence de la Biomédecine; BS, bariatric surgery; CNI, calcineurin inhibitor; CST, corticosteroid; CV, cardiovascular; CYA, cyclosporine; ESLD, end-stage liver disease; HCC, hepatocellular carcinoma; LT, liver transplantation; MS, metabolic syndrome; mTOR-i, mTOR inhibitor; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis
Graphical abstract
Highlights
-
•
We analyzed the rate of histological recurrence in 150 patients transplanted for NAFLD cirrhosis.
-
•
Median follow-up after liver transplantation was 4.7 years.
-
•
Recurrence of the initial disease is frequent and rapid after liver transplantation.
-
•
Grade ≥2 steatosis at 1 year is highly predictive of recurrence of NASH and advanced fibrosis.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is now the most frequent chronic liver disease in the world, with a prevalence of nearly 24.1% in the USA adult population and 23.7% in Europe with many disparities: NAFLD affects an estimated 18.2% of the French population.1,2 NAFLD is a spectrum of disease that ranges from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH) characterized by steatosis, inflammation, hepatocyte ballooning, and varying degrees of hepatic fibrosis, which may progress to cirrhosis and end-stage liver disease.3 Hepatocellular carcinoma (HCC) can develop on a cirrhotic or non-cirrhotic liver. Currently, no specific treatment is available for NAFLD and the only effective treatment is weight lost, for instance after bariatric surgery (BS).4 Liver transplantation (LT) may be indicated in case of decompensated NAFLD-related cirrhosis and/or HCC. LT for NAFLD is a growing indication worldwide: it is the second leading cause of LT in the USA but remains less frequent in Europe.5,6 In 2019, NAFLD represented 12% and 7.9% of all LTs in the UK and France, respectively.7,8 Patient survival at 5 years after LT for NAFLD without HCC has been reported to be 75.4% in Europe compared to 75% for alcohol-associated liver disease and 80% for HBV-related disease.5
Few studies have analyzed the recurrence of NAFLD after LT, especially based on liver biopsy.[9], [10], [11], [12] The persistence of metabolic syndrome (MS) factors after LT, and even its aggravation because of immunosuppressive treatment, may suggest a recurrence of the initial disease on the graft. NAFLD recurrence rates 5 years after LT are therefore estimated to be more than 80%. There is a need to better assess the prevalence of recurrence of NAFLD on the graft, its evolution and associated risk factors.
The aim of the present study, based on a large retrospective cohort, was to describe the recurrence of initial disease (steatosis, NASH and fibrosis) on the graft after LT for NAFLD, based on liver biopsies, and to identify the factors influencing it.
Patients and methods
Study population
We included all adult patients transplanted in all French LT centers and in Geneva (Switzerland), based on the national database of the French Agence de la Biomédecine (ABM) and local databases (the “NASH” item did not exist in the ABM thesaurus before January the 1st 2018). We first selected all patients transplanted between January the 1st 2000 to 31 December 2019 for “other causes of cirrhosis”, “cirrhosis of unknown cause”, “metabolic disease” or “HCC” and “NASH” disease after January 2018 in the ABM database. All medical records were reviewed and patients were finally included after histopathological examination of an available liver biopsy before LT, if there native liver was compatible with NAFLD cirrhosis, they had metabolic risk factors (diabetes, obesity or overweight, arterial hypertension) and no other confounding etiology (alcohol consumption >10 g per week, autoimmune disease, viral hepatitis, Wilson's disease or hemochromatosis). The aim of this study was to investigate the recurrence of the initial disease based on graft biopsies performed ≥6 months after LT: the population is defined as all transplanted patients with a graft biopsy ≥6 months after LT.
This study was conducted in accordance with the Declaration of Helsinki. According to French law (Loi Jardé), retrospective studies do not require Institutional Review Board approval.
Clinical and biological characteristics at the time of listing
Cirrhosis characteristics at the LT registration time were specified including model for end-stage liver disease score, Child-Pugh score and cirrhosis complications. Metabolic characteristics were recorded: dry weight at LT listing (without ascites or after paracentesis), height and highest lifetime BMI were collected. BMI (kg/m2) was calculated from these height and weight values. Lipid profile, glycated hemoglobin (HbA1c) and diabetes treatment history were collected. MS was defined according to the American Heart Association, replacing waist circumference with BMI over 30 kg/m2.13 Data on cardiovascular (CV) events, CV check-up and pulmonary disease before LT were collected.
All patients received grafts from cadaveric or living donors. Donor characteristics (age, weight and BMI) were collected.
Follow-up after LT
Initial immunosuppressive regimen was based on a calcineurin inhibitor (CNI): cyclosporine (CYA) or tacrolimus. Induction therapy by polyclonal antibodies or anti-interleukin-2 receptor antibodies was mainly administered in case of acute kidney injury. Starting on postoperative day 1, methylprednisolone was tapered to reach a maintenance dose of 0 to 5 mg/day at 6 months post-transplantation. Azathioprine, mycophenolate mofetil (MMF) or sirolimus/everolimus (mTOR inhibitor [mTOR-i]) was either administered as part of an initial triple immunosuppressive regimen or introduced during follow-up as a maintenance immunosuppressive agent. Outpatient follow-up visits were usually conducted once a week during the first month after discharge from the hospital, twice a month during the second and third months, monthly for the rest of the first year, and every 3 or 12 months thereafter, regardless of the length of the observation period after LT. Additional visits were made when necessary. A complete laboratory investigation, including hematology, liver parameters, coagulation, electrolytes, total protein, renal parameters, fasting blood glucose, a lipid profile, and blood calcineurin inhibitor trough levels or mTOR-i levels, was conducted at each visit.
The prevalence of arterial hypertension, diabetes, dyslipidemia and specific associated drugs were recorded. CV events after LT (defined by coronary heart disease, stroke or cardiac arrhythmia, and cardiorespiratory arrest) were recorded. The presence of steatosis on non-invasive imaging exams (ultrasound or CT scan, MRI, liver stiffness measurement) was specified.
Biological standards are considered abnormal if they are above or below the laboratory standard. The end of follow-up corresponded to death, the last medical examination or date of loss to follow-up. All data were retrospectively collected until June 30th 2020.
Diagnosis of disease recurrence on liver graft
Histopathological data were collected from available liver graft biopsies. Depending on local center policy, protocol liver biopsy was performed after LT at 1, 2, 3, 5, 10 and 15 years in some. The liver pathology team at each LT center reviewed all biopsy samples. Steatosis was graded on a 0-3 semi-quantitative scale: (0) steatosis absent or in <5% of hepatocytes;1 steatosis in up to one-third of hepatocytes;2 steatosis in one to two-thirds of hepatocytes; and3 steatosis in more than two-thirds of hepatocytes. The grading of NASH (inflammation and hepatocyte ballooning) was performed according to the SAF (steatosis, activity and fibrosis) scoring system.14 The diagnosis of NASH was defined as a SAF activity score (presence of steatosis, ballooning and lobular inflammation according to the flip algorithm) ≥2.14 Liver fibrosis was scored by NASH Clinical Research Network on a five-stage scale: (0) no fibrosis;1 perisinusoidal zone 3 or periportal fibrosis (1A: mild, zone 3, perisinusoidal; 1B: moderate, zone 3, perisinusoidal; 1C: Portal/periportal);2 perisinusoidal fibrosis with portal or periportal fibrosis;3 perisinusoidal fibrosis with portal or periportal fibrosis with focal or porto-central bridging fibrosis; and4 cirrhosis.15 Significant and advanced fibrosis were defined as a liver fibrosis stage ≥F2 and ≥F3, respectively. For the analysis of fibrosis recurrence, we excluded possible factors that could aggravate fibrosis lesions such as cell rejection, or biliary obstacle. We excluded patients with post-LT alcohol consumption >10 g per week from the analysis of recurrence.
Statistical analysis
All data were analyzed using SPSS software, version 23.0 (IBM, Armonk, NY, USA). Data were described in their totality using median (IQR) or mean (SD) for continuous variables and n (%) for categorical variables. Categorical variables were compared with the Chi-square or Fischer’s exact tests and quantitative variables were compared using the Student’s t test or non-parametric tests (Mann-Whitney or Kruskall-Wallis tests) when appropriate.
The main event of interest was NAFLD recurrence. Steatosis, NASH and fibrosis recurrence rates were estimated using the Kaplan-Meier method. Determination of the risk factors for NASH recurrence was performed using log-rank analysis or Mann-Whitney analysis (for quantitative variables). All significant variables in the univariate analysis with a level set at p <0.1 were incorporated into multivariate models analyzed with binary logistic Cox regression. Logistic regression analyses (univariate and multivariate) were used to calculate the risk for liver steatosis, liver fibrosis and various parameters.
Results
Study population and metabolic characteristics at time of listing for LT
One hundred and fifty patients underwent liver biopsy ≥6 months after LT, among our global cohort of 361 patients transplanted from all 19 French LT centers and Geneva (Switzerland) (i.e. 20 French-speaking centers). These patients with available liver biopsy do not differ from (except for the duration of post-LT follow-up) patients without graft biopsy regarding pre- and post-LT characteristics (Table 1). Patients were transplanted between January 2001 and June 2018. Median (IQR) follow-up after LT was 4.7 (2.8-8.1) years (range 0.7-18.7 years). More than half of the patients had MS when listed for LT. The median BMI at time of LT was 30.9 (26.7-33.9) kg/m2 but the median highest lifetime BMI was 35.0 (30.9-39.3) kg/m2. In patients transplanted for HCC, the median BMI at time of LT was 30.3 (26.8-34.5) kg/m2 compared to 31.0 (27.3-33.3) kg/m2 in patients transplanted for end-stage liver disease (ESLD: not statistically significant; p = 0.522). Thirty patients (20.0%) had at least one CV event before LT. Five patients (3.33%) had pre-LT angioplasty.
Table 1.
Characteristics of patients with liver biopsy before and after LT (on patient alive at 1-year n = 286).
| Whole cohort alive at 12 months after LT |
Centers with biopsy protocol |
||||||
|---|---|---|---|---|---|---|---|
| Patient with liver biopsy after LT (n = 150) | Patient without liver biopsy after LT (n = 136) | With liver biopsy (n = 103) | Without liver biopsy (n = 19) | p∗ | p∗∗ | p∗∗∗ | |
| Sex (M/F) | 98/52 | 102/34 | 63/40 | 16/3 | 0.076 | 0.010 | 0.499 |
| Median age at LT (years) (IQR) | 61.3 (54.4-64.6) | 61.9 (57.9-65.7) | 61.1 (57.5-65.5) | 62.7 (57.7-65.8) | 0.066 | 0.178 | 0.770 |
| HCC | 77 (51.3%) | 75 (55.1%) | 45 (43.7%) | 12 (63.2%) | 0.519 | 0.464 | 0.233 |
| Median MELD score at LT listing (IQR) | 13.1 (9.1-21.1) | 14.6 (8.1-21.6) | 14.3 (9.7-21.0) | 12.6 (6.0-23.0) | 0.912 | 0.583 | 0.848 |
| Median Child-Pugh score at LT listing (IQR) | B9 (B7–C12) | B9 (A6-C12) | C10 (B8–C12) | C10 (B7–C12) | 0.448 | 0.587 | 0.498 |
| Median follow-up (years) (IQR) | 4.9 (3.0-8.3) | 3.5 (2.3-6.2) | 4.5 (2.9-7.4) | 3.4 (1.7-4.9) | 0.001 | 0.001 | 0.0001 |
| Characteristics before LT | |||||||
| BMI at LT time (kg/m2) (IQR) | 30.9 (26.7-33.9) | 31.1 (26.8-34.2) | 31.0 (27.2-33.9) | 34.0 (31.2-37.6) | 0.608 | 0.183 | 0.721 |
| Metabolic syndrome | 77 (51.3%) | 84 (61.8%) | 52 (50.5%) | 15 (78.9%) | 0.076 | 0.143 | 0.213 |
| Diabetes mellitus | 112 (74.7%) | 103 (75.7%) | 78 (75.7%) | 17 (89.5) | 0.835 | 0.077 | 0.341 |
| Median HbA1c level (%) (IQR) | 5.8 (5.1-7.3) | 5.7 (5.1-6.7) | 5.9 (5.1-6.6) | 6.4 (5.6-7.2) | 0.423 | 0.727 | 0.278 |
| Arterial hypertension | 122 (81.3%) | 114 (83.8%) | 77 (74.8%) | 19 (100%) | 0.664 | 0.067 | 0.146 |
| Statin therapy | 20 (13.3%) | 16 (11.8%) | 15 (14.6%) | 4 (21.1%) | 0.660 | 0.854 | 0.267 |
| Fibrate therapy | 4 (2.7%) | 3 (2.2%) | 3 (2.9%) | 0 (0.0%) | 0.768 | 0.220 | 0.214 |
| Bariatric surgery | 5 (3.3%) | 3 (2.2%) | 4 (3.9%) | 0 (0.0%) | 0.564 | 0.540 | 0.680 |
| Cardiovascular history | 30 (20.0%) | 30 (22.1%) | 20 (19.4%) | 6 (31.6%) | 0.147 | 0.549 | 0.212 |
| Median serum creatinine level (μmol/L) (IQR) | 80.5 (67.0-100.0) | 85.0 (70.0-107.5) | 80.0 (67.0-100.5) | 77.0 (64.5-114.5) | 0.146 | 0.434 | 0.487 |
| Medium glomerular filtration rate (MDRD) (μmol/L) (IQR) | 82.1 (60.3-106.7) | 77.9 (58.7-100.8) | 80.6 (59.8-106.5) | 90.3 (60.2-104.7) | 0.707 | 0.988 | 0.149 |
| Characteristics after LT | |||||||
| BMI at 1 year after LT (kg/m2) (IQR) | 29.5 (26.6-33.6) | 29.4 (26.4-32.0) | 29.5 (25.8-33.8) | 32.0 (29.9-34.9) | 0.519 | 0.079 | 0.478 |
| Diabetes post-LT | 111 (74.0%) | 99 (72.8%) | 78 (75.7%) | 19 (100%) | 0.669 | 0.038 | 0.352 |
| Arterial hypertension post-LT | 141 (94.0%) | 111 (81.6%) | 96 (93.2%) | 18 (94.7%) | 0.245 | 0.198 | 0.458 |
| Dyslipidemia or lipid drugs | 81 (54.0%) | 93 (68.3%) | 53 (51.5%) | 6 (31.6%) | 0.321 | 0.541 | 0.254 |
| Metabolic syndrome post-LT | 127 (84.7%) | 118 (86.8%) | 86 (83.5%) | 18 (94.7%) | 0.180 | 0.890 | 0.176 |
| Donor characteristics | |||||||
| Median age (years) (IQR) | 58.5 (43-68.3) | 59.0 (47.0-69.0) | 57.0 (44.0-69.5) | 57.0 (53.0-65.0) | 0.452 | 0.130 | 0.170 |
| Sex (M/F) | 73/48 | 70/36 | 45/24 | 7/4 | 0.691 | 0.120 | 0.240 |
| Median BMI (kg/m2) (IQR) | 24.8 (22.5-29.1) | 24.8 (22.6-27.7) | 24.9 (22.6-28.9) | 24.3 (22.3-27.9) | 0.555 | 0.257 | 0.360 |
| Grade ≥1 steatosis | 66 (44.0%) | 42 (30.9%) | 49 (47.6%) | 9 (47.4%) | 0.478 | 0.875 | 0.699 |
| Grade ≥2 steatosis | 13 (8.7%) | 8 (5.9%) | 9 (8.7%) | 1 (5.3%) | 0.247 | 0.743 | 0.547 |
| Stage ≥1 fibrosis | 36 (24.0%) | 25 (18.4%) | 16 (15.5%) | 3 (15.8%) | 0.364 | 0.788 | 0.272 |
| Stage ≥2 Fibrosis | 3 (2.0) | 8 (5.9%) | 2 (1.9%) | 0 (0.0%) | 0.785 | 0.890 | 0.450 |
Univariate analysis was performed using log-rank analysis or Mann-Whitney analysis (for quantitative variables). All significant variables in the univariate analysis with a level set at p <0.1 were incorporated into multivariate models analyzed with binary logistic Cox regression. Values in bold are considered significant (<0.005).
HbA1c, glycated hemoglobin; HCC, hepatocellular carcinoma; LT, liver transplantation; MDRD, Modification of Diet in Renal Disease; MELD, model for end-stage liver disease.
Comparison in whole cohort between patients with liver biopsy vs. no liver biopsy.
Comparison of patients in centers performing protocol biopsies (with and without biopsies).
Comparison of patients with liver biopsy in whole cohort with patients with liver biopsy in centers performing protocol biopsies.
Metabolic syndrome after LT (Table 1 and Table S1)
After LT, 94.0% of patients had hypertension, with 24 cases of de novo arterial hypertension (representing 85.7% of patients at risk). De novo diabetes developed in 28.9% of patients at risk after LT: 74.0% of patients had diabetes in the LT follow-up. After LT, 78.0% had dyslipidemia or were receiving anti-lipid therapy. De novo obstructive sleep apnea syndrome was diagnosed in 10.7% of patients after LT.
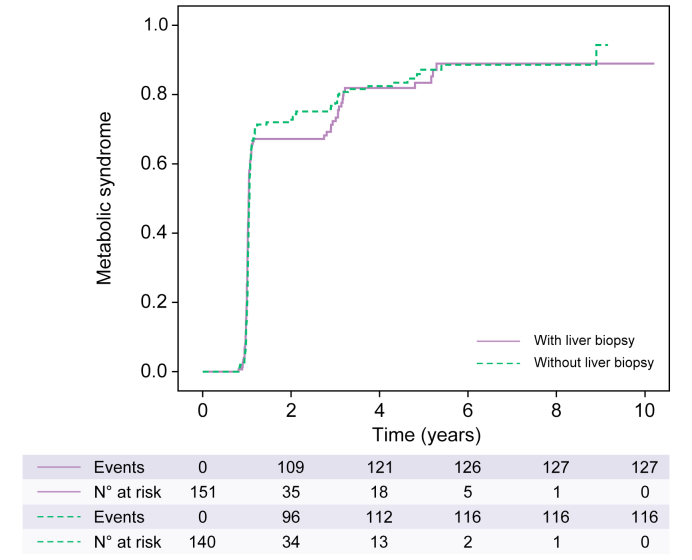
The cumulative incidence of MS after LT at 1, 5 and 10 years was 73.5%, 86.2% and 92.5%, respectively (Fig. 1); 73.1% of patients had a BMI over 25 kg/m2 and 59.3% were obese during follow-up. The median [IQR] BMI at 1 year and 5 years after LT was 29.5 (26.6-33.6) kg/m2 and 32.3 (28.2-35.5) kg/m2, respectively.
Fig. 1.
Cumulative incidence of metabolic syndrome after liver transplantation.
The cumulative incidence of metabolic syndrome at 1, 5 and 10 years was 73.5%, 86.2% and 92.5%, respectively. There was no difference between patients with and without liver biopsy (p = 0.896). Kaplan-Meier analysis.
Five patients (3.3%) had a history of BS: three before LT and two after LT. The median time between BS and LT was 11.0 (8.2-11.8) years and the median weight loss after BS was 20.0 (10.0-35.0) kg. No complication of BS was reported in these patients, including ESLD secondary to BS. Two (1.3%) patients underwent a BS after LT, with the median time between LT and BS of 3.4 (3.2-3.5) years. Sleeve gastrectomy was the only type of BS performed after LT. A patient with NASH recurrence histologically proven at 1 year after LT underwent a sleeve gastrectomy at 3 years after LT, allowing for an improvement of the hepatic histology with complete regression of steatosis, NASH resolution and reduction of fibrosis stage from 3 to 2 on the liver biopsy 5 years after LT. A BS was under discussion for 10 (6.7%) patients at the end of follow-up.
Eight patients (5.3%) entered an intensive nutrition program (defined by specialized rehabilitation services) after LT.
NAFLD recurrence after LT and associated risk factors
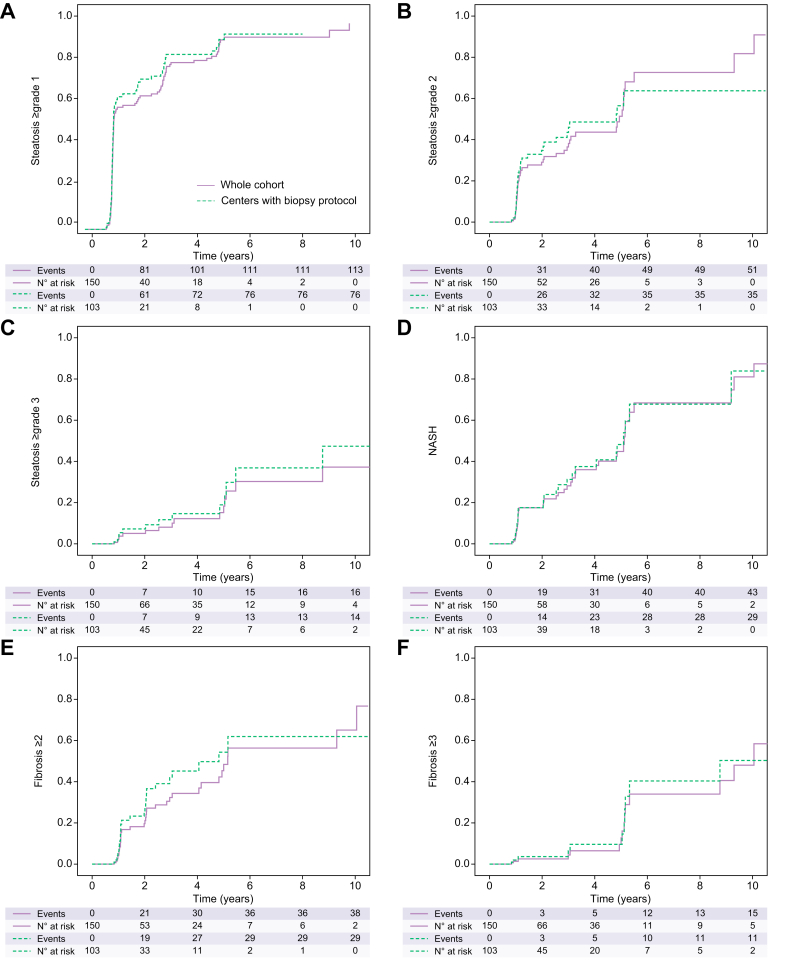
One hundred and fifty patients underwent at least one liver graft biopsy after LT (222 liver biopsies in total, 94.3% performed as protocol biopsy). In centers performing protocol biopsies after LT, a liver graft biopsy was available at 1 year for 103 patients (out of 110 patients with ≥12 months follow-up, i.e 93.6%), 25 at 5 years (out of 28 patients with ≥5 years follow-up, i.e. 89.3%) and 12 at 10 years (out of 13 patients ≥10 years follow-up, i.e. 92.3%) (Table S2). Cumulative incidence rates of steatosis, NASH and advanced fibrosis did not significantly differ when comparing the entire cohort and centers performing protocol biopsies (Fig. 2).
Fig. 2.
Cumulative incidence of steatosis/NASH recurrence and fibrosis after liver transplantation in whole cohort and in centers with a biopsy protocol (based on 150 liver biopsies).
(A) Steatosis ≥1 grade recurrence was 68.0% at 1 year and 85.0% at 5 years in whole cohort; p = 0.381. (B) Steatosis ≥2 grade recurrence was 27.8% at 1 year and 64.5% at 5 years in whole cohort; p = 0.713. (C) Steatosis ≥3 grade recurrence was 5.0% at 1 year and 30.3% at 5 years in whole cohort; p = 0.378. (D) NASH recurrence was 14.9% at 1 year and 60.3% at 5 years in whole cohort; p = 0.823. (E) NAFLD-related ≥grade 2 fibrosis was 18.2% at 1 year and 48.0% at 5 years in whole cohort; p = 0.333. (F) NAFLD-related ≥grade 3 fibrosis was 1.4% at 1 year and 20.0% at 5 years in whole cohort; p = 0.745. Kaplan-Meier analysis. NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Steatosis recurrence on the graft (Fig. 2A-C and Table 2)
Table 2.
Risk factors for steatosis and NASH recurrence.
| Steatosis recurrence |
NASH recurrence |
|||||
|---|---|---|---|---|---|---|
| Univariate p value | Multivariate analysis |
Univariate p value | Multivariate analysis |
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Clinical characteristics before LT | ||||||
| Sex (M/F) | 0.269 | 0.634 | ||||
| Age at LT time | 0.012 | 0.004 | ||||
| Age ≥50 years | 0.145 | 0.285 | ||||
| Age ≥55 years | 0.276 | 0.098 | 1.470 (0.542-3.988)∗ | 0.449∗ | ||
| Age ≥60 years | 0.452 | 0.040 | 1.673 (0.716-3.193)∗ | 0.235∗ | ||
| Age ≥62 years | 0.307 | 0.002 | 0.397 (0.145-1.089)∗ | 0.073∗ | ||
| Age ≥65 years | 0.383 | 0.035 | 0.237 (0.058-0.964)∗ | 0.044∗ | ||
| BMI at LT time | 0.149 | 0.020 | ||||
| ≥20 kg/m2 | 0.809 | 0.564 | ||||
| ≥25 kg/m2 | 0.817 | 0.215 | ||||
| ≥30 kg/m2 | 0.056 | 0.0001 | 5.086 (1.000-28.857)∗∗ | 0.050∗∗ | ||
| ≥31 kg/m2 | 0.002 | 2.727 (1.048-7.098)∗ | 0.040 | 0.0001 | 11.017 (2.073-58.538)∗∗ | 0.005∗∗ |
| ≥32 kg/m2 | 0.003 | 1.766 (0.726-4.297)∗ | 0.210 | 0.001 | 5.715 (1.116-29.270)∗∗ | 0.036∗∗ |
| ≥35 kg/m2 | 0.201 | 0.293 | ||||
| Pre-LT HCC | 0.538 | 0.856 | ||||
| Pre-LT diabetes | 0.108 | 0.892 | ||||
| Pre-LT HbA1c ≥7% | 0.602 | 0.621 | ||||
| Pre-LT insulin therapy | 0.115 | 0.272 | ||||
| Pre-LT arterial hypertension | 0.411 | 0.439 | ||||
| Pre-LT metabolic syndrome | 0.043 | 1.029 (0.501-2.115) | 0.937 | 0.046 | 0.775 (0.286-2.098) | 0.616 |
| Active smoking before LT | 0.810 | 0.836 | ||||
| Donor characteristics | ||||||
| Age of the donor (years) | 0.497 | 0.120 | ||||
| Age ≥60 years | 0.091 | 1.466 (0.832-2.584) | 0.185 | 0.575 | ||
| Age ≥70 years | 0.821 | 0.651 | ||||
| Donor age + recipient age (years) | 0.185 | |||||
| ≥120 years | 0.260 | 0.926 | ||||
| ≥135 years | 0.829 | 0.984 | ||||
| Donor BMI (kg/m2) | 0.378 | 0.333 | ||||
| Graft steatosis (≥5%) | 0.162 | 0.191 | ||||
| Grade ≥2 steatosis | 0.121 | 0.137 | ||||
| Metabolic events after LT | ||||||
| BMI at 1 year after LT | 0.008 | 0.054 | ||||
| ≥30 kg/m2 | 0.0001 | 0.976 (0.426-2.240)∗ | 0.955 | 0.0001 | 0.277 (0.286-1.216)∗∗∗ | 0.089∗∗∗ |
| ≥32 kg/m2 | 0.002 | 1.197 (0.654-2.191)∗ | 0.560 | 0.008 | 0.521 (0.193-1.409)∗∗∗ | 0.199∗∗∗ |
| ≥35 kg/m2 | 0.023 | 1.313 (0.623-2.766)∗ | 0.474 | 0.227 | ||
| Weight difference 1-year post-LT (kg) | 0.148 | 0.805 | ||||
| Diabetes post-LT | 0.974 | 0.635 | ||||
| Arterial hypertension post-LT | 0.452 | 0.587 | ||||
| TG ≥1.7 mmol/L post-LT | 0.248 | 0.527 | ||||
| LDL-c ≥3.70 mmol/L post-LT | 0.474 | 0.566 | ||||
| HDL-c <1.15 mmol/L | 0.402 | 0.050 | 3.463 (1.301-9.220) | 0.013 | ||
| HbA1c ≥6.5% | 0.205 | 0.150 | ||||
| HbA1c ≥7% | 0.333 | 0.330 | ||||
| HbA1c ≥8% | 0.052 | 1.676 (0.939-2.990) | 0.080 | 0.194 | ||
| Metabolic syndrome post-LT | 0.363 | 0.655 | ||||
| Immunosuppressive regimen | ||||||
| Tacrolimus | 0.163 | 0.098 | ||||
| MMF | 0.101 | 0.629 | ||||
| CYA | 0.375 | 0.934 | ||||
| mTOR-i | 0.930 | 0.675 | ||||
| CST | 0.071 | 0.337 (0.074-1.547) | 0.162 | 0.307 | ||
| AZA | 0.377 | 0.673 | ||||
| Specifics therapies after LT | ||||||
| Statin therapy | 0.985 | 0.519 | ||||
| Fibrate therapy | 0.329 | 0.377 | ||||
| Insulin therap | 0.157 | 0.573 | ||||
| Dyslipidemia or lipid drugs | 0.047 | 1.831 (0.883-3.795) | 0.104 | 0.910 | ||
| Complications after LT | ||||||
| CV events | 0.050 | 0.764 (0.444-1.313) | 0.330 | 0.420 | ||
| OSAS | 0.056 | 1.130 (0.605-2.109) | 0.701 | 0.363 | ||
| Acute rejection | 0.627 | 0.587 | ||||
| Disease recurrence on the graft at 1 year | ||||||
| Grade ≥1 steatosis | 0.002 | 10.521 (2.127-52.046)∗∗∗∗ | 0.004∗∗∗∗ | |||
| Grade ≥2 steatosis | 0.0001 | 10.196 (3.553-29.257)∗∗∗∗ | 0.001∗∗∗∗ | |||
| Grade 3 steatosis | 0.0001 | 2.729 (0.845-8.815)∗∗∗∗ | 0.093∗∗∗∗ | |||
Each variable was tested in univariate analysis. All variables with a p value <0.010 were retained for the multivariate model.
Univariate analysis was performed using log-rank analysis or Mann-Whitney analysis (for quantitative variables). All significant variables in the univariate analysis with a level set at p <0.1 were incorporated into multivariate models analyzed with binary logistic Cox regression. Values in bold are considered significant (<0.005).
AZA, azathioprine; CNI, calcineurin inhibitor; CST, corticosteroids; CYA, cyclosporine A; CV, cardiovascular; HbA1c, glycated hemoglobin; HCC, hepatocellular carcinoma; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; LT, liver transplantation; MMF, mycophenolate mofetil; mTOR-i, mTOR inhibitor; TG, triglyceride.
Because these variables are not independent, different multivariate analysis models were tested.
The cumulative recurrence of steatosis (≥5% i.e., all stages) was 68% at 1 year and 85.0% at 5 years (Fig. 2A). The cumulative incidence of grade 2 steatosis recurrence at 1 and 5 years after LT was 27.8% and 64.5%, respectively; one-third of recipients developed at least grade 2 steatosis (Fig. 2B). The cumulative incidence of grade 3 steatosis is shown in Fig. 2C. In multivariate analysis, only the BMI ≥31 kg/m2 at time of LT (OR 2.727; 95% CI 1.048-7.098; p = 0.040) was significantly associated with the risk of steatosis recurrence. There was a trend for patients with unmanaged diabetes (defined as HbA1c ≥8%). We found no relationship with weight gain or BMI at 1 year after LT. At 1 and 5 years after LT, in patients with grade ≥1 steatosis on biopsy, 33.6% and 75.0%, respectively, had steatosis on ultrasound (performed at the same time as the liver biopsy). At 1 and 5 years after LT, in patients with grade ≥2 steatosis on liver biopsy, 63.2% and 100%, respectively, had steatosis on ultrasound (performed at the same time as the liver biopsy). Seven patients (4.7%) had non-invasive assessment of steatosis by controlled-attenuation parameter after LT.
NASH recurrence on the graft (Fig. 2D and Table 2)
The cumulative incidence of NASH recurrence was 14.9% at 1 year and 60.3% at 5 years post-LT (Fig. 2D). The median [IQR] delay between the LT and NASH recurrence was 2.07 (1.1-4.8) years. Of the 43 cases of NASH recurrence (28.7%), 33 patients (76.7%) had steatosis on ultrasound or CT scan.
The median values of alanine aminotransferase, aspartate aminotransferase and gamma-glutamyltransferase at the diagnosis of NASH recurrence were respectively 35 (20-56) IU/L, 32.0 (22-44) IU/L and 43 (28-87) IU/L. Twenty-six (60.5%) patients had gamma-glutamyltransferase above the upper limit, 12 (27.9%) alanine aminotransferase above the upper limit and 10 (23.3%) aspartate aminotransferase above the upper limit at the time of diagnosis of NASH recurrence.
In multivariate analysis, risk factors for NASH recurrence were an age at the time of LT less than 65 years (OR 4.214; 95% CI 1.038-17.108; p = 0.044), a post-LT high-density lipoprotein-cholesterol level less than 1.15 mmol/L (OR 3.463; 95% Cl 1.304-18.780; p = 0.019), grade 1 steatosis on the graft at 1 year after LT (OR 10.521; 95% Cl 2.127-52.046; p = 0.004), or grade 2 steatosis (OR 10.196; 95% Cl 3.553-29.257; p = 0.001). Donor characteristics (age, BMI or steatosis on the graft) were not associated with NASH recurrence..
Recurrence of NAFLD-related fibrosis on the graft (Fig. 2E,F and Table 3)
Table 3.
Risk factors for significant and advanced fibrosis recurrence.
| Significant fibrosis (grade ≥2) |
Advanced fibrosis (grade 3-4) | |||||
|---|---|---|---|---|---|---|
| Univariate p value | Multivariate analysis |
Univariate p value | Multivariate analysis |
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Clinical characteristics before LT | ||||||
| Sex (M/F) | 0.850 | 0.691 | ||||
| Age at LT time | 0.034 | 0.008 | ||||
| Age ≥50 years | 0.105 | 0.0001 | 6.436 (0.316-103.912)∗ | 0.226∗ | ||
| Age ≥55 years | 0.160 | 0.001 | 2.083(0.249-17.411)∗ | 0.498∗ | ||
| Age ≥60 years | 0.068 | 0.422 (0.201-0.889) | 0.023 | 0.022 | 0.492 (0.076-3.186)∗ | 0.457∗ |
| Age ≥62 years | 0.101 | 0.066 | 0.965 (0.153-6.069)∗ | 0.970∗ | ||
| Age ≥65 years | 0.214 | 0.225 | ||||
| BMI at LT time | 0.121 | 0.068 | ||||
| ≥20 kg/m2 | 0.530 | 0.873 | ||||
| ≥25 kg/m2 | 0.628 | 0.059 | ||||
| ≥30 kg/m2 | 0.080 | 1.161 (0.417-3.233)∗ | 0.775∗ | 0.005 | 4.546 (0.154-112.25)∗∗ | 0.952∗∗ |
| ≥31 kg/m2 | 0.013 | 1.569 (0.580-4.243)∗ | 0.375∗ | 0.006 | 3.848 (0.268-55.191)∗∗ | 0.321∗∗ |
| ≥32 kg/m2 | 0.007 | 1.738 (0.657-4.594)∗ | 0.265∗ | 0.005 | 1.591 (0.001-2486)∗∗ | 0.902∗∗ |
| ≥35 kg/m2 | 0.289 | 0.688 | ||||
| HCC pre-LT | 0.285 | 0.016 | 0.175 (0.016-1.896) | 0.152 | ||
| Pre-LT diabetes | 0.378 | 0.413 | ||||
| Pre-LT HbA1c ≥7% | 0.246 | 0.826 | ||||
| Pre-LT insulin therapy | 0.600 | 0.646 | ||||
| Pre-LT arterial hypertension | 0.733 | 0.187 | ||||
| Pre-LT metabolic syndrome | 0.425 | 0.069 | 8.550 (1.125-64.983) | 0.038 | ||
| Active smoking before LT | 0.677 | 0.447 | ||||
| Donor characteristics | ||||||
| Age of the donor (years) | 0.074 | 0.552 | ||||
| Age ≥60 years | 0.713 | 0.828 | ||||
| Age ≥70 years | 0.648 | 0.868 | ||||
| Donor age + recipients age (years) | 0.016 | 0.099 | ||||
| ≥120 years | 0.376 | 0.476 | ||||
| ≥135 years | 0.648 | 0.452 | ||||
| Donor BMI (kg/m2) | 0.170 | 0.161 | ||||
| Graft steatosis (≥5%) | 0.005 | 1.894 (0.915-3.923)∗∗ | 0.086∗∗ | 0.099 | 4.454 (1.257-103.209) | 0.126 |
| Grade ≥2 steatosis | 0.028 | 2.837 (1.266-6.358)∗∗ | 0.011∗∗ | 0.605 | ||
| Metabolic events after LT | ||||||
| BMI at 1 year after LT | 0.121 | 0.019 | ||||
| ≥30 kg/m2 | 0.007 | 1.198 (0.443-3.240)∗∗∗ | 0.723∗∗∗ | 0.001 | 3.578 (0.002-6102) | 0.737 |
| ≥32 kg/m2 | 0.013 | 1.547 (0.679-3.525)∗∗∗ | 0.299∗∗∗ | 0.059 | ||
| ≥35 kg/m2 | 0.130 | 0.160 | ||||
| Weight difference 1-year post-LT (kg) | 0.923 | 0.458 | ||||
| Diabetes post-LT | 0.499 | 0.999 | ||||
| Arterial hypertension post-LT | 0.609 | 0.634 | ||||
| TG ≥1.7 mmol/L post-LT | 0.353 | 0.363 | ||||
| LDL-c ≥3.70 mmol/L post-LT | 0.244 | 0.890 | ||||
| HDL-c <1.15 mmol/L | 0.726 | 0.937 | ||||
| HbA1c ≥6.5% | 0.127 | 0.324 | ||||
| HbA1c ≥7% | 0.190 | 0.606 | ||||
| HbA1c ≥8% | 0.249 | 0.109 | ||||
| Insulin therapy | 0.901 | 0.420 | ||||
| Metabolic syndrome post-LT | 0.641 | 0.739 | ||||
| Immunosuppressive regimen | ||||||
| Tacrolimus | 0.370 | 0.756 | ||||
| MMF | 0.738 | 0.971 | ||||
| CYA | 0.990 | 0.005 | 11.388 (1.257-103.209) | 0.031 | ||
| mTOR-i | 0.554 | 0.466 | ||||
| CST | 0.277 | 0.934 | ||||
| AZA | 0.681 | 0.873 | ||||
| CNI-free therapy | 0.370 | 0.756 | ||||
| Specifics therapies after LT | ||||||
| Statin therapy | 0.290 | 0.840 | ||||
| Fibrate therapy | 0.733 | 0.769 | ||||
| Insulin therapy | 0.901 | 0.420 | ||||
| Dyslipidemia or lipid drugs | 0.681 | 0.680 | ||||
| Complications after LT | ||||||
| OSAS | 0.650 | 0.954 | ||||
| Acute rejection | 0.973 | 0.656 | ||||
| Disease recurrence on the graft at 1 year | ||||||
| Grade ≥1 steatosis | 0.0001 | 5.373 (1.593-18.121)∗∗∗∗ | 0.007∗∗∗∗ | 0.103 | ||
| Grade ≥2 steatosis | 0.0001 | 3.564 (1.605-7.917)∗∗∗∗ | 0.002∗∗∗∗ | 0.001 | 10.720 (1.006-114.259) | 0.049 |
| Grade 3 steatosis | 0.001 | 4.596 (1.630-12.961)∗∗∗∗ | 0.004∗∗∗∗ | 0.109 | ||
| NASH recurrence | 0.0001 | 5.217 (2.013-13.519)∗∗∗∗ | 0.001∗∗∗∗ | 0.131 | ||
Univariate analysis was performed using log-rank analysis or Mann-Whitney analysis (for quantitative variables). All significant variables in the univariate analysis with a level set at p <0.1 were incorporated into multivariate models analyzed with binary logistic Cox regression. Values in bold are considered significant (<0.005).
Each variable was tested in univariate analysis. All variables with a p value < 0.010 were retained for the multivariate model.
AZA, azathioprine; CNI, calcineurin inhibitor; CST, corticosteroids; CYA, cyclosporine A; CV, cardiovascular; HbA1c, glycated hemoglobin; HCC, hepatocellular carcinoma; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; LT, liver transplantation; MMF, mycophenolate mofetil; mTOR-i, mTOR inhibitor; OSAS, obstructive sleep apnea syndrome; TG, triglyceride.
Because these variables are not independent, different multivariate analysis models were tested.
The cumulative incidence of significant fibrosis (stage ≥2) after LT was 18.2% at 1 year, 48.0% at 5 years and 65.0% at 10 years (Fig. 2E). There was no significant difference between the entire cohort and the protocol centers in which biopsies were not clinically directed (p = 0.333). In multivariate analysis, risk factors for NAFLD-related significant fibrosis included a recipient age at time of LT <60 years (OR 2.368; 95% Cl 1.125-4.986; p = 0.023), an initial graft steatosis over grade 2 (OR 2.837; 95% Cl 1.266-6.358; p = 0.011) and the presence at 1 year after LT of grade ≥1 steatosis (OR 5.373; 95% Cl 1.593-18.121; p = 0.007), grade ≥2 steatosis (OR 3.564; 95% Cl 1.605-7.917; p = 0.002), grade ≥3 steatosis (OR 4.596; 95% Cl 1.630-12.961; p = 0.004) or NASH recurrence (OR 5.217; 95% Cl 2.013-13.519; p = 0.001).
Cumulative incidence of advanced fibrosis (F3–F4) was 20.0% at 5 years and 48.0% at 10 years (Fig. 2F). Six patients (4.0%) had recurrent NAFLD cirrhosis after LT after a median delay of 7.1 years; one patient presented decompensated cirrhosis 15 years after LT. Concerning NAFLD-related advanced fibrosis, risk factors identified in multivariate analysis were MS before LT (OR 8.550; 95% Cl 1.125-64.983; p = 0.038), long-term use of CYA (OR 11.388; 95% Cl 1.257-103.209; p = 0.031) and grade 2 steatosis at 1 year (OR 10.720; 95% Cl 1.006-114.259; p = 0.049). Initial graft steatosis does not affect the occurrence of advanced fibrosis. The age of the recipient did not influence the occurrence of advanced fibrosis.
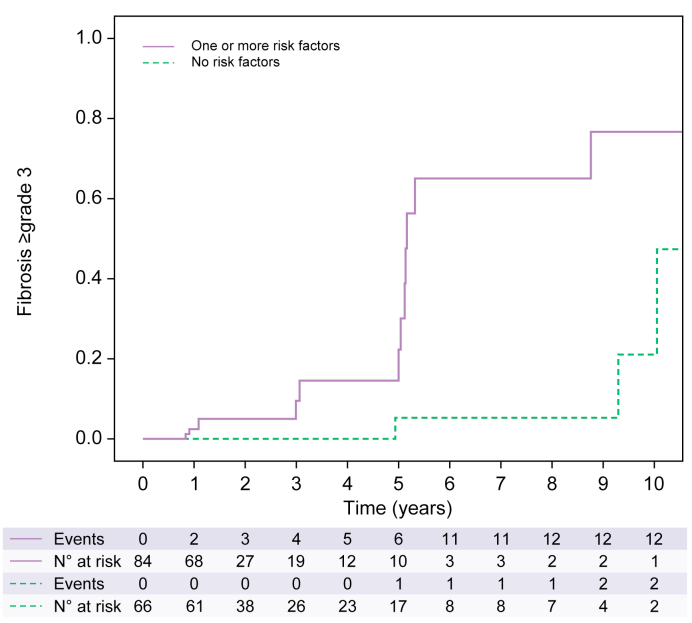
Non-invasive evaluation of fibrosis by liver stiffness measurement was performed in nine patients (6.0%) during follow-up. Fig. 3 shows the cumulative incidence of advanced NAFLD-related fibrosis according to the presence of at least one risk factor; without any risk factor, the risk of advanced fibrosis was estimated at 5.3% at 5 years compared to 58.3% with at list one risk factor (p = 0.001).
Fig. 3.
Cumulative incidence of advanced fibrosis according to the presence of risk factors (based on 150 liver biopsies).
Risk factors considered for analysis: Pre-LT metabolic syndrome, grade ≥2 steatosis at 1 year after LT and long-term use of cyclosporine. In the group with risk factors, incidence of advanced fibrosis was 5.3% at 1 year after LT, 58.3% at 5 years and 77.7% at 10 years. In the group without risk factors, incidence of advanced fibrosis was 0.0% at 1 year, 5.3% at 5 years and 48.4% at 10 years after LT. The difference is significant between the two groups of patients (p = 0.001). Kaplan-Meier analysis. LT, liver transplantation.
Discussion
We report herein the largest available cohort studying recurrence of initial disease after LT for NAFLD, including more than 150 patients with available post-LT liver biopsy. Previous single-center series reported only a few dozen cases.[9], [10], [11],16 We found that recurrence of NAFLD was observed in almost all patients 10 years after LT, that NASH recurrence occurred in more than half of the patients at 5 years, and that NAFLD-related significant and advanced fibrosis occurred in 48.0% and 20.0% at 5 years, respectively. The median follow-up of our patients was 4.7 years: this is because most LTs were performed after 2015 and protocol graft biopsies are usually planned at 1, 5 and 10 years. Our results, despite a median follow-up time of less than 5 years, strongly support a highly frequent recurrence of the initial disease. We previously reported that the survival of the overall cohort was 79.8% at 5 years.17
Since most of our patients had MS at time of LT, our results are not surprising. BMI at time of LT was high in our population: we tried where possible to use the dry, non-ascites weights of these patients. The impact of ascites seems minimal as there is no difference between patients transplanted for HCC and those transplanted for ESLD. The relatively low median Child-Pugh score may explain the small impact of ascites on body weight and BMI before LT. Estimation of MS in patients with ESLD may be underestimated due to possible disappearance of MS components in the advanced stages of liver disease. MS not only usually persists, but may worsen and occur de novo after LT. The aggravation of MS is mainly related to post-LT dyslipidemia: 62.6% of all patients developed de novo dyslipidemia after LT, probably favored by immunosuppressive drugs. In the context of LT (all etiologies of initial liver disease considered) MS incidence after LT is estimated to range between 44-58%.18 Recurrence of MS after LT for NAFLD was estimated to be 62.5% at 5.4 years after LT in a US study, which is lower than our data.19 This high proportion of MS recurrence/occurrence after LT can be explained by the pre-existing MS before LT, associated with immunosuppressive treatment, including CNI, mTOR-i and corticosteroids, which promote diabetes, dyslipidemia, arterial hypertension and weight gain.20 The second explanation could be the very low proportion of patients who have undergone BS or a specialized nutritional rehabilitation program in our cohort.
Herein, we confirm the impact of MS on the recurrence of steatosis, NASH and fibrosis. Available studies come from the US, with only one European study investigating the recurrence of NAFLD on the graft, in 11 patients.11 The largest available study included 34 patients, and disclosed that NAFLD and NASH recurrence occurred in 88% and 44% of patients, respectively, after a median follow-up of 47 months after LT.10 Our study is based on 150 patients with at least one liver biopsy performed after LT: recurrence of steatosis was frequent and early, since the recurrence rate of NASH was 60.3% at 5 years after LT. Not all patients from the global cohort had a liver biopsy, and one of the limitations of our work could be a selection bias regarding the indication for liver biopsies (disturbed liver tests, steatosis on imaging or clinical arguments). Nevertheless, there was no difference between patients who had a biopsy and those who did not, except that the median follow-up was a little bit longer for patients with available biopsy (4.9 vs. 3.5 months). In addition, liver biopsies were in most cases (>90%) protocol biopsies at 1, 5 and 10 years, according to different centers strategy: this high proportion of protocol biopsy reduces the risk of a possible selection bias. There was no difference in recurrence of steatosis, NASH and advanced fibrosis between the whole population with graft biopsy and patients with protocol biopsy after LT (Fig. 2 and Table S2). Finally, recurrence of initial graft disease was diagnosed on liver biopsies interpreted at each of the LT centers. All the biopsies were not reviewed centrally, but histological lesions were graded (and reported) according to standard validated scores. In addition, less than 10 patients underwent non-invasive investigation of steatosis by controlled-attenuation parameter and fibrosis by liver stiffness measurement or blood tests: these tests are not validated after LT21,22 and the small number of patients did not allow for statistical analyses.
In the available literature, studies on NAFLD recurrence need to be interpreted with caution: some studies included cryptogenic cirrhosis and/or assessment of fibrosis/steatosis on the graft by imaging methods, identifying therefore only steatosis (not NASH), with a significant lack of sensitivity. Our study finds a poor correlation between histologically proven steatosis and imaging, especially for grade 1 steatosis. Few studies (and only some patients included in each study) have analyzed results of liver graft biopsies.9,10,23,24 In a meta-analysis including 17 studies (2,378 patients), the cumulative incidence of recurrent NAFLD and NASH was 82% and 38%, respectively, more than 5 years after LT.12 Recurrence of steatosis and NASH has an impact on graft fibrosis. After elimination of other causes of fibrosis, the cumulative incidence of significant fibrosis was 48.0% at 5 years and 65.0% at 10 years in our study. In the study from Bhati et al., NALFD-significant fibrosis recurrence was estimated to be 42.2% at 47 months, and this is consistent with our results.9 No re-transplantation for recurrence of the initial disease was performed in our cohort. This low rate of re-LT is consistent with the literature: in a study of 1,295 patients, only one re-LT was performed and none in the study by Bhati et al..9,25 Recurrence of cirrhosis occurred a median of 7 years after LT in our study, and therefore probably occurs in elderly transplanted patients, over 70 years old, who are generally considered too old for a second LT.
Our study did not aim to compare patients with NAFLD to a non-NAFLD population. However, in the literature, few studies have analyzed recurrence vs. development of de novo NAFLD on the graft (i.e. in patients transplanted for non-NAFLD cirrhosis).11 In a retrospective study, 80 patients, transplanted for non-NAFLD cirrhosis (alcohol-related disease with no relapse after LT, HBV or HCV infection) and presenting de novo NAFLD after LT, were compared to 11 patients with recurrent NAFLD. Advanced fibrosis (71.4% vs. 12.5%) and NASH (71.4% vs. 17.2%) were more frequent and faster in patients with recurrent NAFLD compared to de novo NAFLD. These data were confirmed by a meta-analysis: at 5 years after LT, NASH recurrence was estimated at 38% vs. 17% for de novo NASH.12 A recent study confirmed that the development of recurrent NASH is earlier than de novo NASH (2.8 years vs. 4.8 years, p = 0.02).26
Since our study shows a rapid and frequent recurrence of the initial disease on the graft and an evolution towards advanced fibrosis, it is of great relevance to identify risk factors in order to limit disease recurrence and/or progression. Few studies, which evaluated disease recurrence of NAFLD, are summarized in Table S3: only some of them have identified risk factors for recurrence of steatosis or fibrosis. Factors associated with recurrence of the initial disease on the graft can be separated into three categories: components of the MS (BMI before and after LT, dyslipidemia and diabetes), factors related to immunosuppressive drugs (CYA or corticosteroids) and demographic factors (such as the age at transplantation). Patient factors are not modifiable, and it is therefore necessary to try to manage the other factors to decrease the risk of disease recurrence on the graft. Regarding MS, intensive nutritional management is required along with treatment of diabetes, dyslipidemia and hypertension. The benefit of BS has been demonstrated in non-transplanted patients: in a recent French study, at 5 years after BS, NASH had resolved in 84% of patients with an improvement of liver fibrosis in 76% of patients.4 Several small studies in patients who had received LT are available-though the timing for BS and type of BS has to be defined.[27], [28], [29] BS could probably be discussed in a majority of patients after LT. Current data suggest that sleeve gastrectomy could be performed 6 to 12 months after LT: this BS type allows access to the bile ducts and a good absorption of immunosuppressants. In our study, only five patients had history of BS, including only two patients after LT. According to the French Haute Autorité de Santé criteria, BS is indicated in cases of a BMI greater than 40 kg/m2, or 35 kg/m2 with metabolic complications (NASH, hypertension, obstructive sleep apnea syndrome, type 2 diabetes, disabling osteoarticular diseases).30 Taking these criteria, 41 (27.3%) of our patients met these criteria at 1 year after LT (personal data): from whom 21 have grade 2 steatosis on liver biopsy at 12 months. One of the explanations for the low proportion of BS in our population is probably the relatively old age of the patients: the expected benefit of BS is validated in patients with an age below 65 years.31 Nevertheless, several recent studies have suggested that BS could be performed in patients over 65 years of age, with follow-up of up to 6 years and good efficacy on weight, physical activity and biological parameters.[32], [33], [34] Another possibility needing further evaluation in these “elderly” patients would be endoscopic BS that could also be an alternative approach.35 Another explanation may be reluctance on the part of the patient or surgeon to perform a BS after a LT. Nevertheless, current data, based on series of small numbers, show good results of BS after LT.27,29 The second modifiable factor after LT is immunosuppressive treatments: all maintenance immunosuppressive drug classes (CNI, mTOR-i and corticosteroids) have a deleterious metabolic profile except antimetabolites (MMF and azathioprine). MMF monotherapy is possible but only in selected patients a long time after LT and can therefore not be generally recommended.36 Avoiding steroids and minimizing CNI and/or mTOR-i is feasible but probably has minimal impact. We believe it is essential to reduce the dosage of immunosuppressive drugs to a minimum, and to perform a liver biopsy at 12 months after LT to determine steatosis. Recurrence of grade 2 steatosis on the graft at 12 months seems to be strongly associated with both recurrence of NASH and advanced fibrosis: we suggest discussing BS in these patients, who represent about a quarter of patients transplanted for NAFLD cirrhosis in our population.
In conclusion, we report data on the largest cohort of patients with NAFLD recurrence after LT for whom liver biopsies were available. The recurrence of steatosis and steatohepatitis on the graft was rapid and very frequent. Several associated factors have been identified and need to be confirmed in other large studies. The performance of a protocol biopsy at 12 months after LT appears to be key to identify patients at risk of progression to NASH or advanced fibrosis. In these patients with grade 2 steatosis, representing about 25% of patients, BS should be discussed. The management of metabolic factors and the place of BS need to be evaluated to reduce the risk of recurrence of the initial disease in this exponentially growing population of LT patients.
Financial support
Bourse d’appels offres à recherche 2018-Agence de la Biomédecine.
Authors’ contributions
Jérôme Dumortier had the idea of the project and participated in analysis and interpretation of data. François Villeret collected the data. François Villeret and Domitille Erard performed statistical analysis. François Villeret and Jérôme Dumortier participated in writing of the manuscript. François Villeret Sébastien Dharancy, Domitille Erard, Armand Abergel, Louise Barbier, Camille Besch, Olivier Boillot, Karim Boudjema, Audrey Coilly, Filomena Conti, Christophe Corpechot, Christophe Duvoux, François Faitot, Stéphanie Faure, Claire Francoz, Emiliano Giostra, Jean Gugenheim, Jean Hardwigsen, Marie-Noëlle Hilleret Jean-Baptiste Hiriart, Pauline Houssel-Debry, Nassim Kamar, Guillaume Lassailly, Marianne Latournerie, Georges-Philippe Pageaux, Didier Samuel, Claire Vanlemmens, Faouzi Saliba and Jérôme Dumortier were involved in medical care of the patients and approved the final version of the manuscript.
Data availability statement
Due to the sensitive nature of the questions asked in this study, survey respondents were assured raw data would remain confidential and would not be shared.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank all LT teams that participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100668.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatol Baltim Md. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 2.Nabi O., Lacombe K., Boursier J., Mathurin P., Zins M., Serfaty L. Prevalence and risk factors of nonalcoholic fatty liver disease and advanced fibrosis in general population: the French nationwide NASH-CO study. Gastroenterology. 2020;159:791–793.e2. doi: 10.1053/j.gastro.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a Review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 4.Lassailly G., Caiazzo R., Ntandja-Wandji L.-C., Gnemmi V., Baud G., Verkindt H., et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159:1290–1301.e5. doi: 10.1053/j.gastro.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Haldar D., Kern B., Hodson J., Armstrong M.J., Adam R., Berlakovich G., et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Stepanova M., Ong J., Trimble G., AlQahtani S., Younossi I., et al. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021;19:580–589.e5. doi: 10.1016/j.cgh.2020.05.064. [DOI] [PubMed] [Google Scholar]
- 7.National Health Service Blood and Transplant . 2020. Annual report on liver transplantation. [Google Scholar]
- 8.Agence de la Biomédecine . 2020. Le rapport médical et scientifique 2020 [Internet]https://rams.agence-biomedecine.fr/ Available from: [Google Scholar]
- 9.Bhati C., Idowu M.O., Sanyal A.J., Rivera M., Driscoll C., Stravitz R.T., et al. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation. 2017;101:1867–1874. doi: 10.1097/TP.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 10.Bhagat V., Mindikoglu A.L., Nudo C.G., Schiff E.R., Tzakis A., Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transpl Soc. 2009;15:1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 11.Vallin M., Guillaud O., Boillot O., Hervieu V., Scoazec J.-Y., Dumortier J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transpl Soc. 2014;20:1064–1071. doi: 10.1002/lt.23936. [DOI] [PubMed] [Google Scholar]
- 12.Saeed N., Glass L., Sharma P., Shannon C., Sonnenday C.J., Tincopa M.A. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: Systematic Review and Meta-analysis. Transplantation. 2019;103:e345–e354. doi: 10.1097/TP.0000000000002916. [DOI] [PubMed] [Google Scholar]
- 13.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 14.Bedossa P., Poitou C., Veyrie N., Bouillot J.-L., Basdevant A., Paradis V., et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatol Baltim Md. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol Baltim Md. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Malik S.M., deVera M.E., Fontes P., Shaikh O., Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transpl Off. J. Am. Soc. Transpl Am. Soc. Transpl. Surg. 2009;9:782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 17.Villeret F., Dharancy S., Erard D., Abergel A., Barbier L., Besch C., et al. Liver transplantation for NAFLD cirrhosis: Age and recent coronary angioplasty are major determinants of survival. Liver Int Off J Int Assoc Study Liver. 2022;42:2428–2441. doi: 10.1111/liv.15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui M.S., Sterling R.K. Posttransplant metabolic syndrome. Int J Hepatol. 2012;2012 doi: 10.1155/2012/891516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik S.M., deVera M.E., Fontes P., Shaikh O., Sasatomi E., Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843–1851. doi: 10.1002/lt.21943. [DOI] [PubMed] [Google Scholar]
- 20.Carter D., Dieterich D.T., Chang C. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in liver transplantation. Clin Liver Dis. 2018;22:213–227. doi: 10.1016/j.cld.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui M.S., Idowu M.O., Stromberg K., Sima A., Lee E., Patel S., et al. Diagnostic Performance of Vibration-Controlled Transient Elastography in Liver Transplant Recipients. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021;19:367–374. doi: 10.1016/j.cgh.2020.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhat M., Tazari M., Sebastiani G. Performance of transient elastography and serum fibrosis biomarkers for non-invasive evaluation of recurrent fibrosis after liver transplantation: a meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yalamanchili K., Saadeh S., Klintmalm G.B., Jennings L.W., Davis G.L. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transpl Soc. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 24.Sourianarayanane A., Arikapudi S., McCullough A.J., Humar A. Nonalcoholic steatohepatitis recurrence and rate of fibrosis progression following liver transplantation. Eur J Gastroenterol Hepatol. 2017;29:481–487. doi: 10.1097/MEG.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 25.Agopian V.G., Kaldas F.M., Hong J.C., Whittaker M., Holt C., Rana A., et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–633. doi: 10.1097/SLA.0b013e31826b4b7e. [DOI] [PubMed] [Google Scholar]
- 26.Balitzer D., Tsai J.-H., Gill R.M. Clinicopathologic features of de novo non-alcoholic steatohepatitis in the post-transplant setting. Diagn Pathol. 2022;17:65. doi: 10.1186/s13000-022-01247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrano O.K., Peterson K.J., Vock D.M., Berglund D., Kandaswamy R., Lake J.R., et al. Clinical Impact of Antecedent Bariatric Surgery on Liver Transplant Outcomes: A Retrospective Matched Case-control Study. Transplantation. 2021;105:1280–1284. doi: 10.1097/TP.0000000000003378. [DOI] [PubMed] [Google Scholar]
- 28.Tsamalaidze L., Stauffer J.A., Arasi L.C., Villacreses D.E., Franco J.S.S., Bowers S., et al. Laparoscopic sleeve gastrectomy for morbid obesity in patients after orthotopic liver transplant: a matched case-control study. Obes Surg. 2018;28:444–450. doi: 10.1007/s11695-017-2847-7. [DOI] [PubMed] [Google Scholar]
- 29.Zamora-Valdes D., Watt K.D., Kellogg T.A., Poterucha J.J., Di Cecco S.R., Francisco-Ziller N.M., et al. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatol Baltim Md. 2018;68:485–495. doi: 10.1002/hep.29848. [DOI] [PubMed] [Google Scholar]
- 30.Haute Autorité de Santé . 2009. Obésité:prise en charge chirurgicale chez l’adulte [Internet]https://www.has-sante.fr/upload/docs/application/pdf/2011-10/reco2clics_obesite_adulte_chirurgie.pdf Available from: [Google Scholar]
- 31.Mechanick J.I., Youdim A., Jones D.B., Timothy Garvey W., Hurley D.L., Molly McMahon M., et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American association of clinical endocrinologists, the obesity society, and American society for metabolic & bariatric surgery. Surg Obes Relat Dis. 2013;9:159–191. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Cunha J.B., Fialho M.C.M.P., Arruda S.L.M., Nóbrega O.T., Camargos E.F. Clinical and metabolic improvement after bariatric surgery in older adults: a 6-year follow-up. J Nutr Health Aging. 2020;24:865–869. doi: 10.1007/s12603-020-1406-4. [DOI] [PubMed] [Google Scholar]
- 33.Susmallian S., Barnea R., Weiss Y., Raziel A. Outcome of bariatric surgery in older patients. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2018;14:1705–1713. doi: 10.1016/j.soard.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg I., Yang J., Nie L., Bates A.T., Docimo S., Pryor A.D., et al. Safety of bariatric surgery in patients older than 65 years. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2019;15:1380–1387. doi: 10.1016/j.soard.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Salomone F., Sharaiha R.Z., Boškoski I. Endoscopic bariatric and metabolic therapies for non-alcoholic fatty liver disease: evidence and perspectives. Liver Int. 2020;40:1262–1268. doi: 10.1111/liv.14441. [DOI] [PubMed] [Google Scholar]
- 36.Lassailly G., Dumortier J., Saint-Marcoux F., El Amrani M., Boulanger J., Boleslawski E., et al. Real life experience of mycophenolate mofetil monotherapy in liver transplant patients. Clin Res Hepatol Gastroenterol. 2021;45:101451. doi: 10.1016/j.clinre.2020.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the sensitive nature of the questions asked in this study, survey respondents were assured raw data would remain confidential and would not be shared.