Abstract
Injuries lead to an early systemic inflammatory state with innate immune system activation. Neutrophil extracellular traps (NETs) are a complex of chromatin and proteins released from the activated neutrophils. While initially described as a response to bacterial infections, NETs have also been identified in the sterile post-injury inflammatory state. Peptidylarginine deiminases (PADs) are a group of iso-enzymes that catalyze the conversion of arginine to citrulline, termed citrullination or deimination. PAD2 and PAD4 have been demonstrated to play a role in NET formation through citrullinated histone H3 (CitH3). PAD2 and PAD4 have a variety of substrates with variable organ distribution. Pre-clinical and clinical studies have evaluated the role of PADs and NETs in major trauma, hemorrhage, burns, and traumatic brain injury. NET formation and PAD activation have been shown to contribute to the post-injury inflammatory state leading to a detrimental effect on organ systems. This review describes our current understanding of the of role of PAD and NET formation following injury and burn. This is a new field of study, and the emerging data appears promising for the future development of targeted biomarkers and therapies in trauma.
Keywords: Peptidylarginine deiminase, neutrophil extracellular trap, citrullinated histone H3, trauma, inflammation, traumatic brain injury, burn, hemorrhage
Introduction
Injuries are the leading cause of death for Americans age 1 to 441 and the fourth overall leading cause of death in 2020. 2 Following a major trauma or burn, the innate immune system drives an early systemic inflammatory response syndrome (SIRS). 3 SIRS is a global inflammatory response mediated by pro-inflammatory agents that can occur after trauma or sepsis leading to potentially lethal complications4,5. The immune mechanisms of trauma-induced SIRS likely affect outcomes in ways we are just beginning to investigate. The complexity of changes induced by trauma and injury at the cellular level provide innumerable avenues for ongoing research and development of novel cytoprotective strategies. 6
Pepetidylarginine deiminases (PADs) are a family of enzymes involved in a variety of physiologic processes through post-translational modifications of proteins. 7,8 PADs catalyze a calcium dependent hydrolysis of arginine to citrulline, termed citrullination or deimination. 7,8 The PAD family consists of five iso-enzymes (PAD1–4 and PAD6). The genomic makeup is highly conserved in mammals and PAD expression is distributed in a tissue specific fashion. 8,9 PAD2 is the most widely expressed with distribution in the brain, spinal cord, pancreas, bone marrow, and immune cells, among other sites. Within each tissue location, PADs affect a variety of substrates including both histone and non-histone targets. 8,9 The effects of histone citrullination are vast and are just beginning to be understood. 9 In accordance with their wide distribution, PADs have been implicated in many disease processes including rheumatoid arthritis, 10 multiple sclerosis, 11 cancers, 9 sepsis, 12 ischemia-reperfusion, 13,14 and psoriasis. 15
Extracellular traps (ET) are complexes of chromatin and associated proteins released in response to immune cell activation. 16–18 Neutrophil ETs (NETs) are the most described, and initially discovered as a bactericidal innate immune response. 16 ETs were originally discovered as a neutrophil response, however studies have found that ETs may also be released by other immune cells including macrophages, eosinophils, and mast cells. 17 While NETs provide an antimicrobial immune response, recent evidence has shown that they may also contribute to SIRS and tissue damage in the host. 19,20 Studies have now demonstrated that NET formation (NETosis) plays a role in many additional inflammatory pathologies in both infectious and sterile conditions, including trauma-induced SIRS. 18,21–23
NETosis and extracellular trap formation (ETosis) have been linked to PAD through histone citrullination leading to chromatin decondensation. 24 Prior studies have shown that PAD4 mediated histone citrullination leads to NETosis, 25 while some recent studies also suggest that PAD2 may play a role. 26 At this time, the mechanisms by which PADs and NETs affect the innate immune response in trauma are not fully elucidated. While there has been a well-known association of PADs with sepsis, we are just beginning to investigate the relationship of PADs in non-septic conditions, such as tissue injury. Evaluating NETs and PADs in trauma may be a key step in development of novel therapeutic strategies. This review examines the current understanding of PADs and NETs in trauma and hemorrhage, traumatic brain injury, burn and wound healing, and discusses some future directions in the field.
Trauma and Hemorrhage
Neutrophil Extracellular Traps in Trauma and Hemorrhage
Hemorrhage is the most immediate and life-threatening consideration in trauma. Hemorrhage leads to hypoperfusion , endotheliopathy27, and coagulopathy, that can lead to subsequent ischemia, multi-organ failure (MOF), and death. 6 The management of hemorrhagic shock has remained largely focused on early hemorrhage control and appropriate resuscitation.
Following a major injury, SIRS ensues due to blood loss, tissue damage, and cellular ischemia. 3,28 Pro-inflammatory cytokines and damage-associated molecular patterns (DAMPs) are secreted by immune or necrotic cells further activating the immune system. 28 DAMPs then activate the complement system, monocytes, and neutrophils leading to release of anaphylaxotoxins and interleukins. 3,28 Cell death by apoptosis may result secondary to the immune response and/or tissue ischemia. Further cellular damage may also be caused by the subsequent reperfusion injury with release of reactive oxygen species (ROS). 5 Increased vascular permeability due to endothelial injury, endotheliopathy, and SIRS leads to leakage of plasma proteins and intravascular fluid loss. 29 This post-traumatic endothelial dysfunction contributes to acute lung injury (ALI) and MOF27.
Neutrophils are a known early immune responder to trauma. Prior studies have demonstrated trauma-induced remodeling of neutrophils and changes to intracellular function. 30–32 While neutrophils have a host of beneficial defense mechanisms including phagocytosis, ROS, degranulation, and NETosis, an exaggerated response can worsen post-traumatic SIRS. 31 Additionally, NETs have been shown to increase endothelial permeability through glycocalyx degradation and junction cleavage contributing to vascular leakage and organ edema. 33 Tissue injury and sepsis can both elicit a nearly identical SIRS response (Figure 1A) in the early stages, but they differ in the type of NETosis. Yipp and Kubes summarized two kinds of NETosis: suicidal (non-infectious) and vital (infectious) NET formation (Figure 1B). 34 Suicidal NETosis results from membrane rupture, mediated by Nicotinamide adenine dinucleotide phosphate (NADPH) assisted translocation of neutrophil elastase (NE) and myeloperoxidase (MPO), about two hours after intracellular NET formation. 34,35 Vital NETosis was reported following both direct microbial exposure and lipopolysaccharide (LPS). Infection leads to rapid NET release (< 30 minutes)without subsequent neutrophil death34. The mechanistic differences remain to be fully explored in trauma however, they may prove to be a future therapeutic or diagnostic strategy in differentiating post-traumatic SIRS from sepsis.
FIGURE 1.
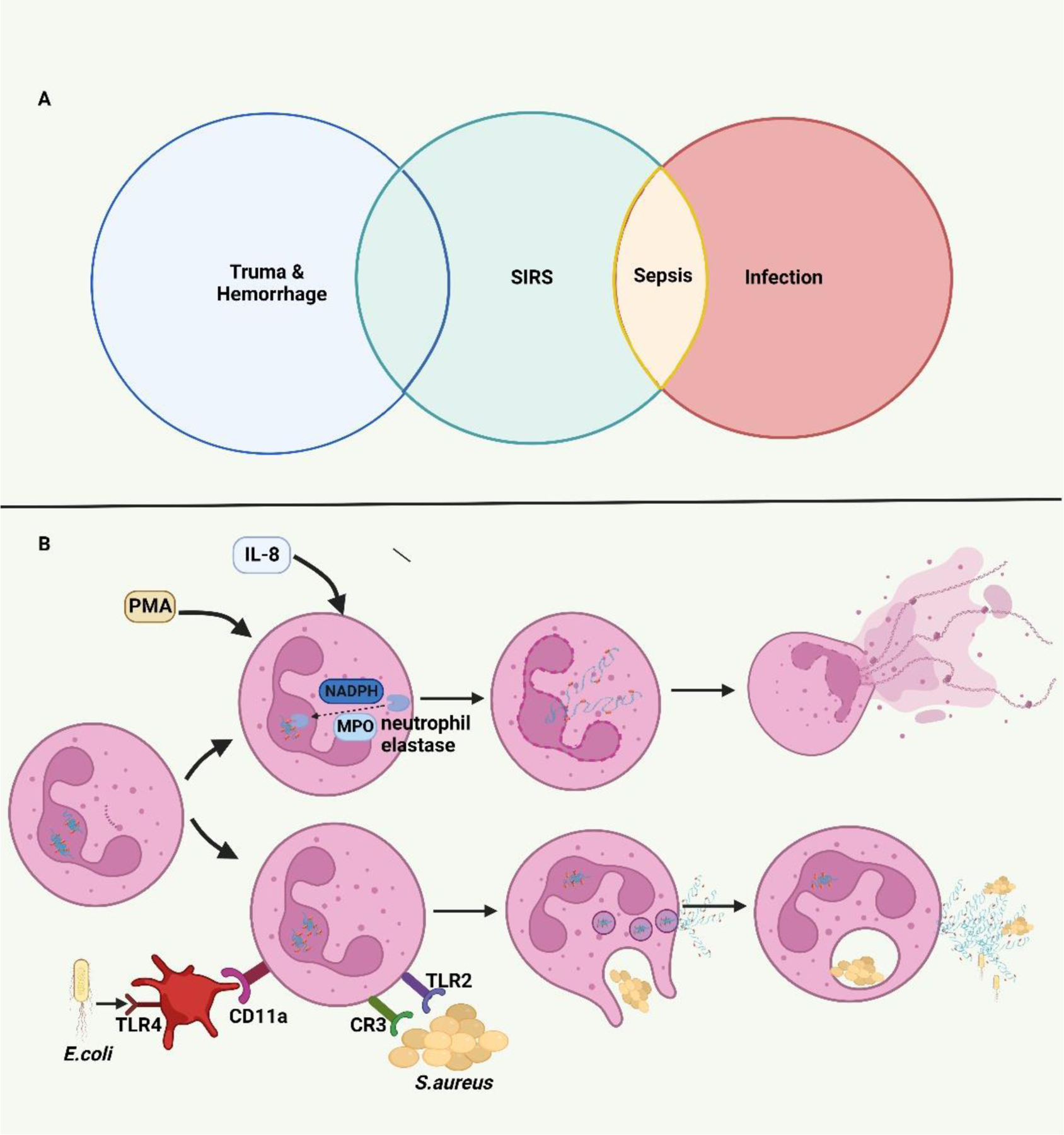
A. Systemic Inflammatory Response Syndrome (SIRS) induced by trauma and infection. Trauma and sepsis experience a similar SIRS state in their early stages. B Mechanistic pathways of NETosis. During the process of suicidal (non-infectious) NETosis, myeloperoxidase (MPO) and nicotinamide adenine dinucleotide phosphate (NADPH) mediate the translocation of neutrophil elastase (NE) from cytosolic granules into the nucleus. By cleaving histones, NE facilities the chromatin breakdown and nuclear envelope breakdown resulting in the rupture of the neutrophil cytomembrane and release of NETs over several hours. The vital (infectious) NETosis induced by direct microbial exposure or neutrophil-platelet interaction can release NETs through nuclear budding rapidly over less than 30 minutes.
NETs have been shown to be released following trauma in both in vitro studies and in critically ill trauma patients. 36,37 NETs have a complex structure containing azurophilic granules, NE, cathepsin G, MPO, DNA, and histones. 16 Thus, circulating NETs can be measured using a variety of assays. Early studies evaluating NETs in trauma used circulating cell-free-DNA (cf-DNA) as a surrogate for NETosis. 38,39 Meng et al found that early after major trauma there is an increase in NETs followed by a decrease to a nadir above normal levels. 37 In patients who subsequently developed sepsis, there was a second peak in NETs corresponding with the onset of infection. 37 Margraf et al similarly demonstrated a post-traumatic increase in cf-DNA, which corelated with patients’ Sequential Organ Failure Assessment (SOFA) and Multiple Organ Dysfunction Score (MODS) scores. 39 Those with higher cf-DNA throughout the hospital course were more likely to have sepsis following trauma. 39 While cf-DNA may not be specific to NETs, additional studies using immunohistochemistry (IHC) to demonstrate NETs have also found NETs in the trauma intensive care unit (ICU) patients. 36
There is also mounting data that cell-free mitochondrial DNA (cf-mtDNA) may be the primary structural component in NETs following trauma. McIlroy et al, one of the early groups to stain and visualize NETs following major trauma, reported that mtDNA was the major structural component of trauma-induced NETs. 22 Mitochondria are known as the site of oxidative phosphorylation affecting adenosine triphosphate (ATP) concentrations, however it also contains mtDNA and its own DAMPs. 40 MtDNA is a potent activator of post-traumatic SIRS and has been linked to development of acute respiratory distress syndrome (ARDS) and MOF in injured patients41. While mtDNA is typically encapsulated, protecting it from activating an immune response, trauma results in cf-mtDNA release adding to the post-traumatic SIRS. 42,43
Citrullinated Histones in Trauma and Hemorrhage
Histone citrullination leads to a conformational change that plays a major role in NET formation. 24,44 Citrullination is a post-translational modification catalyzed by PAD424 and PAD2. 12,26 Citrullinated histones, are then released leading to NETosis. Citrullinated H3 (CitH3) has been the most well studied citrullinated histone and is used as a marker for NETosis. 44 In Hazeldine et al, nuclear DNA associated with CitH3 was used as a marker for NETs in trauma. 45 CitH3 was noted to be elevated during the acute period (~first hour following injury) but not at the subsequent time points. 45 This finding suggests that NET formation is only an acute response to injury, absent at the 4 hours post-injury. Gowami et al, used two different CitH3 enzyme-linked immunosorbent assays (ELISAs), a histone-protein calibrated assay (H3Free) and a nucleosome-based assay (H3NUC) to evaluate NETs in trauma patients at 0 and 6 hours following trauma. 44 NETs were found to be significantly elevated at both time points. 44
While there is compelling data supporting CitH3 as a marker for NET formation in trauma patients, there is also some data to the contrary. In a small observational study with 7 trauma patients, Hirose et al found no elevation of CitH3 or NET formation at the time of ICU admission. 46 Of note, cf-DNA levels were not used as a surrogate for NETs and plasma levels did not correlate with NET formation on IHC staining. Pan et al detected NETs in a 30% controlled hemorrhage mouse model. 47 However, no CitH3 was detected, whereas it was detected in a lipopolysaccharide (LPS) sepsis/endotoxemia model. 47
While CitH3 was quantified using an ELISA, the assay differences may account for the differences in CitH3 levels in the studies. 47 This raises further questions regarding the involvement of CitH3 in NET formation, and the most appropriate assays for quantification. Additionally, there is conflicting data regarding the time course of trauma-induced NETosis that requires further clarification.
Recently, Li and Alam’s group have developed a novel CitH3 antibody. This antibody differs from commercial CitH3 antibody in that it specifically binds to CitH3 released during sepsis. Anti-CitH3 antibodies generated from different CitH3 antigens with varying numbers of citrullines on N-terminal histone H3 can yield different results. 48 The newly developed CitH3 monoclonal antibody (4 Cit) recognizes four citrulline sites: H3 citrullinated R2+R8+R17+R26, while the conventional CitH3 monoclonal antibody (3 Cit) is only designed to identify three citrulline sites: H3 citrullinated R2+R8+R17. Since H3 R26 is a target for PAD2, its addition to 4 Cit leads to a higher likelihood of binding and increased neutralization of CitH3. The additional citrulline recognition site is a likely explanation of the CitH3 discrepancies noted in prior studies given the different assays in each study. As studies have demonstrated that there are both citrullinated and non-citrullinated NETs49 and suicidal and vital NETosis34, the type of NET and the role of CitH3 as a biomarker in trauma remains to be explored.
PADs in Trauma and Hemorrhage
While there are five isozymes of PAD, only PAD250 and PAD424 have been demonstrated to generate CitH3 and induce NETosis. There is relatively little data evaluating PAD activity in trauma. However, given the possible roles of CitH3 and NET formation in trauma, the effects of PADs on citrullination and NETosis in require further investigation.
Studies evaluating PAD in hemorrhagic shock have been carried out in animal models. He et al utilized a rat model of 55% blood volume loss (severe hemorrhagic shock) to assess the effects of YW3–56, a pan-PAD inhibitor. 51 YW3–56 treatment led to a significantly higher 12-hour survival rate (60% treatment vs 20% control). The treatment group was also noted to have less severe acute lung injury (ALI) and attenuated pulmonary MPO levels. Furthermore, in vitro studies demonstrated that macrophages exposed to hypoxia and reoxygenation had increased viability and decreased pro-inflammatory cytokine production following treatment. This was the first study to specifically assess the role of PADs in hemorrhage and in post-hemorrhage ALI. As a follow up, the group evaluated the role of PAD2 in hemorrhagic shock using a similar model in mice. 52 Zhou et al demonstrated a 100% survival of PAD2-/- mice compared to 0% of WT mice at 7 days. 52 A potential mechanism for the protective nature of PAD2 knockout (KO) is cardioprotection. This hypothesis was evaluated in a non-hemorrhage myocardial infarction model, which demonstrated a smaller infarct size in PAD2-/- mice compared to WT and significantly different β-catenin levels, a pro-survival pathway protein. 52 Similarly, this study also redemonstrated an attenuated ALI in PAD2-/- mice. To date, this is the only study that has shown the specific effects of PAD2 in hemorrhagic shock.
Given the known role of PAD4 in NETosis, Biron et al evaluated PAD4 deficiency in a dual insult hemorrhage and cecal ligation and puncture (CLP) model. 53 Mice underwent hemorrhage targeted to a mean arterial pressure (MAP) of 35mmHg followed by CLP 24 hours later to produce sepsis. This model demonstrated a significantly better 14-day survival in PAD4-/- mice (94%) compared to the wild type mice (WT) (59%). In addition, neutrophils from the PAD4-/- mice were unable to undergo NETosis following stimulation. While PAD4 deficiency theoretically leads to increased susceptibility to infections, Biron et al found a decreased bacterial load in the peritoneal cavity in PAD4-/- mice. 53 Similarly, there were decreased pro-inflammatory cytokine levels (IL-6 and TNF-α), MPO, and attenuated ALI in the PAD4-/- mice . The survival differences noted in this study are contrary to a prior study by Martinod et al evaluating sepsis only models in PAD4-/- mice. 54 Their study demonstrated no significant differences in survival rates between PAD4-/- and WT mice in both a high and low-grade CLP. 54 The survival outcome in Biron et al53 is similar to the other sepsis models47 and the He et al51 study. However, both He et al51 and Pan et al47 used a pan-PAD inhibitor instead of KO animals, which notably also inhibits PAD2. Given the conflicting findings, the pathophysiology of the two-hit hemorrhage and sepsis model and PADs remains to be further clarified. The addition of hemorrhagic shock prior to CLP may account for the differences in outcomes among the studies. Further follow up with selective PAD inhibitors and PAD KO in hemorrhagic shock models should be the next step in interpreting these findings.
Trauma-induced ALI is a well-known phenomenon following severe injuries that can progress to ARDS. This can lead to significant morbidity, need for mechanical ventilation, and a prolonged ICU stay. 55 While the pathology is poorly understood at this time, the hypothesized pathogenesis of ALI is associated with the SIRS resulting from a major non-pulmonary injury. The histologic findings of ALI are characterized by activated neutrophils, diffuse alveolar damage, pulmonary edema, fibrin membranes, and microthrombi. Prior studies have demonstrated that sepsis-induced ALI is linked to PAD, with a decreased in pulmonary vascular dysfunction following treatment with a PAD inhibitor YW3–56. 56 .These findings are consistent with the hemorrhagic shock models above demonstrating decreased ALI following inhibition of PAD. Of note, there are varying outcomes in previous articles regarding the changes in ALI in PAD4-/- mice following sepsis. 53,54 Both hemorrhagic shock models above were found to have decreased ALI in the PAD KO or inhibited mice. While this does not fully elucidate the mechanisms by which PAD affects post-traumatic ALI, it does demonstrate involvement of deimination in the pathogenesis of ALI. Furthermore, PAD mediated endothelial dysfunction may be a mechanism for future investigation in evaluating post-trauma ALI.
Coagulation
Coagulation and hemostasis are critical components of trauma induced coagulopathy in the early stages of trauma. 57 In the later phases of trauma, patients are at high risk for deep venous thromboses (DVTs) due to the concurrent presence of all three prongs of Virchow’s triad: hypercoagulable state, endothelial damage, and stasis. The activation of endothelial adhesions and the coagulation cascade also plays a role in the systemic inflammation following trauma. 3 Platelet surface immune receptors, adhesion molecules, and release of a diverse range of platelet derived granules make platelets a key immune modulator in trauma. 58 While there are compelling questions for investigation regarding the role of PAD in trauma induced coagulopathy or thrombosis, there are no current models evaluating this question.
Studies have noted that citrullinated histones and NETosis are pro-thrombotic and associated with DVTs. 59 Fuchs et al showed that the fibrous mesh of NETs provided an intravascular scaffold for platelet adherence. 59 Following an in vitro perfusion of NETs with platelet-rich-plasma, the adherent mesh was visualized on electron microscopy and was notably degraded by DNase treatment. Histones H3 and H4 were found to stimulate platelet aggregation. Furthermore, they demonstrated that von Willebrand Factor (vWF), fibronectin, and fibrinogen bind to the NET. Finally in a baboon model of iliac venous thrombosis, cf-DNA, as surrogate for NETs, was elevated from 2 to 6 days following DVT. 59 These results provide early evidence that NETs contribute to thrombus stabilization.
Given the role of NETs and histones in DVT formation, Martinod et al evaluated the role of PAD4 in thrombosis. PAD4-/- mice were found to have decreased early thrombus formation at 6 hours following inferior vena cava (IVC) ligation compared to WT mice. At 48 hours, fewer than 10% of IVCs in the PAD4-/- mice had thrombus compared to 90% in WT mice (p=0.0002). Infusion of WT neutrophils was then found to restore the thrombosis in PAD4-/- mice. Several studies have begun to evaluate the mechanisms behind the PAD associated thrombosis (Figure 2A). Platelet receptors toll-like receptor 2 (TLR2) and 4 (TLR4) have been shown to mediate histone induced platelet aggregation. 60 NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) has also been found to mediate NETosis and lead to a significantly decreased thrombus progression when inhibited in the IVC ligation model used by the Martinod et al group. 61 In a virus induced NETosis in vivo mouse model, Ai et al demonstrated that pre-treatment with a pan-PAD inhibitor, BB-Cl-Amidine, decreased lung fibrin and thrombus. 62 Sorvillo et al63 assessed the role of a disintegrin and metalloproteinase with thrombospondin typ-1 motif-13 (ADAMTS13), a key enzyme in cleavage or breakdown of vWF. They found that injection of recombinant human PAD4 (rhPAD4) led to formation of vWF platelet strings and that PAD4 citrullinates ADAMTS13. Citrullinated recombinant human ADAMTS13 was then unable to cleave vWF platelet strings in vitro demonstrating that PAD4 mediated citrullination impairs ADAMTS13 activity. In a mesenteric venous injury model, rhPAD4 injection accelerated formation of thrombi in the injured vein, further supporting the role of PAD4 in thrombus formation. 63 In contrast to other studies, Damaiana et al specifically evaluated the effects of PAD2 in addition to PAD4, finding that both PAD2 and PAD4 citrullinate fibrinogen. 64 However, citrullination by PAD2, but not PAD4, was noted to inhibit fibrin polymerization and fibrin fiber diameter in clots. The functional effects of the altered fibrin network remain to be assessed.
FIGURE 2. The role of PADs and NETs in injury.
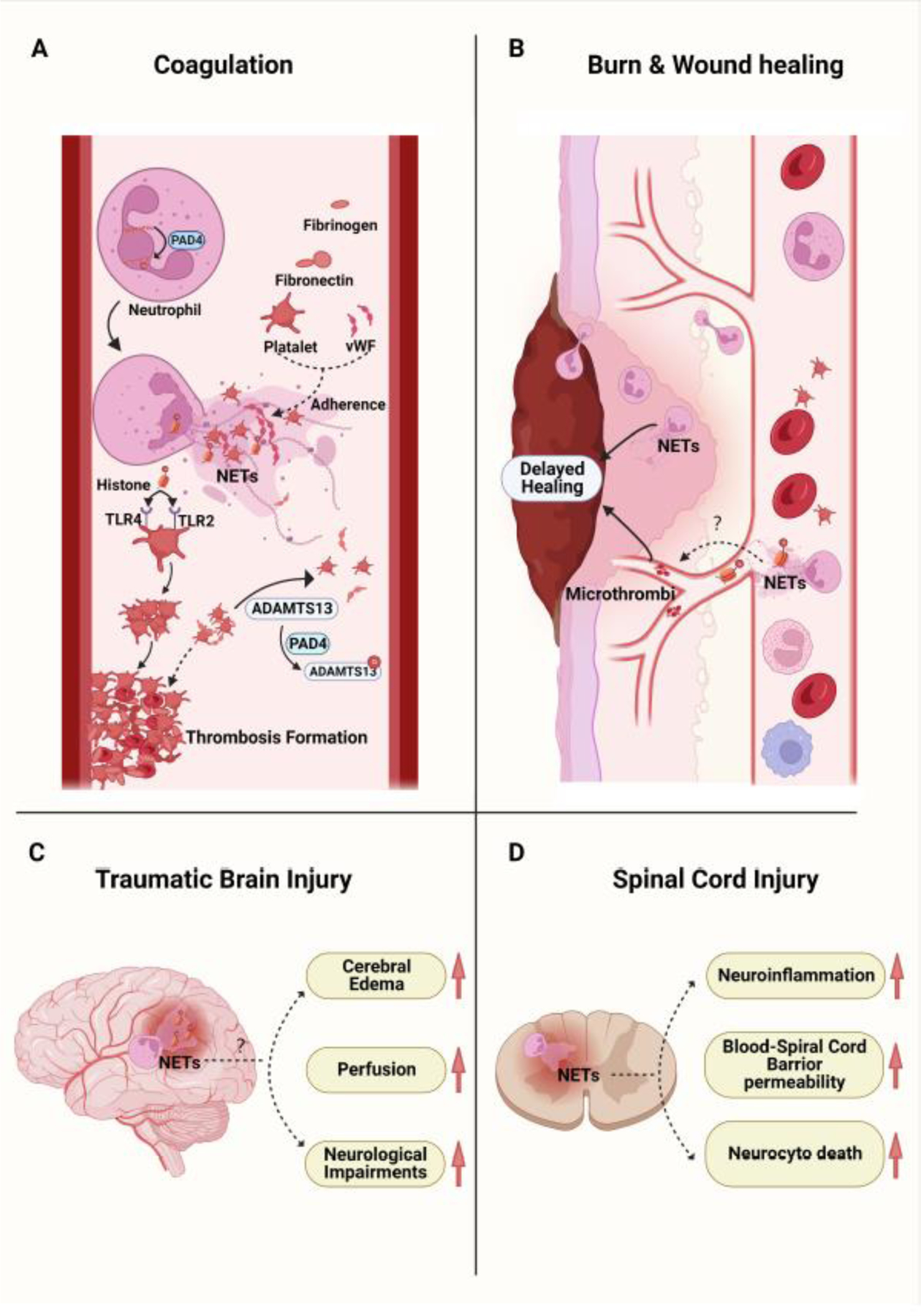
A. Peptidylarginine deiminase 4 (PAD4) mediates the citrullination of histones in neutrophils resulting in NETosis. PADs and NETs have been demonstrated to facilitate the formation of thrombosis after trauma. NETs emit a fibrous mesh that provides an intravascular scaffold for platelets, von Willebrand Factor (vWF), fibronectin, and fibrinogen adherence. Histones released during NETosis induce platelet aggregation through interactions with toll-like receptor 2 (TLR2) and 4 (TLR4) on platelets. PAD4 released during NETosis citrullinates ADAMTS13 impairing its ability to cleave vWF-platelet strings. B. Following a burn or wound, neutrophils infiltrate into the wound tissue and undergo NETosis, which can impair wound healing. A proposed mechanism is through a decreased collagen I to III ratio (higher proportion of immature fibers, collagen III) with poor wound alignment and the formation of microthrombi. C and D. NETosis has been observed in the damaged tissue after traumatic brain injury (TBI) and spinal cord injury (SCI). While PAD inhibitors or DNase1 have been shown to alleviate the local tissue damage, the role of PADs and NETs in TBI and SCI remain to be elucidated.
While the thrombus models are not directly trauma-induced, venous ligation and thrombus formation are relevant to injury. This raises many exciting questions regarding the contribution of PAD to the trauma induced coagulopathy and subsequent hypercoagulable state.
Burn and Wound Healing
Severe burn injuries can lead to significant morbidity and mortality. Burns can lead to tissue damage, inhalation injury, multiorgan failure, and sepsis. Like trauma, burns are also known to cause SIRS and innate immune cell activation leading to electrolyte imbalances and increased cellular metabolism. 65
The understanding of NETosis and the role of PADs in burn injuries is relatively limited at this time (Figure 2B). Similar to in trauma and sepsis, cf-DNA has also been evaluated as a potential biomarker in severely injured burn patients. 66–68 In an early study, Altrichter et al measured cf-DNA levels in patients with at least 25% total body surface area (TBSA) of second-degree burns. 66 There was detectable cf-DNA throughout the 7 days post-injury with the highest level on post-injury day 1. Hampson et al performed a prospective study including patients with ≥15% TBSA burn finding a reduced neutrophil oxidative burst capacity and phagocytosis following injury. 67 The cf-DNA levels were elevated following injury with higher cf-DNA levels in patients who subsequently became septic compared to those who did not. This study confirmed NETosis using the combination of cf-DNA and CitH3. It is notable that the peak cf-DNA levels appear to be post-burn day 7–21 with CitH3 only detected in septic patients. 67 The circulating cf-DNA levels are not specific to NETs and do not provide conclusive evidence for NETosis. 69 Furthermore, the CitH3 may be associated with sepsis rather than the burn injury itself. 67 Kaufman et al used nucleosomes and human neutrophil elastase-DNA complexes (HNE-DNA) as measures of NETosis. Both nucleosomes and HNE-DNA were elevated on admission day 1 and day 4, ultimately with poor correlation with each other and TBSA level. 70 These findings further suggest that while cf-DNA is elevated following burn, the presence of NETosis is not clearly confirmed.
NETosis in burns was further investigated by Korkmaz et al in both rat and swine burn models. 71 NETs were identified using serial staining of CD31 for thrombi, MPO, and CitH3 in the burn wound tissue. In both rats and swine, NETs were identified for up to 60 days post-burn in swine. NETs associated with thrombi were also observed in the burn wounds of severely injured patients. As burns are also associated with a burn-induced hypercoagulable state, microthrombi have been hypothesized to worsen tissue necrosis leading to progression of wounds. 72 This study demonstrates an association of NETs with microthrombi, however the question remains as to the relationship of NETs to the microthrombi. The more precise role of whether PAD activity leads to microthrombi formation versus NETosis as a result of the pre-existing hypercoagulable state is unknown.
Heuer et al used a combination of PAD4-/- and DNaseI-/- mice in both laparotomy and burn wound healing to evaluate both primary and secondary wound healing. 73 PAD4-/- burn wounds closed by secondary intention were noted to have fewer NETs and faster wound closure compared to WT mice. Treatment with DNaseI, previously shown to dissolve NETs, 16 also improved burn wound healing73. Furthermore, there was an increased collagen I to III ratio and alignment in burn scars demonstrating improved stability and tensile strength of the wound following DNaseI treatment and PAD4KO. Healing by primary intention was also noted to be accelerated in PAD4-/- mice. 73 This highlights the connection of PAD4 to NETosis in burn and wound healing. These findings are in line with previous studies in diabetic foot ulcers demonstrating that NETosis impairs wound healing and PAD4 inhibition accelerates wound healing. 74,75
Surolia et al investigates PAD4 in ALI following chemical burn injuries using both PAD4-/- mice and treatment with GSK484, a selective PAD4 inhibitor. 76 That study demonstrated that ALI was attenuated in PAD4-/- and GSK484, with the conclusion that PAD4 plays an essential role in burn-induced ALI. CitH3 spillage in the lungs was measured and noted to be significantly increased in the WT mice compared to PAD4-/- with positive correlation to the degree of lung injury. 76 This finding is in line with prior studies demonstrating the role of NETosis in trauma related ALI. While it is unclear whether burn induced ALI has the same mechanism as trauma induced ALI, this study confirms the prior findings in a burn model.
Ultimately, while there is compelling evidence demonstrating the presence of NETosis and PAD activation in wound healing, burns, and burn-induced ALI, further studies are required to continue exploring the mechanisms and effects of PAD inhibition.
Traumatic Brain Injury
TBI affects millions of Americans each year and is a leading cause of trauma related deaths. 77 Furthermore, TBI can lead to significant morbidity and future disability for patients. Primary treatment for TBI has remained elusive, with the TBI management largely focused on mitigating the secondary brain injury. Thus, further investigating the cellular mechanisms that affect TBI remain critically important to finding effective novel therapies.
Post-translational histone modification and epigenetic changes have long been demonstrated in TBI. 78 However, very little is known about histone deimination and NETosis in the brain and in TBI (Figure 2C). Studies have recently begun to evaluate the role of NETs in stroke-related ischemic brain injury79,80 with few studies focused on TBI. As SIRS develops following trauma, the brain is also affected. The disruption of the blood-brain barrier (BBB) in trauma allows for the infiltration of neutrophils leading to inflammatory changes and microcirculatory dysfunction. 81 As in other pathologies, the neutrophil activation and resulting inflammatory response has been implicated in the worsening of TBI outcomes. 81
Using a controlled cortical impact TBI model in mice, Vaibhav et al demonstrated that NETs were visualized in the contusional and peri-contusional regions of the brain with co-localized CitH3 and NE. 82 DNaseI levels in severe TBI patients were inversely correlated with intracranial pressure and circulating NETs. 82 Administration of recombinant human DNaseI (rhDNaseI) led to reduced cerebral edema, improved perfusion, and improved functional neurologic outcomes at two months following TBI. TLR4 expression was associated with decreased circulating DNaseI levels, NET formation, and decreased NET degradation. 82
Prior stroke models have demonstrated that PAD4 can lead to citrullination and is associated with NET formation in the brain. 83 Kang et al demonstrated increased PAD4 mRNA and protein expression and a significant increase in CitH3 and BBB permeability in ischemic areas. 83 PAD4 overexpressing mice were also found to have more severe functional neurologic impairments. Compared to WT, PAD4-/- mice had no significant differences in brain lesion size but was noted to have fewer CitH3+ neutrophils. This correlation of PAD4 to NETosis in ischemic stroke leads to the possibility that PAD4 may also play a role in TBI. Zeng et al found that NETs were also detected in patients following subarachnoid hemorrhage (SAH) with plasma CitH3 levels correlating with the severity of SAH84. In assessing the role of PAD4, GSK484 administration led to decreased brain edema, fewer functional neurologic impairments, and better performance on the Morris water maze test. 84 In concordance to prior studies in brain injury and other pathologies, administration of DNaseI was able to reduce brain edema and the neuroinflammatory response.
As with trauma and burn, TBI has been linked to a secondary ALI. Gu et al demonstrated the NET involvement in the pathophysiology of TBI induced ALI (TBI-ALI) through a severe TBI model in mice. 85 This study evaluates the protein S100B as a possible regulator of TBI-ALI finding that inhibition of S100B leads to decreased NET formation in the lungs and severity of ALI.
At this time, there are no studies directly examining PAD and TBI. Thus, prior studies examining PADs in brain injury leave questions regarding their applicability to TBI.
Other Injuries
The pathophysiology of NETs in spinal cord injuries (SCIs) was studied by Feng et al. 86 The SCI was induced using a 60 second compression of the spinal cord through a laminectomy. Neutrophils were identified at the region of SCI with NET production at 24 hours. Cl-amidine, a pan-PAD inhibitor reduced NETs, decreased neuroinflammation, neuronal cell death, scar formation, and blood-spinal cord barrier permeability. Furthermore, motor function was significantly improved following Cl-amidine treatment. These findings are consistent with other trauma pathologies as PADs are demonstrated to cause inflammation in many organ systems.
Future Therapies and Perspectives
Post-injury SIRS is a critically important aspect to target as we continue to work towards development of novel therapies in trauma. The rapid response of the innate immune system often leads to further secondary damage, and potential worsening of tissue injury.
Cell-Free DNA
Identification of NETosis was quantified in early studies using circulating cf-DNA levels as a surrogate and a potential biomarker for predicting clinical outcomes. 37,39,66 While cf-DNA is non-specific and should not serve as a marker for NETosis without a confirmatory study, it may have potential as a clinical biomarker. Cf-DNA has also been noted in numerous studies assessing NETosis in other pathologies including septic arthritis and aortic aneurysms, which is likely to affect its utility as a biomarker. 87,88 However, data demonstrating that mt-DNA is the major component of a NET may make cf-mtDNA a better marker for NETosis in trauma. Altrichter et al also noted significantly higher cf-DNA levels in non-survivors of a major burn. 66 Macher et al found that TBI and polytrauma patients present with an elevated cf-DNA. A larger ratio of decrease in cf-DNA levels over the first 24 hours was significantly correlated with survival. Furthermore, the cf-DNA levels were correlated with Injury Severity Score (ISS). While there are some conflicting data, taken together, these studies indicate that cf-DNA may be promising as a marker that correlates with post-traumatic outcomes and sepsis. 37,39,66
Cell-free Mitochondrial DNA
MtDNA has also been demonstrated as the main DNA component in NETs following trauma, surgery, or tissue injury. Plasma cf-mtDNA can be measured using quantitative polymerase chain reaction (qPCR), which allows for a relatively timely result. Gu et al used qPCR to measure plasma mtDNA concentration in trauma patients within 2 hours of admission4. The mtDNA level correlated with the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, ISS, and neutrophil to lymphocyte ratio (NLR). Elevated admission plasma mtDNA levels were found to predict the development of post-traumatic SIRS with an area under the curve (AUC) of 0.725 (95% CI 0.613–0.837). This further demonstrates the potential for mtDNA as a promising clinical biomarker.
CitH3
CitH3 has potential as both a biomarker and a therapeutic target given its strong correlation with NETosis and injury. 47,86 CitH3 has been studied in a variety of pathologies including pancreatitis, 89 aortic dissection, 90 and COVID-1991 as a marker with possible prognostic value. CitH3 has previously been studied as either an independent or confirmatory test for NETosis reflecting inflammation in most studies including for SCI92 and burn. 73,93 A major benefit to CitH3 is that it has been demonstrated in a sepsis/endotoxemia model to be detectable early, within 30 minutes of LPS injection. 47 As previously noted, there is some conflicting evidence regarding the elevation of CitH3 in non-septic insults given the lack of CitH3 noted in hemorrhagic shock. 47 Thus, future studies should continue to evaluate the appropriate usage of CitH3 as a diagnostic or predictor in trauma patients. CitH3 has also been demonstrated to localize to regions of injury and can also be further evaluated as a therapeutic target. 73,86
As previously discussed, each assay’s antibodies’ citrulline binding sites may account for some differences in CitH3 levels among studies. The recently developed antibody 4 Cit has been used to detect elevated CitH3 levels in both non-infectious and infectious shock with higher levels in infectious shock89,94. These results suggest that the 4 Cit antibody is a promising tool to accurately distinguish between infectious and non-infectious inflammatory responses. The clinical and pre-clinical utility of the 4 Cit antibody in early identification of sepsis remains to be further explored. However, it has potential in differentiating between post-traumatic sepsis versus ongoing post-injury SIRS. Initiating earlier treatments for sepsis could lead to decreased morbidity including decreasing mechanical ventilation days, ICU length of stay, and hospital length of stay.
PAD2 and PAD4
PAD4 and PAD2, the major mediators of NET formation have been studied using KO mice and various PAD inhibitors. PAD inhibitors can be selective or non-selective. Both PAD2 and PAD4 have been studied extensively in sepsis and other pathologies. 12 However, the role of PAD2 and PAD4 in trauma are relatively poorly defined at this time. They are clearly associated with the post-traumatic SIRS and with improved injury outcomes following KO or inhibition. PAD is a compelling target for future therapeutics. In particular, selective PAD inhibition would allow for potentially fewer collateral side effects. Furthermore, PAD inhibitors have been recently delivered in the form of targeted intravascular nanoparticles for atherosclerosis95 and cancer therapy96. This may have exciting potential for the development of novel therapies for various pathological processes, including post-traumatic inflammation.
DNaseI
DNaseI is a pancreatic enzyme present in physiologic conditions that digests DNA97. During the early discovery of NETs, Brinkmann et al noted that the NETs were degraded by DNaseI. 16 Given the detrimental effect of NETs in sepsis and trauma, DNaseI has been investigated as both a potential diagnostic and therapeutic tool. Meng et al demonstrated that DNaseI levels were significantly elevated on admission and higher in major trauma patients who developed sepsis. 38 There was no significant difference noted in the days following admission, making it a potential early outcome predictor. 38 DNaseI has also been re-demonstrated to degrade NETs in a thrombus59 and in burns. 73 In both TBI82 and burn, 73 treatment with DNaseI improved outcomes. DNaseI knockout was congruently noted to have impaired degradation of NETs with worsened outcomes. 73 Studies have demonstrated that circulating cf-mtDNA can also be digested by DNaseI98 making it a stronger potential treatment for trauma given the known release and inflammatory potential of mtDNA.
ADAMTS13
ADAMTS13 has been found to be affected by PAD4 through citrullination. 63 Citrullinated ADAMTS13 has been noted to be higher in septic patients compared to controls. 63 Treatment with rhADAMTS13 reversed citrullinated ADAMTS13’s impaired ability to cleave platelet strings and reduced NET formation. Currently, there are no studies evaluating ADAMTS13 citrullination in trauma patients. However, the role of ADAMTS13 in trauma has been investigated. Kleinveld et al demonstrated that increasing ISS’ were associated with higher vWF and lower ADAMTS13 levels. 99 Additionally lower ADAMTS13 levels were demonstrated in patients who subsequently developed MOF. In a rat model of hemorrhagic shock, administration of rhADAMTS13 was noted to limit organ injury and microthrombi formation in the lungs, kidneys, and liver. 99 These studies demonstrate the future potential of ADAMTS13 as a biomarker and potential therapeutic target. Additionally, further studies should continue to evaluate the interaction between PAD4 and ADAMTS13, particularly in trauma.
Conclusion
Post-traumatic inflammation results in a significant inflammatory response activating the innate immune system. PADs and NET formation are increasingly being studied in the setting of trauma and tissue injury. The evidence suggests that excessive NET formation leads to detrimental effects in a variety of conditions including trauma. At this time, many questions remain regarding the mechanisms of the PAD to NETosis pathway and its clinical applications. Given the current body of work, there are many potentially promising directions for developing future diagnostics and therapeutic tools.
Acknowledgements
This work was funded by grants from the National Institute of Health R01 (Grant# RHL155116A) to YL and HA, F32 (Grant #1F32HL162378–01) to JH, and the Joint of-Institute (Grant# U068874) to YL. Figures 1 and 2 are created with BioRender.com.
References
- 1.Rhee P, Joseph B, Pandit V, et al. Increasing trauma deaths in the United States. Ann Surg Jul 2014;260(1):13–21. doi: 10.1097/sla.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 2.10 Leading Causes of Death, United States Centers for Disease Control and Prevention. Accessed 16 August 2022, https://wisqars.cdc.gov/cgi-bin/broker.exe
- 3.Yao YM, Redl H, Bahrami S, Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res May 1998;47(5):201–10. doi: 10.1007/s000110050318 [DOI] [PubMed] [Google Scholar]
- 4.Gu X, Yao Y, Wu G, Lv T, Luo L, Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS One 2013;8(8):e72834. doi: 10.1371/journal.pone.0072834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm 2010;2010:642462. doi: 10.1155/2010/642462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon JW. Hemorrhagic Shock. N Engl J Med Jan 25 2018;378(4):370–379. doi: 10.1056/NEJMra1705649 [DOI] [PubMed] [Google Scholar]
- 7.Bicker KL, Thompson PR. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers Feb 2013;99(2):155–63. doi: 10.1002/bip.22127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta Oct 2013;1829(10):1126–35. doi: 10.1016/j.bbagrm.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu D, Zhang Y, Wang S. Histone citrullination: a new target for tumors. Mol Cancer Jun 11 2021;20(1):90. doi: 10.1186/s12943-021-01373-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Bañuelos E, Shi J, Wang H, et al. ‘Heavy chain constant region usage in antibodies to peptidylarginine deiminase 4 distinguishes disease subsets in rheumatoid arthritis’. Arthritis Rheumatol Jun 8 2022;doi: 10.1002/art.42262 [DOI] [PMC free article] [PubMed]
- 11.Kim Y, Rebman AW, Johnson TP, et al. Peptidylarginine Deiminase 2 Autoantibodies Are Linked to Less Severe Disease in Multiple Sclerosis and Post-treatment Lyme Disease. Front Neurol 2022;13:874211. doi: 10.3389/fneur.2022.874211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian Y, Qu S, Alam HB, et al. Peptidylarginine deiminase 2 has potential as both a biomarker and therapeutic target of sepsis. JCI Insight Oct 15 2020;5(20)doi: 10.1172/jci.insight.138873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du M, Yang L, Gu J, Wu J, Ma Y, Wang T. Inhibition of Peptidyl Arginine Deiminase-4 Prevents Renal Ischemia-Reperfusion-Induced Remote Lung Injury. Mediators Inflamm 2020;2020:1724206. doi: 10.1155/2020/1724206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ham A, Rabadi M, Kim M, et al. Peptidyl arginine deiminase-4 activation exacerbates kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol Nov 1 2014;307(9):F1052–62. doi: 10.1152/ajprenal.00243.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czerwińska J, Kasprowicz-Furmańczyk M, Placek W, Owczarczyk-Saczonek A. Changes in Tumor Necrosis Factor α (TNFα) and Peptidyl Arginine Deiminase 4 (PAD-4) Levels in Serum of General Treated Psoriatic Patients. Int J Environ Res Public Health Jul 18 2022;19(14)doi: 10.3390/ijerph19148723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science Mar 5 2004;303(5663):1532–5. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Li P, Tian Y, et al. Peptidylarginine Deiminase 2 in Host Immunity: Current Insights and Perspectives. Front Immunol 2021;12:761946. doi: 10.3389/fimmu.2021.761946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block H, Rossaint J, Zarbock A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction. Cells Jun 14 2022;11(12)doi: 10.3390/cells11121919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Zhao Y, Lai D, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis May 22 2018;9(6):597. doi: 10.1038/s41419-018-0538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grégoire M, Uhel F, Lesouhaitier M, et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur Respir J Aug 2018;52(2)doi: 10.1183/13993003.02590-2017 [DOI] [PubMed] [Google Scholar]
- 21.Matta B, Battaglia J, Barnes BJ. Detection of neutrophil extracellular traps in patient plasma: method development and validation in systemic lupus erythematosus and healthy donors that carry IRF5 genetic risk. Front Immunol 2022;13:951254. doi: 10.3389/fimmu.2022.951254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIlroy DJ, Jarnicki AG, Au GG, et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care Dec 2014;29(6):1133.e1–5. doi: 10.1016/j.jcrc.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 23.Kim HI, Park J, Konecna B, et al. Plasma and wound fluids from trauma patients suppress neutrophil extracellular respiratory burst. J Trauma Acute Care Surg Feb 1 2022;92(2):330–338. doi: 10.1097/ta.0000000000003461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol Feb 1 2008;180(3):1895–902. doi: 10.4049/jimmunol.180.3.1895 [DOI] [PubMed] [Google Scholar]
- 25.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med Aug 30 2010;207(9):1853–62. doi: 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Deng Q, Pan B, et al. Inhibition of PAD2 Improves Survival in a Mouse Model of Lethal LPS-Induced Endotoxic Shock. Inflammation Aug 2020;43(4):1436–1445. doi: 10.1007/s10753-020-01221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan CY, Zhang J, Wu HL, Li T, Liu LM. Regulatory mechanisms, prophylaxis and treatment of vascular leakage following severe trauma and shock. Mil Med Res 2017;4:11. doi: 10.1186/s40779-017-0117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord JM, Midwinter MJ, Chen YF, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet Oct 18 2014;384(9952):1455–65. doi: 10.1016/s0140-6736(14)60687-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F, Chipman A, Pati S, Miyasawa B, Corash L, Kozar RA. Resuscitative Strategies to Modulate the Endotheliopathy of Trauma: From Cell to Patient. Shock May 2020;53(5):575–584. doi: 10.1097/shk.0000000000001378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazeldine J, Dinsdale RJ, Harrison P, Lord JM. Traumatic Injury and Exposure to Mitochondrial-Derived Damage Associated Molecular Patterns Suppresses Neutrophil Extracellular Trap Formation. Front Immunol 2019;10:685. doi: 10.3389/fimmu.2019.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury Dec 2014;45(12):1824–33. doi: 10.1016/j.injury.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 32.Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery Aug 1995;118(2):358–64; discussion 364–5. doi: 10.1016/s0039-6060(05)80345-9 [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Yang X, Chatterjee V, Meegan JE, Beard RS Jr., Yuan SY. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front Immunol 2019;10:1037. doi: 10.3389/fimmu.2019.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yipp BG, Kubes P. NETosis: how vital is it? Blood Oct 17 2013;122(16):2784–94. doi: 10.1182/blood-2013-04-457671 [DOI] [PubMed] [Google Scholar]
- 35.Pilsczek FH, Salina D, Poon KK, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol Dec 15 2010;185(12):7413–25. doi: 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- 36.Hamaguchi S, Hirose T, Akeda Y, et al. Identification of neutrophil extracellular traps in the blood of patients with systemic inflammatory response syndrome. J Int Med Res Feb 2013;41(1):162–8. doi: 10.1177/0300060513475958 [DOI] [PubMed] [Google Scholar]
- 37.Meng XY, Lu QY, Zhang JF, et al. A Novel Animal Model of Primary Blast Lung Injury and Its Pathological Changes in Mice. J Trauma Acute Care Surg Mar 8 2022;doi: 10.1097/ta.0000000000003571 [DOI] [PMC free article] [PubMed]
- 38.Meng W, Paunel-Görgülü A, Flohé S, et al. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm 2012;2012:149560. doi: 10.1155/2012/149560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock Oct 2008;30(4):352–8. doi: 10.1097/SHK.0b013e31816a6bb1 [DOI] [PubMed] [Google Scholar]
- 40.Thurairajah K, Briggs GD, Balogh ZJ. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur J Trauma Emerg Surg Jun 2018;44(3):325–334. doi: 10.1007/s00068-018-0954-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons JD, Lee Y-L, Mulekar S, et al. Elevated Levels of Plasma Mitochondrial DNA DAMPs Are Linked to Clinical Outcome in Severely Injured Human Subjects. Annals of Surgery 2013;258(4):591–598. doi: 10.1097/SLA.0b013e3182a4ea46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam NY, Rainer TH, Chiu RW, Joynt GM, Lo YM. Plasma mitochondrial DNA concentrations after trauma. Clin Chem Jan 2004;50(1):213–6. doi: 10.1373/clinchem.2003.025783 [DOI] [PubMed] [Google Scholar]
- 43.McIlroy DJ, Bigland M, White AE, et al. Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma surgery. J Trauma Acute Care Surg Feb 2015;78(2):282–8. doi: 10.1097/ta.0000000000000519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goswami J, MacArthur T, Bailey K, et al. Neutrophil Extracellular Trap Formation and Syndecan-1 Shedding Are Increased After Trauma. Shock Sep 1 2021;56(3):433–439. doi: 10.1097/shk.0000000000001741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazeldine J, Naumann DN, Toman E, et al. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med Jul 2017;14(7):e1002338. doi: 10.1371/journal.pmed.1002338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirose T, Hamaguchi S, Matsumoto N, et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS One 2014;9(11):e111755. doi: 10.1371/journal.pone.0111755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan B, Alam HB, Chong W, et al. CitH3: a reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci Rep Aug 21 2017;7(1):8972. doi: 10.1038/s41598-017-09337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng Q, Pan B, Alam HB, et al. Citrullinated Histone H3 as a Therapeutic Target for Endotoxic Shock in Mice. Front Immunol 2019;10:2957. doi: 10.3389/fimmu.2019.02957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes CL, Shim D, Kernien J, Johnson CJ, Nett JE, Shelef MA. Insight into Neutrophil Extracellular Traps through Systematic Evaluation of Citrullination and Peptidylarginine Deiminases. J Immunol Res 2019;2019:2160192. doi: 10.1155/2019/2160192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spengler J, Lugonja B, Ytterberg AJ, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol Dec 2015;67(12):3135–45. doi: 10.1002/art.39313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He W, Zhou P, Chang Z, et al. Inhibition of peptidylarginine deiminase attenuates inflammation and improves survival in a rat model of hemorrhagic shock. J Surg Res Feb 2016;200(2):610–8. doi: 10.1016/j.jss.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J, Biesterveld BE, Li Y, et al. Peptidylarginine Deiminase 2 Knockout Improves Survival in hemorrhagic shock. Shock Oct 2020;54(4):458–463. doi: 10.1097/shk.0000000000001489 [DOI] [PubMed] [Google Scholar]
- 53.Biron BM, Chung CS, Chen Y, et al. PAD4 Deficiency Leads to Decreased Organ Dysfunction and Improved Survival in a Dual Insult Model of Hemorrhagic Shock and Sepsis. J Immunol Mar 1 2018;200(5):1817–1828. doi: 10.4049/jimmunol.1700639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinod K, Fuchs TA, Zitomersky NL, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood Mar 19 2015;125(12):1948–56. doi: 10.1182/blood-2014-07-587709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakowitz M, Bruns B, McCunn M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand J Trauma Resusc Emerg Med Aug 10 2012;20:54. doi: 10.1186/1757-7241-20-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang Y, Pan B, Alam HB, et al. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. Eur J Pharmacol Aug 15 2018;833:432–440. doi: 10.1016/j.ejphar.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore EE, Moore HB, Kornblith LZ, et al. Trauma-induced coagulopathy. Nature Reviews Disease Primers 2021/April/29 2021;7(1):30. doi: 10.1038/s41572-021-00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol Jun 2013;35(3):254–61. doi: 10.1111/ijlh.12084 [DOI] [PubMed] [Google Scholar]
- 59.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A Sep 7 2010;107(36):15880–5. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood Aug 18 2011;118(7):1952–61. doi: 10.1182/blood-2011-03-343061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A May 21 2013;110(21):8674–9. doi: 10.1073/pnas.1301059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ai P, Pan H, Chen K, Zheng J, Gao Z, Jin G. Viral mimetic poly(I:C) induces neutrophil extracellular traps via PAD4 to promote inflammation and thrombosis. Biochem Biophys Res Commun Aug 6 2021;565:64–71. doi: 10.1016/j.bbrc.2021.05.091 [DOI] [PubMed] [Google Scholar]
- 63.Sorvillo N, Mizurini DM, Coxon C, et al. Plasma Peptidylarginine Deiminase IV Promotes VWF-Platelet String Formation and Accelerates Thrombosis After Vessel Injury. Circ Res Aug 16 2019;125(5):507–519. doi: 10.1161/circresaha.118.314571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Damiana T, Damgaard D, Sidelmann JJ, et al. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin Chim Acta Feb 2020;501:6–11. doi: 10.1016/j.cca.2019.10.033 [DOI] [PubMed] [Google Scholar]
- 65.Mulder PPG, Koenen H, Vlig M, Joosten I, de Vries RBM, Boekema B. Burn-Induced Local and Systemic Immune Response: Systematic Review and Meta-Analysis of Animal Studies. J Invest Dermatol May 24 2022;doi: 10.1016/j.jid.2022.05.004 [DOI] [PubMed]
- 66.Altrichter J, Zedler S, Kraft R, et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur J Trauma Emerg Surg Dec 2010;36(6):551–7. doi: 10.1007/s00068-010-0013-1 [DOI] [PubMed] [Google Scholar]
- 67.Hampson P, Dinsdale RJ, Wearn CM, et al. Neutrophil Dysfunction, Immature Granulocytes, and Cell-free DNA are Early Biomarkers of Sepsis in Burn-injured Patients: A Prospective Observational Cohort Study. Ann Surg Jun 2017;265(6):1241–1249. doi: 10.1097/sla.0000000000001807 [DOI] [PubMed] [Google Scholar]
- 68.Shoham Y, Krieger Y, Perry ZH, et al. Admission cell free DNA as a prognostic factor in burns: quantification by use of a direct rapid fluorometric technique. Biomed Res Int 2014;2014:306580. doi: 10.1155/2014/306580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem Dec 2007;53(12):2215. doi: 10.1373/clinchem.2007.092734 [DOI] [PubMed] [Google Scholar]
- 70.Kaufman T, Magosevich D, Moreno MC, et al. Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clin Immunol Oct 2017;183:254–262. doi: 10.1016/j.clim.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 71.Korkmaz HI, Ulrich MMW, Vogels S, et al. Neutrophil extracellular traps coincide with a pro-coagulant status of microcirculatory endothelium in burn wounds. Wound Repair Regen Aug 2017;25(4):609–617. doi: 10.1111/wrr.12560 [DOI] [PubMed] [Google Scholar]
- 72.Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plast Surg Jul 2007;59(1):109–15. doi: 10.1097/01.sap.0000252065.90759.e6 [DOI] [PubMed] [Google Scholar]
- 73.Heuer A, Stiel C, Elrod J, et al. Therapeutic Targeting of Neutrophil Extracellular Traps Improves Primary and Secondary Intention Wound Healing in Mice. Front Immunol 2021;12:614347. doi: 10.3389/fimmu.2021.614347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med Jul 2015;21(7):815–9. doi: 10.1038/nm.3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fadini GP, Menegazzo L, Rigato M, et al. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes Apr 2016;65(4):1061–71. doi: 10.2337/db15-0863 [DOI] [PubMed] [Google Scholar]
- 76.Surolia R, Li FJ, Wang Z, et al. NETosis in the pathogenesis of acute lung injury following cutaneous chemical burns. JCI Insight May 24 2021;6(10)doi: 10.1172/jci.insight.147564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths Centers for Disease Control and Prevention. Accessed 22 August 2022, https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf
- 78.Bertogliat MJ, Morris-Blanco KC, Vemuganti R. Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem Int Feb 2020;133:104642. doi: 10.1016/j.neuint.2019.104642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li C, Xing Y, Zhang Y, Hua Y, Hu J, Bai Y. Neutrophil Extracellular Traps Exacerbate Ischemic Brain Damage. Mol Neurobiol Jan 2022;59(1):643–656. doi: 10.1007/s12035-021-02635-z [DOI] [PubMed] [Google Scholar]
- 80.Denorme F, Portier I, Rustad JL, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest May 16 2022;132(10)doi: 10.1172/jci154225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci Dec 1995;15(12):8223–33. doi: 10.1523/jneurosci.15-12-08223.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaibhav K, Braun M, Alverson K, et al. Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury. Sci Adv May 2020;6(22):eaax8847. doi: 10.1126/sciadv.aax8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang L, Yu H, Yang X, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun May 19 2020;11(1):2488. doi: 10.1038/s41467-020-16191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeng H, Fu X, Cai J, et al. Neutrophil Extracellular Traps may be a Potential Target for Treating Early Brain Injury in Subarachnoid Hemorrhage. Transl Stroke Res Feb 2022;13(1):112–131. doi: 10.1007/s12975-021-00909-1 [DOI] [PubMed] [Google Scholar]
- 85.Gu Z, Li L, Li Q, et al. Polydatin alleviates severe traumatic brain injury induced acute lung injury by inhibiting S100B mediated NETs formation. Int Immunopharmacol Sep 2021;98:107699. doi: 10.1016/j.intimp.2021.107699 [DOI] [PubMed] [Google Scholar]
- 86.Feng Z, Min L, Liang L, et al. Neutrophil Extracellular Traps Exacerbate Secondary Injury via Promoting Neuroinflammation and Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury. Front Immunol 2021;12:698249. doi: 10.3389/fimmu.2021.698249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michalska M, Grochowiecki T, Jakimowicz T, Nazarewski S. A Review of the Impact of Neutrophils and Neutrophil Extracellular Traps (NETs) on the Development of Aortic Aneurysms in Animal and Human Studies. Med Sci Monit Dec 28 2021;27:e935134. doi: 10.12659/msm.935134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lögters T, Paunel-Görgülü A, Zilkens C, et al. Diagnostic accuracy of neutrophil-derived circulating free DNA (cf-DNA/NETs) for septic arthritis. J Orthop Res Nov 2009;27(11):1401–7. doi: 10.1002/jor.20911 [DOI] [PubMed] [Google Scholar]
- 89.Pan B, Li Y, Liu Y, Wang W, Huang G, Ouyang Y. Circulating CitH3 Is a Reliable Diagnostic and Prognostic Biomarker of Septic Patients in Acute Pancreatitis. Front Immunol 2021;12:766391. doi: 10.3389/fimmu.2021.766391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang S, Xiao Y, Du Y, et al. Diagnostic and Prognostic Value of Neutrophil Extracellular Trap Levels in Patients With Acute Aortic Dissection. Front Cardiovasc Med 2021;8:683445. doi: 10.3389/fcvm.2021.683445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim S, Hong KH, Gu JY, In JW, Ahn MY, Kim HK. High Circulating Levels of Neutrophil Extracellular Traps Parameters Predicting Poor Outcome in COVID-19. Ann Clin Lab Sci May 2022;52(3):374–381. [PubMed] [Google Scholar]
- 92.Lange S, Gögel S, Leung KY, et al. Protein deiminases: new players in the developmentally regulated loss of neural regenerative ability. Dev Biol Jul 15 2011;355(2):205–14. doi: 10.1016/j.ydbio.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laggner M, Lingitz MT, Copic D, et al. Severity of thermal burn injury is associated with systemic neutrophil activation. Sci Rep Jan 31 2022;12(1):1654. doi: 10.1038/s41598-022-05768-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian Y, Russo RM, Li Y, et al. Serum citrullinated histone H3 concentrations differentiate patients with septic verses non-septic shock and correlate with disease severity. Infection Feb 2021;49(1):83–93. doi: 10.1007/s15010-020-01528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molinaro R, Yu M, Sausen G, et al. Targeted delivery of protein arginine deiminase-4 inhibitors to limit arterial intimal NETosis and preserve endothelial integrity. Cardiovasc Res Nov 22 2021;117(13):2652–2663. doi: 10.1093/cvr/cvab074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song S, Gui L, Feng Q, et al. TAT-Modified Gold Nanoparticles Enhance the Antitumor Activity of PAD4 Inhibitors. Int J Nanomedicine 2020;15:6659–6671. doi: 10.2147/ijn.S255546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Love JD, Hewitt RR. The relationship between human serum and human pancreatic DNase I. J Biol Chem Dec 25 1979;254(24):12588–94. [PubMed] [Google Scholar]
- 98.Puyo CA, Peruzzi D, Earhart A, et al. Endotracheal tube-induced sore throat pain and inflammation is coupled to the release of mitochondrial DNA. Mol Pain Jan-Dec 2017;13:1744806917731696. doi: 10.1177/1744806917731696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kleinveld DJB, Simons DDG, Dekimpe C, et al. Plasma and rhADAMTS13 reduce trauma-induced organ failure by restoring the ADAMTS13-VWF axis. Blood Adv Sep 14 2021;5(17):3478–3491. doi: 10.1182/bloodadvances.2021004404 [DOI] [PMC free article] [PubMed] [Google Scholar]


