Abstract
The Aryl Hydrocarbon Receptor (AHR) is a ligand-dependent transcription factor able to control complex transcriptional processes in several cell types, which has been correlated with various diseases, including inflammatory bowel diseases (IBD). Numerous studies have described different compounds as ligands of this receptor, like xenobiotics, natural compounds, and several host-derived metabolites.
Dietary (poly)phenols have been studied regarding their pleiotropic activities (e.g., neuroprotective and anti-inflammatory), but their AHR modulatory capabilities have also been considered. However, dietary (poly)phenols are submitted to extensive metabolism in the gut (e.g., gut microbiota). Thus, the resulting gut phenolic metabolites could be key players modulating AHR since they are the ones that reach the cells and may exert effects on the AHR throughout the gut and other organs. This review aims at a comprehensive search for the most abundant gut phenolic metabolites detected and quantified in humans to understand how many have been described as AHR modulators and what could be their impact on inflammatory gut processes. Even though several phenolic compounds have been studied regarding their anti-inflammatory capacities, only 1 gut phenolic metabolite, described as AHR modulator, has been evaluated on intestinal inflammatory models. Searching for AHR ligands could be a novel strategy against IBD.
Keywords: AHR, Dietary (poly)phenols, Microbiota, Inflammatory bowel disease
Graphical abstract
Abbreviations
- 3MI
Skatole
- 5-HT
Serotonin
- AHR
Aryl Hydrocarbon Receptor
- AHRR
AHR Repressor
- ARNT
AHR Nuclear Translocator
- BCL-2
B-cell lymphoma 2
- BMDCs
Primary bone marrow-derived dendritic cells
- BMDMs
Mouse bone marrow derived macrophages
- Caco-2
Human colorectal adenocarcinoma cell line
- CD
Crohn's disease
- Cldn4
Claudin 4
- COX-2
Cyclooxygenase-2 c-SRC Proto-oncogene tyrosine-protein kinase Src
- CUL4B
Cullin 4 B
- CXCL1
Chemokine (C-X-C motif) ligand 1
- CYP
Cytochrome P450
- CYP1A1
Cytochrome P450 Family 1 Subfamily A Member 1
- CYP1A2
Cytochrome P450 Family 1 Subfamily A Member 2
- DC
Dendritic cells
- DHNA
1,4-dihydroxy-2-naphthoic acid
- DOPAC
3′,4′-dihydroxyphenylacetic acid
- HO-1
Heme oxygenase-1
- HSP90
Heat shock protein 90
- IA
Indole-3-aldehyde
- IAA
Indole-3-acetate
- IAC
Indole-3-acrylate
- IAD
Indole-3-acetamide
- IBD
Inflammatory bowel disease
- IECs
Intestinal epithelial cells
- IET
Indole-3-ethanol
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- ILA
Indole-3-lactate
- ILCs
Innate lymphoid cells
- IND
Indole
- IPA
Indole-3-propionate
- IPY
Indole-3-pyruvate
- KLF6
Krueppel-like factor 6
- Lgr5+
Leucine-rich repeat-containing G-protein coupled receptor 5
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- miR-212/132
microRNA cluster 212/132
- miR-335
microRNA 335
- MPO
Myeloperoxidase
- NES
Nuclear Export signal
- bHLH
Basic-helix-loop-helix
- NFκB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLS
Nuclear Localization signal
- Notch 1
Neurogenic locus notch homolog protein 1
- NQO1
NAD(P)H dehydrogenase [quinone] 1
- NRF2
Nuclear factor erythroid 2–related factor 2
- Ocln1
Occludin 1
- p53
Tumour protein 53
- PD-1
Programmed cell death protein 1
- PER
Period circadian protein
- PRR
Pattern Recognition Receptor
- RE
Responsive element
- RNF43
Ring finger protein 43
- RORγt+
Positive t isoform of the retinoic-acid-receptor-related orphan nuclear receptor gamma
- ROS
Reactive oxygen species
- SCFAs
Short-chain fatty acids
- SIM
Single-minded Protein
- SOCS3
Suppressor of cytokine signalling 3
- SOX4
SRY-related HMG-box4
- SPF
Specific pathogen-free
- TA
Tryptamine
- TAD
Transactivation Domain
- TC
Total cholesterol
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TGF-β
Transforming growth factor beta
- TiPARP
TCDD Inducible Poly(ADP-Ribose) Polymerase
- TJs
Tight junctions
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
- TNF-α
Tumour necroses factor alpha
- UC
Ulcerative colitis
- XAP2
Hepatitis B virus X protein associated protein
- XRE
Xenobiotic responsive element
- ZNRF3
Zinc/ring finger protein 3
- ZO-1
Zonula occludens-1
1. The AHR
The Aryl Hydrocarbon Receptor (AHR) is a widely conserved ligand-activated transcription factor that can sense a wide range of molecules, including those from environmental, dietary, and microbial sources. Upon ligand sensing, the AHR integrates information from the different signals and translates it into cellular responses, tailored to a specific ligand, cell type, and tissue context, albeit by not fully understood mechanisms. Notably, the AHR has been reported to crosstalk and be a convergence point with several signalling pathways inside the cell, including those regulating inflammation, immunity, cell proliferation and differentiation, among others. Altogether, the AHR is known to modulate processes highly relevant to cellular, tissue and organism development, maintenance and homeostasis. Furthermore, modulation of the AHR pathway(s) (both canonical and non-canonical) in response to a perturbation of cellular and tissue homeostasis profoundly impacts therapy in diseases such as inflammatory diseases, cancer and microbial infections [[1], [2], [3], [4], [5]].
The AHR was initially discovered as the target receptor for dioxin (e.g., 2,3,7,8-tetraclorodibenzo-p-dioxin, TCDD), being involved in its toxicity [6,7]. Further studies uncovered a variety of AHR ligands [8], including synthetic and naturally occurring molecules. Synthetic ligands, polychlorinated biphenyls, halogenated-dioxins and related compounds are often harmful environmental pollutants [9] (e.g., the potent ligand, TCDD), and are generated from modern industrial and manufacturing processes and have been associated with toxicity. Natural AHR modulators consist of food-derived compounds, microbiome-derived metabolites (including those arising from pathogenic and opportunistic pathogenic microbes), plant-derived, and endogenous tryptophan metabolites [10]. Of note, in this review, whenever ligand binding studies have been performed, confirming direct interaction between the molecule and the AHR, molecules are listed as ligands, otherwise, described as modulators (either agonist or antagonist). Food-derived compounds include flavonoids like quercetin and apigenin [11], microbiome-derived metabolites include indole, indole-3-acetate, and tryptamine [12] and endogenous ligands include tryptophan metabolites such as 6-Formylindolo[3,2-b]carbazole (FICZ) [13], kynurenine [14] and kynurenic acid [15]. The capacity of the AHR to detect diverse molecules from pathogenic and opportunistic pathogens, including pigments (e.g., naphthoquinones and phenazines) and quorum sensing molecules (e.g., homoserine lactones), unveiled its role as a Pattern Recognition Receptor (PRR) [16,17].
The AHR is a member of the family of basic helix-loop-helix/(Period circadian protein (PER) | Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT) | Single-minded Protein (SIM); PER-ARNT-SIM) (bHLH/PAS) transcription factors and consists of an N-terminal bHLH, followed by two PAS domains (PAS A and PAS B) and a C-terminal transactivation domain (TAD) (Fig. 1) [18]. The bHLH domain mediates the binding of AHR to ARNT and DNA, whereas the PAS A domain is required for the interaction between AHR and ARNT [19] and for enhancing DNA binding [20]. The PAS B is the ligand-binding domain [18]. Of note, in this review, we focus on the canonical AHR pathway, its modulators and AHR functions (Fig. 2). In the absence of a ligand, the AHR is localized to the cytoplasm, bound to a chaperone complex, composed of heat shock protein 90 (HSP90) [21], the Hepatitis B virus X protein associated protein 2 (XAP2) [22] and p23 proteins [23]. Upon ligand binding, the AHR undergoes a conformation change, resulting in the dissociation from HSP90 and translocation into the nucleus. In the nucleus, the AHR forms a heterodimer with the ARNT [24] and binds to xenobiotic response elements (XRE, 5′-TA/TGCGTG-3′) [25] on the promoter of its downstream target genes to induce gene expression. Activation of the AHR by different ligands (e.g., TCDD) leads to the expression of different genes, among those are the well-studied members of the xenobiotic-metabolizing cytochrome P450 (CYP) enzymes such as CYP1A1 and CYP1A2 [26]. AHR activation also leads to the expression of aryl-hydrocarbon receptor repressor (AHRR), and TCDD-inducible poly(ADP-ribose) polymerase (TiPARP) [27], resulting in a negative feedback loop. The AHRR negatively regulates AHR signalling by interacting with ARNT, displacing AHR from the AHR:ARNT complex. The displaced AHR is then exported into the cytosol where it is targeted to the 26S proteasome for degradation [15]. TiPARP also promotes the degradation of the AHR [28] via the 26S proteasome (Fig. 2).
Fig. 1.
The AHR protein domains. The AHR contains three major domains, the bHLH domain, the PAS domains and the TAD. The bHLH domain located at the N-terminus is involved in interaction with chaperones, HSP90 and XAP2, dimerization with ARNT and binding to DNA. This is followed by the PAS domain which consists of two repeats, A and B. PAS A is involved in dimerization with ARNT and interaction with HSP90 and XAP2, the PAS B domain is the ligand binding domain. At the C-terminus is the transactivation domain. This domain consists of three subdomains, an acidic residue (glutamate/aspartate) rich subdomain, a glutamate-rich subdomain and a proline/serine/threonine-rich domain. The transactivation domain is involved in nucleocytoplasmic shuttling and interaction with other transcription factors. HSP90- heat shock protein 90; XAP2- Hepatitis B virus X protein associated protein 2; ARNT- AHR Nuclear Translocator; KLF6- Krueppel-like factor 6; NLS- Nuclear Localization signal; NES- Nuclear Export signal; bHLH- basic-helix-loop-helix; TAD- Transactivation Domain; PAS- Period circadian protein (PER) | Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT) | Single-minded Protein (SIM).
Fig. 2.
The AHR signalling pathways. The AHR is found in a complex containing HSP90, p23, XAP2 and c-SRC. Upon ligand binding, AHR dissociates from the complex leading to either canonical or non-canonical downstream signalling. Canonical AHR signalling involves the translocation of AHR into the nucleus where it dimerises with ARNT and binds to specific DNA sequences known as xenobiotic-response elements (XRE). This leads to the expression of canonical AHR target genes, Tiparp, Ahrr and P450 cytochromes such as Cyp1a1 and Cyp1a2. Expression of P450 cytochromes and AHRR act as a negative feedback loop to attenuate AHR signalling. P450 cytochromes degrade AHR ligands and AHRR forms a heterodimer with ARNT, displacing AHR from the AHR-ARNT complex. The displaced AHR is then targeted for proteasomal degradation. The AHR can also carry out non-canonical signalling through both genomic and non-genomic signalling. During non-canonical genomic signalling, the AHR can bind to transcription factors such as KLF6, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) to induce specific downstream gene expression. Regarding non-canonical and non-genomic signalling functions of the AHR, it can act as an E3 ubiquitin ligase in a complex with CUL4B. The AHR/CUL4B ubiquitin ligase ubiquitinates and targets proteins such as steroid receptors for proteasomal degradation, or lead to protein phosphorylation events in a c-SRC-dependent mechanism. AHR- Aryl Hydrocarbon Receptor; c-SRC- Proto-oncogene tyrosine-protein kinase Src; XRE- Xenobiotic responsive element; CYP1A1- Cytochrome P450 Family 1 Subfamily A Member 1; CYP1A2- Cytochrome P450 Family 1 Subfamily A Member 2; AHRR- AHR Repressor; TiPARP- TCDD Inducible Poly(ADP-Ribose) Polymerase; ARNT- AHR Nuclear Translocator; HSP90- Heat Shock Protein 90; XAP2- Hepatitis B virus X protein associated protein 2; KLF6- Krueppel-like factor 6; CUL4B- Cullin 4 B; NFκB- Nuclear factor kappa-light-chain-enhancer of activated B cells; RE- Responsive element. Figure created with BioRender.com.
In the last couple of years, there have been more insights into AHR biology and crosstalk with other signalling pathways. These include the interaction of AHR with RelB to modulate immune responses [29,30], with the Wnt signalling [31] to influence tissue regeneration, with retinoblastoma protein to mediate cell cycle arrest [32] and with KLF6 to modulate cell cycle control and tissue regeneration [33]. Furthermore, the AHR regulates protein phosphorylation and ubiquitination, via its interaction with c-SRC and via its E3 ubiquitin ligase activity, respectively [4,5,34,35]. The details and impacts of the crosstalk of AHR signalling with other signalling pathways have been reviewed by others [4,5,36]. In this review, we will be focusing on the involvement of AHR in inflammation and immunity.
2. AHR in immunity
For many years, the AHR has been mostly the focus of toxicologists due to its role as a receptor for dioxins [37]. The AHR was subsequently found to be important in the regulation of other processes, including immune/inflammatory responses in the skin [38] and gut [39,40], expression of many cytokines [[41], [42], [43], [44]], lipopolysaccharide (LPS)-induced septic shock [42] and disease tolerance [45]. However, observations that activation of the AHR by TCDD inhibited T-cell responses [46], the loss of AHR decreased lymphocyte accumulation in lymph nodes and spleen [47] and increased expression of different cytokines (e.g., IL-12 and IFN-γ) in spleen cells of ovalbumin immunised mice [43], pointed to the involvement of the AHR in immune cell development and regulation. These observations led to further investigations into the role of AHR in immunity (reviewed in detail in Refs. [4,5]). In 2008, two seminal studies demonstrated a role for the AHR in T helper 17 (Th17) and regulatory T (Treg) cell polarization [37,48]. The AHR is highly expressed in Th17 cells and activation of the AHR by FICZ induces Th17 differentiation, expression of IL-17α and IL-22, and worsens experimental autoimmune encephalomyelitis [37,48]. However, when activated by TCDD, the AHR induced Treg differentiation and suppresses experimental autoimmune encephalomyelitis [37]. In line with this, the AHR was found to suppress immune activation, with AHR deficiency leading to autoimmunity in mice [49,50], further evidencing the direct role of the AHR in immune regulation. The involvement of the AHR in T-cell differentiation, maintenance and function also extend to other T-cell types, including T regulatory type 1 (Tr1) cells, gamma-delta (γδ) T-cells and T-cell receptor (TCR) αβ cluster of differentiation (CD) 8αα (TCRαβ+CD8αα) T-cells [51,52].
The AHR also plays a role in the development and function of B-cells. The AHR promotes the maturation and maintenance of B-cells [53] and drives B-cell proliferation via cyclin O [54]. Typical of the context-specific functions of the AHR, and the opposing effects reported to occur upon AHR activation, AHR activation can also inhibit B-cell development [55,56], inhibit B-cells class switching [56] and suppress the IgM secretion in B cells [57].
In addition to the regulation of adaptive immune cell development and function, the AHR is also involved in the function and maturation of different innate immune cells. In macrophages, the AHR suppresses the transcriptional activities and expression of different cytokines, including IL-6, TNF-α [41] and IL-1β secretion, preventing excessive inflammation [42]. The AHR can also affect the recruitment of neutrophils during infection [16,17], wound healing [58], and modulate the antigen uptake of inflammatory bone marrow-derived dendritic cells (BMDCs) [1,44]. The mechanism may be linked to an AHR regulation of neutrophil chemoattractants, including diverse chemokines and cytokines, (e.g., cxcl5, IL-8) [16,17,59]. However, loss of the AHR can also result in increased neutrophil infiltration in an imiquimod-induced skin inflammation model [38]. Recently, Diny et al. demonstrated that the activation of the AHR signalling in the intestinal eosinophils results in a transcriptional adaption of eosinophils, as they migrated from the bone marrow to the intestinal tissue [60]. The AHR can also modulate dendritic cell (DC) activities and influence DC differentiation, in a ligand and context-dependent manner [49,[61], [62], [63]]. Moreover, the AHR plays an important role in the maturation of Langerhans cells via both cell-autonomous and non-cell-autonomous mechanisms [64]. The involvement of the AHR has also been shown to affect the immune function of natural killer (NK) cells, by promoting IL-10 expression [65].
Altogether, and linked to the different roles of AHR in the differentiation and function of various immune cells, several studies demonstrated an important role in host-pathogen interactions. Indeed, infection studies provided direct evidence for the role of the AHR in influencing host-pathogen interaction and infection outcomes. The AHR protects against infections, such as to Citrobacter rodentium [66], Listeria monocytogenes [67], Candida albicans [68], Staphylococcus epidermidis [69], Streptococcus pneumoniae [70], Aspergillus fumigatus [71], Mycobacterium tuberculosis [1,16,17,72], and Pseudomonas aeruginosa [16,17]. On the other hand, activation of the AHR showed detrimental effects on the immune response towards infections by Trypanosoma cruzi [73], Leishmania major [74], Zika virus [75], dengue virus, influenza virus [63], Herpes simplex virus [76], and HIV [77]. One explanation for the opposing effects of the AHR and its elicited functions is the context and the ligand-specific activation of the AHR, and its involvement in the maintenance and differentiation of different immune cells [37]. In the human body, the gut can accommodate an abundance of AHR ligands which can be produced by the metabolism of the microbial community present in the gut, the microbiota. As such, there is much interest in the involvement of AHR in the homeostatic control of gut and intestinal pathologies.
3. AHR and intestinal inflammation
The human gastrointestinal tract has a large area (250–400 m2), continuously exposed to xenobiotics, food and microorganisms, such as bacteria, fungi and parasites. Over thousands of years, the co-evolution of these microbes with the host led to a mutualistic relationship between the host and its gut microbes. In this regard, the microbiome is the dynamic ecosystem composed of the microbiota, their genetic pool, metabolites, viruses and other mobile genetic elements [78], which plays an important role in the development and homeostasis of the gut. Studies using mouse and zebrafish models have demonstrated that the microbiome helps to educate the gut immune system [79], for example, stimulating the proliferation and differentiation of intestinal cells [[80], [81], [82], [83]], influencing the level of neuroendocrine peptides [84], or modulating gut motility [81,85], amongst other functions.
The expression of the AHR has been shown to maintain specific ratios of the various bacterial populations in the caecum [86]. Diets rich in AHR ligands can also influence the gut microbiota in mice. Wild type (WT) mice fed with a diet depleted of natural AHR ligands (e.g., phenols and tryptophan derivatives) have more Actinobacteria, Bacteroidetes and Tenericutes in their intestine compared to AHR−/− mice, whereas the presence of AHR ligands in the diet of mice led to an expansion of Firmicutes and reduction in Bacteroidetes population [87]. The AHR can also modulate the microbiota population by changing the level of mucus production in the gut. This is due to the inhibition of Neurogenic locus notch homolog protein 1 (Notch 1) signalling by AHR which leads to induced differentiation of secretory cells to produce more mucus [88].
In addition to the regulation of the gut microbiome, the AHR is involved in various intestinal immune cell development and associated inflammatory responses in the gut [89], as depicted in Fig. 3. The AHR is thought to influence the immune response in inflammatory bowel diseases (IBD), such as Crohn's disease (CD) and ulcerative colitis (UC). Expression of the AHR is lowered in inflamed tissues of CD patients compared to uninflamed tissues or healthy individuals [90], while AHR ligands have been shown to reduce colitis in mice [91,92]. The single layer of intestinal epithelial cells (IECs) forms a physical and biochemical barrier that acts as the first line of defence against pathogens. The renewal of this epithelial layer is maintained by the proliferation of the leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5+) stem cell population [93], and the WNT-β-catenin pathway is essential for the proliferation of these Lgr5+ stem cells [94]. The AHR modulates the expression of negative regulators of WNT signalling, E3 ubiquitin ligases ring finger protein 43 (RNF43) and zinc/ring finger protein 3 (ZNRF3) to ensure a coordinated IECs regeneration [95]. The coordinated regeneration of IECs by AHR is important to maintain gut homeostasis and prevent intestinal pathologies. AHR deficiency has also been shown to reduce the levels of TCRγδ cells in the small intestine, leading to a reduced turnover of the intestinal epithelial and an increased bacterial load [96]. The AHR deficiency in CD11c + cells leads to a perturbation of the epithelium development [97]. The AHR also inhibits the negative regulation of IL-22 signalling by suppressor of cytokine signalling 3 (SOCS3) [98]. IL-22 stimulation of colonic stem cells is shown to induce cell proliferation [98], and activation of the AHR ensures the production of IL-22, which in turn increases cell proliferation of the IECs to maintain an intact intestinal barrier. Another way that AHR can maintain the intestinal barrier is through the modulation of tight junctions (TJs) proteins. Administration of FICZ, an endogenous AHR ligand [99], attenuates the loss of TJs proteins in a DSS-induced colitis mouse model [100]. Singh et al. also showed that 3,8-Dihydroxy-urolithin (urolithin A), a metabolite of ellagic acid which is found in high levels in pomegranate, acts on the AHR and nuclear factor erythroid 2–related factor 2 (NRF2) signalling pathways to upregulate the expression of TJs proteins and ameliorate colitis [101]. Indoles from bacteria are also known to activate the AHR, reduce inflammation and improve TJs' permeability [102]. Together, these results point to the important role of AHR in the maintenance of gut barriers and function.
Fig. 3.
Schematic representation of key intestinal immunity players and the impact of the AHR. Compounds present in the intestinal lumen can be metabolized either by microbiota or in the enterocytes generating several metabolites. The parent compounds and their metabolites can serve as ligands for the AHR, modulating several cell functions and immune system responses. These include the modulation of IECs proliferation, as well TJs expression and IL-22 production in this and other immune cells; innate lymphocytes' development; T cells differentiation and migration, and eosinophils adherence capacity and degranulation. The expression of the AHR has also been shown to maintain specific ratios of the various bacterial populations in the caecum by modulating the production of antimicrobial peptides and by modulating mucus production and intestinal motility. Abbreviations: AHR – Aryl Hydrocarbon Receptor; IECs – Intestinal epithelial cells; ILCs – Innate lymphoid cells; IL-22 – Interleukin 22; TJs – Tight junctions. Figure created with BioRender.com.
In addition to the IECs, gut health and homeostasis are regulated by a plethora of immune cells in the lamina propria and specialised immune tissues such as the mesenteric lymph nodes and gut-associated lymphoid tissues. These lymphoid tissues are dedicated sites of immune cell priming and include Peyers’ patches in the small intestines and isolated lymphoid follicles, found in both small and large intestines [103]. The AHR is required for the proper development of the lymphoid follicles. The AHR is highly expressed in the positive t isoform of the retinoic-acid-receptor-related orphan nuclear receptor gamma (RORγt+) innate lymphoid cells (ILCs) and drives the postnatal expansion of these cells [66,104,105]. In CD4–RORγt+ T cells, AHR-mediated tyrosine-protein kinase KIT expression leads to its maintenance and expansion, leading to the development of intestinal lymphoid follicles [104]. The maintenance of RORγt + ILCs by AHR was later found to be related to reduced apoptosis of RORγt + ILCs [105], as AHR deficient RORγt + ILCs showed increased Annexin V staining and decreased expression of anti-apoptotic genes, Bcl2 and Bcl2l1 [105]. As such, AHR-deficient mice have a reduced number of IL-22-producing RORγt + ILCs and are unable to produce IL-22 to mount a protective immune response to Citrobacter rodentium infection [66,104]. The susceptibility to C. rodentium infection was also observed in R26Cyp1a1 mice that constitutively express the enzyme CYP1A1 leading to the depletion of endogenous AHR ligands and generating a quasi-AHR-deficient state [106]. The AHR also regulates the expression of the transcription factor Aiolos, suppressing the expression of Programme cell death protein 1 (PD-1) in intestinal type 2 innate lymphoid cells (ILC2s). The absence of AHR resulted in reduced Aiolos and IL-5 expression, and decreased cell proliferation in ILC2s, leading to what the authors described as an “exhaust-like” phenotype [107]. Together, it shows that the AHR plays an important role in the development of ILCs in the gut to maintain homeostasis and prevent microbial-induced pathogenesis. The AHR also modulates the function of T cells in the gut. AHR activation by microbial-converted tryptophan metabolites induces a rare population of CD4+CD8αα+ T cells that promotes tolerance to dietary antigens [92]. In a model of DSS-induced colitis, AHR−/− mice show altered frequencies of the T cell subsets, Th17, Th1, and Tc1 (type1 CD8 ± T cells) in the gut, when compared to those of WT mice [108]. It is suggested that the AHR induces Th17 differentiation and migration into the lamina propria during colitis by modulating the expression of miR-212/132 [109]. The AHR was also recently shown to be important for driving the transcriptional adaption of eosinophils to the intestine. The intestinal gene expression program of AHR−/− eosinophils is impaired, including the expression of extracellular matrix and cell junction-related genes, impairing their capacity to adhere to and degrade extracellular matrix and rendering them more prone to degranulation [60].
Due to the involvement of the AHR in various aspects of gut homeostasis and immunity, many have attempted to modulate the outcome of gut pathology by modulating its activity using AHR ligands. For example, consumption of indole-3-aldehyde (IA) enhances resistance to Candida albicans infection by an AHR-dependent induction of IL-22 [68], while indole-3 carbinol can restore immunity towards C. rodentium infection in mice [106]. Altogether, these results confirm the important role of the AHR, and its direct and indirect modulation by different molecules, in maintaining gut health. Consequently, through the homeostasis regulation of IECs and the control of immune responses to prevent overt inflammation and damage to the gut, and while ensuring sufficient immune response to prevent infections, the ability of AHR ligands to modulate gut inflammation and TJs expression provides an avenue to target the AHR as a therapeutic intervention against intestinal inflammatory diseases.
4. AHR ligands present in the gut
Since the AHR was first described, the number of xenobiotics and other compounds that were depicted to be able to modulate the AHR has greatly increased. As a recognized key regulator of homeostatic processes in the skin, lungs and gut, the search for physiological AHR ligands [4,110] led to the identification of several dietary and microbiota-derived metabolites as AHR modulators, including short-chain fatty acids (SCFAs), tryptophan metabolites, phenolic microbial metabolites, among others [10]. Regarding the compounds that act as AHR modulators, Table 1 highlights endogenous and microbiota-derived metabolites, compounds related to (poly)phenol's metabolism will be detailed below (Table 2). Table 1 comprises compounds identified as agonists and/or antagonists of the AHR canonical pathway, being the metabolites from the tryptophan metabolism and related pathways the most studied so far.
Table 1.
AHR described ligands of host and microbial origina.
| Metabolite Structure | Metabolite Name | Origin | Pathway | Modulatory capacity | References |
|---|---|---|---|---|---|
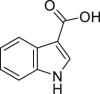 |
Indole-3-carboxylic acid | Host | Tryptophan metabolism | Agonist | [112] |
 |
Kynurenic acid | Host | Tryptophan metabolism | Agonist | [15] |
 |
Xanthurenic acid | Host | Tryptophan metabolism | Agonist | [15,112] |
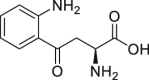 |
l-Kynurenine | Host | Tryptophan metabolism | Agonist | [14,45,113,114] |
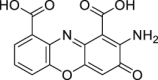 |
Cinnabarinic acid | Host | Tryptophan metabolism | Agonist | [115,116] |
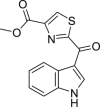 |
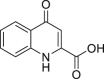
methyl 2-(1H-indole-3-carbonyl)-1,3-thiazole-4-carboxylate (ITE) | Host | Tryptophan metabolism | Agonist | [117] |
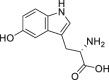 |
5-Hydroxytryptophan | Host | Tryptophan metabolism | Agonist | [118] |
 |
Indirubin | Host | Hepatic metabolism | Agonist | [[119], [120], [121]] |
| Microbial | Tryptophan metabolism | ||||
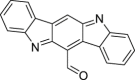 |
6-formylindolo[3,2-b]carbazole (FICZ) | Host | Tryptophan metabolism | Agonist | [[122], [123], [124], [125]] |
| Microbial | Tryptophan metabolism | ||||
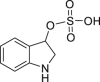 |
Indoxyl sulfate | Microbial + Host | Tryptophan metabolism | Agonist | [126] |
 |
3-Methyloxindole | Microbial | Tryptophan metabolism | Agonist | [112] |
 |
5-Hydroxyindole-3-acetic acid | Microbial | Tryptophan metabolism | Agonist | [112] |
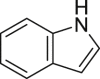 |
Indole (IND) | Microbial | Tryptophan metabolism | Agonist | [111,[127], [128], [129]] |
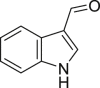 |
Indole-3-aldehyde (IA) | Microbial | Tryptophan metabolism | Agonist (concentration dependent antagonist) | [68,130] |
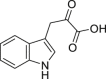 |
Indole-3-pyruvate | Microbial | Tryptophan metabolism | Agonist (concentration dependent antagonist) | [91,111] |
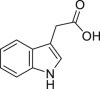 |
Indole-3-acetic acid (IAA) | Microbial | Tryptophan metabolism | Agonist | [12,111,127,131] |
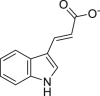 |
Indole-3-acrylate (IAC) | Microbial | Tryptophan metabolism | Agonist | [111,112] |
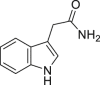 |
Indole-3-acetamide (IAD) | Microbial | Tryptophan metabolism | Agonist (concentration dependent antagonist) | [111] |
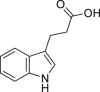 |
Indole-3-propionic acid (IPA) | Microbial | Tryptophan metabolism | Agonist | [111,132] |
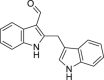 |
Malassezin | Microbial | Tryptophan metabolism | Agonist | [[133], [134], [135]] |
 |
Tryptanthrin | Microbial | Tryptophan metabolism | Agonist | [136] |
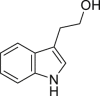 |
Indole-3-ethanol (IET) | Microbial | Tryptophan metabolism | Agonist (concentration dependent antagonist) | [111] |
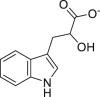 |
Indole-3-lactate (ILA) | Microbial | Tryptophan metabolism | Agonist | [111] |
 |
Skatole (3-methylindole) (3MI) | Microbial | Tryptophan metabolism | Agonist (concentration dependent antagonist) | [111,128,129,137] |
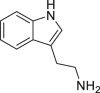 |
Tryptamine (TA) | Microbial | Tryptophan metabolism | Agonist (concentration dependent antagonist) | [12,111,127,136,138] |
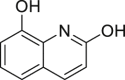 |
2,8-Dihydroxyquinoline | Microbial | Tryptophan metabolism | Agonist | [139] |
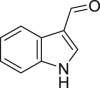 |
Indole-3-carboxaldehyde | Microbial | Tryptophan metabolism | Agonist | [112,140] |
 |
2-Oxindole | Microbial | Tryptophan metabolism | Agonist | [112] |
 |
Bilirubin | Host | Heme metabolism | Agonist | [141,142] |
 |
Biliverdin | Host | Heme metabolism | Agonist | [142] |
| Lipoxin A4 | Host | Arachidonic acid metabolite | Agonist | [143] | |
 |
Prostaglandin G2 | Host | Arachidonic acid metabolite | Agonist | [144] |
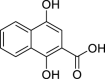 |
1,4-Dihydroxy-2-naphthoic acid | Microbial | Intermediate in the biosynthesis of menaquinone (vitamin K2) | Agonist | [145,146] |
 |
Phenazine 1-carboxamide | Microbial | Shikimic acid pathway | Agonist | [17] |
 |
Phenazine 1-carboxylic acid | Microbial | Shikimic acid pathway | Agonist | [17] |
 |
N-(3-oxodecanoyl)-l-homoserine Lactone (3-oxo-C12-L-HSL) | Microbial | Acyl-homoserine lactones synthase | Antagonist | [16] |
 |
4-hydroxy-2-heptylquinoline (HHQ) | Microbial | 4-hydroxy-2-alkylquinolines pathway | Antagonist | [16] |
 |
2-heptyl-3,4-dihydroxyquinoline (PQS) | Microbial | 4-hydroxy-2-alkylquinolines pathway | Antagonist | [16] |
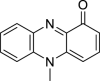 |
Pyocyanin | Microbial | Chorismate metabolization by phz operons | Agonist | [17] |
 |
1-Hydroxyphenazine | Microbial | Chorismate metabolization by phz operons | Agonist | [16,17,147] |
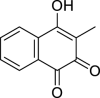 |
Phthiocol | Microbial | Chorismate metabolization | Agonist | [1,17] |
 |
Tapinarof | Microbial | Ketosynthase-directed stilbenoids biosynthesis pathway | Agonist | [148] |
Ligands originated exclusively from the consumption of (poly)phenols or the (poly)phenols metabolism were excluded from this table and described in Table 2.
Table 2.
List of gut phenolic metabolites described as AHR agonists/antagonists.
| Metabolite | Model | Approach | Concentration | AHR modulation | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Benzene-1,3-diol (resorcinol) | In vitro | HaCaT - human keratinocyte cell line | 500, 1000, 3000 μM | Antagonist | ↑NRF2, ↑NQO1, ↑HO-1 | [244] |
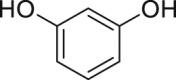 |
↓B[a]P-induced nuclear translocation of the AHR, ↓ROS, ↓Pro-inflammatory cytokines | |||||
| Hepa1.12 cR –murine hepatoma cell line | 0.01–1000 μM | – | No agonist activity on its own, Enhance TCDD-induced activation of the AHR | [250] | ||
| 3,4,5-Trihydroxybenzoic acid (gallic acid) | In silico | Computational approach | – | Identification as a human AHR ligand by molecular docking simulation | [251] | |
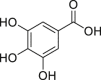 |
Computational approach | – | Identification as a mouse AHR ligand by molecular docking simulation | [252] | ||
| In vitro | Ligand binding | 15, 30, 45 μM | Displacement of [3H]-TCDD from AHR:ARNT heterodimer | [251] | ||
| MDA-MB-231–human breast cancer cell lines | 15, 30, 45, 60, 90 μM | Agonist | ↑Cyp1a1, ↑p53, ↑miR-335, ↓proliferation, migration and invasion, ↓BCL-2, ↓COX-2, ↓SOX4 | |||
| ER-positive T47D – human breast cancer cell lines | 15, 30, 45, 60, 90 μM | Agonist | ↑Cyp1a1, ↑miR-335, ↓proliferation, migration and invasion, ↓BCL-2, ↓COX-2, ↓SOX4 | |||
| Th17 derived cells | 40, 80, 120 μM | Agonist | ↑Cyp1a1, ↑CYP1A1, ↑miR-212/132 | [252] | ||
| Derived macrophage cells | 40, 80, 120 μM | Agonist | ↑Cyp1a1, ↑CYP1A1, ↑miR-212/132 | |||
| Ah-I receptor-binding assay in a free cell system | 0.5–50 μM | – | No inhibitory effect on 0.025 nM of TCDD-induced AHR | [235] | ||
| H1L1 – Murine hepatoma cell line | 0.01 nM−100 μM | – | No induction of AHR dependent luciferase activity | [234] | ||
| In vivo | MDA-MB-231 and ER-positive T47D cell lines injected into athymic nude BALB/c female mice | 3 mg/day, for 2 days, orallya | Agonist | ↑Cyp1a1 in tumour cells ↓growth of breast cancer cells | [251] | |
| Experimental Autoimmune Encephalomyelitis, C57BL/6 female mice | 2 mg/day for 10 days, i.p.b | Agonist | ↑Cyp1a1, ↑TGF-β, ↓IL-6, ↓IL-1β, ↓TNF-α, ↓IL-10, ↓infiltration of CD4+CD45+T cells, ↓infiltration of monocytes into central nervous system | [252] | ||
| 3,4-dihydroxyphenylacetic acid (DOPAC) | In vitro | Hepa1c1c7 - murine hepatoma cell line | 10, 20, 50 μM | Agonist | ↑AHR nuclear translocation, ↑NRF2 nuclear translocation, ↑ALDH activity, ↑Aldh1a1, ↑Aldh2, ↑Aldh3a1 | [253] |
 | ||||||
| 3,8-Dihydroxy-urolithin (urolithin A) | In silico | Computational approach | – | – | Identification as a AHR ligand by molecular docking simulation | [254] |
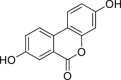 |
In vitro | HepG2 40/6 – human hepatoma cell line | 1–20 μM | Antagonist | ↓Cyp1a1, ↓AHR dependent luciferase activity | [208] |
| Caco-2 – human colorectal adenocarcinoma cell line | 10 μM | Antagonist | ↓Cyp1a1, ↓IL-6, ↓COX-2 | |||
| Hepa1.1 – murine hepatoma cell line | 10 μM | Agonist | ↑AHR dependent luciferase activity | |||
| HT-29 – human colorectal adenocarcinoma cell line | 50 μM | Agonist | ↑CYP1A1, ↑Cyp1a1, ↑NRF2, ↑AHR nuclear translocation, ↑HO-1, ↑Cldn4, ↑ZO-1, ↑Ocln1 ↑NQO1 | [101] | ||
| Bone marrow-derived dendritic murine cells | 5 μM | Agonist | ↑Cyp1a1 ↓BMDCs activation, ↓Th17 differentiation in T cell and DC-CD4+ T cell | [254] | ||
| In vivo | LPS-induced peritonitis C57BL/6J male mice | 20 mg/kg, orally | Agonist | ↓IL-6, ↓TNF-α | [101] | |
| C57BL/6 mice | 20 mg/kg/d for 7 days, orally | Agonist | ↑CYP1A1 | |||
| TNBS-induced colitis model C57BL/6J male mice | 20 mg/kg at 12 h intervals, orally, from t = 12h to t = 60h after TNBS | Agonist | ↑Cldn4 ↓TNBS-induced body weight loss, ↓disease activity index, ↓intestinal permeability, ↓colonic inflammation, ↓neutrophil infiltration, ↓MPO activity, ↓IL-6, ↓TNF-α, ↓CXCL1, ↓IL-1β | |||
| 4 or 20 mg/kg, orally post 12 h of TNBS instillation | Agonist | ↓intestinal permeability ↓TNBS-induced body weight loss, ↓IL-6, ↓TNF-α, ↓CXCL1, ↓IL-1β | ||||
| 20 mg/kg/d for 3 days, orally, after TNBS | Agonist | ↑barrier function ↓intestinal permeability ↓TNBS-induced body weight loss, ↓IL-6, ↓TNF-α | ||||
| 20 mg/kg/d for 7 days, orally, before TNBS treatment | Agonist | ↑barrier function ↓intestinal permeability ↓TNBS-induced body weight loss, ↓IL-6, ↓TNF-α | ||||
| TNBS-induced colitis model C57BL/6J AHR−/− male mice | 20 mg/kg at 12 h intervals, orally, from t = 12h to t = 60h after TNBS | – | No effect on colon length | |||
| No effect on intestinal permeability | ||||||
| No effect on IL-6 levels | ||||||
| DSS-induced colitis C57BL/6J male mice | 20 mg/kg/d, 4th and 6th day after DSS treatment | Agonist | ↑Cldn4, ↑colon length ↓disease activity index, ↓intestinal permeability, ↓IL-6, ↓IL-1β, ↓TNF-α, | |||
| Experimental Autoimmune Encephalomyelitis, C57BL/6 female mice | 25 mg/kg/d, for 30 days orally | Agonist | ↓disease progression, ↓inflammatory cells, ↓demyelination, ↓M1-type microglia, ↓DC, ↓Th1/Th17 | [254] | ||
| 3-Hydroxy-urolithin (urolithin B) | In vitro | HepG2 40/6 – human hepatoma cell line | 1–20 μM | Antagonist | ↓Cyp1a1, ↓AHR dependent luciferase activity | [208] |
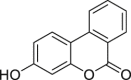 | ||||||
| 3,8,9-Trihydroxy-urolithin (urolithin C) | In vitro | HepG2 40/6 – human hepatoma cell line | 10 μM | Antagonist | ↓ AHR dependent luciferase activity | [208] |
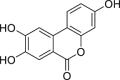 | ||||||
| 3,4,8,9-Tetrahydroxy-urolithin (urolithin D) | In vitro | HepG2 40/6 – human hepatoma cell line | 10 μM | Agonist | ↑ AHR dependent luciferase activity | [208] |
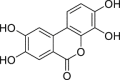 |
Hepa1.1 – murine hepatoma cell line | 10 μM | Agonist | ↑ AHR dependent luciferase activity | ||
| 3,8,9,10-Tetrahydroxy-urolithin (urolithin M6) | In vitro | HepG2 40/6 – human hepatoma cell line | 10 μM | Agonist | ↑ AHR dependent luciferase activity | [208] |
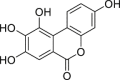 |
Hepa1.1 – murine hepatoma cell line | 10 μM | Agonist | ↑ AHR dependent luciferase activity | ||
Abbreviations: AHR – Aryl Hydrocarbon Receptor; ALDH – Aldehyde dehydrogenase; BCL-2 – B-cell lymphoma 2; BMDCs – Primary bone marrow-derived dendritic cells; BMDMs – Mouse bone marrow derived macrophages; Cldn4 – Claudin 4; COX-2 – Cyclooxygenase-2; CXCL1 – Chemokine (C-X-C motif) ligand 1; CYP1A1 – Cytochrome P450 Family 1 Subfamily A Member 1; DC – Dendritic cells; HO-1- Heme oxygenase-1; I3A – Indole-3-acetic acid; IL-1β – Interleukin 1 beta; IL-6 – Interleukin 6; LPS – Lipopolysaccharide; miR-212/132 – microRNA cluster 212/132; miR-335 – microRNA 335; MPO – Myeloperoxidase; NFκB – Nuclear factor kappa-light-chain-enhancer of activated B cells; NQO1- NAD(P)H dehydrogenase [quinone] 1; NRF2- Nuclear factor erythroid 2–related factor 2; Ocln1 – occludin 1; p53 – Tumour protein 53; ROS – Reactive oxygen species; SOX4 – SRY-related HMG-box4; TC – Total cholesterol; TCDD – 2,3,7,8-Tetrachlorodibenzo-p-dioxin; TGF-β – Transforming growth factor beta; TNBS – 2,4,6-Trinitrobenzenesulfonic acid; TNF-α – Tumour necroses factor alpha; ZO-1 – Zonula occludens-1.
Estimated circulating concentration 12.16 mM.
Estimated circulating concentration 8.11 mM.
Vyhlídalová et al. recently performed a comprehensive in vitro study on the effect of eleven gut microbiota tryptophan metabolites (indole (IND), tryptamine (TA), skatole (3MI), indole-3-pyruvate (IPY), indole-3-lactate (ILA), indole-3-acrylate (IAC), indole-3-propionate (IPA), indole-3-acetamide (IAD), indole-3-acetate (IAA), indole-3-ethanol (IET), and IA) as AHR agonist/antagonist modulators [111]. Their agonist/antagonist capabilities were assessed taking into account: (i) their ability to activate the nuclear translocation of the AHR; (ii) the formation of the AHR:ARNT heterodimer; (iii) the binding of the AHR:ARNT heterodimer to the target gene promoter; (iv) the expression of the AHR target gene Cyp1a1. Furthermore, AHR ligand binding studies were performed using the well-established [3H]-TCDD competitive radio-ligand binding assay using mouse hepatocyte cell extracts. All eleven tryptophan metabolites were classified as low-potency agonists of the human AHR, indicating that are able to activate the nuclear translocation of the AHR and the formation of the AHR:ARNT heterodimer.
Moreover, these authors identified six-strong inducers of Cyp1a1 (IND, TA, 3MI, IAD, IAC, and IPY), and described IAD, IPY, IAC, and IET as AHR ligands [111]. The authors also concluded that the AHR activation is dependent on ligand concentration and that it is cell type-specific, as observed by others [12,129].
In another study, Dong et al. showed that both endogenous and microbiota-derived tryptophan metabolites present in caecal and faecal samples of mice and humans, respectively, can induce AHR activation [112]. Among the thirteen tryptophan metabolites identified from the samples of mice and humans, only five of them were identified as AHR modulators (IAA, 2,8-dihydroxyquinoline, kynurenic acid, 2-oxindole, and IND) at the concentrations found in intestinal contents [112]. Importantly, the fact that Dong et al. did not identify some of the tryptophan metabolites identified by Vyhlídalová et al. as AHR ligands, may reside in the range of concentrations tested. For instance, Vyhlídalová et al. used TA in a wide range of concentrations (EC50 = 83.15 μM) while the study of Dong et al. used a maximum concentration of 5.2 μM. Importantly, in the work of Dong et al. they showed that in germ-free mice, alterations in the production of AHR ligands occur, highlighting the essential role of microbiota in the production of AHR ligands. The authors were able to identify twelve tryptophan metabolites (IAA, IND, IAC, TA, serotonin (5-HT), indole-3-carboxaldehyde, 2,8-dihydroxyquinoline, kynurenic acid, xanthurenic acid, 2-oxindole, indole-3-carboxylic acid and 5-hydroxyindole-3-acetic acid) in specific pathogen-free (SPF) C57Bl/6J mice, while only five (IAA, indole-3-carboxaldehyde, kynurenic acid, indole-3-carboxylic acid, and 5-hydroxyindole-3-acetic acid) were identified in germ-free animals. Out of those five, only two metabolites (indole-3-carboxylic acid and 5-hydroxyindole-3-acetic acid) were present at similar concentrations as those of the conventionally raised mice.
Recently, it has been shown that 5-HT can indirectly modulate the AHR activity by competing for CYP1A1 action with the AHR ligands that are substrates of CYP1A1, preventing ligand metabolism, resulting in the maintenance of the levels of the AHR ligands in the cells [149]. A similar indirect modulation of the AHR has been previously proposed to different dietary (poly)phenols (e.g., quercetin, resveratrol and curcumin), through the inhibition of Cyp1a1 and resulting in a lack of metabolism of AHR ligands [150]. Schiering et al. showed a similar result with Cyp1a1 chemical inhibitors [151].
Dietary tryptophan is also metabolized by mammalian cells through the kynurenine pathway, resulting in several kynurenine derivatives. Among those, kynurenic acid has been described as an AHR ligand capable of mediating immune responses by activation of the AHR signalling pathways, both canonical and non-canonical [15,112,113]. Kynurenic acid has also been previously described to exert the maintenance of the immunosuppressive microenvironment in some cancer types through AHR modulation [152,153].
The AHR is described as capable of assessing the relative abundance of the bacterial communities and modulating the type and strength of the immune response, from induction of inflammatory cytokines and anti-microbial factors to the differentiation of Th17 and Treg cells and promoting bacterial clearance [16,17,37,104,154]. Phenazines, such as pyocyanin and 1-hydroxyphenazine, have been described as AHR ligands, and several negative effects on host cells are associated with the presence of these compounds, such as cytokine production, inhibition of ciliary motion and mucus production [17,155]. An in vitro study identified the Mycobacterium tuberculosis naphthoquinone phthiocol as ligan of the AHR, tus the reported toxic effects due to the reactive oxygen intermediates generated by its degradation it is most likely due to the activation of the transcription of canonical detoxifying genes regulated by the AHR [17].
The precursor of vitamin K2, 1,4-dihydroxy-2-naphthoic acid (DHNA), can exert an inhibitory action on DSS-induced colitis in mice through the modulation of the AHR. It has been shown that DHNA is an agonist of the AHR, promoting the induction of both Cyp1a1 and Cyp1b1 expression, in an AHR dependent manner, and can also act as an antagonist by inhibiting TCDD-induced Cyp1a1 expression in a Caco-2 (human colorectal adenocarcinoma cell line) cells [145]. In a mice model of colitis, feeding the animals with DHNA resulted in an increased expression of Cyp1a1 in the intestine of the animals, improved survival, reduced inflammation score and pro-inflammatory cytokine production (e.g., IL-6 in macrophages). Therefore DHNA is an interesting candidate molecule for the treatment of IBD, considered to be safe as it can be produced by the probiotic bacterium strain Propionibacterium freudenreichii ET-3 [146].
Other microbial metabolites, such as SCFAs, have been initially studied as modulators of the AHR, although it was further demonstrated that the modulation of the AHR occurs via an indirect action of the SCFAs [156]. These small metabolites are the main metabolic products of indigestible fibres from anaerobic bacterial fermentation in the intestine and are involved in several pathways through the modulation of G-protein-coupled receptors, histone deacetylase inhibition, mammalian target of rapamycin (mTOR) activation and hormone secretion, as such affecting epithelial integrity, differentiation, proliferation, and immune responses, as reviewed recently by Visekruna and Luu [156]. In the case of butyrate, there is evidence that it is capable to increase the expression of AHR target genes, such as Cyp1a1, both in mouse colonocytes and on the human Caco-2 cells, in an AHR-dependent manner [157]. Interestingly, butyrate enhanced Cyp1a1 in these cells to a similar extent as other well-known microbiota-derived ligands, such as tryptamine, indole and DHNA. These outcomes appear to be cell-context dependent and did not ascertain a direct interaction of the SCFAs with the AHR, as SCFAs inhibit histone deacetylases, which causes an increase in the AHR ligand-induced responses [157].
A large number of gut metabolites can affect a wide variety of cells and tissues, from intestinal epithelium to neuronal cells. The modulation of the AHR activity by gut metabolites is an area of intense research since AHR activity is involved in several inflammatory outcomes, such as immune cell proliferation and differentiation, among other processes. Nonetheless, the unveiling of the mechanism(s) involved in the interaction of these gut metabolites and the AHR activity is still lacking. Some of these metabolites generate a significant interest towards IBD treatments, due to their capacity to maintain the integrity of the intestinal barrier in animal models of colitis, through the modulation of apical junctional complex and associated actin regulatory proteins in an AHR-dependent manner [158]. Importantly, it is already clear that the gut metabolites’ AHR modulatory properties and the AHR elicited functions, either agonist or antagonist of the AHR canonical pathway, or an AHR direct-ligand, is organism, cell, tissue and context-dependent. This indicates that the conclusions about modulation of the AHR by gut metabolites should be carefully drawn for every single molecule and experimental/disease model.
5. Diet and gut inflammation
The Western diet, characterized by the low consumption of fibre and high levels of refined sugar, animal fats and animal protein, is considered a potentially reversible risk factor in gut inflammation. This pattern of diet is known to increase intestinal permeability, contribute to the overexpression of pro-inflammatory cytokines and induce alterations in the gut microbiota, which altogether can promote low-grade chronic intestinal inflammation [159]. In contrast to Western diets, the consumption of specific diets rich in plant-based foods contributes to a high intake of phytochemicals, including (poly)phenols. In particular, the Mediterranean diet is a good source of plant-based foods, and its key components, such as fruits and vegetables, olive oil or red wine, are (poly)phenols-rich foods. Many studies have shown a direct modulatory effect of these compounds on the gut microbiota and inflammatory diseases [[160], [161], [162]]. Particularly, IBD is considered a chronic intestinal inflammation and an abnormal immune response caused by alterations in gut microbiota, with several repercussions on the host's immune system [163]. In this regard, interventional studies have shown that the intake of bioactive compounds like (poly)phenols provides a potential approach to influence the microbiota–immunity dialogue, alleviating the symptoms in these patients [164,165].
Several clinical trials support that the specific CD exclusion diet and the UC exclusion diet are well-established procedures to achieve remission in those patients [166]. Besides, carbohydrate diet patterns have been shown to help clinical remission in paediatric patients with CD [167,168]. However, these diets are very restrictive diets depriving people of a large number of foods. The Mediterranean diet has gained popularity to prevent and/or treat IBD due to its potential in decreasing inflammatory markers and mortality in IBD patients [169,170]. Regular consumption of a Mediterranean diet in healthy people decreases the risk of developing IBD associated with specific gut microbiome regulation as well as a reduction in gut inflammatory markers (e.g., calprotectin) [171]. The beneficial effects of long-lasting adherence to the Mediterranean diet have been attributed to specific components like (poly)phenols [172], which are very abundant in this diet and are associated with immunomodulatory properties and a reduced incidence of inflammation.
5.1. Dietary (poly)phenols and gut inflammation
Dietary (poly)phenols are phytochemicals extensively studied due to their health-promoting properties. (Poly)phenols have been studied regarding their pleiotropic activities, such as cardio-vascular activity, antioxidant capabilities, neuroprotective, anti-cancer and anti-inflammatory action, as reviewed elsewhere [[173], [174], [175], [176], [177]]. (Poly)phenols are present in a great variety of vegetables and fruits, and in beverages like coffee, tea and wine, making them the most abundant phytochemicals in the human diet (Fig. 4) [178]. Due to their plethora of bioactivities, (poly)phenol-containing products may be appropriate nutraceuticals and supplementary treatments with wide-ranging health benefits [179]. However, most (poly)phenols have very low bioavailability, and to exert a positive effect on health, they need to be absorbed in the human gut and become bioavailable in circulation. These compounds are submitted to extensive metabolism and when they reach the colon, gut microbiota catabolizes them, producing metabolites that are better absorbed. Lastly, they can undergo phase I and phase II metabolism in enterocytes and hepatocytes, and the resulting phenolic metabolites (i.e., glucuronide or sulfate conjugates) can enter the blood circulation or be excreted into the bile, intestinal lumen, or urine.
Fig. 4.
Main flavonoid and non-flavonoids (ellagitannins) classes with nutritional relevance. The bonds and atoms in blue indicate the major characteristics of the classes. Ellagic acid is the product obtained from the hydrolysis of ellagitannins, which are a diverse class of non-flavonoids. In brackets are representative food examples from each class of (poly)phenols. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Thus, the metabolism of (poly)phenols by both the host and the gut microbiota into small molecules creates a wide range of gut phenolic metabolites that can have different biological effects, which may be responsible for health-promoting effects attributed to the consumption of dietary (poly)phenols [173,180,181]. In particular, in the digestive tract where they are mainly produced, the pool and concentration of those resulting metabolites locally will impact the intestinal integrity and could interfere with intestine homeostasis and consequently cause or prevent the disease onset, the inflammatory response and recovery. The health impact associated with the (poly)phenol metabolites can happen through different pathways, either directly or indirectly. Therefore, the study of the impact of these metabolites in the intestine is key to understanding their effect on gut inflammation or related illness.
Several studies aiming to evaluate the effect of (poly)phenols have been performed using in vivo models of colitis, mostly in rodents, by either supplementing their diets with berries or with berry extracts, as berries are a rich source of dietary (poly)phenols, such as phenolic acids, flavonols and anthocyanins [182]. In these studies, reduction in neutrophil infiltration in the colonic mucosa was described, reducing the formation of oxidants linked to inflammation, the restoration of IL-10 levels, the inhibition of NFκB and a decreased expression of genes relevant to the inflammatory response, such as TNF-α, COX-2, IL-1β and IFN-γ [[183], [184], [185], [186]]. The results also showed that not all food sources render the same outcome. For example, supplementation of the diets with blueberry products leads to a reduction of neutrophil infiltration and suppression of oxidative stress markers, which is not mirrored when supplementing a DSS-colitis animal model's diet with black raspberry [183,184]. Bilberries and their anthocyanin extracts also reduce disease severity and the expression of pro-inflammatory cytokines in a model of acute-induced colitis in mice and reduced epithelial cell apoptosis [187]. A reduction in mucosal inflammation, histologic score and faecal calprotectin were observed in a human intervention study with UC patients consuming bilberries. Despite these results, the improvement in these patients was reverted once they stop the consumption of the berries [188], indicating that the chronic consumption of (poly)phenols is needed for the amelioration of the disease symptoms.
Curcumin, a (poly)phenol present in the turmeric plant, has been reported to have anti-inflammatory, anti-cancer and antioxidant activities in a DSS-induced colitis mice model. Curcumin can improve induced-colitis markers, such as weight loss and mucosal inflammation [189,190], most likely due to its capability of inhibiting NFκB activation, as has been observed with a lower secretion of pro-inflammatory cytokine by macrophages and epithelial cells [191,192]. Additionally, the gut microbiota-derived metabolite of curcumin, tetrahydrocurcumin, has been observed to prevent colitis-associated colon cancer in animal models [190,193]. Chlorogenic acid, a (poly)phenol present in a wide variety of foods and beverages, reduced the expression of macrophage inflammatory protein-2, a homolog of human IL-8, in a mouse model of UC [194,195].
In addition, innumerable in vitro studies have been carried out on the role of dietary bioactive compounds pointing them to anti-inflammatory activity. For example, bilberry extracts and single anthocyanins showed, in human monocytic THP-1 cells, inhibition of the secretion of different pro-inflammatory cytokines (e.g., TNF-α) [188,196] Maqui berry extracts decreased the growth of HT-29 (human colorectal adenocarcinoma) and Caco-2 cell lines, correlating with the inhibition of COX-2 and NFκB [197]. TNF-α/IL-1β activation followed by VEGF stimulation was observed as an anti-angiogenic effect of black raspberry extracts, mediated by protein kinase B (Akt), mitogen-activated protein kinase (MAPK), and JNK phosphorylation inhibitions [198]. Chlorogenic acid promotes a decrease in the expression of TNF-α and IL-8 in Caco-2 cells supporting this compound's in vivo effects [194,195]. However, these studies do not have a nutritional relevance, i.e., regardless of bioavailability, metabolism, and distribution of dietary (poly)phenols, since whatever reach these cells, are mainly gut phenolic metabolites and not the parent compounds present in the plants or their extracts.
Overall, the in vitro and in vivo studies indicate that the ability of dietary (poly)phenols to prevent and ameliorate disease markers of IBD is related to their capability of inhibiting oxidative stress by acting as free radical scavengers and, mainly, by the reduction of the expression of pro-inflammatory proteins through modulation of the NFκB signalling pathway [199]. However, the specific potential of their metabolites that could be directly linked to the observed effects remains to be determined. Therefore, in the next sections of this review, we focus on the potential physiologically relevant gut phenolic metabolites that could be the main players in the protective effects of gut inflammation and concerning the AHR.
5.2. (Poly)phenol-derived metabolites produced by gut microbiota
As mentioned, the bioavailability and metabolism of (poly)phenols are key factors to consider since they may increase or limit their beneficial effects. The dietary (poly)phenols, as they are present in fruits and vegetables are mainly conjugated with glycosides and organic acids (e.g., quinic acid), or can occur as oligomers (e.g., condensed tannins). Nonetheless, each of these forms is poorly or not absorbed at all, and is extensively submitted to metabolic reactions in the gut.
In the gut, the microbiota is the main responsible to transform dietary (poly)phenols mainly through three different reactions: (i) hydrolysis; (ii) ring fission, and (iii) reduction (Fig. 5). Flavonoids represent the most abundant family of phenolic compounds in the diet, with more than 8000 flavonoids described so far [200]. Most flavonoids are present as O- or C-glycosides. Although these flavonoids can be absorbed in the small intestine due to the action of glycosidases, such as lactase phloridzin hydrolase or cytosolic β-glucosidase [201,202], these compounds can be hydrolyzed into smaller metabolites prior to absorption by gut microbiota [203]. As an example of this dual metabolism, quercetin glycosides (e.g., quercetin 3-O-glucoside and quercetin 4ʹ-O-glucoside) can be hydrolyzed by phloridzin hydrolase [201], and further catabolized by intestinal bacteria into ring-fission products producing 3′,4′-dihydroxyphenylacetic acid (DOPAC) and 3′-hydroxyphenylacetic acid (OPAC), with a small production of 3,4-dihydroxybenzoic acid (protocatechuic acid) [204]. Another example of a metabolic reaction is the ester hydrolysis by some bacterial species (Fig. 5). For instance, the release of 3′,4′-dihydroxycinnamic acid (caffeic acid) by microbial esterases after incubation of chlorogenic acid [205] or acteoside [206] with human faecal samples has been described. In general terms, ring fission reactions are site-dependent on the structure of the phenolic compound. For instance, ring fission of flavan-3-ols (e.g., catechin) produces 5-(hydroxyphenyl)valeric acid and 5-(hydroxyphenyl)-γ-valerolactones, which are not produced by any other flavonoid classes [207]. The ring fission of flavan-3-ols also produces other phenolic metabolites, such as hydroxycinnamic acids, which are non-specific biomarkers of the dietary (poly)phenols intake. On the other hand, some of the (poly)phenol-derived metabolites are unique metabolites derived from a specific family of (poly)phenols. In this regard, the 6H-dibenzo[b,d]pyran-6-one derivatives (known as urolithins) are unique metabolites from ellagic acid, produced by gut microbiota [208]. The first step to producing urolithins from ellagic acid is the ring cleavage, and then the sequential decarboxylation leads to the formation of different urolithins (Fig. 5). This exclusive class of phenolic metabolites are not further metabolized into smaller compounds, remaining just as they are produced in the gut, although they are conjugated in the liver and/or enterocytes to be excreted in urine as phase II conjugates [209].
Fig. 5.
Main transformation reactions by gut microbiota of dietary (poly)phenols. Hydrolysis (O-glycosylation, ester hydrolysis), ring fission (C-ring cleavage, delactonization) and reduction (dehydroxylation, hydrogenation and decarboxylation) reactions. Examples of dietary (poly)phenols as found in food: flavonols (onions, apples), ellagitannins (berries, nuts), and hydroxycinnamates (coffee, whole grains) are converted into lower molecular weight metabolites by gut microbiota. These gut phenolic metabolites can: 1) be absorbed by intestinal epithelium entering entero-hepatic circulation and being redirected to the gut, 2) undergo direct phase II metabolism reactions into the enterocyte (e.g., glucuronidation, sulfation); 3) be further metabolized into short-chain fatty acids (SCFAs) by gut microbiota. Finally, all these microbiota metabolites and their conjugates can be absorbed and reach systemic circulation. The non-metabolized or non-absorbed dietary (poly)phenols are then excreted in faeces [175,213]. Figure created with BioRender.com.
Nonetheless, it should be pointed out that the smaller metabolites derived from flavonoids can also appear in the gut or circulate in the bloodstream after (poly)phenol-rich foods intake, as they can be absorbed from the food matrix without further metabolism (e.g., caffeic acid). Another important mechanism to note is the metabolic convergence resulting from progressive cleavage into common phenolic metabolites as it has been suggested for different (poly)phenol classes (e.g., benzenes, benzoic acids, and hippuric acids) [181] which leads to high and sustained concentrations in the circulation of these metabolites. In addition, these phenolic metabolites are not exclusively derived from dietary (poly)phenols, as they can be produced in the host by endogenous pathways, such as the phenylalanine metabolic pathway, which generates hippuric acids.
An increasing number of studies are looking for protective benefits against IBD with gut phenolic metabolites, which could have fewer undesirable effects in conditions of acute or chronic intestinal inflammation through the improvement of colonic oxidative and pro-inflammatory status. The production of specific isoflavone-gut microbiota metabolites (e.g., equol) has been associated with differences in the gut microbial ecologies, proposing that specific gut microbes may explain the differences observed and their causal role in health effects related to (poly)phenol consumption. Nevertheless, the core of this review is focused on those originating from ellagitannins, and the main dietary flavonoids—namely anthocyanins, flavones, flavonols, flavanones, and flavan-3-ols because they are well represented in diverse foods and therefore could be ingested daily (e.g., wine, walnuts, berries, oil, apples, etc) [210].
The metabolism by the gut microbiota gives rise to other relevant compounds, like branched-chain amino acids or SCFAs [173,176,211,212]. Moreover, it has been shown that the SCFAs contribute to the maintenance of gut homeostasis, by modulating the equilibrium of interactions between the intestinal epithelium and the host immune system [156].
Taking into account the transformations of dietary (poly)phenols by the gut microbiota, understanding their impact on the health benefits attributed to dietary (poly)phenols should be redirected towards their absorbable gut-derived metabolites. Some specific products of bacterial transformation, such as ring-fission products and reduced metabolites, could exhibit enhanced properties.
These smaller phenolic metabolites derived from the dietary (poly)phenols can also have their origins within the human body from other exogenous sources. Some of the gut microbes, and host enzymes involved in the described biotransformations, are responsible for the general metabolism of any chemical substance not naturally produced by or expected to be present within the human organism (xenobiotic metabolism). Therefore, it is not surprising that some phenolic metabolites could be obtained in humans not only through regular ingestion of dietary (poly)phenols but also by metabolizing other ingested compounds, like residues of industrial chemicals and pharmaceuticals [214]. Some of these compounds are intermediates of xenobiotics biodegradation and metabolism pathways like the bacterial aminobenzoate degradation pathway. Of notice, aminobenzoate metabolites are also derived from tryptophan and other indole ring-containing compounds metabolism [176]. On the other hand, it is also known that some of these metabolites could appear in human circulation from endogenous origin, due to the overlap with endogenous pathways such as the tyrosine or dopamine pathways [176].
6. Role of (poly)phenols and derived metabolites in the AHR modulation
6.1. Dietary (poly)phenols and AHR modulation
Several dietary (poly)phenols have been identified as AHR ligands, either agonists and/or antagonists. Their effect in the AHR modulation can result in several outcomes, as highlighted in Fig. 6, impacting cell fate, proliferation and differentiation, xenobiotic metabolism and inflammatory processes, among others; and is host, cell, tissue type, and context-dependent, as described for other AHR ligands. Although many studies revealed the potential of the dietary (poly)phenols to modulate the AHR, these are not relevant at the organ level due to their extensive metabolism and rapid elimination in the gut as described before. Still, these studies are a good indication of the (poly)phenols' potential to be explored as lead compounds for AHR modulation. However, not only (poly)phenols and their metabolites affect the AHR in the gut, but other compounds like xenobiotics (food contaminants, pollutants or drugs) are also described to affect this pathway. Some examples of xenobiotics that can modulate the AhR include dioxins, polychlorinated biphenyls (PCBs), and certain pesticides [7,53,57]. Concerning xenobiotics, some have been studied regarding their ability to impact gut health and how they affect drug metabolism, efficacy and toxicity, taking a special relevance to the role of the microbiota, as reviewed elsewhere [215,216].
Fig. 6.
Outcomes of AHR modulation by (poly)phenol metabolites. Schematic representation of selected outcomes regulated by the AHR upon modulation by (poly)phenol metabolites. Of note, the classification of ligands/modulators as agonists and antagonists herein adopted is based on their listed effects on the AHR canonical pathway. Complementary information related to the ligand/modulator, host, cell, tissue and context-dependent effects can be seen in Table 2. Abbreviations: ALDH – Aldehyde dehydrogenase; CYP1A1 – Cytochrome P450 Family 1 Subfamily A Member 1; IL-6 – Interleukin 6; Nrf2 – Nuclear factor erythroid 2–related factor 2; TJs – Tight junctions; TNF-α - Tumour necroses factor alpha; ZO-1 – Zonula occludens-1. Figure created with BioRender.com.
Some anthocyanidins and anthocyanins have been studied regarding their role in the AHR-CYP1A signalling pathway. The anthocyanidin pelargonidin was found to be an AHR agonist and induce the expression of the Cyp1a1 in human hepatocellular carcinoma cells and human colon adenocarcinoma cells [217]. Among twenty-one different anthocyanins studied for their ability to modulate AHR, only two, pelargonidin-3-O-rutinoside and cyanidin-3,5-O-diglucoside, could activate AHR in a dose-dependent manner in hepatic and intestinal cancer cells. Those two anthocyanins induced Cyp1a1 mRNA expression in these cells but not protein expression, and no Cyp1a1 mRNA or protein was induced in primary human hepatocytes cultures [218].
Initially, some flavonoids, like epigallocatechin gallate and epigallocatechin, were identified as strong antagonists of the mouse AHR [219], but later research on epigallocatechin gallate established that the previous result was associated with its interaction with the HSP90 protein, demonstrating that this effect occurs by indirect inhibition of the AHR [220,221]. Other subclasses of flavonoids, like flavones, flavonols, and flavanones, inhibit the binding between agonists and the AHR, indicating that they are competitive antagonists of the AHR [222].
Likewise, quercetin was first described as a ligand of both human and mouse AHR [223] with protective effects against inflammation [224,225] and hepatotoxicity [226]. Shortly after, Mohammadi-Bardbori et al. showed that quercetin, resveratrol, and curcumin indirectly activate the AHR [150]. Other authors demonstrate that quercetin is capable of inducing Cyp1a1 in an AHR dependent manner [227] and inhibiting EGFR phosphorylation and UV-associated AHR-mediated signalling [225].
Luteolin, previously described as having anti-inflammatory activity in vitro [228,229], was studied for its ability to induce AHR activation. A dose-dependent activation of the AHR with an optimal concentration of 80 μM was defined for luteolin [230]. Since AHR is considered a sensor that connects the outside environment with cellular processes regulating the immune system, one can speculate that the anti-inflammatory role of luteolin in LPS-induced macrophages may occur due to the AHR activation by this (poly)phenol. Notwithstanding, the biological relevance of luteolin in the gut is arguable due to its rapid metabolization in the colon [230]. Baicalein and 4-O-caffeoylquinic acid showed a dose-dependent AHR activation with an optimal concentration of 320, and 40 μM, respectively [230]. Recently it has been shown that baicalein is capable of protecting the colon from inflammatory damage in the DSS-induced colitis mice model by improving the intestinal epithelial barrier through decreasing intestinal permeability and restoring TJs via AHR/IL-22 pathway, regulating Treg cell differentiation and maintaining immune homeostasis and suppresses NFκB activation [[231], [232], [233]].
A screening on the ability to activate AHR by natural compounds, that include (poly)phenols, was made on hepatoma cells (H1L1). Ninety-five (poly)phenols from five different classes were screened using an in vitro reporter gene assay. In this screening, sixteen dietary compounds were able to activate AHR in this cell line displaying agonist activity [234]. In another study carried out by the same authors, they tested the antagonist effect of thirty-four (poly)phenols from eight different classes [235], using an AHR-Immunoassay. Among these compounds, flavones, flavonols, anthraquinones, piperine, coumestrol, brevifolincarboxylic acid, and resveratrol showed marked inhibitory effects on AHR-based bioassay activation by TCDD, and their effects were dose-dependent [235]. Some of the studied (poly)phenols were revealed to have both agonist and antagonist abilities to modulate AHR [235]. Denison and Nagy reviewed both natural and synthetic compounds that can directly activate and/or inhibit the AHR signalling pathway. They reported that curcumin, diosmin, tangeritin, and tamarixetin have been described as AHR ligands and substrates for CYP1A1 [150,236].
Resveratrol, another minor (poly)phenol in the human diet, was first described as an AHR antagonist by Ciolino et al. and can inhibit directly [237] or indirectly [150] the induction of the CYP1 family members by inhibiting the recruitment of the transcription factors AHR and ARNT to XREs of the enhancer regions of Cyp1a1 and Cyp1b1 genes [238]. Resveratrol has also been described as a partial agonist in human hepatocytes since is capable of inducing CYP1 family members in an AHR dependent manner [239,240].
Throughout the years, the effect of dietary (poly)phenols in the modulation of the AHR has been extensively studied, both in vivo and in vitro, revealing its potential to modulate the AHR. However, since they are submitted to extensive metabolism in the gut and rapid elimination, gut phenolic metabolites are the ones able to exert locally the effects in AHR modulation. Therefore, the trend of the research should be redirected towards evaluating the role of these gut phenolic metabolites, thus increasing the real knowledge of their possible effects in the AHR throughout the gut.
6.2. Gut phenolic metabolites and AHR modulation
Due to the importance to consider the final phenolic metabolites produced after multiple steps involving the gut microbiota, and not the parent compounds, a comprehensive search in Pubmed resorting to a list with 108 (using in the search terms also their respective synonyms + “AHR” or “aryl hydrocarbon receptor”) of the most abundant gut phenolic metabolites identified and/or quantified in human circulation (Supplementary Table S1 [181,241]) was performed. In this list, we have excluded phase II metabolites (i.e., glucuronides and sulphates), and comprise a total of thirteen different compound classes and other (poly)phenol compounds, aiming to understand how many of these metabolites have been described as AHR modulators and having an impact in inflammatory processes.
Among the 108 metabolites, we could only find a link between eight of these metabolites and crosstalk with the AHR (Table 2). The metabolites described as AHR agonists/antagonists have been studied on resourcing in silico, in vitro and in vivo models. Most studies have been done in tissues such as skin, lung and liver, unrelated to the gut, but reveal the potential for these compounds to modulate the AHR. Other gut phenolic metabolites have been evaluated in vitro for their ability to be potential ligands but with no clear AHR modulatory capabilities (see Supplementary Table S2). These results could be related to either the range of concentrations tested, the cell type used, or even can be in fact no AHR ligands. However, due to the limited studies, we decided to exclude them from our discussion and just list the evidence collected in Supplementary Table S2.
Benzene-1,3-diol (resorcinol) has been reported as an anti-melanogenic, anti-cancer, and antibacterial metabolite [242,243], but only recently was published the first report describing its biological activity in the skin [244]. High concentrations of resorcinol (0.5–3 mM) were described to suppress AHR activation by the environmental pollutant B[a]P in human keratinocytes resulting in a lower AHR nuclear translocation and inhibition of Cyp1a1 expression; decrease in B[a]P-induced damaged in human keratinocytes [245]; decrease of ROS production and IL-8 excretion [150]; an increase in the NRF2 nuclear translocation and upregulation of its target genes, although the data indicate that NRF2 activation is AHR-independent [244]. Nevertheless, a crosstalk between the NRF2 and AHR pathways has been previously described [[246], [247], [248], [249]]. In another cell model study by Kruger et al. in Hepa1.12 cR cells, resorcinol, in a wide range of concentrations, acts as a competitive ligand of AHR in the presence of TCDD, although no agonist activity was observed in this study [250]. In conclusion, resorcinol reveals the potential to modulate AHR activity although, due to the limited number of studies, it is not clear if its antagonist/agonist effects are cell specific or dependent on the concentration tested.
3,4,5-trihydroxybenzoic acid (gallic acid), a gut metabolite, but also a parent compound present in some fruits (i.e., berries), is one of the most studied metabolites as an AHR modulator [234,235,251,252]. In silico analysis pointed out gallic acid as a ligand of the AHR [251,252]. In vitro tests in different cell types (breast cancer cells, T cells, macrophages) indicate that gallic acid is an agonist of the AHR, capable of inducing AHR nuclear translocation and expression of AHR target genes, such as Cyp1a1 [251,252]. In an in vitro breast cancer model, gallic acid promoted apoptosis of the cancer cells and decreased proliferation, migration and invasion capacities in an AHR dependent manner [251]. It was also observed that gallic acid can modulate, in an AHR-dependent and -independent manner, the expression of B-cell lymphoma 2, COX-2, SOX4, and tumour protein 53 [251]. Although some in vitro studies point out gallic acid as an AHR agonist, the concentrations tested are far from relevant to reach that type of cell in a nutritional scenario. Moreover, near physiological circulating concentrations tested in liver cells reveal no induction of AHR [234]. In vivo analysis in an autoimmune encephalomyelitis mice model has shown that the animals treated with gallic acid display increased CYP1A1 activity (EROD assay), increased resistance to the disease and TGF-β (transforming growth factor-beta), have lower levels of pro-inflammatory cytokines and reduce infiltration of T cells and monocytes into the central nervous system [252]. In an in vivo orthotopic model, where human breast cancer cells are injected into mice, it was shown that gallic acid is capable of activating the AHR pathway (e.g., inducing Cyp1a1 expression) and reducing the growth of breast cancer cells [251]. In summary, these works show from in silico, in vitro and in vivo data that gallic acid has therapeutical potential as an AHR ligand and is a compound able to reduce inflammatory responses and cancer spread through the activation of the AHR signalling pathway.
DOPAC has previously been identified as an inducer of drug-metabolizing enzymes, an inhibitor of the secretion of pro-inflammatory cytokines and P-selectin in platelets [205,[255], [256], [257]]. In liver cells was shown that pre-treatment with DOPAC can increase total aldehyde dehydrogenases activity and stimulate nuclear translocation of the NRF2 and AHR, both responsible for the expression of phase I and phase II drug-metabolizing enzymes [253].
Several studies pointed to the beneficial effects of urolithins, microbiota metabolites of ellagitannins and ellagic acid, namely due to their anti-inflammatory, anti-cancer, and anti-estrogenic properties [258,259]. A recent work from Bobowska et al. describes urolithin A, as the most potent urolithin to inhibit an inflammatory response, whereas urolithin A phase II conjugates are inactive, hence losing the beneficial effects in macrophages and neutrophils [260]. In a model of experimental autoimmune encephalomyelitis has been shown that urolithin A is an agonist of AHR, and able to reduce the expression of inflammatory genes in DC, resulting in less active cells, and regulating the IL-17 family signalling pathway through the activation of the AHR, blocking Th17 differentiation [254]. In a related study, using both in vitro and in vivo approaches, Singh et al. demonstrated that urolithin A activates and acts via the AHR-NFκB, improving gut barrier function and preventing unnecessary inflammation by the upregulation of epithelial TJs [101]. The authors also confirmed the role of this compound in preventing experimental colitis through AHR in vitro using HT-29 cells, and in vivo using a TNBS-induced colitis model. Moreover, the authors reported that urolithin A is capable of improving intestinal permeability; reducing colonic inflammation (e.g., IL-6, TNF-α, CXCL1, IL-1β); reducing body weight loss and overall disease activity index [101]. In addition, Muku et al. identified in HepG2 and Caco-2 human cells, urolithin A as an AHR antagonist while in Hepa1.1 mouse cells showed agonist potential [208].
Furthermore, Muku et al. also tested several other urolithins using an AHR luciferase reporter assay, namely 3- hydroxy-urolithin (urolithin B), 3,8,9-trihydroxy-urolithin (urolithin C), 3,4,8,9-tetrahydroxy-urolithin (urolithin D) and 3,8,9,10-tetrahydroxy-urolithin (urolithin M6), all were found to be AHR modulators. Urolithin B and urolithin C showed antagonist activity of the AHR in HepG2 cells, whereas urolithin D and urolithin M6 were described as agonists of the AHR, in both HepG2 and Hepa1.1 cells [208]. To the best of our current knowledge, no further studies were performed to ascertain the response of urolithins in the modulation of the inflammatory response in an AHR dependent manner.
Overall, only gallic acid and urolithin A were evaluated for their ability to modulate the AHR in intestinal and immune cells. The lack of studies with other relevant gut phenolic metabolites in the gut reveals the large gap that exists regarding the effect that those metabolites may exert on AHR in the intestine.
7. Gaps in the field of AHR, inflammation and (poly)phenols
The AHR modulation in the gut can be affected by a myriad of compounds. To date, we are only at the beginning of unravelling which physiological metabolites can affect inflammation-related gut diseases acting through this receptor. Although we are starting to understand the complex role(s) of the AHR in gut inflammation, it has become unequivocally clear that the AHR is an important regulator of metabolic and immunological processes. One remarkable role of the AHR in intestinal health is its capacity to “spy” on quorum sensing molecules to monitor the luminal environment, access the bacterial composition and the presence of virulence factors, and promote either tolerogenic or defence responses according to the context [16,17]. The AHR's ability to sense the luminal environment and to activate or inhibit inflammatory responses using microbial metabolites could become an extremely important tool, opening new possibilities for different therapeutical/nutritional approaches aiming at the prevention and control of inflammatory diseases [261].
Several pending aspects should be addressed in future studies. For example, there is a need to explore the effect that the gut phenolic metabolites can exert on the AHR expressed in the intestinal cells, especially considering the quantity and diversity of these metabolites that can occur in the gut. The majority of research has been performed taking into account the availability and concentration of parent dietary (poly)phenol compounds in human circulation. Regarding the availability/concentration of (poly)phenol metabolites in the gut, it is expected to be at higher concentrations than those found in circulation, in the majority of the cases [262,263], due to the convergent metabolism of (poly)phenols, as discussed. Most of the articles resorting to ileostomy volunteers upon ingestion of fruit juices or extracts with high levels of (poly)phenols only resolve the concentration of the most abundant parent dietary (poly)phenol compounds, not giving information on the gut phenolic metabolites generated in the small intestine. Similarly, studies on the colonic availability of (poly)phenols focus on dietary (poly)phenols and not on (poly)phenol metabolites. A research paper studying the presence of ellagitannin and ellagic acid metabolites in colorectal cancer patients and healthy subjects showed that the concentration of their metabolites ranges from nanomolar to micromolar, depending on the metabolite [262].
One characteristic that has been observed among several known AHR ligands is their planar structure, which could indicate that this is an important feature for the ability of the ligands to interact with the AHR [230]. Thus, the planar conformation, and also hydroxyl groups present in the B and C rings of flavonoids (Fig. 4) could be related to these compounds being AHR ligands [264]. Nevertheless, other structurally different molecules as potential AHR modulators should not be disregarded and be evaluated and studied in detail in the future. As mentioned above, the AHR is endorsed with a remarkable capacity to bind to a broad range of structurally different molecules.
Until now, the most studied microbial-derived metabolites involved in AHR modulation have been the tryptophan metabolites. The literature has shown that a significant number of microbial-derived tryptophan metabolites are able to bind to and modulate AHR activity, although only a small subset has been shown to modulate the inflammatory response in an AHR dependent manner. This connection between microbial tryptophan metabolites and human health is promising, albeit further studies are still needed. Considering the gut phenolic metabolites explored in this review (Supplementary Table S1), only a few of them have been studied regarding their AHR modulatory capabilities in different cell types (Table 2), spanning from in vitro breast cancer models to induced-colitis in vivo models. Both in vitro and in vivo results indicate that gut phenolic metabolites have the capacity to modulate inflammatory players by targeting the AHR [101,208,254], and also these metabolites maintain barrier integrity [101]. However, out of the eight gut phenolic compounds referred on Table 2, only one was tested regarding the modulatory action on intestinal inflammatory models.
The gut phenolic metabolites are derived from the metabolism of the dietary (poly)phenols present in vegetables and fruits, among others. As so, a healthy diet rich in fruits and vegetables, with a high concentration of essential substrates for the synthesis of these microbial metabolites, could contribute to the prevention and treatment of the most prevalent inflammatory diseases [173]. The fact that these phenolic metabolites are present in the gut and may have the ability to modulate the AHR and improve gut microbiota ecology opens the avenue to be studied with great therapeutic and nutritional potential.
An important aspect that is frequently overlooked is that several different types of metabolites, besides phenolics, are simultaneously present in the gut and can produce a mixture of effects in the AHR pathway. Another potential pitfall that animal studies can face while trying to establish this connection and be translated to humans is linked to the fact that humans and rodents have different microbiomes and consequently slightly different phenolic metabolism pathways. It would be important to resort to more suitable models that could allow us to take more generalized conclusions, such as the utilization of humanized mice models or human organoid models, where human phenolic metabolites should be evaluated. There is also a need to perform more diet intervention studies on both healthy volunteers and patients. Another issue that can be identified is that some metabolites can act both as agonists or antagonists of the AHR, depending on the concentration of the metabolite and/or the cell type/host, this is also important to consider for extrapolating the results from animal models to human context. Besides, depending on a nutritional approach, careful selection of the physiologically relevant concentrations for the metabolites should be considered for mechanistic studies concerning the biological impact on AHR modulation. For a pharmacological approach, a controlled delivery of a specific concentration can be envisaged to achieve the desired effect on AHR.
There is much potential in gut phenolic metabolites concerning the prevention and/or treatment of intestinal inflammation. Whether intestinal phenolic metabolites can modulate the AHR and its specific effects throughout the gut, is an area that answers to an unmet need to address serious clinical problems such as IBD. Therefore, it is a topic in which knowledge is in its infancy and should arouses great interest in the scientific community due to its great impact on managing these pathologies.
Declaration of competing interest
I declare that there is no competing interests and that funding source was disclosed as requested.
Acknowledgements
This work was supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under grant agreement No 804229. iNOVA4Health Research Unit (LISBOA-01-0145-FEDER-007344), which is co-funded by Fundação para a Ciência e Tecnologia (FCT)/Ministério da Ciência e do Ensino Superior, through national funds, and by FEDER under the PT2020 Partnership Agreement, is acknowledged (UIDB/04462/2020 and UIDP/04462/2020). Y. Lian and P. Moura-Alves acknowledge the support of the Ludwig Institute for Cancer Research- Core Award, and P. Moura-Alves the H2020-WIDESPREAD-2018-951921- ImmunoHUB. Authors would like to acknowledge FCT for financial support of C. J. G. Pinto (2022.11465. BD).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102622.
Contributor Information
Pedro Moura-Alves, Email: pmouraalves@i3s.up.pt.
Cláudia Nunes dos Santos, Email: claudia.nunes.santos@nms.unl.pt.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Puyskens A., Stinn A., van der Vaart M., Kreuchwig A., Protze J., Pei G., Klemm M., Guhlich-Bornhof U., Hurwitz R., Krishnamoorthy G., Schaaf M., Krause G., Meijer A.H., Kaufmann S.H.E., Moura-Alves P. Aryl hydrocarbon receptor modulation by tuberculosis drugs impairs host defense and treatment outcomes. Cell Host Microbe. 2020;27:238–248 e7. doi: 10.1016/j.chom.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 2.D.R. Neavin, D. Liu, B. Ray, R.M. Weinshilboum, Molecular Sciences The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases, (n.d.). 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed]
- 3.Corre S., Tardif N., Mouchet N., Leclair H.M., Boussemart L., Gautron A., Bachelot L., Perrot A., Soshilov A., Rogiers A., Rambow F., Dumontet E., Tarte K., Bessede A., Guillemin G.J., Marine J.-C., Denison M.S., Gilot D., Galibert M.-D. Sustained activation of the Aryl hydrocarbon Receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat. Commun. 2018;9:4775. doi: 10.1038/s41467-018-06951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockinger B., di Meglio P., Gialitakis M., Duarte J.H. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 5.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19:184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 6.Poland A., Glover E., Kende A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976;251:4936–4946. https://www.ncbi.nlm.nih.gov/pubmed/956169 [PubMed] [Google Scholar]
- 7.Burbach K.M., Poland A., Bradfield C.A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen L.P., Bradfield C.A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safe S. Polychlorinated biphenyls (PCBs): mutagenicity and carcinogenicity. Mutat. Res. 1989;220:31–47. doi: 10.1016/0165-1110(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 10.Pernomian L., Duarte-Silva M., de Barros Cardoso C.R. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020;59:382–390. doi: 10.1007/s12016-020-08789-3. [DOI] [PubMed] [Google Scholar]
- 11.Jin U.-H., Park H., Li X., Davidson L.A., Allred C., Patil B., Jayaprakasha G., Orr A.A., Mao L., Chapkin R.S., Jayaraman A., Tamamis P., Safe S. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol. Sci. 2018;164:205–217. doi: 10.1093/toxsci/kfy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin U.H., Lee S.O., Sridharan G., Lee K., Davidson L.A., Jayaraman A., Chapkin R.S., Alaniz R., Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014;85:777–788. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rannug A., Rannug U., Rosenkranz H.S., Winqvist L., Westerholm R., Agurell E., Grafstrom A.K. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 1987:262. doi: 10.1016/s0021-9258(18)47743-5. [DOI] [PubMed] [Google Scholar]
- 14.Opitz C.A., Litzenburger U.M., Sahm F., Ott M., Tritschler I., Trump S., Schumacher T., Jestaedt L., Schrenk D., Weller M., Jugold M., Guillemin G.J., Miller C.L., Lutz C., Radlwimmer B., Lehmann I., von Deimling A., Wick W., Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 15.DiNatale B.C., Murray I.A., Schroeder J.C., Flaveny C.A., Lahoti T.S., Laurenzana E.M., Omiecinski C.J., Perdew G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moura-Alves P., Puyskens A., Stinn A., Klemm M., Guhlich-Bornhof U., Dorhoi A., Furkert J., Kreuchwig A., Protze J., Lozza L., Pei G., Saikali P., Perdomo C., Mollenkopf H.J., Hurwitz R., Kirschhoefer F., Brenner-Weiss G., Weiner J., Oschkinat H., Kolbe M., Krause G., Kaufmann S.H.E. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science. 2019;366 doi: 10.1126/science.aaw1629. 1979. [DOI] [PubMed] [Google Scholar]
- 17.Moura-Alves P., Faé K., Houthuys E., Dorhoi A., Kreuchwig A., Furkert J., Barison N., Diehl A., Munder A., Constant P., Skrahina T., Guhlich-Bornhof U., Klemm M., Koehler A.B., Bandermann S., Goosmann C., Mollenkopf H.J., Hurwitz R., Brinkmann V., Fillatreau S., Daffe M., Tümmler B., Kolbe M., Oschkinat H., Krause G., Kaufmann S.H.E. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 18.Fukunaga B.N., Probst M.R., Reisz-Porszasz S., Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J. Biol. Chem. 1995;270:29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 19.Pongratz I., Antonsson C., Whitelaw M.L., Poellinger L. Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor. Mol. Cell Biol. 1998;18:4079–4088. doi: 10.1128/MCB.18.7.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman-Smith A., Lutwyche J.K., Whitelaw M.L. Contribution of the Per/Arnt/Sim (PAS) domains to DNA binding by the basic helix-loop-helix PAS transcriptional regulators. J. Biol. Chem. 2004;279:5353–5362. doi: 10.1074/jbc.M310041200. [DOI] [PubMed] [Google Scholar]
- 21.Perdew G.H. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 1988;263:13802–13805. https://www.ncbi.nlm.nih.gov/pubmed/2843537 [PubMed] [Google Scholar]
- 22.Meyer B.K., Pray-Grant M.G., vanden Heuvel J.P., Perdew G.H. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol. Cell Biol. 1998;18:978–988. doi: 10.1128/MCB.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazlauskas A., Poellinger L., Pongratz I. Evidence that the Co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. J. Biol. Chem. 1999;274:13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 24.Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. 1979. [DOI] [PubMed] [Google Scholar]
- 25.Denison M.S., Fisher J.M., Whitlock J.P., Jr. The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J. Biol. Chem. 1988;263:17221–17224. https://www.ncbi.nlm.nih.gov/pubmed/2846558 [PubMed] [Google Scholar]
- 26.Wang Z., Snyder M., Kenison J.E., Yang K., Lara B., Lydell E., Bennani K., Novikov O., Federico A., Monti S., Sherr D.H. How the AHR became important in cancer: the role of chronically active AHR in cancer aggression. Int. J. Mol. Sci. 2020;22:387. doi: 10.3390/ijms22010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Q., Baldwin K.T., Renzelli A.J., McDaniel A., Dong L. TCDD-inducible poly(ADP-ribose) polymerase: a novel response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem. Biophys. Res. Commun. 2001;289:499–506. doi: 10.1006/bbrc.2001.5987. [DOI] [PubMed] [Google Scholar]
- 28.Rijo M.P., Diani-Moore S., Yang C., Zhou P., Rifkind A.B. Roles of the ubiquitin ligase CUL4B and ADP-ribosyltransferase TiPARP in TCDD-induced nuclear export and proteasomal degradation of the transcription factor AHR. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel C.F., Sciullo E., Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun. 2007;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel C.F., Sciullo E., Li W., Wong P., Lazennec G., Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew L.K., Sengupta S.S., Ladu J., Andreasen E.A., Tanguay R.L. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. Faseb. J. 2008;22:3087–3096. doi: 10.1096/fj.08-109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puga A., Barnes S.J., Dalton T.P., Chang C., Knudsen E.S., Maier M.A. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J. Biol. Chem. 2000;275:2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 33.Wright E.J., Pereira De Castro K., Joshi A.D., Elferink C.J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr Opin Toxicol. 2017;2:87–92. doi: 10.1016/j.cotox.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohtake F., Fujii-Kuriyama Y., Kato S. AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions. Biochem. Pharmacol. 2009;77:474–484. doi: 10.1016/j.bcp.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 35.Xie G., Peng Z., Raufman J.-P. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G1006–G1015. doi: 10.1152/ajpgi.00427.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puga A., Ma C., Marlowe J.L. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 38.di Meglio P., Duarte J.H., Ahlfors H., Owens N.D., Li Y., Villanova F., Tosi I., Hirota K., Nestle F.O., Mrowietz U., Gilchrist M.J., Stockinger B. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q., Yang K., Han B., Sheng B., Yin J., Pu A., Li L., Sun L., Yu M., Qiu Y., Xiao W., Yang H. Aryl hydrocarbon receptor inhibits inflammation in DSSinduced colitis via the MK2/pMK2/TTP pathway. Int. J. Mol. Med. 2018;41:868–876. doi: 10.3892/ijmm.2017.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv Q., Wang K., Qiao S., Yang L., Xin Y., Dai Y., Wei Z. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018;9:258. doi: 10.1038/s41419-018-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura A., Naka T., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekine H., Mimura J., Oshima M., Okawa H., Kanno J., Igarashi K., Gonzalez F.J., Ikuta T., Kawajiri K., Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol. Cell Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Sosa M., Elizondo G., Lopez-Duran R.M., Rivera I., Gonzalez F.J., Vega L. Over-production of IFN-gamma and IL-12 in AhR-null mice. FEBS Lett. 2005;579:6403–6410. doi: 10.1016/j.febslet.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Bankoti J., Rase B., Simones T., Shepherd D.M. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 2010;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M.C., Macchiarulo A., Vacca C., Iannitti R., Tissi L., Volpi C., Belladonna M.L., Orabona C., Bianchi R., v Lanz T., Platten M., della Fazia M.A., Piobbico D., Zelante T., Funakoshi H., Nakamura T., Gilot D., Denison M.S., Guillemin G.J., Duhadaway J.B., Prendergast G.C., Metz R., Geffard M., Boon L., Pirro M., Iorio A., Veyret B., Romani L., Grohmann U., Fallarino F., Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511 doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerkvliet N.I., Baecher-Steppan L., Smith B.B., Youngberg J.A., Henderson M.C., Buhler D.R. Role of the Ah locus in suppression of cytotoxic T lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): structure-activity relationships and effects in C57Bl/6 mice congenic at the Ah locus. Fund. Appl. Toxicol. 1990;14:532–541. doi: 10.1016/0272-0590(90)90257-k. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Salguero P., Pineau T., Hilbert D.M., McPhail T., Lee S.S., Kimura S., Nebert D.W., Rudikoff S., Ward J.M., Gonzalez F.J. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. 1979. [DOI] [PubMed] [Google Scholar]
- 48.Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 49.Quintana F.J., Murugaiyan G., Farez M.F., Mitsdoerffer M., Tukpah A.M., Burns E.J., Weiner H.L. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinde R., Hezaveh K., Halaby M.J., Kloetgen A., Chakravarthy A., da Silva Medina T., Deol R., Manion K.P., Baglaenko Y., Eldh M., Lamorte S., Wallace D., Chodisetti S.B., Ravishankar B., Liu H., Chaudhary K., Munn D.H., Tsirigos A., Madaio M., Gabrielsson S., Touma Z., Wither J., de Carvalho D.D., McGaha T.L. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol. 2018;19:571–582. doi: 10.1038/s41590-018-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandhi R., Kumar D., Burns E.J., Nadeau M., Dake B., Laroni A., Kozoriz D., Weiner H.L., Quintana F.J. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D., Burns E.J., Sherr D.H., Weiner H.L., Kuchroo V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurmond T.S., Staples J.E., Silverstone A.E., Gasiewicz T.A. The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 2000;165:227–236. doi: 10.1006/taap.2000.8942. [DOI] [PubMed] [Google Scholar]
- 54.Villa M., Gialitakis M., Tolaini M., Ahlfors H., Henderson C.J., Wolf C.R., Brink R., Stockinger B. Aryl hydrocarbon receptor is required for optimal B-cell proliferation. EMBO J. 2017;36:116–128. doi: 10.15252/embj.201695027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Abrew K.N., Kaminski N.E., Thomas R.S. An integrated genomic analysis of aryl hydrocarbon receptor-mediated inhibition of B-cell differentiation. Toxicol. Sci. 2010;118:454–469. doi: 10.1093/toxsci/kfq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaidyanathan B., Chaudhry A., Yewdell W.T., Angeletti D., Yen W.F., Wheatley A.K., Bradfield C.A., McDermott A.B., Yewdell J.W., Rudensky A.Y., Chaudhuri J. The aryl hydrocarbon receptor controls cell-fate decisions in B cells. J. Exp. Med. 2017;214:197–208. doi: 10.1084/jem.20160789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sulentic C.E., Holsapple M.P., Kaminski N.E. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol. Pharmacol. 1998;53:623–629. https://www.ncbi.nlm.nih.gov/pubmed/9547351 [PubMed] [Google Scholar]
- 58.Lozza L., Moura-Alves P., Domaszewska T., Lage Crespo C., Streata I., Kreuchwig A., Puyskens A., Bechtle M., Klemm M., Zedler U., Silviu Ungureanu B., Guhlich-Bornhof U., Koehler A.-B., Stäber M., Mollenkopf H.-J., Hurwitz R., Furkert J., Krause G., Weiner J., Jacinto A., Mihai I., Leite-de-Moraes M., Siebenhaar F., Maurer M., Kaufmann S.H.E. The Henna pigment Lawsone activates the Aryl Hydrocarbon Receptor and impacts skin homeostasis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-47350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith K.J., Boyer J.A., Muku G.E., Murray I.A., Gowda K., Desai D., Amin S.G., Glick A.B., Perdew G.H., Highlight Editor’s. Ah receptor activation potentiates neutrophil chemoattractant (C-X-C motif) ligand 5 expression in keratinocytes and skin. Toxicol. Sci. 2017;160:83–94. doi: 10.1093/toxsci/kfx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diny N.L., Schonfeldova B., Shapiro M., Winder M.L., Varsani-Brown S., Stockinger B. The aryl hydrocarbon receptor contributes to tissue adaptation of intestinal eosinophils in mice. J. Exp. Med. 2022;219 doi: 10.1084/jem.20210970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goudot C., Coillard A., Villani A.C., Gueguen P., Cros A., Sarkizova S., Tang-Huau T.L., Bohec M., Baulande S., Hacohen N., Amigorena S., Segura E. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity. 2017;47:582–596 e6. doi: 10.1016/j.immuni.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Liu H., Ramachandran I., Gabrilovich D.I. Regulation of plasmacytoid dendritic cell development in mice by aryl hydrocarbon receptor. Immunol. Cell Biol. 2014;92:200–203. doi: 10.1038/icb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin G.B., Moore A.J., Head J.L., Neumiller J.J., Lawrence B.P. Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol. Sci. 2010;116:514–522. doi: 10.1093/toxsci/kfq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jux B., Kadow S., Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 65.Wagage S., John B., Krock B.L., Hall A.O., Randall L.M., Karp C.L., Simon M.C., Hunter C.A. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J. Immunol. 2014;192:1661–1670. doi: 10.4049/jimmunol.1300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M., Mantovani A., Kopan R., Bradfield C.A., Newberry R.D., Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2011;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimura A., Abe H., Tsuruta S., Chiba S., Fujii-Kuriyama Y., Sekiya T., Morita R., Yoshimura A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2014;26:209–220. doi: 10.1093/intimm/dxt067. [DOI] [PubMed] [Google Scholar]
- 68.Zelante T., Iannitti R.G., Cunha C., DeLuca A., Giovannini G., Pieraccini G., Zecchi R., D'Angelo C., Massi-Benedetti C., Fallarino F., Carvalho A., Puccetti P., Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Rademacher F., Simanski M., Hesse B., Dombrowsky G., Vent N., Glaser R., Harder J. Staphylococcus epidermidis activates aryl hydrocarbon receptor signaling in human keratinocytes: implications for cutaneous defense. J Innate Immun. 2019;11:125–135. doi: 10.1159/000492162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vorderstrasse B.A., Lawrence B.P. Protection against lethal challenge with Streptococcus pneumoniae is conferred by aryl hydrocarbon receptor activation but is not associated with an enhanced inflammatory response. Infect. Immun. 2006;74:5679–5686. doi: 10.1128/IAI.00837-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L., Jiang N., Zhao G.Q., Peng X.D., Zhu G.Q., Jiang W., Ma J.J. Expression and role of aryl hydrocarbon receptor in Aspergillus fumigatus keratitis. Int. J. Ophthalmol. 2020;13:199–205. doi: 10.18240/ijo.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Memari B., Bouttier M., Dimitrov V., Ouellette M., Behr M.A., Fritz J.H., White J.H. Engagement of the aryl hydrocarbon receptor in Mycobacterium tuberculosis –infected macrophages has pleiotropic effects on innate immune signaling. J. Immunol. 2015;195:4479–4491. doi: 10.4049/jimmunol.1501141. [DOI] [PubMed] [Google Scholar]
- 73.Ambrosio L.F., Insfran C., Volpini X., Acosta Rodriguez E., Serra H.M., Quintana F.J., Cervi L., Motran C.C. Role of aryl hydrocarbon receptor (AhR) in the regulation of immunity and immunopathology during trypanosoma cruzi infection. Front. Immunol. 2019;10:631. doi: 10.3389/fimmu.2019.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elizondo G., Rodriguez-Sosa M., Estrada-Muniz E., Gonzalez F.J., Vega L. Deletion of the aryl hydrocarbon receptor enhances the inflammatory response to Leishmania major infection. Int. J. Biol. Sci. 2011;7:1220–1229. doi: 10.7150/ijbs.7.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovannoni F., Bosch I., Polonio C.M., Torti M.F., Wheeler M.A., Li Z., Romorini L., Rodriguez Varela M.S., Rothhammer V., Barroso A., Tjon E.C., Sanmarco L.M., Takenaka M.C., Modaresi S.M.S., Gutierrez-Vazquez C., Zanluqui N.G., dos Santos N.B., Munhoz C.D., Wang Z., Damonte E.B., Sherr D., Gehrke L., Peron J.P.S., Garcia C.C., Quintana F.J. AHR is a Zika virus host factor and a candidate target for antiviral therapy. Nat. Neurosci. 2020;23:939–951. doi: 10.1038/s41593-020-0664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J., Liang J., Xu H., Liu W., Liu S., Duan L., Li F., Wang Z., Liu Y., McSharry B., Feng C.G., Zhang G. Targeting aryl hydrocarbon receptor signaling enhances type I interferon-independent resistance to Herpes simplex virus. Microbiol. Spectr. 2021;9 doi: 10.1128/Spectrum.00473-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Y.H., Sun L., Chen J., Sun W.W., Ma L., Han Y., Jin X., Zhao Q.X., Li T., Lu H., Qiu X., Wang J.H. Tryptophan metabolism activates aryl hydrocarbon receptor-mediated pathway to promote HIV-1 infection and reactivation. mBio. 2019;10 doi: 10.1128/mBio.02591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berg G., Rybakova D., Fischer D., Cernava T., Vergès M.-C.C., Charles T., Chen X., Cocolin L., Eversole K., Corral G.H., Kazou M., Kinkel L., Lange L., Lima N., Loy A., Macklin J.A., Maguin E., Mauchline T., McClure R., Mitter B., Ryan M., Sarand I., Smidt H., Schelkle B., Roume H., Kiran G.S., Selvin J., de Souza R.S.C., van Overbeek L., Singh B.K., Wagner M., Walsh A., Sessitsch A., Schloter M. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly D., Campbell J.I., King T.P., Grant G., Jansson E.A., Coutts A.G., Pettersson S., Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat. Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 80.Rawls J.F., Samuel B.S., Gordon J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bates J.M., Mittge E., Kuhlman J., Baden K.N., Cheesman S.E., Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 2006;297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Naito T., Mulet C., de Castro C., Molinaro A., Saffarian A., Nigro G., Berard M., Clerc M., Pedersen A.B., Sansonetti P.J., Pedron T. Lipopolysaccharide from crypt-specific core microbiota modulates the colonic epithelial proliferation-to-differentiation balance. mBio. 2017;8 doi: 10.1128/mBio.01680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dougherty M.W., Kudin O., Muhlbauer M., Neu J., Gharaibeh R.Z., Jobin C. Gut microbiota maturation during early human life induces enterocyte proliferation via microbial metabolites. BMC Microbiol. 2020;20:205. doi: 10.1186/s12866-020-01892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uribe A., Alam M., Midtvedt T., Smedfors B., Theodorsson E. Endogenous prostaglandins and microflora modulate DNA synthesis and neuroendocrine peptides in the rat gastrointestinal tract. Scand. J. Gastroenterol. 1997;32:691–699. doi: 10.3109/00365529708996520. [DOI] [PubMed] [Google Scholar]
- 85.Husebye E., Hellstrom P.M., Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig. Dis. Sci. 1994;39:946–956. doi: 10.1007/BF02087542. [DOI] [PubMed] [Google Scholar]
- 86.Murray I.A., Nichols R.G., Zhang L., Patterson A.D., Perdew G.H. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice. Sci. Rep. 2016;6 doi: 10.1038/srep33969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korecka A., Dona A., Lahiri S., Tett A.J., Al-Asmakh M., Braniste V., D'Arienzo R., Abbaspour A., Reichardt N., Fujii-Kuriyama Y., Rafter J., Narbad A., Holmes E., Nicholson J., Arulampalam V., Pettersson S. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes. 2016;2 doi: 10.1038/npjbiofilms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alvarado D.M., Chen B., Iticovici M., Thaker A.I., Dai N., VanDussen K.L., Shaikh N., Lim C.K., Guillemin G.J., Tarr P.I., Ciorba M.A. Epithelial indoleamine 2,3-dioxygenase 1 modulates aryl hydrocarbon receptor and notch signaling to increase differentiation of secretory cells and alter mucus-associated microbiota. Gastroenterology. 2019;157:1093–1108.e11. doi: 10.1053/j.gastro.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamas B., Natividad J.M., Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11:1024–1038. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 90.Monteleone I., Rizzo A., Sarra M., Sica G., Sileri P., Biancone L., MacDonald T.T., Pallone F., Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. doi: 10.1053/j.gastro.2011.04.007. 248 e1. [DOI] [PubMed] [Google Scholar]
- 91.Aoki R., Aoki-Yoshida A., Suzuki C., Takayama Y. Indole-3-Pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J. Immunol. 2018;201:3683–3693. doi: 10.4049/jimmunol.1701734. [DOI] [PubMed] [Google Scholar]
- 92.Cervantes-Barragan L., Chai J.N., Tianero M.D., di Luccia B., Ahern P.P., Merriman J., Cortez V.S., Caparon M.G., Donia M.S., Gilfillan S., Cella M., Gordon J.I., Hsieh C.S., Colonna M. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 94.Schuijers J., Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Metidji A., Omenetti S., Crotta S., Li Y., Nye E., Ross E., Li V., Maradana M.R., Schiering C., Stockinger B. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2018;49:353–362 e5. doi: 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y., Innocentin S., Withers D.R., Roberts N.A., Gallagher A.R., Grigorieva E.F., Wilhelm C., Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 97.Chng S.H., Kundu P., Dominguez-Brauer C., Teo W.L., Kawajiri K., Fujii-Kuriyama Y., Mak T.W., Pettersson S. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Sci. Rep. 2016;6 doi: 10.1038/srep23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han H., Davidson L.A., Fan Y.Y., Landrock K.K., Jayaraman A., Safe S.H., Chapkin R.S. Loss of aryl hydrocarbon receptor suppresses the response of colonic epithelial cells to IL22 signaling by upregulating SOCS3. Am. J. Physiol. Gastrointest. Liver Physiol. 2022;322:G93–G106. doi: 10.1152/ajpgi.00074.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rannug U., Rannug A., Sjöberg U., Li H., Westerholm R., Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem. Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-X. [DOI] [PubMed] [Google Scholar]
- 100.Yu M., Wang Q., Ma Y., Li L., Yu K., Zhang Z., Chen G., Li X., Xiao W., Xu P., Yang H. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int. J. Biol. Sci. 2018;14:69–77. doi: 10.7150/ijbs.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.v., Hegde B., Kotla N.G., Hiwale A.A., Saiyed T., Patel P., Vijay-Kumar M., Langille M.G.I., Douglas G.M., Cheng X., Rouchka E.C., Waigel S.J., Dryden G.W., Alatassi H., Zhang H.-G., Haribabu B., Vemula P.K., Jala V.R. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019;10:89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Junt T., Scandella E., Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat. Rev. Immunol. 2008;8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- 104.Kiss E.A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. 1979. [DOI] [PubMed] [Google Scholar]
- 105.Qiu J., Heller J.J., Guo X., Chen Z.M., Fish K., Fu Y.X., Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schiering C., Wincent E., Metidji A., Iseppon A., Li Y., Potocnik A.J., Omenetti S., Henderson C.J., Wolf C.R., Nebert D.W., Stockinger B. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu J., Zhang J., Ji Y., Sun H., Gu Z., Sun Q., Bai M., Gong J., Tang J., Zhang Y., Li S., Shao Z., Li J., Sheng H., Shen L., Qiu J. Tissue signals imprint Aiolos expression in ILC2s to modulate type 2 immunity. Mucosal Immunol. 2021;14:1306–1322. doi: 10.1038/s41385-021-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brandstatter O., Schanz O., Vorac J., Konig J., Mori T., Maruyama T., Korkowski M., Haarmann-Stemmann T., von Smolinski D., Schultze J.L., Abel J., Esser C., Takeyama H., Weighardt H., Forster I. Balancing intestinal and systemic inflammation through cell type-specific expression of the aryl hydrocarbon receptor repressor. Sci. Rep. 2016;6 doi: 10.1038/srep26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chinen I., Nakahama T., Kimura A., Nguyen N.T., Takemori H., Kumagai A., Kayama H., Takeda K., Lee S., Hanieh H., Ripley B., Millrine D., Dubey P.K., Nyati K.K., Fujii-Kuriyama Y., Chowdhury K., Kishimoto T. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int. Immunol. 2015;27:405–415. doi: 10.1093/intimm/dxv015. [DOI] [PubMed] [Google Scholar]
- 110.McMillan B.J., Bradfield C.A. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol. Pharmacol. 2007;72:487–498. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 111.Vyhlídalová B., Krasulová K., Pečinková P., Marcalíková A., Vrzal R., Zemánková L., Vančo J., Trávníček Z., Vondráček J., Karasová M., Mani S., Dvořák Z. Gut microbial catabolites of tryptophan are ligands and agonists of the aryl hydrocarbon receptor: a detailed characterization. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong F., Hao F., Murray I.A., Smith P.B., Koo I., Tindall A.M., Kris-Etherton P.M., Gowda K., Amin S.G., Patterson A.D., Perdew G.H. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microb. 2020;12:1–24. doi: 10.1080/19490976.2020.1788899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wirthgen E., Hoeflich A., Rebl A., Günther J. Kynurenic Acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018;8 doi: 10.3389/fimmu.2017.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marszalek-Grabska M., Walczak K., Gawel K., Wicha-Komsta K., Wnorowska S., Wnorowski A., Turski W.A. Kynurenine emerges from the shadows – current knowledge on its fate and function. Pharmacol. Ther. 2021;225 doi: 10.1016/j.pharmthera.2021.107845. [DOI] [PubMed] [Google Scholar]
- 116.Lowe M.M., Mold J.E., Kanwar B., Huang Y., Louie A., Pollastri M.P., Wang C., Patel G., Franks D.G., Schlezinger J., Sherr D.H., Silverstone A.E., Hahn M.E., McCune J.M. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henry E.C., Welle S.L., Gasiewicz T.A. TCDD and a putative endogenous AhR ligand, ITE, elicit the same immediate changes in gene expression in mouse lung fibroblasts. Toxicol. Sci. 2010;114:90–100. doi: 10.1093/toxsci/kfp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bittinger M.A., Nguyen L.P., Bradfield C.A. 2003. Aspartate Aminotransferase Generates Proagonists of the Aryl Hydrocarbon Receptor.http://molpharm.aspetjournals.org [DOI] [PubMed] [Google Scholar]
- 119.Adachi J., Mori Y., Matsui S., Takigami H., Fujino J., Kitagawa H., Miller C.A., Kato T., Saeki K., Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 2001;276:31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- 120.Guengerich F.P., Martin M.v., McCormick W.A., Nguyen L.P., Glover E., Bradfield C.A. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch. Biochem. Biophys. 2004;423:309–316. doi: 10.1016/j.abb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 121.Sugihara K., Kitamura S., Yamada T., Okayama T., Ohta S., Yamashita K., Yasuda M., Fujii-Kuriyama Y., Saeki K., Matsui S., Matsuda T. Aryl hydrocarbon receptor-mediated induction of microsomal drug-metabolizing enzyme activity by indirubin and indigo. Biochem. Biophys. Res. Commun. 2004;318:571–578. doi: 10.1016/j.bbrc.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka Y., Uchi H., Hashimoto-Hachiya A., Furue M. Tryptophan photoproduct FICZ upregulates IL1A, IL1B, and IL6 expression via oxidative stress in keratinocytes. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/9298052. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rannug A. How the AHR became important in intestinal homeostasis—a diurnal FICZ/AHR/CYP1A1 feedback controls both immunity and immunopathology. Int. J. Mol. Sci. 2020;21:1–19. doi: 10.3390/IJMS21165681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smirnova A., Wincent E., Vikström Bergander L., Alsberg T., Bergman J., Rannug A., Rannug U. Evidence for new light-independent pathways for generation of the endogenous aryl hydrocarbon receptor agonist FICZ. Chem. Res. Toxicol. 2016;29:75–86. doi: 10.1021/acs.chemrestox.5b00416. [DOI] [PubMed] [Google Scholar]
- 125.Wang J., Wang P., Tian H., Tian F., Zhang Y., Zhang L., Gao X., Wang X. Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immun. 2018;24:297–306. doi: 10.1177/1753425918785016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schroeder J.C., DiNatale B.C., Murray I.A., Flaveny C.A., Liu Q., Laurenzana E.M., Lin J.M., Strom S.C., Omiecinski C.J., Amin S., Perdew G.H. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49:393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miller C.A. 1997. Expression of the Human Aryl Hydrocarbon Receptor Complex in Yeast ACTIVATION of TRANSCRIPTION by INDOLE COMPOUNDS*.http://www.jbc.org/ [DOI] [PubMed] [Google Scholar]
- 128.Hubbard T.D., Murray I.A., Perdew G.H. Special section on drug metabolism and the microbiome - minireview indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metabol. Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hubbard T.D., Murray I.A., Bisson W.H., Lahoti T.S., Gowda K., Amin S.G., Patterson A.D., Perdew G.H. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 2015;5 doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bjeldanes L.F., Kim J.-Y., Grose K.R., Bartholomew4 J.C., Bradfield C.A. 1991. Aromatic Hydrocarbon Responsiveness-Receptor Agonists Generated from Indole-3-Carbinol in Vitro and in Vivo: Comparisons with 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (anticarcinogen/Brassica oleracea/indolo[3,2-b4carbazole) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei Y.L., Chen Y.Q., Gong H., Li N., Wu K.Q., Hu W., Wang B., Liu K.J., Wen L.Z., Xiao X., Chen D.F. Fecal microbiota transplantation ameliorates experimentally induced colitis in mice by upregulating AhR. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park H., Jin U.H., Karki K., Jayaraman A., Allred C., Michelhaugh S.K., Mittal S., Chapkin R.S., Safe S. Dopamine is an aryl hydrocarbon receptor agonist. Biochem. J. 2020;477:3899–3910. doi: 10.1042/BCJ20200440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leibundgut-Landmann S., Sellam A., Lee S.C., Richard M.L., Spatz M. Overview of the potential role of malassezia in gut health and disease. Front. Cell. Infect. Microbiol. 2020;10:201. doi: 10.3389/fcimb.2020.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krämer H.-J., Podobinska M., Bartsch A., Battmann A., Thoma W., Bernd A., Kummer W., Irlinger B., Steglich W., Mayser P. Malassezin, a novel agonist of the aryl hydrocarbon receptor from the yeast malassezia furfur, induces apoptosis in primary human melanocytes. Chembiochem. 2005;6:860–865. doi: 10.1002/cbic.200400247. [DOI] [PubMed] [Google Scholar]
- 135.Gaitanis G., Magiatis P., Stathopoulou K., Bassukas I.D., Alexopoulos E.C., Velegraki A., Skaltsounis A.L. AhR ligands, malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J. Invest. Dermatol. 2008;128:1620–1625. doi: 10.1038/SJ.JID.5701252. [DOI] [PubMed] [Google Scholar]
- 136.Schrenk D., Riebniger2 D., Ti1l2 M., Vetter S., Fiedler H.-P., Schrenk D. 1999. 51 TRYPTANTHRINS AND OTHER TRYPTOPHAN-DERIVED AGONISTS OF THE DIOXIN RECEPTOR. [DOI] [PubMed] [Google Scholar]
- 137.Weems J.M., Yost G.S. 3-methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem. Res. Toxicol. 2010;23:696–704. doi: 10.1021/tx9004506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vlachos C., Schulte B.M., Magiatis P., Adema G.J., Gaitanis G. Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit Toll-like receptor-induced maturation in monocyte-derived dendritic cells. Br. J. Dermatol. 2012;167:496–505. doi: 10.1111/j.1365-2133.2012.11014.x. [DOI] [PubMed] [Google Scholar]
- 139.Hubbard T.D., Liu Q., Murray I.A., Dong F., Miller C., Smith P.B., Gowda K., Lin J.M., Amin S., Patterson A.D., Perdew G.H. Microbiota metabolism promotes synthesis of the human ah receptor agonist 2,8-dihydroxyquinoline. J. Proteome Res. 2019;18:1715–1724. doi: 10.1021/acs.jproteome.8b00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Puccetti M., Pariano M., Borghi M., Barola C., Moretti S., Galarini R., Mosci P., Ricci M., Costantini C., Giovagnoli S. Enteric formulated indole-3-carboxaldehyde targets the aryl hydrocarbon receptor for protection in a murine model of metabolic syndrome. Int. J. Pharm. 2021;602 doi: 10.1016/J.IJPHARM.2021.120610. [DOI] [PubMed] [Google Scholar]
- 141.Sinal C.J., Bend J.R. Aryl hydrocarbon receptor-dependent induction of Cyp1a1 by bilirubin in mouse hepatoma Hepa 1c1c7 cells. Mol. Pharmacol. 1997;52:590–599. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 142.Phelan D., Winter G.M., Rogers W.J., Lam J.C., Denison M.S. 1998. Activation of the Ah Receptor Signal Transduction Pathway by Bilirubin and Biliverdin. [DOI] [PubMed] [Google Scholar]
- 143.Schaldach C.M., Riby J., Bjeldanes L.F. Lipoxin A4: a new class of ligand for the ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/BI982861E. [DOI] [PubMed] [Google Scholar]
- 144.Seidel S.D., Winters G.M., Rogers W.J., Ziccardi M.H., Li V., Keser B., Denison M.S. Activation of the Ah receptor signaling pathway by prostaglandins. J. Biochem. Mol. Toxicol. 2001;15:187–196. doi: 10.1002/JBT.16. [DOI] [PubMed] [Google Scholar]
- 145.Cheng Y., Jin U.-H., Davidson L.A., Chapkin R.S., Jayaraman A., Tamamis P., Orr A., Allred C., Denison M.S., Soshilov A., Weaver E., Safe S., Highlight Editor’s. Microbial-derived 1,4-Dihydroxy-2-naphthoic acid and related compounds as aryl hydrocarbon receptor agonists/antagonists: structure–activity relationships and receptor modeling. Toxicol. Sci. 2017;155:458–473. doi: 10.1093/toxsci/kfw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fukumoto S., Toshimitsu T., Matsuoka S., Maruyama A., Oh‐oka K., Takamura T., Nakamura Y., Ishimaru K., Fujii‐Kuriyama Y., Ikegami S., Itou H., Nakao A. Identification of a probiotic bacteria‐derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 2014;92:460–465. doi: 10.1038/icb.2014.2. [DOI] [PubMed] [Google Scholar]
- 147.Y. Wan, H. Liu, M. Xian, W. Huang, Biosynthesis and metabolic engineering of 1-hydroxyphenazine in Pseudomonas chlororaphis H18, (n.d.). 10.1186/s12934-021-01731-y. [DOI] [PMC free article] [PubMed]
- 148.Smith S.H., Jayawickreme C., Rickard D.J., Nicodeme E., Bui T., Simmons C., Coquery C.M., Neil J., Pryor W.M., Mayhew D., Rajpal D.K., Creech K., Furst S., Lee J., Wu D., Rastinejad F., Willson T.M., Viviani F., Morris D.C., Moore J.T., Cote-Sierra J. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Invest. Dermatol. 2017;137:2110–2119. doi: 10.1016/j.jid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 149.Manzella C.R., Ackerman M., Singhal M., Ticho A.L., Ceh J., Alrefai W.A., Saksena S., Dudeja P.K., Gill R.K. Serotonin modulates AhR activation by interfering with CYP1A1-mediated clearance of AhR ligands. Cell. Physiol. Biochem. 2020;54:126–141. doi: 10.33594/000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mohammadi-Bardbori A., Bengtsson J., Rannug U., Rannug A., Wincent E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR) Chem. Res. Toxicol. 2012;25:1878–1884. doi: 10.1021/tx300169e. [DOI] [PubMed] [Google Scholar]
- 151.Schiering C., Vonk A., Das S., Stockinger B., Wincent E. Cytochrome P4501-inhibiting chemicals amplify aryl hydrocarbon receptor activation and IL-22 production in T helper 17 cells. Biochem. Pharmacol. 2018;151:47–58. doi: 10.1016/j.bcp.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 152.Murray I.A., Patterson A.D., Perdew G.H. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Platten M., von Knebel Doeberitz N., Oezen I., Wick W., Ochs K. Cancer immunotherapy by targeting Ido1/TDO and their downstream effectors. Front. Immunol. 2015;5 doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Esser C., Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 155.Roussel L., LaFayette S., Nguyen D., Baglole C.J., Rousseau S. Differential contribution of the aryl-hydrocarbon receptor and toll-like receptor pathways to IL-8 expression in normal and cystic fibrosis airway epithelial cells exposed to Pseudomonas aeruginosa. Front. Cell Dev. Biol. 2016;4 doi: 10.3389/fcell.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Visekruna A., Luu M. The role of short-chain fatty acids and bile acids in intestinal and liver function, inflammation, and carcinogenesis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.703218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin U.H., Cheng Y., Park H., Davidson L.A., Callaway E.S., Chapkin R.S., Jayaraman A., Asante A., Allred C., Weaver E.A., Safe S. Short chain fatty acids enhance aryl hydrocarbon (ah) responsiveness in mouse colonocytes and caco-2 human colon cancer cells. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-10824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scott S.A., Fu J., Chang P.v. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang E.Y., Devkota S., Moscoso D., Chang E.B., Leone V.A. The role of diet in triggering human inflammatory disorders in the modern age. Microb. Infect. 2013;15:765–774. doi: 10.1016/j.micinf.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 160.Wu M., Luo Q., Nie R., Yang X., Tang Z., Chen H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021;61:2175–2193. doi: 10.1080/10408398.2020.1773390. [DOI] [PubMed] [Google Scholar]
- 161.Machado A.P. da F., Geraldi M.V., do Nascimento R. de P., Moya A.M.T.M., Vezza T., Diez-Echave P., Gálvez J.J., Cazarin C.B.B., Maróstica Júnior M.R. Polyphenols from food by-products: an alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.110018. [DOI] [PubMed] [Google Scholar]
- 162.Zhang W., Qi S., Xue X., al Naggar Y., Wu L., Wang K. Understanding the gastrointestinal protective effects of polyphenols using foodomics-based approaches. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.671150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marchesi J.R., Adams D.H., Fava F., Hermes G.D.A., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., v Thomas L., Zoetendal E.G., Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu F., Li D., Wang X., Cui Y., Li X. Polyphenols intervention is an effective strategy to ameliorate inflammatory bowel disease: a systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2021;72:14–25. doi: 10.1080/09637486.2020.1760220. [DOI] [PubMed] [Google Scholar]
- 165.Scaioli E., Belluzzi A., Ricciardiello L., del Rio D., Rotondo E., Mena P., Derlindati E., Danesi F. Pomegranate juice to reduce fecal calprotectin levels in inflammatory bowel disease patients with a high risk of clinical relapse: study protocol for a randomized controlled trial. Trials. 2019;20:327. doi: 10.1186/s13063-019-3321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cusimano F.A., Damas O.M. Diet as a treatment for inflammatory bowel disease: is it ready for prime time? Curr. Opin. Gastroenterol. 2022;38:358–372. doi: 10.1097/MOG.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Obih C., Wahbeh G., Lee D., Braly K., Giefer M., Shaffer M.L., Nielson H., Suskind D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016;32:418–425. doi: 10.1016/j.nut.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 168.Suskind D.L., Lee D., Kim Y.-M., Wahbeh G., Singh N., Braly K., Nuding M., Nicora C.D., Purvine S.O., Lipton M.S., Jansson J.K., Nelson W.C. The specific carbohydrate diet and diet modification as induction therapy for pediatric Crohn's disease: a randomized diet controlled trial. Nutrients. 2020;12:3749. doi: 10.3390/nu12123749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lo C.-H., Khalili H., Song M., Lochhead P., Burke K.E., Richter J.M., Giovannucci E.L., Chan A.T., Ananthakrishnan A.N. Healthy lifestyle is associated with reduced mortality in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2021;19:87–95.e4. doi: 10.1016/j.cgh.2020.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strisciuglio C., Cenni S., Serra M.R., Dolce P., Martinelli M., Staiano A., Miele E. Effectiveness of mediterranean diet's adherence in children with inflammatory bowel diseases. Nutrients. 2020;12:3206. doi: 10.3390/nu12103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Turpin W., Dong M., Sasson G., Raygoza Garay J.A., Espin-Garcia O., Lee S.-H., Neustaeter A., Smith M.I., Leibovitzh H., Guttman D.S., Goethel A., Griffiths A.M., Huynh H.Q., Dieleman L.A., Panaccione R., Steinhart A.H., Silverberg M.S., Aumais G., Jacobson K., Mack D., Murthy S.K., Marshall J.K., Bernstein C.N., Abreu M.T., Moayyedi P., Paterson A.D., Xu W., Croitoru K., Abreu M., Beck P., Bernstein C., Croitoru K., Dieleman L., Feagan B., Griffiths A., Guttman D., Jacobson K., Kaplan G., Krause D.O., Madsen K., Marshall J., Moayyedi P., Ropeleski M., Seidman E., Silverberg M., Snapper S., Stadnyk A., Steinhart H., Surette M., Turner D., Walters T., Vallance B., Aumais G., Bitton A., Cino M., Critch J., Denson L., Deslandres C., El-Matary W., Herfarth H., Higgins P., Huynh H., Hyams J., Mack D., McGrath J., Otley A., Panancionne R., Aumais G., Baldassano R., Bernstein C., Denson L., Deslandres C., El-Matary W., Griffiths A.M., Hedin C., Herfarth H., Higgins P., Hussey S., Hyams H., Jacobson K., Keljo D., Kevans D., Lees C., Mack D., Marshall J., McGrath J., Murthy S., Otley A., Panaccione R., Parekh N., Plamondon S., Radford-Smith G., Ropeleski M., Rosh J., Rubin D., Schultz M., Seidman E., Siegel C., Snapper S., Steinhart H., Turner D. Mediterranean-like dietary pattern associations with gut microbiome composition and subclinical gastrointestinal inflammation. Gastroenterology. 2022;163:685–698. doi: 10.1053/j.gastro.2022.05.037. [DOI] [PubMed] [Google Scholar]
- 172.Finicelli M., di Salle A., Galderisi U., Peluso G. The mediterranean diet: an update of the clinical trials. Nutrients. 2022;14:2956. doi: 10.3390/nu14142956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 175.Williamson G., Kay C.D., Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018;17:1054–1112. doi: 10.1111/1541-4337.12351. [DOI] [PubMed] [Google Scholar]
- 176.Carecho R., Carregosa D., dos Santos C.N. Low molecular weight (poly)Phenol metabolites across the blood-brain barrier: the underexplored journey. Brain Plast. 2020;6:193–214. doi: 10.3233/bpl-200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Carregosa D., Carecho R., Figueira I., Santos C.N. Low-molecular weight metabolites from polyphenols as effectors for attenuating neuroinflammation. J. Agric. Food Chem. 2020:1790–1807. doi: 10.1021/acs.jafc.9b02155. [DOI] [PubMed] [Google Scholar]
- 178.V. Neveu, J. Perez-Jimé Nez, F. Vos, V. Crespy, L. du Chaffaut, L. Mennen, C. Knox, R. Eisner, J. Cruz, D. Wishart, A. Scalbert, Phenol-Explorer: an online comprehensive database on polyphenol contents in foods, (n.d.). 10.1093/database/bap024. [DOI] [PMC free article] [PubMed]
- 179.Mahabir S. Methodological challenges conducting epidemiological research on nutraceuticals in health and disease. PharmaNutrition. 2014;2:120–125. doi: 10.1016/j.phanu.2013.06.002. [DOI] [Google Scholar]
- 180.González-Sarrías A., Espín J.C., Tomás-Barberán F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017;69:281–288. doi: 10.1016/j.tifs.2017.07.010. [DOI] [Google Scholar]
- 181.Carregosa D., Pinto C., Ávila‐Gálvez M.Á., Bastos P., Berry D., Santos C.N. A look beyond dietary (poly)phenols: the low molecular weight phenolic metabolites and their concentrations in human circulation. Compr. Rev. Food Sci. Food Saf. 2022;21:3931–3962. doi: 10.1111/1541-4337.13006. [DOI] [PubMed] [Google Scholar]
- 182.Lavefve L., Howard L.R., Carbonero F. Food Funct. Royal Society of Chemistry; 2020. Berry polyphenols metabolism and impact on human gut microbiota and health; pp. 45–65. [DOI] [PubMed] [Google Scholar]
- 183.Montrose D.C., Horelik N.A., Madigan J.P., Stoner G.D., Wang L.-S., Bruno R.S., Park H.J., Giardina C., Rosenberg D.W. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis. 2011;32:343–350. doi: 10.1093/carcin/bgq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pervin M., Hasnat MdA., Lim J.-H., Lee Y.-M., Kim E.O., Um B.-H., Lim B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016;28:103–113. doi: 10.1016/j.jnutbio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 185.Osman N., Adawi D., Ahrné S., Jeppsson B., Molin G. Probiotics and blueberry attenuate the severity of dextran sulfate sodium (DSS)-Induced colitis. Dig. Dis. Sci. 2008;53:2464–2473. doi: 10.1007/s10620-007-0174-x. [DOI] [PubMed] [Google Scholar]
- 186.Wu L.-H., Xu Z.-L., Dong D., He S.-A., Yu H. Protective effect of anthocyanins extract from blueberry on TNBS-induced IBD model of mice. Evid. base Compl. Alternative Med. 2011:1–8. doi: 10.1093/ecam/neq040. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Piberger H., Oehme A., Hofmann C., Dreiseitel A., Sand P.G., Obermeier F., Schoelmerich J., Schreier P., Krammer G., Rogler G. Bilberries and their anthocyanins ameliorate experimental colitis. Mol. Nutr. Food Res. 2011;55:1724–1729. doi: 10.1002/mnfr.201100380. [DOI] [PubMed] [Google Scholar]
- 188.Biedermann L., Mwinyi J., Scharl M., Frei P., Zeitz J., Kullak-Ublick G.A., Vavricka S.R., Fried M., Weber A., Humpf H.-U., Peschke S., Jetter A., Krammer G., Rogler G. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis — an open pilot study. J Crohns Colitis. 2013;7:271–279. doi: 10.1016/j.crohns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 189.Sugimoto K., Hanai H., Tozawa K., Aoshi T., Uchijima M., Nagata T., Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid–induced colitis in mice. Gastroenterology. 2002;123:1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 190.Yang J., Zhong X., Kim S.-J., Kim D.-H., Kim H.S., Lee J.-S., Yum H.-W., Lee J., Na H.-K., Surh Y.-J. Comparative effects of curcumin and tetrahydrocurcumin on dextran sulfate sodium-induced colitis and inflammatory signaling in mice. J Cancer Prev. 2018;23:18–24. doi: 10.15430/JCP.2018.23.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pan M.-H., Lin-Shiau S.-Y., Lin J.-K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem. Pharmacol. 2000;60:1665–1676. doi: 10.1016/S0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 192.Wang J., Ghosh S.S., Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017;312:C438–C445. doi: 10.1152/ajpcell.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lai C.-S., Wu J.-C., Yu S.-F., Badmaev V., Nagabhushanam K., Ho C.-T., Pan M.-H. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol. Nutr. Food Res. 2011;55:1819–1828. doi: 10.1002/mnfr.201100290. [DOI] [PubMed] [Google Scholar]
- 194.Zhao Z., Shin H.S., Satsu H., Totsuka M., Shimizu M. 5-Caffeoylquinic acid and caffeic acid down-regulate the oxidative stress- and TNF-α-induced secretion of interleukin-8 from caco-2 cells. J. Agric. Food Chem. 2008;56:3863–3868. doi: 10.1021/jf073168d. [DOI] [PubMed] [Google Scholar]
- 195.Shimizu M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017;25:93–99. doi: 10.1016/j.jfda.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Roth S., Spalinger M.R., Gottier C., Biedermann L., Zeitz J., Lang S., Weber A., Rogler G., Scharl M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Céspedes-Acuña C.L., Xiao J., Wei Z.-J., Chen L., Bastias J.M., Avila J.G., Alarcon-Enos J., Werner-Navarrete E., Kubo I. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. J. Berry Res. 2018;8:275–296. doi: 10.3233/JBR-180356. [DOI] [Google Scholar]
- 198.Medda R., Lyros O., Schmidt J.L., Jovanovic N., Nie L., Link B.J., Otterson M.F., Stoner G.D., Shaker R., Rafiee P. Anti inflammatory and anti angiogenic effect of black raspberry extract on human esophageal and intestinal microvascular endothelial cells. Microvasc. Res. 2015;97:167–180. doi: 10.1016/j.mvr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lu Y., Zamora-Ros R., Chan S., Cross A.J., Ward H., Jakszyn P., Luben R., Opstelten J.L., Oldenburg B., Hallmans G., Karling P., Grip O., Key T., Bergmann M.M., Boeing H., Overvad K., Palli D., Masala G., Khaw K.T., Racine A., Carbonnel F., Boutron-Ruault M.C., Andersen V., Olsen A., Tjonneland A., Kaaks R., Tumino R., Trichopoulou A., Scalbert A., Riboli E., Hart A.R. Dietary polyphenols in the aetiology of Crohn's disease and ulcerative colitis - a multicenter European prospective cohort study (EPIC) Inflamm. Bowel Dis. 2016;23:2072–2082. doi: 10.1097/MIB.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 200.Mutha R.E., Tatiya A.U., Surana S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur. J. Pharm. Sci. 2021;7:25. doi: 10.1186/s43094-020-00161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Day A.J., Cañada F.J., Díaz J.C., Kroon P.A., Mclauchlan R., Faulds C.B., Plumb G.W., Morgan M.R.A., Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/S0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 202.Németh K., Plumb G.W., Berrin J.-G., Juge N., Jacob R., Naim H.Y., Williamson G., Swallow D.M., Kroon P.A. Deglycosylation by small intestinal epithelial cell ?-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 203.Espín J.C., González-Sarrías A., Tomás-Barberán F.A. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 204.Mullen W., Rouanet J.-M., Auger C., Teissèdre P.-L., Caldwell S.T., Hartley R.C., Lean M.E.J., Edwards C.A., Crozier A. Bioavailability of [2- 14 C]Quercetin-4′-glucoside in rats. J. Agric. Food Chem. 2008;56:12127–12137. doi: 10.1021/jf802754s. [DOI] [PubMed] [Google Scholar]
- 205.Rechner A.R., Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005;116:327–334. doi: 10.1016/j.thromres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 206.Cui Q., Pan Y., Xu X., Zhang W., Wu X., Qu S., Liu X. The metabolic profile of acteoside produced by human or rat intestinal bacteria or intestinal enzyme in vitro employed UPLC-Q-TOF–MS. Fitoterapia. 2016;109:67–74. doi: 10.1016/j.fitote.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 207.Mena P., Bresciani L., Brindani N., Ludwig I.A., Pereira-caro G., Angelino D., Llorach R., Calani L., Brighenti F., Cli N., Gill C.I.R., Crozier A., Curti C., Del D. 2018. Natural Product Reports. [DOI] [PubMed] [Google Scholar]
- 208.Muku G., Murray I., Espín J., Perdew G., Urolithin A is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. Metabolites. 2018;8:86. doi: 10.3390/metabo8040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Espín J.C., Larrosa M., García-Conesa M.T., Tomás-Barberán F. 2013. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: the Evidence So Far, Evidence-Based Complementary and Alternative Medicine; pp. 1–15. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rothwell J.A., Perez-Jimenez J., Neveu V., Medina-Remon A., M'Hiri N., Garcia-Lobato P., Manach C., Knox C., Eisner R., Wishart D.S., Scalbert A. 2013. Phenol-Explorer 3.0: a Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content, Database. 2013. bat070–bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stevens J.F., Maier C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochemistry Rev. 2016;15:425–444. doi: 10.1007/s11101-016-9459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Iglesias-Aguirre C.E., Cortés-Martín A., Ávila-Gálvez M.Á., Giménez-Bastida J.A., Selma M.v., González-Sarrías A., Espín J.C. Main drivers of (poly)phenol effects on human health: metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021;12:10324–10355. doi: 10.1039/D1FO02033A. [DOI] [PubMed] [Google Scholar]
- 213.Han X., Shen T., Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007;8:950–988. http://www.mdpi.org/ijms [Google Scholar]
- 214.Koppel N., Maini Rekdal V., Balskus E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017:356. doi: 10.1126/science.aag2770. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lindell A.E., Zimmermann-Kogadeeva M., Patil K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022;20:431–443. doi: 10.1038/s41579-022-00681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Clarke G., Sandhu K.v., Griffin B.T., Dinan T.G., Cryan J.F., Hyland N.P. Gut reactions: breaking down xenobiotic–microbiome interactions. Pharmacol. Rev. 2019;71:198–224. doi: 10.1124/pr.118.015768. [DOI] [PubMed] [Google Scholar]
- 217.Kamenickova A., Anzenbacherova E., Pavek P., Soshilov A.A., Denison M.S., Anzenbacher P., Dvorak Z. Pelargonidin activates the AhR and induces CYP1A1 in primary human hepatocytes and human cancer cell lines HepG2 and LS174T. Toxicol. Lett. 2013;218:253–259. doi: 10.1016/j.toxlet.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kamenickova A., Anzenbacherova E., Pavek P., Soshilov A.A., Denison M.S., Zapletalova M., Anzenbacher P., Dvorak Z. Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines. Toxicol. Lett. 2013;221:1–8. doi: 10.1016/j.toxlet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Palermo C.M., Hernando J.I.M., Dertinger S.D., Kende A.S., Gasiewicz T.A. Identification of potential aryl hydrocarbon receptor antagonists in green tea. Chem. Res. Toxicol. 2003;16:865–872. doi: 10.1021/tx025672c. [DOI] [PubMed] [Google Scholar]
- 220.Yin Z., Henry E.C., Gasiewicz T.A. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry. 2009;48:336–345. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Palermo C.M., Westlake C.A., Gasiewicz T.A. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44:5041–5052. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- 222.Fukuda I., Mukai R., Kawase M., Yoshida K., Ashida H. Interaction between the aryl hydrocarbon receptor and its antagonists, flavonoids. Biochem. Biophys. Res. Commun. 2007;359:822–827. doi: 10.1016/j.bbrc.2007.05.199. [DOI] [PubMed] [Google Scholar]
- 223.Flaveny C.A., Murray I.A., Chiaro C.R., Perdew G.H. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol. Pharmacol. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Choi Y.J., Arzuaga X., Kluemper C.T., Caraballo A., Toborek M., Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ. Int. 2010;36:931–934. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Potapovich A.I., Lulli D., Fidanza P., Kostyuk V.A., de Luca C., Pastore S., Korkina L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR–ERK pathway. Toxicol. Appl. Pharmacol. 2011;255:138–149. doi: 10.1016/j.taap.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 226.Sekaran S., Kandaswamy S., Gunasekaran K., Perumal E., Afsar Basha F.Y., Madhan Mohan B.J., Jagadeesan A. Protective role of quercetin on polychlorinated biphenyls (Aroclor-1254) induced oxidative stress and apoptosis in liver of adult male rats. J. Biochem. Mol. Toxicol. 2012;26:522–532. doi: 10.1002/jbt.21466. [DOI] [PubMed] [Google Scholar]
- 227.Perepechaeva M.L., Seredina T.A., Sidorova Y.A., Pivovarova E.N., Markel A.L., Lyakhovich V.v., Grishanova A.Y. Quercetin attenuates benzo(α)pyrene-induced CYP1A expression. Biomed. Environ. Sci. 2017;30:308–313. doi: 10.3967/bes2017.041. [DOI] [PubMed] [Google Scholar]
- 228.Dzoyem J.P., McGaw L.J., Kuete V., Bakowsky U. Medicinal Spices and Vegetables from Africa. Elsevier; 2017. Anti-inflammatory and anti-nociceptive activities of african medicinal spices and vegetables; pp. 239–270. [DOI] [Google Scholar]
- 229.Mizuno M., Nishitani Y. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease. Elsevier; 2013. Antioxidant, luteolin exhibits anti-inflammatory effect in in vitro gut inflammation model; pp. 227–234. [DOI] [Google Scholar]
- 230.Koper J.E.B., Loonen L.M.P., Wells J.M., Troise A.D., Capuano E., Fogliano V. Polyphenols and tryptophan metabolites activate the aryl hydrocarbon receptor in an in vitro model of colonic fermentation. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li Y., Wang X., Su Y., Wang Q., Huang S., Pan Z., Chen Y., Liang J., Zhang M., Xie X., Wu Z., Chen J., Zhou L., Luo X. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 2021 doi: 10.1038/s41401-021-00781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu A., Wang W., Fang H., Yang Y., Jiang X., Liu S., Hu J., Hu Q., Dahmen U., Dirsch O. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur. J. Pharmacol. 2015;748:45–53. doi: 10.1016/j.ejphar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 233.Liu C., Li Y., Chen Y., Huang S., Wang X., Luo S., Su Y., Zhou L., Luo X. Baicalein restores the balance of Th17/treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediat. Inflamm. 2020:1–19. doi: 10.1155/2020/5918587. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Amakura Yoshiaki, Tomoaki Tsutsumi, Nakamura Masafumi, Hiroko Kitagawa, Fujino Junko, Kumiko Sasaki, Masatake Toyoda, Takashi Yoshida, Tamio Maitani. Activation of the aryl hydrocarbon receptor by some VegetableConstituents determined using in VitroReporter gene assay. Biol. Pharm. Bull. 2003;26:532–539. doi: 10.1248/bpb.26.532. [DOI] [PubMed] [Google Scholar]
- 235.Amakura Yoshiaki, Tomoaki Tsutsumi, Kumiko Sasaki, Takashi Yoshida, Tamio Maitani. Screening of the inhibitory effect of vegetable constituents on the aryl hydrocarbon receptor-mediated activity induced by 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Biol. Pharm. Bull. 2003;26:1754–1760. doi: 10.1248/bpb.26.1754. [DOI] [PubMed] [Google Scholar]
- 236.Denison M.S., Nagy S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 237.Ciolino H.P., Yeh G.C. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol. Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- 238.Beedanagari S.R., Bebenek I., Bui P., Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol. Sci. 2009;110:61–67. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dvorak Z., Vrzal R., Henklova P., Jancova P., Anzenbacherova E., Maurel P., Svecova L., Pavek P., Ehrmann J., Havlik R., Bednar P., Lemr K., Ulrichova J. JNK inhibitor SP600125 is a partial agonist of human aryl hydrocarbon receptor and induces CYP1A1 and CYP1A2 genes in primary human hepatocytes. Biochem. Pharmacol. 2008;75:580–588. doi: 10.1016/j.bcp.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 240.Pastorková B., Vrzalová A., Bachleda P., Dvořák Z. Hydroxystilbenes and methoxystilbenes activate human aryl hydrocarbon receptor and induce CYP1A genes in human hepatoma cells and human hepatocytes. Food Chem. Toxicol. 2017;103:122–132. doi: 10.1016/j.fct.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 241.Tomás-Barberán F.A., González-Sarrías A., García-Villalba R., Núñez-Sánchez M.A., Selma M.v., García-Conesa M.T., Espín J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- 242.Kang M., Park S.-H., Oh S.W., Lee S.E., Yoo J.A., Nho Y.H., Lee S., Han B.S., Cho J.Y., Lee J. Anti-melanogenic effects of resorcinol are mediated by suppression of cAMP signaling and activation of p38 MAPK signaling. Biosci. Biotechnol. Biochem. 2018;82:1188–1196. doi: 10.1080/09168451.2018.1459176. [DOI] [PubMed] [Google Scholar]
- 243.Himejima M., Kubo I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991;39:418–421. doi: 10.1021/jf00002a039. [DOI] [Google Scholar]
- 244.Lee S.E., Kwon K., Oh S.W., Park S.J., Yu E., Kim H., Yang S., Park J.Y., Chung W.-J., Cho J.Y., Lee J. Mechanisms of resorcinol antagonism of benzo[a]pyrene-induced damage to human keratinocytes. Biomol Ther (Seoul) 2021;29:227–233. doi: 10.4062/biomolther.2020.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tsuji G., Takahara M., Uchi H., Takeuchi S., Mitoma C., Moroi Y., Furue M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011 doi: 10.1016/j.jdermsci.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 246.Hayes J.D., Dinkova-Kostova A.T., McMahon M. Cross-talk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicol. Sci. 2009;111:199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 247.Shin S., Wakabayashi N., Misra V., Biswal S., Lee G.H., Agoston E.S., Yamamoto M., Kensler T.W. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tsuji G., Takahara M., Uchi H., Matsuda T., Chiba T., Takeuchi S., Yasukawa F., Moroi Y., Furue M. Identification of ketoconazole as an AhR-nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J. Invest. Dermatol. 2012;132:59–68. doi: 10.1038/JID.2011.194. [DOI] [PubMed] [Google Scholar]
- 249.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005;280:20340–20348. doi: 10.1074/JBC.M412081200. [DOI] [PubMed] [Google Scholar]
- 250.Krüger T., Long M., Bonefeld-Jørgensen E.C. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology. 2008;246:112–123. doi: 10.1016/j.tox.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 251.Hanieh H., Ibrahim H.-I.M., Mohammed M., Alwassil O.I., Abukhalil M.H., Farhan M. Activation of aryl hydrocarbon receptor signaling by gallic acid suppresses progression of human breast cancer in vitro and in vivo. Phytomedicine. 2021 doi: 10.1016/j.phymed.2021.153817. [DOI] [PubMed] [Google Scholar]
- 252.Abdullah A., Maged M., Hairul-Islam M I., Osama I A., Maha H., Manal A., Hamza H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu Y., Kurita A., Nakashima S., Zhu B., Munemasa S., Nakamura T., Murata Y., Nakamura Y. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci. Biotechnol. Biochem. 2017;81:1978–1983. doi: 10.1080/09168451.2017.1361809. [DOI] [PubMed] [Google Scholar]
- 254.Shen P.-X., Li X., Deng S.-Y., Zhao L., Zhang Y.-Y., Deng X., Han B., Yu J., Li Y., Wang Z.-Z., Zhang Y. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine. 2021;64 doi: 10.1016/j.ebiom.2021.103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tang Y., Nakashima S., Saiki S., Myoi Y., Abe N., Kuwazuru S., Zhu B., Ashida H., Murata Y., Nakamura Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016;89:716–723. doi: 10.1016/j.foodres.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 256.Monagas M., Khan N., Andrés-Lacueva C., Urpí-Sardá M., Vázquez-Agell M., Lamuela-Raventós R.M., Estruch R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009;102:201–206. doi: 10.1017/S0007114508162110. [DOI] [PubMed] [Google Scholar]
- 257.Murota K., Nakamura Y., Uehara M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018;82:600–610. doi: 10.1080/09168451.2018.1444467. [DOI] [PubMed] [Google Scholar]
- 258.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 259.Larrosa M., García-Conesa M.T., Espín J.C., Tomás-Barberán F.A. Ellagitannins, ellagic acid and vascular health. Mol. Aspect. Med. 2010;31:513–539. doi: 10.1016/j.mam.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 260.Bobowska A., Granica S., Filipek A., Melzig M.F., Moeslinger Thomas, Zentek J., Kruk A., Jakub ·, Piwowarski P. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur. J. Nutr. 2021;60:1957–1972. doi: 10.1007/s00394-020-02386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gasaly N., de Vos P., Hermoso M.A. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nuñez-Sánchez M.A., García-Villalba R., Monedero-Saiz T., García-Talavera N.v., Gómez-Sánchez M.B., Sánchez-Álvarez C., García-Albert A.M., Rodríguez-Gil F.J., Ruiz-Marín M., Pastor-Quirante F.A., Martínez-Díaz F., Yáñez-Gascón M.J., González-Sarrías A., Tomás-Barberán F.A., Espín J.C. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014;58:1199–1211. doi: 10.1002/mnfr.201300931. [DOI] [PubMed] [Google Scholar]
- 263.di Pede G., Bresciani L., Brighenti F., Clifford M.N., Crozier A., del Rio D., Mena P. In vitro faecal fermentation of monomeric and oligomeric flavan‐3‐ols: catabolic pathways and stoichiometry. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tripathy D.R., Panda A., Dinda A.K., Dasgupta S. Positional preferences in flavonoids for inhibition of ribonuclease A: where “ <scp>OH</scp> ” where? Proteins: Struct., Funct., Bioinf. 2021;89:577–587. doi: 10.1002/prot.26043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.









